Abstract
Whipple's disease is a rare bacterial infection that may involve any organ system in the body. It occurs primarily in Caucasian males older than 40 years. The gastrointestinal tract is the most frequently involved organ, with manifestations such as abdominal pain, malabsorption syndrome with diarrhea, and weight loss. Other signs include low-grade fever, lymphadenopathy, skin hyperpigmentation, endocarditis, pleuritis, seronegative arthritis, uveitis, spondylodiscitis, and neurological manifestations, and these signs may occur in the absence of gastrointestinal manifestations. Due to the wide variability of manifestations, clinical diagnosis is very difficult and is often made only years or even decades after the initial symptoms have appeared. Trimethoprim-sulfamethoxazole for at least 1 year is usually considered adequate to eradicate the infection. The microbiological diagnosis of this insidious disease is rendered difficult by the virtual lack of culture and serodiagnostic methods. It is usually based on the demonstration of periodic acid-Schiff-positive particles in infected tissues and/or the presence of bacteria with an unusual trilaminar cell wall ultrastructure by electron microscopy. Recently, the Whipple bacteria have been characterized at the molecular level by amplification of their 16S rRNA gene(s). Phylogenetic analysis of these sequences revealed a new bacterial species related to the actinomycete branch which was named “Tropheryma whippelli.” Based on its unique 16S ribosomal DNA (rDNA) sequence, species-specific primers were selected for the detection of the organism in clinical specimens by PCR. This technique is currently used as one of the standard methods for establishing the diagnosis of Whipple's disease. Specific and broad-spectrum PCR amplifications mainly but not exclusively from extraintestinal specimens have significantly improved diagnosis, being more sensitive than histopathologic analysis. However, “T. whippelii” DNA has also been found in persons without clinical and histological evidence of Whipple's disease. It is unclear whether these patients are true asymptomatic carriers or whether differences in virulence exist among strains of “T. whippelii” that might account for the variable clinical manifestations. So far, six different “T. whippelii” subtypes have been found by analysis of their 16S-23S rDNA spacer region. Further studies of the pathogen “T. whippelii” as well as the host immune response are needed to fully understand this fascinating disease. The recent cultivation of the organisms is a promising major step in this direction.
Intestinal lipodystrophy, now referred to as Whipple's disease, was first recognized as a new disorder in 1907 by the great American pathologist George Hoyt Whipple (203). This case report is a detailed description of a fatal illness in a patient with weight loss, arthritis, chronic cough, and fever. The illness caused pathological changes in the intestinal mucosa, mesenterium, heart, and lungs. As realized later, the same disorder had previously been described by Allchim and Hebb in 1895 (4), but they had failed to recognize it as a new disease. Based on the presence of unsplit fat in the stools, intestine, and mesenteric glands, a disease of fat metabolism was supposed. “Rod-shaped organisms in silver-stained gland tissue, closely resembling the tubercle bacillus” (203) were observed but not considered the etiology of the disease. However, no other tissue was available for further analysis. The histological criteria for Whipple's disease were summarized by Black-Schaffer in 1949 (18); periodic acid-Schiff reagent (PAS) was used to stain inclusions in macrophages found in the intestines and mesenteric lymph nodes of patients with this disease. With the help of electron microscopy free rod-shaped bodies with an outer membrane were noticed in the lamina propria (32). The authors considered the possibility of virus-like particles. A probable bacterial etiology of Whipple's disease was first considered in 1961 based on light and electron microscopy (28, 211).
Further support for bacteria as the cause of Whipple's disease was provided by the first successful treatment of a patient with chloramphenicol in 1952 (140). Despite numerous attempts, the causative organism remained uncultured until very recently (152, 169) and has never been successfully transferred to experimental animals.
Conclusive evidence for a bacterial infection fulfilling the classical Koch's postulates is still missing, although antibodies against the causative organism have been found in a majority of patients with Whipple's endocarditis. The bacterium associated with Whipple's disease, now known as “Tropheryma whippelii,” was partially characterized at the molecular level by PCR using primers complementary to conserved regions of the bacterial 16S RNA in 1991 for a single patient with Whipple's disease (207). Later, the complete 16S rRNA gene (154) and the 16S–23S intergenic spacer region and 200 bp of the 23S rRNA gene (110) were determined. Recently, a nearly complete rRNA operon sequence of about 5,747 bp was assembled from PCR products of a patient with Whipple's disease (113). Using molecular techniques, additional genes and eventually the entire genome of the Whipple bacillus will be characterized, thereby providing a basis for improved diagnostic tests as well as for a better understanding of the putative virulence mechanisms of this peculiar pathogen.
Despite improved diagnostic methods to recognize the disease, most of the reports published mention the difficulties and the long delay before Whipple's disease was correctly diagnosed. It is important to emphasize that although Whipple's disease is rare, physicians should always consider it in their differential diagnosis since its clinical presentation may be so variable and since it may be lethal for the patient.
In 1987, Dobbins published a comprehensive monograph on Whipple's disease (46) summarizing the current knowledge of not only clinical but also epidemiological and diagnostic aspects. In this review we will therefore focus on the knowledge accumulated since then with the intention of summarizing and updating the excellent description of the disease provided by Dobbins. Because PCR has greatly improved our ability to confirm the clinical suspicion of Whipple's disease, we will concentrate on reports published after the introduction of molecular techniques.
WHIPPLE'S DISEASE
Epidemiology
Very little is known about the epidemiology of Whipple's disease. According to the data published by Dobbins (46), it predominantly affects Caucasian males, with a male-to-female ratio of approximately 8:1 and a mean age of onset around 50 years. The fact that the disease is more frequently diagnosed in older individuals might at least in part be related to the usually significant delay between initial symptoms and diagnosis. A similar sex ratio is also found in the relatively large number of cases published since 1991, i.e., since the first PCR report on Whipple's disease was published by Wilson et al. (207) (Table 1). The mean age of the 363 patients reported in Table 1 is 51 years (range, 4 to 77 years), with an unexplained slight increase for females (from 13% to 20%) compared to earlier data (46). This change in the male-to-female ratio was also reported by Durand et al. (50) and by von Herbay et al. (199). There is no plausible explanation for the significantly higher incidence of Whipple's disease in males than females. If we assume genetic predisposition, the responsible mutation might be X- linked or associated with either an imprinted gene(s) or a reduction of regulatory genes controlling the monoallelic expression of particular cytokines (16, 88). In the German epidemiological study (199), the authors also observed a slight increase in the average age of Whipple's patients diagnosed between 1986 and 1995 to those diagnosed during the previous two decades. It may be speculated whether the apparent increase in the age of Whipple's disease patients is somehow related to the generally widespread use of antibiotics prior to the appearance of the most classical symptoms of the disease.
TABLE 1.
Age and sex of patients and laboratory method used to confirm the diagnosis in cases of Whipple's disease published since 1991a
| Age (yr) | Sexb | Result by:
|
Publication yr (reference) | ||
|---|---|---|---|---|---|
| PAS staining | PCR amplification | Electron microscopy | |||
| 47 | F | + | 1991 (6) | ||
| 64 (mean) | 12M, 2F | + | 1991 (11) | ||
| 35 | M | + | + | 1991 (14) | |
| 59 | F | + | 1991 (42) | ||
| 37 | M | + | + | 1991 (92) | |
| 53 | F | + | + | 1991 (141) | |
| 70 | F | + | + | 1991 (207) | |
| 32 | M | + | + | 1991 (210) | |
| 51 (mean) | 13M, 3F | + | + | 1992 (55) | |
| 36 | M | + | 1992 (145) | ||
| 36 | M | + | + | + | 1992 (154) |
| 51 | M | + | + | ||
| 50 | M | + | + | ||
| 52 | M | + | + | ||
| 48 | M | + | + | + | |
| 36 | M | + | + | 1992 (163) | |
| 62 | M | + | 1992 (187) | ||
| 71 | M | + | 1993 (35) | ||
| 45 | M | + | 1993 (73) | ||
| 58 | M | + | + | + | 1993 (134) |
| 64 | F | + | 1993 (162) | ||
| 39 | M | + | |||
| 45 | M | + | 1993 (180) | ||
| 43 | M | + | |||
| 28 | M | + | 1993 (212) | ||
| 65 | F | + | + | 1994 (13) | |
| 59 | M | + | + | 1994 (33) | |
| 62d | F | + | + | 1994 (82) | |
| 59 | M | + | + | ||
| 47e | M | + | 1994 (106) | ||
| 61 (mean) | 24M, 3F | + | 1994 (120) | ||
| 65 | M | + | 1995 (40) | ||
| 59 | F | + | + | + | 1995 (155) |
| 6 | M | + | − | 1995 (183) | |
| 43 | M | + | + | 1995 (201) | |
| 31 | F | + | + | 1996 (5) | |
| 63 | M | + | + | 1996 (12) | |
| 35 | M | + | 1996 (21) | ||
| 55 | M | + | 1996 (26) | ||
| 60 | M | − | + | 1996 (31) | |
| 49 | M | + | 1996 (38) | ||
| 4 | M | + | 1996 (49) | ||
| 58 | F | − | + | + | 1996 (67) |
| 44 | M | + | + | 1996 (61) | |
| 46 | M | + | + | 1996 (68) | |
| 31 | M | + | 1996 (96) | ||
| 55 | M | + | + | 1996 (105) | |
| 47f | F | − | |||
| 54f | M | − | |||
| 50 | M | + | + | 1996 (168) | |
| 63 | M | + | 1996 (194) | ||
| 55 (mean) | 38M, 14F | + | 1997 (50) | ||
| 40 | M | + | + | + | 1997 (54) |
| 55 | M | + | 1997 (94) | ||
| 47 | F | − | + | 1997 (107) | |
| 60f | M | − | − | ||
| 63f | M | − | − | ||
| 55f | M | − | − | ||
| 48f | W | − | − | ||
| 59 | M | + | + | + | 1997 (108) |
| 50 | M | + | 1997 (116) | ||
| 61 | M | + | + | 1997 (117) | |
| 51 | F | + | 1997 (119) | ||
| 46g | M | ||||
| 58g | M | ||||
| 60 | M | + | + | 1997 (124) | |
| 22 | M | + | + | 1997 (129) | |
| 8 | F | − | + | ||
| 38 | M | + | + | ||
| 72 | F | + | + | ||
| 62 | F | + | + | ||
| 48 | M | + | + | + | 1997 (131) |
| 42 | F | + | + | 1997 (133) | |
| 65 | M | + | + | ||
| 53 | M | + | 1997 (142) | ||
| 70 | M | + | 1997 (144) | ||
| 58 | F | + | + | ||
| 35 | F | + | + | 1997 (149) | |
| 54 (mean) | 13M, 4F | + | + | 1997 (150) | |
| 58 | M | + | 1997 (165) | ||
| 44 | F | + | + | 1997 (174) | |
| 65 | M | + | + | 1997 (193) | |
| 52 (mean) | 94M, 16F | + | (+)c | 1997 (199) | |
| 62 | F | + | + | 1998 (30) | |
| 63 (mean) | 12M | + | 1998 (43) | ||
| 44 | M | + | + | 1998 (98) | |
| 59 | M | + | 1998 (101) | ||
| 32 | M | + | + | 1998 (130) | |
| 34 | M | + | + | 1998 (136) | |
| 58 | M | + | + | + | 1998 (143) |
| 55 | M | + | + | 1998 (166) | |
| 66 | M | + | + | 1998 (167) | |
| 77 | F | + | + | + | 1998 (185) |
| 62 | M | + | + | + | 1998 (204) |
| 54 | F | − | + | 1999 (8) | |
| 36 | M | + | + | 1999 (22) | |
| 40 | M | + | + | 1999 (27) | |
| 55 | M | + | + | ||
| 75 | M | + | 1999 (39) | ||
| 43 | M | + | + | 1999 (57) | |
| 47 | F | + | + | 1999 (78) | |
| 64 | M | + | + | 1999 (79) | |
| 53 | M | + | + | ||
| 55 | M | + | |||
| 55 | F | + | + | ||
| 48 | M | + | 1999 (83) | ||
| 72 | M | + | |||
| 50 | M | − | + | ||
| 32 | F | − | + | ||
| 30 | F | + | |||
| 59 | M | − | + | ||
| 47 | M | + | 1999 (115) | ||
| 36 | M | + | + | 1999 (127) | |
| 41 | M | + | 1999 (138) | ||
| 61 | M | + | + | ||
| 62 | M | + | + | 1999 (148) | |
| 75 | M | + | + | ||
| 50 | M | + | + | ||
| 65 | M | + | + | ||
| 64 | M | + | + | ||
| 29 | F | + | + | ||
| 48 | M | + | + | ||
Patients redundantly described in more than one reference are reported only once (based on the information provided by the authors in the different manuscripts). Laboratory results refer to intestinal or extraintestinal specimens. Spaces in the table indicate “not determined.”
M, male; F, female.
For 39 patients histology was also confirmed by PCR.
Patient with Whipple's disease-associated bacterial organism infection.
Gram-positive rods detected.
Clinically diagnosed.
No data about PAS, PCR, and electron microscopy.
Whipple's disease is rarely found in children, but it is important to note that it may occur at any age (46, 49, 129, 183). Outbreaks of Whipple's disease and patient-to-patient transmission have never been reported.
A worldwide annual incidence of about 12 new cases of Whipple's disease has been estimated (46). However, the recent introduction of PCR to diagnose the infection and the increase in the number of cases diagnosed in nonuniversity hospitals (199) suggested that the disease may be more prevalent than previously suspected. This assumption is supported by the relatively large series of cases published from single laboratories serving small geographic areas (50, 199) as well as by our own experience. Since our very first, unsuspected case of Whipple's disease detected by broad-spectrum PCR and sequencing in a 31-year-old Caucasian woman with spondylodiscitis in late 1995 (5), we have come across at least 16 additional clinically and microbiologically proven cases including 6 cases of endocarditis and 3 cases in patients in whom “T. whippelii” was detected in the cerebrospinal fluid (references 22, 79, and 83 and unpublished observations). This would account for an annual incidence of at least 4.5 cases in the Swiss population of less than 7 million. This number might even be higher, considering that most probably not all of the Swiss cases came to our attention.
The small number of non-Caucasians affected by Whipple's disease might be related to differences in health care structures. However, genetic differences in the susceptibility of various populations or a particular geographic distribution of “T. whippelii” cannot be excluded. This view is supported by two recent studies which investigated the occurrence of “T. whippelii” DNA in Swiss and Malaysian patients undergoing elective gastroscopy but not showing classical signs suggestive of Whipple's disease (for details, see below). In the Swiss study comprising 105 persons, 2 were positive on the basis of duodenal biopsy specimens, 9 were positive on the basis of gastric fluid, and 3 were positive on the basis of both types of specimens (56). In contrast, none of the 108 individuals from Malaysia was positive on the basis of duodenal biopsy specimens (gastric fluid was not available [F. Dutly, T. Pang, B. R. Naidu, and M. Altwegg, unpublished data]). Similar studies are warranted in other countries to confirm these preliminary data, with the intention of determining possible differences in the geographical distribution of “T. whippelii.”
Some studies have shown a statistically significantly higher prevalence of Whipple's disease in farmers than in persons with other occupations (46). This raises the obvious question whether Whipple's disease, like leprosy, is a zoonosis or whether Whipple's disease bacilli are present in the soil. As shown by 16S rDNA sequence comparisons, these organisms belong to the gram-positive actinomycetes. Members of this class are present in a wide range of habitats including the soil, where they are active in the decomposition of organic materials.
There has been only a single report of a gorilla in a zoo that was affected by symptoms very similar to Whipple's disease (190). Furthermore, canine histiocytic ulcerative colitis in dogs is associated with signs comparable to Whipple's disease in humans but without evident bacterial etiology (34, 191). In a first attempt to find whether the pathogen responsible for Whipple's disease may reside in different natural reservoirs, we investigated domestic animals for the presence of “T. whippelii” DNA (F. Dutly, M. Wolf, and M. Altwegg, unpublished data). By analogy to humans, where Whipple's bacilli occur mainly in the small intestine, DNA extracted from intestinal biopsy specimens from a limited number of different domestic animals (20 cattle, 24 pigs, 10 horses, 15 sheep, 13 dogs, 14 cats, and 19 chickens) was analyzed by PCR. None of the specimens became positive. Our results suggest that “T. whippelii” does not have a widespread reservoir in domestic animals. However, the relatively small number of specimens available for each animal species does not exclude the presence of “T. whippelii” in animal hosts. In addition, animals might be colonized in other parts of the body. Therefore, these results should be considered tentative until they are confirmed in larger-scale studies.
Clinical Manifestations
The leading symptoms of Whipple's disease are weight loss, diarrhea, and arthropathy (Table 2). These symptoms may occur simultaneously by the time of diagnosis. Arthropathy, however, may precede gastrointestinal symptoms by many years. Nine percent of patients have granulomas (46), preferentially located in lymph nodes and liver but also found in other involved tissues, and very often the diagnosis of sarcoidosis is considered because clinical samples fail to stain by the PAS method (21, 108, 142, 162, 175, 202). Systemic symptoms like low-grade intermittent fever, night sweats, and lymphadenopathy are quite frequent in Whipple's disease (up to 60% of the reported cases). Skin hyperpigmentation (40 to 60%), particularly of light-exposed areas, is not unusual and is often erroneously diagnosed as Addison's disease (46). Less common (or maybe underestimated) is pulmonary (35 to 65%), cardiac (35 to 60%), skeletal muscle (193), and central nervous system (CNS) (20 to 30% in living patients) involvement. Skeletal involvement (5, 26) and hormonal changes are also described in Whipple's disease (43). Renal manifestations were reported on only a few occasions (35, 180). Since Whipple's disease is uncommon and since the same clinical manifestations may be observed in other diseases as well, laboratory confirmation is compulsory.
TABLE 2.
Signs and symptoms in patients with Whipple's disease
| Gastrointestinal | % of cases | Extraintestinal | % of cases |
|---|---|---|---|
| Common | Common | ||
| Weight loss | 80–90 | Arthralgias, arthritis | 70–90 |
| Diarrhea | 70–85 | Anemia | 75–90 |
| Abdominal pain | 50–90 | Low grade intermittent fever | 40–60 |
| Lymphadenopathy | 40–60 | ||
| Hyperpigmentation | 40–60 | ||
| Less common | Less common | ||
| Abdominal mass | 15–25 | Cardiac | 35–65 |
| Hematochezia | Pericardial friction rub | ||
| Murmurs | |||
| Nonspecific ECG changes | |||
| Pulmonary | 35–60 | ||
| Chronic cough (20%)a | |||
| Pleuritic pain | |||
| CNS | 20–30 | ||
| Cognitive changes (71%)b | |||
| Supranuclear gaze palsy (51%)b | |||
| Altered level of consciousness (50%)b | |||
| Hypothalamic manifestations (31%)b | |||
| Myoclonus (25%)b | |||
| Ataxia (20%)b | |||
| OMM or OFSM (20%)b | |||
| Sensory deficits (12%)b | |||
| Ocular | 5–15 | ||
| Visual changes or loss | |||
| Uveitis | |||
| Retinitis | |||
| Splenomegaly | 5–10 | ||
| Ascites | 5–10 |
Frequency in patients with pulmonary manifestations.
Frequency in patients with CNS manifestations.
Gastrointestinal tract.
Combinations of digestive symptoms such as diarrhea, weight loss, and malabsorption are the most prominent gastrointestinal manifestations (Table 2). The small intestinal mucosa of most patients is characterized by the presence of large foamy macrophages and a loss of microvilli. The macrophages are filled with PAS-positive particles. Other pathologic findings of the intestinal tract include lymphatic obstruction and extensive deposits of extracellular lipids. For this reason Whipple's disease was first named “intestinal lipodystrophy” by Whipple (203). The defect in the intestinal mucosa results in an excess of fat in the stool. The duodenum, jejunum, and ileum are almost always involved in Whipple's disease in patients with gastrointestinal manifestations (46). Liver, esophagus, stomach, and colon involvement has been demonstrated in few cases (46, 116, 143). Granulomas may be present in liver with negative PAS staining (29).
Diarrhea is the most common complaint in patients with Whipple's disease (58, 64, 114, 128) and has the features of steatorrhea, although it may consist of multiple watery stools per day. Weight loss, ranging from 10 to 15 kg in 1 year, is the second most common manifestation (58, 64, 114, 128). However, weight loss and diarrhea seem to be less frequent in patients younger than 40 years (46, 114) Cachexia may result from anorexia and nutritional deficiencies due to malabsorption. Other intestinal symptoms such as abdominal bloating and cramps may be present but are rare. Occasional bleeding, manifested by hematochezia and probably due to intestinal lesions comparable to those seen in untreated celiac disease (gluten-sensitive enteropathy), has been found in patients with Whipple's disease (13, 60, 114).
Intestinal symptoms in Whipple's disease are not specific and may also be observed in other diseases with gastrointestinal involvement such as Crohn's disease, celiac disease, and amyloidosis. Lymphomas can also cause similar gastrointestinal manifestations. The clinical features of infections with nontuberculous mycobacteria such as Mycobacterium avium-intracellulare (74, 100, 147, 161, 192, 195, 206) or M. genavense (3) in AIDS patients may mimic Whipple's disease. This syndrome has consequently been named pseudo-Whipple's disease. A few cases of AIDS patients with Whipple's disease were also described (7, 91, 111). However, no particular differences from the infection in nonimmunosupressed patients have been emphasized.
Arthralgias and arthritis.
Articular symptoms are the rule rather than the exception in patients with Whipple's disease and thus are the most common extraintestinal manifestations, occurring in up to 90% of patients (Table 2). In about one-third of affected patients (64), these symptoms may precede the gastrointestinal and/or other systemic symptoms by several years, rendering the diagnosis of Whipple's disease difficult (12, 21, 35, 46, 59, 67, 94, 106, 138, 162, 165, 189). Descriptions of the articular symptoms are often imprecise because they are usually reported only years later upon specific questioning when the diagnosis is finally made. In general, they were described as transient, intermittent, and migratory. Arthralgias and/or arthritis involve mainly the peripheral joints such as knees, elbows, fingers, ankles, and shoulders (78). Whipple's disease patients with arthritis and fever should be distinguished from patients with adult onset of Still's disease characterized by a salmon-pink rash and a marked neutrophilia (89).
Vertebral involvement is rare. Sarcoiliitis and spondylitis also associated with Whipple's disease (25, 164). So far, only one case of spondylodiscitis, initially detected by broad-spectrum PCR and sequence analysis from an open biopsy specimen and later confirmed by PAS staining (in an ileum biopsy specimen only) and PCR (in ileum, duodenum, sigmoid, and colon biopsy specimens) has been reported (5). Articular attacks are usually acute and last for hours to a few days (77). Chronic pain is uncommon. Joint deformity or destructive joint changes associated with Whipple's disease are very rare (9, 71, 164). For unknown reasons, joint pain often diminishes after intestinal symptoms develop. On radiography, the joints appear normal (103).
Cardiovascular system.
Up to one-third of the patients develop cardiac involvement (64, 93, 123, 209). Associated clinical findings are characterized by the presence of systolic murmurs, a pericardial friction rub, congestive heart failure, and nonspecific electrocardiogram changes (Table 2) (123). The most usual pathological changes are infectious endocarditis with negative blood cultures, presenting with thickened and deformed mitral or aortic valve. Other cardiac presentations include adhesive pericarditis and myocardial fibrosis. Lymphocytic myocarditis is very rare (141, 175). PAS-positive macrophages may be found in affected valves and myocardium and pericardium (94, 201) or may be absent (40). In one case of Whipple's disease, the replaced porcine valve was infiltrated by PAS-positive macrophages requiring a second valve replacement (151). Reviews of postmortem reports on patients with Whipple's disease describe cardiovascular involvement in more than 50% (123). Clinical manifestations of heart disease are less evident (46, 124). Nevertheless, the occurrence of cardiovascular involvement not accompanied by other symptoms is usually considered quite rare (20, 27, 40, 57, 79, 115, 131, 166, 174):
Pulmonary manifestations.
In his original report, Whipple described a chronic cough (203). Since then, lung involvement, occurring in 30 to 40% of the patients (46), has been characterized by pleuritic chest pain, chronic nonproductive cough, and dyspnea (Table 2). The chest X-ray may show a pleural effusion or pulmonary infiltrates (96, 114, 156, 158, 182). If the clinical or radiological pulmonary features are not accompanied by intestinal manifestations, it is difficult to distinguish Whipple's disease from sarcoidosis, which is a granulomatous disorder also associated with nonspecific symptoms such as fatigue, fever, anorexia, and weight loss. The disease can affect almost any organ, with the lungs being involved in almost 90% of patients. There are a number of case reports of Whipple's disease with a sarcoidosis-like presentation (29, 158, 162). In all patients, lung and lymph node biopsy specimens were PAS- negative and the diagnosis of Whipple's disease was established only on the basis of PAS-positive inclusions in duodenal biopsy specimens. PCR was not performed on any of these extraintesinal specimens.
Central nervous system.
CNS symptoms related to Whipple's disease may be present in only 10% to 30% of patients (46, 50, 59). However, postmortem examination of brain and spinal cord specimens revealed CNS lesions in over 90% of both symptomatic and asymptomatic patients (46). CNS involvement in most patients with Whipple's disease was confirmed in a recent study, where testing of cerebrospinal fluid yielded a high rate of positive results (PAS staining or PCR), even in patients without neurological manifestations (198). Focal CNS lesions are characterized by the presence of PAS-positive perivascular macrophages. These lesions are scattered within the cortical and subcortical gray matter of the cerebrum, the nuclear gray matter of the brain stem, and the cortical and nuclear gray matter of the cerebellum (172). Lesions and macrophages are less numerous in the white matter. The gray and white matter may show moderate gliosis at pathological examination (24, 172). Pleocytosis and/or elevated protein levels in cerebrospinal fluid are uncommon. The distribution of CNS lesions accounts for the various clinical symptoms (1, 6, 24, 30, 33, 146, 149, 157, 170, 184, 187, 194, 210). Hemispheric involvement may be responsible for dementia, personality changes, hemiparesis, or seizures. Cerebral ataxia, mesencephalic lesions causing ophthalmoplegia or nystagmus, and Wernicke's encephalopathy have all been reported as complications of Whipple's disease. Hypothalamic involvement causing insomnia, hypersomnia, polyuria, and polydipsia is less common (22, 46, 50, 117, 127). Hypothalamic-pituitary manifestation may be responsible for impairment of sex hormone secretion and hypogonadism (43). Meningeal involvement is uncommon (102). Occasional inclusions within Schwann cells, probably resulting from the late complications in the CNS, have also been reported (46, 50). Neurologic symptoms may appear with or without gastrointestinal (30, 31, 38, 46, 49, 50, 59, 210) or joint (39) manifestations. After initial successful treatment for intestinal manifestations, a number of patients relapsed with progressive neurologic involvement (22, 168, 197, 198). A significant fraction of these patients died (46, 49, 50, 59, 168, 183).
Guidelines for the diagnosis and treatment of CNS Whipple's disease were provided by Louis et al. (105). According to their recommendations, cognitive changes, supranuclear gaze palsy, and an altered level of consciousness are the most frequent neurological manifestations. They are followed by hypothalamic manifestations, myoclonus, seizure, and ataxia (Table 2). Combinations of the different neurological signs are also frequent (105). Oculomasticatory myorhythmia (OMM) and oculofacial-skeletal myorhythmia (OSFM), although rare, have never been documented in other diseases, and for this reason, they are considered pathognomonic for Whipple's disease of the CNS (24, 105).
According to Louis et al. (105), patients must fulfill at least one of the following criteria to clearly establish the diagnosis of neurological Whipple's disease: OMM, OSFM, or PAS- or PCR-positive tissue biopsy specimen. If histological or PCR analyses are not performed on CNS tissues, the patient must also show neurological signs. Patients with possible neurological Whipple's disease must have at least one of the following systemic signs: fever of unknown origin, gastrointestinal symptoms, arthralgias, lymphadenopathy, or night sweats. One neurological sign such as supranuclear vertical gaze palsy, rhythmic myoclonus, dementia, or hypothalamic manifestations should be present. Despite these reported guidelines, 20% of patients with CNS involvement have no systemic symptoms and 11% have only cognitive changes or altered levels of consciousness, for this reason, these patients will not be diagnosed (105).
Eyes.
Ocular symptoms of Whipple's disease are rare (about 5% of patients) and include uveitis, vitritis, retinitis, retrobulbar neuritis, papilledema (46), and direct involvement of the lens epithelium (204). The usual patient complaints are blurred or complete loss of vision (Table 2). In general, ocular manifestations occur in patients who also have gastrointestinal (42) or CNS involvement. Whipple's disease of the eye without or with only minimal CNS involvement is very rare in the absence of intestinal manifestations (8, 46, 136, 155, 204) and, consequently, is very difficult to recognize clinically.
Lymph nodes.
Peripheral lymphadenopathy is frequent (Table 2). The nodes are easily palpable and are clinically indistinguishable from lymphadenopathy due to other infectious diseases, sarcoidosis, or lymphomas (54, 145, 163). However, lymphomas associated with Whipple's disease were also reported (61, 73, 130). A few cases of mediastinal lymphadenopathy have been described (101, 108).
Hematologic manifestations.
Anemia is present in 90% of Whipple's disease patients and is caused by vitamin B12 malabsorption, intestinal blood loss, and iron deficiency. Hypoalbuminemia is frequent and is largely due to malabsorption (46). Thrombocytosis has been reported in some patients (137). In contrast, thrombocytopenia is very rare (129).
Treatment
Numerous antimicrobial drugs have been successfully used, including penicillin, penicillin combined with streptomycin, erythromycin, ampicillin, chloramphenicol, tetracycline, and trimethoprim-sulfamethoxazole (10, 46, 50, 59, 64, 75). Since Whipple's disease is an uncommon systemic illness, it is impossible to determine the optimal antibiotic regimens and the duration of treatment in controlled studies. Resistance of Whipple's disease bacilli to the reported antibiotics is also unknown. After initiation of antibiotic therapy, the clinical manifestations usually improve within a few weeks. At the same time, positive PCR results may become negative, suggesting an efficacy of the antibiotic treatment (22, 133, 148, 150). However, the regression of histopathological findings is slower, and PAS-positive structures may persist for years. This is probably due to degradation of the bacterial DNA prior to resolution of the rigid bacterial cell wall of gram-positive organisms (143, 196, 197, 198). The presence of PAS-positive macrophages alone without clinical deterioration during treatment may not be an indication for active disease or high risk of CNS disease relapse (197, 212). On the other hand, intestinal histological remission alone does not exclude the possibility that the patient will develop later cerebral manifestations (22, 197, 198) or disease affecting other organs. For this reason, cerebrospinal fluid examination (histology and/or PCR) during and after therapy should be considered (198). PCR may be useful to monitor the disappearance of “T. whippelii” DNA in a given tissue or to recognize relapses (150), but it is probably not sufficient to demonstrate complete eradication of the disease (22, 143, 150).
Treatment of Whipple's disease remains empirical. Several investigators recommend trimethoprim-sulfamethoxazole for at least 1 year or, alternatively, an initial parenteral therapy with penicillin and streptomycin for 2 weeks followed by trimethoprim-sulfamethoxazole (46, 59, 63, 64, 75, 95). This treatment has an excellent prognosis in the vast majority of cases, with a very small number of relapses compared to other therapies (Table 3) (50, 63, 75). Furthermore, trimethoprim-sulfamethoxazole, in contrast to tetracycline, crosses the blood-brain barrier, which is important since the majority of Whipple's patients may have CNS involvement. Short-term antibiotic therapy (a few weeks or months) may be sufficient (10, 64). The recommended long treatment is due to prudence rather than to clinical data and aims at avoiding CNS relapses, which have been described after clinically successful short-term treatment (22). Such relapses occur despite presumably sufficient antibiotic treatment (24% of patients [Table 3]) and are associated with recurrence of gastrointestinal symptoms and arthritis. The nonneurologic relapses seem to respond favorably to further treatment with the same antibiotics (67). CNS relapses, however, have a poor prognosis and are associated with a high mortality rate. For this reason, it is important to use drugs with good penetration into the brain right from the beginning. In patients with CNS manifestations who are allergic (168, 204) or do not respond (33, 105) to trimethoprim-sulfamethoxazole, the drugs of choice are chloramphenicol, cefixime, and/or ceftriaxone (2, 8, 33, 50, 105, 141, 149, 168, 198, 204). At the conclusion of antibiotic therapy, long-term follow-up including PCR and PAS staining of affected tissue might be useful to monitor a possible reappearance of the disease (121, 143, 198).
TABLE 3.
Treatment and relapses in patients with Whipple's disease
| Antibioticsb | No. of relapsesa
|
Total no. (%) | ||||
|---|---|---|---|---|---|---|
| Ref. 95 | Ref. 64 | Ref. 10 | Ref. 75 | Ref. 50 | ||
| TCN | 21/49 | 2/14 | 2/12 | 4/8 | 5/28 | 34/111 (30) |
| TCN + Other | 2/15 | 0/8 | 0/2 | 1/4 | 0/0 | 3/29 (10) |
| PCN | 3/8 | 0/2 | 0/0 | 0/0 | 0/0 | 3/10 (30) |
| PCN + STM | 2/5 | 0/1 | 0/0 | 0/0 | 0/0 | 2/6 (33) |
| TMP-SMX | 0/3 | 0/0 | 0/0 | 0/6 | 0/12 | 0/21 (0) |
| Other | 3/8 | 0/0 | 1/5 | 0/0 | 2/12 | 6/25 (24) |
| Total | 31/88 | 2/25 | 3/19 | 5/18 | 7/52 | 48/202 (24) |
Number of relapses per number of treated patients.
TCN, tetracycline; PCN, penicillin; STM, streptomycin; TMP-SMX, trimethoprim-sulfamethoxazole.
Gamma interferon plays an important role in controlling intracellular bacterial infections (72). This cytokine was used successfully in a patient with relapses despite presumably appropriate antibiotic therapy (167). The authors suggested that the use of antibiotics supplemented by IFN-γ might be more successful in avoiding relapses, but further studies are needed to prove the benefit of this therapy.
Immunology
Whipple's disease has fascinated many clinicians, immunologists, and microbiologists since the causative organism seems to affect only certain individuals. It is tempting to suppose that these individuals have some kind of immune defect, considering the following factors: (i) Whipple's disease is rare; (ii) Whipple's disease bacteria might occur in the environment, as suggested by the presence of “T. whippelii” DNA in sewage samples (112) and by the phylogenetic relatedness of “T. whippelii” to actinomycetes, which essentially are environmental organisms (110, 154, 207); and (iii) “T. whippelii” DNA has been found in gastric fluid and/or duodenal biopsy specimens in more than 10% of persons without clinical manifestations typical of Whipple's disease (56) and in the saliva of more than 30% of healthy individuals (181). However, the suspected immunological deficiency in Whipple's disease is probably subtle since these patients are generally not susceptible to infections with other pathogens. Only in a few cases of Whipple's disease were concomitant infections with other organisms reported (14, 46). Coinfection with “T. whippelii” and a related organisms that had previously been detected in a patient with Whipple's disease (82), was described in a patient with CNS involvement (135). This may or may not somehow be related to the immune status of the patients. Furthermore, the most striking immunological changes were detected prior to therapy and tended to disappear during and after therapy (44, 55, 167). Therefore, they must be considered a consequence of the disease rather than its cause.
Familial occurrence of Whipple's disease has been reported on a few occasions (46, 50), suggesting that the infection might be associated with immunogenetic factors. However, no linkage analysis has been undertaken to localize the putative gene(s) responsible for the immunodeficiency which may promote this rare disease. Several authors have suggested that the increased frequency of the HLA-B27 antigen among Whipple's disease patients (26%, compared to 8% in the general population of European and North American Caucasians) might represent a possible genetic factor predisposing to an immunological deficiency (25, 45, 63, 125). However, a direct association between HLA-B27 carriers and decreased resistance to infections has never been shown. Furthermore, a study of a larger number of affected individuals and control persons in a defined population has insinuated some doubts on a defect linked to a given HLA type (11).
Currently, there are no concise data available regarding putative antigens expressed by “T. whippelii” and corresponding antibodies in patients with Whipple's disease. Whipple's disease bacilli seem to express epitopes that are cross-reactive with those found in streptococcal groups B and G and in Shigella flexneri (46). A slight cross-reactivity of macrophages containing Whipple's disease bacilli with bacterial antisera against some Escherichia coli serovars and streptococcal groups C and D has also been described (46).
Probably due to the small number of patients with Whipple's disease and their disparate clinical manifestations, some authors have reported contradictory immunologic findings. In Whipple's disease patients, the lamina propria is often characterized by a massive macrophage infiltration. B-cell numbers are reduced, and T lymphocytes are missing, suggesting a deficient B- and/or T-cell response to the causative agents. A correct humoral response to Whipple's disease bacteria is currently not measurable. Some authors reported normal levels of immunoglobulin G (IgG) and IgM, while IgA concentrations seemed slightly increased before treatment and returned to normal after therapy (46, 108, 121, 180). Plasma cell numbers may be normal, decreased, or increased (46, 54, 55) illustrating how difficult it is to distinguish between changes caused directly by the pathogens and those possibly due to the severe clinical manifestations in untreated patients.
The circulating T-cell population is characterized by an increase in the number of CD8-positive cells, resulting in a reduced CD4/CD8 ratio accompanied by a shift in the T-cell subpopulations (75, 120). These changes are present in ill patients as well as in patients with residual PAS-positive cells but are not found in patients with complete remission (120). In addition, T cells of patients with Whipple's disease have a reduced ability to respond to mitogens like phytohemagglutinin and concanavalin A (46, 121).
Macrophages of Whipple's disease patients also show some dysfunctions: they have a decreased ability to degrade bacterial antigens (proteins and DNA derived from E. coli and Streptococcus pyogenes), although phagocytosis and intracellular killing do not seem to be impaired (17). The latter finding suggested that the presence of large amounts of Whipple antigens may be immunosuppressive itself (46). This seems less probable, however, since enrichment of bacterial antigens from organisms distinct from “T. whippelii” should also predispose to other infectious diseases.
Another dysfunction detected in macrophages is the reduced expression of CD11b markers, a component of complement receptor 3 (CR3). CR3 is a member of the integrin protein family, which facilitates microbial phagocytosis (120). In a single study, Marth et al. (119) were able to demonstrate that monocyte/macrophage production of interleukin-12 (IL-12) is reduced in Whipple's disease patients. They suggested that macrophages and not T cells are directly involved in the immune defect. IL-12 is a cytokine produced by granulocytes, monocytes, macrophages, and dendritic cells, which are the first cells to encounter a foreign antigen during infection. IL-12 then activates natural killer (NK) cells and T cells and induces the production of IFN-γ. IFN-γ and IL-12 drive the T-helper precursor cells, which differentiate into type 1 T helper cells. This cellular switch activates macrophages to eliminate cells infected with pathogens such as parasites, bacteria, and some viruses. The lower monocyte IL-12 production consequently leads to a decreased IFN-γ expression in T cells and subsequently to a decreased activation and function of macrophages. Defective IL-12 production also correlates with chronic or relapsing infections due to organisms of low-grade virulence such as Mycobacterium avium (69). If this observation is confirmed by other groups, it will be possible to develop a more efficient therapy based on IFN-γ to successfully treat Whipple's disease (120, 167). However, all the immunologic studies have not explained whether pathogenic properties of “T. whippelii,” subtle immunologic changes in patients with Whipple's disease, or both are responsible for the fact that clinically manifest disease develops only in a fraction of colonized individuals.
“TROPHERYMA WHIPPELII”
Morphologic Description
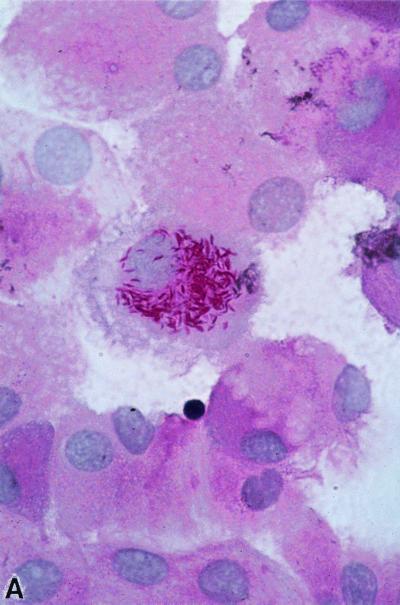
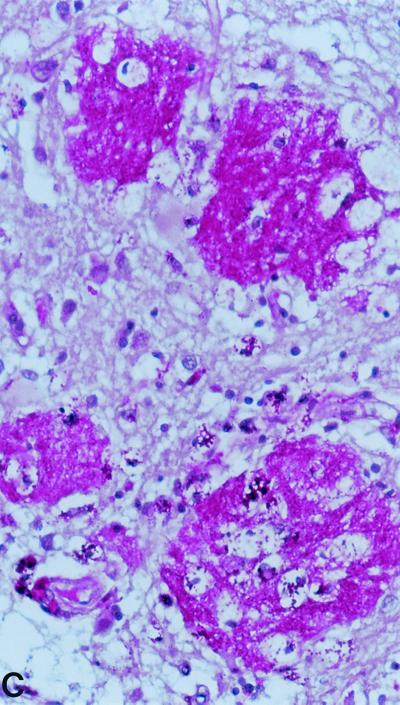
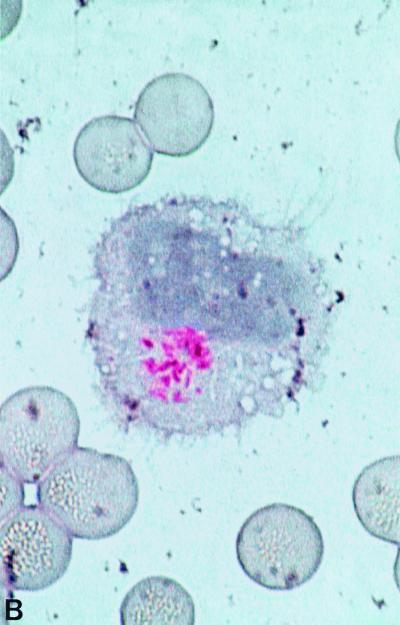
As early as 1907, Whipple described in his case report the presence of silver-stained (Levaditi method) organisms 2 μm long and with a rod-shaped morphology, which were most numerous in the vacuoles of macrophages (203). However, he did not consider a direct association between the organism and the disease. Later, Black-Schaffer (18) showed that macrophages from the intestine and mesenteric lymph nodes of four Whipple's disease patients stained deep scarlet with PAS (Fig. 1). He concluded that based on the staining properties of PAS, the macrophages were filled with an undefined glycoprotein. The PAS-positive particles phagocytosed by these macrophages had a configuration similar to sickled erythrocytes and for this reason were also named sickle-form particles. They can be found in several tissues (171). A possible bacterial etiology of Whipple's disease was proposed after the initial analysis of such tissue specimens by electron microscopy (28, 211). Cohen et al. (32) observed for the first time free dense bodies with a maximal diameter of 0.25 μm in the lamina propria. Some of these bodies appeared to be ingested by macrophages and were subsequently degraded. The PAS-positive material remaining in the foamy macrophages corresponds to the mucopolysaccharide-containing capsule of the bacteria (28). The dimensions and ultrastructural morphology of the extracellular bodies closely resemble those of microorganisms of a bacterial type and not virus-like particles (28, 211). The gram-positive staining and the staining with Giemsa supported the view that these structure are bacteria (28, 211). Thus, the original hypothesis that Whipple's disease might be due to an obscure metabolic fat disorder was discarded. Further descriptions of the bacilli confirmed the initial electron microscopy and histological observations, which had suggested that this particular organism is indeed the etiologic agent. Fluorescence in-situ hybridization might prove useful in demonstrating a direct link between the histologically characterized rod-shaped bacteria and the molecularly characterized organism “T. whippelii.”
FIG. 1.
PAS staining. (A) PAS-positive, diastase-resistant “T. whippelii” bacteria in IL-4-deactivated cultures of human peripheral blood mononuclear cells. (Copyright Gabriele Schoedon, Department of Internal Medicine, University Hospital of Zurich.) (B) Macrophages with PAS-positive inclusions in cerebrospinal fluid of a patient with proven neurologic Whipple's disease. (Copyright Gabriele Schoedon.) (C) Right basal ganglion biopsy specimens filled with numerous PAS-positive inclusions from a patient with clinically proven neurologic Whipple's disease. (Copyright Sebastian Brandner, Institute of Neuropathology, University Hospital of Zurich.)
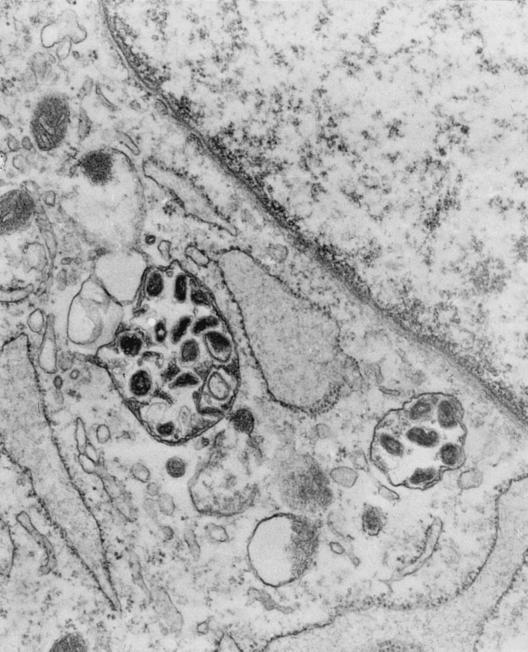
Whipple's disease bacilli show an unusual trilaminar cell wall ultrastructure by electron microscopy (Fig. 2), including an inner dense layer consisting of polysaccharides which are probably stained by the PAS reagents (173), surrounded by an electron-translucent layer covered by an electron-dense outer “membrane” similar to that observed for gram-negative bacteria but with a symmetric appearance (48, 173). Silva et al. (173) argued that the surface membrane of Whipple's disease bacilli might be of either bacterial (occasionally found on gram-positive bacteria) or host origin because the surface membrane of Whipple's disease bacilli and the macrophage plasma membrane are both symmetric, have similar thickness, and do not contain polysaccharides. Dobbins and Kawanishi (48) suggested that this surface membrane may be responsible for the infectious and immunological characteristics of Whipple's disease. Perpendicular cell division has been observed in extracellular bacteria, and growth seems to occur only rarely in macrophages (46). This observation suggests that Whipple's disease bacilli could be extracellular organisms with the ability to invade a large variety of cells while failing to cause injury or to induce an intense immune response (46). Whipple's disease bacteria are usually found as intact organisms extracellularly and at different stages of degeneration in macrophages (33, 68).
FIG. 2.
Electron micrograph of Whipple's disease bacilli cultured with HEL cells. (Copyright Didier Raoult, Unité des Rickettsies, CNRS: UPRESA 6020, Faculté de Médicine, Université de la Méditerranée, Marseille, France.)
The apparently unique ultrastructure of the bacilli associated with Whipple's disease seems to exclude structural similarity to other bacteria (46). This is supported by the fact that “T. whippelii” is not closely related to any other bacterium based on 16S rRNA gene sequences.
Culture
Many attempts to culture “T. whippelii” on artificial media or in cell lines or to transmit the pathogen to laboratory animals have been made without success. Despite numerous reports about successful cultivation of the organism on axenic media, all of them were probably contaminations with bacteria belonging to genera such as Corynebacterium, Streptococcus, Propionibacterium, and Haemophilus, which can easily be cultured (46).
A few years ago, “T. whippelii” was propagated in human blood-derived mononuclear phagocytes inoculated with heart valves from two patients with Whipple's endocarditis (169). IL-4 deactivation of these macrophages reduced the killing mechanism but not phagocytosis, thereby allowing intracellular survival and replication of the Whipple's disease bacteria. Demonstration of intracellular growth of the organism was based on the increased percentage of PAS-positive inclusions (Fig. 1A) and PCR amplification of “T. whippelii” DNA after a number of cell passages sufficient to eliminate DNA detection after a corresponding dilution of the inoculum. However, the authors were not able to establish stable subcultures.
A different approach to culturing “T. whippelii” was published very recently by Raoult et al. (152). They used a human fibroblast cell line (HEL) inoculated with a heart valve specimen of a patient with Whipple's endocarditis. The use of an appropriate cell line that can be kept for several weeks without passaging, the high ratio of bacteria to cultured cells, and the centrifugation procedure (shell vial procedure) with the intent of enhancing the adhesion of the bacteria to the HEL cells have probably facilitated the isolation of Whipple's disease bacilli. After 285 days, the authors obtained 120 heavily infected cell culture flasks, and they calculated that the doubling time of “T. whippelii” is about 18 days, which is even longer than the 12 days for Mycobacterium leprae in animal models. The authors claimed that they were successful in establishing stable subcultures. Confirmation that the passaged isolates indeed were “T. whippelii” was based on the presence of PAS-positive bacilli growing intacellularly in HEL cells. In addition, the amplified 16S rRNA gene was identical to the “T. whippelii” reference sequence, and Whipple's disease bacilli with the typical trilaminar cell wall were demonstrated by electron microscopy (Fig. 2). Furthermore, the bacteria present in infected HEL cells were successfully stained by indirect immunofluorescence with serum specimens from seven of nine Whipple patients and an IgM-specific conjugate. Such IgM antibodies were rare in controls without Whipple's disease. The establishment of stable cultures of “T. whippelii” is a major achievement and will facilitate further investigations to better understand the biology and pathogenicity of this organism, including the development of more sensitive and specific serologic assays and of monoclonal antibodies for immunohistochemical analysis, the establishment of genomic libraries, the identification of putative virulence factors, and the improvement of routine culture techniques. Considering the previous frustrating attempts to culture Whipple's disease bacteria, all these results await further confirmation by other groups.
Molecular Characterization
16S rRNA.
rRNA operons are usually transcribed into a pre-rRNA comprising (in the 5′→3′ direction) the 16S rRNA gene, a spacer sequence often containing one (sometimes more) tRNA gene, the 23S rRNA gene, and the 5S rRNA gene (80). This precursor molecule is then cleaved into the separate functional entities. Both the 16S and, to a lesser extent, the 23S rRNA and 5S rRNA gene sequences have served as molecular clocks to determine the evolutionary relationship among various groups of bacteria (208). Further, they have been used as one of the standard methods to classify bacteria, mainly at the level of families and genera but sometimes also at the species level, because 16S rRNA gene sequences are more readily accessible than DNA-DNA hybridization data. Nevertheless, DNA-DNA hybridization remains the current “gold standard” to delineate species (177). However, closely related species (e.g., Mycobacterium kansasii and M. gastri, M. malmoense and M. szulgai, or Aeromonas trota and A. caviae) may have identical or almost identical 16S rRNA genes (122, 160).
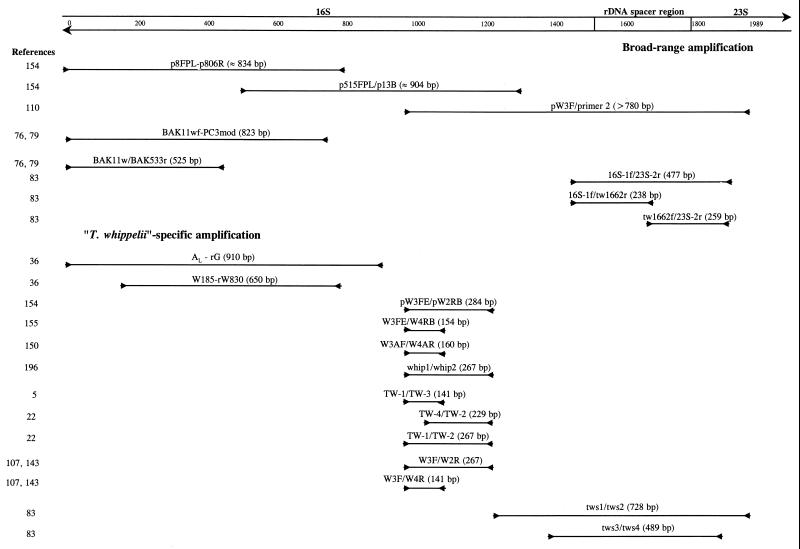
“T. whippelii” has been characterized at the molecular level mainly by PCR using universal bacterial primers for highly conserved regions of the 16S rRNA gene (Fig. 3). Using such an approach, Wilson et al. (207) amplified a fragment of about 700 bp from a small bowel biopsy specimen of a patient with Whipple's disease. Its sequence contained many ambiguous nucleotides but nevertheless seemed not closely related to any known bacterium. At about the same time, Relman et al. (154) used various broad-spectrum primers (p8FPL, p515FPL, p91E, p806R, and p13B) to amplify bacterial 16S rRNA gene sequences from tissue specimens of five independent patients with clinically and histopathologically proven Whipple's disease. Based on the resulting 1,321-bp sequence (i.e., a sequence comprising more than 80% of the entire 16S rRNA gene), a specific PCR-based assay was proposed for the first time as a powerful tool to facilitate the diagnosis of Whipple's disease. Relman et al. (154) proposed the new genus and species designation “Tropheryma whippelii.” This name was derived from the Greek words “trophe” meaning nourishment, “eryma” meaning barrier for the malabsorption syndrome, and “whippelii” to honor George H. Whipple. However, this species designation has not yet been validated by the International Committee on Systematic Bacteriology and thus should always be put between quotation marks. For every uncharacterized microorganism, it is important to establish its evolutionary relationship to other known organisms, thus providing relevant informations about to possible origin and biological behavior. Phylogenetic analysis of this sequence suggested that the organism associated with Whipple's disease can be classified as a member of the actinomycete line of descent (class Actinobacteria [177]). These are gram-positive bacteria with a high G+C content in their chromosome, which usually are isolated from environmental habitats, especially from soil. Some species have been found in freshwater and seawater sediments. Bacteria isolated from these natural environments are known to be difficult to culture on artificial media (200). Some actinomycetes such as coryneforms are part of the normal human skin flora, and species of Actinomyces live in subgingival crevices (70). Actinomycetes also include well-known human pathogens like Mycobacterium tuberculosis, M. leprae (which has never been cultured on artificial media), and Corynebacterium diphtheriae, as well as opportunistic pathogens, e.g., M. avium-intracellulare and Rhodococcus equi (81, 126).
FIG. 3.
Amplification systems used to analyze the 16S and the 16S-23S rDNA spacer region of “T. whippelii.” Expected product sizes are given. The position numbering is based on the reference sequence of Maiwald et al. (110).
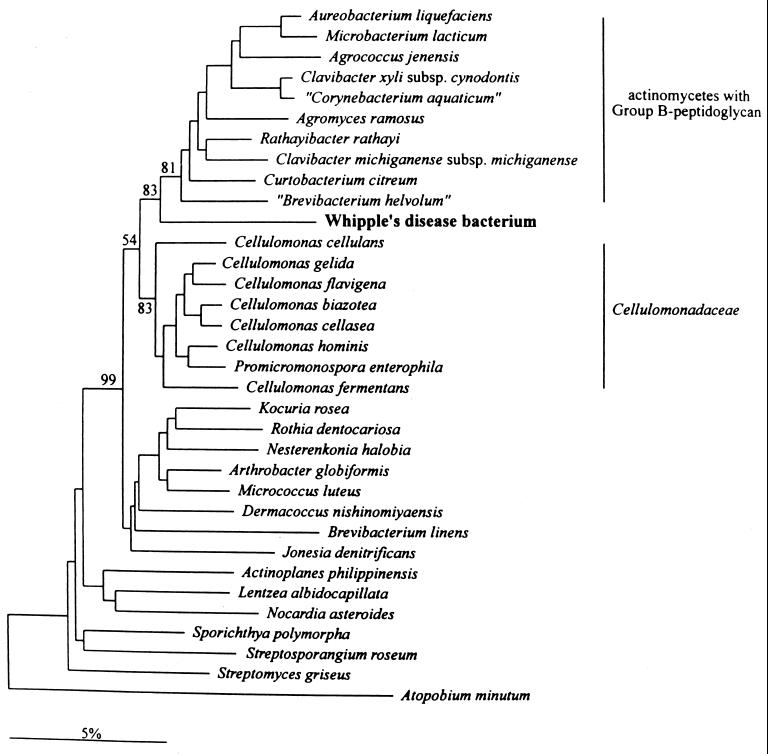
Recent reassessment based on the complete 16S rRNA gene sequence (110) led to “T. whippelii” being phylogenetically located between the Cellulomonadaceae and the actinomycetes with group B peptidoglycan. Most of these organisms are environmental bacteria (Fig. 4). The 16S rRNA sequence similarity of “T. whippelii” to the most closely related species (Cellulomonas cellasea and “Corynebacterium aquaticum”) is only in the range of about 90%. Phylogenetic analysis for 23S and 5S rDNAs placed “T. whippelii” within the Actinobacteria; however, due to the lack of a sufficient number of related sequences in both 23S and 5S rDNA, a more detailed association with 16S rDNA-related species was impossible (113). Variability of the 16S rRNA gene with a difference at a single position was shown for two patients (113).
FIG. 4.
Phylogenetic tree showing the relation of the Whipple's disease bacterium “T. whippelii” to other representatives of the actinomycetes. Reproduced from reference 110 with permission of the publisher.
A putative second causative agent of Whipple's disease, more closely related to Nocardia and other taxa, was suggested based on PCR results with a specimen from a single patient with clinically and histologically verified Whipple's disease (82). Significant sequence differences were found in a short amplicon stretch (19 substitutions over 225 nucleotides) using the 16S rRNA “T. whippelii“-specific primers pW3FE and pW2RB (Fig. 3) (154). A single case of coinfection with “T. whippelii” and the related Whipple's disease-associated bacterial organism has also been reported (135). These results are difficult to interpret since they are based on a very small part of the 16S rRNA only. Intraspecies variation of “T. whippelii” 16S rRNA genes cannot definitely be excluded, and this issue certainly needs further confirmation in additional patients.
Internal transcribed spacer.
The internal transcribed spacer (or the 16S-23S rDNA spacer region) located between the genes coding for the 16S and 23S rRNAs is known to be more variable than the flanking structural genes and was proposed as a promising tool for subtyping strains in various taxonomic groups (80). The sequences of the 16S-23S rDNA spacer region as well as 200 nucleotides of the 23S rRNA were also determined using a “T. whippelii”-specific primer (targeting the 3′ end of the 16S rDNA) in combination with a universal bacterial primer (targeting the 5′ end of the 23S rDNA) (Fig. 3) (110). The reported length of the “T. whippelii” internal transcribed spacer was 294 bp without internal tRNA or 5S rRNA genes. This is comparable to the size and structure described for the majority of gram-positive bacteria with high G+C content (80). As expected, searches for sequence similarities to the spacers of other actinomycetes revealed low homology, but several short stretches with high similarities to other actinomycetes were found (110).
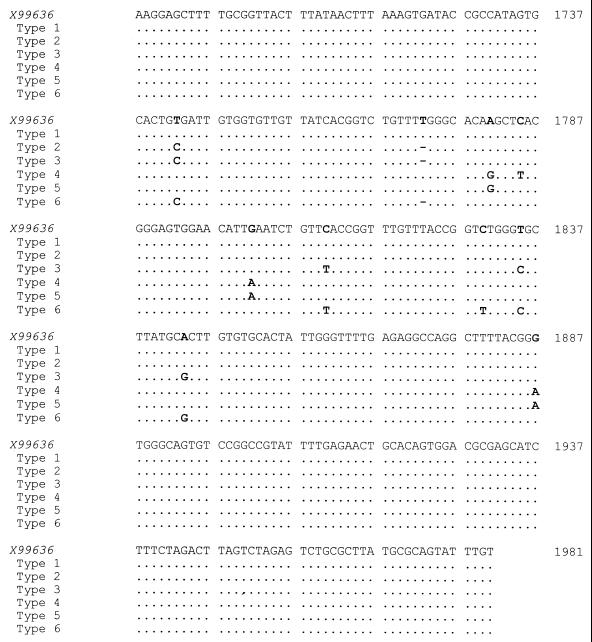
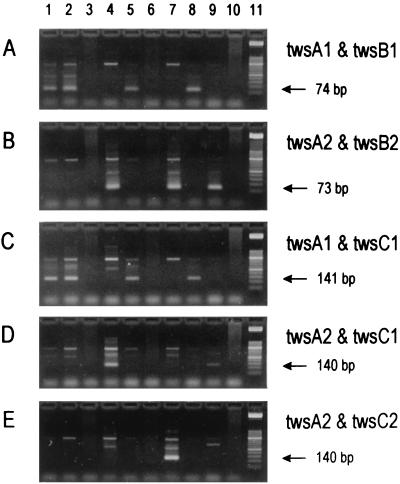
The internal transcribed spacer sequence described by Maiwald et al. (110) was confirmed by the detection of identical sequences in clinical specimens from nine Swiss patients with Whipple's disease, using both bacterial universal primers recognizing the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene and “T. whippelii”-specific primers (Fig. 3) (83). However, sequence differences at the 3′ and 5′ ends of the 16S and 23S rDNAs, respectively, were noticed. It was possible to demonstrate that these differences were due to errors contained in the reference entry and not to the existence of “T. whippelii” subtypes (83). Later, the spacer region was analyzed in specimens from 28 additional patients known to harbor “T. whippelii” as shown by species-specific PCR targeting the 16S rRNA gene. Sequence analysis of the amplicons revealed the existence of five dimorphic sites constituting three different spacer types (Fig. 5) (86). The most frequent type detected, i.e., spacer type 1, perfectly matched the original “T. whippelii” spacer sequence (294 bp) (110). Compared to this reference sequence, spacer types 2 and 3 differed solely at two and five nucleotide positions, respectively. These slight DNA alterations could be confirmed by single-strand conformation polymorphism (SSCP) analysis and by type-specific PCR assays. With SSCP analysis, three distinct SSCP profiles, each corresponding to one of the spacer types, were obtained. Type-specific PCR allowed us to selectively amplify the three different spacer types (Fig. 6). While the type-specific PCR was very helpful in detecting the then known three types, SSCP and sequence analysis have the potential of recognizing additional types not related to the reported five dimorphic sites.
FIG. 5.
Nucleotide sequence of the “T. whippelii” 16S-23S rDNA internal transcribed spacer. The base numbering is that of the reference sequence (X99636) of Maiwald et al. (110); dots and hyphens symbolize identity and alignment gaps, respectively.
FIG. 6.
Representative results of nested PCR assays for direct detection of “T. whippelii” 16S-23S rDNA spacer types in clinical specimens on ethidium bromide-stained agarose gels. PCR products derived from amplification using primer pair tws3 and tws4 (83) were reamplified with various type-specific primer combinations: twsA1 and twsB1 (for spacer type 1) (A), twsA2 and twsB2 (for types 2 and 3) (B), twsA1 and twsC1 (for type 1) (C), twsA2 and twsC1 (for type 2) (D), and twsA2 and twsC2 (for type 3) (E). The expected products are indicated by arrows. Lanes 1 to 10 show clinical specimens positive for “T. whippelii” spacer type 1 (lanes 1, 2, 5, and 8), spacer type 2 (lanes 4 and 9), and spacer type 3 (lane 7) and negative controls (lanes 3, 6, and 10). Lane 11 shows molecular mass markers (50-bp ladder; Boehringer, Mannheim). Reproduced from reference 86 with permission of the publisher.
Very recently, five distinct 16S-23S rDNA spacer types were observed among 43 patients with Whipple's disease (113). Two types corresponded to types 1 and 2 of Hinrikson et al. (86), whereas three types (now referred to as types 4 to 6) had not been described previously. Type 3 of Hinrikson et al. was not found in this study. Compared to type 1, spacer types 4, 5, and 6 differed at four, three, and six positions, respectively (Fig. 5). The existence of the distinct spacer types was confirmed by sequencing and restriction enzyme analysis (113). The six spacer types identified so far do not occur with equal frequencies (Table 4). Types 1 and 2 are predominant, whereas types 3 to 6 are found only occasionally. The relative frequencies of types 1 and 2 differ between the studies of Hinrikson et al. (84, 85) and Maiwald (113). It is unlikely that these differences can be attributed to different geographical origins since in all studies the vast majority of individuals included lived in central Europe. Another explanation might be that the larger Swiss study (85) included not only patients with Whipple's disease but also PCR-positive individuals not having symptoms characteristic of this disease.
TABLE 4.
Frequencies of internal transcribed spacer types found in PCR-positive individuals
| Study | No. of internal transcribed spacer type:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Hinrikson et al. (83) | 9 | |||||
| Hinrikson et al. (86) | 15 | 10 | 3 | |||
| Maiwald et al. (113) | 14 | 26 | 1 | 1 | 1 | |
| Total | 38 (48%) | 36 (45%) | 3 (4%) | 1 (1%) | 1 (1%) | 1 (1%) |
Identical spacer types were found in all independently processed samples from each of the patients with multiple positive specimens (51, 86, 113), with the exception of a single patient with a possible double infection (113). These findings are compatible with the assumption that each patient is infected with only a single strain and also points in the direction of the presence of one single rRNA operon per strain as described for other actinobacteria (80). Some single-base mutations in the rDNA spacer may lead to an inappropriately folded rRNA molecule and should be compensated by further sequence variation to obtain a functional rRNA. Spacer types found in “T. whippelii” indeed seem to have evolved at least in part by pairwise DNA alterations (positions 1551 and 1578 and positions 1643 and 1652).
The variations found in the internal transcribed spacer region raise the question whether they represent six different, closely related species or subtypes of the single species “T. whippelii.” Partial 16S sequences determined for most specimens included in above studies did not reveal any differences from the reference sequence. However, as with other organisms, even complete identity of 16S rDNA sequences does not prove species identity (65) but only suggests a very close relationship (176). To solve the problem, DNA-DNA hybridization studies are definitely needed; however, they are not feasible because “T. whippelii” has not yet been cultured on artificial media. As long as hybridization data are not available, we suggest that the six types found be regarded as subtypes of the single species “T. whippelii” for practical reasons and because of the small number of variable nucleotides, which is similar to or even smaller than the variations found within other species (80). The homogeneity found at the molecular level for the hypervariable Actinobacteria-specific insertion in domain III of the 23S rRNA (see below) supports the concept that the six different 16S-23S rDNA spacers represent subtypes of the single species “T. whippelii” rather than closely related but different species (84, 113).
Insertion in domain III of the 23S rRNA gene.
In actinobacteria, an insertion in domain III of the 23S rDNA of about 100 nucleotides (range, 86 to 116 nucleotides) has been described which does not seem to be present in any other bacterial group (159). This insertion is more variable between species than are the remaining parts of the 23S and the 16S rDNA, but only little variability has been found within given species. We have amplified a part of domain III of “T. whippelii” using broad-range primers targeting the flanking regions of the insertion from nine clinical specimens (85). Sequence analysis revealed the presence of an insertion of about 80 nucleotides (84, 113) and thus confirmed the classification of “T. whippelii” as an actinobacterium. Of 28 patients, 27 harbored identical insertion sequences while the remaining patient had an insertion sequence that differed in a single position most probably located in a loop structure (85). Sequence similarity to other actinobacteria was high (>90%) for the region immediately upstream but only moderate (around 70%) for the region downstream of the insertion (84). Compared to other actinobacteria, which are 42 to 81% related, the insertion sequence itself of “T. whippelii” is smaller (80 versus 86 to 116 nucleotides) and its similarity to those sequences is negligible (84, 113, 159). In addition, it does not contain any of the group-specific sequence elements (159) and, more specifically, there was no significant similarity to the insertion sequences found for the Cellulomonadaceae and the actinomycetes with group B peptidoglycan, i.e., the groups most closely related to “T. whippelii” based on 16S rDNA comparisons (110). The sequence diversity in domain III of the 23S rDNA between various bacteria was also helpful in developing species-specific PCR primers (reference 84 and see below).
Heat shock protein 65 gene (hsp65).
Until very recently, all sequence information available for “T. whippelii” was related to the rRNA operon that now has been entirely sequenced (113). To have a target for a PCR assay completely independent of this operon and in view of a possible recombinant antigen for serodiagnostic purposes, we amplified, cloned, and sequenced a 620-bp fragment of hsp65 from the heart valve of a patient with Whipple's disease endocarditis (132). Heat shock proteins, similar to rRNAs, are mosaic molecules with some relatively constant regions and some variable regions, and thus their genes can often be amplified using broad-range primers directed against their constant portions. With the derived specific primers, a 357-bp fragment was amplified from all 17 clinical specimens previously shown to contain “T. whippelii” DNA by PCR targeting the 16S rDNA and/or the 16S-23S rDNA spacer region but from none of 33 control specimens. Variability within the specific fragment was assessed by sequencing the amplicons from eight positive specimens. Nucleotide substitutions were found at six different locations, but none of these changes affected the amino acid sequence. The variability detected at the DNA level calls for a very careful selection of primers for other specific PCR assays based on hsp65, whereas the apparent homogeneity at the amino acid level supports the idea that HSP65 might provide a useful antigen as previously described for other pathogens (213).
Epidemiology
It has been mentioned above that the worldwide annual incidence of 12 new cases of Whipple's disease as calculated by Dobbins (46) more than 10 years ago may be an underestimation for several reasons, the most important being that PCR is more sensitive than histology or electron microscopy in detecting “T. whippelii” in affected tissues (Table 5). However, the repeated finding of “T. whippelii” DNA in gastrointestinal specimens from two female patients clinically not considered to have Whipple's disease raised some doubts about the clinical significance of positive PCR results from gastrointestinal specimens as well as about the specificity of PCR tests directed against “T. whippelii.” Therefore, we decided to perform a prospective study on the prevalence of positive PCR in duodenal biopsy specimens and gastric fluid of patients without clinical evidence of Whipple's disease (no diarrhea, fever, arthritis, or weight loss) and to compare the results to those obtained by classical histopathology (56). A total of 105 patients (60 males and 45 females) referred for elective gastroscopy were investigated. Histology of duodenal biopsy specimens was not suggestive of Whipple's disease in any of them. Of two biopsy specimens taken for PCR, one was stored at −20°C while the other one was analyzed by “T. whippelii”-specific PCR. PCR was positive in duodenal biopsy specimens from 5 patients (4.8%) and in the gastric fluid from 12 patients (11.4%). For three of these patients, “T. whippelii” PCR was positive in both specimens. The possibility of carryover contamination in the laboratory (97) leading to false-positive PCR results could virtually be excluded by analyzing the second duodenal biopsy specimens from all 14 PCR-positive (gastric fluid and/or biopsy specimens) as well as 24 PCR-negative patients (15, 56). In all 5 patients with initially positive biopsy specimens, PCR was again positive, while the second biopsy specimens of all 9 patients with positive PCR from gastric fluid but negative PCR in duodenal biopsy remained negative, as did those from the 24 patients previously negative in both specimens. In addition, to confirm that the amplified fragments were indeed derived from “T. whippelii,” at least one TW-4/TW-2 fragment per PCR-positive patient was sequenced. All fragments were identical to the published “T. whippelii” reference sequences except for a C missing in a GC-rich region, which most probably reflects a sequencing problem. In contrast to the results obtained with Swiss patients, PCR remained negative with all DNA extracts from duodenal biopsy specimens from 108 Asian patients (Dutly, Pang, et al., unpublished). This study probably reflects the rarity of Whipple's disease in non-Caucasians and, in addition, supports the view that the results obtained with the Swiss patients were not due to laboratory contamination.
TABLE 5.
Comparision of histopathology (PAS-positive inclusions in macrophages) and PCRa for patients with suspected or proven Whipple's disease
| Clinical manifestations | Type of specimen | Result by
|
Reference(s) | |
|---|---|---|---|---|
| PAS staining | PCR amplification | |||
| Spondylodiscitis, occasional fever, no diarrhea | Terminal ileum | + | + | 5 |
| Duodenum | − | + | ||
| Colon | − | + | ||
| Lumbar spine | − | + | ||
| Blurred vision, parkinsonian syndrome | Small bowel | − | + | 8 |
| Neck pain and limb paraesthesia | Cord biopsy | + | NDb | 30 |
| Jejunal biopsy | − | + | ||
| Oculomotoric disorders, nystagmus, fever, weight loss | Cerebrospinal fluid | − | + | 31, 36 |
| Suspected neurological Whipple's disease | Intestine | + | − | 36 |
| Weight loss, abdominal lymphadenopathy, fever, arthritis | Duodenum | −c | ND | 67 |
| Joint fluid | ND | + | ||
| Endocarditis | Aortic valve | + | + | 79 |
| Duodenum | − | + | ||
| Relapsing oligoarthritis | Duodenum | − | + | 83 |
| Arthralgias, chronic colitis | Duodenum | − | + | 83 |
| Ileum | + | ND | ||
| Arthritis | Joint fluid | − | + | 83 |
| Gaze, cognitive changes, depression | Duodenum | − | + | 107 |
| Hepatosplenomegaly, episodic fever, granulomatous lymphadenopathy, no gastroinestinal symptoms | Lymph nodes | + | ND | 129 |
| Duodenum | − | + | ||
| Spleen | + | + | ||
| Blood | ND | + | ||
| Arthropathy, episodic fever, hepatosplenomegaly, granulomatous lymphadenopathy, episodes of intestinal obstruction | Synovium | − | ND | 129 |
| Duodenum | − | + | ||
| Blood | ND | + | ||
| Fever, night sweats, para-aortic lymphadenopathy, no gastrointestinal symptoms | Lymph nodes | + | − | 129 |
| Duodenum | − | + | ||
| Blood | ND | + | ||
| Spastic tetraparesis, no gastrointestinal symptoms | Spinal cord | + | ND | 129 |
| Duodenum | − | + | ||
| Anorexia, arthritis, night sweats, lymphadenopathy | Duodenum | + | + | 133 |
| Antrum | − | + | ||
| Blood | ND | + | ||
| Fever, neurological symptoms, hyperpigmentation | Mesenteric lymph node | + | ND | 133 |
| Duodenum | − | + | ||
| Weight loss, diarrhea, cachexia | Duodenum | + | + | 133 |
| Antrum | + | + | ||
| Stomach | − | + | ||
| Blood | ND | + | ||
| Weight loss, diarrhea, arthralgias, increased skin pigmentation | Duodenum | + | + | 143 |
| Stomach | − | + | ||
| Liver | − | + | ||
| Osteoporosis, anemia, poor appetite, occasional diarrhea, steatorrhea (diagnosed as sprue) | Small bowel | ?d | + | 150 |
| Prolonged episodic diarrhea (diagnosed as sprue) | Small bowel | − | + | 150 |
| Fever, enlarged paraortic lymph nodes | Small bowel | − | + | 150 |
| Lymph nodes | ? | + | ||
| Diarrhea, weight loss | Small bowel | − | + | 150 |
| Bilateral uveitis, arthritis, weight loss | Vitreous fluid | + | + | 155 |
| Duodenum | − | + | ||
Different PCR methods were used.
ND, not done.
Positive by electron microscopy.
?, suspicious histologic findings.
The presence of “T. whippelii” DNA in a considerable fraction of patients without clinical evidence of Whipple's disease was recently confirmed by Street et al. (181), who analyzed the saliva of healthy people by PCR. Of 40 samples, 14 (35%) were positive. Additional samples from six initially PCR-positive patients were positive on many occasions. Six samples were sequenced and shown to be identical to the 16S rDNA reference sequence. Similarly, we have analyzed saliva specimens from the 14 non-Whipple's disease patients with positive PCR results from gastric fluid and/or from duodenal biopsy specimens mentioned above 3 to 6 months after inclusion in this study (51). Of the 14, 6 became PCR positive and in 4 of them the same internal transcribed spacer type was determined as for the previous specimen(s). For the remaining two, the internal transcribed spacer type could be determined for one specimen only. The epidemiological significance of the above findings is unclear because it is not known whether the presence of DNA also reflects the presence of viable organisms. Since Whipple's disease is very rare, it is rather unlikely that a “T. whippelii”-positive PCR indicates an early stage of the disease not yet accompanied by characteristic clinical signs. Consequently, PCR-positive individuals could be true carriers of “T. whippelii.” In turn, this raises the question whether there are nonpathogenic strains of “T. whippelii” lacking important, perhaps plasmid-mediated virulence factors or whether as yet unknown host factors contribute to the development of clinical disease (119, 120). The involvement of host factors might also provide a basis for speculations about the reasons for the significantly different prevalence of Whipple's disease in males and females as well as in the various ethnic groups. Currently we are investigating whether the various clinical manifestations of this disease and/or the geographic origin of the patients correlate with one or the other of the molecular types of “T. whippelii.”
Humans were the only known source of Whipple's disease bacilli until Maiwald et al. (112) demonstrated the presence of “T. whippelii” DNA in 25 of 38 sewage samples by PCR. This finding suggests a possible environmental reservoir of the pathogen but might simply reflect excretion of the organisms as indicated by the presence of “T. whippelii” DNA in the feces of patients with or without Whipple's disease (78, 109). Thus, the habitat, natural growth conditions, and route(s) of infection of “T. whippelii” remain obscure.
Laboratory Diagnosis
Whipple's disease should be suspected in patients with diarrhea, weight loss, arthritis, lymphadenopathy, neurologic disorder, and fever (46, 59). However, these symptoms are nonspecific and may be associated with other diseases as well. In addition, radiological examinations of joints do not reveal characteristic lesions and endoscopy may show anything from normal duodenal mucosa to edematous folds, yellowish merging plaques, and hemorrhage (46, 50, 59, 165, 197). Computed tomography may show thickening of the small bowel folds and large bulky nodes in the mesentery and retroperitoneum (89). In the brain, computed tomography or magnetic resonance imaging may be normal or reveal cerebral atrophy, hydrocephalus, or focal lesions (22, 24, 36, 37, 49, 50, 105, 117, 130, 142, 187, 210). Thus, in the absence of clinical or radiological criteria to establish the diagnosis of Whipple's disease, laboratory methods play a crucial role. These include histology, electron microscopy, and PCR usually on tissue biopsy specimens, joints, and cerebrospinal fluid. Preliminary reports on the detection of “T. whippelii” DNA in stool specimens (78, 109) and on the detection of antibodies (152) have raised hopes for routinely available tests not requiring invasive procedures.
Histology.
For decades, the confirmation of Whipple's disease was based on the demonstration of diastase-resistant, non-acid-fast, PAS-positive inclusions in macrophages detected mainly in the lamina propria of duodenal biopsy specimens (18). However, PAS-positive macrophages may be found in all affected organs, including heart, lungs, CNS (Fig. 1B and C), eyes, liver, spleen, joints, and bone marrow, underlining the systemic nature of the disease (64, 92). Furthermore, they are commonly present in mesenteric lymph nodes, but infiltration of periaortic, inguinal, cervical, and axillary nodes may also occur (46). Due to the presumably patchy distribution of the Whipple's disease bacilli, multiple intestinal specimens may be required to confirm the diagnosis. Gastric or rectal biopsy specimens are not adequate to diagnose Whipple's disease, since faintly PAS-positive lipid-containing macrophages in the stomach and strongly PAS-positive macrophages in the rectum have been observed in patients with other diseases as well. Also, in duodenal biopsy specimens, PAS-positive macrophages can be seen in association with other infectious agents (e.g., M. avium-intracellulare), rendering the diagnosis more difficult (74, 147, 158, 161, 192, 195). To distinguish Whipple's disease from infections due to M. avium and M. intracellulare in AIDS patients, an acid-fast stain of the biopsy specimen is required. Whipple's disease bacilli are not acid fast (47). In addition, synovial tissue and synovial fluid mononuclear cells may contain nonspecific PAS-positive material. On the other hand, granulomas related to Whipple's disease may be PAS negative. Therefore, electron microscopy or PCR is required to confirm the diagnosis (138, 202).
Recently, Bodaghi et al. (19) reported the case of a patient suffering from uveitis and systemic inflammatory manifestations. Whipple's disease was suspected because of the presence of PAS-positive inclusions in macrophages. However, “T. whippelii”-specific PCR remained negative while partial-sequence analysis of a 16S rRNA gene fragment revealed an infection by an Arthrobacter sp. Another case of suspected Whipple's disease with the diagnosis based on the presence of PAS-positive inclusions in the brain was reevaluated when the “T. whippelii”-specific PCR proved to be negative and trimethoprim-sulfamethoxazole therapy failed (185). Later, histiocytosis was diagnosed. These findings suggest that the diagnosis of Whipple's disease should not be made exclusively on the basis of PAS-positive macrophage infiltrates in biopsy specimens nor should it be excluded if histopathology is negative.
Electron microscopy.
In 1961, the PAS-positive inclusions in macrophages associated with Whipple's disease were confirmed by electron microscopy to represent bacteria (28, 211). These bacteria exhibited an unusual trilamellar membrane (Fig. 2) (173) and a cell wall structure similar to that of gram-positive bacteria. Some authors have reported similarities between Whipple's disease bacilli and the mycolic acid-containing mycobaceria (28, 46), which have a cell wall consisting of an inner layer of moderate electron density, an electron-transparent layer, and an outer electron-opaque layer of variable appearance and thickness (23). However, electron microscopy is time-consuming and not readily available in most laboratories. In addition, no data regarding its sensitivity compared to histopathology (and PCR) are available. It is therefore not considered an established tool for diagnosing Whipple's disease but may be a useful adjunct in doubtful cases.
PCR.
An initial characterization of “T. whippelii” was achieved by using broad-spectrum PCR and sequencing of the 16S rRNA gene (154, 207). Similar methods with or without cloning of the amplified fragments are now widely applied not only as research tools but also as routine diagnostic tools (41, 76, 87, 90, 99, 153, 178, 206). This approach resulted in the unexpected detection of “T. whippelii” DNA in removed heart valves of patients with endocarditis (76, 79) or in the lumbar biopsy of a young female patient with spondylodiscitis (5). Broad-spectrum PCR remains an important tool for the analysis of culture-negative specimens from patients with a strong suspicion of bacterial infection and especially for “T. whippelii” because clinicians often do not consider Whipple's disease in their differential diagnosis.
From the 16S rRNA gene sequence, species-specific primers were selected for the detection of “T. whippelii” in clinical specimens by PCR (154). Using primers pW3FE and pW2RB (Fig. 3), Relman et al. (154) were able to amplify a 284-bp product from duodenal biopsy specimens from five patients with Whipple's disease. The complete identity of amplicons with the “T. whippelii” 16S rRNA gene was confirmed by sequencing. The same primers, in conjunction with a specific hybridization probe, were used in another study to confirm the specificity of PCR without any sequence analysis (197). These primers are more sensitive than broad-spectrum primers; however, they cannot be considered absolutely specific since they were also used with two patients to characterize another bacterial organism associated with Whipple's disease which differed from “T. whippelii” in 19 of 225 nucleotides (82, 135).
Very little is known about the analytical sensitivity and specificity of the established PCR assays, except that PCR and hybridization (with primers whip1 and whip2 and the probe whip3) tested on a serial dilution of cloned Whipple's disease bacillus DNA were able to detect as few as 10 copies of the Whipple-specific target (197). However, Lynch et al. (107) were able to detect one to three copies of the 16S “T. whippelii” DNA using the primer pair W3F-W4R. Thus, depending on the primers used and the number of cycles, the PCR sensitivity may show variations (107, 197, 201). Furthermore, amplification of small 16S rRNA fragments seems to be more sensitive than amplification of large fragments (5, 107, 150, 155). It is important to notice that in addition to the length of the amplicons, fresh clinical specimens give better results than formalin-fixed ones with partially degraded DNA (150). Recent data show that PCR from gastrointestinal and other biopsy specimens is more sensitive than histopathologic evaluation (Table 5). Higher PCR sensitivity has also been reported for cerebrospinal fluid samples (198).
The value of PCR to monitor response to therapy is controversial (22, 133, 143, 150, 196). If PCR becomes negative within a few weeks after initiation of antibiotic therapy, this may suggest efficacy of the treatment but it certainly does not imply eradication of the organism from the whole body.
An estimation of the true prevalence of Whipple's disease is not possible at present. However, the use of “T. whippelii”-specific PCR assays has simplified the laboratory diagnosis, especially for patients with CNS manifestation. PAS staining may require more invasive procedures such as cerebral biopsies to confirm the diagnosis, while PCR can be performed using cerebrospinal fluid (22, 31, 198). Detection of “T. whippelii” by PCR is routinely done by using DNA extracts from tissues or fluids of affected sites (gastrointestinal tract, joint, cardiovascular system, and CNS). The suggestion that PCR-based diagnosis of Whipple's disease may be possibly exclusively by using DNA extracted from blood (106, 107, 134) has been questioned (118, 133). For example, Marth et al. (118) did not find any evidence for “T. whippelii” DNA in peripheral blood mononuclear cells in four patients with active Whipple's disease (confirmed by PAS-positive intestinal biopsy specimens) or in four patients with treated inactive disease (PAS-negative biopsy specimens) and five controls.
Distinct oligonucleotides recognizing different parts of the 16S rRNA gene have been proposed to improve and speed the identification of “T. whippelii” in clinical samples (Fig. 3) (22, 36, 107, 150, 155). Nested primers targeting the 16S-23S spacer region (83) or domain III of the 23S rDNA (84) may also be used as diagnostic tools. The “T. whippelii” specific primers targeting domain III of the 23S rDNA are about as sensitive as but more specific than the 16S rDNA primers. In contrast, the 16S-23S spacer region-specific primers have a lower sensitivity (84). However, the specificities of all the newly proposed primer combinations have not yet been fully evaluated. On the other hand, the number of patients with Whipple's disease not corresponding to the classic description of the infection is increasing (5, 22, 30, 79, 107, 117, 129, 144, 150), as is the fraction of patients with negative results in histopathology (Table 5). Furthermore, the recent study done by Ehrbar et al. about the presence of “T. whippelii” DNA in people without Whipple's disease (56) raises some concerns about the diagnostic value of “T. whippelii” PCR alone in clinical practice. These findings certainly need further confirmation behind the fact that “T. whippelii” DNA has also been found in saliva of healthy persons in England (181). At present, it cannot be excluded that these persons represent early stages of the disease, although this seems rather unlikely because of the rarity of Whipple's disease.
Serology.
The recent achievement of stable cultures of “T. whippelii” allowed indirect immunofluorescence assays to be performed on serum samples from patients with Whipple's disease and control subjects (152). Astonishingly, IgG antibodies at titers of ≥1:100 were found almost as frequently in patients as in controls. It might be speculated that the presence of IgG in a majority of individuals analyzed independent of the presence or absence of Whipple's disease reflects the widespread occurrence of the organisms and the putative carrier state (51, 56, 181). Antibodies of the IgM type seemed to be more specific. Titers of ≥1:50 were present in 5 of 7 patients with classic Whipple's disease, 2 of 2 with Whipple's endocarditis, 0 of 10 with endocarditis due to other causes, 2 of 9 with autoimmune disease, and 1 of 20 healthy blood donors. These results are promising, especially since patients with autoimmune disease frequently have false-positive serologic reactions. Nevertheless, the above findings need to be confirmed by other groups with larger series of well-defined patients and with alternative antigens. The cloning of a part of the hsp65 gene promises to be a step in the direction of producing a recombinant antigen for serodiagnostic purposes (132).
OUTLOOK
The identification of agents causing bacterial infections is still largely based on culture methods and serology. Neither is available for the diagnosis of Whipple's disease since “T. whippelii” resists cultivation by standard microbiological methods and thus no antigen for serodiagnostic purposes is readily available. Laboratory diagnosis is obscured by the partially insufficient sensitivity and/or specificity of both microscopic and molecular tests. In addition, clinical manifestations are nonspecific and may also be observed in conjunction with other pathogens. Atypical presentations should not be underestimated. Thus, the diagnosis of Whipple's disease relies on a combination of clinical and laboratory data. The availability of reliable cultures, antigens for serodiagnostic purposes, and molecular tests not requiring invasive procedures (e.g., stool specimens rather than duodenal biopsy specimens) would be most welcome. Activities in all these areas are under way in various laboratories.
Several genome-based typing methods have been described for other microbial pathogens and have allowed us to identify the geographical distribution, habitat, strain differences, and routes of infection (139, 186, 188). The efficiency of epidemiological studies for “T. whippelii,” however, is restricted by our inability to reliably culture the organism and by the few DNA fragments known. Thus, only a very minor fraction of the genome is accessible for analysis. This compromises the detection of heterogeneity significantly compared to using the whole genome. The presence of at least six different 16S-23S rDNA spacer sequences for “T. whippelii” is a first step and certainly stimulates the search for new types and investigations to determine their geographical distribution and their association with certain clinical manifestations or particular hosts.
A possible environmental habitat of Whipple's disease bacteria is indicated by their phylogenetic relationship to organisms found in soil and water sediments. Furthermore, the high proportion of farmers among Whipple's disease patients (46) suggests exposure to soil as at least one possible route of infection. Isolation of M. avium (causing a Whipple's disease-like syndrome in AIDS patients) from soil, water, and amoebae (179) should encourage analysis of environmental samples for the presence of “T. whippelii.” Methodological improvement seems necessary in this area since PCR from soil specimens is often inhibited by substances like humic acid that usually are copurified with DNA (205).
The sequence information available for “T. whippelii” is limited to the rRNA operon. Optimization of the recently established culture of “T. whippelii” within a human fibroblast cell line (152) may offer new ways to enrich bacterial biomass for further molecular characterization. Additional information on the “T. whippelii” genome is very helpful in attempts to develop alternative confirmatory PCR assays and to characterize genes possibly involved in the infectious disease process. To estimate strain variability and to gain further phylogenetic information on “T. whippelii,” it is essential to characterize other target genes such as the elongation factor G, the proton-translocating ATPases, or the heat shock proteins by using broad-range amplifications similar to those widely used for the 16S rDNA. Further characterization of the “T. whippelii” genome might also be achieved by representational difference analysis (66, 104). Furthermore, newly characterized genes coding for putative antigens could be overexpressed in vitro and screened with human sera with the aim of detecting specific antibodies in patients with Whipple's disease. This might eventually result in a serodiagnostic test not requiring invasive procedures to obtain adequate specimens.
Further molecular characterization of the “T. whippelii” genome as well as of patients and carriers should be very helpful in explaining whether the presence of Whipple's disease bacterium DNA in persons without clinical and histological evidence for Whipple's disease (56, 181) is due to the presence of pathogenic or nonpathogenic strains, associated with host factors, or both.
ACKNOWLEDGMENTS
We thank A. von Graevenitz for critically reading the manuscript and M. Schneeman for many helpful discussions and review of the clinical parts of the manuscript.
The work done in the laboratory of M.A. was supported by the Swiss Naitonal Science Foundation (grants 32-40445.94 and 32-50790.97).
ADDENDUM IN PROOF
Since the submission of the revised version of this manuscript in July 2000, two major points have been addressed, as follows. First, the organism tentatively named “Tropheryma whippelii” has been formally described. To conform with correct latinization, Tropheryma whipplei is to be considered the valid name (B. La Scola, F. Fenollar, P.-E. Fournier, M. Altwegg, M. N. Mallett, and D. Raoult, Int. J. Syst. Evol. Microbiol., in press). Second, tissue sections from patients with Whipple's disease have been analyzed by fluorescence in situ hybridization and laser scanning confocal microscopy to determine the location of rRNA, i.e., metabolically active cells (D. N. Fredericks and D. A. Reldman, J. Infect. Dis., 183:1229–1237, 2001). It was found that rRNA is located mainly at the tips of villus structures in duodenal biopsies, whereas PAS-positive inclusions are much more abundant in the deeper mucosa. In addition, there is some evidence that 16S rRNA is present mainly between cells and not in association with the intracellular compartments, suggesting that T. whipplei is probably not an obligate intracellular pathogen.
REFERENCES
- 1.Adams M, Rhyner P A, Day J, DeArmond S, Smuckler E A. Whipple's disease confined to the central nervous system. Ann Neurol. 1987;21:104–108. doi: 10.1002/ana.410210121. [DOI] [PubMed] [Google Scholar]
- 2.Adler C H, Galetta S L. Oculo-facial-skeletal myorhythmia in Whipple disease: treatment with ceftriaxone. Ann Intern Med. 1990;112:467–469. doi: 10.7326/0003-4819-76-3-112-6-467. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht H, Rusch-Gerdes S, Stellbrink H J, Greten H, Jackle S. Disseminated Mycobacterium genavense infection as a cause of pseudo-Whipple's disease and sclerosing cholangitis. Clin Infect Dis. 1997;25:742–743. doi: 10.1086/516941. [DOI] [PubMed] [Google Scholar]
- 4.Allchin W H, Hebb R G. Lymphangiectasis intestini. Trans Pathol Soc London. 1895;46:221–223. [Google Scholar]
- 5.Altwegg M, Fleisch-Marx A, Goldenberger D, Hailemariam S, Schaffner A, Kissling R. Spondylodiscitis caused by Tropheryma whippelii. Schweiz Med Wochenschr. 1996;126:1495–1499. [PubMed] [Google Scholar]
- 6.Amarenco P, Roullet E, Hannoun L, Marteau R. Progressive supranuclear palsy as the sole manifestation of systemic Whipple's disease treated with pefloxacine. J Neurol Neurosurg Psychiatry. 1991;54:1121–1122. doi: 10.1136/jnnp.54.12.1121-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autran B, Gorin B, Leibowitch M, Laroche L, Escande J P, Hewitt J, Marche C. AIDS in an Haitian woman with cardiac Kaposi's sarcoma and Whipple's disease. Lancet. 1983;i:767–768. doi: 10.1016/s0140-6736(83)92058-5. [DOI] [PubMed] [Google Scholar]
- 8.Averbuch-Heller L, Paulson G W, Daroff R B, Leight J R. Whipple's disease mimicking progressive supranuclear palsy: the diagnostic value of eye movement recording. J Neurol Neurosurg Psychiatry. 1999;66:532–535. doi: 10.1136/jnnp.66.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayoub W T, David D E, Torretti D, Viozzi F J. Bone destruction and ankylosis in Whipple's disease. J Rheumatol. 1982;9:930–931. [PubMed] [Google Scholar]
- 10.Bai J C, Crosetti E E, Maurino E C, Martinez C A, Sambuelli A, Boerr L A. Short-term antibiotic treatment in Whipple's disease. J Clin Gastroenterol. 1991;13:303–307. doi: 10.1097/00004836-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bai J C, Mota A H, Mauriño E, Niveloni S, Grossman F, Boerr L A, Fainboim L. Class I and class II HLA antigens in a homogeneous Argentinian population with Whipple's disease: lack of association with HLA-B 27. Am J Gastroenterol. 1991;86:992–994. [PubMed] [Google Scholar]
- 12.Balestrieri G P, Villanacci V, Battocchio S, Sleiman I, Facchetti F, Giustina G. Cutaneous involvement in Whipple's disease. Br J Dermatol. 1996;135:666–668. doi: 10.1111/j.1365-2133.1996.tb03868.x. [DOI] [PubMed] [Google Scholar]
- 13.Bassotti G, Rossodivita M E, Caporali M, Del Favero A. A, Morelli A. Scanning electron microscopic findings in Whipple's disease. J Clin Gastroenterol. 1994;19:175–176. doi: 10.1097/00004836-199409000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Bassotti G, Pelli M A, Ribacchi R, Miglietti M, Cavalletti M L, Rossodivita M E, Giovenali P, Morelli A. Giardia lamblia infestation reveals underlying Whipple's disease in a patient with longstanding constipation. Am J Gastroenterol. 1991;86:371–374. [PubMed] [Google Scholar]
- 15.Bauerfeind P, Koelz H R, Altwegg M. PCR for Tropheryma whippelii. Lancet. 1999;354:1476–1477. doi: 10.1016/S0140-6736(99)01776-6. [DOI] [PubMed] [Google Scholar]
- 16.Bix M, Locksley R M. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–1354. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- 17.Bjerknes R, Ødegaard S, Bjerkvig R, Borkje B, Lærum O D. Whipple's disease. Demonstration of a persisting monocyte and macrophage dysfunction. Scand J Gastroenterol. 1988;23:611–619. doi: 10.3109/00365528809093921. [DOI] [PubMed] [Google Scholar]
- 18.Black-Schaffer B. The tinctorial demonstration of a glycoprotein in Whipple's disease. Proc Soc Exp Biol Med. 1949;72:225–227. doi: 10.3181/00379727-72-17388. [DOI] [PubMed] [Google Scholar]
- 19.Bodaghi B, Dauga C, Cassoux N, Wechsler B, Merle-Beral H, Poveda J D, Piette J C, LeHoang P. Whipple's syndrome (uveitis, B27-negative spondylarthropathy, meningitis, and lymphadenopathy) associated with Arthrobacter sp. infection. Ophthalmology. 1998;105:1891–1896. doi: 10.1016/S0161-6420(98)91036-3. [DOI] [PubMed] [Google Scholar]
- 20.Bostwick D G, Bensch K G, Burke J S, Billingham M E, Miller D C, Smith J C, Keren D F. Whipple's disease presenting as aortic insufficiency. N Engl J Med. 1981;305:995–998. doi: 10.1056/NEJM198110223051708. [DOI] [PubMed] [Google Scholar]
- 21.Bowles K, Muller A F, Ilesley I C. A 35-year-old with swollen knees who had recurrent fever and pericarditis, then diarrhoea before getting better. Lancet. 1996;348:1356. doi: 10.1016/S0140-6736(96)08101-9. [DOI] [PubMed] [Google Scholar]
- 22.Brändle M, Ammann P, Spinas G A, Dutly F, Galeazzi R L, Schmid C, Altwegg M. Relapsing Whipple's disease presenting with hypopituitarism. Clin Endocrinol. 1999;50:399–403. [PubMed] [Google Scholar]
- 23.Brennan P J, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 24.Brown A P, Lane J C, Murayama S, Vollmer D G. Whipple's disease presenting with isolated neurological symptoms. J Neurosurg. 1990;73:623–627. doi: 10.3171/jns.1990.73.4.0623. [DOI] [PubMed] [Google Scholar]
- 25.Canoso J J, Saini M, Hermos J A. Whipple's disease and ankylosing spondylitis. Simultaneous occurrence in HLA-B27 positive male. J Rheumatol. 1978;5:79–84. [PubMed] [Google Scholar]
- 26.Carnevale V, Minisola S, Romagnoli E, Rosso R, Marcheggiano A, Iannoni C, Mazzuoli G. Case report: reversal of decreased bone mass by antibiotic treatment in a patient with Whipple's disease. Am J Med Sci. 1996;311:145–147. doi: 10.1097/00000441-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Celard M, de Gevigney G, Mosnier S, Buttard P, Benito Y, Etienne J, Vandenesch F. Polymerase chain reaction analysis for diagnosis of Tropheryma whippelii infective endocarditis in two patients with no previous evidence of Whipple's disease. Clin Infect Dis. 1999;29:1348–1349. doi: 10.1086/313477. [DOI] [PubMed] [Google Scholar]
- 28.Chears W C, Ashworth C T. Electron microscopy study of the intestinal mucosa in Whipple's disease: demonstration of encapsulated bacilliform bodies in the lesion. Gastroenterology. 1961;41:129–138. [PubMed] [Google Scholar]
- 29.Cho C, Linscheer W G, Hirschkorn M A, Ashutosh K. Sarcoidlike granulomas as an early manifestation of Whipple's disease. Gastroenterology. 1984;87:941–947. [PubMed] [Google Scholar]
- 30.Clarke C E, Falope Z F, Abdelhadi H A, Franks A J. Cervical myelopathy caused by Whipple's disease. Neurology. 1998;50:1505–1506. doi: 10.1212/wnl.50.5.1505-a. [DOI] [PubMed] [Google Scholar]
- 31.Cohen L, Berthet K, Dauga C, Thivart L, Pierrot-Deseilligny C. Polymerase chain reaction of cerebrospinal fluid to diagnose Whipple's disease. Lancet. 1996;347:329. doi: 10.1016/s0140-6736(96)90505-x. [DOI] [PubMed] [Google Scholar]
- 32.Cohen A S, Schimmel E M, Holt P R, Isselbacher K J. Ultrastructural abnormalities in Whipple's disease. Proc Soc Exp Biol Med. 1960;105:411–414. doi: 10.3181/00379727-105-26126. [DOI] [PubMed] [Google Scholar]
- 33.Cooper G S, Blades E W, Remler B F, Salata R A, Bennert K W, Jacobs G H. Central nervous system Whipple's disease: relapse during therapy with trimethoprim-sulfamethoxazole and remission with cefixime. Gastroenterology. 1994;106:782–786. doi: 10.1016/0016-5085(94)90716-1. [DOI] [PubMed] [Google Scholar]
- 34.Cornelius C E. Animal models—a neglected medical resource. N Engl J Med. 1969;281:934–944. doi: 10.1056/NEJM196910232811706. [DOI] [PubMed] [Google Scholar]
- 35.Cruz I, Oliveira A P, Lopes J M, Ricardo J L, de Freitas J. Whipple's disease and renal amyloidosis. Am J Gastroenterol. 1993;88:1954–1956. [PubMed] [Google Scholar]
- 36.Dauga C, Miras I, Grimont P A. Strategy for detection and identification of bacteria based on 16S rRNA genes in suspected cases of Whipple's disease. J Med Microbiol. 1997;46:340–347. doi: 10.1099/00222615-46-4-340. [DOI] [PubMed] [Google Scholar]
- 37.Davion T, Rosat P, Sevestre H, Desablens B, Debussche C, Delamarre J, Capron J P. MR imaging of CNS relapse of Whipple disease. J Comput Assist Tomogr. 1990;14:815–817. doi: 10.1097/00004728-199009000-00029. [DOI] [PubMed] [Google Scholar]
- 38.De Coene B, Gilliard C, Indekeu P, Duprez T, Trigaux J P. Whipple's disease confined to the central nervous system. Neuroradiology. 1996;38:325–327. doi: 10.1007/BF00596579. [DOI] [PubMed] [Google Scholar]
- 39.Delanty N, Georgescu L, Lynch T, Paget S, Stubgen J P. Synovial fluid polymerase chain reaction as an aid to the diagnosis of central nervous system Whipple's disease. Ann Neurol. 1999;45:137–138. doi: 10.1002/1531-8249(199901)45:1<137::aid-art25>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.de Takats P G, de Takats D L, Iqbal T H, Watson R D, Sheppard M N, Cooper B T. Symptomatic cardiomyopathy as a presentation in Whipple's disease. Postgrad Med J. 1995;71:236–239. doi: 10.1136/pgmj.71.834.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dicuonzo G, Lorino G, Lilli D, Rivanera D, Guarino P, Angeletti S, Gherardi G, Filadoro F. Polymerase chain reaction, with sequencing, as a diagnostic tool in culture-negative bacterial meningitis. Clin Microbiol Infect. 1999;5:92–96. doi: 10.1111/j.1469-0691.1999.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 42.Disdier P, Harle J R, Vidal-Morris D, Sahel J, Weiller P J. Chemosis associated with Whipple's disease. Am J Ophthalmol. 1991;112:217–219. doi: 10.1016/s0002-9394(14)76712-1. [DOI] [PubMed] [Google Scholar]
- 43.Di Stefano M, Jorizzo R A, Brusco G, Cecchetti L, Sciarra G, Loperfido S, Brandi G, Gasbarrini G, Corazza G R. Bone mass and metabolism in Whipple's disease: the role of hypogonadism. Scand J Gastroenterol. 1998;33:1180–1185. doi: 10.1080/00365529850172548. [DOI] [PubMed] [Google Scholar]
- 44.Dobbins W O., III Is there an immune deficit in Whipple's disease? Dig Dis Sci. 1981;26:247–252. doi: 10.1007/BF01391638. [DOI] [PubMed] [Google Scholar]
- 45.Dobbins W O., III HLA antigens in Whipple's disease. Arthritis Rheum. 1987;30:102–105. doi: 10.1002/art.1780300115. [DOI] [PubMed] [Google Scholar]
- 46.Dobbins W O., III . Whipple's disease. Springfield, Ill: Charles C Thomas; 1987. [Google Scholar]
- 47.Dobbins W O., III The diagnosis of Whipple's disease. N Engl J Med. 1995;332:390–392. doi: 10.1056/NEJM199502093320611. [DOI] [PubMed] [Google Scholar]
- 48.Dobbins W O, III, Kawanishi H. Bacillary characteristics in Whipple's disease: an electron microscopic study. Gastroenterology. 1981;80:1468–1475. [PubMed] [Google Scholar]
- 49.Duprez T P J, Grandin C B, Bonnier C, Thauvoy C W, Gadisseux J F, Dutrieux J L, Evrard P. Whipple disease confined to the central nervous system in childhood. Am J Neuroradiol. 1996;17:1589–1591. [PMC free article] [PubMed] [Google Scholar]
- 50.Durand D V, Lecomte C, Cathebras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. Medicine. 1997;76:170–84. doi: 10.1097/00005792-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Dutly F, Hinrikson H P, Seidel T, Morgenegg S, Altwegg M, Bauerfeind P. “Tropheryma whippelii” DNA in saliva of patients without Whipple's disease. Infection. 2000;28:219–222. doi: 10.1007/s150100070039. [DOI] [PubMed] [Google Scholar]
- 52.Reference deleted.
- 53.Reference deleted.
- 54.Eck M, Kreipe H, Harmsen D, Müller-Hermelink H K. Invasion and destruction of mucosal plasma cells by Tropheryma whippelii. Hum Pathol. 1997;28:1424–1428. doi: 10.1016/s0046-8177(97)90234-3. [DOI] [PubMed] [Google Scholar]
- 55.Ectors N, Goebes K, De Vos R, Heidbuchel H, Rutgeerts P, Desmet V, Vantrappen G. Whipple's disease: a histological, immunological and electronmicroscopic study of the immune response in the small intestinal mucosa. Histopathology. 1992;21:1–12. doi: 10.1111/j.1365-2559.1992.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 56.Ehrbar H U, Bauerfeind P, Dutly F, Koelz H R, Altwegg M. PCR-positive tests for Tropheryma whippelii in patients without Whipple's disease. Lancet. 1999;353:2214. doi: 10.1016/S0140-6736(99)01776-6. [DOI] [PubMed] [Google Scholar]
- 57.Elkins C, Shuman T A, Pirolo J S. Cardiac Whipple's disease without digestive symptoms. Ann Thorac Surg. 1999;67:250–251. doi: 10.1016/s0003-4975(98)01204-1. [DOI] [PubMed] [Google Scholar]
- 58.Enzinger F M, Helwig E B. Whipple's disease: a review of the literature and report of 15 patients. Virchows Arch Pathol Anat. 1963;336:238–269. [Google Scholar]
- 59.Fantry G T, James S P. Whipple's disease. Dig Dis. 1995;13:108–118. doi: 10.1159/000171492. [DOI] [PubMed] [Google Scholar]
- 60.Feldman M, Price G. Intestinal bleeding in patients with Whipple's disease. Gastroenterology. 1989;96:1207–1209. doi: 10.1016/0016-5085(89)91643-0. [DOI] [PubMed] [Google Scholar]
- 61.Fest T, Pron B, Lefranc M P, Pierre C, Angonin R, de Wazieres B, Soua Z, Dupond J L. Detection of a clonal BCL2 gene rearrangement in tissues from a patient with Whipple disease. Ann Intern Med. 1996;124:738–740. doi: 10.7326/0003-4819-124-8-199604150-00006. [DOI] [PubMed] [Google Scholar]
- 62.Feurle G E, Dorken B, Schopf E, Lenhard V. HLA B27 and defects in the T-cell system in Whipple's disease. Eur J Clin Investig. 1979;9:385–389. doi: 10.1111/j.1365-2362.1979.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 63.Feurle G E, Marth T. An evaluation of antimicrobial treatment for Whipple's disease. Tetracycline versus trimethoprim-sulfamethoxazole. Dig Dis Sci. 1994;39:1642–1648. doi: 10.1007/BF02087770. [DOI] [PubMed] [Google Scholar]
- 64.Fleming J L, Wiesner R H, Shorter R G. Whipple's disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988;63:539–551. doi: 10.1016/s0025-6196(12)64884-8. [DOI] [PubMed] [Google Scholar]
- 65.Fox G E, Wisotzkey J D, Jurtshuk P. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 66.Fredericks D N, Relman D A. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frésard A, Guglielminotti C, Berthelot P, Ros A, Farizon F, Dauga C, Rousset H, Lucht F. Prosthetic joint infection caused by Tropheryma whippelii (Whipple's bacillus) Clin Infect Dis. 1996;22:575–576. doi: 10.1093/clinids/22.3.575. [DOI] [PubMed] [Google Scholar]
- 68.Fricker E J, McDonald T J., Jr Tropheryma whippelii. N Engl J Med. 1996;335:26. doi: 10.1056/NEJM199607043350105. [DOI] [PubMed] [Google Scholar]
- 69.Frucht D M, Holland S M. Defective monocyte costimulation for IFN-γ production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol. 1996;157:411–416. [PubMed] [Google Scholar]
- 70.Funke G, von Graevenitz A, Clarridge III J E, Bernard K A. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gagne M, Brown J, Lussier A, Rola-Pleszczynski M, Camerlain M. Maladie de Whipple sans manifestations digestives: arthropathie de diagnostic tardif. Union Med Can. 1983;112:628–632. [PubMed] [Google Scholar]
- 72.Gallin J L, Farber J M, Holland S M, Nutman T B. Interferon-γ in the management of infectious diseases. Ann Intern Med. 1995;123:216–224. doi: 10.7326/0003-4819-123-3-199508010-00009. [DOI] [PubMed] [Google Scholar]
- 73.Gillen C D, Coddington R, Monteith P G, Taylor R H. Extraintestinal lymphoma in association with Whipple's disease. Gut. 1993;34:1627–1629. doi: 10.1136/gut.34.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillin J S, Urmacher C, West R, Shike M. Disseminated Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome mimicking Whipple's disease. Gastroenterology. 1983;85:1187–1191. [PubMed] [Google Scholar]
- 75.Geboes K, Ectors N, Heidbuchel H, Rutgeerts P, Desmet V, Vantrappen G. Whipple's disease: the value of upper gastrointestinal endoscopy for the diagnosis and follow-up. Acta Gastroenterol Belg. 1992;55:209–219. [PubMed] [Google Scholar]
- 76.Goldenberger D, Kunzli A, Vogt P, Zbinden R, Altwegg M. Molecular diagnosis of bacterial endocarditis by broad-range PCR amplification and direct sequencing. J Clin Microbiol. 1997;35:2733–2739. doi: 10.1128/jcm.35.11.2733-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Good A E. Enteropathic arthritis: Whipple's disease. In: Kelly W N, Harris E D Jr, Ruddy S, Sledge C B, editors. Textbook of rheumatology. Philadelphia, Pa: The W. B. Saunders Co.; 1981. pp. 1071–1075. [Google Scholar]
- 78.Gross M, Jung C, Zoller W G. Detection of Tropheryma whippelii DNA (Whipple's disease) in feces. Ital J Gastroenterol Hepatol. 1999;31:70–72. [PubMed] [Google Scholar]
- 79.Gubler, J. G. H., M. Kuster, F. Dutly, F. Bannwart, M. Krause, H. P. Vögelin, G. Garzoli, and M. Altwegg. Whipple endocarditis without gastrointestinal disease: report of four cases. Ann. Intern. Med. 131:112–116. [DOI] [PubMed]
- 80.Gürtler V, Stanisich V A. New approaches to typing and identification of bacteria using the 16S–23S rDNA spacer region. Microbiology. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 81.Hamrock D, Azmi F H, O'Donnell E, Gunning W T, Philips E R, Zaher A. Infection by Rhodococcus equi in a patient with AIDS: histological appearance mimicking Whipple's disease and Mycobacterium avium-intracellulare infection. J Clin Pathol. 1999;52:68–71. doi: 10.1136/jcp.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harmsen D, Heesemann J, Brabletz T, Kirchner T, Müller-Hermelink H K. Heterogeneity among Whipple's-disease-associated bacteria. Lancet. 1994;343:1288. doi: 10.1016/s0140-6736(94)92176-8. [DOI] [PubMed] [Google Scholar]
- 83.Hinrikson H P, Dutly F, Altwegg M. Homogeneity of 16S–23S ribosomal intergenic spacer regions of Tropheryma whippelii in Swiss patients with Whipple's disease. J Clin Microbiol. 1999;37:152–156. doi: 10.1128/jcm.37.1.152-156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hinrikson H P, Dutly F, Altwegg M. Evaluation of a specific nested PCR targeting domain III of the 23S rRNA gene of “Tropheryma whippelii” and proposal of a classification system for its molecular variants. J Clin Microbiol. 2000;38:595–599. doi: 10.1128/jcm.38.2.595-599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinrikson H P, Dutly F, Altwegg M. Analysis of the actinobacterial insertion in domain III of the 23S rRNA gene of uncultured variants of the bacterium associated with Whipple's disease using broad-range and “Tropheryma whippelii”-specific PCR. Int J Syst Evol Microbiol. 2000;50:1007–1011. doi: 10.1099/00207713-50-3-1007. [DOI] [PubMed] [Google Scholar]
- 86.Hinrikson H P, Dutly F, Nair S, Altwegg M. Detection of three different types of “Tropheryma whippelii” directly from clinical specimens by sequencing, single-strand conformation polymorphism (SSCP) analysis and type-specific PCR of their 16S–23S ribosomal intergenic spacer region. Int J Syst Bacteriol. 1999;49:1701–1706. doi: 10.1099/00207713-49-4-1701. [DOI] [PubMed] [Google Scholar]
- 87.Hitti J, Riley D E, Krohn M A, Hillier S L, Agnew K J, Krieger J N, Eschenbach D A. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 88.Holländer G A, Zuklys S, Morel C, Mizoguchi E, Mobisson K, Simpson S, Terhorst C, Wishart W, Golan D E, Bhan A K, Burakoff S J. Monoallelic expression of the interleukin-2 locus. Science. 1998;279:2118–2121. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- 89.Horton K M, Corl F M, Fishman E K. CT of nonneoplastic diseases of the small bowel: spectrum of disease. J Comput Assist Tomogr. 1999;23:417–428. doi: 10.1097/00004728-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Jalava J, Kotilainen P, Nikkari S, Skurnik M, Vanttinen E, Lehtonen O P, Eerola E, Toivanen P. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin Infect Dis. 1995;21:891–896. doi: 10.1093/clinids/21.4.891. [DOI] [PubMed] [Google Scholar]
- 91.Jankovic J. Whipple's disease of the central nervous system in AIDS. N Engl J Med. 1986;315:1029–1030. doi: 10.1056/NEJM198610163151613. [DOI] [PubMed] [Google Scholar]
- 92.Jarolim D R, Parker G A, Sheehan W W. Bone marrow involvement by Whipple bacillus. J Infect Dis. 1991;163:1169–1170. doi: 10.1093/infdis/163.5.1169. [DOI] [PubMed] [Google Scholar]
- 93.James T N, Bulkley B H. Abnormalities of the coronary arteries in Whipple's disease. Am Heart J. 1983;105:481–491. doi: 10.1016/0002-8703(83)90367-8. [DOI] [PubMed] [Google Scholar]
- 94.Jeserich M, Ihling C, Holubarsch C. Aortic valve endocarditis with Whipple disease. Ann Intern Med. 1997;126:920. doi: 10.7326/0003-4819-126-11-199706010-00029. [DOI] [PubMed] [Google Scholar]
- 95.Keinath R D, Merrell D E, Vlietstra R, Dobbins W O. Antibiotic treatment and release in Whipple disease. Gastroenterology. 1985;88:1867–1873. doi: 10.1016/0016-5085(85)90012-5. [DOI] [PubMed] [Google Scholar]
- 96.Kelly C A, Egan M, Rawlinson J. Whipple's disease presenting with lung involvement. Thorax. 1996;51:343–344. doi: 10.1136/thx.51.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelly P. PCR for Tropheryma whippelii. Lancet. 1999;354:1476. doi: 10.1016/S0140-6736(05)77619-4. [DOI] [PubMed] [Google Scholar]
- 98.Knight S M, Symmons D P M. A man with intermittent fever and arthralgia. Ann Rheum Dis. 1998;57:711–714. doi: 10.1136/ard.57.12.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knox C M, Cevellos V, Dean D. 16S ribosomal DNA typing for identification of pathogens in patients with bacterial keratitis. J Clin Microbiol. 1998;36:3492–3496. doi: 10.1128/jcm.36.12.3492-3496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kooijman C D, Poen H. Whipple-like disease in AIDS. Histopathology. 1984;8:705–708. doi: 10.1111/j.1365-2559.1984.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 101.Kubaska S M, Shepard J A O, Chew F S, Keel S B. Whipple's disease involving the mediastinum. Am J Roentgenol. 1998;171:364. doi: 10.2214/ajr.171.2.9694452. [DOI] [PubMed] [Google Scholar]
- 102.Lapointe L R, Lamarche J, Salloum A, Beaudry R. Meningo-ependymitis in Whipple's disease. Can J Neurol Sci. 1980;7:163–167. doi: 10.1017/s0317167100023556. [DOI] [PubMed] [Google Scholar]
- 103.LeVine M E, Dobbins W O., III Joint changes in Whipple's disease. Semin Arthritis Rheum. 1973;3:79–93. doi: 10.1016/0049-0172(73)90036-x. [DOI] [PubMed] [Google Scholar]
- 104.Lisitsyn N, Lisitsyn N, Wigler M. Cloning the differences between two complex genomes. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 105.Louis E D, Lynch T, Kaufmann P, Fahn S, Odel J. Diagnostic guidelines in central nervous system Whipple's disease. Ann Neurol. 1996;40:561–568. doi: 10.1002/ana.410400404. [DOI] [PubMed] [Google Scholar]
- 106.Lowsky R, Archer G L, Fyles G, Minden M, Curtis J, Messner H, Atkins H, Patterson B, Willey B M, McGeer A. Brief report: diagnosis of Whipple's disease by molecular analysis of peripheral blood. N Engl J Med. 1994;331:1343–1346. doi: 10.1056/NEJM199411173312004. [DOI] [PubMed] [Google Scholar]
- 107.Lynch T, Odel J, Fredericks D N, Louis E D, Forman S, Rotterdam H, Fahn S, Relman D A. Polymerase chain reaction-based detection of Tropheryma whippelii in central nervous system Whipple's disease. Ann Neurol. 1997;42:120–124. doi: 10.1002/ana.410420120. [DOI] [PubMed] [Google Scholar]
- 108.MacDermott R P, Shepard J A Q, Graemecook F M, Bloch K J. A 59-year-old man with anorexia, weight loss, and a mediastinal mass Whipple's-disease involving the small intestine and mesenteric and mediastinal lymph nodes. N Engl J Med. 1997;337:1612–1619. doi: 10.1056/NEJM199711273372208. [DOI] [PubMed] [Google Scholar]
- 109.Maibach R, Dutly F, Altwegg M. Detection of “Tropheryma whippelii” DNA in feces by PCR using a target capture method. J Microbiol Methods. 1999;38:200. doi: 10.1128/JCM.40.7.2466-2471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiwald M, Ditton H J, von Herbay A, Rainey F A, Stackebrandt E. Reassessment of the phylogenetic position of the bacterium associated with Whipple's disease and determination of the 16S–23S ribosomal intergenic spacer sequence. Int J Syst Bacteriol. 1996;46:1078–1082. doi: 10.1099/00207713-46-4-1078. [DOI] [PubMed] [Google Scholar]
- 111.Maiwald M, Meier-Willersen H J, Hartmann M, von Herbay A. Detection of Tropheryma whippelii DNA in a patient with AIDS. J Clin Microbiol. 1995;33:1354–1356. doi: 10.1128/jcm.33.5.1354-1356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maiwald M, Schumacher F, Ditton H J, von Herbay A. Environmental occurrence of the Whipple's disease bacterium (Tropheryma whippelii) Appl Environ Microbiol. 1998;64:760–762. doi: 10.1128/aem.64.2.760-762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maiwald M, von Herbay A, Lepp P W, Relman D A. Organization, structure, and variability of the rRNA operon of the Whipple's disease bacterium (Tropheryma whippelii) J Bacteriol. 2000;182:3292–3297. doi: 10.1128/jb.182.11.3292-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maizel H, Ruffin J M, Dobbins W O., III Whipple's disease: a review of 19 patients from one hospital and a review of the literature since 1950. Medicine. 1970;49:175–205. [PubMed] [Google Scholar]
- 115.Mannaerts H F, Hekker T, Visser C A. A rare case of aortic valve endocarditis caused by Tropheryma whippelii with left coronary cusp perforation diagnosed by transoesophageal echocardiography and PCR. Heart. 1999;81:217. doi: 10.1136/hrt.81.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marcial M A, Villafana M. Whipple's disease with esophageal and colonic involvement: endoscopic and histopathologic findings. Gastrointest Endosc. 1997;46:263–266. doi: 10.1016/s0016-5107(97)70098-1. [DOI] [PubMed] [Google Scholar]
- 117.Marinella M A, Chey W. The syndrome of inappropriate antidiuretic hormone secretion in a patient with Whipple's disease. Am J Med Sci. 1997;313:247–248. doi: 10.1097/00000441-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Marth T, Fredericks D, Strober W, Relman D A. Limited role for PCR-based diagnosis of Whipple's disease from peripheral blood mononuclear cells. Lancet. 1996;348:66–67. doi: 10.1016/s0140-6736(05)64400-5. [DOI] [PubMed] [Google Scholar]
- 119.Marth T, Neurath M, Cuccherini B A, Strober W. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology. 1997;113:442–448. doi: 10.1053/gast.1997.v113.pm9247462. [DOI] [PubMed] [Google Scholar]
- 120.Marth T, Roux M, von Herbay A, Meuer S C, Feurle G E. Persistent reduction of complement receptor 3 alpha-chain expressing mononuclear blood cells and transient inhibitory serum factors in Whipple's disease. Clin Immunol Immunopathol. 1994;72:217–226. doi: 10.1006/clin.1994.1134. [DOI] [PubMed] [Google Scholar]
- 121.Marth T, Strober W. Whipple's disease. Semin Gastrointest Dis. 1996;7:41–48. [PubMed] [Google Scholar]
- 122.Martinez-Murcia A J, Esteve C, Garay E, Collins M D. Aeromonas allosaccharophila sp. nov., a new mesophilic member of the genus Aeromonas. FEMS Microbiol Lett. 1992;70:199–205. doi: 10.1016/0378-1097(92)90698-n. [DOI] [PubMed] [Google Scholar]
- 123.McAllister H A, Jr, Fenoglio J J. Cardiac involvement in Whipple's disease. Circulation. 1975;52:152–156. doi: 10.1161/01.cir.52.1.152. [DOI] [PubMed] [Google Scholar]
- 124.McGettigan P, Mooney E E, Sinnott M, Sweeney E C, Feely J. Sudden death in Whipple's disease. Postgrad Med J. 1997;73:509–511. doi: 10.1136/pgmj.73.862.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKinley R, Grace C S. Whipple's disease in an HLA-B27 positive female. Aust N Z J Med. 1985;15:758–760. [PubMed] [Google Scholar]
- 126.McNeil M M, Brown J M. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mendel E, Khoo L T, Go J L, Hinton D, Zee C S, Apuzzo M L J. Intracerebral Whipple's disease diagnosed by stereotactic biopsy: a case report and review of the literature. Neurosurgery. 1999;44:203–209. doi: 10.1097/00006123-199901000-00123. [DOI] [PubMed] [Google Scholar]
- 128.Miksche L W, Blumcke S, Fritsche D, Kuchemann K, Schuler H W, Grozinger K H. Whipple's disease: etiopathogenesis, treatment, diagnosis, and clinical course. Case report and review of the world literature. Acta Hepato-Gastroenterol. 1974;21:307–326. [PubMed] [Google Scholar]
- 129.Misbah S A, Ozols B, Franks A, Mapstone N. Whipple's disease without malabsorption—new atypical features. Q J Med. 1997;90:765–772. doi: 10.1093/qjmed/90.12.765. [DOI] [PubMed] [Google Scholar]
- 130.Mohm J, Naumann R, Schuler U, Ehninger G. Abdominal lymphomas, convulsive seizure and coma: a case of successfully treated, advanced Whipple's disease with cerebral involvement. Eur J Gastroenterol Hepatol. 1998;10:893–895. [PubMed] [Google Scholar]
- 131.Mooney E E, Kenan D J, Sweeney E C, Gaede J T. Myocarditis in Whipple's disease: an unsuspected cause of symptoms and sudden death. Mod Pathol. 1997;10:524–529. [PubMed] [Google Scholar]
- 132.Morgenegg S, Dutly F, Altwegg M. Cloning and sequencing of a part of the heat shock protein 60 (groEL) gene of “Tropheryma whippelii”. J Clin Microbiol. 2000;38:2248–2253. doi: 10.1128/jcm.38.6.2248-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Müller C, Petermann D, Stain C, Riemer H, Vogelsang H, Schnider P, Zeiler K, Wrba F. Whipple's disease: comparison of histology with diagnosis based on polymerase chain reaction in four consecutive cases. Gut. 1997;40:425–427. doi: 10.1136/gut.40.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Müller C, Stain C, Burghuber O. Tropheryma whippelii in peripheral blood mononuclear cells and cells of pleural effusion. Lancet. 1993;341:701. doi: 10.1016/0140-6736(93)90475-v. [DOI] [PubMed] [Google Scholar]
- 135.Neumann K, Neumann V, Zierz S, Lahl R. Coinfection with Tropheryma whippelii and a Whipple's disease-associated bacterial organism detected in a patient with central nervous system Whipple's disease. J Clin Microbiol. 1997;35:1645. doi: 10.1128/jcm.35.6.1645-1645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishimura J K, Cook B E, Pach J M. Whipple disease presenting as posterior uveitis without prominent gastrointestinal symptoms. Am J Ophthalmol. 1998;126:130–132. doi: 10.1016/s0002-9394(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 137.Nuzum C T, Sandler R S, Paulk H T. Thrombocytosis in Whipple's disease. Gastroenterology. 1981;80:1465–1467. [PubMed] [Google Scholar]
- 138.O'Duffy J D, Griffing W L, Li C Y, Abdelmalek M F, Persing D H. Whipple arthritis. Arthritis Rheum. 1999;42:812–817. doi: 10.1002/1529-0131(199904)42:4<812::AID-ANR27>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 139.Olive D M, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paulley J W. A case of Whipple's disease (intestinal lipodystrophy) Gastroenterology. 1952;22:128–132. [PubMed] [Google Scholar]
- 141.Pelech, T., P. Fric, A. Huslarova, and A. Jirasek. Interstitial lymphocytic myocarditis in Whipple's disease. Lancet 337:553–554. [DOI] [PubMed]
- 142.Peters F P J, Wouters R S M E, de Bruine A P, Stockbrügger R W. Cerebral relapse of sarcoidlike Whipple's disease. Clin Infect Dis. 1997;24:1252–1255. doi: 10.1086/513630. [DOI] [PubMed] [Google Scholar]
- 143.Petrides P E, Müller-Höcker J, Fredricks D N, Relman D A. PCR analysis of T. whippelii DNA in a case of Whipple's disease: effect of antibiotics and correlation with histology. Am J Gastroenterol. 1998;93:1579–1582. doi: 10.1111/j.1572-0241.1998.00416.x. [DOI] [PubMed] [Google Scholar]
- 144.Pianko S, Wells C. Whipple's disease: unusual presentations. Aust N Z J Med. 1997;27:592–593. doi: 10.1111/j.1445-5994.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 145.Playford R J, Schulenburg E, Herrington C S, Hodgson H J. Whipple's disease complicated by a retinal Jarisch-Herxheimer reaction: a case report. Gut. 1992;33:132–134. doi: 10.1136/gut.33.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pollock S, Lewis P D, Kendall B. Whipple's disease confined to the nervous system. J Neurol Neurosurg Psychiatry. 1981;44:1104–1109. doi: 10.1136/jnnp.44.12.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Poorman J C, Katon R M. Small bowel involvement by Mycobacterium avium complex in a patient with AIDS: endoscopic, histologic, and radiographic similarities to Whipple's disease. Gastrointest Endosc. 1994;40:753–759. [PubMed] [Google Scholar]
- 148.Pron B, Poyart C, Abachin E, Fest T, Belanger C, Bonnet C, Capelle P, Bretagne J-F, Fabianek A, Girard L, Hagège H, Berche P. Diagnosis and follow-up of Whipple's disease by amplification of the 16S rRNA gene of Tropheryma whippelii. Eur J Clin Microbiol Infect Dis. 1999;18:62–65. doi: 10.1007/s100960050228. [DOI] [PubMed] [Google Scholar]
- 149.Rajput A H, McHattie J D. Ophthalmoplegia and leg myorhythmia in Whipple's disease: report of a case. Mov Disord. 1997;12:111–114. doi: 10.1002/mds.870120120. [DOI] [PubMed] [Google Scholar]
- 150.Ramzan N N, Loftus Jr E, Burgart L J, Rooney M, Batts K P, Wiesner R H, Fredricks D N, Relman D A, Persing D H. Diagnosis and monitoring of Whipple disease by polymerase chain reaction. Ann Intern Med. 1997;126:520–527. doi: 10.7326/0003-4819-126-7-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 151.Ratliff N B, McMahon J T, Naab T J, Cosgrove D M. Whipple's disease in the porcine leaflets of a Carpentier-Edwards prosthetic mitral valve. N Engl J Med. 1984;311:902–903. doi: 10.1056/NEJM198410043111407. [DOI] [PubMed] [Google Scholar]
- 152.Raoult D, Birg M L, La Scola B, Fournier P E, Enea M, Lepidi H, Roux V, Piette J C, Vandenesch F, Vital-Durand D, Marrie T J. Cultivation of the bacillus of Whipple's disease. N Engl J Med. 2000;342:620–625. doi: 10.1056/NEJM200003023420903. [DOI] [PubMed] [Google Scholar]
- 153.Relman D A. Detection and identification of previously unrecognized microbial pathogens. Emerg Infect Dis. 1998;4:382–389. doi: 10.3201/eid0403.980310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Relman D A, Schmidt T M, MacDermott R P, Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 155.Rickman L S, Freeman W R, Green W R, Feldman S T, Sullivan J, Russack V, Relman D A. Brief report: uveitis caused by Tropheryma whippelii (Whipple's bacillus) N Engl J Med. 1995;322:363–366. doi: 10.1056/NEJM199502093320604. [DOI] [PubMed] [Google Scholar]
- 156.Riemer H, Hainz R, Stain C, Dekan G, Feldner-Busztin M, Schenk P, Müller C, Sertl K, Burghuber O C. Severe pulmonary hypertension reversed by antibiotics in a patient with Whipple's disease. Thorax. 1997;52:1014–1015. doi: 10.1136/thx.52.11.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Robson D K, Faraj B B, Hamal P B, Ironside J W. Whipple's disease with cerebral involvement. Postgrad Med J. 1990;66:724–726. doi: 10.1136/pgmj.66.779.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodarte J R, Garrison C O, Holley K E, Fontana R S. Whipple's disease simulating sarcoidosis. A case with unique clinical and histological features. Arch Intern Med. 1972;129:479–482. doi: 10.1001/archinte.129.3.479. [DOI] [PubMed] [Google Scholar]
- 159.Roller C, Ludwig W, Schleifer K H. Gram-positive bacteria with a high DNA G+C content are characterized by a common insertion within their 23S rRNA genes. J Gen Microbiol. 1992;138:1167–1175. doi: 10.1099/00221287-138-6-1167. [DOI] [PubMed] [Google Scholar]
- 160.Roth A, Fischer M, Hamid M E, Michalke S, Ludwig W, Mauch H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S–23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–147. doi: 10.1128/jcm.36.1.139-147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roth R I, Owen R L, Keren D F, Volberding P A. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple's disease. Dig Dis Sci. 1985;30:497–504. doi: 10.1007/BF01318186. [DOI] [PubMed] [Google Scholar]
- 162.Rouillon A, Menkes C J, Gerster J C, Perez-Sawka I, Forest M. Sarcoid-like forms of Whipple's disease. Report of 2 cases. J Rheumatol. 1993;20:1070–1072. [PubMed] [Google Scholar]
- 163.Saleh H, Williams T M, Minda J M, Gupta P K. Whipple's disease involving the mesenteric lymph nodes diagnosed by fine-needle aspiration. Diagn Cytopathol. 1992;8:177–180. doi: 10.1002/dc.2840080217. [DOI] [PubMed] [Google Scholar]
- 164.Scheib J S, Quinet R J. Whipple's disease with axial and peripheral joint destruction. South Med J. 1990;83:684–687. doi: 10.1097/00007611-199006000-00024. [DOI] [PubMed] [Google Scholar]
- 165.Schilling D, Adamek H E, Kaufmann V, Maier M, Riemann J F. Arthralgia as an early extraintestinal symptom of Whipple's disease. Report of five cases. J Clin Gastroenterol. 1997;24:18–20. doi: 10.1097/00004836-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 166.Schneider T, Salamon-Looijen M, von Herbay A, Schwerdt H, Weg-Remers S, Stallmach A, Zeitz M. Whipple's disease with aortic regurgitation requiring aortic valve replacement. Infection. 1998;26:178–180. doi: 10.1007/BF02771847. [DOI] [PubMed] [Google Scholar]
- 167.Schneider T, Stallmach A, von Herbay A, Marth T, Strober W, Zeitz M. Treatment of refractory Whipple disease with interferon-γ. Ann Intern Med. 1998;129:875–877. doi: 10.7326/0003-4819-129-11_part_1-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 168.Schnider P J, Reisinger E C, Gerschlager W, Müller C, Berger T, Krejs G J, Auff E. Long-term follow-up in cerebral Whipple's disease. Eur J Gastroenterol Hepatol. 1996;8:899–903. [PubMed] [Google Scholar]
- 169.Schoedon G, Goldenberger D, Forrer R, Gunz A, Dutly F, Höchli M, Altwegg M, Schaffner A. Deactivation of macrophages with IL-4 is the key to the isolation of Tropheryma whippelii. J Infect Dis. 1997;176:672–677. doi: 10.1086/514089. [DOI] [PubMed] [Google Scholar]
- 170.Selhorst J B, Schwartz M A. Cerebral manifestations of Whipple's disease. Mayo Clin Proc. 1988;63:1057. doi: 10.1016/s0025-6196(12)64926-x. [DOI] [PubMed] [Google Scholar]
- 171.Sieracki J C. Whipple's disease; observation on systemic involvement. Arch Pathol. 1958;66:464–467. [PubMed] [Google Scholar]
- 172.Silbert S W, Parker E, Horenstein S. Whipple's disease of the central nervous system. Acta Neuropathol. 1976;36:31–38. doi: 10.1007/BF00685145. [DOI] [PubMed] [Google Scholar]
- 173.Silva M T, Macedo P M, Moura Nunes J F. Ultrastructure of bacilli and the bacillary origin of the macrophagic inclusions in Whipple's disease. J Gen Microbiol. 1985;131:1001–1013. doi: 10.1099/00221287-131-5-1001. [DOI] [PubMed] [Google Scholar]
- 174.Silvestry F E, Kim B, Pollack B J, Haimowitz J E, Murray R K, Furth E E, Nisenbaum H L, Kochman M L, Freedman N, Pine R, Herrmann H C. Cardiac Whipple disease: identification of Whipple bacillus by electron microscopy of a patient before death. Ann Intern Med. 1997;126:214–216. doi: 10.7326/0003-4819-126-3-199702010-00006. [DOI] [PubMed] [Google Scholar]
- 175.Southern J F, Moscicki R A, Magro C, Dickersin G R, Fallon J T, Bloch K J. Lymphedema, lymphocytic myocarditis, and sarcoidlike granulomatosis. Manifestations of Whipple's disease. JAMA. 1989;261:1467–1470. [PubMed] [Google Scholar]
- 176.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 177.Stackebrandt E, Rainey F A, Ward-Rainey N L. Proposal for a new hierarchic classification system. Actinobacteria classis nov. Int J Syst Bacteriol. 1997;47:479–491. [Google Scholar]
- 178.Stähelin J, Goldenberger D, Gnehm H E, Altwegg M. Polymerase chain reaction diagnosis of Kingella kingae arthritis in a young child. Clin Infect Dis. 1998;27:1328–1329. doi: 10.1093/clinids/27.5.1328. [DOI] [PubMed] [Google Scholar]
- 179.Steinert M, Birkness K, White E, Fields B, Quinn F. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol. 1998;64:2256–2261. doi: 10.1128/aem.64.6.2256-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stoll T, Keusch G, Jost R, Burger H, Oelz O. IgA nephropathy and hypercalcemia in Whipple's disease. Nephron. 1993;63:222–225. doi: 10.1159/000187187. [DOI] [PubMed] [Google Scholar]
- 181.Street S, Donoghue H D, Neild G H. Tropheryma whippelii DNA in saliva of healthy people. Lancet. 1999;354:1178–1179. doi: 10.1016/s0140-6736(99)03065-2. [DOI] [PubMed] [Google Scholar]
- 182.Symmons D P, Shepherd A N, Boardman P L, Bacon P A. Pulmonary manifestations of Whipple's disease. Q J Med. 1985;56:497–504. [PubMed] [Google Scholar]
- 183.Tan T Q, Vogel H, Tharp B R, Carrol C L, Kaplan S L. Presumed central nervous system Whipple's disease in a child: case report. Clin Infect Dis. 1995;20:883–889. doi: 10.1093/clinids/20.4.883. [DOI] [PubMed] [Google Scholar]
- 184.Tarter R E, Edwards N, Hays A, Van Thiel D H. Neuropsychiatric dysfunction in a patient with Whipple's disease: effects of antibiotic treatment. Psychosomatics. 1990;31:225–230. doi: 10.1016/S0033-3182(90)72202-5. [DOI] [PubMed] [Google Scholar]
- 185.Taskén K, Schulz T, Elgjo K, Skullerud K, Relman D, Brubbakk O. Diagnostic utility of the polymerase chain reaction in 2 cases of suspected Whipple disease. Arch Intern Med. 1998;158:801–803. doi: 10.1001/archinte.158.7.801. [DOI] [PubMed] [Google Scholar]
- 186.Tenover F C, Arbeit R D, Goering R V the Molecular Typing Working Group of the Society for Healthcare Epidemiology of America. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18:426–439. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 187.Uldry P A, Bogousslavsky J. Partially reversible parkinsonism in Whipple's disease with antibiotherapy. Eur Neurol. 1992;32:151–153. doi: 10.1159/000116813. [DOI] [PubMed] [Google Scholar]
- 188.van Belkum A. DNA fingerprinting of medically important microorganisms by use of PCR. Clin Microbiol Rev. 1994;7:174–184. doi: 10.1128/cmr.7.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vanderschueren D, Dequeker J, Geboes K. Whipple's disease in a patient with longstanding seronegative polyarthritis. Scand J Rheumatol. 1988;17:423–426. doi: 10.3109/03009748809105282. [DOI] [PubMed] [Google Scholar]
- 190.Van Kruiningen H J, Dobbins W O, John G. Bacterial histiocytic colitis in a lowland gorilla (Gorilla gorilla gorilla) Vet Pathol. 1991;28:544–546. doi: 10.1177/030098589102800616. [DOI] [PubMed] [Google Scholar]
- 191.Van Kruiningen H J, Montali R J, Strandberg J D, Kirk R W. A granulomatous colitis of dogs with histologic resemblance to Whipple's disease. Pathol Vet. 1965;2:521–544. doi: 10.1177/030098586500200601. [DOI] [PubMed] [Google Scholar]
- 192.Vazquez-Iglesias J L, Yanez J, Durana J, Arnal F. Infection by Mycobacterium avium intracellulare in AIDS: endoscopic duodenal appearance mimicking Whipple's disease. Endoscopy. 1988;20:279–280. doi: 10.1055/s-2007-1018194. [DOI] [PubMed] [Google Scholar]
- 193.Venmans B J, Claessen F A. Presentation of Whipple's disease. Lancet. 1997;349:433–434. doi: 10.1016/S0140-6736(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 194.Verhagen W I M, Huygen P L M, Dalman J E, Schuurmans M M J. Whipple's disease and the central nervous system a case report and a review of the literature. Clin Neurol Neurosurg. 1996;98:299–304. doi: 10.1016/0303-8467(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 195.Vincent M E, Robbins A H. Mycobacterium avium-intracellulare complex enteritis: pseudo-Whipple disease in AIDS. Am J Roentgenol. 1985;144:921–922. doi: 10.2214/ajr.144.5.921. [DOI] [PubMed] [Google Scholar]
- 196.von Herbay A, Ditton H J, Maiwald M. Diagnostic application of a polymerase chain reaction assay for the Whipple's disease bacterium to intestinal biopsies. Gastroenterology. 1996;110:1735–1743. doi: 10.1053/gast.1996.v110.pm8964398. [DOI] [PubMed] [Google Scholar]
- 197.von Herbay A, Maiwald M, Ditton H J, Otto H F. Histology of intestinal Whipple's disease revisited. A study of 48 patients. Virchows Arch. 1996;429:335–343. doi: 10.1007/BF00198437. [DOI] [PubMed] [Google Scholar]
- 198.von Herbay A, Ditton H J, Schuhmacher F, Maiwald M. Whipple's disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434–441. doi: 10.1053/gast.1997.v113.pm9247461. [DOI] [PubMed] [Google Scholar]
- 199.von Herbay A, Otto H F, Stolte M, Borchard F, Kirchner T, Ditton H J, Maiwald M. Epidemiology of Whipple's disease in Germany. Analysis of 110 patients diagnosed in 1965-95. Scand J Gastroenterol. 1997;32:52–57. doi: 10.3109/00365529709025063. [DOI] [PubMed] [Google Scholar]
- 200.Ward D, M, Weller R, Bateson M M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990;345:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- 201.Wendler D, Mendoza E, Schleiffer T, Zander M, Maier M. Tropheryma whippelii endocarditis confirmed by polymerase chain reaction. Eur Heart J. 1995;16:424–425. doi: 10.1093/oxfordjournals.eurheartj.a060928. [DOI] [PubMed] [Google Scholar]
- 202.Wilcox G M, Tronic B S, Schecter D J, Arron M J, Righi D F, Weiner N J. Periodic acid-Schiff-negative granulomatous lymphadenopathy in patients with Whipple's disease. Am J Med. 1987;83:165–170. doi: 10.1016/0002-9343(87)90514-6. [DOI] [PubMed] [Google Scholar]
- 203.Whipple G H. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp. 1907;18:382–391. [Google Scholar]
- 204.Williams J G, Edward D P, Tessler H H, Persing D H, Mitchell P S, Goldstein D A. Ocular manifestations of Whipple disease—an atypical presentation. Arch Ophthalmol. 1998;116:1232–1234. doi: 10.1001/archopht.116.9.1232. [DOI] [PubMed] [Google Scholar]
- 205.Wilson I G. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wilson K H. Detection of culture-resistant bacterial pathogens by amplification and sequencing of ribosomal DNA. Clin Infect Dis. 1994;18:958–962. doi: 10.1093/clinids/18.6.958. [DOI] [PubMed] [Google Scholar]
- 207.Wilson K H, Blitchington R, Frothingham R, Wilson J A. Phylogeny of the Whipple's-disease-associated bacterium. Lancet. 1991;338:474–475. doi: 10.1016/0140-6736(91)90545-z. [DOI] [PubMed] [Google Scholar]
- 208.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wright C B, Hiratzka L F, Crossland S, Isner J, Snow J A. Aortic insufficiency requiring valve replacement in Whipple's disease. Ann Thorac Surg. 1978;25:466–469. doi: 10.1016/s0003-4975(10)63589-8. [DOI] [PubMed] [Google Scholar]
- 210.Wroe S J, Pires M, Harding B, Youl B D, Shorvon S. Whipple's disease confined to the CNS presenting with multiple intracerebral mass lesions. J Neurol Neurosurg Psychiatry. 1991;54:989–992. doi: 10.1136/jnnp.54.11.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yardley J H, Hendrix T R. Combined electron microscopy and light microscopy in Whipple's disease: demonstration of “bacillary bodies” in the intestine. Bull Johns Hopkins Hosp. 1961;109:80–98. [PubMed] [Google Scholar]
- 212.Zighelboim J, Carpenter H A, Talley N J. A patient with diarrhea, arthralgias, and fever. Gastroenterology. 1993;105:923–930. doi: 10.1016/0016-5085(93)90913-w. [DOI] [PubMed] [Google Scholar]
- 213.Zügel U, Kaufmann S H E. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]