Abstract
Although there is no shortage of research on the markers for stroke, to our knowledge, there are no clear markers that can meet the needs of clinical prediction and treatment. The inflammatory cascade is a critical process that persists and functions throughout the stroke process, ultimately worsening stroke outcomes and increasing mortality. Numerous inflammatory factors, including tumor necrosis factor (TNF), are involved in this process. These inflammatory factors play a dual role during stroke, and their mechanisms are complex. As one of the representatives, TNF is the primary regulator of the immune system and plays an essential role in the spread of inflammation. In researches done over the last few years, tumor necrosis factor-alpha (TNF-α) has emerged as a potential marker for stroke because of its essential role in stroke. This review summarizes the latest research on TNF-α in stroke and explores its potential as a therapeutic target.
1. Introduction
Stroke is the leading cause of death and long-term disability worldwide, and its incidence is increasing at younger ages [1, 2]. The high mortality and disability rates place a severe burden on society [3, 4]. Thus, the search for biomarkers that can predict disease prognosis or targeted therapy is significant to improve the treatment and reduce the disability rate [5]. However, there are no specific markers that can provide predictive and therapeutic information as far as we know. Previous studies by Simats et al. have summarized the role of inflammatory biomarkers in helping predict outcomes in stroke patients which may even become therapeutic targets [6]. The inflammatory response process runs through the entire stroke course [7]. In this cascade of inflammatory changes, cytokines like interleukin (IL), TNF, and interferon (IFN) act as central mediators in the inflammatory cascade and are considered as a therapeutic target and prognostic biomarker [8]. The researchers observed changes in the concentrations of several types of these cytokines in the cerebrospinal fluid and blood of stroke patients, and these changes were associated with prognosis [9–12]. TNF-α is an emerging molecule that is a kind of pleiotropic cytokine as the primary regulatory factor of the immune system that can be produced by a variety of cell types and is involved in a wide range of pathological processes [13, 14]. It plays a homeostasis and pathophysiological role in the central nervous system. Under pathological conditions, microglia release large amounts of TNF-α, which is a crucial component of the neuroinflammatory response associated with various neurological diseases [15]. Based on several robust pieces of evidence, changes in TNF-α were associated with stroke injury and stroke recovery [16–18]. For example, Tuttolomondo et al. reported that TNF-α expression was elevated after stroke, which stimulated the expression of tissue factors and leukocyte adhesion molecules and inhibited the fibrinolytic system [19]. Although several studies have reported contrary results, the use of TNF-α as a marker of stroke remains promising. In addition to the role of TNF-α in stroke, anti-TNF-α-based antistroke therapies have received increasing attention from the researchers. In a preclinical study, TNF-α receptor inhibitors reduce brain damage by reducing inflammatory responses in a rat model of ischemic stroke [20]. Therefore, this article reviews the research progress of TNF-α and its antagonists and discusses its application prospect in the treatment of stroke.
2. TNF-α Molecule and Its Receptor
TNF-α is produced by various cells, but its primary source is the cells of the immune system, such as macrophages, lymphoid cells, and mast cells [14, 21]. In these cells, TNF-α is first synthesized into transmembrane protein (tmTNF-α), which is then cleaved by matrix metalloproteinase TNF-α-converting enzyme (TACE) to release soluble TNF-α (sTNF-α) homotrimer and can bind to two types of receptors, namely, TNF receptor (TNFR) type 1 (TNFR1) and type 1 (TNFR2) [15, 21–23]. These two receptors are expressed differently in various cells and differ functionally [24]. Unlike TNFR1, which is ubiquitously expressed in all cell types, TNFR2 is expressed by some immune cells and preferentially by some Treg cells, some endothelial cells, and nerve tissue cells [13, 25]. The TNF-signaling complex structure enables TNF-α to induce inflammation and cell death or to induce tolerance to ischemia after stroke [26]. The main role of TNFR1 is to initiate apoptosis through its death domain and also to induce cell survival mechanisms [27]. Activation of the TNFR2 pathway by TNF-α contributes to immune response and inflammation [28]. It can affect the activation of many intracellular signaling pathways and ultimately lead to cell survival, cell migration, apoptosis, and necrosis (Figure 1) [29–31].
Figure 1.
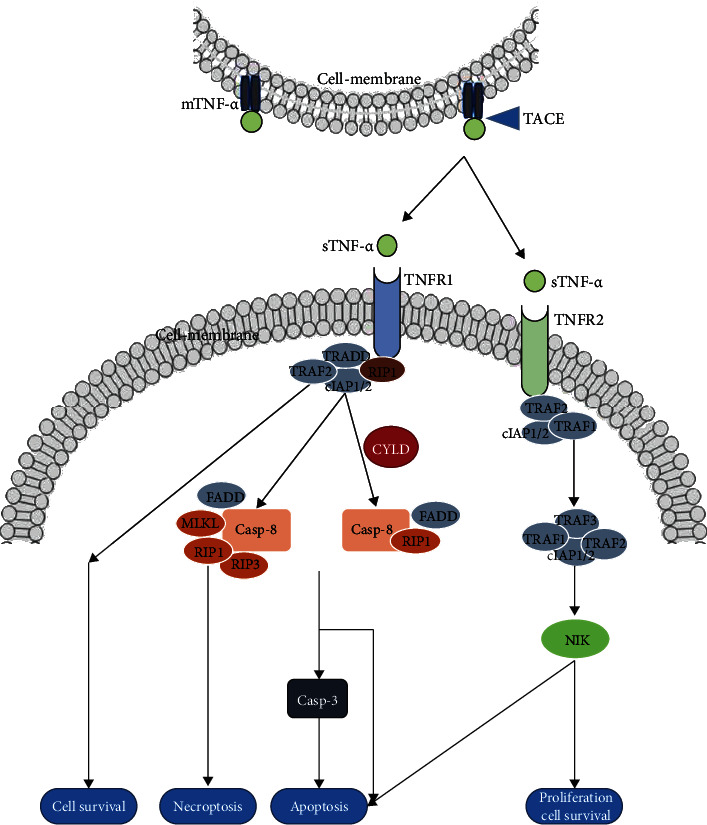
TNF-α binds to receptors and affects intracellular signal transduction. MTNF-α is hydrolyzed and cleaved by TACE to produce STNF-α. STNF-α binds to TNFR1 and TNFR2 through different signaling pathways, ultimately leading to a series of outcomes, including necrosis, apoptosis, survival, and proliferation.
Although TNF-α has a higher affinity for TNFR2 than for TNFR1, most of the biological activities of TNF-α are initiated by TNFR1 [32]. The structure of TNFR1 includes a death domain (DD), which is constitutively expressed in most cell types and is activated by TNF-α in the form of membrane binding (mTNF-α) or soluble (sTNF-α) [33]. Activation of TNFR1 leads to trimer formation, which promotes DD recruitment of TNFR1-associated death domains (TRADD), and TRADD further recruits serine/threonine-protein kinase (RIPK) and TNFR-associated factor (TRAF) 2 [34–36]. The specific process can be described as TNFR1 binds to trimer TNF-α to release death domain silencer (SODD) protein. The TNFR-associated death domain (TRADD) binds to the TNFR1 death domain (DD) and recruits adapter protein receptor-interacting protein (RIP), TNFR-associated factor 2 (TRAF2), and Fas-associated death domain (FADD). When TNFR1 signals apoptosis, FADD binds to procaspase-8 and activates it, eventually initiating the protease cascade reaction. Activation of endonuclease (such as EndoG) mediates DNA breakage and leads to apoptosis. When TNFR1 signals survival, TRAF2 is recruited to the complex, inhibiting apoptosis by cytoplasmic apoptotic protein inhibitor (cIAP). Activation of TRAF2 results in activation of cFos/cJun transcription factors through mitogen-activated protein kinase (MAPK) and cJun N-terminal kinase (JNK) [37, 38]. The TNFR1 core signaling complex is thus formed and stabilized by RIPK1 ubiquitination, which ultimately mediates a cellular response. For example, cytokine signaling and cell survival are induced by activation of the NF-κB, JNK, and p38 pathways [39, 40]. The apoptotic pathway would be activated in the absence of complete ubiquitination of RIPK1, leading to cell apoptosis or necrosis [41].
TNFR2 has no dead domain and is only fully activated by mTNF-α [42]. TNFR2 forms trimer and directly recruits TRAF2, TRAF1, or TRAF3 [43]. The nuclear factor kappa-light chain enhancer (NF-κB), Akt (protein kinase B), and mitogen-activated protein kinase (MAPK) of B cells are then activated to initiate their biological function [44, 45]. For example, it promotes cell activation, migration, and proliferation; plays a protective role in cells; affects the amplification and function of Treg; and also mediates apoptosis through its cooperation with TNFR1 [45–47].
3. Physiological Role of TNF-α Molecule in the Central Nervous System
In the adult brain, TNF is mainly derived from glia, astrocytes, and microglia, and its levels are low, but its role in the central nervous system (CNS) is complex and multipotent [48–50]. First, TNF-α regulates normal neurotransmitter processes in different ways. For example, it not only can induce a rapid increase in AMPA receptors but also can decrease AMPAR levels in cortical surface and hippocampal neurons (a process achieved in the striatum through the elimination of Ca2+ permeability inhibition) and enhance tetrodotoxin insensitive Na+ channel currents in the plasma membrane of dorsal root ganglion (DRG) neurons. Furthermore, it also regulates the release of glutamate by astrocytes [51–57]. Second, TNF-α plays a dual role in neurogenesis through different inductive environments and receptor subtypes [58]. For example, TNF-α can cause progenitor cell death by abruptly stopping cell division [59]. It exerts neuroprotective effects when it binds to TNFR2 receptors expressed by human neural stem cells [60]. Third, TNF-α can affect endothelial cells in CNS. These pathways include influencing the morphology of endothelial cells, thereby affecting BBB permeability, enhancing the adhesion between leukocytes and endothelial cells, thereby facilitating leukocyte migration to the central nervous system and inducing angiogenic mediators that affect vascular endothelial cells proliferation [61–63].
4. TNF-α in Stroke
The etiology of vascular lesions is obviously redox reaction and stress-dependent [64]. In stroke, neurovascular units can become dysfunctional due to the lack of oxygen and nutrients [65]. During ischemia, changes in the brain include the release of glutamate, the production of reactive oxygen species (ROS) that cause oxidative stress, and activation of microglia, which can affect the secretion of proinflammatory mediators [66, 67]. Oxidative stress and inflammatory response have bidirectional effects on the whole stroke process. When blood vessels are occluded or underperfused, the immune response begins near the ischemic parenchyma and then extends to the ischemic zone, eventually spreading throughout the body, and microglia are activated and promote the release of TNF-α [68, 69]. Studies have shown that levels of TNF-α in brain tissue may continue to rise 1 day after ischemic injury and correlate with their severity [70, 71]. TNF-α is a core mediator in the immune processes of infection control, autoimmunity, allergic diseases, and antitumor activity [15]. The mechanism of TNF-α's influence on vascular endothelium includes stimulating the expression of tissue factors and leukocyte adhesion molecules, activating matrix metalloproteinases, and producing oxidative stress through xanthine oxidase [61, 72]. These actions trigger local segments of blood vessels and lead to local inflammation, thrombosis, and bleeding [73]. Other studies have shown that TNF-α can disrupt the protective barrier between brain circulation. These effects include, first, stimulating the activation and proliferation of astrocytes and microglia and, second, regulating apoptosis factors, such as cysteine. Third, matrix metalloproteinase (MMP) transcription is induction in ischemia and penumbra inflammation. The last induced transcription of cytokines, such as IL-1 and IL-6 [74–77]. In addition, TNF can also induce ischemia tolerance and regulate the signal transduction of cerebral hypoxia and ischemia tolerance [78, 79]. In stroke outcomes, TNF-α is associated with epileptic seizures, movement disorders, spasms, aphasia, pain, depression, and cognitive impairment [80–83]. Zaremba et al. found that the level of TNF-α in cerebrospinal fluid (CSF) was significantly increased in stroke patients, and the increase of CSF and SERUM TNF-α in the first 24 hours of stroke was also significantly associated with the severity of a neurological stroke and the degree of dysfunction according to SSS and BI scores [84]. However, in a clinical study, the researchers found that the level of TNF-α was not associated with functional outcomes after acute stroke [85]. We speculate that this is because of how TNF-α plays a role in stroke prognosis, which is complex and diverse, and these specific mechanisms need to be further investigated. Doll et al. reviewed several preclinical and clinical studies suggesting that TNF-α has neurotoxic or neuroprotective effects in stroke. There were also conflicting findings when TNF-α was used to predict prognosis. These seem to indicate that the action of TNF-α is complex and bidirectional [26]. Because TNF-α ligand-receptor interactions are involved in almost every aspect of stroke-induced brain injury, it is a promising direction to use TNF-α as an inflammatory marker to predict the outcome of stroke. On the other hand, when TNF-α is used as a potential therapeutic target for stroke, blocking TNF-α can reduce focal ischemic injury and improve clinical outcomes [83, 86].
5. TNF-α Inhibitors
Ischemic stroke is a catastrophic disease. Unfortunately, because of the limited time window for treatment, only a small number of patients receive tissue plasminogen activator (tPA), which is the primary treatment; as a result, most patients receive only supportive care [6, 26]. It is urgent to renew the therapeutic drugs in the clinic. The positive effects of treatment targeting TNF-α in stroke have been demonstrated in preclinical studies over the past few years (Table 1). There are three effective ways to interfere with TNF-α action by blocking receptors, interfering with TNF-α signal transduction, and removing TNF-α protein in effectors [87]. Currently, TNF-α inhibitors, including enanercib, infliximab, adalimumab, pertuzumab, and golimumab, are mainly used to treat autoimmune diseases or inflammatory diseases [87–89]. Intraventricular injection of TNFR1 decoy receptors or anti-TNF-α antibodies, as well as systemic injection of TACE inhibitors, can reduce ischemic brain damage in stroke [90, 91]. After injecting TNF-α receptor inhibitor R-7050 into stroke rats, Lin et al. found that R-7050 reversed neuronal changes, TNF-α receptor/NF-κB inflammatory signaling, and BBB destruction and ultimately reduced the area of cerebral infarction [20]. In another study, in older animals, mice treated with adalimumab (TNF-α-inhibiting antibody) reduced poststroke defects and improved poststroke survival [92]. When the preclinical experiment is transformed into clinical application, the researchers must overcome the adverse reactions. These include the most worrisome severe infections, malignancies, heart failure, and nerve demyelination, as well as other general side effects, such as headache, rash, anemia, pharyngitis, diarrhea, nausea, and abdominal pain [88, 93, 94]. Finally, the safety of anti-TNF-α agents during pregnancy or lactation needs to be further explored [88]. In the meantime, the researchers are still working to develop other types of inhibitors to improve stroke outcomes. For example, the IL-2/IL-2R antibody complex enhances Treg-induced neuroprotective effects by inhibiting TNF-α induced inflammation [95]. Contreras et al. proposed that the trimer TNF-R2 extracellular domain might be an innovative TNF-α antagonist [96]. Targeting P2X4 receptors improves postcentral stroke pain through the TNF-α/TNFR1/GABAAR pathway [97]. Given the fact that TNF-α inhibitors are less effective at penetrating BBB, the researchers are also looking for new types of inhibitors that can more easily move through BBB and act more effectively in the damaged areas [98]. These emerging studies provide new research ideas for anti-TNF-α treatment of stroke.
Table 1.
Current research reports on use of TNF inhibitors in stroke.
| Drug name | Drug type | Research type | Describe | Ref. | Year |
|---|---|---|---|---|---|
| R-7050 | TNF-α receptor inhibitors | Preclinical | Using a rat model of permanent cerebral ischemia, pretreatment with R-7050 offered protection against poststroke neurological deficits, brain infarction, edema, oxidative stress, and caspase 3 activations. | [20] | 2021 |
| Adalimumab | TNF-α-neutralizing antibody | Preclinical | Older animals treated with adalimumab show a tendency to reduce poststroke defects and improve survival in older animals after stroke. | [92] | 2021 |
| Infliximab | TNF-α inhibitor | Preclinical | Improving stroke outcomes in a mouse model of rheumatoid arthritis. | [18] | 2019 |
| Alpha-lipoic acid and etanercept | Free radical scavenger/TNF-α inhibitor | Preclinical | By inhibiting peripheral TNF-α and downregulating microglia activation, it has protective effect on ischemic stroke rats. | [99] | 2015 |
| Infliximab and etanercept | TNF-α inhibitor | Preclinical | Compared with untreated rats, the volume of cerebral infarction was significantly reduced in the etanercept or infliximab group. | [86] | 2015 |
| Etanercept | TNF-α inhibitor | Preclinical | Decreased middle cerebral artery remodeling but increased cerebral ischemia injury in hypertensive rats. | [100] | 2014 |
| CNTO5048 | TNF-α antibody | Preclinical | In a mouse model of intracerebral hemorrhage, posttraumatic treatment with CNTO5048 reduced neuroinflammation and improved functional outcomes. | [101] | 2013 |
| Etanercept | TNF-α inhibitor | Clinical | Perispinal administration of etanercept improves clinical symptoms in patients with chronic neurological dysfunction following stroke and traumatic brain injury. | [102] | 2012 |
| CTfRMAb-TNFR | Fusion protein | Preclinical | CTfRMAb-TNFR fusion protein treatment can reduce hemispheric, cortical, and subcortical stroke volume and neurological deficits and prevent stroke. | [103] | 2012 |
6. Conclusion
In conclusion, although some current studies do not support TNF-α as a clear marker of stroke, we still believe that it is desirable to focus on TNF-α in the following studies, considering that TNF-α is involved in the occurrence, development, and prognosis of stroke and has an indicative effect on the disease. Therefore, it is a promising research direction to use TNF-α as a biomarker of stroke development process or prognosis. At the same time, anti-TNF-α therapy can reduce brain damage in stroke, and it is also worth exploring as a therapeutic target. To make TNF-α be a reliable marker of stroke, the specific role and mechanism it plays in stroke, the protective effect and mechanism of anti-TNF-α treatment against brain injury, and how to reduce the side effects of antibodies are the primary issues that need to be further studied and solved by the researchers. There is a reason to believe that the next marker of stroke is on the horizon with the ongoing research.
Acknowledgments
This study was supported by grants from the China Postdoctoral Science Foundation (Nos. 2019M660921 and 2020T130436); Science Foundation for Post Doctorate Research of the Beijing (Nos. 2017-ZZ-123 and 2020-ZZ-005); and Natural Science Foundation of Tianjin (No. 19JCYBJC26600).
Contributor Information
Wen-Jun Tu, Email: tuwenjun@irm-cams.ac.cn.
Jizong Zhao, Email: zhaojz205@163.com.
Data Availability
Please contact the corresponding author (Pro. Tu) for the data request.
Ethical Approval
Ethical approval is not applicable.
Consent
Consent is not applicable.
Conflicts of Interest
The authors have no conflict of interest relevant to this study.
References
- 1.Benjamin E. J., Muntner P., Alonso A., et al. Heart disease, and stroke statistics-2019 update: a report from the American Heart Association. Circulation . 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Johnson C. O., Nguyen M., Roth G. A., et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology . 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsalta-Mladenov M., Andonova S. Health-related quality of life after ischemic stroke: impact of sociodemographic and clinical factors. Neurological Research . 2021;43(7):553–561. doi: 10.1080/01616412.2021.1893563. [DOI] [PubMed] [Google Scholar]
- 4.Tu W. J., Chao B. H., Ma L., et al. Case-fatality, disability and recurrence rates after first-ever stroke: a study from bigdata observatory platform for stroke of China. Brain Research Bulletin . 2021;175:130–135. doi: 10.1016/j.brainresbull.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Dolati S., Soleymani J., Kazem Shakouri S., Mobed A. The trends in nanomaterial-based biosensors for detecting critical biomarkers in stroke. Clinica Chimica Acta . 2021;514:107–121. doi: 10.1016/j.cca.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Simats A., García-Berrocoso T., Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochimica et Biophysica Acta . 2016;1862(3):411–424. doi: 10.1016/j.bbadis.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Faura J., Bustamante A., Miró-Mur F., Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. Journal of Neuroinflammation . 2021;18(1):127–127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teymuri Kheravi M., Nayebifar S., Aletaha S. M., Sarhadi S. The effect of two types of exercise preconditioning on the expression of TrkB, TNF-α, and MMP2 genes in rats with stroke. BioMed Research International . 2021;2021:7. doi: 10.1155/2021/5595368.5595368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarkowski E., Rosengren L., Blomstrand C., et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke . 1995;26:8. doi: 10.1161/01.STR.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 10.Fassbender K., Rossol S., Kammer T., et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. Journal of the Neurological Sciences . 1994;122(2):135–139. doi: 10.1016/0022-510X(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 11.Vila N., Castillo J.´., Dávalos A., Chamorro A.´. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke . 2000;31(10):2325–2329. doi: 10.1161/01.STR.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 12.Vila N., Castillo J.´., Dávalos A., Esteve A., Planas A. M., Chamorro A.´. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke . 2003;34(3):671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X., Shen Y., Li R. Targeting TNF: a therapeutic strategy for Alzheimer's disease. Drug Discovery Today . 2014;19(11):1822–1827. doi: 10.1016/j.drudis.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal B. B. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Annals of the Rheumatic Diseases . 2000;59(90001) doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olmos G., Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators of Inflammation . 2014;2014:12. doi: 10.1155/2014/861231.861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer R., Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Medicine and Cellular Longevity . 2015;2015:18. doi: 10.1155/2015/610813.610813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barone F. C., Arvin B., White R. F., et al. Tumor necrosis factor-α. Stroke . 1997;28(6):1233–1244. doi: 10.1161/01.STR.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 18.Bonetti N. R., Diaz-Cañestro C., Liberale L., et al. Tumour necrosis factor-α inhibition improves stroke outcome in a mouse model of rheumatoid arthritis. Scientific Reports . 2019;9(1):p. 2173. doi: 10.1038/s41598-019-38670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuttolomondo A., di Raimondo D., di Sciacca R., Pinto A., Licata G. Inflammatory cytokines in acute ischemic stroke. Current Pharmaceutical Design . 2008;14(33):3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 20.Lin S.-Y., Wang Y. Y., Chang C. Y., et al. TNF-α receptor inhibitor alleviates metabolic and inflammatory changes in a rat model of ischemic stroke. Antioxidants . 2021;10(6):p. 851. doi: 10.3390/antiox10060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein Shochet G., Brook E., Israeli-Shani L., Edelstein E., Shitrit D. Fibroblast paracrine TNF-α signaling elevates integrin A5 expression in idiopathic pulmonary fibrosis (IPF) Respiratory Research . 2017;18(1):p. 122. doi: 10.1186/s12931-017-0606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates R. C., Mercurio A. M. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Molecular Biology of the Cell . 2003;14(5):1790–1800. doi: 10.1091/mbc.e02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engwerda C. R., Ato M., Stäger S., Alexander C. E., Stanley A. C., Kaye P. M. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. The American Journal of Pathology . 2004;165(6):2123–2133. doi: 10.1016/S0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nature Reviews Immunology . 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Bäumel M., Männel D. N., Howard O. M. Z., Oppenheim J. J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. Journal of Immunology . 2007;179(1):154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 26.Doll D. N., Barr T. L., Simpkins J. W. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging and Disease . 2014;5(5):294–306. doi: 10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Horssen R., Ten Hagen T. L., Eggermont A. M. TNF-α in cancer treatment: molecular insights, antitumor effects, and clinical utility. The Oncologist . 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 28.Laha D., Grant R., Mishra P., Nilubol N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Frontiers in Immunology . 2021;12, article 656908 doi: 10.3389/fimmu.2021.656908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware C. F. Network communications: lymphotoxins, LIGHT, and TNF. Annual Review of Immunology . 2005;23(1):787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 30.McCoy M. K., Tansey M. G. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of Neuroinflammation . 2008;5(1):45–45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harashima S., Horiuchi T., Hatta N., et al. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. Journal of Immunology . 2001;166(1):130–136. doi: 10.4049/jimmunol.166.1.130. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia L. A., Goeddel D. V. Two TNF receptors. Immunology Today . 1992;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 33.Diaz Arguello O. A., Haisma H. J. Apoptosis-inducing TNF superfamily ligands for cancer therapy. Cancers . 2021;13(7) doi: 10.3390/cancers13071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banner D. W., D'Arcy A., Janes W., et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell . 1993;73(3):431–445. doi: 10.1016/0092-8674(93)90132-A. [DOI] [PubMed] [Google Scholar]
- 35.Hsu H., Shu H. B., Pan M. G., Goeddel D. V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell . 1996;84(2):299–308. doi: 10.1016/S0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 36.Hsu H., Huang J., Shu H. B., Baichwal V., Goeddel D. V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity . 1996;4(4):387–396. doi: 10.1016/S1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 37.Shu H. B., Takeuchi M., Goeddel D. V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proceedings of the National Academy of Sciences of the United States of America . 1996;93(24):13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vince J. E., Pantaki D., Feltham R., et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. The Journal of Biological Chemistry . 2009;284(51):35906–35915. doi: 10.1074/jbc.M109.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dostert C., Grusdat M., Letellier E., Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiological Reviews . 2019;99(1):115–160. doi: 10.1152/physrev.00045.2017. [DOI] [PubMed] [Google Scholar]
- 40.Haas T. L., Emmerich C. H., Gerlach B., et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Molecular Cell . 2009;36(5):831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Gough P., Myles I. A. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Frontiers in Immunology . 2020;11, article 585880 doi: 10.3389/fimmu.2020.585880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faustman D., Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nature Reviews. Drug Discovery . 2010;9(6):482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 43.Mukai Y., Nakamura T., Yoshikawa M., et al. Solution of the structure of the TNF-TNFR2 complex. Science Signaling . 2010;3(148) doi: 10.1126/scisignal.2000954. [DOI] [PubMed] [Google Scholar]
- 44.Yang S., Wang J., Brand D. D., Zheng S. G. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Frontiers in Immunology . 2018;9:784–784. doi: 10.3389/fimmu.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng Y., Li F., Qin Z. TNF receptor 2 makes tumor necrosis factor a friend of tumors. Frontiers in Immunology . 2018;9:1170–1170. doi: 10.3389/fimmu.2018.01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad S., Azid N. A., Boer J. C., et al. The key role of TNF-TNFR2 interactions in the modulation of allergic inflammation: a review. Frontiers in Immunology . 2018;9:2572–2572. doi: 10.3389/fimmu.2018.02572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medler J., Wajant H. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opinion on Therapeutic Targets . 2019;23(4):295–307. doi: 10.1080/14728222.2019.1586886. [DOI] [PubMed] [Google Scholar]
- 48.Boulanger L. M. Immune proteins in brain development and synaptic plasticity. Neuron . 2009;64(1):93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Flynn J. L., Goldstein M. M., Chan J., et al. Tumor necrosis factor-α is required in the protective immune response against mycobacterium tuberculosis in mice. Immunity . 1995;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 50.Fresegna D., Bullitta S., Musella A., et al. Re-examining the role of TNF in MS pathogenesis and therapy. Cell . 2020;9(10):p. 2290. doi: 10.3390/cells9102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis M., Tartaglia L. A., Lee A., et al. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proceedings of the National Academy of Sciences of the United States of America . 1991;88(7):2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stellwagen D., Malenka R. C. Synaptic scaling mediated by glial TNF-α. Nature . 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 53.Beattie E. C., Stellwagen D., Morishita W., et al. Control of synaptic strength by glial TNFalpha. Science . 2002;295(5563):2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 54.Ogoshi F., Yin H. Z., Kuppumbatti Y., Song B., Amindari S., Weiss J. H. Tumor necrosis-factor-alpha (TNF-α) induces rapid insertion of Ca2+-permeable α-amino-3-hydroxyl-5-methyl-4-isoxazole- propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Experimental Neurology . 2005;193(2):384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 55.He P., Liu Q., Wu J., Shen Y. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. The FASEB Journal . 2012;26(1):334–345. doi: 10.1096/fj.11-192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perea G., Navarrete M., Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends in Neurosciences . 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Jin X., Gereau RW 4th Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. The Journal of Neuroscience . 2006;26(1):246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montgomery S. L., Bowers W. J. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. Journal of Neuroimmune Pharmacology . 2012;7(1):42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 59.Cacci E., Claasen J. H., Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells In Vitro. Journal of Neuroscience Research . 2005;80(6):789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- 60.Heldmann U., Thored P., Claasen J. H., Arvidsson A., Kokaia Z., Lindvall O. TNF-α antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Experimental Neurology . 2005;196(1):204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 61.Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiological Reviews . 1990;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 62.Sato N., Goto T., Haranaka K., et al. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. Journal of the National Cancer Institute . 1986;76(6):1113–1121. [PubMed] [Google Scholar]
- 63.Pan W., Zadina J. E., Harlan R. E., TJO W., Banks W. A., Kastin A. J. Tumor necrosis factor-α: a neuromodulator in the CNS. Neuroscience and Biobehavioral Reviews . 1997;21(5):603–613. doi: 10.1016/S0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 64.Maciejczyk M., Bielas M., Zalewska A., Gerreth K. Salivary biomarkers of oxidative stress and inflammation in stroke patients: from basic research to clinical practice. Oxidative Medicine and Cellular Longevity . 2021;2021:22. doi: 10.1155/2021/5545330.5545330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.del Zoppo G. J. Stroke and neurovascular protection. The New England Journal of Medicine . 2006;354(6):553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 66.Dirnagl U., Iadecola C., Moskowitz M. A. Pathobiology of ischaemic stroke: an integrated view. Trends in Neurosciences . 1999;22(9):391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 67.Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science . 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 68.Zhou X., Yu F., Feng X., et al. Immunity and inflammation predictors for short-term outcome of stroke in young adults. The International Journal of Neuroscience . 2018;128(7):634–639. doi: 10.1080/00207454.2017.1408614. [DOI] [PubMed] [Google Scholar]
- 69.Clausen B. H., Degn M., Martin N. A., et al. Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ischemia. Journal of Neuroinflammation . 2014;11(1):p. 203. doi: 10.1186/s12974-014-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu T., Clark R. K., McDonnell P. C., et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke . 1994;25(7):1481–1488. doi: 10.1161/01.STR.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 71.Zaremba J., Losy J. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurologica Scandinavica . 2001;104(5):288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 72.Mark K. S., Trickler W. J., Miller D. W. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. The Journal of Pharmacology and Experimental Therapeutics . 2001;297(3):1051–1058. [PubMed] [Google Scholar]
- 73.Hallenbeck J. M. The many faces of tumor necrosis factor in stroke. Nature Medicine . 2002;8(12):1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 74.Badiola N., Malagelada C., Llecha N., et al. Activation of caspase-8 by tumour necrosis factor receptor 1 is necessary for caspase-3 activation and apoptosis in oxygen-glucose deprived cultured cortical cells. Neurobiology of Disease . 2009;35(3):438–447. doi: 10.1016/j.nbd.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology . 1988;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- 76.Loppnow H., Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. The Journal of Clinical Investigation . 1990;85(3):731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yong V. W., Power C., Forsyth P., Edwards D. R. Metalloproteinases in biology and pathology of the nervous system. Nature Reviews. Neuroscience . 2001;2(7):502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nawashiro H., Tasaki K., Ruetzler C. A., Hallenbeck J. M. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. Journal of Cerebral Blood Flow and Metabolism . 1997;17(5):483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Cheng B., Christakos S., Mattson M. P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron . 1994;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 80.Liang M., Zhang L., Geng Z. Advances in the development of biomarkers for poststroke epilepsy. BioMed Research International . 2021;2021:8. doi: 10.1155/2021/5567046.5567046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y., Pu J., Liu Y., et al. Pro-inflammatory cytokines are associated with the development of post-stroke depression in the acute stage of stroke: a meta-analysis. Topics in Stroke Rehabilitation . 2020;27(8):620–629. doi: 10.1080/10749357.2020.1755813. [DOI] [PubMed] [Google Scholar]
- 82.Grigolashvili M. A., Mustafina R. M. The role of the inflammatory process in the development of post-stroke cognitive impairment. Zh Nevrol Psikhiatr Im S S Korsakova . 2021;121(3):16–21. doi: 10.17116/jnevro202112103216. [DOI] [PubMed] [Google Scholar]
- 83.Tobinick E., Kim N. M., Reyzin G., Rodriguez-Romanacce H., DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury. CNS Drugs . 2012;26(12):1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 84.Zaremba J., Losy J. Early TNF-α levels correlate with ischaemic stroke severity. Acta Neurologica Scandinavica . 2001;104(5):288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 85.Flores-Cantú H., Góngora-Rivera F., Lavalle-González F., et al. Tumor necrosis factor alpha, prognosis and stroke subtype etiology. Medicina Universitaria . 2016;18(73):194–200. [Google Scholar]
- 86.Arango-Dávila C. A., Vera A., Londoño A. C., et al. Soluble or soluble/membrane TNF-± inhibitors protect the brain from focal ischemic injury in rats. The International Journal of Neuroscience . 2015;125(12):936–940. doi: 10.3109/00207454.2014.980906. [DOI] [PubMed] [Google Scholar]
- 87.Frankola A. K., Greig H. N., Luo W., Tweedie D. Targeting TNF-Alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS & Neurological Disorders Drug Targets . 2011;10(3):391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerriets V., Bansal P., Goyal A., Khaddour K. Tumor necrosis factor inhibitors . Treasure Island (FL): StatPearls Publishing LLC.; 2021. [PubMed] [Google Scholar]
- 89.Present D. H., Rutgeerts P., Targan S., et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. The New England Journal of Medicine . 1999;340(18):1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 90.Wang X., Feuerstein G. Z., Xu L., et al. Inhibition of tumor necrosis factor-α-converting enzyme by a selective antagonist protects brain from focal ischemic injury in rats. Molecular Pharmacology . 2004;65(4):890–896. doi: 10.1124/mol.65.4.890. [DOI] [PubMed] [Google Scholar]
- 91.Nawashiro H., Martin D., Hallenbeck J. M. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Research . 1997;778(2):265–271. doi: 10.1016/S0006-8993(97)00981-5. [DOI] [PubMed] [Google Scholar]
- 92.Liberale L., Bonetti N. R., Puspitasari Y. M., et al. TNF-α antagonism rescues the effect of ageing on stroke: perspectives for targeting inflamm-ageing. European Journal of Clinical Investigation . 2021;51(11, article e13600) doi: 10.1111/eci.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mariette X., Matucci-Cerinic M., Pavelka K., et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Annals of the Rheumatic Diseases . 2011;70(11):1895–1904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 94.Wolfe F., Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis and Rheumatism . 2007;56(9):2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 95.Borlongan M. C., Kingsbury C., Salazar F. E., et al. IL-2/IL-2R antibody complex enhances Treg-induced neuroprotection by dampening TNF-α inflammation in an In Vitro stroke model. Neuromolecular Medicine . 2021;23(4):540–548. doi: 10.1007/s12017-021-08656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Contreras M. A., Macaya L., Manrique V., et al. A trivalentTNF‐R2as a new tumor necrosis factor alpha‐blocking molecule. Proteins . 2021;89(11):1557–1564. doi: 10.1002/prot.26177. [DOI] [PubMed] [Google Scholar]
- 97.Lu J., Guo X., Yan M., et al. P2X4R contributes to central disinhibition via TNF-α/TNFR1/GABAaR pathway in post-stroke pain rats. The Journal of Pain . 2021;22(8):968–980. doi: 10.1016/j.jpain.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Manrique-Suárez V., Macaya L., Contreras M. A., et al. Design and characterization of a novel dimeric blood-brain barrier penetrating TNFα inhibitor. Proteins . 2021;89(11):1508–1521. doi: 10.1002/prot.26173. [DOI] [PubMed] [Google Scholar]
- 99.Wu M.-H., Huang C. C., Chio C. C., et al. Inhibition of peripheral TNF-α and downregulation of microglial activation by alpha-lipoic acid and etanercept protect rat brain against ischemic stroke. Molecular Neurobiology . 2016;53(7):4961–4971. doi: 10.1007/s12035-015-9418-5. [DOI] [PubMed] [Google Scholar]
- 100.Pires P. W., Girgla S. S., Moreno G., McClain J. L., Dorrance A. M. Tumor necrosis factor-α inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. American Journal of Physiology. Heart and Circulatory Physiology . 2014;307(5):H658–H669. doi: 10.1152/ajpheart.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lei B., Dawson H. N., Roulhac-Wilson B., Wang H., Laskowitz D. T., James M. L. Tumor necrosis factor alpha antagonism improves neurological recovery in murine intracerebral hemorrhage. Journal of Neuroinflammation . 2013;10(1) doi: 10.1186/1742-2094-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tobinick E., Kim N. M., Reyzin G., Rodriguez-Romanacce H., DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs . 2012;26(12):1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- 103.Sumbria R. K., Boado R. J., Pardridge W. M. Brain protection from stroke with intravenous TNFα decoy receptor-Trojan horse fusion protein. Journal of Cerebral Blood Flow and Metabolism . 2012;32(10):1933–1938. doi: 10.1038/jcbfm.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author (Pro. Tu) for the data request.


