Abstract
Background
Cryptosporidium spp. are obligate intracellular apicomplexan parasites transmitted to humans and other animals by contaminated water, food, or direct contact. They mainly cause gastrointestinal symptoms, although subclinical infections are also common. Cats are primarily infected by host-adapted Cryptosporidium felis while C. parvum and C. muris have also been detected in some cases. In this study, the molecular prevalence of Cryptosporidium spp. was investigated by screening 399 fecal samples collected from stray cats using nested PCR targeting the 18S rRNA gene for the first time in Turkey. Additionally, Cryptosporidium PCR-positive samples were genotyped by nested PCR- restriction fragment length polymorphism (RFLP), and subsequently, amplicons of 18S SSU rRNA were sequenced. They were further subtyped by amplification and sequencing of the gp60 gene.
Results
Among fecal samples screened, 12 of them (3%) were found to be Cryptosporidium-positive, and according to RFLP and sequencing of 18S rRNA gene, all positive samples were identified as C. felis. Subtyping analyses at the gp60 gene showed that C. felis isolates belonged to the XIXa subtype family, which are closely related to human subtypes of the parasite.
Conclusions
The results of this study are important in terms of indicating the potential role of stray cats for transmission of Cryptosporidium spp. to humans or other animals. Also, the presence of XIXa, which is the dominant subtype family of C. felis in cats and humans was shown for the first time in stray cats of İzmir, Turkey.
Keywords: Cryptosporidium felis, 18S rRNA, gp60, XIXa, Cats, Turkey
Background
Cryptosporidium spp. are obligate intracellular apicomplexan parasites distributed worldwide and infecting all vertebrates including humans and domestic animals [1–3]. The main transmission routes of Cryptosporidium are consumption of contaminated water and food or direct contact with infected animals and humans [3]. In humans, Cryptosporidium causes gastrointestinal disturbance presented with diarrhea, although subclinical cases are also common. It may cause serious disseminated infections in immunocompromised individuals [3]. Cryptosporidium infection is generally asymptomatic in cats but symptoms like diarrhea may occur especially in young and newborn kittens. Additionally, the degeneration of host epithelial cells, loss of microvilli, and atrophy of the villi often occur in severe infections [4].
Among the 19 Cryptosporidium species (C. hominis, C. parvum C. meleagridis, C. canis, C. felis, C. ubiquitum, C. cuniculus, C. viatorum, C. muris, C. andersoni, C. erinacei, C. tyzzeri, C. bovis, C. suis, C. scrofarum, C. occultus, C. xiaoi, C. fayeri and C. ditrichi) and 4 genotypes (Cryptosporidium chipmunk genotype I, mink genotype, skunk genotype and horse genotype), C. hominis and C. parvum are the most prevalently found in humans [3]. C. parvum is a zoonotic species whereas mainly anthroponotic C. hominis has been detected in different non-human animal species [5, 6]. Cryptosporidium canis, C. felis, C. meleagridis, and C. ubiquitum are other significant zoonotic pathogens causing diseases in humans [3]. In cats, host-adapted C. felis is the main Cryptosporidium species, although C. parvum and C. muris have also been detected [7–12].
During the diagnosis of Cryptosporidium, microscopic staining methods such as Ziehl–Neelsen, Kinyoun, and Giemsa are used to identify parasite oocysts in the feces [7]. Antigen detection methods targeting the surface proteins of the parasite in stool samples are also used [13]. Microscopic and antigen detection methods are not useful for species and genotype identification due to morphologically identical oocysts and shared antigens [8].
Molecular methods including polymerase chain reaction (PCR), DNA sequencing, and restriction fragment length polymorphism (RFLP) based on the analysis of the small subunit 18S rRNA gene are used to detect the parasite DNA, and identify species and genotypes in animal, human, and environmental samples [7]. During the species identification and genotyping of Cryptosporidium spp. by an RFLP method, an 830-bp fragment of the 18S rRNA gene is digested using SspI and VspI restriction enzymes [14–16]. Additionally, a gene encoding 60-kDa glycoprotein (gp60) in the subtyping of C. parvum, C. hominis, C. meleagridis, C. ubiquitum, C. viatorum, C. fayeri, C. ryanae, C. canis and C. felis has been used as a marker [3, 5, 17–20].
The study region İzmir harbors several stray cats that have close contact with humans and do not have sufficient veterinary care to prevent Cryptosporidium spp. transmission. Many important zoonotic parasites such as Toxoplasma gondii, Leishmania spp., and Blastocystis spp. have been detected in stray cats of İzmir in our previous studies [21–25]. This study initially investigated Cryptosporidium spp. in stray cats by a comprehensive PCR screening performed in 399 fecal samples. Secondly, species identification and subtyping analyses have been performed in Cryptosporidium-positive samples by RFLP and sequencing of the 18S rRNA and gp60 genes.
Results
Molecular prevalence of Cryptosporidium spp.
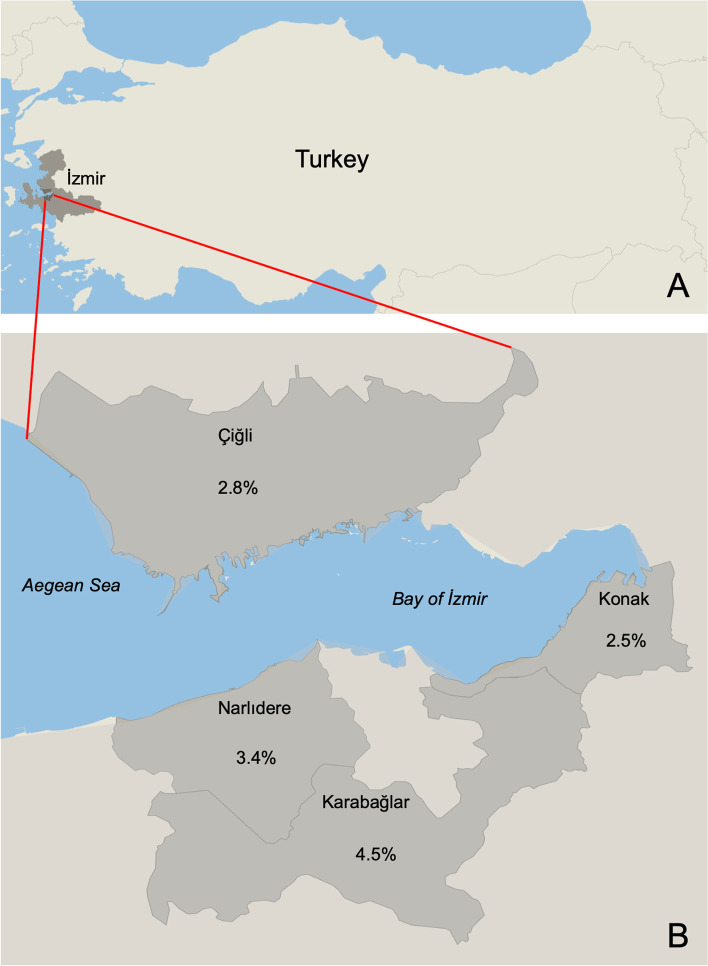
Cryptosporidium spp. DNA was detected in 12 of 399 fecal samples collected from stray cats by a nested PCR targeting the 18S rRNA gene. Accordingly, the molecular prevalence was 3%. Among the prevalence values of the regions, the highest prevalence value was observed in Karabağlar district with 4.5% which was followed by Narlıdere district with 3.4% (Fig. 1).
Fig. 1.
The map shows the prevalence values among different regions of Izmir, Turkey. Datawrapper (https://www.datawrapper.de/) was used to create the map
Species identification
Species identification of Cryptosporidium PCR-positive samples was performed by two different approaches including RFLP and Sanger sequencing analyses. According to the results, all Cryptosporidium-positive samples were identified as C. felis. After digestion with SspI, two fragments 426 bp and 390 bp were detected for all C. felis positive samples. Three fragments 444 bp, 247 bp, and 106 bp were detected for C. parvum which was used as the positive control. Sequencing of 18S rRNA gene also confirmed that all Cryptosporidium-positive samples were C. felis (Table 1). Similarity rates of C. felis samples identified in this study varied between 91 and 98% compared to reference C. felis isolates deposited in GenBank (Table 1).
Table 1.
BLAST results of C. felis isolates based on 18S rRNA gene
| Sample | Accession number | Percentage of nucleotide identity |
|---|---|---|
| C. felis cat isolate 1 | KJ194110.1 | 725/780 (93%) |
| C. felis cat isolate 2 | AB694730.1 | 468/491 (95%) |
| C. felis cat isolate 3 | AB694730.1 | 658/722 (91%) |
| C. felis cat isolate 4 | KJ194110.1 | 780/795 (98%) |
| C. felis cat isolate 5 | KJ194110.1 | 774/794 (97%) |
| C. felis cat isolate 6 | KJ194110.1 | 771/797 (97%) |
| C. felis cat isolate 7 | KJ194110.1 | 768/806 (95%) |
| C. felis cat isolate 8 | KJ194110.1 | 797/813 (98%) |
| C. felis cat isolate 9 | MH115431.1 | 743/780 (95%) |
| C. felis cat isolate 10 | MK982512.1 | 734/784 (94%) |
| C. felis cat isolate 11 | AF159113.1 | 766/818 (94%) |
| C. felis cat isolate 12 | AF159113.1 | 716/769 (93%) |
Subtyping of C. felis
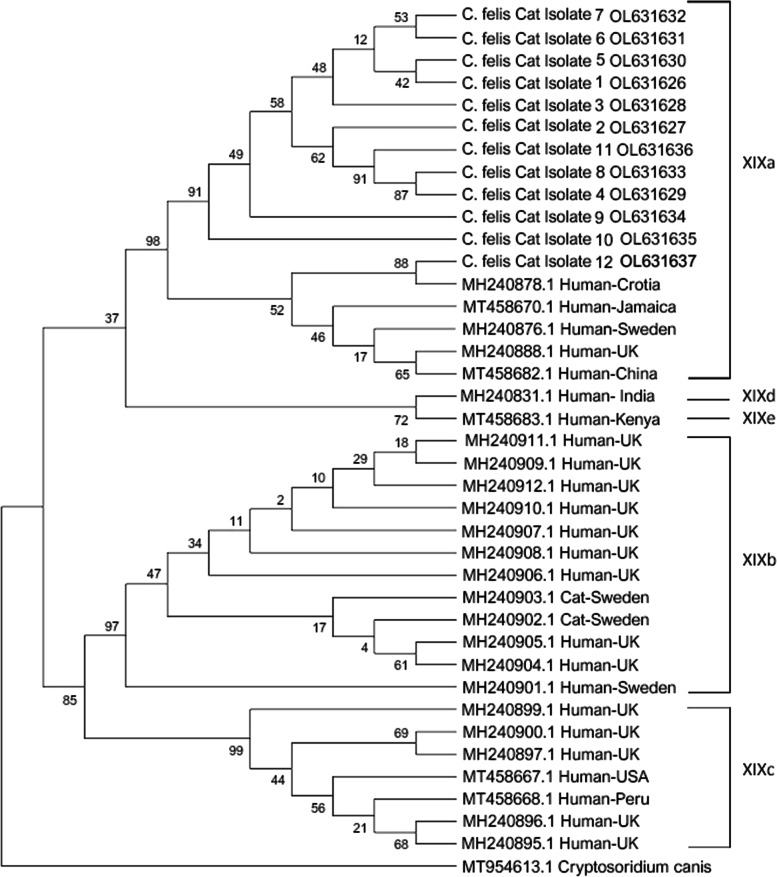
Subtypes of C. felis isolates were identified by Sanger sequencing of the gp60 gene. The scheme defined by Jiang et al., (2020) [26] was used for subtyping. The relation of subtypes was shown by phylogenetic analysis performed with reference C. felis isolates subtyped as XIXa, XIXb, XIXc, XIXd, or XIXe. According to this scheme, a repeat sequence with one copy called R1 (CCACCTAGTGGCGGTAGTGGCGTGTCCCCTGCT) and a 9 bp deletion were detected in all C. felis isolates. Also, two indels, which are located between positions 706-711 bp, and 1015–1068 bp, were not available. Another repeat sequence called R2 (AGCACAACTACGGCTACAGCGAGCACTGCGAGTTCGACA) was detected as one or two copies whereas the GGT sequence among positions 1156 and 1167 was detected as two, three, or four copies. These findings were compatible with the subtype family named as XIXa (Table 2). Also, all C. felis samples were clustered within the subtype family XIXa in the phylogenetic tree (Fig. 2).
Table 2.
Subtyping of C. felis isolates using reference positions based on gp60 gene
| Sample | R1 (463–556) |
Deletion (667–679) |
Indel (706–711) |
R2 (770–910) |
Indel (1015–1068) |
Indel (1156–1167) |
Subtype family |
|---|---|---|---|---|---|---|---|
| C. felis cat isolate 1 | 1 copy | 9 bp | - | 2 copy | - | 4 GGT | XIXa |
| C. felis cat isolate 2 | 1 copy | 9 bp | - | 1 copy | - | a | XIXa |
| C. felis cat isolate 3 | 1 copy | 9 bp | - | 2 copy | - | a | XIXa |
| C. felis cat isolate 4 | 1 copy | 9 bp | - | 2 copy | - | a | XIXa |
| C. felis cat isolate 5 | 1 copy | 9 bp | - | 2 copy | - | 3 GGT | XIXa |
| C. felis cat isolate 6 | 1 copy | 9 bp | - | 1 copy | - | 4 GGT | XIXa |
| C. felis cat isolate 7 | 1 copy | 9 bp | - | 1 copy | - | 4 GGT | XIXa |
| C. felis cat isolate 8 | 1 copy | 9 bp | - | 1 copy | - | 2 GGT | XIXa |
| C. felis cat isolate 9 | 1 copy | 9 bp | - | 2 copy | - | 3 GGT | XIXa |
| C. felis cat isolate 10 | 1 copy | 9 bp | - | 2 copy | - | 2 GGT | XIXa |
| C. felis cat isolate 11 | 1 copy | 9 bp | - | 2 copy | - | 4 GGT | XIXa |
| C. felis cat isolate 12 | 1 copy | 9 bp | - | 1 copy | - | 4 GGT | XIXa |
| Reference isolate 1 | 1–3 copies | 3 or 9 bp | - | 1–4 copies | - | 2–5 GGT | XIXa |
| Reference isolate 2 | 1 or 2 copies | 6 or 15 bp | - | 2–4 copies | 18 bp | 4 GGT | XIXb |
| Reference isolate 3 | 2 copies | 15 bp | - | 3 copies | - | 3 GGT | XIXc |
| Reference isolate 4 | 1 copy | - | - | 1 copy | - | 4 GGT | XIXd |
| Reference isolate 5 | 1 copy | - | 6 bp | 1 copy | 36 bp | 4 GGT | XIXe |
Reference positions are from Jiang et al. 2020 [26]
ashows the unevaluated sequences because of sequencing errors
R1: 33-bp tandem repeat (CCACCTAGTGGCGGTAGTGGCGTGTCCCCTGCT)
R2: 39-bp tandem repeat (AGCACAACTACGGCTACAGCGAGCACTGCGAGTTCGACA)
Fig. 2.
Phylogenetic tree shows C. felis isolates clustering with XIXa subtype family
Discussion
Cats are reservoirs of different infectious diseases caused by parasites or bacteria with zoonotic potential [27–29]. To date, the presence of important zoonotic parasites such as T. gondii, Blastocystis spp., and Leishmania spp. in stray cats living in İzmir (our study region) have been reported using molecular or serological methods with a prevalence of 14.4%, 3.7%, and 11.1%, respectively [22, 23, 25]. Although different Cryptosporidium species have been detected in cats, which play a role as reservoir host, the prevalence of Cryptosporidium spp., as well as species and subtypes in stray cats of İzmir are not known in detail. However, the prevalence of Cryptosporidium in humans has been frequently investigated by microscopy or immunological methods rather than molecular methods that enable species identification or subtyping in İzmir. According to obtained results, although the prevalence of Cryptosporidium spp. changed based on the sample number analyzed or method used, high prevalence rates varying from 33.5% to 37.4% were detected in İzmir [30, 31].
In this comprehensive study, three significant findings have been reported for the first time in Turkey. Firstly, the molecular prevalence of Cryptosporidium spp. was found to be 3% (12/399) in stray cats. Although this prevalence is low, it is rather important in terms of indicating that stray cats can act as a potential source of human and other animal infections in İzmir, Turkey. Secondly, all Cryptosporidium-positive samples were identified as C. felis by nested PCR–RFLP and Sanger sequencing. Cryptosporidium felis causes infections in cats and in some cases, in cows and humans as well [1, 3, 32]. Human cryptosporidiosis cases caused by C. felis have been detected frequently in developing countries and a zoonotic transmission from a household cat to its owner was reported [2, 17, 33–36]. However, there are uncertainties about the zoonotic transmission from cats into humans because of the limited number of cases and lack of sufficient information about C. felis isolated from cats at the genetic level [17]. In line with this, in a study investigating household transmission of zoonotic Cryptosporidium spp. from pet dogs and cats to their owners, it was emphasized that domestic cats do not play an important role as the source of human cryptosporidiosis [37].
The molecular prevalence of C. felis in cats varied from 0.3% to 11% in studies conducted in different regions (Table 3). Similarly, C. felis was detected in humans using molecular methods with different prevalence rates ranging from 0.4% to 4.5% (Table 3). Although any prevalence study associated with cryptosporidiosis in cats except our study showing a 3% C. felis prevalence has not been conducted in Turkey, a recent study identified for the first time a C. felis isolate (ANK_1) (NCBI accession number: MN394123)by molecular methods in a house cat [38]. Additionally, C. felis has been identified in surface water samples in the province of Samsun, Turkey [39]. This finding is relevant because it highlights the capability of infected cats of contaminating the environment (soil, waters) with their feces.
Table 3.
Molecular prevalence and subtyping data of C. felis isolates detected in cats and humans from different countries
| Molecular prevalence | Target gene | Subtyping gene | Subtype family | Host | Country | Reference |
|---|---|---|---|---|---|---|
| 2.0% (7/357) | 18S rRNA | - | - | household cats | Japan | [40] |
| 0.3% (1/329) | 18S rRNA | - | - | pet shop kittens | Japan | [40] |
| 2.5% (2/80) | 18S rRNA | - | - | cats | Thailand | [41] |
| 4.5% (7/155) | 18S rRNA | - | - | HIV-infected patients | Thailand | [42] |
| 1.7% (18/1063) | actin | - | - | cats | Australia | [43] |
| 11% (5/46) | 18S rRNA | - | - | cats | Colombia | [44] |
| 5.4% (3/55) | 18S rRNA | - | - | cats | Brazil | [45] |
| 4.8% (12/250) | 18S rRNA | - | - | domestic cats | Mississippi and Alabama | [46] |
| 1.92% (1/52) | 18S rRNA | - | - | cats | China | [10] |
| 5% (21/418) | 18S rRNA | - | - | cats | China | [47] |
| 0.49% (3/609) | 18S rRNA | - | - | humans | China | [48] |
| 0.35% (1/283) | 18S rRNA | - | - | immunodeficient patients | Poland | [49] |
| 3.3% (1/30) | 18S rRNA | - | - | humans | Sweden | [50] |
| 0.9% (1/108) | 18S rRNA | - | - | patients | Mozambique | [51] |
| 1% (4/398) | 18S rRNA | gp60 | XIXa | patients | Sweden | [52] |
| - | 18S rRNA | gp60 | XIXa | pet shelter/shop cats, stray cats | China | [17] |
| - | 18S rRNA | gp60 | XIXa | cats | Peru, USA, Slovakia, Australia | [26] |
| - | 18S rRNA | gp60 | XIXa, XIXc, XIXd, XIXe | humans | Peru, USA, Jamaica, Portugal, Nigeria, China, Kenya | [26] |
In subtyping studies based on gp60 gene variations of C. felis, five subtype families (XIXa, XIXb, XIXc, XIXd, and XIXe) have been identified using 200 C. felis isolates from all around the world [17]. Among the five subtype families, the XIXa subtype family has been reported as the dominant subtype family in cats and humans in previous studies [17, 26, 52]. The third important finding in our study is the detection of subtype family XIXa in stray cats, which is consistent with previous results (Table 2; Fig. 2). This result also indicates that the XIXa subtype family is the dominant subtype in circulation causing the transmission between stray cats and humans in İzmir.
Conclusion
The presence of C. felis in stray cats was demonstrated for the first time by analyzing a huge number of fecal samples in Turkey. Detection of the dominant XIXa subtype family in cats and humans indicated the possible transmission risk between stray cats and humans in İzmir. Accordingly, it was thought that the stool of stray cats should be analyzed for the presence of C. felis and positive stray cats should be treated to reduce the zoonotic transmission from cats to humans by the municipality veterinarians during their routine testing before sterilization operation.
Materials and methods
Fecal samples
Fecal samples (n = 399) collected from stray cats which were brought for routine surgical sterilization process performed by the Veterinary clinics of the Municipality of İzmir were included in this study. Regions that fecal samples were collected were Konak (n = 159), Narlıdere (n = 148), Çiğli (n = 70) and Karabağlar (n = 22) districts. Before surgical sterilization and receiving any treatment, the fecal samples were collected from stray cats which were kept in separate cages.
Nested PCR–RFLP
DNA was extracted by a commercial stool DNA isolation kit (RTA Labs, Turkey) according to the manufacturer’s instructions. During DNA isolation, 100 mg of fecal sample was used, and they were eluted with 100 µl elution buffer [23]. A nested PCR targeting small subunit 18S rRNA gene was applied as described before [14]. In the initial reaction, 5'-TTCTAGAGCTAATACATGCG-3' and 5'-CCCTAATCCTTCGAAACAGGA-3' primers were used to amplify a 1325 bp gene fragment. In the second reaction, 5'-GGAAGGGTTGTATTTATTAGATAAAG-3' and 5'-AAGGAGTAAGGAACAACCTCCA-3' primers were used to amplify gene fragments with a molecular size approximately 826–864 bp from the initial reaction product. In the initial reaction of nested PCR, 100 μl amplification reaction included 2 μl template DNA, 2 μl primers, 16 μl MgCI2 (25 nM), 2 μl dNTP, 10 μl Taq buffer, and 0,5 μl Taq DNA Polymerase (ThermoFisher). In the second step of nested PCR, 4 μl PCR product as template DNA and 4 μl MgCI2 (25 nM) was used as different from the first step. The nested PCR was performed using the following protocol for both steps: 3 min initial denaturation step at 94 °C, followed by 35 cycles of 45 s at 94 °C, 45 s at 55 °C, and 1 min at 72 °C, and a final extension of 7 min at 72 °C. After amplification, PCR products were visualized using 1% agarose gel. Then, PCR products were purified by Qiaquick PCR Purification Kit (Qiagen, USA), sequenced, and compared with the NCBI database (https://www.ncbi.nlm.nih.gov) by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For sequencing of the 18S rRNA gene, forward primer (5'-GGAAGGGTTGTATTTATTAGATAAAG-3') was used.
For species identification of Cryptosporidium-positive samples, PCR products with a size of 826–864 bp were digested by SspI restriction enzyme (ThermoFisher) for 4 h [14–16]. During digestion, 20 μl total reaction volume included 10 μl amplified PCR product, 1.5 μl restriction enzyme, 2 μl restriction buffer (10x), and 6.5 μl distilled water. After digestion, PCR products were visualized using 2.5% agarose gel electrophoresis. After digesting by SspI enzyme, the expected product sizes for C. felis are 426 bp and 390 bp whereas [2, 16] the expected products for C. parvum are 444 bp, 247 bp, and 106 bp [16].
Subtyping
Cryptosporidium felis isolates were subtyped by sequencing of the gp60 gene as described previously [5]. In the initial reaction, GP60CF_F1 (5'-TTTCCGTTATTGTTGCAGTTGCA-3') and GP60CF_R1 (5'-ATCGGAATCCCACCATCGAAC-3') primers were used to amplify a 1200 bp gene fragment. In the second reaction, GP60CF_F2 (5'-GGGCGTTCTGAAGGATGTAA-3') and GP60CF_R2 (5'-CGGTGGTCTCCTCAGTCTTC-3') primers were used to amplify a 900 bp gene fragment from the initial reaction product. In the initial reaction of nested PCR, 20 μl amplification reaction included 3 μl template DNA, 0.5 μl primers, and 12.5 μl Dye Master Mix II (GeneMark). The second step of nested PCR was the same as the first step except the 1 μl PCR product that was used as template DNA. The nested PCR was performed using the following protocol for both steps: 4 min initial denaturation step at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and 1.5 min at 72 °C, and a final extension of 7 min at 72 °C. Obtained PCR products were visualized using 1% agarose gel. Then, PCR products purified by Qiaquick PCR Purification Kit (Qiagen, USA) were sequenced, and generated sequences belonging to the gp60 gene were aligned with reference sequences subtyped as XIXa, XIXb, XIXc, XIXd, or XIXe by Jiang et al., 2020. For sequencing the gp60 gene, forward primer (5'-GGGCGTTCTGAAGGATGTAA-3') was used. Subtype determination was performed by a scheme defined by Jiang et al. [26]. Next, a phylogenetic tree was constructed by the Maximum Likelihood method using the Kimura-3 parameter with 1000 bootstraps in order to confirm subtype results.
Acknowledgements
Not Applicable.
Abbreviations
- C. felis
Cryptosporidium felis
- PCR
Polymerase chain reaction
- gp60
60-KDa glycoprotein gene
- RFLP
Restriction fragment length polymorphism
- 18S rRNA
Small subunit rRNA gene
Authors’ contributions
Conceptualization: HC, and CÜ; Methodology: HC, and CÜ; Formal analysis and investigation: AEK, MG, PBM, HC, MD, ADD and MK; Writing-original draft preparation: HC; Writing-review and editing: HC, CÜ, MD, ADD and AYG; Funding acquisition: HC; Supervision: HC, and CÜ. All authors read and approved the final manuscript.
Funding
This study was supported by a project given by the Ege University Scientific Research Projects Coordination Unit (Project number: TGA-2021–22513) to H.C.
Availability of data and materials
All sequences obtained from 18S rRNA and gp60 genes of Cryptosporidium isolates were deposited into GenBank (National Center for Biotechnology Information Search database) under accession numbers OL615014- OL615025 (for the 18S rRNA gene) and OL631626- OL631637 (for gp60).
Declarations
Ethics approval and consent to participate
The protocol for collecting fecal samples from stray cats was declared as exempt according to the instructions and approval of the Institutional Animal Care and Use Committee (IACUC) of Ege University. All methods were performed in accordance with the relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huber F, da Silva S, Bomfim TC, Teixeira KR, Bello AR. Genotypic characterization and phylogenetic analysis of Cryptosporidium sp. from domestic animals in Brazil. Vet Parasitol. 2007;150(1–2):65–74. doi: 10.1016/j.vetpar.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Cieloszyk J, Goni P, García A, Remacha MA, Sánchez E, Clavel A. Two cases of zoonotic cryptosporidiosis in Spain by the unusual species Cryptosporidium ubiquitum and Cryptosporidium felis. Enferm Infecc Microbiol Clin. 2012;30:549–551. doi: 10.1016/j.eimc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Ryan U, Zahedi A, Feng Y, Xiao L. An Update on Zoonotic Cryptosporidium Species and Genotypes in Humans. Animals. 2021;11(11):3307. doi: 10.3390/ani11113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scorza V, Tangtrongsup S. Update on the diagnosis and management of Cryptosporidium spp infections in dogs and cats. Top Companion Anim Med. 2010;25(3):163–169. doi: 10.1053/j.tcam.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Rojas-Lopez L, Elwin K, Chalmers RM, Enemark HL, Beser J, Troell K. Development of a gp60-subtyping method for Cryptosporidium felis. Parasit Vectors. 2020;13(1):39. doi: 10.1186/s13071-020-3906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmer G, Köster PC, Carmena D. Cryptosporidium hominis infections in non-human animal species: revisiting the concept of host specificity. Int J Parasitol. 2020;50(4):253–262. doi: 10.1016/j.ijpara.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Alves MEM, Martins FDC, Bräunig P, Pivoto FL, Sangioni LA, Vogel FSF. Molecular detection of Cryptosporidium spp. and the occurrence of intestinal parasites in fecal samples of naturally infected dogs and cats. Parasitol Res. 2018;117:3033–3038. doi: 10.1007/s00436-018-5986-4. [DOI] [PubMed] [Google Scholar]
- 8.Lucio-Forster A, Griffiths JK, Cama VA, Xiao L, Bowman DD. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010;26(4):174–179. doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Ying JL, Monis P, Ryan U. Molecular characterisation of Cryptosporidium and Giardia in cats (Felis catus) in Western Australia. Exp Parasitol. 2015;155:13–18. doi: 10.1016/j.exppara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Li Y, Song M, Lu Y, Yang J, Tao W, Jiang Y, Wan Q, Zhang S, Xiao L. Prevalence and genetic characteristics of Cryptosporidium, Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang province. China Vet Parasitol. 2015;208(3–4):125–134. doi: 10.1016/j.vetpar.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Gil H, Cano L, de Lucio A, Bailo B, de Mingo MH, Cardona GA, Fernández-Basterra JA, Aramburu-Aguirre J, López-Molina N, Carmena D. Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in Northern Spain. Infect Genet Evol. 2017;50:62–69. doi: 10.1016/j.meegid.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Liu X, Gu Y, Liu J, Luo J. Prevalence of Cryptosporidium, Giardia, Blastocystis, and trichomonads in domestic cats in East China. J Vet Med Sci. 2019;81(6):890–896. doi: 10.1292/jvms.19-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omoruyi BE, Nwodo UU, Udem CS, Okonkwo FO. Comparative diagnostic techniques for cryptosporidium infection. Molecules. 2014;19(2):2674–2683. doi: 10.3390/molecules19022674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65(4):1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W, Cabrera L, Gilman RH, Lal AA. Identification of 5 types of Cryptosporidium parasites in children in Lima. Peru J Infect Dis. 2001;183(3):492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 16.Das P, Roy SS, MitraDhar K, Dutta P, Bhattacharya MK, Sen A, Ganguly S, Bhattacharya SK, Lal AA, Xiao L. Molecular characterization of Cryptosporidium spp. from children in Kolkata, India. J Clin Microbiol. 2006;44:4246–4249. doi: 10.1128/JCM.00091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Yang F, Liang R, Guo S, Guo Y, Li N, Feng Y, Xiao L. Subtype Characterization and Zoonotic Potential of Cryptosporidium felis in Cats in Guangdong and Shanghai, China. Pathogens. 2021;10(2):89. doi: 10.3390/pathogens10020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power ML, Cheung-Kwok-Sang C, Slade M, Williamson S. Cryptosporidium fayeri: diversity within the GP60 locus of isolates from different marsupial hosts. Exp Parasitol. 2009;121(3):219–223. doi: 10.1016/j.exppara.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Huang N, Jiang W, Wang X, Li N, Guo Y, Kváč M, Feng Y, Xiao L. Subtyping Cryptosporidium ryanae: A Common Pathogen in Bovine Animals. Microorganisms. 2020;8:1107. doi: 10.3390/microorganisms8081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W, Roellig DM, Guo Y, Li N, Feng Y, Xiao L. Development of a Subtyping Tool for Zoonotic Pathogen Cryptosporidium canis. J Clin Microbiol. 2021;59(3):e02474–e2520. doi: 10.1128/JCM.02474-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Can H, Döşkaya M, Ajzenberg D, Özdemir HG, Caner A, İz SG, Döşkaya AD, Atalay E, Çetinkaya Ç, Ürgen S, Karaçalı S, Ün C, Dardé ML, Gürüz Y. Genetic characterization of Toxoplasma gondii isolates and toxoplasmosis seroprevalence in stray cats of Izmir. Turkey. PLoS One. 2014;9:e104930. doi: 10.1371/journal.pone.0104930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Can H, Döşkaya M, Özdemir HG, Şahar EA, Karakavuk M, Pektaş B, Karakuş M, Töz S, Caner A, Döşkaya AD, İz SG, Özbel Y, Gürüz Y. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir. Turkey Exp Parasitol. 2016;167:109–114. doi: 10.1016/j.exppara.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Karakavuk M, Can H, Selim N, Yeşilsiraz B, Atlı E, Atalay Şahar E, Demir F, Gül A, Özdemir HG, Alan N, Yalçın M, Özkurt O, Aras M, Çelik T, Can Ş, Değirmenci Döşkaya A, Gürüz AY, Döşkaya M. Investigation of the role of stray cats for transmission of toxoplasmosis to humans and animals living in İzmir. Turkey J Infect Dev Ctries. 2021;15:155–162. doi: 10.3855/jidc.13932. [DOI] [PubMed] [Google Scholar]
- 24.Karakavuk M, Selim N, Yeşilsiraz B, Atlı E, Özdemir H, Alan N, Yalçın M, Özkurt O, Aras M, Çelik T, Can Ş, Değirmenci Döşkaya A, Köseoğlu A, Erkunt Alak S, Karakavuk T, Un C, Gürüz A, Döşkaya M, Can H. Prevalence of Gastrointestinal Parasites in Stray Cats of İzmir. Anim Health Product Hyg. 2021;10:6–11. [Google Scholar]
- 25.Can H, Köseoğlu AE, ErkuntAlak S, Güvendi M, Ün C, Karakavuk M, DeğirmenciDöşkaya A, Aykur M, AksoyGökmen A, Gürüz AY, Döşkaya M. Molecular prevalence and subtyping of Blastocystis sp. isolates in stray cats of İzmir, Turkey: First report of “ST4 allele 42” in cats. Pol J Vet Sci. 2021;24:217–223. doi: 10.24425/pjvs.2021.137656. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Roellig DM, Lebbad M, Beser J, Troell K, Guo Y, Li N, Xiao L, Feng Y. Subtype distribution of zoonotic pathogen Cryptosporidium felis in humans and animals in several countries. Emerg Microbes Infect. 2020;9(1):2446–2454. doi: 10.1080/22221751.2020.1840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhold RW, Jessup DA. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health. 2013;60(3):189–195. doi: 10.1111/j.1863-2378.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- 28.Diakou A, Di Cesare A, Accettura PM, Barros L, Iorio R, Paoletti B, di Frangipane Regalbono A, Halos L, Beugnet F, Traversa D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl Trop Dis. 2017;11(1):e0005335. doi: 10.1371/journal.pntd.0005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak D, Seo MG. Genetic Analysis of Zoonotic Gastrointestinal Protozoa and Microsporidia in Shelter Cats in South Korea. Pathogens. 2020;9(11):894. doi: 10.3390/pathogens9110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turgay N, Ünver-Yolasigmaz A, Oyur T, Özcem SB, Töz S. Monthly distribution of intestinal parasites detected in a part of western Turkey between May 2009-April 2010-results of acid fast and modified trichrome staining methods. Türkiye Parazitol Derg. 2012;36:71–74. doi: 10.5152/tpd.2012.18. [DOI] [PubMed] [Google Scholar]
- 31.Ulusan O, Zorbozan O, Yetismis K, Töz S, Ünver A, Turgay N. The distribution of the intestinal parasites detected in Ege University Medical Faculty Parasitology Direct Diagnosis Laboratory; 10-years evaluation. Türk Mikrobiyoloji Cem Derg. 2019;49:86–91. [Google Scholar]
- 32.Cardona GA, de Lucio A, Bailo B, Cano L, de Fuentes I, Carmena D. Unexpected finding of feline-specific Giardia duodenalis assemblage F and Cryptosporidium felis in asymptomatic adult cattle in Northern Spain. Vet Parasitol. 2015;209(3–4):258–263. doi: 10.1016/j.vetpar.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Cacciò S, Pinter E, Fantini R, Mezzaroma I, Pozio E. Human infection with Cryptosporidium felis: case report and literature review. Emerg Infect Dis. 2002;8:85. doi: 10.3201/eid0801.010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Beser J, Toresson L, Eitrem R, Troell K, Winiecka-Krusnell J, Lebbad M. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect Ecol Epidemiol. 2015;5:28463. doi: 10.3402/iee.v5.28463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lucio A, Merino FJ, Martínez-Ruiz R, Bailo B, Aguilera M, Fuentes I, Carmena D. Molecular genotyping and sub-genotyping of Cryptosporidium spp. isolates from symptomatic individuals attending two major public hospitals in Madrid. Spain. Infect Genet Evol. 2016;37:49–56. doi: 10.1016/j.meegid.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 37.de Lucio A, Bailo B, Aguilera M, Cardona GA, Fernández-Crespo JC, Carmena D. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Álava. Northern Spain. Acta Trop. 2017;170:48–56. doi: 10.1016/j.actatropica.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Sursal N, Simsek E, Yildiz K. Occurrence and First Molecular Characterization of Cryptosporidium felis in a Cat in Turkey. Kafkas Univ Vet Fak Derg. 2020;26:833–837. [Google Scholar]
- 39.Koloren Z, Ayaz E. Genotyping of Cryptosporidium spp. in environmental water in Turkey. Acta Parasitol. 2016;61(4):671–679. doi: 10.1515/ap-2016-0094. [DOI] [PubMed] [Google Scholar]
- 40.Ito Y, Itoh N, Iijima Y, Kimura Y. Molecular prevalence of Cryptosporidium species among household cats and pet shop kittens in Japan. JFMS Open Rep. 2017;3(2):2055116917730719. doi: 10.1177/2055116917730719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koompapong K, Mori H, Thammasonthijarern N, Prasertbun R, Pintong AR, Popruk S, Rojekittikhun W, Chaisiri K, Sukthana Y, Mahittikorn A. Molecular identification of Cryptosporidium spp. in seagulls, pigeons, dogs, and cats in Thailand. Parasite. 2014;21:52. doi: 10.1051/parasite/2014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sannella AR, Suputtamongkol Y, Wongsawat E, Cacciò SM. A retrospective molecular study of Cryptosporidium species and genotypes in HIV-infected patients from Thailand. Parasit Vectors. 2019;12:1–6. doi: 10.1186/s13071-019-3348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer CS, Traub RJ, Robertson ID, Devlin G, Rees R, Thompson RC. Determining the zoonotic significance of Giardia and Cryptosporidium in Australian dogs and cats. Vet Parasitol. 2008;154(1–2):142–147. doi: 10.1016/j.vetpar.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Santín M, Trout JM, Vecino JA, Dubey JP, Fayer R. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet Parasitol. 2006;141(3–4):334–339. doi: 10.1016/j.vetpar.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 45.de Oliveira AGL, Sudré AP, do Bergamo Bomfim TC, Santos HLC. Molecular characterization of Cryptosporidium spp. in dogs and cats in the city of Rio de Janeiro, Brazil, reveals potentially zoonotic species and genotype. PLoS One. 2021;16:e0255087. doi: 10.1371/journal.pone.0255087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballweber LR, Panuska C, Huston CL, Vasilopulos R, Pharr GT, Mackin A. Prevalence of and risk factors associated with shedding of Cryptosporidium felis in domestic cats of Mississippi and Alabama. Vet Parasitol. 2009;160:306–310. doi: 10.1016/j.vetpar.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Dan X, Zhu K, Li N, Guo Y, Zheng Z, Feng Y, Xiao L. Genetic characterization of Cryptosporidium spp. and Giardia duodenalis in dogs and cats in Guangdong, China. Parasit Vectors. 2019;12(1):571. doi: 10.1186/s13071-019-3822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Wei Z, Zhang Y, Zhang Q, Zhang L, Yu F, Qi M, Zhao W. Cryptosporidium in asymptomatic children in Southern Xinjiang, China and the potential of zoonotic transmission. 2021. [Google Scholar]
- 49.Bednarska M, Jankowska I, Pawelas A, Piwczyńska K, Bajer A, Wolska-Kuśnierz B, Wielopolska M, Welc-Falęciak R. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res. 2018;117:2869–2879. doi: 10.1007/s00436-018-5976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gherasim A, Lebbad M, Insulander M, Decraene V, Kling A, Hjertqvist M, Wallensten A. Two geographically separated food-borne outbreaks in Sweden linked by an unusual Cryptosporidium parvum subtype, October 2010. Euro Surveill. 2012;17(46):20318. doi: 10.2807/ese.17.46.20318-en. [DOI] [PubMed] [Google Scholar]
- 51.Casmo V, Lebbad M, Maungate S, Lindh J. Occurrence of Cryptosporidium spp. and Cystoisospora belli among adult patients with diarrhoea in Maputo. Mozambique. Heliyon. 2018;4:e00769. doi: 10.1016/j.heliyon.2018.e00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lebbad M, Winiecka-Krusnell J, Stensvold CR, Beser J. High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad. Pathogens. 2021;10:523. doi: 10.3390/pathogens10050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences obtained from 18S rRNA and gp60 genes of Cryptosporidium isolates were deposited into GenBank (National Center for Biotechnology Information Search database) under accession numbers OL615014- OL615025 (for the 18S rRNA gene) and OL631626- OL631637 (for gp60).