Abstract
Data on treatment and survival of patients with advanced unresectable esophageal squamous cell carcinoma (ESCC) from Western populations are limited. Here we describe treatment and survival in patients with advanced unresectable ESCC: patients with cT4b disease without metastases (cT4b), metastases limited to the supraclavicular lymph nodes (SCLNM) or distant metastatic ESCC at the population level. All patients with unresectable (cT4b) or synchronous metastatic ESCC at primary diagnosis (2015‐2018) or patients with metachronous metastases after primary non‐metastatic diagnosis in 2015‐2016 were selected from the Netherlands Cancer Registry. Fifteen percent of patients had cT4b disease (n = 146), 12% SCLNM (n = 118) and 72% distant metastases (n = 681). Median overall survival (OS) time was 6.3, 11.2, and 4.4 months in patients with cT4b, SCLNM, and distant metastases, respectively (P < .001). Multivariable Cox regression showed that patients with cT4b (hazard ratio 1.44, 95% CI 1.04‐1.99) and patients with distant metastases (hazard ratio 1.42, 95% CI 1.12‐1.80) had a worse survival time compared with patients with SCLNM. Among patients who received chemoradiotherapy and/or underwent resection (primary tumor and/or metastases), median OS was 11.9, 16.1, and 14.0 months in patients with cT4b, SCLNM, and distant metastases, respectively (P = .76). Patients with SCLNM had a better survival time compared with patients with cT4b and patients with distant metastases. Survival of patients with advanced unresectable ESCC in clinical practice was poor, even in patients treated with curative intent.
Keywords: distant metastases, esophageal squamous cell carcinoma, palliative treatment, supraclavicular lymph node metastases, unresectable advanced disease
Patients with metastases limited to the supraclavicular lymph nodes had a better survival time compared with patients with a cT4b tumor (without metastases) and patients with distant metastases. Survival of patients with advanced unresectable ESCC in clinical practice was poor, even in patients treated with curative intent.

Abbreviations
- ESCC
esophageal squamous cell carcinoma
- Gy
gray
- IQR
interquartile range
- NCR
Netherlands Cancer Registry
- OS
overall survival
- SBRT
stereotactic body radiotherapy
- SCLNM
metastases limited to the supraclavicular lymph nodes
1. INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) accounts for the vast majority of esophageal carcinoma incidences worldwide (~90%). 1 Curative options are generally no longer available if patients present with distant metastases and palliative systemic treatment is the treatment of choice. 2 , 3 However, patients with metastases limited to the supraclavicular lymph nodes, who are diagnosed as M1 disease, could still be eligible for treatment with curative intent consisting of definitive chemoradiation. 4 , 5 , 6 Definitive chemoradiation or palliative treatment is recommended for patients with T4b disease without metastases, as these tumors are generally unresectable.
Until recently, evidence for systemic treatment in ESCC was scarce. However, preliminary results from the phase III CheckMate 648 trial in advanced ESCC showed improved survival of patients treated with nivolumab plus ipilimumab (13.2 months) or nivolumab plus chemotherapy (12.8 months) compared with chemotherapy alone (10.7 months). 7 Second‐line phase III trials in advanced ESCC also showed improved survival of 1.1‐2.5 months in patients receiving PD‐1 inhibitors (with or without chemotherapy) compared with chemotherapy alone. 8 , 9 , 10 , 11 A major limitation of these studies is the limited inclusion of patients from Western countries. This might be of relevance as, at genomic level, ESCC differs between Asian and Caucasian populations and the effect of systemic treatment appears to be distinct. 12 , 13 , 14
To provide data on patients with advanced ESCC in a Western population and put the recent studies on checkpoint inhibition in a real‐world context, this study aimed to describe treatment patterns and survival in ESCC patients with cT4b disease without metastases, patients with supraclavicular lymph node metastases, and patients with distant metastatic ESCC in a nationwide population‐based study.
2. MATERIALS AND METHODS
2.1. Study population
Patients diagnosed with squamous cell carcinoma (ICD‐O3 morphology codes 8050‐8084) of the esophagus or gastroesophageal junction/cardia diagnosed with clinical stages invading adjacent structures without metastases (cT4bcNallcM0; 2015‐2018), synchronous metastatic disease (cTallcNallcM1; 2015‐2018), or metachronous metastatic disease after primary diagnosis of non‐metastatic disease treated with curative intent (cT1‐4a,XcNallcM0; 2015‐2016) were selected from the Netherlands Cancer Registry (NCR) (Figure 1). 15
FIGURE 1.
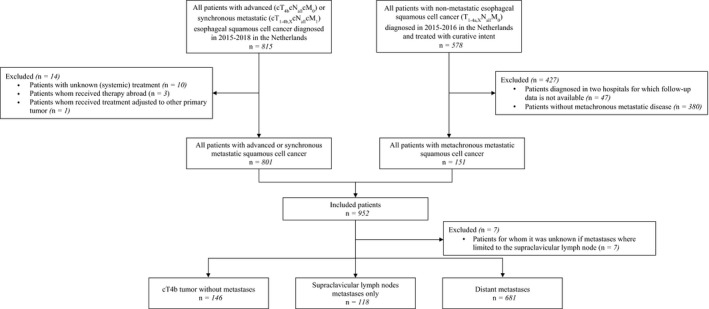
Flowchart of patient selection
Synchronous metastatic disease was defined as diagnosis of metastases before the start of treatment or, to account for delay in pathological conformation of metastases, within the first 5 days of start of treatment. In patients who had had surgery without preoperative treatment, metastases had to be diagnosed before or during surgery. Metachronous metastases were defined as diagnosis of metastases after either resection (endoscopic or surgical resection) or definitive chemoradiotherapy (chemotherapy with concurrent radiotherapy consisting of ≥28 fractions and/or total radiation dose of ≥50 gray (Gy)) for primary non‐metastatic disease. In case of resection, metastases needed to be diagnosed at least five days after resection to account for any delay in pathological confirmation of metastases. Patients with a diagnosis of metastases during treatment with curative intent were considered interval metastases and were beyond the scope of this study and presented elsewhere. 16
The NCR is a nationwide population‐based cancer registry that covers the total Dutch population and includes all newly diagnosed malignancies. Specially trained data managers from the NCR routinely extract information on diagnosis, tumor stage, and treatment from medical records. All data used in this study have been registered in the NCR and collected by trained data managers from the NCR by extraction from patients' medical records according to strict coding manuals. Data on progression of disease (including metachronous metastases) were not routinely registered in the NCR and were collected in the second half of 2019 from medical records by data managers from the NCR. Complete follow‐up was available for patients with advanced or synchronous metastatic disease who had been diagnosed in 2015‐2017, and for patients with metachronous metastatic disease after prior primary non‐metastatic diagnosis in 2015‐2016. For patients with metastases limited to the head and neck lymph nodes, data managers from the NCR extracted information from medical records on whether the metastases were located and limited to the supraclavicular lymph nodes. Information on vital status was available by linking the NCR with the Dutch Personal Records Database and updated until 1 February 2021. According to the Central Committee on Research involving Human Subjects, this type of study did not require approval from an ethics committee in The Netherlands. This study was approved by the Privacy Review Board of the NCR and the scientific committee of the Dutch Upper GI Cancer Group.
Patients were classified into three groups: patients with cT4b disease without distant metastases (cT4b), patients with metastases limited to the supraclavicular lymph node (SCLNM; including patients with cT4b tumor and SCLNM), and patients with other distant metastases (including patients with cT4b tumor and metastases not limited to the supraclavicular lymph node). Patients with SCLNM or distant metastases were further classified into synchronous or metachronous metastatic disease.
In patients with non‐metastatic disease, 47 patients were excluded, as follow‐up was unavailable in two hospitals due to logistical constraints. Patients without metachronous metastatic disease were excluded (n = 380). In patients with advanced or synchronous metastatic disease, patients with unknown (systemic) treatment (n = 10), and patients whom received treatment abroad (n = 3) were excluded. One patient was excluded because of a secondary primary tumor that prompted a change in their treatment plan. Lastly, patients for whom it was unknown if metastases were limited to the supraclavicular lymph nodes were excluded (n = 7).
2.2. Treatment definitions
Treatment after diagnosis of unresectable or metastatic disease was classified into: resection of primary tumor and/or metastases (with or without [neo]adjuvant therapy), chemoradiotherapy (chemotherapy with concurrent radiotherapy with a maximum of 2.2 Gy per dosage not followed by resection of primary tumor), stereotactic body radiotherapy (SBRT) for metastases (≥10 Gy per fraction if ≥1 fraction, ≥7 Gy per fraction if ≥5 fractions or ≥5 Gy per fraction if ≥12 fractions), and systemic therapy (chemotherapy without concurrent radiotherapy). A systemic treatment regimen was defined as all chemotherapy and targeted agents that started within three days of each other and were given until suspension. 17 Treatment groups were not mutually exclusive and were based on the treatment received after primary diagnosis (cT4b and synchronous disease) or first metastatic site (metachronous disease). If patients did not receive any of the above‐mentioned treatments, they were grouped under best supportive care.
2.3. Statistical analysis
Characteristics and type of treatment were compared using ANOVA, chi‐square test or Fisher exact test when appropriate. Overall survival (OS) was assessed from primary diagnosis (cT4b and synchronous disease) or date of first metastasis (metachronous disease). OS was assessed until death or end of follow‐up. OS was analyzed using Kaplan‐Meier methods with log‐rank tests as well as univariate and multivariable Cox proportional hazard analyses. The multivariable models were adjusted for clinically relevant patient and tumor characteristics, as well as type of treatment. Time‐dependent covariates were created as a function of survival time to test the proportional hazard assumption. If time‐dependent covariates were significant against survival time, the Schoenfeld residual plots were graphically inspected and if the residuals of the covariates changed over time the covariates were deemed nonproportional and the Cox model was stratified, instead of adjusted for these covariates. Two‐sided P‐values of less than .05 were considered statistically significant. All analyses were conducted using SAS® version 9.4 (SAS Institute).
3. RESULTS
3.1. Patient characteristics
In total, 945 patients were included (Figure 1). Median age was 68 years and 60% of patients were male (Table 1). Fifteen percent of patients were diagnosed with cT4b (n = 146), 12% with SCLNM (n = 118), and 72% with distant metastases (n = 681). Among patients with metachronous metastatic disease, median time since end of treatment after primary non‐metastatic disease until metastatic disease was 8.7 months (interquartile range [IQR] 5.0‐17.8). The characteristics of patients with synchronous or metachronous metastatic disease are listed in Table S1.
TABLE 1.
Baseline characteristics and type of treatment of all patients subdivided by subgroup
| All patients (N = 945) | cT4b disease without metastases (N = 146) | Supraclavicular lymph node disease (N = 118) | Distant metastatic disease (N = 681) | P‐value | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 570 (60%) | 93 (64%) | 62 (53%) | 415 (61%) | .151 a |
| Female | 375 (40%) | 53 (36%) | 56 (47%) | 266 (39%) | |
| Age | |||||
| Median (IQR) | 68 (62‐74) | 66 (61‐73) | 69 (62‐73) | 68 (62‐74) | .381 b |
| Type of metastatic disease, n (%) | |||||
| Synchronous | 794 (84%) | 146 (100%) | 104 (88%) | 544 (80%) | < .001 a |
| Metachronous | 151 (16%) | 0 (0%) | 14 (12%) | 137 (20%) | |
| Comorbidities, n (%) | |||||
| 0 | 401 (42%) | 60 (41%) | 52 (44%) | 289 (42%) | .04 c |
| 1 | 311 (33%) | 45 (31%) | 41 (35%) | 225 (33%) | |
| ≥2 | 179 (19%) | 23 (16%) | 22 (19%) | 134 (20%) | |
| Unknown | 54 (6%) | 18 (12%) | 3 (3%) | 33 (5%) | |
| Performance status, n (%) | |||||
| 0‐1 | 430 (46%) | 65 (45%) | 82 (69%) | 283 (42%) | < .001 a |
| ≥2 | 176 (19%) | 29 (20%) | 11 (9%) | 136 (20%) | |
| Unknown | 339 (36%) | 52 (36%) | 25 (21%) | 262 (38%) | |
| Tumor location at primary diagnosis, n (%) | |||||
| Cervical thoracal esophagus | 19 (2%) | 9 (6%) | 1 (1%) | 9 (1%) | < .001 a |
| Proximal thoracal esophagus | 165 (17%) | 41 (28%) | 35 (30%) | 89 (13%) | |
| Mid thoracal esophagus | 362 (38%) | 66 (45%) | 53 (45%) | 243 (36%) | |
| Distal thoracal esophagus | 295 (31%) | 16 (11%) | 17 (14%) | 262 (38%) | |
| Overlapping/unknown esophagus | 95 (10%) | 14 (10%) | 12 (10%) | 69 (10%) | |
| Gastroesophageal junction | 9 (1%) | 0 (0%) | 0 (0%) | 9 (1%) | |
| Tumor differentiation at primary diagnosis, n (%) | |||||
| Well/moderate | 352 (37%) | 62 (42%) | 47 (40%) | 243 (36%) | .095 a |
| Poorly/undifferentiated | 289 (31%) | 31 (21%) | 37 (31%) | 221 (32%) | |
| Unknown | 304 (32%) | 53 (36%) | 34 (29%) | 217 (32%) | |
| cT stage at primary diagnosis, n (%) | |||||
| cT1 | 4 (0%) | 0 (0%) | 1 (1%) | 3 (0%) | < .001 c |
| cT2 | 233 (25%) | 0 (0%) | 24 (20%) | 209 (31%) | |
| cT3 | 296 (31%) | 0 (0%) | 54 (46%) | 242 (36%) | |
| cT4 | 262 (28%) | 146 (100%) | 21 (18%) | 95 (14%) | |
| cTX | 150 (16%) | 0 (0%) | 18 (15%) | 132 (19%) | |
| cN stage at primary diagnosis, n (%) | |||||
| cN0 | 167 (18%) | 32 (22%) | 18 (15%) | 117 (17%) | .799 c |
| cN1 | 342 (36%) | 55 (38%) | 40 (34%) | 247 (36%) | |
| cN2 | 327 (35%) | 47 (32%) | 44 (37%) | 236 (35%) | |
| cN3 | 73 (8%) | 8 (5%) | 11 (9%) | 54 (8%) | |
| cNX | 36 (4%) | 4 (3%) | 5 (4%) | 27 (4%) | |
| Distant metastatic sites, n (%) | |||||
| 0‐1 | 638 (68%) | 146 (100%) | 118 (100%) | 374 (55%) | < .001 a |
| 2 | 197 (21%) | 0 (0%) | 0 (0%) | 197 (29%) | |
| ≥3 | 110 (12%) | 0 (0%) | 0 (0%) | 110 (16%) | |
| Resection primary tumor or metastasis, n (%) | 37 (4%) | 10 (7%) | 10 (8%) | 17 (2%) | .001 a |
| Chemoradiotherapy (without resection of primary tumor) n (%) | 141 (15%) | 53 (36%) | 48 (41%) | 40 (6%) | < .001 a |
| <50.4 Gy | 24 (17%) | 6 (11%) | 7 (15%) | 11 (28%) | .119 c |
| 50.4 Gy | 100 (71%) | 41 (77%) | 32 (67%) | 27 (68%) | |
| >50.4 Gy | 17 (12%) | 6 (11%) | 9 (19%) | 2 (5%) | |
| SBRT for metastasis, n (%) | 9 (1%) | 0 (0%) | 0 (0%) | 9 (1%) | .172 a |
| Systemic therapy, n (%) | 185 (20%) | 8 (5%) | 15 (13%) | 162 (24%) | < .001 a |
| Mono | 7 (4%) | 0 (0%) | 1 (7%) | 6 (4%) | .878 c |
| Doublet | 165 (89%) | 8 (100%) | 13 (87%) | 144 (89%) | |
| Triplet | 11 (6%) | 0 (0%) | 1 (7%) | 10 (6%) | |
| Targeted | 2 (1%) | 0 (0%) | 0 (0%) | 2 (1%) | |
| Best supportive care, n (%) | 584 (62%) | 76 (52%) | 48 (41%) | 460 (68%) | < .001 a |
| Radiotherapy for symptom control, n (%) | 268 (46%) | 30 (39%) | 25 (52%) | 213 (46%) | .362 a |
| Stent placement, n (%) | 93 (16%) | 17 (22%) | 9 (19%) | 67 (15%) | .194 a |
All patients with unresectable (cT4b) or synchronous metastatic esophageal squamous cell carcinoma at primary diagnosis (2015‐2018) or patients with metachronous metastases after prior primary non‐metastatic diagnosis (2015‐2016) were included.
Chi‐square P‐value.
ANOVA F‐test P‐value.
Fisher Exact P‐value.
Among patients with distant metastases, the primary tumor was more often located in the distal esophagus (38%) compared with patients with cT4b (11%) or SCLNM (14%; P < .001). Seventeen percent and 10% of patients with SCLNM and distant metastases had a cT4b tumor, respectively. In patients with distant metastases, the four most common metastatic locations were the nonregional lymph nodes (48%), lung (37%), liver (33%) and bone (19%).
3.2. Treatment
Sixty‐two percent of patients received best supportive care (Table 1), of whom 46% received radiotherapy for symptom control and 16% received a stent. A small proportion of patients had resection of primary tumor and/or metastases: 7%, 8%, and 2% of patients with cT4b, SCLNM, and distant metastases, respectively (P = .001). Patients with cT4b and SCLNM more often received chemoradiotherapy (without resection of primary tumor; 36% and 41%, respectively) compared with patients with distant metastases (6%; P < .001). The majority (71%) of these patients received a dose of 50.4 Gy and the most common chemotherapy regimen was carboplatin plus paclitaxel (97%). The other chemotherapy regimens were cisplatin (1%), cisplatin plus capecitabine (1%), and cisplatin plus paclitaxel (1%).
Systemic therapy was administered in 20% of patients, most often doublet therapy (89%; Table 1). Patients with distant metastases (24%) more often received systemic therapy compared with patients with cT4b (5%) and SCLNM (13%; P < .001). In total, 15 different systemic regimens were administered (Figure 2), most frequently capecitabine or 5‐FU plus oxaliplatin (48%), and carboplatin plus paclitaxel (37%). For patients with complete follow‐up (n = 141), 23% received a subsequent systemic regimen after failure of the first regimen, most frequently paclitaxel monotherapy (22%) and capecitabine plus oxaliplatin (19%). None of these patients received docetaxel monotherapy.
FIGURE 2.

Word cloud of the 15 systemic treatment regimens that were administered. Font size of the word corresponds to the number of patients that received the regimen. CapOx, capecitabine and oxaliplatin; CarboPac, carboplatin and paclitaxel; ECC, epirubicin, cisplatin, and capecitabine; EOX, epirubicin, oxaliplatin, and capecitabine; FOLFOX, fluorouracil and oxaliplatin
3.3. Survival
Median OS time in all patients was 5.3 months (IQR 2.3‐10.9). Median OS was 6.3 (IQR 2.8‐13.1), 11.2 (IQR 5.6‐22.5), and 4.4 months (IQR 1.9‐9.3) in patients with cT4b, SCLNM, and distant metastases, respectively (P < .001; Figure 3). Among patients who received chemoradiotherapy and/or had resection, median OS was 11.9 (IQR 6.4‐39.0), 16.1 (IQR 7.9‐25.9), and 14.0 months (IQR 9.2‐23.5) in patients with cT4b, SCLNM, and distant metastases, respectively (P = .76; Figure 4). Among patients who received best supportive care, median OS was 3.1 (IQR 1.3‐7.1), 6.2 (IQR 3.3‐12.1), and 3.0 months (IQR 1.5‐6.2) in patients with cT4b, SCLNM, and distant metastases, respectively (P = .003; Figure 5). Among patients with distant metastases receiving systemic therapy (and whom did not have resection), median OS was 7.5 months (IQR 4.8‐12.6). For patients with distant metastases receiving a second systemic treatment regimen, OS from start of second‐line treatment was 6.2 months (IQR 4.0‐9.1).
FIGURE 3.
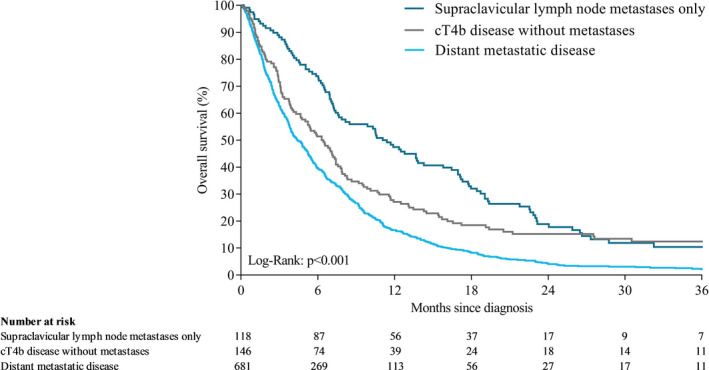
Overall survival of patients with cT4b, SCLNM, or distant metastases after unresectable or metastatic diagnosis
FIGURE 4.
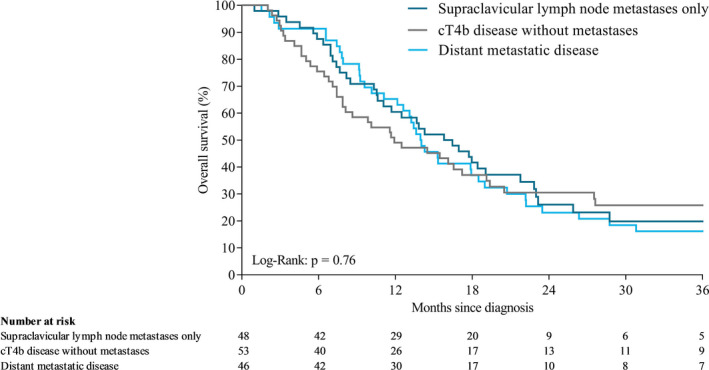
Overall survival of patients with cT4b, SCLNM or distant metastases who received chemoradiotherapy and/or underwent resection after unresectable or metastatic diagnosis
FIGURE 5.
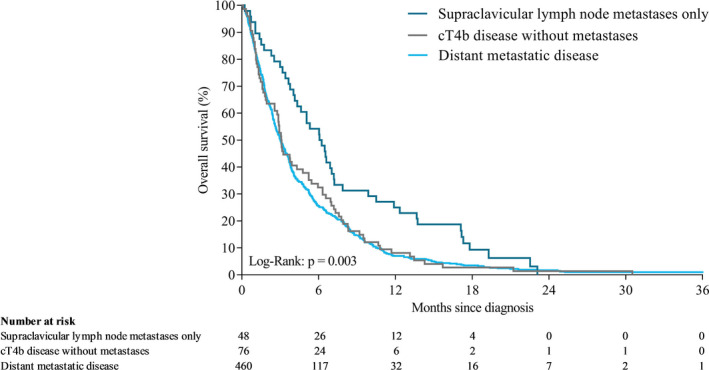
Overall survival of patients with cT4b, SCLNM, or distant metastases who received best supportive care after unresectable or metastatic diagnosis
Multivariable analyses showed that patients with cT4b (hazard ratio 1.44, 95% CI 1.04‐1.99) and patients with distant metastases (hazard ratio 1.42, 95% CI 1.12‐1.80) had a worse survival compared with patients with SCLNM (Table 2).
TABLE 2.
Cox regression for overall survival in patients with T4b disease without metastases, supraclavicular lymph nodes metastases only or distant metastases after unresectable or metastatic diagnosis a
| Variable | Number of patients | Events | Median OS | Univariable regression | Multivariable regression | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | ||||
| Type of disease | |||||||
| T4b disease without metastases | 146 | 129 | 6.3 | 1.38 (1.05‐1.81) | .019 | 1.44 (1.04‐1.99) | .028 |
| Supraclavicular lymph nodes metastases only | 118 | 100 | 11.2 | Ref | Ref | ||
| Distant metastatic disease | 681 | 671 | 4.4 | 1.52 (1.22‐1.90) | < .001 | 1.42 (1.12‐1.80) | .004 |
All patients with unresectable (cT4b) or synchronous metastatic esophageal squamous cell carcinoma at primary diagnosis (2015‐2018) or patients with metachronous metastases after primary non‐metastatic diagnosis (2015‐2016) were included.
Sex, age, number of comorbidities, tumor location at primary diagnosis, cT stage at primary diagnosis, cN stage at primary diagnosis, tumor differentiation at primary diagnosis, and number of metastatic sites all met the proportional hazard assumptions and the multivariable Cox regression analyses were adjusted for these variables. Type of treatment and performance status did not meet the proportional hazard assumptions and the multivariable model was stratified for these variables.
4. DISCUSSION
Our study showed that Western patients with advanced unresectable ESCC are a diverse patient population in terms of characteristics and treatment, as well as survival. Our main finding was that patients with metastases limited to the supraclavicular lymph nodes, who are diagnosed as M1 disease, had a considerably better survival compared with patients with cT4b and patients with other distant metastases.
In previous studies of patients with esophageal cancer without other distant metastases than the supraclavicular lymph node and treated with chemoradiotherapy, involvement of the supraclavicular lymph node was not identified as a negative prognostic factor for survival. 5 , 18 In contrast with the TNM classification, the Japanese Classification of Esophageal Cancer considers supraclavicular lymph nodes as regional lymph nodes, depending on the location of the primary tumor. 19 In our study, we found a superior survival of all patients with SCLNM compared with patients with cT4b and patients with distant metastases, independent of treatment. The survival time among patients who received chemoradiotherapy and/or had resection with SCLNM (16.1 months) or distant metastases (14.0 months) was not significantly different. Most likely, patients with distant metastases who received this type of treatment had oligometastatic disease for whom prolonged survival could be achieved. 20
Twenty‐three percent of patients with SCLNM who received chemoradiotherapy and/or had resection were still alive after 3 years. The 3‐year survival rate was higher in previous studies of patients with esophageal cancer who had received definitive chemoradiotherapy (36%‐46%). 5 , 21 A population‐based study of the CROSS regimen reported 5‐year survival rates of 62% and 38%, depending on response after neoadjuvant chemoradiotherapy. 22 Similarly, survival after definitive chemoradiotherapy predominantly depended on response, with complete responders showing a 3‐year survival rate of 58%. 23 To improve outcomes after definitive chemoradiotherapy, radiation dose escalation was considered an option until recently. As the phase III ARTDECO study showed that dose escalation up to 61.6 Gy did not improve outcomes in patients with esophageal cancer, including patients with ESCC. 24 Currently, clinical trials are underway to explore the addition of PD‐1 inhibitors and/or TGF‐β to definitive chemoradiotherapy and will hopefully improve outcomes for patients with ESCC who are eligible for definitive chemoradiotherapy. 25 , 26
The phase III CheckMate 648 trial reported a survival time of 10.7 months for patients receiving chemotherapy alone, and an increased survival of 12.8 and 13.2 months for patients receiving nivolumab plus chemotherapy and nivolumab plus ipilimumab, respectively. 7 In our study, among patients with distant metastases receiving systemic therapy, survival was 7.5 months. In CheckMate 648, no information was provided on inclusion of patients with SCLNM. The inclusion of patients with SCLNM, locoregional recurrent, or unresectable advanced disease in CheckMate 648 could potentially explain the longer survival observed in the chemotherapy alone group compared with our population. The percentage of patients in our study with distant metastatic ESCC who received systemic therapy (24%) was lower compared with patients with metastatic esophagogastric adenocarcinoma (39%). 27 Possible explanations could be that patients with ESCC are less fit compared with patients with adenocarcinoma due to differences in etiology, including lifestyle, or because evidence for palliative systemic therapy in ESCC has been limited until recently. Hopefully, with novel treatment strategies becoming available, more patients will be able to benefit from systemic therapy and checkpoint inhibition could potentially improve survival in this population. However, the question remains how checkpoint inhibitors will perform in the real‐world situation.
Survival from the start of second‐line treatment in our study of patients with distant metastases (6.2 months) was comparable with the control arms (ie, chemotherapy only) of the second‐line ESCORT, RATIONALE 302, and KEYNOTE 181 trials, with a reported OS of 6.2, 6.3, and 7.1 months. 9 , 10 , 11 In ATTRACTION‐3, survival time of patients in the control arm (all receiving taxane monotherapy) was higher at 8.4 months. 8 In a real‐world study of patients with ESCC, the survival of patients receiving second‐line taxane monotherapy (7.3 months) was longer compared with patients receiving other second‐line regimens (5.1 months). 28 In our study, 22% of patients received second‐line taxane monotherapy and this could explain the shorter survival time compared with ATTRACTION‐3. In our population, approximately one‐third of patients received carboplatin plus paclitaxel as first‐line treatment and were therefore ineligible for second‐line taxane monotherapy.
The major strength of this study was the use of population‐based data and the relatively large sample size of patients with ESCC from a Western population. This study also has some limitations. This is a retrospective study and data for certain variables, eg, performance status, were incomplete. In patients with cT4b it is unknown which adjacent structure(s) were invaded and the definition of cT4b could differ between hospitals. In addition, classification of supraclavicular lymph nodes might have been inconsistent, as it is possible that these lymph nodes were sometimes considered regional cervical lymph nodes in clinical practice. Lastly, the reason for choice of treatment was unknown and could have provided more insight into the choice of treatment.
In conclusion, in this population‐based study of patients with advanced unresectable ESCC, we showed that characteristics, treatment patterns, and survival differed between patients with cT4b, SCLNM, and distant metastases. The majority of patients were treated with best supportive care. Survival of patients with advanced ESCC in clinical practice is poor, even in patients treated with curative intent.
CONFLICT OF INTEREST
JdV has served as a consultant for Amgen, AstraZeneca, MSD, Pierre Fabre, and Servier, and has received institutional research funding from Servier. HvL reports grants from Roche, has served as a consultant for BMS, Celgene, Lilly, and Nordic and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, and Roche. RV reports grants from BMS and Roche. MP, PV, MH, SG, and PJ have no disclosures to declare.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the NCR. The authors thank all participating hospitals in The Netherlands.
Pape M, Vissers PAJ, de Vos‐Geelen J, et al. Treatment patterns and survival in advanced unresectable esophageal squamous cell cancer: A population‐based study. Cancer Sci. 2022;113:1038–1046. doi: 10.1111/cas.15262
The work for this manuscript was carried out at IKNL, Godebaldkwartier 419, 3511 DT Utrecht, The Netherlands.
Funding information
This work was supported by Bristol Myers Squibb (CA209‐77E). The funder has financed part of the data collection. The funder of the study had no role in the study design, analysis, and interpretation of the data, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381‐387. [DOI] [PubMed] [Google Scholar]
- 2. Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The efficacy and safety of first‐line chemotherapy in advanced esophagogastric cancer: a network meta‐analysis. J Natl Cancer Inst. 2016;108:djw166. [DOI] [PubMed] [Google Scholar]
- 3. van Kleef JJ, Ter Veer E, van den Boorn HG, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta‐analysis. J Natl Cancer Inst. 2020;112:12‐29. [DOI] [PubMed] [Google Scholar]
- 4. TNM . Classification of Malignant Tumours, 8th edn: Wiley‐Blackwell, International Union Against Cancer (UICC); 2017. [Google Scholar]
- 5. Jeene PM, Versteijne E, van Berge Henegouwen MI, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation. Acta Oncol. 2017;56:33‐38. [DOI] [PubMed] [Google Scholar]
- 6. Honma Y, Hokamura N, Nagashima K, et al. Clinical outcomes of resectable esophageal cancer with supraclavicular lymph node metastases treated with curative intent. Anticancer Res. 2017;37:3741‐3749. [DOI] [PubMed] [Google Scholar]
- 7. Ajani JA, Kato K, Doki Y, et al. CheckMate 648: a randomized phase 3 study of nivolumab plus ipilimumab or nivolumab combined with fluorouracil plus cisplatin versus fluorouracil plus cisplatin in patients with unresectable advanced, recurrent, or metastatic previously untreated esophageal squamous cell carcinoma. J Clin Oncol. 2018;36:TPS193‐TPS193. [Google Scholar]
- 8. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:1506‐1517. [DOI] [PubMed] [Google Scholar]
- 9. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second‐line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open‐label, phase 3 study. Lancet Oncol. 2020;21:832‐842. [DOI] [PubMed] [Google Scholar]
- 10. Shen L, Kato K, Kim S‐B, et al. RATIONALE 302: randomized, phase 3 study of tislelizumab versus chemotherapy as second‐line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol. 2021;39:4012‐4012. [Google Scholar]
- 11. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE‐181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138‐4148. [DOI] [PubMed] [Google Scholar]
- 12. Deng J, Chen H, Zhou D, et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun. 2017;8:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narumiya K, Bollschweiler E, Holscher AH, et al. Different response rates to chemotherapy between Japanese and German esophageal squamous cell carcinoma: patients may be influenced by ERCC1 or ABCB1. Future Oncol. 2020;16:2075‐2087. [DOI] [PubMed] [Google Scholar]
- 15. Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology: ICD‐O, 3rd edn. World Health Organization; 2000. [Google Scholar]
- 16. Kroese TE, Dijksterhuis WPM, van Rossum PSN, et al. Prognosis of interval distant metastases after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Thorac Surg. 2022;113(2):482‐490. 10.1016/j.athoracsur.2021.01.061 [DOI] [PubMed] [Google Scholar]
- 17. Dijksterhuis WPM, Verhoeven RHA, Slingerland M, et al. Heterogeneity of first‐line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: a real‐world evidence study. Int J Cancer. 2020;146:1889‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YH, Lu HI, Lo CM, et al. The clinical impact of supraclavicular lymph node metastasis in patients with locally advanced esophageal squamous cell carcinoma receiving curative concurrent chemoradiotherapy. PLoS One. 2018;13:e0198800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Japan Esophageal Society . Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schizas D, Mylonas KS, Kapsampelis P, et al. Patients undergoing surgery for oligometastatic oesophageal cancer survive for more than 2 years: bootstrapping systematic review data. Interact Cardiovasc Thorac Surg. 2020;31:299‐304. [DOI] [PubMed] [Google Scholar]
- 21. de Vos‐Geelen J, Hoebers FJP, Geurts SME, et al. A national study to assess outcomes of definitive chemoradiation regimens in proximal esophageal cancer. Acta Oncol. 2020;59:895‐903. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Kaabi A, van der Post RS, van der Werf LR, et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long‐term outcome of esophageal cancer: a population‐based study. Acta Oncol. 2021;60(4):497‐504. [DOI] [PubMed] [Google Scholar]
- 23. de Vos‐Geelen J, Geurts SME, Nieuwenhuijzen GAP, et al. Patterns of recurrence following definitive chemoradiation for patients with proximal esophageal cancer. Eur J Surg Oncol. 2021;47:2016‐2022. [DOI] [PubMed] [Google Scholar]
- 24. Hulshof M, Geijsen ED, Rozema T, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO Study). J Clin Oncol. 2021;39:JCO2003697. [DOI] [PubMed] [Google Scholar]
- 25. Veen L, Hulshof MCCM, Jimenez CR, et al. TGF‐β and PD‐L1 inhibition combined with definitive chemoradiotherapy in esophageal squamous cell carcinoma: a phase II clinical trial (NCT04595149). J Clin Oncol. 2021;39:TPS4154‐TPS4154. [Google Scholar]
- 26. Xi M, Zhu Y, Li Q‐Q, et al. The efficacy and safety of toripalimab combined with definitive chemoradiotherapy for patients with locally advanced esophageal squamous cell carcinoma. J Clin Oncol. 2021;39:e16043–e16043. [Google Scholar]
- 27. Pape M, Vissers PAJ & Bertwistle D et al. A population‐based study in synchronous versus metachronous metastatic esophagogastric adenocarcinoma. 2021. [Manuscript submitted for publication]. [DOI] [PMC free article] [PubMed]
- 28. Abraham P, Gricar J, Zhang Y, Shankaran V. Real‐world treatment patterns and outcomes in patients receiving second‐line therapy for advanced/metastatic esophageal squamous cell carcinoma. Adv Ther. 2020;37:3392‐3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


