Abstract
Background
COVID-19 vaccination is considered as an effective intervention for controlling the burden of the pandemic. However, vaccine hesitation is increasing and hindering efforts targeting to reduce the burden of the COVID-19 disease. Hence, determining COVID-19 vaccine acceptance and identifying determinants that would hinder people to vaccinate against COVID-19 is crucial to effectively improve COVID-19 vaccine uptake. In Ethiopia, the pooled proportion of COVID-19 vaccine acceptance and its determinants is not well known. Thus, the aim of this study is to estimate the pooled proportion of COVID-19 vaccine acceptance and its determinants in Ethiopia.
Methods
A systematic search of articles was conducted from PubMed, Scopus, Web of Science, MEDLINE, CINAHL, Science Direct and Cochrane Library. Data were extracted using a data extraction tool which was adapted from the Joanna Briggs Institute. The quality of each included primary studies was evaluated using the Newcastle-Ottawa scale tool. Data analysis was performed using STATA 14. Heterogeneity in studies was assessed using Cochrane Q and I2 test. Publication bias was assessed using visual inspection of funnel plots and Egger's test. A random effects model was applied to determine the pooled estimates if heterogeneity was exhibited; otherwise, a fixed-effects model was used.
Results
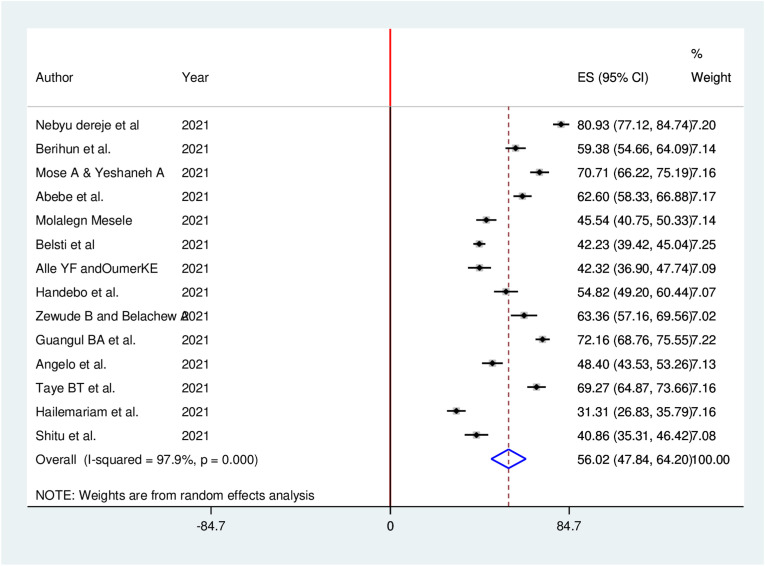
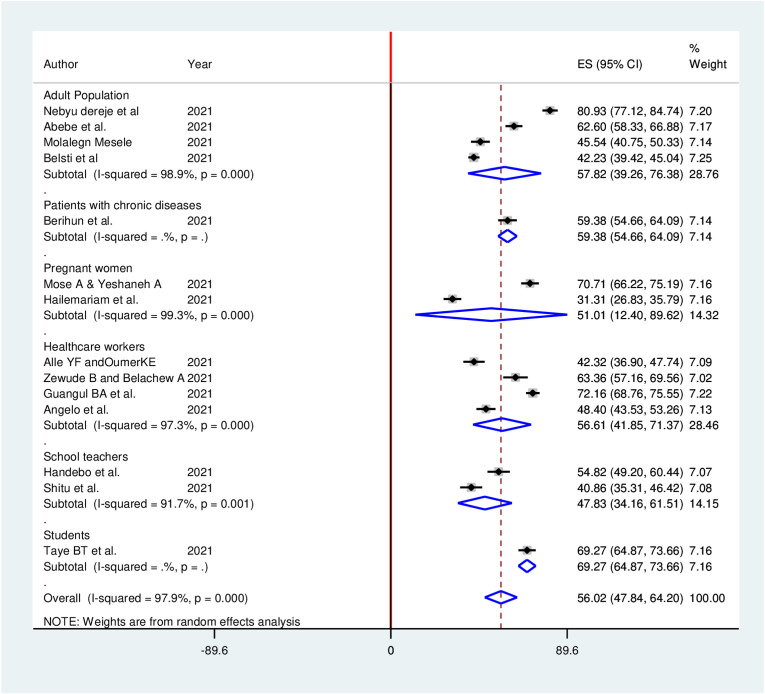
A total of 14 studies involving 6373 participants were included for the final analysis. The pooled proportion of COVID-19 vaccine acceptance in Ethiopia was 56.02% (95% CI: 47.84, 64.20). The likelihood of COVID-19 vaccine acceptance was higher among participants who had history of chronic disease (AOR = 1.33, 95% CI: 1.09, 2.97), good knowledge (AOR = 2.13, 95% CI: 1.59, 4.97), positive attitude (AOR = 2.23, 95% CI: 1.21, 4.66), good COVID-19 preventive practice (AOR = 1.97, 95% CI: 1.82, 2.12), and high perceived seriousness of COVID-19 (AOR = 3.21, 95% CI: 2.32, 5.98).
Conclusion
More than half participants were willing to accept COVID-19 vaccine. Thus, awareness creation battles about the efficacy and safety of the COVID-19 vaccine should be provided to the community. Besides, policy-makers, health planners and other stakeholders should encourage COVID-19 vaccine uptake behaviors by providing trusted information.
Systematic review and meta-analysis registration: PROSPERO CRD42021264708.
Keywords: Acceptance, COVID-19, Risk factors, Vaccine, Ethiopia
1. Background
The World Health Organization (WHO) began a global campaign of prevention, early diagnosis, and medical treatment of the coronavirus disease 2019 (COVID-19) following the outbreak that was declared as a pandemic in early 2020.1 Subsequently, multiple COVID-19 vaccines were developed and tested across diverse populations in different clinical trials.2 , 3
Vaccination is considered as effective intervention for controlling pandemics and most preventable infectious diseases.4 The vaccination utilization level must be high in order to be successful.5 Additionally, distribution and equitable access of safe and effective vaccine strongly desires methods to improve vaccine acceptance and sufficient health system capacity.6
In recent times, several literatures have reported the effectiveness and safety of COVID-19 vaccines.2 , 6 , 7 The efficacy of the mRNA-1273 vaccine was reported to be 94.1%,8 the BNT162b2 mRNA vaccine has been documented to be 95%,9 that of the ChAdOx1 nCoV-19 vaccine has been reported to be 70.4%,10 and the Gam-COVID-Vac has been documented to be 91.6%.11
Public perceptions, misconceptions and rumors on the vaccines may result in vaccine hesitancy.12 , 13 Over again, vaccine hesitancy would result decreasing vaccine coverage, which further could lead to disease outbreaks including COVID-19.14 Hence, understanding vaccine acceptance is crucial as high vaccine hesitancy for existing vaccines could lead to relatively low vaccination coverage.15
Despite the efforts to control COVID-19 through vaccination, vaccine hesitation is increasing and hindering efforts targeting to reduce the burden of the disease.16 Globally, vaccine hesitancy is considered as a major public health issue because of its substantial increase.17 Wrong impression due to accelerated development the vaccine, misinformation, and multiple myths would potentially affect the willingness of population to accept COVID-19 vaccine.18 , 19
COVID-19 vaccination acceptance have been investigated and reported in previous studies.20, 21, 22, 23 A systematic review and meta-analysis has been reported that the pooled proportion of willingness to COVID-19 vaccination among the general population was 81.65%.20 Another systematic review and meta-analysis conducted has revealed that the pooled proportion of COVID-19 vaccination willingness among healthcare workers was 51%.21 However, these studies have not been included findings from Ethiopia.
Similar to all other parts of the world, many African countries have been implemented COVID-19 vaccines trials to contribute to the global search for vaccines against the COVID-19 pandemic.24 Africa need about 1.5 billion doses of COVID-19 vaccine to vaccinate 60% of its population.25 Most African countries including Ethiopia need COVID-19 vaccines from different donating countries to achieve the estimated target.26
Though Ethiopia is gaining vaccines from different donating countries, the proportion of COVID-19 vaccine acceptance in the general population is not well known. Except for fragmented studies with varying reports, there are no national prevalence studies conducted on COVID-19 vaccine acceptance in Ethiopia. Moreover, investigating determinants that would hinder people to vaccinate against COVID-19 is not well investigated. It is crucial to warrant the population to have access to reliable and sufficient information regarding this vaccine to raise its acceptance rate.2 Hence, by determining COVID-19 vaccine acceptance and identifying associated factors, governments policy-makers, and health authorities, and other stakeholders can design specific vaccine campaigns and the development of evidence-based guidelines to effectively improve COVID-19 vaccine uptake. Thus, the aim of this systematic review and meta-analysis is to estimate the pooled proportion of COVID-19 vaccine acceptance and identify factors associated with COVID- 19 vaccine acceptance.
2. Methods
The methods adopted for this systematic review and meta-analysis are consistent with the guidelines detailed on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.27 The systematic review was prospectively registered on International Prospective Register of Systematic Reviews (PROSPERO) with unique of number CRD42021264708.
3. Eligibility criteria
The inclusion criteria for this study were the following1: Studies that investigated COVID-19 vaccine acceptance and/or its determinants in Ethiopia,2 observational studies which contain relevant outcomes were included,3 studies that had been performed on healthcare workers,4 studies that had been conducted and reported in English language, and5 articles that had a full-text article publicly available were included. The exclusion criteria of the study were the following1: studies that were qualitative studies, reviews, case reports and letters to editors, and3 studies did not have full-text available publicly.
4. Information sources and search strategy
A comprehensive search and article retrieval strategy were performed to find potentially relevant articles in the following databases: PubMed, Scopus, Web of Science, MEDLINE, CINAHL, Science Direct and Cochrane Library. In addition, the reference lists of all articles that were considered and searched. Google Scholar, input of content experts and Institutional Digital Library were also searched for gray literature and unpublished papers. The full electronic search strategy was searched using the following search terms: ‘COVID-19’, ‘SARS-CoV-2’, ‘Coronavirus’, ‘vaccine’, ‘vaccination’, ‘immunization’, ‘acceptance’, ‘willingness’, ‘intention’, ‘accept’, ‘factors’, ‘determinates’, ‘reasons’, ‘predictors’ and ‘Ethiopia’. A combination of appropriate Boolean operators (AND, OR), truncation, and the MeSH terms were used. The searching of articles was conducted from 20th of August to September 10th, 2021.
5. Study selection
EndNote X7 (Thomson Reuters, New York, USA) software was used to manage identified and retrieved studies. After removing the duplicated articles from EndNote Library, the titles and abstracts of the remaining articles were assessed independently by two reviewers (BDM and BAM). The full texts of articles were reviewed to confirm eligibility criteria match. Disagreements between the two reviewers were resolved by discussion. The PRISMA flow diagram was used to summarize the study selection processes.
6. Data extraction and main data items
All required data from included articles were extracted using a standardized, pre-piloted data extraction format. Two reviewers (BDM and BAM) independently extracted the data using the Joanna Briggs Institute (JBI) data extraction form.28 Disagreement during data extraction was resolved by discussion and consensus. Relevant information was collected for each study, including: the first authors' name, region, study year, publication year, study design, study setting, participants, sample size, data collection methods, sampling method, response rate, outcome measures (COVID-19 vaccine acceptance), determinants of vaccine acceptance.
7. Quality assessment
The methodologic quality and risk of bias of each studies were assessed using the Newcastle-Ottawa scale (NOS) tool. Two authors (BDM and BAM independently evaluated the risk of bias and any disparity was resolved through discussion and reviewing the articles together. The quality assessment tool was adapted from a nonrandomized study which developed for the quality assessments of meta-analytic results.29 The quality appraisal tool has three main themes with a maximum of 10 stars: The first them of the tool focuses on selection (representativeness of the sample, sample size, response rate, sampling technique, ascertainment of the exposure of COVID-19 vaccine) with a maximum of 4 stars; the second them of the tool focuses on comparability (in the context of participant distribution and analyses) with a maximum of 2 stars; and the last them of the tool concerned with outcome (assessment of the outcome; and statistical tests assessment) with a maximum of 3 stars.
8. Measurement of outcomes
COVID-19 vaccine acceptance was the main outcomes of this study, which is defined as the willingness to take the COVID-19 vaccine. In the primary studies, participants were asked their willingness to COVID-19 vaccination. Accordingly, participants who respond “yes” were considered as having the willingness to accept the COVID-19 vaccine, whereas participants who respond “No” were considered as having no willingness to accept the COVID-19 vaccine. In this study, identify factors associated with COVID-19 vaccine acceptance was the second outcome of interest, which were measured in the form of the odds ratio (OR). The odds ratio for each identified factor was calculated based on the binary outcome data reported by each original study.
9. Data processing and analysis
The individual primary studies were described succinctly using a summary table. Findings were presented using forest plot graphical representation with 95% confidence interval (CI). We used STATA version 14 statistical software to conduct this meta-analysis. A random-effects model was used to pool the estimate of COVID-19 vaccine acceptance if substantial heterogeneity was exhibited between studies; otherwise, a fixed-effects model was used. The Cochran's Q test and I2 were used to detect heterogeneity between the studies. The variation in the pooled estimates of the proportion of COVID-19 vaccine acceptance was adjusted through subgroup analysis. As a result, subgroup analysis was done based on study region, study population, and data collection methods to reduce the random variations among the point estimates of the primary study. In addition, evidence of publication bias was checked using visual inspection of funnel plots asymmetry, and weighted Egger's regression test with p - value of less than 0.05 as a cutoff point to declare the presence of publication bias. The results indicated that evidence of publication bias was not observed. Furthermore, a sensitivity analysis was conducted to check the stability of the summary estimate.
10. Results
10.1. Study selection
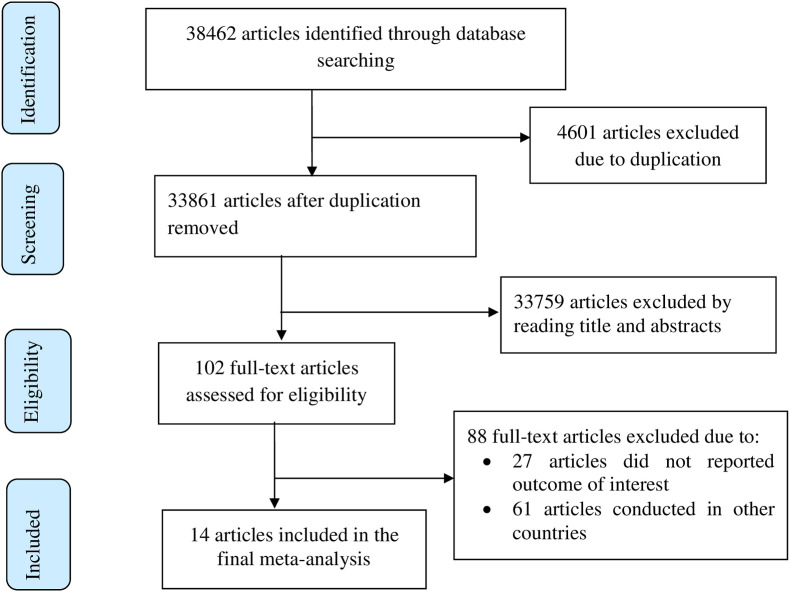
Overall, 38462 potentially relevant articles were retrieved from which 33861 unique studies. Two reviewers (BDM and BAM) independently assessed articles based on their titles and abstracts which result in the exclusion of 33759 articles. One hundred two full-text articles were assessed for eligibility based on the inclusion and exclusion criteria, which result further exclusion of 88 articles. Finally, 14 articles were included in the final analysis (Fig. 1 ).
Fig. 1.
Flow chart of study selection for systematic review and meta-analysis of COVID-19 vaccine acceptance in Ethiopia, 2021.
10.2. Study characteristics
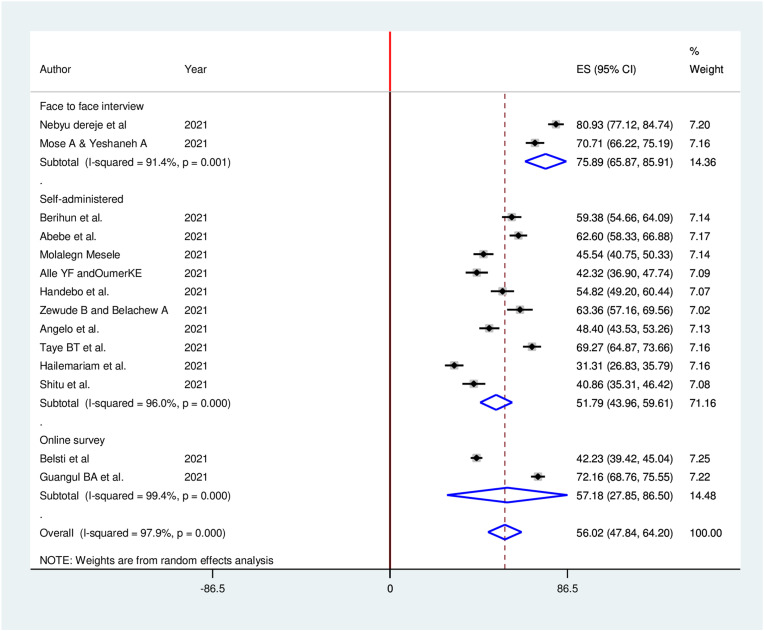
All of the articles included in this meta-analysis were cross-sectional designs. Of fourteen studies included, 5 were from SNNPR region,30, 31, 32, 33, 34 5 were from Amhara region,35, 36, 37, 38, 39 3 were nationwide studies40, 41, 42 and 1 was from Addis Ababa city administration.43 All articles were published in 2021. In this study, a total of 6373 participants were included from an estimated 7064 sample size, yielding 90.2% response rate. The proportion of COVID-19 vaccine acceptance reported in the selected studies varied from 31.3% to 80.9%. As for the evaluation of COVID-19 vaccine acceptance, ten of the included studies utilized self-designed questionnaires, two studies used face to face interview, and the other two used online survey. More than half8 of studies had high quality, and the remaining 6 studies had medium quality (Table 1 ).
Table 1.
Characteristics of studies included in the systematic review and meta-analysis of COVID-19 vaccine acceptance in Ethiopia, 2021.
| Author | Year | Region | Study design | Study area | Study population | Data collection method | Sample size | Participants | Vaccine acceptance (%) | Quality of studies |
|---|---|---|---|---|---|---|---|---|---|---|
| Nebyu D et al.43 | 2021 | Addis Ababa | Cross sectional | Akaki kality | Adult Population | Face to face interview | 422 | 409 | 80.9 | High |
| Berihun et al.35 | 2021 | Amhara | Cross sectional | Dessie | Patients with chronic diseases | Self-administered | 422 | 416 | 59.4 | High |
| Mose A and Yeshaneh A33 | 2021 | SNNPR | Cross sectional | Gurage zone | Pregnant women | Face to face interview | 396 | 396 | 70.7 | Medium |
| Abebe et al.30 | 2021 | SNNPR | Cross sectional | Gurage zone | Adult Population | Self-administered | 501 | 492 | 62.6 | High |
| Molalegn Mesele31 | 2021 | SNNPR | Cross sectional | Sodo town | Adult Population | Self-administered | 424 | 415 | 45.5 | High |
| Belsti et al.40 | 2021 | Ethiopia | Cross sectional | Ethiopia | Adult Population | Online survey | 1184 | 1184 | 42.2 | High |
| Alle YF and Oumer36 | 2021 | Amhara | Cross sectional | Debre tabor | Healthcare workers | Self-administered | 327 | 319 | 42.3 | Medium |
| Handebo et al.39 | 2021 | Amhara | Cross sectional | Gondar | School teachers | Self-administered | 323 | 301 | 54.8 | Medium |
| Zewude B, Belachew A41 | 2021 | Ethiopia | Cross sectional | Ethiopia | Healthcare workers | Self-administered | 384 | 232 | 63.4 | Medium |
| Guangul BA et al.42 | 2021 | Ethiopia | Cross sectional | Ethiopia | Healthcare workers | Online survey | 1110 | 668 | 72.2 | Medium |
| Angelo et al.32 | 2021 | SNNPR | Cross sectional | Mizan tepi | Healthcare workers | Self-administered | 423 | 405 | 48.4 | High |
| Taye BT et al.37 | 2021 | Amhara | Cross sectional | Debre Berhan | Students | Self-administered | 423 | 423 | 69.3 | High |
| Hailemariam et al.34 | 2021 | SNNPR | Cross sectional | Bench-sheko zone | Pregnant women | Self-administered | 423 | 412 | 31.3 | High |
| Shitu et al.38 | 2021 | Amhara | Cross sectional | Gondar | School teachers | Self-administered | 302 | 301 | 40.9 | Medium |
10.3. Publication bias and sensitivity analysis
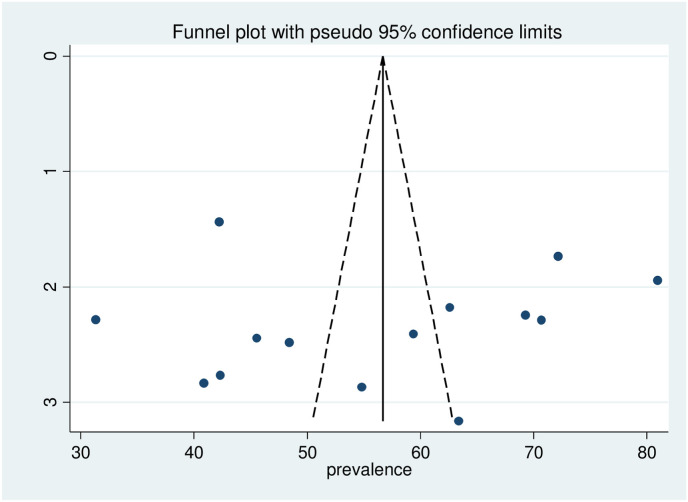
In this systematic review and meta-analysis, sensitivity analysis was conducted using the random-effects model for the estimates of COVID-19 vaccine acceptance. The results of the sensitivity analysis suggested that there is no influential study as none of the points estimate outside of the overall 95% confidence interval. Visual inspection of the funnel plot indicated that there is symmetrical distribution of studies included in the review, and Egger's test was not statistically significant (P = 0.821) suggesting the absence of publication bias for COVID-19 vaccine acceptance (Fig. 2 ).
Fig. 2.
Funnel plot assessed for publication bias in the studies conducted on of COVID-19 vaccine acceptance in Ethiopia, 2021.
10.4. Meta-analysis of COVID-19 vaccine acceptance
Fourteen studies reported the acceptance of COVID-19 vaccine in a total of 6373 study participants. The pooled proportion of COVID-19 vaccine acceptance in Ethiopia was found to be 56.02% (95% CI: 47.84, 64.20). In this meta-analysis, a random effects model was executed as high heterogeneity (I2 = 97.9%, P ≤ 0.001) was detected within the included studies (Fig. 3 ).
Fig. 3.
Forest plot of the pooled proportion of COVID-19 vaccine acceptance in Ethiopia, 2021.
10.5. Subgroup analysis of COVID-19 vaccination acceptance
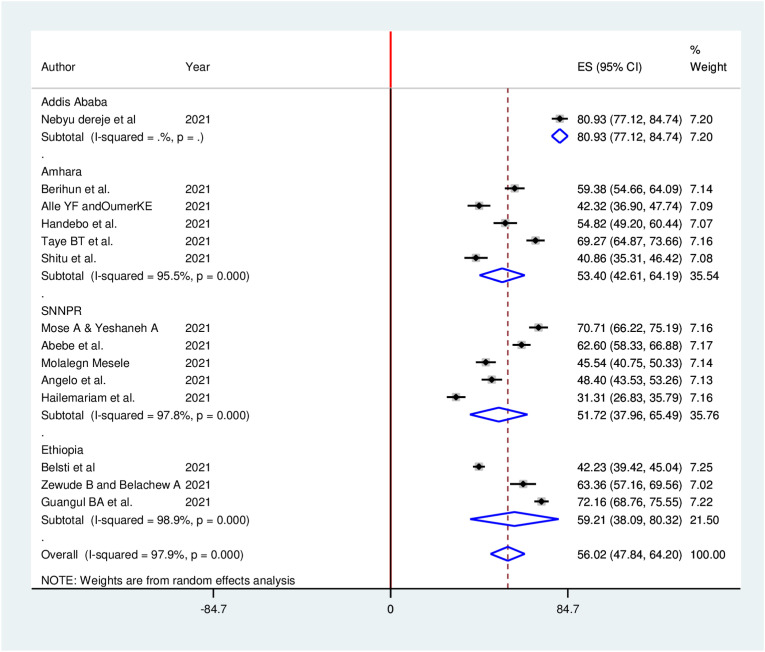
Subgroup analyses for the meta-analysis was implemented to clarify the source of heterogeneity Because of severe heterogeneity observed among selected studies. The subgroup analysis was implemented based on study region, study population, and data collection methods. However, no considerable difference between subgroup heterogeneity and overall heterogeneity exhibited in subgroup analysis, indicating that the heterogeneity was not associated with study region, study population, and data collection methods (Fig. 4 , Fig. 5 , Fig. 6 ).
Fig. 4.
Sub-group analysis (by region) of the pooled proportion of COVID-19 vaccine acceptance in Ethiopia, 2021.
Fig. 5.
Sub-group analysis (by study population) of the pooled proportion of COVID-19 vaccine acceptance in Ethiopia, 2021.
Fig. 6.
Sub-group analysis (by data collection method) of the pooled proportion of COVID-19 vaccine acceptance in Ethiopia, 2021.
10.6. Meta-analysis of factors associated with COVID-19 vaccine acceptance
In this meta-analysis, variables common to all participants were pooled quantitatively to examine if they are significantly associated with COVID-19 vaccine acceptance. Nevertheless, some factors were not pooled because of inconsistent categorization in the primary studies. Those determinants reported in more than one original studies were included in this meta-analysis. Accordingly, having good knowledge of the COVID-19 vaccine, having a positive attitude towards COVID-19 vaccine, good preventive practices, history of chronic illness, and having high perceived seriousness of COVID-19 were significant factors associated with COVID-19 vaccine acceptance.
Study participants with a history of chronic disease were 1.33 times (AOR = 1.33, 95% CI: 1.09, 2.97) more likely to accept the COVID-19 vaccine than study participants without a history of chronic disease. Study participants who had good knowledge about the COVID-19 vaccine were 2.13 times (AOR = 2.13, 95% CI: 1.59, 4.97) more likely to accept the COVID- 19 vaccine as compared with study participants with poor knowledge. Study participants who had positive attitude towards the COVID-19 vaccine were 2.23 times (AOR = 2.23, 95% CI: 1.21, 4.66) more likely to accept the COVID- 19 vaccine than those study participants with negative attitude. The odds of COVID- 19 vaccine acceptance was 3.21 times (AOR = 3.21, 95% CI: 2.32, 5.98) among study participants who had high perceived seriousness of COVID-19 than those study participants who had low perceived seriousness of COVID-19. The likelihood of COVID- 19 vaccine acceptance was 1.97 times (AOR = 1.97, 95% CI: 1.82, 2.12) among study participants who had good preventive practice of COVID-19 than those study participants who had poor preventive practice of COVID-19 (Table 2 ).
Table 2.
Factors associated with COVID-19 vaccine acceptance in Ethiopia: a systematic review and meta-analysis, 2021.
| Variables | Included studies | OR (95%CI) | Pooled OR (95%CI) | Heterogeneity (I2, p-value) |
|---|---|---|---|---|
| History Chronic disease | Abebe et al. Angelo et al. |
1.40 (.07, 2.11) 1.15 (1.06, 2.10) |
1.33 (1.09, 2.97) | 0.0%, 0.667 |
| Good knowledge | Berihun et al. Mose A & Yeshaneh A Abebe et al. Taye BT et al. Hailemariam et al. |
1.93 (1.36, 2.45) 1.78 (1.38, 2.19) 2.41 (1.47, 3.87) 2.15 (1.32, 3.14) 1.52 (1.12, 3.14) |
2.13 (1.59, 4.97) | 75.6%, 0.003 |
| Positive attitude |
Berihun et al. Angelo et al. |
2.06 (1.19, 3.83) 2.64 (1.77, 5.37) |
2.23 (1.21, 4.66) | 0.0%, 0.088 |
| High perceived severity | Hailemariam et al. Shitu et al. |
3.52 (2.41, 5.78) 2.42 (1.96, 5.88) |
3.21 (2.32, 5.98) | 0.0%, 0.397 |
| Goo preventive practice | Mose A & Yeshaneh A Angelo et al. Taye BT et al. |
2.21 (2.05, 2.38) 1.12 (1.06, 2.03) 1.12 (1.04, 2.86) |
1.97 (1.82, 2.12) | 96.1%, 0.000 |
11. Discussion
The findings of this study indicated that more than half of the participants were willing to accept COVID-19 vaccine. This observed low COVID-19 vaccine acceptance could attribute to public exposure to misconception and concerns over the safety of the COVID-19 vaccine. Evidence suggests that public exposure to misinformation and concerns over the safety of COVID-19 vaccines could be contributing to low intentions to be vaccinated.44, 45, 46 It is believed that the wide uptake of COVID-19 vaccine could contribute to the development of herd immunity and guard the public against COVID-19.47 Hence, this finding implies the need for better enactment to address public trust, acceptability, benefit and concern over the safety of the approved COVID-19 vaccine.
Results of this systematic review and meta-analysis indicated that the pooled proportion of COVID-19 vaccine acceptance in Ethiopia was found to be 56.02%. The result of this study was in line with different systematic review and meta-analysis which reported pooled proportion of COVID-19 vaccine acceptance as 60.1%48 and 60%.23 However, the finding was lower than a systematic review and meta-analysis that reported the pooled proportion of willingness to COVID-19 vaccination among the general population to be 81.65%.20 This could attribute to variations in the spread and burden of the COVID-19 pandemic across the countries. The discrepancy could be also due differences in the respondents' local norms and cultures, and exposure to credible social media disseminating factual information regarding COVID-19 vaccine. Moreover, the variation could be explained by differences in awareness on the severity of COVID-19, access to health care service.
Results of the subgroup analysis indicated that the pooled proportion of COVID-19 vaccine acceptance among healthcare workers in Ethiopia was 56.61%. The result of this study was in line with a systematic review and meta-analysis which reported pooled proportion of COVID-19 vaccine acceptance as 51%.21 This implies that a considerable proportion of healthcare workers were hesitant towards COVID-19 vaccine, which hinder their recommendation of vaccination to their patients. Evidence indicates that the attitude of the healthcare workers toward COVID-19 vaccine were found to influence their intention to suggest the vaccine to their patients and general population.49 Hence, future prioritized education needs to involve healthcare workers to influence their own use of the vaccine and to be accepted by the population.
This study identified that having history of chronic disease had significantly associated with COVID vaccine acceptance. Participants with a history of chronic disease were more likely to accept the COVID-19 vaccine than study participants without a history of chronic disease. This result is supported by a report from WHO,50 and a study conducted in Australia.51 This finding highlights the need to create platform that could enable individuals with history of chronic disease to be vaccinated against COVID-19 vaccine.
Having good knowledge about the COVID-19 vaccine had significantly associated with willingness to accept the COVID vaccine. Participants who had good knowledge about the COVID-19 vaccine were more likely to accept the COVID- 19 vaccine as compared with study participants with poor knowledge. This finding implies that improving knowledge regarding the benefit, effectiveness and safety of the COVID-19 vaccine could be one of the strategies for achieving targeted vaccination coverage in the general population. This finding is also explained by having good knowledge considered as an engine to take actions regarding a certain behaviour, as it helps individuals to understand the seriousness of the disease, and to know the benefit of the vaccination program. This result is supported by survey carried out in England52 and Southeast Asia.53
Positive attitude towards the COVID-19 vaccine was another significant factor associated with COVID-19 vaccine acceptance. Study participants who had positive attitude towards the COVID-19 vaccine were more likely to accept the COVID- 19 vaccine than those study participants with negative attitude. This implies that the role of positive attitude towards COVID- 19 vaccination is crucial. This study also identified that participants with high perceived seriousness of COVID-19 infection were more likely to accept the COVID- 19 vaccination than those with low perceived seriousness of COVID-19 infection. This finding is in line with studies reported from Indonesia54 China.55 This could be due to the fact that people would take measures to avoid any risk if they think they are vulnerable to it. Evidence indicated that individuals' readiness of taking the recommended action would be influence by their level of perception to the positive consequences that are caused by taking an action.56
Furthermore, having good preventive practice towards COVID-19 disease was significantly associated with COVID-19 vaccine acceptance. The likelihood of COVID- 19 vaccine acceptance was higher among study participants who had good preventive practice of COVID-19 than those study participants who had poor preventive practice of COVID-19. This finding is in line with studies reported from the Democratic Republic of the Congo57 and Middle Eastern Population.58 This could be due to individuals who had good practice knowing the burden of COVID-19 infection on the health of the general population.
Lastly, the study has the following limitations. First, generalizability of the finding may not be established as the representability of the sample size was uncertain in most studies, and uncertainty of Internet access to complete an online survey. Hence, subgroup analysis was performed according to the sampling method to have acceptable validity. Second, some determinants were not examined due to inconsistent categorization, and limitations of the data in the primary studies. Besides, temporal relationships between outcome variable and predictors couldn't be established as all included studies were cross sectional. Finally, the study may not be representative of the national reaction to the COVID-19 vaccine as it didn't include studies from all regions of the country.
12. Conclusions
This study showed that more than half participants were willing to the accept COVID-19 vaccine. However, substantial number of people were not to be vaccinated against COVID-19, which indicated that vaccine acceptability still needs more attention. The likelihood of COVID-19 vaccine acceptance was higher among participants who had history of chronic disease, good knowledge, positive attitude, good COVID-19 preventive practice, and with high perceived seriousness of COVID-19. Thus, awareness creation battles about the efficacy and safety of the COVID- 19 vaccine should be provided to the community with collaboration effort of all COVID- 19 vaccine taskforce established by the Ethiopia's Ministry of Health, regional and zonal health offices, and public health institute. Besides, policy-makers, health planners and other stakeholders should encourage COVID-19 vaccine uptake behaviors by providing trusted information about the COVID-19 vaccine in all regions of Ethiopia. Moreover, better public health messaging and health education targeting increasing knowledge, and changing attitude towards COVID-19 vaccine should be disseminated to the general population.
Funding
No funding has been received for this systematic review and meta-analysis.
Availability of data and materials
All relevant data generated and analyzed in the analysis process is included in this article.
Ethics approval and consent to participate
Not applicable, since this is systematic review and meta-analysis.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no competing interest.
Acknowledgements
The authors would like to thank the authors of the included primary studies, which used as source of information to conduct this systematic review and meta-analysis.
References
- 1.Sage W. 2020. WHO SAGE Values Framework for the Allocation and Prioritization of COVID-19 Vaccination. [Google Scholar]
- 2.Dror A.A., Eisenbach N., Taiber S., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrotri M., Swinnen T., Kampmann B., Parker E.P. An interactive website tracking COVID-19 vaccine development. Lancet Global Health. 2021;9(5):e590–e592. doi: 10.1016/S2214-109X(21)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajj Hussein I., Chams N., Chams S., et al. Vaccines through centuries: major cornerstones of global health. Front Public Health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betsch C., Böhm R., Korn L., Holtmann C. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1–6. [Google Scholar]
- 6.Lazarus J.V., Ratzan S.C., Palayew A., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg M.J. Public misunderstanding of science? Reframing the problem of vaccine hesitancy. Perspect Sci. 2016;24(5):552–581. [Google Scholar]
- 13.Troiano G., Nardi A. Public Health; 2021. Vaccine Hesitancy in the Era of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubé E., Gagnon D., Nickels E., Jeram S., Schuster M. Mapping vaccine hesitancy—country-specific characteristics of a global phenomenon. Vaccine. 2014;32(49):6649–6654. doi: 10.1016/j.vaccine.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afolabi A.A., Ilesanmi O.S. Dealing with vaccine hesitancy in Africa: the prospective COVID-19 vaccine context. Pan Afr Med J. 2021;38 doi: 10.11604/pamj.2021.38.3.27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crescitelli M.D., Ghirotto L., Sisson H., et al. A meta-synthesis study of the key elements involved in childhood vaccine hesitancy. Publ Health. 2020;180:38–45. doi: 10.1016/j.puhe.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Paul E., Steptoe A., Fancourt D. The Lancet Regional Health—Europe Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg Health Eur. 2021;1 doi: 10.1016/j.lanepe.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullah I., Khan K.S., Tahir M.J., Ahmed A., Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas. 2021;22(2):93–97. doi: 10.1016/j.vacun.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Yang L., Jin H., Lin L. Vaccination against COVID-19: a systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021:106694. doi: 10.1016/j.ypmed.2021.106694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo C., Yang Y., Liu Y., et al. Intention to COVID-19 vaccination and associated factors among health care workers: a systematic review and meta-analysis of cross-sectional studies. Am J Infect Control. 2021 doi: 10.1016/j.ajic.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdulmoneim S.A., Aboelsaad I.A.F., Hafez D.M.H., et al. medRxiv; 2021. Systematic Review and Meta-Analysis on COVID-19 Vaccine Hesitancy. [Google Scholar]
- 23.Robinson E., Jones A., Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. medRxiv. 2020 doi: 10.1016/j.vaccine.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makoni M. COVID-19 vaccine trials in Africa. Lancet Respir Med. 2020;8(11):e79–e80. doi: 10.1016/S2213-2600(20)30401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gostin L.O., Karim S.A., Mason Meier B. Facilitating access to a COVID-19 vaccine through global health law. J Law Med Ethics. 2020;48(3):622–626. doi: 10.1177/1073110520958892. [DOI] [PubMed] [Google Scholar]
- 26.Nkengasong J.N., Ndembi N., Tshangela A., Raji T. Nature Publishing Group; 2020. COVID-19 Vaccines: How to Ensure Africa Has Access. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D. The preferred reporting Items for systematic reviews and meta-analyses group.(2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Munn Z., Tufanaru C., Aromataris E. JBI's systematic reviews: data extraction and synthesis. AJN Am J Nurs. 2014;114(7):49–54. doi: 10.1097/01.NAJ.0000451683.66447.89. [DOI] [PubMed] [Google Scholar]
- 29.Wells G., Shea B., O'Connell D., Peterson J., Welch V. 2012. Losos Met Al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [Google Scholar]
- 30.Abebe H., Shitu S., Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. 2021;14:2015. doi: 10.2147/IDR.S312116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesele M. COVID-19 vaccination acceptance and its associated factors in Sodo Town, Wolaita Zone, Southern Ethiopia: cross-sectional study. Infect Drug Resist. 2021;14:2361. doi: 10.2147/IDR.S320771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelo A.T., Alemayehu D.S., Dachew A.M. Health care workers intention to accept COVID-19 vaccine and associated factors in southwestern Ethiopia, 2021. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mose A., Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in southwest Ethiopia: Institutional-based cross-sectional study. Int J Gen Med. 2021;14:2385. doi: 10.2147/IJGM.S314346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hailemariam S., Mekonnen B., Shifera N., et al. Predictors of pregnant women's intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE Med. 2021;9 doi: 10.1177/20503121211038454. 20503121211038454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berihun G., Walle Z., Berhanu L., Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting dessie comprehensive specialized hospital, Northeastern Ethiopia. Patient Prefer Adherence. 2021;15:1795. doi: 10.2147/PPA.S324564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alle Y.F., Oumer K.E. Attitude and associated factors of COVID-19 vaccine acceptance among health professionals in Debre Tabor Comprehensive Specialized Hospital, North Central Ethiopia; 2021: cross-sectional study. Virusdisease. 2021;32(2):272–278. doi: 10.1007/s13337-021-00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taye B.T., Amogne F.K., Demisse T.L., et al. Coronavirus disease 2019 vaccine acceptance and perceived barriers among university students in northeast Ethiopia: a cross-sectional study. Clin Epidemiol Glob Health. 2021;12:100848. doi: 10.1016/j.cegh.2021.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shitu K., Wolde M., Handebo S., Kassie A. Acceptance and willingness to pay for COVID-19 vaccine among school teachers in Gondar City, Northwest Ethiopia. Trop Med Health. 2021;49(1):1–12. doi: 10.1186/s41182-021-00337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handebo S., Wolde M., Shitu K., Kassie A. Determinant of intention to receive COVID-19 vaccine among school teachers in Gondar City, Northwest Ethiopia. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belsti Y., Gela Y.Y., Akalu Y., et al. Willingness of Ethiopian population to receive COVID-19 vaccine. J Multidiscip Healthc. 2021;14:1233. doi: 10.2147/JMDH.S312637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zewude B., Belachew A. Intention to receive the second round of COVID-19 vaccine among healthcare workers in Eastern Ethiopia. Infect Drug Resist. 2021;14:3071. doi: 10.2147/IDR.S326055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guangul B.A., Georgescu G., Osman M., et al. Healthcare workers attitude towards SARS-COVID-2 vaccine, Ethiopia. Glob J Infect Dis Clin Research. 2021;7(1) 043-048. [Google Scholar]
- 43.Dereje N., Tesfaye A., Tamene B., et al. medRxiv; 2021. COVID-19 Vaccine Hesitancy in Addis Ababa, Ethiopia: A Mixed-Methods Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daly M., Robinson E. medRxiv; 2020. Willingness to Vaccinate against COVID-19 in the US: Longitudinal Evidence from a Nationally Representative Sample of Adults from April-October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul E, Steptoe A, Fancourt D. Anti-vaccine Attitudes and Risk Factors for Not Agreeing to Vaccination against COVID-19 Amongst 32,361 UK Adults: Implications for Public Health Communications. Available at SSRN 3716874. 2020.
- 46.Roozenbeek J., Schneider C.R., Dryhurst S., et al. Susceptibility to misinformation about COVID-19 around the world. R Soc Open Sci. 2020;7(10):201199. doi: 10.1098/rsos.201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dodd R., Cvejic E., Bonner C., Pickles K., McCaffery K. Sydney health literacy lab COVID-19 group. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30559-4. 30559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nehal K.R., Steendam L.M., Campos Ponce M., van der Hoeven M., Smit G.S.A. Worldwide vaccination willingness for COVID-19: a systematic review and meta-analysis. Vaccines. 2021;9(10):1071. doi: 10.3390/vaccines9101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iguacel I., Maldonado A.L., Ruiz-Cabello A.L., et al. Attitudes of healthcare professionals and general population toward vaccines and the intention to Be vaccinated against COVID-19 in Spain. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.739003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.BIO S . 2021. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. [Google Scholar]
- 51.Rhodes A., Hoq M., Measey M.-A., Danchin M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2021;21(5):e110. doi: 10.1016/S1473-3099(20)30724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherman S.M., Smith L.E., Sim J., et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccines Immunother. 2021;17(6):1612–1621. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2021;49(1):21–29. doi: 10.1016/j.ajic.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harapan H., Wagner A.L., Yufika A., et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum Vaccines Immunother. 2020;16(12):3074–3080. doi: 10.1080/21645515.2020.1819741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Jing R., Lai X., et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8(3):482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gellman M.D., Turner J.R. Springer; New York, NY, USA: 2013. Encyclopedia of Behavioral Medicine. [Google Scholar]
- 57.Nzaji M.K., Ngombe L.K., Mwamba G.N., et al. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. Pragmatic Observational Res. 2020;11:103. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Qerem W.A., Jarab A.S. COVID-19 vaccination acceptance and its associated factors among a Middle Eastern population. Front Public Health. 2021;9:34. doi: 10.3389/fpubh.2021.632914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data generated and analyzed in the analysis process is included in this article.