Highlights
-
•
Soluble co-inhibitory immune checkpoint molecules are increased in basal cell carcinoma.
-
•
Pre-therapy measurement of these checkpoints may have prognostic potential.
-
•
Measuring soluble immune checkpoint molecules might be of value in patient selection.
-
•
Immune checkpoint blockade may be of value in early disease.
-
•
Co-blockade of PD-1 and TIM-3 may hold particular promise.
Keywords: Basal cell carcinoma, Co-inhibitory immune checkpoints, C-reactive protein, Immunosuppression, Vitamin D
Abbreviations: BCC, Basal cell carcinoma; CRP, C-reactive protein; VD, Vitamin D
Abstract
Although co-inhibitory immune checkpoint proteins are primarily involved in promoting cell-cell interactions that suppress adaptive immunity, especially tumor immunity, the soluble cell-free variants of these molecules are also detectable in the circulation of cancer patients where they retain immunosuppressive activity. Nevertheless, little is known about the systemic levels of these soluble co-inhibitory immune checkpoints in patients with various subtypes of basal cell carcinoma (BCC), which is the most invasive and treatment-resistant type of this most commonly-occurring malignancy. In the current study, we have measured the systemic concentrations of five prominent co-inhibitory immune checkpoints, namely CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3, as well as those of C-reactive protein (CRP) and vitamin D (VD), in a cohort of patients (n = 40) with BCC, relative to those of a group of control participants, using the combination of multiplex bead array, laser nephelometry and ELISA technologies, respectively. The median systemic concentrations of CRP and VD were comparable between the two groups; however, those of all five immune checkpoints were significantly elevated (P = 0.0184 - P = < 0.00001), with those of CTLA-4 and PD-1 being highly correlated (r = 0.87; P < 0.00001). This seemingly novel finding not only identifies the existence of significant systemic immunosuppression in BCC, but also underscores the therapeutic promise of immune checkpoint targeted therapy, as well as the potential of these proteins to serve as prognostic/predictive biomarkers in BCC.
Introduction
Basal cell carcinoma (BCC) is the most common malignancy, comprising about 75% of all cases of skin cancer, and the incidence is rising [1,2]. The main risk factors include ultraviolet (UV) radiation exposure, male sex, light skin type, advanced age, an individual or family history of BCC, and long-term immunosuppression [2]. BCC rarely metastasizes and the mortality rate is low; however, the disease is associated with substantial morbidity. The majority of BCC patients can be successfully treated with standard surgery or, in selected cases, with topical treatment [3]. Locally advanced and metastasized BCCs are rare and can be treated with radiation or systemic therapy. The hedgehog intracellular signaling pathway regulates cell growth, and aberrant activation of this pathway leads to BCC development [3]. The hedgehog inhibitors vismodegib and sonidegib are currently approved for systemic therapy of BCC in Europe [3], [4], [5]. Hedgehog-dependent tumors are characterized by a highly immunosuppressive microenvironment associated with increased infiltration of various types of suppressive immune cells, such as M2-like tumor-associated macrophages (M2-TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells, as well as the presence of cancer-associated fibroblasts (CAF) [6], [7], [8], [9], [10], [11], [12], [13], [14].
Checkpoint proteins are critical for maintaining self-tolerance and modulating the immune responses of effector cells in normal tissues to minimize tissue damage. These proteins also modulate the immune infiltrates in the tumor microenvironment (TME). Cancer cells exploit the up-regulation or down-regulation of these proteins to evade the anti-tumor immune response [15,16]. In this context, soluble forms of immune checkpoint molecules (ICMs) have recently been identified and can be measured in human plasma; however, their biological and clinical significance remains essentially unknown [17,18]. In the case of soluble co-inhibitory ICMs, however, it does appear that these proteins not only retain their immunosuppressive activities, but may also counter the therapeutic activity of monoclonal antibodies (mAbs) that target negative checkpoints, underscoring their unfavorable predictive/prognostic potential in certain types of cancer [17], [18], [19], [20], [21]. Nevertheless, other than the existence of several case reports focused on the therapeutic activity of mAbs that target programmed cell death protein 1 (PD-1) and its ligand (PD-L1) in advanced and metastatic BCC [22], [23], [24], relatively little is known about the presence and types of systemic, soluble ICMs in this malignancy. Accordingly, the present study aimed to measure the pre-treatment plasma levels of the prominent soluble ICMs, PD-1, PDL-1, cytotoxic T-lymphocyte-associated protein (CTLA-4), lymphocyte activation gene 3 (LAG-3) and T cell and mucin-domain containing 3 (TIM-3) in newly diagnosed BCC patients relative to those of healthy controls. Importantly, these biomarkers were selected because they are five of the most prominent co-inhibitory immune checkpoints that have been researched in the field of cancer immunotherapy. These malignancies include non-small cell lung cancer, melanoma, triple-negative breast cancer, hepatocellular carcinoma and others [16,25]. In addition, measurement of plasma concentrations of vitamin D (VD) and C-reactive protein (CRP) was also undertaken as a strategy to detect possible immunosuppression resulting from depletion of VD and systemic inflammation, respectively [26,27].
Patients
Ethics approval was granted by the Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Ethics Committee Approval Number: 356/2020). Advance written informed consent was obtained from all patients and control participants. The study population consisted of a total of 40 South African patients (12F:28M; mean age ± SD: 69.13 ± 11.20 years) with BCC attending the Dermatology Screening Clinic at Steve Biko Academic Hospital, Pretoria, South Africa. Patients aged 18 years and older, with a histologically confirmed diagnosis of BCC of varying subtypes were included. Those with known active acute or chronic infections were excluded. Patients with a diagnosis of other malignant tumors, which could potentially elevate the plasma levels of soluble ICMs, were also excluded. Patients were almost exclusively of Caucasian ethnicity (n = 38; 11F:27M), as well as one female and one male of African and Asian ethnicity, respectively. The various histological diagnoses [28] and anatomical sites of the malignancy are shown in Tables 1 and 2, respectively. The control group consisted of 20 participants (5F:15M; mean age ± SD: 49.95 ± 14.59 years). The age difference between the patients and control participants was significantly different (P = 0.00001), underscoring the difficulty in recruiting healthy, older age-matched control subjects.
Table 1.
Numbers of patients with distinct clinical types of basal cell carcinoma (BCC).
| Clinical subtype of BCC |
| Adenoid (n = 1)* |
| Basosquamous (n = 3) |
| Infiltrating (n = 22) |
| Infiltrating with squamous differentiation (n = 4) |
| Keratotic (n = 1) |
| Micronodular (n = 2) |
| Nodular (n = 5) |
| Pigmented (n = 1)+ |
| Superficial (n = 1)o |
Numbers of patients are shown in parenthesis.
African patient.
Asian patient.
Table 2.
Numbers of patients with basal cell carcinomas at distinct anatomical sites.
| Anatomical site |
| Cheek (n = 3)*,+ |
| Chest (n = 2) |
| Ear (n = 4) |
| Forearm (n = 4) |
| Forehead (n = 2) |
| Lower limb (n = 5) |
| Neck (n = 2) |
| Nose (n = 13)o |
| Shoulder (n = 1) |
| Temple (n = 2) |
| Upper anterior chest (n = 2) |
Numbers of patients are shown in parenthesis.
African patient.
Asian patient.
Methods
Venous blood was collected in ethylenediaminetetracetic acid (EDTA) vacutainers and processed within 30 min to separate the plasma component by centrifugation, which was then aliquoted and stored at -70 ⁰C. Plasma was used as the matrix for analysis of the co-inhibitory immune checkpoint proteins, vitamin D (VD) and C-reactive protein (CRP) as described below.
Measurement of the soluble co-inhibitory immune checkpoint proteins
A Human Immuno-Oncology Checkpoint Protein Panel [Milliplex® MAP Kit (HCKP1–11 K), Merck, KGaA, Darmstadt, Germany] was used to simultaneously determine the plasma concentrations of five soluble co-inhibitory immune checkpoint proteins, CTLA-4, PD-1, PD-L1, LAG-3 and TIM-3 with lower detection limits of 12.2, 24.4, 4.88, 122 and 4.88 picograms per milliliter (pg/mL), respectively. The methodology was followed as per the manufacturer's instructions. Briefly, samples were thawed at room temperature and mixed gently. Diluted plasma samples (1:2) were added to the appropriately designated wells. The conjugated beads [25 microliters (μL)] were added and the plate was sealed and incubated, protected from light, for two hours at 22 ⁰C with gentle agitation on an orbital plate shaker (Stuart Scientific Orbital Shaker SO3, Wilford, Nottingham, UK).
Following the incubation period, the 96-well plate was washed three times with 200 μL wash buffer using a Bio-Plex Pro Wash Station Magnetic Plate Washer (Bio-Rad Laboratories Inc., Hercules, CA, USA). Thereafter, 25 μL of detection antibodies were added to each well. The plate was then sealed and incubated with gentle agitation on a plate shaker for 1 h at 22 ⁰C. This was followed by the addition of 25 μL streptavidin-phycoerythrin to each well. The plate was sealed and incubated for a final period of 30 min as described above. The plate was then washed a further three times with 200 μL wash buffer using a Bio-Plex Pro Wash Station Magnetic Plate Washer. Sheath fluid (150 μL) was added to all wells and the beads were resuspended on a plate shaker (Thomas Scientific, Swedesboro, NJ, USA) for 2 min prior to being assayed on a Bio-Plex Suspension Array platform (Bio-Rad Laboratories Inc., Hercules, CA, USA). The Bio-Plex Manager software 6.0 was used for bead acquisition and analysis of median fluorescence intensity. The results are reported as pg/mL plasma.
Measurement of plasma vitamin D (VD)
A 25-OH Vitamin D enzyme-linked immunosorbent assay (ELISA) kit [EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany; (EQ6411–9601)] was used to measure the plasma vitamin D levels for both the participants and control samples. The lower detection limit of the assay was 1.6 nanograms (ng)/mL. The methodology was followed as outlined by the manufacturer. Briefly, samples were thawed at room temperature (22 ⁰C) and mixed gently prior to use (Genie 2 vortex, Scientific Industries, New York, USA). The samples were diluted 26-fold, and added to the appropriately designated wells of a 96-well microplate. The plate was sealed and incubated on a plate shaker (Stuart Scientific Orbital Shaker SO3) for 2 h at 22 ⁰C. Following the incubation period, the plate was washed three times with 200 mL wash buffer using an automated plate washer (BioTek Instruments, Inc., Winooski, VT, USA). Enzyme conjugate [100 (µL)] was then added to each well followed by an additional incubation for 30 min at room temperature. The plate was then washed a further three times, as described above, followed by the addition of 100 µL of chromagen/substrate. The plate was sealed and incubated for 15 min at room temperature protected from light. The reaction was stopped by the addition of 100 µL stop solution and the optical density determined immediately using a PowerWaveX plate spectrophotometer (BioTek Instruments, Inc., Winooski. VT, USA) set at a wavelength of 450 nanometers (nm) with the reference wavelength set at 620 nm. The results are reported as ng/mL plasma.
Measurement of C-reactive protein (CRP)
Plasma concentrations of CRP were measured using high-sensitivity laser nephelometry [Siemens Healthcare Diagnostics (10,446,091), Atellica NEPH 630 Nephelometer, Newark, NJ, USA] according to the manufacturer's specifications. Briefly, plasma samples were diluted 1:20 and 150 µL aliquoted into the appropriate tubes and placed into the instrument where they were mixed with polystyrene particles coated with monoclonal antibodies reactive with human CRP. In the presence of CRP, the particles aggregate and scatter a beam of light the magnitude of which is directly proportional to the concentration of CRP in the sample. Reference curves were generated by a multi-point calibration; serial dilutions of N Rheumatology Standard SL were automatically prepared by the nephelometer, using N diluent. The results are expressed as micrograms (μg)/mL plasma with values of > 2.5 μg/mL considered to be elevated.
Expression and statistical analysis of results
The primary hypothesis was that there was a significant difference in the plasma levels of the soluble co-inhibitory immune checkpoints between BCC patients and healthy controls. Descriptive statistics were used to tabulate patient characteristics. The Mann Whitney U-test was used to compare levels of the various test biomarkers between BCC patients and healthy controls. The area under the ROC curve (AUC) was used as a measure of discriminatory ability of the biomarkers. The Youden index, a summary measure of the ROC curve, was used as an agnostic method for choosing an optimal cut-off value on the biomarker value to illustrate potential clinical usefulness. A correlation matrix report was used to identify correlations between variables (or subsets of variables) within the subset, using Spearman P-values to define significance. A P value of < 0.05 was considered statistically significant. NCSS 2021 software for Windows (USA) was used for statistical analyses.
Results
Soluble immune checkpoints
These results are shown in Table 3. The plasma concentrations of all five soluble co-inhibitory checkpoint molecules were significantly elevated in the cohort of BCC patients relative to those of the group of control participants (P ≤ 0.0184 -P ≤ 0.00001). The fold increases in the median values were 5.67, 36.1, 4.38, 6.2 and 7.1 for CTLA-4, LAG-3, PD-1, PD-L1 and TIM-3, respectively. The corresponding mean fold increases for these five co-inhibitory ICMs were 3.26, 2.09, 3.34, 3.15 and 3.36. Most notable were the differences in the median plasma levels of TIM-3 in BCC patients (7978 pg/mL) compared to healthy controls 1129 pg/mL; P < 0.00001) and those of PD-1 (11,303 pg/mL compared to 2575 pg/mL; P < 0.0002).
Table 3.
Comparison of the systemic concentrations of soluble CTLA-4, LAG-3, PD-1, PD-L1 and TIM-3 in patients with basal cell carcinoma and control participants.
| Soluble immune checkpoints (pg/mL) | Patients with basal cell carcinoma (n = 40) | Control participants (n = 20) | P ≤ |
| CTLA-4 | 749 (326–1924)* | 148 (50.75–444) | 0.0022 |
| LAG-3 | 401,252(4467–843,050) | 11,115 (635.77–528,229) | 0.0184 |
| PD-1 | 11,303 (3946–31,514) | 2575 (500–9955) | 0.0002 |
| PD-L1 | 1422 (185–7243) | 230 (21–1099) | 0.00433 |
| TIM-3 | 7978 (4956–10,105) | 1129 (21–4842) | 0.00001 |
Results are expressed as the median values with 25%−75% interquartile ranges in parenthesis.
ROCs/heat maps/correlations
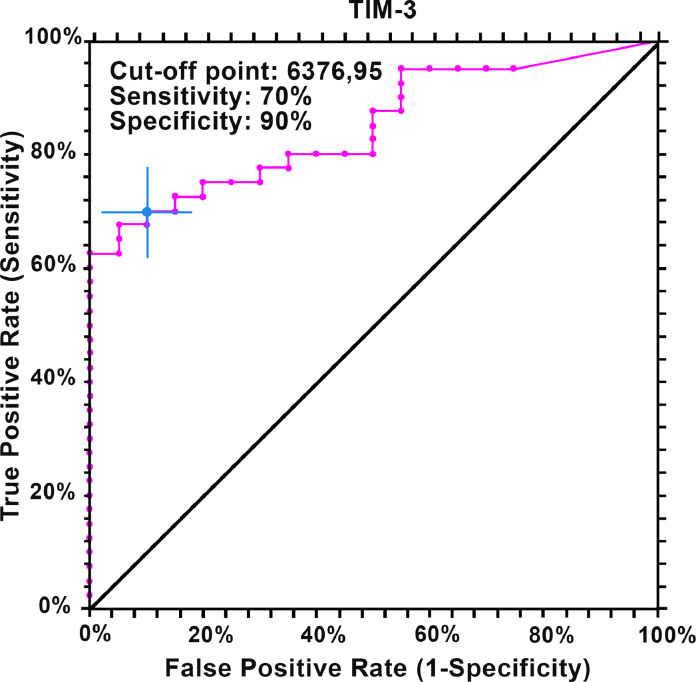
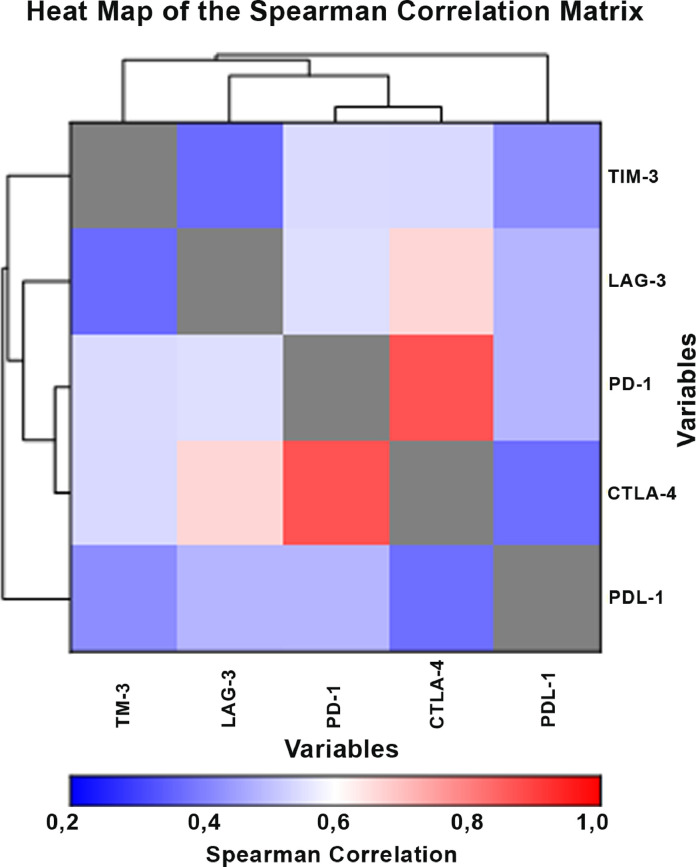
The ROC curve for TIM-3 is shown in Fig. 1, while the ROC-derived data for all five soluble ICMs is summarized in Table 4, which shows that all five biomarkers have predictive ability to discriminate BCC patients from healthy controls. The cut-off point for TIM-3 was calculated at 6377 pg/mL (sensitivity 70%, specificity 90%) and the area under the curve at 85% (95% CI 72–92; P < 0.00001), suggesting that TIM-3 is an effective measure of prediction. Additionally, the results for LAG-3 (AUC - 72%, P < 0.0004), PD-1 (AUC - 75%, P < 0.0001), CTLA-4 (AUC - 76%, P < 0.0001) and PD-L1 (AUC - 68%, P < 0.0052) indicated that these molecules can be considered for meaningful clinical interpretation regarding prognostic/predictive biomarkers and possible use for targeted therapy. The heat map shown in Fig. 2 reveals strong positive correlations between the co-inhibitory immune checkpoints, particularly CTLA-4 with PD-1, CTLA-4 with LAG-3 and PD-1 with LAG-3. These associations are summarized in Table 5.
Fig. 1.
ROC curve of TIM-3.
Table 4.
ROC curve cut-off values (using Youden Index) and AUC (95% CI) for immune checkpoint molecules.
| Soluble immune checkpoints (pg/mL) | AUC (CI 95%) | Cut-off point (pg/mL) | Sensitivity (TPR) | Specificity (TNR) | P≤ |
| CTLA-4 | 0.757 (0.597–0.859) | 324 | 75% | 70% | 0.0001 |
| LAG-3 | 0.724 (0.565–0.831) | 345,396 | 70% | 70% | 0.0004 |
| PD-1 | 0.753 (0.594–0.855) | 4915 | 73% | 65% | 0.0001 |
| PD-L1 | 0.681 (0.517–0.797) | 498 | 73% | 65% | 0.0052 |
| TIM-3 | 0.848 (0.721–0.919) | 6377 | 70% | 90% | 0.00001 |
Fig. 2.
Heatmap of the Spearman correlation matrix.
Table 5.
Matrix correlations of soluble co-inhibitory immune checkpoints.
| CTLA-4 | LAG-3 | PD-1 | PD-L1 | TIM-3 | |
| CTLA-4 | - | 0.73+,* (0.00001) | 0.85* (0.00001) | 0.27 (0.12) | 0.13 (0.47) |
| LAG-3 | - | 0.60* (0.0003) | 0.50* (0.003) | 0.22 (0.22) | |
| PD-1 | - | 0.47* (0.006) | 0.13 (0.47) | ||
| PD-L1 | - | 0.17 (0.36) | |||
| TIM-3 | - |
The paired values represent the correlation coefficients uppermost with the corresponding P value in parenthesis.
Denotes statistical significance.
A heatmap of the plasma levels of the soluble co-inhibitory ICMs of the BCC patients is shown in Fig. 2 and a summary of the correlation coefficients in Table 5. These revealed a high correlation of CTLA-4 with PD-1 (P < 0.00001), as well as CTLA-4 with LAG-3 (P < 0.00001). Correlations between PD-L1 with LAG-3 (P < 0.003) and PD-1 with LAG-3 (P < 0.003) were also significant.
Vitamin D (VD)
The median concentrations (with interquartile ranges) of plasma VD for the cohort of patients with BCC and the group of control participants were 28.02 (23.29–29.47) ng/mL and 26.0 (21.72–30.51) ng/mL, respectively (not significantly different).
C-reactive protein (CRP)
The median concentrations of plasma CRP, although higher in the group of patients with BCC, were also not significantly different from those of the control participants, the values being 2.47 (1.26–5.90) µg/mL and 1.45 (0.41–3.29) µg/mL, respectively.
Discussion
The present study has shown a statistically significant increase in the concentrations of soluble CTLA-4, PD-1, PD-L1, TIM-3 and LAG-3 in the plasma of patients with BCC compared to healthy controls. Additionally, positive correlations were detected between the various soluble co-inhibitory immune checkpoints in the group of BCC patients. The collective increase in the systemic levels of the five prominent co-inhibitory soluble ICMs in patients with BCC described in the current study is seemingly indicative of the existence of extensive immunosuppression [17] that is likely to contribute to tumor persistence and invasion, as well as increasing the risk of development of other cancers [29]. Other potentially pro-tumorigenic, immunosuppressive mechanisms such as decreased plasma levels of VD or chronic or sub-clinical inflammation do not appear to be of major pathogenetic significance in the setting of advanced BCC, but, in the case of the latter, may be eclipsed by the predominant, cumulative impact of the co-inhibitory soluble ICMs.
Although the anatomical and cellular origins of the various co-inhibitory checkpoints may be diverse, diffusion from the TME appears to represent the most plausible cause [17,18,21]. In this setting, co-inhibitory soluble ICMs are derived from tumor cells per se, as well as from various types of immune suppressor cells. In this context, the five co-inhibitory ICMs investigated in the current study primarily target the anti-tumor activity of cytotoxic tumor-infiltrating lymphocytes (TILs), both directly and indirectly. In the case of CTLA-4, this ICM, which is constitutively expressed by Tregs, attenuates the activation of antigen-presenting dendritic cells (DCs) and macrophages via interaction with the co-stimulatory molecules CD80 and CD86, as well as by several other mechanisms that are dependent on the Treg subtype [30,31]. These include induction of suppressive M2-like TAMs and MDSCs [30,31]. In contrast to CTLA-4, PD-1 has a broader cellular distribution and is expressed not only by activated T cells, but also by B cells and cells of the myeloid lineage [32]. Interaction of PD-1 expressed by activated anti-tumor CD4+and CD8+TILs with PD-L1 expressed by tumor cells, antigen-presenting cells and structural cells such as cancer-associated fibroblasts in the TME, attenuates the effector phase of T cell activation, resulting in failure of cellular proliferation, impaired production of immunostimulatory cytokines and induction of apoptosis [32].
With respect to LAG-3 and TIM-3, the former is expressed by T cells, including Tregs, as well as by B cells, natural killer (NK) cells and plasmacytoid DCs [33]. LAG-3 interacts with several types of ligand, including MHC class II and galectin-3. Via their interactions with MHC II expressed by T cells, tumor-infiltrating, LAG-3-expressing, tolerogenic plasmacytoid DCs not only inhibit T cell proliferation, but also promote differentiation of these cells towards a Treg phenotype; LAG-3-expressing Tregs, in turn, interact with non-tolerogenic MHC II+DCs, suppressing their maturation and immunostimulatory activities [33]. Like LAG-3, TIM-3 is expressed by activated CD4+and CD8+T cells and also interacts with several different ligands including galectin −9, which is expressed on tumor cells and Tregs [34,35]. Engagement of TIM-3 expressed on activated T cells results primarily in attenuation of production of interferon-γ, a key anti-tumor cytokine [34,35].
With respect to management of BCC, current treatments include surgery, local treatment with topical creams, radiation therapy, and targeted therapies with hedgehog inhibitors [36]. Although most cases of BCC can be effectively managed with standard surgery, topical treatment of selected patients with imiquimod and fluorouracil creams has been approved by the US Food and Drug Administration (FDA) to treat superficial BCCs [2,37]. However, locally advanced and metastasized BCC must be treated with radiation or systemic therapy. Radiation is also an option for older patients in whom surgery is contraindicated, while the hedgehog inhibitors, vismodegib and sonidegib, are currently approved for systemic treatment of BCC in Europe [37].
In the case of immunotherapy, Lipson et al. have shown that most patients with BCC have a high level of expression of PD-L1 in either tumor cells or the TME [22]. Their study, which involved analysis of 40 BCC biopsy specimens, demonstrated that PD-L1 was expressed on tumor cells in 9/40 (22%) specimens, while expression of this checkpoint was detected on TILs and associated macrophages in 33/40 (82%) of specimens [22]. This study provided a rationale for testing cemiplimab, a PD-1 inhibitor, in patients with advanced, refractory BCC. In a phase 2 study, this immunotherapeutic agent demonstrated favorable activity in patients with metastatic BCC, as well as in those with locally advanced BCC who had progressed on, or who were intolerant to, previous hedgehog inhibitor therapy as second-line therapy. Objective responses were observed in 26 (31%; 95% CI 21–42) of 84 patients. However, no biomarker analysis for the prediction of response to cemiplimab was included in the study [38]. In February 2021, based on the results of this phase 2 study, the US Food and Drug Administration granted regular approval to cemiplimab-rwlc for the treatment of patients with locally advanced BCC previously treated with a hedgehog pathway inhibitor, or for whom treatment with hedgehog inhibitors is not appropriate [39]. This treatment was also approved in Europe in June 2021 [40].
Future studies should assess the role of anti-PD-1-targeted mAbs in combination with hedgehog inhibitors, or in combination with mAbs that target other co-inhibitory ICMs such as CTLA-4 or LAG-3 or TIM-3 that may enable effective co-blockade. In this context, and as alluded to in the current study, co-targeting of PD-1 and TIM-3 appears to be a particularly promising strategy. This contention is based on the following: (i) the presence of both PD-1 and TIM-3 on dysfunctional anti-tumor CD4+ and CD8+ T cells; (ii) the efficacy of dual PD-1/TIM-3-targeted immunotherapy relative to that of PD-1 alone in preclinical models of experimental tumorigenesis; (iii) ongoing clinical trials, which are focused on both safety and efficacy of PD-1/TIM-3 co-blockade in various types of malignancy including, but not limited to, hepatocellular carcinoma, non-small lung cancer, melanoma and squamous cell carcinoma; and iv) the development of adaptive resistance to PD-1-targeted anti-cancer immunotherapy due to upregulated expression of TIM-3 [35,[41], [42], [43], [44]].
In the context of LAG-3, a recent study in patients with metastatic malignant melanoma, which was focused on dual monoclonal antibody-mediated immunotherapy targeting PD-1 and LAG-3 with nivolumab and relatlimab, respectively, is noteworthy [45]. Patients recruited to this trial, known as the Phase II/III RELATIVITY-047 trial, were randomized to a fixed dose combination of nivolumab and relatlimab at 160 and 480 mg (mg), respectively, or nivolumab alone every four weeks. Treatment was administered until disease progression or development of unacceptable toxicity. Median progression-free survival (PFS) was 10.1 months [95% confidence interval (CI) = 6.4–15.7 months] in the nivolumab/relatlimab group compared with 4.6 months (95% CI, 3.4–5.6 months) in the nivolumab only group [hazard ratio (HR) for progression to death = 0.75, 95% CI = 0.62–0.92, P = 0.006]. The respective PFS rates at 12 months were 47.7% (95% CI = 41.8%−53.2%) vs 36.0% (95% CI = 30.5−41.6%). No new safety signals were detected using the combination of nivolumab and relatlimab. The RELATIVITY-047 TRIAL supports the rationale of dual blockade of the PD-1 LAG-3 pathways that contribute to T cell exhaustion in cancer [45]. Given the finding of high levels of soluble PD-1/PD-L1 and LAG-3 detected in BCC patients in the current study, a similar rationale for the dual targeting PD-1 and LAG-3 may be of therapeutic benefit in this condition.
Conclusions
High concentrations of co-inhibitory ICMs were detected in patients with BCC, seemingly indicative of significant pro-tumorigenic immunosuppression. The role of soluble negative immune checkpoint molecules should therefore be investigated as possible predictors of response to treatment and as prognostic biomarkers in patients with BCC. The therapeutic potential of dual targeting of PD-1 and TIM-3 or LAG-3 in this condition, as well as treatment with checkpoint inhibitors early in the course of disease, is warranted.
Funding
The study was supported by research funds provided by the University of Pretoria.
Data availability
Access to data will be provided on request.
CRediT authorship contribution statement
Nonkululeko Z. Malinga: Investigation, Project administration, Data curation, Writing – original draft, Writing – review & editing, Validation. Shalete C. Siwele: Investigation, Project administration, Data curation, Writing – original draft, Writing – review & editing, Validation. Helen C. Steel: Investigation, Supervision, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Luyanda L.I. Kwofie: Investigation, Writing – review & editing. Pieter W.A. Meyer: Formal analysis, Data curation, Writing – review & editing. Teresa Smit: Formal analysis, Data curation, Writing – review & editing. Ronald Anderson: Project administration, Funding acquisition, Writing – original draft, Writing – review & editing. Bernardo L. Rapoport: Project administration, Funding acquisition, Writing – original draft, Writing – review & editing. Mahlatse C.M. Kgokolo: Investigation, Project administration, Data curation, Writing – original draft, Writing – review & editing, Validation, Supervision.
Declaration of Competing Interest
None. This applies to all the authors.
References
- 1.Verkouteren J.A.C., Ramdas K.H.R., Wakkee M. Nijsten, T Epidemiology of basal cell carcinoma: scholarly review. Br. J. Dermatol. 2017;177(2):359–372. doi: 10.1111/bjd.15321. PMID: 28220485. [DOI] [PubMed] [Google Scholar]
- 2.Seidl-Philipp M., Frischhut N., Höllweger N., Schmuth M., Nguyen V.A. Known and new facts on basal cell carcinoma. J. Dtsch. Dermatol. Ges. 2021;19(7):1021–1041. doi: 10.1111/ddg.14580. PMID: 34288482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fania L., Didona D., Morese R., Campana I., Coco V., Di Pietro F.R., Ricci F., Pallotta S., Candi E., Abeni D., Dellambra E. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8(11):449. doi: 10.3390/biomedicines8110449. PMID: 33113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D.P., Kus K.J.B., Ruiz E. Basal cell carcinoma review. Hematol. Oncol. Clin. North Am. 2019;33(1):13–24. doi: 10.1016/j.hoc.2018.09.004. PMID: 30497670. [DOI] [PubMed] [Google Scholar]
- 5.Tanese K. Diagnosis and management of basal cell carcinoma. Curr. Treat. Options Oncol. 2019;20(2):13. doi: 10.1007/s11864-019-0610-0. PMID: 30741348 Review. [DOI] [PubMed] [Google Scholar]
- 6.Petty A.J., Li A., Wang X., Dai R., Heyman B., Hsu D., Huang X., Yang Y. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J. Clin. Invest. 2019;129(12):5151–5162. doi: 10.1172/JCI128644. PMID: 31638600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinshaw D.C., Hanna A., Lama-Sherpa T., Metge B., Kammerud S.C., Benavides G.A., Kumar A., Alsheikh H.A., Mota M., Chen D., Ballinger S.W., Rathmell J.C., Ponnazhagan S., Darley-Usmar V., Samant R.S., Shevde L.A. Hedgehog signaling regulates metabolism and polarization of mammary tumor-associated macrophages. Cancer Res. 2021;81(21):5425–5437. doi: 10.1158/0008-5472.CAN-20-1723. PMID: 34289986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J. The hedgehog's trick for escaping immunosurveillance: the molecular mechanisms driving myeloid-derived suppressor cell recruitment in hedgehog signaling-dependent tumors. Oncoimmunology. 2014;3:e29180. doi: 10.4161/onci.29180. PMID: 25054089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grund-Gröschke S., Stockmaier G., Aberger F. Hedgehog/GLI signaling in tumor immunity - new therapeutic opportunities and clinical implications. Cell Commun. Signal. 2019;17(1):172. doi: 10.1186/s12964-019-0459-7. PMID: 31878932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenti G., Quinn H.M., Heynen G.J.J.E., Lan L., Holland J.D., Vogel R., Wulf-Goldenberg A., Birchmeier W. Cancer stem cells regulate cancer-associated fibroblasts via activation of hedgehog signaling in mammary gland tumors. Cancer Res. 2017;77(8):2134–2147. doi: 10.1158/0008-5472.CAN-15-3490. PMID: 28202523. [DOI] [PubMed] [Google Scholar]
- 11.Omland S.H., Nielsen P.S., Gjerdrum L.M., Gniadecki R. Immunosuppressive environment in basal cell carcinoma: the role of regulatory T cells. Acta Derm. Venereol. 2016;96(7):917–921. doi: 10.2340/00015555-2440. PMID: 27117439. [DOI] [PubMed] [Google Scholar]
- 12.Omland S.H., Hamrouni A., Gniadecki R. High diversity of the T-cell receptor repertoire of tumor-infiltrating lymphocytes in basal cell carcinoma. Exp. Dermatol. 2017;26(5):454–456. doi: 10.1111/exd.13240. PMID: 27714856. [DOI] [PubMed] [Google Scholar]
- 13.Omland S.H., Wettergren E.E., Mollerup S., Asplund M., Mourier T., Hansen A.J., Gniadecki R. Cancer associated fibroblasts (CAFs) are activated in cutaneous basal cell carcinoma and in the peritumoural skin. BMC Cancer. 2017;17(1):675. doi: 10.1186/s12885-017-3663-0. PMID: 28987144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omland S.H. Local immune response in cutaneous basal cell carcinoma. Dan. Med. J. 2017;64(10):B5412. PMID: 28975891. [PubMed] [Google Scholar]
- 15.Zhang Y., Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv. Exp. Med. Biol. 2020;1248:201–226. doi: 10.1007/978-981-15-3266-5_9. PMID: 32185712. [DOI] [PubMed] [Google Scholar]
- 16.Tu L., Guan R., Yang H., Zhou Y., Hong W., Ma L., Zhao G., Yu M. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int. J. Cancer. 2020;147(2):423–439. doi: 10.1002/ijc.32785. PMID: 31721169. [DOI] [PubMed] [Google Scholar]
- 17.Gu D., Ao X., Yang Y., Chen Z., Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J. Immunother. Cancer. 2018;6(1):132. doi: 10.1186/s40425-018-0449-0. PMID: 30482248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti R., Kapse B., Mukherjee G. Soluble immune checkpoint molecules: serum markers for cancer diagnosis and prognosis. Cancer Rep. 2019;2(4):e1160. doi: 10.1002/cnr2.1160. (Hoboken)PMID: 32721130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Zhang J., Tu H., Liang D., Chang D.W., Ye Y., Wu X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer. 2019;7(1):334. doi: 10.1186/s40425-019-0810-y. PMID: 31783776; PMCID: PMC6884764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han B., Dong L., Zhou J., Yang Y., Guo J., Xuan Q., Gao K., Xu Z., Lei W., Wang J., Zhang Q. The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte. Cancer Immunol. Immunother. 2021;70(10):2893–2909. doi: 10.1007/s00262-021-02898-4. PMID: 33688997; PMCID: PMC8423647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machiraju D., Wiecken M., Lang N., Hülsmeyer I., Roth J., Schank T.E., Eurich R., Halama N., Enk A., Hassel J.C. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology. 2021;10(1) doi: 10.1080/2162402X.2021.1926762. PMID: 34104542; PMCID: PMC8158029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipson E.J., Lilo M.T., Ogurtsova A., Esandrio J., Xu H., Brothers P., Schollenberger M., Sharfman W.H., Taube J.M. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J. Immunother. Cancer. 2017;5:23. doi: 10.1186/s40425-017-0228-3. PMID: 28344809; PMCID: PMC5360064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang A.L.S., Tran D.C., Cannon J.G.D., Li S., Jeng M., Patel R., Van der Bokke L., Pague A., Brotherton R., Rieger K.E., Satpathy A.T., Yost K.E., Reddy S., Sarin K., Colevas A.D. Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study. J. Am. Acad. Dermatol. 2019;80(2):564–566. doi: 10.1016/j.jaad.2018.08.017. PMID: 30145186; PMCID: PMC6839543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moujaess E., Merhy R., Kattan J., Sarkis A.S., Tomb R. Immune checkpoint inhibitors for advanced or metastatic basal cell carcinoma: how much evidence do we need? Immunotherapy. 2021;13(15):1293–1304. doi: 10.2217/imt-2021-0089. PMID: 34463126. [DOI] [PubMed] [Google Scholar]
- 25.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. Epub 2018 Mar 22. PMID: 29567705; PMCID: PMC7391259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karkeni E., Morin S.O., Bou Tayeh B., Goubard A., Josselin E., Castellano R., Fauriat C., Guittard G., Olive D., Nunès J.A. Vitamin D controls tumor growth and CD8+ T cell infiltration in breast cancer. Front. Immunol. 2019;10:1307. doi: 10.3389/fimmu.2019.01307. PMID: 31244851; PMCID: PMC6563618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allin K.H., Nordestgaard B.G. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab. Sci. 2011;48(4):155–170. doi: 10.3109/10408363.2011.599831. PMID: 22035340. [DOI] [PubMed] [Google Scholar]
- 28.Sexton M., Jones D.B., Maloney M.E. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J. Am. Acad. Dermatol. 1990;23:1118–1126. doi: 10.1016/0190-9622(90)70344-h. 6 Pt 1PMID: 2273112. [DOI] [PubMed] [Google Scholar]
- 29.Puig S., Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin. Transl. Oncol. 2015;17(7):497–503. doi: 10.1007/s12094-014-1272-9. PMID: 25643667; PMCID: PMC4495248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jørgensen N., Persson G., Hviid T.V.F. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front. Immunol. 2019;10:911. doi: 10.3389/fimmu.2019.00911. PMID: 31134056; PMCID: PMC6517506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson R., Theron A.J., Rapoport B.L. Immunopathogenesis of immune checkpoint inhibitor-related adverse events: roles of the intestinal microbiome and Th17 cells. Front. Immunol. 2019;10:2254. doi: 10.3389/fimmu.2019.02254. PMID: 31616428; PMCID: PMC6775220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchbinder E.I., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239. PMID: 26558876; PMCID: PMC4892769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graydon C.G., Mohideen S., Fowke K.R. LAG3′s enigmatic mechanism of action. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.615317. PMID: 33488626; PMCID: PMC7820757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang R., Rangachari M., Kuchroo V.K. Tim-3: a co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019;42 doi: 10.1016/j.smim.2019.101302. PMID: 31604535. [DOI] [PubMed] [Google Scholar]
- 35.Wolf Y., Anderson A.C., Kuchroo V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020;20(3):173–185. doi: 10.1038/s41577-019-0224-6. PMID: 31676858; PMCID: PMC7327798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peris K., Fargnoli M.C., Garbe C., Kaufmann R., Bastholt L., Seguin N.B., Bataille V., Marmol V.D., Dummer R., Harwood C.A., Hauschild A., Höller C., Haedersdal M., Malvehy J., Middleton M.R., Morton C.A., Nagore E., Stratigos A.J., Szeimies R.M., Tagliaferri L., Trakatelli M., Zalaudek I., Eggermont A., Grob J.J., European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC) Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. PMID: 31288208. [DOI] [PubMed] [Google Scholar]
- 37.Love W.E., Bernhard J.D., Bordeaux J.S. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch. Dermatol. 2009;145(12):1431–1438. doi: 10.1001/archdermatol.2009.291. PMID: 20026854. [DOI] [PubMed] [Google Scholar]
- 38.Stratigos A.J., Sekulic A., Peris K., Bechter O., Prey S., Kaatz M., Lewis K.D., Basset-Seguin N., Chang A.L.S., Dalle S., Orland A.F., Licitra L., Robert C., Ulrich C., Hauschild A., Migden M.R., Dummer R., Li S., Yoo S.Y., Mohan K., Coates E., Jankovic V., Fiaschi N., Okoye E., Bassukas I.D., Loquai C., De Giorgi V., Eroglu Z., Gutzmer R., Ulrich J., Puig S., Seebach F., Thurston G., Weinreich D.M., Yancopoulos G.D., Lowy I., Bowler T., Fury M.G. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848–857. doi: 10.1016/S1470-2045(21)00126-1. PMID: 34000246. [DOI] [PubMed] [Google Scholar]
- 39.FDA (U.S. Food & Drug Administration). FDA approves cemiplimab-rwlc for locally advanced and metastatic basal cell carcinoma. Content current as of: 02/09/2021. Last accessed on the 28 November 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvescemiplimab-rwlc-locally-advanced-andmetastatic-basal-cell-carcinoma.
- 40.Libtayo® (Cemiplimab) approved by the European Commission as the first immunotherapy indicated for patients with advanced basal cell carcinoma. BioSpace. June 25, 2021. Accessed November 28, 2021. https://www.biospace.com/article/libtayocemiplimab-approved-by-the-european-commission-as-the-first-immunotherapyindicatedfor-patients-with-advanced-basal-cell-carcinoma/.
- 41.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. PMID: 20819927; PMCID: PMC2947065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama S., Akbay E.A., Li Y.Y., Herter-Sprie G.S., Buczkowski K.A., Richards W.G., Gandhi L., Redig A.J., Rodig S.J., Asahina H., Jones R.E., Kulkarni M.M., Kuraguchi M., Palakurthi S., Fecci P.E., Johnson B.E., Janne P.A., Engelman J.A., Gangadharan S.P., Costa D.B., Freeman G.J., Bueno R., Hodi F.S., Dranoff G., Wong K.K., Hammerman P.S. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016;7:10501. doi: 10.1038/ncomms10501. PMID: 26883990; PMCID: PMC4757784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J.E., Patel M.A., Mangraviti A., Kim E.S., Theodros D., Velarde E., Liu A., Sankey E.W., Tam A., Xu H., Mathios D., Jackson C.M., Harris-Bookman S., Garzon-Muvdi T., Sheu M., Martin A.M., Tyler B.M., Tran P.T., Ye X., Olivi A., Taube J.M., Burger P.C., Drake C.G., Brem H., Pardoll D.M., Lim M. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 2017;23(1):124–136. doi: 10.1158/1078-0432.CCR-15-1535. PMID: 27358487; PMCID: PMC5735836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian T., Li Z. Targeting Tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.731175. PMID: 34631560; PMCID: PMC8492972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tawbi H.A., Schadendorf D., Lipson E.J., Ascierto P.A., Matamala L., Castillo Gutiérrez E., Rutkowski P., Gogas H.J., Lao C.D., De Menezes J.J., Dalle S., Arance A., Grob J.J., Srivastava S., Abaskharoun M., Hamilton M., Keidel S., Simonsen K.L., Sobiesk A.M., Li B., Hodi F.S., GV; Long, RELATIVITY-047 Investigators Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. PMID: 34986285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to data will be provided on request.