Abstract
Primary gastrointestinal T‐cell lymphomas are rare. Presenting symptoms can be non‐specific, and imaging studies can show overlap with nonmalignant processes. Definitive diagnosis requires clinical suspicion and histologic evaluation with ancillary studies for appropriate disease classification and therapeutic intervention.
Keywords: bowel perforation, Intestinal T‐cell lymphoma
Intestinal T‐cell lymphomas are rare and should be considered in patients with abdominal pain. Imaging studies may show bowel perforation. Diagnosis requires tissue biopsy with histologic evaluation and ancillary studies.
1. INTRODUCTION
Malignant lymphoma arises within lymph nodes or develops outside of nodal tissues (extranodal). Primary lymphoma of the gastrointestinal (GI) tract accounts for 30–40% of extranodal lymphomas, 10–15% of non‐Hodgkin lymphoma (NHL), and 1–4% of all GI malignancies. 1 The stomach is the most common site of involvement (60–75%) followed by the small bowel (20%–50%) and colorectum (4%–6%). 1 , 2 The majority of primary GI lymphomas are NHLs of B cell lineage; T‐cell lymphomas comprise only 4%–6% of all primary GI lymphomas. 2 , 3 According to the 2017 World Health Organization (WHO) classification system, intestinal T‐cell lymphomas encompass four subtypes, enteropathy‐associated T‐cell lymphoma (EATL), monomorphic and epitheliotropic intestinal T‐cell lymphoma (MEITL), intestinal T‐cell lymphoma, not otherwise specified (NOS) and a provisional entity indolent T‐cell lymphoproliferative disorder of the gastrointestinal tract. 4 , 5 , 6 The differentiation between EATL and MEITL is based on risk factors (e.g., celiac disease), cellular morphology, immunophenotype of neoplastic T cells, and molecular genetic features. Intestinal T‐cell lymphomas that do not meet criteria for EATL or MEITL are designated as intestinal T‐cell lymphoma, NOS. 5 Indolent T‐cell lymphoproliferative disorder of the gastrointestinal tract is a clonal T‐cell disorder with an indolent clinical course. 7
Patients with intestinal T‐cell lymphoma may present with non‐specific symptoms, the most common are fever, diarrhea/hematochezia, or abdominal pain, and acute perforation is a common complication. 8 , 9 , 10 Computed tomography (CT) scans may reveal thickening of the luminal wall. 8 , 10 , 11 With the exception of indolent T‐cell lymphoproliferative disorder of the gastrointestinal tract, intestinal T‐cell lymphomas are clinically aggressive malignancies. We present a rare case of intestinal T‐cell lymphoma presenting with severe acute upper abdominal pain and imaging studies suggestive of possible bowel perforation with mesenteritis. Exploratory laparotomy unexpectantly revealed a firm mass encasing the superior mesenteric vessels. The final diagnosis of intestinal T‐cell lymphoma required open tissue biopsy with immunophenotypic and molecular studies.
2. CASE REPORT
A 73‐year‐old woman with history of rheumatoid arthritis and celiac sprue presented to the emergency department (ED) complaining of right upper quadrant abdominal pain radiating to the back for the past two weeks. She reported nausea but no emesis. Laboratory tests were notable for hyponatremia (Na: 123 mmol/L), hypochloremia (Cl: 88 mmol/L), and elevated lactate dehydrogenase level (LD: 505 IU/L). At admission, a CT of the abdomen with contrast showed significant retroperitoneal inflammation and fat stranding in the mid abdominal region at the level of the ¾ portion of the duodenum and pancreatic head abutting the superior mesenteric artery and superior mesenteric vein. An upper GI with KUB showed a small collection of contrast at the junction of the 4th portion of the duodenum and ligament of Treitz. Overall, the imaging studies were suspicious for a sealed duodenal perforation. The initial clinical impression was that the abdominal pain was due to an inflammatory process with secondary inflammation of the pancreatic head. She was managed non‐operatively due to delayed presentation of perforation, radiographic evidence that the ulcer had sealed, clinical hemodynamic stability and complex, risky surgery inherent in operating on the third and fourth portion of the duodenum. Surgical options at this time included: resection of the distal duodenum with enteric bypass, wide local drainage with enteric bypass, wide local drainage with distal feeding access and omental patch repair of the duodenum. Surgical approaches would have required challenging dissection of fibrotic and inflamed tissue abutting the pancreas, aorta, superior mesenteric artery, and superior mesenteric vein. After a period of bowel rest, IV antibiotics, and proton‐pump inhibitors, repeat CT scan showed inflammatory changes consistent with a contained duodenal perforation. The patient was discharged in stable condition with oral antibiotics, proton‐pump inhibitors, and close outpatient follow‐up.
The patient presented to the ED the next day with severe left upper quadrant pain. CT of the abdomen with contrast showed relatively stable phlegmonous changes centered within the mesentery adjacent to the 3rd and 4th portion of the duodenum, with involvement of the head and body of the pancreas as well as the superior mesenteric artery and superior mesenteric vein without evidence of thrombosis. Scattered reactive mesenteric lymph nodes were described without suspicious intrabdominal masses or lymphadenopathy. The decision was made for urgent exploratory laparotomy due to the patient's marked increase in severe abdominal pain and diffuse abdominal tenderness. Intraoperatively, the surgical team noted a retroperitoneal mass encasing the small bowel mesenteric stalk and aorta, chylous ascites, and intraperitoneal and retroperitoneal adenopathy. Intraoperative esophagogastroduodenoscopy (EGD) revealed extrinsic compression of the distal duodenum and intraluminal pathology. A biopsy submitted as retroperitoneal mass was sent for intraoperative consultation and showed undifferentiated, atypical cells and necrosis (touch imprints; Figure 1A). Subsequent histologic evaluation of hematoxylin and eosin (H&E) stained permanent sections of the retroperitoneal mass showed soft tissue fragments with large atypical cells embedded in a dense sclerotic matrix, abundant apoptotic cells, and tumor necrosis; no intestinal mucosa or residual lymphoid tissue was identified (Figure 1B‐C). Immunohistochemical stains performed on the retroperitoneal mass demonstrated that neoplastic cells expressed CD45, CD43, and CD8 (Figure 1D‐E). The tumor cells were negative for other lymphoid‐associated antigens (CD3, CD4, CD5, CD7, CD1a, CD56, CD30, CD20, PAX5, CD138, MUM, BCL6, BCL2, BCL1, c‐MYC, and TdT), CD34, CD68, myeloperoxidase, CD163, lysozyme, CD117, CD61, pan‐cytokeratin, S‐100, and in situ hybridization studies for Epstein‐Barr encoded RNA (EBER). Molecular studies were positive for a clonal rearrangement of the T‐cell receptor (TCR) gamma gene. Based on the T‐cell immunophenotypic and molecular features, the diagnosis of T‐cell lymphoma, NOS, was rendered. A biopsy of a mesenteric lymph node obtained intraoperatively was also sent for pathologic evaluation. The H&E‐stained sections showed cauterized fibrofatty tissue with focal crushed large cells, consistent with involvement by lymphoma. Post‐operative imaging studies were negative for hepatosplenomegaly and lymphadenopathy in the chest. After appropriate pain control, the patient was discharged with palliative care and passed away ten days later, precluding bone marrow evaluation.
FIGURE 1.
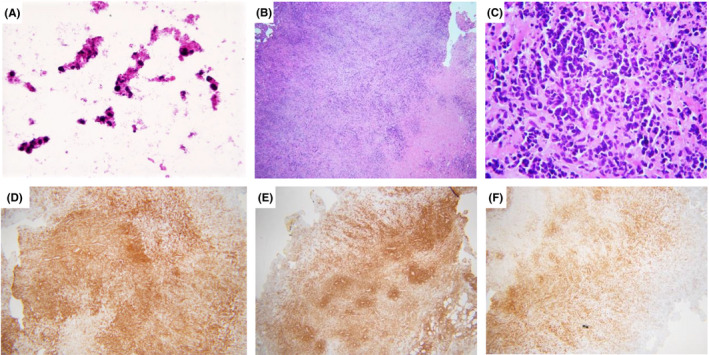
(A) Touch imprints of retroperitoneal mass showed necrosis and undifferentiated atypical cells. (B‐C) The H&E‐stained sections of the retroperitoneal mass showed large atypical cells embedded in a dense sclerotic matrix with abundant apoptotic cells and tumor necrosis. By immunohistochemistry, neoplastic cells positive for CD45 (A), CD43 (B), and CD8 (C). Original magnifications; A: 40× B, D‐F: 4×; C: 40×
3. DISCUSSION
Mature T‐cell lymphomas are relatively uncommon, comprising ~10% of all NHL on a worldwide basis. Although the GI tract is the most frequent site of extranodal NHL, the majority are of B‐cell origin. 2 , 3 Primary intestinal T‐cell lymphoma is rare.
Intestinal T‐cell lymphomas are subclassified according to the 2017 WHO classification scheme as enteropathy‐associated T‐cell lymphoma (EATL), monomorphic epitheliotropic intestinal TCL (MEITL), indolent T‐cell lymphoproliferative disorder of the gastrointestinal tract and intestinal T‐cell lymphoma, NOS. 5 EATL comprises the most common subtype of primary intestinal T‐cell lymphoma, accounting for approximately two thirds of cases. 12 EATL is uncommon in Asian countries and seen in greater frequencies in Europe and North America. 5 , 9 , 12 Primary intestinal T‐cell lymphoma is classified as EATL if there is clinical evidence of celiac disease, including malabsorption or villous atrophy of the mucosa. 4 Most cases of EATL express CD3, cytotoxic‐granule‐associated proteins, and T‐cell receptor (TCR) αβ. 5 MEITL shows no association with celiac disease and appears with an increased incidence in Asian and Hispanic populations. 5 , 13 MEITL has a distinct immunophenotype, positive for CD3, CD8, and CD56, and tumor cells are derived from γδ T cells. 4 Indolent T‐cell lymphoproliferative disorder of the gastrointestinal tract is a clonal T‐cell disorder with a chronic, relapsing clinical course. This disease usually presents as one or more shallow ulcers with erythema or multiple small polyps, may involve the colon and congruent lesions may resemble idiopathic bowel disease endoscopically. 7 In contrast to EATL and MEITL, histologic evaluation shows small mature‐appearing neoplastic lymphocytes without destructive lesions. 7 Intestinal T‐cell lymphoma, NOS, has no specific immunohistochemical expression pattern, although a cytotoxic (CD8 positive) T‐cell phenotype is most common, and is a diagnosis of exclusion. 5 In one case series, intestinal T‐cell lymphoma, NOS, presented as a solitary ulcer in the small intestine and the average survival period was 15 months. 10 Intestinal T‐cell lymphoma, NOS, may present in either the small or large bowel; if nodal or extranodal sites of disease are present, it may be difficult to confirm the intestine as the primary site. 5 In such cases, the differential diagnosis may include peripheral T‐cell lymphoma (PTCL), NOS, a heterologous category of nodal and extranodal mature T‐cell lymphomas that do not correspond to any of the specifically defined entities of mature T‐cell lymphomas. 14 Most patients with PTCL, NOS, present with peripheral lymph node involvement and advanced disease with B symptoms, although the skin and GI tract can be involved. 14 In nodal PTCL, NOS, a CD4+/CD8‐ phenotype predominates and T‐cell receptor beta is usually expressed. 14
The diagnosis of intestinal T‐cell lymphoma, NOS, is based on histology, immunophenotype, and molecular studies and requires the exclusion of other T‐cell lymphomas. Our patient had a history of had a history of celiac sprue, although there was no known history of malabsorption or diarrhea to support a diagnosis of EATL. Preoperative imaging studies and intraoperative findings were consistent with a healed/sealed bowel perforation with the presence of inflammatory adhesions, bacterial contamination, and chylous ascites. The retroperitoneum appeared encased in firm tissue by a mass involving critical vessels. Intraoperative endoscopy showed luminal pathology, suggestive of tumoral involvement of the duodenum. A biopsy of the retroperitoneal mass sent for intraoperative histologic evaluation revealed tumor necrosis and large cells with pleomorphic and hyperchromatic nuclei encased in a sclerotic background; no intestinal mucosa or residual lymph node tissue was identified. By immunohistochemical stains, the neoplastic cells showed an aberrant T‐cell phenotype (CD3 and CD7 negative) with coexpression of CD8 and absence of CD56 expression. Molecular studies were positive for a clonal TCR gamma gene rearrangement. Histologic review of a mesenteric lymph node biopsy showed neoplastic cells. Overall, these features were most consistent with locally advanced intestinal T‐cell lymphoma, NOS. Intestinal T‐cell lymphoma, NOS, may present with abdominal pain and are clinically aggressive. 5 Perforation is a poor predictive factor, 9 and our patient survived <2 months after diagnosis.
4. CONCLUSION
This case illustrates the challenges in diagnosing primary intestinal T‐cell lymphoma due to the rarity of this entity and non‐specific clinical presentation and radiographic findings. Accurate diagnosis requires clinical awareness and adequate biopsy material with ancillary studies, including immunohistochemistry and molecular studies.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
AM, EFQ, and JM contributed to data collection and drafting of the manuscript. GRP contributed to final revision of the manuscript. All authors read and approved the final version on the manuscript.
ETHICAL APPROVAL
The Institutional Review Board Director reviewed the case and determined that this project is not human subject research as defined in 45 CFR 46.102, and therefore, it does not fall under the jurisdiction of the IRB review process.
CONSENT
Written informed consent was obtained from the husband of patient for the publication of this case report.
ACKNOWLEDGEMENT
None.
Mashayekhi A, Quiroga EF, Margolick JF, Post GR. Intestinal T‐cell lymphoma: A rare entity presenting with severe acute upper quadrant pain. Clin Case Rep. 2022;10:e05546. doi: 10.1002/ccr3.5546
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Bautista‐Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3(3):209‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohri M, Tsukasaki K, Akuzawa Y, et al. Peripheral T‐cell lymphoma with gastrointestinal involvement and indolent T‐lymphoproliferative disorders of the gastrointestinal tract. Leuk Res. 2020;91:106336. [DOI] [PubMed] [Google Scholar]
- 3. Shirwaikar Thomas A, Schwartz M, Quigley E. Gastrointestinal lymphoma: the new mimic. BMJ Open Gastroenterol. 2019;6(1):e000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaffe ES, Chott A, Ott G, et al. Intestinal T‐cell lymphoma. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. IARC Press; 2017:372‐380. [Google Scholar]
- 6. van Vliet C, Spagnolo DV. T‐ and NK‐cell lymphoproliferative disorders of the gastrointestinal tract: review and update. Pathology. 2020;52(1):128‐141. [DOI] [PubMed] [Google Scholar]
- 7. Perry AM, Warnke RA, Hu Q, et al. Indolent T‐cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122(22):3599‐3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chun HB, Baek IH, Lee MS, et al. Jejunocolic fistula associated with an intestinal T cell lymphoma. Gut Liv. 2011;5(3):387‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun ZH, Zhou HM, Song GX, Zhou ZX, Bai L. Intestinal T‐cell lymphomas: a retrospective analysis of 68 cases in China. World J Gastroenterol. 2014;20(1):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang XF, Yang L, Duan S, Guo H, Guo QN. Intestinal T‐cell and NK/T‐cell lymphomas: a clinicopathological study of 27 Chinese patients. Ann Diagn Pathol. 2018;37:107‐117. [DOI] [PubMed] [Google Scholar]
- 11. Tellez‐Avila FI, Garcia‐Osogobio S, Chavez‐Tapia NC, et al. Utility of endoscopy in patients with incidental gastrointestinal luminal wall thickening detected with CT. Surg Endosc. 2009;23(10):2191. [DOI] [PubMed] [Google Scholar]
- 12. Delabie J, Holte H, Vose JM, et al. Enteropathy‐associated T‐cell lymphoma: clinical and histological findings from the international peripheral T‐cell lymphoma project. Blood. 2011;118(1):148‐155. [DOI] [PubMed] [Google Scholar]
- 13. Tse E, Gill H, Loong F, et al. Type II enteropathy‐associated T‐cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol. 2012;87(7):663‐668. [DOI] [PubMed] [Google Scholar]
- 14. Pileri SA, Weisenburger DD, Sng I, et al. Peripheral T‐cell lymphoma, NOS WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. IARC Press; 2017:403‐407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


