Abstract
Myxoid liposarcoma (MLPS) is genetically characterized by FUS‐DDIT3 or EWSR1‐DDIT3 gene fusion and the high frequency of hotspot mutations (C228T or C250T) in the promoter region of telomerase reverse transcriptase (TERT) that encodes the TERT protein. The latter leads to telomerase reactivation, a mechanism of telomere maintenance. Although the TERT promoter hotspot mutation is a poor prognostic factor in various tumors, its effect on MLPS has not been reported in detail. In the present study, we examined the clinicopathological characteristics, prognosis, and telomere maintenance mechanisms in 83 primary tumor samples of MLPS, which were resected surgically at the Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan, from 2008 to 2020. TERT promoter hotspot mutations were observed in 77% (63/82) cases, and alternative lengthening of telomeres (ALT) was absent in all cases. Among the cases without TERT promoter hotspot mutations, TERT rearrangements, and minor point mutations in the TERT promoter region were found in 3 and 2 cases, respectively. TERT mRNA expression was observed consistently even in patients for whom no genomic TERT aberrations were detected, and the presence of TERT promoter hotspot mutation did not correlate significantly with either overall and metastasis‐free survival (P = .56, P = .83, respectively) or clinicopathological features. Therefore, patients with MLPS characteristically shows TERT expression and a high prevalence of TERT aberrations. Our findings suggest that TERT aberration is not prognostic factor, but might occur at an early stage and play a key role in tumorigenesis.
Keywords: myxoid liposarcoma, telomerase, telomere, TERT, TERT promoter
In this study, we investigated the 2 types of telomere maintenance mechanisms in a large cohort of MLPS cases and examined the relationship between the telomere maintenance mechanism and disease prognosis or clinicopathological parameters in patients with MLPS. Their findings suggest that TERT aberrations are not prognostic factors, but might occur at an early stage and play key roles in tumorigenesis.
Abbreviations
- ALT
alternative lengthening of telomeres
- BAC
bacterial artificial chromosome
- Cl
confidence interval
- DDLPS
dedifferentiated liposarcoma
- ETS
E‐twenty six
- FFPE
formalin‐fixed paraffin‐embedded
- FNCLCC
French Fédération Nationale des Centers de Lutte Contre le Cancer
- HR
hazard ratio
- JFCR
Japanese Foundation for Cancer Research
- LI
labeling index
- MFS
metastasis‐free survival
- MLPS
myxoid liposarcoma
- OS
overall survival
- PML
promyelocytic leukemia
- PNA
peptide nucleic acid
- SCR
single copy reference
- SFT
solitary fibrous tumor
- TERC
telomerase RNA component
- TERT
telomerase reverse transcriptase
- TERTp
TERT promoter
- TL
telomere length
- TMA
tissue microarray
1. INTRODUCTION
Telomeres, which play a crucial role in cellular survival, shorten with each cell cycle, which leads to senescence or apoptosis. 1 In vertebrates, they consist of non‐coding tandem repeats of 5′‐TTAGGG‐3′ at the 3ʹ ends of chromosomes, which protect the chromosome ends from progressive degradation. 2 , 3 By maintaining telomere length (TL) via 2 known mechanisms: reactivation of telomerase and telomerase‐independent alternative lengthening of telomeres (ALT), 1 tumor cells can avoid senescence and apoptosis caused by telomere shortening and obtain immortality.
Up to 90% of human cancers are reported to possess reactivated telomerase. 4 Telomerase, a reverse transcriptase, adds a telomere repeat sequence to the 3′ end of telomeres. It is composed of a catalytic protein subunit, telomerase reverse transcriptase (TERT) encoded by TERT, and a telomerase RNA component (TERC) encoded by TERC, which is used as a template for elongating telomeres. 5 Telomerase activity is observed in the germline and stem cells, but is absent in most somatic cells. 6 In normal human somatic cells, TERT is repressed epigenetically, while TERC is ubiquitously expressed, and TERT re‐expression is regarded as a limiting factor for controlling telomerase activity. 7 , 8 , 9 , 10 In human cancer, 2 hotspot mutations in the TERT promoter (c. −124 C>T and c. −146 C>T), also called C228T and C250T, respectively, are known to be the most prevalent genetic changes that upregulate TERT mRNA expression by creating a binding site for E‐twenty six (ETS) transcription factors. 5 , 11 , 12
Most of the remaining 10%‐15% cancers achieve immortalization via a telomerase‐independent ALT pathway. 13 , 14 ALT is based on homologous recombination of telomeric sequences and is associated with deactivating mutations in the chromatin remodeling genes, ATRX and its binding partner DAXX. 13 , 15 Phenotypically, ALT‐positive cells are characterized by highly heterogeneous TLs, presence of ALT‐associated PML protein nuclear bodies and extrachromosomal telomeric circles, and genomic instability. 13 , 16 , 17 , 18
Myxoid liposarcoma (MLPS) accounts for 5% of all adult soft tissue sarcomas and 20%‐30% of liposarcomas. 19 It exhibits the reciprocal translocation, t(12;16)(q13;p11), in approximately 95% of patients, which results in the fusion of FUS‐DDIT3, and t(12;22)(q13;q12) in nearly 5% of patients, resulting in the formation of EWSR1‐DDIT3. 20 , 21 Soft tissue sarcomas generally show a high frequency of ALT, while MLPS is the only exception and is known to have a high prevalence of TERT promoter (TERTp) mutations (22.2%‐79.1%). 5 , 14 , 22 , 23 , 24 , 25 , 26 It has also been reported that 5%‐18% of patients with MLPS have ALT, 27 , 28 and telomerase activation and ALT are not mutually exclusive. 27 Costa et al 27 have reported that ALT is associated with aggressive behavior in the study of all types of liposarcoma, including a limited number of MLPS cases. However, the relationship between telomere maintenance mechanisms and prognosis in MLPS still remains unclear.
Therefore, in the present study, we investigated the 2 types of telomere maintenance mechanism, telomerase reactivation and ALT, in a large number of patients with MLPS. Then, we examined the relationship between the telomere maintenance mechanism and disease prognosis or clinicopathological parameters in patients with MLPS.
2. MATERIALS AND METHODS
2.1. Patient and tumor selection
In total, 83 MLPS samples included in the present study were resected surgically from each patient at the Cancer Institute Hospital, Japanese Foundation for Cancer Research (JFCR), Tokyo, Japan, between 2008 and 2020. All samples were obtained from primary lesions in patients, 3 of whom were treated preoperatively with chemotherapy, radiation therapy, or chemoradiotherapy. Medical records from January 2008 to October 2020 were reviewed retrospectively to obtain clinical information such as sex, age, treatment history, follow‐up data, and tumor size. Primary cases of 36 patients with dedifferentiated liposarcoma (DDLPS) were retrieved from the pathology files of the Cancer Institute Hospital, JFCR, between 2009 and 2017. DDLPS cases were used as a control for ALT and telomere length analyses. Unstained sections from formalin‐fixed paraffin‐ embedded (FFPE) specimens were available for all 83 MLPS cases and 36 DDLPS cases, and frozen tumor specimens were available for 75 MLPS cases. This study was conducted in accordance with the principles embodied in the Declaration of Helsinki, and was approved by the Institutional Review Board of the JFCR (IRB number 2020‐1120, August 18, 2020).
2.2. Histological review
The slides of all MLPS cases were reviewed by 2 pathologists (JK and KY) using the French Fédération Nationale des Centers de Lutte Contre le Cancer (FNCLCC) system. 29 Examined factors in the grading system were as follows: tumor differentiation (MLPS or high‐grade MLPS), mitotic activity (0‐9, 10‐19, or ≥20 mitoses per 10 high‐power fields), and tumor necrosis (absent, <50%, or ≥50%). High‐grade MLPS was defined by the presence of ≥5% round cell component, which was characterized by marked increase in cellularity, reduction in myxoid matrix, and the presence of round cells with nuclear overlap. 30
2.3. Tissue microarrays
Tumor tissues were fixed in 20% neutral buffered formalin and embedded in paraffin. Three histologically representative sites were selected from each MLPS case and 2 representative areas were selected from each DDLPS case. Tissue microarrays (TMAs) were generated as described previously. 31 Briefly, selected sites were punched with a 2 mm‐diameter coring needle on the donor paraffin blocks and displaced to the array in the recipient block.
2.4. Immunohistochemistry
For immunohistochemistry, the 4‐μm thick TMA sections of MLPS were stained with antibodies against p53, FOXM1, NY‐ESO‐1, and Ki‐67. Primary antibodies are listed in Table S1. For p53 and NY‐ESO‐1, nuclear staining of ≥10% tumor cells was required for a case to be positive. A cut‐off point of ≥10% nuclear staining for p53 was based on previous reports. 20 , 32 For FOXM1, tumors with moderate to strong nuclear staining in ≥1% tumor cells were recorded as positive. The Ki‐67 labeling index (LI) was calculated as the percentage of positive cells. For statistical analyses, we divided the patients into 2 groups: Ki‐67 low (LI <5%) and Ki‐67 high (LI ≥5%).
2.5. Fluorescence in situ hybridization
To identify rearrangements of DDIT3, FUS, EWSR1, and TERT, and amplification of MDM2 and TERT, fluorescence in situ hybridization (FISH) was performed using bacterial artificial chromosome (BAC) clone‐derived DNA probes. DNA from the BAC clones was extracted using PI‐80X (Kurabo) and labeled fluorescently with the nick translation kit (Abbott Molecular Inc). The names of the BAC clones used will be provided upon request. The unstained 4‐μm thick TMA sections were hybridized with fluorescent DNA probes and the hybridized slides were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) and examined using a BX51 fluorescence microscope (Olympus).
2.6. Telomere‐specific immunostaining FISH
For assessing ALT, combined telomere‐specific FISH and immunofluorescence labeling of PML were performed on unstained 4‐μm thick TMA sections as described previously. 33 , 34 Tissue sections were hybridized with peptide nucleic acid (PNA) probes for the telomere and the centromere (Table S2). After post‐hybridization washes, tissue sections were incubated with an anti‐PML antibody (Table S1). They were then incubated with biotinylated anti‐mouse immunoglobulins (1:1000 dilution; Dako), followed by incubation with Alexa Fluor 647 streptavidin (1:1000 dilution; Thermo Fisher Scientific). The nuclei were stained with DAPI. The slides were examined under a BX51 fluorescence microscope at ×600 magnification. The criteria used for interpreting the FISH results were the same as those mentioned previously. 35 Briefly, the sample was considered ALT positive if ≥1% of tumor cells displayed ultrabright intranuclear foci of telomere FISH signals. At least 500 cells were assessed in each sample. Telomere‐PML colocalization was used to support classifying cases as ALT positive.
2.7. DNA extraction
Genomic DNA was extracted from fresh‐frozen specimens using the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's protocol. In cases for which fresh‐frozen specimens were unavailable, DNA was extracted from 3‐10 (depending on the size of the tumor sample) 10‐µm thick sections of FFPE specimens using the RecoverAll Total Nucleic Acid Isolation Kit (Thermo Fisher Scientific).
2.8. DNA sequencing of the TERT promoter region
For detection of TERTp hotspot mutations, C228T and C250T, we performed genomic PCR and Sanger sequencing as described previously. 36 Briefly, PCR was performed using PrimeSTAR® GXL DNA polymerase (TaKaRa Bio). In addition, to detect other minor TERTp point mutations, a broader TERT promoter region (−280 to +80 bp from the ATG start site) was amplified. The primers are listed in Table S2.
2.9. TERT mRNA expression analysis
Relative TERT mRNA expression of each RNA sample (MLPS and non‐tumor soft tissue) was assessed using real‐time reverse transcription PCR (real‐time RT‐PCR). Total RNA was isolated from fresh‐frozen specimens using the RNeasy Mini Kit (Qiagen) and was converted to cDNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) and random primers. Real‐time RT‐PCR was conducted in triplicate using the TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific). The previously reported primer‐probe sets (Table S2) and TaqMan Gene Expression Assays (#Hs99999905_m1; Thermo Fisher Scientific) were used for TERT and GAPDH, respectively. 37 TERT expression levels were normalized using GAPDH, and relative expression was calculated using the method.
2.10. Measurement of absolute telomere length
The mean TL of each DNA sample (MLPS, DDLPS, and normal tissue) was measured using the Absolute Human Telomere Length Quantification qPCR Assay Kit (ScienCell Research Laboratories) according to the manufacturer's instructions.
2.11. Statistical analysis
All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing). 38 Significance was defined as P < .05. The Mann‐Whitney U test was used to compare the relative values of TERT mRNA and TL. Overall survival (OS) was defined as the interval between the date of tumor resection and the date of death or last follow‐up, and metastasis‐free survival (MFS) was defined as the duration from the date of tumor resection to the date of identification of distant metastasis. Cox univariate regression models were used to evaluate the association between the potential clinicopathological factors and OS or MFS. Survival curves were estimated using the Kaplan‐Meier method and compared statistically using the log‐rank test. Fisher exact test was used for the comparison of categorical variables between the 2 groups, and paired t test was used for comparison between the 2 corresponding groups.
3. RESULTS
3.1. Clinicopathological characteristics
Table 1 summarizes the clinical and pathological features of the patients. In total, 83 cases of MLPS comprised 52 men and 31 women, with a median age of 42 y (range: 21‐76 y). The mean and median tumor sizes were 10.1 and 9.5 cm, respectively (range: 3.0‐27.0 cm). Twenty‐three patients (28%) had high‐grade MLPS (Figure 1A,B). In the FNCLCC grading system, 41 cases were grade I, 37 cases were grade II, and 5 cases were grade III. Positive results for p53, FOXM1, and NY‐ESO‐1 were observed in 27 (33%), 42 (51%), and 65 (78%) patients, respectively. The number of Ki‐67 LI high cases was 30 (36%).
TABLE 1.
Clinical and pathological features
| n | % | |
|---|---|---|
| Sex | ||
| Male | 52 | 63 |
| Female | 31 | 37 |
| Age (y) | ||
| <50 | 62 | 75 |
| ≥50 | 21 | 25 |
| Median age (y; range) | 42 (21‐76) | |
| Size (cm) | ||
| <10 | 46 | 55 |
| ≥10 | 37 | 45 |
| Median tumor size (cm; range) | 9.5 (3‐27) | |
| Location | ||
| Thigh | 48 | 58 |
| Lower leg | 12 | 14 |
| Knee | 6 | 7 |
| Buttock | 4 | 5 |
| Inguinal region | 3 | 4 |
| Other | 10 | 12 |
| Histotype | ||
| MLPS | 60 | 72 |
| High‐grade MLPS | 23 | 28 |
| Necrosis (%) | ||
| None | 49 | 59 |
| <50 | 29 | 35 |
| ≥50 | 5 | 6 |
| Mitotic count | ||
| 0‐9/10 HPF | 79 | 95 |
| 10‐19/10 HPF | 4 | 5 |
| ≥20/10 HPF | 0 | 0 |
| FNCLCC grade | ||
| I | 41 | 49 |
| II | 37 | 45 |
| III | 5 | 6 |
| Fusion gene | ||
| FUS‐DDIT3 | 74 | 89 |
| EWSR1‐DDIT3 | 9 | 11 |
Abbreviations: FNCLCC, French Fédération of Cancer Centers; HPF, high‐power fields; MLPS, myxoid liposarcoma.
FIGURE 1.
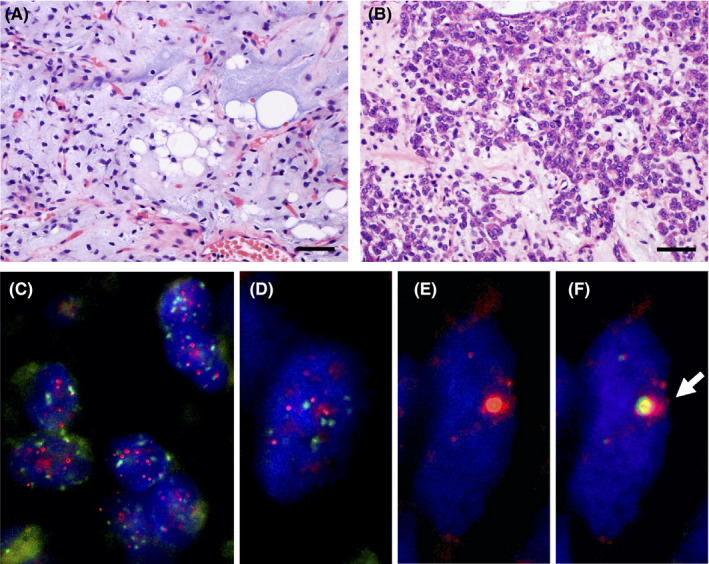
Histology and telomere‐specific immunostaining fluorescence in situ hybridization (FISH). A, B, Representative histology of myxoid liposarcoma (MLPS). Scale bar, 50 µm. A, Traditional area of MLPS; B, round cell components. C‐F, Representative images of telomere‐specific FISH in alternative lengthening of telomeres (ALT)‐positive and ALT‐negative cases. C, D, The ALT‐negative case of MLPS (C) and dedifferentiated liposarcoma (DDLPS) (D). The centromere signals (green) are approximately the same intensity as the telomere signals (red). E, F, The ALT‐positive case of DDLPS. E, The large and bright red telomere signals indicated ALT. F, The green signals of promyelocytic leukemia protein colocalized with the ALT‐associated highly bright telomere signals (arrow)
3.2. Genetic background of tumors
A break‐apart in DDIT3 was confirmed in all 83 MLPS patients: 74 had FUS (FUS‐DDIT3 fusion) and 9 had EWSR1 break‐apart (EWSR1‐DDIT3 fusion) (Table 1). MDM2 amplification was confirmed using FISH in all 36 DDLPS cases.
3.3. Assessment of alternative lengthening of telomeres
The telomere‐specific FISH of 83 MLPS and 36 DDLPS cases (Figure 1C‐F) showed that all MLPS cases were ALT negative and 31% (11/36) DDLPS cases were ALT positive. Telomere/PML colocalization was also confirmed in all 11 ALT‐positive DDLPS cases. ALT‐negative cases showed relatively uniform intensity of tumor telomeric signals (Figure 1C,D), while ALT‐positive cases showed a marked heterogeneity in telomere signal intensity (Figure 1E,F).
3.4. Evaluation of TERT promoter hotspot mutation and TERT expression
DNA sequencing around the TERTp mutation hotspot was performed using 82 MLPS samples, as the PCR reaction failed for 1 sample because of poor DNA quality. We detected TERTp hotspot mutations in 63/82 cases (77%), C228T mutation in 58 cases, and C250T mutation in 5 cases. Next, we performed real‐time RT‐PCR for 42 samples whose RNA were available: 31 MLPS tumor samples (16 with TERTp hotspot mutation and 15 without TERTp hotspot mutation), and 11 non‐tumor soft tissue samples (5 muscle tissue and 6 adipose tissue). TERT mRNA expression was detected in all 31 MLPS cases, and the expression levels of both MLPS samples with or without a TERTp hotspot mutation were significantly higher than those in the non‐tumor soft tissue samples (P ≤ .001, respectively) (Figure 2). In the non‐tumor soft tissue, TERT mRNA expression was detectable in 1/11 samples. The expression levels in cases with TERTp hotspot mutations were significantly higher than those in cases without the mutation (P = .006). Cases in which TERT mRNA expression levels were higher than the lowest level of TERT mRNA expression in cases with TERTp hotspot mutation were classified as the high expression group, while the other cases were classified as the low expression group. In cases without TERTp hotspot mutation, 7 cases were classified in the high expression group and 8 cases were classified in the low expression group (Table 2).
FIGURE 2.
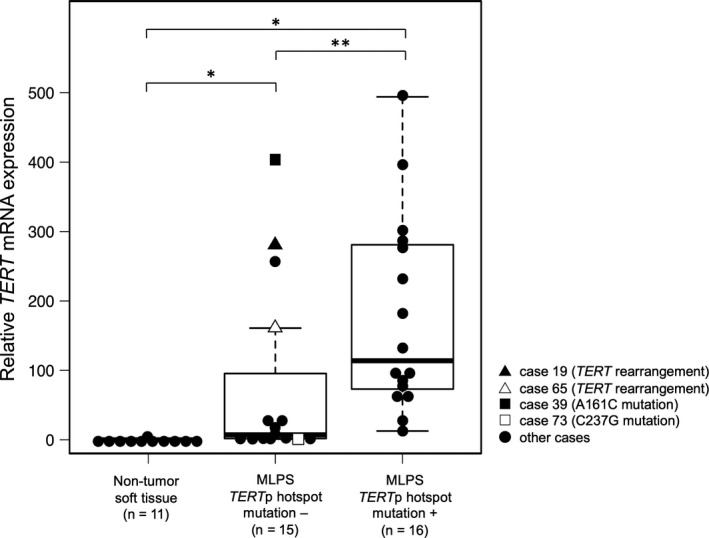
Relative TERT mRNA expression. TERT mRNA expression was detected in all 31 patients with myxoid liposarcoma (MLPS), whereas it was not detected in non‐tumor soft tissue samples, with the exception of 1 case. Data are shown as box plots that represent the first and third quartiles of the distribution. The median is shown in the center and the whiskers cover data within 1.5× of the interquartile range from the box. *P‐value < .001, **P‐value = .006 (Mann‐Whitney U test)
TABLE 2.
Relative TERT mRNA expression
| Relative TERT mRNA expression level | MLPS | Non‐tumor soft tissue (n = 11) | |
|---|---|---|---|
| TERTp hotspot mutation + (n = 16) | TERTp hotspot mutation − (n = 15) | ||
| ND | 0 | 0 | 10 |
| Low | 0 | 8 | 1 |
| High | 16 | 7 | 0 |
Abbreviations: MLPS, myxoid liposarcoma; ND, not detected; TERTp, TERT promoter.
3.5. Analysis of TERT structural change and other minor TERT promoter point mutations
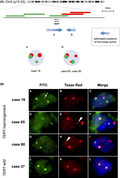
TERT mRNA expression was observed irrespective of TERTp hotspot mutations; therefore, we searched for other TERT or TERTp aberrations. The original probe for TERT split FISH was designed to estimate roughly the location of the break point (Figure 3A). FISH analysis detected TERT rearrangements in 3/19 MLPS (16%) cases without TERTp hotspot mutations (Figure 3B). Two signal patterns were observed and the break points were estimated to be located in 1 of the 2 regions upstream or around TERT (Figure 3A). Among the 3 cases, cases 19 and 65 showed the high expression of TERT mRNA (Figure 2), while case 80 was not analyzed as frozen material was not available. Clinicopathologically, cases 19 and 80 were classified as high‐grade MLPS, and cases 19 and 65 experienced distant metastases (Table S3). TERT rearrangement was not observed in cases with TERTp hotspot mutations. TERT amplification was not observed in any MLPS case. Next, we performed direct sequencing analysis to detect other minor TERTp mutations reported previously. 24 , 39 Among 19 MLPS cases without TERTp hotspot mutation, 16 cases were analyzed by direct sequencing of the DNA from the frozen material. In addition to the hotspot TERTp mutations (C228T and C250T), A161C and C237G mutations in the TERT promoter region were detected in 2 cases (Figure 4). The other 14 cases did not harbor any mutations. The A161C mutated case expressed high levels of TERT mRNA, while the C237G mutated case showed low TERT expression (Figure 2).
FIGURE 3.
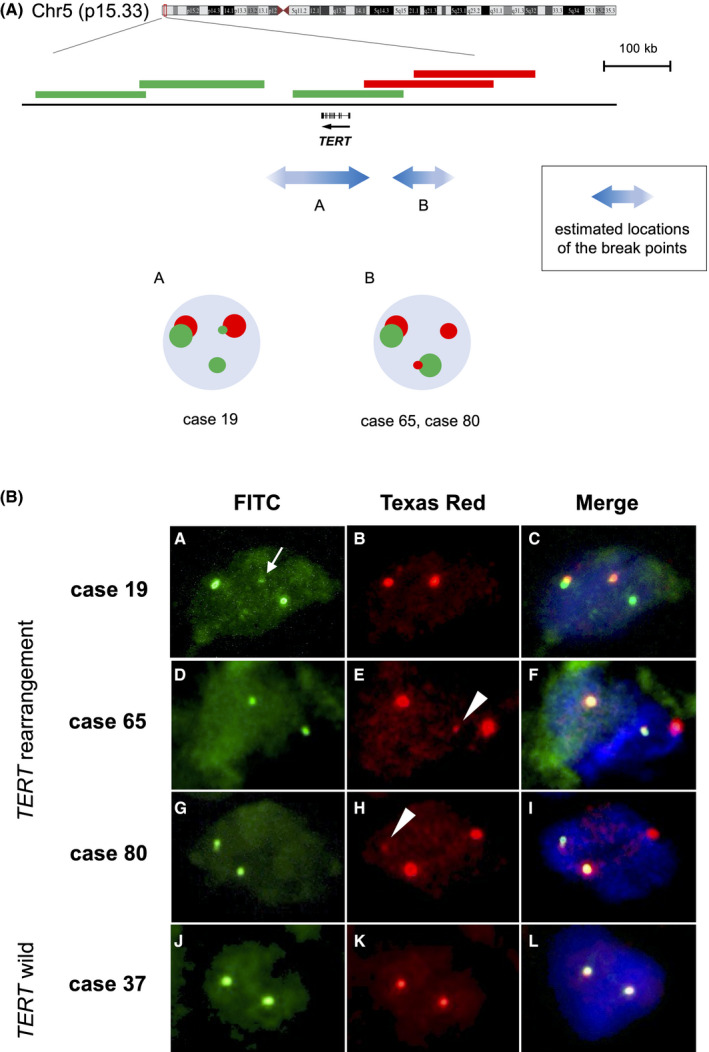
Fluorescence in situ hybridization (FISH) analysis of TERT rearrangements. A, Probe design for TERT split FISH and the obtained signal patterns. TERT is shown with the direction of transcription, indicated by the arrow. Three bacterial artificial chromosome (BAC) clones located around or downstream of TERT were labeled with FITC (green), and 2 BAC clones that cover the upstream region of TERT were labeled with Texas Red (red). Small green or red signal indicates structural variation in the corresponding covered genomic region. The double‐headed arrows with the letters A, B indicate the suspected locations of the break points estimated based on the observed signal patterns. B, Detection of TERT rearrangements using TERT split FISH. Three cases harbored TERT rearrangements (case 19 B a‐c; case 65 d‐f; case 80 g‐i). (a‐c) In case 19, a small green signal (arrow) overlapped with a red signal, which indicated TERT rearrangement. (d‐f, g‐i) In case 65 and case 80, a small red signal (arrowheads) overlapped with a green signal, indicative of TERT rearrangement. (j‐l) Representative images of cases without TERT rearrangement
FIGURE 4.
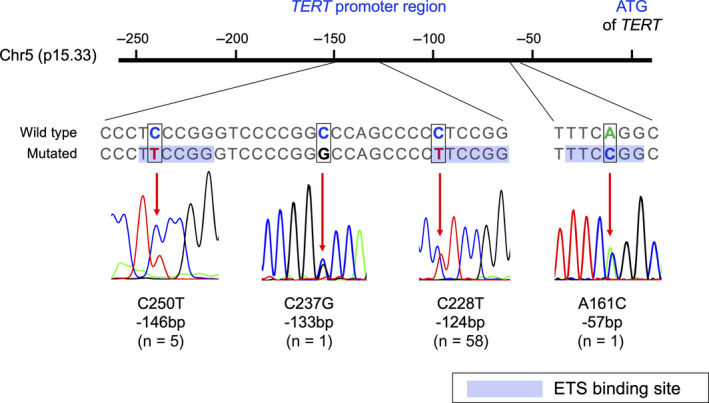
TERT promoter mutations in myxoid liposarcoma samples. Schematic figure showing the TERT promoter region on chromosome 5 and the genomic DNA sequence of the mutational region with a wild‐type strand and a mutated strand. Newly created E‐twenty six (ETS) binding sites are shown. Representative sequencing chromatograms show each heterozygous mutation (indicated by red arrows)
3.6. Analysis of telomere length
Telomere length was analyzed in 50 samples using DNA extracted from frozen materials: 30 tumor samples from 16 MLPS cases with TERTp hotspot mutation, 8 MLPS cases without TERTp hotspot mutation, 3 ALT‐positive and 3 ALT‐negative DDLPS cases, and 20 normal tissue samples. Normal tissue samples, which were non‐tumor soft tissue obtained from the same patient, were used as a control. Matching tumor and normal tissue samples were available from 15 patients with TERTp hotspot mutations and 5 patients without the mutations (Table 3). The mean TL was as follows: 6.12 ± 4.68 kb in MLPS, 7.78 ± 1.78 kb in normal tissue, and 10.2 ± 7.15 kb in DDLPS. The mean TL of the MLPS cases did not differ significantly between TERTp hotspot mutation‐positive and mutation‐negative cases (P = .74) (Figure 5). Irrespective of the TERTp hotspot mutation, the mean TL of the MLPS tumor samples was shorter than that of the matched normal samples (Table 3). However, a paired t test did not reveal any significant difference between tumor samples and their matched normal tissue samples in both groups with or without TERTp hotspot mutations (P = .37, P = .46, respectively). In DDLPS cases, the mean TL of the ALT‐positive cases (16.8 ± 3.44 kb) was markedly longer than that of the ALT‐negative cases (3.49 ± 1.23 kb) (Table 3).
TABLE 3.
Mean telomere length
| Mean telomere length (kb) ± SD | ||
|---|---|---|
| Tumor | Normal tissue | |
| MLPS | ||
| TERTp hotspot mutation + | 5.91 ± 4.84 (n = 16) | 7.52 ± 1.81 (n = 15) |
| TERTp hotspot mutation − | 6.53 ± 4.29 (n = 8) | 8.54 ± 1.42 (n = 5) |
| DDLPS | ||
| ALT + | 16.8 ± 3.44 (n = 3) | |
| ALT − | 3.49 ± 1.23 (n = 3) | |
Abbreviations: ALT, alternative lengthening of telomeres; DDLPS, dedifferentiated liposarcoma; MLPS, myxoid liposarcoma; SD, standard deviation; TERTp, TERT promoter.
FIGURE 5.
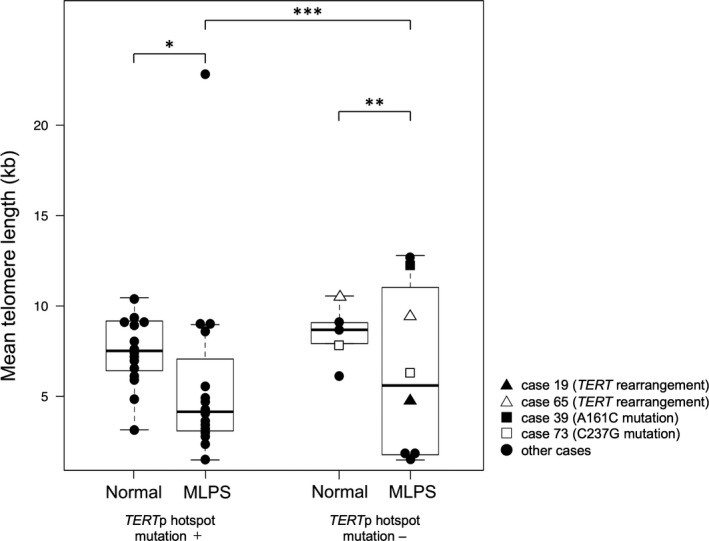
Telomere length of myxoid liposarcoma (MLPS). The mean telomere length of MLPS samples was compared with that of matched normal tissues. Data are shown as box plots that represent the first and third quartiles of the distribution. The median is shown in the center and the whiskers cover data within 1.5× of the interquartile range from the box. *P‐value = .009, **P‐value = .52, ***P‐value = .74 (Mann‐Whitney U test)
3.7. Prognostic analysis
We assessed the impact of TERTp hotspot mutations and all TERT genomic aberrations on survival rate and distant metastasis. The median follow‐up time was 74 mo (range, 4‐146 mo), and 10/83 patients died due to the disease. The 5‐ and 10‐y OS were 91% and 82%, respectively. Kaplan‐Meier and log‐rank analysis showed that the TERTp hotspot mutations did not significantly affect OS (P = .56) and MFS (P = .83) (Figure 6), and that the TERT aberrations did not differ significantly in terms of OS (P = .85) and MFS (P = .73). Clinicopathologically, age ≥50 y (P = .040), tumor size ≥10 cm (P = .012), presence of necrosis (P = .012), and FNCLCC grade (P = .002) were significantly associated with OS (Table 4). Immunohistochemically, there was no significant correlation between MFS or OS and p53, FOXM1, NY‐ESO‐1, or Ki‐67 positivity (Table S4). In contrast, positive correlations were suggested between p53, FOXM1, NY‐ESO‐1, and Ki‐67 positivity and the aforementioned poor prognostic factors: p53 and high FNCLCC grade (P = .004), p53 and necrosis (P = .028), FOXM1 and high FNCLCC grade (P = .038), NY‐ESO‐1 and age (P = .033), NY‐ESO‐1 and necrosis (P = .011), and high Ki‐67 LI and histotype (P = .005) (Table S5). Finally, TERTp hotspot mutation was not related to prognosis and was not associated with clinicopathological and immunohistochemical prognostic factors (Table 5).
FIGURE 6.
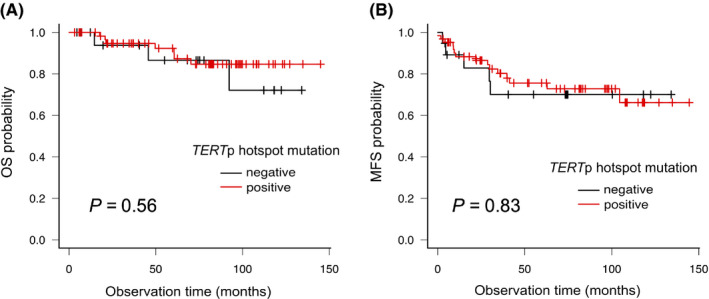
Analysis of outcomes for patients with TERT promoter (TERTp) hotspot mutation. A, B, Kaplan‐Meier curves of the groups with or without TERTp hotspot mutation. A, Analysis of overall survival (OS). B, Analysis of metastasis‐free survival (MFS)
TABLE 4.
Cox analysis for metastasis‐free survival and overall survival in myxoid liposarcoma
| Metastasis‐free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age (y): ≥50 (vs <50) | 2.41 | 0.98‐5.92 | .055 | 3.68 | 1.06‐12.7 | .040 |
| Sex: male (vs female) | 2.36 | 0.79‐7.06 | .13 | 2.36 | 0.56‐11.1 | .28 |
| Size (cm): ≥10 (vs <10) | 3.70 | 1.41‐9.71 | .008 | 14.1 | 1.78‐111.9 | .012 |
| Location: trunk (vs limb) | 0.81 | 0.24‐2.77 | .74 | 1.31 | 0.28‐6.16 | .73 |
| Histotype: high‐grade MLPS (vs MLPS) | 1.55 | 0.61‐3.90 | .36 | 1.32 | 0.34‐5.16 | .69 |
| Necrosis: present (vs absent) | 3.69 | 1.46‐9.30 | .006 | 7.30 | 1.54‐34.6 | .012 |
| Mitotic score (/10 HPF): ≥10 (vs <10) | 2.88 | 0.66‐12.5 | .16 | 3.22 | 0.39‐26.6 | .28 |
| FNCLCC grade: I, II, III | 3.37 | 1.71‐6.62 | <.001 | 5.38 | 1.89‐15.4 | .002 |
| TERTp hotspot mutation: present (vs absent) | 0.90 | 0.33‐2.47 | .83 | 0.67 | 0.17‐2.59 | .56 |
Abbreviations: CI, confidence interval; FNCLCC, French Fédération of Cancer Centers; HPF, high‐power fields; HR, hazard ratio; MLPS, myxoid liposarcoma; TERTp, TERT promoter. Bold values show significant difference.
TABLE 5.
The correlation between TERTp hotspot mutation status and clinicopathologic parameters
| TERTp hotspot mutation | |||
|---|---|---|---|
| Absent (n = 19) | Present (n = 63) | P‐value | |
| Sex | |||
| Male | 13 | 39 | .79 |
| Female | 6 | 24 | |
| Age (y) | |||
| <50 | 16 | 46 | .38 |
| ≥50 | 3 | 17 | |
| Size (cm) | |||
| <10 | 10 | 35 | 1.0 |
| ≥10 | 9 | 28 | |
| Histotype | |||
| MLPS | 15 | 44 | .57 |
| High‐grade MLPS | 4 | 19 | |
| Mitotic score | |||
| <10/10 HPF | 18 | 60 | 1.0 |
| ≥10/10 HPF | 1 | 3 | |
| Necrosis (%) | |||
| None | 12 | 36 | .70 |
| <50 | 7 | 22 | |
| ≥50 | 0 | 5 | |
| FNCLCC grade | |||
| I | 11 | 29 | .76 |
| II | 7 | 30 | |
| III | 1 | 4 | |
| p53 | |||
| Negative | 11 | 44 | .41 |
| Positive | 8 | 19 | |
| FOXM1 | |||
| Negative | 9 | 31 | 1.0 |
| Positive | 10 | 32 | |
| NY‐ESO‐1 | |||
| Negative | 5 | 13 | .75 |
| Positive | 14 | 50 | |
| Ki‐67 LI | |||
| Low | 12 | 40 | 1.0 |
| High | 7 | 23 | |
Abbreviations: FNCLCC, French Fédération of Cancer Centers; HPF, high‐power fields; LI, labeling index; MLPS, myxoid liposarcoma; TERTp, TERT promoter.
4. DISCUSSION
In the present study, 82% (68/83) of MLPS cases harbored TERT/TERTp aberrations, including TERTp hotspot mutations (63 cases), TERTp minor point mutations (2 cases), and TERT rearrangements (3 cases). In the remaining 18% (15/83) of cases, TERT/TERTp aberrations were not detected and TERT mRNA expression was present in all 11 cases with available RNA. No samples were ALT positive.
The prevalence of TERTp mutations in MLPS here was slightly higher or similar to that reported previously. 5 , 22 , 23 , 24 , 25 , 26 However, unlike previous studies, no ALT‐positive MLPS cases were observed, regardless of using a similar FISH method. 27 , 28 The reason for this discrepancy is unclear, although this may be because all patients in our study were in a molecularly homogeneous group harboring DDIT3 rearrangement. Using telomere‐specific FISH, the most commonly used technique for detecting ALT, 5 the frequency of ALT in DDLPS was similar to that reported previously. 27 , 28 Furthermore, the mean TL in DDLPS cases with ALT was strikingly longer than that in those without ALT, which was as expected. Therefore, the method we used for detecting ALT was sufficiently sensitive.
Telomerase is upregulated mainly by TERTp hotspot mutations; however, some genetic aberrations, including TERT rearrangement and amplification, TERC amplification, and epigenetic changes are also involved in telomerase activation. 5 , 40 Among TERTp point mutations, the A161C with high level of TERT mRNA and C237G mutations were detected. A161C creates an ETS binding site within the TERT promoter region, which has strong transcriptional activities. 39 C237G is a previously unreported mutation and does not form an ETS binding site. 39 , 41 Three cases harbored TERT rearrangement and the 2 for which total RNA could be extracted showed high TERT mRNA expression levels.
Here, we report for the first time TERT rearrangement in MLPS. In previous studies, TERT rearrangements were suggested to activate TERT transcription via juxtaposition of superenhancers in neuroblastoma and pheochromocytoma. 42 , 43 , 44 The positions of breakpoints were identified as both upstream and downstream of TERT. 42 , 45 Furthermore, TERT gene fusions were reported in some tumors, including sarcomas. 40 , 46 , 47 , 48 In our study, the 2 patterns of break points were estimated to be located upstream or around TERT, respectively, as reported previously. 42 , 45 Therefore, it was suggested that TERT transcription was activated by the juxtaposition of superenhancers or due to the creation of TERT gene fusion in the 3 cases with TERT rearrangement.
Although TERTp mutations are linked to adverse clinical parameters in various tumor entities, 5 , 26 few studies have examined the association between telomerase activation status and prognosis in soft tissue and bone tumors. These studies include the association of the TERTp mutation with increased risk in solitary fibrous tumors (SFTs) 49 and the correlation between TERT mRNA expression and poor survival in osteosarcoma. 50 In contrast, we found that survival and metastasis rate did not differ significantly, irrespective of the TERTp hotspot mutation in MLPS. In addition, the clinicopathological parameters (age, tumor size, necrosis, and FNCLCC grade), which were significantly associated with worse prognosis, did not correlate with TERTp mutations. Immunohistochemically, there was no association between TERTp hotspot mutation and p53 and NY‐ESO‐1 expression, which have been reported as adverse prognostic factors in MLPS, 20 , 32 , 51 as well as with FOXM1, which correlated with a worse prognosis in various carcinomas and sarcomas. 52 In contrast, 3 cases with TERT rearrangement tended to be graded higher both clinically or pathologically, this significance was unclear because of the small number of cases. To our knowledge, this study is the first to demonstrate a relationship between telomere maintenance mechanism and prognosis in MLPS in detail.
Our results suggested that for the following 3 reasons telomerase activation mechanisms could be present in almost all MLPS cases. First, TERT mRNA expression was observed even in cases with no TERTp mutation or TERT rearrangement. After MLPS, SFT has been reported to have the second highest frequency of TERTp mutations in soft tissue tumors. 5 , 22 , 23 In a previous study, approximately 28% SFT cases harbored a TERTp mutation, whereas the remaining 72% cases without TERTp mutations showed low or undetectable TERT mRNA expression. 49 Contrary to SFT, we found that MLPS was a soft tissue tumor characteristically showing TERT expression. Second, although TERTp mutation is associated with a poor prognosis in many tumors, 5 , 26 prognosis did not differ significantly irrespective of TERTp hotspot mutation or other TERT aberrations in the present study. In addition, the clinicopathological features were not associated with TERTp hotspot mutations. Third, the mean TL of the MLPS samples was shorter than that of matched normal tissues, irrespective of the TERTp hotspot mutation. Analysis of 31 cancer types in The Cancer Genome Atlas showed that the TL of TERT‐expressing cancer tissue was relatively shorter than that of matched normal tissue in many cancers, including sarcomas, and that the TL of cancer tissue without TERT expression or ALT was slightly longer than that of matched normal tissue in sarcomas. 40 Therefore, the patients with MLPS for whom we did not find any TERT/TERTp structural abnormalities may also possess some telomere maintenance mechanisms such as epigenetic change in TERT or TERC amplification. However, further investigations are necessary to confirm this hypothesis.
In MLPS, gene fusion is a driver genetic alteration required for its tumorigenesis; and an observational study indicated that the TERTp mutation may be a secondary change occurring during tumor progression. 26 In contrast, our results suggested that TERT abnormalities may be earlier genetic events and essential for tumorigenesis in MLPS, because almost all MLPS cases were suggested to possess telomerase reactivation mechanisms. In mouse models, FUS‐DDIT3 fusion is insufficient for malignant transformation on its own. 53 Furthermore, FUS‐DDIT3 fusion in human mesenchymal stromal/stem cells immortalized by oncogenic hits including TERT overexpression were able to drive MLPS formation. 54 TERTp mutations were also suggested to appear as early tumorigenic events in urothelial carcinoma, cutaneous melanoma, and basal cell carcinoma. 55 Chiba et al 56 reported that TERTp mutations contributed to tumorigenesis by a two‐step mechanism in malignant melanoma. First, those mutations extended cellular life span by healing the shortest telomeres. Next, the critically short telomeres led to genome instability and telomerase was further upregulated to sustain cell proliferation.
There are several limitations to this study. First, we evaluated TERT mRNA expression, and not TERT protein expression or telomerase activity, to determine the telomerase‐dependent telomere maintenance mechanism. Although it is unclear whether TERT mRNA expression directly leads to telomerase reactivation, recent studies have shown a positive correlation between telomerase activity and TERT mRNA expression. 40 , 41 , 57 Second, although there are several methods for measuring the occurrence of ALT, including the C‐circle assay, only 1 method was used in our study. 14 , 58 Third, this was a retrospective study. Finally, the number of patients with poor outcome was not so large that the statistical power was limited.
In conclusion, TERT re‐expression is an important and unique feature of MLPS among sarcomas, the majority of which have ALT. Our results demonstrated that telomerase activation mechanisms may play a critical role in tumorigenesis rather than in progression. Further research is warranted to confirm our findings and to examine the potential for interventions targeting telomerase reactivation mechanism in patients with MLPS.
DISCLOSURE
The authors have no conflict of interest. Kengo Takeuchi is an associate editor of Cancer Science.
Supporting information
Table S1‐S5
ACKNOWLEDGMENTS
The authors thank Tomoyo Kakita and Motoyoshi Iwakoshi for their excellent technical assistance.
Kunieda J, Yamashita K, Togashi Y, et al. High prevalence of TERT aberrations in myxoid liposarcoma: TERT reactivation may play a crucial role in tumorigenesis. Cancer Sci. 2022;113:1078–1089. doi: 10.1111/cas.15256
Funding information
This study was supported by JSPS KAKENHI Grant Number JP 19K16570 (KY)
REFERENCES
- 1. Okamoto K, Seimiya H. Revisiting telomere shortening in cancer. Cells. 2019;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611‐622. [DOI] [PubMed] [Google Scholar]
- 3. Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Annu Rev Pathol Mech Dis. 2013;8:49‐78. [DOI] [PubMed] [Google Scholar]
- 4. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787‐791. [DOI] [PubMed] [Google Scholar]
- 5. Gaspar TB, Sá A, Lopes JM, Sobrinho‐Simões M, Soares P, Vinagre J. Telomere maintenance mechanisms in cancer. Genes (Basel). 2018;9:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173‐179. [DOI] [PubMed] [Google Scholar]
- 7. Feng J, Funk WD, Wang S‐S, et al. The RNA component of human telomerase. Science. 1995;269:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955‐959. [DOI] [PubMed] [Google Scholar]
- 9. Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up‐regulated in tumor cells and during immortalization. Cell. 1997;90:785‐795. [DOI] [PubMed] [Google Scholar]
- 10. Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38:6172‐6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu T, Yuan X, Xu D. Cancer‐specific telomerase reverse transcriptase (Tert) promoter mutations: biological and clinical implications. Genes (Basel). 2016;7:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319‐330. [DOI] [PubMed] [Google Scholar]
- 14. Henson JD, Reddel RR. Assaying and investigating alternative lengthening of telomeres activity in human cells and cancers. FEBS Lett. 2010;584:3800‐3811. [DOI] [PubMed] [Google Scholar]
- 15. Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryan TM, Englezou A, Dalla‐Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor‐derived cell lines. Nat Med. 1997;3:1271‐1274. [DOI] [PubMed] [Google Scholar]
- 17. Perrem K, Colgin LM, Neumann AA, Yeager TR, Reddel RR. Coexistence of alternative lengthening of telomeres and telomerase in hTERT‐transfected GM847 cells. Mol Cell Biol. 2001;21:3862‐3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lovejoy CA, Li W, Reisenweber S, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8:12‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours. 5th ed. IARC Press; 2020. [Google Scholar]
- 20. Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status, TLS‐CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977‐3987. [PubMed] [Google Scholar]
- 21. Conyers R, Young S, Thomas DM. Liposarcoma: molecular genetics and therapeutics. Sarcoma. 2011;2011:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self‐renewal. Proc Natl Acad Sci USA. 2013;110:6021‐6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koelsche C, Renner M, Hartmann W, et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J Exp Clin Cancer Res. 2014;33:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saito T, Akaike K, Kurisaki‐arakawa A, et al. TERT promoter mutations are rare in bone and soft tissue sarcomas of Japanese patients. Mol Clin Oncol. 2016;4:61‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campanella NC, Penna V, Abrahão‐Machado LF, et al. TERT promoter mutations in soft tissue sarcomas. Int J Biol Markers. 2016;31:e62‐e67. [DOI] [PubMed] [Google Scholar]
- 26. Ventura Ferreira M, Crysandt M, Braunschweig T, et al. Presence of TERT promoter mutations is a secondary event and associates with elongated telomere length in myxoid liposarcomas. Int J Mol Sci. 2018;19:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costa A, Daidone MG, Daprai L, et al. Telomere maintenance mechanisms in liposarcomas: association with histologic subtypes and disease progression. Cancer Res. 2006;66:8918‐8924. [DOI] [PubMed] [Google Scholar]
- 28. Lee J‐C, Jeng Y‐M, Liau J‐Y, Tsai J‐H, Hsu H‐H, Yang C‐Y. Alternative lengthening of telomeres and loss of ATRX are frequent events in pleomorphic and dedifferentiated liposarcomas. Mod Pathol. 2015;28:1064‐1073. [DOI] [PubMed] [Google Scholar]
- 29. Fletcher CDM, Bridge J, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press; 2013. [Google Scholar]
- 30. Smith TA, Easley KA, Goldblum JR. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol. 1996;20:171‐180. [DOI] [PubMed] [Google Scholar]
- 31. Hiramatsu M, Ninomiya H, Inamura K, et al. Activation status of receptor tyrosine kinase downstream pathways in primary lung adenocarcinoma with reference of KRAS and EGFR mutations. Lung Cancer. 2010;70:94‐102. [DOI] [PubMed] [Google Scholar]
- 32. Oda Y, Yamamoto H, Takahira T, et al. Frequent alteration of p16INK4a/p14ARF and p53 pathways in the round cell component of myxoid/round cell liposarcoma: p53 gene alterations and reduced p14ARF expression both correlate with poor prognosis. J Pathol. 2005;207:410‐421. [DOI] [PubMed] [Google Scholar]
- 33. Meeker AK, Gage WR, Hicks JL, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aida J, Izumiyama‐Shimomura N, Nakamura K‐I, et al. Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol. 2007;38:1192‐1200. [DOI] [PubMed] [Google Scholar]
- 35. Heaphy CM, Subhawong AP, Hong S‐M, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebina A, Togashi Y, Baba S, et al. TERT promoter mutation and extent of thyroidectomy in patients with 1–4 cm intrathyroidal papillary carcinoma. Cancers (Basel). 2020;12:2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dome JS, Bockhold CA, Li SM, et al. High telomerase RNA expression level is an adverse prognostic factor for favorable‐histology Wilms’ tumor. J Clin Oncol. 2005;23:9138‐9145. [DOI] [PubMed] [Google Scholar]
- 38. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang D‐S, Wang Z, He X‐J, et al. Recurrent TERT promoter mutations identified in a large‐scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer. 2015;51:969‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49:349‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borah S, Xi L, Zaug AJ, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valentijn LJ, Koster J, Zwijnenburg DA, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411‐1414. [DOI] [PubMed] [Google Scholar]
- 43. Peifer M, Hertwig F, Roels F, et al. Telomerase activation by genomic rearrangements in high‐risk neuroblastoma. Nature. 2015;526:700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dwight T, Flynn A, Amarasinghe K, et al. TERT structural rearrangements in metastatic pheochromocytomas. Endocr Relat Cancer. 2018;25:1‐9. [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Roberts ND, Wala JA, et al. Patterns of somatic structural variation in human cancer genomes. Nature. 2020;578:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Delespaul L, Lesluyes T, Pérot G, et al. Recurrent TRIO fusion in nontranslocation‐related sarcomas. Clin Cancer Res. 2017;23:857‐867. [DOI] [PubMed] [Google Scholar]
- 47. Suster DI, Deshpande V, Chebib I, et al. Spindle cell liposarcoma with a TRIO‐TERT fusion transcript. Virchows Arch. 2019;475:391‐394. [DOI] [PubMed] [Google Scholar]
- 48. Occidental M, Shen G, Feng X, et al. Novel CTNND2‐TERT fusion in a spindle cell liposarcoma. Genes Chromosom Cancer. 2020;59:544‐548. [DOI] [PubMed] [Google Scholar]
- 49. Bahrami A, Lee S, Schaefer I‐M, et al. TERT promoter mutations and prognosis in solitary fibrous tumor. Mod Pathol. 2016;29:1511‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanders RP, Drissi R, Billups CA, Daw NC, Valentine MB, Dome JS. Telomerase expression predicts unfavorable outcome in osteosarcoma. J Clin Oncol. 2004;22:3790‐3797. [DOI] [PubMed] [Google Scholar]
- 51. Iura K, Kohashi K, Hotokebuchi Y, et al. Cancer‐testis antigens PRAME and NY‐ESO‐1 correlate with tumour grade and poor prognosis in myxoid liposarcoma. J Pathol Clin Res. 2015;1:144‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maekawa A, Kohashi K, Kuda M, et al. Prognostic significance of FOXM1 expression and antitumor effect of FOXM1 inhibition in synovial sarcomas. BMC Cancer. 2016;16:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodriguez R, Rubio R, Gutierrez‐Aranda I, et al. FUS‐CHOP fusion protein expression coupled to p53 deficiency induces liposarcoma in mouse but not in human adipose‐derived mesenchymal stem/stromal cells. Stem Cells. 2011;29:179‐192. [DOI] [PubMed] [Google Scholar]
- 54. Rodriguez R, Tornin J, Suarez C, et al. Expression of FUS‐CHOP fusion protein in immortalized/transformed human mesenchymal stem cells drives mixoid liposarcoma formation. Stem Cells. 2013;31:2061‐2072. [DOI] [PubMed] [Google Scholar]
- 55. Vinagre J, Pinto V, Celestino R, et al. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch. 2014;465:119‐133. [DOI] [PubMed] [Google Scholar]
- 56. Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two‐step mechanism. Science. 2017;357:1416‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dome JS, Chung S, Bergemann T, et al. High telomerase reverse transcriptase (hTERT) messenger RNA level correlates with tumor recurrence in patients with favorable histology Wilms’ tumor. Cancer Res. 1999;59:4301‐4307. [PubMed] [Google Scholar]
- 58. Henson JD, Cao Y, Huschtscha LI, et al. DNA C‐circles are specific and quantifiable markers of alternative‐lengthening‐of‐telomeres activity. Nat Biotechnol. 2009;27:1181‐1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S5


