Abstract
Isotope labelling, particularly deuteration, is an important tool for the development of new drugs, specifically for identification and quantification of metabolites. For this purpose, many efficient methodologies have been developed that allow for the small-scale synthesis of selectively deuterated compounds. Due to the development of deuterated compounds as active drug ingredients, there is a growing interest in scalable methods for deuteration. The development of methodologies for large-scale deuterium labelling in industrial settings requires technologies that are reliable, robust and scalable. Here we show that a nanostructured iron catalyst, prepared by combining cellulose with abundant iron salts, permits the selective deuteration of (hetero)arenes including anilines, phenols, indoles and other heterocycles, using inexpensive D2O under hydrogen pressure. This methodology represents an easily scalable deuteration (demonstrated by the synthesis of deuterium-containing products on the kilogram scale) and the air- and water-stable catalyst enables efficient labelling in a straightforward manner with high quality control.

Subject terms: Homogeneous catalysis, Synthetic chemistry methodology, Synthetic chemistry methodology, Heterogeneous catalysis
A method for the selective deuteration of anilines, indoles, phenols and heterocyclic compounds, including natural products and other bioactive molecules, has been developed. The nanostructured iron catalyst that underpins this process is prepared by combining cellulose with iron salts and has been used for the preparation of deuterated compounds on up to a kilogram scale.
Main
Isotope labelling methodologies play an essential part in the development of new pharmaceuticals and agrochemicals1 (Fig. 1a). For example, in the pharmaceutical industry, isotopes of active drugs are commonly prepared to understand their metabolism and to identify specific metabolites (Fig. 1b). The most common isotopic labels are deuterium atoms, which are also well suited to determine kinetic isotope effects (KIEs) in fundamental mechanistic investigations that result from differences in the rate of C–H versus C–D bond cleavage2,3 (Fig. 1c). Deuterium-labelled compounds show virtually identical physical behaviour to that of their hydrogen analogues, whilst differing in molecular mass, and thus are the primary source for the preparation of internal standards for liquid chromatography–mass spectrometry (LC–MS) analysis in the investigation of environmental, animal and human samples4,5 (Fig. 1d). Accordingly, deuteration facilitates advancements in metabolomics including metabolite identification and quantification, in toxicogenomic studies for the related reactive metabolites and in proteomics studies.
Fig. 1. Applications of deuteration and methods for H/D exchange.
a, Deuterated drug molecules: Austedo has been approved by the FDA and BMS-986165 is under trial. b, Identification of specific metabolites using LC–MS: accurate analysis of metabolites based on stable isotope labelling coupled with LC–MS analysis. c, KIE investigations based on C–H versus C–D bond cleavage. d, Preparation of internal standards for LC–MS analysis. The isotopically labelled internal standards can be used for quantitative determination. e, Acid-mediated H/D exchange. f, Transition-metal-catalysed H/D exchange. DG, directing group. g, This work: nanostructured iron catalyst for H/D exchange of (hetero)arenes using D2O.
Due to the potentially improved pharmacokinetic and pharmacological properties of deuterium-labelled compounds, while retaining almost the same chemical structure and physical properties as the unlabelled counterparts, in recent years this class of compounds has gained increasing interest as actual medications6,7. Notably, in 2017 the Food and Drug Administration (FDA) cleared the first deuterated drug, Austedo, for the treatment of Huntington’s-disease-related disorders8. Meanwhile, several deuterated compounds are in clinical trials for various applications. Based on these developments, there is growing potential and interest in accessing selectively deuterated building blocks on a larger scale. In this context, specific and practical labelling methodologies for arenes/heteroarenes as well as amines, which are found in most small-molecule-based drugs, are of increasing importance. Critical parameters for such applications are the availability and price of the labelling reagent, catalysts and so on, and the feasibility of the process in an industrial setting. Notably, precise control of impurities, degree of deuteration and consistency are also prerequisites for any real-life implementation9.
Acid-mediated hydrogen–deuterium exchange reactions (H/D exchange) are among the oldest methods known for labelling of arenes. Nevertheless, they allow selective incorporation of deuterium only for simple substrates following an electrophilic aromatic substitution mechanism (Fig. 1e)10. In most of the known protocols, the necessity to use high temperatures and stoichiometric amounts of concentrated strong acids leads to poor functional group tolerances and safety risks, especially on a larger scale11.
Based on advances in homogeneous metal-catalysed C–H activation, a variety of organometallic complexes have evolved for catalytic H/D exchange reactions of arenes, as well as aliphatic amines in the α or β positions (Fig. 1f)12–15. For example, homogeneous iridium-based Crabtree and Kerr catalysts have been used for C(sp2)–H hydrogen isotope exchange reactions using D2 gas16,17. Chirik and co-workers first reported the use of a molecularly defined iron catalyst for the tritiation/deuteration of pharmaceutical drugs at aromatic C–H moieties using D2 and T2 gas18. More recently, the MacMillan group developed a photochemical-catalysed hydrogen-isotope-exchange (HIE) method for the selective labelling of N-alkylamine-based drugs19.
Apart from photocatalysts and defined organometallic complexes, heterogeneous materials have also been studied in labelling reactions. So far, Pd/C and Pt/C are known to catalyse multi H/D exchange of arenes and heterocyclic amines20. In addition, ruthenium- and iridium-based catalysts in the presence of D2 have been developed with promising activity21,22. Unfortunately, in all these cases the selectivity and the tolerance of easily reducible functional groups and halogens is challenging. Furthermore, besides those based on nickel23, all heterogeneous catalysts known for deuterations rely on expensive precious metals, which hamper their use in the agrochemical, pharmaceutical or food industries as those metals must be removed completely from the final products according to regulations. Apart from the catalyst and the reaction conditions, the source of deuterium is critical for the application of such methodologies. This is especially true for the preparation of labelled building blocks on the multi-gram or even kilogram scale. In this respect, cheap, safe and operationally convenient D2O is the ideal source for such transformations because it is basically the parent compound for all other deuteration reagents, including D2.
In this article we report a unique heterogeneous iron catalyst for general and practical deuteration of arenes and heteroarenes. Using D2O in the presence of a biomass-derived iron catalyst under hydrogen pressure allows for the preparation of >90 selectively deuterated building blocks, representative drugs and natural products with high and reliable deuterium incorporation (Fig. 1g).
Results and discussion
Reaction development
Initially, we tested standard, commercially available heterogeneous catalysts and tailor-made supported nanoparticles (NPs) for selective deuteration of 4-phenylmorpholine in D2O (Supplementary Table 1). This benchmark substrate was chosen because it permits labelling both at the nitrogen-containing heterocycle and the phenyl ring. In all cases, the extent of isotopic exchange was determined using 1H NMR spectroscopy. In agreement with previous works24, Pd/C led to deuterium incorporation at the α position of the nitrogen atom on the morpholine ring (Supplementary Table 1, entry 1). Recently, we introduced a variety of supported 3d-metal NPs for selective hydrogenation and oxidation reactions25,26. In this context, iron-based NPs are particularly attractive to us due to the abundance, low cost and negligible safety concerns of iron salts. Much to our surprise, pyrolysis of Fe(NO3)3·9H2O with cellulose resulted in highly active and selective catalytic systems for deuteration of the phenyl ring in the ortho and para positions, which even outperformed commercial catalysts such as Pt/C, Au/C and Ru/C (Supplementary Table 1, entry 10 versus entries 2 and 4). The deuterium incorporation could be further improved under hydrogen pressure (Supplementary Table 1, entry 12). Furthermore, the stability and recyclability of the catalyst under H2 are better (Supplementary Information, section 5). Pseudo in situ X-ray photoelectron spectroscopy (XPS) studies show that the iron oxides formed on the surface of the catalyst during the catalysis could be partially reduced to Fe(0) when heating the sample under H2 (Supplementary Fig. 6). Finally, the benchmark reaction performed with D2O in the presence of hydrogen at 120 °C gave nearly quantitative deuteration (see Supplementary Table 1 for more details).
Catalyst characterization
To understand the structure of the most active material (Fe-Cellulose-1000), powder X-ray diffraction, XPS, scanning transmission electron microscopy (STEM) and X-ray absorption spectroscopy (XAS) investigations were performed. These results show that the freshly pyrolysed catalyst consists of Fe/Fe3C particles 20–50 nm in size, covered by a shell of up to 30 graphene layers with a overall thickness of 6–10 nm. Embedding the particles in the carbon matrix prevents them from undesired aggregation. During the early stages of the catalytic reaction, the graphene cover is partly removed, thus enabling the contact of the iron surface with reactants. Then, during the reaction, Fe3C is partly converted to metallic iron which is considered as active phase in the reaction (for details see Supplementary Information, section 6).
Mechanistic studies
Next, investigations of KIE and electron paramagnetic resonance (EPR) studies were performed to obtain a better mechanistic understanding (Supplementary Information, section 7). According to comparison experiments of the model substrate, the reaction is roughly four times faster in H2O than in D2O. Apparently, the cleavage of the D–OD bond is the rate-limiting step in this process (Fig. 2a and Supplementary Fig. 13). Interestingly, by comparing the deuteration of aniline and 3,5-dideuterioaniline, a minor secondary KIE is also observed (Fig. 2b and Supplementary Fig. 14). This kinetically relevant result may be explained by the slightly different coordination of the deuterated and non-deuterated substrate to active catalyst centres on the surface. To check whether radicals are formed upon cleavage of the D–OD bond, EPR measurements with the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) have been performed (Supplementary Information, section 7.2). In a blind experiment, the catalyst was heated in D2O before DMPO was added, and an EPR spectrum was recorded immediately after quenching the reaction to room temperature. This spectrum showed only a weak but characteristic hyperfine structure quartet signal of DMPO–OD spin adducts27 which results from trapping •OD radicals by DMPO (Fig. 2c black line). This shows that the iron catalyst can promote to a small extent homolytic splitting of D2O. However, in the presence of 4-phenylmorpholine, an additional hyperfine structure sextet characteristic of a DMPO–R spin adduct28 was detected (Fig. 2c, blue line), suggesting that *OD subtract hydrogen atoms from the substrate, forming HOD, leaving behind •R radicals. Interestingly, no •R radicals were detected when the iron catalyst and the 4-phenylmorpholine substrate were heated in toluene, which indicates that formation of radical intermediates is a consequence of homolytic D–OD scission initiated by the iron catalyst. Considering the well-known fact that •OD radicals are very reactive and unselective, the observed high selectivity is surprising.
Fig. 2. Mechanistic studies and proposed mechanism.
a, Aniline in D2O and aniline-2,4,6-d3 in H2O were used for the KIE studies. The reaction in H2O is roughly four times faster than the reaction in D2O. b, Deuteration of aniline and 3,5-dideuterioaniline in D2O were performed for KIE studies, and a minor secondary KIE was observed. c, EPR studies: •OD radical trapping using DMPO and detection of the DMPO–R spin adduct. This indicates that formation of radical intermediates is a consequence of homolytic D–OD scission initiated by the iron catalyst. d, Proposed mechanism: the multiple graphene layer is removed during the early stages of the catalytic reaction, thus enabling contact of the iron surface with D2O and reactants. The key step of the reaction is D2O splitting by the iron catalyst. The resulting radicals are not liberated into the solution but remain adsorbed on the catalyst surface.
Proposed mechanism
We propose a concerted mechanism in which D2O is split homogeneously by the iron catalyst, yet the resulting radicals are not liberated into the solution but remain adsorbed on the catalyst surface as activated D* and *OD species. *OD abstracts a hydrogen atom from the phenyl ring, forming HDO and the corresponding phenyl* species29,30, which subsequently produces D–R (Fig. 2c,d). The observed high ortho/para selectivity may be a result of electron density transfer from the electron-rich metallic iron particle to the adsorbed aromatic ring because this is well known to promote electrophilic ortho/para substitutions.
Synthetic scope
Building blocks
Having established the optimized conditions, we then evaluated the substrate scope of the system and its tolerance towards functional groups. Because anilines are used for the synthesis of diverse building blocks for agrochemicals and pharmaceuticals, we started to explore the deuteration of functionalized anilines. Indeed, Fe-Cellulose-1000 permitted smooth deuteration of 35 different anilines with excellent chemo- and regioselectivity (Table 1, 3–37b).
Table 1.
Nanostructured iron catalyst for deuteration of anilines and phenols
Deuterium content determined by quantitative 1H NMR. aThe reaction was performed on the gram scale. bThe reaction time was 72 h.
In general, the transformations can be easily run on the gram-scale (4b and 23b). Diverse halogen-containing (for example, chlorine and iodine) anilines afforded the deuterated products (7b, 8b, 11–14b and 16–19b) without notable dehalogenation side-reactions, which is a common problem of precious-metal-based catalysts. We demonstrated the isotopic purity of chloro-, bromo- and iodo-containing compounds by LC–MS (Supplementary Information, section 8.3). Furthermore, deuteration of some phenols in the presence of the iron catalyst showed deuterium incorporation after an extended time (Table 1, 38–43b). Like the model system, negligible or no deuterium incorporation is observed for several representative substrates (3a, 4a, 38a, 44a, 52a and 67a) in the absence of the iron catalyst (Supplementary Fig. 16).
Next, deuteration of different nitrogen-containing heteroarenes such as pyridines, tetrahydroquinolines, phenothiazines, phenoxazines, indoles, indolines and quinolines, and even those bearing two nitrogen atoms, were investigated. Such heterocycles are representative structural components of modern pharmaceuticals: 59% of FDA-approved drugs contain a nitrogen heterocyclic motif31. As shown in Table 2, catalytic labelling provided the corresponding products (44–66b). Notably, for anilines and phenols the regioselectivity of the labelling reaction is in accordance with an electrophilic aromatic substitution mechanism. However, using pyridines, indoles or indolines, different reactivity patterns are observed, which hints towards a different process.
Table 2.
Nanostructured iron catalyst for deuteration of heteroarenes
Deuterium content determined by quantitative 1H NMR. aThe reaction was performed at 140 °C for 24 h. The pink circles and numbers denote the positions of the C–H bonds that are labelled and the percentage incorporation of the hydrogen isotope, respectively.
Natural products and pharmaceuticals
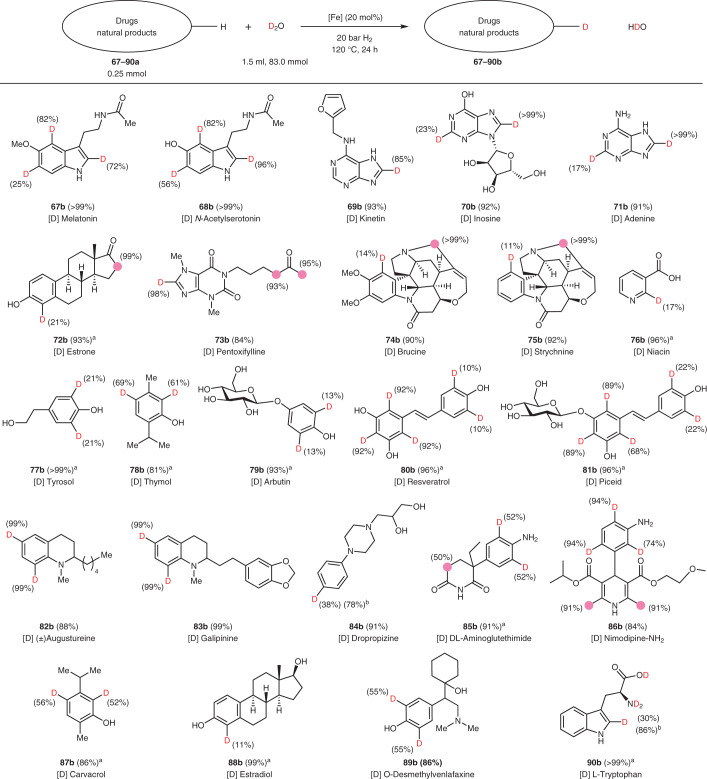
To further showcase the utility of iron-catalysed H/D exchange reactions, late-stage deuteration of representative drugs and natural products was investigated (Table 3). For example, melatonin is converted into the deuterated 67b with high levels of deuterium incorporation. Similarly, N-acetylserotonin is deuterated to give 68b. Purine-containing compounds, such as nucleoside analogues kinetin, inosine, pentoxifylline and the nucleobase adenine, are labelled to the products 69–71b and 73b. Alkaloids, for example, brucine and strychnine, are deuterated at both aromatic rings and in the heterocyclic ring in the α position to the nitrogen (74b and 75b). In the case of nicotinic acid, labelling occurred with relatively lower deuterium incorporation selectively to give 76b. Furthermore, natural phenol derivatives, for example, tyrosol, resveratrol, thymol, arbutin and piceid, were evaluated (77–81b). Tetrahydroquinoline alkaloids, for example, augustureine and galipinine, also proceeded with deuterium labelling (82b and 83b). Aniline derivatives such as dropropizine, dl-aminoglutethimide and nimodipine-NH2 were deuterated with good selectivity (84–86b). Notably, medications such as carvacrol, estradiol and O-desmethylvenlafaxine gave the deuterium analogues 87–89b. As an example, for selective deuteration of an aromatic amino acid, l-tryptophan was employed to directly give 90b. In all cases shown above the standard catalytic protocol was used and no further optimization was performed. However, it should be noted that the deuterium incorporation can be substantially improved at higher temperature (84b and 90b).
Table 3.
Nanostructured iron catalyst for deuteration of natural products and pharmaceuticals
Representative drugs, hormones, nucleobases, steroids, alkaloids, amino acids, upscale and applications. Deuterium content determined by quantitative 1H NMR. aThe reaction time was 72 h. bThe reaction was performed at 140 °C for 24 h. The pink circles and numbers denote the positions of the C–H bonds that are labelled and the percentage incorporation of the hydrogen isotope, respectively.
Following standard conditions, deuteration of N-acylated anilines is more difficult; however, such labelled products can be conveniently prepared from the corresponding deuterated anilines (Fig. 3a). For instance, 2,6-D-labelled paracetamol 92b was readily synthesized with excellent deuterium incorporation. Deuterated lidocaine 93b, herbicides fluometuron 94b and chlortoluron 95b as well as fungicide boscalid 96b were obtained from the corresponding deuterated anilines.
Fig. 3. Synthetic applications and large-scale synthesis.
a, Preparation of deuterated N-acylated anilines. Deuterated drugs, herbicides and fungicide were prepared from the corresponding deuterated anilines. b, Dedeuteration of perdeuterated aniline using H2O to 3,5-dideuterioaniline. c, Scale-up reactions: reuse of the iron catalyst on the 2.0 mol scale. Overall, 1.005 kg 4-phenylmorpholine (6.17 mol) was deuterated using the same catalyst batch; >95% deuterium-labelled 4-phenylmorpholine and 1,2,3,4-tetrahydroquinoline were produced.
All these cases demonstrate that the presented methodology works well with amino- and/or hydroxyl-substituted arenes as well as heteroarenes; however, less electron-rich benzenes, for example, 1-bromo-, 1-chloro- and 1-fluoro-4-methoxybenzene and 1-methyl-4-(trifluoromethyl)benzene, showed no notable deuterium incorporation under standard conditions. In case of successful labelling as described above deuteration occurred at the most electron-rich positions. Obviously, this limits the possibilities to obtain deuterated compounds at other positions. For example, 2,4,6-trideuterated anilines are conveniently available, while selective deuteration at the 3- and 5-positions are apparently not possible. However, we envisioned a convenient strategy to overcome this limitation: non-selective deuteration of anilines is known in the presence of Pt/C (ref. 32). Subsequent selective D/H exchange using our Fe-Cellulose-1000 catalyst in water makes it possible to selectively obtain deuterium isomers at positions that are not prone to electrophilic substitution reactions. Thus, 3,5-dideuterioaniline 2a is obtained from aniline via the perdeuterated derivative (Fig. 3b).
Having established complementary approaches for selective deuteration of a variety of substrates, we then evaluated some practical aspects of our catalyst system. It should be noted that most of the reactions shown above have been performed on a scale typically used in drug discovery. However, as shown in Fig. 3c it is possible to conveniently run such labelling reactions on the 20 to >300 g scale (Supplementary Information, section 9), with the only limitation being the size of the commercial autoclave. In general, stability, reusability and avoidance of metal contamination are intrinsic advantages to the use of any heterogeneous catalyst. To demonstrate these benefits, the iron catalyst was recycled up to five times for the benchmark reaction. As depicted in Supplementary Fig. 18, no substantial loss of activity was observed. During deuteration reactions, an oxidic structure composed of Fe2+ and Fe3+ was formed as proven by XPS (Supplementary Fig. 5). With respect to metal contamination of the product, inductively coupled plasma optical emission spectrometry measurements of the D2O solution detected no iron leaching in any of these runs (Supplementary Table 8). Notably, the recycled catalyst system can also be used for different substrates, which is important in the context of multipurpose batch reactors, which dominate in the pharmaceutical industry. Thus, labelled products with high deuterium incorporation were produced on >1 kg scale from the same catalyst batch (Fig. 3c and Supplementary Information, section 9).
In summary, we have developed a general methodology for heterogeneous iron-catalysed deuteration reactions. We believe this protocol paves the way for practical labelling processes and the large-scale synthesis of specific deuterated building blocks. Although the quality of different deuteration reactions was performed as accurately as possible (Supplementary Tables 5 and 6), all presented developments took place under conditions that did not conform to good manufacturing practice. The optimal biomass-derived catalyst allows for an activation and utilization of low-cost D2O. The performance of this catalyst system is improved in the presence of hydrogen, which leads to in situ reduction of iron oxides on the surface as indicated by pseudo in situ XPS measurements (Supplementary Fig. 6). The presented system is effective for the selective deuteration of anilines, indoles, phenols and other heterocyclic compounds, including late-stage labelling of natural products and bioactive molecules, and can be readily used for the preparation of deuterated compounds on the kilogram scale. By using complementary approaches, different positional deuterated products can also be obtained in a practical manner.
Methods
Determination of deuterium incorporation
The positions and percentage of deuterium incorporation were determined by 1H NMR. The equation below was used to determine the degree of deuterium incorporation; peaks were calibrated against a signal corresponding to a unlabelled position. The labelling position was determined by 1H NMR according to the chemical shifts and peak multiplicity. In addition, deuterium incorporations were confirmed using high-resolution MS by comparison of all the labelled and unlabelled compounds (note that high-resolution MS serves here to substantiate the results of quantitative NMR analysis).
Procedure for the preparation of the catalyst
A 250 ml oven-dried single-necked round-bottomed flask equipped with a reflux condenser and a Teflon-coated, egg-shaped magnetic stir bar (40 mm × 18 mm) was charged with Fe(NO3)3·9H2O (404 mg, 1.0 mmol) dissolved in ethanol (150 ml). Then, this solution was heated to 80 °C (r.t. to 80 °C) in an oil bath and stirred for 1 h. To the reaction solution, 6.0 g cellulose was added via a glass funnel, and the resulting heterogeneous mixture was stirred at 450 r.p.m. for 15 h at 80 °C. The flask was taken out from the bath and cooled to ambient temperature. The solvent was removed in vacuum and then dried under an oil pump vacuum for 4 h to give a yellow solid. The sample was transferred to a ceramic crucible and placed in an oven. The latter was evacuated to ∼5 mbar and then flushed with argon three times. The furnace was heated to 1,000 °C at a rate of 25 °C min−1 and held at 1,000 °C for 2 h under argon atmosphere. After the heating was switched off, the oven was allowed to reach r.t., giving the Fe-Cellulose-1000 catalyst as a black powder (note that throughout the process, argon was constantly passed through the oven).
General procedure for the deuteration reactions
In a 4 ml vial fitted with a magnetic stir bar and septum cap, iron catalyst (60 mg, 20 mol%) and substrate (0.25 mmol) were added. Then, a needle was inserted in the septum, allowing gaseous reagents to enter. After adding the solvent deuterium oxide (1.5 ml), the vials (up to eight) were set in an alloy plate and then placed into a 300 ml steel Parr autoclave. The autoclave was flushed with hydrogen six times at 10 bar and finally pressurized to the desired value (20 bar). Then, it was placed into an aluminium block and heated to the desired temperature. At the end of the reaction, the autoclave was quickly cooled down to r.t. with an ice bath and vented. Finally, the samples were removed from the autoclave, and ethyl acetate was added to the crude mixture. This mixture was centrifuged, and the organic layer was removed from the vials (three times). After removal of all volatiles in vacuo, the desired products were obtained. In case of anilines with deuterium labelling on the nitrogen, 1 ml H2O was added during work up and N–D was replaced by N–H.
General procedure for the >300 g scale reactions
To a 2 l steel Parr autoclave containing 28.0 g Fe-Cellulose-1000 catalyst (1 mol%), 340 g 4-phenylmorpholine (2.09 mol) and deuterium oxide (1,500 ml, 83 mol) were added. The autoclave was flushed with hydrogen six times at 10 bar and finally pressurized to the desired value (20 bar). Then, it was placed into an equipment (see Supplementary Section 9 for more details) for the autoclave and heated to 120 °C, and then held at 120 °C for 120 h. At the end of the reaction, the autoclave was quickly cooled down to r.t. with an ice bath and vented. Finally, the crude reaction was added to ethyl acetate (1.5 l). The reaction mixture was filtered with filter paper. The D2O layer was removed from the mixture and washed with ethyl acetate (1.5 l, three times). After removal of all volatiles in vacuo, 341 g of deuterium product was obtained. It is important to note that during the cool down, trace amounts of gas can stay in the solid phase (product and catalyst). Thus, the solid phase should be dissolved slowly in ethyl acetate.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-021-00846-4.
Supplementary information
General remarks, procedure details for the preparation of the catalyst, deuteration reactions, reaction development and optimization, catalyst characterization, mechanistic studies, control experiments, scale-up reactions, catalyst recycling and characterization data of the substrates and products, Supplementary Figs. 1–18 and Tables 1–8.
Acknowledgements
We thank the analytical staff of the Leibniz-Institute for Catalysis, Rostock, for their excellent service, specifically W. Baumann for his accurate quantitative 1H NMR analysis, R Jackstell for offering the 2 l autoclave and D.K. Leonard for assistance in the preparation of the manuscript. We gratefully acknowledge support from the Federal Ministry of Education and Research (BMBF), the State of Mecklenburg-Vorpommern, EU (FLIX project) and the European Research Council (ERC; project NoNaCat 670986).
benzen-2,4,6-d3-amine

- PubChemID:
445468662
- InChIKey
PAYRUJLWNCNPSJ-NHPOFCFZSA-N
- MDL Molfile
- 41557_2021_846_MOESM1_ESM.mol (1,013B, mol)
- Chemdraw file
- 41557_2021_846_MOESM2_ESM.cdx (2.2KB, cdx)
benzen-d5-amine

- PubChemID:
445468663
- InChIKey
PAYRUJLWNCNPSJ-RALIUCGRSA-N
- MDL Molfile
- 41557_2021_846_MOESM3_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM4_ESM.cdx (2.5KB, cdx)
4-(phenyl-2,4,6-d3)morpholine

- PubChemID:
445468664
- InChIKey
FHQRDEDZJIFJAL-NHPOFCFZSA-N
- MDL Molfile
- 41557_2021_846_MOESM5_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM6_ESM.cdx (2.7KB, cdx)
4-methoxybenzen-2,6-d2-amine

- PubChemID:
445468665
- InChIKey
BHAAPTBBJKJZER-PBNXXWCMSA-N
- MDL Molfile
- 41557_2021_846_MOESM7_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM8_ESM.cdx (2.5KB, cdx)
benzene-d4-1,4-diamine

- PubChemID:
445468666
- InChIKey
CBCKQZAAMUWICA-RHQRLBAQSA-N
- MDL Molfile
- 41557_2021_846_MOESM9_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM10_ESM.cdx (2.6KB, cdx)
4-aminophen-2,3,5,6-d4-ol

- PubChemID:
445468667
- InChIKey
PLIKAWJENQZMHA-RHQRLBAQSA-N
- MDL Molfile
- 41557_2021_846_MOESM11_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM12_ESM.cdx (2.6KB, cdx)
4-iodobenzen-2,6-d2-amine

- PubChemID:
445468668
- InChIKey
VLVCDUSVTXIWGW-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM13_ESM.mol (1,013B, mol)
- Chemdraw file
- 41557_2021_846_MOESM14_ESM.cdx (2.2KB, cdx)
4-chlorobenzen-2,6-d2-amine

- PubChemID:
445468669
- InChIKey
QSNSCYSYFYORTR-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM15_ESM.mol (1,013B, mol)
- Chemdraw file
- 41557_2021_846_MOESM16_ESM.cdx (2.2KB, cdx)
4-(benzyloxy)benzen-2,6-d2-amine

- PubChemID:
445468670
- InChIKey
FIIDVVUUWRJXLF-QFIQSOQBSA-N
- MDL Molfile
- 41557_2021_846_MOESM17_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM18_ESM.cdx (2.8KB, cdx)
3-(trifluoromethyl)benzen-4,6-d2-amine

- PubChemID:
445468671
- InChIKey
VIUDTWATMPPKEL-PBNXXWCMSA-N
- MDL Molfile
- 41557_2021_846_MOESM19_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM20_ESM.cdx (2.8KB, cdx)
3-iodobenzen-2,4,6-d3-amine

- PubChemID:
445468672
- InChIKey
FFCSRWGYGMRBGD-NRUYWUNFSA-N
- MDL Molfile
- 41557_2021_846_MOESM21_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM22_ESM.cdx (2.4KB, cdx)
2-fluorobenzen-4,6-d2-amine

- PubChemID:
445468673
- InChIKey
FTZQXOJYPFINKJ-DRSKVUCWSA-N
- MDL Molfile
- 41557_2021_846_MOESM23_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM24_ESM.cdx (2.2KB, cdx)
2-iodobenzen-4,6-d2-amine

- PubChemID:
445468674
- InChIKey
UBPDKIDWEADHPP-DRSKVUCWSA-N
- MDL Molfile
- 41557_2021_846_MOESM25_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM26_ESM.cdx (2.2KB, cdx)
4'-chloro-[1,1'-biphenyl]-3,5-d2-2-amine

- PubChemID:
445468675
- InChIKey
JPBWZIPCMDZOPM-DRSKVUCWSA-N
- MDL Molfile
- 41557_2021_846_MOESM27_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM28_ESM.cdx (2.7KB, cdx)
2-ethoxybenzen-4,6-d2-amine

- PubChemID:
445468676
- InChIKey
ULHFFAFDSSHFDA-KFRNQKGQSA-N
- MDL Molfile
- 41557_2021_846_MOESM29_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM30_ESM.cdx (2.5KB, cdx)
3-chloro-2,6-dimethylbenzen-4-d-amine

- PubChemID:
445468677
- InChIKey
FGMXFOTYCHZCLA-QYKNYGDISA-N
- MDL Molfile
- 41557_2021_846_MOESM31_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM32_ESM.cdx (2.2KB, cdx)
3-chloro-4-methylbenzen-2,6-d2-amine

- PubChemID:
445468678
- InChIKey
RQKFYFNZSHWXAW-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM33_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM34_ESM.cdx (2.3KB, cdx)
2-amino-5-chlorophen-3,4,6-d3-ol

- PubChemID:
445468679
- InChIKey
FZCQMIRJCGWWCL-CBYSEHNBSA-N
- MDL Molfile
- 41557_2021_846_MOESM35_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM36_ESM.cdx (2.6KB, cdx)
2-amino-4-chlorophen-3,5,6-d3-ol

- PubChemID:
445468680
- InChIKey
SWFNPENEBHAHEB-CBYSEHNBSA-N
- MDL Molfile
- 41557_2021_846_MOESM37_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM38_ESM.cdx (2.6KB, cdx)
2-amino-5-methylphen-3,4,6-d3-ol

- PubChemID:
445468681
- InChIKey
HCPJEHJGFKWRFM-NRUYWUNFSA-N
- MDL Molfile
- 41557_2021_846_MOESM39_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM40_ESM.cdx (2.4KB, cdx)
3-amino-[1,1'-biphenyl]-2,5,6-d3-4-ol

- PubChemID:
445468682
- InChIKey
IGIDZGNPFWGICD-AYBVGXBASA-N
- MDL Molfile
- 41557_2021_846_MOESM41_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM42_ESM.cdx (2.9KB, cdx)
4-methylbenzene-2,6-d2-1,3-diamine

- PubChemID:
445468683
- InChIKey
VOZKAJLKRJDJLL-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM43_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM44_ESM.cdx (2.3KB, cdx)
2,6-dimethylbenzen-4-d-amine

- PubChemID:
445468684
- InChIKey
UFFBMTHBGFGIHF-WFVSFCRTSA-N
- MDL Molfile
- 41557_2021_846_MOESM45_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM46_ESM.cdx (2KB, cdx)
4,4'-oxybis(benzen-2,6-d2-amine)

- PubChemID:
445468685
- InChIKey
HLBLWEWZXPIGSM-RHQRLBAQSA-N
- MDL Molfile
- 41557_2021_846_MOESM47_ESM.mol (1.8KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM48_ESM.cdx (3.2KB, cdx)
9H-fluoren-1,3-d2-2-amine

- PubChemID:
445468686
- InChIKey
CFRFHWQYWJMEJN-VBCVTXNDSA-N
- MDL Molfile
- 41557_2021_846_MOESM49_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM50_ESM.cdx (2.6KB, cdx)
3-aminonaphthalen-1,4-d2-2-ol

- PubChemID:
445468687
- InChIKey
ZHVPTERSBUMMHK-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM51_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM52_ESM.cdx (2.6KB, cdx)
bis(phenyl-2,4,6-d3)amine

- PubChemID:
445468688
- InChIKey
DMBHHRLKUKUOEG-MWJWXFQUSA-N
- MDL Molfile
- 41557_2021_846_MOESM53_ESM.mol (1.8KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM54_ESM.cdx (3.2KB, cdx)
3-fluoro-N-methylbenzen-2,4,6-d3-amine

- PubChemID:
445468689
- InChIKey
FHYDHJXZZQCXOX-QGZYMEECSA-N
- MDL Molfile
- 41557_2021_846_MOESM55_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM56_ESM.cdx (2.4KB, cdx)
N,4-dimethylbenzen-2,6-d2-amine

- PubChemID:
445468690
- InChIKey
QCIFLGSATTWUQJ-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM57_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM58_ESM.cdx (2.2KB, cdx)
N-methyl-3-(trifluoromethyl)benzen-2,4,6-d3-amine

- PubChemID:
445468691
- InChIKey
SRTKIHVQZYXHHJ-QGZYMEECSA-N
- MDL Molfile
- 41557_2021_846_MOESM59_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM60_ESM.cdx (3KB, cdx)
2-((phenyl-2,4,6-d3)amino)ethan-1-ol

- PubChemID:
445468692
- InChIKey
MWGATWIBSKHFMR-NHPOFCFZSA-N
- MDL Molfile
- 41557_2021_846_MOESM61_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM62_ESM.cdx (2.5KB, cdx)
N-butylbenzen-2,4,6-d3-amine

- PubChemID:
445468693
- InChIKey
VSHTWPWTCXQLQN-WUNMZEPUSA-N
- MDL Molfile
- 41557_2021_846_MOESM63_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM64_ESM.cdx (2.5KB, cdx)
1-(phenyl-2,4,6-d3)pyrrolidine

- PubChemID:
445468694
- InChIKey
VDQQJMHXZCMNMU-HVRNSXJSSA-N
- MDL Molfile
- 41557_2021_846_MOESM65_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM66_ESM.cdx (2.5KB, cdx)
3-morpholinophen-2,4,6-d3-ol

- PubChemID:
445468695
- InChIKey
BMGSGGYIUOQZBZ-HRYXNQDDSA-N
- MDL Molfile
- 41557_2021_846_MOESM67_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM68_ESM.cdx (2.8KB, cdx)
4-(4-methylphenyl-2,6-d2)morpholine

- PubChemID:
445468696
- InChIKey
OLAFVASCPJETBP-KFRNQKGQSA-N
- MDL Molfile
- 41557_2021_846_MOESM69_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM70_ESM.cdx (2.6KB, cdx)
4-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl-2,4,6-d3)morpholine

- PubChemID:
445468697
- InChIKey
NCJDKFFODGZRRL-DHMPXPLHSA-N
- MDL Molfile
- 41557_2021_846_MOESM71_ESM.mol (2.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM72_ESM.cdx (3.6KB, cdx)
1-(4-(phenyl-2,4,6-d3)piperazin-1-yl)ethan-1-one

- PubChemID:
445468698
- InChIKey
YFBOBXSXWBMZCY-UJESMPABSA-N
- MDL Molfile
- 41557_2021_846_MOESM73_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM74_ESM.cdx (3KB, cdx)
3,4-dimethylphen-2,6-d2-ol

- PubChemID:
445468699
- InChIKey
YCOXTKKNXUZSKD-KFRNQKGQSA-N
- MDL Molfile
- 41557_2021_846_MOESM75_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM76_ESM.cdx (2.2KB, cdx)
2-methoxy-4-methylphen-6-d-ol

- PubChemID:
445468700
- InChIKey
PETRWTHZSKVLRE-QYKNYGDISA-N
- MDL Molfile
- 41557_2021_846_MOESM77_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM78_ESM.cdx (2.4KB, cdx)
2,4-dimethylphen-6-d-ol

- PubChemID:
445468701
- InChIKey
KUFFULVDNCHOFZ-QYKNYGDISA-N
- MDL Molfile
- 41557_2021_846_MOESM79_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM80_ESM.cdx (2KB, cdx)
2,6-dimethoxyphen-3,4,5-d3-ol

- PubChemID:
445468702
- InChIKey
KLIDCXVFHGNTTM-QGZYMEECSA-N
- MDL Molfile
- 41557_2021_846_MOESM81_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM82_ESM.cdx (3KB, cdx)
4-methylbenzene-3,5,6-d3-1,2-diol

- PubChemID:
445468703
- InChIKey
ZBCATMYQYDCTIZ-NRUYWUNFSA-N
- MDL Molfile
- 41557_2021_846_MOESM83_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM84_ESM.cdx (2.4KB, cdx)
4-(tert-butyl)benzene-3,5,6-d3-1,2-diol

- PubChemID:
445468704
- InChIKey
XESZUVZBAMCAEJ-WVALGTIDSA-N
- MDL Molfile
- 41557_2021_846_MOESM85_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM86_ESM.cdx (2.6KB, cdx)
1H-indazol-3,4,6-d3-5-amine

- PubChemID:
445468705
- InChIKey
XBTOSRUBOXQWBO-IWDQAABOSA-N
- MDL Molfile
- 41557_2021_846_MOESM87_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM88_ESM.cdx (2.7KB, cdx)
benzo[c][1,2,5]thiadiazol-5,7-d2-4-amine

- PubChemID:
445468706
- InChIKey
DRLGIZIAMHIQHL-PBNXXWCMSA-N
- MDL Molfile
- 41557_2021_846_MOESM89_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM90_ESM.cdx (2.6KB, cdx)
2-aminopyridin-4,6-d2-3-ol

- PubChemID:
445468707
- InChIKey
BMTSZVZQNMNPCT-PBNXXWCMSA-N
- MDL Molfile
- 41557_2021_846_MOESM91_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM92_ESM.cdx (2.3KB, cdx)
N,N-dimethylpyridin-4-amine-2,6-d2

- PubChemID:
445468708
- InChIKey
VHYFNPMBLIVWCW-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM93_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM94_ESM.cdx (2.3KB, cdx)
pyridin-2,6-d2-4-amine

- PubChemID:
445468709
- InChIKey
NUKYPUAOHBNCPY-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM95_ESM.mol (923B, mol)
- Chemdraw file
- 41557_2021_846_MOESM96_ESM.cdx (2.2KB, cdx)
(S)-3-(1-methylpyrrolidin-2-yl)pyridine-2-d

- PubChemID:
445468710
- InChIKey
SNICXCGAKADSCV-QNICBWFLSA-N
- MDL Molfile
- 41557_2021_846_MOESM97_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM98_ESM.cdx (2.5KB, cdx)
indoline-4,5,6,7-d4

- PubChemID:
445468711
- InChIKey
LPAGFVYQRIESJQ-RHQRLBAQSA-N
- MDL Molfile
- 41557_2021_846_MOESM99_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM100_ESM.cdx (2.5KB, cdx)
2-methylindoline-4,5,6,7-d4

- PubChemID:
445468712
- InChIKey
QRWRJDVVXAXGBT-QFFDRWTDSA-N
- MDL Molfile
- 41557_2021_846_MOESM101_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM102_ESM.cdx (2.6KB, cdx)
1,2,3,4-tetrahydroquinoline-6,8-d2

- PubChemID:
445468713
- InChIKey
LBUJPTNKIBCYBY-UPXKZQJYSA-N
- MDL Molfile
- 41557_2021_846_MOESM103_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM104_ESM.cdx (2.3KB, cdx)
2-methyl-1,2,3,4-tetrahydroquinoline-6,8-d2

- PubChemID:
445468714
- InChIKey
JZICUKPOZUKZLL-GSUVGXDCSA-N
- MDL Molfile
- 41557_2021_846_MOESM105_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM106_ESM.cdx (2.4KB, cdx)
10H-phenothiazine-3,7-d2

- PubChemID:
445468715
- InChIKey
WJFKNYWRSNBZNX-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM107_ESM.mol (1.6KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM108_ESM.cdx (2.7KB, cdx)
10H-phenoxazine-1,3,7,9-d4

- PubChemID:
445468716
- InChIKey
TZMSYXZUNZXBOL-LNFUJOGGSA-N
- MDL Molfile
- 41557_2021_846_MOESM109_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM110_ESM.cdx (3.1KB, cdx)
1H-pyrrolo[2,3-b]pyridine-3-d

- PubChemID:
445468717
- InChIKey
MVXVYAKCVDQRLW-WFVSFCRTSA-N
- MDL Molfile
- 41557_2021_846_MOESM111_ESM.mol (1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM112_ESM.cdx (2.2KB, cdx)
1H-indol-2,3,4,5,7-d5-6-amine

- PubChemID:
445468718
- InChIKey
MIMYTSWNVBMNRH-RALIUCGRSA-N
- MDL Molfile
- 41557_2021_846_MOESM113_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM114_ESM.cdx (2.9KB, cdx)
5-methyl-1H-indole-2,3,4,6,7-d5

- PubChemID:
445468719
- InChIKey
YPKBCLZFIYBSHK-VIQYUKPQSA-N
- MDL Molfile
- 41557_2021_846_MOESM115_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM116_ESM.cdx (2.8KB, cdx)
2-methyl-1H-indol-3,4,6-d3-5-ol

- PubChemID:
445468720
- InChIKey
MDWJZBVEVLTXDE-KXXDXSMKSA-N
- MDL Molfile
- 41557_2021_846_MOESM117_ESM.mol (1.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM118_ESM.cdx (2.6KB, cdx)
3-methyl-1H-indole-2,4,5,6,7-d5

- PubChemID:
445468721
- InChIKey
ZFRKQXVRDFCRJG-VIQYUKPQSA-N
- MDL Molfile
- 41557_2021_846_MOESM119_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM120_ESM.cdx (2.8KB, cdx)
2-phenyl-1H-indole-3-d

- PubChemID:
445468722
- InChIKey
KLLLJCACIRKBDT-MMIHMFRQSA-N
- MDL Molfile
- 41557_2021_846_MOESM121_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM122_ESM.cdx (2.7KB, cdx)
5-bromo-1H-indole-3-d

- PubChemID:
445468723
- InChIKey
VXWVFZFZYXOBTA-WFVSFCRTSA-N
- MDL Molfile
- 41557_2021_846_MOESM123_ESM.mol (1.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM124_ESM.cdx (2.3KB, cdx)
2,3-dimethyl-1H-indole-4,5,6,7-d4

- PubChemID:
445468724
- InChIKey
PYFVEIDRTLBMHG-LNFUJOGGSA-N
- MDL Molfile
- 41557_2021_846_MOESM125_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM126_ESM.cdx (2.7KB, cdx)
quinolin-5,7-d2-8-amine

- PubChemID:
445468725
- InChIKey
WREVVZMUNPAPOV-XBTCESIDSA-N
- MDL Molfile
- 41557_2021_846_MOESM127_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM128_ESM.cdx (2.5KB, cdx)
2-methylquinolin-3-d-4-amine

- PubChemID:
445468726
- InChIKey
COCFIBRMFPWUDW-RAMDWTOOSA-N
- MDL Molfile
- 41557_2021_846_MOESM129_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM130_ESM.cdx (2.4KB, cdx)
quinolin-3-d-4-ol

- PubChemID:
445468727
- InChIKey
PMZDQRJGMBOQBF-UICOGKGYSA-N
- MDL Molfile
- 41557_2021_846_MOESM131_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM132_ESM.cdx (2.3KB, cdx)
[D] Melatonin

- PubChemID:
445468728
- InChIKey
DRLFMBDRBRZALE-LESCGEGLSA-N
- MDL Molfile
- 41557_2021_846_MOESM133_ESM.mol (2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM134_ESM.cdx (3.4KB, cdx)
[D] N-Acetylserotonin

- PubChemID:
445468729
- InChIKey
MVAWJSIDNICKHF-COJKPNSOSA-N
- MDL Molfile
- 41557_2021_846_MOESM135_ESM.mol (1.8KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM136_ESM.cdx (5.4KB, cdx)
[D] Kinetin

- PubChemID:
445468730
- InChIKey
QANMHLXAZMSUEX-UICOGKGYSA-N
- MDL Molfile
- 41557_2021_846_MOESM137_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM138_ESM.cdx (4.3KB, cdx)
[D] Inosine

- PubChemID:
445468731
- InChIKey
UGQMRVRMYYASKQ-MEGDDXBKSA-N
- MDL Molfile
- 41557_2021_846_MOESM139_ESM.mol (2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM140_ESM.cdx (3.8KB, cdx)
[D] Adenine

- PubChemID:
445468732
- InChIKey
GFFGJBXGBJISGV-QDNHWIQGSA-N
- MDL Molfile
- 41557_2021_846_MOESM141_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM142_ESM.cdx (2.7KB, cdx)
[D] Estrone

- PubChemID:
445468733
- InChIKey
DNXHEGUUPJUMQT-AAKDDEGHSA-N
- MDL Molfile
- 41557_2021_846_MOESM143_ESM.mol (2.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM144_ESM.cdx (3.5KB, cdx)
[D] Pentoxifylline

- PubChemID:
445468734
- InChIKey
BYPFEZZEUUWMEJ-BNEYPBHNSA-N
- MDL Molfile
- 41557_2021_846_MOESM145_ESM.mol (2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM146_ESM.cdx (3.3KB, cdx)
[D] Brucine

- PubChemID:
445468735
- InChIKey
RRKTZKIUPZVBMF-OCUHVGHDSA-N
- MDL Molfile
- 41557_2021_846_MOESM147_ESM.mol (3.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM148_ESM.cdx (5.2KB, cdx)
[D] Strychnine

- PubChemID:
445468736
- InChIKey
QMGVPVSNSZLJIA-UQYZGTHLSA-N
- MDL Molfile
- 41557_2021_846_MOESM149_ESM.mol (2.9KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM150_ESM.cdx (4.4KB, cdx)
[D] Niacin

- PubChemID:
445468737
- InChIKey
PVNIIMVLHYAWGP-QYKNYGDISA-N
- MDL Molfile
- 41557_2021_846_MOESM151_ESM.mol (1,014B, mol)
- Chemdraw file
- 41557_2021_846_MOESM152_ESM.cdx (2.2KB, cdx)
[D] Tyrosol

- PubChemID:
445468738
- InChIKey
YCCILVSKPBXVIP-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM153_ESM.mol (1.2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM154_ESM.cdx (2.3KB, cdx)
[D] Thymol

- PubChemID:
445468739
- InChIKey
MGSRCZKZVOBKFT-QRZVFWKLSA-N
- MDL Molfile
- 41557_2021_846_MOESM155_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM156_ESM.cdx (2.3KB, cdx)
[D] Arbutin

- PubChemID:
445468740
- InChIKey
BJRNKVDFDLYUGJ-RCBWPJCFSA-N
- MDL Molfile
- 41557_2021_846_MOESM157_ESM.mol (2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM158_ESM.cdx (3.6KB, cdx)
[D] Resveratrol

- PubChemID:
445468741
- InChIKey
LUKBXSAWLPMMSZ-BIFZJDEASA-N
- MDL Molfile
- 41557_2021_846_MOESM159_ESM.mol (2.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM160_ESM.cdx (3.5KB, cdx)
[D] Piceid

- PubChemID:
445468742
- InChIKey
HSTZMXCBWJGKHG-XNFYHSJESA-N
- MDL Molfile
- 41557_2021_846_MOESM161_ESM.mol (3.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM162_ESM.cdx (4.9KB, cdx)
[D] (±)Augustureine

- PubChemID:
445468743
- InChIKey
KSQZVAWGIAAZHJ-NXTQXPHYSA-N
- MDL Molfile
- 41557_2021_846_MOESM163_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM164_ESM.cdx (2.6KB, cdx)
[D] Galipinine

- PubChemID:
445468744
- InChIKey
QRYQKXWZOXSQAJ-GSUVGXDCSA-N
- MDL Molfile
- 41557_2021_846_MOESM165_ESM.mol (2.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM166_ESM.cdx (3.4KB, cdx)
[D] Dropropizine

- PubChemID:
445468745
- InChIKey
PTVWPYVOOKLBCG-MICDWDOJSA-N
- MDL Molfile
- 41557_2021_846_MOESM167_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM168_ESM.cdx (2.9KB, cdx)
[D] DL-Aminoglutethimide

- PubChemID:
445468746
- InChIKey
ROBVIMPUHSLWNV-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM169_ESM.mol (1.8KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM170_ESM.cdx (3KB, cdx)
[D] Nimodipine-NH2

- PubChemID:
445468747
- InChIKey
GUJDTMSUOZMUQI-LAJMJUAESA-N
- MDL Molfile
- 41557_2021_846_MOESM171_ESM.mol (2.9KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM172_ESM.cdx (4.2KB, cdx)
[D] Carvacrol

- PubChemID:
445468748
- InChIKey
RECUKUPTGUEGMW-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM173_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM174_ESM.cdx (2.3KB, cdx)
[D] Estradiol

- PubChemID:
445468749
- InChIKey
VOXZDWNPVJITMN-JZTNAQEWSA-N
- MDL Molfile
- 41557_2021_846_MOESM175_ESM.mol (2.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM176_ESM.cdx (3.5KB, cdx)
[D] O-Desmethylvenlafaxine

- PubChemID:
445468750
- InChIKey
KYYIDSXMWOZKMP-XETGXLELSA-N
- MDL Molfile
- 41557_2021_846_MOESM177_ESM.mol (2KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM178_ESM.cdx (3KB, cdx)
[D] L-Tryptophan

- PubChemID:
445468751
- InChIKey
QIVBCDIJIAJPQS-RZRKSHIUSA-N
- MDL Molfile
- 41557_2021_846_MOESM179_ESM.mol (2.1KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM180_ESM.cdx (3.7KB, cdx)
N-(4-methoxyphenyl-2,6-d2)acetamide

- PubChemID:
445468752
- InChIKey
XVAIDCNLVLTVFM-NMQOAUCRSA-N
- MDL Molfile
- 41557_2021_846_MOESM181_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM182_ESM.cdx (2.8KB, cdx)
[D] Paracetamol

- PubChemID:
445468753
- InChIKey
RZVAJINKPMORJF-PBNXXWCMSA-N
- MDL Molfile
- 41557_2021_846_MOESM183_ESM.mol (1.3KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM184_ESM.cdx (2.5KB, cdx)
[D] Lidocaine

- PubChemID:
445468754
- InChIKey
NNJVILVZKWQKPM-WHRKIXHSSA-N
- MDL Molfile
- 41557_2021_846_MOESM185_ESM.mol (1.7KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM186_ESM.cdx (2.7KB, cdx)
[D] Fluometuron

- PubChemID:
445468755
- InChIKey
RZILCCPWPBTYDO-WVALGTIDSA-N
- MDL Molfile
- 41557_2021_846_MOESM187_ESM.mol (1.9KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM188_ESM.cdx (5.2KB, cdx)
[D] Clortoluron

- PubChemID:
445468756
- InChIKey
JXCGFZXSOMJFOA-KCZCTXNHSA-N
- MDL Molfile
- 41557_2021_846_MOESM189_ESM.mol (1.5KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM190_ESM.cdx (3.6KB, cdx)
[D] Boscalid

- PubChemID:
445468757
- InChIKey
WYEMLYFITZORAB-UPXKZQJYSA-N
- MDL Molfile
- 41557_2021_846_MOESM191_ESM.mol (2.4KB, mol)
- Chemdraw file
- 41557_2021_846_MOESM192_ESM.cdx (6.9KB, cdx)
Author contributions
M.B. and W.L. conceived and designed the project. W.L. and F.B developed and prepared the catalysts and performed all catalytic experiments and analysed the data. A.B. and J.R. were responsible for the EPR experiments. D.Y. and A.L. were responsible for the XAFS experiments. C.K and N.R. were responsible for the TEM experiments. H.L. was responsible for the X-ray diffraction experiments. S.B. was responsible for the XPS experiments. K.J., A.-E.S. and A.B. participated in the discussions and supported the project. W.L., J.R., A.B. and M.B. co-wrote the paper.
Peer review
Peer review information
Nature Chemistry thanks Marc Reid and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All data generated or analysed in this study are available in this article and its Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Angelika Brückner, Email: angelika.brueckner@catalysis.de.
Aiwen Lei, Email: aiwenlei@whu.edu.cn.
Matthias Beller, Email: matthias.beller@catalysis.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-021-00846-4.
References
- 1.Prechtl MH, Teltewskoi M, Dimitrov A, Kemnitz E, Braun T. Catalytic C–H bond activation at nanoscale Lewis acidic aluminium fluorides: H/D exchange reactions at aromatic and aliphatic hydrocarbons. Chem. Eur. J. 2011;17:14385–14388. doi: 10.1002/chem.201102853. [DOI] [PubMed] [Google Scholar]
- 2.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 3.Zhan M, et al. A simple and cost-effective method for the regioselective deuteration of phenols. Eur. J. Org. Chem. 2015;2015:3370–3373. doi: 10.1002/ejoc.201500192. [DOI] [Google Scholar]
- 4.Smith JA, et al. Preparation of cyclohexene isotopologues and stereoisotopomers from benzene. Nature. 2020;581:288–293. doi: 10.1038/s41586-020-2268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atzrodt J, Derdau V, Fey T, Zimmermann J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 2007;46:7744–7765. doi: 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson K. Big interest in heavy drugs. Nature. 2009;458:269. doi: 10.1038/458269a. [DOI] [PubMed] [Google Scholar]
- 7.Katsnelson A. Heavy drugs draw heavy interest from pharma backers. Nat. Med. 2013;19:656. doi: 10.1038/nm0613-656. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt C. First deuterated drug approved. Nat. Biotechnol. 2017;35:493–494. doi: 10.1038/nbt0617-493. [DOI] [PubMed] [Google Scholar]
- 9.Czeskis B, et al. Deuterated active pharmaceutical ingredients: a science-based proposal for synthesis, analysis, and control. Part 1: Framing the problem. J. Labelled Comp. Radiopharm. 2019;62:690–694. doi: 10.1002/jlcr.3743. [DOI] [PubMed] [Google Scholar]
- 10.Martins A, Lautens M. A simple, cost-effective method for the regioselective deuteration of anilines. Org. Lett. 2008;10:4351–4353. doi: 10.1021/ol801763j. [DOI] [PubMed] [Google Scholar]
- 11.Magano J, Dunetz JR. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 12.Atzrodt J, Derdau V, Kerr WJ, Reid M. C–H functionalisation for hydrogen isotope exchange. Angew. Chem. Int. Ed. 2018;57:3022–3047. doi: 10.1002/anie.201708903. [DOI] [PubMed] [Google Scholar]
- 13.Hesk D. Highlights of C(sp2)–H hydrogen isotope exchange reactions. J. Labelled Comp. Radiopharm. 2020;63:247–265. doi: 10.1002/jlcr.3801. [DOI] [PubMed] [Google Scholar]
- 14.Qiao-Xia G, Bao-Jian S, Hai-Qing G, Tamotsu T. Aromatic H/D exchange reaction catalyzed by groups 5 and 6 metal chlorides. Chin. J. Chem. 2005;23:341–344. doi: 10.1002/cjoc.200590341. [DOI] [Google Scholar]
- 15.Müller V, Weck R, Derdau V, Ackermann L. Ruthenium(ii)-catalyzed hydrogen isotope exchange of pharmaceutical drugs by C−H deuteration and C−H tritiation. ChemCatChem. 2019;12:100–104. doi: 10.1002/cctc.201902051. [DOI] [Google Scholar]
- 16.Crabtree RH, Felkin H, Morris GE. Cationic iridium diolefin complexes as alkene hydrogenation catalysts and the isolation of some related hydrido complexes. J. Organomet. Chem. 1977;141:205–215. doi: 10.1016/S0022-328X(00)92273-3. [DOI] [Google Scholar]
- 17.Nilsson GN, Kerr WJ. The development and use of novel iridium complexes as catalysts for ortho-directed hydrogen isotope exchange reactions. J. Labelled Comp. Radiopharm. 2010;53:662–667. doi: 10.1002/jlcr.1817. [DOI] [Google Scholar]
- 18.Yu RP, Hesk D, Rivera N, Pelczer I, Chirik PJ. Iron-catalysed tritiation of pharmaceuticals. Nature. 2016;529:195–199. doi: 10.1038/nature16464. [DOI] [PubMed] [Google Scholar]
- 19.Loh YY, et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science. 2017;358:1182–1187. doi: 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajiki H, Sawama Y, Monguchi Y. Efficient H–D exchange reactions using heterogeneous platinum-group metal on carbon–H2–D2O System. Synlett. 2012;23:959–972. doi: 10.1055/s-0031-1289696. [DOI] [Google Scholar]
- 21.Pieters G, et al. Regioselective and stereospecific deuteration of bioactive aza compounds by the use of ruthenium nanoparticles. Angew. Chem. Int. Ed. 2014;53:230–234. doi: 10.1002/anie.201307930. [DOI] [PubMed] [Google Scholar]
- 22.Valero M, et al. NHC-stabilized iridium nanoparticles as catalysts in hydrogen isotope exchange reactions of anilines. Angew. Chem. Int. Ed. 2020;59:3517–3522. doi: 10.1002/anie.201914369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heys JR. Nickel-catalyzed hydrogen isotope exchange. J. Labelled Comp. Radiopharm. 2010;53:716–721. doi: 10.1002/jlcr.1822. [DOI] [Google Scholar]
- 24.Sajiki H, et al. Efficient and selective deuteration of phenylalanine derivatives catalyzed by Pd/C. Synlett. 2005;2005:0845–0847. doi: 10.1055/s-2005-863730. [DOI] [Google Scholar]
- 25.Jagadeesh RV, et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science. 2013;342:1073–1076. doi: 10.1126/science.1242005. [DOI] [PubMed] [Google Scholar]
- 26.Cui X, et al. Synthesis and characterization of iron-nitrogen-doped graphene/core-shell catalysts: efficient oxidative dehydrogenation of N-heterocycles. J. Am. Chem. Soc. 2015;137:10652–10658. doi: 10.1021/jacs.5b05674. [DOI] [PubMed] [Google Scholar]
- 27.Belhadj H, Melchers S, Robertson PKJ, Bahnemann DW. Pathways of the photocatalytic reaction of acetate in H2O and D2O: a combined EPR and ATR-FTIR study. J. Catal. 2016;344:831–840. doi: 10.1016/j.jcat.2016.08.006. [DOI] [Google Scholar]
- 28.Dai X, et al. Sustainable co-synthesis of glycolic acid, formamides and formates from 1,3-dihydroxyacetone by a Cu/Al2O3 catalyst with a single active sites. Angew. Chem. Int. Ed. 2019;58:5251–5255. doi: 10.1002/anie.201814050. [DOI] [PubMed] [Google Scholar]
- 29.Mader EA, Davidson ER, Mayer JM. Large ground-state entropy changes for hydrogen atom transfer reactions of iron complexes. J. Am. Chem. Soc. 2007;129:5153–5166. doi: 10.1021/ja0686918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barckholtz C, Barckholtz TA, Hadad CM. A mechanistic study of the reactions of H, O (3P), and OH with monocyclic aromatic hydrocarbons by density functional theory. J. Phys. Chem. A. 2001;105:140–152. doi: 10.1021/jp001884b. [DOI] [Google Scholar]
- 31.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 32.Ito N, et al. Efficient and selective Pt/C-catalyzed H–D exchange reaction of aromatic rings. Bull. Chem. Soc. Jpn. 2008;81:278–286. doi: 10.1246/bcsj.81.278. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General remarks, procedure details for the preparation of the catalyst, deuteration reactions, reaction development and optimization, catalyst characterization, mechanistic studies, control experiments, scale-up reactions, catalyst recycling and characterization data of the substrates and products, Supplementary Figs. 1–18 and Tables 1–8.
Data Availability Statement
All data generated or analysed in this study are available in this article and its Supplementary Information.