Abstract
Nanoparticles (NPs) modified by cell membranes represent an emerging biomimetic platform that can mimic the innate biological functions resulting from the various cell membranes in biological systems. researchers focus on constructing the cell membrane camouflaged NPs using a wide variety of cells, such as red blood cell membranes (RBC), macrophages and cancer cells. Cell membrane camouflaged NPs (CMNPs) inherit the composition of cell membranes, including specific receptors, antigens, proteins, for target delivering to the tumor, escaping immune from clearance, and prolonging the blood circulation time, etc. Combining cell membrane-derived biological functions and the NP cores acted cargo carriers to encapsulate the imaging agents, CMNPs are widely developed to apply in tumor imaging techniques, including computed tomography (CT), magnetic resonance imaging (MRI), fluorescence imaging (FL) and photoacoustic imaging (PA). Herein, in this review, we systematically summarize the superior functions of various CMNPs in tumor imaging, especially highlighting the advanced applications in different imaging techniques, which is to provide the theoretical supports for the development of precise guided imaging and tumor treatment.
Keywords: Nanoparticles, Cell membrane, Biomimetic, Tumor, Imaging, Therapy
Graphical abstract
Nomenclature
Abbreviations
- BM
bacterial membrane
- BBB
blood-brain barrier
- CCM
cancer cell membranes
- CCL2
C–C chemokine ligand
- CM
cell membrane
- CDT
chemodynamic therapy
- CTCs
circulating tumor cells
- CT
computed tomography
- DOX
doxorubicin
- EPR
enhanced permeability and retention
- FL
fluorescence
- FA
folic acid
- FDA
food and drug administration
- Gd
gadolinium
- GSH
glutathione
- HB
hypocrellins B
- ICG
indocyanine Green
- MM
macrophage membrane
- MRI
magnetic resonance imaging
- MSC
mesenchymal stem cell
- MPS
monocyte phagocyte system
- CNs
nanocarbons
- NPs
nanoparticles/nanoprobes
- NIR
near infrared
- NM
neutrophil membranes
- ODV
optical droplet vaporization
- PTX
paclitaxel
- PFP
perfluoropentane
- PA
photoacoustic
- PDT
photodynamic therapy
- PTT
photothermal therapy
- PLM
platelet membrane
- PET
positron emission tomography
- PB
prussian blue
- QE
quercetin
- ROS
reactive oxygen species
- RBC
red blood cell
- RBCM
red blood cell membranes
- RES
reticuloendothelial system
- SIRP-α
signal-regulated protein-α
- SPECT
single-photon emission computed tomography
- SCM
stem cell membrane
- US
ultrasound
- UCL
upconversion luminescence
- WJ
wharton's jelly
1. Introduction
Cancer is one of the major diseases that threaten human life and health, with thousands of people losing their lives every year due to the deterioration of disease [1]. It is estimated that there will be approximately 19.3 million new cases and nearly 10 million deaths worldwide in 2020 (which does not include non-melanoma skin cancers). Moreover, the incidence and mortality rates of cancer are increasing rapidly worldwide. Therefore, there is an urgent need to improve the efficiency of cancer treatment.
Currently, the main treatments for cancer are surgical removal, chemotherapy, radiotherapy, etc [2]. In order to gain an effective cure for cancer, precise localization of lesion site prior to treatment are urgent required. Image-assisted oncology is a promising approach to enable precise localization of tumors for highly localized treatment. There are various imaging techniques, such as CT, MRI, FL and PA, to guide treatments such as PDT, PTT and CDT [[3], [4], [5]]. These imaging techniques exhibit their unique imaging superiority along with some certain drawbacks. For example, MRI is a powerful imaging tool with high spatial resolution, excellent depth of penetration. However, MRI still has the disadvantage of being less sensitive in distinguishing small lesions from surrounding normal tissue [[6], [7], [8]]. Although FL can be scanned quickly and in real-time, the use of optical imaging, especially in deep tissue and solid tumors, is severely hampered by the low depth of light penetration [9,10]. PA is suitable for high optical contrast and spatial resolution vascular observation, but due to the low medium laser energy, only a small area can be imaged [11]. As a result, the imaging of the cancerous area is greatly affected, which limits the effective cure for cancer.
In the imaging, the main problem is resulting from the lack of specific targeting and effective delivery of the developer to the tumor area. To alleviate the inadequate target, researchers have been studying the use of NPs for delivering contrast agents. In fact, NPs have been widely used in drug delivery [12], imaging [13] and nucleic acid delivery [14]. Usually, NPs are beneficial for passively target delivery to the tumor through the mechanism of EPR, i.e., the incompact tumor vasculature facilitates NPs leakage, and subsequently reduces lymphatic clearance, which allows nanoscale carriers to be effectively retained within the tumor lesion [15]. However, it is extremely difficult to obtain a stable targeting function during the actual circulation in vivo. In response, researchers have gradually diverted their attention to active targeting strategies. Active targeting strategies are based on surface modification of functional groups to recognize receptors or antigens overexpressed on the tumor surface. The advantages of using actively targeted NPs for drug or contrast delivery are as follows: (1) continuous and concentrated drug delivery through in vivo targeting, (2) significant reduction in systemic side effects and toxicity, (3) improved targeting performance through modification of ligands, etc [16,17]. Despite this, only a small number of NPs have been evaluated in clinical trials and finally successfully approved for clinical translation by the FDA. The main reason in-clinic loss is caused by the RES, which recognizes and subsequently leads to NPs clearance [18]. Hence, the “foreign” property has greatly hindered the further application of NPs in clinics.
In the ideal case, the effective nanocarriers should own the autogenous properties for long-term in vivo retention, immune escape, targeted delivery and specific barriers crossing in vivo [19]. However, the tumor-active targeting properties of the most engineered NPs are very inefficient, with the controversial enhanced permeability and retention effect in preclinical and clinical trials [[20], [21], [22]]. A tumor-active targeting approach based on ligand-receptor interactions has also been applied to enhance the tumor accumulation of NPs. However, the in vivo off-target effect of NPs, active clearance by macrophages and low immunocompatibility limit their further biomedical applications [23,24]. The CM-based bionanotechnology strategy proposed by Zhang's group offers another opportunity to address the active targeting of engineered NPs [25]. To this end, biomimetic CM camouflaged NPs (CMNPs) have attracted a lot of attention in recent years [26]. Since CMNPs retain the antigens and structures from CM, NPs tend to accept by the organism, providing an effective delivery platform for imaging contrast agents, drugs, vaccination, through their specific functions such as ligand recognition and targeted delivery, prolonging blood circulation and immune escape [[27], [28], [29]]. The CM camouflaging technique is a simple and feasible top-down approach to construct the cell or CM-based carriers for improving safe and efficient target delivery of NPs without special limitations of the core nanomaterials [30]. Furthermore, because of the structural and functional similarity to the host cell, CMNPs show the inherited specific functional protein for effective targeted delivery without rare clearance by RES [31]. Considering these attractive advantages, numerous research about CMNPs have been developed for tumor imaging. For example, Chen et al. [27] fabricated an MCF-7 CCM encapsulated and ICG loaded NPs (ICNPs), exhibiting good dispersity, PTT responsiveness and excellent FL/PA imaging properties with specific homologous targeting to cancer cells. It may be ascribed to the homologous binding adhesion molecules on CCM, which promoted endocytosis and homologous target delivery and enhanced tumorous accumulation. Furthermore, ICNPs were significantly reduced the interception by the liver and kidneys due to their camouflaged biological surface as cells. For NIR-FL/PA dual-modality imaging, ICNPs are able to exhibit high-resolution and deep-penetrating in vivo real-time monitoring. Nowadays, except for the application of CCM in tumor imaging, various CMs, such as RBCM [32], PLM [33] and MM [34], are widely investigated for tumor imaging (Table 1).
Table 1.
Cell membrane biomimetic nanoparticles for tumor imaging.
| Cell Membrane | Core | Imaging approach | Tumor model | Reference |
|---|---|---|---|---|
| RBCM | FA and Cy5-modified nanoscale vesicles | FL | 4T1 cancer | [35] |
| ICG | FL | SKBR3 breast cancer | [36] | |
| Fe3O4 magnetic NPs | MRI | MCF-7 human breast tumor | [37] | |
| DOX-loaded Prussian blue nano-composites | PA/FL/PTT | HeLa tumor | [38] | |
| Cyp-superparamagnetic nanoclusters | MRI | Colorectal tumor | [39] | |
| Fe3O4@Cu2-xS | MRI | Human cervical cancer (Hela) | [40] | |
| CCM | Upconversion NPs | FL/MRI/PET | Triple negative breast cancer | [41] |
| Ir–B–TiO2 | PA/PTT | HeLa tumor | [42] | |
| Dendritic mesoporous silica NPs-DNA probe | PA | MCF-7 tumor | [43] | |
| Mesoporous copper/manganese silicate nanospheres | MRI | MCF-7 tumor | [44] | |
| styrene and acrylic acid-crosslinked SPION | MRI/FL | SMMC-7721 tumor | [45] | |
| Dox and IGCG-loaded hollow copper sulfide NPs | PA | B16F10 tumor | [46] | |
| MM | Quercetin-loaded hollow Bi2Se3 | CT | Breast cancer | [47] |
| Upconversion NPs | FL | MCF-7 tumor | [48] | |
| PLM | IR 1048 dye-loaded liposomes | PA | C6, SW1990 and 4T1 tumor | [49] |
| Dox-PFP-CNs@PLGA/PM NPs | PA/US/FL | 4T1 tumor | [50] | |
| W18O49 NPs and metformin | FL | Raji lymphoma | [51] | |
| C3F8 gas and ICG | US/FL | 4T1 tumor | [52] | |
| Antibody-drug and scFvGPIIb/IIIa-monomethyl auristatin E | FL | MDA-MB-231 | [53] | |
| SCM | Fe3O4@PDA-siRNA NPs | MRI | DU145 cells | [54] |
| Mn2+ and Gd3+ co-doped CuInS2–ZnS nanocrystals | FL/MRI/SPECT | B16F10 tumor | [55] | |
| BM | Magnetosomes | MRI | orthotopic breast cancer | [56] |
| ZGGO@SiO2@LRM | FL | Colorectal cancer | [57] | |
| Cancer-macrophage hybrid membrane | IR825/Ir ZGGO@SiO2 | FL | CT26 tumor | [58] |
| Bacterial-cancer hybrid membrane | HPDA@[OMV-CC] NPs | PTT | Melanoma | [59] |
| Erythrocyte-cancer hybrid membrane | Melanin NPs | PA | MCF-7 tumor | [60] |
Hence, this paper focuses on the recent advances in CM biomimetic NPs for tumor imaging, systematically summarizes the construction of CMNPs using various CM for applications in tumor imaging, especially highlights the strategies and functions of various CM in imaging techniques, and anticipates their future perspective.
2. Cell membrane biomimetic nanoparticles for imaging applications
The conventional delivery of imaging contrast agents/drugs is mainly by intravenous injection. However, in this delivery method, contrast agent/drug would pass through the body's circulatory system and subsequently be largely eliminated by the kidneys, affecting the kinetic parameters in the body. From last decades, NPs have been expoited to effectively overcome those problems. Synthetic NPs have the advantages of targeted delivery and diverse functionality. As a result, they have been widely studied and used in biomedicine [61]. However, there are still some problems such as immune resistance in the application of NPs [62]. Initially, NPs were often coated by various polymeric molecules, such as natural substances like polysaccharides or semi-synthetic substances like copolymers, making NPs as biocompatible and immune escapable as possible. However, the use of polymers as coatings (e.g. polyethylene glycol) does not completely prevent clearance by the immune system and thus NPs can still activate the body's immune compensation system [63,64]. Considering the biological interactions of NPs in the body, cell membrane camouflaged biomimetic NPs become an effective design solution due to their natural structural advantages.
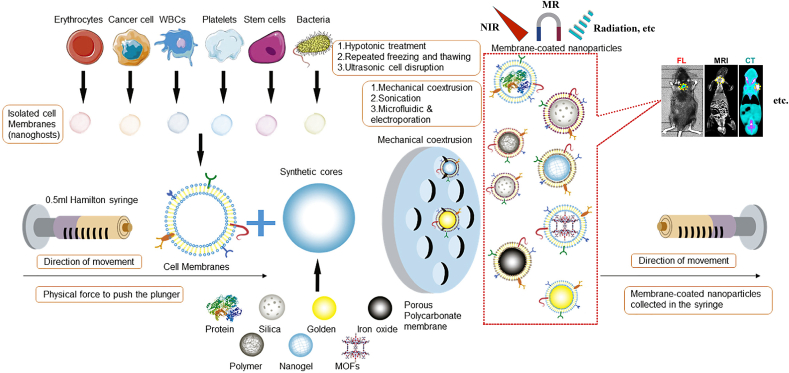
Owing to the fundamental unit of life activity, the individual cell is vital to the functioning of the organism. As a result, researchers have taken inspiration from natural cells and mimicked them to build bionic transporters. Initially, Hu et al. [65]. established a bionic system wrapped by cell membranes. They successfully achieved improved biocompatibility of NPs using RBCM coated on the surface of polymeric NPs. Nowadays, the applications of CMNPs are mainly focused on drug transport, detoxification and immunomodulation [66]. The preparation of biomimetic NPs mainly contains the membrane and the inner core nanocarrier. After obtaining the membrane and the inner core nanocarrier, the two materials must be fused so that the membrane can cover the surface of the core and generate cell membrane biomimetic NPs. The origins of membrane coating technology can be traced back to 2011 when it was first reported by Zhang et al. [25], who used an up-down strategy to cover NPs with intact cell membranes. At present, there are three fusion methods: membrane extrusion, ultrasonic fusion and electroporation [67]. Among them, physical co-extrusion is one of the efficient methods, including three main steps, i.e., membrane extraction, preparation of core NPs and fusion (Fig. 1) [[68], [69], [70], [71], [72], [73]]. For cell membrane fusion, both membrane carriers and inner core nanomaterials can be repeatedly extruded through nanoscale polycarbonate porous membranes using an Avanti micro-extruder. During this extrusion process, mechanical forces lead to the membrane coating of NPs [74]. Although the method is difficult to prepare on a large scale, it is simple and effective. On the other hand, as the research progression, a strong interaction between tumor cells and various cells, demonstrating unique advantages of CMNPs with a wide potential application in tumor imaging. Thus, according to the previous reports, six kinds of CM, including RBCM, CCM, MM, PLM, SCM, BM and hybrid membranes, have been widely investigated to coat NPs through physical co-extrusion method for tumor imaging applications.
Fig. 1.
Physical co-extrusion method for membrane coating fabrication and the biomimetic NPs application on tumor imaging. Copyright © 2018, Elsevier Publishing Group. Replicated with permission from Ref. [70].
2.1. Red blood cell membrane
RBC is the most common form in blood cells with a diameter between 7–8 μm and a thickness of approximately 1 μm at the center. Because of the enormous content and size property, RBC can be easily separated from the blood, as well-known as an easy-to-obtain membrane [75]. Furthermore, because the lifetime of RBC is up to more than 120 days, RBC has been exploited as an excellent natural long-circulation carrier. In fact, RBC is rich in the “self-tagged” proteins (e.g., CD 47), glycans and acidic sialic acid fractions on the surface, which effectively avoid phagocytosis by the immune system [[76], [77], [78], [79]]. Among them, CD 47 is the key substance to act immune escape performance resulting from serials of the signal path, i.e., CD 47 binding to SIRP-α and inducing phosphorylation of the SIRP-α tail, which result in protein phosphatase activation to block phagocytosis by inhibiting the accumulation of the motor protein myosin IIA at phagocytic synapses [80]. Thus, inheriting the innate biological functions, RBCM is improved as a novel candidate to coat on the surface of NPs in the “right-side-out” orientation (i.e., front-facing outward containing multiple surface antigens) for significantly inhibiting immune system phagocytosis in the efficient cargo delivery.
Besides the camouflaging function to avoid clearance by the immune system, RBCM based CMNPs are capable of reducing the toxic side effects, prolonging the circulation time, and enhancing drug retention at the lesion. Additionally, RBCM based CMNPs are able to integrate the flexibility of synergistic materials and the functionality of RBCM, endowing the combined advantages for the advanced drug delivery system. For example, Pei et al. [81] reported RBCM camouflaged NPs (RBC(M(TPC-PTX))) for synergistic chemotherapy and photodynamic therapy (PDT). In vivo studies also confirmed that RBC(M(TPC-PTX)) was capable of effectively prolonging the circulation time, promoting drug accumulation in the lesion, and enhancing the synergetic efficacy of chemotherapy and PDT for safe and efficient anticancer therapy.
In addition, RBCM is also developed to encapsulate the contrast agents for tumor imaging. The widely used imaging contrast agents include ICG [82], PB [83], Fe3O4 [84] and others. However, because of their unpredictable metabolic pathways and potential long-term accumulation of toxicity in the body, the usage of contrast agents is still cautious and limited in clinics [85,86]. To address this problem, Xiao et al. [87] developed the DOX-loaded multimodal PB nanocomposites with RBCM coats and FA functionalization, developing as a target multimodal bioimaging agent for PTT, FL and PA imaging of tumor.
Similar to upconversion NPs (UCNPs), a class of synthetic NPs, that have been applied in MRI and UCL imaging for a long term, are easy to recognize as the extraneous invaders by the innate immune system, and subsequently eliminate by the RES/MPS [88,89]. To solve the “foreign” loss, Li et al. [90] reconstituted the vesicles (RBC-vesicles) to encapsulat UCNPs. These RBC vesicles based biomimetic NPs with short half-life radionuclides could escape the immune clearance, prolong the blood circulation time, and significantly enhance tumor target delivery for precise PET imaging to 4T1 tumors. In the same year, She et al. [91]. reported that an RBCM coated FeS2 NPs (FeS2@RBCs). FeS2@RBCs show not only the prolonged circulation and negligible immune response, but also self-enhanced MRI under imaging-guided laser irradiation after reaction with H2O2 in the tumor area. It is shown that FeS2 encapsulated by RBCM could enhance MRI signals and enable PTT-CDT imaging-guided synergistic therapy.
Generally, because of the innate superiority in long-term blood circulation and “stealth” functions, RBCM based CMNPs are able to inherit the biological function for prolonging blood circulation time and enhancing tumor target imaging agent delivery for promoting the precise tumor imaging and/or therapy, as well as reducing the side effects to normal cells and tissues. Critically, because of lack of target performance resulting from EPR mediated the passive target effect, RBCM based CMNPs usually further functionalize through the active target modification on the surface of RBC to significantly improve the target efficiency for tumor lesion.
2.2. Cancer cell membrane
Compared with the RBC, cancer cells exhibit unique properties to facilitate binding the homologous cancer cells because of the homologous adhesion molecules expressed on the surface of cancer cells(e.g., lectin, integrins, cadherins, selectins and protein [92]. After being engineered by CCM, CMNPs endow the strong homologous target ability without any cumbersome surface modification for tumor-specific targeted delivery of drugs or contrast agents. Additionally, CCM has been proved as one of the most powerful ways to enhance the biocompatibility of NPs. Hence, owing to the target functional protein components on the surface [45], CCM camouflaged NPs can not only promote endogenous biomimic for “stealth” delivery in vivo, but also enhance cellular uptake, tumor targeting and accumulation.
Because of the unique target functional proteins on CCM endowing immune escape and homologous binding to cancer cells, CCM-based CMNPs can be exploited as the biomimetic carriers for efficient tumor imaging and therapy [93]. For example, Rao et al. [94] reported a CCM membrane camouflaged NPs (CC-UCNPs), which inherited the immune escape and homologous targeting from the source cancer cells. In vitro and in vivo UCL imaging studies as well as Y3+ measurements further confirmed that MDA-UCNPs exhibited the strongest fluorescent signal for BALB/c nude mice modeling MDA-MB-435 mammary tumor allografts. Therefore, CCM-based CMNPs with homologous target property inaugurate a simple and feasible way to significantly expand the novel design for the application in tumor imaging/therapy.
Recently, because of their homologous targeting and immune escape abilities, CCM has gained much attention and exploited wide application in precise tumor imaging, including MR, PA, NIR, etc. (Table 1). For example, the degradable mesoporous copper/manganese silicate NPs (mCMSN) coated by MCF-7 CM were developed as a hypoxic-responsive Fenton reagent analog and photosensitizer for MRI-guided CDT/PDT synergistic anti-tumor therapy, exhibiting favorable target cargo delivery to tumor both in vitro and in vivo [44]. Furthermore, pathological GSH could trigger mCMSNs degradation to release Cu+ and Mn2+ in the lesion, where Mn2+ exhibited high T1 relaxation acting as an MRI contrast agent to monitor the synergistic CDT/PDT treatment for the tumor. For PA imaging, owing to low sensitivity has greatly hindered the development in practical applications, CCM-based CMNPs should be a feasible candidate to improve homologous tumor imaging.
Wu et al. [46] fabricated the imaging and therapeutic synergistic theragnostic agents (ID-HCuSNP@B16 F10) from the B16 F10 CM camouflaged hollow copper sulfide NPs loading with DOX and ICG for homology target melanoma. Both in vitro and in vivo experiments confirmed that ID-HCuSNP@B16 F10 inherited the highly specific homologous target property from the source cells to enhance the PA imaging and therapy for B16 F10 tumor cells. Compared to RBCs, cancer cells have a unique unlimited replication potential and homologous targeting ability. Due to the proliferative capacity of cancer cells, it is easy to obtain cancer cells through in vitro cell culture rather than from autologous plasma or donors [95]. Biomimetic NPs can disguise the contrast agent as cancer cells and actively target the contrast agent to the lesion by exploiting the properties of mutual recognition and adhesion of molecules on the surface of cancer cells, resulting in effective imaging. More importantly, even in the case of heterogeneous tumors, the same tumor cells can still achieve highly tumor-selective self-targeting of homologous tumors in vivo [96].
2.3. Platelet membrane
Platelet is the smallest circulating blood cells from mature megakaryocytes in the bone marrow. Usually, the content of platelet is approximately 150,000–350,000 cells/mL in blood, and an average lifetime is about 8–9 days [97,98]. Particularly, platelets play a vital role in vascular injury, wound healing, inflammatory response and haemostasis after thrombosis [99], which provide an innate and wonderful potency to involved in the biomimetic NPs for enhancing the target cargo delivery. Over the past few years, many studies have confirmed the relationship between the platelets induced hemostatic properties and the cancer metastasis as well as cancer progression. For example, in tumor angiogenesis, platelets facilitate cancer cells to survive in the blood and enhance tumor cell-vascular interactions [[100], [101], [102]]. In this case, the recognition and interaction between CTCs and platelets play a crucial role, i.e., the activated platelets will change their shape, and release serials of granules, including growth factors, chemokines and proteases, to increase adhesion to CTCs and form CTCs & leukocytes heterodimers for further promoting tumor metastasis and progression [103]. Based on the strong innate interaction between platelets and tumor metastasis, the platelet-involved biomimetic strategy for tumor-targeted drug/contrast delivery has drawn much attention. Moreover, different from the ‘homing’ targeting of cancer cell membranes, platelets are recalled and accumulate mainly through the inflammatory characteristics of the tumor site [104]. It allows platelet membrane-encapsulated nanoparticles to effectively target more than one tumor model. Inspired by these properties of platelets, researchers have developed imaging nanoparticles using platelet membrane modifications for enhanced imaging of tumor sites.
For example, Geng et al. [105] reported that the PLM was involved to constructed the NIR-II phototherapy NPs (BLIPO-1048), which were able to not only evade phagocytosis by macrophages, but also specifically combine with CD44 on the surface of most cancer cells. According to the results, BLIPO-1048 showed an excellent PA imaging capability to significantly improve the PTT conversion efficiency in the NIR-II window. Moreover, BLIPO-1048 was capable of actively and aggressively targeting different tumors, such as pancreatic cancer, breast cancer and glioma. Owing to the strong broad-spectrum target efficacy to tumors, BLIPO-1048 was introduced to investigate a variety of tumor models for NIR-II PA imaging in vivo. On the other hand, in the BLIPO-1048 treated group, the PA signal was evenly distributed imaging in the complete tumor location and boundary for all the tumor models. For the orthotopic allogeneic breast cancer and glioma models, the PA signal of the BLIPO-1048 treated group was almost 2.01-fold and 2.83-fold higher than that of the LIPO-1048 group at 12 h post-injection, which suggested that PLM modified NPs were beneficial for enhancing the active target cargo delivery to the tumor. Notably, the PA imaging depth of the orthotopic heterogeneous glioma model was up to 2.6 mm, indicating that BLIPO-1048 could effectively deliver into the tumor substance, revealing that PLM camouflaged NPs exhibited an excellent tumor target activity for precise tumor imaging and therapy.
The above examples indicate that PLM-mimetic NPs can exploit the properties in the inflammatory environment at the tumor site to achieve NPs accumulation, which can enhance the imaging capabilities of contrast agents/fluorescein for different tumors.
2.4. Macrophages membrane
Macrophage, a type of leukocyte mostly associated with tumors, is larger than RBC with diameters ranging from 7 to 20 μm. Similar to most leukocytes, macrophages can migrate between blood vessels and extravascular tissues, extremely easily crossing through ameboid motion. Therefore, macrophages are widely found in blood vessels and lymphatic vessels as well as other inflammation tissues. One of the main characteristics of cancer is chronic inflammation with various cytokines and chemokines to attract macrophages immigration into tumor lesions [106]. Moreover, macrophages accumulating into the tumor inflammatory environment will facilitate the formation of tumor blood vessels and the metastasis of cancer cells, accelerating the tumor pathological deterioration [107,108]. Because of the innate inflammatory immigration tendency of macrophages, macrophage-coated NPs can inherit target ability and biomimetic “stealth”. Additionally, macrophage-coated NPs are able to efficiently cross the vascular barrier and selectively recognize tumor cells resulting from the biological functions from the source macrophages [109]. Thus, MM camouflaged NPs could be a promising way to enhance the tumor chemotherapy and high-signal imaging through the inherent active target mechanism.
Recently, Rao et al. [48] constructed the MM camouflaged UCNPs (MM-UCNPs) using MM-derived vesicles to inherit the cancer-targeting ability from source macrophages. After injection 48 h in the mice tumor model, the MM-UCNPs treated group exhibited bright UCL at the location of tumor lesion, suggesting their favorable tumor-targeting ability. In the UCL ex vivo imaging, the MM-UCNPs treated group showed a stronger FL signal than the UCNPs treated group, further confirming that the cancer target property of MM-UCNPs was a result of the biological function of MM. In order to quantify the biodistribution, ICP-AES was introduced to measure the Y3+ contents of tumors and major organs. Compared with UCNPs, MM-UCNPs could significantly enhance accumulation in tumors lesion and reduce liver and spleen uptake, confirming that the MM coat endowed NPs with the excellent cancer target capability for efficient cancer imaging.
Moreover, Zhao et al. [47] reported the MM coated hollow Bi2Se3 NPs loading with QE (M@BS-QE NPs). Owing to the biological function of immune escape and CCL2 mediated recruitment phenomenon from MM, compared with the uncoated BS-QE NPs, M@BS-QE NPs had a longer cycle life and enhanced the local tumor accumulation resulting from the active target property of MM, exhibited the promoted CT and NIR-FL imaging performances.
Although MM coated NPs can significantly improve tumor imaging, the mechanism for tumor homing remains highly controversial [110], which represents a promising research field for further development of the MM mediated targeted tumor imaging/therapy applications.
2.5. Stem cell membrane
MSC is one of the representative cells among stem cells because of its superiority in self-renew, easy isolation and culture in vitro. Moreover, MSCM has a complex composition of the target ligands and surface antigens on the surface, regarding as one of the feasible candidates applied for NPs coats for target delivery to tumor lesions [111,112].
Yao et al. [54] developed the MSCM coated Fe3O4@PDA (Fe3O4@PDA-siRNA@MSCs) NPs as a platform for MRI-guided PTT and siRNA delivery. Fe3O4@PDA-siRNA@MSCs NPs showed excellent cancer-targeting ability and PTT conversion efficiency in vitro. Besides, after 24 h treatment, tumor darkening in MRI was observed in the Fe3O4@PDA-siRNA@MSCs NPs group according to the in vivo imaging studies, while no significant change was observed in other groups, which confirmed that Fe3O4@PDA-siRNA@MSCs NPs could be the efficient MRI probes to selectively imaging the tumor lesion in vivo. From the ICP-AES results of the major organs and tumors after 48 h treatment. The quantitative accumulation efficacy of Fe3O4@PDA-siRNA@MSCs NPs at tumor lesion was much higher than that of Fe3O4@PDA, further demonstrating the efficient tumor target ability and imaging capacity of MSCM coated Fe3O4@PDA-siRNA NPs.
MSCM have gained widespread interest in target cargo delivery for cancer therapy because of their inherent tumor homing ability. However, owing to the lack of intrinsic MSCM-specific markers and the FDA-approved relevant genetic modifications, it remains a great challenge to track MSCM by FL in situ hybridization, immunohistochemistry and flow cytometry techniques and translational clinical applications. Chetty et al.[55] prepared WJ-derived MSC (WJ-MSCs) to camouflage CuInS2–ZnS (CIS-ZMGS) NPs, which was regarded as the new-generation, biocompatible and multimodal imaging product to detect early and deep subcutaneous carriage of B16 F10 melanoma in C57BL/6 mice within 6 h through common imaging modalities (NIR-FL, MR and CT imaging). The WJ-MSCs camouflaged CIS-ZMGS NPs exhibited a high efficiency of imaging without significant leakage influenced by exocytosis, migratory behavior and the small changes in protein or gene expression during proliferation & multidirectional specific differentiation. Therefore, WJ-MSCs camouflaged CIS-ZMGS NPs could be an effective imaging agent applied in NIR-FL, MR and CT for early and deep tumor imaging.
In addition to their superior ability to target tumor cells, MSCs are present in a wide range of tissues and retain their original biological properties after MSCM extraction, even after a serial succession of cultures and cryopreservation. Therefore, MSCM has unique advantages in the preparation of tumor imaging biomimetic NPs.
2.6. Bacterial membrane
Bacteria have formed symbiotic relationships with human organisms throughout their long evolutionary history. The pathogenesis, progression and treatment of a wide range of complex diseases are closely linked to the composition of the microbial community, as evidenced by the important role of a wide range of bacteria in infectious diseases. In addition, a recent study demonstrating the presence of bacteria in human tumors highlights the close relationship between bacteria and human disease [113]. With a better understanding of bacteria and their associated ecosystems, there is an emerging trend to use bacteria for biomedical applications. Based on the fact that BM contains a variety of bacterial components, including nucleic acids, proteins and lipopolysaccharides [114], BM-encapsulated NPs inherit the tumor tissue-loving properties of their parental bacteria and therefore can be ideal carriers for targeting tumors. BM as nanocarriers shows several advantages as they obtain a rigid membrane, which confers stability and reduces leakage in the circulation. In addition, BM is safe as they are cell-free and can be used in very small amounts in vivo. Importantly, BM could be customized to carry the required payload and can be easily produced in large quantities using the fermentation and purification procedures previously optimized on a pilot scale. These advantages offered by BM are significantly demonstrated when considering that bacteria can be easily genetically modified to produce vectors that can be used for bioimaging, targeted delivery [[115], [116], [117]].
Today, there is an emerging trend to combine bacterial-based delivery systems with novel imaging agents to improve the resolution and sensitivity of early disease detection and diagnosis through bacterial-mediated targeted and spatiotemporal delivery of imaging agents. Inspired by the dependence of anaerobic bacteria on hypoxic conditions, Luo et al. [118]. proposed two strategies based on anaerobic bacteria to achieve targeted drug delivery to hypoxic regions of tumors. First, by adsorbing nanoparticles onto the surface of Bifidobacterium breve bacteria through electrostatic interactions, NPs can hitchhike through the bacteria to reach the hypoxic tumor region. Secondly, based on the principle that Clostridium only germinate can only survive and multiply in a hypoxic environment. In this environment, NPs modified with antibodies against germinating bacteria can achieve specific accumulation in the hypoxic zone of tumor tissue. Using UCNP and Au NPs, increased accumulation of NPs at the tumor site was demonstrated, which was attributed to the active homing ability of anaerobic bacteria, resulting in high-resolution imaging capabilities. In addition, Au NPs with thermal conversion produced thermal ablation of the tumor after irradiation, resulting in negligible side effects on normal tissue. In addition to being used as tumor-targeting carriers for contrast agents, bacteria can also be used for tumor imaging and therapy by converting light into heat. Gujrati et al. [116]. applied BM to construct bionanoplasmic NPs for tumor therapeutics. Engineered bacteria can secrete BM containing large amounts of melanin with high photothermal conversion efficiency to mediate PA imaging and photothermal therapy of 4T1 breast tumors. Furthermore, the PA signal induced by the BM-biomimetic NPs could last for at least 24 h, allowing for relatively long-term tumor monitoring. Notably, PA signal intensity is positively correlated with melanin concentration in tumor tissue.
Despite growing interests in the field and significant advances in preclinical and clinical research, significant challenges remain in utilizing engineered bacteria as delivery systems or for clinical translation, including the scaling up manufacturing, dose determination and potential biosafety [[119], [120], [121]]. These challenges need to be explored by researchers to expand the use of BM-based NPs in tumor imaging through biological and chemical engineering strategies for advanced imaging and therapy.
2.7. Hybrid membranes
Besides the use of individual CM, recently, combining multiple types of CM are emerging to develop hybrid membranes with the enhanced integrated functions encapsulating contrast agent or/and drug for target tumor imaging and therapy applications.
To achieve the improvement of imaging-guidance tumor therapy, Jiang et al. [60] developed a dual-membrane coated melanin NPs (Melanin@RBCM) by fusing the membranes of RBC and MCF-7 cells (human breast cancer cell line), which could effectively combine the characteristics of both RBC and MCF-7 cells retaining the proteins and biological functions of the parent membranes, i.e., the long-term blood circulation resulting from RBCM and the homologous target resulting from MCF-7 CCM. Notably, increasing the content of the MCF-7 membrane fraction could significantly enhance the homologous target to tumor lesion, while increasing the content of the RBCM fraction would effectively reduce the cellular uptake by macrophages and prolonged the blood circulation time. After 4 h treatment, the PA signal intensity of Melanin@RBCM reached a peak in MCF-7 tumor-bearing mice, confirming that Melanin@RBCM (64 nm) was beneficial for enhancing tumor target delivery for efficient PA-guided PTT treatment.
The versatile functional combination of hybrid membranes can also be developed in the precise treatment and real-time monitoring of tumors for promoting therapy efficacy and extending patient survival time. Recently, Wang et al. [58] prepared the cancer cell-macrophage hybrid membrane camouflaged NPs for the trackable long afterglow NIR luminescent application in colorectal cancer chemotherapy and imaging-guided PTT. This cancer cell-macrophage hybrid membrane coated NPs integrated the superiorities of macrophage and tumor cell with the enhanced homologous tumor target and immune escape, resulting in a significantly long metabolic half-life and a dramatically high tumor accumulation in vivo. Hence, these biomimetic NPs exhibited excellent biological tracer and tumor imaging capabilities, which could provide a promising platform for the accurate guidance PTT with the enhanced therapy efficacy, and also reduce the thermal damage and the undesirable accumulation of toxic side effects of chemotherapeutic drugs to the normal tissues in vivo, including liver and spleen.
This shows that hybrid films are multi-functional and have the potential to outperform their single film counterparts. But the complexity of the preparation process limits the widespread use of hybrid membranes.
3. Cell membrane-based biomimetic nanoparticles for cancer imaging
Among the different visualization techniques for cancer imaging and treatment, the commonly used techniques are FL, PA, PTT, CT and MRI. However, in clinic applications, a traditional contrast agent usually has a short half-life and suffers undesirable immune clearance, significantly reducing the imaging efficacy and precision. For example, MRI is usually introduced to detect the boundary of tumor tissue before surgery or further image the tumor morphology using Gd chelate during surgery. However, because of the short half-life of Gd, it is necessary to dose frequently to maintain its concentration for efficient imaging, which is a common phenomenon but obviously not reasonable [122]. To address this problem, cell or CM-based biomimetic NPs provide the natural superiorities for prolonging the blood circulation time and the active target delivery. i.e., the CM camouflaged NPs loading with the imaging agent can not only inherit the innate “stealth” function to effectively reduce the undesirable clearance by the immune system, resulting in long-term blood circulation [123], but also inherit the active target function from the source cells for precise tumor target imaging and effective tumor therapy [124]. Hence, in this section, the application of CM coated NPs in different tumor imaging techniques was systematically discussed (As shown in Table 2).
Table 2.
Different imaging approach with cell membrane.
| Imaging approach | Cell membrane | Core | Reference |
|---|---|---|---|
| FL | RBCM | FA and Cy5-modified nanoscale vesicles | [35] |
| RBCM | ICG | [36] | |
| MM | Upconversion NPs | [48] | |
| PLM | W18O49 NPs and metformin | [51] | |
| PLM | Antibody-drug and scFvGPIIb/IIIa-monomethyl auristatin E | [53] | |
| Cancer-macrophage hybrid membrane | IR825/Ir ZGGO@SiO2 | [58] | |
| PA | CCM | Dendritic mesoporous silica NPs-DNA probe | [43] |
| PLM | IR 1048 dye-loaded liposomes | [49] | |
| Erythrocyte-cancer hybrid membrane | Melanin NPs | [60] | |
| MRI | RBCM | Fe3O4 magnetic NPs | [37] |
| RBCM | Cyp-superparamagnetic nanoclusters | [39] | |
| CCM | Mesoporous copper/manganese silicate nanospheres | [44] | |
| SCM | Fe3O4@PDA-siRNA NPs | [54] | |
| PTT | Bacterial-cancer hybrid membrane | HPDA@[OMV-CC] NPs | [59] |
| CT | MM | Quercetin-loaded hollow Bi2Se3 | [47] |
| BM | Magnetosomes | [56] | |
| PA/FL/PTT | RBCM | DOX-loaded Prussian blue nano-composites | [38] |
| FL/MRI/PET | CCM | Upconversion NPs | [41] |
| PA/PTT | CCM | Ir–B–TiO2 | [42] |
| MRI/FL | CCM | Styrene and acrylic acid-crosslinked SPION | [45] |
| FL/MRI/CT | SCM | Mn2+ and Gd3+ co-doped CuInS2–ZnS nanocrystals | [55] |
| PA/FL | PLM | Dox-PFP-CNs@PLGA/PM NPs | [50] |
3.1. Photothermal imaging
PTT has been widely used as a cancer treatment strategy, and many multimodal NPs with PTT imaging and therapeutic functions are also being developed with the rapid development of the PTT technology [[125], [126], [127], [128]]. Owing to its enhanced convenience, selectivity, remote control and efficiency, the PTT imaging-guided nano-system with simultaneously monitoring and killing tumor functions has attracted extensive attention [129]. The operability and high spatio-temporal resolution of light can significantly improve the precise tumor therapy [130]. However, similar to the nanodrug delivery systems, traditional PTT NPs are easily recognized and cleared by RES, resulting in a low bioavailability for tumor lesion imaging [131]. Most surface modifications of PTT NPs are able to alleviate this problem, but the efficacy is limited because of the “foreign” property of NPs [25].
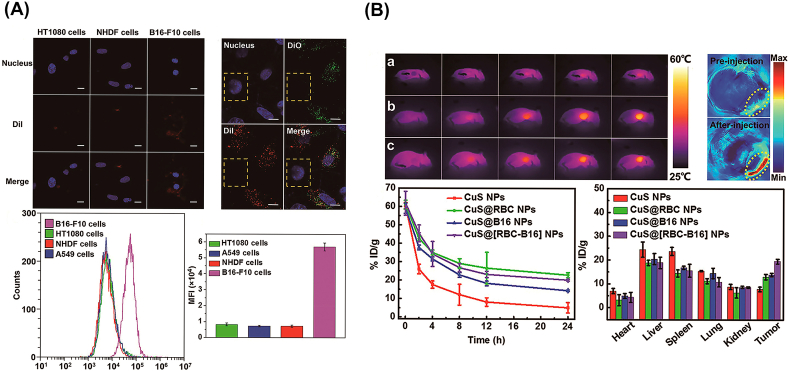
CM coated strategy has emerged as an effective biomimetic method for camouflaging NPs to optimize cancer imaging and therapy efficiency [133]. Owing to the innate biological functions resulting from the adhesion proteins, antigens and membrane structure of the source CM on the surface [131], CMNPs are capable of inheriting the biological functions such as “stealth”, active target. For instance, Wang et al. [132] fused RBC and melanoma cells (B16–F10 cells) to harvest a hybrid CM (RBC-B16) for further coating the hollow copper sulphide NPs (DCuS@[RBC-B16] NPs) loading with DOX (Fig. 2). DCuS@[RBC-B16] NPs preferred to exhibit highly specific homogenous recognition of the source cell line in vitro experiment, and also prolonged circulation time and the homologous target tumor model in vivo investigations. In PTT imaging studies, after irradiated with a NIR laser (1064 nm, 1 W/cm2) for 5 min, DCuS@[RBC-B16] NPs could significantly increase the local temperature at the tumor lesion, which was consistent with PA imaging results, exhibiting the excellent thermogenic performance and the enhanced tumor-targeted accumulation for promoting the precise imaging and therapeutic efficacy at tumor lesion. In another study, Sun et al. [134] prepared biomimetic NPs (CDAuNs) with 4T1 CCM coated on the shell layer. Surprisingly, CDAuNs integrated the homotypic targeting of the source CCM and the heat-sensitive of Au NPs. In vivo PTT imaging, after 1 h treatment, the temperature of CDAuNs group was significantly increased along with the irradiation time lasting, indicating excellent PTT imaging and tumor target accumulation properties. Similarly, Zhang et al. [135] constructed NIR-responsive NPs (DIC3NPs) coated with CCM, aiming at the efficient tumor target and the controllable intracellular drug release to significantly enhance the therapeutic effect to the tumor. According to results of the infrared PTT imaging in vitro, DIC3NPs were selectively delivered and accumulated into the tumor lesion and subsequently generated a high temperature up to 52.4 °C, which led to irreversible damage as well as the high-resolution PTT imaging signal to tumors. Obviously, CM camouflaged strategy provide a feasible platform to further improve PTT imaging in tumor applications.
Fig. 2.
(A) Highly specific self-targeting adhesion interactions of source cells in vitro. (B) In vivo PTT imaging and antitumor activity. In vivo biological imaging. Copyright © 2018, ACS Publishing Group. Replicated with permission from Ref. [132].
3.2. Computed tomography
Owing to the wide availability, cost-effectiveness, high spatial resolution, short scanning time and simplicity, CT is the widely used non-invasive method for both advanced scientific research and in clinical imaging modalities. Moreover, the combined functional exploration is also the hot spot for developing novel hybrid imaging systems, such as PET/CT and SPECT/CT [136,137]. In clinic application of CT imaging, the contrast agents commonly used in CT are barium sulphate suspension and water-soluble aromatic iodinated compounds. The usage of barium sulphate suspension in gastrointestinal imaging is greatly limited due to the toxicity of Ba2+. And the aromatic iodinated compound as an injectable CT contrast agent is also hampered by the short lifetime in blood circulation, which hinders the wide application of CT. In recent, iodinated agents are considered as the clinically safe contrast agent but sometimes cause serious side effects due to their high permeability and viscosity [138,139]. In addition, CT imaging has a high spatial resolution for cell level quantification, but the sensitivity is extremely limited [140]. Over the past years, with the rapid development of nanotechnology, various nanomaterials have been introduced into CT applications, especially the biomimetic nano-system used as the advanced contrast agent to not only reduce the immune clearance and the toxic side effects, but also enhance target delivery to the tumor [141].
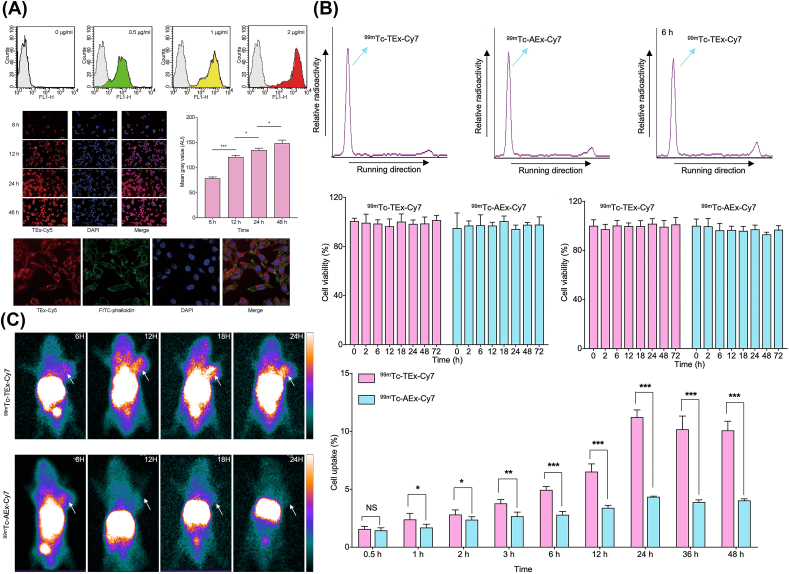
Jing et al. [142] developed an exosome-based NPs (99mTc-Tex-Cy7) via the hydrophobic effect introduced encapsulation mechanism for SPECT and NIR imaging in colon cancer (Fig. 3). According to the imaging results, the nanoprobe exhibit an SPECT imaging with strong penetration and sensitivity, as well as a NIR imaging with high temporal resolution and spatial resolution. 99mTc-Tex-Cy7, the tumor cell-derived exosome probe, displayed high affinity to tumor cells in vitro study and efficient cellular uptake as well as significant accumulation into tumor lesion in the tumor-bearing mice model. Therefore, the tumor cell-derived exosomes were demonstrated to be a promising strategy for improving precise imaging. Additionally, to address the issues of rapid blood clearance and immune limitation for UCNPs in practical applications, Li et al. [90] engineered the UCNPs by coating RBCM on their surface to harvest the short half-life nucleophile-labeled biomimetic NPs for accurate PET imaging in 4T1 tumors.
Fig. 3.
(A) Tumor-binding ability of Tex. (B) The representative radiochemical purity and cell uptakes of 99mTc-Tex-Cy7/99mTc-Aex-Cy7. (C) SPECT imaging of tumor-bearing nude mice. Copyright © 2021, Springer nature Publishing Group. Replicated with permission from Ref. [142].
3.3. Fluorescence imaging
FL imaging has been used as an indispensable tool to exploit and monitor the fundamental processes in life science, such as conformational dynamics, interactions and the distribution of biomolecules in organelles, cells or tissues [143]. However, traditional dyes for FL imaging have some inherent disadvantages, such as low absorption, poor photostability, which is hard to meet the demands of high-sensitivity imaging and high-throughput assay. For example, ICG, the only NIR activator approved by the FDA for specific clinical diagnostic applications, has strong optical imaging capabilities, but is coupled with the short plasma half-life (3–5 min) and non-specific interactions with various biomolecules. To develop the FL imaging, Chen et al. [27] developed a theranostic nanoplatform (ICNPs) by CCM coated NPs. In this design, these biomimetic NPs were characterized with the core-shell nanostructure, i.e., ICG was encapsulated into the core layer to prolong its lifetime, and a CCM was coated as the shell layer to enhance the specific homologous target to cancer cells. Except for cell biomimetic “stealth” to reduce the undesirable accumulation in the liver and kidney, ICNPs exhibited the excellent mono-dispersity and FL/PA imaging efficacy, which was exploited to develop the NIR-FL/PA dual-modality imaging for the real-time monitoring of the dynamic distribution of tumors with high spatial resolution and depth penetration in vivo.
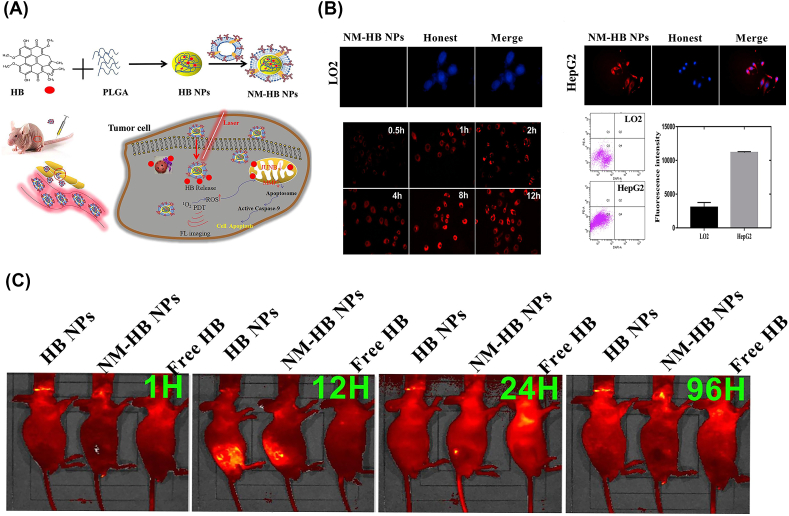
Recently, based on the inflammatory environment in tumor lesions, Zhang et al. [144] developed a biomimetic HB NPs coated with NM (NM-HB NPs) (Fig. 4). HB, a naturally occurring photosensitizer, was widely used in the PDT/PTT treatment, and also generated FL under NIR excitation for in vivo tumor imaging [145]. In the cell uptake assay in vitro, NM-HB NPs were able to effectively enhance target interaction with cancer cells. Compared with the HB NPs without cell coating, NM-HB NPs could selectively accumulate into tumor lesions according to the in vivo FL imaging because of the tumor-inflammation mediated target cargo delivery, suggesting the efficient therapeutic agent and FL imaging for cancer.
Fig. 4.
(A) Schematic diagram of a NM camouflaged HB NPs for cancer therapy and FL imaging. (B) Cellular uptake of NM-HB NPs by different cells. (C) FL imaging of the HCC tumor mice. Copyright © 2021, Elsevier Publishing Group. Replicated with permission from Ref. [144].
3.4. Photoacoustic imaging
PA is an emerging optical imaging modality for imaging that combines photoexcitation and US signal detection based on the PA effect. Compared with the traditional FL imaging modalities, PA overcomes the limitation of the strong light scattering in biological tissues, providing the high spatial resolution and deep penetration PA signal up to 3 cm to visualize the pathological biomarkers at the molecular level in deep tissue in vivo [146]. However, the further application of PA imaging is limited by lack of the selective and activatable probes [147].
To this end, Zhang et al. [148] constructed CCM (MCF-7 cells) camouflaged the dendritic mesoporous silica NPs (DMSN-DP@CM). The NPs were further functionalized with DNA PA probes (DNA-PA) and glutathione (GSH)-responsive DNA dye chains to amplify PA imaging signals for efficient applications in tumors (Fig. 5). According to the investigation, DMSN-DP@CM could escape the immune recognition and clearance, prolong the blood circulation time, and enhance the tumor target accumulation. Moreover, miRNAs could trigger the entropy-driven multiple PA fluorescent probe disassembly from bursters using GSH-responsive DNA fuel strands, resulting in a significant enhancement in PA signal ratio. For the oncogenic gene miRNA-21 as a model, the ratio between miRNA-21 concentration and PA showed a linear relationship in the dynamic range of 10 × 10−12 to 100 × 10−9 M, even with a minimum low limit up to 11.69 × 10−12 M. Therefore, DMSN-DP@CM can be used to accurately and dynamically image miRNA-21 changes in tumor applications. Similarly, Zheng et al. [149] reported RBCM coated low bandgap electron donor-acceptor-conjugated SPNs (SPN@RBCM) to enhance PA imaging and PTT efficiency. In the following study, SPN@RBCM exhibited extremely strong and stable near-infrared light absorption, revealing the efficient performance in PA imaging. In particular, SPN@RBCM could not only be “stealth” in the blood circulation, but also significantly accumulate and deeply penetrate into the tumor tissue for enhancing PA signal, providing a valuable design for safe and efficient tumor PA imaging.
Fig. 5.
(A) PA photographs of DMSN-DP in response to miRNA-21 at different concentrations. (B) The ratiometric PA signal (PA780/PA725) of DMSN-DP. Copyright © 2019, John Wiley & Sons Publishing Group. Replicated with permission from Ref. [148].
3.5. Magnetic resonance imaging
Over other imaging modalities, the main advantage of MRI is favorable spatial resolution, but the limited sensitivity of probes. Therefore, the focus is to improve the applicative probe for enhancing MRI applications in molecular imaging [150]. However, Fe3O4@Cu2-xS and Fe3O4@CuS, the regularly used probes for MRI-guided PTT treatments, are limited to be applied in biological applications because of the poor compatibility in the aqueous environment [151]. In addition, as the “foreign” property of NPs, these probes will be rapidly cleared by the immune system in vivo, resulting in the great loss of cargo and the limited tumor target efficacy [152]. For this reason, Lin et al. [40] designed the imaging/PTT NPs (SCS@RBCM) coating with RBCM on the surface. SCS@RBCM could maintain the stable nanostructure, the prolonged blood circulation time and the magnetic field targeted MRI capability. Guided by the external magnetic field, SCS@RBCM was able to enhance the tumor target delivery and accumulation significantly, exhibiting efficient MRI and anti-tumor efficacy through the PTT effect under the tunable NIR II irradiation.
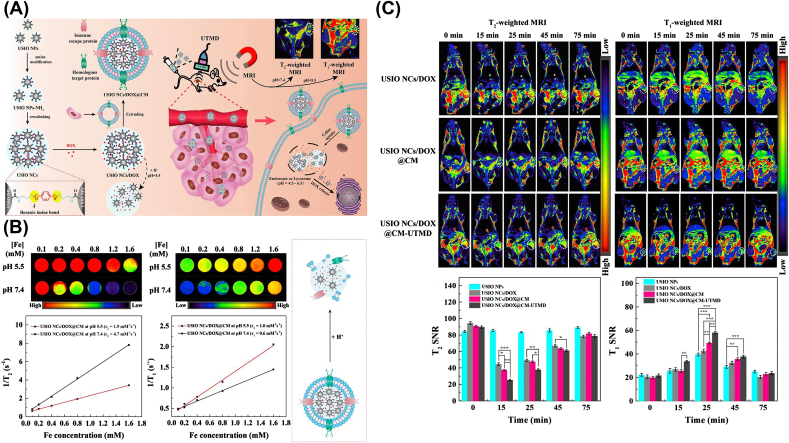
Furthermore, the ideal case for the novel therapeutic diagnostic nanoplatforms should integrate the precise dynamic imaging modalities, tumor-target and synergetic tumor therapy. To this end, Jia et al. [153] reported a simple strategy to fabricate the pH-responsive ultrasmall iron oxide NPs (USIO NCs/DOX@CM) camouflaged with the CCM shell. In this design, the amino group on the surface allowed USIO NCs/DOX@CM with pH-responsivity, and CCM membrane endowed the anti-macrophage uptake and the homologous cancer cell target function (Fig. 6). Once under the slight acid environment in the tumor, the T2 MR imaging USIO NCs/DOX@CM could transfer into the individual NPs with efficient T1 MRI for T2/T1 MR dynamic imaging switching of tumors. In addition, the dynamic MRI and tumor chemotherapy could be also further enhanced by the US-induced phono-hole effect.
Fig. 6.
(A) Illustration of the fabrication process of USIO NCs/DOX@CM. (B) Switchable T2/T1 MR relaxometry. (C) Dynamic T2/T1 MRI of tumors in vivo. Copyright © 2020, Elsevier Publishing Group. Replicated with permission from Ref. [153].
3.6. Multi-modal imaging
Although each individual imaging technique shows its unique advantages, the same as its limitations, such as MR is a powerful imaging tool with high spatial resolution, excellent depth of penetration and superior soft tissue spatial resolution [154], but low sensitivity in differentiating small lesions from surrounding normal tissue [155]. CT imaging has a high spatial resolution for cells quantification, but the low sensitivity and poor efficiency for soft tissue imaging [156]. FL allows for rapid real-time scanning, but has low spatial resolution and poor depth penetration [10]. PA is suitable for vascular viewing with the high optical contrast and spatial resolution, but the limited imaging vision owing to moderate laser energy [11]. Generally, the individual diagnostic probe suits only one imaging technique (e.g., PA, CT or MR), which is highly susceptible to result in poor imaging accuracy and incomplete diagnostic information. For this reason, multi-modal imaging systems have been seasonably exploited to compensate for the limitations of the individual imaging method for improving the accurate, reliable and versatile images applied in the tumor.
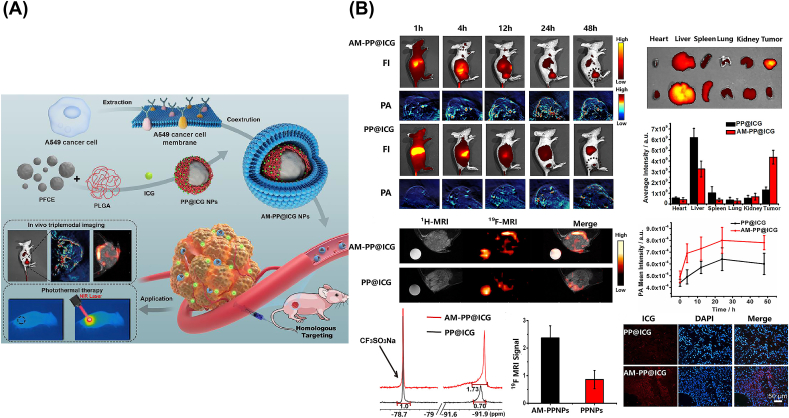
For instance (Fig. 7), Li et al. [157] designed the biomimetic NPs camouflaged with A549 lung CCM (AMs), which consist of two parts. The one was a PLGA-encapsulated Perfluoro-15-crown-5-ether (PFCE) as the core layer, loaded with ICG (PP@ICGNPs) for not only 19F MRI signals but also NIR-FL and PA signals, improving the multi-modal tumor imaging with high contrast and spatial resolution. The other one was the A549 CM as the shell layer, conferred homologous PP@ICGNPs target ability, improving the tumor target diagnostic and therapeutic efficiency. According to the in vivo studies, the CCM camouflaged PP@ICGNPs exhibited the effective homologous targeting ability, excellent biocompatibility and prolonged blood circulation. Benefiting from the triple-modality imaging, it allowed the accurate tumor imaging and therapy, i.e., FL showing the highly sensitive and time-dependent tumor accumulation, 19F MRI locating tumor without any background signal interference, and PA demonstrating the heterogeneous distribution into the tumor with high spatial resolution. Considering the multi-modal imaging, it was much easier to obtain the accurate location and the precise ablation of tumors.
Fig. 7.
(A) Diagram of Preparation and Application of AM-PP@ICGNPs. (B) In vivo triple-modal imaging of AM-PP@ICGNPs. Copyright © 2020, ACS Publishing Group. Replicated with permission from Ref. [157].
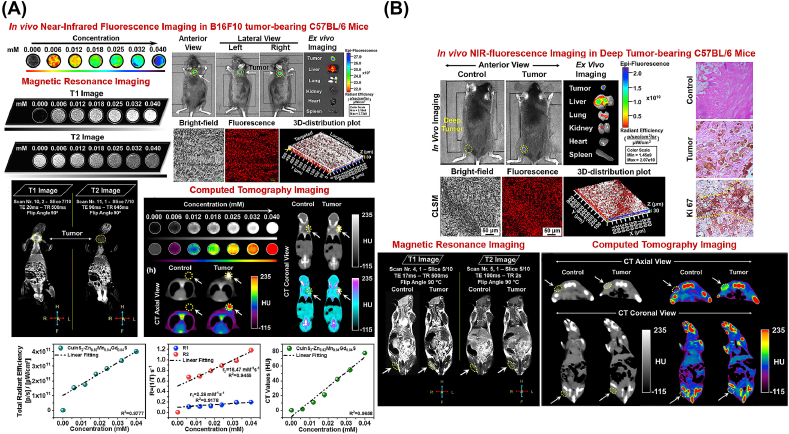
In another study [55], CuInS2–ZnS (CIS-ZMGS) NPs were camouflaged WJ-derived MSC (WJ-MSCs) for FL, CT and MRI (Fig. 8). The multimodal imaging nanoparticle CIS-ZMGS NC was found to exhibit significant NIR-FL, magnetic relativity and X-ray attenuation for early non-invasive multi-modal imaging of subcutaneous melanoma in the B16F10 tumor-bearing C57BL/6 mouse model. It can be used in imaging modalities for stem cell-assisted anti-cancer therapy and for tracking tissue/organ regeneration.
Fig. 8.
(A) FL, (B) MR and CT imaging of Subcutaneous Melanoma in C57BL/6 Mice Models. Copyright © 2020, ACS Publishing Group. Replicated with permission from Ref. [55].
In conclusion, the CM coated biomimetic NPs with multi-modal imaging capabilities not only promote immune escape, homologous target and long-lasting circulation, but also compensate for the shortcomings of the individual imaging modality, developing for the ultimately precise imaging and efficient treatment of tumor lesions.
4. Conclusion and perspectives
For further development of traditional imaging, CMNPs have been widely exploited to improve cancer imaging applications, including PA, FL, MR, etc. A series of CMs have attracted great interest because of the innate biological properties derived from source cells, including RBC, macrophages, platelets, stem cells and cancer cells. Top-down strategy is engineered to develop biomimetic nanoplatforms to inherit the superiorities, such as prolonging the blood circulation times, immune escape and the diverse tumor target capabilities.
Despite the current advances in CM biomimetic nanoplatforms for tumor imaging, it is a long way to be applied in clinics. Firstly, the complex and inefficient process during the preparation of membrane-coated NPs has limited further usage. In addition, the certain mechanisms of the structural units and their specific functional proteins on CM need to be further confirmed. For example, although thousands of proteins exist on the surface of CMNPs, only a few proteins are well-known as the specific antigens for tumor targeting. Furthermore, the immunogenicity and potential cytotoxicity still need to be further investigated before CMNPs are applied in clinics. Additionally, the current synthesis of biomimetic NPs for imaging involves multiple steps that may introduce multiple processes of variability. Some important characteristics, such as purity and integrity, in particular, need further study and elucidation. And have no studies reported on the yield, loading capacity or efficacy of biomimetic NPs. At present, the application of membrane coating technologies such as engineering cell membranes and various immune cell membranes in tumor imaging has not been effectively developed. It needs to actively develop these types of cell membranes for imaging studies in the future. If successfully addressed these challenges, it would allow such novel NPs to be used in the imaging of tumors for precise treatment.
In conclusion, CM biomimetic nanoplatforms for cancer imaging and treatment is novel but still in infancy. Lots of challenges must be overcome before the transition from the laboratory to the clinic. Much more innovative and systematic works for CM-based nanoplatforms will be continuously promoted to support cancer imaging, diagnostic and therapy for the benefit of human health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (31971301, 32171324, 51901160), Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0149), Fundamental Research Funds for Central Universities (2020CDJQY-A061, 2018CDHB1B08).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100228.
Contributor Information
Da Sun, Email: sunday@wzu.edu.cn.
Wei Wu, Email: david2015@cqu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Li S., Liu J., Sun M., Wang J., Sun Y. Cell membrane-camouflaged nanocarriers for cancer diagnostic and therapeutic. Front. Pharmacol. 2020;11:24. doi: 10.3389/fphar.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B., Li C., Chen G., Liu B., Deng X., Wei Y., Xia J., Xing B., Ma P.a., Lin J. Synthesis and optimization of MoS2@Fe3O4-ICG/Pt(IV) nanoflowers for MR/IR/PA bioimaging and combined PTT/PDT/chemotherapy triggered by 808 nm laser. Adv. Sci. 2017;4(8):1600540. doi: 10.1002/advs.201600540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pu Y., Zhu Y., Qiao Z., Xin N., Chen S., Sun J., Jin R., Nie Y., Fan H. A Gd-doped polydopamine (PDA)-based theranostic nanoplatform as a strong MR/PA dual-modal imaging agent for PTT/PDT synergistic therapy. J. Mater. Chem. B. 2021;9(7):1846–1857. doi: 10.1039/d0tb02725a. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z., Xie J., Ma S., Luo X., Liu J., Wang S., Chen Y., Yan J., Luo F. Construction of smart nanotheranostic platform Bi-Ag@PVP: multimodal CT/PA imaging-guided PDT/PTT for cancer therapy. ACS Omega. 2021;6(16):10723–10734. doi: 10.1021/acsomega.1c00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harald U., Christina J., Marc S., László D., Rudolf U., Jasmin M., Erik r., Tamás F., Tobias B., János S. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: a biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017;12:5223–5238. doi: 10.2147/IJN.S138108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin R., Lin B., Li D., Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr. Opin. Pharmacol. 2014;18:18–27. doi: 10.1016/j.coph.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B., Li Q., Yin P., Rui Y., Qiu Y., Wang Y., Shi D. Ultrasound-Triggered BSA/SPION hybrid nanoclusters for liver-specific magnetic resonance imaging. ACS Appl. Mater. Interfaces. 2012;4(12):6479–6486. doi: 10.1021/am301301f. [DOI] [PubMed] [Google Scholar]
- 9.Ammar A.Y., Sierra D., Merola F., Hildebrandt N., Guével X. Self-Assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery-ESI. ACS Nano. 2016;10(2):2591–2599. doi: 10.1021/acsnano.5b07596. [DOI] [PubMed] [Google Scholar]
- 10.Hong G., Antaris A.L., Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017;1(1) [Google Scholar]
- 11.Lee D., Beack S., Yoo J., Kim S.-K., Lee C., Kwon W., Hahn S.K., Kim C. In vivo photoacoustic imaging of livers using biodegradable hyaluronic acid-conjugated silica nanoparticles. Adv. Funct. Mater. 2018;28(22):1800941. [Google Scholar]
- 12.Naahidi S., Jafari M., alat F.E., Raymond K., Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J. Contr. Release. 2013;166(2):182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Shi S., Chen F., Goel S., Graves S.A., Luo H., Theuer C.P., Engle J.W., Cai W. In vivo tumor-targeted dual-modality PET/optical imaging with a yolk/shell-structured silica nanosystem. Nano-Micro Lett. 2018;10(4):65. doi: 10.1007/s40820-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agasti S.S., Rana S., Park M.H., Kim C.K., Rotello V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010;62(3):316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose T., Latawiec D., Mondal P.P., Mandal S. Overview of nano-drugs characteristics for clinical application: the journey from the entry to the exit point. J. Nanoparticle Res. 2014;16(8):2527. [Google Scholar]
- 16.Jin S., Ye K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol. Prog. 2007;23(1):32–41. doi: 10.1021/bp060348j. [DOI] [PubMed] [Google Scholar]
- 17.Peng J.Q., Fumoto S., Suga T., Miyamoto H., Kuroda N., Kawakami S., ishida K.N. Targeted co-delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J. Contr. Release. 2019;302:42–53. doi: 10.1016/j.jconrel.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Parodi A., Molinaro R., Sushnitha M., Evangelopoulos M., Martinez J.O., Arrighetti N., Corbo C., Tasciotti E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Thanuja M.Y., Anupama C., Ranganath S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: so near and yet so far. Adv. Drug Deliv. Rev. 2018;132:57–80. doi: 10.1016/j.addr.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 20.de Lázaro I., Mooney D.J. A nanoparticle's pathway into tumours. Nat. Mater. 2020;19(5):486–487. doi: 10.1038/s41563-020-0669-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang X.-L., Xue C., Kong N., Wu Z., Zhang J., Wang X., Zhou R., Lin H., Li Y., Li D.-S., Wu T. Molecular modulation of a molybdenum–selenium cluster by sulfur substitution to enhance the hydrogen evolution reaction. Inorg. Chem. 2019;58(18):12415–12421. doi: 10.1021/acs.inorgchem.9b02099. [DOI] [PubMed] [Google Scholar]
- 22.Pandit S., Dutta D., Nie S. Active transcytosis and new opportunities for cancer nanomedicine. Nat. Mater. 2020;19(5):478–480. doi: 10.1038/s41563-020-0672-1. [DOI] [PubMed] [Google Scholar]
- 23.Cassetta L., Pollard J.W. Targeting macrophages: therapeutic approaches in cancer, Nature reviews. Drug Discov. 2018;17(12):887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 24.Fan K., Jia X., Zhou M., Wang K., Conde J., He J., Tian J., Yan X. Ferritin nanocarrier traverses the blood brain barrier and Kills glioma. ACS Nano. 2018;12(5):4105–4115. doi: 10.1021/acsnano.7b06969. [DOI] [PubMed] [Google Scholar]
- 25.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R., He Y., Zhang S., Qin J., Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B. 2018;8(1):14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P., Gao G., Pan H., Liu L., Ma A., Cui H., Ma Y., Cai L. Cancer cell membrane–biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 28.Ding H., Lv Y., Ni D., Wang J., Tian Z., Wei W., Ma G. Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale. 2015;7(21):9806–9815. doi: 10.1039/c5nr02470f. [DOI] [PubMed] [Google Scholar]
- 29.Hu C.-M.J., Fang R.H., Luk B.T., Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013;8(12):933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang R.H., Jiang Y., Fang J.C., Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi: 10.1016/j.biomaterials.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., Yang X.Q., An J., Cheng K., Hou X.L., Zhang X.S., Hu Y.G., Liu B., Zhao Y.D. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy. Theranostics. 2020;10(2):867–879. doi: 10.7150/thno.37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Bremner D.H., Wu K., Gong X., Zhu L.M. Platelet membrane biomimetic bufalin-loaded hollow MnO2 nanoparticles for MRI-guided chemo-chemodynamic combined therapy of cancer. Chem. Eng. J. 2019;382:122848. [Google Scholar]
- 34.Xuan M., Shao J., Dai L., Li J., He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces. 2016:9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 35.Chen M., Liu A., Chen B., Zhu D.M., Xie W., Deng F.F., Ji L.W., Chen L.B., Huang H.M., Fu Y.R., Liu W., Wang F.B. Erythrocyte-derived vesicles for circulating tumor cell capture and specific tumor imaging. Nanoscale. 2019;11(25):12388–12396. doi: 10.1039/c9nr01805k. [DOI] [PubMed] [Google Scholar]
- 36.Burns J.M., Vankayala R., Mac J.T., Anvari B. Erythrocyte-derived theranostic nanoplatforms for near infrared fluorescence imaging and photodestruction of tumors. ACS Appl. Mater. Interfaces. 2018;10(33):27621–27630. doi: 10.1021/acsami.8b08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao L., Cai B., Bu L.L., Liao Q.Q., Guo S.S., Zhao X.Z., Dong W.F., Liu W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi: 10.1021/acsnano.7b00133. [DOI] [PubMed] [Google Scholar]
- 38.Xiao F., Fan J., Tong C., Xiao C., Wang Z., Liu B., Daniyal M., Wang W. An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging. RSC Adv. 2019;9(48):27911–27926. doi: 10.1039/c9ra05867b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S., Yin Y., Song W., Zhang Q., Yang Z., Dong Z., Xu Y., Cai S., Wang K., Yang W., Wang X., Pang Z., Feng L. Red-blood-cell-membrane-enveloped magnetic nanoclusters as a biomimetic theranostic nanoplatform for bimodal imaging-guided cancer photothermal therapy. J. Mater. Chem. B. 2020;8(4):803–812. doi: 10.1039/c9tb01829h. [DOI] [PubMed] [Google Scholar]
- 40.Lin K., Cao Y., Zheng D., Li Q., Liu H., Yu P., Li J., Xue Y., Wu M. Facile phase transfer of hydrophobic Fe3O4@Cu2-xS nanoparticles by red blood cell membrane for MRI and phototherapy in the second near-infrared window. J. Mater. Chem. B. 2020;8(6):1202–1211. doi: 10.1039/c9tb02766a. [DOI] [PubMed] [Google Scholar]
- 41.Fang H., Li M., Liu Q., Gai Y., Yuan L., Wang S., Zhang X., Ye M., Zhang Y., Gao M., Hou Y., Lan X. Ultra-sensitive nanoprobe modified with tumor cell membrane for UCL/MRI/PET multimodality precise imaging of triple-negative breast cancer. Nano-Micro Lett. 2020;12(1):62. doi: 10.1007/s40820-020-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J., Karges J., Xiong K., Chen Y., Ji L., Chao H. Cancer cell membrane camouflaged iridium complexes functionalized black-titanium nanoparticles for hierarchical-targeted synergistic NIR-II photothermal and sonodynamic therapy. Biomaterials. 2021;275:120979. doi: 10.1016/j.biomaterials.2021.120979. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K., Meng X., Yang Z., Cao Y., Cheng Y., Wang D., Lu H., Shi Z., Dong H., Zhang X. Cancer cell membrane camouflaged nanoprobe for catalytic ratiometric photoacoustic imaging of MicroRNA in living mice. Adv. Mater. 2019;31(12):1807888. doi: 10.1002/adma.201807888. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Wang D., Zhang S., Cheng Y., Yang F., Xing Y., Xu T., Dong H., Zhang X. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano. 2019;13(4):4267–4277. doi: 10.1021/acsnano.8b09387. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Wang X., Zheng D., Lin X., Wei Z., Zhang D., Li Z., Zhang Y., Wu M., Liu X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater. Sci. 2018;6(7):1834–1845. doi: 10.1039/c8bm00343b. [DOI] [PubMed] [Google Scholar]
- 46.Wu M., Mei T., Lin C., Wang Y., Chen J., Le W., Sun M., Xu J., Dai H., Zhang Y., Xue C., Liu Z., Chen B. Melanoma cell membrane biomimetic versatile CuS nanoprobes for homologous targeting photoacoustic imaging and photothermal chemotherapy. ACS Appl. Mater. Interfaces. 2020;12(14):16031–16039. doi: 10.1021/acsami.9b23177. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H., Li L., Zhang J., Zheng C., Ding K., Xiao H., Wang L., Zhang Z. C-C chemokine ligand 2 (CCL2) recruits macrophage-membrane-camouflaged hollow bismuth selenide nanoparticles to facilitate photothermal sensitivity and inhibit lung metastasis of breast cancer. ACS Appl. Mater. Interfaces. 2018;10(37):31124–31135. doi: 10.1021/acsami.8b11645. [DOI] [PubMed] [Google Scholar]
- 48.Rao L., He Z., Meng Q.F., Zhou Z., Bu L.L., Guo S.S., Liu W., Zhao X.Z. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J. Biomed. Mater. Res. 2017;105(2):521–530. doi: 10.1002/jbm.a.35927. [DOI] [PubMed] [Google Scholar]
- 49.Geng X., Gao D., Hu D., Liu Q., Liu C., Yuan Z., Zhang X., Liu X., Sheng Z., Wang X., Zheng H. Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl. Mater. Interfaces. 2020;12(50):55624–55637. doi: 10.1021/acsami.0c16872. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Fu J., Wang X., Chen Q., Zhang W., Cao Y., Ran H. Biomimetic "nanoplatelets" as a targeted drug delivery platform for breast cancer theranostics. ACS Appl. Mater. Interfaces. 2021;13(3):3605–3621. doi: 10.1021/acsami.0c19259. [DOI] [PubMed] [Google Scholar]
- 51.Zuo H., Tao J., Shi H., He J., Zhou Z., Zhang C. Platelet-mimicking nanoparticles co-loaded with W18O49 and metformin alleviate tumor hypoxia for enhanced photodynamic therapy and photothermal therapy. Acta Biomater. 2018;80:296–307. doi: 10.1016/j.actbio.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Gao C., Bhattarai P., Zhou Y., Zhang N., Hameed S., Yue X., Zhao B. Harnessing platelets as functional vectors for contrast enhanced ultrasound imaging and fluorescence imaging. RSC Adv. 2019;9(72):41993–41999. doi: 10.1039/c9ra05118j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yap M.L., McFadyen J.D., Wang X., Ziegler M., Chen Y.C., Willcox A., Nowell C.J., Scott A.M., Sloan E.K., Hogarth P.M., Pietersz G.A., Peter K. Activated platelets in the tumor microenvironment for targeting of antibody-drug conjugates to tumors and metastases. Theranostics. 2019;9(4):1154–1169. doi: 10.7150/thno.29146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mu X., Li J., Yan S., Zhang H., Zhang W., Zhang F., Jiang J. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater. Sci. Eng. 2018;4(11):3895–3905. doi: 10.1021/acsbiomaterials.8b00858. [DOI] [PubMed] [Google Scholar]
- 55.Chetty S.S., Praneetha S., Vadivel Murugan A., Govarthanan K., Verma R.S. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells labeled with Mn2+ and Gd3+ Co-doped CuInS2-ZnS nanocrystals for multimodality imaging in a tumor mice model. ACS Appl. Mater. Interfaces. 2020;12(3):3415–3429. doi: 10.1021/acsami.9b19054. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y., Ni Q., Xu C., Wan B., Geng Y., Zheng G., Yang Z., Tao J., Zhao Y., Wen J., Zhang J., Wang S., Tang Y., Li Y., Zhang Q., Liu L., Teng Z., Lu G. Smart bacterial magnetic nanoparticles for tumor-targeting magnetic resonance imaging of HER2-positive breast cancers. ACS Appl. Mater. Interfaces. 2019;11(4):3654–3665. doi: 10.1021/acsami.8b15838. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z.-H., Liu J.-M., Li C.-Y., Wang D., Lv H., Lv S.-W., Zhao N., Ma H., Wang S. Bacterial biofilm bioinspired persistent luminescence nanoparticles with gut-oriented drug delivery for colorectal cancer imaging and chemotherapy. ACS Appl. Mater. Interfaces. 2019;11(40):36409–36419. doi: 10.1021/acsami.9b12853. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z.H., Liu J.M., Zhao N., Li C.Y., Wang S. Cancer cell macrophage membrane-camouflaged persistent-luminescent nanoparticles for imaging-guided photothermal therapy of colorectal cancer. ACS Appl. Nano Mater. 2020;3(7):7105–7118. [Google Scholar]
- 59.Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl. Mater. Interfaces. 2020;12(37):41138–41147. doi: 10.1021/acsami.0c13169. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Q., Liu Y., Guo R., Yao X., Sung S., Pang Z., Yang W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi: 10.1016/j.biomaterials.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 61.Swain S., Sahu P.K., Beg S., Babu S.M. Nanoparticles for cancer targeting: current and future directions. Curr. Drug Deliv. 2016;13(8):1290–1302. doi: 10.2174/1567201813666160713121122. [DOI] [PubMed] [Google Scholar]
- 62.Blackman L.D., Qu Y., Cass P., Locock K.E.S. Approaches for the inhibition and elimination of microbial biofilms using macromolecular agents. Chem. Soc. Rev. 2021;50(3):1587–1616. doi: 10.1039/d0cs00986e. [DOI] [PubMed] [Google Scholar]
- 63.van de Ven A.L., Kim P., Haley O., Fakhoury J.R., Adriani G., Schmulen J., Moloney P., Hussain F., Ferrari M., Liu X., Yun S.H., Decuzzi P. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Contr. Release : Off. J. Contr. Release Soc. 2012;158(1):148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tasciotti E., Liu X., Bhavane R., Plant K., Leonard A.D., Price B.K., Cheng M.M., Decuzzi P., Tour J.M., Robertson F., Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 65.Hu C.M.J., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chxa B., Pjya B., Ycza B., Dxha B., Hua W., Cyya B. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Luo J., Chen X., Liu W., Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 2019;11(1):100. doi: 10.1007/s40820-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao L., Zhao S.K., Wen C., Tian R., Lin L., Cai B., Sun Y., Kang F., Yang Z., He L., Mu J., Meng Q.F., Yao G., Xie N., Chen X. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv. Mater. 2020;32(47) doi: 10.1002/adma.202004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meng Q.F., Zhao Y., Dong C., Liu L., Pan Y., Lai J., Liu Z., Yu G.T., Chen X., Rao L. Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew. Chem. Int. Ed. 2021;60(50):26320–26326. doi: 10.1002/anie.202108342. [DOI] [PubMed] [Google Scholar]
- 70.Wu H.H., Zhou Y., Tabata Y., Gao J.Q. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J. Contr. Release. 2019;294:102–113. doi: 10.1016/j.jconrel.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Rao L., Bu L.L., Ma L., Wang W., Liu H., Wan D., Liu J.F., Li A., Guo S.S., Zhang L., Zhang W.F., Zhao X.Z., Sun Z.J., Liu W. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew. Chem. Int. Ed. 2018;57(4):986–991. doi: 10.1002/anie.201709457. [DOI] [PubMed] [Google Scholar]
- 72.Lyu M., Chen M., Liu L., Zhu D., Wu X., Li Y., Rao L., Bao Z. A platelet-mimicking theranostic platform for cancer interstitial brachytherapy. Theranostics. 2021;11(15):7589–7599. doi: 10.7150/thno.61259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng Q.F., Tian R., Long H., Wu X., Lai J., Zharkova O., Wang J.W., Chen X., Rao L. Capturing cytokines with advanced materials: a potential strategy to tackle COVID-19 cytokine storm. Adv. Mater. 2021;33(20):2100012. doi: 10.1002/adma.202100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu T., Shi C., Duan L., Zhang Z., Luo L., Goel S., Cai W., Chen T. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J. Mater. Chem. B. 2018;6(29):4756–4764. doi: 10.1039/C8TB01398E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y.X., Tuo W.W., Wang D., Kang L.L., Chen X.Y., Luo M. Restoring the youth of aged red blood cells and extending their lifespan in circulation by remodelling membrane sialic acid. J. Cell Mol. Med. 2016;20(2):294–301. doi: 10.1111/jcmm.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qie Y., Yuan H., Roemeling C.V., Chen Y., Liu X., Shih K.D., Knight J.A., Tun H.W., Wharen R.E., Jiang W. Erratum: corrigendum: Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 2016;6(1):30663. doi: 10.1038/srep30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng J.R., Yang Q., Li W., Tan L., Xiao Y., Lijuan C., Hao Y., Qian Z. Erythrocyte-membrane-coated prussian blue/manganese dioxide nanoparticles as H2O2-responsive oxygen generators to enhance cancer chemotherapy/photothermal therapy. ACS Appl. Mater. Interfaces. 2017:44410–44422. doi: 10.1021/acsami.7b17022. [DOI] [PubMed] [Google Scholar]
- 78.Oldenborg Per-Arne, Zheleznyak Alex, Yi-Fu Fang, Lagenaur Carl F., Gresham Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051. doi: 10.1126/science.288.5473.2051. 2051. [DOI] [PubMed] [Google Scholar]
- 79.Zheleznyak A., Ikotun O.F., Dimitry J., Frazier W.A., Lapi S.E. Imaging of CD47 expression in xenograft and allograft tumor models. Mol. Imag. 2013;12(8):525–534. doi: 10.2310/7290.2013.00069. [DOI] [PubMed] [Google Scholar]
- 80.Lee J.Y., Vyas C.K., Kim G.G., Choi P.S., Park J.H. Red blood cell membrane bioengineered Zr-89 labelled hollow mesoporous silica nanosphere for overcoming phagocytosis. Sci. Rep. 2019;9(1):7419. doi: 10.1038/s41598-019-43969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pei Q., Hu X., Zheng X., Liu S., Li Y., Jing X., Xie Z. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano. 2018;12(2):1630–1641. doi: 10.1021/acsnano.7b08219. [DOI] [PubMed] [Google Scholar]
- 82.Thawani J.P., Amirshaghaghi A., Yan L., Stein J.M., Liu J., Tsourkas A. Photoacoustic-guided surgery with indocyanine green-coated superparamagnetic iron oxide nanoparticle clusters. Small. 2017;13(37):1701300. doi: 10.1002/smll.201701300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim T., Lemaster J.E., Chen F., Li J., Jokerst J.V. Photoacoustic imaging of human mesenchymal stem cells labeled with prussian blue-poly(L-lysine) nanocomplexes. ACS Nano. 2017;11(9):9022. doi: 10.1021/acsnano.7b03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharifi S., Seyednejad H., Laurent S., Atyabi F., Saei A.A., Mahmoudi M. Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. Contrast Media Mol. Imaging. 2015;10(5):329–355. doi: 10.1002/cmmi.1638. [DOI] [PubMed] [Google Scholar]
- 85.Yang J., Choi J., Bang D., Kim E., Haam S. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells. Angew. Chem. Int. Ed. 2011;50(2):441–444. doi: 10.1002/anie.201005075. [DOI] [PubMed] [Google Scholar]
- 86.Tong C., Zhang X., Fan J., Li B., Liu B., Daniyal M., Wang W. PEGylated mBPEI-rGO nanocomposites facilitate hepotocarcinoma treatment combining photothermal therapy and chemotherapy. Sci. Bull. 2018;63(14):935–946. doi: 10.1016/j.scib.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Xiao F., Fan J., Tong C., Xiao C., Wang W. An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging. RSC Adv. 2019;9(48):27911–27926. doi: 10.1039/c9ra05867b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zolnik B.S., González-Fernández A., Sadrieh N., Dobrovolskaia M.A. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh R., Pantarotto D., Lacerda L., Pastorin G., Klumpp C., Prato M., Bianco A., Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl. Acad. Sci. 2006;103(9):3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M., Fang H., Liu Q., Gai Y., Yuan L., Wang S., Li H., Hou Y., Gao M., Lan X. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater. Sci. 2020;8(7):1802–1814. doi: 10.1039/d0bm00029a. [DOI] [PubMed] [Google Scholar]
- 91.She D., Peng S., Liu L., Huang H., Zheng Y., Lu Y., Geng D., Yin B. Biomimic FeS2 nanodrug with hypothermal photothermal effect by clinical approved NIR-Ⅱ light for augmented chemodynamic therapy. Chem. Eng. J. 2020;400:125933. [Google Scholar]
- 92.Iurisci I., Cumashi A., Sherman A.A., Tsvetkov Y.E., Nifantiev N.E. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009;29(1):403–410. [PubMed] [Google Scholar]
- 93.Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 94.Rao L., Bu L.L., Cai B., Xu J.H., Li A., Zhang W.F., Sun Z.J., Guo S.S., Liu W., Wang T.H. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv. Mater. 2016;28(18):3460–3466. doi: 10.1002/adma.201506086. [DOI] [PubMed] [Google Scholar]
- 95.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 96.Zhu J.Y., Zheng D.W., Zhang M.K., Yu W.Y., Qiu W.X., Hu J.J., Feng J., Zhang X.Z. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16(9):5895–5901. doi: 10.1021/acs.nanolett.6b02786. [DOI] [PubMed] [Google Scholar]
- 97.Hu C.-M.J., Fang R.H., Wang K.-C., Luk B.T., Thamphiwatana S., Dehaini D., Nguyen P., Angsantikul P., Wen C.H., Kroll A.V., Carpenter C., Ramesh M., Qu V., Patel S.H., Zhu J., Shi W., Hofman F.M., Chen T.C., Gao W., Zhang K., Chien S., Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harker L.A., Roskos L.K., Marzec U.M., Carter R.A., Sheri Da N W. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood. 2000;95(8):2514–2522. [PubMed] [Google Scholar]
- 99.Wagner D.D., Burger P.C. Platelets in inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2003;23(12):2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 100.Erpenbeck L., Schn M.P. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jurasz P., Alonso-Escolano D., Radomski M.W. Platelet--cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol. 2010;143(7):819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 2011;11(2):123. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nash G.F., Turner L.F., Scully M.F., Kakkar A.K. Platelets and cancer. Lancet Oncol. 2002;3(7):425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 104.Guo M., Xia C., Wu Y., Zhou N., Chen Z., Li W. Research progress on cell membrane-coated biomimetic delivery systems. Front. Bioeng. Biotechnol. 2021;9:772522. doi: 10.3389/fbioe.2021.772522. 772522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geng X., Gao D., Hu D., Liu Q., Liu C., Yuan Z., Zhang X., Liu X., Sheng Z., Wang X., Zheng H. Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl. Mater. Interfaces. 2020;12(50):55624–55637. doi: 10.1021/acsami.0c16872. [DOI] [PubMed] [Google Scholar]
- 106.Wahl L.M., Kleinman H.K. Tumor-associated macrophages as targets for cancer therapy. J. Natl. Cancer Inst. 1998;21:1583–1584. doi: 10.1093/jnci/90.21.1583. [DOI] [PubMed] [Google Scholar]
- 107.Schoppmann S.F., Birner P., Stockl J., Kalt R., Ullrich R., Caucig C., Kriehuber E., Nagy K., Alitalo K., Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ono M., Torisu H., Fukushi J.I., Nishie A., Kuwano M. Biological implication of macrophage infiltration in human tumor angiogenesis. Cancer Chemother. Pharmacol. 1999;43:S69–S71. doi: 10.1007/s002800051101. Suppl(7) [DOI] [PubMed] [Google Scholar]
- 109.Xuan M., Shao J., Dai L., He Q., Li J. Nanocapsules: macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthc. Mater. 2015;4(11):1578. doi: 10.1002/adhm.201500129. 1578. [DOI] [PubMed] [Google Scholar]
- 110.Middleton J., Patterson A.M., Gardner L., Schmutz C., Ashton B.A. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100(12):3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 111.Hu Y.L., Fu Y.H., Tabata Y., Gao J.Q. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Contr. Release. 2010;147(2):154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 112.Dennis J.E., Cohen N., Goldberg V.M., Caplan A.I. Targeted delivery of progenitor cells for cartilage repair. J. Orthop. Res. 2004;22(4):735–741. doi: 10.1016/j.orthres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 113.Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L.T., Rotter-Maskowitz A., Weiser R., Mallel G., Gigi E., Meltser A., Douglas G.M., Kamer I., Gopalakrishnan V., Dadosh T., Levin-Zaidman S., Avnet S., Atlan T., Cooper Z.A., Arora R., Cogdill A.P., Khan M.A.W., Ologun G., Bussi Y., Weinberger A., Lotan-Pompan M., Golani O., Perry G., Rokah M., Bahar-Shany K., Rozeman E.A., Blank C.U., Ronai A., Shaoul R., Amit A., Dorfman T., Kremer R., Cohen Z.R., Harnof S., Siegal T., Yehuda-Shnaidman E., Gal-Yam E.N., Shapira H., Baldini N., Langille M.G.I., Ben-Nun A., Kaufman B., Nissan A., Golan T., Dadiani M., Levanon K., Bar J., Yust-Katz S., Barshack I., Peeper D.S., Raz D.J., Segal E., Wargo J.A., Sandbank J., Shental N., Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gnopo Y.M.D., Watkins H.C., Stevenson T.C., DeLisa M.P., Putnam D. Designer outer membrane vesicles as immunomodulatory systems-Reprogramming bacteria for vaccine delivery. Adv. Drug Deliv. Rev. 2017;114:132–142. doi: 10.1016/j.addr.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Chen Q., Rozovsky S., Chen W. Engineering multi-functional bacterial outer membrane vesicles as modular nanodevices for biosensing and bioimaging. Chem. Commun. 2017;53(54):7569–7572. doi: 10.1039/c7cc04246a. [DOI] [PubMed] [Google Scholar]
- 116.Gujrati V., Kim S., Kim S.-H., Min J.J., Choy H.E., Kim S.C., Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 117.Kim O.Y., Park H.T., Dinh N.T.H., Choi S.J., Lee J., Kim J.H., Lee S.-W., Gho Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun. 2017;8(1):626. doi: 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo C.H., Huang C.T., Su C.H., Yeh C.S. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–3499. doi: 10.1021/acs.nanolett.6b00262. [DOI] [PubMed] [Google Scholar]
- 119.Piraner D.I., Abedi M.H., Moser B.A., Lee-Gosselin A., Shapiro M.G. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol. 2017;13(1):75–80. doi: 10.1038/nchembio.2233. [DOI] [PubMed] [Google Scholar]
- 120.Riglar D.T., Silver P.A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 2018;16(4):214–225. doi: 10.1038/nrmicro.2017.172. [DOI] [PubMed] [Google Scholar]
- 121.Hosseinidoust Z., Mostaghaci B., Yasa O., Park B.W., Singh A.V., Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 2016;106(Pt A):27–44. doi: 10.1016/j.addr.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Mukhtar M., Bilal M., Rahdar A., Barani M., Bungau S.G. Nanomaterials for diagnosis and treatment of brain cancer: recent updates. Sens. Rev. 2020;8(4):117. [Google Scholar]
- 123.Bose R.J.C., Paulmurugan R., Moon J., Lee S.-H., Park H. Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discov. Today. 2018;23(4):891–899. doi: 10.1016/j.drudis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 124.Li S.-Y., Cheng H., Qiu W.-X., Zhang L., Wan S.-S., Zeng J.-Y., Zhang X.-Z. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149–161. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L., Gao S., Zhang F., Yang K., Ma Q., Zhu L. Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano. 2014;8(12):12250–12258. doi: 10.1021/nn506130t. [DOI] [PubMed] [Google Scholar]
- 126.Zhao X., Yang C.X., Chen L.G., Yan X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017;8:14998. doi: 10.1038/ncomms14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Radionuclide (131)I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials. 2015;66:21–28. doi: 10.1016/j.biomaterials.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 128.Li W.T., Peng J.R., Tan L.W., Jing W., Qian Z.Y. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by lyp-1 modified docetaxel/IR820 Co-loaded micelles. Biomaterials. 2016;106:119–133. doi: 10.1016/j.biomaterials.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Yu J., Yang C., Li J., Ding Y., Zhang L., Yousaf M.Z., Lin J., Pang R., Wei L., Xu L., Sheng F., Li C., Li G., Zhao L., Hou Y. Multifunctional Fe5C2 nanoparticles: a targeted theranostic platform for magnetic resonance imaging and photoacoustic tomography-guided photothermal therapy. Adv. Mater. 2014;26(24):4114–4120. doi: 10.1002/adma.201305811. [DOI] [PubMed] [Google Scholar]
- 130.Li J., Zhen X., Lyu Y., Jiang Y., Huang J., Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12(8):8520–8530. doi: 10.1021/acsnano.8b04066. [DOI] [PubMed] [Google Scholar]
- 131.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P., Gao G., Pan H., Liu L., Ma A., Cui H., Ma Y., Cai L. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 132.Wang D., Dong H., Li M., Cao Y., Yang F., Zhang K., Dai W., Wang C., Zhang X. Erythrocyte–cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi: 10.1021/acsnano.7b08355. [DOI] [PubMed] [Google Scholar]
- 133.Yu B., Goel S., Ni D., Ellison P.A., Siamof C.M., Jiang D., Cheng L., Kang L., Yu F., Liu Z., Barnhart T.E., He Q., Zhang H., Cai W. Reassembly of (89) Zr-labeled cancer cell membranes into multicompartment membrane-derived liposomes for PET-trackable tumor-targeted theranostics. Adv. Mater. 2018;30(13) doi: 10.1002/adma.201704934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W., Zhang Z., Yu H., Zhang P., Wang S., Li Y. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv. Funct. Mater. 2017;27(3):1604300. [Google Scholar]
- 135.Zhang N., Li M., Sun X., Jia H., Liu W. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials. 2018;159:25–36. doi: 10.1016/j.biomaterials.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Alberini J.L., Edeline V., Giraudet A.L., Champion L., Paulmier B., Madar O., Poinsignon A., Bellet D., Pecking A.P. Single photon emission tomography/computed tomography (SPET/CT) and positron emission tomography/computed tomography (PET/CT) to image cancer. J. Surg. Oncol. 2011;103(6):602–606. doi: 10.1002/jso.21695. [DOI] [PubMed] [Google Scholar]
- 137.Cao J., Wei Y., Zhang Y., Wang G., Ji X., Zhong Z. Iodine-rich polymersomes enable versatile SPECT/CT imaging and potent radioisotope therapy for tumor in vivo. ACS Appl. Mater. Interfaces. 2019;11(21):18953–18959. doi: 10.1021/acsami.9b04294. [DOI] [PubMed] [Google Scholar]
- 138.Yu S.B., Watson A.D. Metal-based X-ray contrast media. Chem. Rev. 1999;99(9):2353–2378. doi: 10.1021/cr980441p. [DOI] [PubMed] [Google Scholar]
- 139.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: an update. Curr. Probl. Diagn. Radiol. 2006;35(4):164–169. doi: 10.1067/j.cpradiol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 140.Huang J., Huang J.H., Bao H., Ning X., Yu C., Chen Z., Chao J., Zhang Z. CT/MR dual-modality imaging tracking of mesenchymal stem cells labeled with a Au/GdNC@SiO2 nanotracer in pulmonary fibrosis. ACS Appl. Bio Mater. 2020;3(4):2489–2498. doi: 10.1021/acsabm.0c00195. [DOI] [PubMed] [Google Scholar]
- 141.Gangadaran P., Hong C.M., Oh J.M., Rajendran R.L., Kalimuthu S., Son S.H., Gopal A., Zhu L., Baek S.H., Jeong S.Y., Lee S.W., Lee J., Ahn B.C. In vivo non-invasive imaging of radio-labeled exosome-mimetics derived from red blood cells in mice. Front. Pharmacol. 2018;9(817) doi: 10.3389/fphar.2018.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jing B., Gai Y., Qian R., Liu Z., Zhu Z., Gao Y., Lan X., An R. Hydrophobic insertion-based engineering of tumor cell-derived exosomes for SPECT/NIRF imaging of colon cancer. J. Nanobiotechnol. 2021;19(1):7. doi: 10.1186/s12951-020-00746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu C., Chiu D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Ed. 2013;52(11):3086–3109. doi: 10.1002/anie.201205133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Z., Li D., Cao Y., Wang Y., Wang F., Zhang F., Zheng S. Biodegradable Hypocrellin B nanoparticles coated with neutrophil membranes for hepatocellular carcinoma photodynamics therapy effectively via JUNB/ROS signaling. Int. Immunopharm. 2021;99:107624. doi: 10.1016/j.intimp.2021.107624. [DOI] [PubMed] [Google Scholar]
- 145.Zhang C., Wu J., Liu W., Zheng X., Wang P. Natural-origin hypocrellin-HSA assembly for highly efficient NIR light-responsive phototheranostics against hypoxic tumors. ACS Appl. Mater. Interfaces. 2019;11(48):44989–44998. doi: 10.1021/acsami.9b18345. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H.F., Maslov K., Stoica G., Wang L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006;24(7):848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 147.Zhang J., Smaga L.P., Satyavolu N.S.R., Chan J., Lu Y. DNA aptamer-based activatable probes for photoacoustic imaging in living mice. J. Am. Chem. Soc. 2017;139(48):17225–17228. doi: 10.1021/jacs.7b07913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang K., Meng X., Yang Z., Cao Y., Cheng Y., Wang D., Lu H., Shi Z., Dong H., Zhang X. Cancer cell membrane camouflaged nanoprobe for catalytic ratiometric photoacoustic imaging of MicroRNA in living mice. Adv. Mater. 2019;31(12) doi: 10.1002/adma.201807888. [DOI] [PubMed] [Google Scholar]
- 149.Zheng D., Yu P., Wei Z., Zhong C., Wu M., Liu X. RBC membrane camouflaged semiconducting polymer nanoparticles for near-infrared photoacoustic imaging and photothermal therapy. Nano-Micro Lett. 2020;12(1):94. doi: 10.1007/s40820-020-00429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terreno E., Castelli D.D., Viale A., Aime S. Challenges for molecular magnetic resonance imaging. Chem. Rev. 2010;110(5):3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 151.Wu Z.C., Li W.P., Luo C.H., Su C.H., Yeh C.S. Rattle-type Fe3O4@CuS developed to conduct magnetically guided photoinduced hyperthermia at first and second NIR biological windows. Adv. Funct. Mater. 2015;25(41):6527–6537. [Google Scholar]
- 152.Matsumoto Y., Nichols J.W., Toh K., Nomoto T., Cabral H., Miura Y., Christie R.J., Yamada N., Ogura T., Kano M.R., Matsumura Y., Nishiyama N., Yamasoba T., Bae Y.H., Kataoka K. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016;11(6):533–538. doi: 10.1038/nnano.2015.342. [DOI] [PubMed] [Google Scholar]
- 153.Jia L., Li X., Liu H., Xia J., Shi X., Shen M. Ultrasound-enhanced precision tumor theranostics using cell membrane-coated and pH-responsive nanoclusters assembled from ultrasmall iron oxide nanoparticles. Nano Today. 2021;36:101022. [Google Scholar]
- 154.Jin R., Lin B., Li D., Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr. Opin. Pharmacol. 2014;18:18–27. doi: 10.1016/j.coph.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 155.Unterweger H., Janko C., Schwarz M., Dézsi L., Urbanics R., Matuszak J., Őrfi E., Fülöp T., Bäuerle T., Szebeni J., Journé C., Boccaccini A.R., Alexiou C., Lyer S., Cicha I. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: a biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017;12:5223–5238. doi: 10.2147/IJN.S138108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jakhmola A., Anton N., Anton H., Messaddeq N., Hallouard F., Klymchenko A., Mely Y., Vandamme T.F. Poly-ε-caprolactone tungsten oxide nanoparticles as a contrast agent for X-ray computed tomography. Biomaterials. 2014;35(9):2981–2986. doi: 10.1016/j.biomaterials.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 157.Li S., Jiang W., Yuan Y., Sui M., Yang Y., Huang L., Jiang L., Liu M., Chen S., Zhou X. Delicately designed cancer cell membrane-camouflaged nanoparticles for targeted 19F MR/PA/FL imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2020;12(51):57290–57301. doi: 10.1021/acsami.0c13865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.