Abstract
The health of women over the entire span of their reproductive years is crucial — beginning in adolescence and extending through the postpartum period. This paper provides a scoping review of the relevant literature on risk factors for gestational diabetes mellitus (GDM) and progression from GDM to type 2 diabetes mellitus (T2DM), particularly among women of Native Hawaiian and Pacific Islander (NHPI) and Asian racial/ethnic backgrounds in Hawai‘i, using the PubMed database (July 2010 to July 2020). NHPI and Asian populations have a greater likelihood of developing GDM compared to their White counterparts. Risk factors such as advanced maternal age, high maternal body mass index, and lack of education about GDM have varying levels of impact on GDM diagnosis between ethnic populations. Mothers who have a history of GDM are also at higher risk of developing T2DM. Common risk factors include greater increase in postpartum body mass index and use of diabetes medications during pregnancy. However, few studies investigate the progression from GDM to T2DM in Hawai‘i’s Asian and NHPI populations, and no studies present upstream preconception care programs to prevent an initial GDM diagnosis among Hawai‘i’s women. Thus, updated reports are necessary for optimal early interventions to prevent the onset of GDM and break the intergenerational cycle of increased susceptibility to T2DM and GDM in both mother and child. Further attention to the development of culturally sensitive interventions may reduce disparities in GDM and improve the health for all affected by this condition.
Keywords: gestational diabetes mellitus, Native Hawaiian or Pacific Islander, Asian, minority populations, health disparities, intergenerational prevention, scoping review
Introduction
Gestational diabetes mellitus (GDM) refers to “any degree of glucose intolerance with onset or first recognition during pregnancy”1 and increases the risk of adverse pregnancy outcomes for both mother (ie, preeclampsia, cesarean delivery, preterm delivery) and neonate (ie, shoulder dystocia, macrosomia, neonatal hypoglycemia, and birth trauma).2–4 These issues are of increasing importance as GDM affects 5% to 9% of pregnancies in the United States (US), with the prevalence steadily increasing over the last 20 years.5,6 As GDM incidence and prevalence increase, the racial/ethnic disparities gap continues to widen between Whites and non-White minority women diagnosed with GDM. Previous studies have shown that American Indians, Native Hawaiians (NH), Hispanics, Asians and African Americans experience higher rates of GDM compared to White women.7
GDM is also of particular concern because it is associated with an increased risk of developing type 2 diabetes mellitus (T2DM), obesity, and other adverse metabolic effects on metabolism later in a mother’s lifetime.8–10 T2DM is a major health concern in the US, especially among racial/ethnic groups such as Native Hawaiians and Pacific Islanders (NHPI) and Asians. In Hawai‘i, 27% of the population self-identifies as NHPI and 24.2% as multiracial. Among Asians in Hawai‘i, 37.6% self-identify as Asian only, although the state reports 56.4% of its 1.4 million residents are of diverse Asian backgrounds.11 From 2017 to 2019, 14.4% of NH, 18.5% of Pacific Islander (PI), and 8.4%-13.0% of adults from various Asian races in Hawai‘i reported a diagnosis of T2DM, and this prevalence has continued to increase.12
Furthermore, ample epidemiologic and experimental evidence supports the phenomenon of “fetal programming,” in which the intrauterine environment predisposes the fetus to adult onset cardiometabolic conditions later in life.13–16 Infants born to mothers with GDM are themselves at increased risk for T2DM as adults, in part due to epigenetic changes induced by the diabetic intrauterine environment.13–16 Thus, existing health disparities in GDM in the current generation may exacerbate T2DM disparities in future generations. This lends urgency and importance to the prevention of GDM in today’s women of reproductive age.
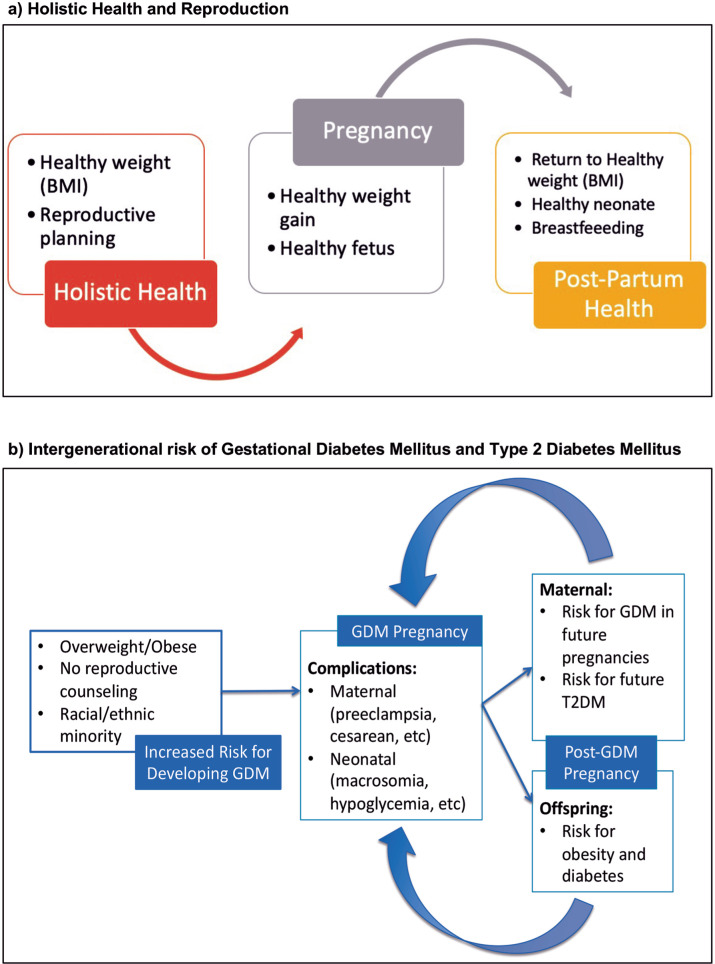
Given the increasing risk of both GDM and T2DM among racial/ethnic minority populations as well as the increased risk for T2DM in the offspring of pregnancies complicated by GDM, the need to understand how best to stop the intergenerational risk for developing GDM, and hence T2DM, is imperative to break this cycle (Figure 1). Thus, the purpose of this paper was to conduct a scoping review to evaluate the current literature relevant to Hawai‘i’s multi-ethnic women and adolescent females of NHPI and Asian (Chinese, Japanese, Korean, Vietnamese, Filipino) ethnic groups who are at highest risk of developing GDM and subsequent T2DM.
Figure 1.
Proposed Concept of Holistic Health and Reproduction compared with Intergenerational Risk of Gestational Diabetes Mellitus for At-risk Women
Methods
Database searches were performed in PubMed for the time period between July 1, 2010 and July 1, 2020 for English language publications. Two authors independently reviewed all articles’ abstracts and discussed the selection until consensus was reached,
The search was conducted with two primary areas of focus. The first search aimed to include literature on GDM risk and risk factors among Asian and NHPI women. The search terms used included gestational diabetes combined with the following terms using an AND function: Hawai’i, Pacific Islander, and Asian. Articles were excluded if they (1) were reviews of previous literature, (2) did not distinguish between pre-gestational and gestational diabetes, (3) were limited either to neonatal outcomes/populations other than Asians and/or NHPIs, or (4) included a population exclusively outside of the US. After review of all retrieved articles and application of exclusion criteria, a final selection of 15 peer-reviewed papers were included (Figure 2a).
Figure 2a.

Flow diagram of literature screening process for GDM risk and risk factors among Asian and NHPI women
The second portion of the search included literature on the progression of GDM to T2DM. The following search terms were used: gestational diabetes, type 2 diabetes, progression, Hawai’i, Pacific Islander, and Asian (Figure 2b). Articles were excluded if they (1) were reviews of previous literature, (2) did not address the progression between GDM and T2DM, or (3) included a population exclusively outside of the US. All retrieved articles were reviewed and exclusion criteria were applied, yielding a selection of 14 papers. Thus, a total of 29 articles were included in this scoping review.
Figure 2b.
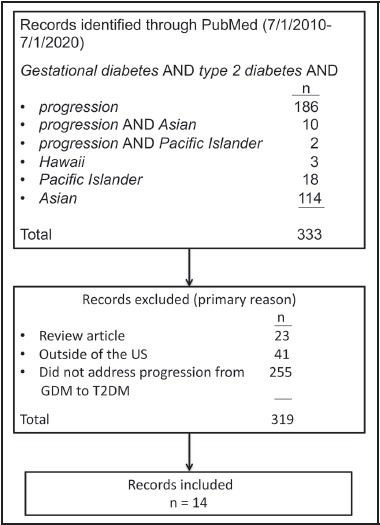
Flow diagram of literature screening process for Progression of GDM to T2DM
Results
Studies on Risk for Developing GDM and Characteristics of GDM Pregnancies (Table 1A)
The 15 studies highlighted racial disparities in GDM as well as differing associations between GDM and risk factors among Asian and NHPI women compared to other racial groups. Key findings include the following:
Epidemiology and Racial/Ethnic Disparities (Table 1.A.1)
Multiple studies from varying cohorts within the US demonstrated increased risk of GDM among Asian women.17–21 For example, one study found that GDM prevalence was higher in Asian American subgroups (Filipino: 19.0%, Asian Indian: 19.3%, Chinese: 15.3%, Korean: 12.9%, Vietnamese: 18.8%) compared with non-Hispanic Whites (7.0%).20 Similar findings of increased rates of GDM among Asian women were present in a Los Angeles cohort.17 Also, Hunsberger et al found that Asian women had a 16.4% prevalence of GDM while PI women had an 11.7% prevalence, both of which were increased compared to White women (6.0%).21 However, it is important to note that such comparisons may be affected by different diagnostic criteria used to diagnose GDM and/or variable access to care.
Among women in Hawai‘i in 2013, Tsai et al found the highest GDM prevalence to be among Filipino women (13.1%) and NHPI women (12.1%), with lowest prevalence in White women (7.4%). Overall, Asian and PI women had a 50% increased odds of developing GDM compared to their White counterparts.22 In 2015, Chang et al looked at PI subpopulations and found that NHs (9.3%), Micronesians (8.5%), Samoans (11.8%), and other PIs (13.7%) had higher rates of GDM compared to Whites (5.8%).3 Such findings also demonstrate the heterogeneity even within Asian and PI populations and speak to the need to disaggregate ethnic groups from larger racial classifications, especially if there are substantial differences within racial classifications that can inform interventions targeted to specific cultures.
Clinical, Lifestyle, Nutrition, and Environmental Factors (Table 1.A.2–3)
Several studies identified common risk factors for developing GDM, including advanced maternal age, high pre-pregnancy body mass index (BMI), a history of a GDM pregnancy, and non-White race.17,21–25
Despite the commonality among GDM risk factors, differences between and within racial/ethnic groups were observed. For example, Pu et al found the effects of advanced maternal age to be more significant for non-Hispanic Whites and Hispanics compared to Asian Americans, suggesting that the pathophysiology of GDM might differ by racial/ethnic group.20 Similarly, BMI was less predictive for GDM among Asians,19 and overweight/obese BMI contributed less to GDM risk in Asian and PI women compared to other racial groups.23–25 Conversely, an inverse association between maternal height and GDM risk was strongest in Asian women.26 Another study found that individuals of Asian or PI ancestry have a higher susceptibility to pancreatic beta cell dysfunction when exposed to volatile organic compounds.27
Associations between immigration and acculturation and GDM risk have been investigated with conflicting results.17,20,28–30 Pu et al found that Chinese and Filipino women born outside the US had a higher risk of developing GDM compared to their US-born counterparts,20 and Chen et al also found increased GDM among Asian women with less acculturation.17 Likewise, in a California cohort, Pacific Islander, Filipina, and Chinese women born outside the US had higher rates of GDM compared to those born in the US. However, in that same cohort, Japanese and Korean women born outside of the US had decreased risks of GDM compared to those who were born in the US.28 Other cohorts have not found GDM rates to change with country of birth or markers of acculturation.29,30 These inconsistencies highlight the complexity of the relationships between immigration, acculturation, and GDM. Immigration from various Asian and Pacific Island locations to Hawai‘i, both historical and current, highlights the need for careful ongoing evaluation of GDM disparities.20
Studies on Risk for Developing T2DM following GDM Pregnancy (Table 1B)
Women with a history of GDM pregnancy are at an increased risk of developing T2DM later in life. A clear limitation of those findings is the unknown rates of T2DM among those who do not receive postpartum testing. Key findings from the 14 reviewed articles include the following:
Clinical, Lifestyle, and Metabolic Signatures (Table 1.B.1–3)
Risk factors for the subsequent diagnosis of T2DM after GDM include low intensity and short duration of lactation, earlier gestational age at GDM diagnosis, use of insulin or oral diabetes medications during pregnancy, higher pre-pregnancy BMI (>25 kg/m2), advanced age, lower physical activity, higher dietary consumption of animal fat, greater intakes of total iron, lack of healthy lifestyle behaviors, adverse newborn outcomes, and greater postpartum BMI increase.31–37 For example, the Diabetes and Women’s Health cohort study found that women who gained greater than 5 kg (11 lbs) after a GDM pregnancy had a 43-times higher risk of developing T2DM compared to those gaining less than 5 kg (11 lbs).6 Other risk factors included lipid dysmetabolism and the presence of metabolic signatures such as certain amino acids, medium-chain acylcarnitines, and reduced levels of sphingolipid metabolism.38–41
Racial/Ethnic Disparities (Table 1.B.4)
Racial/ethnic disparities have been observed in the development of T2DM after GDM. Gunderson et al reported that Hispanic (41%) and Asian (32%) women were at a higher risk for developing T2DM after a GDM pregnancy compared to non-Hispanic Whites (15%).31 However, recent investigations regarding the progression from GDM to T2DM among Hawai.i’s Asian and NHPI women were lacking.
Prevention Interventions and Postpartum Follow-up Care (Table 1.B.5)
GDM represents a strong risk factor for later development of T2DM. Despite the importance of screening, suboptimal follow-up prevents many women from obtaining T2DM screening and education.42 Reasons for not obtaining postpartum T2DM screening include missed appointments, false assumptions about the low risk for T2DM, inconvenient or unpleasant tests, fear of a T2DM diagnosis, time constraints, and lack of physician awareness and communication.38
For those who do receive follow-up care, it was found that postpartum intervention implementation reduces the progression to T2DM. One study reported that diets consisting of fruits, vegetables, and low-fat dairy products, were associated with a 15-35% reduction in incidence of T2DM after GDM.6 Other studies also found that intensive lifestyle interventions and metformin were highly effective in delaying and preventing subsequent T2DM.10
Discussion
This scoping review confirms the disproportionate burden of risk factors leading to GDM or subsequent development of T2DM in NHPIs and Asians. These findings are supported by other studies in the literature, which demonstrate that GDM disproportionately affects Asian, African American, and Hispanic women compared to White women, and that the risk for developing GDM is 3-fold higher for Asian women: a trend that increases exponentially with increasing BMI.4,43–45
In addition to the 2 studies in this review that investigated GDM prevalence among Asian and NHPI populations in Hawai‘i,3,22 the most recent PRAMS data reported that 14.4% of new mothers in Hawai‘i self-reported a GDM diagnosis in 2015, including 27.1% of Samoan and 20.2% of Filipino mothers, compared to 6.6% of White mothers. This review demonstrates the growing number of pregnancies complicated by GDM in Hawai‘i, which is up from 4.8% in 1995. Work done prior to this scoping review found similar racial/ethnic disparities in GDM prevalence.45 This further highlights the longstanding disparities in GDM prevalence that are still present today.
The time before and during pregnancy is an important time in a woman’s life to prevent an initial GDM diagnosis, or to mitigate the risk of a future T2DM diagnosis for both herself and her offspring.16,46 A substantial number of women, especially those of high-risk racial/ethnic groups, are unaware of the risk factors that could lead to the development of GDM and subsequent T2DM. For example, one study showed that 42% of a cohort of Samoan women were either unsure or not aware that diabetes could first occur during pregnancy. Furthermore, only 25% of participants identified pre-pregnancy obesity as a risk factor for GDM, which was the most widely identified risk factor in the study.46 Another study interviewed American Indian and Alaska Native women who stated they wished they had been aware of the risk factors for GDM prior to pregnancy. Participants also voiced that intervention programs should be cognizant of cultural preferences when encouraging diet and lifestyle modifications.47
Therefore, culturally appropriate preconception planning programs are important to provide education counseling to women of reproductive age who may face language barriers when seeking reproductive health guidance.,5,8,9,47 Populations that may benefit from such programs include immigrants to higher-income countries, who are traditionally underweight or of normal weight, yet have higher rates of obesity and thus are at increased risk of developing GDM.20,43,48,49 For example, a program for a disadvantaged Mexican-American cohort partnered coaches with diabetic patients based on language and ethnicity. These coaches helped to promote patient engagement during medical appointments.9 Other studies proposed and implemented programs that educated women about GDM, GDM-associated risk factors, reproductive health, nutrition label interpretation, and how to include diabetes care amidst family and social situations.8,9,28
Prior work has reported success in preventative interventions for GDM during pregnancy. One study of high-risk Finnish women in early pregnancy using a lifestyle intervention was shown to reduce the development of GDM by 39%.50 In addition, 2 meta-analyses found physical activity programs in pregnancy reduce the development of GDM.51,52 while not all preventative interventions demonstrated such positive results,16 these encouraging data suggest that modifiable risk factors can be effectively addressed to reduce GDM. Thus, based on our review findings highlighting disparities and challenges regarding GDM particular to NHPI and Asian women, preventative interventions targeted to these high-risk groups are warranted.
Because a recent meta-analysis found that women with previous GDM have a 10-fold increased risk of developing T2DM compared to women without a history of GDM,53 and the prevalence of T2DM following GDM ranged from 26% to 36% with the risk of T2DM increasing linearly by 10% every 10 years in other cohorts, the necessity to manage care after an initial GDM diagnosis is critical to avoid long-term health complications.54,55 A common theme across other reviews shows that early and consistent postpartum screening for T2DM plays an essential role in delaying and preventing the onset of T2DM after GDM.56 However, similar to the reviewed literature, other sources cite inadequate GDM screening resources and inadequate training for staff, difficulty scheduling appointments or tests, and family-related practicalities as reasons why patients are unable to receive proper follow-up screening.43,57
while various risk factors have been established for the progression of GDM to T2DM, few studies focus on Asian and NHPI women, particularly in Hawai‘i. As previous studies showed that PIs had the highest need for insulin to treat GDM, and the use of insulin, as a marker of greater hyperglycemia, is associated with a 3.5 times higher risk for postpartum T2DM. Similar investigation of disparities in other risk factors would offer the opportunity to develop a tailored-approach to preventative care for high-risk populations.49
Study Limitations
There were several common limitations that posed difficulties in reviewing and interpreting the published literature. First, inconsistent criteria used to clinically diagnose GDM hindered comparisons between, and possibly even within, cohorts.17–21 GDM risk factors may also vary depending on the criteria used to diagnose the condition.
In addition, due to the large number of multiracial women in Hawai‘i, classifying individuals into a single racial/ethnic group is challenging. Aggregating racial/ethnic groups and subgroups may assist in classification, however, heterogeneity within racial/ethnic groups may obscure important differences between ethnic groups that are clinically relevant.3,20–22
Finally, many of the studies relied on retrospective data, such as birth certificate data, that may underestimate GDM prevalence and lack important variables.3 On the other hand, GDM diagnoses in women prior to 20 weeks’ gestation suggest the presence of pre-existing undiagnosed diabetes that was only identified when tested during pregnancy, thereby overestimating true GDM prevalence.3 Other studies also used self-report data for variables such as GDM, BMI, and ethnicity, which may also be subject to bias.22
Conclusion
This review shows that there is a paucity of recent literature on the ethnic disparities in GDM and subsequent development of T2DM, particularly for Hawai‘i’s Asian and NHPI populations, despite these populations’ known increased risk for GDM. Given the relationships between GDM, subsequent T2DM, and increased adult-onset metabolic disease in offspring exposed to in-utero GDM/T2DM, these disparities are likely to worsen without intervention. Periodic reviews of present disparities and the healthcare system’s response to the increase of GDM and T2DM are necessary to help design upstream ethnic-specific intervention and education programs in order to stop the perpetual intergenerational cycle of GDM and T2DM in Asian and NHPI populations. Identifying high-risk women prior to and early in pregnancy offers unique advantages during a “window of opportunity” in women’s reproductive lifecycles to implement interventions and educational programs aimed at healthy lifestyle behaviors that may reduce the risk of GDM and subsequent T2DM.
Future studies should also look at individuals who identify with a particular mix of races/ethnicities that independently are at increased risk for GDM or subsequent T2DM. This would provide a more accurate representation of Hawai.i’s multi-ethnic population and could inform the development of ethnic-specific approaches to dietary preferences and cultural-based physical activities. In addition, it is critical to establish consistent screening with standard diagnostic criteria to better detect and compare the development of GDM in vulnerable populations.5
Lastly, several risk factors for GDM were shown to vary by race/ethnicity, such as high BMI and maternal age. Future studies should continue to investigate other modifiable and health system risk factors, such as follow-up screening and post-partum care of GDM pregnant mothers aimed at reducing the progression from GDM to T2DM that may vary between populations.
Table 1.
Summary of Scoping Review on Risk Associated with Developing Gestational Diabetes Mellitus and Subsequent Type 2 Diabetes Mellitus
| Study Categories | Studies | Number of Participants | Study Time Period | Location | Purpose of Study | Methods | Key Findings Relevant to Hawai‘i's Multi-ethnic Population |
| A) Studies on Risk for Developing GDM and Characteristics of GDM Pregnancies (n=15) | |||||||
| 1) Epidemiology and Racial/Ethnic Disparities | Chang et al. (2015) | n = 5,510 (Native Hawaiian=6,662, Micronesian=1,548, Samoan=897, Other Pacific Islanders=539) |
2010-2011 | Hawai‘i | Quantify obstetric outcomes of Pacific Islander subgroups in Hawai‘i | Retrospective cohort study | Outcome differences were observed between Pacific Islander subpopulations and when compared to White women. Of Pacific Islander subgroups, Micronesians had the highest risk of cesarean sections and Native Hawaiians had the highest risk of low-birthweight infants Samoans had a higher risk of macrosomia compared to Whites, while Native Hawaiians and Micronesians were significantly less likely to have an infant with macrosomia compared to Whites |
| Chen et al. (2019) | n = 5,562 | 2007 | Los Angeles, California | Assess GDM prevalence among Asian Americans and association with acculturation | Cross-sectional study | Compared to non-Hispanic White women, Asian women had a higher risk of GDM (OR 2.44 [95%CI 1.81–3.29]) Acculturation negatively associated with GDM |
|
| Hunsberger et al. (2010) | n = 3,883 (API=617) |
2004–2005 | Oregon | Explore racial/ethnic disparities in the prevalence of GDM | Retrospective cohort study | Asian/Pacific Islander women (both normal and high BMI) had the highest prevalence of GDM (14.8%) among all women studied Asian women were more likely to have GDM compared to Pacific Islander women |
|
| Liu et al. (2020) | 16,258 (Asian n=620) | 2010–2015 | Detroit, Michigan | Investigate relationships between maternal race/ethnicity and age with GDM | Retrospective cohort study | Asian women had a significantly higher GDM risk compared to White women (OR 2.53 [95%CI 2.10–3.10]) Older maternal age increased GDM risk and affected risk interactively with race/ethnicity (smaller effect of age among African American compared to non-African American women) |
|
| Pu et al. (2015) | n = 24,195 (Chinese=3,218), Filipino=1,088, Japanese=677, Korean=460, Vietnamese=460) |
2007–2012 | California | Assess racial/ethnic differences in risk factors for GDM | Retrospective cohort study | GDM was most prevalent among Asian American subgroups: Asian Indian (19.3%), Chinese (15.3%), Filipino (19.0%), Korean (12.9%) and Vietnamese (18.8%) Family history and foreign-born status were important risk factors for Asian subgroups Risk attributed to advanced maternal age was modified by race/ethnicity |
|
| Tsai et al. (2013) | n = 4,735 (Native Hawaiian/Pacific Islander=20,851, Filipina=9,922, Other Asian=9,225) |
2009–2011 | Hawai‘i | Examine the relationship between ethnicity, GDM, and macrosomia in Hawai‘i | Retrospective cohort study | Overall prevalence of GDM in Hawai‘i was 10.9% The highest prevalence of GDM was in Filipina (13.1%) and Native Hawaiian/Pacific Islander (12.1%) women, while the lowest prevalence was in White women (7.4%) Asian/Pacific Islander women had a 50% increased odds of having GDM compared to White women |
|
| 2) Clinical Risk Factors | Brite et al. (2014) | n=135,861 (Asian=4,190) |
2002–2008 | Consortium on Safe Labor study (11 states and District of Columbia) | Evaluate associations between maternal height and GDM across racial/ethnic groups | Retrospective cohort study | Maternal height was inversely correlated to GDM risk, with the strongest association among Asians |
| Hedderson et al. (2012) | n=123,040 (Asian=18,497, Filipina=9,636) |
1995–2006 | Northern California | Examine associations between GDM and BMI by racial/ethnic groups | Retrospective cohort study | Asian and Filipina women had an increased risk of GDM at a lower BMI compared to non-Hispanic White and African American women | |
| Kim et al. (2012) | n=656,925 (API=16,799) |
2004–2007 | Florida | Evaluate percentages of GDM attributable to overweight and obesity across racial/ethnic groups | Cross-sectional study | API women had a higher GDM prevalence (9.9% vs 4.0% for Black women). API women had the lowest percentage of GDM attributable to overweight and obesity compared to other racial groups |
|
| Kim et al. (2013) | n=1,228,265 (API=168,933) |
2007–2009 | California | Evaluate percentages of GDM attributable to overweight and obesity across racial/ethnic groups | Cross-sectional study | API women had a higher GDM prevalence (11.9% vs 5.4% for White women). API women had the lowest percentage of GDM attributable to overweight and obesity compared to other racial groups |
|
| Shah et al. (2011) | 24,325 (Asian n=7,404) |
1988–200 | San Francisco, California | Examine body mass index as a screening tool for GDM among racial/ethnic groups | Retrospective cohort study | Asian women had increased risk of GDM compared to White women regardless of body mass index Body mass index had the poorest sensitivity for GDM among Asian women compared to other racial groups |
|
| 3) Lifestyle, Nutrition, and Environmental Factors | Hedderson et al. (2010) | n=216,089 | 1995–2004 | Northern California | Compare GDM risk by country of birth (US or non-US) among racial/ethnic groups | Retrospective cohort study | Being born outside of the US associated with increased risk of GDM among Filipina, Chinese, and Pacific Islander women, but a decreased risk of GDM in Japanese and Korean women |
| Janevic et al. (2014) | n=89,703 (Chinese=10,603) |
2001–2002 | New York City | Evaluate associations between living in an ethnic enclave and risk for GDM | Cross-sectional study | Among Chinese women, no association between living in an ethnic enclave and GDM risk | |
| Ramadhani et al. (2011) | n=6,463 (API=198) |
1997–2005 | National Birth Defects Prevention Study (10 states in the US) | Examine differences in pregnancy-related risk factors among US versus non-US born women across racial/ethnic groups | Retrospective cohort study | No difference in GDM between US and non-US born API women | |
| Williams et al. (2019) | n=220,065 (API=9,068) |
2002–2008 | Consortium on Safe Labor study (11 states and District of Columbia) | Understand racial/ethnic disparities in GDM by looking at exposure to volatile organic compounds (VOCs) | Retrospective cohort study | Exposure to high VOCs was associated with increased odds of GDM among whites and Asian/Pacific Islanders GDM risk was significantly higher for Asian/Pacific Islanders compared to whites for most compounds |
|
| B) Studies on Risk for Developing T2DM following GDM pregnancy (n=14) | |||||||
| 1) Clinical Factors | Bao et al. (2015) | n=1,695 | 1991–2001 | Nurse’s Health Study II cohort (14 states in US) | Examine how adiposity and weight change influences the long-term risk of developing T2DM after GDM | Prospective cohort study | Baseline BMI, most recent BMI, and weight gain after GDM pregnancy were significantly and positively associated with risk of progression from GDM to T2DM |
| Gunderson et al. (2014) | n=1,007 (Asian=362) |
2008–2011 | SWIFT cohort; Northern California: Asian, Non-Hispanic White, Non-Hispanic Black, Hispanic, Other | Investigate whether higher lactation intensity is related to more favorable blood lipids, lipoproteins, and adipokines after a GDM pregnancy | Prospective cohort study | Higher lactation intensity was associated with more favorable biomarkers for T2DM except for lower plasma adiponectin after GDM delivery | |
| Zhang et al. (2019) | n=7,759 | 2012–2016 | Denmark and US | Investigate the genetic and environmental factors that are implicated in the progression from GDM to T2DM | Hybrid Prospective cohort study combined with existing data | Progression from GDM to T2DM ranged from 23.1% to 27.2% of the study population Women with a history of GDM have a greater risk of hypertension and cardiovascular disease A healthful diet, lifestyle factors, and weight control correlated with a lower risk of T2DM, hypertension, and cardiovascular disease |
|
| 2) Lifestyle Factors | Allalou et al. (2016) | n=1,035 (Asian=340) |
2008–2011 | Non-Hispanic White, Non-Hispanic Black, Hispanic, Asian, Other | Identify early diagnostic biomarkers to predict risk and etiology of developing T2DM following GDM | Prospective cohort study | Several amino acids and all hexose sugars play important roles in T2DM development and impaired fasting glucose levels Women who developed T2DM had a more T2DM-like metabolite profile within 6-9 weeks postpartum and were more likely to have been treated with insulin or oral medication during pregnancy |
| Bao et al. (2014) | n=4,554 | 1991–2007 | Nurse’s Health Study II cohort (14 states in US) | Examine the role of physical activity and sedentary behaviors in the progression from GDM to T2DM | Prospective cohort study | Increased physical activity may lower the risk of progression from GDM to T2DM | |
| Bao, Chavarro et al. (2016) | n=3976 | 1991–2009 | Nurse’s Health Study II cohort (14 states in US) | Examine the association of habitual iron intake with long-term risk of T2DM among women with previous GDM | Prospective cohort study | Greater intakes of total iron, dietary heme iron, dietary heme iron and supplemental iron were associated with increased risk for T2DM among women with a history of GDM | |
| Bao, Li et al. (2016) | n=4,502 | 1991–2011 | Nurse’s Health Study II cohort (14 states in US) | Examine the long-term effects of a low-carbohydrate diet on GDM to T2DM progression | Prospective Cohort Study | Low-carbohydrate diet with high protein and fat intake from animal-source foods is associated with higher risk of T2DM, while a low-carbohydrate diet with high protein and fat intake from plant-source foods is not significantly associated with risk of T2DM | |
| Brown et al. (2016) | n=1,463 (Chinese=168, Filipina=165, South Asian=144, Other Asian=153) |
2011–2013 | Northern California (Black, Latina, Non-Hispanic white, Chinese, Filipina, South Asian, Other Asian) | Examine whether strength of attachment to one’s ethnic group could account for variation in lifestyle behaviors in women with previous GDM who are at high risk for T2DM | Cross-sectional study | Ethnic group attachment is associated with certain lifestyle behaviors that may promote behaviors associated with risk of developing T2DM | |
| 3) Metabolic Signatures | Batchuluun et al. (2018) | n=24 | Not specified | SWIFT cohort; Northern California: Asian, Non-Hispanic White, Non-Hispanic Black, Hispanic, Other | Investigate the link between acylC species and ß-cell dysfunction / onset of diabetes after GDM | Prospective cohort study | Serum medium-chain (M)-acylCs are associated with GDM and early TD2M onset, which directly impairs ß-cell function |
| Khan et al. (2019) | n=1,035 (Asian=362) |
2008–2011 | SWIFT cohort; Northern California: Asian, Non-Hispanic White, Non-Hispanic Black, Hispanic, Other | Identify a predictive signature and early-stage pathophysiology of the transition from GDM to TD2M | Prospective cohort study | Predictive signature of reduced sphingolipids is associated with the pathophysiology of transition from GDM to T2DM | |
| Lai et al. (2020) | n=658 (Asian=196) |
2008–2011 | California: White, Black, Asian, Hispanic | Further the understanding of the pathology underlying the transition from GDM to T2DM | Nested case-control study | Amino acid and lipid dysmetabolism among women with a history of GDM can serve as a metabolic signature to predict the transition from GDM to T2DM in the early postpartum period | |
| 4) Racial/Ethnic Disparities | Gunderson et al. (2015) | n=1,035 (Asian=362) |
2008–2011 | SWIFT cohort; Northern California: Asian, Non-Hispanic White, Non-Hispanic Black, Hispanic, Other | Evaluate lactation and 2-year incidence of T2DM after GDM pregnancy | Prospective observational cohort study | Lower lactation intensity and shorter duration; Hispanic/Asian ethnicity, adverse newborn outcomes, use of insulin or oral medications during pregnancy, and higher dietary consumption of animal fat were associated with higher 2-year incidences of T2DM following a GDM pregnancy |
| 5) Prevention Interventions and Postpartum Follow-up Care | Aroda et al. (2015) | n=350 (GDM) n=1,416 (no GDM) |
1996–2009 | Caucasian, African American, Hispanic, American Indian, and Asian American (n=47) | Evaluate the impacts of Intensive Lifestyle (ILS) and metformin interventions in women with and without a history of GDM using Diabetes Prevention Program (DPP) cohort | Randomized controlled clinical trial with observational follow-up | Among women with a history of GDM, both lifestyle and metformin interventions were effective in reducing the progression to diabetes over a 10-year period Among women without a history of GDM, lifestyle interventions only reduced the progression to T2DM |
| Bernstein et al. (2019) | n=12,622 (Asian=123/1,091 women who conceived within 3 years of index pregnancy) |
2006–2012 | United States; Asian, Black, Hispanic, White | Assess the impact of GDM recurrence and/or delivery interval on follow-up care and T2DM onset | Secondary analysis | GDM severity in the index pregnancy was a strong predictor of subsequent T2DM onset Regardless of interval between deliveries, GDM reoccurs in 50% of subsequent pregnancies A short interval (<1 year, compared to >2 years) between the initial GDM delivery and subsequent pregnancy increased the likelihood of early onset T2DM, suggesting the importance of contraceptive counseling after a GDM pregnancy |
|
Acknowledgements
This paper was supported, in part, by the Research Division, Department of Native Hawaiian Health (MKM, S21 MD 000228). The authors would like to thank Ms. Kim Spencer-Tolentino for bibliographic support and Ms. Mona-Ann Cardejon for coordination support.
Acronyms
- BMI
body mass index
- GDM
gestational diabetes mellitus
- NH
Native Hawaiian
- NHPI
Native Hawaiian and Pacific Islander
- PI
Pacific Islander
- T2DM
Type 2 Diabetes Mellitus
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1(Suppl 1)):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiyama MS, Cash HL, Roseveare C, Reklai R, Basilius K, Madraisau S. Assessment of gestational diabetes and associated risk factors and outcomes in the pacific island nation of Palau. Matern Child Health J. 2017;21((10)):1961–1966. doi: 10.1007/s10995-017-2313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang AL, Hurwitz E, Miyamura J, Kaneshiro B, Sentell T. Maternal risk factors and perinatal outcomes among pacific islander groups in Hawaii: a retrospective cohort study using statewide hospital data. BMC Pregnancy Childbirth. 2015;15:239. doi: 10.1186/s12884-015-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18((1)):494. doi: 10.1186/s12884-018-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth - United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67((43)):1201–1207. doi: 10.15585/mmwr.mm6743a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Olsen SF, Hinkle SN, et al. Diabetes & Women’s Health (DWH) Study: an observational study of long-term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open. 2019;9((4)):e025517. doi: 10.1136/bmjopen-2018-025517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plows J, Stanley J, Baker P, Reynolds C, Vickers M. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19((11)):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore K, Stotz S, Fischl A, et al. Pregnancy and gestational diabetes mellitus (GDM) in North American Indian adolescents and young adults (AYA): implications for girls and stopping GDM. Curr Diab Rep. 2019;19((11)):113. doi: 10.1007/s11892-019-1241-3. [DOI] [PubMed] [Google Scholar]
- 9.Daneshmand SS, Stortz S, Morrisey R, Faksh A. Bridging gaps and understanding disparities in gestational diabetes mellitus to improve perinatal outcomes. Diabetes Spectrum. 2019;32((4)):317–323. doi: 10.2337/ds19-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aroda VR, Christophi CA, Edelstein SL, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100((4)):1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Census Bureau Hawaii population characteristics 2019. Published online June 25, 2020. https://census.hawaii.gov/wp-content/uploads/2020/06/Hawaii-Population-Characteristics-2019.pdf.
- 12.Hawaii State Department of Health, Hawaii Health Data Warehouse, Behavioral Risk Factor Surveillance System Diabetes Prevalence, adult by race/ethnicity (DOH) 2017-2019. Published online August 6, 2021. http://ibis.hhdw.org/ibisph-view/indicator/view/DXDiabetesAA.RacEthDOH.html.
- 13.Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta. 2016;48(Suppl 1):S54–S60. doi: 10.1016/j.placenta.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Yang H. Gestational diabetes mellitus, programing and epigenetics. J Matern-Fetal Neonatal Med. 2014;27((12)):1266–1269. doi: 10.3109/14767058.2013.853733. [DOI] [PubMed] [Google Scholar]
- 15.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reprod Camb Engl. 2014;148((6)):R111–120. doi: 10.1530/REP-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. 2016;59((7)):1385–1390. doi: 10.1007/s00125-016-3979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Shi L, Zhang D, Chao SM. Influence of acculturation on risk for gestational diabetes among Asian women. Prev Chronic Dis. 2019;16:E158. doi: 10.5888/pcd16.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Lamerato LE, Misra DP. A retrospective analysis of the relationship between race/ethnicity, age at delivery and the risk of gestational diabetes mellitus. J Matern-Fetal Neonatal Med. 2020;33((17)):2961–2969. doi: 10.1080/14767058.2019.1566310. [DOI] [PubMed] [Google Scholar]
- 19.Shah A, Stotland NE, Cheng YW, Ramos GA, Caughey AB. The association between body mass index and gestational diabetes mellitus varies by race/ethnicity. Am J Perinatol. 2011;28((7)):515–520. doi: 10.1055/s-0031-1272968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu J, Zhao B, Wang EJ, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol. 2015;29((5)):436–443. doi: 10.1111/ppe.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunsberger M, Rosenberg KD, Donatelle RJ. Racial/ethnic disparities in gestational diabetes mellitus: findings from a population-based survey. Womens Health Issues. 2010;20((5)):323–328. doi: 10.1016/j.whi.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Tsai P-JS, Roberson E, Dye T. Gestational diabetes and macrosomia by race/ethnicity in Hawaii. BMC Res Notes. 2013;6:395. doi: 10.1186/1756-0500-6-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, England L, Sappenfield W, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004-2007. Prev Chronic Dis. 2012;9:E88. doi: 10.5888/pcd9.110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007-2009. Am J Public Health. 2013;103((10)):e65–72. doi: 10.2105/AJPH.2013.301469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35((7)):1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brite J, Shiroma EJ, Bowers K, et al. Height and the risk of gestational diabetes: variations by race/ethnicity. Diabet Med. 2014;31((3)):332–340. doi: 10.1111/dme.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams AD, Grantz KL, Zhang C, Nobles C, Sherman S, Mendola P. Ambient volatile organic compounds and racial/ethnic disparities in gestational diabetes mellitus: are Asian/Pacific Islander women at greater risk? Am J Epidemiol. 2019;188((2)):389–397. doi: 10.1093/aje/kwy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24((5)):441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadhani TA, Canfield MA, Farag NH, et al. Do foreign- and U.S.-born mothers across racial/ethnic groups have a similar risk profile for selected sociodemographic and periconceptional factors? Birt Defects Res A Clin Mol Teratol. 2011;91((9)):823–830. doi: 10.1002/bdra.20839. [DOI] [PubMed] [Google Scholar]
- 30.Janevic T, Borrell LN, Savitz DA, Echeverria SE, Rundle A. Ethnic enclaves and gestational diabetes among immigrant women in New York City. Soc Sci Med 1982. 2014;120:180–189. doi: 10.1016/j.socscimed.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Gunderson EP, Hurston SR, Ning X, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;163((12)):889–898. doi: 10.7326/M15-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunderson EP, Kim C, Quesenberry CPJ, et al. Lactation intensity and fasting plasma lipids, lipoproteins, non-esterified free fatty acids, leptin and adiponectin in postpartum women with recent gestational diabetes mellitus: the SWIFT cohort. Metabolism. 2014;63((7)):941–950. doi: 10.1016/j.metabol.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao W, Li S, Chavarro JE, et al. Low carbohydrate-diet scores and long-term risk of type 2 diabetes among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2016;39((1)):43–49. doi: 10.2337/dc15-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao W, Tobias DK, Bowers K, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014;174((7)):1047–1055. doi: 10.1001/jamainternmed.2014.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao W, Yeung E, Tobias DK, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia. 2015;58((6)):1212–1219. doi: 10.1007/s00125-015-3537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SD, Ehrlich SF, Kubo A, et al. Lifestyle behaviors and ethnic identity among diverse women at high risk for type 2 diabetes. Soc Sci Med 1982. 2016;160:87–93. doi: 10.1016/j.socscimed.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao W, Chavarro JE, Tobias DK, et al. Long-term risk of type 2 diabetes in relation to habitual iron intake in women with a history of gestational diabetes: a prospective cohort study. Am J Clin Nutr. 2016;103((2)):375–381. doi: 10.3945/ajcn.115.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allalou A, Nalla A, Prentice KJ, et al. A predictive metabolic signature for the transition from gestational diabetes mellitus to type 2 diabetes. Diabetes. 2016;65((9)):2529–2539. doi: 10.2337/db15-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai M, Liu Y, Ronnett GV, et al. Amino acid and lipid metabolism in post-gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med. 2020;17((5)):e1003112. doi: 10.1371/journal.pmed.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batchuluun B, Al Rijjal D, Prentice KJ, et al. Elevated medium-chain acylcarnitines are associated with gestational diabetes mellitus and early progression to type 2 diabetes and induce pancreatic β-Cell dysfunction. Diabetes. 2018;67((5)):885–897. doi: 10.2337/db17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan SR, Mohan H, Liu Y, et al. The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes. Diabetologia. 2019;62((4)):687–703. doi: 10.1007/s00125-018-4800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein J, Lee-Parritz A, Quinn E, et al. After gestational diabetes: impact of pregnancy interval on recurrence and type 2 diabetes. BioResearch Open Access. 2019;8((1)):59–64. doi: 10.1089/biores.2018.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuen L, Wong VW, Simmons D. Ethnic disparities in gestational diabetes. Curr Diab Rep. 2018;18((9)):68. doi: 10.1007/s11892-018-1040-2. [DOI] [PubMed] [Google Scholar]
- 44.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278((13)):1078–1083. [PubMed] [Google Scholar]
- 45.Silva JK, Kaholokula JK, Ratner R, Mau M. Ethnic differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care. 2006;29((9)):2058–2063. doi: 10.2337/dc06-0458. [DOI] [PubMed] [Google Scholar]
- 46.Price LA, Lock LJ, Archer LE, Ahmed Z. Awareness of gestational diabetes and its risk factors among pregnant women in Samoa. Hawaii J Med Public Health. 2017;76((2)):48–54. [PMC free article] [PubMed] [Google Scholar]
- 47.Stotz S, Charron-Prochownik D, Terry MA, Gonzales K, Moore K. Reducing risk for gestational diabetes mellitus (GDM) through a preconception counseling program for American Indian/Alaska Native girls: perceptions from women with type 2 diabetes or a history of GDM. Diabetes Educ. 2019;45((2)):137–145. doi: 10.1177/0145721718821663. [DOI] [PubMed] [Google Scholar]
- 48.Anand SS, Gupta M, Teo KK, et al. Causes and consequences of gestational diabetes in South Asians living in Canada: results from a prospective cohort study. CMAJ Open. 2017;5((3)):E604–E611. doi: 10.9778/cmajo.20170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuen L, Wong VW. Gestational diabetes mellitus: Challenges for different ethnic groups. World J Diabetes. 2015;6((8)):1024–1032. doi: 10.4239/wjd.v6.i8.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koivusalo SB, Rönö K, Klemetti MM, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish gestational diabetes prevention study (RADIEL): a randomized controlled trial. Diabetes Care. 2016;39((1)):24–30. doi: 10.2337/dc15-0511. [DOI] [PubMed] [Google Scholar]
- 51.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125((3)):576–582. doi: 10.1097/AOG.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 52.Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, Álvarez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG Int J Obstet Gynaecol. 2015;122((9)):1167–1174. doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 53.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16((1)):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Cheng Y, Wang D, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review and meta-analysis of 170,139 women. J Diabetes Res. 2020;2020:3076463. doi: 10.1155/2020/3076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon JH, Kwak SH, Jang HC. Prevention of type 2 diabetes mellitus in women with previous gestational diabetes mellitus. Korean J Intern Med. 2017;32((1)):26–41. doi: 10.3904/kjim.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennison RA, Fox RA, Ward RJ, Griffin SJ, Usher-Smith JA. Women’s views on screening for type 2 diabetes after gestational diabetes: a systematic review, qualitative synthesis and recommendations for increasing uptake. Diabet Med J. 2020;37((1)):29–43. doi: 10.1111/dme.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]