Abstract
Detecting rare circulating tumor cells (CTCs) in the bloodstream is extremely challenging. We had previously developed a novel polymeric microfluidic device, “CTC‐chip,” for capturing CTCs and have shown high capture efficiency in lung cancer cell lines by conjugating Abs against epithelial cell adhesion molecules (EpCAM). This study aimed to optimize the EpCAM‐chip and clarify the prognostic impact of CTCs in lung cancer patients. Of 123 patients with pathologically proven lung cancer, both progression‐free survival (P = .037) and cancer‐specific survival (P = .0041) were predominantly poor when CTCs were detected before treatment. After classification into surgical and chemotherapy groups, progression‐free survival was worse in CTC‐positive patients in both groups (surgery, P = .115; chemotherapy, P = .012), indicating that the detection of baseline CTCs is a risk factor for recurrence and progression. Furthermore, we recovered captured CTCs using micromanipulators and undertook mutation analysis using PCR. Thus, the EpCAM‐chip is a highly sensitive system for detecting CTCs that contributes to the prediction of recurrence and progression and enables genetic analysis of captured CTCs, which could open new diagnostic, therapeutic, and prognostic options for lung cancer patients.
Keywords: circulating tumor cell, CTC‐chip, EpCAM, lung cancer, polymeric microfluidic device
This study aimed to optimize the epithelial cell adhesion molecule (EpCAM)‐chip and clarify the prognostic impact of circulating tumor cells (CTCs) in lung cancer patients. The EpCAM‐chip is a highly sensitive system for detecting CTCs. The EpCAM‐chip contributes to the prediction of recurrence and progression and enables genetic analysis of captured CTCs, which could open new diagnostic, therapeutic, and prognostic options for lung cancer patients.

Abbreviations
- CK
cytokeratin
- CSS
cancer‐specific survival
- CSV
cell surface vimentin
- CTC
circulating tumor cell
- EGFR
epidermal growth factor receptor
- EMT
epithelial‐mesenchymal transformation
- EpCAM
epithelial cell adhesion molecules
- MPM
malignant pleural mesothelioma
- PD‐L1
programmed death‐ligand 1
- PFS
progression‐free survival
- WGA
whole‐genome amplification
1. INTRODUCTION
Metastatic spread of cancer is one of the leading causes of cancer‐associated morbidity and mortality. 1 Circulating tumor cells form the very basis of this process, and the detection and molecular biological analysis of CTCs could help enhance the diagnosis and treatment of cancer patients. 2 , 3 , 4 However, CTCs are rare in the bloodstream and the detection of CTC contamination in many normal hematologic cells has been elusive. 5 , 6 , 7 In various CTC isolation technologies, most of the data pertaining to the clinical utility of CTCs was collected utilizing CellSearch (Veridex), the only FDA‐approved technology for CTC‐enumeration for breast, colon, and prostate cancers. 8 CellSearch is a semiautomated CTC‐capture system that uses a ferrofluid coated with an Ab against EpCAM, and has showed clinical relevance in many types of epithelial cancers. 9 , 10 , 11 , 12 , 13 Our previous study using CellSearch showed that CTCs could be a useful surrogate marker of distant metastasis in primary lung cancer, but also indicated the need for a more sensitive system. 14 A microfluidic system, called a “CTC‐chip,” could capture CTCs with Abs attached to the surface of numerous microposts within a microflow chamber, and the CTC‐chip conjugated with anti‐EpCAM Ab showed high sensitivity in the detection of CTCs. 15 , 16 Based on this conventional CTC‐chip, we developed a novel polymeric microfluidic device CTC‐chip with lower cost, higher durability, and improved transparency. 17 Furthermore, the most important advantage of our novel CTC‐chip is its capability of capturing various types of CTCs by arbitrarily coating antibody against expressed in the targeted CTC, which we named “universal CTC‐chip”. 18 We have reported highly sensitive capture of lung cancer and MPM cell lines using this universal CTC‐chip coated with the appropriate Abs (lung cancer, anti‐EpCAM Ab; 18 MPM, anti‐podoplanin Ab 18 , 19 , 20 ), and have subsequently investigated clinical cases. In the current study, based on accumulated clinical cases, we confirmed the superiority of the cell capture efficiency of the CTC‐chip compared to CellSearch, and clarified the prognostic impact of CTCs in lung cancer patients. Furthermore, as the biggest concern of the CTC‐chip was whether the cells captured with the CTC‐chip were true CTCs, we validated the cells by capturing them with a micromanipulator and carrying out genetic analysis.
2. MATERIALS AND METHODS
This research was carried out with the approval of the Ethics Committee of the University of Occupational and Environmental Health, Japan (approval no. H26‐15). The patients and healthy volunteers provided formal written informed consent to participate in this study.
2.1. Cell lines
Four human lung cancer cell lines were used in this study. PC9 (Riken BioResource Research Center), NCI‐H441 (ATCC), and NCI‐H1975 (ATCC) were cultured in RPMI‐1640 medium (Wako Pure Chemical Industries) supplemented with 10% FBS (Thermo Fisher Scientific) at 37°C and 5% CO2. A549 (ATCC) was cultured in EMEM (Wako Pure Chemical Industries) supplemented with 10% FBS and 1% nonessential amino acids (Wako Pure Chemical Industries) at 37°C and 5% CO2.
2.2. Flow cytometry analysis
To analyze the EpCAM expression, cell lines were incubated with anti‐EpCAM Ab (1:100 dilution, clone HEA125; Santa Cruz Biotechnology) for 1 hour at room temperature, and were subsequently incubated with goat anti‐mouse IgG Ab conjugated with fluorescein isothiocyanate (1:20 dilution; BD Biosciences). Flow cytometry analysis was carried out using an EC800 Cell Analyzer (Sony Biotechnology), and the data were analyzed using FlowJo software (Becton, Dickinson and Company). Mean fluorescence intensity was calculated as the ratio of positive control to negative control.
2.3. Preparation of CTC‐chip and evaluation of cell‐capture efficiency in lung cancer cell lines
Preparation of the CTC‐chip and evaluation of cell capture efficiency were carried out as described previously. 18 The CTC‐chip was first incubated with goat anti‐mouse IgG (SouthernBiotech) as the base Ab overnight at 4°C and then with mouse anti‐human EpCAM Ab as the capture Ab at room temperature for 1 hour (“EpCAM‐chip”). For the evaluation of cell‐capture efficiency, 1 mL (100 cells/mL) of blood collected from healthy volunteers was mixed with tumor cells labeled using the CellTrace CSFE Cell Growth Kit (Thermo Fisher Scientific). The sample was then applied to the CTC‐chip at a constant flow rate (1.0 mL/h) and monitored using a fluorescence microscope (CKX41; Olympus). The total number of cells applied to the CTC‐chip (N‐total) was determined by counting the cells that passed through the inlet of the CTC‐chip, and the number of captured cells (N‐captured) was determined by counting the cells that remained on the CTC‐chip. Cell‐capture efficiency was calculated as the N‐captured / N‐total ratio, and the average and standard errors were calculated for each sample. The blood collection tube used for the CTC‐chip was a BD Vacutainer EDTA‐2K (Becton, Dickinson and Company), and each experiment was carried out in triplicate.
2.4. Clinical evaluation of CTCs in lung cancer patients
Peripheral blood was sampled from patients with a pathological diagnosis of lung cancer, and 1 mL blood was applied to the EpCAM‐chip. Cells captured on CTC‐chips were incubated with primary Abs, rabbit anti‐CK Ab (ab9377; Abcam) and rat anti‐CD45 Ab (clone YTH24.5; Abcam), for 1 hour at room temperature, followed by incubation for 30 minutes at room temperature with a secondary Ab, Alexa Fluor 594 anti‐rabbit IgG Ab (Thermo Fisher Scientific) and Alexa Fluor 488 anti‐rat IgG Ab (Thermo Fisher Scientific) containing 1 µg/mL Hoechst 33 342 (Cell Signaling Technology). Cells with round‐to‐oval morphology, Hoechst 33342‐positive nuclei, CK‐positive staining in the cytoplasm, and CD45‐negative staining were identified as CTCs. Circulating tumor cell identification was independently examined by two of the investigators without any knowledge of the clinical data. In case of discrepancy between the two investigators, a consensus was reached by simultaneous examination. For CTC detection in the CellSearch system to compare the sensitivity, CTCs were quantitatively evaluated in 7.5 mL blood per standard manufacturer protocol. The number of CTCs in 1 mL blood was indicated as the CTC count for the CTC‐chip and that in 7.5 mL blood was indicated as the CTC count for the CellSearch system. Additionally, to evaluate nonspecific detection, peripheral blood samples were collected from 10 healthy individuals and used to detect CTCs in the same manner.
2.5. Genetic analysis for validation of captured cells with EpCAM‐chip
For the validation of captured cells with the EpCAM‐chip, 1 mL (100 cells/mL) of blood collected from healthy volunteers containing PC9 (EGFR exon 19 deletion), NCI‐H441 (KRAS G12V mutation), and NCI‐H1975 (EGFR exon 21 L858R and exon 20 T790M mutations) was applied to the CTC‐chip system in the above method. After tumor cell identification, captured single cells were collected into 200‐μL tubes (including 2 μL PBS) by micromanipulator M‐152 (Narishige Group), and was submitted for WGA using the PicoPLEX Single Cell WGA Kit version 3 (Takara Bio) according to the manufacturer’s instructions, in order to obtain a sample suitable for sequencing analysis. Mutational analysis of EGFR exons 19 and 21 and KRAS codon 12 was based on our previous reports. 21 Polymerase chain reaction was carried out by using Taq polymerase (Takara Taq; Takara Bio) and sequences of the primers are as follows: EGFR exon 19, 5′‐GTCTTCCTTCTCTCTCTGTCATAG‐3′ (sense) and 5′‐CCACACAGCAAAGCAGAAACTCAC‐3′ (antisense); EGFR exon 21, 5′‐TCAAGATCACAGATTTTGGGCG‐3′ (sense) and 5′‐CATCCTCCCCTGCATGTGTTAAAC‐3′ (antisense); and KRAS codon 12, 5′‐AAACTTGTGGTAGTTGGACCT‐3′ (sense) and 5′‐CTATTGTTGGATCATATTCG‐3′ (antisense).
A fraction of KRAS PCR product was subsequently digested with BstNI (New England Biolabs) to identify codon 12 mutation. All PCR reactions were carried out on the GeneAmp PCR System 9700 (Thermo Fisher Scientific). Amplification of the intended fragments was confirmed by electrophoresis on 3.0% agarose gel (Nippon Gene Co., Ltd.) containing 0.5 μg/mL ethidium bromide (Genesee Scientific).
2.6. Statistical analysis
Differences in categorical variables were evaluated using Pearson’s χ 2 test or Fisher’s exact test, as appropriate. Differences in continuous variables were evaluated using a t test (Student’s t test or Welch’s t test) for normally distributed data and the Mann‐Whitney U test for nonnormally distributed data. Cancer‐specific survival was calculated from the date of the CTC test until cancer‐specific mortality or the last follow‐up. Progression‐free survival was calculated from the date of the CTC test until recurrence, mortality, or the last follow‐up. The Kaplan‐Meier method was used to estimate CSS and PFS, and survival differences were compared between the two groups using the log‐rank test. The analysis of prognostic factors was undertaken using the Cox proportional hazards regression model. A P value of less than .05 was considered statistically significant. All statistical analyses were carried out using SPSS (version 27.0; IBM).
3. RESULTS
3.1. Optimizing EpCAM‐chip and cell‐capture efficiency in lung cancer cell lines
In the previous study, with a base Ab concentration of 20 μg/mL and a capture Ab concentration of 20 μg/mL, PC9 cells spiked in blood were captured with an average capture rate of 88%. 18 When the concentration of the base Ab was increased to 200 μg/mL to achieve a higher capture rate, PC9 cells were captured at approximately 100% (P = .022; Figure S1A), and we adopted this condition for further experiments. NCI‐H441 cells with positive EpCAM expression were effectively captured with the optimized EpCAM‐chip (average capture efficiency, 88.8%). In contrast, A549 cells with low EpCAM expression were not effectively captured with the EpCAM‐chip (average capture efficiency, 13.4%) (Figure S1B).
3.2. Comparison of CTC detection sensitivity of EpCAM‐chip and CellSearch in lung cancer patients
Overall, 31 peripheral blood samples drawn from individual lung cancer patients were subjected to quantitative analyses for CTCs using the EpCAM‐chip (Figure S2) and CellSearch system. Patients’ characteristics and distribution of CTC count with the EpCAM‐chip and CellSearch system for each sample are summarized in Table 1 and Figure 1A. Circulating tumor cells were detected in 58.1% samples (18/31 patients) with the EpCAM‐chip and in 25.8% samples (8/31 patients) with the CellSearch platform. Median CTC counts with the EpCAM‐chip and CellSearch system were 1/1 mL (range, 0‐11) and 0/7.5 mL (range, 0‐19), respectively, and average CTC counts were 1.88/1 mL and 1.74/7.5 mL, respectively. Despite no significant difference being observed between the two devices (P = .257), the results suggested that the EpCAM‐chip has better performance in CTC detection. In 10 healthy participants, no cells were detected, and there was no obvious nonspecific detection (Figure 1B).
TABLE 1.
Characteristics of 31 patients with lung cancer
| Characteristics | Patients (n = 31) |
|---|---|
| Age (y); median (range) | 69.0 (55‐91) |
| Sex | |
| Male | 19 (61.3) |
| Female | 12 (38.7) |
| TNM stage | |
| IA/IB | 2 (6.5)/7 (22.6) |
| IIA/IIB | 2 (6.5)/1 (3.2) |
| IIIA/IIIB | 9 (29.0)/2 (6.5) |
| IV | 8 (25.8) |
| Histology | |
| Adenocarcinoma | 16 (51.6) |
| Squamous cell carcinoma | 12 (38.7) |
| Others | 3 (9.7) |
Data are shown as n (%) unless otherwise indicated.
FIGURE 1.
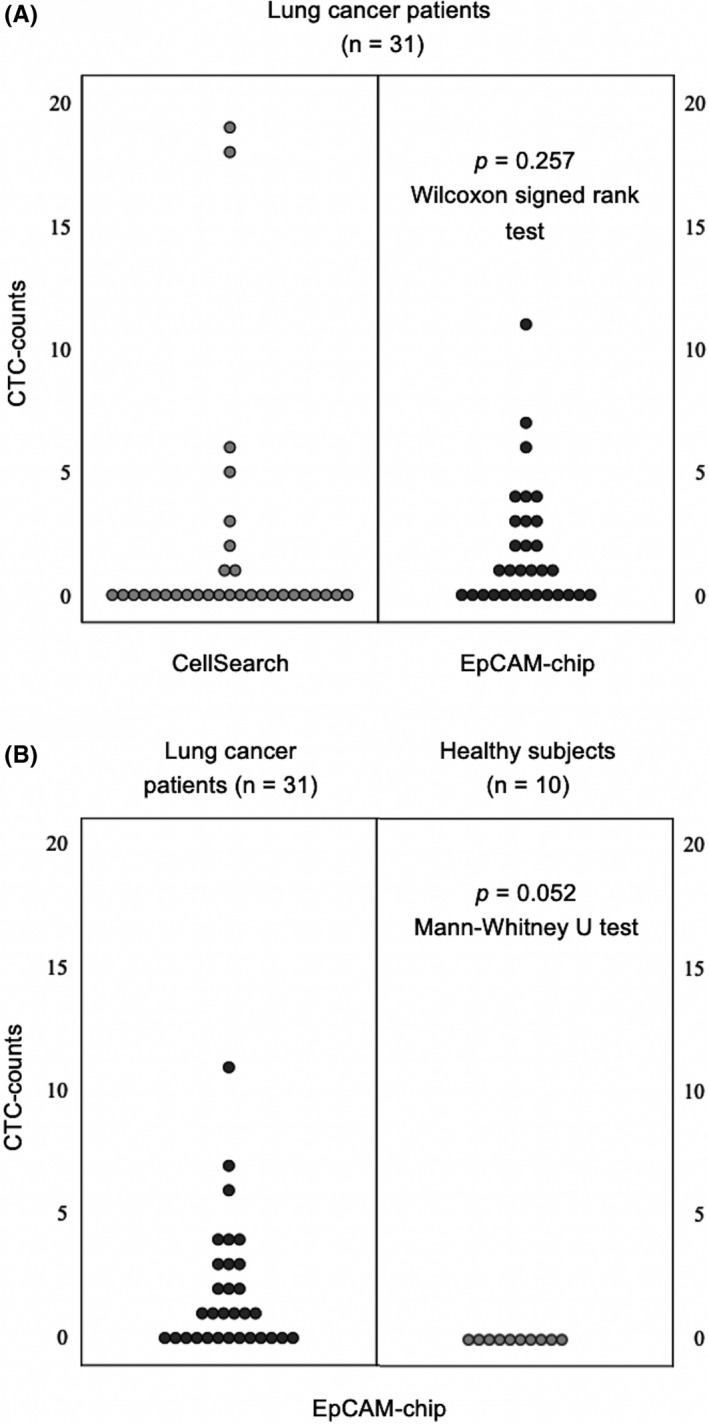
A, Distribution of circulating tumor cells (CTCs) detected using the CellSearch system and epithelial cell adhesion molecule (EpCAM)‐chip according to their count in 7.5 mL and 1 mL peripheral blood collected from lung cancer patients. B, Distribution of CTCs detected using the EpCAM‐chip according to their count in 1 mL peripheral blood collected from lung cancer patients and healthy participants
3.3. Clinical impact of CTCs detected with EpCAM‐chip in lung cancer patients
To evaluate the clinical implications of CTCs detected using the EpCAM‐chip, we further analyzed additional blood samples drawn from a total of 123 lung cancer patients prior to treatment, especially surgery (n = 71, including postinduction therapy [n = 3]) or chemotherapy (n = 52, including more than second‐line therapy [n = 8]) (Figure 2). Patients’ characteristics are described in Table 2. Circulating tumor cells were detected in 58 patients (47.2%), and the median and average CTC counts were 0 and 1.73 (range, 0‐29), respectively, in the overall population. Circulating tumor cell positivity according to the stage was as follows: 26.5% (9/34) in stage I, 56.3% (9/16) in stage II, 50.0% (12/24) in stage III, and 57.1% (28/49) in stage IV. The detection rate of CTCs was significantly higher in chemotherapy group patients than in surgery group patients (P = .0062). These results suggested that CTCs are more easily detected in inoperable patients due to lymph node metastasis or distant metastasis, and that CTCs might contribute to tumor progression. The survival analysis is presented in Figure 3. The median follow‐up was 984 (range, 13‐1684) days. The 1‐year/3‐year CSS rates for the CTC‐positive and CTC‐negative groups were 69.8%/60.3% and 90.1%/72.3%, respectively. The 1‐year/3‐year PFS rates for the CTC‐positive and CTC‐negative groups were 39.5%/23.9% and 68.5%/50.5%, respectively. Both CSS (P = .037) and PFS (P = .0041) were significantly better in the CTC‐negative group (Figure 3A). Patient characteristics for the surgical group, excluding postinduction therapy, (n = 68) are described in Table S1. Circulating tumor cells were detected in 24 patients (35.3%), and the median and average CTC counts were 0 and 1.53 (range, 0‐29), respectively. In the CTC‐negative group, 54.5% patients were stage I, whereas 37.5% were stage I patients in the CTC‐positive group. The 1‐year/3‐year CSS rates for the CTC‐positive and CTC‐negative groups were 91.3%/95.1% and 81.9%/80.7%, respectively. The 1‐year/3‐year PFS rates for the CTC‐positive and CTC‐negative groups were 69.9%/38.6% and 75.6%/60.7%, respectively. Cancer‐specific survival was similar between the CTC‐positive and CTC‐negative groups (P = .841); PFS tended to be better in the CTC‐negative group, although the difference was not statistically significant (P = .115) (Figure 3B). Patient characteristics (n = 52) for the chemotherapy group are described in Table S2. Circulating tumor cells were detected in 32 patients (61.5%), and the median and average CTC counts were 1 and 1.88 (range, 0‐12), respectively. The 1‐year/3‐year CSS rates for the CTC‐positive and CTC‐negative groups were 79.4%/50.0% and 59.7%/39.4%, respectively. The 1‐year/3‐year PFS rates for the CTC‐positive and CTC‐negative groups were 52.9%/27.5% and 13.3%/6.7%, respectively. In the CTC‐positive group, CSS (P = .060) and PFS (P = .012) were associated with poor prognosis, and the difference in PFS was statistically significant (Figure 3C). Furthermore, in the analysis of patients with (17 patients; EGFR tyrosine kinase inhibitors, 14 patients; anaplastic lymphoma kinase inhibitors, 3 patients) and without (35 patients) the use of molecular targeted agents, there was no significant difference in CSS (P = .567) or PFS (P = .973) in patients with molecular targeted agents (Figure S3A). In patients without molecular targeted agents, both CSS (P = .095) and PFS (P = .0089) were poor in the CTC‐positive group, with a significant difference in PFS (Figure S3B).
FIGURE 2.
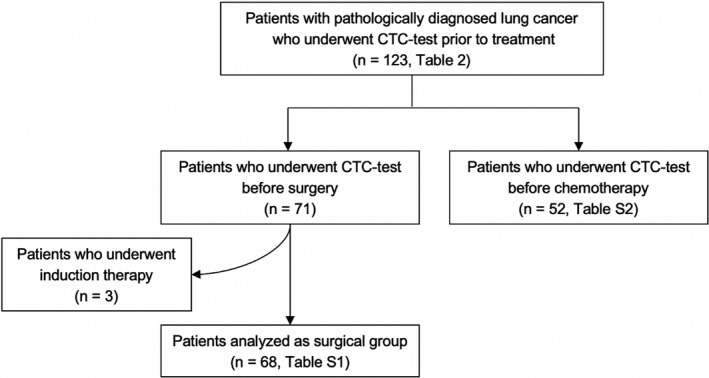
CONSORT diagram of the study. CTC, circulating tumor cell
TABLE 2.
Characteristics of 123 lung cancer cases according to circulating tumor cell (CTC) counts (CTC ≥ 1/CTC = 0)
| Characteristic | CTC ≥ 1 (n = 58) | CTC = 0 (n = 65) | P value |
|---|---|---|---|
| Age (y); median (range) | 74.0 (42‐91) | 73.0 (49‐91) | .883 |
| Sex | |||
| Male | 36 (62.1) | 43 (66.2) | .637 |
| Female | 22 (37.9) | 22 (33.8) | |
| Tumor laterality | |||
| Right | 32 (55.2) | 41 (63.1) | .373 |
| Left | 26 (44.9) | 24 (36.9) | |
| Histology | |||
| Adenocarcinoma | 34 (58.6) | 45 (69.2) | .206 |
| Squamous cell carcinoma | 17 (29.3) | 13 (20.0) | |
| Small cell carcinoma | 3 (5.2) | 4 (6.2) | |
| Others | 4 (6.9) | 3 (4.6) | |
| TNM stage | |||
| IA/IB | 5 (8.6)/4 (6.9) | 13 (20.0)/12 (18.5) | .217 |
| IIA/IIB | 2 (3.4)/7 (12.1) | 5 (7.7)/2 (3.1) | |
| IIIA/IIIB/IIIC | 6 (10.3)/4 (6.9)/2 (3.4) | 9 (13.8)/2 (3.1)/1 (1.5) | |
| IVA/IVB | 15 (25.9)/13 (22.4) | 11 (16.9)/10 (15.4) | |
| Classification | |||
| Surgery | 26 (44.8) | 45 (69.2) | .0062 |
| Postadjuvant therapy | 2 (7.7) | 1 (2.2) | |
| Chemotherapy | 32 (55.2) | 20 (30.8) | |
| First line | 29 (90.6) | 16 (80.0) | |
| More than second line | 3 (9.4) | 4 (20.0) | |
| CTC counts (median, average) | 2, 3.67 | 0, 0 | |
Data are shown as n (%) unless otherwise indicated.
FIGURE 3.
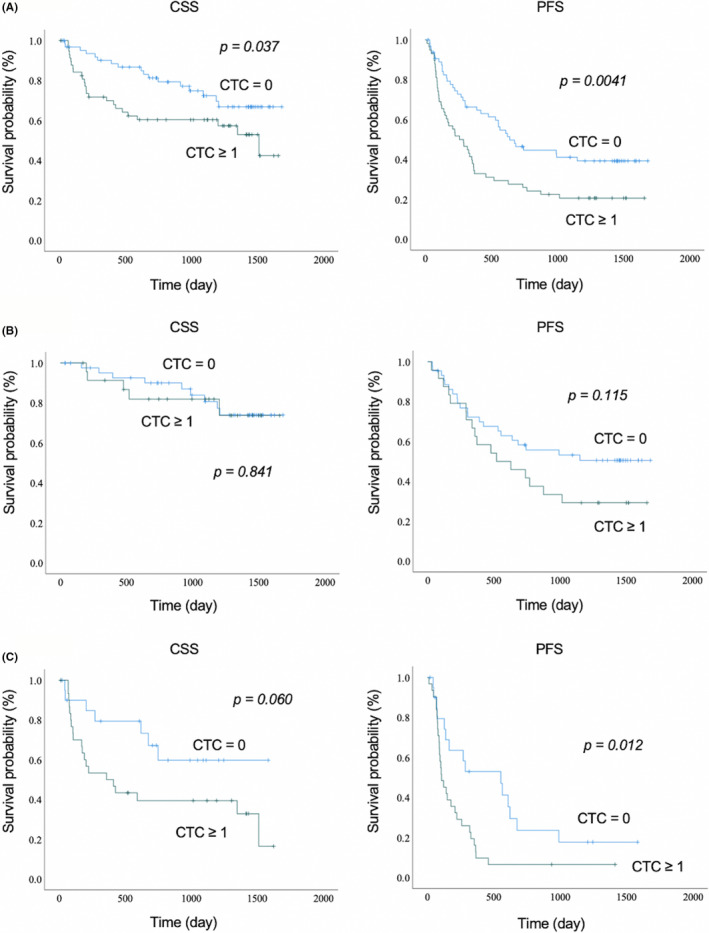
Cancer‐specific survival (CSS) and progression‐free survival (PFS) according to circulating tumor cell (CTC) count (CTC ≥ 1/CTC = 0) in (A) all cases, (B) surgery patients, and (C) chemotherapy patients
3.4. Genetic analysis for validation of cells captured with EpCAM‐chip
The detection of cells from the CTC‐chip is described in Figure 4, and the results of electrophoresis of cells captured by the CTC‐chip are presented in Figure 5. The PCR products containing exon 19 revealed a 147‐bp band when the allele was a WT and a shorter band (132 bp) when the allele was a deletion type. To detect a point mutation of the second base of codon 858 in exon 21, mutant allele‐specific amplification was carried out, and only the DNA sample with a mutation revealed a band. Following BstNI (New England Biolabs) digestion, the 94‐bp product of the WT KRAS was cut into 74 bp and 20 bp fragments. If there is any mutation at the second position of codon 12 of KRAS, BstNI digestion is not possible. In cell line analysis, genetic mutations in each cell line were detected in the cells captured by the CTC‐chip. Therefore, subsequent examinations of clinical specimens from lung cancer patients with EGFR exon 19 deletions (78‐year‐old woman with recurrence of intrapulmonary metastasis and pleural dissemination after treatment with EGFR tyrosine kinase inhibitor osimertinib for pT4 [ipsilateral intrapulmonary metastasis] N0M1a stage 4a) revealed that the captured cells had EGFR exon 19 deletions (CTCs) (Figure 6).
FIGURE 4.
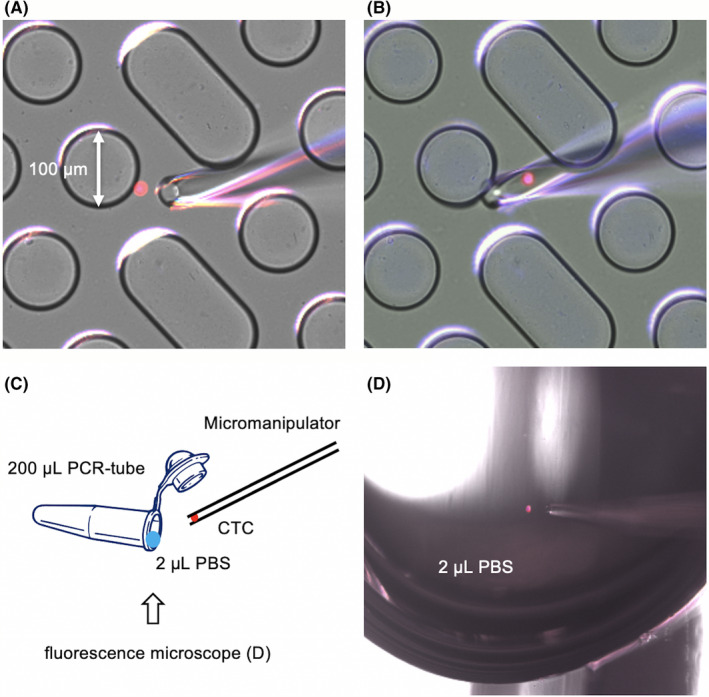
Images of cells trapped in the epithelial cell adhesion molecule (EpCAM)‐chip being collected using a micromanipulator. CTC, circulating tumor cell
FIGURE 5.
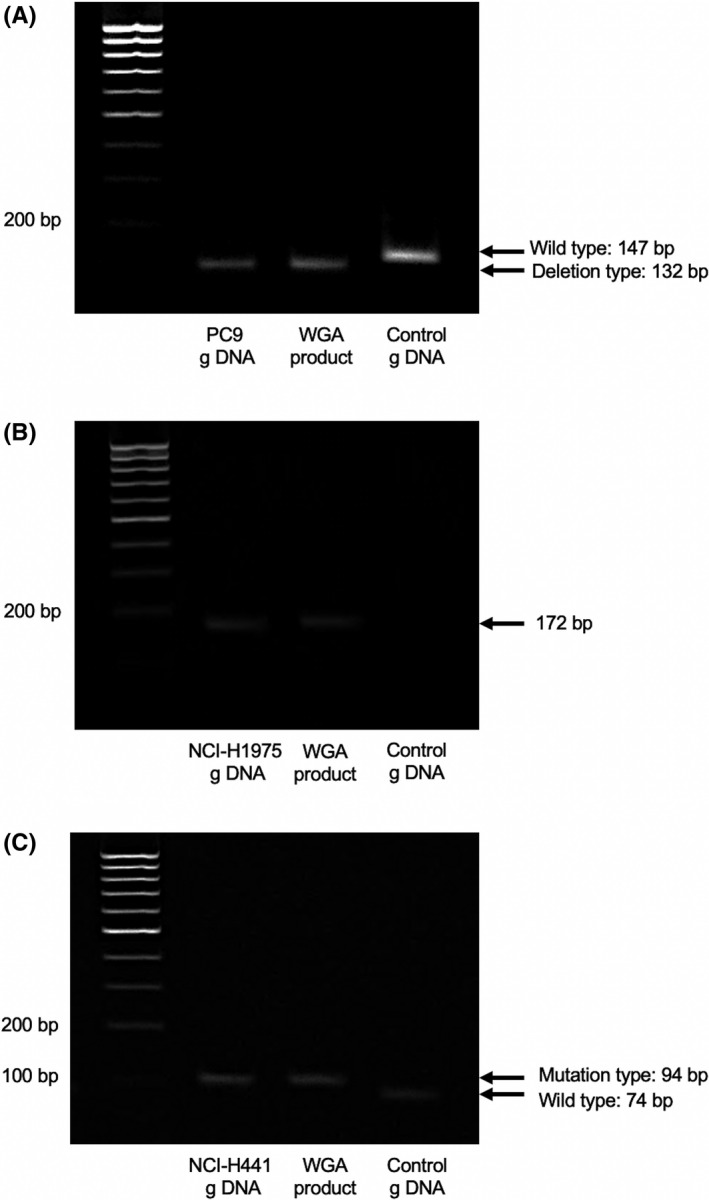
Results of electrophoresis for cells. (A) PC9 (EGFR exon 19 deletion), (B) NCI‐H1975 (EGFR exon 21 L858R and exon 20 T790 M mutations), and (C) NCI‐H441 (KRAS G12V mutation) captured using the epithelial cell adhesion molecule (EpCAM)‐chip. g DNA, genomic DNA; WGA, whole‐genome amplification
FIGURE 6.
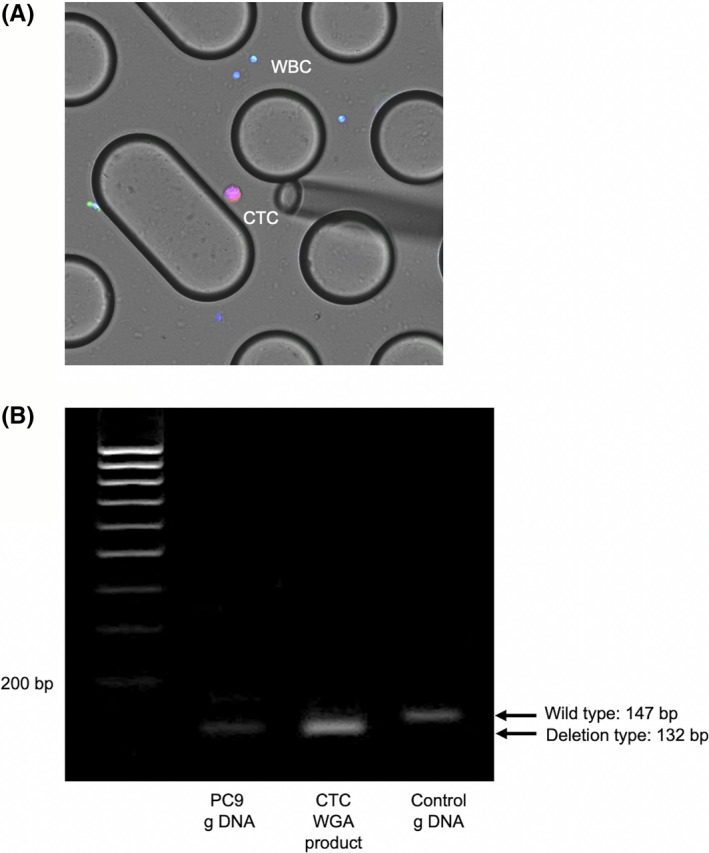
A, Image of immunohistochemical staining of cells in the peripheral blood of lung cancer patients with EGFR exon 19 deletion captured using the epithelial cell adhesion molecule (EpCAM)‐chip. B, Electrophoresis results of these cells. CTC, circulating tumor cell; g DNA, genomic DNA; WBC, white blood cell; WGA, whole‐genome amplification
4. DISCUSSION
In our study, we first optimized the EpCAM‐chip by increasing the concentration of the base Ab that showed better performance than the CellSearch system in clinical cases. Second, PFS was worse in CTC‐positive patients in both surgical and chemotherapy cases, indicating that the detection of baseline CTCs is a risk factor for recurrence and progression of lung cancer. Finally, we determined that the cells captured with the CTC‐chip were true CTCs by recovering them using a micromanipulator and undertaking genetic mutation analysis.
Enumeration of CTCs can provide prognostic information for cancer management. 9 , 10 , 22 However, it is extremely difficult to detect CTCs present in very small amounts in the blood, and even the CellSearch system, the only method approved for clinical use by the US FDA, has been suggested to have insufficient detection sensitivity. 8 , 12 , 14 Therefore, we developed a new microfluidic device, the CTC‐chip, as a more sensitive detection system. 17 , 18 In our study, the EpCAM‐chip was optimized and showed higher capture efficiency than the CellSearch system with clinical specimens. Furthermore, pretreated CTC‐positive patients evaluated with the optimized EpCAM‐chip had predominantly poor PFS and CSS, suggesting the clinical significance of baseline CTC count as a prognostic marker. In particular, CTCs could be useful in assessing the risk of recurrence and progression as CTC‐positive patients had poor PFS even after stratification by surgery and chemotherapy. These results indicated that a CTC test could be useful in detecting and predicting the presence of minimal residual disease, which could cause tumor recurrence after complete surgical resection, and in prescribing optimal postoperative therapy. Additionally, the evaluation of risk factors for recurrence might also be useful in selecting the appropriate chemotherapy. Although there was no difference in CSS or PFS between CTC‐positive and CTC‐negative patients treated with molecular targeted agents, the number of cases was small at 17, and five of them (CTC ≥1, 2 cases; CTC = 0, 3 cases) were more than second‐line treatment. Therefore, further studies are needed to validate these results with more accumulated cases.
Recent studies have recognized that CTCs are useful not only in providing various information about the primary tumor and distant metastasis but also in elucidating molecular mechanisms involved in cancer evolution. 23 , 24 , 25 In addition to the previous information on the number of CTCs, genetic analysis of captured CTCs is expected to provide new insights, which should be validated in future prospective studies.
There are several limitations to this study. First, the detection of CTCs was essentially done only once for each patient. Monitoring CTCs during and after treatment might provide unique information for the clinical management of an individual cancer patient and allow an early induction or change in therapy before the appearance of overt metastases signals. Therefore, we are currently collecting peripheral blood samples from patients who are newly diagnosed or treated for lung cancer, which will reveal the prognostic and predictive value of our CTC detection system. Second, the molecular biological analysis of captured CTCs has not been fully performed. There is increasing evidence that the phenotype and genotype of primary and metastatic cancer cells are discordant and that the molecular analysis of CTCs isolated from peripheral blood will reveal characteristics of metastatic cancer cells. 2 , 3 , 4 We are currently staining captured CTCs for PD‐L1 and undertaking genetic analysis using next‐generation sequencers to characterize the molecular characteristics of CTCs, which will help us to evaluate treatment resistance and elucidate the mechanism of resistance. Finally, the EpCAM‐chip showed more sensitive detection than the CellSearch system; however, CTCs were not detected in approximately 40% of patients with stage IV lung cancer. This result suggested that the EpCAM‐chip might overlook CTCs undergoing EMT, 26 , 27 which is considered an important metastasis‐causing cell subtype. 28 , 29 Therefore, we are currently integrating clinical specimens using the CTC‐chip with Abs against CSV as an EMT‐CTC marker, 30 , 31 , 32 , 33 which might be a more prognostically correlated indicator than EpCAM.
In conclusion, this study showed that the EpCAM‐chip has a high performance in detecting CTCs in lung cancer patients, and the number of detected CTCs is a useful risk factor for recurrence and progression. Our current efforts to capture CTCs using CSV and elucidation of the molecular characteristics of captured CTCs using additional staining for PD‐L1 and genetic analysis will reveal the further clinical value of CTCs. We believe that the universal CTC‐chip can become an indispensable tool for developing new diagnostic, therapeutic, and prognostic options for lung cancer patients.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Fig S1‐S3
Tab S1‐S2
ACKNOWLEDGMENTS
We thank every patient who participated in the study. We also want to express our sincere gratitude to all clinical staff members and researchers who contributed to the study. This work was supported by JSPS KAKENHI Grant Number JP 19K09293.
Kanayama M, Kuwata T, Mori M, et al. Prognostic impact of circulating tumor cells detected with the microfluidic “universal CTC‐chip” for primary lung cancer. Cancer Sci. 2022;113:1028–1037. doi: 10.1111/cas.15255
Funding information
Japan Society for the Promotion of Science, Grant/Award Number: 19K09293
REFERENCES
- 1. Alix‐Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623‐631. [DOI] [PubMed] [Google Scholar]
- 2. Alix‐Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013‐5021. [DOI] [PubMed] [Google Scholar]
- 3. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531‐548. [DOI] [PubMed] [Google Scholar]
- 4. Cabel L, Proudhon C, Gortais H, et al. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421‐430. [DOI] [PubMed] [Google Scholar]
- 5. Dotan E, Cohen SJ, Alpaugh KR, Meropol NJ. Circulating tumor cells: evolving evidence and future challenges. Oncologist. 2009;14:1070‐1082. [DOI] [PubMed] [Google Scholar]
- 6. Xu T, Lu B, Tai YC, Goldkorn A. A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter. Cancer Res. 2010;70:6420‐6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takakura M, Kyo S, Nakamura M, et al. Circulating tumour cells detected by a novel adenovirus‐mediated system may be a potent therapeutic marker in gynaecological cancers. Br J Cancer. 2012;107:448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897‐6904. [DOI] [PubMed] [Google Scholar]
- 9. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781‐791. [DOI] [PubMed] [Google Scholar]
- 10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213‐3221. [DOI] [PubMed] [Google Scholar]
- 11. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration‐resistant prostate cancer. Clin Cancer Res. 2008;14:6302‐6309. [DOI] [PubMed] [Google Scholar]
- 12. Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non‐small‐cell lung cancer. J Clin Oncol. 2011;29:1556‐1563. [DOI] [PubMed] [Google Scholar]
- 13. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15:6980‐6986. [DOI] [PubMed] [Google Scholar]
- 15. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung‐cancer cells. N Engl J Med. 2008;359:366‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohnaga T, Shimada Y, Moriyama M, et al. Polymeric microfluidic devices exhibiting sufficient capture of cancer cell line for isolation of circulating tumor cells. Biomed Microdevices. 2013;15:611‐616. [DOI] [PubMed] [Google Scholar]
- 18. Chikaishi Y, Yoneda K, Ohnaga T, Tanaka F. EpCAM‐independent capture of circulating tumor cells with a 'universal CTC‐chip'. Oncol Rep. 2017;37:77‐82. [DOI] [PubMed] [Google Scholar]
- 19. Yoneda K, Kuwata T, Chikaishi Y, et al. Detection of circulating tumor cells with a novel microfluidic system in malignant pleural mesothelioma. Cancer Sci. 2019;110:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuwata T, Yoneda K, Mori M, et al. Detection of circulating tumor cells (CTCs) in malignant pleural mesothelioma (MPM) with the "Universal" CTC‐Chip and An Anti‐Podoplanin Antibody NZ‐1.2. Cells. 2020;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugio K, Uramoto H, Ono K, et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer. 2006;94:896‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poveda A, Kaye SB, McCormack R, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol. 2011;122:567‐572. [DOI] [PubMed] [Google Scholar]
- 23. Kaganoi J, Shimada Y, Kano M, Okumura T, Watanabe G, Imamura M. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg. 2004;91:1055‐1060. [DOI] [PubMed] [Google Scholar]
- 24. Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small‐cell lung cancer. J Clin Oncol. 2012;30:525‐532. [DOI] [PubMed] [Google Scholar]
- 25. Pilati P, Mocellin S, Bertazza L, et al. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann Surg Oncol. 2012;19(2):402‐408. [DOI] [PubMed] [Google Scholar]
- 26. Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM‐based detection due to epithelial‐to‐mesenchymal transition. BMC Cancer. 2012;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peeters DJ, van Dam PJ, Van den Eynden GG, et al. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Brit J Cancer. 2014;110:375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao R, Cai Z, Li S, et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget. 2017;8:9293‐9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satelli A, Mitra A, Cutrera JJ, et al. Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer Res. 2014;74:1645‐1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating tumor cell enumeration with a combination of epithelial cell adhesion molecule‐ and cell‐surface vimentin‐based methods for monitoring breast cancer therapeutic response. Clin Chem. 2015;61:259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Satelli A, Mitra A, Brownlee Z, et al. Epithelial‐mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res. 2015;21:899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Satelli A, Batth I, Brownlee Z, et al. EMT circulating tumor cells detected by cell‐surface vimentin are associated with prostate cancer progression. Oncotarget. 2017;8:49329‐49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Tab S1‐S2


