Abstract
Background:
Evidence suggests that poor sleep increases risk of delirium. Because delirium is associated with poor outcomes, institutions have developed protocols to improve sleep in critically ill patients.
Objective:
To assess the impact of implementing a multicomponent sleep protocol.
Methods:
In this prospective, preimplementation and postimplementation evaluation, adult patients admitted to the medical intensive care unit (ICU) over 42 days were included. Outcomes evaluated included median delirium-free days, median Richards-Campbell Sleep Questionnaire (RCSQ) score, median optimal sleep nights, duration of mechanical ventilation (MV), ICU and hospital length of stay (LOS), and in-hospital mortality.
Results:
The preimplementation group included 78 patients and postimplementation group, 84 patients. There was no difference in median delirium-free days (1 day [interquartile range, IQR, = 0–2.5] vs 1 day [IQR = 0–2]; P = 0.48), median RCSQ score (59.4 [IQR = 43.2–71.6] vs 61.2 [IQR = 49.9–75.5]; P = 0.20), median optimal sleep nights (1 night [IQR = 0–2] vs 1 night [IQR = 0–2]; P = 0.95), and in-hospital mortality (16.7% vs 17.9%, P = 1.00). Duration of MV (8 days [IQR = 4–10] vs 4 days [IQR = 2–7]; P = 0.03) and hospital LOS (13 days [IQR = 7–22.3] vs 8 days [IQR = 6–17]; P = 0.05) were shorter in the postimplementation group, but both were similar between groups after adjusting for age and severity of illness.
Conclusions and Relevance:
This report demonstrates that implementation of a multicomponent sleep protocol in everyday ICU care is feasible, but limitations exist when evaluating impact on measurable outcomes. Additional evaluations are needed to identify the most meaningful interventions and best practices for quantifying impact on patient outcomes.
Keywords: critical care, sleep disorders, sedatives, mechanical ventilators, quality assurance
Introduction
Disruptions in sleep are common in critically ill adults. Both quantity and quality of sleep are poor, with disruptions occurring secondary to environmental noise, bright lights, medical interventions, psychoactive medications, pain, and general critical illness.1 Additionally, previous studies have described altered sleep-wake cycles in which most sleep occurs over short intervals and during the daytime.2 Sleep architecture of critically ill patients is severely fragmented with reductions in both slow wave and rapid eye movement sleep.1 Secretion of melatonin, a key hormone involved in the regulation of sleep, is also altered in the critically ill, with a delay in secretion and lower peaks compared with healthy controls.3–5
Poor sleep quality can have detrimental effects on patient outcomes. Sleep deprivation can lead to increased upper airway collapsibility, hormone imbalance, and decreased levels of energy, which in turn can alter ventilation, metabolic function, and ability to participate in physical activity.6 Recently, increasing evidence suggests that poor sleep increases the risk of delirium.7,8 This finding could be crucial to patient care given that delirium is common in critically ill adults and is associated with numerous poor outcomes such as increased mortality, longer duration of mechanical ventilation (MV), and increased long-term cognitive dysfunction after discharge.9
Researchers have attempted to improve sleep quality in critically ill patients with targeted interventions, such as offering earplugs and eye masks in addition to implementation of sleep-promoting multicomponent protocols.10–14 The 2018 Clinical Practice Guidelines for Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the Intensive Care Unit (ICU) introduce this as one of the core components and suggest implementing a multicomponent protocol to improve sleep.15
In an effort to optimize patients’ sleep and improve their clinical outcomes, we devised a quality improvement initiative to develop and implement a multicomponent sleep protocol in the medical intensive care unit (MICU). We sought to quantify the effect of this intervention on various outcomes, including median delirium-free days, median Richards-Campbell Sleep Questionnaire (RCSQ) score, median optimal sleep nights, duration of MV, ICU and hospital length of stay (LOS), and in-hospital mortality.
Materials and Methods
We conducted a prospective quality improvement project within an MICU at an academic medical center. All adult patients admitted to the MICU at our center from November 1, 2016, to November 23, 2016, and January 23, 2017, to February 12, 2017, were included. We excluded patients who required frequent (every 2 to 4 hour) neurological assessments, had a diagnosis of status epilepticus, were undergoing a barbiturate-induced coma, or receiving continuous paralytics. If patients were readmitted to the ICU during the same hospitalization, they were counted as a new, separate patient.
Sleep Protocol Planning and Implementation
The sleep protocol was implemented in a 30-bed MICU within an 803-bed academic medical center. Approximately 160 patients with acute or chronic illnesses are admitted each month to this unit. This protocol was developed by a multidisciplinary team, including physicians, pharmacists, and nursing staff. The sleep protocol was implemented as part of the delirium monitoring and management component of the awakening and breathing coordination, delirium monitoring and management, early mobility, family engagement and empowerment (ABCDEF) bundle. The ABCDEF bundle is intended to provide clinicians an organized approach to deliver comprehensive, evidence-based care for critically ill patients with the goal of improving both practice and patient outcomes.16,17 At the time of implementation, the awakening and breathing coordination component and early mobility component had been implemented in the MICU.
Staff training took place over an 8-week time frame after preimplementation data were collected and focused on the delirium monitoring and management component of the ABCDEF bundle. Key aspects of this training included appropriate assessment of sedation and delirium using the Richmond Agitation and Sedation Scale (RASS) and Confusion Assessment Method for the ICU (CAM-ICU), respectively, and proper implementation of the sleep protocol. Nursing education consisted of a 30-minute didactic session and one-on-one education using case examples and the teach-back method for competency in assessing RASS and CAM-ICU. Nurses were asked to assess RASS every 4 hours and CAM-ICU at least once per 12-hour shift or if a change in a patient’s mental status occurred, including while patients were mechanically ventilated. Sleep protocol interventions were completed throughout each shift. Laminated cards including the RASS, CAM-ICU, and sleep protocol were placed outside each patient room for reference and documentation. Nurses were educated to report their patients’ complete delirium assessment during daily rounds.
Sleep Protocol Process Overview
The sleep protocol consists of 3 successive stages that focus on optimizing sleep hygiene and providing patients with nonpharmacological and pharmacological interventions when sleep quality remains poor despite optimized environmental conditions (Figure 1).
Figure 1.
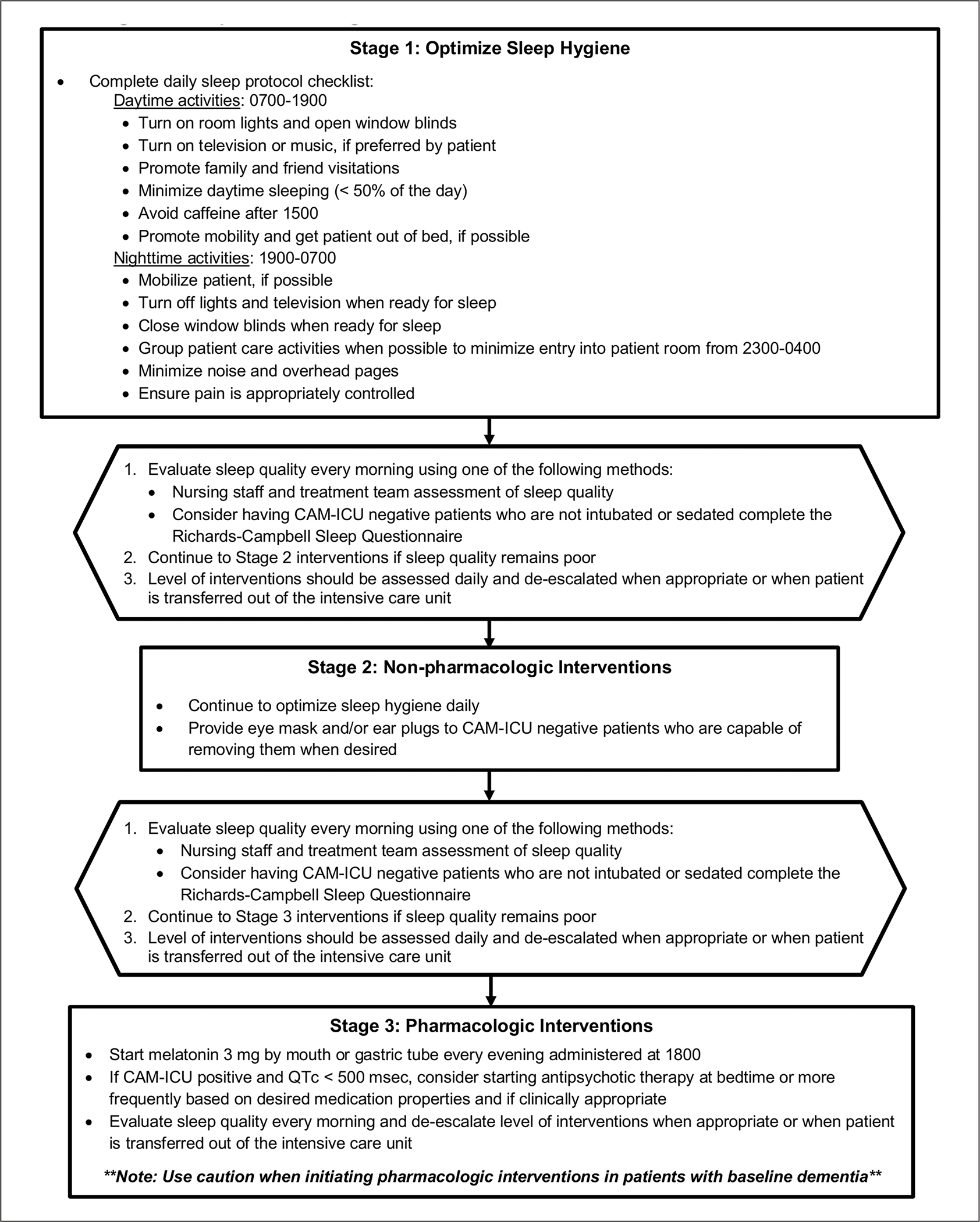
Sleep promotion algorithm for medical intensive care unit patients: Interventions provided during 3 progressive stages of sleep protocol.
Abbreviation: CAM-ICU, Confusion Assessment Method for the intensive care unit.
Stage 1 interventions were initiated on the first day of ICU admission and tracked by nursing staff using a daily checklist that included daytime and nighttime activities (Figure 2). Activities on this checklist were compiled based on feasibility and potential benefits to promote sleep and reduce delirium.10–14,18–22 Daytime interventions included promoting a lighted environment, turning on music or the television if preferred by the patient, encouraging family and friend visitations, mobility, minimization of daytime sleeping, and avoidance of caffeine after 3:00 PM. Nighttime interventions included encouraging mobilization until bedtime, reducing light and noise, grouping patient care activities to minimize entry into the room from 11:00 PM to 4:00 AM, assessing pain, and performing a spontaneous awakening trial (SAT) and spontaneous breathing trial (SBT) for appropriate mechanically ventilated patients between 4:00 AM and 6:00 AM per unit protocol. Starting on the second day of ICU admission, sleep quality was assessed using the RCSQ. The RCSQ was completed daily by the patient prior to morning rounds when able or by the overnight nurse prior to shift change in patients deemed unable to participate by nursing staff.23,24
Figure 2.
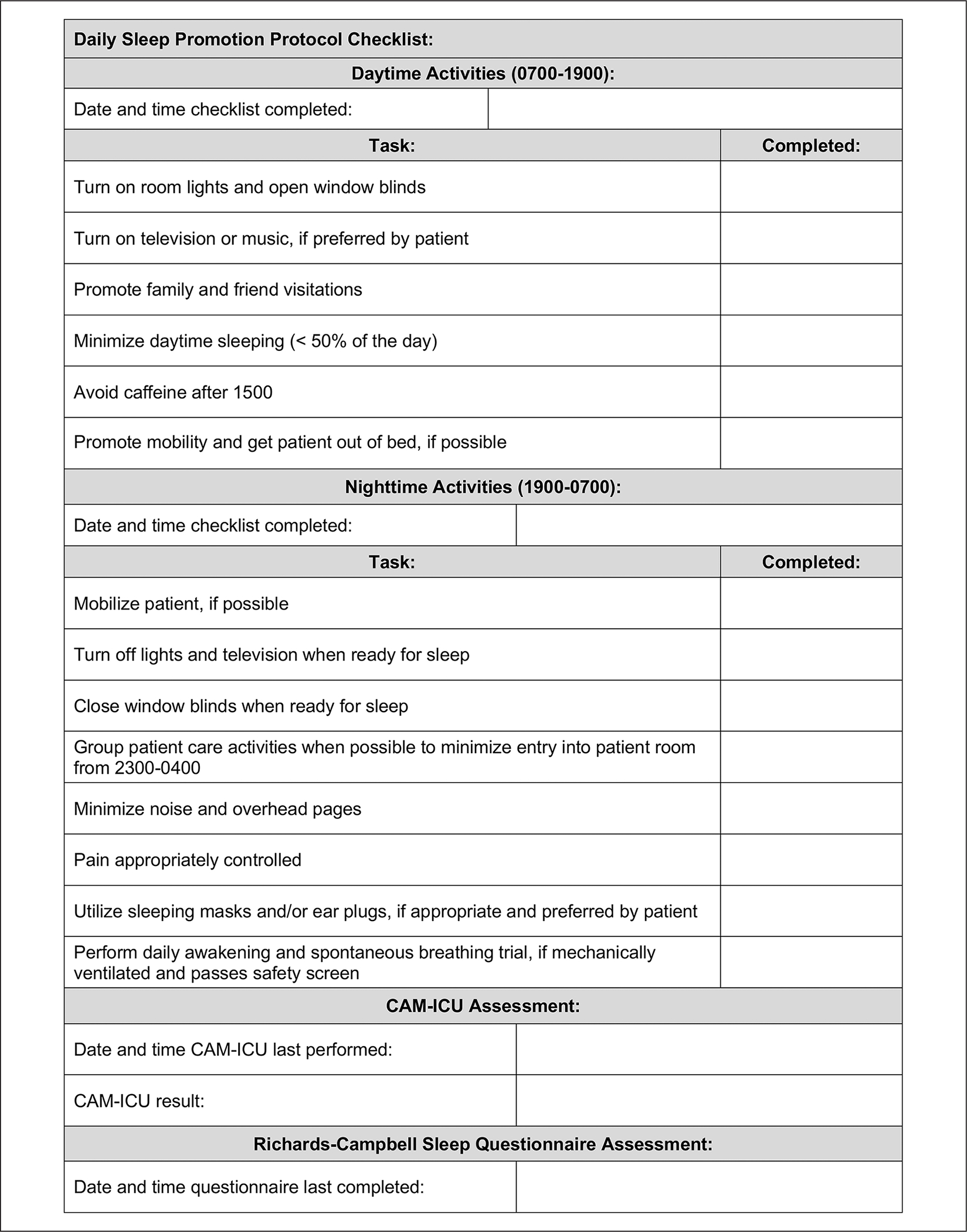
Daily nursing sleep protocol checklist: Bedside checklist utilized by nursing staff to track sleep interventions provided.
Abbreviation: CAM-ICU, Confusion Assessment Method for the intensive care unit.
Stage 2 interventions were implemented in patients with suboptimal sleep (Figure 1). Stage 2 interventions included the use of nonpharmacological agents, such as sleep masks and/or earplugs, to further minimize sleep disruptions throughout the night. Only patients who were nondelirious with the capability of removing these items were offered them.
Stage 3 interventions were implemented in patients who continued to have suboptimal sleep (Figure 1). Stage 3 interventions included the use of pharmacological agents, which included melatonin and/or antipsychotics in appropriate patients. When administered, melatonin was dosed at 3 mg by mouth (or by gastric tube), timed nightly at 6:00 PM. This dose was chosen based on pharmacokinetic studies performed in critically ill patients suggesting that lower doses achieve ideal pharmacological levels and higher doses may lead to elevated levels the next day.25 Additionally, 6:00 PM was selected as the administration time to account for the pharmacokinetics of orally administered melatonin, while balancing nursing workflows and timing of SATs/SBTs the next morning.26,27
For patients who were delirious with no clinical contraindications, atypical antipsychotics (haloperidol, olanzapine, quetiapine, or risperidone) were considered.28–32 Once patients had 2 negative CAM-ICU assessments, the team discontinued or de-escalated neuroleptic agents. For example, if a patient was prescribed quetiapine dosed every 12 hours, the team would taper the medication dose to once daily at night with plans to discontinue as the patient’s delirium resolved.
Sleep Protocol Evaluation
The sleep protocol was evaluated using a preimplementation and postimplementation design. The institutional review board waived full review of the protocol because it was minimal risk to the patient and consistent with a quality improvement initiative. All adult patients who were admitted to the MICU for >2 nights and met the eligibility criteria for the sleep protocol were included in the analysis. Baseline demographics were collected at the time of ICU admission from the electronic health record (EHR).
Compliance with the daytime and nighttime sleep hygiene checklist was assessed in the preimplementation and postimplementation groups on sleep protocol days by interviewing nursing staff. Compliance was assessed in the preimplementation group to better understand the baseline use of sleep hygiene methods by nursing staff before education. Sleep protocol days began on the second full day of ICU admission and continued until ICU discharge. Melatonin, antipsychotic, opioid, and sedation administration information were collected from the EHR on all sleep protocol days.
The primary outcome of interest was median number of delirium-free days, which was assessed on sleep protocol days. Patients were considered to have a delirium-free day if they had negative CAM-ICU assessments or if they had at least 2 consecutive negative CAM-ICU assessments after a previous positive assessment. Patients were assumed to remain delirium free thereafter unless a change was recorded by nursing staff.
Secondary outcomes evaluated included median RCSQ score, median number of optimal sleep nights based on RCSQ score >70, duration of MV, ICU and hospital LOS, and in-hospital mortality. Median RCSQ score and median number of optimal sleep nights were evaluated on sleep protocol days. Duration of MV, ICU and hospital LOS, and in-hospital mortality were evaluated on all admission days.
Data were analyzed using median and interquartile range (IQR) for continuous variables and proportions for categorical variables. Continuous variables were compared using the Wilcoxon Rank-Sum test, and categorical variables were compared using the χ2 test. Multivariate linear regression was used to account for potential effect modifiers of hospital LOS, ICU LOS, and duration of MV. This model adjusted for severity of illness via utilization of the Sequential Organ Failure Assessment (SOFA) score and age.33 An α <0.05 was considered significant. All analyses were performed using SPSS v 25 (IBM Corp, Armonk, NY).
Results
Sleep Protocol Planning and Implementation
A total of 54% of MICU nurses were educated during one of the 30-minute didactic sessions, and 83% of nurses completed one-on-one training sessions. The preimplementation group consisted of 78 patients and the postimplementation group, 84 patients. Baseline demographics were similar between groups (Table 1). The median age was 59 years (IQR = 48–68), and most patients were admitted for respiratory failure and nonpulmonary sepsis. The median SOFA score on ICU admission was 6 (IQR = 3.3–8), indicating a 21.5% predicted mortality rate. A total of 70 patients (43.2%) required MV during their ICU stay.
Table 1.
Baseline Demographics on ICU Admission.
| Cohort (n = 162) | Preimplementation (n = 78) | Preimplementation (n = 84) | P value | |
|---|---|---|---|---|
|
| ||||
| Median age, years [IQR] | 59 [48–68] | 69 [50–70.8] | 58 [47–67] | 0.231 |
| Male sex, n (%) | 83 (51.2) | 43 (55.1) | 40 (47.6) | 0.339 |
| Race, n (%) | 0.845 | |||
| Caucasian | 84 (51.9) | 40 (51.3) | 44 (52.4) | |
| African American | 51 (31.5) | 23 (29.5) | 28 (33.3) | |
| Asian | 2 (1.2) | 1 (1.3) | 1 (1.2) | |
| Other | 25 (15.4) | 14 (17.9) | 11 (13.1) | |
| Median SOFA score on ICU admission [IQR] | 6 [3.25–8] | 6 [3–8.75] | 5.5 [4–8] | 0.472 |
| ICU admission diagnosis, n (%) | 0.351 | |||
| Respiratory failure | 48 (29.6) | 18 (23.0) | 30 (35.7) | |
| Gastrointestinal | 32 (19.8) | 19 (24.4) | 13 (15.5) | |
| Nonpulmonary sepsis | 34 (20.9) | 16 (20.5) | 18 (21.4) | |
| Cardiovascular | 10 (6.2) | 6 (7.7) | 4 (4.8) | |
| Other | 38 (23.5) | 19 (24.4) | 19 (22.6) | |
| History of sleep disorder listed in past medical history, n (%) | 21 (13.0) | 10 (12.8) | 11 (13.1) | 0.959 |
| Required MV during ICU stay, n (%) | 70 (43.2) | 35 (44.9) | 35 (41.7) | 0.681 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; SOFA, Sequential Organ Failure Assessment.
The preimplementation group consisted of 308 sleep protocol days, and the postimplementation group consisted of 295 sleep protocol days for assessment. The median number of sleep protocol days per patient was similar between the preimplementation and postimplementation groups (3 days [IQR = 1–6] vs 2 days [IQR = 1–4], respectively; P = 0.319). Compliance with stage 1 sleep hygiene interventions were assessed between groups (Table 2). There was no improvement between groups in compliance rates in performing SAT/SBT in ventilated patients, opening window blinds, and turning on lights during the daytime, and closing window blinds and turning off lights during the nighttime. Stage 2 nonpharmacological interventions were not offered in the preimplementation group, and only 2 patients (0.9%) utilized eye masks and/or earplugs in the postimplementation group. Stage 3 pharmacological interventions were completed in a moderate percentage of patients in the preimplementation and postimplementation groups (Table 3). Melatonin administration was significantly lower in the preimplementation versus postimplementation group (16.7% vs 38.1%, respectively; P = 0.002). Use of atypical antipsychotics was similar between groups, except that significantly fewer patients received risperidone in the preimplementation versus postimplementation group (0% vs 7.1%, respectively; P = 0.03). There was no difference in the percentage of patients who received opioids and sedatives on sleep protocol days. The median dose of propofol administered was significantly lower in the preimplementation group (836.5 mg [IQR = 486.5–4398.3] vs 7999.0 mg [IQR = 1569.0–14 577.0]; P = 0.037), but median doses administered of opioids, benzodiazepines, and dexmedetomidine were similar between groups. Finally, median RASS score was not different between the preimplementation and postimplementation groups.
Table 2.
Protocol Compliance on Sleep Protocol Days.
| Preimplementation (n = 308 sleep protocol days) |
Postimplementation (n = 295 sleep protocol days) |
|
|---|---|---|
|
| ||
| Daytime checklist completion days, n (%) | 245 (79.5) | 182 (61.7) |
| SAT/SBT performed in MV patients, n (%) | 77/132 (58.3) | 42/112 (37.5) |
| Lights/blinds open, n (%) | 222 (90.6) | 153 (84.1) |
| Television/music on, n (%) | 164 (66.9) | 135 (74.2) |
| Visitations, n (%) | 184 (75.1) | 155 (85.2) |
| Minimize daytime sleeping, n (%) | 157 (64.0) | 128 (70.3) |
| Avoid caffeine, n (%) | — | 173 (95.1) |
| Mobility, n (%) | 82 (33.5) | 94 (51.6) |
| Evening checklist completion days, n (%) | 260 (84.4) | 213 (72.2) |
| Mobility, n (%) | 84 (32.3) | 79 (37.1) |
| Lights/television off, n (%) | 244 (93.8) | 197 (92.5) |
| Blinds closed, n (%) | 216 (83.1) | 164 (77.0) |
| Group patient care activities, n (%) | 247 (95.0) | 205 (96.2) |
| Minimize overhead pages, n (%) | 258 (99.2) | 211 (99.1) |
| Pain controlled, n (%) | — | 194 (91.1) |
| Mask/ear plugs, n (%) | — | 2 (0.9) |
| CAM-ICU assessment compliance (≥2 per day), n (%) | 53 (17.2) | 93 (31.5) |
| RASS assessment compliance (≥6 per day), n (%) | 154 (50.0) | 130 (44.0) |
Abbreviations: CAM-ICU, Confusion Assessment Methods for the Intensive Care Unit; MV, mechanical ventilation; RASS, Richmond Agitation Sedation Score; SAT, spontaneous awakening trial; SBT, spontaneous breathing trial.
Table 3.
Medication Administration Per Patient on Sleep Protocol Days.
| Preimplementation (n = 78) | Postimplementation (n = 84) | P value | |
|---|---|---|---|
|
| |||
| Melatonin administered, n (%) | 13 (16.7) | 32 (38.1) | 0.002 |
| Antipsychotics administered, n (%) | |||
| Haloperidol, IV/IM/oral | 14 (17.9) | 20 (23.8) | 0.36 |
| Quetiapine, oral | 8 (10.2) | 10 (11.9) | 0.81 |
| Olanzapine, oral | 7 (8.9) | 12 (14.3) | 0.34 |
| Risperidone, oral | 0 (0) | 6 (7.1) | 0.03 |
| Opioids administered, IV/oral, n (%) | 53 (67.9) | 56 (66.7) | 0.862 |
| Median morphine equivalents administered, mg [IQR] | 204.0 [56.0–503.3] | 75.3 [26.8–789.8] | 0.349 |
| Benzodiazepines administered, IV/oral, n (%) | 28 (35.9) | 31 (36.9) | 0.894 |
| Median lorazepam equivalents administered, mg [IQR] | 7.5 [2.1–31.1] | 2.0 [1.0–16.0] | 0.050 |
| Propofol administered, IV, n (%) | 8 (10.3) | 13 (15.5) | 0.323 |
| Median propofol administered, mg [IQR] | 836.5 [486.5–4398.3] | 7999.0 [1569.0–14 577.0] | 0.037 |
| Dexmedetomidine administered, IV, n (%) | 9 (11.5) | 11 (13.1) | 0.763 |
| Median dexmedetomidine administered, μg [IQR] | 2717.6 [1252.8–5252.4] | 1284.4 [520.8–3640.0] | 0.230 |
| Median daily RASS score [IQR] | −0.3 [−0.9 to 0]a | −0.2 [−0.7 to 0]b | 0.31 |
Abbreviations: IM, intramuscular; IQR, interquartile range; IV, intravenous; RASS, Richmond Agitation Sedation Scale.
n = 75.
n = 82.
Sleep Protocol Evaluation
The median number of delirium-free days on sleep protocol days was compared between groups, in which 9 patients in the preimplementation group and 4 patients in the postimplementation group were excluded because a CAM-ICU assessment was not completed during their ICU admission. There was no significant difference in median number of delirium-free days between the preimplementation and postimplementation groups (1 day [IQR = 0–2.5] vs 1 day [IQR = 0–2], respectively, P = 0.48; Table 4). A total of 23 patients (29.5%) in the preimplementation group and 33 patients (39.3%) in the postimplementation group had at least 1 CAM-ICU assessment performed on all sleep protocol days. A total of 49 patients (62.8%) in the preimplementation group and 62 patients (73.8%) in the postimplementation group had at least 1 CAM-ICU assessment performed on ≥50% of all sleep protocol days.
Table 4.
Outcomes Per Patient on Sleep Protocol and All Admission Days.
| Preimplementation (n = 78) | Postimplementation (n = 84) | P value | |
|---|---|---|---|
|
| |||
| Outcomes evaluated on sleep protocol days | |||
| Median number of delirium-free days [IQR] | 1 [0–2.5]a | 1 [0–2]b | 0.48 |
| Median RCSQ score [IQR] | 59.4 [43.2–71.6]c | 61.2 [49.9–75.5]c | 0.20 |
| Median number of optimal sleep nights [IQR] | 1 [0–2]c | 1 [0–2]c | 0.95 |
| Outcomes evaluated on all admission days | |||
| Median duration of MV, days [IQR] | 8 [4–10]d | 4 [2–7]d | 0.03 |
| Median duration of MV, days [IQR], excluding mortality patients | 7 [3–9]e | 3 [2–5]f | 0.01 |
| Median ICU length of stay, days [IQR] | 5.5 [4–10] | 4 [3–7] | 0.10 |
| Median ICU length of stay, days [IQR], excluding mortality patients | 5 [3.5–8.5]g | 4 [3–6]a | 0.12 |
| Median hospital length of stay, days [IQR] | 13 [7–22.3] | 8 [6–17] | 0.05 |
| Median hospital length of stay, days [IQR], excluding mortality patients | 15 [7–23.5]g | 8 [6–15]a | 0.02 |
| In-hospital mortality, n (%) | 13 (16.7) | 15 (17.9) | 1.00 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; RCSQ, Richards-Campbell Sleep Questionnaire.
n = 69.
n = 80.
n = 74.
n = 35.
n = 27.
n = 25.
n = 65.
An RCSQ was completed in a high percentage of total sleep protocol days in the preimplementation and postimplementation groups (77.6% vs 72.2%, respectively). A total of 4 patients in the preimplementation group and 10 patients in the postimplementation group were excluded from this analysis because an RCSQ was not completed during their ICU admission. The median RCSQ score on sleep protocol days was not significantly different between groups (59.4 [IQR = 43.2–71.6] vs 61.2 [IQR = 49.9–75.5], P = 0.20; Table 4). Additionally, the median number of optimal sleep nights (RCSQ score > 70) on sleep protocol days was not significantly different between groups (1 night [IQR = 0–2] vs 1 night [IQR = 0–2]; P = 0.95).
Median duration of MV on all admission days was significantly longer in the preimplementation versus postimplementation group (8 days [IQR = 4–10] vs 4 days [IQR = 2–7], respectively, P = 0.03; Table 4). This result was also demonstrated when patients who died during their ICU admission were excluded from the analysis. When adjusted for age and severity of illness, the postimplementation group had a reduction in duration of MV of 0.33 days (95% CI = −1.69 to 0.94; P = 0.387).
ICU and hospital LOS on all admission days was examined. There was no difference in median ICU LOS between groups (5.5 days [IQR = 4–10] vs 4 days [IQR = 3–7], P = 0.10; Table 4). However, a significantly shorter median hospital LOS in the postimplementation group (13 days [IQR = 7–22.3] vs 8 days [IQR = 6–17]; P = 0.05) was observed. This result was also demonstrated when patients who died during their hospitalization were excluded from the analysis. When adjusted for age and severity of illness, the postimplementation group had a reduction of ICU LOS by 0.76 days (95% CI = −2.45 to 0.94; P = 0.387) and hospital LOS by 3.25 days (95% CI = −9.10 to 2.60; P = 0.110).
There was no difference in in-hospital mortality on all admission days between groups (16.7% vs 17.9%, P = 1.00; Table 4).
Discussion
The relationship between poor sleep quality and patient outcomes is a growing area of interest in the management of critically ill patients. The 2018 Clinical Practice Guidelines for Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU support the use of multicomponent protocols to improve sleep quality. This sleep protocol intervention was associated with decreases in duration of MV and shorter hospital LOS, but these findings were attenuated when adjusted for age and severity of illness.
The interventions included in this protocol were multifaceted and based on previously published studies with positive outcomes.10–14,18–22,26,28–32 Kamdar et al11 demonstrated no difference in reported sleep quality but a significant improvement in delirium-free days after implementing a multitier protocol that optimized sleep hygiene, and nonpharmacological and pharmacological interventions to promote sleep. One main difference between the protocol of Kamdar et al and this protocol was the medication recommended in nondelirious patients. Kamdar et al used zolpidem, whereas this protocol recommended the use of melatonin. Melatonin was chosen for this protocol given the known alterations in melatonin secretion in critically ill patients and the desire to avoid or minimize all medications with any deleriogenic properties.2,25,26,34,35 Additionally, melatonin was administered at 6:00 PM, which is unique to this protocol. Other investigators have opted to administer melatonin at 8:00 PM.25,26 Previous literature demonstrates that peak concentrations are achieved approximately 15 to 50 minutes after oral administration of melatonin, and levels persist for up to 10 hours.26,27 An earlier administration time was selected to ensure that melatonin levels would be sufficiently elevated prior to bedtime while minimizing the risk for residual levels affecting SAT/SBTs at 4:00 to 6:00 AM. This time also avoided medication administration at nursing shift change.
Although the RCSQ was used, which has been validated against polysomnography in critically ill adults, sleep measurement in this patient population remains extremely difficult.8,36–40 The current methods of measuring sleep may be too insensitive to quantify these differences in this evaluation. Unlike the Kamdar et al11 study, a difference in delirium-free days was not observed. It is unclear if improvement in sleep quality directly affects development of delirium or if the calmer environment reduces the incidence of delirium.
Nishikimi et al41 demonstrated a significant reduction in ICU LOS in individuals taking ramelteon compared with placebo. ICU LOS after implementation of this sleep protocol was unchanged, but this duration can be affected by a number of administrative factors, including bed availability. Perhaps, a better assessment would have been to calculate ICU LOS based on status change from ICU status to step down or floor status; unfortunately, there is currently no effective way to collect these data from the EHR. Duration of MV and hospital LOS are less prone to inflation because of administrative factors, but a difference in these durations after adjusting for age and severity of illness was not identified. This was likely because the preimplementation group had multiple outliers with very high SOFA scores.
The daily checklist compliance rates were similar between the preimplementation and postimplementation groups, making it difficult to quantify the impact of this protocol on outcomes. The use of direct interviews with nursing staff could have affected the preimplementation data, in which overreporting of checklist implementation may have occurred. Direct observation may have been a more appropriate method of collecting compliance rates. Alternatively, these results may highlight that many of the interventions included in sleep protocols have become standard of care, and thus it may be difficult to quantify the impact on outcomes outside of randomized clinical trials. It is also unclear why there were fewer SAT/SBTs performed in the postimplementation group. Perhaps as more elements of the ABCDEF bundle were implemented, it became more difficult to perform each component. Finally, the low use of ear plugs and eye masks may be attributed to patients being ineligible for this intervention. Eye masks and earplugs were also newly acquired items available for use in the MICU, and some nurses may not have been aware that they were available. Overall, the use of eye masks and earplugs by patients was slightly lower in this evaluation compared with other published protocols (1%−9% utilization).11,25
Limitations of this analysis include the single-center design and use of nonrandomized cohorts. Although there were no significant differences in baseline characteristics and attempts were made to reduce selection bias by collecting preimplementation group data immediately before implementation, it is possible that preexisting temporal or seasonal differences could have influenced results. Second, data for the primary outcome were collected in a retrospective manner from the EHR after the implementation data collection periods, so nursing staff were critical to document this information. In this evaluation, delirium-free days were calculated by carrying the last reported CAM-ICU value forward until a different assessment value was recorded by the nurse. Only 56 patients (34.6%) had at least 1 CAM-ICU assessment performed on all sleep protocol days and 111 patients (68.5%) on ≥50% of all sleep protocol days. This retrospective process could lead to underreporting of delirium and heavily affect the results of this evaluation compared to the prospective collection method utilized in the Kamdar et al11 study. Future sleep protocol evaluations should ensure that nursing compliance with CAM-ICU documentation is high prior to implementation if evaluating delirium-free days, or this metric should be tracked and targeted during plan-do-check-act cycles after implementation to improve compliance. Third, we allowed nursing staff to complete the RCSQ for patients who were unable to participate in the survey (eg, delirious or receiving deep continuous sedation). Prior investigators have used and evaluated the accuracy of these methods, but we recognize that this surrogate reporting process could lead to under- or overreporting of sleep quality when compared with scores reported directly by the patient.23,24 Finally, protocol implementation and data collection required the involvement and participation of nursing staff. Therefore, the potential for the Hawthorne effect (ie, alteration of one’s behavior resulting from the presence of an observer) cannot be excluded.42
Conclusion and Relevance
Implementation of a multicomponent protocol to promote sleep in everyday ICU care is feasible, but there are limitations that make it difficult to demonstrate impact on measurable outcomes. There is growing evidence to support the implementation of protocols that improve sleep in critically ill patients. More real-life experiences with implementation of sleep protocols are needed to better determine which interventions are most impactful and best practices for quantifying impact on patient outcomes.
Acknowledgments
The authors would like to thank Medical Intensive Care Unit nurses Dana Raines, Stephen Jernigan, and Nathan Myers for assistance in protocol development and implementation; Dr. Richards for permission to use the Richards-Campbell Sleep Questionnaire; and Mary Pat Bulfin for assistance with data collection. We thank Data Analytics at the University of North Carolina Medical Center Department of Pharmacy. We acknowledge the assistance of the North Carolina Translational and Clinical Sciences, which is supported by the National Center for Advancing Translations Sciences at the National Institutes of Health, through Grant Award Number UL1T4002489.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Elliot R, McKinley S, Cistulli P, Fien M. Characterization of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17:R46. doi: 10.1186/cc12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–281. doi: 10.1097/00000441-199905000-00002 [DOI] [PubMed] [Google Scholar]
- 3.Seifman MA, Gomes K, Nguyen PN, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front Neurol. 2014;5:237. doi: 10.3389/fneur.2014.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisk U, Olsson J, Nylen P, Hahn RG. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond). 2004;107:47–53. doi: 10.1042/CS20030374 [DOI] [PubMed] [Google Scholar]
- 5.Boyko Y, Ording H, Jennum P. Sleep disturbances in critically ill patients in ICU: how much do we know? Acta Anaesthesiol Scand. 2012;56:950–958. doi: 10.1111/j.1399-6576.2012.02672.x [DOI] [PubMed] [Google Scholar]
- 6.Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. 2012;27:97–111. doi: 10.1177/0885066610394322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35:781–795. doi: 10.1007/s00134-009-1397-4 [DOI] [PubMed] [Google Scholar]
- 8.Kamdar BB, Knauert MP, Jones SF, Parsons EC, Parthasarathy S, Pisani MA; Sleep in the ICU Task Force. Perceptions and practices regarding sleep in the intensive care unit: a survey of 1223 critical care providers. Ann Am Thorac Soc. 2016;13:1370–1377. doi: 10.1513/AnnalsATS.201601-087OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson P, Khan A. Delirium in critically ill patients. Crit Care Clin. 2015;31:589–603. doi: 10.1016/j.ccc.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 10.Demoule A, Carreira S, Lavault S, et al. Impact of earplugs and eye mask on sleep in critically ill patients: a prospective randomized study. Crit Care. 2017;21:284. doi: 10.1186/s13054-017-1865-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamdar BB, King LM, Collop NA, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–809. doi: 10.1097/CCM.0b013e3182746442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14:R66. doi: 10.1186/cc8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SY, Wang TJ, Wu SFV, Liang SY, Tung HH. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J Clin Nurs. 2011;20:396–407. doi: 10.1111/j.1365-2702.2010.03507.x [DOI] [PubMed] [Google Scholar]
- 14.Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69:540–549. doi: 10.1111/anae.12638 [DOI] [PubMed] [Google Scholar]
- 15.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 16.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33:225–243. doi: 10.1016/j.ccc.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn DM, Cook TE, Carlisle CC, Nelson DL, Kramer NR, Millman RP. Identification and modification of environmental noise in an ICU setting. Chest. 1998;114:535–540. doi: 10.1378/chest.114.2.535 [DOI] [PubMed] [Google Scholar]
- 19.Walder B, Francioli D, Meyer JJ, Lancon M, Romand JA. Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels. Crit Care Med. 2000;28:2242–2247. doi: 10.1097/00003246-200007000-00010 [DOI] [PubMed] [Google Scholar]
- 20.Monsen MG, Edell-Gustafsson UM. Noise and sleep disturbance factors before and after implementation of a behavioural modification programme. Intensive Crit Care Nurs. 2005;21:208–219. doi: 10.1016/j.iccn.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Wallace CJ, Robins J, Alvord LS, Walker JM. The effect of earplugs on sleep measures during exposure to simulated intensive care unit noise. Am J Crit Care. 1999;8:210–219. [PubMed] [Google Scholar]
- 22.Richardson A, Allsop M, Coghill E, Turnock C. Earplugs and eye masks: do they improve critical care patients’ sleep? Nurs Crit Care. 2007;12:278–286. doi: 10.1111/j.1478-5153.2007.00243.x [DOI] [PubMed] [Google Scholar]
- 23.Frisk U, Nodström G. Patients’ sleep in an intensive care unit—patients’ and nurses’ perception. Intensive Crit Care Nurs. 2003;19:342–349. doi: 10.1016/s0964-3397(03)00076-4 [DOI] [PubMed] [Google Scholar]
- 24.Nicolás A, Aizitarte E, Iruarrizaga A, Vázquez M, Margall A, Asiain C. Perception of night-time sleep by surgical patients in an intensive care unit. Nurs Crit Care. 2008;13:25–33. doi: 10.1111/j.1478-5153.2007.00255.x [DOI] [PubMed] [Google Scholar]
- 25.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistraletti G, Sabbatini G, Taverna M, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–147. doi: 10.1111/j.1600-079X.2009.00737.x [DOI] [PubMed] [Google Scholar]
- 27.Harpsoe NG, Andersen LP, Gogenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71:901–909. doi: 10.1007/s00228-015-1873-4 [DOI] [PubMed] [Google Scholar]
- 28.Rea RS, Battistone S, Fong JJ, Devlin JW. Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27:588–594. doi: 10.1592/phco.27.4.588 [DOI] [PubMed] [Google Scholar]
- 29.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–427. doi: 10.1097/CCM.0b013e3181b9e302 [DOI] [PubMed] [Google Scholar]
- 30.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449. doi: 10.1007/s00134-003-2117-0 [DOI] [PubMed] [Google Scholar]
- 31.Maneeton B, Maneeton N, Srisurapanont M, Chittawatanarat K. Quetiapine versus haloperidol in the treatment of delirium: a double-blind, randomized, controlled trial. Drug Des Devel Ther. 2013;7:657–667. doi: 10.2147/DDDT.S45575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serafim RB, Bozza FA, Soares M, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: a systematic review. J Crit Care. 2015;30:799–807. doi: 10.1016/j.jcrc.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 34.Foster J, Burry LD, Thabane L, et al. Melatonin and melatonin agonists to prevent and treat delirium in critical illness: a systematic review protocol. Syst Rev. 2016;5:199. doi: 10.1186/s13643-016-0378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shilo L, Dagan Y, Smorjik Y, et al. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int. 2000;17:71–76. doi: 10.1081/cbi-100101033 [DOI] [PubMed] [Google Scholar]
- 36.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226. doi: 10.1186/cc5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litton E, Elliott R, Thompson K, Watts N, Seppelt I, Webb SAR; ANZICS Clinical Trials Group and The George Institute for Global Health. Using clinically accessible tools to measure sound levels and sleep disruption in the ICU: a prospective multicenter observational study. Crit Care Med. 2017;45:966–971. doi: 10.1097/CCM.0000000000002405 [DOI] [PubMed] [Google Scholar]
- 38.Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131–144. [PubMed] [Google Scholar]
- 39.Watson PL. Measuring sleep in critically ill patients: beware the pitfalls. Crit Care. 2007;11:159. doi: 10.1186/cc6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flannery AH, Oyler DR, Weinhouse GL. The impact of interventions to improve sleep on delirium in the ICU: a systematic review and research framework. Crit Care Med. 2016;44:2231–2240. doi: 10.1097/CCM.0000000000001952 [DOI] [PubMed] [Google Scholar]
- 41.Nishikimi M, Numaguchi A, Takahashi K, et al. Effect of administration of Ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single-center randomized placebo-controlled trial. Crit Care Med. 2018;46:1099–1105. doi: 10.1097/CCM.0000000000003132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe F, Michaud K. The Hawthorne effect, sponsored trials, and the overestimation of treatment effectiveness. J Rheumatol. 2010;37:2216–2220. doi: 10.3899/jrheum.100497 [DOI] [PubMed] [Google Scholar]


