Abstract
Viruses are important causes of nosocomial infection, but the fact that hospital outbreaks often result from introduction(s) from community-based epidemics, together with the need to initiate specific laboratory testing, means that there are usually insufficient data to allow the monitoring of trends in incidences. The most important defenses against nosocomial transmission of viruses are detailed and continuing education of staff and strict adherence to infection control policies. Protocols must be available to assist in the management of patients with suspected or confirmed viral infection in the health care setting. In this review, we present details on general measures to prevent the spread of viral infection in hospitals and other health care environments. These include principles of accommodation of infected patients and approaches to good hygiene and patient management. They provide detail on individual viral diseases accompanied in each case with specific information on control of the infection and, where appropriate, details of preventive and therapeutic measures. The important areas of nosocomial infection due to blood-borne viruses have been extensively reviewed previously and are summarized here briefly, with citation of selected review articles. Human prion diseases, which present management problems very different from those of viral infection, are not included.
Nosocomial infections are infections that develop as a result of a stay in hospital or are produced by microorganisms and viruses acquired during hospitalization. They may be endogenous, arising from an infectious agent present within a patient's body, or exogenous, transmitted from another source within the hospital. In addition to patient-to-patient spread, others may be involved, including staff, students, visitors, and voluntary workers. Infections that are in the incubation period at the time of admission to hospital are not classed as nosocomial, but community-acquired infection of patients or staff can be an important source of nosocomial infection.
Viruses are important causes of nosocomial infection, but apart from specific situations such as the follow-up of known or suspected exposure to blood-borne viruses or the investigation of outbreaks, there are usually insufficient data to allow the monitoring of trends in incidence. When routine monitoring has been carried out, 5% of all nosocomial infections have been attributed to viruses (157), and this figure is likely to be an underestimate (160). Pediatric units (75, 124, 158) and wards with elderly patients (116, 161) are particularly prone to seasonal introductions and nosocomial spread of viral infections; in one study, 32% of pediatric nosocomial infection were attributed to viruses, of which respiratory syncytial virus (RSV) was the most common (157). Nosocomial spread of viruses often parallels outbreaks in the community, and the frequency of asymptomatic infections, together with the possibility of repeated introductions of infection, can cause major problems in detecting and monitoring the spread. Detailed study of the time of onset of disease in patients, in the light of knowledge of their admission date and range of incubation times for specific viruses, can assist in distinguishing between nosocomial and community-acquired infections. Application of laboratory techniques to characterize viruses, phenotypically or genotypically, can provide definitive evidence of transmission routes.
This review is concerned with the main viral agents responsible for nosocomial transmission. The blood-borne viruses, which have been reviewed extensively elsewhere, are described only briefly. The agents of transmissible spongiform encephalopathies (prions) have physicochemical and biological characteristics very different from those of viruses and are not included in this review.
GENERAL MEASURES TO PREVENT SPREAD OF VIRUSES
The most important defense against nosocomial transmission of viruses and other infectious agents is detailed and continuing education of staff and strict adherence to infection control policies. The doctor, nurse, or medical student, who may have rapid contact with a succession of patients of varying degrees of vulnerability, provides an excellent potential vector for virus carriage and transmission. All involved in patient care should be aware of the potential dangers to patients of continuing to work while suffering from a respiratory infection, cold sores, or other viral disease. The mundane but critically important role of adequate handwashing after examining every patient must be emphasized, as must the potential risks to the workers, and to their patients, of breaks in hygienic practice such as eating, drinking, smoking, applying cosmetics, or inserting contact lenses in clinical or laboratory areas and from touching their mouth or eyes during the course of their work. All health care workers should have access to an employee health (occupational health) department or infection control nurse in addition to immediate access to a clinical virologist or microbiologist to provide advice and emergency coverage after normal working hours. By providing this infrastructure, the immune status of employees can be checked and, when appropriate, supplemented by immunization. In addition, specific advice in emergency situations, e.g., recommendations for postexposure prophylaxis, can be provided, as well as specialist advice on individual patient management in controlling nosocomial infections.
ISOLATION AND COHORTING
A single patient with a known or suspected viral infection may present a potential risk to other patients and/or staff and could serve as the point source of a nosocomial outbreak. Awareness of the potential risk and knowledge of the nature of the virus concerned and its likely transmissibility may lead to the need for immediate single-room isolation (Table 1).
TABLE 1.
Methods of isolation and measures for preventing nosocomial transmission of viruses
| Measure | Respiratory precautions | Enteric precautions | Blood precautions |
|---|---|---|---|
| Single room | Necessary. Keep door shut. | Necessary for adults who are symptomatic and/or incontinent. Necessary for children. | Advisable if patient is bleeding or has open wounds. |
| Hands | Wash with soap and water 1 min before leaving room. Alcohol-based handrub when outside room. | Wash with soap and water 1 min before leaving room. Alcohol-based handrub when outside room. | Cover cuts and abrasions with waterproof dressings. Wash with soap and water 1 min before leaving room and after contact with blood or body fluids. |
| Gloves | Not necessary unless in contact with respiratory secretions. | Wear if stool or vomitus contact likely. | Wear if contact with blood or other body fluids is likely. |
| Apron | Wear when in direct contact with patient or bed linen. | Wear when in contact with stool or vomitus. | Wear when in contact with blood or other body fluids. |
| Mask | Not necessary | Not necessary | Wear, together with visor or goggles, if splashing into mouth or conjunctivae is possible. |
| Postexposure measures | None necessary | None necessary | May be necessary if inoculation injury or mucous membrane exposure. |
| Visitors | Wash hands before leaving room. Children should not normally be admitted to room. | No need for protective clothing but should wash hands before leaving. | If isolated for reasons given above, wash hands before leaving room. |
In an outbreak situation, whether the origin is within the hospital or in the community, it may be necessary to consider cohort isolation. This normally involves separately accommodating groups of infected and apparently uninfected individuals. It may also involve a third group of individuals who have been exposed and are at risk of developing the infection. If one of the exposed or the “uninfected” group develops the disease, they are immediately moved to the infected cohort.
In an ideal world, the two (or three) cohorts would be accommodated in geographically separate rooms, with each group having its own dedicated nursing staff. In reality, shortage of beds and staff usually necessitates compromise. This may involve selecting different sections or bays of a single ward for each of the cohorts and, if staff numbers are inadequate to avoid the need to work in more than one area, ensuring that infection control protocols are strictly adhered to. Each cohort should have access to dedicated equipment and adequate handwashing and immediate acess to disposable gloves and aprons. For medical staff and others whose patient access cannot be easily restricted, a recommendation to visit patients in the “uninfected” area before visiting those with overt disease is obviously sensible. Knowledge of the immune status of staff members may be of great value in allocating staff to caring for patients with specific viral infections. A good example is the need to ensure that staff have antibody to varicella-zoster virus (VZV) before allowing access to patients with chickenpox or shingles.
At times of high incidence of viral disease, particularly in pediatric units during winter outbreaks of respiratory disease, it may be necessary to cohort new admissions and in-patients on the basis of symptoms alone. This will assist in protecting the apparently uninfected patient population, but if more than one virus is circulating, patients in the infected cohort may be at risk of acquiring a second infection. Access to rapid viral diagnostic techniques will allow identification of the infection in individuals, and this may lead to more rational cohorting of patients with the same infection.
In addition to staff awareness of the potential transmission of viruses from patients to other patients and from staff to patients (and vice versa), visitors to patient areas should be regarded as a potential source of nosocomial outbreaks. Although variations in vulnerability of patients are likely to determine the degree of alerting of visitors to the dangers of spreading virus infections, it is obviously important to exclude those with potentially dangerous viruses such as VZV and prudent to exclude people with obvious respiratory symptoms until they are convalescent.
VIRUSES SPREAD BY RESPIRATORY ROUTE
Respiratory viruses (RSV, influenza viruses A and B, parainfluenza viruses 1 to 3, rhinoviruses, and adenoviruses) are increasingly recognized as significant pathogens; given the relative ease with which they spread and their relatively short incubation times (usually between 1 and 8 days), these viruses can result in significant nosocomial problems. Transmission occurs via spread of either small (median diameter, <5 μm) or large droplets. Typically, small droplets containing infectious virus particles are generated by coughing, sneezing, or talking and are readily transmitted over considerable distances. This is in contrast to large droplets, for which transmission usually only follows close person-to-person contact and results from direct inoculation of virus-laden droplets onto the mucous membranes (e.g., eye and nose) of the susceptible host. Autoinoculation can also lead to infection and results from the transfer of virus from hands to mucous membranes. Transmission between patients on the hands of health care workers may occur if there is a failure to wash hands between patients. While most other nosocomial infections occur throughout the year, the transmission of respiratory viruses in the hospital is seasonal, with the peak incidence occurring in the winter months, mirroring the disease activity in the community. Figure 1 summarizes the data on respiratory virus identification (influenza A virus, parainfluenza viruses, and RSV) from our center (St. Bartholomew's and Royal London Hospital) over the winter of 1999–2000.
FIG. 1.
Incidence of respiratory viruses, September 1999 to March 2000, at St. Bartholomew's and the Royal London Hospitals.
The prevention of spread of respiratory virus infections within a hospital or other institution (e.g., old people's home or psychiatric home) depends on the early diagnosis of the infection. This is most conveniently achieved by direct immunofluorescence on nasopharyngeal epithelial cells. Good communication between the laboratory and the infection control team and effective procedures such as source isolation, the wearing of protective clothing, and handwashing to interrupt transmission are also of major importance (114).
Respiratory Syncytial Virus
RSV is a major cause of morbidity in infants and young children (109) and has been reported to be responsible for approximately 45% of all hospital admissions for acute respiratory disease in those under the age of 2 years (74). Bronchiolitis and pneumonia are the most common manifestations in young children, with most children having been exposed by 5 years of age. However, immunity to reinfection is not permanent, with older children and immunocompetent adults developing recurrent episodes of mild upper respiratory tract infections throughout life (77). RSV is seasonal, occurring in the winter months (usually November to February or March) in temperate climates, and may result from the cocirculation of two antigenically distinct types of the virus within the community.
Over the last 10 years, the seriousness of RSV infections in immunocompromized patients has been realized. There are numerous reports of severe lower respiratory tract disease (pneumonia and pneumonitis) following bone marrow transplantation (BMT) and solid-organ transplantation and in patients with lymphoma and acute leukemia (105, 169, 170). The virus spreads from the upper to the lower respiratory tract in up to 50% of these patients, resulting in severe disease with an associated mortality of between 30 and 100% (13). Factors influencing outcome in BMT patients include the type of treatment used and whether the patients are pre- or postengraftment (13), with more severe disease occurring preengraftment. Even though most patients develop evidence of lower respiratory tract disease (hypoxia or lung infiltrations on chest x-ray), the outcome is more variable following solid-organ transplantation, with lung transplant recipients being at the greatest risk of severe disease (165). This increased risk is partly due to the surgery (suppression of the cough reflex secondary to denervation of the transplanted lung, disruption to the lymphatic drainage, and impaired mucociliary clearance) and bronchiolitis obliterans following episodes of rejection (105). Infants with underlying bronchopulmonary dysplasia or congenital heart disease are also at risk of severe disease, often requiring hospitalization and thus acting as a focus for further nosocomial spread.
RSV is spread by close contact with infectious respiratory secretions inoculated into the eyes or nose either via large droplets or from fomites. Infected infants shed large amounts of virus in their respiratory secretions, usually for about 7 days (range, 1 to 21 days) (74), and it is likely that transmission occurs via the ungloved or unwashed hands of health care workers or relatives. Contamination of the environment is another important source of nosocomial infections. Hall et al. have shown that RSV can persist on skin and porous surfaces such as gowns and paper tissues for up to 30 min and for up to 6 h on nonporous surfaces, e.g., gloves and countertops (76). Subsequent transfer and persistence of virus from these surfaces on skin were also demonstrated, supporting the idea that contaminated hands are important in the nosocomial transmission of this infection. Small-droplet spread is much less common but can occur when the source and recipient are in close contact (within 1 m), e.g., coughing, sneezing, or aspiration of infected secretions. Cross-infection on pediatric wards is a common problem, with over 40% of children becoming infected if hospitalized during the winter months for more than 7 days, and each year approximately 50% of pediatric staff acquire the infection (119). Outbreaks on oncology and BMT wards have also been reported (78, 118), often with multiple sources (as assessed by genotyping the virus isolates) despite the identification of an obvious source patient. In one outbreak in Toronto, Canada, three distinct sources of RSV with transmission of each strain within the ward were identified, resulting in death of four of the eight patients (118).
A high standard of infection control practices is essential to prevent both nosocomial transmission and reintroduction of RSV infection into a ward. The role of asymptomatic carriage is also of concern. Adams et al. reported an 8% carriage rate in asymptomatic pediatric BMT recipients and a 40% rate in the staff members during the winter of 1995–1996 (3). From our own experience and that of others, these high rates of asymptomatic infection have not been confirmed (113), with most patients having some symptoms, however mild, attributable to the respiratory tract infection.
Control of spread.
Numerous studies have been conducted to determine the best strategy to prevent the nosocomial transmission of RSV. There is general agreement that rapid laboratory diagnosis (direct antigen detection in nasopharyngeal cells), the wearing of aprons or gowns and gloves (for close contact with infected infants), and source isolation (or cohorting of infected patients) are essential. Whereas efficient handwashing, even with proprietary soap preparations, is extremely effective in reducing transmission (38), many studies have shown that handwashing practices even in intensive care units are suboptimal. Repeated handwashing may result in chapped, irritated skin, and this may deter the practice. Gloves are a practical alternative, although they are uncomfortable if worn for long periods and may engender a false sense of security. Contamination of the hands may still occur when the gloves are removed or through small holes in the latex. Gloves should be changed after patient contact and before contact with another patient. Source isolation of all infected cases in a single room is often not possible over the winter months, when the demand for a limited number of side rooms is at its highest, and it may be necessary to institute cohorting of patients as described earlier. The need for masks is less clear. There is some evidence that their use in conjunction with other infection control procedures may reduce nosocomial transmissions to high-risk patients (1). Eye-nose goggles have been used, but because of their inconvenience and limited availability, they are not recommended for routine use. Other measures, such as prohibiting symptomatic staff or relatives from working with or visiting high-risk patients and limiting visits by children under the age of 12 during the winter months, may also reduce nosocomial transmission in these groups (13). Aerosolized ribavirin decreases the quantity and duration of RSV shedding and may prevent severe disease in immunocompromised patients, providing the infection is limited to the upper airway (3), but whether this affects the rate of nosocomial infection is unknown. Problems with its use include the need for special equipment (small-particle aerosol generator) and the theoretical risk of teratogenicity, limiting access to patients receiving ribavirin therapy to nonpregnant staff.
To interrupt nosocomial transmission, it is essential that all possible cases of hospital acquired infection be identified early and that the infection control team be informed so that the appropriate procedures are implemented. Jones et al. partly attributed an outbreak in a BMT unit to a delay in informing the infection control team (93). In our hospital, the laboratory liaises directly with the infection control team, informing them of significant results on a daily basis. They are not only important in reducing the morbidity of patients; infection control measures may also be associated with significant cost savings. By preventing 14 cases of nosocomial RSV infection, Karanfil and coworkers demonstrated an annual saving of U.S. $84,000 (96).
Influenza Viruses A and B
Influenza A and B virus infections are characterized by the sudden onset of fever, coryza, sore throat, headache, and profound myalgia. The symptoms typically last about 7 days in the immunocompetent host, with some patients developing a protracted cough. The period of infectivity is taken as either 7 days from the onset of symptoms or until symptoms cease, whichever is the longest. During major epidemics, severe illness and death from primary viral or secondary bacterial pneumonia can occur, usually in the elderly, those with underlying disease (cardiac, pulmonary, renal, or metabolic), and the immunocompromised. In hospitalized BMT patients, influenza is often complicated by pneumonia, with an associated mortality of 50%. Unlike RSV, however, only half of the pneumonias are viral in origin, the others being secondary to bacterial or fungal superinfections (169). Severe disease has also been reported in solid-organ transplant recipients, particularly in the early posttransplantation period (7). The clinical course in patients with AIDS is usually the same as in the immunocompetent host.
Influenza virus infections occur mainly in the winter months and, depending on the level of immunity in the community and the nature of the main circulating viruses, may result in sporadic infections or epidemics. Pandemics result from the genetic reassortment (antigenic shift) in influenza A viruses and, as there is little or no immunity in the community after such an event, usually affect a large proportion of the population.
Influenza viruses are highly infectious, being readily transmitted in both large and small droplets. It is the small-droplet spread which accounts for the explosive nature of influenza outbreaks in closed environments, where one infected person can potentially infect a large number of susceptible hosts. Numerous nosocomial outbreaks involving long-stay facilities for the elderly have been reported (116). These outbreaks are often exacerbated by poor vaccine coverage. In addition, outbreaks involving medical, pediatric, and BMT units have also been reported (159, 168).
Control of spread.
Measures to control the nosocomial spread of influenza virus infections are based on those outlined for RSV, with particular emphasis on droplet precautions, including the wearing of masks. Isolation in a single room with negative pressure is best for known or suspected cases (147), but cohorting may also be used when large numbers of patients are infected. As for RSV, additional measures such as restricting visitors and excluding infected staff (particularly those working with immunocompromized patients) can be implemented depending on the severity of the outbreak. In contrast to RSV, the precautions taken to prevent the nosocomial spread of influenza can be maximized by the use of immunization and antiviral drug prophylaxis. In the United Kingdom (U.K.), annual influenza immunization is recommended for all patients with underlying chronic cardiac or respiratory disease, diabetes mellitus, chronic renal disease, or immunosuppression (due to disease or treatment) and for those over the age of 65 or living in long-stay care facilities (45). In the United States (U.S.), those over the age of 50 are offered immunization (32). Although not a routine, immunization of health care workers, especially those working with the immunocompromised, is also recommended in many hospitals. Immunization of staff and/or patients may still be of benefit even during an outbreak, as an immune response is usually detectable within about 14 days in adults (16). Specific antiviral therapy with amantadine has also been used in the following situations: (i) as short-term prophylaxis to cover late immunization during a ward outbreak for staff or patients, (ii) for prophylaxis if immunization is contraindicated, and (iii) for immunocompromised patients not likely to respond to immunization, e.g., BMT recipients within 6 months of transplantation and patients with graft-versus-host disease.
Amantadine is not, however, without problems. Side effects, including central nervous system (CNS) and gastrointestinal disturbances, are more common in the elderly, although these can be reduced by halving the daily dose to 100 mg. Resistance develops readily when amantadine is used to treat influenza A, and while there is no evidence to suggest that resistant strains are more virulent, they can be transmitted to others. This is potentially a problem if infected patients receiving amantadine as treatment are not separated from those receiving the drug as prophylaxis (23). Rimantadine is an alternative to amantadine; it has comparable efficacy and, because of a lower incidence of side effects, is better tolerated. Newer agents such as zanamavir (a neuraminidase inhibitor) offer the advantage that they are extremely active against both influenza A and B viruses (unlike amantadine, which is only active against influenza A virus), and so far only one case of resistance has been reported in an immunocompromised child with severe influenza B virus infection (71). The role of these new agents in interrupting nosocomial transmission of influenza virus infection needs to be established.
Parainfluenza Virus Infections
There are four serotypes of human parainfluenza virus, with types 1, 2, and 3 being the most important. They are the major cause of laryngotracheobronchitis (croup) in children and are also responsible for upper respiratory tract infections (types 1, 2, and 3) and bronchiolitis/pneumonia (type 3). Typically, infections with parainfluenza virus types 1 and 2 occur in the autumn, and type 3 infections occur throughout the year. Virtually all children have been infected with parainfluenza virus type 3 by the age of 2 years, with infections due to types 1 and 2 occurring at a lower rate; 74 and 59% of children have been infected with types 1 and 2, respectively, by the age of 5 years. Most infections are self-limiting in the immunocompetent host, but severe lower respiratory tract infection has been reported in up to two-thirds of BMT patients with parainfluenza virus infections (164). Death and respiratory failure occurred in one-third of these patients. There are isolated reports of severe parainfluenza virus infections in children infected with human immunodeficiency virus (HIV), but in most instances there was usually coinfection with another pathogen, such as Pneumocystis carinii (100).
Whereas parainfluenza virus type 1 and 2 infections are often community acquired, type 3 is the most common serotype causing nosocomial infections and pneumonias in the immunocompromised host (164). Nosocomial transmission of type 3 has also been reported in neonatal units and homes for the elderly (19, 126), and there is some evidence to suggest that outbreaks are more likely to be due to transmission between patients rather than the continuous reintroduction of different strains by staff or visitors (97). Transmission of these viruses is similar to that of RSV, and direct contact with respiratory secretions via fomites or large-droplet spread is the main route. Survival of the viruses for up to 10 h on nonabsorptive surfaces (stainless steel) and 4 h on absorptive ones (laboratory coats and gowns) has been reported (15). However, survival on fingerpads is poor, with only 5% of the virus being recoverable after 10 min (6).
Control of spread.
As for the other respiratory viruses, the prevention of nosocomial transmission of parainfluenza virus infections relies on rapid diagnosis and effective communication with the infection control team. The measures used to interrupt droplet transmission are the same, and the importance of handwashing can only be reemphasized. Cleaning the environment with hospital cleaners and disinfectants virtually eliminates surface contamination (15).
Rhinoviruses and Coronaviruses
Rhinoviruses and coronaviruses are responsible for the common cold in 20 to 40% and 10 to 15% of cases, respectively. Typical symptoms include coryza, sneezing, lacrimation, and chilliness and last from 2 to 7 days. No fatalities have been reported, but these infections may predispose individuals to more serious complications such, as sinusitis, otitis media, and asthma. A recent report has suggested that pneumonia may follow an upper respiratory tract rhinovirus infection in BMT patients analogous to that seen with RSV (64).
Control of spread.
These viruses are readily transmitted following close contact with infected respiratory secretions and fomites. Like RSV, they are transmitted by contaminated hands carrying virus to the mucous membranes of the nose or eye. There is also evidence supporting the role of aerosols in the transmission of rhinovirus infections (47). Reinfections are common because there are over 100 different serotypes and the immune response is short-lived. Handwashing is the most effective way of minimizing transmission. Large- and small-droplet precautions should be used in an outbreak situation to control spread, but laboratory confirmation is rarely achieved, as direct antigen detection of rhinoviruses or coronaviruses is not routine, and coronaviruses cannot be cultured in standard cell lines. However, with the development of antipicornaviral agents such as pleconaril, rapid diagnosis will be required if therapy is to be started within 24 to 48 h of the onset of symptoms (70).
Adenoviruses
Adenoviruses differ from the other community-acquired respiratory viruses because they can be acquired both exogenously and endogenously, following reactivation. There are over 40 different serotypes causing a range of syndromes, including acute respiratory disease (51), epidemic keratoconjunctivitis (88), and pharyngoconjunctival fever (11). Fecal-oral transmission also occurs and accounts for cases of sporadic diarrhea seen in children. Severe disease can develop following bone marrow or solid-organ transplantation and in patients with AIDS. Typical presentations in the immunocompromised include pneumonitis, enterocolitis, hemorrhagic cystitis, hepatitis, encephalitis, and disseminated disease (100). In one study, 46% of BMT patients with adenovirus infections died seven patients with pneumonia and six with disseminated disease); ribavirin therapy did not offer any benefit in these severe cases (171). Risk factors for severe disease included identification of virus at multiple sites and prolonged excretion. Adenovirus-induced hepatitis in liver transplant patients is associated with significant mortality (∼40%) and can result in massive hepatic necrosis (18). Various therapies have been tried in the past, including reductions in immunosuppression and ganciclovir. Currently there are no specific therapies, although cidofovir, an anticytomegalovirus (CMV) agent, has in vitro activity against some adenovirus types and may be of some clinical benefit.
Adenovirus infections can be identified in the community throughout the year. These viruses are highly stable and can be transmitted via large droplets and also via small aerosol droplets. Nosocomial adenovirus infections have been well described and can affect both patients and staff. A fatal case of disseminated adenovirus 3a infection in an immunocompromised patient resulted in 38 staff members becoming infected (108). Those in the intensive care unit were at highest risk, probably as a result of direct contact with respiratory secretions following intubation, although prolonged or frequent contact with the patients was also important. Large outbreaks of epidemic keratoconjunctivitis in ophthalmology clinics have been reported (148). Using restriction enzyme analysis, a point source is usually identified, with virus being transmitted on the hands of health care workers or via infected equipment, e.g., tonometer heads.
Control of spread.
Droplet precautions in addition to standard isolation should be used. Infected patients should be placed in a single room. Staff should wear gloves and gowns when coming into contact with infected patients or when handling body secretions. Handwashing should be emphasized, and contaminated linen should be handled with gloves. Infected staff members should not work until symptoms have resolved, as excretion can be prolonged. As adenoviruses are very stable and can survive for prolonged periods, it is essential to decontaminate equipment thoroughly. This is particularly relevant to ophthalmology equipment, for which thorough cleaning followed by steam sterilization is recommended.
Measles, Mumps, and Rubella Viruses
The incidence of measles, mumps, and rubella in the U.K. has been dramatically reduced following the introduction of universal immunization of infants in 1988. Within the last few years, community outbreaks of measles and mumps have been reported. In 1999 there were 34 cases of measles, 262 cases of mumps, and 47 cases of rubella in the U.K. These have resulted from a build-up of a susceptible cohort due either to vaccine failure or to parental refusal to accept immunization. Despite a “catch-up” program in 1996 targeted at schoolchildren, further small outbreaks have continued to occur in the orthodox Jewish communities of east London and Manchester, where vaccine uptake is poor. Genotyping of these viruses has established a link between these two outbreaks and some cases in New York, U.S. (B. Cohen, personal communication). A recent outbreak of mumps in east London was largely restricted to a group of new immigrants who had never received mumps immunization. Nosocomial transmissions of mumps, measles, and rubella have all been documented in the past (134, 143). Paradoxically, as the incidence of these infections falls, the risk of nosocomial infection increases, due to a failure to recognize these infections in the early stages. Infected children may therefore remain on an open ward for prolonged periods. Rubella may be complicated by encephalitis and thrombocytopenia, and mumps by aseptic meningitis and orchitis or oophoritis, but neither of these infections is likely to be worse in the immunocompromised host. Measles virus infection can result in neurological complications and pneumonia. Immunocompromised patients can develop severe progressive measles infection associated with giant cell pneumonia, with an associated mortality of 70%. Primary rubella is of particular concern in pregnant women, in whom the incidence of congenital rubella syndrome can reach 80% if the infection is acquired in the first 8 weeks of pregnancy.
Control of spread.
The incubation times, periods of infectivity, and recommended precautions are shown in Table 2. As measles, mumps, and rubella viruses are all transmitted by direct contact and droplet spread, the index case should be isolated and respiratory precautions (gown and gloves) should be used for patient contact. Ideally, the immune status of the staff should be known, but if not, immunization with a single dose of the trivalent mumps-measles-rubella vaccine as soon as possible (within 72 h) after the exposure is indicated. Although this may not prevent mumps or rubella in susceptible contacts, it will provide protection for the future in those who do not develop the disease. Prompt measles vaccination may prevent disease, as the incubation period of vaccine measles virus infection is shorter (approximately 7 days) than that for the natural infection (approximately 10 days) (45). Measles vaccination is contraindicated in pregnant women and immunocompromised individuals. In contacts for whom live vaccine is contraindicated, prophylaxis for measles consists of two doses of normal human immunoglobulin (Ig) given 48 h apart (0.06 to 0.12 μl/kg). If possible, measles contacts should be discharged home, and exposed members of staff should not have patient contact between days 5 and 21 after exposure. The local infection control team should be informed of all suspected or confirmed cases to ensure compliance with the infection control precautions. They will also be responsible for drawing up a list of contacts (staff and patient) to allow early detection of any further cases. The employee health (occupational health) department will be responsible for following up staff members. In the U.K., the Consultant in Communicable Diseases Control should be informed of all confirmed cases and the relevant statutory notifications should be completed. Although congenital rubella is now extremely rare, only staff immune to rubella should deliver an infant born to a woman who has had rubella infection in pregnancy. Following delivery, an infected infant will excrete large amounts of rubella virus, and therefore isolation of mother and baby until discharge is recommended. The baby with congenital rubella virus infection should be isolated if admitted to hospital in the following 2 years, as virus excretion continues for a long period after birth.
TABLE 2.
Infection control precautions for Measles, mumps, and rubella
| Virus | Incubation period (days) | Duration of infectivity | Precautions |
|---|---|---|---|
| Measles virus | 10–14 | 4 days before to 7 days after rash appears | Respiratory isolation, immunization of close contacts (staff, patients) if immune status unknown or susceptible |
| Mumps virus | 14–21 | 2 days before to 7 days after onset of parotitis | Respiratory isolation, immunization of close contacts (staff, patients) if immune status unknown or susceptible |
| Rubella virus | 14–21 | 7 days before to 10 days after rash onset | Respiratory isolation, immunization of close contacts (staff, patients) if immune status unknown or susceptible, special consideration if contact is pregnant (refer to clinical virologist/infectious disease physician) |
Parvovirus B19
Parvovirus B19 is associated with erythema infectiosum or fifth disease (slapped cheek syndrome) in children, arthralgia and arthritis in adults, hydrops fetalis (3% risk for women infected between 9 and 20 weeks of pregnancy), and second-trimester abortions (9% risk for women infected in first 20 weeks of pregnancy), transient aplastic crises in patients with hemaglobinopathies, and chronic anemia in immunocompromised patients. It is a common infection, with between 40 and 60% of the adult population having serological evidence of previous exposure, with most of the infections having been acquired before the age of 10 years (129). The diagnosis is often confused with the other viral exanthema, such as rubella and measles, and in up to 30% of people the disease is asymptomatic. Typical infection results in a biphasic illness. Occurring about 5 days after exposure, the initial viremic phase is when the host is infectious to others. About a week later, the immunological phenomena, rash and arthralgia, develop, and at this stage most infected individuals will have detectable parvovirus IgM in their serum. Protection from further infection is life-long in the immunocompetent host, but chronic or recurrent anemia can occur in the immunocompromised. The epidemic cycle is around 4 years, with one or two epidemic years followed by two or three years when the infection is much less frequent.
It is not completely clear how parvovirus B19 is spread. Transmission is thought to occur following close contact during the period of maximum infectivity (7 days before the appearance of the rash) and via large droplets or fomites (40). People with aplastic crises are infectious for up to 1 week after the onset of symptoms, and those with chronic infection may remain infectious for prolonged periods. Environmental contamination also occurs, particularly following the birth of an infected infant. Overall, the transmissibility is low, with the most significant risk factor in the community being contact with school-age children. The annual seroconversion rate in elementary school employees is around 5%, compared to 0.5% in hospital employees even during epidemic years (4). In household settings where contact is intense, around 50% of susceptible contacts develop the infection. Attack rates of between 0 and 30% have been reported in susceptible hospital staff (12, 103, 133); however, the high rates may be an overestimate, as the authors of these reports did not allow for possible acquisition of the infection in the community, and it is likely that the risk to staff or patients is not higher than the risk in the community at any given time.
Control of spread.
The U.S. Centers for Disease Control and Prevention (CDC) do not recommend any restrictions for personnel exposed to parvovirus B19 even if they are pregnant. This may be because a case is usually identified when the rash appears and the patient is no longer infectious. In the U.K., respiratory precautions and handwashing are recommended for contacts with a patient with suspected or confirmed parvovirus B19 infection. Only those members of staff or patients who are immunocompromised or pregnant (<20 weeks) and who have had significant contact with the index case during the period of maximum infectivity (7 days before onset of the rash) need to be followed up. A significant contact is defined, as for VZV (which is likely to be much more infectious), as one who is in the same room (classroom, house, two- to four-bed hospital bay) for >15 min or who has had face-to-face contact. Usually a stored sample (either from an occupational health or antenatal booking sample) can be tested to determine susceptibility. Serological follow-up of seronegative individuals can then be arranged. Early identification of hydrops fetalis and the application of intrauterine blood transfusion reduces the rate of fetal loss by 38% (54). Human normal immunoglobulin is of benefit in treating the chronic anemia which can develop in the immunocompromised (58).
VIRUSES TRANSMITTED BY FECAL-ORAL ROUTE
In this section, viruses that cause gastroenteritis and other viruses, including enteroviruses, that are able to replicate in the intestines will be considered. While transmission is predominantly by the fecal-oral route, other transmission routes may occasionally lead to transfer of infection. The high rate of horizontal transmission of small round-structured viruses (SRSVs) following an episode of vomiting raises the possibility of transmission from contact with aerosols and/or contaminated fomites.
Rotavirus
Rotaviruses are recognized as an important cause of nosocomial infection, particularly in infants and children under the age of 5 years and in the elderly. They may also cause nosocomial gastroenteritis in the immunocompromised. In outbreaks in hospital nurseries, 33 to 70% of infants have been shown to shed rotavirus in the stool, and 8 to 28% of the shedders have clinical symptoms (14, 89). Rotaviruses have been found to account for nearly 50% of all cases of nosocomially acquired infectious gastroenteritis in pediatric patients (14, 57, 163). Steele et al. reported that 45% of pediatric rotavirus infections in hospitalized children in Pretoria, South Africa, were acquired nosocomially (145). Studies using electropherotyping in Chile (59) and London (145) demonstrated transfer of infection within single rooms or wards but showed no evidence of transfer from one room or ward to another. Thus, in these particular outbreaks, there was no evidence that staff were acting as vectors of infection within the hospitals.
Rotaviruses have been identified as the cause of diarrhea outbreaks in elderly hospitalized patients (41,98). Although adults with normal immune systems rarely have symptomatic infection, Yolken et al. reported rotaviruses as the causative agent in 9 of 31 patients with infectious diarrhea in a BMT unit (177).
Rotavirus infection usually produces sudden onset of fever, abdominal pain, and vomiting, followed by watery diarrhea that lasts for 4 to 7 days. The incubation period is short (1 to 2 days). Laboratory diagnosis is relatively easy as large numbers of viral particles are shed in feces. The main techniques used are enzyme-linked immunosorbent assays (ELISAs), passive particle agglutination tests, and, if a wider search for gastroenteritis viruses is required, electron microscopy. Oligonucleotide primers complementary to common and type-specific regions of the VP7 and VP4 genes allow sensitive detection and typing of the G and P types of the viruses, respectively, using reverse-transcription-PCR (RT-PCR) (61, 67).
Control of spread.
The principle for control of rotaviruses, as with other enteric viral infections, is the institution of single-room or cohort isolation with enteric precautions. Although sodium hypochlorite is used for general disinfection, 70 or 95% alcohol handrubs are recommended as an adjunct to handwashing to prevent patient-to-patient transmission. Vaccines have been under development for some years. In August 1998, although a tetravalent human-rhesus rotavirus vaccine was approved by the Food and Drug Administration in the U.S., the vaccine was later withdrawn due to an increased incidence of intussusception in vaccinees.
Small Round-Structured Viruses
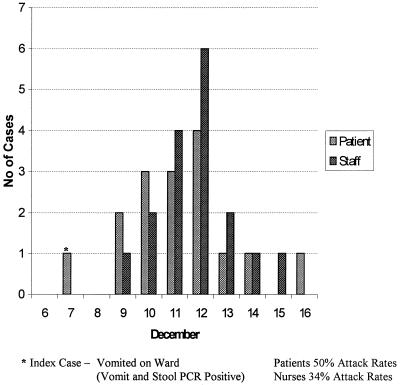
The first recognition of SRSVs occurred as a result of an outbreak of gastroenteritis in an elementary school in Norwalk, Ohio, in 1968. Subsequent cloning and sequencing of the Norwalk agent led to its classification as a calicivirus. Further outbreaks of similar disease have been attributed to Norwalk virus-like agents (caliciviruses, 30 to 40 nm in diameter) or astroviruses (28 to 30 nm); collectively these agents are known as SRSVs (for reviews, see references 42 and 94). Outbreaks of gastroenteritis due to SRSVs occur in schools, families, and hospitals and can involve any age group. Vomiting is often a prominent feature, and the high virus titers demonstrable in vomitus are thought to account for the widespread transmission which may occur in a ward or clinic setting (60) and in other enclosed areas such as holiday coaches (34) and cruise ships (95). The time course of such an outbreak in a single ward in our own hospital is shown in Fig. 2.
FIG. 2.
SRSV outbreak in a general medical ward at St. Bartholomew's and the Royal London Hospitals.
The clinical picture of calicivirus and astrovirus gastroenteritis is similar to that of rotavirus gastroenteritis. In the laboratory, both virus families can be recognized and diagnosed by electron microscopy, ELISA, or, more recently, RT-PCR techniques (46).
Control of spread.
Methods of control for SRSVs are as described for rotaviruses.
Enteroviruses
The enteroviruses are a genus of the Picornavirus family and include coxsackieviruses A and B, echoviruses, polioviruses, and enteroviruses 68 to 71. They are transmitted by the fecal-oral route, but coxsackievirus A21 has been reported to spread by droplet transmission, and other enteroviruses are probably spread by this route (120). Virus shedding in the oropharynx and in feces may continue for at least 1 month after infection. Infection is common in the general population, with most disease in temperate climates presenting in the summer and autumn months. In tropical countries, the seasonal pattern is not seen, and the diseases are present thoughout the year.
Asymptomatic infection is common with all enteroviruses, and they are associated with a wide spectrum of clinical syndromes. These range from nonspecific febrile illness and rashes to the devastating paralysis of spinal or bulbar poliomyelitis. Specific, clinically recognized syndromes occur with coxsackieviruses (e.g., type A—herpangina, hand, and foot-and-mouth disease, and type B—Bornholm disease and myocarditis) and nosocomial outbreaks of non-polio enteroviruses have been particularly severe and problematic in neonatal units and nurseries. In neonates, transmission from an infected mother to her newborn baby may occur during delivery, and this may represent the index case of an outbreak which may present as meningitis, encephalitis, and myocarditis in this vulnerable patient population. Enterovirus infections can be diagnosed in the laboratory by virus isolation from pharynx, cerebrospinal fluid, or feces, followed by identification of the virus type by neutralization tests. Coxsackievirus infection can be diagnosed serologically using IgM antibody capture techniques, and the heterotypic nature of antibody responses to enteroviruses may lead to a positive IgM response for other viruses in the group. Recently, advances in molecular genetic technology have led to the introduction of hybridization probes for identifying enteroviruses in cell culture and tissues, and the use of PCR with primers homologous to conserved regions of the genomes has provided genus-specific, sensitive diagnostic tests (123).
Control of spread.
As with other enteric viral infections, isolation or cohorting of infected individuals is critical in the control of non-polio enterovirus infections. Applying rigorous handwashing and virological surveillance to monitor swiftly any cross-infection, Isaacs et al. (86) demonstrated the control of an outbreak of echovirus type 11 in the special-care baby unit in Oxford, U.K. These actions allowed them to keep the unit open to admissions.
Poliovirus is unlikely to pose a problem of nosocomial infection in the industrialized world, and with imminent global eradication of polio, the problem should cease to exist in all countries. In the meantime, the higher risk of vaccine-induced paralytic poliomyelitis in adults than in children means that unvaccinated parents of children who have received oral polio vaccine and any unvaccinated ward staff should be immunized (45). Immunocompromised contacts should be offered inactivated polio vaccine. In the U.S., continued occurrence of vaccine-associated paralytic poliomyelitis has led to recommendations for a progressive change to the use of inactivated polio vaccine for childhood immunization (33).
Hepatitis A Virus
Previously designated enterovirus 72, hepatitis A virus (HAV) has now been reclassified into its own genus (Hepatovirus) within the Picornaviridae. Nosocomial transmission of hepatitis A has for years been associated with poor hygienic practices and crowded conditions in institutions and wards for the developmentally disabled (116). Transmission in general hospital wards has been reported, but it is unusual. Transmission from patients to staff has occurred, usually as a result of contact with asymptomatically infected children or from older children or adults with vomiting, diarrhea, and fecal incontinence. Doebbeling et al. (48) reported an outbreak in a burn unit in which a father and son, both with severe burns, transmitted HAV to 11 health care workers and another patient, all of whom developed clinical hepatitis. As in other episodes of nosocomial transmission, in this outbreak infection control in the unit was suboptimal, and food had been shared and consumed in clinical areas. Food-borne outbreaks of HAV in hospitals have usually involved staff and often also small numbers of patients.
As with other types of hepatitis, the clinical effects of HAV infection range from asymptomatic attacks to nonspecific febrile illness to classical acute hepatitis with jaundice. A laboratory diagnosis is usually achieved serologically by demonstration of seroconversion or the presence of HAV-specific IgM.
Control of spread.
Maximum fecal shedding of HAV occurs in the late incubation period, and once a symptomatic patient has been admitted to the hospital, the risk of transmission is low. Care should be taken in handling excreta, and single-room isolation should be used if the patient is incontinent of feces.
Human normal immunoglobulin may be administered prophylactically in the event of significant exposure to HAV-positive material (45). There is growing evidence to support the use of HAV vaccine for prophylaxis following exposure to the infection.
Hepatitis A vaccination may be considered for personnel working in health care settings where HAV infection is endemic.
Hepatitis E Virus
Hepatitis E virus is rarely encountered in industrialized countries and has not been associated with nosocomial transmission as yet. The clinical effects of this virus are similar to those of HAV, except that it may cause particularly severe disease during pregnancy. Classified as a calicivirus, it is transmitted by fecally contaminated water and food. No vaccine is available at present, and hospital management is conducted as for other fecal-oral infections.
HERPESVIRUSES
The members of the herpesvirus family include herpes simplex virus (HSV) types 1 and 2, VZV, CMV, Epstein Barr virus (EBV), and human herpesviruses (HHVs) 6, 7, and 8. The human herpesviruses are ubiquitous, with most of the population having serological evidence of exposure to at least one member of the family. The typical clinical course of all the infections consists of a primary infection, which may be symptomatic, followed by repeated reactivations, often asymptomatic. Nosocomial infections are rare and usually the result of direct inoculation from person to person. VZV is the only member of this family affecting humans that has the potential to be transmitted by aerosols. Immunocompromised patients (transplant recipients and those infected with HIV) are susceptible to reactivations and, because of the immunosuppression, are more likely to have symptomatic disease. Early treatment with appropriate antiviral chemotherapy is indicated to prevent dissemination of the infection.
Herpes Simplex Viruses
The most common clinical manifestations of HSV infections include oral (predominantly HSV-1) and genital (predominantly HSV-2) ulceration, keratitis, neurological disease (encephalitis, meningoencephalitis, and meningitis), herpetic whitlow, and neonatal infection. The immunocompromised can also develop life-threatening disseminated infection.
Direct contact either with lesions or saliva is the most efficient way of transmitting HSV. Contamination of the hands is common in individuals with herpes labialis, and even though the virus survives only poorly outside the body (150), this route has been implicated in cross-infection outbreaks (111). Transmission from patients to staff is also well recognized. Before gloves were worn routinely in dental practice, herpetic whitlows often resulted from direct inoculation of the virus into the fingers following contact with infected saliva in the patient's mouth (69). Neonatal herpes usually results from mother-to-baby transmission at the time of delivery. The risk to the infant is reduced if the baby is delivered by cesarean section and if the infection is a reactivation rather than a primary infection.
Rapid diagnosis can be achieved by electron microscopy of vesicle fluid or immunofluorescence microscopy of skin scrapings with specific monoclonal antibodies.
Control of spread.
Good standards of hygiene are sufficient to prevent transmission. Gloves should be worn when handling potentially infectious body secretions, and frequent handwashing should be encouraged. Patients with active lesions should be nursed away from high-risk groups, i.e., neonates, the immunocompromised, and those with burns and severe eczema, until the lesions have crusted over. A new mother with active lesions should not have contact with the other babies in the nursery; she should wash her hands before holding her own baby and should keep active lesions covered (mask, gloves, etc.). Breast-feeding is contraindicated if there are active lesions around the nipple. The same rules apply to infected members of staff; during active infection they should restrict their contact with high-risk patients. If there is evidence of active genital herpes infection in the mother during the delivery of an infant, intervention should be kept to a minimum and scalp electrodes should not be used. Acyclovir can be used to reduce the period of virus shedding and hasten healing and, in the immunocompromised, to prevent dissemination.
Varicella-Zoster Virus
VZV is the most infectious of the herpesviruses and is the cause of chickenpox and shingles. Primary infection (chickenpox) is usually a mild self-limiting illness, with the majority of cases occurring in children. Complications are rare, with <2 deaths per 100,000 cases (136). In adults, the disease is more severe, with a higher incidence of pneumonitis, encephalitis, and death (135). Additional complications of VZV in pregnancy include the congenital varicella syndrome (first 20 weeks), premature labor, and neonatal varicella if the infection occurs around the time of delivery (52, 122, 128). Severe disease is also more common in the immunocompromised, among whom mortality rates may approach 15% (55). In temperate climates, chickenpox is more common in the winter and early spring, and infection is almost universal, with 90 to 95% of the adult population having serological evidence of past exposure. This is not the same in developing countries, where up to 60% of the population may remain susceptible into adulthood. Protection is life-long, and reinfection is rare.
VZV is spread by direct contact with an infected patient. Transmission occurs following contact with the infected lesions (chickenpox and shingles) and by droplet spread from respiratory secretions (chickenpox). Contact with articles contaminated with vesicle fluid may also be important. VZV is highly infectious, with an 80% attack rate in the household setting. Nosocomial outbreaks have been well described (104), with aerosolization of the virus accounting for some of the outbreaks (107). The importance of this route was confirmed by Sawyer et al., who were able to detect VZV DNA in air samples up to 2.5 m from the beds of patients with chickenpox and in some instances even outside the patients' rooms (140). Patients with chickenpox are infectious via the respiratory route from 2 days before the rash appears until it crusts over (usually about 5 days). Symptoms usually develop about 14 days following an exposure (range, 10 to 21 days). A presumptive diagnosis of chickenpox or shingles is usually based on recognition of the typical clinical features. It can be confirmed (as for HSV) by electron microscopy or immunofluorescence.
Control of spread.
Patients with uncomplicated chickenpox or shingles should, if possible, be nursed at home. If this is not possible or symptoms are severe, chickenpox cases should be placed in isolation (preferably negative pressure). Isolation of shingles cases is also preferable, but if no single rooms are available and the affected dermatome does not involve an exposed part of the body, this may not be necessary. Standard respiratory precautions are recommended. Following the identification of a case of VZV infection in the hospital, the infection control team should be informed, to coordinate the follow-up of staff and patients. Those patients and staff who have had close contact with the index case (in the same room for >15 min, in the same two- to four-bed bay, or face-to-face conversation) should have their VZV immune status determined. Only patients who have not had chickenpox or who are not sure of their immune status need to have a blood test to confirm their VZV antibody status. Most staff members have been screened preemployment and, if seronegative, ideally should not have contact with varicella-zoster cases. For patients and staff found to be seronegative, prophylaxis with varicella-zoster immune globulin (VZIG) is available for (i) immunocompromised patients (including BMT patients for up to 6 months transplantation), (ii) pregnant women, and (iii) neonates, if maternal rash develops within 7 days either side of delivery.
VZIG is administered to attenuate rather than prevent infection and ideally should be given within 96 h of exposure. It may, however, still of be of benefit up to 10 days after exposure. If possible, all other seronegative patients should be discharged home and their general practitioner should be warned about the possibility of chickenpox developing in the 3 weeks following exposure. Patients who cannot be discharged should be isolated between days 10 and 21 (day 28 if VZIG is administered) following exposure. If working with high-risk patients, seronegative members of staff should be either redeployed or sent home during the incubation period.
In the U.S., varicella vaccination is now part of the routine immunization schedule for infants aged 12 to 18 months. The Advisory Committee on Immunization Practices (ACIP) also recommend its use for susceptible adults who have close contact with high-risk patients, either at work or at home (30). Postvaccination testing of hospital staff is not required, as 99% of adults seroconvert after two doses given 4 to 8 weeks apart (30). Although the duration of immunity is still not known, 70% of adult vaccinees were still protected following a household contact 2 years after immunization (63). Although there is evidence to suggest that immunization within 72 h of varicella exposure may offer protection in children (8), there are no data to support its use in adults, and there has been no formal trial of the currently licensed vaccine for this use. Therefore, varicella vaccine is not currently recommended for prophylaxis of adult contacts exposed to nosocomial varicella. Acyclovir may also be useful in preventing varicella infection (9). Concerns that the immune response may be blunted by the antiviral drug have not been substantiated (106), but because the optimal dose and timing of administration are still not known, its use is not currently recommended.
Cytomegalovirus
CMV is spread by direct contact with body fluids, including vaginal secretions, semen, saliva, and whole blood. Primary infection is usually asymptomatic, although some individuals may develop a glandular fever-like illness. Asymptomatic reactivations are common and facilitate transmission.
Severe disease in immunocompromised patients (solid-organ transplant recipients, BMT recipients, and AIDS patients) and congenital infections can result from both primary infection and reactivation of latent virus. Although up to 40% of pregnant women with primary infection transmit CMV to the fetus, only about 5% of their infants have clinical features of congenital infection at the time of birth. An additional 5 to 10% of infected infants who appear normal at birth will develop long-term sequelae such as deafness.
Control of spread.
Although there is concern that CMV may be transmitted nosocomially, there is little evidence to support this. Studies of hospital pediatric nurses caring for children excreting CMV showed that they were not more likely to acquire CMV than women of a similar age working in other occupations (176). Similarly, restriction enzyme analysis of isolates has failed to confirm cross-infection when a member of the staff has been exposed to CMV and subsequently acquired the virus (10, 130). Although transmission between children has been reported in the hospital setting, it is likely that this horizontal transmission is due to close contact between the children, as in the day care center setting, rather than from a breakdown in infection control procedures (44). Although there is little evidence to suggest that CMV is transmitted in hospitals, basic precautions (gloves and aprons or gowns) should be employed when handling body fluids or contaminated linen or diapers.
Epstein-Barr Virus and Human Herpesviruses
The remaining members of the herpesvirus family, EBV, HHV6, HHV7, and HHV8, have not been associated with nosocomial transmissions, although EBV, HHV6, and HHV7 are shed in the oropharynx (66, 83). As for CMV, basic precautions used for contact with body fluids of all patients should be taken, i.e., gloves and apron or gown.
BLOOD-BORNE VIRUSES
Although many viruses may be present in the bloodstream during the acute phase of infection, a group of genetically unrelated viruses that are capable of inducing persistent viremia, or a carrier state, have been collectively termed blood-borne viruses. Hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV-1 have all been associated with nosocomial infections, with transmission from patient to patient, from patients to health care workers, and from infected health care workers to patients. Transmission of these viruses is dependent on transfer of blood, other body fluids, or tissue from an infected to an uninfected individual, and patient-to-patient transmission in health care is only likely to arise when there has been a breakdown in instrument decontamination or other deficiency in infection control procedures. Reduction of risks of transmission from patients to health care workers and vice versa has been the subject of a number of important guideline documents (20, 21, 31, 151–156, 174), and the reader is referred to these for detailed information.
Hepatitis B Virus
In the unvaccinated health care worker, the rate of transmission of the virus after percutaneous exposure to HBV e antigen (HBeAg)-positive blood may be higher than 30% (68, 84, 166). In studies in the U.S. before the introduction of HBV vaccine, surgeons and other health care workers exposed to the risk of inoculation injury were shown to be four to five times more likely than other workers to be infected with HBV (167). Routine immunization and monitoring of immune status of all health care workers exposed to the risk of percutaneous inoculation of blood and other body fluids have produced a major reduction in patient-to-staff transmission of HBV. Staff who are carriers of HBV have been shown to present a risk of transmission to their patients during the course of surgical or dental procedures (56, 110). In the U.K. during the period from 1975 to 1990, 12 outbreaks of HBV infection in patients were associated with an infected health care worker as the source of the outbreak (81). A total of 95 infected patients were identified in follow-up studies of these incidents. Surgeons were responsible for 11 of the outbreaks, and the other 1 resulted from an HBeAg-positive heart-lung perfusion technician. In all but one of these outbreaks, the source health care worker was HBeAg positive, and the status of one surgeon was unknown. Published transmission rates of HBV from surgical and dental procedures involving infected health care workers are shown in Table 3.
TABLE 3.
HBV transmission from infected health care workers to patients
| Specialty | No. infected/no. of patients tested (% infected) | Reference |
|---|---|---|
| Cardiac surgery | 5/69 (7.2) | 73 |
| Coronary artery bypass grafting | 18/231 (7.3) | 137 |
| Orthopedic surgery | 49/1532 (3.2) | Johnston et al.a |
| Obstetrics and gynecology | 25/250 (10) | 162 |
| Hysterectomy | 10/42 (24) | 162 |
| Caesarean section | 10/51 (19.5) | 162 |
| General dentistry | 23/711 (3.2) | 141 |
| Oral surgery | 6/395 (1.5) | 72 |
| 52/570 (9.1) | 65 | |
| 52/511 (10.2) | 138 |
B. L. Johnston, D. B. Langille, and J. C. Le Blanc, Conf. Prevent. Transm. Blood-Borne Pathogens Surg. Obstet., 1994, abstr. A52.
Control of spread.
Routine HBV immunization and monitoring of immune status ensure that health care workers are protected against the risks of HBV transmission. Nonresponders to the vaccine are offered hepatitis B-specific immunoglobulin following exposure to a known HBV surface antigen (HbsAg)-positive or unidentified source (45).
In the U.K., guidelines recommending exclusion of HbeAg-positive health care workers from participating in exposure-prone procedures were introduced in 1993 (151). Exposure-prone procedures are defined as invasive procedures that pose a risk that injury to the worker may result in exposure of the patient's open tissues to the blood of the worker. These include procedures where the worker's gloved hands may be in contact with sharp instruments, needle tips, or sharp tissues, e.g., spicules of bone or teeth inside a patient's body cavity, wound, or confined anatomical space where the hands or fingertips may not be completely visible at all times. Since then, transmission of HBV from surgeons who were HbeAg-negative carriers of the virus has been demonstrated (5). This has led to an evaluation of HBV DNA testing of HBV-infected health care workers who may have precore mutants and, despite the absence of HBeAg, may have high infectivity levels. Health care workers who are HbeAg-negative carriers of HBV and have been proven to have transmitted the virus to patients should also cease carrying out exposure-prone procedures. Guidelines currently being issued in the U.K. indicate the need to carry out HBV DNA testing of HbeAg-negative health care workers who are HbsAg- positive if they are participating in exposure-prone procedures.
Single-room isolation is not routinely required for patients with acute HBV infection or carriers unless the patient is incontinent of feces or is bleeding. Careful attention to infection control procedures, including glove and gown wearing, careful disposal of sharp instruments and needles, and satisfactory decontamination procedures, is the hallmarks of preventing transmission in the clinical setting.
Hepatitis C Virus
There is a documented risk of nosocomial transmission of HCV from patients to health care workers. The risk of infection from a single percutaneous injury involving HCV-positive blood is usually estimated to be approximately 3%. The presence of HCV RNA in the blood of a source patient is associated with a greater risk of transmission to a health care worker than if the individual is RNA negative. In two studies from Japan, the transmission rate was reported as 2.7 and 10%, respectively (101, 125). The first of these studies was based on the detection of antibody to C100-3, a nonstructural region of the virus. Of 110 people exposed, 4 developed clinical hepatitis and 3 became anti-HCV positive. In the second study, 7 of 68 exposed health care workers showed specific evidence of HCV infection (all 7 were positive for HCV RNA, and 5 of these also developed antibody to C100-3).
In a survey of New York dentists conducted on stored, frozen sera collected from 1985 to 1987, 8 of 456 (1.75%) were positive for anti-HCV antibodies, compared with 1 of 723 (0.14%) control volunteer blood donors (102). Oral surgeons were more likely to be positive (4 of 43) than those in other dental specialties. In a survey of 3,267 North American orthopedic surgeons, 0.8% were found to be have antibody to HCV (149). This increased with age from 0% in the 20- to 29-year age group to 1.4% in those over the age of 60 years.
Control of spread.
There have been two published incidents of transmission of HCV from health care workers engaged in exposure-prone invasive procedures to their patients (31, 50). Further cases of health care worker-to-patient transmission of HCV in the U.K. are currently under investigation. No guidelines have been issued from the U.K. Health Departments to date, but health care workers who are shown to have transmitted the virus during surgical procedures are advised to cease engaging in exposure-prone procedures. During the investigation of suspected nosocomial transmission of HCV (as with other blood-borne viruses), molecular genetic techniques, including genotyping by restriction fragment length polymorphism analysis and genetic sequencing, assist in confirming the likelihood of a transmission event.
To emphasize the point made earlier about the importance of optimal infection control procedures to prevent patient-to-patient transmission, HCV infection has been reported in five patients successively undergoing minor surgery in a clinic in Australia (35). The mode of transmission is unknown, but the authors postulate either the reuse of a needle or syringe on successive patients or contamination of anesthetic equipment as the likely route.
Human Immunodeficiency Virus Type 1
On the basis of data reported in 25 prospective studies, the average risk of transmission of HIV following percutaneous inoculation involving blood from infected individuals was calculated to be 0.32% (95% confidence interval [C.I.], 0.18 to 0.46%); this was based on 21 infections resulting from 6,498 inoculations (85). The current estimate of the infection risk following mucocutaneous exposure to blood is 0.03% (1 infection following 2,885 exposures to mucous membranes and/or nonintact skin) (62, 82). The results of a case-control study published by the CDC in Atlanta, Ga., provided evidence of factors that may enhance the transmission risk (28). Three of these factors, deep injury, visible blood on the device, and inoculation with a needle previously used in a blood vessel, increase the odds ratio for infection to 16.1 (95% C.I., 6.1 to 44.6), 5.2 (95% C.I., 1.8 to 17.7), and 5.1 (95% C.I., 1.9 to 14.8), respectively. These factors are likely to have resulted in a larger transfer of blood to the recipient than would occur in less severe injuries or those involving solid needles or other sharp instruments. The belief that the high virus loads present in the later stages of HIV infection are likely to enhance the transmission rate was supported by the odds ratio of 6.4 (95% C.I., 2.2 to 18.9) if the source patient was known to have had terminal AIDS. Importantly, the use of postexposure prophylaxis with zidovudine was associated with an 80% reduction in transmission (odds ratio, 0.2; 95% C.I., 0.1 to 0.6) in this case-control study.
In July 1990, the CDC published the first of a series of reports describing transmission of HIV from a health care worker, a dental surgeon with AIDS, to six of his patients (22, 25, 36, 127). Since then, suspected transmission of HIV from an orthopedic surgeon to a patient during surgery has been reported (112).
Patient-to-patient transmission of HIV in health care settings has resulted from deficiencies in technique and/or decontamination procedures. Inadvertant reuse of a syringe and needle previously used for nuclear medicine investigations on an HIV-positive patient transmitted infection to another patient despite prompt administration of prophylactic antiretroviral drugs.
Control of spread.
In the U.K., in the interests of protecting the public, guidelines issued by the Health Departments in 1994 recommend exclusion from exposure-prone procedures of all HIV-positive health care workers (152). The principles of controlling nosocomial infection with HIV are similar to those applied to other blood-borne viruses, and for detail, the reader is referred to the guideline documents referred to in the introduction to this section.
Evidence of a protective effect of an antiretroviral drug used for postexposure prophylaxis, together with data from animal studies and evidence of the value of zidovudine in preventing mother-to-baby transmission of HIV, supports the continued recommendation for postexposure prophylaxis for health care workers exposed to HIV-positive material (31, 80, 153).
EXOTIC VIRUSES
Viral Hemorrhagic Fevers
Viral hemorrhagic fevers (VHFs) are a group of severe life-threatening diseases caused by a range of viruses. Most are endemic in certain parts of the world, including Africa, some parts of South America, and the U.S. and rural parts of the Middle East and eastern Europe. With the exception of the rodent-borne hantaviruses, VHFs are concentrated in tropical and subtropical areas and have only rarely been imported into industrialized countries. This apparent lack of spread is most easily explained by the fact that most of the individuals at greatest risk are unlikely or financially unable to travel internationally by air. However, this may not always be the case; as travel and commerce expand, more and more people travel internationally, and it is likely that more cases of VHFs will be seen in countries where they are not endemic. A summary of the main agents of VHF, their known vectors, recent estimates of the number of cases, and incubation times is given in Table 4. Typical clinical features for the VHFs include fever, myalgia, and headache. Leukopenia and thrombocytopenia are also common, with proteinuria following generalized capillary dilatation and leakage. Damage to parenchymal organs varies from virtually none to life-threatening, and the level of viremia may vary from virtually absent to extremely high. It is the viremic VHFs that are associated with the greatest risk of nosocomial transmission. These include the filoviruses Ebola virus and Marburg virus and the arenaviruses, particularly Lassa virus, and the Bunyavirus Congo Crimean hemorrhagic fever virus. These are addressed in greater detail below.
TABLE 4.
Main agents of VHFs
| Virus | No. of cases/yr | Geographic distributiona | Zoonotic element | Incubation period (days) | Mortality (%) |
|---|---|---|---|---|---|
| Arenaviridae | |||||
| Junin virus | 20–200 | N/C Argentina | Mouse | 7–14 | 1–10 |
| Machupo virus | <10 | NE Bolivia | Mouse | 7–14 | 10–20 |
| Guanarito virus | 0–100 | C Venezuela | Mouse | ? | 25 |
| Lassa virus | 10,000 | W Africa | Mouse | 7–16 | 10–30 |
| Bunyaviridae | |||||
| Congo Crimean hemorrhagic fever virus | 10–100 | Africa, Middle East, W China, N Asia, Balkans | Cows, hares, ticks | 2–6 | 10–40 |
| Hantaan | 100,000–200,000 | Europe, Scandinavia | Mice, rats | 14–30 | <1–10 |
| Hantavirus Sin Nombre virus | 125 since 1993 | N America | Mice | 7–14? | 40–50 |
| Rift Valley fever virus | 200,000–1,000,000 | Africa | Cattle mosquito | 2–6 | 30–50 |
| Filoviridae | |||||
| Ebola and Marburg viruses | 5–300 | Sub-Saharan Africa | ? | 5–12 | 50–90 |
| Flaviviridae | |||||
| Dengue virus 1–4 | 5,000–50,000 | SE Asia, Caribbean, S America | Mosquito | 2–5 | 2–10 |
| Yellow fever virus | 100–20,000 | Tropical Africa, Amazon | Primates | 2–5 | 10–30 |
| Kyasanur forest disease virus | 100–400 | India | Mosquito, cattle, ticks, birds, monkeys | 3–7 | ? |
| Omsk hemorrhagic fever virus | None recent | W Siberia | Vole, ticks | 3–7 | <1–3 |
N, north; S, south; E, east; W, west; C, central.
Ebola and Marburg viruses.
Currently, four serotypes of Ebola viruses (Zaire, Sudan, Reston, and Ivory Coast) and one Marburg virus have been identified. All have a similar appearance by electron microscopy, and all are human pathogens, with the exception of the Reston subtype. Marburg virus was the first member of this family to appear in Germany in 1967 (115). Thirty-one cases occurred in laboratory workers handling infected African green monkeys that had been imported to provide kidney cells to produce polio vaccine. The monkeys, some of which had been shipped to Frankfurt and Belgrade, were euthanized, and the epidemic was contained; however, this was not before secondary transmissions to hospital staff and family members had occurred. The significant mortality (23%) and a general lack of knowledge regarding the life cycle and natural hosts of the virus resulted in the implementation of strict quarantine procedures for monkeys to prevent a recurrence (43). Tests to detect Marburg virus in vaccine substrates have also been implemented (131). Over the following 20 years, only three isolated cases were detected, in 1975, 1982, and 1987. All were in humans traveling in rural Africa, two of whom had visited Kitum cave in the Mt. Elgon region of Kenya, and no secondary cases occurred (37, 92, 142). In May 1999, an outbreak of Marburg disease was confirmed in the Democratic Republic of Congo (DRC) (175). Although the natural reservoir is unknown, contact with infected monkeys is likely to be important. Person-to-person transmission is most likely to follow contact with infected blood; however, aerosol transmission has not been discounted, and sexual intercourse in the convalescent phase of the infection has resulted in a secondary case.
Ebola virus was identified in the late 1970s as the causative agent of two simultaneous outbreaks of hemorrhagic fever in the DRC and Sudan (172, 173). In the DRC there were 318 cases, resulting in 280 deaths (88% mortality), and in Sudan there were 299 cases, with 150 deaths (50% mortality). In the Madiri hospital in the Sudan, 76 of the 230 medical staff became infected, and 41 died. Secondary transmission was linked to the reuse of unsterilized needles and direct contact with body fluids. Inadvertent inoculation with material from this outbreak resulted in a serious but nonfatal laboratory infection in the U.K. A further sporadic case was reported from the DRC in 1977, and 34 cases were reported from Sudan in 1979.
In 1989 Ebola virus was isolated from cynomolgus monkeys in quarantine facilities in Reston, Va. (87); further epidemics occurred in this facility in 1992 and 1996. In 1996 an outbreak in Siena, Italy, was associated with another batch of cynomolgus monkeys (139). Although no human cases were identified, there was serological evidence of asymptomatic infection in 15 individuals. The batches of monkeys were all linked to one Filipino exporter, but the actual source of the virus remains a mystery. This strain of Ebola is of Asian origin and appears to be less pathogenic for humans and macaques than other Ebola viruses. The current quarantine procedures for imported primates have prevented spread into the general population. Between 1994 and 1996, five independent sites of Ebola virus transmission were identified: Côte d'Ivoire in 1994, DRC in 1995, and Gabon in 1994, 1995, and 1996. All occurred in sites at or near tropical forests. The case in the Côte d'Ivoire was a Swiss ethnologist who contracted the infection during her investigation of the unusually high mortality in a chimpanzee troupe that she was studying. During the postmortem, she was the only one to wear household instead of latex gloves; thus, it is likely that she came into direct contact with infected body fluids. Her two colleagues who took part in the postmortem remained well. She recovered following repatriation to Switzerland, and there was no evidence of transmission to 18 contacts in the Côte d'Ivoire, 52 medical personnel in Switzerland, or members in the air ambulance service. The episode in Kikwit, DRC, was intensively reported by the world press. Over 300 cases were identified, with a 77% mortality, and 50% of the cases were among hospital staff or caregivers responsible for known cases. The international community first became aware of the problem in Kikwit in May 1995; however, retrospective epidemiological investigations suggested that the virus had been circulating since January of that year (99). The index case was identified as a charcoal worker and farmer who died of a hemorrhagic illness 7 days after his admission to hospital in January. Three members of his family and 10 secondary contacts died. Towards the end of April, a nosocomial outbreak among theater staff was linked to a laparotomy performed on an infected laboratory worker presumed to have a perforated viscus. International scientific and medical teams were dispatched in May 1995 to implement infection control measures and monitor the outbreak. Adequate sanitation and electricity supplies were reconnected, safe disposal of sharps was implemented, and oral as opposed to intravenous rehydration was encouraged when possible. A quarantine facility was also established. These measures terminated further nosocomial transmission (17). Factors identified as important in transmission included direct physical contact with an infected person during the symptomatic phase. Twenty secondary cases had touched an ill person, while none of the 78 household members without physical contact became infected.
Contact with infected body fluids, blood, stool, vomitus, and urine was also identified as important, and high-titered virus has been demonstrated in the urine of nonhuman primates (49). Indirect contacts following percutaneous exposures and laboratory accidents are also important in the hospital setting. Exposure to an infected person in the early stages of infection prehospitalization may also result in infection. Obviously this has potential public health implications in situations such as contact with a mildly ill patient on an airplane. The role of aerosol transmission is still not clear. While there is experimental evidence to support droplet transmission in rhesus monkeys (90), there is no clear evidence of its importance in human populations. Its potential importance should not be underestimated, however, and the relative risk may depend on the strain of the virus (132).
Lassa fever virus.
The first outbreak of Lassa fever occurred in 1969 in a small hospital in Lassa, in northern Nigeria. The first case was a nurse who died about 14 days after the onset of her symptoms. Two further nurses who cared for her also contracted the infection; one died, and the other was repatriated to the U.S. and eventually recovered after a prolonged illness. Two laboratory workers also became ill; one had handled infected tissue cultures, and the other was based in another laboratory in the same building. Over the years, at least 12 patients have contracted Lassa fever and have been airlifted to Europe or the U.S. Despite a range of containment levels used, there has not been any serological evidence of secondary cases in over 200 apparently healthy contacts (39). The most recent imported case in the U.K. was a British aid worker based in Daru, eastern Sierra Leone. He became unwell on 21 February 2000 and was transferred to London on 6 March. Following the development of hemorrhagic complications, he was transferred to the high-security facility at Coppetts Wood Hospital in London, where he subsequently died.
In its natural host (the multimammate rat, Mastomys natalensis), Lassa fever virus causes a chronic asymptomatic infection, with excretion of virus in the urine. In rural west Africa, direct contact with the infected urine via scratches or abrasions or indirect contact with contaminated foods or materials probably results in transmission. Person-to-person transfer results from contact with infected body fluids or following accidental exposure to blood via contaminated sharps. Intimate contact may also result in transmission; this may be a problem, as viral excretion may continue for a few months postrecovery. The high level of endemicity of Lassa fever in west Africa is illustrated by the high annual incidences reported in Table 3.
Congo Crimean hemorrhagic fever virus.
This virus has a wide distribution and has been found in Russia, the central Asian republics, Dubai, Iraq, South Africa, Pakistan, Greece, Albania, Turkey, Afghanistan, and India. Geographic variation in virulence has been observed, with more severe disease following infection with Asian than with African strains. Natural infection results from a tick bite, but person-to-person transmission can occur following contact with infected body fluids. Secondary cases have also occurred following exposure to cases requiring resuscitation (146).
Control of spread.
The most common cause of fever in patients returning to Europe from Africa is malaria. Other causes include typhoid fever, dengue, and rickettsial infections. Although in practical terms most febrile patients returning from Africa will not have a VHF, it is essential to assess their risk so that appropriate infection control procedures can be implemented. Both the CDC (29) and the Advisory Committee on Dangerous Pathogens (ACDP), U.K. (2), have defined important criteria on which to base a risk assessment for an individual febrile patient: (i) residence in an area where these viruses are endemic during the 21 days before the onset of symptoms; (ii) direct contact with blood, other body fluids, secretions, and excretions of a person or animal with or strongly suspected of having a VHF (this includes hospital staff, laboratory workers, and household contacts); and (iii) residence in areas adjacent to regions where these viruses are endemic and development of organ failure and/or hemorrhagic complications, which could be due to a VHF, within 21 days.
Based on these criteria, the ACDP guidelines then categorize patients into minimum, moderate, and high risk. Patients in the minimum-risk category are extremely unlikely to have a VHF and can be managed in a general hospital or infectious disease unit using standard universal precautions. Patients in the moderate category can be managed in either a high-security infectious disease unit (HSIDU) or intermediate isolation facility, whereas those in the high-risk category should be transferred to an HSIDU. The two HSIDU facilities in the U.K. are on constant alert to receive patients with known or suspected VHFs and are equipped with plastic isolators for high-security isolation of patients. An initial sample to exclude malaria can be tested locally, providing the blood film is prepared in a class 1 cabinet and the technician wears gloves and protective clothing. The slide is flooded with methanol and then ethanol and, after examination, is disposed of immediately into a sharps box. Any further samples taken for ongoing management from patients in these categories should be processed in the high-security laboratories attached to the HSIDUs. The CDC guidelines do not stratify the patients according to risk. All patients should be isolated, preferably in a negative-pressure room, although this may not be necessary in the early stages of the infection. All staff entering the room should wear gloves and gowns, and for those with close patient contact, goggles and face protection are recommended. If the patient has prominent respiratory symptoms, vomiting, diarrhea, or hemorrhage, additional protection with personal respirators is recommended. In the U.S., clinical samples are handled in a class 2 cabinet following biosafety level 3 practices. It is recommended that serum be treated with 10 μl of 10% Triton X-100 per ml of serum for 1 h. This will reduce but not eliminate infectious virus. Automated analyzers should be disinfected as recommended by the manufacturer or with 1:100 sodium hypochloride after use.
Both the CDC and ACDP recommended the following: (i) environmental decontamination with household bleach (10,000 ppm available chlorine); (ii) use of disposable linen (decontamination in an autoclave and washing in a washing machine with bleach are alternatives); (iii) autoclaving, incineration, or treatment with household bleach for >5 min of all waste materials from the patients; (iv) if a patient dies, the body should be handled minimally and placed in a sealable plastic body bag; it should be placed in a robust coffin and stored separately before burial or cremation; (v) persons with accidental percutaneous or mucous membrane exposure should wash the area immediately with copious amounts of soap and water. Expert advice concerning the possible prophylactic use of antiviral drugs should be sought urgently.
The diagnosis of a VHF can be confirmed by antibody testing or by the detection of viral RNA in blood or other body fluids. In the U.K., virus isolation is usually also attempted.
Rabies Virus
Rabies virus infection results in an almost uniformly fatal viral encephalitis in both humans and animals. Rabies was first recognized in Babylon in the 23rd century BC, and early attempts to treat rabies included wound cautery and cleansing. These recommendations continued until Pasteur introduced immunization in 1885. On a global scale, rabies exposure has serious medical and economic implications, accounting for up to 40,000 deaths a year, mainly in resource-deprived countries; as many as 4 million people receive postexposure prophylaxis per annum (121). Although they have minimal side effects and are highly immunogenic, the current human diploid cell vaccines (HDCV) are expensive. In poorer countries, this often leads to the use of older, animal-derived vaccines that are associated with CNS complications in up to 1 in 200 vaccinees.
Virus-laden saliva from a rabid animal is introduced following a scratch or bite or, less commonly, following mucous membrane exposure. Humans are more resistant to rabies infection than other mammals—only about 40% of individuals exposed to the infection develop the disease. Theoretically, nosocomial transmission could occur following contact with saliva from an infected individual; however, to date this has not occurred, and the only reports of nosocomial transmission have been for two patients who received corneal grafts from donors with “obscure neurological conditions”; one had a Guillain-Barre-type syndrome, and the other had flaccid paralysis (79).
Laboratory personnel working with bats or other rabid animals are also at risk. Aerosolization of laboratory strains has been reported, resulting in revised safety recommendations for laboratory personnel working with rabies virus (26).
Control of spread.
Standard isolation is recommended for patients with proven or suspected rabies. The diagnosis can be confirmed with immunofluorescence staining of a skin biopsy from the nape of the neck above the hairline. Staff should be warned of the potential hazard of infection from saliva and take appropriate precautions to avoid exposure. This may include masks if the patient is coughing saliva. Immunization with HDCV is recommended for persons with a documented exposure, for those with a possible exposure, and for preexposure prophylaxis for laboratory workers and others whose occupation may expose them to rabid animals, e.g., veterinarians and biologists. Postexposure prophylaxis of hospital personnel needs to be judged on an individual basis according to established guidelines (121). In addition to vaccination, local wounds should be throughly cleaned with soap and water, and if possible, the wound should be infiltrated with rabies immunoglobulin.
CONCLUSIONS
The main defenses against nosocomial transmission of viral diseases are education, awareness of transmission routes, and strict adherence to infection control procedures. Recent developments in diagnostic techniques and improvements in ward and clinic design assist in preventing or reducing the number of contact cases.
Many of the documented instances of nosocomial transmission of viral disease have been atrributable to clear deficiences in infection control. The economic implications of outbreaks of viral disease in hospitals are usually less easily defined than those due to bacterial disease, as they frequently represent the overspill of community-based epidemics. Although it may be identifiable as a problem for years to come, the relatively low incidence of resistance of viruses to currently available antiviral drugs means that this is currently less of a problem than in the control of nosocomial bacterial infections.
ACKNOWLEDGMENTS
We are pleased to acknowledge the assistance of the PHLS Communicable Disease Surveillance Centre, London NW9 5EQ, U.K., who provided recent data on measles, mumps, and rubella incidence in the U.K.
REFERENCES
- 1.Abbas J, Whimbey E. Infection control of nosocomial respiratory viral disease in the immunocompromised host. Am J Med. 1997;102:48–52. doi: 10.1016/s0002-9343(97)00011-9. [DOI] [PubMed] [Google Scholar]
- 2.ACDP. Management and control of viral hemorrhagic fevers. London, United Kingdom: London Stationery Office; 1996. [Google Scholar]
- 3.Adams R H, Christenson J C, Petersen F B, Beatty P G. Pre-emptive use of aerosolised ribavirin in the treatment of asymptomatic pediatric marrow transplant patients testing positive for RSV. Bone Marrow Transpl. 1999;24:661–664. doi: 10.1038/sj.bmt.1701959. [DOI] [PubMed] [Google Scholar]
- 4.Adler S P, Manganello A M, Koch W C, Hempfling S H, Best A M. Risk of human parvovirus B19 infections among school and hospital employees during endemic periods. J Infect Dis. 1993;168:361–368. doi: 10.1093/infdis/168.2.361. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. Transmission of hepatitis B to patients from four infected surgeons without hepatitis B e antigen. New Eng J Med. 1997;336:178–184. doi: 10.1056/NEJM199701163360304. [DOI] [PubMed] [Google Scholar]
- 6.Ansari S A, Springthorpe V S, Sattar S A, Rivard S, Rahman M. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29:2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apalsch A M, Green M, Ledesma-Medina J, Nour B, Wald E R. Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Clin Infect Dis. 1995;20:394–399. doi: 10.1093/clinids/20.2.394. [DOI] [PubMed] [Google Scholar]
- 8.Arbeter A M, Starr S E, Preblud S R, Ihara T, Paciorek P M, Miller D S, Zelson C M, Procter E A, Plotkin S A. Varicella vaccine trials in healthy children: a summary of comparative follow-up studies. Am J Dis Child. 1984;138:434–438. doi: 10.1001/archpedi.1984.02140430012004. [DOI] [PubMed] [Google Scholar]
- 9.Asano Y, Yoshikawa T, Suga S, Kobayashi I, Nakashima T, Yazaki T, Osaki T, Yamada A, Imanishi J. Post exposure prophylaxis of varicella in family contact by oral acyclovir. Pediatrics. 1993;92:219–222. [PubMed] [Google Scholar]
- 10.Balcarek K B, Bagley R, Cloud G A, Pass R F. Cytomegalovirus infection among employees of a childrens hospital: no evidence for increased risk associated with patient care. JAMA. 1990;263:840–844. [PubMed] [Google Scholar]
- 11.Bell J A, Rowe W P, Engler J I, Parrott R H, Huebner R J. Pharyngoconjunctival fever: epidemiological studies of a recently recognized disease entity. JAMA. 1955;157:1083–1092. doi: 10.1001/jama.1955.02950300011003. [DOI] [PubMed] [Google Scholar]
- 12.Bell L M, Naides S J, Stoffman P, Hodinka R L, Plotkin S A. Human parvovirus B19 infection among hospital staff members after contact with infected patients. N Engl J Med. 1989;321:485–491. doi: 10.1056/NEJM198908243210801. [DOI] [PubMed] [Google Scholar]
- 13.Bowden R A. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 14.Brady M T, Pacini D L, Budde C T, Connell M. Diagnostic studies of nosocomial diarrhoea in children: assessing their use and value. Am J Infect Control. 1989;17:77–82. doi: 10.1016/0196-6553(89)90021-7. [DOI] [PubMed] [Google Scholar]
- 15.Brady M T, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18:18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 16.Brokstad K A, Cox R J, Olofsson J, Jonsson R, Haaheim L R. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- 17.Bwaka M, Bonnet M J, Calain P, Colebunders R, De Roo A, Guimard Y, Katwiki K R, Kabadi K, Kipasa M A, Kuvula K J, Mapanda B B, Massanda M, Mupapa K D, Muyembe-Tamfum J J, Ndaberey E, Peters C J, Rollin P E, Van den Enden E. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(suppl. 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 18.Carnes B, Rahier J, Burtomboy G, de Ville de Goyet J, Reding R, Lamy M, Otte J B, Sokal E M. Acute adenovirus hepatitis in liver transplant recipients. J Pediatr. 1992;120:33–37. doi: 10.1016/s0022-3476(05)80593-1. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control. Parainfluenza outbreaks in extended-care facilities. Morb Mortal Wkly Rep. 1978;27:475. [Google Scholar]
- 20.Centers for Disease Control. Recommendations for preventions of HIV transmission in health care settings. Morb Mortal Wkly Rep. 1987;36:1–8S. [Google Scholar]
- 21.Centers for Disease Control. Universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. Morb Mortal Wkly Rep. 1988;37:377–82. , 387–388. [PubMed] [Google Scholar]
- 22.Centers for Disease Control. Possible transmission of human immunodeficiency virus to a patient during an invasive dental procedure. Morb Mortal Wkly Rep. 1990;39:489–493. [PubMed] [Google Scholar]
- 23.Centers for Disease Control. Control of influenza A outbreaks in nursing homes: amantadine as adjunct to vaccine, 1989–1990. Morb Mortal Wkly Rep. 1991;40:841–844. [PubMed] [Google Scholar]
- 24.Centers for Disease Control. Update: transmission of HIV infection during an invasive dental procedure—Florida. Morb Mortal Wkly Rep. 1991;40:21–27. , 33. [PubMed] [Google Scholar]
- 25.Centers for Disease Control. Update: transmission of HIV infection during invasive dental procedures—Florida. Morb Mortal Wkly Rep. 1991;40:377–381. [PubMed] [Google Scholar]
- 26.Centers for Disease Control. Recommendations of the immunization Practices Advisory Committee (ACIP): rabies prevention—United States. Morb Mortal Wkly Rep. 1991;401(RR-3):1–16. [Google Scholar]
- 27.Centers for Disease Control. Update: investigations of persons treated by HIV-infected health care workers—United States. Morb Mortal Wkly Rep. 1993;42:329–337. [PubMed] [Google Scholar]
- 28.Centers for Disease Control. Case-control study of HIV seroconversion in health-care workers after percutaneous exposures to HIV-infected blood—France, United Kingdom and United States, January 1988–August 1994. Morb Mortal Wkly Rep. 1995;44:929. [PubMed] [Google Scholar]
- 29.Centers for Disease Control. Update: outbreak of Ebola viral hemorrhagic fever—Zaire. Morb Mortal Wkly Rep. 1995;44:468–469. , 475. [PubMed] [Google Scholar]
- 30.Centers for Disease Control. Varicella prevention: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 1996;45(RR-11):1–37. [PubMed] [Google Scholar]
- 31.Centers for Disease Control. Public health Service guidelines for the management of health care workers, exposures to HIV and recommendations for post exposure prophylaxis. Morb Mortal Wkly Rep. 1998;47(RR-7):1–33. [PubMed] [Google Scholar]
- 32.Centers for Disease Control. 2000. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49(RR-3). [PubMed]
- 33.Centers for Disease Control. Poliomyelitis prevention in the United States: updated recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 2000;49(RR-5):1–22. [PubMed] [Google Scholar]
- 34.Chadwick P R, Walker M, Rees A E. Airborne transmission of a small round structured virus. Lancet. 1994;343:171. doi: 10.1016/s0140-6736(94)90959-8. [DOI] [PubMed] [Google Scholar]
- 35.Chant K, Kociuba K, Munro R. Investigation of possible patient-to-patient transmission of hepatitis C in a hospital. New South Wales Public Health Bull. 1994;5:47–51. [Google Scholar]
- 36.Ciesielski C, Marianos D, Ou C Y, Dumbaugh R, Witte J, Berkelman R, Gooch B, Myers G, Luo C C, Schochetman G. Transmission of human immunodeficiency virus in a dental practice. Ann Intern Med. 1992;116:798–805. doi: 10.7326/0003-4819-116-10-798. [DOI] [PubMed] [Google Scholar]
- 37.Conrad J L, Isaacson M, Smith E B, Wulff H, Crees M, Geldenhuys P, Johnston J. Epidemiological investigation of Marburg virus disease, Southern Africa, 1975. Am J Trop Med Hyg. 1978;27:1210–1215. doi: 10.4269/ajtmh.1978.27.1210. [DOI] [PubMed] [Google Scholar]
- 38.Contreras P A, Sami I R, Darnell M E, Ottolini M E, Prince G A. Inactivation of respiratory syncytial virus by generic hand dishwashing detergents and antibacterial hand soaps. Infect Cont Hosp Epidemiol. 1999;20:57–58. doi: 10.1086/501550. [DOI] [PubMed] [Google Scholar]
- 39.Cooper C B, Gransden W, Webster M, King M, O'Mahony M, Young S, Banatvala J E. A case of Lassa fever: experience at St. Thomas's Hospital. Br Med J. 1982;285:1003–1005. doi: 10.1136/bmj.285.6347.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cossart Y E, Field A M, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 41.Cubitt W D, Holzel H. An outbreak of rotavirus infections in a long-stay ward of a geriatrics hospital. J Clin Pathol. 1980;33:306–309. doi: 10.1136/jcp.33.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cubitt W D. Caliciviruses. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. New York, N.Y: Marcel Dekker; 1994. pp. 549–568. [Google Scholar]
- 43.De Marcus T A, Tipple M A, Ostrowiski S R. US policies for disease control among imported nonhuman primates. J Infect Dis. 1999;179(Suppl. I):S282–S283. doi: 10.1086/514279. [DOI] [PubMed] [Google Scholar]
- 44.Demmler G J, Yow M D, Spector S. Nosocomial cytomegalovirus infections within two hospitals caring for infants and children. J Infect Dis. 1987;156:9–16. doi: 10.1093/infdis/156.1.9. [DOI] [PubMed] [Google Scholar]
- 45.Department of Health, Welsh Office Department of Health, DHSS (Northern Ireland) Immunization against infectious disease. London, United Kingdom: HMSO; 1996. [Google Scholar]
- 46.Desselburger U. Viruses associated with acute diarrhoeal diseases. In: Zuckerman A J, Banatvala J E, Pattison J R, editors. Principles and practice of clinical virology. 4th ed. Chichester, United Kingdom: John Wiley and Sons; 2000. pp. 235–252. [Google Scholar]
- 47.Dick E C, Jennings L C, Mink K A, Wartgow C D, Inhorn S L. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156:442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 48.Doebbeling B N, Li N, Wenzel R P. An outbreak of hepatitis A among health care workers: risk factors for transmission. Am J Public Health. 1993;83:1679–1684. doi: 10.2105/ajph.83.12.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowell S F, Mukunu R, Ksiazek T G, Khan A S, Rollin C J, Peters Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of Congo, 1995. J Infect Dis. 1999;179:S87–S89. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 50.Duckworth G J, Heptonstall J, Aitken C, Curminis A, Gaya H, Gill O N, Gorman A, Graneek B, Jeffries D, Simmonds P, Taylor P, Tedder R S, Teo C G, van den Bosh C, Wright J E C. Transmission of hepatitis C virus from a surgeon to a patient. Commun Dis Public Health. 1999;2:188–192. [PubMed] [Google Scholar]
- 51.Dudding B A, Top F H, Winter P E, Buescher E L, Lamson T H, Leibovitz A. Acute respiratory disease in military trainees. The adenovirus surveillance program 1966–1971. Am J Epidemiol. 1973;97:187–98. doi: 10.1093/oxfordjournals.aje.a121499. [DOI] [PubMed] [Google Scholar]
- 52.Enders G, Miller E, Craddock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548–1551. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 53.Esteban J L, Gomez J, Martell M, Cabot B, Quer J, Camps J, Gonzalez A, Otero T, Moya A, Esteban R. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555–560. doi: 10.1056/NEJM199602293340902. [DOI] [PubMed] [Google Scholar]
- 54.Fairley C K, Smoleniec J S, Caul O E, Miller E. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet. 1995;346:1335–1337. doi: 10.1016/s0140-6736(95)92346-2. [DOI] [PubMed] [Google Scholar]
- 55.Feldman S, Hughes W T, Daniel C B. Varicella in children with cancer: seventy seven cases. Pediatrics. 1975;56:388–397. [PubMed] [Google Scholar]
- 56.Foley F E, Gutheim R N. Serum hepatitis following dental procedures: a presentation of 15 cases, including 3 fatalities. Ann Intern Med. 1956;45:369–380. doi: 10.7326/0003-4819-45-3-369. [DOI] [PubMed] [Google Scholar]
- 57.Ford-Jones E L, Mindorff C M, Gold R, Petric M. The incidence of viral associated diarrhoea after admission to a pediatric hospital. Am J Epidemiol. 1990;131:711–718. doi: 10.1093/oxfordjournals.aje.a115555. [DOI] [PubMed] [Google Scholar]
- 58.Frickhofen N, Abkowitz J L, Safford M, Berry J M, Antunez-de-Mayolo J, Astrow A, Cohen R, Halperin J, King L, Mintzer D. Persistent parvovirus B19 infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anaemia in AIDS. Ann Intern Med. 1990;113:926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 59.Gaggero A, Avendano L F, Fernadez J, Spencer E. Nosocomial transmission of rotavirus from patients admitted with diarrhoea, J. Clin Microbiol. 1992;30:3294–3297. doi: 10.1128/jcm.30.12.3294-3297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gellert G A, Waterman S H, Ewert D, Oshiro L, Giles M P, Monroe S S, Gonelkin L, Glass R I. An outbreak of gastroenteritis caused by a small round structured virus in a geriatric convalescent facility. Infect Control Hosp Epidemiol. 1990;11:459–464. doi: 10.1086/646212. [DOI] [PubMed] [Google Scholar]
- 61.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerberding J L. Management of occupational exposures to bloodborne viruses. N Engl J Med. 1995;332:444. doi: 10.1056/NEJM199502163320707. [DOI] [PubMed] [Google Scholar]
- 63.Gershon A A. Human responses to live attenuated varicella vaccine. Rev Infect Dis. 1991;13(Suppl.):957–959. doi: 10.1093/clind/13.supplement_11.s957. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S, Champlin R, Couch R, England J, Raad I, Malik S, Luna M, Whimbey E. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis. 1999;29:528–532. doi: 10.1086/598627. [DOI] [PubMed] [Google Scholar]
- 65.Goodman R A, Ahtone J L, Finton R J. Hepatitis B transmission from dental personnel to patients unfinished business. Ann Intern Med. 1982;96:119. doi: 10.7326/0003-4819-96-1-119_1. [DOI] [PubMed] [Google Scholar]
- 66.Gopal M R, Thompson B J, Fox J, Tedder R S, Honess R W. Detection by PCR of HHV6 and EBV DNA in blood and oropharynx of healthy adults and HIV seropositives. Lancet. 1990;335:1598–1599. doi: 10.1016/0140-6736(90)91433-b. [DOI] [PubMed] [Google Scholar]
- 67.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark G F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;18:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grady G F, Lee V A, Prince A M, Gitnick G L, Fawaz K A, Vyas G N, Levitt M D, Senior J R, Galambos J T, Bynum T E, Singleton J W, Clowdus B F, Akdamar K, Aach R D, Winkelman E L, Schiff G M, Hersh T. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicentre controlled trial. J Infect Dis. 1978;138:625–638. doi: 10.1093/infdis/138.5.625. [DOI] [PubMed] [Google Scholar]
- 69.Greaves W L. The problem of herpetic whitlow among hospital personnel. Infect Control. 1980;1:381. [Google Scholar]
- 70.Groarke J M, Peviar D C. Attenuated virulence of pleconaril-resistant coxsackie virus B3 variants. J Infect Dis. 1999;179:1538–1541. doi: 10.1086/314758. [DOI] [PubMed] [Google Scholar]
- 71.Gubareva L V, Matrosovich M N, Brenner M K, Bethell R C, Webster R G. Evidence for zanamavir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis. 1998;178:1257–1262. doi: 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- 72.Hadler S C, Sorley D L, Acree K H, Webster H M, Schable C A, Francis D P, Maynard J E. An outbreak of hepatitis B in a dental practice. Ann Intern Med. 1981;95:133–138. doi: 10.7326/0003-4819-95-2-133. [DOI] [PubMed] [Google Scholar]
- 73.Haerem J W, Siebke J-C, Ulstrup J, Geiran O, Helle I. HBsAg transmission from a cardiac surgeon incubating hepatitis B resulting in chronic antigenaemia in four patients. Acta Med Scand. 1981;210:389–392. doi: 10.1111/j.0954-6820.1981.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 74.Hall C B. The shedding and spreading of respiratory syncytial virus. Pediatr Res. 1977;11:236. [PubMed] [Google Scholar]
- 75.Hall C B. Nosocomial viral respiratory infections: perennial weeds on pediatric wards. Am J Med. 1981;70:670–676. doi: 10.1016/0002-9343(81)90594-5. [DOI] [PubMed] [Google Scholar]
- 76.Hall C B, Douglas G, German J. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141:98–101. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 77.Hall C B, McCarthy C A. Respiratory syncytial virus. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1501–1519. [Google Scholar]
- 78.Harrington R D, Hooton T M, Hackman R C, Starch G A, Osborne B, Gleaves C A, Benson A, Meyers J D. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 79.Helmick C G, Tauxe R V, Vernon A A. Is there risk to contacts of patients with rabies? Rev Infect Dis. 1986;9:511–518. doi: 10.1093/clinids/9.3.511. [DOI] [PubMed] [Google Scholar]
- 80.Henderson D K. Post exposure chemoprophylaxis for occupational exposures to the human immunodeficiency virus, J. Am Med Assoc. 1999;281:931–936. doi: 10.1001/jama.281.10.931. [DOI] [PubMed] [Google Scholar]
- 81.Heptonstall J. Outbreaks of hepatitis B virus infection associated with infected surgical staff. PHLS Commun Dis Rep. 1991;1:R81–R85. [PubMed] [Google Scholar]
- 82.Heptonstall J, Porter K, Gill O N. Occupational HIV: summary of published reports. London, United Kingdom: Public Health Laboratory Service Communicable Disease Surveillance Centre; 1995. [Google Scholar]
- 83.Hidaka Y, Liu Y, Yamomoto M, Mori R, Miyazaki C, Kusuhara K, Okada K, Euda K. Frequent isolation of human herpes 7 from saliva samples. J Med Virol. 1993;40:343. doi: 10.1002/jmv.1890400416. [DOI] [PubMed] [Google Scholar]
- 84.Hoofnagle J H, Seeff L B, Buskell Bales Z, Wright E C, Zimmerman H J. Veterans Administration Cooperative Study Group. Passive-active immunity from hepatitis B immune globulin: re-analysis of a Veterans Administration cooperative study of needlestick hepatitis. Ann Intern Med. 1979;91:813–818. doi: 10.7326/0003-4819-91-6-813. [DOI] [PubMed] [Google Scholar]
- 85.Ippolito G, Puro V, de Carli G. The risk of occupational human immunodeficiency virus infection in health care workers. Italian Multicentre Study. The Italian Study Group on Occupational Risk of HIV Infection. Arch Intern Med. 1993;153:1451. [PubMed] [Google Scholar]
- 86.Issacs D, Doubloon S R M, Wilkinson A R, Hope D L, Eglin R, Moxon E R. Conservative management of echovirus 11 outbreak in a neonatal unit. Lancet. 1989;1:543–545. doi: 10.1016/s0140-6736(89)90078-0. [DOI] [PubMed] [Google Scholar]
- 87.Jahrling P B, Geisbert T W, Dalgard D W, Johnson E D, Ksiazek T E, Hall W C, Peters C J. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet. 1990;335:502–505. doi: 10.1016/0140-6736(90)90737-p. [DOI] [PubMed] [Google Scholar]
- 88.Jawetz E. The story of shipyard eye. Br Med J. 1959;1:873–876. doi: 10.1136/bmj.1.5126.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jayashree S, Bhan M K, Raj P, Kumar R, Svensson L, Stinzing L, Bhandari N. Neonatal rotavirus infection and its relation to cord blood antibodies. Scand J Infect Dis. 1988;20:249–253. doi: 10.3109/00365548809032447. [DOI] [PubMed] [Google Scholar]
- 90.Johnson E, Jaax N, White J, Jahrling P. Lethal infections of rhesus monkeys by aerosolised Ebola virus. Int J Exp Pathol. 1995;76:227–236. [PMC free article] [PubMed] [Google Scholar]
- 91.Reference deleted.
- 92.Johnson E D, Johnson B K, Silverstein D, Tukei P, Geisbert T W, Sanchez A N, Jahrling P B. Characterisation of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch Virol Suppl. 1996;11:101–114. doi: 10.1007/978-3-7091-7482-1_10. [DOI] [PubMed] [Google Scholar]
- 93.Jones B L, Clark S, Curran E T, McNamee S, Horne G, Thakker B, Hood J. Control of an outbreak of respiratory syncytial virus infection in immunocompromised adults. J Hosp Infect. 2000;44:53–57. doi: 10.1053/jhin.1999.0666. [DOI] [PubMed] [Google Scholar]
- 94.Kapikian A Z, Estes M K, Chanock R M. Norwalk group of viruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 783–810. [Google Scholar]
- 95.Kaplan J E, Feldman R, Campbell D S, Lookabaugh C, Gary G W. The frequency of a Norwalk-like pattern of illness in outbreaks of acute gastroenteritis. Am J Public Health. 1982;72:1329–1322. doi: 10.2105/ajph.72.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karanfil L, Conlon M, Lykens K, Masters C F, Forman M, Griffith M E, Townsend T R, Perl T M. Reducing the rate of nosocomially transmitted respiratory syncytial virus. Am J Infect Control. 1999;27:91–96. doi: 10.1016/s0196-6553(99)70087-8. [DOI] [PubMed] [Google Scholar]
- 97.Karron R A, O'Brien K L, Froelich J L, Brown V A. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167:1441–1445. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- 98.Kelly T W, Patrick M R, Hillman K M. Study of diarrhoea in critically ill patients. Crit Care Med. 1983;11:7–9. doi: 10.1097/00003246-198301000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Khan A S, Tshioko F K, Heyman D L, Le Guenno B, Nabeth P, Kerstiens B, Fleerackers Y, Kilmarx P H, Rodier G R, Nkuku O, Rollin P E, Sanchez A, Zaki S R, Swanepoel R, Tomori O, Nichol S T, Peters C J, Muyembe-Tamfum J J, Ksiazek T G. The re-emergence of Ebola hemorrhagic fever in Democratic Republic of Congo 1995. J Infect Dis. 1999;179(Suppl. 1):S76–S86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 100.King J C. Community respiratory viruses in individuals with human immunodeficiency virus infection. Am J Med. 1997;102:19–23. doi: 10.1016/S0002-9343(97)80005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kiyosawa K, Sodeyama T, Tanaka E, Nakano Y, Furuta S, Nishioka K, Purcell R H, Alter H J. Hepatitis C in hospital employees with needlestick injuries. Ann Intern Med. 1991;115:367–369. doi: 10.7326/0003-4819-115-5-367. [DOI] [PubMed] [Google Scholar]
- 102.Klein R S, Freeman K, Taylor P E, Stevens C. Occupational risk for hepatitis C infection among New York City dentists. Lancet. 1991;338:1540–1542. doi: 10.1016/0140-6736(91)92369-d. [DOI] [PubMed] [Google Scholar]
- 103.Koziol D E, Kurtzman G, Ayub J, Young N S, Henderson D K. Nosocomial human parvovirus B19 infection: lack of transmission from a chronically infected patient to hospital staff. Infect Control Hosp Epidemiol. 1992;13:343–348. doi: 10.1086/646542. [DOI] [PubMed] [Google Scholar]
- 104.Krasinski K, Holzman R S, LaCouture R, Florman A. Hospital experience with varicella zoster virus. Infect Control. 1986;7:312–316. doi: 10.1017/s019594170006433x. [DOI] [PubMed] [Google Scholar]
- 105.Krinzman S, Basgoz N, Kradin R, Shepard J A, Flieder D B, Wright C D, Wain J C, Ginns L C. Respiratory syncytial virus associated infections in adult recipients of solid organ transplants. J Heart Lung Transpl. 1998;17:202–210. [PubMed] [Google Scholar]
- 106.Kumagai T, Kamada M, Igarashi C, Yuri K, Furukawa H, Chjiba S, Kojima H, Sainto A, Okui T, Yano S. Varicella-Zoster virus-specific cellular immunity in subjects given acyclovir after household chickenpox exposure. J Infect Dis. 1999;180:834–837. doi: 10.1086/314950. [DOI] [PubMed] [Google Scholar]
- 107.Leclair J M, Zaia J A, Levin M J, Congdon R G, Goldmann D A. Airborne transmission of chickenpox in a hospital. N Engl J Med. 1980;302:450–453. doi: 10.1056/NEJM198002213020807. [DOI] [PubMed] [Google Scholar]
- 108.Levandowski R A, Rubenis M. Nosocomial conjunctivitis caused by adenovirus type 4. J Infect Dis. 1981;143:28–31. doi: 10.1093/infdis/143.1.28. [DOI] [PubMed] [Google Scholar]
- 109.Levin M J. Treatment and prevention options for respiratory syncytial infections. J Pediatr. 1994;124:S22–S27. doi: 10.1016/s0022-3476(94)70187-3. [DOI] [PubMed] [Google Scholar]
- 110.Levin M L, Maddrey W C, Wands J R, Mendeloff A I. Hepatitis B transmission by dentists. JAMA. 1974;228:1139–1140. [PubMed] [Google Scholar]
- 111.Linnemann C C, Jr, Buchman T G, Light I J, Ballard J L. Transmission of herpes simplex virus type 1 in a nursery for the newborn. Identification of viral isolates by DNA fingerprinting. Lancet. 1978;1:964–966. doi: 10.1016/s0140-6736(78)90251-9. [DOI] [PubMed] [Google Scholar]
- 112.Lot F, Segvier J C, Fegueux S, Astragneau P, Simon P, Aggoune M, van Amerongen P, Ruch M, Cheron M, Brucker G, Desenclos J C, Drucker J. Probable transmission of HIV from an orthopaedic surgeon to a patient in France. Ann Intern Med. 1999;130:1–6. doi: 10.7326/0003-4819-130-1-199901050-00002. [DOI] [PubMed] [Google Scholar]
- 113.Lungman P, Gleaves C A, Meyers J D. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4:34–40. [PubMed] [Google Scholar]
- 114.Madge P, Paton J, McColl J H, Mackie P L K. Prospective controlled study of four infection control procedures to prevent nosocomial infection with respiratory syncytial virus. Lancet. 1992;340:1079–1083. doi: 10.1016/0140-6736(92)93088-5. [DOI] [PubMed] [Google Scholar]
- 115.Martini G A, Siegert R, editors. Marburg virus disease. Berlin, Germany: Springer-Verlag; 1971. [Google Scholar]
- 116.Mathur U, Bentley D W, Hall C B. Concurrent respiratory syncytial virus and influenza A infections in the institutionalised elderly and chronically ill. Ann Intern Med. 1980;93:49. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- 117.Matthew E B, Dietzman D E, Madden D L, Newman S J, Seuer J L, Nagler B, Bouton S M, Rostafinski M. A major epidemic of infectious hepatitis in an institution for the mentally retarded. Am J Epidemiol. 1973;98:199–215. doi: 10.1093/oxfordjournals.aje.a121549. [DOI] [PubMed] [Google Scholar]
- 118.Mazzuli T, Peret T C, McGeer A, Cann D, MacDonald K S, Chua R, Erdman D D, Anderson L J. Molecular characterisation of a nosocomial outbreak of human respiratory syncytial virus on an adult leukemia/lymphoma ward. J Infect Dis. 1999;180:1686–1689. doi: 10.1086/315085. [DOI] [PubMed] [Google Scholar]
- 119.McCarthy C A, Hall C. Prevention of nosocomial respiratory syncytial virus in the pediatric patient. Infect Med. 1990;7:4. [Google Scholar]
- 120.Melnick J L. Enteroviruses. In: Evans A S, editor. Viral infections in humans: epidemiology and control. 3rd ed. New York, N.Y: Plenum; 1991. pp. 191–263. [Google Scholar]
- 121.Meslin F-X, Fishbein D B, Matter H C. Rationale and prospects for rabies elimination in developing countries. In: Rupprecht C E, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin, Germany: Springer-Verlag; 1994. pp. 49–53. [DOI] [PubMed] [Google Scholar]
- 122.Meyers J D. Congenital varicella in term infants: risk reconsidered. J Infect Dis. 1974;129:215–217. doi: 10.1093/infdis/129.2.215. [DOI] [PubMed] [Google Scholar]
- 123.Minor P D, Morgan-Capner P, Muir P. Enteroviruses. In: Zuckerman A J, Banatvala J E, Pattison J R, editors. Principles and practice of clinical virology. Chichester, United Kingdom: Wiley; 2000. pp. 427–449. [Google Scholar]
- 124.Mintz L, Ballard R A, Sniderman S H, Roth R S, Drew W L. Nosocomial respiratory syncytial virus infection in an Intensive Care Nursery; rapid diagnoses by immunofluorescence. Paedatrics. 1979;64:149–153. [PubMed] [Google Scholar]
- 125.Mitsui T, Iwano K, Masuko K, Yamazaki C, Okamoto H, Tsuda F, Tanaka T, Mishire S. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology. 1992;16:1109–1114. [PubMed] [Google Scholar]
- 126.Moisiuk S E, Robson D, Klass L, Kliewer G, Wasylink W, Davi M, Plourde P. Outbreak of parainfluenza virus type 3 in an intermediate care neonatal nursery. Pediatr Infect Dis. 1998;17:49–53. doi: 10.1097/00006454-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 127.Ou C Y, Ciesielski C A, Myers G, Bandea C I, Luo C C, Korber C T, Mullins J I, Schochetman G, Berkelman R L, Economan A N. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992;256:1165–1171. doi: 10.1126/science.256.5060.1165. [DOI] [PubMed] [Google Scholar]
- 128.Pastuszak A L, Levy M, Schick B, Zuber C, Feldkamp M, Gladstone J, Bar-Levy F, Jackson E, Donnenfeld A, Meschino W. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. N Engl J Med. 1994;330:901–905. doi: 10.1056/NEJM199403313301305. [DOI] [PubMed] [Google Scholar]
- 129.Pattison J R. Parvovirus: medical and biological aspects. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1765–1786. [Google Scholar]
- 130.Peckham C S, Garrett A S, Chinn K S, Preece P M, Nelson D B, Warren D E. Restriction enzyme analysis of cytomegalovirus DNA to study transmission of infection. J Clin Pathol. 1986;39:318–324. doi: 10.1136/jcp.39.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peters C J, Sanchez A, Rollin P, Zsiazek G, Mirphy F A. Filoviridae: Marburg and Ebola viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 816–820. [Google Scholar]
- 132.Peters C J, Jahrling P B, Ksiazek T G, Lupton H. Filovirus contamination of cell cultures. Dev Biol Stand. 1992;76:267–274. [PubMed] [Google Scholar]
- 133.Pillay D, Patou G, Hurt S, Kibbler C C, Griffiths P D. Parvovirus B19 outbreak in a children's ward. Lancet. 1992;339:107–10. doi: 10.1016/0140-6736(92)91009-w. [DOI] [PubMed] [Google Scholar]
- 134.Poland G A, Nichol K. Medical students as sources of rubella and measles outbreaks. Arch Intern Med. 1990;150:44. [PubMed] [Google Scholar]
- 135.Preblud S R. Age specific risks of varicella complications. Pediatrics. 1981;68:14–17. [PubMed] [Google Scholar]
- 136.Preblud S R. Varicella: complications and costs. Pediatrics. 1986;78(Suppl.):728–735. [PubMed] [Google Scholar]
- 137.Prentice M B, Flower A J E, Morgan G M, Nicholson K G, Rana B, Firmin R K, Mitchell C J. Infection with hepatitis B virus after open heart surgery. Br Med J. 1992;304:761–764. doi: 10.1136/bmj.304.6829.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reingold A L, Kane M A, Murphy B L, Checko P, Francis D P, Maynard J E. Transmission of hepatitis B by an oral surgeon. J Infect Dis. 1982;145:262–268. doi: 10.1093/infdis/145.2.262. [DOI] [PubMed] [Google Scholar]
- 139.Rollin P E, Williams R J, Bressler D S, Pearson S, Cottingham M, Pucak G, Sanchez A, Trappier S G, Peters R L, Greer P W, Zaki S, Demarcus T, Hendricks K, Kelley M, Simpson D, Geisbert T W, Jahrling P J, Peters C J, Ksiazek T G. Ebola (subtype Reston) virus among quarantined nonhuman primates recently imported from the Philippines to the United States. J Infect Dis. 1999;179(Suppl. 1):S108–S114. doi: 10.1086/514303. [DOI] [PubMed] [Google Scholar]
- 140.Sawyer M H, Chamberlin C J, Wu Y N, Aintablian N, Wallace M R. Detection of varicella zoster virus DNA in air samples from hospital rooms. J Infect Dis. 1994;169:9–4. doi: 10.1093/infdis/169.1.91. [DOI] [PubMed] [Google Scholar]
- 141.Shaw F E, Barrett C L, Hamm R, Pearce R B, Coleman P J, Hadler S C, Fields H A, Maenad J E. Lethal outbreak of hepatitis B in a dental practice. JAMA. 1986;255:3260–3264. [PubMed] [Google Scholar]
- 142.Smith D H, Johnson B K, Isaacson M, Swanapoel R, Johnson K M, Killey M, Bagshawe A, Siongok T, Keruga W K. Marburg Virus Disease in Kenya. Lancet. 1982;1:816–820. doi: 10.1016/s0140-6736(82)91871-2. [DOI] [PubMed] [Google Scholar]
- 143.Sparling D. Transmission of mumps. N Engl J Med. 1979;280:276. doi: 10.1056/NEJM196901302800524. [DOI] [PubMed] [Google Scholar]
- 144.Steel H M, Garnham S, Beards G M, Brown D W G. Investigation of an outbreak of rotavirus infection in geriatric patients by sero-typing and polyacrylamide gel electrophoresis (PAGE) J Med Virol. 1992;37:132–136. doi: 10.1002/jmv.1890370211. [DOI] [PubMed] [Google Scholar]
- 145.Steele A D, Minisi Y N, Williams M M, Bos P, Aspinall S. Electrophoretic typing of nosocomial rotavirus infection in general pediatric unit showing the continual introduction of community strains. J Med Virol. 1993;40:126–132. doi: 10.1002/jmv.1890400209. [DOI] [PubMed] [Google Scholar]
- 146.Suleiman M N H, Muscat-Baron J M, Harries J R, Satti A G, Platt G S, Bowen E T, Simpson D I. Congo/Crimean hemorrhagic fever in Dubai: an outbreak at the Rashid hospital. Lancet. 1980;2:939–941. [PubMed] [Google Scholar]
- 147.Tablan O C, Anderson L J, Arden N H, Breiman R F, Butler J C, McNeil M M. Guideline for prevention of nosocomial pneumonia. The Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. Am J Infect Control. 1994;22:247–292. doi: 10.1016/0196-6553(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 148.Takeuchi R, Nomura Y, Kojima M, Uchio E, Kobayashi N, Matumoto M. A nosocomial outbreak of epidemic keratoconjunctivitis due to adenovirus type 37. Microbiol Immunol. 1990;34:749–754. doi: 10.1111/j.1348-0421.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 149.Tokars J I, Camberland M, Sapiro C. Epidemic Intelligence Service 41st Annual Conference, Atlanta, Ga. 1992. Infection with human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), among orthopaedic surgeons; pp. 45–46. [Google Scholar]
- 150.Turner R, Shehab Z, Osborne K, Hendley J O. Shedding and survival of herpes simplex virus from “fever blisters.”. Pediatrics. 1982;70:547–549. [PubMed] [Google Scholar]
- 151.U. K. Health Departments. Protecting health care workers and patients from hepatitis B. London, United Kingdom: Department of Health; 1993. [Google Scholar]
- 152.U.K. Health Departments. AIDS/HIV-infected health care workers; guidance on the management of infected health care workers. London, United Kingdom: Department of Health; 1994. [Google Scholar]
- 153.U.K. Health Departments. Guidelines on post-exposure phrophylaxis for health care workers occupationally exposed to HIV: recommendations of the expert advisory group on AIDS. London, United Kingdom: Department of Health; 1997. [Google Scholar]
- 154.U.K. Health Departments. Guidance for clinical health care workers: protection against infection with bloodborne viruses: recommendations of the expert advisory group on AIDS and the advisory group on hepatitis. London, United Kingdom: Department of Health; 1998. [Google Scholar]
- 155.U.K. Health Departments. Guidance for clinical health care workers: protection against infection with bloodborne viruses. London, United Kingdom: Department of Health; 1998. [Google Scholar]
- 156.U.K. Health Departments. AIDS/HIV infected health care workers: guidance on the management of infected health care workers and patient notification. London, United Kingdom: Department of Health; 1998. [Google Scholar]
- 157.Valenti W M, Menegus M A, Hall C B, Pincus P H, Douglas R G., Jr Nosocomial viral infection. 1. Epidemiology and significance. Infect Control. 1980;1:33–37. doi: 10.1017/s0195941700052371. [DOI] [PubMed] [Google Scholar]
- 158.Valenti W M, Clarke T A, Hall C B, Menegus M A, Shapiro D L. Concurrent outbreaks of rhinovirus and respiratory syncytial virus in an intensive care nursery: epidemiology and associated risk factors. J Pediatr. 1982;100:722–726. doi: 10.1016/s0022-3476(82)80571-4. [DOI] [PubMed] [Google Scholar]
- 159.Valenti W M. Influenza viruses. In: Mayhall C G, editor. Hospital epidemiology and infection control. Baltimore, Md: Williams and Wilkins; 1996. pp. 479–485. [Google Scholar]
- 160.Valenti W M. Selected viruses of nosocomial importance. In: Bennett J V, Brachman P S, editors. Hospital infections. 4th ed. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 637–642. [Google Scholar]
- 161.Van Voris L P, Belshe R B, Shaffer J L. Nosocomial influenza B in the elderly. Ann Intern Med. 1982;96:153–158. doi: 10.7326/0003-4819-96-2-153. [DOI] [PubMed] [Google Scholar]
- 162.Welch J, Webster M, Tilzey A J, Noah N D, Banatvala J E. Hepatitis B infections after gynaecological surgery. Lancet. 1989;1:205–207. doi: 10.1016/s0140-6736(89)91213-0. [DOI] [PubMed] [Google Scholar]
- 163.Welliver R C, McLaughlin S. Unique epidemiology of nosocomial diarrhoea in a children's hospital. Am J Dis Child. 1984;138:131–135. doi: 10.1001/archpedi.1984.02140400017004. [DOI] [PubMed] [Google Scholar]
- 164.Wendt C H, Weisdorf D J, Jordan M C, Balfour H H, Jr, Hertz M I. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326:921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 165.Wendt C H, Fox J M K, Hertz M I. Paramyxovirus infection in lung transplant recipients. J Heart Lung Transplant. 1995;14:479–485. [PubMed] [Google Scholar]
- 166.Werner B G, Grady G F. Accidental hepatitis B surface antigen positive inoculations: use of e antigen to estimate infectivity. Ann Intern Med. 1982;97:367–369. doi: 10.7326/0003-4819-97-3-367. [DOI] [PubMed] [Google Scholar]
- 167.West D J. The risk of hepatitis B infection among health professionals in the United States: a review. Am J Med Sci. 1984;287:26–33. doi: 10.1097/00000441-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 168.Whimbey E, Elting L S, Couch R B, Lo W, Williams L, Champlin R E, Bodey G P. Influenza A virus infection among hospitalised adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]
- 169.Whimbey E, Champlin R E, Couch R B, Englund J A, Goodrich J M, Raad I, Przepiorka D, Lewis V A, Mirza N, Yousof H, Tarrand J J, Bodey G P. Community respiratory virus infections among adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 170.Whimbey E, Couch R B, Englund J A, Andreeff M, Goodrich J M, Raad I I, Lewis V, Mirza N, Luna M A, Baxter B. Respiratory syncytial virus pneumonia in hospitalised adult patients with leukaemia. Clin Infect Dis. 1995;21:376–379. doi: 10.1093/clinids/21.2.376. [DOI] [PubMed] [Google Scholar]
- 171.Whimbey E, England J A, Couch R B. Community respiratory virus infectious in immunocompromised patients with cancer. Am J Med. 1997;1007:10–18. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.World Health Organisation. Ebola haemmorrhagic fever in Zaire, 1976: report of an international commission. Bull WHO. 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- 173.World Health Organisation. Ebola haemmorrhagic fever in Sudan, 1976, report of a World Health Organisation International Study Team. Bull WHO. 1978;56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 174.World Health Organisation. Report of the consultation on action to be taken after occupational exposure of health care workers to HIV. Geneva, Switzerland: World Health Organisation; 1989. [Google Scholar]
- 175.World Health Organisation. Viral hemorrhagic fever/Marburg, Democratic Republic of Congo. Wkly Epidemiol Rec. 1999;74:157–158. [Google Scholar]
- 176.Yeager A S. Longitudinal study of cytomegalovirus in nurses and personnel without patient contact. J Clin Microbiol. 1975;2:448–452. doi: 10.1128/jcm.2.5.448-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yolken R H, Bishop C A, Townsend T R, Bolyard E A, Bartlett J, Santos G W, Sara R. Infectious gastroenteritis in bone marrow transplant recipients. N Engl J Med. 1982;306:1010–1012. doi: 10.1056/NEJM198204293061701. [DOI] [PubMed] [Google Scholar]