Abstract
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a novel prothrombotic disorder characterized by thrombosis, thrombocytopenia, and disseminated intravascular coagulation identified in hundreds of recipients of ChAdOx1 nCoV-19 (Oxford/AstraZeneca), an adenovirus vector coronavirus disease 2019 (COVID-19) vaccine. VITT resembles heparin-induced thrombocytopenia (HIT) in that patients have platelet-activating anti-platelet factor 4 antibodies; however, whereas heparin typically enhances platelet activation by HIT antibodies, VITT antibody-induced platelet activation is often inhibited in vitro by pharmacological concentrations of heparin. Further, the thrombotic complications in VITT feature much higher frequencies of atypical thrombosis, most notably cerebral vein thrombosis and splanchnic vein thrombosis, compared with HIT. In this review, we outline the treatments that have been used to manage this novel condition since its recognition in March 2021, including anticoagulation, high-dose intravenous immune globulin, therapeutic plasma exchange, corticosteroids, rituximab, and eculizumab. We discuss the controversial issue of whether heparin, which often inhibits VITT antibody-induced platelet activation, is harmful in the treatment of VITT. We also describe a case of “long VITT,” describing the treatment challenges resulting from platelet-activating anti-PF4 antibodies that persisted for more than 9 months.
Keywords: Heparin, High-dose immune globulin (IVIG), Long VITT, Therapeutic plasma exchange (TPE), Vaccine-induced immune thrombotic thrombocytopenia (VITT)
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT), also known as thrombosis with thrombocytopenia syndrome (TTS), was first identified in March 2021 following vaccination with the ChAdOx1 nCoV-19 (AstraZeneca) vaccine, an adenovirus vector COVID-19 (coronavirus disease 2019) vaccine [1]. Multiple peer-reviewed publications [2], [3], [4] subsequently confirmed the initial observations, showing that this syndrome is characterized by thrombosis and thrombocytopenia, and as in heparin-induced thrombocytopenia (HIT), involves platelet-activating antibodies against platelet factor 4 (PF4).
Treatments that have been used in the management of VITT include anticoagulation, high-dose intravenous immune globulin (IVIG), therapeutic plasma exchange (TPE), corticosteroids, rituximab, and eculizumab. Further, many patients have received supportive blood product transfusions, including platelets, fibrinogen (concentrates, cryoprecipitate), and plasma. The primary objective of our article is to review these various treatments of VITT. We will focus on the 2 major therapies appropriate for most or all patients, namely anticoagulation and high-dose IVIG. We will address the issue of whether heparin may be as effective as non-heparin anticoagulation for this novel anti-PF4, HIT-mimicking disorder. Many aspects of VITT treatment remain uncertain, including the controversy of heparin vs non-heparin anticoagulation, when salvage therapy such as TPE might be indicated, the role of corticosteroids, and duration of anticoagulation, among other issues. We will conclude our review with considerations of what the legacy of VITT will be regarding better understanding of certain aspects of HIT management. Table lists some noteworthy treatment controversies and paradoxes applicable to HIT and VITT.
Table 1.
Treatment controversies and paradoxes in HIT and VITT.
| Treatment controversy or paradox | Comment |
|---|---|
| Heparin vs non-heparin anticoagulation | Heparin may promote platelet activation if heparin-dependent antibodies are present (characteristic of HIT); however, VITT features PF4-dependent, rather than heparin-dependent, platelet activation (rather, heparin in pharmacological concentrations can inhibit VITT serum-induced platelet activation) |
| IVIG—prothrombotic or antithrombotic | IVIG inhibits platelet activation in HIT and VITT, and thus helps to deescalate antibody-induced hypercoagulability (in contrast, IVIG treatment of ITP and drug-induced ITP is rarely complicated by acute thrombosis) |
| Treatment of “isolated” HIT and VITT | As per recommendations for treatment of “isolated” HIT (ie, strongly suspected HIT without apparent thrombosis), patients with strongly suspected VITT recognized by thrombocytopenia alone should receive therapeutic-dose anticoagulation; high-dose IVIG is also indicated for VITT |
| Corticosteroid therapy | Corticosteroids decrease reticuloendothelial (macrophage) clearance of antibody-coated platelets, and thus their use could shift platelet clearance towards platelet activation (theoretical deleterious effect of corticosteroids) |
| Avoidance of vitamin K antagonist therapy in acute HIT and VITT | Increased risk of venous limb gangrene and skin necrosis due to depletion of protein C and ongoing thrombin generation |
References for this table are found in the relevant sections of the text.
General considerations
As with patients with severe HIT [5,6], VITT can be viewed as a highly prothrombotic form of disseminated intravascular coagulation (DIC). One distinction between HIT and VITT is that the former is often identified through platelet count monitoring only; in 1 study [7], approximately half of patients ultimately proven to have HIT were recognized following initial HIT-associated thrombosis, while the remaining patients with so-called “isolated HIT” were recognized because of an unexpected platelet count fall. In contrast, VITT patients are almost always recognized because of symptoms and signs of thrombosis, beginning 5 to 45 days following vaccination with an adenovirus vector vaccine (most often, between 5 and 30 days post-vaccination, with median time from vaccination to presentation 14 days; day of vaccination = day 0) [8,9]. Of course, not all post-vaccination thrombotic events reflect the occurrence of VITT, so the role of the clinician when assessing a patient with post-vaccination thrombosis is to distinguish between VITT and non-VITT thrombosis.
In a patient who develops symptoms or signs of post-vaccine thrombosis, the presence of thrombocytopenia is an important clue pointing towards a potential diagnosis of VITT. However, there are other non-vaccine explanations for the combination of thrombosis and thrombocytopenia, including (among others) cancer-associated DIC [10], antiphospholipid syndrome [11,12], pulmonary embolism with DIC [13], chronic immune thrombocytopenia (ITP) [14], paroxysmal nocturnal hemoglobinuria [15], thrombotic thrombocytopenic purpura [16], and even “spontaneous HIT syndrome” [17]. Perhaps most common, on purely statistical grounds, are simply patients who have mild chronic thrombocytopenia of diverse explanations (low-normal range spectrum, hereditary, hypersplenism, myelodysplasia, etc.) who subsequently develop thrombosis following vaccination, either coincidentally or perhaps related to (non-VITT) proinflammatory effects of vaccination. These are important considerations, given that the clinician may need to make initial treatment decisions in the absence of a clear diagnosis of VITT vis-à-vis other possibilities.
The anatomical location of the thrombosis is also an important clue pointing towards a diagnosis of VITT. Just as there are certain unusual thrombotic events that point to a diagnosis of HIT in a patient receiving heparin (eg, bilateral lower-limb DVT, pulmonary embolism, acute limb artery thrombosis, adrenal hemorrhagic infarction, and so forth) [18], so too unusual thrombi, most notably cerebral venous (sinus) thrombosis (CVT) and splanchnic vein thrombosis (SVT) are characteristic thrombi seen in approximately 50% and 20% of patients, respectively, with recognized VITT [8,9].
Anticoagulation
The principles of the treatment of VITT have been extrapolated from clinical experience with HIT. Similar to HIT, VITT is a highly prothrombotic DIC disorder, and therapeutic anticoagulation is one of the mainstays of treatment. Given the involvement of anti-PF4 antibodies in VITT, current treatment guidelines [19], [20], [21], [22] recommend against the use of heparin-based anticoagulation, and for the use of non-heparin anticoagulants, including fondaparinux, direct oral anticoagulants (DOACs), and direct thrombin inhibitors (argatroban, bivalirudin). While there is clinical uncertainty in this area, current evidence—both clinical and laboratory—suggests that the use of heparin-based anticoagulation may be safe for the management of VITT.
In vitro studies using VITT sera have demonstrated that platelet activation is usually inhibited by the addition of pharmacological (i.e., clinically relevant) concentrations of heparin [2], [3], [4],23,24]; this is in contrast to what is seen using HIT patient sera, where pharmacological concentrations of heparin typically enhance reactivity in vitro in washed platelet activation assays, including the serotonin-release assay (SRA) [25] as well as the heparin-induced platelet activation (HIPA) test [26]. Further, in HIT, there are known situations in which heparin administration is harmful, most notably “heparin-associated anaphylactoid reactions,” in which patients develop abrupt complications—including thrombotic events—within 5 to 30 minutes after receiving a heparin bolus [27]. To our knowledge, similar abrupt thrombotic or anaphylactoid events associated with abrupt heparin-induced platelet count declines have not been reported in VITT.
Biochemical data also support the concept that heparin may not be harmful for VITT patients, based on the discovery that the target antigens of VITT antibodies differ from HIT [28]. HIT antibodies bind to 1 or more heparin-dependent antigen sites on PF4 that are only revealed upon heparin binding to the heparin-binding site on PF4. In contrast, the target of VITT antibodies appears to be the heparin-binding site itself; thus, the addition of heparin can displace VITT antibodies, resulting in inhibition of platelet activation by VITT sera. Further, heparin was also shown to inhibit directly the binding of VITT antibodies to PF4 [28].
Currently published case series do not appear to demonstrate a significant increase in mortality in VITT patients treated with heparin as opposed to non-heparin-based anticoagulation [8,29,30]. A recent meta-analysis [31] found no significant difference in mortality between heparin- and non-heparin-based anticoagulation treatments (risk ratio, 0.84; 95% CI, 0.47-1.50; P = .80). However, these data are challenging to interpret given numerous confounders, including variability in concurrent treatments patients received (IVIG, corticosteroids, TPE), and some patients treated with both heparin and non-heparin anticoagulation.
Another problematic aspect of trying to ascertain benefit/risk of heparin vs non-heparin anticoagulation is that single endpoints, such as mortality, may be too crude to determine adverse effects of treatment. Further, case-series typically do not provide details regarding granular aspects of anticoagulation effects, such as changes in platelet count or alterations in hypercoagulability. Nevertheless, in a study of 3 VITT patients that included a detailed assessment of platelet count changes and coagulation parameters, there did not appear to be an abrupt decline in platelet count upon starting heparin, as would be expected if the patients had heparin-dependent, activation-enhancing antibodies as is characteristic of HIT; moreover, heparin was associated with a decrease in consumptive coagulopathy, as shown by improvements in fibrinogen concentrations (Fig. 1 ) [24]. Given the uncertainty in this area, non-heparin anticoagulants should continue to be used preferentially over heparin anticoagulants in the management of cases of proven or suspected VITT.
Fig. 1.
Platelet counts in relation to heparin treatment in 3 patients with VITT. For patients 1 and 2, heparin treatment was given prior to clinical recognition of VITT; for patient 3 heparin treatment was given for an 8-day period during which high-dose IVIG and TPE were administered, and after demonstration in vitro that the patient's serum did not enhance platelet activation in the presence of heparin. None of the patients showed an abrupt drop in platelet count upon starting heparin; rather, platelet counts increased in all 3 patients (although the platelet counts returned to pre-heparin baseline in patient 2). For patients 1 and 2, available fibrinogen data indicated that heparin administration was associated with a decrease in consumptive coagulopathy, as shown by rising fibrinogen levels. Data obtained from 3 patients reported elsewhere [24]. Data for patients 1 and 2 reprinted with permission of the Massachusetts Medical Society (data for patient 3 in Fig. 1 has not been reported previously). aPTT, activated partial thromboplastin time; IV, intravenous; IVIG, intravenous immune globulin; SC, subcutaneous; TPE, therapeutic plasma exchange; U, units; UFH, unfractionated heparin.
Heparin and non-heparin anticoagulation: Activated partial thromboplastin time (aPTT) confounding
In addition to the uncertainty regarding the safety of heparin-based anticoagulation in the management of VITT, there is also uncertainty regarding whether aPTT-adjusted therapies, such as heparin, are optimally effective in a DIC state. Non-heparin anticoagulants, such as argatroban and bivalirudin, are also monitored by aPTT. A DIC state often leads to prolongation of the aPTT, which thereby alters the anticoagulant/aPTT dosing relationship when using aPTT-guided treatment nomograms, leading to potential systematic under-treatment with anticoagulation. This phenomenon, called “aPTT confounding” [32,33], could explain why aPTT-adjusted therapies often fail in cases of severe HIT and could also explain failure in severe VITT cases. Another explanation for aPTT confounding relevant to critical illness is that very high factor VIII levels (explained by factor VIII being an acute-phase reactant) interfere with the relationship between anticoagulant levels and aPTT values [34].
Corroborating data for this concept of aPTT confounding was presented in a recent study examining the use of argatroban in HIT and VITT patients, which demonstrated significant discordance between aPTT levels and argatroban concentrations [34]. The authors proposed that direct assessment of argatroban levels, using a dilute thrombin time (dTT), to be preferable to aPTT monitoring. The sole use of aPTT levels to monitor argatroban in the treatment of VITT was also discouraged in a recently published guideline [35]. Taking this into account, non-aPTT-adjusted therapies, including fondaparinux, danaparoid, and direct oral anticoagulants (DOACs; either Xa or IIa-inhibiting), may be preferred in the treatment of VITT and HIT on theoretical grounds. A small case-series (n = 6) describing use of danaparoid (often administered by subcutaneous injection) from Finland reported generally favorable outcomes, with recovery in 5 of 6 patients; the single fatal outcome was the first patient in Finland diagnosed with VITT, who presented with myocardial infarction and CVT with cerebral hemorrhages, and in whom high-dose IVIG therapy was not administered up-front [36].
A further theoretical advantage of non-aPTT-adjusted therapies that target factor Xa (eg, fondaparinux, danaparoid, rivaroxaban, apixaban, edoxaban) is that they downregulate thrombin generation but do not interfere with thrombin-enhanced activation of protein C [37,38]. The paradoxical concept that an anticoagulant that is otherwise effective in most situations might be deleterious in an extreme hypercoagulability state is illustrated in the subsequent section that discusses warfarin-associated microthrombosis.
Avoidance of vitamin K antagonists in acute HIT/VITT
In the acute setting, vitamin K antagonists such as warfarin should be avoided in HIT, as this treatment has been shown to trigger microthrombosis, resulting in venous limb gangrene and skin necrosis [39,40]. HIT patient plasma following treatment with vitamin K antagonists in acute HIT has been shown to have ongoing thrombin generation and a significant reduction in protein C levels, with an estimated increased risk of vitamin K antagonist-associated venous limb gangrene of 5% to 20% [41]. Given the similar prothrombotic nature of VITT, vitamin K antagonists should be avoided in the acute setting based on clinical experience with HIT.
High-dose IVIG
Prior to the recognition of VITT, it had been found that high-dose IVIG was effective for the treatment of severe HIT, including patients with atypical clinical features (delayed-onset HIT, persisting/refractory HIT); such patients are known to have high-titre HIT antibodies that possess both heparin-dependent and heparin-independent platelet-activating properties [6,42]. Moreover, both in vitro and ex vivo data showed that high-dose IVIG inhibits HIT antibody-induced platelet activation in a dose-dependent fashion, and without any effects on EIA reactivity [42], [43], [44], [45], [46]. These data provided a scientific and clinical rationale for the use of high-dose IVIG to treat VITT.
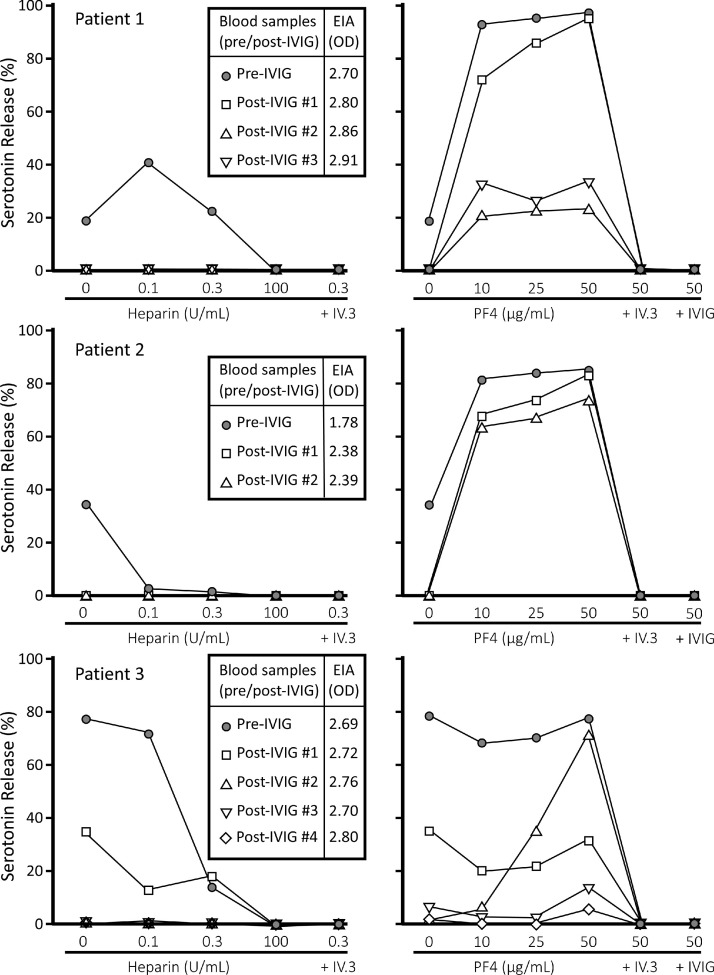
Indeed, observational data support the concept that high-dose IVIG is also effective in the management of VITT [2], [3], [4],23,24,29,[47], [48], [49], [50]. The probable efficacy is based on several clinically-relevant observations: first, the platelet count usually rises abruptly following administration of IVIG; second, laboratory markers of hypercoagulability are improved (eg, rising fibrinogen levels, falling d-dimer levels) [23,24,48,49]; and third, serial blood samples obtained in VITT patients before and after 2 or 3 doses of IVIG have demonstrated reversion to a weaker or negative PF4-enhanced SRA (PF4-SRA)—especially in the absence of added PF4—albeit with persistence of strong EIA reactivity (Fig. 2 ) [23,24]. These ex vivo data indicate that the mechanism of action of IVIG is competitive inhibition of platelet activation by VITT antibodies at the level of the platelet FcγIIa receptors, rather than inhibition of binding of the antibodies to their PF4 target [8,23,24,28,42]. These data also corroborate in vitro data, in which HIT or VITT serum-induced platelet activation in the SRA (or PF4-SRA) is inhibited by IVIG in a dose-dependent fashion [45,46]. Similar ex vivo and in vitro data have also been reported for patients with spontaneous HIT syndrome treated with high-dose IVIG [46,51].
Fig. 2.
Inhibition of platelet activation by high-dose IVIG. Platelet activation (as shown by percent serotonin-release) in the standard serotonin-release assay (SRA) (leftmost panel) and in the PF4-enhanced SRA (PF4-SRA; rightmost panel) for serial samples obtained for 3 patients—samples obtained before and after 2 to 4 doses of high-dose intravenous immune globulin (IVIG). In all 3 patients, samples obtained post-IVIG showed substantial decrease in serum-induced platelet activation. In contrast, there was no inhibition of EIA reactivity, shown as units of optical density (OD); boxed insets in leftmost panel). These data indicate that IVIG works by inhibiting platelet activation through platelet FcγIIa receptors, rather than by inhibiting interaction between the antibodies and the antigen target(s) on PF4. Reprinted (with modifications) [24] with permission of the Massachusetts Medical Society. EIA, enzyme-immunoassay; IV.3 indicates an Fc receptor-blocking monoclonal antibody; IVIG, intravenous immune globulin; OD, optical density; PF4, platelet factor 4; U, units.
Current guidelines recommend that IVIG be administered up-front in the treatment of probable and confirmed cases of VITT, at a dose of 1 gram/kilogram body weight for 2 days, with the dosing based on actual body weight [19], [20], [21], [22]. Given the dose-dependent effect of IVIG on reducing antibody-mediated platelet activation, actual body weight dosing is preferred to ideal body weight dosing [44]. In certain resistant cases, repeat administration of IVIG has been required, based upon recurrence of a platelet count decline after an initial increase [8,24,52].
Therapeutic Plasma Exchange (TPE)
TPE has been used in the treatment of refractory cases of VITT, with evidence of clinical benefit [9,52,53]. Plasma exchange results in the removal of VITT anti-PF4 antibodies [9]. In a prospective cohort study of suspected VITT cases, 17 patients with severe disease, defined in the study as CVT, thrombosis at multiple sites, or both, were treated with plasma exchange, with a survival rate of 90% [8]. In a case series of 3 VITT patients refractory to initial treatment with IVIG and anticoagulation, TPE was continued until there was a sustained increase in platelet count; 1 patient had an increase in platelet count and decrease in d-dimer with TPE alone; 1 patient had a demonstrated increase in platelet count and decrease in d-dimer with TPE and rituximab; 1 patient did not have an increase in platelet count until additional doses of IVIG were administered concurrently with TPE [52]. For 2 of these patients, the replacement fluid was plasma, and for 1 patient the replacement fluid was half plasma and half albumin [52]. In another reported case, a VITT patient who was refractory to treatment with anticoagulation, IVIG, and rituximab, had subsequent sustained improvement of thrombocytopenia following TPE with plasma used as the replacement fluid [53]. Further study on the optimal timing of initiation of TPE in refractory disease, and its potential frontline use in severe VITT is required. As with HIT, plasma is the preferred replacement fluid as it results in greater inhibition of HIT antibody-mediated platelet activation compared to albumin [54].
Corticosteroid, rituximab, eculizumab
Corticosteroids are sometimes used to treat VITT based on treatment parallels with other immune-mediated disorders, such as ITP. For example, corticosteroids are believed to raise the platelet count in chronic ITP by decreasing clearance of antibody-coated platelets by macrophages of the reticuloendothelial system [55]. However, corticosteroids could theoretically be deleterious in disorders such as HIT and VITT if such corticosteroid-induced inhibition of “benign” platelet clearance by macrophages results in a shift towards greater antibody-induced platelet activation. Also, corticosteroids may not result in a decrease in VITT antibody levels, given observations that many VITT patients continue to have strong positive EIA test results for many months following the development of VITT, irrespective of whether or not they received corticosteroids. Based on these considerations, we do not routinely use corticosteroids to treat VITT.
Rituximab, a monoclonal antibody that targets CD20 on B-cells, has also been used in the treatment of VITT, though the clinical efficacy of this adjunct therapy remains unclear [3,8,9,29,52,53]. Rituximab should be used with caution in the context of an ongoing pandemic, particularly when there exists uncertainty as to its clinical benefit, as its use can limit vaccine immune response [56], [57], [58].
Eculizumab, a monoclonal antibody which targets the complement system via its activity against C5, has also been used in the treatment of VITT. In a case series of 5 VITT patients, 2 were treated with eculizumab [30]. One of these patients was treated with eculizumab because of renal failure and evidence of thrombotic microangiopathy, in combination with heparin. The second patient, who had received argatroban and IVIG, had eculizumab administered as salvage therapy due to ongoing thrombocytopenia post-IVIG. Both patients recovered, though the influence of eculizumab on their clinical course and its role in this prothrombotic condition remains uncertain.
Blood product replacement (platelets, fibrinogen)
Clinical guidelines for the treatment of VITT suggest that prophylactic platelet transfusions should be avoided, as in HIT, as this could further fuel the prothrombotic state of this disorder [9,[19], [20], [21]. Platelet transfusions administered to VITT patients have been associated with progression of thrombosis [4], and an increased mortality rate, with 1 paper demonstrating an 84% mortality rate in VITT patients with CVT after receiving platelet transfusions (n = 25), compared to 27% of patients who did not receive platelet transfusions (n = 45) [29]. However, in the case of severe bleeding or severe thrombocytopenia and the need for emergency surgery, platelet transfusions have been administered in VITT patients [3,8].
The fibrinogen level is often reduced in VITT patients [2,3,4,48,59]. Transfusion to correct hypofibrinogenemia has been performed in patients with VITT [9,29]. Given the possibility of increasing thrombotic risk with fibrinogen replacement, this should be reserved for patients with bleeding or who need surgical intervention with a fibrinogen level less than 1.5 g/L.
Treatment duration
A remarkable feature of HIT is the usual transient nature of anti-PF4/heparin antibodies, with a median time to loss of assay reactivity of 40 and 100 days, respectively, for platelet activation and immunoassays [60]. In contrast, however, VITT antibody reactivity appears to persist for much longer, including in platelet activation assays [61]. Thus, relapse of thrombocytopenia with or without thrombotic complications is a major risk of VITT, if the effects of IVIG or TPE have waned, especially if anticoagulation is interrupted. Thus, even in patients with VITT-associated thrombosis who have good initial responses to high-dose IVIG and anticoagulation, follow-up outpatient management should include ongoing platelet count, fibrinogen, and d-dimer monitoring, to determine if the effects of IVIG are waning, and/or whether hypercoagulability is exacerbated. As illustrated in the next section, some patients with VITT can have antibody-induced hypercoagulability that persists for several months, and which can prove refractory to high-dose IVIG and/or anticoagulation.
Long VITT
Günther and colleagues [62] reported a 54-year-old male who developed severe VITT as shown by symptomatic stroke (CVT with secondary cerebral hemorrhage requiring emergency craniectomy), bilateral adrenal hemorrhages, thrombocytopenia (platelet count, 37 × 109/L), and DIC (fibrinogen, 0.94 g/L; d-dimer >30 mg/L), with initial presentation 12 days following first dose of vaccination with ChAdOx1 nCoV-19. The patient tested strongly positive for VITT antibodies, both by EIA as well as PF4-dependent washed platelet activation assay. Interestingly, these assays were strongly positive both when an initial sample was studied from admission, as well as a day 90 sample. The patient's clinical course was complex, and he was managed with several anticoagulants (argatroban, tinzaparin [low-molecular-weight heparin], danaparoid, fondaparinux), with the platelet count falling on several occasions after the effects of a preceding course of high-dose IVIG had waned. This case of “long VITT” illustrates that platelet-activating anti-PF4 antibodies associated with VITT can persist for more than 3 months, thus explaining recurrence of thrombocytopenia when platelet activation-inhibiting effects of IVIG wear off.
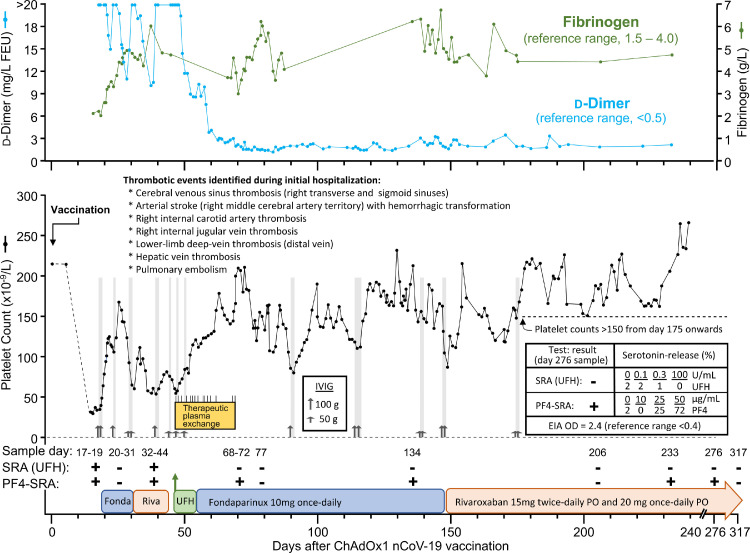
We have also encountered a patient with “long VITT” who had platelet-activating anti-PF4 antibodies detectable for more than 9 months following a diagnosis of VITT at our center (Fig. 3 ). The 69-year-old male patient was hospitalized beginning 1 week following vaccination with ChAdOx1 nCoV-19, with complaints of headache and progressive neurological symptoms indicating stroke (the first several weeks of hospitalization have been reported elsewhere [24]). Investigation of associated thrombocytopenia resulted in a confirmed diagnosis of VITT. Imaging studies performed during the first week of hospitalization demonstrated numerous venous and arterial thrombotic events (Fig. 3). Repeat administration of high-dose IVIG resulted in generally transient platelet count increases on several occasions. The patient also received TPE from day 47 to day 69 (15 TPE procedures with replacement using solvent-detergent plasma, total 374 units of plasma administered).
Fig. 3.
Patient with "long VITT.” The figure shows serial laboratory values (platelet counts, d-dimer, and fibrinogen), treatments given—including anticoagulants (fondaparinux, rivaroxaban, unfractionated heparin), high-dose intravenous immune globulin (IVIG; up-pointing arrows), and therapeutic plasma exchange (TPE). In addition, results of serial platelet activation assays—including conventional serotonin-release assay (SRA) performed with different concentrations of unfractionated heparin (UFH), indicated as “SRA (UFH)” as well as the PF4-enhanced SRA, indicated as “PF4-SRA.” Results of serial polyspecific anti-PF4/polyvinyl sulfonate EIAs are not shown, as these were uniformly strong (all >2.0 units of optical density) throughout the entire period from post-vaccination days 17 through 317. In general, administration of high-dose IVIG raised the platelet counts over the subsequent several days, although response was generally transient, and appeared to become refractory (approximately days 25-47), resulting in the decision to initiate TPE on day 47 because of recurrent thrombocytopenia and concern regarding possible new subacute infarct in the right putamen. The patient continued to have detectable platelet-activating antibodies on day 276, but tested negative on day 317. See text for further details regarding the case, including clinical and laboratory follow-up until day 317. FEU, fibrinogen-equivalent units; Fonda, fondaparinux; IVIG, intravenous immunoglobulin; PF4-SRA, PF4-enhanced SRA; PO, per os; Riva, rivaroxaban; SRA (UFH), conventional SRA performed at different concentrations of heparin; UFH, unfractionated heparin; + = positive platelet activation test result; - = negative platelet activation test result.
Serial platelet counts in this patient showed gradually increasing values over time, with all platelet counts after day 175 measuring greater than 150 × 109/L (except for a transient period of thrombocytopenia from days 302 to 308 related to invasive line-related polymicrobial bacteremia associated with septic shock; not shown in Fig. 3). Throughout the patient's hospital stay serial PF4-dependent EIAs (assessed by commercial polyspecific PF4/polyvinyl sulfonate EIA) have remained strongly positive (all >2.0 units of optical density [OD]). Serial platelet activation assays have also been generally positive, although some samples tested negative (for some samples, temporally related to recent administration of high-dose IVIG). As shown in Fig. 3, both the EIA and the PF4-SRA (but not the conventional SRA) remained positive on day 276, indicating long-term persistence of platelet-activating anti-PF4 antibodies. The next available blood sample from day 317 tested negative in both the SRA and PF4-SRA, although the EIA remained strongly positive (2.31 OD units). This case illustrates the challenges of managing patients with long VITT.
The legacy of VITT—a better understanding of HIT treatment
Since the initial identification of VITT in March 2021, the scientific community has gained considerable experience in the diagnosis and treatment of this novel disease. One of the likely unexpected legacies of VITT is that there will be a better appreciation of certain clinical and treatment aspects of HIT itself. Clinical experience with VITT has yielded further insights into HIT, including better recognition of this condition as a DIC state. IVIG has previously been shown to decrease hypercoagulability in cases of severe HIT [42], a concept that is further exemplified by the generally positive outcomes following treatment of VITT with IVIG. IVIG and TPE have demonstrated to have some clinical benefit in cases of refractory VITT, and through this clinical experience may become more widely used in the treatment of severe HIT.
Ongoing observational research is required to further explore the long-term outcomes of these conditions, and the ideal treatment regimen to maximize good clinical outcomes in these prothrombotic disorders.
Acknowledgments
The authors thank Jo-Ann I. Sheppard for assistance in preparation of the figures.
Conflicts of interest
Theodore (Ted) E. Warkentin, has received lecture honoraria from Alexion and Instrumentation Laboratory, and royalties from Informa (Taylor & Francis); has provided consulting services to Aspen Canada, Aspen Global, CSL Behring, Ergomed, Paradigm Pharmaceuticals, and Octapharma; has received research funding from Werfen (Instrumentation Laboratory); and has provided expert witness testimony relating to heparin-induced thrombocytopenia (HIT) and non-HIT thrombocytopenic and coagulopathic disorders. The remaining authors have no conflicts of interest to disclose.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. DOI: 10.21203/rs.3.rs-362354/v1 Posted Mar 29, 2021 on Research Square [preprint server]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(7):502–506. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 6.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101(5):502–507. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 8.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2021;9(1):e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warkentin TE, Cook RJ, Sarode R, Sloane DA, Crowther MA. Warfarin-induced venous limb ischemia/gangrene complicating cancer: a novel and clinically distinct syndrome. Blood. 2015;126(4):486–493. doi: 10.1182/blood-2015-01-622787. [DOI] [PubMed] [Google Scholar]
- 11.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 12.James TE, Martin LJ, Warkentin TE, Crowther MA. Catastrophic antiphospholipid syndrome refractory to high-dose intravenous immunoglobulin responsive to therapeutic plasma exchange. Platelets. 2021;32(6):828–831. doi: 10.1080/09537104.2020.1802414. [DOI] [PubMed] [Google Scholar]
- 13.Stahl RL, Javid JP, Lackner H. Unrecognized pulmonary embolism presenting as disseminated intravascular coagulation. Am J Med. 1984;76:772–778. doi: 10.1016/0002-9343(84)90985-9. [DOI] [PubMed] [Google Scholar]
- 14.Sarpatwari A, Bennett D, Logie JW, et al. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica. 2010;95(7):1167–1175. doi: 10.3324/haematol.2009.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patriquin CJ, Kiss T, Caplan S, et al. How we treat paroxysmal nocturnal hemoglobinuria: a consensus statement of the Canadian PNH Network and review of the national registry. Eur J Haematol. 2019;102(1):36–52. doi: 10.1111/ejh.13176. [DOI] [PubMed] [Google Scholar]
- 16.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40–51. doi: 10.1016/j.thromres.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin TE. In: Heparin-induced thrombocytopenia. 5th edn. Warkentin TE, Greinacher A, editors. CRC Press; Boca Raton, FL: 2013. Clinical picture of heparin-induced thrombocytopenia; pp. 24–76. [Google Scholar]
- 19.Warkentin TE, Cuker A. COVID-19: vaccine-induced immune thrombotic thrombocytopenia (VITT). UpToDate; 2021.Available: https://www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt. Accessed date 16 Mar 2022.
- 20.Guidance produced from the Expert Haematology Panel (EHP) focused on syndrome of thrombosis and thrombocytopenia occurring after coronavirus vaccination. London (UK): British Society for Haematology; 2021. Available: https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/, Accessed date 16 Mar 2022.
- 21.Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). World Health Organization; 2021. Available: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf. Accessed date 16 Mar 2022. [PubMed]
- 22.Pai M, Chan B, Stall NM, et al.; Drugs & Biologics Clinical Practice Guidelines Working Group and the Ontario COVID-19 Science Advisory Table. Vaccine induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination. Ontario: Ontario COVID-19 Science Advisory Table; 2021. Available: https://covid19-sciencetable.ca/sciencebrief/vaccine-induced-immune-thrombotic-thrombocytopenia-vitt-following-adenovirus-vector-covid-19-vaccination/. Accessed date 16 Mar 2022.
- 23.Gabarin N, Patterson S, Pai M, et al. Venous thromboembolism and mild thrombocytopenia after ChAdOx1 nCoV-1 vaccination. Thromb Haemost. 2021;121(12):1677–1680. doi: 10.1055/a-1585-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(8):720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 26.Greinacher A, Ittermann T, Bagemühl J, et al. Heparin-induced thrombocytopenia: towards standardization of platelet factor 4/heparin antigen tests. J Thromb Haemost. 2010;8(9):2025–2031. doi: 10.1111/j.1538-7836.2010.03974.x. [DOI] [PubMed] [Google Scholar]
- 27.Warkentin TE, Greinacher A. Heparin-induced anaphylactic and anaphylactoid reactions: two distinct but overlapping syndromes. Expert Opin Drug Saf. 2009;8(2):129–144. doi: 10.1517/14740330902778180. [DOI] [PubMed] [Google Scholar]
- 28.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 29.Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398(10306):1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiede A, Sachs UJ, Czalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138(4):350–353. doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim AY, Woo W, Yon DK, et al. Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a systematic review and meta-analysis. Int J Infect Dis 2022 Mar 23;S1201-9712(22)00171-0. doi: 10.1016/j.ijid.2022.03.034. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 32.Warkentin TE. Anticoagulant failure in coagulopathic patients: PTT confounding and other pitfalls. Exp Opin Drug Saf. 2014;13(1):25–43. doi: 10.1517/14740338.2013.823946. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143–149. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 34.Guy S, Kitchen S, Makris M, M Maclean R, Saccullo G, Vanveen JJ. Caution in using the activated partial thromboplastin time to monitor argatroban in COVID-19 and vaccine-induced immune thrombocytopenia and thrombosis (VITT) Clin Appl Thromb Hemost. 2021;27 doi: 10.1177/10760296211066945. 10760296211066945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siguret V, Boissier E, De Maistre E, et al. GFHT proposals on the practical use of argatroban - With specifics regarding vaccine-induced immune thrombotic thrombocytopaenia (VITT) Anaesth Crit Care Pain Med. 2021;40(6) doi: 10.1016/j.accpm.2021.100963. [DOI] [PubMed] [Google Scholar]
- 36.Myllylahti L, Pitkänen H, Magnani H, Lassila R. Experience of danaparoid to treat vaccine-induced immune thrombocytopenia and thrombosis, VITT. Thromb J. 2022;20(1):4. doi: 10.1186/s12959-021-00362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoppensteadt D, Cunanan J, Lewis BE, Fareed J. Effect of dabigatran and rivaroxaban on thrombomodulin mediated activation of protein C and thrombin activated fibrinolysis inhibitor (TAFI)- potential clinical implications. Blood. 2013;122(21):3641. [Abstract] [Google Scholar]
- 38.Furugohri T, Sugiyama N, Morishima Y, Shibano T. Antithrombin-independent thrombin inhibitors, but not direct factor Xa inhibitors, enhance thrombin generation in plasma through inhibition of thrombin-thrombomodulin-protein C system. Thromb Haemost. 2011;106(6):1076–1083. doi: 10.1160/TH11-06-0382. [DOI] [PubMed] [Google Scholar]
- 39.Warkentin TE, Elavathil LJ, Hayward CPM, Johnston MA, Russett JI, Kelton JG. The pathogenesis of venous limb gangrene associated with heparin-induced thrombocytopenia. Ann Intern Med. 1997;127(9):804–812. doi: 10.7326/0003-4819-127-9-199711010-00005. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan AF, Rice L, Bartholomew JR, et al. Warfarin-induced skin necrosis and venous limb gangrene in the setting of heparin-induced thrombocytopenia. Arch Intern Med. 2004;164(1):66–70. doi: 10.1001/archinte.164.1.66. [DOI] [PubMed] [Google Scholar]
- 41.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) 2008;133(6 Suppl.):340S-80S. [DOI] [PubMed]
- 42.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Exp Rev Hematol. 2019;12(8):685–698. doi: 10.1080/17474086.2019.1636645. [DOI] [PubMed] [Google Scholar]
- 43.Padmanabhan A, Jones CG, Pechauer SM, et al. IVIg for treatment of severe refractory heparin-induced thrombocytopenia. Chest. 2017;152(3):478–485. doi: 10.1016/j.chest.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warkentin TE, Climans TH, Morin PA. Intravenous immune globulin to prevent heparin-induced thrombocytopenia. N Engl J Med. 2018;378(19):1845–1848. doi: 10.1056/NEJMc1801799. [DOI] [PubMed] [Google Scholar]
- 45.Arcinas LA, Manji RA, Hrymak C, et al. Autoimmune heparin-induced thrombocytopenia and venous limb gangrene following aortic dissection repair: in vitro and in vivo effects of intravenous immunoglobulin. Transfusion. 2019;59(6):1924–1933. doi: 10.1111/trf.15263. [DOI] [PubMed] [Google Scholar]
- 46.Mohanty E, Nazir S, Sheppard JI, Forman DA, Warkentin TE. High-dose intravenous immunoglobulin to treat spontaneous heparin-induced thrombocytopenia syndrome. J Thromb Haemost. 2019;17(5):841–844. doi: 10.1111/jth.14411. [DOI] [PubMed] [Google Scholar]
- 47.Jones M, Boisvert A, Landry J, Petrasek PF. Limb ischemia and pulmonary artery thrombosis after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine: a case of vaccine-induced immune thrombotic thrombocytopenia. CMAJ. 2021;193(24):E906–E910. doi: 10.1503/cmaj.210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douxfils J, Vayne C, Pouplard C, et al. Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic thrombocytopenia syndrome after initial successful therapy with intravenous immunoglobulins - a rational for monitoring immunoglobulin G levels. Haematologica. 2021 doi: 10.3324/haematol.279509. [published online ahead of print, 2021 Aug 12] 10.3324/haematol.2021.279509. [DOI] [PubMed] [Google Scholar]
- 49.Uzun G, Althaus K, Singh A, et al. The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(11):992–996. doi: 10.1182/blood.2021012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lentz SR. Cooling down VITT with IVIG. Blood. 2021;138(11):921–922. doi: 10.1182/blood.2021012819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irani M, Siegel E, Jella A, Aster R, Padmanabhan A. Use of intravenous immunoglobulin G to treat spontaneous heparin-induced thrombocytopenia. Transfusion. 2019;59(3):931–934. doi: 10.1111/trf.15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patriquin CJ, Laroche V, Selby R, et al. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(9):857–859. doi: 10.1056/NEJMc2109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Major A, Carll T, Chan CW, et al. Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) managed with delayed therapeutic plasma exchange (TPE) J Clin Apher. 2021 doi: 10.1002/jca.21945. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Jones CG, Pechauer SM, Curtis BR, Bougie DW, Aster RH, Padmanabhan A. Normal plasma IgG inhibits HIT antibody-mediated platelet activation: implications for therapeutic plasma exchange. Blood. 2018;131(6):703–706. doi: 10.1182/blood-2017-08-803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handin RI, Stossel TP. Effect of corticosteroid therapy on the phagocytosis of antibodycoated platelets by human leukocytes. Blood. 1978;51(5):771–779. [PubMed] [Google Scholar]
- 56.Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80(10):1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 57.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 58.Chilimuri S, Mantri N, Zahid M, Sun H. COVID-19 vaccine failure in a patient on rituximab therapy. Rheumatol Adv Pract. 2021;5(2) doi: 10.1093/rap/rkab038. rkab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine-induced immune thrombocytopenia and thrombosis (VITT) Res Pract Thromb Haemost. 2021;5(5):e12529. doi: 10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):720–728. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 61.Schönborn L, Thiele T, Kaderali L, Greinacher A. Decline in pathogenic antibodies over time in VITT. N Engl J Med. 2021;385(19):1815–1816. doi: 10.1056/NEJMc2112760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Günther A, Brämer D, Pletz MW, et al. Complicated long term vaccine induced thrombotic immune thrombocytopenia-a case report. Vaccines (Basel) 2021;9(11):1344. doi: 10.3390/vaccines9111344. [DOI] [PMC free article] [PubMed] [Google Scholar]