Abstract
Global stock markets react positively when different phases of human clinical trials on COVID-19 vaccines begin. The average abnormal stock return on the first day of the trials is both statistically and economically significant at 8.08 basis points. The increase in the average abnormal stock return is threefold higher for leading vaccine candidates. The positive reaction is more pronounced upon the start of phase III trials, and it is also stronger for vaccine candidates developed by the U.S. and China.
Keywords: COVID-19, Pandemics, Stock markets, Vaccines
1. Introduction
Prior studies show that COVID-19 negatively affects liquidity (O’Hara and Zhou, 2021), aggregate equity markets (Gormsen and Koijen, 2020; Smales, 2021; Yarovaya et al., 2021), cross-sectional stock returns (Ramelli and Wagner, 2020; Ding et al., 2021), cryptocurrency markets (Caferra and Vidal-Tomás, 2021; Corbet et al., 2022), real estate markets (Chong and Phillips, 2022; Qian et al., 2021), sovereign credit risk (Augustin et al., 2022), trade credit (Luo, 2021) and firm performance (Haque et al., 2021).1 Bao et al. (2021), Demir et al. (2021), Khalfaoui et al. (2021) and Rouatbi et al. (2021) find vaccine inoculation positively affect the stock market, while Acharya et al. (2021) show that the value of the vaccine is worth 5-15% of capital stocks. Hong et al. (2021) develop a model that suggests an earnings crash and lower earnings growth until vaccine arrives in late 2020. This study contributes to the literature by examining the development progress of COVID-19 vaccines, and its impact on global stock markets.
Using a dataset collected by the World Health Organization (WHO), we identify the start dates of three key human clinical trial phases conducted for 83 COVID-19 vaccine candidates developed worldwide from January 2020 to April 2021.2 The start of each phase marks a milestone in vaccine development and indicates the successful completion of the previous phase, which is one step closer to obtain approval for large-scale inoculation. We contend that prior to public inoculation, the development of COVID-19 vaccines has a positive impact on stock markets around the globe. In other words, any potential breakthrough documented during the development of a vaccine reflects the potential economic and social benefits (e.g., minimal cross-border closure) of the vaccine, especially at times when pandemics such as COVID-19 occur, leading to a positive impact on the global stock markets.3
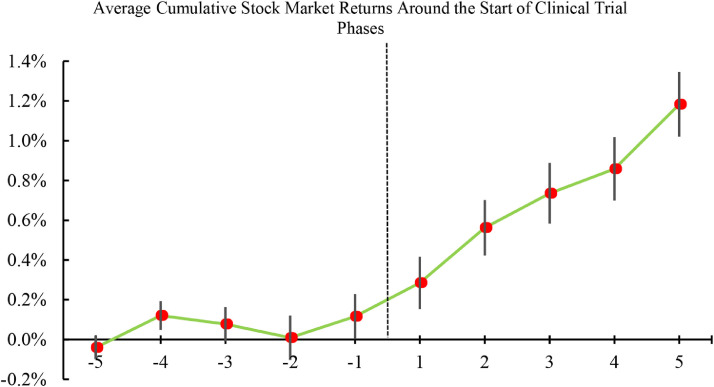
We provide empirical support to the above proposition. Upon the start of the clinical trials, global stock markets react positively with an average abnormal return of 8.08 basis points (bps). This result is economically meaningful: the 8.08 bps average abnormal return translates to an increase of USD46.4 billion in total market capitalization. To underscore our finding, Fig. 1 shows the average cumulative returns on all stock markets in a [-5, 5]-trading-day event window surrounding the start of clinical trial phases. As the figure shows, average stock market return increases significantly after the first day of each clinical trial phase. While there is no discernible pattern in the cumulative stock returns in days leading to “day + 1,” the average increase in returns persists for several days after “day + 1.” In short, Fig. 1 shows that global stock markets view clinical trials positively in terms of their impacts on the global economy.
Fig. 1.
Cumulative stock returns around first day of clinical trial phases.
The figure plots the average cumulative returns of all 50 stock markets over the [-5, +5] event window around the start of clinical trial phases of all 83 vaccine candidates. The first day of each clinical trial phase is marked as “day + 1” in the event window. The cumulative return is calculated for each event window, and then averaged across all country indices and clinical trials. The 95% confidence intervals are also reported with vertical black lines. The dotted line indicates the time cutoff before and after the start of clinical trial phase.
Further analysis shows that the stock market reaction is stronger when clinical trials progress to the final phase III, with an average day-one abnormal return of 16.55 bps. We also analyze a group of leading vaccine candidates of which trials began early in the pandemic and have been subsequently approved for mass inoculation by the end of the sample period. These unique candidates, labelled as “first movers,” include the usual suspects such as Pfizer, Moderna, and AstraZeneca. Since first movers are at the forefront in the race to develop an effective vaccine, we expect a stronger stock market reaction on the first day of the trials for these leading candidates. The empirical finding supports our conjecture: the average day-one abnormal stock market return in response to the first movers is substantially higher at 40.33 bps for phase III.
We further show that the day-one impact of clinical trials in phases II and III is stronger for developed economies relative to emerging economies. Additionally, we find that the stock market reaction is conditional on the vaccine origins: the average day-one abnormal return in all phases is the highest for vaccines developed in China (and in the U.S. if we focus only on phase III). In contrast, the stock market reactions are relatively modest for vaccines produced by developers based in other countries.4
2. Data
We retrieve information (including the start dates of clinical trial phases) about the vaccine candidates from an official document issued by the WHO: “COVID-19 Vaccine Tracker and Landscape.”5 We pinpoint 83 vaccine candidates that have had human clinical trials from January 2, 2020, to April 30, 2021, with the earliest trial beginning in mid-March 2020. Table 1 describes all 83 vaccine candidates. These vaccine candidates were developed in 24 countries, and we label them as “vac-countries.” Most of the vaccines developed in vac-countries are from the U.S. (26), followed by China (17). We also identify 30 “non-vac-countries” that did not have any vaccine undergoing human clinical trials during the sample period.
Table 1.
Information on Vaccine Candidates, This table lists all 83 COVID-19 vaccine candidates that had started human clinical trials as of April 30, 2021. The table includes information on vaccine developers and the country where the vaccine developer is domiciled (referred to as “vac-country”). The last three columns report the earliest start dates of the clinical trial phases I, II, and III, respectively. Blank cells indicate that the clinical trials of a certain phase either do not exist or had not begun as of April 30, 2021. Rows tagged with an asterisk (*) in the first column are first movers.
| No. | Vaccine developer/manufacturer | Vac-country | Phase I | Phase II | Phase III |
|---|---|---|---|---|---|
| 1* | Pfizer/ BioNTech/ Fosun Pharma# | Germany/ US/ Mainland China | 2020-04-23 | 2020-04-29 | |
| 2* | AstraZeneca/ University of Oxford | UK | 2020-04-23 | 2020-05-28 | |
| 3* | Sinopharm/ China National Biotec Group Co/ Wuhan Institute of Biological Products | Mainland China | 2020-04-11 | 2020-07-16 | |
| 4* | Sinopharm/ China National Biotec Group Co/ Beijing Institute of Biological Products | Mainland China | 2020-04-28 | 2020-07-16 | |
| 5* | Sinovac Research and Development Co., Ltd | Mainland China | 2020-04-16 | 2020-07-21 | |
| 6* | Moderna/ National Institute of Allergy and Infectious Diseases (NIAID) | US | 2020-03-16 | 2020-05-29 | 2020-07-27 |
| 7* | Gamaleya Research Institute/ Health Ministry of the Russian Federation | Russia | 2020-06-17 | 2020-09-07 | |
| 8* | Janssen Pharmaceutical | US | 2020-07-15 | 2020-09-07 | |
| 9* | CanSino Biological Inc./ Beijing Institute of Biotechnology | Mainland China | 2020-03-16 | 2020-04-12 | 2020-09-11 |
| 10 | Novavax | US | 2020-05-25 | 2020-09-28 | |
| 11* | Bharat Biotech International Limited | India | 2020-07-15 | 2020-11-16 | |
| 12* | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology "Vector" | Russia | 2020-07-27 | 2020-11-18 | |
| 13 | Medicago Inc. | Canada | 2020-07-10 | 2020-11-19 | |
| 14 | AnGes/ Takara Bio/ Osaka University | Japan | 2020-06-29 | 2020-11-23 | |
| 15 | Inovio Pharmaceuticals/ International Vaccine Institute/ Advaccine (Suzhou) Biopharmaceutical Co., Ltd | US/ Korea/ Mainland China | 2020-04-03 | 2020-07-15 | 2020-11-30 |
| 16 | CureVac AG | Germany | 2020-06-18 | 2020-08-17 | 2020-12-14 |
| 17* | Anhui Zhifei Longcom Biopharmaceutical/ Institute of Microbiology, Chinese Academy of Sciences | Mainland China | 2020-06-22 | 2020-07-12 | 2020-12-16 |
| 18* | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Kazakhstan | 2020-09-19 | 2020-12-25 | |
| 19 | Institute of Medical Biology/ Chinese Academy of Medical Sciences | Mainland China | 2020-05-15 | 2021-01-28 | |
| 20 | Shifa Pharmed Industrial Co | Iran | 2020-12-21 | 2021-03-14 | |
| 21 | ReiThera/ Leukocare/ Univercells | Italy/ Germany/ Belgium | 2020-08-10 | 2021-03-15 | |
| 22 | Valneva/ National Institute for Health Research, United Kingdom | France/ UK | 2020-12-16 | 2021-04-26 | |
| 23 | Genexine Consortium | Korea | 2020-06-17 | ||
| 24 | Zydus Cadila | India | 2020-07-13 | ||
| 25 | Arcturus Therapeutics | US/ Singapore | 2020-08-04 | ||
| 26 | Serum Institute of India/ Accelagen Pty/ SpyBiotech | UK/ Australia/ India | 2020-08-17 | ||
| 27 | Instituto Finlay de Vacunas | Cuba | 2020-08-24 | ||
| 28 | Sanofi Pasteur/ GSK | France/ UK | 2020-09-03 | ||
| 29 | Beijing Minhai Biotechnology Co | Mainland China | 2020-10-07 | 2020-10-27 | |
| 30 | Israel Institute for Biological Research | Israel | 2020-10-28 | ||
| 31 | Biological E. Limited | India | 2020-11-16 | ||
| 32 | West China Hospital/ Sichuan University | Mainland China | 2020-08-28 | 2020-11-17 | |
| 33 | University of Hong Kong/ Xiamen University/ Beijing Wantai Biological Pharmacy | Hong Kong SAR/ Mainland China | 2020-09-01 | 2020-11-17 | |
| 34 | Nanogen Pharmaceutical Biotechnology | Vietnam | 2020-12-10 | ||
| 35 | Shionogi | Japan | 2020-12-16 | ||
| 36 | GeneOne Life Science, Inc. | Korea | 2020-12-23 | ||
| 37 | Cellid Co., Ltd. | Korea | 2020-12-29 | ||
| 38 | Medigen Vaccine Biologics/ Dynavax/ National Institute of Allergy and Infectious Diseases (NIAID) | Taiwan Region/ US | 2020-10-07 | 2020-12-30 | |
| 39 | Kentucky Bioprocessing Inc. | US | 2020-12-30 | ||
| 40 | SK Bioscience Co., Ltd./ CEPI | Korea | 2021-01-20 | ||
| 41 | Vaxxinity | US | 2020-09-25 | 2021-01-30 | |
| 42 | Takis/ Rottapharm Biotech | Italy/ US | 2021-02-03 | ||
| 43 | Erciyes University | Turkey | 2020-11-05 | 2021-02-10 | |
| 44 | POP Biotechnologies/ EuBiologics Co.,Ltd | US/ Korea | 2021-02-23 | ||
| 45 | KM Biologics Co., Ltd. | Japan | 2021-03-02 | ||
| 46 | Institute of Vaccines and Medical Biologicals, Vietnam | Vietnam | 2021-03-10 | ||
| 47 | Sanofi Pasteur/ Translate Bio | France/ US | 2021-03-12 | ||
| 48 | Daiichi Sankyo Co., Ltd. | Japan | 2021-03-15 | ||
| 49 | VBI Vaccines Inc. | US | 2021-03-15 | ||
| 50 | The Government Pharmaceutical Organization (GPO)/ PATH/ Dynavax | Thailand/ US | 2021-03-20 | ||
| 51 | Entos Pharmaceuticals Inc. | Canada | 2021-04-07 | ||
| 52 | Razi Vaccine and Serum Research Institute | Iran | 2021-01-29 | 2021-04-21 | |
| 53 | National Vaccine and Serum Institute, China | Mainland China | 2021-04-25 | ||
| 54 | Elixirgen Therapeutics, Inc | US | 2021-04-28 | ||
| 55 | Imperial College London | UK | 2020-06-16 | ||
| 56 | Clover Biopharmaceuticals Inc./ GSK/ Dynavax | Mainland China/ UK/ US | 2020-06-19 | ||
| 57 | Vaxine Pty Ltd. | Australia | 2020-06-30 | ||
| 58 | The University of Queensland | Australia | 2020-07-13 | ||
| 59 | Adimmune Corporation | Taiwan Region | 2020-08-24 | ||
| 60 | Vaxart | US | 2020-09-21 | ||
| 61 | University of Munich (Ludwig-Maximilians) | Germany | 2020-10-05 | ||
| 62 | ImmunityBio, Inc | US | 2020-10-19 | ||
| 63 | Academy of Military Science (AMS)/ Walvax Biotechnology/ Suzhou Abogen Biosciences | Mainland China | 2020-10-28 | ||
| 64 | Symvivo Corporation | Canada | 2020-11-02 | ||
| 65 | University Hospital Tuebingen | Germany | 2020-11-27 | ||
| 66 | City of Hope Medical Center/ National Cancer Institute | US | 2020-12-11 | ||
| 67 | Codagenix/ Serum Institute of India | US/ India | 2020-12-11 | ||
| 68 | SK Bioscience Co., Ltd. | Korea | 2020-12-17 | ||
| 69 | Providence Health & Services | US | 2020-12-30 | ||
| 70 | Providence Therapeutics | Canada | 2021-01-14 | ||
| 71 | University of Saskatchewan | Canada | 2021-02-10 | ||
| 72 | GlaxoSmithKline | UK | 2021-02-15 | ||
| 73 | Guangdong Provincial Center for Disease Control and Prevention/ Gaozhou Center for Disease Control and Prevention | Mainland China | 2021-02-22 | ||
| 74 | Altimmune, Inc. | US | 2021-02-25 | ||
| 75 | Organization of Defensive Innovation and Research | Iran | 2021-03-10 | ||
| 76 | Radboud University | Netherlands | 2021-03-11 | ||
| 77 | Kocak Farma | Turkey | 2021-03-19 | ||
| 78 | Gritstone Oncology | US | 2021-03-25 | ||
| 79 | Shanghai East Hospital/ Stemirna Therapeutics | Mainland China | 2021-03-25 | ||
| 80 | The Scientific and Technological Research Council of Turkey | Turkey | 2021-03-27 | ||
| 81 | Walter Reed Army Institute of Research (WRAIR) | US | 2021-04-05 | ||
| 82 | Meissa Vaccines, Inc. | US | 2021-04-12 | ||
| 83 | Jiangsu Rec-Biotechnology | Mainland China | 2021-06-18 |
# The vaccine is quite commonly referred to as the “Pfizer-BioNTech” vaccine, given most research and development stages for the vaccine were conducted in the U.S. and Germany. We follow the WHO document “COVID-19 Vaccine Tracker and Landscape” to define who the vaccine developers are. Our results are not sensitive to whether to include China as developer in this vaccine.
From the Morgan Stanley Capital International (MSCI) database, we use the MSCI All Country World Index (ACWI) to proxy for the aggregate global equity market. The ACWI consists of 23 developed economies and 27 emerging economies as of April 2021; together, these markets make up about 90% of the world's gross domestic product.6 We use the MSCI Investible Market Index (IMI) to measure the stock market return on individual country i.
3. Empirical findings
We begin by estimating the following panel regression:
| (1) |
where the daily abnormal return (ARi,t) of country i on day t is calculated as:
Both α and β are estimated from a market model over the daily estimation window from January to December 2019, and Rm,t is the daily return on ACWI. In Eq. (1), the key variable of interest is Dt, which takes the value of 1 on the first day of the clinical trial of any phase, and 0 otherwise. We posit a positive stock market return on the first day when the clinical trial phase begins, leading to a prediction that ϕ is positive. The ηi variable is the country fixed effect, and X refer to control variables (see Table 2 ) commonly used by prior studies that examine the impacts of COVID-19.
Table 2.
Global stock market reactions on the first day of clinical trial phases.
| Panel A: All vaccine candidates | Panel B: First movers | |||
| (1) | (2) | (3) | (4) | |
| Dt | 0.0808*** | 0.2914*** | ||
| (3.57) | (5.59) | |||
| DII,t | 0.0803** | 0.1597*** | ||
| (2.67) | (3.41) | |||
| DIII,t | 0.1655*** | 0.4033*** | ||
| (4.08) | (6.44) | |||
| VIXt | -0.0050** | -0.0046** | -0.0050** | -0.0044** |
| (-2.46) | (-2.32) | (-2.49) | (-2.22) | |
| BBsprdt | 0.1491** | 0.1595** | 0.2197*** | 0.2233*** |
| (2.19) | (2.31) | (3.09) | (3.25) | |
| Pct_casesi,t | -1.1296*** | -1.1298*** | -1.1434*** | -1.1281*** |
| (-5.00) | (-5.01) | (-5.03) | (-5.01) | |
| Pct_deathsi,t | 0.6142* | 0.6056* | 0.5980* | 0.6027* |
| (1.93) | (1.90) | (1.88) | (1.89) | |
| ARi,t-1 | -0.0950*** | -0.0951*** | -0.0954*** | -0.0958*** |
| (-4.47) | (-4.47) | (-4.48) | (-4.49) | |
| Country FE | Yes | Yes | Yes | Yes |
| # of obs | 17350 | 17350 | 17350 | 17350 |
| Adj. R2 | 0.0192 | 0.0196 | 0.0208 | 0.0212 |
| ϕIII minus ϕII | N/A | 0.0852 | N/A | 0.2436*** |
| (1.57) | (2.99) | |||
The table reports the results of stock market reactions on the first day of vaccine clinical trial phases with the parenthesized t-statistics computed using standard errors clustered at the country level. Panel A reports the results for all vaccines. Panel B reports the results for 13 “first mover” vaccines that had gained approval in at least one governing body as of April 30, 2021, but the regressions are estimated on all 50 countries. The control variables are:
Pct_casesi,t is daily growth rate of COVID-19-confirmed cases, estimated as ln(1+confirmed casesi,t) - ln(1+confirmed casesi,t-1). Following Ding et al. (2021), we collect the number of confirmed cases in all 50 countries from the WHO's COVID-19 Dashboard (https://covid19.who.int/).
Pct_dealthi,t is daily growth rate of COVID-19-related death cases, estimated as ln(1+deathi,t) - ln(1+deathi,t-1).
CBOE VIX is a proxy of investor “fear gauge” around the globe (Whaley, 1993).
Bull-bear spread (BBsprd) is the American Association of Individual Investors Sentiment Survey bull-bear spread, estimated by subtracting the percentage of pessimistic investors who believe that the market would go bearish from the percentage of optimistic investors who believe the market would go bullish.
The lag of abnormal returns (ARi,t-1) control for short-term reversal effect (Pástor and Stambaugh, 2003).
The last row reports the coefficient differences between DIII,t and DII,t dummy variables with t-statistics are parenthesized. The sample period covers from January 2, 2020, to April 30, 2021. *, **, *** denote significance levels at 10%, 5%, and 1%, respectively.
Column (1) of Table 2 shows that ϕ = 0.0808 (t-statistic = 3.57); this suggests that the abnormal stock market return increases significantly by 8.08 bps, on average, when various clinical trial phases begin. The total market capitalization of all 50 countries is around USD57.4 trillion before the pandemic, so the 8.08 bps regression estimate translates to an average USD46.4 billion increase in the market cap on the first day of the clinical trials.
We now test the stock market reactions to different phases. To this end, we separate the Dt dichotomous variable in (1) into D II ,t (a 0/1 dummy variable on the first day of phase II) and D III ,t (a 0/1 dummy variable on the first day of phase III) for the following reasons. First, untabulated analysis shows that 39 vaccines (out of 83 candidates) have concurrent phases I and II and thus, by omitting the dummy variable corresponding to phase I, we can focus on the differential impact between phases II and III. Second, unreported experiment shows that the addition of the 0/1 dummy variable on the first day of phase I carries little explanatory power and its loading is statistically insignificant. Third, phase II is arguably more challenging than phase I and likewise, the beginning of phase III marks an even more significant milestone than that of Phase II. Thus, we predict that the stock markets react more strongly to the beginning of phase III than to the start of phase II. These arguments lead us to conduct the following panel regression:
| (2) |
Column (2) of Table 2 reports results. Consistent with our prediction, the market reaction is 8.03 bps upon the start of phase II, and this estimate doubles to 16.55 bps for phase III.
Of all 83 vaccine candidates, 13 were approved by the WHO and/or the regulators of the respective countries by the end of the sample period. These unique candidates, which we label as “first movers”, include Pfizer, Moderna, and AstraZeneca, and their corresponding clinical trials were initiated early in the pandemic (see Table 1). As such, first movers are at the forefront in the race to develop an effective vaccine, and we posit a stronger stock market reaction on day one of the trials for the first movers relative to other vaccine candidates.
The results reported in Columns (3) and (4) of Table 2 are consistent with our prediction for the first movers. Column (3) shows that the day-one clinical trial effect of the first movers is more than threefold stronger than the case we include all vaccines in Column (1). When we use Eq. (2) and analyze the effect of each specific phase, the impact of first-movers is almost 16 bps (t-statistic = 3.41) in phase II and 40.33 bps (t-statistic = 6.43) in phase III, and both numbers are much larger than those from all vaccine candidates. Taken together, our findings show that the day-one average abnormal stock return is much larger for first-mover vaccine clinical trials.
We build on the above analysis by partitioning the 50 stock markets into vac-countries and non-vac-countries, and within each group, we further sort the sample markets into developed and emerging markets. We re-estimate regression Eq. (2) and report the results in Table 3 . To save space, the table only report two key parameters of interest, ϕII and ϕIII as well as the difference between ϕII and ϕIII; with the regression estimates for the control variables available upon request.
Table 3.
Stock market reactions on the first day of clinical trial phases by country group.
| Panel A: 20 vac-countries | Panel B: 30 non-vac-countries | |||
| 13 developed economies | 7 emerging economies | 10 developed economies | 20 emerging economies | |
| (1) | (2) | (3) | (4) | |
| DII,t | 0.0906* | 0.0655 | 0.1483* | 0.0474 |
| (2.07) | (0.48) | (2.14) | (1.14) | |
| DIII,t | 0.1328* | -0.0025 | 0.2192*** | 0.2155*** |
| (1.89) | (-0.02) | (3.46) | (3.12) | |
| ϕIII minus ϕII | 0.0422 | -0.068 | 0.0709 | 0.1681* |
| (0.60) | (0.31) | (0.61) | (1.83) | |
The table reports the results of stock market reactions, by various groupings of countries, on the first day of vaccines’ clinical trial phases with the parenthesized t-statistics computed using standard errors clustered at the country level. Panel A reports the results for 20 vac-countries, and panel B tabulates the results for 30 non-vac-countries. The MSCI ACWI classifies the following countries as “developed economies” - Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Hong Kong SAR, Ireland, Israel, Italy, Japan, Netherlands, New Zealand, Norway, Portugal, Singapore, Spain, Sweden, Switzerland, U.K., and U.S, - and the following countries as “emerging economies” - Argentina, Brazil, Chile, China, Colombia, Czech Republic, Egypt, Greece, Hungary, India, Indonesia, Korea, Kuwait, Malaysia, Mexico, Pakistan, Peru, Philippines, Poland, Qatar, Russia, Saudi Arabia, South Africa, Taiwan Region, Thailand, Turkey, and UAE. Four vac-countries — Cuba, Iran, Kazakhstan, and Vietnam — are not part of the MSCI ACWI classification. The last row of each panel reports the coefficient differences between DIII,t and DII,t dummy variables with t-statistics presented in parentheses. The sample period covers from January 2, 2020, to April 30, 2021. *, **, *** denote significance levels at 10%, 5%, and 1%, respectively.
Table 3 shows that the economic impacts of clinical trials that began in phases II and III are stronger in developed markets relative to emerging markets, and this finding continues to hold irrespective of whether vaccine developments in human clinical trials were initiated in the respective countries. For developed-vac-countries, Column (1) of Table 3 shows that the average day-one abnormal return is 9.06 bps (t-statistic = 2.07) for phase II and 13.28 bps (t-statistic = 1.89) for phase III. For emerging-vac-countries, the stock markets appear to have a muted response to vaccine development (see Column (2)). For developed-non-vac-countries, the stock markets also react positively to the start of the clinical trials in phases II and III; indeed, their reactions are stronger than those from developed-vac-countries (see Columns (3) and (4)). A possible explanation is that vaccine development is perceived to yield more benefits to countries in which vaccine projects have yet to progress to human clinical trials, or they have yet to initiate any vaccine projects. This finding is also consistent with the expectation that the COVID-19 vaccine is part of “public goods” of which the research-and-development cost is mostly borne by vac-countries, but the benefits are “shared” by both vac- and non-vac-countries.
The U.S. and China are widely regarded as being in the forefront in the race to develop COVID-19 vaccines. In addition, vaccines differ from each other in terms of medical fundamentals and success rates. For example, US-developed vaccines are perceived as safer and have a higher efficacy rate than vaccines developed by other countries because of U.S.’ track record, advancement in medical research and its domination in the global pharmaceutical industry. Conversely, it is also possible that China has a higher success rate than other countries in developing a safe and effective COVID-19 vaccine because of its prior experience in dealing with the 2002–2004 severe acute respiratory syndrome (SARS). Therefore, we expect global stock markets to react heterogeneously to vaccines developed in the U.S. and China, versus in other countries, on the first day of clinical trial phases.
To this end, we modify Eq. (1) by replacing Dt with DUS,t, which is equal to 1 on the first day when vaccine candidates developed by pharmaceutical companies domiciled in the U.S. began their clinical trials in a generic phase, and 0 otherwise. Analogously, we substitute DUS,t with DChina,t for pharmaceutical companies domiciled in China, and D others ,t for pharmaceutical companies domiciled elsewhere. We also modify Eq. (2) by replacing Dj ={II,III}, t with Dj={II,II}, US ,t, Dj ={II,II},China, t, and Dj ={II,II},others, t, one at a time. The Dj ={II,II},US, t variable, for example, is equal to 1 on the first day of phase j for vaccines developed in the U.S., and 0 otherwise. In all the analyses, we test the abnormal returns on 50 stock markets.
Table 4 reports the results. The result of the modified Eq. (1) shows that the average increase in abnormal stock market returns is highest for vaccines developed in China (13.32 bps, t-statistic = 3.56). Turning to the modified Eq. (2), the results show that the abnormal stock market reaction in phase III is strongest for vaccines developed in the U.S. (17.73 bps, t-statistic = 2.00). Also, the abnormal stock market returns on day-one of phase III of vaccines developed in the U.S. and other countries are significantly higher than those at the start of phase II. For example, Column (2) in Panel A reports that the average day-one abnormal return increases by 17.73 bps when US-developed vaccines enter phase III versus -1.90 bps in phase II, for a return differential of 19.64 bps (t-statistic = 1.91). In short, stock market reactions are heterogeneous and conditional on clinical trial phases and the vaccine origins.
Table 4.
Global stock market reactions on the first day of clinical trial phases of vaccines developed in different countries.
| Panel A: k = US | Panel B: k = China | Panel C: k = Others | ||||
| (1) | (2) | (1) | (2) | (1) | (2) | |
| Dk,t | -0.0238 | 0.1332*** | 0.0804*** | |||
| (-0.64) | (3.56) | (3.73) | ||||
| DII,k,t | -0.0190 | 0.3176*** | 0.0722* | |||
| (-0.35) | (5.75) | (1.95) | ||||
| DIII,k,t | 0.1773* | 0.1664** | 0.1652*** | |||
| (2.00) | (2.66) | (4.20) | ||||
| ϕIII,k minus ϕII,k | N/A | 0.1964* | N/A | -0.1512** | N/A | 0.0930* |
| (1.91) | (-2.07) | (1.74) | ||||
The table reports the empirical estimates of regression Eqs. (1) and (2), except that in the current table, Dt and Dj,t are replaced by Dk,t and Dj,k,t with k = US in Panel A, China in Panel B and other countries in Panel C. For example, DUS,t takes the value of 1 if day t is the first day of clinical trial phases and the vac-country is the U.S. In all the columns of each panel, to construct Dk,t and Dj,k,t we use all vaccines developed by that vac-country k. The last row reports the differences between the loading on phase II and phase III dummy variables with t-statistics presented in parentheses. The sample period covers from January 2, 2020, to April 30, 2021. *, **, *** denote significance levels at 10%, 5%, and 1%, respectively.
4. Conclusion
Recent work has investigated the different impacts of the COVID-19 pandemic on various social, economic, and financial aspects. This study offers new insights to whether global stock markets react when human clinical trials for COVID-19 vaccine candidates begin. We show that they do: upon the start of vaccine clinical trials, the average abnormal return of global stock markets increase by 8.08 basis points, and this increase is both economically and statistically significant. Our findings also suggest that global stock markets convey important information about market-wide expectations on the economic value of the development of COVID-19 vaccines even before public vaccine inoculation begins.
CRediT authorship contribution statement
Kam Fong Chan: Writing – original draft, Writing – review & editing, Methodology, Supervision. Zhuo Chen: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Methodology. Yuanji Wen: Writing – original draft, Writing – review & editing, Supervision, Methodology. Tong Xu: Formal analysis, Data curation, Methodology, Software, Validation.
Acknowledgment
We gratefully acknowledge Samuel Vigne (journal editor-in-chief), the anonymous journal referee, Lee Smales and Antonio Gargano for helpful many comments that have substantially improved the paper. Zhuo Chen acknowledges financial support from National Natural Science Foundation of China (grant no. 71790605, 71790591, 71903106 ).
Footnotes
Further, Acharya and Steffen (2020), Fahlenbrach et al., 2021, and Halling et al. (2020) provide evidence that COVID-19 influences firm policies, investment, and financing decisions.
Human clinical trials in a vaccine development have three important phases. In Phase I, the objective is to ascertain the minimum dose required to create an optimal immune response in the test subjects. Phase II involves more volunteers with different demographics to evaluate the safety and efficacy of the vaccine. In phase III, a clinical trial on a larger scale ensues. This phase has the longest duration because it occurs in “natural disease conditions”; that is, the vaccine is administered to test subjects who are exposed to natural conditions of the disease.
In an earlier version of this paper, we develop a theoretical framework commonly used in capital budgeting to formalize the proposition that the beginning of each clinical trial phase has a positive effect on global stock markets.
Unreported experiment shows that stock market reactions are stronger for countries with higher work-from-home capacity (Dingel and Neiman, 2020). In a related study, Bakry et al. (2021) show that investors from developed and emerging markets react heterogeneously to the daily release of COVID-19 announcements.
The caption in Table 3 provides a list of all the developed and emerging countries.
References
- Acharya V., Steffen S. The risk of being a fallen angel and the corporate dash for cash in the midst of COVID. Rev. Corp. Financ. Stud. 2020;9(3):430–471. [Google Scholar]
- Augustin P., Sokolovski V., Subrahmanyam M., Tomio D. In sickness and in debt: the COVID-19 impact on sovereign credit risk. J. Financ. Econ. 2022;143(3):1251–1274. doi: 10.1016/j.jfineco.2021.05.009. Article in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya, V., Johnson, T., Sundaresan, S., and Zheng, S., 2021. The value of a cure: an asset pricing perspective. Available at SSRN: https://ssrn.com/abstract=3731098.
- Bao, C., Bao, K., Tam, V., and Phuong, T., 2021. Vaccine initiation rate and volatility in the international stock market during COVID-19. Available at SSRN: https://ssrn.com/abstract=3945810.
- Bakry W., Kavalmthara P., Saverimuttu V., Liu Y., Cyril S. Response of stock market volatility to COVID-19 announcements and stringency measures: a comparison of developed and emerging markets. Financ. Res. Lett. 2021 doi: 10.1016/j.frl.2021.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caferra R., Vidal-Tomás D. Who raised from the abyss? A comparison between cryptocurrency and stock market dynamics during the COVID-19 pandemic. Financ. Res. Lett. 2021;43 doi: 10.1016/j.frl.2021.101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Phillips G. COVID-19 losses to the real estate market: an equity analysis. Financ. Res. Lett. 2022;45 [Google Scholar]
- Corbet S., Hou Y., Hu Y., Larkin C., Lucey B., Oxley L. Cryptocurrency liquidity and volatility interrelationships during the COVID-19 pandemic. Financ. Res. Lett. 2022;45 doi: 10.1016/j.frl.2021.102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E., Kizys R., Rouatbi W., Zaremba A. COVID-19 vaccinations and the volatility of energy companies in international markets. J. Risk Financ. Manag. 2021;14(2) doi: 10.1016/j.irfa.2021.101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W., Levine R., Lin C., Xie W. Corporate immunity to the COVID-19 pandemic. J. Financ. Econ. 2021;141(2):802–830. doi: 10.1016/j.jfineco.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingel J., Neiman B. How many jobs can be done at home? J. Public Econ. 2020;189 doi: 10.1016/j.jpubeco.2020.104235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlenbrach R., Rageth K., Stulz R. How valuable is financial flexibility when revenue stops? Evidence from the COVID-19 crisis. Rev. Financ. Stud. 2021;34(11):5474–5521. [Google Scholar]
- Gormsen N., Koijen R. Coronavirus: impact on stock prices and growth expectations. Rev. Asset Pricing Stud. 2020;10(4):574–597. [Google Scholar]
- Halling M., Yu J., Zechner J. How did COVID-19 affect firms’ access to public capital markets? Rev. Corp. Financ. Stud. 2020;9(3):501–533. [Google Scholar]
- Haque M., Choi B., Lee D., Wright S. Insider vs. outsider CEO and firm performance: evidence from the Covid-19 pandemic. Financ. Res. Lett. 2021 doi: 10.1016/j.frl.2021.102609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H., Kubik J., Wang, N., Xu, X., and Yang, J., 2021. Pandemics, vaccines and an earnings damage function. Available at SSRN: https://ssrn.com/abstract=3689939.
- Khalfaoui R., Nammouri H., Labidi O., Ben Jabeur S. Is the COVID-19 vaccine effective on the US financial market? Public Health. 2021;198:177–179. doi: 10.1016/j.puhe.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. COVID-19 and trade credit speed of adjustment. Financ. Res. Lett. 2021 doi: 10.1016/j.frl.2021.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara M., Zhou X. Anatomy of a liquidity crisis: Corporate bonds in the COVID-19 crisis. 2021;142(1):46–68. doi: 10.1016/j.jfineco.2021.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pástor L., Stambaugh R. Liquidity risk and expected stock returns. J. Polit. Econ. 2003;111(3):642–685. [Google Scholar]
- Qian X., Qiu S., Zhang G. The impact of COVID-19 on housing price: evidence from China. Financ. Res. Lett. 2021;43 doi: 10.1016/j.frl.2021.101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramelli S., Wagner A. Feverish stock price reactions to COVID-19. Rev. Corp. Financ. Stud. 2020;9(3):622–655. [Google Scholar]
- Rouatbi W., Demir E., Kizys R., Zaremba A. Immunizing markets against the pandemic: COVID-19 vaccinations and stock volatility around the world. Int. Rev. Financ. Anal. 2021;77 doi: 10.1016/j.irfa.2021.101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smales L. Investor attention and global market returns during the COVID-19 crisis. Int. Rev. Financ. Anal. 2021;73 [Google Scholar]
- Whaley R. Derivatives on market volatility: hedging tools long overdue. J. Deriv. 1993;1:71–84. [Google Scholar]
- Yarovaya L., Elsayed A., Hammoudeh S. Determinants of spillovers between Islamic and conventional financial markets: exploring the safe haven assets during the COVID-19 pandemic. Financ. Res. Lett. 2021;43 [Google Scholar]