Abstract
Over the past decade, CRISPR has rapidly made its way from the bench to the bedside, providing a newfound therapeutic avenue to not only treat genetic diseases but also permanently cure them. Although there are several clinical trials in early stages, there are so far no CRISPR-based clinical trials for cutaneous disease. In this review, we describe multiple cutaneous diseases that represent ideal targets for CRISPR-based therapeutics owing to known single gene‒causing mutations. We also explore the potential of CRISPR nucleases to treat inflammatory disorders such as eczema and psoriasis, which are not classically categorized as genodermatoses. We describe the therapeutic solutions for these diseases that are guided by various CRISPR-associated (Cas) effector proteins, for example, using Cas9 to permanently edit the DNA of somatic cells, Cas3 to target foreign DNA to combat viral/bacterial skin infections, and Cas13 to edit mutated RNA transcripts in diseases where permanent DNA editing is untenable. Furthermore, we discuss various drug delivery modalities for CRISPR therapeutics, including transdermal patches and microneedles, which are uniquely suited for dermatological diseases. In summary, we highlight the potential of CRISPR-based therapeutics to revolutionize the treatment of cutaneous disease with a goal of being accessible to the practicing dermatologist.
Abbreviations: AD, atopic dermatitis; Cas, CRISPR-associated; EB, epidermolysis bullosa; RDEB, recessive dystrophic epidermolysis bullosa
Introduction
The standard of care for the majority of cutaneous diseases, including genodermatoses, inflammatory disorders, and bacterial skin infections, largely involve treatment of symptoms rather than the underlying cause of disease. Although dermatologists have a repertoire of pharmacotherapy available to prescribe to patients (e.g., topical steroids, anti-inflammatory biologics, or antibiotics), these treatment options are often short-term solutions for long-term chronic problems and come with undesirable side effects. For example, corticosteroids for atopic dermatitis (AD) can result in stretch marks and thinning and darkening of the skin, or chronic use of antibiotics for acne vulgaris can lead to resistance and poor outcomes. In addition, although biologics have revolutionized the way severe inflammatory skin diseases are treated, a major drawback is that patients typically need to take the medication for life. Hence, there is an urgent need for treatment modalities to target the underlying cause of disease rather than focus on symptomatic management.
Recent advances in genetics and molecular biology have revealed that many cutaneous diseases stem from changes in DNA—either DNA mutations in the host (genodermatoses [Ko et al., 2019] and inflammatory disorders [Bowcock and Cookson, 2004]) or pathogenic DNA in viruses (de Buhr and Lebbink, 2018) and bacteria (Greene, 2018; Pursey et al., 2018; Viertel et al., 2014) (skin infections)—opening a new avenue to target these diseases. Specifically, the discovery of CRISPR and corresponding CRISPR-associated (Cas) nucleases has enabled the editing of precise molecular targets (e.g., DNA and RNA sequences) in a variety of clinically applicable contexts (Doudna and Charpentier, 2014; Fellmann et al., 2017). Although several clinical trials using CRISPR nucleases, including Cas9 and Cas3, are in progress for blood disorders (Frangoul et al., 2021, 2020), cancers (Lacey and Fraietta, 2020; Lu et al., 2020; Stadtmauer et al., 2020), eye diseases, chronic infections (Lenneman et al., 2021), and protein-folding disorders, there are so far no CRISPR-based clinical trials for dermatological diseases.
To that end, CRISPR-based therapies have enormous implications for three classes of cutaneous disorders: genodermatoses, inflammatory disorders, and bacterial infections (overview of the strategy is presented in Figure 1). Specifically, many genodermatoses are monogenic, that is, they are associated with a mutation in a single gene (for example, recessive dystrophic epidermolysis bullosa [(RDEB] and congenital ichthyosis) and present ideal candidates for CRISPR targeting. Beyond monogenic disorders, other therapeutic targets for CRISPR include inflammatory disorders, such as AD, in which certain disease-causing mutations are well-known (Wan et al., 2021). Furthermore, other Cas nucleases such as CRISPR-Cas3 have recently been the focus of clinical trials for treating bacterial urinary tract infections (Lenneman et al., 2021); this opens another avenue for using CRISPR nucleases to treat antibiotic-resistant or latent bacterial (Pursey et al., 2018; Viertel et al., 2014) or viral (de Buhr and Lebbink, 2018) skin infections.
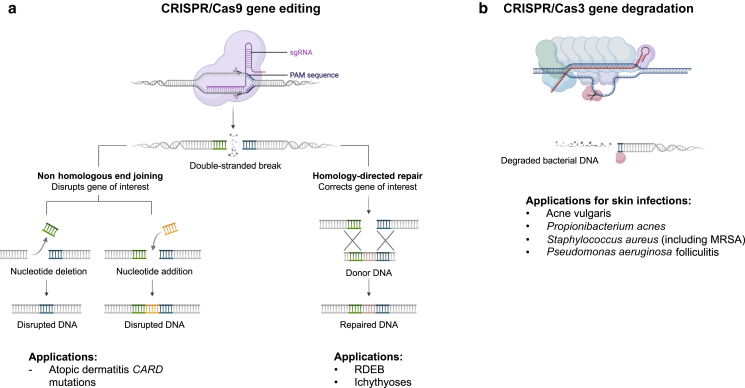
Figure 1.
Overview of CRISPR-based treatment strategies for cutaneous diseases. Strategy for targeted treatment of (a) inherited cutaneous disorders by CRISPR/Cas9 or (b) bacterial infections by CRISPR/Cas3. Cas9 loaded with an sgRNA recognizes the PAM, hybridizes at a specific genomic locus, and generates a double-stranded break in DNA. At this point, there are two options for editing: (left) disrupting a gene of interest through nonhomologous end joining or (right) correcting a gene of interest through homology-directed repair through the integration of a donor DNA template carrying the correct sequence. Whereas Cas9 is a single protein that has both DNA-targeting and -cutting activity, CRISPR/Cas3 involves a complex of multiple proteins called the Cascade complex and recruits a trans- nuclease helicase called Cas3 to make the initial cut in DNA. After making a cut, Cas3 can use ATP to processively degrade DNA, making it useful for the cleavage of long segments of bacterial DNA. Delivery strategies for components are discussed in Figure 2. ATP, adenosine triphosphate; Cas, CRISPR-associated; MRSA, methicillin-resistant Staphylococcus aureus; PAM, Protospacer Adjacent Motif sequence; RDEB, recessive dystrophic epidermolysis bullosa; sgRNA, short guide RNA.
Recently, several informative review articles in dermatology journals have focused on ex vivo DNA/gene-editing therapies for rare genodermatoses (De Rosa et al., 2020; Jayarajan et al., 2021; March et al., 2018). In this study, we include both ex vivo and in vivo gene-editing applications in dermatology and further focus our attention on incorporating all classes of CRISPR nucleases—DNA and RNA targeting—into the repertoire of clinical tools available to the dermatologist. Finally, given the unique structural barriers of the epidermis for drug delivery, we discuss advances in delivering CRISPR-based therapies to the skin and challenges that will need to be overcome to bring CRISPR into the clinic. In summary, we highlight the potential of CRISPR-based therapeutics to revolutionize the treatment of cutaneous disease with a goal of being accessible to the practicing dermatologist.
Targeted Treatment of Genodermatoses and AD
Treatment of genetic diseases that affect the skin, including keratinization disorders (e.g., ichthyoses and Darier disease), blistering disorders (e.g., epidermolysis bullosa [EB]), or subtypes of inflammatory dermatitis (e.g., eczema and psoriasis caused by various CARD mutations), has traditionally consisted of medications that treat symptoms rather than the underlying cause of the disease. These treatment options generally have included potent topical and systemic steroids, immune-suppressive agents, and emollients, which provide short-term relief and thereby limit long-term improvement in patient satisfaction. In addition, cases refractory to treatment are at risk of breaking the protective stratum corneum layer and thus are at a higher risk of developing secondary bacterial infections. Despite the urgent need, there are no cures for genodermatoses, and treatment options for AD are limited. Newer IL-4 inhibitor‒based therapies for AD (e.g., Dupixent [Beck et al., 2014]) show promising clinical benefits, but patients must remain on the medication for life and often experience significant side effects. Recently, genetic mutations associated with AD (Wan et al., 2021) and mutations underlying several monogenic genodermatoses (Kocher et al., 2017; Shinkuma et al., 2016) have been the focus of CRISPR/Cas9-mediated therapy in mouse and cellular models (Benati et al., 2018; Hainzl et al., 2017; Shinkuma et al., 2016; Wan et al., 2021; Webber et al., 2016). Although much of this work is still in the early stages, there is now precedence for Cas9-mediated therapies in nondermatological conditions (Frangoul et al., 2021) and non-CRISPR gene therapy for dermatological indications (reviewed elsewhere [Ain et al., 2021]) to treat patients and improve outcomes. In this section, we discuss recent progress on utilizing CRISPR/Cas9 to successfully repair DNA mutations in genetic cutaneous diseases.
Although Cas9 has not made it into the dermatology clinical trials, alternative gene therapy methods repairing damaged DNA in cutaneous disease have largely focused on ex vivo therapies, namely extracting and modifying cells from patients before re-engraftment back to the body. For example, current clinical trials are underway using gene-replacement strategies for RDEB(NCT04186650), Netherton syndrome (NCT01545323), and congenital ichthyosis (NCT04047732). Many of these clinical trials have been successful in treating these skin disorders by taking a biopsy and expanding keratinocytes, correcting the gene mutation responsible for the disease, and regrafting skin equivalents into patients (NCT04186650). Similar ex vivo delivery of CRISPR would be indicated for genodermatoses in which there is severe skin involvement. Although CRISPR for treating genodermatoses has been explored in animal and cellular models, virtually all progress has been focused on one severe skin blistering disease, EB. Specifically, researchers have used Cas9 to successfully restore enough gene function (of full-length type VII collagen gene, COL7A1) in rodent and cellular models of EB that are considered sufficient for a scarless phenotype after engraftment onto a human body (Benati et al., 2018; Bonafont et al., 2019; Izmiryan et al., 2018; Jacków et al., 2019; Kocher et al., 2020). Nevertheless, it remains to be seen how CRISPR/Cas9-based therapy for EB compares with existing gene therapies. Future work will determine the safety and efficacy of Cas9-mediated therapy for EB and eventually other genodermatoses such as Netherton syndrome, congenital ichthyosis, and other monogenic skin disorders.
Beyond monogenic skin disorders, there is growing interest in understanding the molecular genetics underlying inflammatory skin diseases, particularly eczema and psoriasis (Bieber, 2008; Weidinger and Novak, 2016), to research new ways to target these mutations for therapy. Many subtypes of psoriasis harbor gene mutations in CARD14, which results in the upregulation of inflammatory cytokines (Capon, 2017), and similarly, eczema carries similar causative mutations in CARD11 (Ma et al., 2017). In contrast to cases carrying gene mutations in CARD, one of the most common associations of genetic mutation with AD is in the FLG gene encoding FLG (Irvine et al., 2011; O’Regan and Irvine, 2008). Finally, another genetic similarity between eczema and psoriasis is in an inflammasome called NLRP3, which when activated leads to a greater inflammatory response and more resistance to glucocorticoid therapy. It was recently shown that codelivering Cas9-targeting NLRP3 with dexamethasone in mouse models alleviated symptoms—reduction in skin edema, reduced infiltration of mast cells, and overall improvement in inflammatory activity—in comparison with the Cas9‒NLRP3 treatment alone or the dexamethasone treatment alone (Wan et al., 2021).
Furthermore, the advent of RNA editing using CRISPR/Cas13 (Abudayyeh et al., 2017) may enable further treatment options for genetic diseases where permanently editing the DNA might not be tenable or dangerous owing to unintended off-target effects. Taking the example stated earlier, knocking out the NLRP3 gene through Cas9 may be effective but may also cause unintended off-target effects that would be permanent for the duration of the cell lifetime. In contrast, editing the mRNA product before translation would obviate the need to target the genome directly and instead prevent the expression of the proinflammatory protein. This type of precision and targeted therapy would be useful in cases where systemic corticosteroid therapy is untenable owing to adverse side effects or resistance. In summary, targeted treatment through Cas9 of genodermatoses and AD has been incredibly promising in animal and cellular models of disease and poses a hopeful opportunity to translate these findings into humans.
Antibiotic-Free Treatment of Bacterial Skin Infections
Needless to say, the mainstay treatment of bacterial infections, from acne or skin infections caused by bacteria, has centered around prescribing antibiotics. Although effective in many cases, antibiotic resistance is a significant problem, and alternative, innovative options for treating infections are needed. Although antibiotics typically target the bacterial cell machinery involved in essential growth processes (e.g., protein synthesis, transcription of RNA, etc.), an alternative approach is to specifically target bacterial genome sequences to disable the pathogen, prevent replication, and treat the infection. Recently, researchers have repurposed a CRISPR/Cas system (Cas3) to target bacterial infections (Lenneman et al., 2021; Selle et al., 2020) by delivering CRISPR machinery packaged within viral vectors (bacteriophage) that exclusively infect bacterial cells and not human cells. Rather than using CRISPR to modify or edit host DNA, the central tenet of the CRISPR/Cas3 strategy is to make thousands of cuts in bacterial DNA and leave human DNA unmodified. In contrast to Cas9, which makes a single cut in DNA (Jinek et al., 2012), Cas3 is a processive nuclease and helicase that uses adenosine triphosphate to unwind DNA and successively degrade long segments of DNA (Hochstrasser et al., 2014; Redding et al., 2015). As a result, the bacteria cannot replicate with its genetic code disabled.
CRISPR/Cas complexes with short guide RNAs complementary in sequence to pathogenic bacterial sequences can be repurposed to target and kill specific bacterial species. For example, Cas9 was recently used to exclusively target one of two strains of E. coli in a mouse model (Lam et al., 2021), and Cas3 was recently used to target Clostridium difficile (Selle et al., 2020) in vivo; furthermore, Cas3 is currently in clinical trials for E. coli urinary tract infections (e.g., NCT04191148). Although the latter study has confirmed the safety and efficacy of this strategy of antimicrobial targeting, challenges in delivery remain to be optimized. Namely, the current Cas3 clinical trial is testing the administration of the phage treatment directly into patients’ bladders (through catheterization) with an immediate next goal of intravenous or intramuscular delivery, all of which largely require treatment by medical professionals.
Nevertheless, the Cas3 clinical trial sets an important precedent for considering CRISPR/Cas treatment for bacterial skin infections. Specifically, applications in dermatology where CRISPR/Cas-mediated antimicrobial treatment would be beneficial include skin infections such as Staphylococcus aureus (including methicillin-resistant S. aureus) or Pseudomonas aeruginosa folliculitis. Instances in which a patient cannot tolerate the standard antibiotic owing to an allergy or is refractory to treatment owing to antibiotic resistance would be indicators for using CRISPR-mediated therapy. Whereas antibiotics (even relatively selective ones) may kill both bad bacteria and normal flora that exists on the skin, the advantage of the CRISPR/Cas strategy is that normal flora can be spared by programing the CRISPR machinery to target bacterial genes conserved within a strain or even a specific species. Furthermore, acne has a wide range of treatment options, ranging from oral contraceptives to topical creams to antibiotics to isotretinoin. Some patients either do not respond to treatment or are concerned about significant side effects (e.g., birth defects, liver failure, etc.) and therefore would be good candidates for incorporating CRISPR-mediated treatment for acne exacerbated by bacteria. Because the cause of acne is multifactorial, CRISPR will not be a monotherapy and will be administered with already existing topical acne therapies, which are often combined with antimicrobial therapy.
In summary, CRISPR-guided targeting of bacterial infections adds another layer of antimicrobial strategies for managing infections. In practice, a combination of approaches may be beneficial for treatment, for example, targeting bacterial replication through CRISPR targeting of the DNA in addition to mild topical creams and facial cleansing solutions that can work synergistically.
Delivering CRISPR to the Skin
The success of any treatment depends on the efficiency of uptake and downstream bioavailability of the drug. Delivery of CRISPR to treat cutaneous diseases can be divided into two large categories: (i) ex vivo, in which primary cells are treated outside the body and reintroduced to patients on gene correction, and (ii) in vivo, in which the CRISPR components are directly delivered to patients (Figure 2). Furthermore, viral vectors (Kimura et al., 2019) and nonviral delivery systems (e.g., lipid-based [Buck et al., 2019] and polymeric [Malloggi et al., 2015] nanoparticles, electroporation [Labala et al., 2017], ultrasound [Lifshiz Zimon et al., 2018; Pereira et al., 2017], and microneedles [Dul et al., 2017]) have been utilized to deliver gene therapies and similarly hold promise for CRISPR-based therapeutics. In this section, we discuss the emerging technologies undergoing development to improve drug delivery for dermatological diseases and how this can potentially be applied for the delivery of CRISPR in the skin.
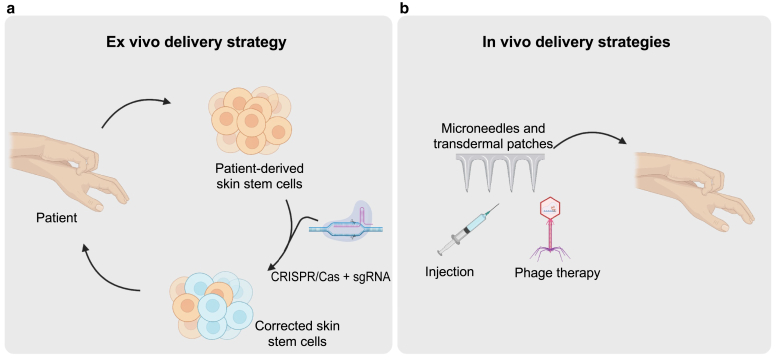
Figure 2.
CRISPR delivery strategies to the skin. (a) Ex vivo delivery strategy involves deriving patient skin stem cells, treating with CRISPR/Cas + sgRNA against the targeted gene, and reintegrating the corrected skin stem cells into patients. (b) Effective in vivo delivery strategies for CRISPR may include hollow, dissolvable microneedles that penetrate the epidermis, hypodermic needles, and phage delivery (for bacterial infection applications). sgRNA, short guide RNA.
The skin presents unique advantages owing to the accessibility of the epidermis as well as presents unique challenges owing to the relatively impermeable barrier of the stratum corneum and the size of this organ. Topical creams spread over the surface of the skin rely on diffusion to penetrate the skin barrier, resulting in 1‒5% bioavailability (Surber and Davis, 2002). Another strategy is transdermal drug delivery, which still must overcome the resilient barrier of the epidermis (Alkilani et al., 2015; Jeong et al., 2021; Prausnitz and Langer, 2008). Physical delivery methods through hypodermic needles are direct approaches to improve bioavailability but require penetrance into the dermis layer and are particularly painful, thereby reducing patient compliance. Laser-assisted drug delivery creates microscopic ablation zones, with vertical channels penetrating past the stratum corneum and accessing deep into the dermis (Haedersdal et al., 2016). Although this strategy has largely focused on the delivery of drugs such as methotrexate (Lee et al., 2008) and methyl aminolevulinate (Haedersdal et al., 2014), it remains to be shown whether laser-assisted drug delivery can be repurposed for delivering gene therapy vehicles such as CRISPR/Cas9.
Recent work has been done in delivering CRISPR-based therapeutics in animal models and in patients through clinical trials. Viral vector‒based methods such as adeno-associated viral vectors in theory could deliver CRISPR/Cas9, guide RNA, and a donor template (with the corrected mutation) into cells (Yang et al., 2016) but require more than one vector to simultaneously deliver the components, thereby reducing the efficiency of targeting. Another viral-based method for delivering CRISPR is phage therapy (Lam et al., 2021; Lenneman et al., 2021; Selle et al., 2020) for the treatment of bacterial infections. The central tenet in this situation is that bacteriophages selectively infect bacterial cells and can target a specific bacterial species DNA when packaged with CRISPR/Cas3 as has been successfully done in a recent clinical trial for the treatment of lower urinary tract infections caused by E. coli (NCT04191148).
Nonviral methods for CRISPR delivery have also gained traction. For example, intradermal injections followed by electroporation of CRISPR/Cas9 complexes targeting the gene encoding type VII collagen facilitated the transfection of skin stem cells and improved skin adhesion in a mouse model of EB (Wu et al., 2017). Hypodermic needles or intradermal injections (Jacków et al., 2019) are physical methods to directly deliver CRISPR therapeutics past the epidermis, but administration to patients can be painful and dampen clinical utility. Relatively new and innovative methods include microneedle technology (Dul et al., 2017; Wan et al., 2021), which utilize hollow and dissolvable microneedles to create small pores in the epidermis and successfully deliver drugs into the dermal layer. Specifically, CRISPR/Cas9 complexes targeting a proinflammatory gene NLPR3 in combination with dexamethasone were delivered through a microneedle patch in a mouse model of AD (Wan et al., 2021). In addition, to facilitate delivery, the microneedle was loaded with a polymer-encapsulated Cas9 and a dexamethasone-containing polymeric nanoparticle (Wan et al., 2021). Furthermore, this approach comes with markedly less pain because the microneedle does not encounter nociceptors in the dermis layer. Future work will be needed to show the safety and efficacy of this delivery strategy in patients and also the feasibility of extending this approach to whole-body administration. Furthermore, although many of these studies have been studied within the context of in vitro and animal models, it is our view that continued progress in this area will enable these therapeutics to reach human patients.
Limitations of CRISPR
Several concerns exist for the implementation of CRISPR for gene editing, which we briefly list in this paper. First, there are concerns of off-target effects; although some studies have shown that there are insignificant or even undetectable levels of off-target effects (Long et al., 2016), others have clear documentation of large insertions or deletions that may result in unintended consequences (Shin et al., 2017a). Second, immunogenicity against CRISPR proteins would need to be monitored given observations of pre-existing serum antibodies to Cas9 in some donors (Charlesworth et al., 2019) or prevalence of Cas9-reactive T cells in some patients (Wagner et al., 2019). The development of CRISPR inhibitors offers a solution to immunogenicity by disabling the gene-editing enzymes after DNA cleavage (Shin et al., 2017b). Finally, most CRISPR-mediated gene therapy has focused on ex vivo treatment, namely editing of stem cells outside the body and subsequent reintroduction of corrected cells back into patients. Although this strategy has been done to edit human epidermal stem cells or induce pluripotent stem cells from patients (Jayarajan et al., 2021) and successfully regraft corrected cells into mice, in vivo editing directly on the skin has been limited to a modicum of studies. Furthermore, gene editing of stem cells in vivo would target both stem and differentiated cells—this is likely not a limitation because stem cells will persist, and the differentiated skin cells will eventually die and slough off through the natural course of skin cell maturation. Another challenge includes treating wide areas of skin where whole-body involvement and treatment by CRISPR might not be tenable. Future work will need to be done to determine how efficiently emerging technologies in dermatology (i.e., microneedle drug delivery) will improve in vivo gene-editing capabilities in human tissue.
Conclusion
Treating the underlying cause of certain skin diseases through CRISPR/Cas gene editing in combination with symptomatic management will be crucial for better long-term patient outcomes. To that end, we are proposing that CRISPR will not altogether replace current therapies but rather enable dermatologists to provide a higher level of care to patients. For example, phototherapy is life changing for patients with severe AD (Rodenbeck et al., 2016), but they still may be at risk for bacterial skin infections, and chronic use of antibiotics might not be tenable, thereby requiring local administration of CRISPR therapy to treat skin infection. Nevertheless, the burgeoning field of CRISPR has only been around for a decade, with many open questions and room for improvement. Although CRISPR has tangible progress for localized administration in dermatological disease, further work will be needed to address the delivery strategies for disorders in which the whole body is affected (including multiorgan involvement). Furthermore, gene-editing efficiency is not 100%, thereby generating a heterogeneous mixture of cells that contain repaired DNA and the original, mutated DNA. Ongoing work will be needed to investigate what level of efficiency is enough to significantly improve long-term patient outcomes. We expect that recent advances in increasing editing efficiency and decreasing undesirable off-target effects (Donohoue et al., 2021) will bring us closer to incorporating CRISPR-mediated therapies in the dermatological setting.
ORCIDs
Prashant Bhat: http://orcid.org/0000-0003-3832-4871
Lilit Garibyan: http://orcid.org/0000-0002-9266-0887
Author Contributions
Conceptualization: PB, LG; Writing - Original Draft Preparation: PB; Writing - Review and Editing: PB, LG
Acknowledgments
PB is supported by the University of California, Los Angeles-Caltech Medical Scientist Training Program, National Institutes of Health F30CA247447, a Chen Graduate Innovator Grant, and the Josephine de Kármán Fellowship Trust. The figures were created using BioRender.
Conflict of Interest
LG received gift/grant support from Advancing Innovation in Dermatology and LEO Pharma for the Virtual Magic Wand program. Magic Wand is a service mark of The General Hospital Corporation, the owner of Massachusetts General Hospital. The remaining author states no conflict of interest.
accepted XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2022;X:100103
References
- Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., et al. RNA targeting with CRISPR–Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain Q.U., Campos E.V.R., Huynh A., Witzigmann D., Hedtrich S. Gene delivery to the skin – how far have we come? Trends Biotechnol. 2021;39:474–487. doi: 10.1016/j.tibtech.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani A.Z., McCrudden M.T.C., Donnelly R.F. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck L.A., Thaçi D., Hamilton J.D., Graham N.M., Bieber T., Rocklin R., et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- Benati D., Miselli F., Cocchiarella F., Patrizi C., Carretero M., Baldassarri S., et al. CRISPR/Cas9-mediated in situ correction of LAMB3 gene in keratinocytes derived from a junctional epidermolysis bullosa patient. Mol Ther. 2018;26:2592–2603. doi: 10.1016/j.ymthe.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Bonafont J., Mencía Á., García M., Torres R., Rodríguez S., Carretero M., et al. Clinically relevant correction of recessive dystrophic epidermolysis bullosa by dual sgRNA CRISPR/Cas9-mediated gene editing. Mol Ther. 2019;27:986–998. doi: 10.1016/j.ymthe.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowcock A.M., Cookson W.O. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum Mol Genet. 2004 doi: 10.1093/hmg/ddh094. 13 Spec No 1:R43–55. [DOI] [PubMed] [Google Scholar]
- Buck J., Grossen P., Cullis P.R., Huwyler J., Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13:3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- Capon F. The genetic basis of psoriasis. Int J Mol Sci. 2017;18:2526. doi: 10.3390/ijms18122526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth C.T., Deshpande P.S., Dever D.P., Camarena J., Lemgart V.T., Cromer M.K., et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 2019;25:249–254. doi: 10.1038/s41591-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Buhr H., Lebbink R.J. Harnessing CRISPR to combat human viral infections. Curr Opin Immunol. 2018;54:123–129. doi: 10.1016/j.coi.2018.06.002. [DOI] [PubMed] [Google Scholar]
- De Rosa L., Latella M.C., Secone Seconetti A., Cattelani C., Bauer J.W., Bondanza S., et al. Toward combined cell and gene therapy for genodermatoses. Cold Spring Harb Perspect Biol. 2020;12:a035667. doi: 10.1101/cshperspect.a035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoue P.D., Pacesa M., Lau E., Vidal B., Irby M.J., Nyer D.B., et al. Conformational control of Cas9 by CRISPR hybrid RNA-DNA guides mitigates off-target activity in T cells. Mol Cell. 2021;81:3637–3649.e5. doi: 10.1016/j.molcel.2021.07.035. [DOI] [PubMed] [Google Scholar]
- Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Dul M., Stefanidou M., Porta P., Serve J., O’Mahony C., Malissen B., et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J Control Release. 2017;265:120–131. doi: 10.1016/j.jconrel.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Fellmann C., Gowen B.G., Lin P.C., Doudna J.A., Corn J.E. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16:89–100. doi: 10.1038/nrd.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangoul H., Bobruff Y., Cappellini M.D., Corbacioglu S., Fernandez C.M., de la Fuente J., et al. Safety and efficacy of CTX001 in patients with transfusion-dependent β-thalassemia and sickle cell disease: early results from the climb THAL-111 and climb SCD-121 studies of autologous CRISPR-CAS9–modified CD34+ hematopoietic stem and progenitor cells. Blood. 2020;136:3–4. [Google Scholar]
- Frangoul H., Altshuler D., Cappellini M.D., Chen Y.S., Domm J., Eustace B.K., et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- Greene A.C. CRISPR-based antibacterials: transforming bacterial defense into offense [published correction appears in Trends Biotechnol 2018;36:1299] Trends Biotechnol. 2018;36:127–130. doi: 10.1016/j.tibtech.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Haedersdal M., Erlendsson A.M., Paasch U., Anderson R.R. Translational medicine in the field of ablative fractional laser (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981–1004. doi: 10.1016/j.jaad.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Haedersdal M., Sakamoto F.H., Farinelli W.A., Doukas A.G., Tam J., Anderson R.R. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL) Lasers Surg Med. 2014;46:462–469. doi: 10.1002/lsm.22259. [DOI] [PubMed] [Google Scholar]
- Hainzl S., Peking P., Kocher T., Murauer E.M., Larcher F., Del Rio M., et al. COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Mol Ther. 2017;25:2573–2584. doi: 10.1016/j.ymthe.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M.L., Taylor D.W., Bhat P., Guegler C.K., Sternberg S.H., Nogales E., et al. CasA mediates Cas3-catalyzed target degradation during CRISPR RNA-guided interference. Proc Natl Acad Sci USA. 2014;111:6618–6623. doi: 10.1073/pnas.1405079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine A.D., McLean W.H.I., Leung D.Y.M. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Izmiryan A., Ganier C., Bovolenta M., Schmitt A., Mavilio F., Hovnanian A. Ex vivo COL7A1 correction for recessive dystrophic epidermolysis bullosa using CRISPR/Cas9 and homology-directed repair. Mol Ther Nucleic Acids. 2018;12:554–567. doi: 10.1016/j.omtn.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacków J., Guo Z., Hansen C., Abaci H.E., Doucet Y.S., Shin J.U., et al. CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc Natl Acad Sci USA. 2019;116:26846–26852. doi: 10.1073/pnas.1907081116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarajan V., Kounatidou E., Qasim W., Di W.L. Ex vivo gene modification therapy for genetic skin diseases—recent advances in gene modification technologies and delivery. Exp Dermatol. 2021;30:887–896. doi: 10.1111/exd.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W.Y., Kwon M., Choi H.E., Kim K.S. Recent advances in transdermal drug delivery systems: a review. Biomater Res. 2021;25:24. doi: 10.1186/s40824-021-00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Ferran B., Tsukahara Y., Shang Q., Desai S., Fedoce A., et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci Rep. 2019;9:13601. doi: 10.1038/s41598-019-49624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C.J., Atzmony L., Lim Y., McNiff J.M., Craiglow B.G., Antaya R.J., et al. Review of genodermatoses with characteristic histopathology and potential diagnostic delay. J Cutan Pathol. 2019;46:756–765. doi: 10.1111/cup.13520. [DOI] [PubMed] [Google Scholar]
- Kocher T., March O.P., Bischof J., Liemberger B., Hainzl S., Klausegger A., et al. Predictable CRISPR/Cas9-mediated COL7A1 reframing for dystrophic epidermolysis bullosa. J Invest Dermatol. 2020;140:1985–1993.e5. doi: 10.1016/j.jid.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Kocher T., Peking P., Klausegger A., Murauer E.M., Hofbauer J.P., Wally V., et al. Cut and paste: efficient homology-directed repair of a dominant negative KRT14 mutation via CRISPR/Cas9 nickases. Mol Ther. 2017;25:2585–2598. doi: 10.1016/j.ymthe.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labala S., Jose A., Chawla S.R., Khan M.S., Bhatnagar S., Kulkarni O.P., et al. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int J Pharm. 2017;525:407–417. doi: 10.1016/j.ijpharm.2017.03.087. [DOI] [PubMed] [Google Scholar]
- Lacey S.F., Fraietta J.A. First trial of CRISPR-edited T cells in lung cancer. Trends Mol Med. 2020;26:713–715. doi: 10.1016/j.molmed.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Lam K.N., Spanogiannopoulos P., Soto-Perez P., Alexander M., Nalley M.J., Bisanz J.E., et al. Phage-delivered CRISPR-Cas9 for strain-specific depletion and genomic deletions in the gut microbiome. Cell Rep. 2021;37:109930. doi: 10.1016/j.celrep.2021.109930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.R., Shen S.C., Fang C.L., Zhuo R.Z., Fang J.Y. Topical delivery of methotrexate via skin pretreated with physical enhancement techniques: low-fluence erbium:YAG laser and electroporation. Lasers Surg Med. 2008;40:468–476. doi: 10.1002/lsm.20655. [DOI] [PubMed] [Google Scholar]
- Lenneman B.R., Fernbach J., Loessner M.J., Lu T.K., Kilcher S. Enhancing phage therapy through synthetic biology and genome engineering. Curr Opin Biotechnol. 2021;68:151–159. doi: 10.1016/j.copbio.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshiz Zimon R., Lerman G., Elharrar E., Meningher T., Barzilai A., Masalha M., et al. Ultrasound targeting of Q-starch/miR-197 complexes for topical treatment of psoriasis. J Control Release. 2018;284:103–111. doi: 10.1016/j.jconrel.2018.05.040. [DOI] [PubMed] [Google Scholar]
- Long C., Amoasii L., Mireault A.A., McAnally J.R., Li H., Sanchez-Ortiz E., et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351:400–403. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Xue J., Deng T., Zhou X., Yu K., Deng L., et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer [published correction appears in Nat Med 2020;26:1149] Nat Med. 2020;26:732–740. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- Ma C.A., Stinson J.R., Zhang Y., Abbott J.K., Weinreich M.A., Hauk P.J., et al. Germline hypomorphic CARD11 mutations in severe atopic disease [published correction appears in Nat Genet 2017;49:1661] Nat Genet. 2017;49:1192–1201. doi: 10.1038/ng.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloggi C., Pezzoli D., Magagnin L., De Nardo L., Mantovani D., Tallarita E., et al. Comparative evaluation and optimization of off-the-shelf cationic polymers for gene delivery purposes. Polym Chem. 2015;6:6325–6339. [Google Scholar]
- March O.P., Reichelt J., Koller U. Gene editing for skin diseases: designer nucleases as tools for gene therapy of skin fragility disorders. Exp Physiol. 2018;103:449–455. doi: 10.1113/EP086044. [DOI] [PubMed] [Google Scholar]
- O’Regan G.M., Irvine A.D. The role of filaggrin loss-of-function mutations in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2008;8:406–410. doi: 10.1097/ACI.0b013e32830e6fb2. [DOI] [PubMed] [Google Scholar]
- Pereira T.A., Ramos D.N., Lopez R.F.V. Hydrogel increases localized transport regions and skin permeability during low frequency ultrasound treatment. Sci Rep. 2017;7:44236. doi: 10.1038/srep44236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz M.R., Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey E., Sünderhauf D., Gaze W.H., Westra E.R., van Houte S. CRISPR-Cas antimicrobials: challenges and future prospects. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding S., Sternberg S.H., Marshall M., Gibb B., Bhat P., Guegler C.K., et al. Surveillance and processing of foreign DNA by the Escherichia coli CRISPR-Cas system. Cell. 2015;163:854–865. doi: 10.1016/j.cell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenbeck D.L., Silverberg J.I., Silverberg N.B. Phototherapy for atopic dermatitis. Clin Dermatol. 2016;34:607–613. doi: 10.1016/j.clindermatol.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Selle K., Fletcher J.R., Tuson H., Schmitt D.S., McMillan L., Vridhambal G.S., et al. In vivo targeting of Clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. mBio. 2020;11:e00019–e00020. doi: 10.1128/mBio.00019-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.Y., Wang C., Lee H.K., Yoo K.H., Zeng X., Kuhns T., et al. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat Commun. 2017;8:15464. doi: 10.1038/ncomms15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Jiang F., Liu J.J., Bray N.L., Rauch B.J., Baik S.H., et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv. 2017;3 doi: 10.1126/sciadv.1701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkuma S., Guo Z., Christiano A.M. Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc Natl Acad Sci USA. 2016;113:5676–5681. doi: 10.1073/pnas.1512028113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367 doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surber C., Davis A.F. In: Dermatological transdermal formulations. Walters K.A., editor. CRC Press; Boca Raton, FL: 2002. Bioavailability and bioequivalence of dermatological formulations; pp. 401–498. [Google Scholar]
- Viertel T.M., Ritter K., Horz H.P. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother. 2014;69:2326–2336. doi: 10.1093/jac/dku173. [DOI] [PubMed] [Google Scholar]
- Wagner D.L., Amini L., Wendering D.J., Burkhardt L.M., Akyüz L., Reinke P., et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat Med. 2019;25:242–248. doi: 10.1038/s41591-018-0204-6. [DOI] [PubMed] [Google Scholar]
- Wan T., Pan Q., Ping Y. Microneedle-assisted genome editing: a transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber B.R., Osborn M.J., McElroy A.N., Twaroski K., Lonetree C.L., DeFeo A.P., et al. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regen Med. 2016;1:16014. doi: 10.1038/npjregenmed.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger S., Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- Wu W., Lu Z., Li F., Wang W., Qian N., Duan J., et al. Efficient in vivo gene editing using ribonucleoproteins in skin stem cells of recessive dystrophic epidermolysis bullosa mouse model. Proc Natl Acad Sci USA. 2017;114:1660–1665. doi: 10.1073/pnas.1614775114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang L., Bell P., McMenamin D., He Z., White J., et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 2016;34:334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]