Abstract
The gram-positive bacterium Listeria monocytogenes is the causative agent of listeriosis, a highly fatal opportunistic foodborne infection. Pregnant women, neonates, the elderly, and debilitated or immunocompromised patients in general are predominantly affected, although the disease can also develop in normal individuals. Clinical manifestations of invasive listeriosis are usually severe and include abortion, sepsis, and meningoencephalitis. Listeriosis can also manifest as a febrile gastroenteritis syndrome. In addition to humans, L. monocytogenes affects many vertebrate species, including birds. Listeria ivanovii, a second pathogenic species of the genus, is specific for ruminants. Our current view of the pathophysiology of listeriosis derives largely from studies with the mouse infection model. Pathogenic listeriae enter the host primarily through the intestine. The liver is thought to be their first target organ after intestinal translocation. In the liver, listeriae actively multiply until the infection is controlled by a cell-mediated immune response. This initial, subclinical step of listeriosis is thought to be common due to the frequent presence of pathogenic L. monocytogenes in food. In normal indivuals, the continual exposure to listerial antigens probably contributes to the maintenance of anti-Listeria memory T cells. However, in debilitated and immunocompromised patients, the unrestricted proliferation of listeriae in the liver may result in prolonged low-level bacteremia, leading to invasion of the preferred secondary target organs (the brain and the gravid uterus) and to overt clinical disease. L. monocytogenes and L. ivanovii are facultative intracellular parasites able to survive in macrophages and to invade a variety of normally nonphagocytic cells, such as epithelial cells, hepatocytes, and endothelial cells. In all these cell types, pathogenic listeriae go through an intracellular life cycle involving early escape from the phagocytic vacuole, rapid intracytoplasmic multiplication, bacterially induced actin-based motility, and direct spread to neighboring cells, in which they reinitiate the cycle. In this way, listeriae disseminate in host tissues sheltered from the humoral arm of the immune system. Over the last 15 years, a number of virulence factors involved in key steps of this intracellular life cycle have been identified. This review describes in detail the molecular determinants of Listeria virulence and their mechanism of action and summarizes the current knowledge on the pathophysiology of listeriosis and the cell biology and host cell responses to Listeria infection. This article provides an updated perspective of the development of our understanding of Listeria pathogenesis from the first molecular genetic analyses of virulence mechanisms reported in 1985 until the start of the genomic era of Listeria research.
The genus Listeria consists of a group of gram-positive bacteria of low G+C content closely related to Bacillus, Clostridium, Enterococcus, Streptococcus, and Staphylococcus. Listeria spp. are facultative anaerobic rods of 0.4 by 1 to 1.5 μm that do not form spores, have no capsule, and are motile at 10 to 25°C (98, 552, 579). Listeria spp. are isolated from a diversity of environmental sources, including soil, water, effluents, a large variety of foods, and the feces of humans and animals. The natural habitat of these bacteria is thought to be decomposing plant matter, in which they live as saprophytes. Domesticated ruminants probably play a key role in the maintenance of Listeria spp. in the rural environment via a continuous fecal-oral enrichment cycle (178, 318, 420, 559, 682, 687, 692). The genus Listeria currently includes six species: L. monocytogenes, L. ivanovii, L. seeligeri, L. innocua, L. welshimeri, and L. grayi. Two of these species, L. monocytogenes and L. ivanovii, are potentially pathogenic. The infectious disease caused by these bacteria is known as listeriosis. L. monocytogenes causes serious localized and generalized infections in humans and a variety of other vertebrates, including domesticated and wild birds and mammals. The official discovery of Listeria microorganisms dates back to 1924, when E. G. D. Murray, R. A. Webb, and M. B. R. Swann isolated L. monocytogenes as the etiological agent of a septicemic disease affecting rabbits and guinea pigs in their laboratory at Cambridge in England (458). The first cases of human listeriosis were reported in 1929 in Denmark (481). However, the first recorded culture of L. monocytogenes dates from 1921, with the bacterium isolated in France by Dumont and Cotoni from a patient with meningitis (159, 604). L. ivanovii (formerly known as L. monocytogenes serotype 5) was first isolated in Bulgaria in 1955 from lambs with congenital listeriosis (299). Human cases of L. ivanovii infection are rare (116), the vast majority of reported isolations of this species being from abortions, stillbirths, and neonatal septicemias in sheep and cattle (4, 90, 134, 529, 610, 693). A third species, L. seeligeri, is considered nonpathogenic (555), although it has been implicated in at least one case of human listeriosis (556).
For many years, clinical Listeria isolates were a mere laboratory rarity, and the epidemiology of the disease was an unresolved mystery (603, 604). However, at the end of the 1970s and the start of the 1980s, the number of reports on Listeria isolations begun to increase, and from 1983 onwards, a series of epidemic outbreaks in humans in North America and Europe clearly established listeriosis as an important food-borne infection (46, 180, 386, 421, 572). The foods most frequently implicated are soft cheeses and dairy products, pâtés and sausages, smoked fish, salads, “delicatessen,” and in general industrially produced, refrigerated ready-to-eat products that are eaten without cooking or reheating (176, 425–427, 551, 572). In ruminants, Listeria infection is transmitted by consumption of spoiled silage, in which these bacteria multiply readily, resulting in herd outbreaks (178, 666, 671, 693). Listeria organisms are widely disseminated in the rural environment and, consequently, contaminate the raw materials used in the preparation of industrially processed foods and the production plants as well (237). These bacteria are well equipped to survive food-processing technologies. For example, they tolerate high concentrations of salt and relatively low pHs, and worst of all, they are able to multiply at refrigeration temperatures (145, 362, 393, 426). This makes Listeria microorganisms a serious threat to food safety and ranks them among the microorganisms that most concern the food industry.
Researchers in immunology were interested in L. monocytogenes long before its importance as a risk to public health and food safety was recognized, because an infection highly reminiscent of human listeriosis was easily reproducible in laboratory rodents and protection could be transferred in syngeneic mice through spleen cells. Since the pioneering work of Mackaness in the early 1960s, which demonstrated that L. monocytogenes is able to survive and multiply in macrophages, this bacterium has been used in immunological research as a prototype intracellular parasite (401). For decades, the experimental model of Listeria infection in the mouse has made a significant contribution to our understanding of the cellular immune response (615). For example, key concepts such as the inability of antibodies to protect against infections produced by intracellular pathogens, the importance of activated macrophages in the elimination of intracellular parasites, and that the T cell is the macrophage-activating element required for cell-mediated immunity were established based on studies with the murine model of listeriosis (405, 406, 443, 478, 479).
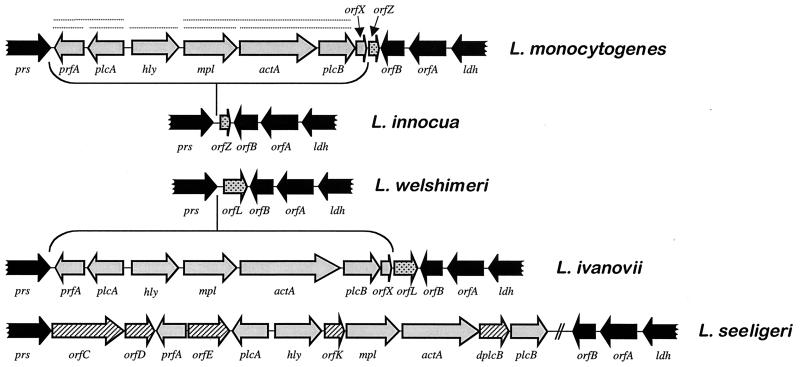
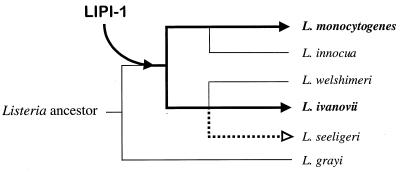
In addition to the emergence of L. monocytogenes as a major food-borne pathogen, the 1980s also marked the start of investigations into the molecular mechanisms underlying Listeria virulence. The attention of researchers was first drawn to hemolytic activity, classically considered a virulence marker because it was present in the pathogenic species but not (with the exception of L. seeligeri) in the nonpathogenic species. These studies led between 1986 and 1989 to the discovery of the hemolysin gene, hly, and to elucidation of the key role that hemolysin plays in escape from destruction inside phagosomes, a prerequisite for intracellular bacterial proliferation. So, a decade ago, hemolysin became not only the first Listeria virulence factor to have its gene characterized, but also the first bacterial gene product to which a function critical to the survival of a parasite within the cells of the eukaryote host was attributed.
L. monocytogenes has since become not only an important paradigm for immunological investigation but also an important model system for analysis of the molecular mechanisms of intracellular parasitism (107). The identification of the hly gene was rapidly followed by a succession of discoveries resulting in complete characterization of the genetic locus to which this gene maps. This locus is a 9-kb virulence gene cluster that is involved in functions essential to intracellular survival. The internalin locus, inlAB, was also identified and characterized at this time (193). This locus encodes the first invasin described in a gram-positive bacterium, implicated in internalization by cells that are not usually phagocytic, such as epithelial and endothelial cells and hepatocytes. The progress made in the molecular characterization of listerial virulence factors during this period was summarized in a minireview published in 1992 (517). Many advances have since been made that have significantly increased our understanding of the molecular mechanisms of Listeria intracellular parasitism. The sequencing of the genomes of L. monocytogenes and L. innocua is currently finished (European Listeria Genome Consortium, unpublished data), with the expected outcome that very soon the panorama of research into the molecular pathogenesis of Listeria spp. will change dramatically.
The aim of this article is to review the knowledge gathered during the pregenomic era of Listeria research about the pathogenesis of listeriosis and the molecular virulence determinants involved. Readers interested more specifically in immunopathogenesis and the immune response should consult reference 497a. Those interested in issues related to taxonomy, clinical diagnosis and disease management, molecular typing, epidemiology, ecology, and food safety are also referred to other publications (145, 176, 289, 395, 550, 551, 573, 594, 605). For a historical perspective on Listeria spp. and listeriosis, we recommend the book Listeriosis by the pioneer listeriologist Heinz P. R. Seeliger (602) and the classical review, “Listeria monocytogenes and listeric infections,” by Gray and Killinger (238).
PATHOPHYSIOLOGY OF LISTERIA INFECTION
Clinical Features
The clinical signs of L. monocytogenes infection are very similar in all susceptible hosts. Two basic forms of presentation can be distinguished: perinatal listeriosis and listeriosis in the adult patient. In both instances, the predominant clinical forms correspond to disseminated infection or to local infection in the central nervous system (CNS). Listeriosis is usually a very severe disease—in fact, one of the most deadly bacterial infections currently known—with a mean mortality rate in humans of 20 to 30% or higher despite early antibiotic treatment (422, 423, 554, 594).
Fetomaternal and neonatal listeriosis.
This form of presentation mainly results from invasion of the fetus via the placenta and develops as chorioamnionitis. Its consequence is abortion, usually from 5 months of gestation onwards, or the birth of a baby or stillborn fetus with generalized infection, a clinical syndrome known as granulomatosis infantiseptica and characterized by the presence of pyogranulomatous microabscesses disseminated over the body and a high mortality (336a) (Fig. 1). The infection is usually asymptomatic in the mother or may present as a mild flu-like syndrome with chills, fatigue, headache, and muscular and joint pain about 2 to 14 days before miscarriage. Less frequently (10 to 15% of perinatal cases), late neonatal listeriosis is observed. It generally occurs 1 to 8 weeks postpartum and involves a febrile syndrome accompanied by meningitis and, in some cases, gastroenteritis and pneumonia. It presumably results from aspiration of contaminated maternal exudates during delivery, although hospital-acquired cases involving horizontal transmission by fomites or medical personnel in neonatal units have been reported (177, 554, 628). The mortality of late-onset neonatal listeriosis is lower (10 to 20%), but like early-onset neonatal listeriosis, it may have sequelae such as hydrocephalus or psychomotor retardation (173). L. monocytogenes is one of the three principal causes of bacterial meningitis in neonates (391, 554, 594, 645).
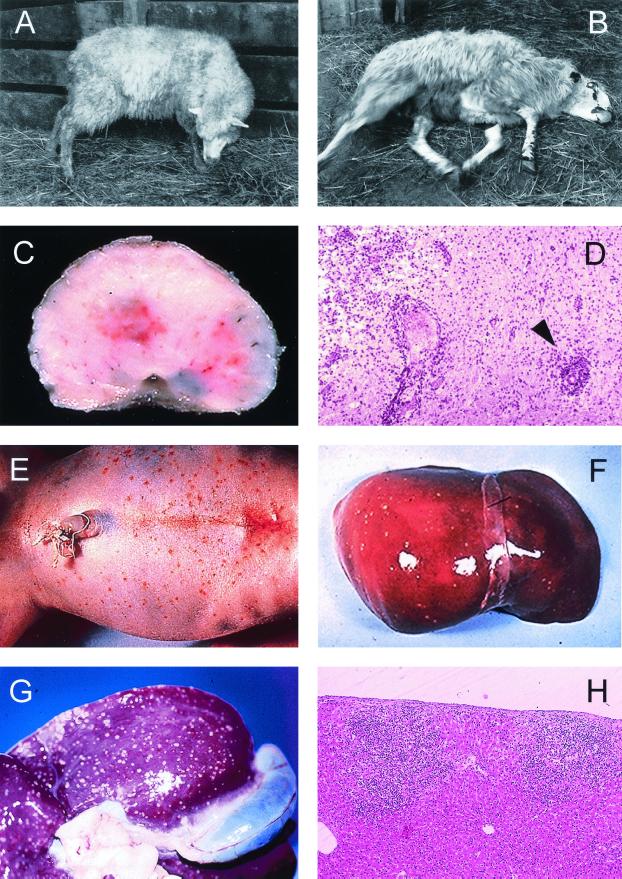
FIG. 1.
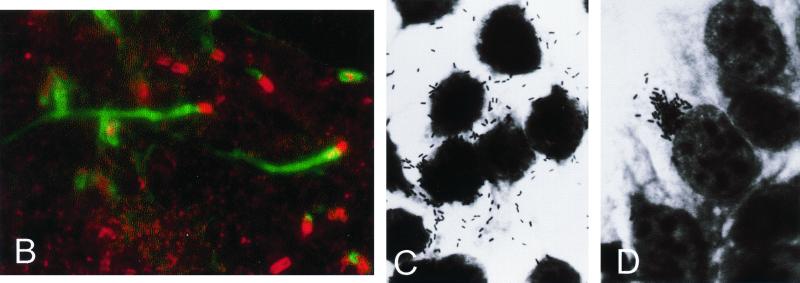
Clinical and pathological characteristics of Listeria infection in animals and humans. (A to D) Neuromeningeal listeriosis in sheep. (A) Typical aspect of circling disease, first described by Gill in 1933 in New Zealand (215, 216) and the most characteristic clinical manifestation of listeriosis in ruminants; the syndrome is characterized by involuntary torticollis and walking aimlessly in circles as a result of brainstem lesions. (B) In a further step of the infectious process, animals lie on the ground with evident signs of uncoordination (paddling movements) and cranial nerve paralysis (strabismus, salivation, etc.). (C) Section of the medulla oblongata of a sheep with listerial rhombencephalitis, showing clear inflammatory lesions in the brain tissue. (D) Microscopic preparation of the brainstem showing extensive parenchymal inflammatory infiltration and typical perivascular cuffing (one is indicated by an arrowhead) (panels C and D copyright M. Domingo, Barcelona, Spain). (E and F) Stillborn with generalized L. monocytogenes infection, a clinical condition also known as granulomatosis infantiseptica and characterized by the presence of military-disseminated pyogranulomatous lesions on the body surface (E) and internal organs (F, liver of the fetus in E) and a high mortality. (G and H) Extensive pyogranulomatous hepatitis in a lamb experimentally infected with L. ivanovii, with multiple necrotic foci in the liver surface (G) and the parenchyma (H, hematoxylin/eosin-stained cut from the liver in G, showing two subcapsular pyogranulomes [magnification, ×60]).
Listeriosis in adults.
The listerial infection most frequently reported in nonpregnant adults is that affecting the CNS (55 to 70% of cases). Pure meningeal forms are observed in some cases, but infection normally develops as a meningoencephalitis accompanied by severe changes in consciousness, movement disorders, and, in some cases, paralysis of the cranial nerves (Fig. 1). The encephalitic form, in which Listeria organisms are isolated with difficulty from the cerebrospinal fluid (CSF), is common in animals (Fig. 1A to D) but rare in humans (see below). Its course is usually biphasic, with an initial subfebrile phase lasting 3 to 10 days in which there may be headache, vomiting, visual disorders, and general malaise, followed in a second phase by the onset of severe signs of rhombencephalitis. The mortality rate for CNS infection is around 20% but may be as high as 40 to 60% if associated with concurrent, underlying debilitating disease. It has been estimated that L. monocytogenes accounts for 10% of community-acquired bacterial meningitis. Due to effective vaccination against Haemophilus influenzae, L. monocytogenes is now the fourth most common cause of meningeal infection in adults after Streptococcus pneumoniae, Neisseria meningitidis, and group B streptococci. However, in certain high-risk groups, such as cancer patients, L. monocytogenes is the most common cause of bacterial meningitis (391, 473, 593). Another frequent form of listeriosis (in some series of patients reported, even more frequent than CNS infection) is bacteremia or septicemia (15 to 50% of cases), with a high mortality rate (up to 70%) if it is associated with severe underlying debilitating conditions (391) (see below). There are other atypical clinical forms (5 to 10% of cases), such as endocarditis (the third most frequent form), myocarditis, arteritis, pneumonia, pleuritis, hepatitis, colecystitis, peritonitis, localized abscesses (e.g., brain abscess, which accounts for about 10% of CNS infections by Listeria spp.), arthritis, osteomyelitis, sinusitis, otitis, conjunctivitis, ophthalmitis, and, in cows, mastitis (48, 176, 199, 201, 390, 391, 395, 422–424, 550, 628).
An association between clinical episodes of invasive listeriosis and a history of gastrointestinal symptoms, including diarrhea, vomiting, and fever, was noticed some time ago (286, 542, 599). Investigations of recent food-borne outbreaks have provided compelling evidence that a febrile gastroenteritis syndrome may indeed be the main clinical manifestation of L. monocytogenes infection (19, 118, 442, 577, 628). The lesson to be learned from these epidemics is that L. monocytogenes should be sought as a possible etiologic agent in cases of diarrheagenic disease in humans. The potential enteropathogenicity of L. monocytogenes has also been recognized in animals, with outbreaks of diarrhea and gastroenteritis having been reported in sheep (52, 219, 395).
There is also a primary cutaneous form of Listeria infection characterized by a pyogranulomatous rash. This form occurs sporadically among farmers and veterinarians and is contracted by direct contact with the genital tract or placenta from cows having had a miscarriage due to Listeria infection (6, 428).
Pathogenesis
The pathophysiology of Listeria infection in humans and animals is still poorly understood. Most of the available information is derived from interpretation of epidemiological, clinical, and histopathological findings and observations made in experimental infections in animals, particularly in the murine model. As contaminated food is the major source of infection in both epidemic and sporadic cases (176, 512), the gastrointestinal tract is thought to be the primary site of entry of pathogenic Listeria organisms into the host. The clinical course of infection usually begins about 20 h after the ingestion of heavily contaminated food in cases of gastroenteritis (118), whereas the incubation period for the invasive illness is generally much longer, around 20 to 30 days (386, 542). Similar incubation periods have been reported in animals for both gastroenteric and invasive disease (138, 219, 671).
Ingestion of L. monocytogenes is likely to be a very common event, given the ubiquitous distribution of these bacteria and the high frequency of contamination of raw and industrially processed foods. However, the incidence of human listeriosis is very low, normally around 2 to 8 sporadic cases annually per million population in Europe and the United States (176, 301, 554, 649). Higher incidence rates have been reported for sporadic listeriosis (for example, 10.9 cases per million population per year in Barcelona, Spain [476], and 14.7 in France [234]), but these are exceptional. In epidemic situations, the incidence in the target population increases by a factor of 3 to 10 (46, 386, 587, 599). Thus, L. monocytogenes seems to have a lower pathogenic potential than other food-borne pathogens. This is consistent with the relatively high 50% lethal dose (LD50) values reported for mice infected experimentally by the oral (109 [18, 485]) or parenteral (105 to 106 [18, 333, 553]) routes. The minimum dose required to cause clinical infection in humans has not been determined, but the large numbers of L. monocytogenes bacteria detected in foods responsible for epidemic and sporadic cases of listeriosis (typically 106) suggest that it is high. However, these data should be interpreted with caution, given the long incubation period of invasive listeriosis and the time normally elapsed between diagnosis and analysis of the food eaten, during which Listeria organisms can have multiplied in the patient's refrigerator. It therefore cannot be excluded that low doses may be able to cause infection, at least in immunosuppressed persons, old people, and pregnant women. Indeed, levels of contamination as low as 102 to 104 L. monocytogenes cells per g of food have been associated with listeriosis in humans (176, 425). Obviously, the infectious dose may vary depending upon the pathogenicity and virulence of the L. monocytogenes strain involved and the host risk factors.
Pathogenicity of L. monocytogenes.
Heterogeneity in the virulence of L. monocytogenes has been observed in several in vivo (mice) and in vitro (cell culture) studies (68, 129, 550, 663), but in most cases a clear correlation between the level of virulence and the origin or type characteristics of the strain could not be established. However, a recent study has found statistically significant differences in virulence between strains of food and clinical origin, the latter having slightly lower lethal doses (477). On the other hand, there is ample circumstantial evidence for an association between antigenic composition and pathogenicity in Listeria spp. The clearest example is provided by the L. ivanovii-specific serovar 5, which is recovered almost exclusively from ruminants, especially sheep. In these animals, serovar 5 strains cause perinatal infections but not encephalitis, the most typical clinical manifestations of ovine listeriosis (134, 397, 606, 610, 693) (Fig. 1A to D). Further evidence comes from the fact that only 3 of the 12 known serovars of L. monocytogenes, 1/2a, 1/2b, and 4b, account for more than 90% of human and animal cases of listeriosis (176, 397, 594), although other serovars, such as 1/2c, are often found as food contaminants (172, 176). Among the listeriosis-associated serovars, 4b strains cause over 50% of listeriosis cases worldwide, but strains of antigenic group 1/2 (1/2a, 1/2b, and 1/2c) predominate in food isolates (52, 172, 550, 557, 591). This suggests that serovar 4b strains are more adapted to mammalian host tissues than strains from serogroup 1/2. A phenotypically and genomically closely related group of serovar 4b isolates was found to be responsible for major outbreaks of food-borne human listeriosis in California in 1985 (386), Switzerland from 1983 to 1987 (46), Denmark from 1985 to 1987 (580), and France in 1992 (233), supporting the notion that some clones of L. monocytogenes may be particularly pathogenic (302, 510).
A number of observations suggest that there may be differences in pathogenic tropism between L. monocytogenes' strains. In humans, for example, serovar 4b strains have been found to occur more frequently in fetomaternal cases than in cases not associated with pregnancy (424). In sheep, the two major clinical forms of L. monocytogenes infection, meningoencephalitis and abortion, do not tend to occur simultaneously in the same flock (13, 397, 671). Molecular epidemiological evidence for such pathogenic tropism has been presented recently by Wiedman et al. (696). These authors found a correlation between the three serovar-related evolutionary branches into which L. monocytogenes isolates can be grouped (44, 510, 531, 678) and the pathogenic potential for humans and animals. Thus, one group of strains (serovars 1/2b and 4b) contained all isolates from human food-borne epidemics and isolates from sporadic cases in humans and animals, another (serovars 1/2a, 1/2c, and 3a) contained strains from both human and animal cases but no isolates from human food-borne epidemics, while a third group (serovar 4a) contained only animal isolates. A specific ribotype in the first group comprised all the serovar 4b strains associated with human food-borne epidemics but less than 10% of the ruminant isolates, suggesting a possible pathogenic tropism for humans (696). This possible tropism for humans of certain clones of L. monocytogenes serovar 4b may explain, for example, the observations made in Scotland, where during the same time period serovar 4b was most commonly isolated from human cases, whereas most ruminant cases were due to serovar 1/2a strains (397). There are also indications of adaptation to cause a particular clinical form in L. monocytogenes serovar 4b strains, as deduced from the analysis of two food-borne outbreaks of human listeriosis in France in 1992 and 1993, each associated with a different phage type of this serovar. In these outbreaks, the target population was similar but there was a significant difference in the percentage of fetomaternal cases (33 versus 80%) (557, 558).
Host risk factors.
Host susceptibility plays a major role in the presentation of clinical disease upon exposure to L. monocytogenes. Thus, most listeriosis patients have a physiological or pathological defect that affects T-cell-mediated immunity. This justifies the classification of L. monocytogenes as an opportunisitic pathogen. The groups at risk for listeriosis are pregnant women and neonates, the elderly (55 to 60 years and older), and immunocompromised or debilitated adults with underlying diseases. Listeriosis in nonpregnant adults is associated in most cases (>75%) with at least one of the following conditions: malignancies (leukemia, lymphoma, or sarcoma) and antineoplastic chemotherapy, immunosuppresant therapy (organ transplantation or corticosteroid use), chronic liver disease (cirrhosis or alcoholism), kidney disease, diabetes, and collagen disease (lupus) (176, 423, 551, 554, 594).
Human immunodeficiency virus (HIV) infection is also a significant risk factor for listeriosis. AIDS is the underlying predisposing condition in 5 to 20% of listeriosis cases in nonpregnant adults. It has been estimated that the risk of contracting listeriosis is 300 to 1,000 times higher for AIDS patients than for the general population. Nevertheless, listeriosis remains a relatively rare AIDS-associated infection, probably due to the preventive dietary measures taken by HIV-infected patients (avoidance of high-risk foods), the antimicrobial treatments that they receive regularly to treat or prevent opportunistic infections, and the fact that HIV infection does not significantly reduce the activity of the major effectors of immunity of Listeria spp. (innate immune mechanisms and the CD8+ T-cell subset [266]) (35, 306, 316, 592).
The health status of the patient greatly influences the outcome of listeriosis. Immunocompetent patients usually survive listeriosis, whereas those with underlying debilitating diseases often succumb to the infection (mean mortality rate for this group, >30 to 40%) (235, 627). Although most listeriosis cases are associated with underlying risk factors, there are also a few adult patients for whom no obvious predisposing condition can be identified (177, 233, 235). This shows that L. monocytogenes has the potential to infect immunocompetent individuals. In some epidemics, all the nonpregnant adult patients had preexisting illnesses or immunosuppresive conditions (180, 386), whereas in others no such predisposing factors were found in any of the nonperinatal cases (587). This may be due to differences in the bacterial load present in the contaminated food involved or to the degree of virulence of the corresponding strains of L. monocytogenes.
Entry and colonization of host tissues.
(i) Crossing the intestinal barrier.
Before reaching the intestine, the ingested Listeria organisms must withstand the adverse environment of the stomach (Fig. 2). Oral infective doses are lower for cimetidine-treated experimental animals than for untreated animals (586a), and the use of antacids and H2-blocking agents has been reported to be a risk factor for listeriosis (286, 592). This indicates that gastric acidity may destroy a significant number of the Listeria organisms ingested with contaminated food.
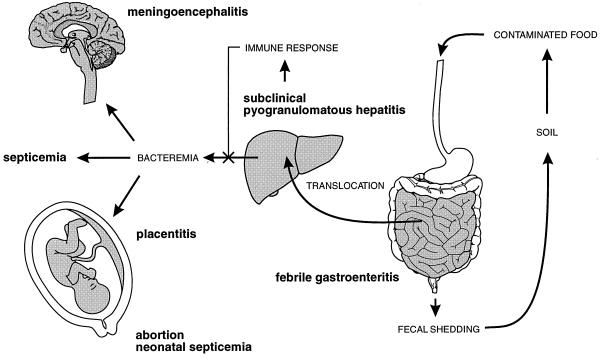
FIG. 2.
Schematic representation of the pathophysiology of Listeria infection. See text for details.
There is controversy concerning the point of entry and the mechanism of intestinal translocation used by L. monocytogenes. In an early study by Racz et al. with guinea pigs infected intragastrically with 1010 L. monocytogenes, detailed histological analyses revealed that all the animals developed enteritis (526). In the initial stages, bacteria were detected mostly in the absorptive epithelial cells of the apical area of the villi, whereas in later phases most were inside macrophages of the stroma of the villi, suggesting that L. monocytogenes penetrates the host by invading the intestinal epithelium (526). This is consistent with the observation that L. monocytogenes is able to penetrate the apical surface of polarized, differentiated human enterocyte-like Caco-2 cells with a brush border (322) (see Fig. 3B and C). In other studies using mice inoculated per os with 108 to 109 bacteria, no invasion of the intestinal villous epithelium was observed; instead, there was colonization of the Peyer's patches (400, 410, 411), suggesting that L. monocytogenes uses the M-cell epithelium as an entry portal, as reported for other bacterial pathogens (623). Direct evidence that L. monocytogenes may indeed penetrate the host via the M cells overlying the Peyer's patches has been provided by a recent study using a murine ligated-loop model and scanning electron microscopy (308). However, listerial penetration at this level appears not to be very efficient, because only small numbers of bacteria are observed in association with the follicle-associated epithelium overlying Peyer's patches (411).
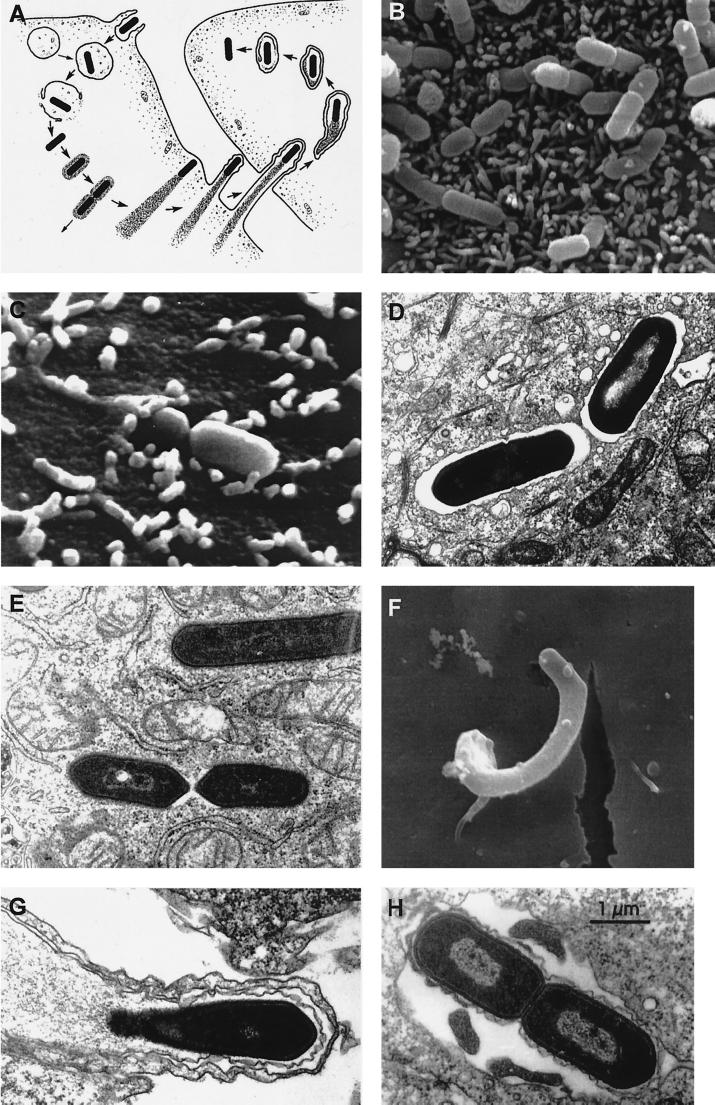
FIG. 3.
Stages of listerial intracellular parasitism. (A) Scheme of the intracellular life cycle of pathogenic Listeria spp. (reproduced from reference 655 with permission of the Rockefeller University Press). In anticlockwise order: entry into the host cell by induced phagocytosis, transient residence within a phagocytic vacuole, escape from the phagosome into the cytoplasm, cytosolic replication and recruitment of host cell actin onto the bacterial surface, actin-based motility, formation of pseudopods, phagocytosis of the pseudopods by neighboring cells, formation of a double-membrane phagosome, escape from this secondary phagosome, and reinitiation of the cycle. (B to H) Scanning and transmission electron micrographs of cell monolayers infected with L. monocytogenes (B) Numerous bacteria adhering to the microvilli of a Caco-2 cell (30 min after infection). (C) Two bacteria in the process of invasion (Caco-2 cell, 30 min postinfection). (D) Two intracellular bacteria soon after phagocytosis, still surrounded by the membranes of the phagocytic vacuole (Caco-2 cell, 1 h postinfection). (E) Intracellular Listeria cells free in the host cell cytoplasm after escape from the phagosome (Caco-2 cell, 2 h postinfection). (F) Pseudopod-like membrane protrusion induced by moving Listeria cells, with the bacterium being evident at the tip (brain microvascular endothelial cell, 4 h postinfection; taken from reference 245 with permission). (G) Section of a pseudopod-like structure in which a thin cytoplasmic extension of an infected cell is protruding into a neighboring noninfected cell (notice that the protrusion is covered by two membranes) (Caco-2 cell, 4 h postinfection). (H) Bacteria in a double-membrane vacuole formed during cell-to-cell spread (Caco-2 cell, 4 h postinfection).
Intestinal translocation of pathogenic listeriae occurs without the formation of gross macroscopic or histological lesions in the gut of mice (411). This suggests that an epithelial phase involving bacterial multiplication in the intestinal mucosa is not required by L. monocytogenes for systemic infection. Indeed, a recent study using a rat ileal loop model of intestinal infection (523) has shown that Listeria organisms are translocated to deep organs very rapidly (within a few minutes), demonstrating that crossing of the intestinal barrier occurs in the absence of prior intraepithelial replication. This study also showed that defined mutants defective in virulence factors known to be involved in epithelial cell invasion (InlA and InlB), intracellular survival (Hly), and cell-to-cell spread (ActA) (see below), and even the nonpathogenic species L. innocua, translocated at the same rate as wild-type L. monocytogenes. Translocation was dose dependent, and the presence of Peyer's patches in ligated loops did not affect the rate of translocation, levels of uptake being similarly low for Peyer's patches and villous intestine (50 to 250 bacteria per cm2 of tissue after inoculation of the loop with 109 bacteria) (523). These findings favor the view that listerial translocation is a passive, nonspecific process similar to that observed for other bacteria (36), at least in mice and rats. However, L. monocytogenes proliferated in the intestinal wall, whereas L. innocua did not. The preferential site for bacterial replication was the Peyer's patches, and the essential listerial virulence factor Hly (hemolysin) was indispensable for this process, showing that L. monocytogenes establishes an active local infection in these lymphoid structures of the intestine. The foci of infection consisted of pyogranulomatous reactions in the subepithelial follicular tissue, with bacteria visible inside permissive mononuclear cells, presumably nonactivated resident macrophages and dendritic cells (522a, 523). Thus, antigen presentation events probably already take place in the intestine during the early phases of host colonization, and this may play an important role in the acquired resistance to subsequent reinfection that develops after primary oral exposure to L. monocytogenes (394, 400). A recent study has shown that invasion of intestinal epithelial cells by L. monocytogenes induces the activation of NF-κB and the subsequent upregulation of interleukin-15 (IL-15), and that at early stages of an oral infection with L. monocytogenes in mice and rats, intestinal intraepithelial lymphocytes become activated and produce Th1-type cytokines. This suggests that the interaction between intestinal epithelial and lymphoid cells may be relevant in the local host defence against listerial infection in the gut (706).
Experimental observations made with the mouse and rat models of intestinal translocation do not, however, explain how L. monocytogenes causes enteritis. The association of gastroenteric symptoms with fever is consistent with invasive intestinal disease, as observed by Racz in the guinea pig model (526). Pathogenic Listeria organisms pass directly from cell to cell by a mechanism involving host cell actin polymerization (see below). Therefore, regardless of the mechanism of entry used, the bacteria that penetrate the intestinal wall might then invade neighboring enterocytes by basolateral spread, leading to enteritis. This is consistent with in vitro experimental data showing that L. monocytogenes enters polarized Caco-2 cells predominantly via the basolateral surface (196). Gross intestinal lesions develop in experimental animals only if large oral doses of L. monocytogenes are given (400, 523). Similarly, episodes of listerial gastroenteritis in humans occur in the form of outbreaks with very short incubation periods and high attack rates among immunocompetent adults (118, 577), consistent with the ingestion of a very high dose of bacteria (as high as 2.9 × 1011, as estimated for one of these oubreaks, caused by the consumption of heavily contaminated chocolate milk [118]). Thus, intestinal invasion and the ensuing febrile gastroenteritis syndrome probably result from extensive exposure of the intestine to pathogenic Listeria organisms. However, the possibility that some L. monocytogenes strains have a greater enteropathogenic potential and cause intestinal damage at a lower dose cannot be ruled out.
(ii) Multiplication in the liver.
The Listeria organisms that cross the intestinal barrier are carried by the lymph or blood to the mesenteric lymph nodes, the spleen, and the liver (411, 523) (Fig. 2). As stated above, this initial step of host tissue colonization by L. monocytogenes is rapid. The unusually long incubation period required by L. monocytogenes for the development of symptomatic systemic infection after oral exposure in relation to that for other food-borne pathogens is therefore puzzling and indicates that listerial colonization of host tissues involves a silent, subclinical phase, many of the events and underlying mechanisms of which are unknown.
Experimental infections of mice via the intravenous route have shown that L. monocytogenes bacteria are rapidly cleared from the bloodstream by resident macrophages in the spleen and liver (99, 112a, 405). Most (90%) of the bacterial load accumulates in the liver, presumably captured by the Kupffer cells that line the sinusoids. These resident macrophages kill most of the ingested bacteria, as shown by in vivo depletion experiments (161), resulting in a decrease in the size of the viable bacterial population in the liver during the first 6 h after infection. Kupffer cells are believed to initiate the development of antilisterial immunity by inducing the antigen-dependent proliferation of T lymphocytes and the secretion of cytokines (243). Not all Listeria cells are destroyed by tissue macrophages, and the surviving bacteria start to grow, increasing in numbers for 2 to 5 days in mouse organs (93, 99, 128, 181, 381, 408, 448).
The principal site of bacterial multiplication in the liver is the hepatocyte (100, 112a, 197, 241, 241a, 563). This finding has led to the dismissal of the long-held idea that the major host niche for the parasitic life of L. monocytogenes is the macrophage population. There are two possible ways for L. monocytogenes to gain access to the liver parenchyma after its intestinal translocation and carriage by the portal or arterial bloodstream: via Kupffer cells, by cell to cell spread, or by the direct invasion of hepatocytes from the Disse space after crossing the fenestrated endothelial barrier lining the sinusoids. L. monocytogenes has been shown to efficiently invade hepatocytes in vitro (149, 701).
Electron microscopy of hepatic tissue from infected mice suggests that L. monocytogenes goes through the complete intracellular infectious cycle in hepatocytes (197, 621), including actin-based intercellular spread (see below). Direct passage from hepatocyte to hepatocyte would lead to the formation of infectious foci in which L. monocytogenes disseminates through the liver parenchyma without coming into contact with the humoral effectors of the immune system. This may explain why antibodies play no major role in anti-Listeria immunity (516).
During the early steps of liver colonization, polymorphonuclear neutrophils are recruited at the sites of infection, forming discrete microabscesses. Neutrophils have been shown to play an important role in controlling the acute phase of Listeria infection (561) and in mediating the destruction of Listeria-infected hepatocytes in vivo (99). Hepatocytes respond to Listeria infection by releasing neutrophil chemoattractants and exhibiting an increase in adhesion to neutrophils, resulting in microabscess formation (560). They also respond by initiating an apoptosis program that may be critical for removing Listeria-infected cells from the liver tissue at early stages of infection (448a, 560). Using transgenic mice expressing a degradation-resistant IκB transgene, it has been shown recently that NF-κB activation in mouse hepatocytes is essential to clear L. monocytogenes from the liver, possibly by coordinating local innate immune response (368). Two to four days after infection, neutrophils are gradually replaced by blood-derived mononuclear cells together with lymphocytes to form the characteristic granulomas (283, 408) (Fig. 1G and H). These granulomas are the histomorphological correlate of cell-mediated immunity and presumably act as true physical barriers that confine the infectious foci, impeding further bacterial dissemination by direct cell-to-cell passage (516).
Between days 5 and 7 postinfection, L. monocytogenes bacteria start to disappear from mouse organs until their complete clearance as a result of gamma interferon (IFN-γ)-mediated macrophage activation and the induction of an acquired immune response primarily mediated by CD8+ lymphocytes, which together destroy Listeria-infected cells (241a, 268, 328, 441). These cytotoxic T lymphocytes (CTL) are directed against listerial epitopes present in secreted virulence-associated proteins, such as Hly (59, 71, 266, 281, 625, 677), the metalloprotease Mpl (79), and the surface protein p60 (203, 204, 267, 624), and possibly also in somatic housekeeping proteins such as superoxide dismutase (280). Protection against L. monocytogenes also involves a vigorous Th1-biased CD4+ T-cell response (125, 203) and innate mechanisms such as the activation of IFN-γ-producing natural killer (NK) cells in response to IL-12 and tumor necrosis factor alpha (TNF-α) secretion by infected macrophages (125, 166, 268, 290). Hepatocytes may contribute to protection by becoming less permissive to intracellular proliferation of Listeria organisms upon exposure to IFN-γ (244), especially if costimulated with other cytokines (646).
The above course of events is accelerated in immune animals, resulting in rapid elimination of L. monocytogenes from the liver (Fig. 2). This is probably the most common outcome of L. monocytogenes infection in humans and animals in normal conditions, given the potentially high frequency of exposure to the pathogen via contaminated food and the relatively rare occurrence of clinical disease. Indeed, T lymphocytes directed against Listeria antigens are commonly found in healthy individuals (457), probably due to chronic stimulation of the immune system by L. monocytogenes antigens that are presumably continuously delivered to the immune system via food. A recent study has shown that the nonpathogenic species L. innocua can enhance a previously established L. monocytogenes-specific T-cell memory via recognition of cross-reactive p60 epitopes shared by the two species (204). L. innocua is commonly found in food (573), and as stated above, it is translocated to the deep organs of mice as efficiently as L. monocytogenes. Therefore, repeated contact with L. innocua may boost protective immunity against pathogenic Listeria spp., possibly explaining in part the rare occurrence of listeriosis in the general population.
If the infection is not controlled by an adequate immune response in the liver, as may occur in immunocompromised individuals, unlimited proliferation of L. monocytogenes in the liver parenchyma may result in the release of bacteria into the circulation (Fig. 2). L. monocytogenes is a multisystemic pathogen that can infect a wide range of host tissues, as indicated by its capacity to cause septicemia involving multiple organs and by the variety of potential sites of localized Listeria infection (see above). However, the principal clinical forms of listeriosis clearly show that L. monocytogenes has a pathogenic tropism towards the gravid uterus and the CNS (Fig. 2).
(iii) Colonization of gravid uterus and fetus.
Abortion and stillbirth due to Listeria spp. have been reproduced experimentally by intravenous, oral, and respiratory inoculation in naturally susceptible gestating animal hosts, such as sheep, cattle, rabbits, and guinea pigs, as well in pregnant mice and rats (1, 238, 239, 329, 450, 487, 488, 586). This shows that L. monocytogenes gains access to the fetus by hematogenous penetration of the placental barrier (Fig. 2). In pregnant mice, the blood-borne bacteria first invade the decidua basalis and then progress to the placental villi, where they cause diffuse inflammatory infiltration and necrosis (1, 535, 536). Macrophages appear to be excluded from the murine placenta, neutrophils acting as the main antilisterial effector cell population (251a). Using homozygous mutant mice, it has been shown recently that colony-stimulating factor-1 is required for the recruitment of neutrophils to the infectious foci in the decidua basalis. This occurs via induction of neutrophil chemoattractant synthesis by the trophoblast (251a). In humans, placental infection is characterized by numerous microabscesses and focal necrotizing villitis (502, 582). Colonization of the trophoblast layer followed by translocation across the endothelial barrier would enable the bacteria to reach the fetal bloodstream, leading to generalized infection and subsequent death of the fetus in utero or to premature birth of a severely infected neonate with miliary pyogranulomatous lesions (the above-mentioned granulomatosis infantiseptica) (Fig. 1E and F).
The depression of cell-mediated immunity during pregnancy (686) presumably plays an important role in the development of listeriosis. In laboratory rodents, pregnancy reduces resistance to L. monocytogenes (58, 399) and significantly prolongs the course of primary infection in the liver (1). The T-cell-dependent elimination of L. monocytogenes from the organs of pregnant mice is delayed in late pregnancy. This correlates with a failure in the production of IFN-γ and results in listerial invasion of placental and fetal tissues (463). Abnormally high physiological levels of estrogenic hormones, such as those that occur during late pregnancy, may account for the disruption of T-cell-mediated resistance to infection, as treatment of mice with steroids inhibits the proliferative response to L. monocytogenes of splenic T cells and increases susceptibility to Listeria infection (524). This correlates with a decrease in the production of IL-2 (524), a cytokine that stimulates resistance to Listeria spp. in mice (255). The intracellular killing function of macrophages has also been shown to be decreased by β-estradiol, which is associated with inhibition of both IL-12 and TNF-α secretion in vivo (578). Local depression in the cellular immune response in the placenta, which physiologically is important for preventing rejection of the fetus, may also contribute to the higher susceptibility to uterine infection by L. monocytogenes (398, 535, 536). It is not known why mothers suffering Listeria-induced miscarriage never develop CNS infection or overt septicemic disease.
(iv) Invasion of the brain.
In humans, CNS infection by Listeria spp. presents primarily in the form of meningitis. This meningitis, however, is often associated with the presence of infectious foci in the brain parenchyma, especially in the brain stem (391, 473), suggesting L. monocytogenes has a tropism for nerve tissue. The neurotropism and special predilection of L. monocytogenes for the rhombencephalon are shown most clearly in ruminants, in which listerial CNS infection, in contrast to the situation in humans, develops mainly as primary encephalitis. In these animals, infectious foci are restricted to the pons, medulla oblongata, and spinal cord (Fig. 1C). Although there is inflammatory lymphocyte or mononuclear cell infiltration of the meninges, this condition occurs as an extension of the brain process, and macroscopic lesions may not even be evident or may be restricted to basal areas, midbrain, and cerebellum. Unilateral cranial nerve paralysis is a characteristic of listerial rhombencephalitis in ruminants, leading to the well-known circling disease syndrome (92, 103, 215, 216, 312, 534, 666, 693) (Fig. 1A and B). In humans, primary nonmeningeal brain infection is seldom observed. However, as in ruminants, it develops as cerebritis involving the rhombencephalon (15, 20, 69, 390, 391, 473).
Brain lesions in listerial meningoencephalitis are typical and very similar in humans and animals. They consist of perivascular cuffs of inflammatory infiltrates composed of mononuclear cells and scattered neutrophils and lymphocytes (Fig. 1D). Bacteria are generally absent from these perivascular areas of inflammation. Parenchymal microabscesses and foci of necrosis and malacia are also typically present (Fig. 1D). Bacteria are relatively abundant in these lesions, within phagocytes or free in the brain parenchyma around the necrotic areas (92, 103, 312, 315, 412, 489). Depletion experiments in mice using a neutrophil-specific monoclonal antibody have shown that neutrophils play a critical role in eliminating L. monocytogenes from infectious foci in the brain (389a). Less commonly, bacteria are observed within neurons in both natural (412, 489) and experimentally induced (588) infections. This is consistent with in vitro data showing that the invasion of cultured neurons is a relatively rare event (151, 505). However, neurons are efficiently invaded in vitro by direct cell-to-cell spread from infected macrophages or microgial cells (151). A recent study (146) has shown that L. monocytogenes can efficiently invade sensory neurons of rat dorsal root ganglia but not hippocampal neurons, suggesting that there may be differences in the susceptibility to infection among different types of neurons. The relevance of neuronal invasion to the pathogenesis of Listeria encephalitis is subject to speculation, as discussed below.
The mechanisms by which L. monocytogenes infects the CNS are still largely unknown. Numerous attempts have been made to reproduce listerial meningoencephalitis in susceptible animal hosts and laboratory rodents using various routes of inoculation, with variable success. Intravenous delivery of the inoculum does not usually result in CNS infection in sheep (103). This, and the usual absence of visceral involvement in natural cases of ovine listerial encephalitis, seems to argue against a hematogenous route of infection. The topographic distribution of the lesions in sheep, strictly confined to the brainstem, and the frequent unilateral involvement of the trigeminal nerve, with neuritis affecting distal portions of a single or a few fasciculi in only one of the nerve branches, led to the hypothesis that L. monocytogenes invades the brain by centripetal migration along cranial nerves (17, 92, 661). To test this hypothesis, Asahi and coworkers (17) scarified oral, nasal, and labial mucosae of mice with L. monocytogenes. Most mice developed neurological signs, such as torticollis, rolling movements, and paralysis of the hindlegs, 5 days after infection. These signs correlated with the presence of mononuclear and round cell infiltrations along the trigeminal nerve, in the corresponding nerve ganglion, and in the medulla oblongata, suggesting retrograde bacterial ascension along the cranial nerve to the brainstem. In another experiment, 4 of 11 goats inoculated in the lip developed listerial encephalitis 17 to 28 days after inoculation, with microscopic lesions identical to those found in natural cases of listeriosis, with ganglioneuritis of the trigeminal nerve (17). In spontaneous cases of ovine listerial encephalits, bacteria have been observed in linear arrays in individual nerve fibers, inside axons, and with actin tails (91, 92, 489), suggesting active intra-axonal movement. Experiments using a double-chamber cell culture system and dorsal root ganglia neurons provided evidence that L. monocytogenes can infect axons and spread along them towards the nerve cell body (146).
Experimental evidence has been obtained that intra-axonal bacterial transport may indeed occur in vivo. Otter and Blakemore (489) developed a mouse model in which L. monocytogenes was injected distally into the sciatic nerve. Paralysis of the leg into which the bacteria were injected occurred 7 to 12 days later, and examination of the spinal cord revealed lesions very similar to those found in the brains of naturally affected sheep. Bacteria were seen in these lesions in neutrophils and, in some cases, in axons (489). The feasibility of ascending intra-axonal transport via the termini of the trigeminal nerve was tested by experimentally injecting L. monocytogenes directly into the dental pulp of sheep through a hole drilled in the tooth crown (22). Six of 21 sheep developed neurological signs 20 to 40 days after challenge, and most animals showed histological encephalitis and trigeminal ganglioneuritis on the side of inoculation. These observations favor the view that L. monocytogenes may directly invade exposed sensory terminal ramifications of the cranial nerve in the mouth and reach the brain by centripetal spread. They also demonstrate that cases of mild CNS invasion by Listeria organisms may occur without overt clinical signs. This is important because it provides one possible explanation for the relatively small number of cases with neurological manifestations that are observed among animals exposed to contaminated silage in a herd affected by listerial encephalitis (and, by extension, to humans exposed to Listeria-contaminated food). However, in another study in which brains from natural cases of sheep with meningoencephalitis were analyzed, inflammatory infiltration was observed only in the intracranial portion of the trigeminal tract, always associated with meningitis adjacent to proximal parts of cranial nerves. The authors were of the opinion that cranial nerve invasion was only secondary to a hematogenous brain infection and occurred by centrifugal spread from the brainstem (103). The above-mentioned experiments with the two-chamber model (146) suggested that both retrograde and anterograde transport of L. monocytogenes may occur inside infected neurons.
Although intravenous inoculation only occasionally gives positive results, intracarotid inoculation consistently results in encephalitis in sheep (103, 314, 486). This supports the notion that L. monocytogtenes may indeed reach the brain via the blood. Strong evidence that meningoencephalitis in ruminants has a vascular basis is provided by the nature of the brain lesions themselves, with extensive perivascular infiltrates and mononuclear microabscesses and necrotic foci developing in close proximity to brain capillaries (103) (Fig. 1D). In contrast to intravenous inoculation, intracarotid delivery provides the inoculum direct access to the vascular system of the brain, enabling bacteria to bypass the clearance mechanisms of organs such as the spleen and liver. In an immunocompetent host, these clearance mechanisms should be sufficient to eliminate rapidly a single-dose intravenous inoculum, preventing the bacteria from invading the brain. Intracarotid inoculation in sheep causes CNS lesions involving the choroid plexus and the ependyma of the cerebral ventricles and aqueduct, resulting in primary meningitis and secondary lesions in the adjoining cerebral tissues (17). These brain structures are only rarely affected in natural cases of listerial meningoencephalitis in ruminants (103). The reason for these differences between natural and experimental lesion patterns probably resides in the concentration of bacteria in the blood, as this is an important factor affecting the mode by which bacterial pathogens gain access to the CNS (659, 660). This concentration would reach an extraordinarily high peak in the brain after intracarotid delivery, and high-grade bacteremia is more likely to cause meningitis due to massive bacterial penetration across the epithelium lining the choroid plexus, which has an exceptionally high rate of blood flow. In contrast, low-grade bacteremia, such as that resulting from the release of bacteria into the blood from infectious foci located in distant organs (e.g., the liver), tends to be associated with multiple foci in the brain parenchyma due to individual penetrations along the extensive microvascular endothelial bed (660).
Experimental infections in small laboratory animals also provide evidence for a hematogenous route of CNS invasion for L. monocytogenes. Intravenous inoculation in mice results in meningoencephalitis in a significant proportion of animals that survive the initial systemic phase of infection. Lesions include choroiditis, meningitis, and the characteristic perivascular cuffing and parenchymal pyogranulomatous foci in the brainstem (31, 103). No brain invasion is detected in mice challenged with low infective doses that induce only transient bacteremia, even if there is significant bacterial multiplication in the spleen and liver. However, brain invasion is achieved with high infective doses causing severe systemic disease and prolonged bacteremia (31). This suggests that the persistence of large numbers of bacteria in the blood is essential for the induction of meningoencephalitis by L. monocytogenes. This conclusion is consistent with a recent study by Blanot and coworkers (47), who adapted a model of otitis media in gerbils to study listerial CNS invasion. With a low infective dose of L. monocytogenes (103/ear), these authors induced persistent low-level bacteremia (102 bacteria/ml) which was associated with the development of rhombencephalitis in gerbils mimicking that observed during human listerial meningoencephalitis, with leptomeningitis, parenchymal foci, and perivascular sheaths. The number of bacteria in the liver and spleen of gerbils decreased by day 5 postinoculation, but growth in brain tissue was unrestricted (47), suggesting that nerve tissue is a privileged niche for the multiplication of L. monocytogenes. CNS invasion was also regularly observed in mice after oral or subcutaneous inoculation (9, 17, 522). These infection routes imply systemic involvement and, clearly, also lymphohematogenous dissemination of bacteria to the brain.
In experiments with orally or subcutaneously infected mice, parenchymal brain lesions are irregularly observed, but marked inflammatory lesions in the meninges and ventricular system (choroid plexus, lateral, third and fourth ventricles, and aqueduct) are consistently recorded (9, 522). This striking affinity of L. monocytogenes for mouse ventricular structures was also noted after direct intracerebral inoculation (588), which prevents primary blood-borne exposure of the ependyma via the choroid plexus. In contrast, no involvement of the ventricular system was noticed in the gerbil model (47). These observations suggest that there may be anatomical and physiological differences in the brains of the various animal species that affect L. monocytogenes neuropathogenesis. Indeed, this may account for the differential characteristics of listerial CNS infection in humans and ruminants. However, it cannot be excluded that the peculiar characteristics of listerial meningoencephalitis in ruminants result from the fact that an alternative primary nonvascular mechanism of CNS invasion, which may perfectly coexist with hematogenous dissemination, has specifically evolved in these animals. Ruminants eat plant material, the physical characteristics of which may lead to small breaches of the oral mucosa. If animals are fed contaminated silage, these small wounds are repeatedly exposed to L. monocytogenes during rumination, favoring invasion of the trigeminal nerve terminals and subsequent intraneural, direct spread to the brain stem.
Bacterial pathogens such as H. influenzae, N. meningitidis, S. pneumoniae, and Streptococcus suis cross the blood-brain barrier (BBB) primarily via the choroid plexus. This enables them to reach the CSF and create purulent meningitis by spreading through the subarachnoid space (525, 659, 660, 697). Although in human listerial infections of the CNS, purulent meningitis also occurs and bacteria may also be detected in the CSF, it is clear that L. monocytogenes, unlike other meningitis-causing bacterial pathogens, tends to affect brain parenchymal tissue. This may reflect a tropism for the microvascular endothelium, in particular that lining the capillary bed of the rhombencephalon, resulting in significant or preferential (in ruminants) crossing of the BBB at this point. Direct uptake by endothelial cells of bacteria circulating free in the blood is one possible mechanism by which L. monocytogenes may cross the microvascular BBB. Evidence for this mechanism is provided by an electron microscopic study of human brainstem tissue, in which bacteria have been observed within endothelial cells or adhering to the luminal face of the microvascular endothelium (335). This mechanism is also consistent with in vitro data showing that L. monocytogenes is able to invade cultured human brain microvascular endothelial cells (BMEC) (245, 246). The listerial surface protein InlB, of the internalin family (see below), has been shown to be required for invasion of endothelial cells in vitro (246, 498), indicating that specific bacterial molecules are actively involved in the interaction with the BBB. L. monocytogenes efficiently replicates for long periods of time within brain microvascular cells without causing any evident damage, creating heavily infected foci from which bacteria spread to neighboring cells by actin-based motility (246). In this way, L. monocytogenes may reach and disseminate easily into the protected spaces of the CNS.
L. monocytogenes infection induces a potent response in cultured endothelial cells which is accompanied by the upregulation of endothelial adhesion molecules (P- and E-selectin, ICAM-1, and VCAM-1) (see below), leading to an increase in the binding of polymorphonuclear leukocytes to infected endothelial cells (152, 247, 330, 600, 618). Heterologous plaque assays using infected macrophages as vectors have demonstrated that L. monocytogenes can spread efficiently from macrophages into endothelial cells (157, 246). Therefore, the recruitment of phagocytes to the infected endothelium, primarily a host defense response, may also be used by L. monocytogenes, in addition to direct invasion, to cross the BBB. Evidence for a significant role in vivo for such a Trojan horse mechanism of CNS invasion, presumably involving direct bacterial cell-to-cell spread from infected phagocytes carried by the blood after adhesion to endothelial cells, has been provided recently by Drevets (154). This author showed that phagocyte-associated L. monocytogenes accounted for 30% of the total bacterial population circulating in the bloodstream after intravenous inoculation. These entrapped bacteria spread to endothelial cells in vitro, and Listeria-infected peripheral blood leukocytes successfully established brain colonization in vivo (154). It is unknown whether trafficking of infected phagocytes across the BBB, as part of the inflammatory influx induced by endothelial and underlying brain tissue infection, occurs in vivo during CNS invasion by L. monocytogenes.
Hypothetical scenario.
From the information presented above, the following hypothetical scenario for the pathogenesis of listeriosis can be proposed. Clinical outcome of Listeria infection depends on three major variables: (i) the number of bacteria ingested with food, (ii) the pathogenic properties of the strain, and (iii) the immunological status of the host. In immunocompetent individuals with no predisposing conditions, ingestion of low doses of L. monocytogenes will probably have no effect other than the development or boosting of antilisterial protective immunity. In contrast, oral exposure to large doses is likely to result in an episode of gastroenteritis and fever and, depending on the virulence of the strain, possible invasive disease. Immunocompromised and debilitated individuals, however, cannot mount an immune response strong enough to control bacterial proliferation in the liver, the primary target organ of L. monocytogenes, and are therefore susceptible to invasive disease following the ingestion of a lower inoculum. Inefficiently restricted growth of L. monocytogenes in the hepatocytes in these individuals is likely to result in an increase in the critical mass of bacteria and their release into the bloodstream. The ensuing prolonged bacteremia will result in local infections in secondary target organs (particularly the brain and placenta) or in septicemic disease in severely immunocompromised hosts. Figure 2 summarizes the key steps of the pathophysiology of Listeria infection.
INTRACELLULAR INFECTIOUS CYCLE
In addition to multiplying within macrophages, Listeria organisms are invasive pathogens that can induce their own internalization in various types of cell that are not normally phagocytic. These include epithelial cells (194, 435, 518), fibroblasts (359, 641), hepatocytes (149, 242, 701), endothelial cells (157, 245, 498), and various types of nerve cell, including neurons (151). Listeria organisms have been also shown to be taken up by and survive within dentritic cells (253, 345). In the interior of all cells that L. monocytogenes is able to penetrate, whether macrophages or nonprofessional phagocytes, it develops an intracellular life cycle with common characteristics (Fig. 3).
Internalization
The cycle begins with adhesion to the surface of the eukaryote cell and subsequent penetration of the bacterium into the host cell. The invasion of nonphagocytic cells involves a zipper-type mechanism, in that the bacterium gradually sinks into diplike structures of the host cell surface until it is finally engulfed. During this process, the target cell membrane closely surrounds the bacterial cell and does not form the spectacular local processes or membrane ruffles characteristic of invasion by Salmonella and Shigella spp. (147, 322, 358, 435, 642) (Fig. 3B and C). The structures, mechanisms, and signal transduction cascades involved in the interaction between the bacterium and the cell during phagocytosis are only beginning to be elucidated.
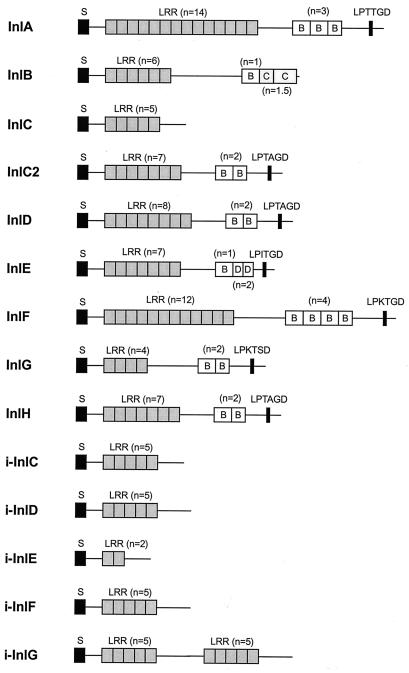
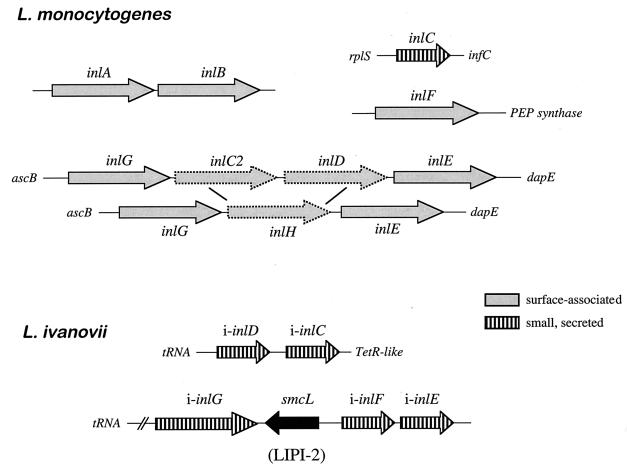
The multisystemic nature of listerial infection indicates that L. monocytogenes probably recognizes a number of different eukaryotic receptors. The C3bi and C1q complement receptors have been reported to be involved in L. monocytogenes uptake by phagocytic cells (10, 115, 155, 156). L. monocytogenes is also efficiently internalized in the absence of serum, indicating that nonopsonic receptor-ligand interactions are also involved in host cell recognition by Listeria spp. (509). The eukaryote cell receptors used by Listeria spp. include the transmembrane glycoprotein E-cadherin (435), the C1q complement fraction receptor (61), the Met receptor for hepatocyte growth factor (HGF) (615a), and components of the extracellular matrix (ECM) such as heparan sulfate proteoglycans (HSPG) (12) and fibronectin (217). The macrophage scavenger receptor was shown to bind L. monocytogenes lipoteichoic acids and hence may also be involved in Listeria-macrophage interactions (160, 240). The bacterial ligands identified to date are all surface proteins, such as the internalins InlA and InlB, the actin-polymerizing protein ActA, and p60 (see below). Recently, several putative adhesion factors of L. monocytogenes have been identified by screening transposon mutants for defective attachment to eukaryotic cells. Among these putative adhesins are the surface protein Ami, an autolysin with a C-terminal cell wall-anchoring domain similar to InlB (60, 429, 445) (see below), and Lap, a 104-kDa surface protein involved in attachment to Caco-2 cells (581a). Also recently, a 24.6-kDa fibronectin-binding protein has been identified in L. monocytogenes by screening a genomic library in Escherichia coli for fibronectin binding (215). Bacterium-host cell interactions involving lectins (482) may also be involved in L. monocytogenes adhesion to eukaryotic cells (174). Indirect evidence has been obtained for an L. monocytogenes adhesin containing α-d-galactose, involved in uptake by mouse CB1 dendritic cells (253) and human HepG22 hepatocarcinoma cells (114) upon recognition of a carbohydrate receptor at the eukaryotic cell surface. The presence in L. monocytogenes of lectin-like ligands mediating binding to d-glucosamine, l-fucosylamine, and p-aminophenyl-α-d-mannopyranoside moieties has also been indirectly demonstrated (110).
Intracellular Proliferation and Intercellular Spread
During invasion, Listeria bacteria become engulfed within a phagocytic vacuole (194) (Fig. 3D). Little is known about the characteristics of the Listeria-containing vacuolar compartment, but the vacuoles become acidified soon after uptake (25). There is evidence that L. monocytogenes ensures its intravacuolar viability by preventing phagosome maturation to the phagolysosomal stage (11). Thirty minutes after entry, the bacteria begin to disrupt the phagosome membrane (194), and within 2 h, about 50% of the intracellular bacterial population is free in the cytoplasm (655) (Fig. 3E). This membrane disruption step is essential for listerial intracellular survival and proliferation (220) and is mediated by the hemolysin in combination with phospholipases (see below).
Once in the cytosol, bacteria multiply (Fig. 3E), with a doubling time of approximately 1 h (194, 518), i.e., approximately three times slower than in rich medium. In L. monocytogenes, intracellular growth does not involve the upregulation of known stress proteins (261) (see below), and auxotrophic mutants requiring aromatic amino acids or purines for growth in minimal medium grow inside cells at rates similar to those of the wild-type parent strain (413). This indicates that the cytoplasmic compartment is permissive for Listeria proliferation. However, three metabolic genes (purH, purD, and pyrE, involved in purine and pyrimidine biosynthesis) and an arginine ABC transporter (arpJ) have been shown to be induced within cells (336). Mutation of these genes had no major effect on intracellular proliferation and virulence (336), which can mean that certain nutrients are present in the host cell cytoplasm at concentrations that, although not limiting, are sufficiently low that L. monocytogenes has to upregulate certain metabolic genes to grow efficiently within cells. Recent experimental evidence indicates that pathogenic Listeria spp. may exploit hexose phosphates from the host cell cytoplasm for efficient intracellular growth (543) (see below). Evidence has also been recently presented that antimicrobial peptides from the host cell cytosol, such as ubiquicidin found in macrophages (283a), may play a role in restraining intracellular proliferation of L. monocytogenes. Nothing is known about the listerial mechanisms counteracting these inhibitory peptides.
Intracytoplasmic bacteria are immediately surrounded by a cloud of a fine, fuzzy, fibrillar material composed of actin filaments, which later (around 2 h postinfection) rearranges to form an actin tail of up to 40 μm at one of the poles of the bacterium (see Fig. 6B). This actin tail consists of two populations of cross-linked actin filaments, one formed by relatively long, axially arranged actin bundles and the other by shorter, randomly arranged filaments. The polar assembly of actin filaments propels the bacterial cell in the cytoplasm with a mean speed of 0.3 μm/s, in a fashion reminiscent of the “action-reaction” principle that governs rocket movement. Moving Listeria cells leave an evanescent trail of F-actin undergoing depolymerization at the distal end, the steady-state length of which is proportional to the speed of bacterial movement. This movement is random, so some bacteria eventually reach the cell periphery, come into contact with the membrane, and push it, leading to the formation of finger-like protrusions with a bacterium at the tip (Fig. 3F and G). These pseudopods penetrate uninfected neighboring cells and are in turn engulfed by phagocytosis, resulting in the formation of a secondary phagosome delimited by a double membrane (Fig. 3H), with the inner membrane originating from the donor cell (Fig. 3G). Bacteria escape rapidly (within 5 min) from the newly formed vacuole by dissolving its double membrane, reach the cytoplasm, and initiate a new round of intracellular proliferation and direct intercellular spread (117, 453, 549, 601, 652, 654–656) (Fig. 3A). In tissue culture, this infectious cycle is reflected in the formation of plaques in the cell monolayer (641, 672). Actin-based intracytoplasmic movement and cell-to-cell spread are mediated by the listerial surface protein ActA (see below).
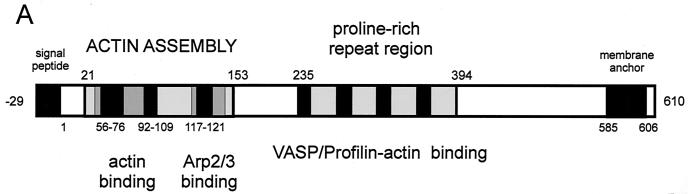
FIG. 6.
Actin-based intracellular motility. (A) Schematic structure and functional motifs of ActA (see text for details; amino acids numbered according to references 339 and 672). (B) Cos-1 cells infected with L. monocytogenes and labeled with FITC-phalloidin (actin stain, green) and rhodamine-conjugated anti-Listeria antibody (red) at 4 h postinfection; moving bacteria with actin tails are visible. (C and D) Giemsa stain of Caco-2 cells infected with L. monocytogenes wild-type (C) and ΔactA mutant (D) at 4 h postinfection; ΔactA mutant bacteria do not move intracellularly and do not spread to neighboring cells, growing in the cytoplasm as a microcolony close to the nucleus.
VIRULENCE FACTORS
Hemolysin
The hemolysin gene, hly, was the first virulence determinant to be identified and sequenced in Listeria spp. Subsequent characterization of the hly locus led to discovery of the chromosomal virulence gene cluster in which most of the genetic determinants required for the intracellular life cycle of pathogenic Listeria spp. reside (see below). The hly product, Hly, was also the first virulence factor for which a precise role in the pathogenesis of Listeria infection was demonstrated. Hly is a key virulence factor essential for pathogenicity, having a vital role not only in intracellular parasitism but also in several other functions in the interaction of listeriae with their vertebrate host.
Characterization and role in escape from phagosome.
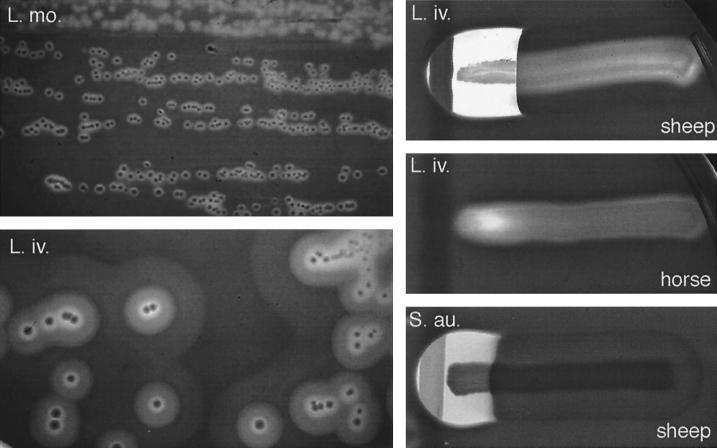
In 1941, Harvey and Faber (269) demonstrated for the first time the production of a soluble hemolysin by L. monocytogenes. During the 1960s and 1970s, various researchers tried to purify this hemolysin and characterize its biochemical and toxic properties (218, 303, 305, 475, 620, 683). Jenkins et al. (303) were the first to provide evidence that the Listeria hemolysin is similar in function and antigenicity to streptolysin O (SLO) from Streptococcus pyogenes. Kingdon and Sword (334) showed that the hemolysin was inhibited by cholesterol, that its optimum pH was below 7, and that it had cytotoxic properties in phagocytic cells. They were also the first to suggest that the hemolysin might be involved in the disruption of phagosomal membranes (15, 334). In 1985, Vicente et al. (676) reported the molecular cloning of a chromosomal fragment from L. monocytogenes that conferred hemolytic activity on E. coli. These authors were the first to use molecular genetics in the study of listerial virulence factors. Finally, in 1987, Geoffroy et al. (207) provided the first unambiguous evidence that the hemolysin of L. monocytogenes is an SLO-related cytolysin belonging to the family of cholesterol-dependent, pore-forming toxins (CDTX). This toxin was given the name listeriolysin O (LLO), and one of its key characteristics was determined, its low optimum pH (5.5) and the narrow pH range at which it is active (4.5 to 6.5) (207). Later, the 58-kDa CDTX hemolysin of L. ivanovii, ivanolysin O (ILO), was purified and characterized (669), and it was also shown that the weakly hemolytic but nonpathogenic species L. seeligeri produces, albeit in small amounts, an LLO-related CDTX (208, 376).
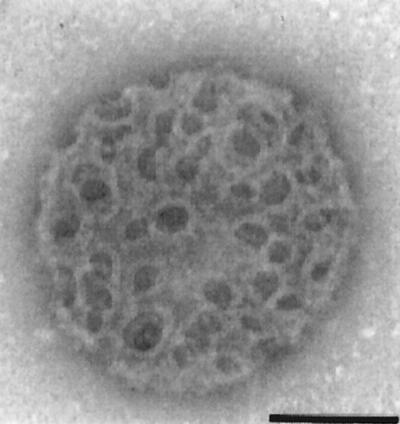
The strong correlation between hemolytic activity and pathogenicity in the genus Listeria (the pathogenic species L. monocytogenes and L. ivanovii are hemolytic [see Fig. 5], whereas the nonpathogenic species are not; the exceptional case of L. seeligeri will be discussed below) (250, 605, 626) and the observation that the spontaneous loss of hemolysin production results in avirulence (288) led Gaillard et al. (195) and various other groups to generate isogenic hemolysin mutants of L. monocytogenes by transposon mutagenesis (108, 323, 518). These mutants were much less virulent in mice (increase of >4 log units in the LD50). Spontaneous revertants from which the transposon was lost recovered both hemolytic activity and pathogenicity, and reintroduction of the hly gene in trans into the nonhemolytic mutants restored virulence to wild-type levels (108, 195, 323, 518). Genetic analysis of the point of insertion for one of these mutants led, in 1987, to the identification and characterization of the hemolysin gene (432, 436). Cell culture studies of the effects of hly inactivation showed that hemolysin is required for the survival and proliferation of L. monocytogenes within macrophages and nonprofessional phagocytes (194, 359, 518). Electron microscopy of L. monocytogenes-infected cultured epithelial cells revealed that hly mutants remained within the vacuolar compartment, whereas hemolytic bacteria were free in the cytoplasm (194). The heterologous expression of hly in Bacillus subtilis conferred on this nonpathogenic bacterium the ability to escape from the phagocytic vacuole and to proliferate within cells (although it did not become pathogenic, indicating that virulence factors other than LLO were required for infection) (45). These studies demonstrated the key role played by LLO in the intracellular infectious cycle of pathogenic Listeria spp., as a mediator of phagosome membrane disruption. LLO not only mediates lysis of the primary phagosomes formed after the uptake of extracellular bacteria but is also required for the efficient escape of L. monocytogenes from the double-membrane vacuole that forms upon cell-to-cell spread (202) (Fig. 3D and H). The pores or membrane lesions caused by LLO (Fig. 4) probably facilitate the access of Listeria phospholipases to their substrates (see following section), leading to total dissolution of the physical barrier that delimits the phagosomal compartment.
FIG. 5.
Hemolytic activities of pathogenic Listeria spp. Left panels, spontaneous hemolysis of L. monocytogenes (L. mo.) and L. ivanovii (L. iv.) on sheep blood agar; compare the narrow ring of β-hemolysis produced by L. monocytogenes (here exceptionally evident) with the striking multizonal lytic activity of L. ivanovii, which is due to the production of an additional cytolytic factor (the sphingomyelinase SmcL). Bacteria were cultured at 37°C for 36 h and then left at 4°C for 48 h. Right panels, synergistic (CAMP-like) hemolysis of L. ivanovii and β-toxin (sphingomyelinase)-producing S. aureus (S. au.) with R. equi (vertical streak); L. ivanovii and S. aureus elicit identical shovel-shaped patches of synergistic hemolysis, reflecting functional relatedness of the sphingomyelinases C produced by these two bacteria (see text and reference 226 for details). The CAMP-like effect results from the total lysis of the halo of sphingomyelinase-damaged erythrocytes exposed to a cholesterol oxidase from R. equi (467). As shown in the two upper right panels, the L. ivanovii sphingomyelinase is active towards sheep erythrocytes (which contain 51% sphingomyelin in the membrane) but not towards horse erythrocytes (13.5% sphingomyelin only). Note that the inner zone of spontaneous hemolysis that surrounds L. ivanovii colonies, due to the activity of the hemolysin (ivanolysin O), is not influenced by the source of erythrocytes. Bacteria were cultured at 37°C for 24 h.
FIG. 4.
Pore-forming activity of LLO. Sheep erythrocyte ghost after treatment with purified LLO, showing the ring-shaped oligomeric structures of the toxin attached to the membrane. (Bar, 100 nm.) (Reproduced from reference 300 with permission of the publisher).
Sequencing of the genes for LLO (436), ILO, and the L. seeligeri hemolysin (seeligerilysin [LSO]) (257) revealed a high degree of similarity at both the protein (86 to 91%) and nucleotide (76 to 78%) levels and confirmed that the deduced proteins are members of the CDTX family of cytolysins. This family of toxins has 23 recognized members from different gram-positive genera, all of which are very similar in structure (40 to 70% amino acid sequence similarity) and show antigenic cross-reactivity (7, 8, 633). Portnoy et al. (519) demonstrated that, despite their structural and functional similarity, not all LLO-related toxins can mediate escape from phagosomes. These authors expressed the genes encoding SLO and perfringolysin O (PFO, the CDTX from Clostridium perfringens) in B. subtilis and observed that only PFO facilitated intracellular growth. However, unlike LLO, PFO damaged host cells (519). The pfo gene was also unable to complement an hly mutation in L. monocytogenes for virulence in mice. The resulting strain was hemolytic and able to escape from phagosomes, but PFO again damaged the host cell, preventing the intracellular proliferation of the bacteria (310). This indicates that LLO has evolved structural features to prevent cytoxicity which are not present in PFO from an extracellular pathogen. By selecting for pfo-expressing L. monocytogenes mutants capable of normal intracellular growth, the same research group identified PFO mutations capable of mediating lysis of the vacuole but devoid of toxic effects on the infected host cell (311). One type of mutation decreased the cytosolic half-life of PFO (see below). Another type of mutation, very interestingly, modified the pH optimum of PFO, making it similar to that of LLO, with low levels of activity at pH 7.4 (a pH similar to that of the eukaryotic cell cytoplasm). This low optimal pH, similar to that within phagosomes, is thought to provide the basis for compartmentalized activity of LLO in an acidified vacuole and for protection against the potential deleterious effect on cell membranes of leakage of the toxin into the cytoplasm. This is probably a key element in the adaptation of L. monocytogenes to intracellular parasitism. Direct evidence for a link between the low optimum pH and compartment-specific pore-forming activity of LLO in macrophage phagosomes has been provided by a recent study using the membrane-nonpermeating fluorophore 8-hydroxypyrene-1,3,6-trisulfonic acid (25). It was shown that L. monocytogenes-containing phagosomes acidify rapidly after bacterial uptake. This was followed by an increase in pH and dye release from the vacuole. Loss of the fluorophore was prevented by lysosomotropic agents, such as ammonium chloride and bafilomycin A1 (25). Bafilomycin A1 was also shown to block L. monocytogenes escape from the primary vacuole in epithelial cells (102). These experiments indicate that a lowering in pH results in activation of LLO present in the phagosome lumen, leading to membrane destabilization, pH equilibration, and consequent deactivation of LLO.
Recent experimental evidence has shown that a PEST-like sequence present at the N terminus of LLO and absent in PFO is responsible for the rapid degradation of the listerial toxin in the host cell cytosol (127a). PEST-like sequences are thought to target eukaryotic proteins for phosphorylation and degradation, and deletion of this sequence in L. monocytogenes LLO led to increased cytotoxicity and lower virulence. When the sequence was introduced in PFO and the chimeric toxin was expressed in L. monocytogenes, bacteria were less cytotoxic than those expressing wild-type PFO and were able to replicate intracellularly. These data suggest that adoption of a PEST motif in LLO is another strategy used by L. monocytogenes, together with the low pH optimum, to restrict the activity of the toxin to the host cell vacuole, thereby preserving the intracellular niche for bacterial proliferation (127a). A recent report has also shown that L. monocytogenes mutants expressing a PEST-deleted hly allele, although fully hemolytic, are strongly impaired in the ability to escape from the phagocytic vacuole. This suggests that the PEST motif in LLO may also play a specific role in the disruption of the phagosomal membrane, e.g., by serving as a ligand for targeting to that membrane other factors required for its efficient disruption (381a).
Structure-function.
CDTXs typically have a conserved undecapeptide, ECTGLAWEWWR, in the C-terminal region (7, 8). Single-amino-acid substitutions in this undecapeptide in LLO produce L. monocytogenes strains with various degrees of attenuation, proportional to the loss of cytolytic activity of the respective hemolysin variants (440). Mutational analyses of the undecapeptide in several other CDTXs have also demonstrated the importance of this motif for activity (511, 583, 608). LSO, the L. seeligeri hemolysin, has an Ala-to-Phe substitution in this conserved motif (257), and this may be one of the reasons for the lower hemolytic activity of this species. The undecapeptide contains the Cys residue that confers on CDTXs the characteristic after which this group of toxins was formerly designated (i.e., “thiol activated or oxygen labile”), that is, inhibition by oxidation or thiol-reacting compounds and reactivation by thiol-reducing agents (8, 633). It was formerly assumed that activation by thiol-reducing agents was due to the breakage of an intramolecular bond. However, once the sequences of many of these toxins became available, it was clear that only one Cys residue was present in most of the toxins of the family. It was also thought that this residue was essential for activity, presumably serving as the ligand in the interaction with membrane cholesterol (see below) (8, 633). However, replacement of this residue with Ala in LLO (440) and in other toxins of the family (511, 583) did not significantly affect activity, although it did render the molecule insensitive to inactivation by oxidation or thiol-alkylating agents. Replacement with Gly or bulkier amino acids, however, abolished most of the activity, indicating that the mechanism underlying Cys-mediated reversible inhibition of cytolytic function probably involves steric hindrance due to formation of toxin dimers or heterodimers with other proteins via disulfide bridges. Indeed, ILO copurifies with a major 27-kDa protein from the culture supernatant of L. ivanovii (349, 669) (the small, secreted internalin i-InlE [169]), and this heterodimer is resolved by treatment with thiol-reducing agents (669). Its nonessential role for cytolytic activity in vitro raises the question of why there is such strong selection pressure for conservation of the Cys residue among CDTXs. This residue may have a major role in vivo, for example, as a modulator of toxin activity (activation in low pH, reducing environments) or as a facilitator of host membrane targeting for other virulence-associated proteins (for example, i-InlE) that may associate with the CDTX in a heterodimer.
Cholesterol irreversibly inhibits the cytolytic activity of CDTXs, and these toxins are active only against cholesterol-containing membranes, reflecting that this membrane compound is a key toxin target (8, 633). The observation that soluble CDTXs form highly stable amphiphilic complexes with cholesterol led to the development of a simple one-step method to purify these toxins from the culture supernatant by selective precipitation with cholesterol (669, 670). With this technique, it was shown that a cytolytically inactive, truncated form of LLO lacking the C-terminal region including the undecapeptide was nonetheless able to form cholesterol-toxin complexes. The conclusion was that LLO possesses functional domains for cholesterol binding located outside the C-terminal region and independent from those involved in cytolysis. It also appeared clear that the undecapeptide containing the Cys residue was not essential for interaction of the toxin with cholesterol (670). Unfortunately, the ability of these truncated LLO molecules to bind to membranes was not tested. Such an experiment would have indicated whether cholesterol acts as the primary binding receptor for LLO. If this were so, then prior treatment of the toxin with cholesterol should block the cholesterol-binding sites on the molecule and prevent the interaction of the toxin with the target membrane. However, recent experiments have demonstrated that preincubation with cholesterol does not impede the binding of LLO-cholesterol complexes to target membranes (300). This is consistent with experimental data showing that PFO mutations in critical residues of the undecapeptide resulting in impaired binding to membranes (see below) did not affect binding specificity for cholesterol (608). The mechanism by which CDTXs disrupt membranes involves the transition from monomeric, water-soluble toxin to noncovalently bound oligomeric, insoluble arc- and ring-shaped toxin structures that insert into the membrane, forming large pores about 20 to 30 nm in diameter (8, 41, 42, 452, 609, 633) (Fig. 4). An interesting observation was that although cholesterol-complexed LLO binds to target membranes, the toxin does not oligomerize to form pores (300). All these findings suggest that cholesterol is involved not in the initial binding of soluble toxin monomers to the membrane but at a subsequent stage of membrane-toxin interaction that leads to polymerization and membrane intercalation. Indeed, early observations of CDTX activity, showing that at 4°C cytolysis does not occur although binding is unaffected (8, 633), did already indicate that membrane binding and disruption are independent events in CDTX function. This is consistent with experimental data showing that most of the anti-LLO monoclonal antibodies that neutralize cytolytic activity do not affect binding to target membranes (123, 464). The ability of noncytolytic LLO-cholesterol complexes to bind cell membranes is also consistent with such complexes being able to induce cytokine expression (474), IL-1 release (707), and the induction of lipid second messengers (618), host cell responses that require prior interaction of the toxin with membrane receptors. This observation raises the interesting possibility that LLO released extracellularly and rendered noncytolytic by the formation of complexes with free cholesterol present in body fluids may be targeted to the cell membranes and mediate signaling events upon binding.
Membrane binding was not affected in LLO mutants with impaired cytolytic activity due to amino acid substitutions in the ECTGLAWEWWR undecapeptide, including Trp-to-Ala substitutions, which almost totally abolish toxin function (440). These data suggested that the motif itself is not primarily involved in the initial interaction of the toxin monomer with the target membrane. This contrasts with other experimental data showing that weakly hemolytic PFO mutants with Phe replacements in the three Trp residues of the Cys-containing region (Trp436, Trp438, or Trp439) had significantly lower erythrocyte membrane-binding activities (608). Circular dichroism spectral analyses of the membrane-associated form of these PFO mutant toxins suggested that both Trp438 and Trp439 are involved in a conformational change that occurs during oligomerization and pore formation (462). Thus, the impaired interaction of PFO mutant toxins with the membrane may reflect either that the motif is directly involved in the interaction with the membrane or that it is required for correct toxin folding during oligomerization and membrane insertion during pore formation. The latter interpretation is consistent with the observed inhibition of pneumolysin (PLY; the CDTX from Streptococcus pneumoniae) self-interaction by derivatization of the single Cys residue with the thiol-active agent dithio(bis)nitrobenzoic acid (213), suggesting that the domain carrying the undecapeptide is involved in toxin autoaggregation. The precise role of the conserved undecapeptide in CDTX function is still not understood and further research on this aspect is clearly needed.
A picture of the possible mechanism underlying membrane disruption and cytolysis by CDTXs has begun to emerge in the last few years from crystallographic studies, functional modeling, and detailed structural analyses performed with SLO, PLY, and, in particular, PFO (214, 461, 493, 494, 566, 611). Given the high degree of sequence similarity between members of this toxin family it can be assumed with a reasonable degree of certainty that most of the predicted functional features are extrapolable to LLO. CDTXs are composed of four domains of which the first three would be involved in toxin oligomerization and membrane disruption and the fourth primarily in binding to the membrane. This is consistent with experimental data showing that the membrane-binding activity is associated with the C-terminal half of the toxin molecule (491) and that neutralizing antibodies that map to domain 4 inhibit binding to cellular membranes (130), whereas neutralizing epitopes that do not inhibit binding to membranes map to domain 1 (123). Membrane contact would facilitate the interaction of toxin molecules with cholesterol, which is buried in the membrane. Binding to cholesterol would then trigger a conformational change in the protein, increasing its hydrophobicity and membrane affinity, favoring a more intimate interaction between toxin monomers and the lipid layer. The resulting hydrophobic monomers would become partially embedded in the membrane, diffuse laterally, and collide with other monomers to form polymeric aggregates. This would explain why initial binding of CDTXs to the membrane is reversible and temperature independent whereas the subsequent steps are irreversible and are blocked at 4°C. At this low temperature, membrane dynamics are presumably restricted, resulting in kinetic energy requirements incompatible with membrane penetration, lateral diffusion, and oligomerization. A region that interacts with the membrane has recently been identified in domain 3. This region contains two transmembrane domains that undergo an α-helix to β-strand transition, creating a pair of hairpin structures that penetrate the membrane. There is some debate over the extent of membrane penetration; some studies claim that the pore is not a hole in the bilayer but instead an unusual phospholipid phase structure (214), whereas others favor the view that the the two amphipathic β-hairpins completely span the bilayer, creating a true pore (611).
Other roles in infection.
Evidence is accumulating that LLO is a multifunctional virulence factor with many important roles in the host-parasite interaction other than phagosomal membrane disruption. Exogenous and endogenous exposure to LLO may induce a number of host cell responses, such as cell proliferation and focus formation in transfected fibroblasts (131), activation of the Raf–Mek–mitogen-activated protein (MAP) kinase pathway in epithelial cells (647, 647a, 685), mucus exocytosis induction in intestinal cells (97), modulation of internalization via calcium signaling (680) and cytokine expression in macrophages (355, 474), degranulation and leukotriene formation in neutrophils (619), apotosis in dendritic cells (252), phosphoinositide metabolism (618), lipid mediator generation (618), NF-κB activation (330), and expression of cell adhesion molecules in infected endothelial cells (153, 330, 351). More details of these aspects of LLO can be found below.
LLO also plays a critical role in the protective immune response to L. monocytogenes infection in two ways. First, the LLO-mediated release of bacteria into the cytosol and subsequent intracellular growth are essential for major histocompatibility complex (MHC) class I-restricted listerial antigen presentation and the induction of specific protective cytotoxic CD8+ T cells (23, 33, 71, 284, 285). Second, LLO is itself a major protective antigen recognized by Listeria-specific CD8+ CTLs during listerial infection (32, 59, 281, 574, 625). LLO has been shown to be processed very efficiently into peptides that are presented by MHC class I molecules (677). One LLO peptide, nonamer LLO 91–99, is an immunodominant epitope that induces CD8+ CTLs, which protect in vivo against L. monocytogenes infection and confer significant anti-Listeria immunity on naive mice upon passive transfer (265, 495). Pore formation by exogenous LLO has also been shown to mediate the delivery of soluble antigens to the TAP-dependent cytosolic MHC class I antigen presentation pathway (121, 122). This may be an additional mechanism involved in the generation of CD8+ CTLs against antigens secreted by extracellular L. monocytogenes bacteria. LLO also elicits a potent humoral response, and several studies have shown that the detection of LLO-specific antibodies can be used in the serodiagnosis of L. monocytogenes infection in humans and animals (34, 211, 212, 248, 382, 394, 396). An old paradigm of cell-mediated immunity, that antibodies are not involved in protection against intracellular parasites (328), has recently been challenged by Edelson et al. (163), who showed that a murine anti-LLO neutralizing monoclonal antibody increases resistance to Listeria infection in mice. It is therefore possible that the humoral arm of the immune system is also involved in protection against L. monocytogenes infection and that LLO plays a key role here also.
Phospholipases
Pathogenic Listeria spp. produce three different enzymes with phospholipase C (PLC) activity that are involved in virulence. Two, PlcA and PlcB, are present in L. monocytogenes and L. ivanovii; the third, SmcL, is specific to L. ivanovii.
Characterization.
Production of phospholipase activity by L. monocytogenes was first described in 1962 by Fuzi and Pillis (191), who reported opacity reactions in egg yolk agar that correlated in intensity with the hemolytic activity of the strains tested (see Fig. 12). This activity was detected in hemolytic fractions of L. monocytogenes culture supernatants and was at first wrongly identified as being due to the hemolysin itself (303, 304, 332). However, the application of careful fractionation procedures led to separation of the hemolytic and phospholipase activities (305, 620). The L. monocytogenes phospholipase was characterized as a secreted phosphatidylcholine (PC) cholinephosphohydrolase (PC-PLC;EC 3.1.4.3) similar to that responsible for the lecithinase activities of Bacillus cereus and C. perfringens (375). The purified PC-PLC/lecithinase is a zinc-dependent enzyme with an apparent molecular mass of 29 kDa, active over a wide pH range (5.5 to 8.0), and with a broad substrate spectrum (206, 223). On lipid micellar dispersions, this PLC rapidly hydrolyzes PC, phosphatidylethanolamine (PE), and phosphatidylserine (PS). It also, to a lesser extent, hydrolyzes sphingomyelin (SM) but does not or only very weakly hydrolyzes phosphatidylinositol (PI) (206, 223). However, PI is rapidly hydrolyzed in erythrocyte ghosts and in PI-PC-cholesterol liposomes, leading to the formation of inositol-l-phosphate (223). The purified enzyme is atoxic for mice; it is weakly hemolytic in guinea pig, horse, and human erythrocytes but not in sheep erythrocytes (206).
FIG. 12.
Hemolysin (Hly) and lecithinase (PlcB) phenotypes of L. monocytogenes. (A and B) Sheep blood agar; (C) egg yolk agar. (A) Original plate from which prfA* mutants of L. monocytogenes were first isolated and identified; the culture shows a mixture of colonies of the wild-type isolate P14 (weakly hemolytic) and its spontaneous prfA* derivative, P14-A (strongly hemolytic) (see text and references 544 and 545 for details). (B and C) Hly and PlcB phenotypes of L. monocytogenes (streaks 1, 2, and 3) compared with those of L. ivanovii (streak 4). 1, weak to undetectable hemolytic and lecithinase activities typical of the L. monocytogenes wild type (clinical strain P37); 2, strong hemolytic and lecithinase activities of prfA* mutant (strains P14-A and NCTC 7973); 3, intermediate variant phenotype found in strain L028, of unknown molecular basis (L028 has a wild-type prfA); 4, strong hemolytic and lecithinase activities by L. ivanovii (clinical isolate P26); all the strains of this species characteristically overexpress PrfA-dependent virulence genes to levels similar to those of prfA* mutants of L. monocytogenes.
Lecithinase activity, like hemolytic activity, is an easily recognizable phenotypic marker closely associated with pathogenicity in the genus Listeria (528) (see Fig. 12). As for hly, transposon mutagenesis was used to identify the lecithinase gene. Two types of lecithinase-deficient, attenuated-virulence mutants were isolated. In the first type, the transposon was inserted into mpl (532), which encodes a zinc-metalloprotease (140), and the first gene of a 5.7-kb operon located immediately downstream from hly (434) (see Fig. 8). The second type of lecithinase-deficient mutant resulted from transposon insertion into the actA gene, located downstream from mpl in the operon and encoding the actin nucleator ActA (672) (see following section). Cloning of the DNA region targeted by the transposons in this mutant led to identification of the structural gene encoding the nonspecific listerial PLC, plcB. This gene occupies the third position in the 5.7-kb operon, called the lecithinase operon (see Fig. 8). The sequence of the plcB product, PlcB, is very similar to those of the PC-PLC precursor of B. cereus (38.7% identity over 253 amino acids) and the α-toxin precursor from C. perfringens (22.4% identity over 223 amino acids) (672). Western immunoblotting of PlcB in the L. monocytogenes culture supernatant detects two antigenically related bands of 33 and 29 to 30 kDa (206, 470, 532). This indicates that PlcB, like the B. cereus and C. perfringens PC-PLCs (309, 657), is secreted as an inactive proenzyme that is processed in the extracellular medium by proteolytic cleavage. The secreted PlcB proenzyme contains 264 amino acids, including a 26-residue N-terminal propeptide of 2.9 kDa, which is cleaved to give the faster-migrating mature, active enzyme. PlcB must be secreted in an inactive form to prevent bacterial membrane damage due to degradation of its phospholipids. The lecithinase-deficient mpl insertion mutant described above produced only the larger PlcB band corresponding to the inactive proenzyme (532), and complementation of this mutant with mpl restored the lecithinase phenotype and production of the active 29- to 30-kDa PlcB form (520). Thus, Mpl, which is encoded by the first gene of the lecithinase operon and, like PlcB, is also a zinc-dependent metalloenzyme, is involved in processing of the PC-PLC/lecithinase into its mature and active form in the L. monocytogenes culture supernatant.
FIG. 8.
Physical and transcriptional organization of the central virulence gene cluster (LIPI-1) of L. monocytogenes and structure of the locus in other Listeria spp. Genes belonging to LIPI-1 are in grey, and the flanking loci are in black. Dotted lines above L. monocytogenes LIPI-1 genes indicate known transcripts. LIPI-1 is inserted in a chromosomal region delimited by the prs and ldh genes, encoding the housekeeping enzymes phosphoribosyl-pyrophosphate synthase and lactate dehydrogenase, respectively. In the plcB-ldh intergenic region, two ORFs, orfA and orfB, are found in all Listeria spp., indicating that the insertion point of LIPI-1 is between the prs and orfB loci. In the plcB-orfB intergenic region of L. monocytogenes, there are two small ORFs, orfX and orfZ (stippled), which delimit the putative excision point of LIPI-1 in L. innocua. In the plcB-orfB intergenic region of L. ivanovii, two small ORFs, orfX (encoding a homolog of the orfX product from L. monocytogenes) and orfL (stippled), are also present, which, like those in L. monocytogenes, delimit the deletion point of LIPI-1 in the nonpathogenic species L. welshimeri. orfZ and orfL encode polypeptides with significant similarity to bacteriophage proteins (respectively Orf6 of the A511 Listeria phage and a serine/threonine protein phosphatase from λ [88a, 670a]), suggesting that LIPI-1 was originally acquired by phage transduction (see text for details). The additional ORFs found in the L. seeligeri virulence gene cluster are hatched (the orfK product shows 39% similarity with the phosphatidylinositol phospholipase PlcA and dplcB is a truncated duplicate of the plcB gene). The figure has been constructed using data from references 88a, 232, 350, and 672 and unpublished results from J.K. (L. seeligeri) and B.G.-Z. and J.-A.V.-B. (L. ivanovii). (Adapted from reference 670a with permission of the publisher.)
The plcA gene, which encodes a PI-specific PLC, was identified in L. monocytogenes earlier than plcB by chromosome walking in the region upstream from hly (82, 377, 431) (see Fig. 8). Its secreted product, PlcA, is a 33-kDa polypeptide similar to the PI-PLCs from Bacillus thuringiensis, B. cereus, and Staphylococcus aureus (around 30% primary sequence identity) and eukaryotic PI-PLCs, such as that produced by Trypanosoma brucei (431). The purified PlcA enzyme is highly specific for PI, with a pH optimum between 5.5 and 6.5 in Triton X-100-mixed micelles, suggesting that, like LLO, it would be active in acidified phagocytic vesicles. No hydrolyzing activity was observed with PC, PS, PE, PI 4-phosphate, or PI 4,5-bisphosphate (222). The enzyme is able to hydrolyze glycosyl PI (GPI)-anchored eukaryotic membrane proteins (222, 431), although much less efficiently than the orthologous enzyme from B. thuringiensis (200).
The animal pathogen L. ivanovii produces an additional PLC that is specific for SM. This SMase was purified from L. ivanovii culture supernatant after selective removal of ILO by sequestration with cholesterol and shown to contribute to the hemolytic activity of this species (669). In contrast to L. monocytogenes, which is weakly hemolytic, L. ivanovii produces a strong bizonal lytic effect on sheep blood agar, with an inner halo of complete hemolysis surrounded by a ring of incomplete hemolysis similar to that for β-toxin-producing S. aureus and B. cereus (546, 607, 669) (Fig. 5). The outer ring of incomplete hemolysis is completely lysed if erythrocytes are exposed to a soluble product released by the actinomycete Rhodococcus equi (546, 626, 667, 668) (a cholesterol oxidase [467]), giving rise to a characteristic shovel-shaped patch of synergistic hemolysis (Fig. 5). This CAMP-like hemolytic reaction is routinely used for the identification of L. ivanovii (605, 606, 668). The gene encoding the L. ivanovii SMase, smcL, has recently been identified and sequenced. Its predicted protein product, SmcL, is highly similar (around 50% identity) to the known bacterial SMases from S. aureus (β-toxin), B. cereus, and Leptospira interrogans (226). Gene disruption and complementation studies have demonstrated that smcL is the determinant responsible for the differential hemolytic properties of L. ivanovii (225, 226). SmcL is functionally very similar to the S. aureus β-toxin in terms of membrane-damaging activity, as revealed by the similar ability of the two molecules to elicit shovel-shaped CAMP-like reactions with R. equi and the lytic selectivity for SM-rich membranes (both enzymes lyse sheep erythrocytes, in which SM accounts for 51% of total membrane phospholipids, but not horse erythrocytes, in which the SM content is only 13.5%) (Fig. 5). However, SmcL does not cause the hot-cold lysis typical of β-toxin (unpublished data), indicating that, despite their structural and functional similarities, there are differences in the mechanisms underlying membrane damage between these two homologous bacterial SMases.
Role in virulence.
The observation that Hly− mutants of L. monocytogenes are able to escape to the cytosol and grow within certain human epithelial cells (Henle 407 and HeLa cells) suggested that listerial membrane-active products other than Hly may be involved in phagosome disruption (518). Such a role for a listerial phospholipase was first shown for PlcB. Sequential insertional mutagenesis of the lecithinase operon of L. monocytogenes revealed that a plcB mutant formed smaller plaques in infected J774 macrophage monolayers and accumulated within double-membrane vacuoles (672). This indicated that the PlcB phospholipase is required for efficient lysis of the secondary phagosomes formed after listerial cell-to-cell spread (Fig. 3H). Using in-frame deletion mutants, Hly was shown to be dispensable for efficient lysis of the primary vacuole of Henle 407 cells as long as PlcB was produced, suggesting that PlcB also mediates escape from primary phagosomes in these cells (414). Immunofluorescence studies with infected cells showed that PlcB accumulates in L. monocytogenes-containing vacuoles, colocalizing with the endosomal-lysosomal marker Lamp1 (415). In the absence of Hly, a Δmpl mutation caused defects in escape from primary phagosomes and cell-to-cell spread similar to those in a ΔplcB mutant or a double Δmpl ΔplcB mutant, consistent with Mpl's being involved in the activation of pro-PlcB (414, 415). Using the Δmpl mutant, it was shown that intracellular activation of proPlcB is mediated not only by Mpl, but also via an Mpl-independent pathway presumably mediated by a lysosomal cysteine protease. PlcB activation by both pathways is sensitive to bafilomycin A1, an inhibitor of the vacuolar proton pump ATPase, indicating that this activation is, as for LLO, low-pH dependent and hence vacuolar compartment specific (415). A recent study has shown that intracellularly multiplying bacteria contain pools of preformed pro-PlcB. These pools are suddenly secreted when pH decreases below 7.0 by an unknown mechanism involving the Mpl-mediated release of active enzyme (416). The amount of the processed form of PlcB, but not that of proPlcB, is significantly lower in cells infected with an ActA− mutant, indicating that proPlcB proteolytic activation occurs predominantly in secondary vacuoles (415). This is consistent with PlcB's playing a key role in cell-to-cell spread. PlcB has recently been shown to be required for intercellular spread from macrophages to different types of mammalian cells, including brain microvascular endothelial cells in vitro (246), and for spread in murine brain tissue in vivo (589). This provides experimental evidence for a major role of PlcB-facilitated cell-to-cell spread in the in vivo physiopathogenesis of Listeria infection.
plcA-deficient strains were initially isolated during a search for molecular determinants involved in cell-to-cell spread based on the screening of a library of transposon-induced mutants for small-plaque formation (82, 641). These plcA insertion mutants were 1,000-fold less virulent in mice, failed to grow in mouse organs, and were defective in intracellular proliferation in macrophages (82). However, this severe virulence deficiency was due to a polar effect on the downstream gene prfA, which encodes the transcriptional activator of Listeria virulence genes with which plcA is cotranscribed (see below). Subsequent studies with an in-frame plcA deletion mutant revealed only a slight reduction in virulence and in the ability to escape from the primary phagosomes of murine bone marrow macrophages (83) (see below). Another study showed that the expression of plcA in the nonpathogenic species L. innocua promotes transient intraphagolysosomal multiplication of this otherwise nonintracellularly replicating bacterium (597). The relevance of this observation to the role of plcA in pathogenesis is, however, unknown. Insight into the contribution of PlcA to virulence could only be obtained by systematic comparison of the behavior of single and double plcA and plcB deletion mutants in mice and cell culture infection assays (629). As expected, the absence of PlcA led only to a minor defect in in vivo virulence (threefold increase in mouse LD50) and in escape from primary vacuoles. Cell-to-cell spread was unaffected. The lack of PlcB, on the other hand, resulted in more significant attenuation (20-fold increase in LD50) and, as noted earlier, defective cell-to-cell spread, but escape from primary phagosomes of bone marrow macrophages was not impaired. However, a double ΔplcA ΔplcB mutant was found to be severely impaired in virulence (500-fold increase in LD50) and in its ability to escape from the primary phagosome and to spread from cell to cell (414). In a Δhly background, PlcB was found to be essential, but PlcA made only a minor contribution to escape from primary vacuoles (414). Overall, these data indicated that PlcA has only a minor individual role in virulence but acts synergistically with PlcB and Mpl (which themselves also have an accessory role in virulence) to achieve, in conjunction with LLO, optimal levels of escape from primary and secondary phagosomes.
Recent experimental evidence has indicated that the L. ivanovii SMase also plays an accessory role in pathogenesis similar to that of PlcA and PlcB. A lack of SMase production in L. ivanovii results in a moderate decrease in virulence (fourfold increase in mouse LD50) and in intracellular growth rate in bovine epithelial cells. Complementation with the smcL gene facilitated intracellular survival of the nonpathogenic species L. innocua in macrophages and conferred on an L. monocytogenes mutant devoid of any known membrane-damaging virulence determinant (hly, plcA, and mpl/plcB) as well as of the actA gene the ability to proliferate within epithelial cells. Electron microscopy of these cells showed that SmcL mediates the disruption of primary vacuoles and the release of bacteria into the cytosol (225, 226).
There are several possible reasons to explain the presence in pathogenic Listeria spp. of such a panoply of phospholipases with redundant functions in phagocytic vacuole destabilization. First, the relative amounts of membrane phospholipids differ between cells (5). Second, in a given cell type, membrane phospholipids are asymmetrically distributed in the lipid bilayer, with some mostly present in the outer (SM) or inner (PE, PS, and PI) lipid leaflets and others equally represented in the two lipid layers (PC) (5). Third, membrane asymmetry is inverted during phagocytosis, and in double-membrane phagosomes, there is presumably a mixed situation, with bacteria bound by membranes with normal and inverted lipid asymmetries. Last, transmembrane asymmetry is also modified in a number of physiological and pathological conditions, such as during apoptosis or during cellular activation associated with increased levels of intracellular Ca2+ (39, 360), so it is likely that variations in phagosome membrane lipid composition occur during the host cell adaptive response to infection. Consequently, (i) the contribution of each phospholipase to phagosome disruption may differ according to cell type and (ii), in the same cell type, the sequential or combined action of several phospholipases with different substrate specificities and mechanisms of action may be important for optimal bacterial escape from phagosomes with variable membrane lipid bilayer topology and composition. In this scenario, the PlcB phospholipase, which is able to degrade most membrane phospholipids, is likely to be a key player in phagosome disruption whatever the composition and distribution of lipids in the membrane, leading to complete dissolution of the physical barrier that separates bacteria from their cytoplasmic niche. The role of PlcA, in contrast, is more difficult to understand if we consider that PlcB has been reported to efficiently hydrolyze PI in biological membranes and liposomes in vitro (see above). PI is moreover a minor lipid in eukaryotic membranes. However, it is clear that PlcA potentiates the membrane-damaging activity of PlcB, which may not depend exclusively on enzymatic activity itself but on particular structural features of the enzyme molecule affecting its membrane insertion and the accessibility to its specific substrate in natural conditions. The role in pathogenesis played by the cleavage of GPI-anchored surface proteins by PlcA is unknown. The production by L. ivanovii of an additional phospholipase specific for SM is particularly intriguing if we take into account that plcA and plcB are sufficient for pathogenesis in L. monocytogenes. It is possible that the hemolysin and the PlcA/PlcB phospholipase tandem of L. ivanovii are less efficient in disrupting the phagocytic vacuole and that SmcL is required to facilitate disruption of the SM-rich luminal leaflet of phagosomes. Another possibility, suggested by the selective membrane-damaging activity of the enzyme for sheep erythrocytes, is that SmcL is involved in the pathogenic tropism of L. ivanovii for ruminants (226). An attractive hypothesis is that SmcL has specifically evolved in L. ivanovii because some of the ruminant cell types or vacuolar compartments important in pathogenesis have, like ruminant erythrocytes, membranes containing large amounts of SM. Consistent with a role for SmcL in host adaptation, expression of smcL was shown to promote intracellular growth of the L. monocytogenes mutant devoid of any membrane-damaging factor in bovine MDBK cells but not in canine MDCK cells, a similar kidney-derived epithelial cell line from a mammalian species that is not naturally susceptible to L. ivanovii (225).
Besides their mechanical function in escape from the phagosome, listerial PLCs may also play an important role in pathogenesis by subverting the host signaling pathways mediated by phospholipid hydrolysis products such as diacylglycerol (DAG), ceramide (CER), and inositol phosphates. These lipid metabolites regulate key cell processes, including cell growth and differentiation, apoptosis, and the synthesis of cytokines and chemokines (346, 596), and hence their generation during Listeria infection may play an important role in adapting host cell and tissue inflammatory responses to the needs for intracellular life and host tissue colonization. Higher levels of lipid turnover with accumulation of DAG and CER have been shown to occur 3 to 4 h after infection of murine J774 macrophages by L. monocytogenes. An involvement of bacterial PLCs in this DAG and CER generation activity was suggested by the fact that cells infected with a ΔplcA ΔplcB mutant showed a smaller increase in the levels of these mediators (629). However, this double mutant still induced the generation of DAG and CER (629), suggesting the involvement of endogenous phospholipases in this host cell response. Pathogenic Listeria spp. also induce phosphoinositide metabolism and DAG accumulation in infected human umbilical vein endothelial cells (HUVECs), and this response was shown, using mutant bacteria and exogenously added purified proteins, to be dependent on the synergistic interaction between LLO and PlcA (617). Since prior treatment with LLO was a prerequisite for the observation of such a response upon external stimulation of the cells, it was suggested that LLO-induced pores provide PlcA with access to PI in the inner membrane leaflet (617). Similar LLO-PlcA cooperation-dependent induction of phosphoinositide hydrolysis and concomitant cell activation has been observed in neutrophils (619). The listerial phospholipases PlcA and PlcB have also been shown to be required for persistent NF-κB activation in P388D1 macrophages (272) and for full induction of CER generation associated with NF-κB activation and enhanced E-selectin expression and neutrophil rolling and adhesion in HUVECs (600). An association between PlcA and PlcB expression, intracellular Ca2+ signaling, and listerial internalization has been reported in J774 macrophages during early time points of infection (680), but the underlying mechanism and true relevance of these observations are unknown. Recently, using L. ivanovii isogenic mutants and heterologous expression in an L. monocytogenes double plcA plcB mutant, it has been shown that the SmcL SMase mediates CER generation in infected host cells. This required the previous release of bacteria into cytosol and was associated with increased apoptosis of infected MDBK cells (González-Zorn et al., unpublished data).
ActA
A great deal of attention has been paid in recent years to the study of the actin-based intracellular motility of pathogenic Listeria spp. (27, 87, 105, 105a, 147, 366a, 401, 402, 515, 581, 630, 636). No classical motor proteins such as myosin have been found to be involved in bacterial intracellular motility, and the actin tail of moving Listeria organisms is functionally similar to the poorly understood actin polymerization process observed in lamellipodia, responsible for the locomotion of eukaryotic cells. Therefore, interest in listerial actin-based motility has transcended microbial pathogenicity, as it provides cell biologists with a relatively simple model for dissecting the molecular mechanisms underlying actin cytoskeleton assembly and function. This model has the enormous advantage that a single bacterial protein is sufficient to promote the actin recruitment and polymerization events responsible for intracellular movement. This protein is ActA (142, 339), the product of the actA gene (672).
The central role of ActA in listerial intracellular motility and virulence was initially revealed by the unusual phenotype of an isogenic actA mutant of L. monocytogenes in infected tissue culture cells (339). After entry, these bacteria were capable of escaping into the host cytoplasm, but could not move intracellularly and accumulated as microcolonies in the perinuclear area of the cell (Fig. 6). The mutant was unable to recruit actin, as shown by staining with fluorescein isothiocyanate-labeled phalloidin, a fungal toxin that binds to F-actin and paralyzes the actin cytoskeleton. In addition, the actA mutant was highly attenuated in the mouse infection model (142, 339).
The actA gene encodes a secreted 639-amino-acid protein with a 610-residue mature form that is attached to the bacterial cell wall via its C-terminal region (Fig. 6). Several lines of experimental evidence have demonstrated the essential role of ActA in actin-based bacterial motility. Transfection experiments in epithelial cells showed that ActA is targeted to the mitochondrial surface and that these organelles recruit actin filaments in a way similar to actA-expressing bacteria (513). Microfilament-associated proteins such as α-actinin and fimbrin were also recruited to the ActA-decorated mitochondria in transfected cells. Thus, ActA, whether anchored to the bacterial or mitochondrial membrane, can induce the formation of an actin cytoskeleton. Although transfection experiments conclusively demonstrated that ActA alone was essential for actin filament recruitment, they did not show how this protein generates intracellular bacterial motility. This was achieved by heterologous expression of the actA gene in L. innocua and S. pneumoniae, bacteria that are otherwise incapable of actin-based motility. This demonstrated that ActA itself mediates actin polymerization-driven bacterial movement (340, 631).
Mature ActA from L. monocytogenes can be arbitrarily divided into three distinct domains: (i) N-terminal domain (amino acids 1 to 234), which is rich in cationic residues; (ii) central region of proline-rich repeats (amino acids 235 to 394); and (iii) C-terminal domain (amino acids 395 to 610), with a highly hydrophobic stretch spanning residues 585 to 606 that anchors the protein to the bacterial surface (Fig. 6). Early structure-function studies with actA deletion constructs expressed in transfected cells (189, 513) or in a Listeria background and tested in Xenopus egg extracts (365, 409) permitted an initial attribution of specific functions to these domains of the ActA protein. The N-terminal region plays an essential role, as it contains all the information necessary to initiate F-actin assembly and bacterial movement (Fig. 6). A domain rich in positively charged residues (amino acids 129 to 153) in this region was identified as being strictly required for actin assembly. Amino acids 117 to 121 were shown to be critical for filament elongation, and deletion of amino acids 21 to 97 led to discontinuous actin tail formation, suggesting a role in maintenance of the dynamics of actin-based motility for this domain. Interestingly, a synthetic peptide corresponding to part of this domain (residues 33 to 74) was shown to interact with F-actin in vitro (365). In the central region, a domain of proline-rich repeats (amino acids 265 to 396) is required for binding of the focal contact proteins vasodilator-stimulated phosphoprotein (VASP) and Mena (see below). Listeria mutants lacking this region of ActA are still able to recruit actin to the bacterial surface and to move intracellularly, albeit at significantly lower speeds. This indicates that while the VASP and Mena proteins are not essential for the initiation of actin assembly on the bacterial surface, they are important for the efficiency of the process (366, 472, 632). There is significant structural and functional similarity between the C-terminal half of ActA (including the proline-rich repeat region) and zyxin (224), a eukaryotic protein associated with focal adhesions and actin stress fibers that may be considered a eukaryotic homolog of the listerial surface protein.
The mechanism by which ActA induces actin polymerization and bacterial motility is now beginning to be understood. ActA is not detected in the actin tail (341, 471), so clearly the listerial surface protein is not necessary for stabilization of the actin meshwork left behind by moving Listeria cells. In the proximal region of this tail, F-actin filaments tend to be unidirectionally polarized, with barbed ends oriented towards the bacterial cell surface, indicating that actin nucleation is occurring at this point (655, 656). This suggested that ActA could act as an actin nucleator. However, globular (G)-actin monomer-binding activity could not be unequivocally demonstrated in ActA until very recently (96, 708), so the question arose whether ActA must be modified in vivo in the mammalian cell to have adequate actin-binding function (e.g., by phosphorylation [70]), or whether another host molecule interacts with ActA and cooperates with it to trigger actin nucleation. Systematic searches for such a host factor among known actin cytoskeleton-binding, cross-linking, and regulatory proteins revealed that many, including α-actinin, tropomyosin, talin, vinculin, fimbrin (plastin), filamin, villin, gelsolin, ezrin/radixin, cofilin, profilin, coronin, Rac, CapZ, Arp3, and VASP, colocalize with Listeria-associated actin filaments (86, 105, 117, 126, 139, 338, 361, 409, 514, 564, 650, 653).
Profilin and VASP were found exclusively at the point of actin assembly, in close association with the bacterial cell (88, 653), suggesting that they are actively involved in initiation of the Listeria-induced actin polymerization process. Profilin is a G-actin-binding protein, and its function in actin assembly is to bring ATP-actin to the barbed end of the filament in a complex that lowers the critical concentration for polymerization. Profilin interacts with polyproline (503), and poly-l-proline beads have been used to deplete profilin from cell extracts, resulting in the abolition of actin tail formation and bacterial motility (653). As profilin colocalizes with ActA at the site of actin polymerization and ActA has four polyproline motifs, it was thought that profilin was involved in actin nucleation by recruiting G-actin to the site of ActA expression on the bacterial surface. However, profilin was shown to associate only with bacteria in cell extracts or within infected cells, and not with ActA-expressing bacteria grown in culture medium, indicating that it requires an additional host factor to interact with ActA (653). VASP, in contrast, was shown to interact directly with ActA in vitro (88). VASP and its homolog Mena, which is also present between the bacterial surface and the growing actin tail (210), normally colocalize with actin filaments in focal contacts and in dynamic membrane processes (515, 538). VASP and Mena belong to the Drosophila Enabled (Ena)/VASP family of proteins, known to be involved in neural development. They are organized into three domains, an EVH1 (Ena-VASP homology 1) domain, a proline-rich central domain, and an EVH2 C-terminal domain. VASP has been shown to act as a ligand for profilin, vinculin, and zyxin (88, 210, 319, 537). Thus, by binding to ActA and profilin, VASP and Mena may establish a direct connection between intracellular Listeria cells and the cytoskeletal components of the host cell (210, 367). The EVH1 domain of the Ena/VASP-like proteins has been shown to bind directly to the proline-rich motif FPPPP, repeated three to four times in ActA, depending on the L. monocytogenes strain. This proline-rich ActA sequence is a novel polyproline motif that mimics the natural interaction between Ena/VASP proteins with their endogenous ligands in the focal adhesion proteins zyxin and vinculin. VASP has also been shown to interact with filamentous (F)-actin via its C-terminal EVH2 domain (85, 367, 472). These data strongly suggest that VASP may act as a bridge between polymerization-competent profilin/actin complexes and ActA and between ActA and the actin tail itself, thereby favoring assembly of the actin network that is used for bacterial propulsion across the cytosol. However, profilin is not essential for listerial actin-based motility (409), and the same is true for Ena/VASP-like proteins because, as indicated above, deletion of their ligand motif on ActA does not prevent (although it does impair) listerial actin-based motility.
A major clue to the mechanism by which ActA induces actin polymerization came from recent experiments showing that the N-terminal 264 amino acids of the protein interact with the Arp2/3 complex, resulting in a dramatic increase in actin nucelation activity (690, 691). First identified in Acanthamoeba, the Arp2/3 complex, which includes two actin-related proteins, Arp2 and Arp3, caps pointed ends, cross-links actin filaments, and has weak actin nucleation activity (403, 456). This complex was shown to initiate actin assembly on the surface of Listeria cells in a reconstituted cell-free system (689). The Arp2/3 complex is activated by proteins of the Wiskott-Aldrich syndrome protein (WASP)/Scar family, which connect the cytoskeleton with the signaling pathways involved in the dynamic remodeling of the actin framework (404, 562, 700, 704). It has been demonstrated that the p21-Arc subunit of the Arp2/3 complex binds to the C-terminal domain of Scar1 and the related WASP. Strikingly, the N-terminal 264 amino acids of ActA, the C-terminal p21-Arc-binding fragment of Scar1, and the C-terminal fragment of WASP are similar in sequence, and all increase the nucleation activity of the purified Arp2/3 complex (404). Indeed, C-terminal fragments of Scar1 that bind Arp2/3 completely block actin tail formation and Listeria motility in cell extracts and in cells overexpressing the corresponding Scar1 constructs, although Listeria organisms are able to initiate actin cloud formation. Motility is restored by adding purified Arp2/3 complex (419). Thus, Arp2/3 is clearly an essential host cell factor for actin-based motility, and the major role of ActA is probably to regulate the activity of this multiprotein complex. Recent studies have demonstrated that the N-terminal region of ActA plays a critical role in binding and stimulating the nucleation activity of the Arp2/3 complex (514a, 626a, 708). Two domains in this ActA region (residues 56 to 76 and 92 to 109), with sequence similarity to WASP homology 2 domains, bind to actin monomers (708) (Fig. 6). A third N-terminal domain (residues 115 to 150, in particular the positively charged motif 117-KKRRK-121), with sequence similarity to the Arp2/3 homology region of WASP family proteins, binds Arp2/3 and competes for this binding with the the WASP family proteins N-WASP and Scar1 (514a, 708) (Fig. 6). It thus appears that ActA uses a mechanism similar to that of the endogenous WASP family proteins to promote Arp2/3-dependent actin nucleation.
However, using pure proteins in solution with assembled F-actin in the presence of ATP (the hydrolysis of which releases free energy for actin assembly), Loisel et al. (389) demonstrated recently that Arp2/3 in combination with VASP or WASP is not sufficient for actin-based motility. Wild-type movement was only reconstituted upon addition of capping protein and actin depolymerizing factor (ADF or cofilin), previously shown to be required for the depolymerization of actin tails (564) and to increase the rate of propulsion of L. monocytogenes in highly diluted, ADF-limited cell extract (86). In accordance with these observations, a cofilin homology sequence similar to that in WASP family proteins has been identified in the N-terminal fragment of ActA and shown to be critical for stimulating actin nucleation with the Arp2/3 complex in vitro and for actin-based motility in cells (626a). These data indicate that adequate turnover and the maintenance of high levels of ATP-G-actin and profilin-actin are essential for elongation of the actin tail and motility (389).
Gelsolin, a protein that caps barbed ends and severs actin filaments and which concentrates behind motile bacteria, was also shown to increase the length of actin tails and to support higher intracellular velocities. However, unlike ADF, gelsolin is not essential for Listeria motility (361). Loisel et al. (389) also confirmed that neither profilin nor VASP is essential for actin-based motility, although their absence causes the rate of bacterial movement to decrease 3- to 10-fold. Similarly, the actin filament cross-linking protein α-actinin, which is uniformly distributed over the actin tail (117), is not required for movement, but in its absence there is an apparent lack of rigidity in the actin tail and a greater tendency for bacteria to drift in solution (389). These findings emphasize that while relatively few proteins are sufficient to build the core of the actin motor of L. monocytogenes, many other host cell proteins, some with as-yet-unknown functions, may be involved in the actin cytoskeletal processes that lead to ActA-mediated listerial intracellular motility. Among these proteins is LaXp180 (506), recently identified using the yeast two-hybrid system aproach and shown to colocalize with intracellular Listeria cells. LaXp180 is a putative binding partner of the phosphoprotein stathmin, involved in the regulation of microtubule dynamics. By immunofluorescence it was demonstrated that stathmin also colocalizes with intracellular ActA-expressing L. monocytogenes (506). The role in infection of these newly identified proteins that are recruited to the listerial cell surface remains to be elucidated.
The motile force for intracellular bacterial movement is thought to be provided by the continuous deposition of actin monomers at the interface between the bacterial cell surface and the growing tail of tangled actin filaments immobilized in the cytosol that serves as a support structure for propulsion. To be effective, this motion system requires that actin deposition occur at only one pole of the bacterial cell, pushing it forward. This is indeed the case, as shown by the fact that the actin tail extends only from one end of the bacterium. This unidirectional actin polymerization appears to be related to the asymmetrical distribution of ActA on the bacterial cell. ActA accumulates at one of the bacterial poles and is totally absent from the other (the newly formed pole after bacterial division), which corresponds to the leading edge of movement opposite the place where actin tail formation occurs (341, 656). The basis of this polar ActA expression is not fully understood, but it clearly depends on the bacterial cell cycle and septum formation. Recent experiments with S. pneumoniae cells and polystyrene beads hemispherically decorated with purified ActA have provided convincing evidence that asymmetric distribution of ActA is indeed sufficient for directional actin assembly and movement (81, 631).
Because actin-based intracellular motility and intercellular spread are crucial for Listeria virulence, it is not surprising that the second pathogenic species of the genus, L. ivanovii, also possesses these features (320). The actA gene from L. ivanovii, i-actA, encodes a protein larger than ActA from L. monocytogenes. The similarity between the two proteins is also limited (34% identity) (230, 348). However, ActA and i-ActA have a similar overall structure (charged N-terminal region, central region of polyproline motifs, and C-terminal membrane anchor region), and the functional domains required for actin recruitment and VASP binding are also conserved (209). The two proteins do indeed have the same function, and i-actA restores actin tail formation in an actA mutant of L. monocytogenes (230). Remarkably, the ActA proteins are the only polypeptides encoded by the central virulence gene cluster that have the same function but a high degree of sequence divergence between L. monocytogenes and L. ivanovii (see below). Differences in the interactions of the two species with the eukaryote host may have resulted in different selection pressures for conservation of actA genes in L. monocytogenes and L. ivanovii. Alternatively, actin-based motility may only require a set of conserved functional modules correctly exposed in space, the core protein carrying these modules tolerating significant deviations and even the insertion of other species-specific functional motifs. L. seeligeri has also been shown to contain actA-homologous sequences (232). However, this species cannot induce actin polymerization in mammalian cells even if it is manipulated so that it can escape from the phagosomes of infected cells (321). The avirulence of this strain may have led to the mutational corruption of a protein with a function that is no longer required.
Two other intracellular bacteria, Shigella flexneri and Rickettsia spp., and vaccinia virus have similar mechanisms of intracellular movement and cell-to-cell spread by continuous polar actin assembly (38, 190, 231, 278, 389). Bacterially induced actin polymerization has also been studied in great detail in S. flexneri, and interestingly, the bacterial protein involved in this process, IcsA, has no sequence similarity to ActA. IcsA also has a polar distribution on the bacterial surface and induces polar actin deposition but uses a different mechanism of actin nucleation, involving activation of the Cdc42 effector WASP to form a ternary IcsA-WASP-Arp2/3 complex (105a, 164). Thus, it appears that ActA and IcsA have evolved convergently, with both subverting the signaling pathway that controls actin cytoskeletal dynamics but by different strategies, accounting for their structural differences.
Recently, evidence has been presented that ActA may also be involved in the entry of L. monocytogenes into eukaryotic cells, probably by recognition of an HSPG receptor (12). Indeed, putative heparan sulfate (HS)-binding motifs, typically consisting of clusters of positively charged amino acids with interdispersed hydrophobic residues, have been identified in the N-terminal region of ActA. One such motif is very similar to the HS ligand of the Plasmodium circumsporozoite protein, and treatment of target cells with a synthetic peptide containing this malarial HS-binding motif significantly inhibited listerial attachment. In addition, by using CHO cells deficient in HS synthesis, it was shown that the absence of ActA reduced the binding and uptake of L. monocytogenes only in cells containing this glycosaminoglycan. A family of HSPGs, the syndecans, are bound to cell membranes via a membrane-spanning core protein, colocalize with actin bundles at the eukaryotic cell surface, and are thought to act as bridges between the ECM and the cortical cytoskeleton (530, 710). It therefore seems plausible that the interaction of ActA with an HSPG receptor induces transmembrane signaling events leading to cytoskeletal rearrangements and phagocytosis. Definitive confirmation that ActA plays a major role in L. monocytogenes internalization by host cells has recently been obtained by using an in-frame ΔactA mutant. Loss of ActA production in this mutant resulted in a decrease in invasiveness of up to a factor of 60 to 70 in epithelial cells, which was fully recovered upon complementation with the actA gene (Suárez et al., submitted for publication).
Although it is a major surface-exposed virulence factor, ActA is not presented by either MHC class I or class II molecules. This is only achieved if ActA is manipulated so that it is delivered in soluble form to the cytosolic MHC class I-processing compartment. However, the CD8+ CTLs so elicited against the listerial surface protein are not protective in vivo (120).
Internalins
Internalins are the protein products of a family of virulence-associated genes found in pathogenic Listeria spp. The first members of this family to be characterized, InlA and InlB, encoded by the inlAB operon, were identified in L. monocytogenes by screening a bank of transposon-induced mutants for impaired invasiveness in Caco-2 cell monolayers. InlA was shown to function as an invasin, mediating bacterial internalization by these normally nonphagocytic epithelial cells, and was therefore named internalin (193). A large number of internalin homologs have since been identified in both L. monocytogenes and L. ivanovii (150, 168, 169, 527) (Fig. 7). An element common to all internalins is a leucine-rich repeat (LRR) domain consisting of a tandem repeat arrangement of an amino acid sequence with leucine residues in fixed positions (317). The typical LRR unit of internalins consists of 22 amino acids, with leucine or isoleucine residues at positions 3, 6, 9, 11, 16, 19, and 22 (— —L— —L— —L—L— —N—I— —I/L— —L). This sequence forms a novel, right-handed helix designated parallel β-helix, with a turn after every LRR unit, first identified in the pectate lyase of Erwinia chrysanthemi (277, 705). The LRR domain is thought to be involved in specific protein-protein interactions. LRR domain proteins are numerous among eukaryotes, in which they are involved in a variety of functions, such as cell adhesion, as is the case for decorin, fibromodulin, and the glycoprotein Ibg, or receptor-ligand interactions and signal transduction pathways, as is the case for Trk, CD14, and the adenylate cyclase of Saccharomyces cerevisiae (337). LRR proteins are less frequent among prokaryotes and most are virulence factors, like YopM of Yersinia pestis and Yersinia pseudotuberculosis, IpaH of Shigella flexneri, and the filamentous hemagglutinin of Bordetella pertussis (55, 264, 407). The occurrence in pathogenic Listeria spp. of a multigene family of LRR-containing proteins is unusual among prokaryotes.
FIG. 7.
Structure of the members of the internalin multigene family from L. monocytogenes and L. ivanovii (reproduced with modifications from reference 358 with permission of the publisher with data from Dominguez-Bernal et al. [unpublished data]). S, signal peptide; B, B-repeats; C, Csa domain repeats C-repeat; D, D-repeats. See text for details.
The internalins best characterized in terms of structure and function are InlA and InlB. InlA comprises 800 amino acids, which can be subdivided into two functional regions. The N-terminal half of InlA contains, in addition to a signal peptide (a feature common to all internalins), 15 LRR units (A repeats), whereas the C-terminal half contains three longer repeat sequences (B repeats) and a cell wall anchor comprising the sorting motif LPXTG followed by a hydrophobic membrane-spanning region of approximately 20 amino acids and a few positively charged amino acids (Fig. 7). This distal LPTXG motif, like similar motifs in other surface proteins covalently linked to the peptidoglycan in gram-positive bacteria, has been shown to be responsible for the attachment of InlA to the bacterial cell envelope in a process mediated by the enzyme sortase (105b, 135, 179, 371, 465, 466, 590). InlB comprises 630 amino acids with seven LRR units in the N-terminal part (149). The LPXTG motif and the hydrophobic tail are absent from the C-terminal part of InlB. Instead, InlB has a 232-amino-acid region consisting of tandemly arranged repeats about 80 amino acids long, each starting with the sequence GW. This domain, known as the cell surface anchor (Csa) domain, is responsible for the attachment of InlB to the bacterial surface (60). The Csa domain mediates a novel mechanism of protein-bacterial surface association in which InlB is partially buried in the cell wall, interacts with the cytoplasmic membrane, and uses lipoteichoic acid as a ligand (313). This type of association appears to be relatively weak, as significant amounts of InlB are found in the supernatant of L. monocytogenes cultures and InlB is released from the bacterial surface upon incubation in buffers with high Tris-HCl concentrations (60, 455). Another surface protein of L. monocytogenes, the putative autolysin/adhesin Ami, also associates with the bacterial cell wall via a Csa domain (313).
inlAB mutants are severely impaired in host cell invasion, and both InlA and InlB have been shown to be required for entry of L. monocytogenes into normally susceptible nonphagocytic cells in vitro (143, 149, 193, 196, 197, 242, 246, 385). The invasion processes mediated by InlA and InlB are apparently similar from a mechanistic point of view (of the zipper type [417, 642]), but the two proteins follow different signaling pathways to achieve entry (see below). InlA and InlB are sufficient to trigger internalization by appropriate host cells, as shown by their ability to confer invasiveness on normally noninvasive bacteria, such as L. innocua and Streptococcus faecalis, and to induce the internalization of latex beads coated with the purified proteins (62, 63, 373, 455).
Functional analysis of InlA has shown that the N-terminal region encompassing the LRR domain and the interrepeat region is necessary and sufficient for bacterial entry into permissive cells (373). Monoclonal antibodies directed against the LRR domain block InlA-mediated entry, indicating that this structure is essential in the entry process (435). The host cell receptor for InlA is E-cadherin, a calcium-dependent intercellular adhesion glycoprotein composed of five extracellular domains and a cytoplasmic tail (205, 703). This receptor was identified by affinity chromatography on a column with covalently coupled InlA onto which a Caco-2 cell extract was loaded. Definitive evidence that E-cadherin is a principal receptor for InlA was obtained by transfection experiments using fibroblasts nonpermissive for invasion by L. monocytogenes; expression of the gene of the chicken homolog of E-cadherin, L-CAM, made these fibroblasts fully susceptible to invasion by recombinant L. innocua expressing the inlA gene but not to invasion by wild-type L. innocua or by a ΔinlA mutant of L. monocytogenes (435). E-cadherin accumulates at the gap junctions between intestinal epithelial cells but is also present over the entire basolateral face of these cells, consistent with experimental data showing that L. monocytogenes preferentially penetrates polarized intestinal Caco-2 cells via their basolateral surface (196, 650). E-cadherin is also present on the surface of hepatocytes, dendritic cells, brain microvascular endothelial cells, and the epithelial cells lining the choroid plexus and placental chorionic villi, all of which are potential targets during Listeria infection. The cadherin specific to the nervous system, N-cadherin, does not promote InlA-mediated entry (435), which correlates with the observation that direct invasion of neurons by L. monocytogenes is not efficient (151). However, certain sensory neurons seem to be permissive for InlA-mediated internalization of L. monocytogenes because they may express E-cadherin (146). InlA presumably interacts, via its LRR region, with the first extracellular domain of E-cadherin (372). This interaction leads to bacterial adherence, but entry is mediated by the intracytoplasmic domain of E-cadherin, which presumably leads to actin cytoskeleton rearrangement via α- and β-catenins (372a).
Upon contact with susceptible eukaryotic cells, InlB induces membrane ruffling and tyrosine phosphorylation of several proteins, including Gabl. Cbl, and Shc, implicated in membrane localization and activation of the phosphatidylinositol 3-kinase (PI3K) p85/p110. This PI3K, known to be involved in the control of the actin cytoskeleton, is stimulated by InlB, resulting in activation of PLC-γ1 and in increased levels of phosphoinositide second messengers. Inhibition studies have shown that p85/p110 PI3K does not participate in InlA-mediated uptake but is indispensable for InlB-mediated phagocytosis (45a, 296, 297). Met, a receptor tyrosine kinase which physiologically serves as ligand for hepatocyte growth factor (HGF), has been recently identified as a signaling receptor for InlB. Interaction with InlB tyrosine phosphorylates the 145-kDa subunit of Met, a binding partner of PI3K. Met-deficient cell lines are unable to mediate InlB-dependent entry, suggesting a major role for this receptor in L. monocytogenes invasion (615a). Using affinity chromatography, a second receptor for InlB has been identified in gC1q-R, the cellular ligand of the globular part of the C1q complement fraction. Pretreatment of target cells with soluble C1q or anti-gC1q-R antibodies impaired InlB-mediated entry, and transfection of the gC1q-R gene in nonpermissive cells promoted internalization of InlB-coated beads (61). However, gC1q-R lacks a transmembrane domain, suggesting that it may function as a coreceptor rather than a signaling receptor. As for InlA, the LRR region of InlB is sufficient for entry into mammalian cells (62, 297). The X-ray crystal structure of the InlB LRR domain has been recently determined: it consists of an elongated structure resembling a bowed tube with an extensive solvent-exposed surface area. Three regions were identified as potential candidates for mediating protein-protein interaction: two were in the concave face of the tube, consisting, respectively, of clusters of hydrophobic and negatively charged amino acids; the other was at the tip and contained two calcium ions, which may serve as bridging molecules in the interaction with host cell receptors (412a).
Evidence indicates that InlA and InlB have different cell specificities and may therefore play an important role in cell and host tropism. Dramsi et al. (149) analyzed in detail the effect of in-frame inlA and inlB deletions and corresponding gene complementations in an internalin double mutant on L. monocytogenes entry into various cell types. They showed that InlA but not InlB mediates entry into human Caco-2 intestinal cells and that InlB is necessary for invasion of murine TIB73 hepatocytes, whereas InlA is not. However, in the human hepatocyte cell line HepG-2, both InlA and InlB were required for internalization. More recently, the entry of L. monocytogenes into HUVECs and brain microvascular endothelial cells has been shown to be dependent on InlB but not on InlA (157, 246, 247, 498). The available data indicate that the role of InlA in invasion is restricted to E-cadherin-expressing cells, whereas InlB mediates entry into a wider range of cell types from various animal species, including certain epithelial and fibroblast cell lines, such as Vero, HEp-2, HeLa, CHO, L2, and S180 cells, as well as hepatocytes and endothelial cells (150, 246, 435, 455, 498). InlA has also been shown to contribute to at least 50% of serum-independent phagocytosis by macrophages (584), indicating that at least one macrophage receptor recognizes this listerial surface protein.
A major breakthrough in our understanding of InlA function has been the recent discovery by Lecuit et al. (372) that a Pro residue at position 16 in the first extracellular domain of E-cadherin is critical for this interaction with InlA. The presence of a Pro residue at position 16 of E-cadherin correlated with permissivity to invasion of the host cell. Thus, cells expressing mouse and rat E-cadherin, both of which have a Glu residue at this position, do not allow InlA-dependent entry, whereas cells expressing human E-cadherin, L-CAM, and guinea pig E-cadherin, with Pro16, are totally permissive to invasion by InlA-expressing bacteria. A Pro16→Glu substitution in human E-cadherin abolished interaction with InlA, whereas replacement of the Glu16 residue with a Pro in mouse E-cadherin resulted in a gain of function (372). These findings are important in that they are the first to identify a possible molecular basis for host specificity in a bacterial pathogen. They also make necessary reevaluation of the available experimental information on the role of InlA and InlB in virulence, in particular, data obtained from in vivo studies in mice and rats, in which no clear pathophysiological role of the inlAB determinant could be identified despite its proven involvement in cell invasion in vitro. In such in vivo studies, for example, the inlAB locus has been shown to affect only very slightly the persistence of L. monocytogenes in mouse liver and not to be essential for the invasion of hepatocytes in vivo (14, 149, 150, 197, 242, 385). Similarly, no role for the inlAB-encoded internalins was detected in intestinal invasion and translocation in mouse and rat ligated ileal loop models (119, 523) or in the development of murine cerebral listeriosis (589). Despite the different cell specificities of InlA and InlB, a double ΔinlAB mutant was 10 times less invasive than a single inlA mutant in Caco-2 cells, indicating that the two internalins synergize to optimize invasiveness. Such synergistic activity of InlA and InlB is also evident in human hepatocytes, with ΔinlA, ΔinlB, and ΔinlAB mutations reducing the rate of entry by factors of 2 to 5, 10, and 100, respectively (149). However, the heterologous expression of inlB did not confer on L. innocua the ability to invade murine hepatocytes (149). It is therefore possible that the absence of InlA recognition by E-cadherin in mouse tissues impairs the targetting of L. monocytogenes to certain critical cell types in vivo, thereby preventing the appropriate interaction of InlB with host cell surface receptors.
The study of Lecuit et al. (372) raises important questions about the usefulness of the widely used mouse model, at least for those aspects in which InlA and InlB are potentially involved. Indeed, as described above, oral infection with L. monocytogenes leads to invasion of the intestinal epithelium in guinea pigs, which have the permissive type of E-cadherin, but not in mice and rats, which express a Listeria-nonpermissive isoform of the intercellular adhesion molecule.
The observation that InlB alone is not sufficient to mediate entry of L. innocua into murine hepatocytes whereas complementation with the inlB gene restores full invasiveness to a ΔinlAB mutant (149) clearly shows that other L. monocytogenes-specific products are involved in the interaction with host cells. This is also indicated by the significant level of residual invasiveness of inlAB mutants both in vitro (143, 150, 157, 196, 197, 247, 253, 385, 435) and, especially, in vivo (14, 149, 196, 242, 385, 589). It seems very likely that other members of the Inl repertoire play a role similar to that of InlA and InlB in the interaction with the eukaryotic cell surface. In addition to the inlAB operon, six other genes encoding putative surface-associated internalins (inlC2, inlD, inlE, inlF, inlG, and inlH) have been identified in L. monocytogenes. These internalin genes are distributed in three loci, two of which contain three internalin genes each and correspond to a single internalin islet that has presumably evolved differently in two isolates of L. monocytogenes serovar 1/2a (150, 527) (see Fig. 7 and below). All these newly identified internalins have signal peptides and LRR domains with various numbers of repeat units in their N-terminal halves and, like InlA, an LPXTG motif at their C-terminal ends, suggesting that they are covalently bound to the bacterial cell wall. Deletion of the inlGHE gene cluster did not affect entry into a number of mammalian cell lines, suggesting that these internalins, unlike InlA and InlB, are not involved in invasion. However, counts of the ΔinlGHE mutant in the liver and spleen were 3 and 2.5 log10 units below those of the wild-type parental strain, indicating that the missing internalins are somehow important for host tissue colonization in vivo (527). Deletion of the inlGHE-homologous inlC2DE gene cluster and of the additional inlF gene identified in the other strain also had no effect on entry into various cell types; however, here the absence of these internalin loci did not affect the bacterial counts in mouse organs (150). This, together with evidence indicating that internalin gene homologs are also present in the nonpathogenic species L. innocua (193), suggests that members of this multigene family may have biological functions not exclusively related to virulence.
All the above-mentioned internalins are large, cell wall-associated proteins which, until very recently (see below), had been found exclusively in L. monocytogenes. Their genes are preferentially expressed extracellularly and, with the exception of the inlAB operon, are independent of the listerial virulence regulator PrfA (75, 527). A new group of LRR proteins has recently been identified in both L. monocytogenes and L. ivanovii. Structurally, they are very similar to the large internalins but lack the C-terminal (B) repeat region, the LPXTG motif, and the membrane anchor (Fig. 7). The internalin proteins of this class are therefore generally smaller (around 30 kDa versus the 80 and 71 kDa of InlA and InlB, respectively) and are released in soluble form into the culture supernatant. InlC (167), also called IrpA (143), is the prototype of this group of small, secreted internalins (167) and is the only secreted internalin detected to date in L. monocytogenes. Unlike the genes encoding the large, cell wall-associated internalins, all the genes encoding these secreted internalins are PrfA dependent. Indeed, this property was fundamental to the discovery of this subfamily of internalins: InlC was identified in the culture supernatant as a major 30-kDa protein that was overproduced in L. monocytogenes after complementation with the prfA gene in multicopy, which results in the artificial upregulation of PrfA-dependent proteins (143, 167, 384). In L. ivanovii, five PrfA-dependent secreted internalins (i-InlC, i-InlD, i-InlE, i-InlF, and i-InlG) have been reported (168, 169, 350), three of which (i-InlE, i-InlF, and i-InlG) are encoded in a large virulence gene cluster containing additional genes encoding secreted internalins (225; Dominguez-Bernal et al., unpublished; see also below). Internalization and intracellular growth were found to be similar for an L. monocytogenes inlC deletion mutant and the parental wild-type strain when tested in several cell types in vitro. The inlC mutation, however, resulted in significantly lower virulence in mice (decrease in 1 log10 in the bacterial load in organs) after infection by the intravenous or oral route (143, 167). A significant loss of virulence in mice has also been observed for the Δi-inlE and Δi-inlF mutants of L. ivanovii (169). Like most PrfA-dependent genes (see below), inlC is preferentially expressed in the cytoplasm, especially at late stages of infection, when bacteria are in the process of active intercellular spread (75, 167). This suggests that InlC may be involved in the dissemination of infection. However, cell-to-cell spread was found not to be affected in an inlC mutant (143, 167, 246). No intracellular target has yet been identified for InlC, and the role in virulence of the small, secreted internalins remains unknown.
Other Virulence Factors
The listerial proteins reviewed so far are true virulence factors because they primarily accomplish tasks specifically required for parasitization of the vertebrate host and have evolved exclusively in pathogenic Listeria species. In addition to these virulence factors, other listerial products have been identified that also contribute to survival within the host. Although some of them have a major influence on the outcome of the host-parasite interaction, their participation in pathogenesis is more indirect, as they are probably involved in general houskeeping functions also necessary for saprophytic life. This does not exclude the possibility that some of these bacterial products may have evolved specific characteristics and differ in certain aspects from their counterparts in nonpathogenic Listeria spp. as a result of adaption to the lifestyle of a facultative intracellular pathogen.
p60 (iap).
On agar plates, L. monocytogenes gives rise spontaneously to colonies with an altered, stable, rough phenotype. These bacteria form long filamentous structures composed of chains of individual cells (354). Although these rough mutants produce wild-type levels of Hly, their virulence is attenuated (288). This lack of virulence correlates with impaired invasiveness, particularly in fibroblasts (354). This invasion deficit results from a defect in the production of a major 60-kDa extracellular protein, p60, that is found both in the culture supernatant (354) and associated with the cell wall (570) and which, when added exogenously, disaggregates the bacterial chains and restores invasiveness (354). The p60 protein is encoded by the iap (for invasion-associated protein) gene, which in L. monocytogenes encodes a 484-residue polypeptide containing a central repeat region of Thr-Asn units (343). The expression of iap is independent of PrfA (72, 75) and is controlled at the posttranscriptional level (342). p60-homologous proteins are synthesized by all Listeria spp., but in each species there are specific differences in amino acid sequence that can be used for identification purposes in PCR-based and immunological assays (74, 77). Only the L. monocytogenes protein is able to restore invasiveness to p60-defective rough mutants (73). There is some experimental evidence supporting a role of p60 in intestinal invasion and in vivo survival (279, 282). A recent study using recombinant p60 from L. monocytogenes has shown that the protein binds specifically to Caco-2 intestinal cells (499). The p60 protein has a murein hydrolase activity required for normal septum formation and essential for cell viability (702), which makes it difficult to determine the precise role of p60 in virulence because iap mutations are lethal. It has recently been shown that p60 is a major antigen in the protective response against L. monocytogenes (203, 204, 267).
Antioxidant factors.
Macrophages become listericidal after their immunological activation by cytokines such as IFN-γ or by engagement of complement receptor 3 during C3b-facilitated opsonic phagocytosis of L. monocytogenes (84, 115, 146, 181). Although it is unclear how macrophages kill intracellular parasites such as L. monocytogenes, it is thought that the generation during the oxidative burst of reactive oxygen intermediates (ROI) and, via interaction of the superoxide anion with nitric oxide (NO), reactive nitrogen intermediates (RNI) plays a key role. In vitro experimental data indeed suggest that ROI and RNI contribute significantly to the macrophage-mediated killing of L. monocytogenes (28, 37, 57, 294, 483, 484). Some studies suggest a major involvement of ROI (294, 454, 484), whereas others indicate that both ROI and RNI are required and may even act in synergy (3, 490). Iron is required for the catalytic reactions leading to the generation of ROI and RNI, and the correct intracellular homeostasis of this element has been reported to be essential for adequate macrophage listericidal activity (181). In vivo, inducible NO synthase (NOS) has been shown to be expressed during listerial encephalitis by macrophages in microabscesses at the sites of active bacterial multiplication (315). Inhibition of NO synthesis by a specific NOS inhibitor was also found to exacerbate listerial infection in mice (28, 56).
In spite of this evidence, catalase and superoxide dismutase (SOD), enzymes that are produced by bacteria primarily to detoxify the endogenous free oxygen radicals generated during prokaryotic oxidative metabolism but which also play a key role in counteracting the oxygen-dependent microbicidal mechanism of the host during infection (175, 185, 256, 258, 445, 658), appear to have only a minor involvement, if any, in Listeria virulence. Thus, loss of catalase activity in an L. monocytogenes transposon-induced mutant had no measurable effect on proliferation in mouse organs (369). Studies of a cat deletion mutant confirmed that catalase is not involved in L. monocytogenes virulence in the mouse model. This lack of a significant role for catalase in pathogenesis is consistent with the reported isolation of spontaneous catalase-negative L. monocytogenes mutants from listeriosis patients (76, 165, 643). Similarly, loss of SOD activity by deletion of the sod gene (65) led to at most a slight decrease in the proliferation capacity of L. monocytogenes in vitro in mouse bone marrow-derived macrophages (which exhibit a clearly detectable oxidative burst in response to this pathogen) and in the organs of experimentally infected mice. These effects were slightly more pronounced with a cat sod double mutant (Brehm et al., unpublished data), indicating that catalase and SOD may together provide some levels of resistance to bacterial killing.
The insignificant protective role of catalase and SOD in virulence is consistent with the kinetics of L. monocytogenes populations within macrophages, with most bacteria being killed very rapidly (within 3 min) in the vacuolar compartment after uptake (127, 533). It is also consistent with the particular characteristics of L. monocytogenes intracellular parasitism, in which bacteria multiply in the cytoplasm, a comparment where toxic concentrations of ROI and RNI are unlikely to be present, thereby rendering catalase and SOD activities unnecessary for bacterial survival. Indeed, in listerial intracellular parasitism, only a small percentage of bacteria need to survive destruction in the phagosome and reach the cytoplasm to initiate a productive infection (128). For this “minimal” survival, Listeria cells may exploit other more specialized resources to cope with oxidative stress, such as stress response mechanisms (see below). Microbicidal mechanisms other than the production of ROI or RNI, such as lysosomal enzymes (270), defensins (374, 469), and cytosolic antimicrobial peptides (283a), may play a critical role in the host defense against Listeria infection (262, 616), but nothing is known about the relevance to pathogenesis of such oxygen-independent killing mechanisms and the putative listerial factors that may counteract them.
Metal ion uptake.
Iron is a key element for all living cells, including prokaryotic cells, serving as cofactor for a large number of enzymes and essential proteins involved in electron transport processes. In animal host tissues, iron is not freely available because it is sequestered by ferric transferrin in serum and by ferritin and heme compounds within cells. Thus, bacterial pathogens have evolved specialized mechanisms to capture iron for growth in host tissues, and these mechanisms play an important role in virulence (502a). In L. monocytogenes, iron not only stimulates growth in synthetic medium but, if administered to infected mice in salt form, also increases bacterial proliferation rates in the liver and spleen and decreases the LD50 (639, 644). In humans, hypersideremia after massive blood transfusions has been identified as an important risk factor for listeriosis (594). Unlike many other bacteria, Listeria does not seem to secrete extracellular iron scavengers (siderophores).
Three different iron uptake systems have been described in L. monocytogenes. One involves the direct transport of ferric citrate to the bacterial cell by a citrate-inducible system (2). Another system involves an extracellular ferric iron reductase, which uses as a substrate naturally occurring iron-loaded catecholamines and siderophores (2, 21, 111, 112, 133). The third system may involve a bacterial cell surface-located transferrin-binding protein (263), although the existence of such a mechanism has been questioned (43). No experimental evidence is currently available on the contribution of these iron uptake systems to the pathogenesis of L. monocytogenes infection.
In addition to its role as an essential nutrient, iron is also used by pathogenic bacteria as a signal molecule for the regulation of virulence gene expression. This sensory system is based on the marked differences in free iron concentrations between the environment and intestinal lumen (high) and host tissues (low) (387). Expression of hly has been shown to be induced in conditions of iron limitation, indicating that this metal ion plays a role in the regulation of virulence gene expression in L. monocytogenes (see below).
Recently, a P-type ATPase involved in copper transport, CtpA, has been identified in L. monocytogenes. A ctpA knockout mutant was impaired in in vivo survival in tissues of infected mice but had normal intracellular growth in HeLa and J774 cell monolayers (182). Copper is a cofactor in a variety of redox enzymes, such as SOD, that are involved in the disposal by bacteria of toxic ROI generated during the oxidative burst of macrophages (2a). However, the marginal implication of SOD in L. monocytogenes virulence (see above) indicates that the involvement of CtpA in pathogenesis may be more indirect and related to the regulation of metal ion homeostasis.
Stress response mediators.
As indicated above, a small proportion of L. monocytogenes cells survive the stressful, hostile conditions of the phagocytic vacuole and reach the cytoplasm, where they proliferate, leading to the spread of the infection (128). Survival under stress involves an adaptive response mediated by a set of conserved proteins that are upregulated in vitro upon exposure to heat shock, low pH, oxidative agents, toxic chemical compounds, starvation, and, in general, any situation in which bacterial growth is arrested. These proteins may be chaperones that assist in the proper refolding or assembly of stress-damaged proteins or proteases that degrade damaged proteins, ensuring that essential physiological pathways function correctly in stressed cells (229).
Analysis of the stress response of L. monocytogenes, including that to heat and cold shock conditions, has revealed similarities to that of other prokaryotes (24, 261, 507, 508). In facultative intracellular pathogenic bacteria, for example, Salmonella, general stress proteins have been shown to be induced upon infection of macrophages (78, 95). In L. monocytogenes, in contrast, stress proteins appear not to be required for intracellular proliferation. This has been shown by Hanawa et al. (261), who found that none of the 32 listerial proteins selectively induced in J774 macrophages infected for 5 h were the same as any of the stress proteins produced when bacteria were subjected to various environemental stresses. It has also been shown that the alternative sigma factor ςB is not involved in L. monocytogenes virulence, although its expression is activated in stress conditions and is required for osmotolerance (26, 695). This may be explained by the particular characteristics of L. monocytogenes intracellular parasitism, which develops mainly in a nonaggressive environment, the host cell cytoplasm, much less hostile than that of the phagolysosome in which Salmonella bacteria proliferate. However, adaptation to sublethal environmental stresses has been shown to protect L. monocytogenes against the lethal action of toxic compounds (392), indicating that in this bacterium there is a “stress-hardening” adaptive response that may be important for survival in hostile conditions. There is evidence that the adaptive response of L. monocytogenes to acidic conditions, such as those encountered in the stomach and in certain foods, may be relevant to pathogenesis. Thus, development of acid tolerance results in increased internalization by Caco-2 cells and higher survival rates in lipopolysaccharide-activated macrophages (101a). Acid adaptation also results in higher loads of L. monocytogenes bacteria in the intestine and the mesenteric lymph nodes after oral infection in mice (576a). Stressful conditions are also likely to be encountered during the transient residence in the phagolysosome. Consistent with this, the stress response of L. monocytogenes involves the induction of hly, which encodes the principal factor involved in escape from the phagocytic vacuole (634, 635) (see below).
Recently, a group of virulence-associated stress mediators involved in intravacuolar survival and subsequent escape to the cytoplasm have been identified in L. monocytogenes. All these stress proteins have highly conserved counterparts in B. subtilis, in which they have stress tolerance-related functions and are involved in the signaling cascade of sporulation. One of these stress proteins is ClpC, an ATPase belonging to the heat shock protein-100 (HSP-100)/Clp family, a class of highly conserved proteins involved in stress tolerance in many prokaryotic and eukaryotic organisms (585, 638, 684). The locus encoding this protein, clpC, was identified by Rouquette et al. (569) by transposon mutagenesis and selection for iron-dependent growth in synthetic medium. The original intention was to identify genes involved in iron uptake (567). However, the phenotype of the mutants isolated, the mutations of which all mapped to the clpC locus, had nothing to do with iron metabolism but was instead related to a defect in resistance to the nutritional stress caused by iron deprivation (569). clpC is upregulated in heat shock conditions (42°C) and preferentially expressed during stationary phase, a pattern consistent with this gene's being induced by general stress conditions. The clpC mutants were found to be highly susceptible to several stresses, including high temperature and high osmolarity in addition to iron limitation. Their growth in bone marrow macrophages and in vivo survival in the organs of infected mice were also severely impaired, resulting in a substantial loss of virulence (decrease of 3 log10 units in bacterial counts) (568, 569). Electron microscopy showed that clpC mutants remained confined to the phagocytic vacuole, indicating either that ClpC is involved in lysis of the phagosomal membrane (568) or, more probably, that the loss of ClpC reduces bacterial fitness to the extent that L. monocytogenes is no longer capable of surviving within the phagosome or producing sufficient quantities of active membrane-damaging virulence factors.
Expression of clpC was found to be almost abolished in an L. monocytogenes prfA* mutant, in which the PrfA regulon is constitutively overexpressed (see below), and in culture conditions that trigger the activation of PrfA. This suggests that the listerial virulence gene transcriptional activator protein negatively controls ClpC. This repressor effect is apparently released as L. monocytogenes enters the stationary phase, indicating that the stress-induced clpC-activating regulatory pathways prevail over PrfA (548). No canonical PrfA boxes (the binding site for PrfA in PrfA-regulated promoters) were identified in the promoter region controlling the expression of clpC, suggesting that expressional crosstalk between PrfA and ClpC is mediated by an intermediary negative regulator activated by PrfA. Expressional interplay between a true virulence determinant and a stress mediator has been described in other facultative intracellular pathogens, such as Salmonella spp. In these bacteria, the stress response mediator RpoS induces the synthesis of the positive transcriptional regulator SpvR, which controls spv plasmid virulence genes important for intracellular survival and the development of systemic infection (95, 251). In L. monocytogenes the situation appears to be the converse, because activation of the central virulence regulator PrfA triggers the downregulation of a general stress response effector. As discussed above, these differences probably stem from the differences in intracellular behavior of the two bacteria. Thus, Salmonella organisms proliferate within a vacuole, presumably a hostile environment in which stress proteins and virulence factors involved in intraphagolysosomal survival would be simultaneously required. In contrast, Listeria cells rapidly escape from the phagosome and actively multiply in the more permissive environment of the host cell cytoplasm. The repression of clpC by PrfA is entirely consistent with the observed activation of the PrfA system when bacteria enter the cytoplasm (see below) and with the nonrequirement for stress proteins for the proliferation of L. monocytogenes in this cellular compartment (see above). The coupling of PrfA activation with ClpC downregulation in L. monocytogenes is a good example of the clever strategies that facultative intracellular pathogens have evolved to coordinate gene expression and to ensure that genes encoding factors superfluous at a given step of the infectious cycle are appropriately turned off. It has been recently shown that ClpC is required for adhesion and invasion of L. monocytogenes, possibly by modulating the expression of InlA, InlB, and ActA (460a).
ClpE is, like ClpC, a member of the Clp family of HSP-100 stress proteins and is also required for prolonged survival at 42°C and involved in the virulence of L. monocytogenes (460). A double clpC clpE mutant was found to be totally avirulent in the mouse model and was readily eliminated from liver tissue. clpE expression was found to be upregulated in a clpC mutant, suggesting that ClpC and ClpE have redundant roles in stress tolerance and that the absence of one is compensated for by upregulation of the other (460).
ClpP is a 22-kDa protein belonging to a family of stress proteases highly conserved in prokaryotes and eukaryotes (198). Deletion of clpP showed that ClpP is required for growth under stress conditions and for survival in macrophages in vitro and in mouse tissues in vivo. In the clpP mutant, LLO production was low under stress conditions, and virulence and hemolytic activity were totally restored by complementation with clpP. This suggests that the ClpP protease is important for the observed stress-induced upregulation of Hly (see above).
ClpC, ClpE, and ClpP are regulated by CtsR, the first gene of the operon including clpC (459). L. monocytogenes CtsR is a homolog of the B. subtilis CtsR repressor of stress response genes. A ctsR deletion mutant of L. monocytogenes displayed enhanced survival under stress conditions and normal virulence in mice, whereas a wild-type strain engineered to overexpress ctsR had significantly attenuated virulence, presumably due to a restricted stress response (459). This is consistent with CtsR being a negative regulator of Clp proteins. It would be interesting to investigate whether repression of clpC is due to PrfA-mediated activation of ctsR, resulting in silencing of the CtsR-controlled stress response regulon.
Another locus that may be involved in stress tolerance and virulence in L. monocytogenes has recently been identified. This locus, lisRK, encodes a two-component system and was identified in an acid-tolerant transposon-induced mutant of L. monocytogenes. Deletion of the gene encoding the histidine kinase component of this LisRK system, lisK, resulted in greater resistance to acid and high ethanol concentrations in the stationary phase and, apparently, also in lower virulence (109). The mechanism underlying the phenotype of the mutant is unclear.
GENETIC ORGANIZATION AND EVOLUTION OF VIRULENCE DETERMINANTS
Although plasmids have been reported in Listeria spp. (370, 504, 521), all the listerial virulence determinants identified to date are chromosomally encoded. The virulence genes of Listeria spp., as in other pathogenic bacteria, are mostly organized into discrete genetic units known as pathogenicity islands (PAIs). PAIs are presumably acquired by bacteria via horizontal gene transfer, sometimes as part of mobile genetic elements, and consequently play a key role in the evolution of bacterial virulence (49, 249, 259, 260).
Central Virulencee Gene Cluster
Six of the virulence factors responsible for key steps of L. monocytogenes intracellular parasitism (prfA, plcA, hly, mpl, actA, and plcB) are physically linked in a 9-kb chromosomal island formerly known as the hly or PrfA-dependent virulence gene cluster (88a, 350) and now referred to as LIPI-1 (for Listeria pathogenicity island 1) (670a). Figure 8 shows the physical and transcriptional organization of this “intracellular life” gene cassette. The hly monocistron, encoding LLO, occupies the central position in this locus. Downstream from hly and transcribed in the same orientation is the lecithinase operon, comprising the mpl, actA, and plcB genes (434, 672). These genes are transcribed either as a single 5.7-kb mRNA under control of the mpl promoter or as shorter transcripts, one specific for mpl and the other starting at a promoter immediately upstream from actA and covering the actA and plcB genes and three additional small open reading frames (ORFs). The plcA-prfA operon is located upstream from hly and is transcribed in the opposite orientation, either as a bicistronic mRNA of 2.1 kb or in monocistronic transcripts of 1.2 and 0.8 to 0.9 kb, respectively (187, 188, 433).
Phylogenetic analyses based on the sequence of the 16S and 23S rRNA have revealed that the genus Listeria comprises two evolutionary branches, one relatively distant, corresponding to L. grayi, and the other containing the remaining species. The latter are in turn divided into two sublines of descent, one comprising L. monocytogenes and the closely related nonpathogenic species L. innocua, and the other containing L. ivanovii and the nonpathogenic species L. seeligeri and L. welshimeri (98, 579) (Fig. 9). L. ivanovii, which has an intracellular life cycle similar to that of L. monocytogenes (320), carries a copy of LIPI-1 (232, 350, 670a) (Fig. 8). The genetic structures of LIPI-1 from L. monocytogenes and L. ivanovii are identical, but the corresponding DNA sequences exhibit only 73 to 78% similarity (350, 670a), a degree of divergence compatible with the genetic distance that separates the two species. One gene of LIPI-1, actA, is specially dissimilar, reflecting a divergent evolution in these two Listeria spp. This lack of similarity is also reflected at the amino acid level (34% identity) even though the corresponding ActA proteins have similar functions in actin-based motility (230, 348). LIPI-1 is absent from the genomes of the nonpathogenic Listeria spp. with one exception, L. seeligeri (88a, 350, 670a). Although in this species LIPI-1 genes are present in a structurally intact form (232, 257), they are not expressed correctly due to the insertion of a divergently transcribed ORF (orfE) between plcA and prfA, disrupting the positive autoregulatory loop crucial for adequate PrfA-dependent virulence gene activation (321, 350) (Fig. 8) (see below). The L. seeligeri LIPI-1 contains additional ORFs (orfCD) and duplications of the plcA and plcB genes (350, 670a). In L. monocytogenes, L. ivanovii, and L. seeligeri, LIPI-1 is stably inserted at the same chromosomal position (Fig. 8), has a DNA composition similar to that of the core genome, lacks any obvious traces of mobility factors (integrases, transposases, or insertion sequences [IS]), and its insertion point is devoid of typical integration signals (direct repeats or tRNA genes) (88a, 350, 670a). These data are consistent with LIPI-1's having been acquired a long time ago by a common listerial ancestor (350, 670a).
FIG. 9.
Evolution of the central virulence gene cluster (LIPI-1) in the genus Listeria. The presence of LIPI-1 at the same chromosomal position in various species belonging to distinct evolutionary branches of the genus suggests that the virulence gene cluster was acquired before speciation (see text for details). Thick lines indicate the evolutionary pathway followed by LIPI-1; solid arrows indicate conservation of a functional LIPI-1 (in L. monocytogenes and L. ivanovii), whereas the dotted lines with an open arrowhead indicate functional corruption of the virulence gene cluster (in L. seeligeri). L. innocua and L. welshimeri presumably arose by excision of LIPI-1 in the corresponding evolutionary branches. The dendrogram is a schematic reconstruction of the phylogeny of Listeria according to references 98 and 579. (Adapted from reference 670 with permission of the publisher.)
It is clear that LIPI-1 provides the host bacterium with access to a new niche, animal host tissues, which imposes selection pressures totally different from those encountered in the environment. The evolutionary history of the genus Listeria can thus be interpreted such that the acquisition of LIPI-1 by a clone of the primordial listerial ancestor was a crucial event that led to separation of the genus into two different phylogenetic branches, one corresponding to bacteria that remained saprophytic, resulting in the current species L. grayi, and the other to bacteria which, by virtue of the intracellular life cassette, became parasitic (350, 670a) (Fig. 9). It is reasonable to assume that the outcome of LIPI-1 in each of the two lines of descent of the parasitic branch also played a key role in the further diversification of the genus, as follows: (i) stabilization, in L. monocytogenes and L. ivanovii; (ii) early deletion (possibly when LIPI-1 still had its original mobility functions), in L. innocua and L. welshimeri; and (iii) functional inactivation, in L. seeligeri (Fig. 9). The persistence of the LIPI-1 genes in intact form in L. seeligeri is intriguing. Because these genes no longer confer any virulence properties on the host bacterium, one would expect them to be eliminated or corrupted during evolution. One explanation for their conservation is that the prfA-inactivating insertion was a relatively recent event in the evolution of L. seeligeri, the additional ORFs present in LIPI-1 of this species representing subsequent gene insertions that were tolerated by the afunctional PAI. Alternatively, the gene cluster present in L. seeligeri may represent an ancestral form of LIPI-1 which is still evolving and which, in its present form, provides an advantage in a specific natural habitat other than the mammalian host but which also requires phagosome escape functions for survival (e.g., in protists that take up bacteria from soil) (350).
Two virulence determinants present in LIPI-1, hly and plcB, have homologs which are exclusively present in low-G+C gram-positive bacteria closely related to Listeria, such as Bacillus and Clostridium. Homologs of two other LIPI-1 genes, plcA and the thermolysin-related zinc-metalloprotease gene mpl, are also common in this branch of gram-positive bacteria. This clearly suggests that the source of LIPI-1 genes is within the same phylogenetic division to which the genus Listeria belongs. PrfA, a member of the Crp/Fnr superfamily of transcription factors, is widespread in both gram-negative and gram-positive bacteria (221). ActA is the only LIPI-1-encoded protein for which no obvious structural homolog has been identified yet in prokaryotes. ActA carries functional domains present in eukaryotic proteins involved in the assembly of the actin cytoskeleton, such as vinculin, zyxin, and the WASP family of proteins (see above). It remains to be determined whether this reflects convergent evolution or indicates horizontal gene transfer between Listeria and eukaryotic cells. Whether LIPI-1 was acquired in a single recombinational event or whether it specifically evolved in the Listeria ancestor by a stepwise assembly process also remains unclear. Recent sequence analyses of the prs-ldh intergenic region of L. innocua and L. welshimeri have provided evidence that LIPI-1 was indeed once present in the chromosome of these species and that it became entirely excised, possibly in a single event, from the chromosome of these bacteria. Consistent with this, two small ORFs at the right end of LIPI-1 have been shown to exhibit significant similarity to bacteriophage or viral proteins, suggesting that the gene cluster (or at least part of it) was originally mobilizable by phage transduction (88a, 670a) (Fig. 8).
Internalin Islands
Internalins belong to a multigene family exclusive to Listeria. Except for the inlC and inlF genes of L. monocytogenes, the members of this family are always found in clusters of two to several genes, all oriented in the same direction (Fig. 10). The largest of the known internalin islands is LIPI-2, a chromosomal locus specific to L. ivanovii which contains 10 inl genes (see below). All inl genes have very similar sequences and encode highly homologous proteins with variable numbers of LRR repeats and, in the case of the large, surface-associated internalins, B and Csa (or GW) repeats. These repetitive DNA sequences are naturally prone to recombination, suggesting that the diversity of inl genes on the listerial chromosome is the result of gene duplications and intra- and intergenic rearrangements. There is evidence for such recombination events in inl genes. Comparison of the same internalin locus in two different L. monocytogenes strains revealed a different gene organization. The two strains shared only two structurally identical inl genes (inlG and E), and two internalin genes present in one isolate (inlC2 and inlD) (150) presumably recombined to generate a new internalin gene in the other (inlH) (527) (Fig. 10). In L. ivanovii, a tandem fusion of two small, secreted internalin genes in LIPI-2 has led to the generation of a hybrid gene encoding a larger, secreted internalin, i-inlG (Dominguez-Bernal et al., unpublished). (Fig. 7).
FIG. 10.
Internalin islands and islets of L. monocytogenes and L. ivanovii. The scheme shows the different gene arrangements found for the same internalin locus in two isolates of L. monocytogenes, possibly resulting from rearrangements between inl genes (inlH presumably arose by recombination of the 5′-terminal part of inlC2 and the 3′-terminal part of inlD [350, 527]). See text for details.
The origin of the inl genes is intriguing. LRR domains are widespread in eukaryotic proteins but rare in bacterial proteins, suggesting that they are a relatively recent acquisition by prokaryotes. inl genes are also found in the nonpathogenic species L. innocua (193; European Listeria Genome Consortium, unpublished). The presence of members of the internalin multigene family in Listeria spp. representing distinct phylogenetic branches of the genus suggests that inl genes were already present in the common listerial ancestor and, consequently, that evolutionarily they are probably as old as LIPI-1. However, inl loci have a different distribution, gene organization, and location in the different Listeria spp. The inlG(C2D/H)H internalin islet of L. monocytogenes is present between two housekeeping genes (ascB and dapE) that are contiguous in L. ivanovii and other Listeria spp. (350). Also, inlC of L. monocytogenes is inserted alone, whereas its counterpart in L. ivanovii, i-inlC, is adjacent to another inl gene (i-inlD), and both are inserted at a different place of the chromosome (168). Another example is the large internalin island LIPI-2 of L. ivanovii, which is inserted between two housekeeping genes that are otherwise contiguous in L. monocytogenes. The analysis of the insertion points of i-inlCD and LIPI-2 revealed that these two loci are associated with tRNA genes (168, 225). tRNA loci are commonly used as a target for integration of lysogenic phages and other mobile elements (539) and are frequently found at the insertion sites of PAIs in gram-negative bacteria (259, 260). Consequently, inl genes have probably been not only transmitted vertically during evolution but also exchanged by horizontal transfer within the genus Listeria, possibly carried by phages, which in these bacteria are abundant, have interspecies infectivity, and are capable of DNA transduction (287, 338).
The conservation of such a repertoire of inl homologs in the genome of pathogenic Listeria spp. must necessarily reflect that each of the individual internalins plays a role in the biology of these bacteria. It has been suggested that variability in the exposed face of internalin LRR motifs is important to create a variety of specific protein-protein interactions (412b). Consistent with this notion, a direct involvement in receptor-facilitated invasion of permissive host cells has been demonstrated for the LRR domains of InlA and InlB (62, 373) and a role in cell and host tropism has been demonstrated for these internalins (149, 372) (see above). In L. monocytogenes, genes encoding large, surface-anchored internalins are abundant (150, 193, 527), whereas there seem to be very few genes encoding small, secreted internalins (only one, inlC, has been identified to date) (167). In L. ivanovii, the reverse seems to be true, with many genes encoding small, secreted internalins and no genes encoding large, surface-associated internalins (168, 169, 226; Dominguez-Bernal et al., unpublished). It is therefore possible that the different gene complements in these two Listeria spp. and the structural diversity of the internalins they encode facilitate the recognition of a variety of host receptors and are thus at least partly responsible for their different pathogenic characteristics.
In line with this assumption is the recent discovery in L. ivanovii of LIPI-2, a large (22 kb) gene cluster composed of many small, secreted internalin genes and the smcL gene, encoding the L. ivanovii SMase (225). LIPI-2 is specifically present in L. ivanovii within the genus Listeria and may play a significant role in the pathogenic tropism of this species for ruminants. At least for the smcL product, there are indications for such a role in host tropism, as it selectively lyses sphingomyelin-rich membranes (e.g., those from sheep erythrocytes) (226) and promotes intracellular proliferation in bovine MDBK cells but not in canine MDCK cells (225). Most of the LIPI-2 inl genes are PrfA dependent, whereas smcL is absolutely independent of PrfA and is transcribed in the opposite orientation (226). This suggests that LIPI-2 was formed by the insertion of smcL into a preexisting core of inl genes. The smcL-encoded enzyme is homologous to the S. aureus β-toxin and the B. cereus SMase (226), suggesting that the gene was acquired by L. ivanovii via horizontal transfer from either of these two low-G+C gram-positive bacteria. LIPI-2 has a DNA composition significantly different from that of the genome of L. ivanovii and is unstable, leading to a 17-kb deletion that affects the right part of the PAI. These characteristics, together with the expressional subordination of most of its genes to the LIPI-1-encoded PrfA protein, indicate that LIPI-2 is a more recent development that LIPI-1 in the evolutionary history of the genus Listeria. Spontaneous LIPI-2 deletion mutants are significantly impaired in virulence for mice and sheep and exhibit altered organ tropism in the latter animals (Dominguez-Bernal et al., unpublished).
REGULATION OF VIRULENCE GENE EXPRESSION
Evidence for coordinate regulation of virulence genes in L. monocytogenes was first provided by the analysis of spontaneous and transposon-induced mutants that were noninvasive and had a combined deficiency in hemolysin (Hly) and lecithinase (PlcB) production (325). Genetic analysis of these mutants revealed that they were affected in the expression of the prfA gene, located immediately downstream from plcA in LIPI-1 (Fig. 8) (228, 379, 433). Trans-complementation experiments in these mutants and in a heterologous host, B. subtilis, confirmed that the prfA gene product, a 27-kDa protein called PrfA (for positive regulatory factor A), was required for the expression of hly and the other genes of LIPI-1 (89, 188, 380, 433). Accordingly, PrfA− mutants had a markedly higher LD50 in mice (around 3 log units) and were unable to grow in host tissues (89, 433, 613). Spontaneous prfA deletion mutants arise frequently in L. monocytogenes strains kept in laboratory conditions. Indeed, the current type strain of the species, SLCC 53T (also called CIP 82110T and ATCC 15313), one of the original strains isolated by Murray et al. in 1926 (458) (see Introduction), is one such mutant (324, 380, 433).
PrfA, Master Regulator of Virulence
PrfA is the only regulator identified to date in Listeria spp. which is directly involved in the control of virulence gene expression. This protein is the main switch of a regulon including the majority of the known listerial virulence genes. Some of the members of the regulon, including LIPI-1 genes (with prfA itself; see below) and several genes of the subfamily of secreted internalins (e.g., inlC of L. monocytogenes and i-inlE of L. ivanovii), are tighly regulated by PrfA; others, such as the inlAB operon, are only partially regulated by PrfA (148, 167, 350, 385, 433, 544). However, the expression of some listerial virulence genes is totally independent of PrfA, as is the case for the SMase gene of L. ivanovii, smcL (226), and the inlGHE internalin locus of L. monocytogenes (527). There is evidence that PrfA also negatively regulates some L. monocytogenes genes, such as the stress response mediator gene clpC (548) and the motility-associated genes motA (439) and flaA (544). This suggests that PrfA has a more global regulatory role in L. monocytogenes.
The deduced amino acid sequence of the PrfA protein shows similarities (20% overall identity) to that of the cyclic AMP (cAMP) receptor protein of E. coli (Crp, also known as CAP), on the basis of which the listerial regulator has been included in the Crp/Fnr family of bacterial transcription factors (347, 364). Crp/Fnr-like proteins have a role in virulence gene regulation in other mammalian and plant pathogens (221, 347, 694). Although most members of the Crp family of transcription factors are from gram-negative bacteria, Crp/Fnr-related transcriptional regulators have been also described in gram-positive bacteria (298). The PrfA homologs encoded by the prfA genes of L. ivanovii and L. seeligeri have a primary structure 80% identical to that of the L. monocytogenes virulence regulator protein. In L. ivanovii, PrfA also plays a central role in the coordinate regulation of virulence gene expression. In L. seeligeri, virulence genes are not expressed, but complementation with the plcA-prfA bicistron from L. monocytogenes activates transcription of the seeligerilysin O (slo) gene, leading to bacterial escape from the phagosome and intracellular bacterial multiplication (321). This suggests that the avirulence of this Listeria species is at least partly due to the insertion of the divergently transcribed orfE between the plcA and prfA genes (Fig. 8), resulting in a PrfA− phenotype even if prfA is intact and the corresponding PrfA protein is potentially active (see preceding section). Whether the complemented L. seeligeri strain is virulent has not yet been tested.
Two structural features that are important for Crp function are also present at the corresponding positions in PrfA (Fig. 11). One is a helix-turn-helix (HTH) motif in the C-terminal region (amino acids 171 to 191) which, as in Crp, mediates the interaction of the protein with specific DNA sequences in target promoters (612). These target sequences, called PrfA-boxes, are 14-bp palindromes centered on position −41.5 relative to the transcription start site in PrfA-dependent promoters (187, 437, 672) (Fig. 11). DNA footprinting experiments have shown that PrfA binds in vitro to the PrfA-box (136). The second structural feature of Crp that is shared by PrfA is a series of short antiparallel β-strands delimited by Gly residues, which form a β-roll structure involving most of the N-terminal half of the protein (residues 19 to 99) (Fig. 11). In Crp, this structure forms the binding site for the activating cofactor, cAMP (344). However, cAMP is not detected and is not known to function as an intracellular messenger in gram-positive bacteria (291). Accordingly, most of the Crp residues involved in cAMP binding are not conserved in PrfA, and the addition of exogenous cAMP to L. monocytogenes does not lead to PrfA-mediated transcriptional activation (673). The role of this putative β-roll structure in PrfA is currently unknown. Two other functionally important regions of Crp, the D α-helix (amino acids 138 to 155), involved in transmission of the allosteric effect from the cAMP-binding N-terminal domain to the DNA-binding C-terminal domain, and activation region 1 (residues 156 to 164), involved in Crp-RNA polymerase interaction with class II promoters, are also particularly well conserved in PrfA (Fig. 11). PrfA does have some features that are unique among the members of the Crp-related family of transcriptional regulators, an additional HTH motif in the N-terminal region of unknown function, and a 25-amino-acid extension at its C terminus, which may form a leucine zipper (Fig. 11). Mutant forms of PrfA lacking this putative leucine zipper motif were found to be inactive (221).
FIG. 11.
Structure of PrfA (A) and its target DNA sequences in PrfA-regulated promoters (B). See text for details. In PrfA, amino acid coordinates correspond to the peptide sequence deduced from the prfA gene (position 1 corresponds to the Met residue of the first triplet), whereas in Crp they correspond to the actual amino acid position in the primary structure of the native protein, which lacks the residue encoded by the first triplet. Therefore, the amino acid numbering of Crp is shifted one position with respect to that of PrfA, and hence, position 144 of Crp, where the Crp* mutation Ala→Thr lies, aligns exactly with position 145 of PrfA, where the Gly→Ser substitution leading to the PrfA* mutant phenotype is located. In Crp, A to F designate the α-helical stretches of the protein and AR1 is activating region 1 (two other activating regions [AR2 and AR3] are embedded within the β-roll structure). In PrfA, structures specific for this protein are in light gray. (B) N indicates any nucleotide. (Adapted from references 66 and 221.)
Environmental Control of Virulence Gene Expression
Virulence factors are primarily required to colonize animal tissues and to overcome the host defense mechanisms brought into play during infection. Therefore, their synthesis outside the host may represent a burden compromising the ability of L. monocytogenes to survive in the natural environment and limiting its potential for transmission. Evidence is accumulating that L. monocytogenes has evolved signal-sensing and signal-transducing mechanisms to exploit the diverse physicochemical environments it may encounter to adapt virulence gene expression to the particular needs of saprophytic versus parasitic lifestyles. Temperature is one such environmental variables. Thus, PrfA-dependent transcription is weak below 30°C (environmental temperature) but becomes induced at 37°C (body temperature of warm-blooded animals) (124, 378, 544). The mechanism underlying this thermoregulation is unknown.
An increase in temperature is, however, not sufficient for maximal levels of transcriptional activation of prfA and the genes of the PrfA-dependent regulon to be reached in L. monocytogenes. Wild-type strains express PrfA-regulated genes very weakly in rich media (e.g., brain heart infusion [BHI]) at 37°C, but transcription of these genes is strongly activated if the bacteria are cultured in BHI supplemented with activated charcoal (543–545, 548). The presence of this absorbent does not affect bacterial growth, indicating that the effect on virulence gene transcription involves a signal-sensing regulatory mechanism rather than a nutritional stress response due to sequestration of essential nutrients (545). One possible explanation for the “charcoal effect” is that activated charcoal absorbs a substance that is present in BHI but not in infected tissues and that allows L. monocytogenes to detect that it is growing outside an adequate host compartment. One such substance might be iron, because low free iron availability defines the environment of host tissues (387) and there is evidence that hly expression is activated in conditions of iron limitation (101, 113, 207). The specific binding of PrfA to target sequences in the hly and actA promoters is also strongly inhibited under high-iron conditions (51). However, charcoal-treated BHI does not retain its virulence-activating properties after removal of the charcoal by filtration (Novella et al., unpublished data), suggesting that the mechanism responsible is not related to the sequestration of a component of the culture medium.
Although the mechanism of charcoal-mediated activation of virulence genes remains unknown, the charcoal effect clearly demonstrates that signals from the growth medium play a critical role in regulating the expression of the PrfA regulon. It also shows that L. monocytogenes needs to sense a suitable combination of environmental signals of physical and, possibly, chemical nature to upregulate the PrfA regulon. This may represent a fail-safe mechanism by which Listeria bacteria prevent the expression of virulence genes in situations in which they are not required even if the temperature rises above the critical activation threshold. An induction of prfA and PrfA-dependent virulence genes similar to that observed with charcoal takes place when L. monocytogenes bacteria growing in BHI are transferred to minimal essential medium (MEM) (54, 635). MEM does not support normal growth of L. monocytogenes (635), so it is unclear whether the virulence gene-activating effect is due to a sensory mechanism involving chemical signals, a stress response to starvation, or both.
L. monocytogenes has been shown to respond to various stresses, such as exposure to high temperature (42°C) and oxidants or entry into stationary phase, by increasing, via induction of prfA, the expression of a set of genes including hly (634, 635). The upstream mediator involved in the activation of prfA under stress conditions is unknown. Detailed transcriptional analysis of the genes of LIPI-1 at 42°C revealed selective induction of hly and plcA, transcription of the genes of the lecithinase operon (mpl, actA, and plcB) remaining at basal levels (Ripio et al., unpublished). This expression pattern is identical to that observed if bacteria are confined within a phagocytic vacuole (75). It is therefore possible that a transient stress response involving the transcriptional activation of some genes of the PrfA regulon (those requiring lysis of the phagocytic vacuole) takes place when Listeria cells are still confined within the acidified phagosome, during the early stages of cell infection after invasion (see below). A simple decrease in pH to between 5.0 and 6.0 (i.e., the pH of acidified phagosomes) reduces hly expression in vitro (30, 124), indicating that other growth-arresting stress conditions found within phagosomes may be required for the induction of hly and the synergistic phagosome-disrupting virulence determinant plcA. It is also possible that de novo synthesis of hly plays a lesser role in phagosome disruption than acid-induced release of preformed intracellular stocks of the toxin, as postulated for PlcB (416).
Although wild-type L. monocytogenes cells produce low to undetectable levels of virulence factors in BHI, they are as virulent in mice as prfA* strains (545), which overexpress virulence genes constitutively in vitro (see below). As described above, wild-type strains can strongly induce PrfA-dependent virulence genes, up to expression levels similar to those in constitutive prfA* mutants, if grown with charcoal or in MEM. These observations led to the suggestion that the high levels of virulence gene induction reached by wild-type strains in vitro upon exposure to charcoal or MEM may correspond to the levels of expression required in vivo by L. monocytogenes to survive and proliferate in infected host tissues (545). A recent study has provided support to this hypothesis by showing that, despite their differences in spontaneous levels of expression in vitro, there is no significant difference in intracellular virulence gene activation between a wild-type strain and a prfA* mutant (75). Indeed, several PrfA-dependent virulence genes, such as plcA (336), actA (54, 137, 451), and inlC (167), and PrfA synthesis itself (540), have been shown to be highly induced intracellularly. Investigation of L. monocytogenes intracellular gene expression using the green fluorescent protein as a reporter revealed that the actA promoter is activated within 30 min to 1 h of infection. This activation depended upon the ability of L. monocytogenes to reach the host cytosol, as it was not observed in a Δhly mutant unable to escape from the phagocytic vacuole (185). All these findings indicate that the expression of prfA and PrfA-dependent genes is upregulated if L. monocytogenes senses activatory signals specific to the host cytosolic compartment.
Carbon Source Regulation of Virulence Genes
Fermentable carbohydrates cause a strong repression of virulence genes in L. monocytogenes (30, 67, 124, 444, 543). This downregulation occurs only if the carbohydrate is added in amounts sufficient to promote bacterial growth, suggesting that the underlying regulatory mechanism is related to catabolite repression (CR) (444). However, an L. monocytogenes mutant lacking CcpA, a central mediator of CR in gram-positive bacteria, did not show any alteration in carbon source regulation of virulence genes (29). Sugar-mediated repression affects not only the divergently transcribed virulence genes, plcA and hly, which have overlapping promoters, but also plcB, belonging to another transcriptional unit with no immediate physical link to plcA and hly and the promoter of which (PactA) is strictly dependent on PrfA (50, 67, 544) (Fig. 8). This suggests that repression is mediated by a trans-acting pleiotropic factor and somehow involves PrfA. Fermentable sugars such as cellobiose (336, 444, 541) and glucose (unpublished data) reduce PrfA-dependent virulence gene expression without affecting the levels of PrfA protein, indicating that this regulation is mediated by a repressor mechanism that either reduces the activity of the PrfA protein or blocks the binding of PrfA to its target promoters. The repressor mechanism appears to be situated hierarchically over the positive control pathway that activates the PrfA virulence regulon (see below), because fermentable carbohydrates annulate the induction of virulence genes by charcoal treatment (67, 543). Control of the synthesis of virulence-associated products by carbon source has also been reported for other pathogenic bacteria, such as E. coli (STb heat stable enterotoxin) (80), Actinobacillus actinomycetemcomitans (leukotoxin) (449), and Pseudomonas aeruginosa (PlcH hemolytic phospholipase) (575). Such a CR-like regulatory mechanism therefore seems to be widespread among pathogenic bacteria, suggesting that it may be important for the coordinate control of bacterial virulence. The actual relevance of this regulatory mechanism to the modulation of virulence expression in vivo is presently unknown.
An interesting observation is that in strain NCTC 7973 of L. monocytogenes, virulence gene expression was downregulated by the β-glucosides cellobiose and arbutin but not by common sugars, such as glucose, mannose, or fructose (67, 500, 501). Although the behavior of NCTC 7973 is anomalous and there is evidence that this strain has accumulated several regulatory mutations (30, 67, 543), it suggests that sugars may use two independent mechanisms to bring about virulence gene repression in L. monocytogenes, one responding to any carbon source and another specific for β-glucosides. This is also suggested by the recent characterization of a mutant derived from the wild-type strain EGD, in which a clear uncoupling between glucose- and β-glucoside-induced PrfA-dependent virulence factor expression was observed (67). In addition, PlcB production is inhibited in NCTC 7973 by cellobiose and arbutin, but not by salicin (67), indicating that repression by β-glucosides is a complex phenomenon in which various sugar-sensing mechanisms, with different substrate specificities, may be involved. Recently, a 4-kb operon specific to L. monocytogenes, bvrABC, encoding a putative β-glucoside-specific phosphoenolpyruvate-sugar phosphotransferase system similar to that of the bgl and arb operons of E. coli and Erwinia chrysanthemi and the bgl regulon of B. subtilis, has been reported to be involved in virulence gene repression by cellobiose and salicin (67). Transcription of the enzyme II permease gene, bvrB, was found to be induced by cellobiose and salicin but not arbutin, and a bvrAB knockout mutation abolished the repression exerted by cellobiose and salicin. β-Glucosides are unique to the plant kingdom and are presumably abundant in decaying vegetation. Thus, Bvr-mediated sensing of β-glucosides may allow L. monocytogenes to recognize its presence in a soil environment and consequently switch off virulence genes. It is presently unknown how the the bvr locus triggers repression of PrfA-dependent genes. Another locus possibly involved in regulation of hly expression by cellobiose has also recently been identified by transposon mutagenesis (292).
In contrast to glucose and other common fermentable carbohydrates, glucose-1-phosphate (G1P) (in general, any hexose phosphate) efficiently stimulates the growth of L. monocytogenes without causing virulence gene repression (543). Sugar phosphate utilization is not constitutive in L. monocytogenes; it is a strictly PrfA-dependent phenotype that is coexpressed with other virulence factors (e.g., Hly, ActA, and PlcB), suggesting a role in pathogenesis. Consistent with this, the capacity to grow on hexose phosphate as a sole carbon source is also present in the pathogenic species L. ivanovi (as a PrfA-dependent phenotype as well) but absent from nonpathogenic Listeria spp. As previously mentioned, PrfA-dependent genes become selectively activated in the cytoplasm of the host cell. In this compartment, the sugar phosphates G1P and glucose 6-phosphate are available as exogenous carbon sources for bacterial growth as a result of glycogen mentabolism. A role of hexose phosphate uptake in Listeria intracellular proliferation has recently been confirmed with the identification and mutagenesis of the PrfA-dependent gene hpt, encoding a sugar phosphate transporter (Chico-Calero et al., submitted for publication). The lack of repression of virulence genes observed with hexose phosphates indicates that uptake of these sugars bypasses the regulatory circuit responsible for triggering the CR response.
Regulatory Mechanism of PrfA
The clearest evidence that PrfA is a functional homolog of Crp was provided by the characterization of prfA* mutants of L. monocytogenes (544). These mutants were first identified by Ripio et al. (545) when testing a panel of L. monocytogenes strains for Hly and PlcB production as markers of PrfA-dependent virulence gene expression. Three different phenotypes were observed (Fig. 12). Phenotype 1 was exhibited by most of the strains tested and was characterized by low to undetectable levels of virulence factor production in BHI and the capacity to substantially (by 10- to 25-fold) increase this production upon culture medium supplementation with charcoal. Phenotype 2 was exhibited by only a few strains and was characterized by spontaneously high levels of virulence factor production and by unresponsiveness to charcoal, the levels of virulence factor production already being maximal. The third phenotype was exhibited by only one strain (LO28) and was characterized by intermediate levels of spontaneous Hly and PlcB production on BHI and responsiveness to charcoal. It was concluded that phenotype 1 corresponded to the wild type of L. monocytogenes and that phenotypes 2 and 3 represented mutant or variant phenotypes (545) (Fig. 12).
All phenotype 2 variants were strains that had been kept under laboratory conditions for a long time. Some were well-known collection strains widely used in research on listerial virulence, such as NCTC 7973 (Fig. 12) and the EGD Mackaness strain used in the laboratory of one of us (P.B.), originally obtained from R. J. North at the Trudeau Institute and thereafter renamed EGD-A to prevent confusion with the wild-type EGD strain currently used as a reference strain by the European Listeria Genome Consortium (545). Phenotype 2 variants were probably introduced inadvertently into research as model strains because their conspicuous hyperhemolytic phenotype facilitated the selection of Hly− mutants during early studies on the role of hemolysin in Listeria virulence. Phenotype 2 variants arise spontaneously at a very low frequency from wild-type strains during subculture in the laboratory and may be induced by chemical mutagenesis (171, 326, 545). The prfA genes in all phenotype 2 strains tested to date have a point mutation at codon 145, leading to an amino acid substitution (Gly→Ser) in the PrfA protein. Complementation studies have shown that this mutation is responsible for the constitutive overexpression of virulence genes exhibited by phenotype 2 strains (544).
A PrfA protein with the Gly145Ser substitution renders the virulence regulon insensitive to a variety of environmental repressor signals, such as growth in rich medium, low temperature, and the presence of readily fermentable carbon sources (30, 543, 544). Gly-145 of PrfA aligns with Ala-144 of Crp (544, 673), a position at which any bulkier substitution (e.g., Ala144Thr) results in a mutant Crp* protein that no longer requires the cofactor cAMP to become transcriptionally active (344) (Fig. 11). Like Crp* mutant forms, the Gly145Ser PrfA protein has greater binding affinity for the specific target DNA sequence (673). The Gly145Ser mutation also increases the capacity of the PrfA-RNA polymerase complex to initiate transcription in vitro (Vega et al., unpublished data). From these observations, it has been suggested that PrfA functions via a cofactor-mediated allosteric transition mechanism similar to that of Crp and that the Gly145Ser mutation represents a cofactor-independent PrfA* form that is “frozen” in an active conformation (544, 673).
The regulatory model currently proposed for PrfA is depicted in Fig. 13. This model is based on two premises. The first is that PrfA has two functional states, inactive and active, and shifts from one to the other upon binding of a low-molecular-weight cofactor, not yet identified, that transduces the stimulatory environmental signals to the PrfA transcriptional machinery (544, 673). Evidence for the existence of this PrfA-activating cofactor has been obtained by the fractionation of bacterial extracts on sucrose gradients, in which a low-molecular-weight component seems to stimulate PrfA-target DNA complex formation (221). The second premise is that PrfA is always present, at least in minimal amounts, in the bacterial cytoplasm, which is also the case. prfA is expressed in two ways: (i) under the control of growth-regulated promoters in the plcA-prfA intergenic region, generating a monocistronic transcript from which PrfA is synthesized in low but detectable amounts even if bacteria are exposed to PrfA regulon-nonactivating conditions (186, 187, 433, 540, 541, 673); and (ii) under the control of a PrfA-dependent promoter in front of the plcA gene, which generates a plcA-prfA bicistronic transcript (83, 186, 187, 433) (Fig. 13; see also Fig. 8), creating an autoregulatory loop which is critical for the adequate activation of prfA (378, 433, 541, 543, 544, 673). In the model proposed, the levels of virulence gene expression depend primarily on the functional status of the PrfA protein. This in turn controls the levels of prfA expression via positive feedback, resulting in strong PrfA-dependent transcriptional activation due to a rapid increase in PrfA in the active conformation, provided that the intracellular levels of the environmentally regulated stimulatory cofactor remain high (see the legend to Fig. 13). This positive-control model is highly versatile and makes possible a fine-tuned adaptive response to rapidly changing environmental conditions, such as would be encountered by L. monocytogenes during its transition from free to parasitic life and during passage across the various physiological barriers, tissues, and compartments of the infected host. The model provides a coherent explanation of all the available experimental data on PrfA regulation and is also compatible with the possible existence of additional regulatory elements modulating the activity of PrfA or its interaction with the target DNA. These elements include the so-called PrfA-activating factor (Paf), a proteinaceous substance that may be a fraction of the RNA polymerase and that facilitates the formation of the PrfA/RNA polymerase/target DNA complex (50, 51, 136, 221), and the putative mediators of carbon source regulation of virulence gene expression (see above).
FIG. 13.
Model for the mechanism of PrfA-mediated regulation. This model predicts that PrfA can undergo an allosteric transition between inactive (light gray) and active (dark gray) forms upon interaction with a low-molecular-weight hypothetical cofactor (see text for details). (A) Resting PrfA system. There is no cofactor, and the PrfA protein is synthesized at low, basal levels from the monocistronic transcripts generated from growth phase-regulated promoters in front of the prfA gene (small light gray arrow). (B) Activated PrfA system. If L. monocytogenes senses a suitable combination of environmental signals (i.e., 37°C and cytoplasmic environment), the intracellular concentration of the cofactor increases, leading to activation of the PrfA protein (a), which binds with increased affinity to the PrfA-boxes (black squares) in PrfA-regulated promoters (b); the transcriptionally active PrfA form causes the synthesis of more PrfA (in active conformation) via the positive autoregulatory loop generated by the PrfA-dependent bicistronic plcA-prfA transcript (c), thereby inducing the transcription of all PrfA-dependent genes (d) (dark gray arrows represent PrfA-dependent transcripts; the empty rectangle on the right represents any PrfA-dependent gene). The PrfA regulon remains switched on as long as the cofactor is present in the bacterial cytoplasm, but is rapidly switched off if the activating environmental signals cease and the concentration of the cofactor drops. (Reproduced from reference 673 with permission of the publisher.)
Fine Regulation of Virulence Determinants
A second level of regulation is provided by the differential response of PrfA-dependent promoters according to the structure of their PrfA-boxes (Fig. 11). Binding affinity to PrfA-boxes is affected by the number of nucleotide mismatches they carry, becoming weaker as the sequence diverges from the perfect inverted repeat. Promoters with perfectly symmetrical palindromes (e.g., Phly and PplcA) are more sensitive and respond more efficiently to activation by PrfA than those with nucleotide mismatches (e.g., Pmpl, PactA/plcB, and PinlA) (Fig. 11), which require larger amounts of the regulatory protein for full activation (53, 72, 186, 187, 544, 613, 698). This complementary cis-acting regulatory mechanism appears to be critical for the temporal and spatial expression of virulence genes and consequently for the correct development of the intracellular infectious cycle of L. monocytogenes. Early work by Freitag et al. (186, 187) and Camilli et al. (83) using different combinations of mutants affected in the monocistronic or bicistronic expression of prfA demonstrated that transcription of prfA from its own promoters in the plcA-prfA intergenic region was sufficient to mediate escape from the phagocytic vacuole and subsequent intracellular proliferation, but not to mediate cell-to-cell spread, which required a functional autoregulatory loop. These observations suggested that small amounts of PrfA synthesized during the early stages of bacterium-cell interaction are sufficient to activate rapidly and fully the high-affinity promoters of the hly and plcA genes, the products of which are involved in phagosome disruption. In contrast, activation of the low-affinity actA promoter, which directs the expression of the actA and plcB determinants involved in cell-to-cell spread, requires higher levels of PrfA, as provided by induction of the bicistronic transcript in the cytoplasm. Consistent with this, a recent study comparing wild-type bacteria with hly and actA mutants (which are confined in the phagosome and host cell cytoplasm, respectively) has shown that the PrfA-dependent membrane-damaging determinants hly and plcA are predominantly expressed in the phagosomal compartment, whereas the actA and mpl genes are selectively induced in the cytoplasm of the infected host cell (75).
As described above, experimental evidence has recently been obtained that PrfA synthesis is indeed activated in the host cell cytoplasm, correlating with higher activity of the PrfA-dependent plcA promoter that directs the expression of the plcA-prfA bicistron (540). However, prfA* mutants, in which PrfA-dependent expression is constitutively activated, are highly cytotoxic for the host cell (unpublished data). This may result from excess, unrestricted production of toxic virulence factors such as Hly and the phospholipases. In other words, activation of the PrfA system in the cytoplasm may be deleterious and incompatible with intracellular parasitism unless modulated by additional regulatory mechanisms (especially when L. monocytogenes reaches high intracellular population densities after multiplication). prfA transcript levels are surprisingly low within host cells (75), suggesting that a high turnover of prfA mRNA may be a mechanism that helps limit PrfA synthesis within host cells. The reported short half-life of Hly (127a, 311, 677) and PlcB (415) in the host cell cytoplasm may also play an important role in posttranslationally regulating the intracellular levels of cytotoxic virulence factors. This is clearly suggested by the observation that, whereas the levels of actA transcription in the host cell cytoplasm are only three times higher than those of hly, the amounts of the corresponding proteins are very different, with 70 times more ActA than Hly (451). Additional negative control mechanisms acting at the transcriptional level may also contribute to modulate prfA expression within the host cell. For example, uptake of glucose from the host cell cytoplasm may counteract, by carbon source-mediated repression (see above), the stimulatory effect of the intracellular environment on PrfA function. Freitag et al. (186, 187) reported that in the absence of PrfA, transcription from the two promoters immediately upstream from prfA is increased, and deletion of the −35 region of one of these two promoters (P2) (see Fig. 11 and 13), with a structure reminiscent of a PrfA-box, leads to higher levels of prfA transcription. This suggests that, by binding to the plcA-prfA intergenic region, PrfA may short-circuit the autoamplification loop, thereby downregulating its own expression. One study did not confirm the existence of such a PrfA-mediated negative autoregulatory mechanism (613), but a more recent study has shown that the transcription of a reporter gene under the control of the P2prfA promoter is significantly repressed by PrfA (72).
Some L. monocytogenes virulence genes, although part of the PrfA regulon, can also be expressed independently of PrfA. This may provide additional expressional characteristics important for the specific role of individual virulence factors in pathogenesis. A clear example is the internalin operon, inlAB, which is expressed from three promoters, two of which are PrfA independent. The third promoter is PrfA dependent, but its PrfA-box has two substitutions, resulting in a low binding affinity and inefficient induction by the listerial virulence regulator (53, 136, 148, 613). This promoter configuration is obviously responsible for the peculiar expression characteristics of the inlAB operon, which, in contrast to other PrfA-regulated loci, is significantly expressed during extracellular growth in broth medium at 37°C and positively regulated by elevated iron concentrations (i.e., PrfA-downregulating conditions) but poorly expressed within the cell cytoplasm or in MEM (i.e., PrfA-activating conditions) (53, 75, 101, 167). This is consistent with the role of the products of this operon, the internalins InlA and InlB, as invasins mediating internalization from the extracellular space and with no known involvement in intracellular proliferation.
The hly gene, which is primarily PrfA dependent, has an alternative means of expression via a weak PrfA-independent promoter (141). This explains why L. monocytogenes prfA deletion mutants have weak hemolytic activity (141, 544) and low-level expression of hly is detectable in L. monocytogenes in conditions of PrfA downregulation, such as extracellular growth in broth culture. As discussed earlier (617, 619), low concentrations of exogenous Hly released by extracellular bacteria may contribute to pathogenesis by triggering cell responses, and the PrfA-independent promoter of the hly gene may be important in this respect.
HOST CELL RESPONSES TO INFECTION
Signal Transduction Pathways Associated with Epithelial Cell Invasion
Little is known about the signaling cascades triggered by Listeria organisms during entry into cells that are normally nonphagocytic. InlB has been shown to be an agonist of the lipid kinase p85/p110 (PI 3-kinase [PI3K], and the InlB-mediated uptake of L. monocytogenes by Vero cells has been shown to be associated with activation of the PI3K (296, 297). In addition to InlB, this activation also requires tyrosine phosphorylation in the host cell, because uptake is inhibited by genistein treatment, which blocks tyrosine-specific protein kinases. The mechanism by which the PI-3 kinase mediates uptake is unknown. However, the products of the reaction catalyzed by PI3K, phosphoinositide-3,4-bisphosphate and phosphoinositide-3,4,5-triphosphate, may directly interfere with the actin cytoskeleton by uncapping the barbed ends of actin filaments, thereby affecting cytoskeleton dynamics. In addition to phosphoinositide-phosphates, leukotrienes are also thought to be involved in L. monocytogenes signaling during the invasion of epithelial cells. Nordihydroguaretic acid, an inhibitor of 5-lipooxygenase activity, which generates leukotrienes from arachidonic acid, also inhibits the invasion by L. monocytogenes of Hep-2 cells (430). However, it is not yet known at which step of the putative signaling cascade leukotrienes are involved, or whether the leukotriene signal is initiated by InlA- or InlB-receptor interactions.
The treatment of various types of cell with the protein kinase C (PKC) inhibitor genistein (or staurosporine) inhibits invasion by L. monocytogenes, suggesting that PKC activity is critical for regulating the cytoskeletal changes necessary for listerial uptake (253, 353, 648, 675). It has recently been reported (648) that the invasion of HeLa cells by L. monocytogenes activates not only ERK-1 and ERK-2, but also two other MAP kinases (p38 and JNK) and the MAP kinase kinase MEK-1. ERK-2 is also phosphorylated upon invasion by LLO-negative mutants and is a downstream target of MEK-1. These data suggest that MEK-1/ERK-2 activation is one step in the signaling cascade leading to L. monocytogenes uptake by host epithelial cells. ERK-2 activation is not sensitive to wortmannin treatment, showing that it is not a downstream event of PI3K activation. Obviously, the PI3K and MEK-1/ERK-2 pathways may form two components of converging or independent signal transduction systems required for L. monocytogenes invasion. Another protein tyrosine kinase, pp60c-src, is clearly activated during the entry of L. monocytogenes into epithelial cells because specific inhibition of pp60c-src by herbimycin blocks inlAB-dependent entry (664). Treatment of host cells with TcdB toxins led to a dramatic breakdown of the normal actin cytoskeleton but did not abrogate L. monocytogenes invasion (or actin-based motility), indicating that the small GTP-binding proteins of the Rho and Ras subfamilies are not involved in the entry process (162).
The L. monocytogenes invasion process has been found to be sensitive to treatment with cytochalasin D for all cell types tested, demonstrating the importance of microfilaments for internalization (194, 359). Several recent reports have demonstrated that microtubules also play some role in invasion. The uptake of L. monocytogenes by mouse dendritic cells, mouse bone marrow-derived macrophages, and human brain endothelial cells is sensitive to nocodazole treatment, which disrupts microtubules (246, 253, 352).
Activation of NF-κB and Modulation of Host Gene Expression in Macrophages
Differential gene expression in eukaryotic cells depends largely on modification of the transactivating activity of inducible transcription factors. NF-κB, which is involved in regulation of the expression of many immunologically important genes (622), is the prototype of a family of transcription factors. NF-κB DNA-binding activity in response to L. monocytogenes infection has been studied in the macrophage-like cell line P388D1 (272, 273), endothelial cells (330, 600), and epithelial cells (271). A rapid invasion-independent increase of the NF-κB RelA/p50 DNA-binding activity is observed in macrophages within 10 to 20 min of addition of the bacteria. Studies with in-frame deletion mutants have shown that NF-κB is induced with biphasic kinetics upon L. monocytogenes infection. The first transient induction of NF-κB requires only the adhesion of L. monocytogenes to the P388D1 cells. This induction also occurs upon infection with mutants of L. monocytogenes that have lost one or more of the known virulence genes and involves lipoteichoic acid, a cell surface component of L. monocytogenes. A second, permanent induction of NF-κB is detectable after release of the Listeria bacteria into the cytoplasm of the host cell. This occurs exclusively with virulent L. monocytogenes strains and requires the synthesis of the bacterial phospholipases PlcA and PlcB in the infected host cell. It has been speculated that DAG and CER, likely products of the two enzymes (see above), act as second messengers in the induction of persistent NF-κB activation.
The expression of cytokines upon treatment of mammalian cells with L. monocytogenes or L. monocytogenes-derived products has been analyzed in many different cell systems. Infections were performed with defined cell populations, such as mouse bone marrow-derived macrophages (BMM), macrophage-like cell lines, and complex mixtures of cells, like spleen cell preparations (356, 357). We will briefly review some of the studies and focus on those in which defined cell types were used as hosts for L. monocytogenes.
Early studies by E. A. Havell (274–276) showed that murine fibroblasts infected with L. monocytogenes produce significant amounts of alpha/beta interferon (IFN-α/β) and IL-6 and that mouse alveolar macrophages respond to L. monocytogenes infection by producing IL-6 and TNF-α. Differences in the induction of cytokine expression were assessed for macrophages infected with live and killed L. monocytogenes-infected macrophages. Live and killed bacteria differ in IL-1, IL-6, and TNF-α production (447, 709), whereas IL-12 is induced in vitro by live and killed Listeria cells (94).
Using various well-defined mutants of L. monocytogenes, it has been shown that viable bacteria rapidly induce IL-1α, IL-1β, IL-6, and TNF-α mRNAs in the mouse macrophage-like cell line P388D1, whereas killed bacteria induce only IL-1β mRNA (355). Hly− mutants are unable to induce IL-1α, IL-6, and TNF-α but do induce IL-1β mRNA. The infection of BMM with L. monocytogenes also induces the proinflammatory cytokines (132). However, an Hly− L. monocytogenes mutant was found to induce the same types and amount of cytokines as the wild-type strain, indicating that intracellular growth is not necessary for the transcriptional induction of these cytokines in BMM.
Not all the bacterial products mediating the induction of cytokines upon L. monocytogenes infection have been identified. The bacterial cell wall polymer lipoteichoic acid has been shown to induce the expression of the proinflammatory cytokines IL-1α/β, IL-6, and TNF-α in BMM (353) and in human monocytes (40). Nishibori et al. (474) analyzed cytokine gene expression upon treatment of spleen cells with purified LLO. Total spleen cell preparations containing macrophages and NK cells responded rapidly with increases in the synthesis of IL-1α, IL-6, IL-10, IL-12, TNF-α, and IFN-γ mRNAs. By depleting the cell preparations of either NK cells or macrophages, the contribution and interaction of the two cell types to cytokine production were assessed. It was found that IL-1α, IL-12, and TNF-α were produced mainly by macrophages, whereas IFN-γ was clearly produced by NK cells stimulated by macrophages, probably via TNF-α and IL-12.
The synthesis of cytokine receptor mRNAs in BMM infected with L. monocytogenes seems to be regulated inversely to the corresponding cytokines (132). TNF receptor type I and IFN-γ receptor are transcriptionally downregulated following the infection of BMM with L. monocytogenes, whereas IL-1 receptor type II mRNA is unaffected. In contrast, TNF receptor type II expression is upregulated. The bacterial requirements for this downregulation of cytokine receptor expression are unknown, but the downregulation is specific for infection with L. monocytogenes and was not found upon infection with nonpathogenic L. innocua strains.
Phagocytosis of L. monocytogenes is required for transcriptional induction of the heat shock protein 70 (hsp70) gene in macrophages. The synthesis of LLO seems to accentuate the hsp70 response (598). In contrast, expression of the gene encoding MHC class II antigen I-Ab is downregulated upon phagocytosis, at a time when the bacteria are still located mainly in the phagosomal compartment (595). Again, LLO seems to be important for this host response. Another important host cell gene that is downregulated on infection of macrophages by L. monocytogenes encodes antigen H-2K, a major component of the class I MHC molecule. Unlike downregulation of the gene encoding antigen I-Ab from the class II MHC, this event requires not only LLO, but also the expression of one or more genes of the listerial lecithinase operon (595).
Host Responses during Infection of Endothelial Cells
Host cell responses elicited in endothelial cells during infection or by treatment with listerial exotoxins include lipid second-messenger generation (617, 618), NF-κB activation (330, 600), stimulation of cytokines and chemokines, and induction of cell adhesion molecule expression (152, 153, 247, 330, 351, 699). HUVECs were shown to respond to L. monocytogenes infection or treatment with purified LLO by increasing phosphoinositide metabolism and inducing the production of the vasoactive inflammatory mediators platelet-activating factor and prostaglandin I2. This LLO-triggered second-messenger induction is further stimulated by the phospholipase PlcA, which acts in synergy with LLO (see above). It has been speculated that the pore-forming activity of LLO might facilitate the uptake of PlcB by the HUVECs, resulting in DAG and inositol phosphate generation (617). Listerial phospholipase-mediated increased levels of CER were also found in HUVECs during L. monocytogenes infection. This increase is followed by an elevation in the activity of the transcription factor NF-κB (152, 600). The cell adhesion molecules for which expression was analyzed in L. monocytogenes-infected HUVECs and BMECs include E-selectin, P-selectin, VCAM-1, and ICAM-1 (152, 153, 351, 699). P-selectin expression peaked 30 to 60 min after infection, whereas the induction of E- selectin, ICAM-1, and VCAM-1 started 4 to 8 h postinfection. Cell adhesion molecule expression is partly dependent on LLO expression (351). E-selectin expression and the concomitant induced adhesion of polymorphonuclear leukocytes to HUVECs is particularly dependent on PlcA and PlcB synthesis by L. monocytogenes. These data show that the phospholipases are important virulence factors in the L. monocytogenes-endothelial cell interaction, interfering with signal transduction pathways mediated by lipid second messengers and causing changes in gene expression (600). The expression of IL-6 and IL-8 is also induced during the infection of HUVECs, but no data are available concerning the bacterial requirements for the induction of cytokine and chemokine expression upon infection (247).
Apoptosis
L. monocytogenes has recently been shown to induce apoptosis in hepatocytes (560), Caco-2 epithelial cells (662), and dendritic cells, in which it was also demonstrated that LLO is the bacterial component triggering this event (252). L. monocytogenes-infected hepatocytes undergo apoptosis in vitro and in the infected mouse. It has been suggested that hepatocyte apoptosis, which is linked to neutrophil recruitment, eliminates infected cells rapidly from the tissue. Thus, apoptosis would inhibit the spread of L. monocytogenes to the neighboring cells and simultaneously would make the intracellular Listeria cells accessible to effector cells. In dendritic cells, which are important antigen-presenting cells, LLO-induced apoptosis should result in lower levels of antigen presentation to immune cells, thereby downregulating the immune response.
Figure 14 summarizes the information available on the signaling cascades involved in the host cell response to Listeria infection.
FIG. 14.
Summary of modulated signal transduction pathways and host cell responses identified during L. monocytogenes infection of murine bone marrow-derived macrophages, murine P388D1 and J774 cell line macrophages, human Caco-2 and HeLa epithelial cells, mouse dendritic cells, and human umbilical vein endothelial cells. Abbreviations: aSMase, acidic sphingomyelinase; gC1q-R, complement C1q receptor; Hsp70, heat shock protein 70; Hsp90, heat shock protein 90; HSPG-R, HSPG receptor; ICAM-1, intercellular adhesion molecule 1; IFN-γR, IFN-γ receptor; IPx, inositolphosphates; LTA, lipoteichoic acid; MEK-1, mitogen-activated protein kinase kinase 1; Met, receptor tyrosine kinase for HGF; MKP-1, mitogen-activated protein kinase phosphatase 1; PAF, platelet-activating factor; PGI2, prostaglandin I2; PIP2, phosphatidylinositol-(3,4)-bisphosphate; PIP3, phosphatidylinositol-(3,4,5)-trisphosphate; PI-PLC, phosphatidylinositol-specific phospholipase C; SR, scavenger receptor; TNF-RI, TNF receptor type 1; VCAM-1, vascular cell adhesion molecule-1. Reproduced with modifications from reference 357 with permission of Elsevier Science.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
In the last 15 years, listeriosis has undergone a transformation from an infectious disease of limited importance to one of the most topical food-borne infections and a source of major concern for health authorities and the food industry alike. In the same period of time, the work of various groups in Europe and the United States has made the causal agent of this disease, L. monocytogenes, one of the best-characterized intracellular parasites at both the molecular and cellular levels. One particular aspect of the interaction between L. monocytogenes and the eukaryotic host cell, the actin-based motility mediated by the surface protein ActA, is today a true paradigm of cell biology. Despite the major progress made towards understanding the mechanisms of virulence of Listeria spp., our current insight into the process of pathogenesis is still very partial and fragmentary. We have unraveled fundamental details of the intracellular parasitism practiced by these bacteria and the molecular determinants responsible for key stages in their life cycle within the cells of the host. However, we know very little about the mechanisms used by pathogenic Listeria spp. to penetrate the organism, to cross the host's physiological barriers, and to interact with the target cells in which they proliferate, causing disease. The same holds true for the strategies used by Listeria spp. to resist and counteract the defense mechanisms of the host for long enough after intestinal translocation to produce invasive disease in the target organs. Only about 15 listerial genetic determinants have been found to date to be associated with pathogenesis, including true virulence genes and housekeeping genes required for survival in the host. This number is very small compared with the nearly 3,000 genes predicted for the 2.9-Mbp genome of L. monocytogenes (438, 679; European Listeria Genome Consortium, unpublished). It is therefore clear that there must be other, as yet unknown loci in the Listeria chromosome that are required for the development of infection.
To date, studies of the molecular determinants of Listeria virulence were mainly based on the analysis of mutants affected in conspicuous phenotypes displayed under in vitro conditions. The clearest illustration of this is the central virulence gene cluster, which was discovered by genetic analysis of transposon mutants affected in hemolytic and lecithinase activities, two phenotypes easily detectable on agar plates. With hindsight, it was a clear stroke of luck that the mutated hly and plcB genes were located in the same chromosomal locus together with other essential virulence genes such as prfA and actA. In other cases, the in vitro phenotype used to identify potential virulence genes during mutagenesis was a priori more directly related to functions relevant to Listeria pathogenesis, such as loss of invasiveness in eukaryotic cells. The extensively studied inlAB operon was identified in this way. However, although the inlAB products have been clearly shown to have an invasin function in cell monolayers in vitro, no clear role for this locus in pathogenesis has been demonstrated yet in infection models in vivo. This illustrates the limitations inherent to the approaches used to date in the molecular analysis of Listeria virulence, particularly when combined with in vitro models of infection that do not necessarily reproduce reliably the natural conditions encountered by L. monocytogenes in the tissues of the host.
This scenario is likely to change radically in the immediate future with the availability of the complete sequence of the genome of L. monocytogenes. This will make it possible to shift from an investigation largely based on reductionist analysis of the role in virulence of individual microbial products in an isolated form, to a holistic approach to Listeria pathogenesis. In conjunction with appropriate in vivo expression technologies, which are beginning to be applied to Listeria spp. with promising results (158, 192), the genomic approach will make it possible to identify systematically the entire set of genes required by L. monocytogenes for its survival and proliferation in the various tissues and compartments of the host. The comparative genomic investigation of pathogenic versus nonpathogenic Listeria species, and of environmental versus epidemic strains of L. monocytogenes, will lead to the discovery of new pathogenicity determinants, providing us at the same time with insight into the evolution of virulence in this bacterial genus. Progress in human genomics will also make it possible to define the host components and processes engaged during Listeria infection, as shown by a recent study in which cellular responses to L. monocytogenes were monitored using oligonucleotide microarrays (97a). Characterization of the expression profiles of both the host cell and the pathogen at various stages of the infection process will undoubtedly represent a quantum leap in our understanding of host-microparasite interaction.
The shift towards genomic technology as the primary approach for analyzing the molecular determinants of Listeria pathogenesis will not make reductionist strategies of investigation less important. Indeed, functional analysis of the genome of L. monocytogenes will bring to light a growing number of potential virulence factors, and the intimate mechanisms of interaction of these factors with the molecules of the host will still require investigation by the classical techniques of molecular and cell biology. The importance of this reductionist approach is illustrated by the recent study by Lecuit et al. (372), suggesting that the host tropism of L. monocytogenes may depend on a single residue of E-cadherin, the cell receptor for InlA. It has required the continuous efforts of various research groups over several years to make the first steps towards elucidating the molecular mechanisms of actin-based motility, which involves only one Listeria protein. This indicates the enormity of the task ahead of us if we are to understand in precise molecular terms the way in which Listeria interacts with its various vertebrate hosts to produce infection.
Fundamental research on Listeria molecular biology and pathogenesis can be exploited in the rational design of novel biotechnological tools to fight disase. The characterization of the listerial intracellular life cycle and of the role that hemolysin plays in it, together with the knowledge accumulated concerning the immunobiology of experimental listeriosis in the mouse, has made L. monocytogenes a most promising candidate as antigen delivery system for the development of novel recombinant oral live vaccines (254). By its phagosome-disrupting activity, Hly permits the antigens secreted by the producer bacteria to be released into the cytoplasm and thus to be presented by MHC class I molecules, inducing a cytotoxic response useful for combating intracellular parasite infections or tumoral disease (327). Indeed, successful vaccination of mice using L. monocytogenes-based recombinant live vaccines has been reported for several viral and tumor model systems (183, 227, 293, 307, 418, 492, 496, 497, 614, 688). Recently, a plasmid containing model antigen genes under the control of a eukaryotic promoter and an endolysin gene from a Listeria phage under the control of the strictly PrfA-dependent promoter of the actA gene (which is selectively activated in the host cell cytoplasm) were introduced into an L. monocytogenes strain attenuated by a deletion in the lecithinase operon. Bacteria lysed upon entry into the cytosol and generated an MHC class I-restricted immune response against the model antigens expressed from the plasmid (137). These and similar experiments (104) show that L. monocytogenes is also a good potential candidate for a vector to transfer DNA into eukaryotic cells, of potential value for DNA vaccines or for gene therapy (637). Increasing our knowledge of the molecular and cellular mechanisms of listerial pathogenesis by genomic research will make it possible to improve these Listeria-based biotechnological tools by endowing them with optimal biosafety and target cell specificity characteristics. Progress in Listeria genomics and comprehensive understanding of the complete physiological pathways of L. monocytogenes may also enable us to identify targets for the development of novel antimicrobial agents to treat listeriosis and other bacterial infections, or of inhibitory substances for selectively controlling the survival and growth of L. monocytogenes in foods.
We are still very far from completely understanding at the molecular level how pathogenic Listeria spp. interact with the host to cause infection. Many questions remain unanswered. For example, what is the true role of the large internalin multigene family in Listeria biology and pathogenesis? Why does L. monocytogenes preferentially infect the CNS and the placenta? Why is it that only certain serovars of L. monocytogenes are associated with listeriosis and certain clones of serovar 4b are associated with epidemic episodes of invasive disease? Why do pregnant women develop neither meningoencephalitis nor septicemic disease while suffering miscarriage due to Listeria infection? What mechanisms are involved in the direct invasion of neighboring cells during cell-to-cell spread? What pathways are involved in transduction of the signals involved in the induction of phagocytosis during cell invasion? In the coming years of the new era of postgenomic Listeria research, we will certainly witness spectacular progress in understanding of the complex molecular mechanisms used by these versatile bacteria to survive as parasites in the tissues and cells of the vertebrate host, and we will no doubt find answers to these and many other questions.
ACKNOWLEDGMENTS
This work was supported by the BIOMED 2 program of the European Commission (grant BMH4-CT96-0659). J.-A.V.-B. thanks the Complutense University of Madrid for the sabbatical leave awarded, which has been critical for the completion of this review.
REFERENCES
- 1.Abram M, Doric M. Primary Listeria monocytogenes infection in gestating mice. Folia Microbiol. 1997;42:65–71. doi: 10.1007/BF02898648. [DOI] [PubMed] [Google Scholar]
- 2.Adams T J, Vartivarian S, Cowart R E. Iron acquisition systems of Listeria monocytogenes. Infect Immun. 1990;58:2715–2718. doi: 10.1128/iai.58.8.2715-2718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Agranof D D, Krishna S. Metal ion homeostasis and intracellular parasitism. Mol Microbiol. 1998;28:403–412. doi: 10.1046/j.1365-2958.1998.00790.x. [DOI] [PubMed] [Google Scholar]
- 3.Akaki T, Sato K, Shimizu T, Sano C, Kajitani H, Dekio S, Tomioka H. Effector molecules in expression of the antimicrobial activity of macrophages against Mycobacterium avium complex: roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids. J Leukocyte Biol. 1997;62:795–804. doi: 10.1002/jlb.62.6.795. [DOI] [PubMed] [Google Scholar]
- 4.Alexander A V, Walker R L, Johnson B J, Charlton B R, Woods L W. Bovine abortions attributable to Listeria ivanovii: four cases (1988–1990) J Am Vet Med Assoc. 1992;200:711–714. [PubMed] [Google Scholar]
- 5.Allan D. Mapping the lipid distribution in the membranes of BHK cells. Mol Membr Biol. 1996;13:81–84. doi: 10.3109/09687689609160580. [DOI] [PubMed] [Google Scholar]
- 6.Allcock J. Cutaneous listeriosis. Vet Rec. 1992;130:18–19. doi: 10.1136/vr.130.1.18-a. [DOI] [PubMed] [Google Scholar]
- 7.Alouf J E. Introduction to the family of the structurally related cholesterol-binding cytolysins (“sulfhydryl-activated” toxins) In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 443–456. [Google Scholar]
- 8.Alouf J E, Geoffroy C. The family of the antigenically-related, cholesterol-binding (‘sulfhydryl-activated’) cytolytic toxins. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, U.K: Academic Press; 1991. pp. 147–186. [Google Scholar]
- 9.Altimira J, Prats N, López S, Domíngo M, Briones V, Domínguez L, Marco A. Repeated oral dosing with Listeria monocytogenes in mice as a model of central nervous system listeriosis in man. J Comp Pathol. 1999;121:117–125. doi: 10.1053/jcpa.1999.0303. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Domínguez C, Carrasco-Marín E, Leyva-Cobián F. Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell lines. Infect Immun. 1993;61:3664–3672. doi: 10.1128/iai.61.9.3664-3672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Domínguez C, Roberts R, Stahl P D. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J Cell Sci. 1997;110:731–743. doi: 10.1242/jcs.110.6.731. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Domínguez C, Vázquez-Boland J A, Carrasco-Marín E, López-Mato P, Leyva-Cobián F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anonymous. Listeria infections in farm animals. Vet Rec. 1983;112:314. [Google Scholar]
- 14.Appelberg R, Leal I S. Mutants of Listeria monocytogenes defective in in vitro invasion and cell-to-cell spreading still invade and proliferate in hepatocytes of neutropenic mice. Infect Immun. 2000;68:912–914. doi: 10.1128/iai.68.2.912-914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong B A, Sword C P. Electron microscopy of Listeria monocytogenes-infected mouse spleen. J Bacteriol. 1966;91:1346–1355. doi: 10.1128/jb.91.3.1346-1355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong R W, Fung P C. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review of the literature. Clin Infect Dis. 1993;16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 17.Asahi O, Hosoda T, Akiyama Y. Studies on the mechanism of infection of the brain with Listeria monocytogenes. Am J Vet Res. 1957;18:147–157. [PubMed] [Google Scholar]
- 18.Audurier A, Pardon P, Marly J, Lantier F. Experimental infection of mice with Listeria monocytogenes and L. innocua. Ann Microbiol. 1980;131B:47–57. [PubMed] [Google Scholar]
- 19.Aureli P, Fioruci G C, Caroli D, Marchiaro G, Novara O, Leone L, Salmaso S. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N Engl J Med. 2000;342:1236–1241. doi: 10.1056/NEJM200004273421702. [DOI] [PubMed] [Google Scholar]
- 20.Bach M C, Davis K M. Listeria rhombencephalitis mimicking tuberculous meningitis. Rev Infect Dis. 1987;9:130–133. doi: 10.1093/clinids/9.1.130. [DOI] [PubMed] [Google Scholar]
- 21.Barchini E, Cowart R E. Extracellular iron reductase activity produced by Listeria monocytogenes. Arch Microbiol. 1996;166:51–57. doi: 10.1007/s002030050354. [DOI] [PubMed] [Google Scholar]
- 22.Barlow R M, McGorum B. Ovine listerial encephalitis: analysis, hypotheses and synthesis. Vet Rec. 1985;116:233–236. doi: 10.1136/vr.116.9.233. [DOI] [PubMed] [Google Scholar]
- 23.Barry R A, Bouwer H G A, Portnoy D A, Hinrichs D J. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992;60:1625–1632. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayles D O, Annous B A, Wilkinson B J. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl Environ Microbiol. 1996;62:1116–1119. doi: 10.1128/aem.62.3.1116-1119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauregard K E, Lee K D, Collier R J, Swanson J A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker L A, Çetin M S, Hutkins R W, Benson A W. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckerle M C. Spatial control of actin filament assembly: lessons from Listeria. Cell. 1998;95:741–748. doi: 10.1016/s0092-8674(00)81697-9. [DOI] [PubMed] [Google Scholar]
- 28.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, Daniel M L, Unanue E R. Release of nitric oxide during T-cell independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 29.Behari J, Youngman P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J Bacteriol. 1998;180:6316–6324. doi: 10.1128/jb.180.23.6316-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behari J, Youngman P. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect Immun. 1998;66:3635–3642. doi: 10.1128/iai.66.8.3635-3642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berche P. Bacteriemia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 32.Berche P, Gaillard J-L, Geoffroy C, Alouf J E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987;139:3813–3821. [PubMed] [Google Scholar]
- 33.Berche P, Gaillard J-L, Sansonetti P. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- 34.Berche P, Reich K A, Bonnichon M, Beretti J-L, Geoffroy C, Ravenau J, Cossart P, Gaillard J-L, Geslin P, Kreis H, Veron M. Detection of anti-listeriolysin O for serodiagnosis of human listeriosis. Lancet. 1990;335:624–627. doi: 10.1016/0140-6736(90)90411-w. [DOI] [PubMed] [Google Scholar]
- 35.Berenguer J, Solera J, Díaz M D, Moreno S, López-Herce J A, Bouza E. Listeriosis in patients infected with human immunodeficiency virus. Rev Infect Dis. 1991;13:115–119. doi: 10.1093/clinids/13.1.115. [DOI] [PubMed] [Google Scholar]
- 36.Berg R D. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez L E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages: the role of nitric oxide. Clin Exp Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bevers E M, Comfurius P, Dekkers D W, Harmsma M, Zwaal R F. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus. 1998;7:S126–S131. doi: 10.1177/096120339800700228. [DOI] [PubMed] [Google Scholar]
- 40.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhakdi S, Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–233. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- 42.Bhakdi S, Tranum-Jensen J, Sziegoleit A. Mechanism of membrane damage by streptolysin O. Infect Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatt R, Ala'Aldeen D A, Baldwin T, Boriello S P. The 126 kDa iron-regulated protein of Listeria monocytogenes is not a transferrin binding protein. FEMS Microbiol Lett. 1994;123:119–123. doi: 10.1111/j.1574-6968.1994.tb07210.x. [DOI] [PubMed] [Google Scholar]
- 44.Bibb W F, Gellin B G, Weaver R, Schwartz B, Plikaytis B D, Reeves M W, Pinner R W, Broome C V. Analysis of clinical and food-borne isolates of Listeria monocytogenes in the United States by multilocus enzyme electrophoresis and application of the method to epidemiological investigations. Appl Environ Microbiol. 1990;56:2133–2141. doi: 10.1128/aem.56.7.2133-2141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 45a.Bierne H, Dramsi S, Gratacap M P, Randriamampita C, Carpenter G, Payrastre B, Cossart P. The invasion protein InlB from Listeria monocytogenes activates PLC-γ1 downstream from PI 3-kinase. Cell Microbiol. 2000;2:465–476. doi: 10.1046/j.1462-5822.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 46.Bille J. Epidemiology of human listeriosis in Europe, whith special reference to the Swiss outbreak. In: Miller A J, Smith J L, Somkuti G A, editors. Foodborne listeriosis. New York, N.Y: Elsevier; 1990. pp. 71–74. [Google Scholar]
- 47.Blanot S, Muffat Joly M, Vilde F, Jaubert F, Clement O, Frija G, Berche P. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microb Pathog. 1997;23:39–48. doi: 10.1006/mpat.1997.0131. [DOI] [PubMed] [Google Scholar]
- 48.Blenden D C, Kampelmacher E H, Torres-Anjel M J. Listeriosis. J Am Vet Med Assoc. 1987;191:1546–1551. [PubMed] [Google Scholar]
- 49.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excission of large DNA-regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böckmann R, Dickneite C, Goebel W, Bohne J. PrfA mediates specific binding to RNA polymerase of Listeria monocytogenes to PrfA-dependent viruelence gene promoters resulting in a transcriptionally active complex. Mol Microbiol. 2000;36:487–497. doi: 10.1046/j.1365-2958.2000.01868.x. [DOI] [PubMed] [Google Scholar]
- 51.Böckmann R, Dickneite C, Middendorf B, Goebel W, Sokolovic Z. Specific binding of the Listeria monocytogenes transcriptional regulator protein PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol Microbiol. 1996;22:643–653. doi: 10.1046/j.1365-2958.1996.d01-1722.x. [DOI] [PubMed] [Google Scholar]
- 52.Boerlin P, Piffaretti J-C. Typing of human, animal, food and environmental isolates of Listeria monocytogenes by multilocus enzyme electrophoresis. Appl Environ Microbiol. 1991;57:1624–1629. doi: 10.1128/aem.57.6.1624-1629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohne J, Kestler H, Uebele C, Sokolovic Z, Goebel W. Differential regulation of the virulence genes of Listeria monocytogenes. Mol Microbiol. 1996;20:1189–1198. doi: 10.1111/j.1365-2958.1996.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 54.Bohne J, Sokolovic Z, Goebel W. Transcriptional regulation of prfA and PrfA-regulated virulence genes in L. monocytogenes. Mol Microbiol. 1994;11:1141–1150. doi: 10.1111/j.1365-2958.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 55.Boland A, Havaux S, Cornelis G R. Heterogeneity of the Yersinia YopM protein. Microb Pathog. 1998;25:343–348. doi: 10.1006/mpat.1998.0247. [DOI] [PubMed] [Google Scholar]
- 56.Boockvar K S, Granger D L, Poston R M, Maybodi M, Washington M K, Hibbs J B, Jr, Kurlander R L. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bortolussi R, Vandenbroucke-Grauls C M J E, van Asbeck B S, Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987;55:3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bortolussi R, Campbell N, Krause V. Dynamics of Listeria monocytogenes type 4b infection in pregnant and infant rats. Clin Investig Med. 1984;7:273–279. [PubMed] [Google Scholar]
- 59.Bouwer H G A, Nelson C S, Gibbins B L, Portnoy D A, Hinrichs D J. Listeriolysin O is the target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175:1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 61.Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a Clq-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun L, Nato F, Payrastre B, Mazié J-C, Cossart P. The 213-amino-acid leucine-rich repeat region of the Listeria monocytogenes InlB protein is sufficient for entry into mammalian cells, stimulation of PI 3-kinase and membrane ruffling. Mol Microbiol. 1999;34:10–23. doi: 10.1046/j.1365-2958.1999.01560.x. [DOI] [PubMed] [Google Scholar]
- 63.Braun L, Ohayon H, Cossart P. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 64.Reference deleted.
- 65.Brehm K, Haas A, Goebel W, Kreft J. A gene encoding a superoxide dismutase of the facultative intracellular bacterium Listeria monocytogenes. Gene. 1992;118:121–125. doi: 10.1016/0378-1119(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 66.Brehm K, Kreft J, Ripio M-T, Vazquez-Boland J-A. Regulation of virulence gene expression in pathogenic Listeria. Microbiol SEM. 1996;12:219–236. [PubMed] [Google Scholar]
- 67.Brehm K, Ripio M-T, Kreft J, Vázquez-Boland J-A. The bvr locus of Listeria monocytogenes mediates virulence gene repression by β-glucosides. J Bacteriol. 1999;181:5024–5032. doi: 10.1128/jb.181.16.5024-5032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brosch R, Catimel B, Milon G, Buchrieser C, Vindel E, Rocourt J. Virulence heterogeneity of Listeria monocytogenes strains from various sources (food, human, animal) in immunocompetent mice and its association with typing characteristics. J Food Protect. 1993;56:296–301. doi: 10.4315/0362-028X-56.4.297. [DOI] [PubMed] [Google Scholar]
- 69.Brun-Buisson C J, De Gialluly E, Gherardi R, Otterbein G, Gray F, Rapin M. Fatal nonmeningitic Listeria rhombencephalitis: reports of two cases. Arch Intern Med. 1985;145:1982–1985. [PubMed] [Google Scholar]
- 70.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 72.Bubert A, Kestler H, Götz M, Böckmann R, Goebel W. The Listeria monocytogenes iap gene as an indicator gene for the study of PrfA-dependent regulation. Mol Gen Genet. 1997;256:54–62. doi: 10.1007/s004380050545. [DOI] [PubMed] [Google Scholar]
- 73.Bubert A, Kuhn M, Goebel W, Köhler S. Structural and functional properties of the p60 proteins from different Listeria species. J Bacteriol. 1992;174:8166–8171. doi: 10.1128/jb.174.24.8166-8171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bubert A, Köhler S, Goebel W. The homologous and heterologous regions within the iap gene allows genus- and species-specific identification of Listeria spp. by polymerase chain reaction. Appl Environ Microbiol. 1992;58:2625–2632. doi: 10.1128/aem.58.8.2625-2632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bubert A, Sokolovic Z, Chun S-K, Papatheodorou L, Simm A, Goebel W. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 76.Bubert A, Riebe J, Schnitzler N, Schönberg A, Goebel W, Schubert P. Isolation of catalase-negative Listeria monocytogenes strains from listeriosis patients and their rapid identification by anti-p60 antibodies and/or PCR. J Clin Microbiol. 1997;35:179–183. doi: 10.1128/jcm.35.1.179-183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bubert A, Schubert P, Köhler S, Frank R, Goebel W. Synthetic peptides derived from the Listeria monocytogenes p60 protein as antigens for the generation of polyclonal antibodies specific for secreted cell-free L. monocytogenes p60 proteins. Appl Environ Microbiol. 1994;60:3120–3127. doi: 10.1128/aem.60.9.3120-3127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 79.Busch D H, Bouwer H G A, Hinrichs D, Pamer E G. A nonamer peptide derived from Listeria monocytogenes metalloprotease is presented to cytolytic T lymphocytes. Infect Immun. 1997;65:5326–5329. doi: 10.1128/iai.65.12.5326-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Busque P, Letellier A, Harel J, Dubreuil J D. Production of Escherichia coli STb enterotoxin is subjected to catabolite repression. Microbiology. 1995;141:1621–1627. doi: 10.1099/13500872-141-7-1621. [DOI] [PubMed] [Google Scholar]
- 81.Cameron L A, Footer M J, van Oudenaarden A, Theriot J A. Motility of ActA protein-coated microspheres driven by actin polymerization. Proc Natl Acad Sci USA. 1999;96:4908–4913. doi: 10.1073/pnas.96.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Camilli A, Goldfine H, Portnoy D A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991;173:751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell P A. Macrophage-Listeria interactions. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker Inc.; 1993. pp. 313–328. [Google Scholar]
- 85.Carl U D, Pollmann M, Orr E, Gertler F B, Chakraborty T, Wehland J. Aromatic and basic residues within the EVH1 domain of VASP specify its interaction with proline-rich ligands. Curr Biol. 1999;9:715–718. doi: 10.1016/s0960-9822(99)80315-7. [DOI] [PubMed] [Google Scholar]
- 86.Carlier M F, Laurent V, Santolini J, Melki R, Didry D, Xia G-X, Hong Y, Chua N-H, Pantaloni D. Actin depolimerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakraborty T. The molecular mechanisms of actin-based intracellular motility by Listeria monocytogenes. Microbiol SEM. 1996;12:237–244. [PubMed] [Google Scholar]
- 88.Chakraborty T, Ebel F, Domann E, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. A novel focal adhesion factor directly linking intracellular motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88a.Chakraborty T, Hain T, Domann E. Genome organization and the evolution of the virulence gene locus in Listeria species. Int J Med Microbiol. 2000;290:167–174. doi: 10.1016/S1438-4221(00)80086-7. [DOI] [PubMed] [Google Scholar]
- 89.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chand P, Sadana J R. Outbreak of Listeria ivanovii abortion in sheep in India. Vet Rec. 1999;145:83–84. doi: 10.1136/vr.145.3.83. [DOI] [PubMed] [Google Scholar]
- 91.Charlton K M. Spontanelous listeric encephalitis in sheep. Electron microscopic studies. Vet Pathol. 1977;14:429–434. doi: 10.1177/030098587701400501. [DOI] [PubMed] [Google Scholar]
- 92.Charlton K M, Garcia M M. Spontaneous listeric encephalitis and neuritis in sheep. Vet Pathol. 1977;14:297–313. doi: 10.1177/030098587701400401. [DOI] [PubMed] [Google Scholar]
- 93.Cheers C, McKenzie I F C, Pavlov H, Waid C, York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant and susceptible mice. Infect Immun. 1978;19:763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheers C, Zhan Y. How do macrophages distinguish the living from the dead? Trends Microbiol. 1996;4:453–455. doi: 10.1016/0966-842x(96)10067-6. [DOI] [PubMed] [Google Scholar]
- 95.Chen C-Y, Buchmeier N A, Libby S, Fang F C, Krause M, Guiney D G. Central regulatory role for the RpoS sigma factor in expression of Salmonella dublin plasmid virulence genes. J Bacteriol. 1995;177:5303–5309. doi: 10.1128/jb.177.18.5303-5309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cicchetti G, Maurer P, Wagener P, Kocks C. Actin and phosphoinositide binding by the ActA protein of the bacterial pathogen Listeria monocytogenes. J Biol Chem. 1999;274:33616–33626. doi: 10.1074/jbc.274.47.33616. [DOI] [PubMed] [Google Scholar]
- 97.Coconnier M-H, Dlissi E, Robard M, Laboisse C L, Gaillard J-L, Servin A L. Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O. Infect Immun. 1998;66:3673–3681. doi: 10.1128/iai.66.8.3673-3681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97a.Cohen P, Bouaboula M, Bellis M, Baron V, Jbilo O, Poinot-Chazel C, Galiegue S, Hadibi E H, Casellas P. Monitoring cellular responses to Listeria monocytogenes with oligonucleotide arrays. J Biol Chem. 2000;275:11181–11190. doi: 10.1074/jbc.275.15.11181. [DOI] [PubMed] [Google Scholar]
- 98.Collins M D, Wallbanks S, Lane D J, Shah J, Nietupski R, Smida J, Dorsch M, Stackebrandt E. Phylogenetic analysis of the genus Listeria based on reverse transcriptase sequencing of 16S rRNA. Int J Syst Bacteriol. 1991;41:240–246. doi: 10.1099/00207713-41-2-240. [DOI] [PubMed] [Google Scholar]
- 99.Conlan J C, North R J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conlan J W, North R J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conte M P, Longhi C, Polidoro M, Petrone G, Buonfiglio V, Di Santo S, Papi E, Seganti L, Visca P, Valenti P. Iron availability affects entry of Listeria monocytogenes into enterocytelike cell line Caco-2. Infect Immun. 1996;64:3925–3929. doi: 10.1128/iai.64.9.3925-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101a.Conte M P, Petrone G, Di Biase A M, Ammendolia M G, Superti F, Seganti L. Acid tolerance in listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb Pathog. 2000;29:137–144. doi: 10.1006/mpat.2000.0379. [DOI] [PubMed] [Google Scholar]
- 102.Conte M P, Petrone G, Longhi C, Valenti P, Morelli R, Superti F, Seganti L. The effects of inhibitors of vacuolar acidification on the release of Listeria monocytogenes from phagosomes of Caco-2 cells. J Med Microbiol. 1996;44:418–424. doi: 10.1099/00222615-44-6-418. [DOI] [PubMed] [Google Scholar]
- 103.Cordy D R, Osebold J W. The neuropathogenesis of Listeria encephalomyelitis in sheep and mice. J Infect Dis. 1959;104:164–173. doi: 10.1093/infdis/104.2.164. [DOI] [PubMed] [Google Scholar]
- 104.Cornell K A, Bouwer H G, Hinrichs D J, Barry R A. Genetic immunization of mice against Listeria monoctogenes using plasmid DNA encoding listeriolysin O. J Immunol. 1999;163:322–329. [PubMed] [Google Scholar]
- 105.Cossart P. Actin-based bacterial motility. Curr Opin Cell Biol. 1995;7:94–101. doi: 10.1016/0955-0674(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 105a.Cossart P. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell Microbiol. 2000;2:195–205. doi: 10.1046/j.1462-5822.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 105b.Cossart P, Jonquières R. Sortase, a universal target for therapeutic agents against Gram-positive bacteria? Proc Natl Acad Sci USA. 2000;97:5013–5015. doi: 10.1073/pnas.97.10.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cossart P, Lecuit M. Interaction of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cossart P, Mengaud J. Listeria monocytogenes, a model system for the molecular study of intracellular parasitism. Mol Biol Med. 1989;6:463–474. [PubMed] [Google Scholar]
- 108.Cossart P, Vincente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cotter P D, Emerson N, Gahan C G M, Hill C. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J Bacteriol. 1999;181:6840–6843. doi: 10.1128/jb.181.21.6840-6843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cottin J, Loiseau O, Robert R, Mahaza C, Carbonelle B, Senet J M. Surface Listeria monocytogenes carbohydrate-binding components revealed by agglutination with neoglycoproteins. FEMS Microbiol Lett. 1990;68:301–306. doi: 10.1016/s0378-1097(05)80058-8. [DOI] [PubMed] [Google Scholar]
- 111.Coulanges V, Andre P, Vidon D J. Effects of siderophores, catecholamines, and catechol compounds on Listeria spp. Growth in iron-complexed medium. Biochem Biophys Res Commun. 1998;249:526–530. doi: 10.1006/bbrc.1998.9184. [DOI] [PubMed] [Google Scholar]
- 112.Coulanges V, Andre P, Ziegler O, Buchheit L, Vidon D J. Utilization of iron-catecholamine complexes involving ferric reductase activity in Listeria monocytogenes. Infect Immun. 1997;65:2778–2785. doi: 10.1128/iai.65.7.2778-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112a.Cousens L P, Wing E J. Innate defenses in the liver during Listeria infection. Immunol Rev. 2000;174:150–159. doi: 10.1034/j.1600-0528.2002.017407.x. [DOI] [PubMed] [Google Scholar]
- 113.Cowart R E, Foster B G. The role of iron in the production of haemolysin by Listeria monocytogenes. Curr Microbiol. 1981;6:287–290. [Google Scholar]
- 114.Cowart R E, Lashmet J, McIntosh M E, Adams T J. Adherence of virulent strains of Listeria monocytogenes to the surface of hepatocarcinoma cell line via lectin-substrate interaction. Arch Microbiol. 1990;153:282–286. doi: 10.1007/BF00249083. [DOI] [PubMed] [Google Scholar]
- 115.Croize J, Arvieux J, Berche P, Colomb M G. Activation of the human complement alternative pathway by Listeria monocytogenes: evidence for direct binding and proteolysis of the C3 component on bacteria. Infect Immun. 1993;61:5134–5139. doi: 10.1128/iai.61.12.5134-5139.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cummins A J, Fielding A K, McLauchlin J. Listeria ivanovii infection in a patient with AIDS. J Infect. 1994;28:89–91. doi: 10.1016/s0163-4453(94)94347-8. [DOI] [PubMed] [Google Scholar]
- 117.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 119.Daniels J J, Autenrieth I B, Goebel W. Interaction of Listeria monocytogenes with the intestinal epithelium. FEMS Microbiol Lett. 2000;190:323–328. doi: 10.1111/j.1574-6968.2000.tb09306.x. [DOI] [PubMed] [Google Scholar]
- 120.Darji A, Bruder D, zur Lage S, Gerstel B, Chakraborty T, Wehland J, Weiss S. The role of the bacterial membrane protein ActA in immunity and protection against Listeria monocytogenes. J Immunol. 1998;161:2414–2420. [PubMed] [Google Scholar]
- 121.Darji A, Chakraborty T, Wehland J, Weiss S. Listeriolysin generates a route for the presentation of exogenous antigens by major histocompatibility complex class I. Eur J Immunol. 1995;25:2967–2971. doi: 10.1002/eji.1830251038. [DOI] [PubMed] [Google Scholar]
- 122.Darji A, Chakraborty T, Wehland J, Weiss S. TAP-dependent major histocompatibility complex class I presentation of soluble proteins using listeriolysin. Eur J Immunol. 1997;27:1353–1359. doi: 10.1002/eji.1830270609. [DOI] [PubMed] [Google Scholar]
- 123.Darji A, Niebuhr K, Hense M, Wehland J, Chakraborty T, Weiss S. Neutralizing monoclonal antibodies against listeriolysin: mapping of epitopes involved in pore formation. Infect Immun. 1996;64:2356–2358. doi: 10.1128/iai.64.6.2356-2358.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Datta A R, Kothary M H. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:3495–3497. doi: 10.1128/aem.59.10.3495-3497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daugelat S, Kaufmann S H E. Role of Th1 and Th2 cells in bacterial infections. Chem Immunol. 1996;63:66–97. [PubMed] [Google Scholar]
- 126.David V, Gouin E, Troys M V, Grogan A, Segal A W, Ampe C, Cossart P. Identification of cofilin, coronin, Rac and CapZ in actin tails using a Listeria affinity approach. J Cell Sci. 1998;111:2877–2884. doi: 10.1242/jcs.111.19.2877. [DOI] [PubMed] [Google Scholar]
- 127.Davies W A. Kinetics of killing Listeria monocytogenes by macrophages: rapid killing accompanying phagocytosis. J Reticuloendothel Soc. 1983;34:131–141. [PubMed] [Google Scholar]
- 127a.Decatur A L, Portnoy D A. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi: 10.1126/science.290.5493.992. [DOI] [PubMed] [Google Scholar]
- 128.De Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Del Corral F, Buchanan R L, Bencivengo M M, Cooke P H. Quantitative comparison of selected virulence associated characteristics in food and clinical isolates of Listeria. J Food Protect. 1990;53:1003–1009. doi: 10.4315/0362-028X-53.12.1003. [DOI] [PubMed] [Google Scholar]
- 130.De los Toyos J R, Méndez F J, Aparicio J F, Vázquez F, García Suárez M M, Fleites A, Hardisson C, Morgan P J, Andrew P W, Mitchell T J. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect Immun. 1996;64:480–484. doi: 10.1128/iai.64.2.480-484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Demuth A, Chakraborty T, Krohne G, Goebel W. Mammalian cells transfected with the listeriolysin gene exhibit enhanced proliferation and focus formation. Infect Immun. 1994;62:5102–5111. doi: 10.1128/iai.62.11.5102-5111.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Demuth A, Goebel W, Beuscher H U, Kuhn M. Differential regulation of cytokine and cytokine receptor mRNA expression upon infection of bone marrow-derived macrophages with Listeria monocytogenes. Infect Immun. 1996;64:3475–3483. doi: 10.1128/iai.64.9.3475-3483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deneer H G, Healey V, Boychuk I. Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology. 1995;141:1985–1992. doi: 10.1099/13500872-141-8-1985. [DOI] [PubMed] [Google Scholar]
- 134.Dennis S M. Perinatal lamb mortality in Western Australia. 6. Listeric infection. Aust Vet J. 1975;51:75–79. doi: 10.1111/j.1751-0813.1975.tb09409.x. [DOI] [PubMed] [Google Scholar]
- 135.Dhar G, Faull K F, Schneewind O. Anchor structure of cell wall surface proteins in Listeria monocytogenes. Biochemistry. 2000;39:3725–3733. doi: 10.1021/bi992347o. [DOI] [PubMed] [Google Scholar]
- 136.Dickneite C, Böckmann R, Spory A, Goebel W, Sokolovic Z. Differential interaction of the transcription factor PrfA and the PrfA-activating factor (Paf) of Listeria monocytogenes with target sequences. Mol Microbiol. 1998;27:915–928. doi: 10.1046/j.1365-2958.1998.00736.x. [DOI] [PubMed] [Google Scholar]
- 137.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, Kaufmann S H E, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 138.Dijkstra R G. Listeriosis in animals: clinical signs, diagnosis and treatment. In: Schönberg A, editor. Listeriosis: Joint WHO/ROI consultation on prevention and control. VetMed Hefte 5/1987. Berlin, Germany: Institut für Veterinärmedizin des Bundesgesundheitsamtes; 1987. pp. 68–76. [Google Scholar]
- 139.Dold F G, Sanger J M, Sanger J W. Intact alpha-actinin molecules are needed for both the assembly of actin into tails and the locomotion of Listeria monocytogenes inside infected cells. Cell Motil Cytoskelet. 1994;28:97–107. doi: 10.1002/cm.970280202. [DOI] [PubMed] [Google Scholar]
- 140.Domann E, Leimeister-Wächter M, Goebel W, Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991;59:65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Domann E, Wehland J, Niebuhr K, Haffner C, Leimeister-Wächter M, Chakraborty T. Detection of a prfA-independent promoter responsible for listeriolysin gene expression in mutant Listeria monocytogenes strains lacking the PrfA regulator. Infect Immun. 1993;61:3073–3075. doi: 10.1128/iai.61.7.3073-3075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wächter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Domann E, Zechel S, Lingnau A, Hain T, Darji A, Nichterlein T, Wehland J, Chakraborty T. Identification and characterization of a novel PrfA-regulated gene in Listeria monocytogenes whose product, IrpA, is highly homologous to internalin proteins, which contain leucine-rich repeats. Infect Immun. 1997;65:101–109. doi: 10.1128/iai.65.1.101-109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reference deleted.
- 145.Donnelly C W. Listeria monocytogenes. In: Hui Y H, Gorham J R, Murrell K D, Cliver D O, editors. Foodborne disease handbook: diseases caused by bacteria. Vol. 1. New York, N.Y: Marcel Dekker; 1994. pp. 215–252. [Google Scholar]
- 146.Dons L, Weclewicz K, Jin Y, Bindseil E, Olsen J E, Kristenson K. Rat dorsal root ganglia neurons as a model for Listeria monocytogenes infections in culture. Med Microbiol Immunol. 1999;188:15–21. doi: 10.1007/s004300050100. [DOI] [PubMed] [Google Scholar]
- 147.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 148.Dramsi S, Kocks C, Forestier C, Cossart P. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator PrfA. Mol Microbiol. 1993;9:931–941. doi: 10.1111/j.1365-2958.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 149.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires the expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 150.Dramsi S, Dehoux P, Lebrun M, Goossens P L, Cossart P. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect Immun. 1997;65:1615–1625. doi: 10.1128/iai.65.5.1615-1625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dramsi S, Lévi S, Thriller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun. 1998;66:4461–4468. doi: 10.1128/iai.66.9.4461-4468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drevets D A. Listeria monocytogenes infection of cultured endothelial cells stimulates neutrophil adhesion and adhesion molecule expression. J Immunol. 1997;158:5305–5313. [PubMed] [Google Scholar]
- 153.Drevets D A. Listeria monocytogenes virulence factors that stimulate endothelial cells. Infect Immun. 1998;66:232–238. doi: 10.1128/iai.66.1.232-238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drevets D A. Dissemination of Listeria monocytogenes by infected phgagocytes. Infect Immun. 1999;67:3512–3517. doi: 10.1128/iai.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Drevets D A, Campbell P A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991;59:2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Drevets D A, Leenen P J M, Campbell P A. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol. 1993;151:5431–5439. [PubMed] [Google Scholar]
- 157.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dubail I, Berche P, Charbit A. Listeriolysin O as a reporter to identify constitutive and in vivo-inducible promoters in the pathogen Listeria monocytogenes. Infect Immun. 2000;68:3242–3250. doi: 10.1128/iai.68.6.3242-3250.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dumont J, Cotoni L. Bacille semblable à celui du rouget du porc rencontré dans le L.C.R. d'un méningitique. Ann Inst Pasteur. 1921;35:625–633. [Google Scholar]
- 160.Dunne D W, Resnick D, Greenberg J, Krieger M, Joiner K A. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 162.Ebel F, von Eichel-Streiber C, Wehland J, Chakraborty T. Entry and intracellular motility of Listeria monocytogenes are not dependent on the small GTP-binding proteins of the Rho- and Rac-subfamilies. FEMS Microbiol Lett. 1999;176:117–124. doi: 10.1111/j.1574-6968.1999.tb13651.x. [DOI] [PubMed] [Google Scholar]
- 163.Edelson B T, Cossart P, Unanue E R. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol. 1999;15:163. :4087–4090. [PubMed] [Google Scholar]
- 164.Egile C, Loisel T P, Laurent V, Li R, Pantaloni D, Sansonetti P J, Carlier M F. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elsner H-A, Sobottka I, Bubert A, Albrecht H, Laufs R, Mack D. Catalase-negative Listeria monocytogenes causing lethal sepsis and meningitis in an adult hematologic patient. Eur J Clin Microbiol Infect Dis. 1996;15:965–966. doi: 10.1007/BF01690521. [DOI] [PubMed] [Google Scholar]
- 166.Emoto Y, Emoto M, Kaufmann S H E. Transient control of interleukin-4-producing natural killer T cells in the livers of Listeria monocytogenes-infected mice by interleukin-12. Infect Immun. 1997;65:5003–5009. doi: 10.1128/iai.65.12.5003-5009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Engelbrecht F, Chun S-K, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovic Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 168.Engelbrecht F, Dickneite C, Lampidis R, Götz M, DasGupta U, Goebel W. Sequence comparison of the chromosomal regions encompassing the internalin C genes (inlC) of Listeria monocytogenes and Listeria ivanovii. Mol Gen Genet. 1998;257:186–197. doi: 10.1007/s004380050638. [DOI] [PubMed] [Google Scholar]
- 169.Engelbrecht F, Dominguez-Bernal G, Dickneite C, Hess J, Greiffenberg L, Lampidis R, Raffelsbauer D, Kaufmann S H E, Kreft J, Vazquez-Boland J-A, Goebel W. A novel PrfA-regulated chromosomal locus of Listeria ivanovii encoding two small, secreted internalins is essential for virulence in mice. Mol Microbiol. 1998;30:405–417. doi: 10.1046/j.1365-2958.1998.01076.x. [DOI] [PubMed] [Google Scholar]
- 170.Reference deleted.
- 171.Ermolaeva S, Varfolomeeva N, Belyi Y, Tartakovskii I. Isolation and characterization of a Listeria monocytogenes mutant strain hyperproducing virulence factors. FEMS Microbiol Lett. 1997;150:189–195. doi: 10.1016/s0378-1097(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 172.Espaze E P, Rocourt J, Courtieu A L. La listériose en France en 1989. Étude à partir des souches adressées au centre national de référence. Bull Epidémiol Hebdom. 1991;3:9–10. [Google Scholar]
- 173.Evans J R, Allen A C, Bortolussi R, Issekutz T B, Stinson D A. Follow-up study of survivors of fetal and early-onset neonatal listeriosis. Clin Investig Med. 1984;7:329–334. [PubMed] [Google Scholar]
- 174.Facinelli B, Giovanetti E, Magi G, Biavasco F, Varaldo P E. Lectin reactivity and virulence among strains of Listeria monocytogenes determined in vitro using enterocyte-like cell line Caco-2. Microbiol. 1998;144:109–118. doi: 10.1099/00221287-144-1-109. [DOI] [PubMed] [Google Scholar]
- 175.Fang F C. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Farber J M, Peterkin P I, Carter A O, Varughese P V, Ashton F E, Ewan E P. Neonatal listeriosis due to cross-infection confirmed by isoenzyme typing and DNA fingerprinting. J Infect Dis. 1991;163:927–928. doi: 10.1093/infdis/163.4.927. [DOI] [PubMed] [Google Scholar]
- 178.Fenlon D R. Listeria monocytogenes in the natural environment. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 21–37. [Google Scholar]
- 179.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface protein from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 180.Fleming D W, Cochi M D, MacDonald K L, Brondum J, Hayes P S, Plikaytis B D, Holmes M B, Audurier A, Broome C V, Reingold A L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 181.Fleming S D, Campbell P A. Some macrophages kill Listeria monocytogenes while others do not. Immunol Rev. 1997;158:69–77. doi: 10.1111/j.1600-065x.1997.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 182.Francis M S, Thomas C J. Mutants in the CtpA copper transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microb Pathog. 1997;22:67–78. doi: 10.1006/mpat.1996.0092. [DOI] [PubMed] [Google Scholar]
- 183.Frankel F R, Hedge S, Lieberman J, Paterson Y. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 gag protein by using Listeria monocytogenes as a live vaccine vector. J Immunol. 1995;155:4775–4782. [PubMed] [Google Scholar]
- 184.Franzon V L, Arondel J, Sansonetti P J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990;58:529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Freitag N E, Jacobs K E. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aquorea victoria. Infect Immun. 1999;67:1844–1852. doi: 10.1128/iai.67.4.1844-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Freitag N E, Portnoy D A. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol Microbiol. 1994;12:845–853. doi: 10.1111/j.1365-2958.1994.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 187.Freitag N E, Rong L, Portnoy D A. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect Immun. 1993;61:2537–2544. doi: 10.1128/iai.61.6.2537-2544.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Freitag N E, Youngman P, Portnoy D A. Transcriptional activation of the Listeria monocytogenes hemolysin gene in Bacillus subtilis. J Bacteriol. 1992;174:1293–1298. doi: 10.1128/jb.174.4.1293-1298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Friederich E, Gouin E, Hellio R, Kocks C, Cossart P, Louvard D. Targeting of Listeria monocytogenes to the plasma membran as a tool to dissect both actin-based cell morphogenesis and ActA function. EMBO J. 1995;14:2731–2744. doi: 10.1002/j.1460-2075.1995.tb07274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 191.Füzi M, Pillis I. Production of opacity in egg-yolk medium by Listeria monocytogenes. Nature. 1962;13:195. [Google Scholar]
- 192.Gahan C G, Hill C. The use of listeriolysin to identify in vivo induced genes in the gram-positive intracellular pathogen Listeria monocytogenes. Mol Microbiol. 2000;36:498–507. doi: 10.1046/j.1365-2958.2000.01869.x. [DOI] [PubMed] [Google Scholar]
- 193.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 194.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gaillard J-L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gaillard J-L, Finlay B B. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1996;64:1299–1308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gaillard J-L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gaillot O, Pellegrini E, Bregenholt S, Nair S, Berche P. The serine-protease ClpP is essential for the intracellular parasitism of the pathogen Listeria monocytogenes. Mol Microbiol. 2000;35:1286–1294. doi: 10.1046/j.1365-2958.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 199.Gallaguer P G, Watakunakorn C. Listeria monocytogenes endocarditis: a review of the literature 1950–1986. Scand J Infect Dis. 1988;20:359–368. doi: 10.3109/00365548809032469. [DOI] [PubMed] [Google Scholar]
- 200.Gandhi A J, Perussia B, Goldfine H. Listeria monocytogenes phosphatidylinositol (PI)-specific phospholipase C has low activity on glycosyl-PI-anchored proteins. J Bacteriol. 1993;175:8014–8017. doi: 10.1128/jb.175.24.8014-8017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gauto A R, Cone L A, Woodward D R, Mahler R J, Lynch R D, Stolzman D H. Arterial infections due to Listeria monocytogenes: report of four cases and review of the world literature. Clin Infect Dis. 1992;14:23–28. doi: 10.1093/clinids/14.1.23. [DOI] [PubMed] [Google Scholar]
- 202.Gedde M M, Higgins D E, Tilney L G, Portnoy D A. Role of listeriolysin o in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Geginat G, Lalic M, Kretschmar M, Goebel W, Hof H, Palm D, Bubert A. Th1 cells specific for a secreted protein of Listeria monocytogenes are protective in vivo. J Immunol. 1998;160:6046–6055. [PubMed] [Google Scholar]
- 204.Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Mülthaler M, Goebel W, Bubert A. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J Immunol. 1999;162:4781–4789. [PubMed] [Google Scholar]
- 205.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 206.Geoffroy C, Raveneau J, Beretti J-L, Lecroisey A, Vazquez-Boland J-A, Alouf J E, Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991;59:2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geoffroy C, Gaillard J-L, Alouf J E, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Geoffroy C, Gaillard J-L, Alouf J E, Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J Gen Microbiol. 1989;135:481–487. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- 209.Gerstel B, Gröbe L, Pistor S, Chakraborty T, Wehland J. The ActA polypeptides of Listeria ivanovii and Listeria monocytogenes harbor related binding sites for host microfilament proteins. Infect Immun. 1996;64:1929–1936. doi: 10.1128/iai.64.6.1929-1936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gertler F B, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 211.Gholizadeh Y, Juvin M, Beretti J-L, Berche P, Gaillard J-L. Culture-negative listeriosis of the central nervous system diagnosed by detection of antibodies to listeriolysin O. Eur J Clin Microbiol Infect Dis. 1997;16:176–178. doi: 10.1007/BF01709483. [DOI] [PubMed] [Google Scholar]
- 212.Gholizadeh Y, Poyart C, Juvin M, Beretti J-L, Croizé J, Berche P, Gaillard J-L. Serodiagnosis of listeriosis based upon detection of antibodies against recombinant truncated forms of listeriolysin O. J Clin Microbiol. 1996;34:1391–1395. doi: 10.1128/jcm.34.6.1391-1395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gilbert R J, Rossjohn J, Parker M W, Tweten R K, Morgan P J, Mitchell T J, Errington N, Rowe A J, Andrew P W, Byron O. Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. J Mol Biol. 1998;284:1223–1237. doi: 10.1006/jmbi.1998.2258. [DOI] [PubMed] [Google Scholar]
- 214.Gilbert R J C, Jiménez J L, Chen S, Tickel I J, Rossjohn J, Parker M, Andrew P W, Saibil H R. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell. 1999;97:647–655. doi: 10.1016/s0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 215.Gill D A. “Circling” disease: a meningoencephalitis of sheep in New Zealand. Vet J. 1933;89:258–270. [Google Scholar]
- 216.Gill D A. Ovine bacterial encephalitis (circling disease) and the bacterial genus Listerella. Aust Vet J. 1937;13:46–56. [Google Scholar]
- 217.Gilot P, André P, Content J. Listeria monocytogenes possesses adhesins for fibronectin. Infect Immun. 1999;67:6698–6701. doi: 10.1128/iai.67.12.6698-6701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Girard K F, Sbarra A J, Bardawil W A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gitter M. A changing pattern of ovine listeriosis in Gt. Britain. In: Courtieu A L, Espaze E P, Reynaud A E, editors. Listeria-listeriosis 1985–1986. Nantes, France: University of Nantes; 1986. pp. 294–299. [Google Scholar]
- 220.Goebel W, Kreft J. Cytolysins and the intracellular life of bacteria. Trends Microbiol. 1997;5:86–88. doi: 10.1016/S0966-842X(96)30044-9. [DOI] [PubMed] [Google Scholar]
- 221.Goebel W, Kreft J, Böckmann R. Regulation of virulence genes in pathogenic Listeria. In: Fischetti V A, et al., editors. Gram-positive pathogens. Washington, D.C.: American Society for Microbiology; 2000. pp. 499–506. [Google Scholar]
- 222.Goldfine H, Knob C. Purification and characterization of Listeria monocytogenes phosphatidylinositol-specific phospholipase C. Infect Immun. 1992;60:4059–4067. doi: 10.1128/iai.60.10.4059-4067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Goldfine H, Johnston N C, Knob C. Nonspecific phospholipase C of Listeria monocytogenes: activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J Bacteriol. 1993;175:4298–4306. doi: 10.1128/jb.175.14.4298-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Golsteyn R M, Beckerle M C, Koay T, Friederich E. Structural and functional similarities between the human cytoskeletal protein zyxin and the ActA protein of Listeria monocytogenes. J Cell Sci. 1997;110:1893–1906. doi: 10.1242/jcs.110.16.1893. [DOI] [PubMed] [Google Scholar]
- 225.González-Zorn B, Domínguez-Bernal G, Suárez M, Ripio M-T, Vega Y, Novella S, Rodriguez A, Chico I, Tierrez A, Vázquez-Boland J-A. SmcL, a novel membrane-damaging virulence factor in Listeria. Int J Med Microbiol Infect Dis. 2000;290:369–374. doi: 10.1016/S1438-4221(00)80044-2. [DOI] [PubMed] [Google Scholar]
- 226.González-Zorn B, Dominguez-Bernal G, Suarez M, Ripio M T, Vega Y, Novella S, Vázquez-Boland J-A. The smcL gene of Listeria ivanovii encodes a sphingomyelinase C that mediates bacterial escape from the phagocytic vacuole. Mol Microbiol. 1999;33:510–523. doi: 10.1046/j.1365-2958.1999.01486.x. [DOI] [PubMed] [Google Scholar]
- 227.Goossens P L, Milon G, Cossart P, Saron M F. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int Immunol. 1995;7:797–805. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 228.Gormley E, Mengaud J, Cossart P. Sequences homologous to the listeriolysin O gene region of Listeria monocytogenes are present in virulent and avirulent haemolytic species of the genus Listeria. Res Microbiol. 1989;140:631–643. doi: 10.1016/0923-2508(89)90195-2. [DOI] [PubMed] [Google Scholar]
- 229.Gottesmann S, Wickner S, Maurizi M R. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 230.Gouin E, Dehoux P, Mengaud J, Kocks C, Cossart P. iactA of Listeria ivanovii, although distantly related to Listeria monocytogenes actA, restores actin tail formation in an L. monocytogenes actA mutant. Infect Immun. 1995;63:2729–2737. doi: 10.1128/iai.63.7.2729-2737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti P J, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 232.Gouin E, Mengaud J, Cossart P. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanoii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infect Immun. 1994;62:3550–3553. doi: 10.1128/iai.62.8.3550-3553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Goulet V, Lepoutre A, Rocourt J, Courtieu A-L, Dehaumont P, Veit P. Épidémie de listériose en France. Bilan final et résultats de l'enquète épidémiologique. Bull Épidemiol Hebdom. 1993;4/1993:13–14. [Google Scholar]
- 234.Goulet V, Brohier S. Listeriosis in France in 1986: survey of hospital laboratories. Pathol Biol. 1989;37:206–211. [PubMed] [Google Scholar]
- 235.Goulet V, Le Magny F, Rebiere I, Espaze E P. La listériose en France en 1987. Etude rétrospective à partir d'un échantillon d'hôpitaux publics. Bull Epidémiol Hebdom. 1989;12:45–46. [Google Scholar]
- 236.Graham T, Goldsteyn-Thomas E J, Gannon V P J, Thomas J E. Genus-and species-specific detection of Listeria monocytogenes using polymerase chain reaction assays targeting the 16S/23S intergenic spacer region of the rRNA operon. Can J Microbiol. 1996;42:1155–1162. doi: 10.1139/m96-147. [DOI] [PubMed] [Google Scholar]
- 237.Gravani R. Incidence and control of Listeria in food-processing facilities. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 657–709. [Google Scholar]
- 238.Gray M L, Killinger A M. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:304–328. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gray M L, Singh C, Thorp F., Jr Abortion, stillbirth, early death of young rabbits by Listeria monocytogenes. II. Oral exposure. Proc Soc Exp Biol Med. 1955;89:169–175. doi: 10.3181/00379727-89-21747. [DOI] [PubMed] [Google Scholar]
- 240.Greenberg J W, Fischer W, Joiner K A. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect Immun. 1996;64:3318–3325. doi: 10.1128/iai.64.8.3318-3325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gregory S H, Barczynski L K, Wing E J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infections. J Leukocyte Biol. 1992;51:421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- 241a.Gregory S H, Liu C C. CD8+ T-cell-mediated response to Listeria monocytogenes taken up in the liver and replicating within hepatocytes. Immunol Rev. 2000;174:112–122. doi: 10.1034/j.1600-0528.2002.017405.x. [DOI] [PubMed] [Google Scholar]
- 242.Gregory S H, Sagnimeni A J, Wing E J. Expression of the inlAB operon by Listeria monocytogenes is not required for entry into hepatic cell s in vivo. Infect Immun. 1996;64:3983–3986. doi: 10.1128/iai.64.10.3983-3986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gregory S H, Wing E J. Accessory function of Kupffer cells in the antigen-specific blastogenic response of an L3T4+ T-lymphocyte clone to Listeria monocytogenes. Infect Immun. 1990;58:2313–2319. doi: 10.1128/iai.58.7.2313-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gregory S H, Wing E J. IFN-γ inhibits the replication of Listeria monocytogenes in hepatocytes. J Immunol. 1993;151:1401–1409. [PubMed] [Google Scholar]
- 245.Greiffenberg L, Goebel W, Kim K S, Daniels J, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: an electron microscopic study. Infect Immun. 2000;68:3275–3279. doi: 10.1128/iai.68.6.3275-3279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Greiffenberg L, Sokolovic Z, Schnittler H-J, Spory A, Böckmann R, Goebel W, Kuhn M. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol Lett. 1997;157:163–170. doi: 10.1111/j.1574-6968.1997.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 248.Grenningloh R, Darji A, Wehland J, Chakraborty T, Weiss S. Listeriolysin and IrpA are major protein targets of the human humoral reponse against Listeria monocytogenes. Infect Immun. 1998;65:3976–3980. doi: 10.1128/iai.65.9.3976-3980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Groisman E A, Ochman H. Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87:791–794. doi: 10.1016/s0092-8674(00)81985-6. [DOI] [PubMed] [Google Scholar]
- 250.Groves R D, Welshimer H J. Separation of pathogenic from apathogenic Listeria monocytogenes by three in vitro reactions. J Clin Microbiol. 1977;5:559–563. doi: 10.1128/jcm.5.6.559-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Guiney D G, Libby S, Fang F C, Krause M, Fierer J. Growth-phase regulation of plasmid virulence genes in Salmonella. Trends Microbiol. 1995;3:275–279. doi: 10.1016/s0966-842x(00)88944-1. [DOI] [PubMed] [Google Scholar]
- 251a.Guleria I, Pollard J W. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 252.Guzman C A, Domann E, Rhode M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 253.Guzman C A, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K N. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Guzman C A, Weiss S, Chakraborty T. Listeria monocytogenes—a promising vaccine carrier to evoke cellular immune responses. In: Pozzi G, Wells J, editors. Gram-positive bacteria as vaccine vehicles for mucosal immunization. Austin, Tex: Landes Biosciences; 1997. pp. 145–173. [Google Scholar]
- 255.Haak-Frendscho M, Czuprynski C J. Use of recombinant interleukin-2 to enchance adoptive transfer of resistance to Listeria monocytogenes infection. Infect Immun. 1992;60:1406–1414. doi: 10.1128/iai.60.4.1406-1414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Haas A, Brehm K. Superoxide dismutases and catalases-biochemistry, molecular biology and some biomedical aspects. Genet Eng Biotechnol. 1993;13:243–269. [Google Scholar]
- 257.Haas A, Dumbsky M, Kreft J. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim Biophys Acta. 1992;1130:81–84. doi: 10.1016/0167-4781(92)90466-d. [DOI] [PubMed] [Google Scholar]
- 258.Haas A, Goebel W. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radical Res Commun. 1992;16:137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- 259.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 260.Hacker J, Kaper J. The concept of pathogenicity islands. In: Kaper J, Hacker J, editors. Pathogenicity islands of virulent bacteria: structure, function, and impact on microbial evolution. Washington, D.C.: American Society for Microbiology; 2000. pp. 1–12. [Google Scholar]
- 261.Hanawa T, Yamamoto T, Kamiya S. Listeria monocytogenes can grow in macrophages without the aid of proteins induced by environmental stresses. Infect Immun. 1995;63:4595–4599. doi: 10.1128/iai.63.12.4595-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Haponsaph R, Czuprynski C J. Inhibition of the multiplication of Listeria monocytogenes in a murine hepatocyte cell line (ATCC TIB73) by IFN-gamma and TNF-alpha. Microb Pathog. 1996;20:287–295. doi: 10.1006/mpat.1996.0027. [DOI] [PubMed] [Google Scholar]
- 263.Hartford T, O'Brien S, Andrew P W, Jones D, Roberts I S. Utilization of transferrin-bound iron by Listeria monocytogenes. FEMS Microbiol Lett. 1993;108:311–318. doi: 10.1111/j.1574-6968.1993.tb06121.x. [DOI] [PubMed] [Google Scholar]
- 264.Hartman A B, Venkatesan M, Oaks E V, Buysse J M. Sequence and molecular characterization of a multicopy invasion plasmid antigene, ipaH, of Shigella flexneri. J Bacteriol. 1990;172:1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1540. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Harty J T, Lenz L L, Bevan M J. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–5330. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Harty J T, Pamer E G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes. J Immunol. 1995;154:4642–4650. [PubMed] [Google Scholar]
- 268.Harty J T, Schreiber R D, Bevan M J. CD8 T cells can protect against an intracellular bacterium in an interferon γ-independent fashion. Proc Natl Acad Sci USA. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Harvey P C, Faber J E. Some biochemical reactions of the Listerella group. J Bacteriol. 1941;41:45–46. doi: 10.1128/jb.42.5.677-687.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hashimoto S, Nomoto K, Yokokura T. The role of superoxide anion and lysosomal enzymes in anti-listerial activity of elicited peritoneal macrophages. Scand J Immunol. 1986;24:429–436. doi: 10.1111/j.1365-3083.1986.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 271.Hauf N, Goebel W, Fiedler F, Kuhn M. Listeria monocytogenes infection of Caco-2 epithelial cells induces activation of transcription factor NF-κB/Rel-like DNA binding activities. FEMS Microbiol Lett. 1999;178:117–122. doi: 10.1111/j.1574-6968.1999.tb13766.x. [DOI] [PubMed] [Google Scholar]
- 272.Hauf N, Goebel W, Fiedler F, Sokolovic Z, Kuhn M. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc Natl Acad Sci USA. 1997;94:9394–9399. doi: 10.1073/pnas.94.17.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hauf N, Goebel W, Serfling E, Kuhn M. Listeria monocytogenes infection enhances transcription factor NF-κB in P388D1 macrophage-like cells. Infect Immun. 1994;62:2740–2747. doi: 10.1128/iai.62.7.2740-2747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Havell E A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986;54:787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Havell E A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989;143:2894–2899. [PubMed] [Google Scholar]
- 276.Havell E A, Sehgal P B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991;146:756–761. [PubMed] [Google Scholar]
- 277.Heffron S, Moe G R, Sieber V, Mengaud J, Cossart P, Vitali J, Jurnak F. Sequence profile of the parallel beta helix in the pectate lyase superfamily. J Struct Biol. 1998;122:223–235. doi: 10.1006/jsbi.1998.3978. [DOI] [PubMed] [Google Scholar]
- 278.Heinzen R A, Grieshaber S S, Van Kirk L S, Devin C J. Dynamics of actin-based movement by Rickettsia rickettsii in vero cells. Infect Immun. 1999;67:4201–4207. doi: 10.1128/iai.67.8.4201-4207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hess J, Dreher A, Gentschev I, Goebel W, Ladel C, Miko D, Kaufmann S H. Protein p60 participates in intestinal host invasion by Listeria monocytogenes. Zentralbl Bakteriol. 1996;284:263–272. doi: 10.1016/s0934-8840(96)80102-2. [DOI] [PubMed] [Google Scholar]
- 280.Hess J, Dietrich G, Gentschev I, Miko D, Goebel W, Kaufmann S H. Protection against murine listeriosis by an attenuated recombinant Salmonella typhimurium vaccine strain that secretes the naturally somatic antigen superoxide dismutase. Infect Immun. 1997;65:1286–1292. doi: 10.1128/iai.65.4.1286-1292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hess J, Gentschev I, Miko D, Welzel M, Ladel C, Goebel W, Kaufmann S H. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci USA. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hess J, Gentschev I, Szalay G, Ladel C, Bubert A, Goebel W, Kaufmann S H E. Listeria monocytogenes p60 supports host cell invasion by and in vivo survival of attenuated Salmonella typhimurium. Infect Immun. 1995;63:2047–2053. doi: 10.1128/iai.63.5.2047-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Heymer B, Wirsing von König C H, Finger H, Hof H, Emmerling P. Histomorphology of experimental listeriosis. Infection. 1988;16:S106–S111. doi: 10.1007/BF01639731. [DOI] [PubMed] [Google Scholar]
- 283a.Hiemstra P S, van den Barselaar M T, Roest M, Nibbering P H, van Furth R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J Leukocyte Biol. 1999;66:423–428. doi: 10.1002/jlb.66.3.423. [DOI] [PubMed] [Google Scholar]
- 284.Hitbold E M, Ziegler H K. Mechanisms of processing and presentation of the antigens of Listeria monocytogenes. Infect Agents Dis. 1994;2:314–323. [PubMed] [Google Scholar]
- 285.Hitbold E M, Safley S A, Ziegler H K. The presentation of classI and classII epitopes of listeriolysin O is regulaged by intracellular localization and by intercellular spread of Listeria monocytogenes. J Immunol. 1996;157:1163–1175. [PubMed] [Google Scholar]
- 286.Ho J L, Shands K N, Friedland G, Eckind P, Fraser D W. An outbreak of type 4b Listeria monocytogenes infection involving patients from eight Boston hospitals. Arch Intern Med. 1986;146:520–524. [PubMed] [Google Scholar]
- 287.Hodgson D A. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol Microbiol. 2000;35:312–323. doi: 10.1046/j.1365-2958.2000.01643.x. [DOI] [PubMed] [Google Scholar]
- 288.Hof H. Virulence of different strains of Listeria monocytogenes serovar 1/2a. Med Microbiol Immunol. 1984;173:207–218. doi: 10.1007/BF02122112. [DOI] [PubMed] [Google Scholar]
- 289.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 291.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the Gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 292.Huillet E, Larpin S, Pardon P, Berche P. Identification of a new locus in Listeria monocytogenes involved in cellobiose-dependent repression of hly expression. FEMS Microbiol Lett. 1999;174:265–272. doi: 10.1111/j.1574-6968.1999.tb13578.x. [DOI] [PubMed] [Google Scholar]
- 293.Ikonomidis G, Portnoy D A, Gerhard W, Paterson Y. Influenza-specific immunity induced by recombinant Listeria monocytogenes vaccines. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 294.Inoue S, Itagaki S-I, Amano F. Intracellular killing of Listeria monocytogenes in the J774.1 macrophage-like cell line and the lipopolysaccharide (LPS)-resistant mutant LPS1916 cell line defective in the generation of reactive oxygen intermediates after LPS treatment. Infect Immun. 1995;63:1876–1886. doi: 10.1128/iai.63.5.1876-1886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ireton K, Cossart P. Host-pathogen interactions during entry and actin-based movement of Listeria monocytogenes. Annu Rev Genet. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]
- 296.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 297.Ireton K, Payrastre B, Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J Biol Chem. 1999;274:17025–17032. doi: 10.1074/jbc.274.24.17025. [DOI] [PubMed] [Google Scholar]
- 298.Irvine A S, Guest J R. Lactobacillus casei contains a member of the CRP-FNR family. Nucleic Acids Res. 1993;21:753. doi: 10.1093/nar/21.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ivanov I. Untersuchungen über die Listeriose der Schaffe in Bulgarien Monatsh. Vet Med. 1962;17:729–736. [Google Scholar]
- 300.Jacobs T, Darji A, Frahm N, Rohde M, Wehland J, Chakraborty T, Weiss S. Listeriolysin O: cholesterol inhibits cytolysis but not binding to cellular membranes. Mol Microbiol. 1998;28:1081–1089. doi: 10.1046/j.1365-2958.1998.00858.x. [DOI] [PubMed] [Google Scholar]
- 301.Jacquet C, Miegeville A-F, Catimel B, Huyn G, Courtieu A-L, Rocourt J. La listériose humaine en France en 1991, 1992 et 1993. Bull Epidémiol Hebdom. 1994;28:123–125. [Google Scholar]
- 302.Jacquet C, Catimel B, Brosch R, Buchrieser C, Dehaumont P, Goulet V, Lepoutre A, Veit P, Rocourt J. Investigations related to the epidemic strain involved in the French listeriosis outbreak in 1992. Appl Environ Microbiol. 1995;61:2242–2246. doi: 10.1128/aem.61.6.2242-2246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jenkins E M, Njoku-Obi A N, Adams E W. Purification of the soluble hemolysin of Listeria monocytogenes. J Bacteriol. 1964;88:418–424. doi: 10.1128/jb.88.2.418-424.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jenkins E M, Watson B B. Serum protein changes: an activity of the extracellular hemolytic-lipolytic protein from Listeria monocytogenes. Am J Vet Res. 1969;30:669–677. [PubMed] [Google Scholar]
- 305.Jenkins E M, Watson B B. Extracellular antigens from Listeria monocytogenes. I. Purification and resolution of hemolytic and lipolytic antigens from culture filtrates of Listeria monocytogenes. Infect Immun. 1971;3:589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jensen A, Fredriksen W, Gerner-Smidt P. Risk factors for listeriosis in Denmark 1989–1990. Scand J Infect Dis. 1994;26:171–178. doi: 10.3109/00365549409011781. [DOI] [PubMed] [Google Scholar]
- 307.Jensen E R, Selvakumar R, Shen H, Ahmed R, Wettstein F O, Miller J F. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J Virol. 1997;71:8467–8474. doi: 10.1128/jvi.71.11.8467-8474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jensen V B, Harty J T, Jones B D. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer's patches. Infect Immun. 1998;66:3758–3766. doi: 10.1128/iai.66.8.3758-3766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Johansen T, Holm T, Guddal P H, Sletten K, Haugli F B, Little C. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C from Bacillus cereus. Gene. 1988;65:293–304. doi: 10.1016/0378-1119(88)90466-0. [DOI] [PubMed] [Google Scholar]
- 310.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jones S, Preiter K, Portnoy D A. Conversion of an extracellular cytolysin into a phagosome-specific lysin which supports the growth of an intracellular pathogen. Mol Microbiol. 1996;21:1219–1225. doi: 10.1046/j.1365-2958.1996.00074.x. [DOI] [PubMed] [Google Scholar]
- 312.Jones T C, Hunt R D. Veterinary pathology. 5th ed. Philadelphia, Pa: Lea and Febiger; 1983. pp. 631–634. [Google Scholar]
- 313.Jonquières R, Bierne H, Fiedler F, Gounon P, Cossart P. Interaction between the protein InlB of Listeria monocytogenes and lipotheicoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol Microbiol. 1999;34:902–914. doi: 10.1046/j.1365-2958.1999.01652.x. [DOI] [PubMed] [Google Scholar]
- 314.Jungherr E. Ovine encephalomyelitis associated with Listerella infection. J Am Vet Med Assoc. 1937;91:73–87. [Google Scholar]
- 315.Jungi T W, Pfister H, Sager H, Fatzer R, Vandevelde M, Zurbriggen A. Comparison of inducible nitric oxide synthase expression in the brains of Listeria monocytogenes-infected cattle, sheep, and goats and in macrophages stimulated in vitro. Infect Immun. 1997;65:5279–5288. doi: 10.1128/iai.65.12.5279-5288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jurado R L, Farley M M, Pereira E, Harvey R C, Schuchat A, Wenger J D, Stephens D S. Increased risk of meningitis and bacteremia due to Listeria monocytogenes in patients with human immunodeficiency virus infection. Clin Infect Dis. 1993;17:224–227. doi: 10.1093/clinids/17.2.224. [DOI] [PubMed] [Google Scholar]
- 317.Kajava A V. Structural diversity of leucine-rich repeat proteins. J Mol Biol. 1998;277:519–527. doi: 10.1006/jmbi.1998.1643. [DOI] [PubMed] [Google Scholar]
- 318.Kampelmacher E H, Van Noorle Jansen L. Listeriosis in humans and animals in the Netherlands (1958–1977) Zentbl Baktenol Hyg I Abt Orig A. 1980;246:211–227. [PubMed] [Google Scholar]
- 319.Kang F, Laine R O, Bubb M R, Southwick F S, Purich D L. Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): implications for actin-based Listeria motility. Biochemistry. 1997;36:8384–8392. doi: 10.1021/bi970065n. [DOI] [PubMed] [Google Scholar]
- 320.Karunasagar I, Krohne G, Goebel W. Listeria ivanovii is capable of cell-to-cell spread involving actin polymerization. Infect Immun. 1993;61:162–169. doi: 10.1128/iai.61.1.162-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Karunasagar I, Lampidis R, Goebel W, Kreft J. Complementation of Listeria seeligeri with the plcA-prfA genes from L. monocytogenes activates transcription of seeligerolysin and leads to bacterial escape from the phagosome of infected mammalian cells. FEMS Microbiol Lett. 1997;146:303–310. doi: 10.1111/j.1574-6968.1997.tb10209.x. [DOI] [PubMed] [Google Scholar]
- 322.Karunasagar I, Senghaas B, Krohne G, Goebel W. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect Immun. 1994;62:3554–3558. doi: 10.1128/iai.62.8.3554-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kathariou S, Pine L. The type strain(s) of Listeria monocytogenes: a source of continuing difficulties. Int J Syst Bacteriol. 1991;41:328–330. doi: 10.1099/00207713-41-2-328. [DOI] [PubMed] [Google Scholar]
- 325.Kathariou S, Pine L, George V, Carlone G M, Holloway B P. Nonhemolytic Listeria monocytogenes mutants that are also noninvasive for mammalian cells: evidence for coordinate regulation of virulence. Infect Immun. 1990;58:3988–3995. doi: 10.1128/iai.58.12.3988-3995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kathariou S, Rocourt J, Hof H, Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections in mice. Infect Immun. 1988;56:534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kaufmann S H, Hess J. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett. 1999;65:81–84. doi: 10.1016/s0165-2478(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 328.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 329.Kautter D A, Silverman S J, Roessler W G, Drawdy J F. Virulence of Listeria monocytogenes for experimental animals. J Infect Dis. 1963;112:167–180. doi: 10.1093/infdis/112.2.167. [DOI] [PubMed] [Google Scholar]
- 330.Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31:1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- 331.Reference deleted.
- 332.Khan M A, Seaman S, Woodbine W. Listeria monocytogenes haemolysin: lecithinase. Acta Microbiol Acad Sci Hung. 1972;19:341–352. [PubMed] [Google Scholar]
- 333.Khan M A, Seaman S, Woodbine W. The pathogenicity of Listeria monocytogenes. Zentbl Bakteriol Hyg 1 Abt Orig A. 1973;224:355–361. [PubMed] [Google Scholar]
- 334.Kingdom G C, Sword C P. Effects of Listeria monocytogenes hemolysin on phagocytic cells and lysosomes. Infect Immun. 1970;1:356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kirk J. Diagnostic ultrastructure of Listeria monocytogenes in human central nervous tissue. Ultrastruct Pathol. 1993;17:583–592. doi: 10.3109/01913129309027794. [DOI] [PubMed] [Google Scholar]
- 336.Klarsfeld A D, Goossens P L, Cossart P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 336a.Klatt E C, Pavlova Z, Teberg A J, Yonekura M L. Epidemic neonatal listeriosis at autopsy. Hum Pathol. 1986;17:1278–1281. doi: 10.1016/s0046-8177(86)80572-x. [DOI] [PubMed] [Google Scholar]
- 337.Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–421. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 338.Kocks C, Cossart P. Directional actin assembly by Listeria monocytogenes at the site of polar surface expression of the actA gene product involving the actin-bundling protein plastin (fimbrin) Infect Agents Dis. 1994;2:207–209. [PubMed] [Google Scholar]
- 339.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 340.Kocks C, Marchand J B, Gouin E, d'Hauteville H, Sansonetti P J, Carlier M-F, Cossart P. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol Microbiol. 1995;18:413–423. doi: 10.1111/j.1365-2958.1995.mmi_18030413.x. [DOI] [PubMed] [Google Scholar]
- 341.Kocks C, Hellio R, Gounon P, Ohayon H, Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993;105:699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- 342.Köhler S, Bubert A, Vogel M, Goebel W. Expression of the iap gene coding for protein p60 of Listeria monocytogenes is controlled on the posttranscriptional level. J Bacteriol. 1991;173:4668–4674. doi: 10.1128/jb.173.15.4668-4674.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Köhler S, Leimeister-Wächter M, Chakraborty T, Lottspeich F, Goebel W. The gene coding for protein p60 of Listeria monocytogenes and its use as a specific probe for Listeria monocytogenes. Infect Immun. 1990;58:1943–1950. doi: 10.1128/iai.58.6.1943-1950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 345.Kolb-Maurer A, Gentschev I, Fries H W, Fiedler F, Brocker E B, Kampgen E, Goebel W. Listeria monocytogenes-infected human dendritic cells: uptake and host cell response. Infect Immun. 2000;68:3680–3688. doi: 10.1128/iai.68.6.3680-3688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kolesnick R N, Krönke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 347.Kreft J, Bohne J, Gross R, Kestler H, Sokolovic Z, Goebel W. Control of Listeria monocytogenes virulence by the transcriptional regulator PrfA. In: Rappuoli R, Scarlato V, Arico B, editors. Signal tranduction and bacterial virulence. R. G. Austin, Tex: Landes Company; 1995. pp. 129–142. [Google Scholar]
- 348.Kreft J, Dumbsky M, Theiss S. The actin-polymerization protein from Listeria ivanovii is a large repeat protein which shows only limited amino acid sequence homology to ActA from Listeria monocytogenes. FEMS Microbiol Lett. 1995;126:113–122. doi: 10.1111/j.1574-6968.1995.tb07403.x. [DOI] [PubMed] [Google Scholar]
- 349.Kreft J, Funke D, Haas A, Lottspeich F, Goebel W. Production, purification and characterization of hemolysins from Listeria ivanovii and Listeria monocytogenes sv4b. FEMS Microbiol Lett. 1989;57:197–202. doi: 10.1111/j.1574-6968.1989.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 350.Kreft J, Vazquez-Boland J-A, Ng E, Goebel W. Virulence gene clusters and putative pathogenicity islands in Listeria. In: Kaper J, Hacker J, editors. Pathogenicity islands and other mobile genetic elements. Washington. D.C: American Society for Microbiology; 1999. pp. 219–232. [Google Scholar]
- 351.Krüll M, Nost R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J Immunol. 1997;159:1970–1976. [PubMed] [Google Scholar]
- 352.Kuhn M. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol Lett. 1998;60:87–90. doi: 10.1111/j.1574-6968.1998.tb12895.x. [DOI] [PubMed] [Google Scholar]
- 353.Kuhn M, Engelbrecht F, Sokolovic Z, Kügler S, Schüller S, Bubert A, Karunasagar I, Böckmann R, Hauf N, Demuth A, Kreft J, Goebel W. Interaction of intracellular bacteria with mammalian host cells and host cell responses. Nova Acta Leopold. 1997;301:207–221. [Google Scholar]
- 354.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kuhn M, Goebel W. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect Immun. 1994;62:348–356. doi: 10.1128/iai.62.2.348-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kuhn M, Goebel W. Responses by murine macrophages infected with Listeria monocytogenes crucial for the development of immunity to this pathogen. Immunol Rev. 1997;158:57–67. doi: 10.1111/j.1600-065x.1997.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 357.Kuhn M, Goebel W. Host cell signalling during Listeria monocytogenes infection. Trends Microbiol. 1998;6:11–15. doi: 10.1016/S0966-842X(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 358.Kuhn M, Goebel W. Internalization of Listeria monocytogenes by nonprofessional and professional phagocytes. Subcell Biochem. 2000;33:411–436. doi: 10.1007/978-1-4757-4580-1_16. [DOI] [PubMed] [Google Scholar]
- 359.Kuhn M, Kathariou S, Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988;56:79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Kuypers F A. Phospholipid asymmetry in health and disease. Curr Opin Hematol. 1998;5:122–131. doi: 10.1097/00062752-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 361.Laine R O, Phaneuf K L, Cunningham C C, Kwiatkowski D, Azuma T, Southwick F S. Gelsolin, a protein that caps the barbed ends and severs actin filaments, enhances the actin-based motility of Listeria monocytogenes in host cells. Infect Immun. 1998;66:3775–3782. doi: 10.1128/iai.66.8.3775-3782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lammerding A M, Doyle M P. Stability of Listeria monocytogenes to non-thermal processing conditions. In: Miller A J, Smith J L, Somkuti G A, editors. Foodborne listeriosis. New York, N.Y: Elsevier; 1990. pp. 195–202. [Google Scholar]
- 363.Reference deleted.
- 364.Lampidis R, Gross R, Sokolovic Z, Goebel W, Kreft J. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol Microbiol. 1994;13:141–151. doi: 10.1111/j.1365-2958.1994.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 365.Lasa I, David V, Gouin E, Goethals M, Vancompernolle K, David V, Vandekerckhove J, Cossart P. Identification of two regions in the N-terminal domain of ActA involved in the actin comet tail formation by Listeria monocytogenes. EMBO J. 1997;16:1531–1540. doi: 10.1093/emboj/16.7.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lasa I, David V, Gouin E, Marchand J-B, Cossart P. The amino-terminal part of ActA is critical for actin-based motility of Listeria monocytogenes; the central prolin-rich region acts as a stimulator. Mol Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- 366a.Lasa I, Dehoux P, Cossart P. Actin polymerization and bacterial movement. Biochim Biophys Acta. 1998;1402:217–228. doi: 10.1016/s0167-4889(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 367.Laurent V, Loisel T P, Harbeck B, Wehman A, Grobe L, Jockusch B M, Wehland J, Gertler F B, Carlier M F. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lavon I, Goldberg I, Amit S, Landssman L, Jung S, Tsuberi B Z, Barshack I, Kopolovic J, Galun E, Bujard H, Ben-Neriah Y. High susceptibility to bacterial infection, but no liver disfunction, in mice compromised for hepatocyte NF-kappaB activation. Nat Med. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 369.Leblond-Francillard M, Gaillard J-L, Berche P. Loss of catalase activity in Tn1545-induced mutants does not reduce growth of Listeria monocytogenes in vivo. Infect Immun. 1989;57:2569–2573. doi: 10.1128/iai.57.8.2569-2573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lebrun M, Audurier A, Cossart P. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are similar to cadA and cadC of Staphylococcus aureus and are induced by cadmium. J Bacteriol. 1994;176:3040–3048. doi: 10.1128/jb.176.10.3040-3048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lebrun M, Mengaud J, Ohayon H, Nato F, Cossart P. Internalin must be on the bacterial surface to mediate entry of Listeria monocytogenes into epithelial cells. Mol Microbiol. 1996;21:579–592. doi: 10.1111/j.1365-2958.1996.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 372.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372a.Lecuit M, Hurme R, Pizarro-Cerda J, Ohayon H, Geiger B, Cossart P. A role for alpha- and beta-catenins in bacterial uptake. Proc Natl Acad Sci USA. 2000;97:10008–10013. doi: 10.1073/pnas.97.18.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun. 1997;65:5309–5319. doi: 10.1128/iai.65.12.5309-5319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 375.Leighton I, Threlfall D R, Oakley C L. Phospholipase C activity in culture filtrates from Listeria monocytogenes. In: Woodbine M, editor. Problems of listeriosis. Leicester, England: Leicester University Press; 1975. pp. 239–241. [Google Scholar]
- 376.Leimeister-Wächter M, Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989;57:2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Leimeister-Wächter M, Domann E, Chakraborty T. Detection of a gene encoding a phospatidylinositol-specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991;5:361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 378.Leimeister-Wächter M, Domann E, Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol. 1992;174:947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Leimeister-Wächter M, Goebel W, Chakraborty T. Mutations affecting hemolysin production in Listeria monocytogenes located outside the listeriolysin gene. FEMS Microbiol Lett. 1989;65:23–30. doi: 10.1016/0378-1097(89)90360-1. [DOI] [PubMed] [Google Scholar]
- 380.Leimeister-Wächter M, Haffner C, Domann E, Goebel W, Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc Natl Acad Sci USA. 1990;87:8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Lepay D A, Nathan C F, Steinman R M, Murray H W, Cohn Z A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985;161:1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381a.Lety M-A, Frehel C, Dubail I, Beretti J-L, Kayal S, Berche P, Charbit A. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence in Listeria monocytogenes. Mol Microbiol. 2001;39:1124–1139. doi: 10.1111/j.1365-2958.2001.02281.x. [DOI] [PubMed] [Google Scholar]
- 382.L'hopital S, Marly J, Pardon P, Berche P. Kinetics of antibody production against listeriolysin O in sheep with listeriosis. J Clin Microbiol. 1993;31:1537–1540. doi: 10.1128/jcm.31.6.1537-1540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Lindsay J A, Ruzin A, Ross H F, Kurepina N, Novick R P. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 384.Lingnau A, Chakraborty T, Niebuhr K, Domann E, Wehland J. Identification and purification of novel internalin-related proteins in Listeria monocytogenes and Listeria ivanovii. Infect Immun. 1996;64:1002–1006. doi: 10.1128/iai.64.3.1002-1006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun. 1995;64:1002–1006. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Linnan M J, Mascola L, Lou X D, Goulet V, May S, Salminen C, Hird D W, Yonekura M L, Hayes P, Weaver R, Audurier A, Plikaytis B D, Fannin S L, Kleks A, Broome C V. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988;319:823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 387.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Loessner M J, Busse M. Bacteriophage typing of Listeria species. Appl Environ Microbiol. 1990;56:1912–1918. doi: 10.1128/aem.56.6.1912-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Loisel T P, Boujemaa R, Pantaloni D, Carlier M-F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:603–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 389a.López S, Marco A J, Prats N, Czuprynski C J. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infect Immun. 2000;68:4789–4791. doi: 10.1128/iai.68.8.4789-4791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Lorber B. Clinical listeriosis—implications for pathogenesis. In: Miller A J, Smith J L, Somkuti G A, editors. Foodborne listeriosis. New York, N.Y: Elsevier; 1990. pp. 41–49. [Google Scholar]
- 391.Lorber B. Listeriosis. Clin Infect Dis. 1996;24:1–11. doi: 10.1093/clinids/24.1.1. [DOI] [PubMed] [Google Scholar]
- 392.Lou Y, Yousef A. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl Environ Microbiol. 1997;63:1252–1255. doi: 10.1128/aem.63.4.1252-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lou Y, Yousef A E. Characteristics of Listeria monocytogenes important to food processors. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 131–224. [Google Scholar]
- 394.Low J C, Donachie W. Clinical and serum antibody responses in lambs to infection by Listeria monocytogenes. Res Vet Sci. 1991;51:185–192. doi: 10.1016/0034-5288(91)90012-d. [DOI] [PubMed] [Google Scholar]
- 395.Low J C, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J. 1997;153:9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 396.Low J C, Davies R C, Donachie W. Purification of listeriolysin O and development of an immunoassay for diagnosis of listeric infections in sheep. J Clin Microbiol. 1992;30:2705–2708. doi: 10.1128/jcm.30.10.2705-2708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Low J C, Wright F, McLauchlin J, Donachie W. Serotyping and distribution of Listeria isolates from cases of ovine listeriosis. Vet Rec. 1993;133:165–166. doi: 10.1136/vr.133.7.165. [DOI] [PubMed] [Google Scholar]
- 398.Lu C Y, Redline R W, Shea C M, Dustin L B, McKay D B. Pregnancy as a natural model of allograft tolerance. Interactions between adherent macrophages and trophoblast populations. Transplantation. 1989;48:848–855. doi: 10.1097/00007890-198911000-00025. [DOI] [PubMed] [Google Scholar]
- 399.Luft B J, Remington J S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.MacDonald T T, Carter P B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516–52233. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Machesky L M. Cell motility: complex dynamics at the leading edge. Curr Biol. 1997;7:R164–R167. doi: 10.1016/s0960-9822(97)70079-4. [DOI] [PubMed] [Google Scholar]
- 402.Machesky L M. Rocket-based motility a universal mechanism? Nat Cell Biol. 1999;1:E29–E31. doi: 10.1038/10020. [DOI] [PubMed] [Google Scholar]
- 403.Machesky L M, Gould K L. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 404.Machesky L M, Mullins R D, Higgs HN, Kaiser D A, Blanchoin L, May R C, Hall M E, Pollard T D. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 406.Mackaness G B. The influence of immunologically committed lymphoid cells on macrophage activity in vitro. J Exp Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis: a bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta-strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 408.Mandel T F, Cheers C. Resistance and susceptibility to bacterial infection. III. Histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980;30:851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Marchand J B, Moreau P, Paoletti A, Cossart P, Carlier M F, Pantaloni D. Actin-based movement of Listeria monocytogenes in Xenopus egg extracts is due to local uncapping of the barbed ends of actin filaments at the bacterial surface and resulting shift in steady state of actin assembly. J Cell Biol. 1995;130:331–343. doi: 10.1083/jcb.130.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Marco A J, Altimira J, Prats N, Lopez S, Domínguez L, Domingo M, Briones V. Penetration of Listeria monocytogenes in mice infected by the oral route. Microb Pathog. 1997;23:255–263. doi: 10.1006/mpat.1997.0144. [DOI] [PubMed] [Google Scholar]
- 411.Marco A J, Prats N, Ramos J A, Briones V, Blanco M, Domínguez L, Domingo M. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J Comp Pathol. 1992;107:1–9. doi: 10.1016/0021-9975(92)90090-h. [DOI] [PubMed] [Google Scholar]
- 412.Marco A, Ramos J A, Domínguez L, Domingo M, González L. Immunocytochemical detection of Listeria monocytogenes in tissue with the peroxidase-antiperoxidase technique. Vet Pathol. 1988;25:385–387. doi: 10.1177/030098588802500508. [DOI] [PubMed] [Google Scholar]
- 412a.Marino M, Braun L, Cossart P, Ghosh P. Structure of the InlB leucine-rich repeats, a domain that triggers host cell invasion by the bacterial pathogen L. monocytogenes. Mol Cell. 1999;4:1063–1072. doi: 10.1016/s1097-2765(00)80234-8. [DOI] [PubMed] [Google Scholar]
- 412b.Marino M, Braun L, Cossart P, Ghosh P. A framework for interpreting the leucine-rich repeats of Listeria internalins. Proc Natl Acad Sci USA. 2000;97:9784–9788. doi: 10.1073/pnas.97.16.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Marquis H, Bouwer H G, Hinrichs D J, Portnoy D A. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61:3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Marquis H, Doshi V, Portnoy D A. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Marquis H, Goldfine H, Portnoy D A. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Marquis H, Hager E J. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol Microbiol. 2000;35:289–298. doi: 10.1046/j.1365-2958.2000.01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Marra A, Isberg R R. Common entry mechanisms: bacterial pathogenesis. Curr Biol. 1996;6:1084–1086. doi: 10.1016/s0960-9822(02)70672-6. [DOI] [PubMed] [Google Scholar]
- 418.Mata M, Paterson Y. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogeneous listerial antigens. J Immunol. 1999;163:1449–1456. [PubMed] [Google Scholar]
- 419.May R C, Hall M E, Higgs H N, Pollard T D, Chakraborty T, Wehland J, Machesky L M, Sechi S A. The Arp2/3 complex is essential for actin-based motility of Listeria monocytogenes. Curr Biol. 1999;9:759–762. doi: 10.1016/s0960-9822(99)80337-6. [DOI] [PubMed] [Google Scholar]
- 420.McCarthy S A. Listeria in the environment. In: Miller A J, Smith J L, Somkuti G A, editors. Foodborne listeriosis. New York, N.Y: Elsevier; 1990. pp. 25–29. [Google Scholar]
- 421.McLauchlin J. Listeria monocytogenes, recent advances in taxonomy and epidemiology of listeriosis in humans. J Appl Microbiol. 1987;63:1–11. doi: 10.1111/j.1365-2672.1987.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 422.McLauchlin J. Human listeriosis in Britain, 1967–1985, a summary of 722 cases. 1. Listeriosis during pregnancy and in the newborn. Epidemiol Infect. 1990;104:181–189. doi: 10.1017/s0950268800059343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.McLauchlin J. Human listeriosis in Britain, 1967–1985, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol Infect. 1990;104:191–201. doi: 10.1017/s0950268800059355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.McLauchlin J. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur J Clin Microbiol Infect Dis. 1990;9:210–213. doi: 10.1007/BF01963840. [DOI] [PubMed] [Google Scholar]
- 425.McLauchlin J. Epidemiology of listeriosis in Britain. In: Amgar A, editor. Proceedings of the International Conference on Listeria and Food Safety. Laval, France: Aseptic Processing Association; 1991. pp. 38–47. [Google Scholar]
- 426.McLauchlin J, Greenwood M H, Pini P N. The occurrence of Listeria monocytogenes in cheese from a manufacturer associated with a case of listeriosis. Int J Food Microbiol. 1990;10:255–262. doi: 10.1016/0168-1605(90)90073-e. [DOI] [PubMed] [Google Scholar]
- 427.McLauchlin J, Hall S M, Velani S K, Gilbert R J. Human listeriosis and paté: a possible association. Br Med J. 1991;303:773–775. doi: 10.1136/bmj.303.6805.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.McLauchlin J, Low J C. Primary cutaneous listeriosis in adults: an occupational disease of veterinarians and farmers. Vet Rec. 1994;135:615–617. [PubMed] [Google Scholar]
- 429.McLaughlan A M, Foster S J. Molecular characterization of an autolytic amidase of Listeria monocytogenes EGD. Microbiology. 1998;144:1359–1367. doi: 10.1099/00221287-144-5-1359. [DOI] [PubMed] [Google Scholar]
- 430.Mecsas J, Raupach B, Falkow S. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol Microbiol. 1998;28:1269–1281. doi: 10.1046/j.1365-2958.1998.00891.x. [DOI] [PubMed] [Google Scholar]
- 431.Mengaud J, Braun-Breton C, Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991;5:367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 432.Mengaud J, Chenevert J, Geoffroy C, Gaillard J L, Cossart P. Identification of the structural gene encoding the SH-activated hemolysin of Listeria monocytogenes: listeriolysin O is homologous to streptolysin O and pneumolysin. Infect Immun. 1987;56:766–772. doi: 10.1128/iai.55.12.3225-3227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Mengaud J, Dramsi S, Gouin E, Vazquez-Boland J A, Milon G, Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol Microbiol. 1991;5:2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 434.Mengaud J, Geoffroy C, Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991;59:1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 436.Mengaud J, Vicente M-F, Chenevert J, Pereira J M, Geoffroy C, Gicquel-Sanzey B, Baquero F, Perez-Diaz J-C, Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988;56:766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Mengaud J, Vicente M-F, Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989;57:3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Michel E, Cossart P. Physical map of the Listeria monocytogenes chromosome. J Bacteriol. 1992;174:7098–7103. doi: 10.1128/jb.174.22.7098-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Michel E, Mengaud J, Galsworthy S, Cossart P. Characterization of a large motility gene cluster containing the cheR, motAB genes of Listeria monocytogenes and evidence that PrfA downregulates motility genes. FEMS Microbiol Lett. 1998;169:341–347. doi: 10.1111/j.1574-6968.1998.tb13338.x. [DOI] [PubMed] [Google Scholar]
- 440.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:3609–3619. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 441.Mielke M E, Ehlers S, Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ in acquired resistance. Infect Immun. 1988;56:1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Miettinen M K, Siitonen A, Heiskanen P, Haajanen H, Bjorkroth K J, Korkaela H J. Molecular epidemiology of an outbreak of febrile gastroenteritis caused by Listeria monocytogenes in cold-smoked rainbow trout. J Clin Microbiol. 1999;37:2358–2360. doi: 10.1128/jcm.37.7.2358-2360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Miki K, Mackaness G B. The passive transfer of acquired resistance to Listeria monocytogenes J. Exp Med. 1964;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Milenbachs A A, Brown D P, Moors M, Youngman P. Carbon source regulation of virulence gene expression in Listeria monocytogenes. Mol Microbiol. 1997;23:1075–1085. doi: 10.1046/j.1365-2958.1997.2711634.x. [DOI] [PubMed] [Google Scholar]
- 445.Miller R, Britigan B E. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445b.Milohanic E, Jonquieres R, Cossart P, Berche P, Gaillard J-L. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol Microbiol. 2001;39:1212–1224. doi: 10.1111/j.1365-2958.2001.02208.x. [DOI] [PubMed] [Google Scholar]
- 446.Milohanic E, Pron B, Berche P, Gaillard J-L The European Listeria Genome Consortium. Identification of new loci involved in adhesion of Listeria monocytogenes to eukaryotic cells. Microbiology. 2000;146:731–739. doi: 10.1099/00221287-146-3-731. [DOI] [PubMed] [Google Scholar]
- 447.Mitsuyama M, Igarashi K, Kawamura I, Ohmori T, Nomoto K. Difference in the induction of macrophage interleukin-1 between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990;58:1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Mitsuyama M, Takeya K, Nomoto K, Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978;106:165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- 448a.Miura T, Nishikawa S, Sasaki S, Yamada K, Hasegawa S, Mizuki D, Mizuki M, Hatayama I, Sekikawa S, Tagawa Y, Iwakura Y, Nakane A. Roles of endogenous cytokines in liver apoptosis of mice in lethal Listeria monocytogenes infection. FEMS Immunol Med Microbiol. 2000;28:335–341. doi: 10.1111/j.1574-695X.2000.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 449.Mizoguchi K, Ohta H, Miyagi A, Kurihara H, Takashiba S, Kato K, Murayama Y, Fukui K. The regulatory effect of fermentable sugar levels on the production of leukotoxin by Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1997;146:161–166. doi: 10.1111/j.1574-6968.1997.tb10187.x. [DOI] [PubMed] [Google Scholar]
- 450.Molello J A, Jensen R. Plancental pathology. IV. Placental lesions of sheep experimentally infected with Listeria monocytogenes. Am J Vet Res. 1964;25:441–449. [PubMed] [Google Scholar]
- 451.Moors M A, Levitt B, Youngman P, Portnoy D A. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect Immun. 1999;67:131–139. doi: 10.1128/iai.67.1.131-139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Morgan P J, Hyman S C, Byron O, Andrew P W, Mitchell T J, Rowe A J. Modelling the bacterial protein toxin, pneumolysin, in its monomeric and oligomeric form. J Biol Chem. 1994;269:25315–25320. [PubMed] [Google Scholar]
- 453.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti P J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Müller M, Althaus R, Frohlich D, Frei K, Eugster H P. Reduced antilisterial activity of TNF-deficient bone marrow-derived macrophages is due to impaired superoxide production. Eur J Immunol. 1999;29:3089–3097. doi: 10.1002/(SICI)1521-4141(199910)29:10<3089::AID-IMMU3089>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 455.Müller S, Hain T, Pashalidis P, Lingnau A, Domann E, Chakraborty T, Wehland J. Purification of the inlB gene product of Listeria monocytogenes and demonstration of its biological activity. Infect Immun. 1998;66:3128–3133. doi: 10.1128/iai.66.7.3128-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Mullins R D, Kelleher J K, Pollard T J. Actin like actin? Trends Cell Biol. 1996;6:208–212. doi: 10.1016/0962-8924(96)20017-0. [DOI] [PubMed] [Google Scholar]
- 457.Munk M E, Kaufmann S H E. Listeria monocytogenes reactive T lymphocytes in healthy individuals. Microb Pathog. 1988;5:49–54. doi: 10.1016/0882-4010(88)90080-0. [DOI] [PubMed] [Google Scholar]
- 458.Murray E G D, Webb R A, Swann M B R. A disease of rabbits characterised by a large mononuclear leukocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n. sp.) J Pathol Bacteriol. 1926;29:407–439. [Google Scholar]
- 459.Nair S, Debré I, Msadek T, Gaillot O, Berche P. CtsR controls classIII heat shock gene expression in the human pathogen Listeria monocytogenes. Mol Microbiol. 2000;35:800–811. doi: 10.1046/j.1365-2958.2000.01752.x. [DOI] [PubMed] [Google Scholar]
- 460.Nair S, Frehel C, Nguyen L, Escuyer V, Berche P. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol Microbiol. 1999;31:185–196. doi: 10.1046/j.1365-2958.1999.01159.x. [DOI] [PubMed] [Google Scholar]
- 460a.Nair S, Milohanic E, Berche P. ClpC ATPase is required for cell adhesion and invasion of Listeria monocytogenes. Infect Immun. 2000;68:7061–7068. doi: 10.1128/iai.68.12.7061-7068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Nakamura M, Sekino N, Iwamoto M, Ohno-Iwashita Y. Interaction of theta-toxin (perfringolysin O), a cholesterol-binding cytolysin, with liposomal membranes: change in the aromatic side chains upon binding and insertion. Biochemistry. 1995;34:6513–6520. doi: 10.1021/bi00019a032. [DOI] [PubMed] [Google Scholar]
- 462.Nakamura M, Sekino-Suzuki N, Mitsui K, Ohno-Iwashita Y. Contribution of tryptophan residues to the structural changes in perfringolysin O during interaction with liposomal membranes. J Biochem. 1998;123:1145–1155. doi: 10.1093/oxfordjournals.jbchem.a022054. [DOI] [PubMed] [Google Scholar]
- 463.Nakane A, Minagawa T, Yasuda I. Induction of alpha/beta interferon and gamma interferon in mice infected with Listeria monocytogenes during pregnancy. Infect Immun. 1985;50:877–880. doi: 10.1128/iai.50.3.877-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Nato F, Reich K, Lhopital S, Rouyre S, Geoffroy C, Mazie J C, Cossart P. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun. 1991;59:4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTGX motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 466.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Navas, J., B. González-Zorn, N. Ladrón, P. Garrido, and J. A. Vázquez-Boland. 1999. Identification and mutagenesis by allelic exchange of choE, encoding a cholesterol oxidase of the intracellular pathogen Rhodococcus equi. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 468.Reference deleted.
- 469.Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu Rev Microbiol. 1995;49:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- 470.Niebuhr K, Chakraborty T, Köllner P, Wehland J. Production of monoclonal antibodies to the phosphatidyl-choline-specific phospholipase C of Listeria monocytogenes, a virulence factor for this species. Med Microbiol Lett. 1993;2:9–16. [Google Scholar]
- 471.Niebubr K, Chakraborty T, Rohde M, Gazlig T, Jansen B, Köllner P, Wehland J. Localization of the ActA polypeptide of Listeria monocytogenes in infected tisue culture cell lines: ActA is not associated with actin “comets.”. Infect Immun. 1993;61:2793–2802. doi: 10.1128/iai.61.7.2793-2802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl U D, Walter U, Gertler F B, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Nieman R E, Lorber B. Listeriosis in adults: a changing pattern. Report of eight cases and review of the literature, 1968–1978. Rev Infect Dis. 1980;2:207–227. doi: 10.1093/clinids/2.2.207. [DOI] [PubMed] [Google Scholar]
- 474.Nishibori T, Xiong H, Kawamura I, Arakaea M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996;64:3188–3195. doi: 10.1128/iai.64.8.3188-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Njoku-Obi A N, Jenkins E M, Njoku-Obi J C, Adams J, Covington V. Production and nature of Listeria monocytogenes hemolysins. J Bacteriol. 1963;86:1–8. doi: 10.1128/jb.86.1.1-8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Nolla-Salas J, Anto J M, Almela M, Coll P, Gasser I, Plasencia A. Incidence of listeriosis in Barcelona, Spain, in 1990. The collaborative study group of listeriosis of Barcelona. Eur J Clin Microbiol Infect Dis. 1993;12:157–161. doi: 10.1007/BF01967105. [DOI] [PubMed] [Google Scholar]
- 477.Norrung B, Andersen J K. Variations in virulence between different electrophoretic types of Listeria monocytogenes. Lett Appl Microbiol. 2000;30:228–232. doi: 10.1046/j.1472-765x.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 478.North R J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970;132:521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.North R J. The concept of the activated macrophage. J Immunol. 1978;121:806–809. [PMC free article] [PubMed] [Google Scholar]
- 480.Reference deleted.
- 481.Nyfelt A. Etiologie de la mononucléose infectieuse. C R Soc Biol. 1929;101:590–591. [Google Scholar]
- 482.Ofek I, Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988;56:539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ohya S, Tanabe Y, Makino M, Nomura T, Xiong H, Arakawa M, Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect Immun. 1998;66:4043–4049. doi: 10.1128/iai.66.9.4043-4049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Ohya S, Xiong H, Tanabe Y, Arakawa M, Mitsuyama M. Killing mechanisms of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J Med Microbiol. 1998;47:211–215. doi: 10.1099/00222615-47-3-211. [DOI] [PubMed] [Google Scholar]
- 485.Okamoto M, Nakane A, Minagawa T. Host resistance to an intragastric infection with Listeria monocytogenes in mice dependes on cellular immunity and intestinal bacterial flora. Infect Immun. 1994;62:3080–3085. doi: 10.1128/iai.62.8.3080-3085.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Olson C, Crook R H, Blore I C. The reaction of blood cells in experimental listeriosis in sheep. Am J Vet Res. 1950;11:29–40. [Google Scholar]
- 487.Osebold J W, Inouye T. Pathogenesis of Listeria monocytogenes infections in natural hosts. I. Rabbit studies. J Infect Dis. 1954;94:52–66. doi: 10.1093/infdis/95.1.52. [DOI] [PubMed] [Google Scholar]
- 488.Osebold J W, Kendrick J W, Njoku-Obi A. Abortion of cattle experimentally with Listeria monocytogenes. J Am Vet Med Assoc. 1960;137:227–233. [PubMed] [Google Scholar]
- 489.Otter A, Blakemore W F. Observation on the presence of Listeria monocytogenes in axons. Acta Microbiol Hung. 1989;36:125–131. [PubMed] [Google Scholar]
- 490.Ouadrhiri Y, Scorneaux B, Sibille Y, Tulkens P M. Mechanisms of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob Agents Chemother. 1999;43:1242–1251. doi: 10.1128/aac.43.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Owen R G H, Boulnois G J, Andrew P W, Mitchell T J. A role in cell binding for the C-terminus of pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae. FEMS Microbiol Lett. 1994;121:217–221. doi: 10.1111/j.1574-6968.1994.tb07101.x. [DOI] [PubMed] [Google Scholar]
- 492.Paglia P, Arioli I, Frahm N, Chakraborty T, Colombo M P, Guzman C A. The defined attenuated Listeria monocytogenes Δmp12 mutant is an effective oral vaccine carrier to trigger a long-lasting immune response against a mouse fibrosarcoma. Eur J Immunol. 1997;27:1570–1575. doi: 10.1002/eji.1830270637. [DOI] [PubMed] [Google Scholar]
- 493.Palmer M, Vulicevic I, Saweljew P, Valeva P, Kehoe M, Bhakdi S. Streptolysin O: A proposed model of allosteric interaction between a pore-forming protein and its target lipid bilayer. Biochemistry. 1998;37:2378–2383. doi: 10.1021/bi9720890. [DOI] [PubMed] [Google Scholar]
- 494.Palmer M, Harris R, Freytag C, Kehoe M, Tranum-Jensen J, Bhakdi S. Assembly mechanism of the oligomeric streptolysin O pore: the early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 1998;6:1598–1605. doi: 10.1093/emboj/17.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Pamer E G, Harty J T, Bevan M. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Pan Z K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 497.Pan Z K, Ikonomidis G, Pardoll D, Paterson Y. Regression of established cancer mediated by the oral delivery of a recombinant Listeria monocytogenes vaccine expressing a model tumour antigen. Cancer Res. 1995;55:4776–4779. [PubMed] [Google Scholar]
- 497a.Parham P, Unanue E R, editors. Immunity to L. monocytogenes: a model intracellular pathogen. Immunol Rev. 1997;158:1–169. [Google Scholar]
- 498.Parida S K, Domann E, Rohde M, Müller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 499.Park J, Lee Y, Lim Y, Kwon S, Lee C, Yoon B. Specific binding of recombinant Listeria monocytogenes p60 protein in Caco-2 cells. FEMS Microbiol Lett. 2000;186:35–40. doi: 10.1111/j.1574-6968.2000.tb09078.x. [DOI] [PubMed] [Google Scholar]
- 500.Park S F. The repression of listeriolysin O expression in Listeria monocytogenes by the phenolic β-d-glucoside arbutin. Lett Appl Microbiol. 1994;19:258–260. doi: 10.1111/j.1472-765x.1994.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 501.Park S F, Kroll R G. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol Microbiol. 1993;8:653–661. doi: 10.1111/j.1365-2958.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 502.Parkassh V, Morotti R A, Joshi V, Cartun R, Rauch C A, West A B. Immunohistochemical detection of Listeria antigen in the placenta in perinatal listeriosis. Int J Gynecol Pathol. 1998;17:343–350. doi: 10.1097/00004347-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 502a.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 503.Perelroizen I, Marchand J B, Blanchoin L, Didry D, Carlier M F. Interaction of profilin with G-actin and poly(l-proline) Biochemistry. 1994;33:8472–8478. doi: 10.1021/bi00194a011. [DOI] [PubMed] [Google Scholar]
- 504.Pérez-Díaz J C, Vicente M F, Baquero F. Plasmids in Listeria. Plasmid. 1982;8:112–118. doi: 10.1016/0147-619x(82)90049-x. [DOI] [PubMed] [Google Scholar]
- 505.Peters M, Hewicker-Trautwein M. Studies on the cell tropism of Listeria monocytogenes in ovine fetal brain cell cultures. Vet Microbiol. 1996;49:169–179. doi: 10.1016/0378-1135(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 506.Pfeuffer T, Goebel W, Laubinger J, Bachmann M, Kuhn M. LaXp180, a mammalian ActA-binding protein, identified with the yeast two-hybrid system colocalizes with intracellular Listeria monocytogenes. Cell Microbiol. 2000;2:101–114. doi: 10.1046/j.1462-5822.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- 507.Phan-Thanh L, Gormon T. Analysis of heat and cold shock proteins in Listeria by two-dimensional electrophoresis. Electrophoresis. 1995;16:444–450. doi: 10.1002/elps.1150160172. [DOI] [PubMed] [Google Scholar]
- 508.Phan-Thanh L, Gormon T. Stress proteins in Listeria monocytogenes. Electrophoresis. 1997;18:1464–1471. doi: 10.1002/elps.1150180821. [DOI] [PubMed] [Google Scholar]
- 509.Pierce M M, Gibson R E, Rodgers F G. Opsonin-independent adherence and phagocytosis of Listeria monocytogenes by murine peritoneal macrophages. J Med Microbiol. 1996;45:248–262. doi: 10.1099/00222615-45-4-258. [DOI] [PubMed] [Google Scholar]
- 510.Piffaretti J-C, Kressebuch H, Aeschbacher M, Bille J, Bannermann E, Musser J M, Selander R K, Rocourt J. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc Natl Acad Sci USA. 1989;86:3818–3822. doi: 10.1073/pnas.86.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Pinkney M, Beachey E, Kehoe M. The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect Immun. 1989;57:2553–2558. doi: 10.1128/iai.57.8.2553-2558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Pinner R W, Schuchat A, Swaminathan B, Hayes P S, Deaver K A, Weaver R E, Plikaytis B D, Reeves M, Broome C V, Wenger J D the Listeria Study Group. Role of foods in sporadic listeriosis. II. Microbiologic and epidemiologic investigation. J Am Med Assoc. 1992;267:2046–2050. [PubMed] [Google Scholar]
- 513.Pistor S, Chakraborty T, Niebuhr K, Domann E, Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994;13:758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Pistor S, Chakraborty T, Walter U, Wehland J. The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr Biol. 1995;5:517–525. doi: 10.1016/s0960-9822(95)00104-7. [DOI] [PubMed] [Google Scholar]
- 514a.Pistor S, Grobe L, Sechi A S, Domann E, Gerstel B, Machesky L M, Chakraborty T, Wehland J. Mutations of arginine residues within the 146-KKRRK-150 motif of the ActA protein of Listeria monocytogenes abolish intracellular motility by interfering with the recruitment of the Arp2/3 complex. J Cell Sci. 2000;113:3277–3287. doi: 10.1242/jcs.113.18.3277. [DOI] [PubMed] [Google Scholar]
- 515.Pollard T D. Missing link for intracellular bacterial motility? Curr Biol. 1995;5:837–840. doi: 10.1016/s0960-9822(95)00167-9. [DOI] [PubMed] [Google Scholar]
- 516.Portnoy D A. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992;4:20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- 517.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Portnoy D A, Tweten R K, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and prefringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Poyart C, Abachin E, Razafimanantsoa I, Berche P. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect Immun. 1993;61:1576–1580. doi: 10.1128/iai.61.4.1576-1580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Poyart-Salmeron C, Carlier C, Trieu-Cuot P, Courtieu A-L, Courvalin P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet. 1990;335:1422–1426. doi: 10.1016/0140-6736(90)91447-i. [DOI] [PubMed] [Google Scholar]
- 522.Prats N, Briones V, Blanco M M, Altimira J, Ramos J A, Domínguez L, Marco A. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1992;11:5–8. doi: 10.1007/BF01989983. [DOI] [PubMed] [Google Scholar]
- 522a.Pron B, Boumaila C, Jaubert F, Berche P, Milon G, Geissmann F, Gaillard J L. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 2001;3:331–340. doi: 10.1046/j.1462-5822.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 523.Pron B, Boumaila C, Jaubert F, Sarnacki S, Monnet J-P, Berche P, Gaillard J-L. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect Immun. 1998;66:747–755. doi: 10.1128/iai.66.2.747-755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Pung O C, Tucker A N, Vore S J, Luster M I. Influence of estrogen on host resistance: increased susceptibility of mice to Listeria monocytogenes correlates with depressed production of interleukin 2. Infect Immun. 1985;50:91–96. doi: 10.1128/iai.50.1.91-96.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 526.Rácz P, Tenner K, Mérö E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental Listeria infection. Lab Investig. 1972;26:694–700. [PubMed] [Google Scholar]
- 527.Raffelsbauer D, Bubert A, Engelbrecht F, Scheinpflug J, Simm A, Hess J, Kaufmann S H E, Goebel W. The gene cluster inlC2DE of Listeria monocytogenes contains additional new internalin genes and is important for virulence in mice. Mol Gen Genet. 1998;260:144–158. doi: 10.1007/s004380050880. [DOI] [PubMed] [Google Scholar]
- 528.Ralovich B, Emödy L, Merö E. Biological properties of virulent and weakly virulent L. monocytogenes strains. Acta Microbiol Acad Sci Hung. 1972;19:323–326. [PubMed] [Google Scholar]
- 529.Ramage C P, Low J C, McLauchlin J, Donachie W. Characterisation of Listeria ivanovii isolates from the UK using pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1999;15:349–353. doi: 10.1111/j.1574-6968.1999.tb13394.x. [DOI] [PubMed] [Google Scholar]
- 530.Rapraeger A C, Ott V L. Molecular interactions of the syndecan core proteins. Curr Opin Cell Biol. 1998;10:620–628. doi: 10.1016/s0955-0674(98)80038-0. [DOI] [PubMed] [Google Scholar]
- 531.Rasmussen O F, Skouboe P, Dons L, Rossen L, Olsen J E. Listeria monocytogenes exists in at least three evolutionary lines: evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology. 1995;141:2053–2061. doi: 10.1099/13500872-141-9-2053. [DOI] [PubMed] [Google Scholar]
- 532.Raveneau J, Geoffroy C, Beretti J-L, Gaillard J-L, Alouf J E, Berche P. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun. 1992;60:916–921. doi: 10.1128/iai.60.3.916-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Raybourne R B, Bunning V K. Bacterium-host cell interactions at the cellular level: fluorescent labeling of bacteria and analysis of short-term bacterium-phagocyte interaction by flow cytometry. Infect Immun. 1994;62:665–672. doi: 10.1128/iai.62.2.665-672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Rebhun W C, deLahunta A. Diagnosis and treatment of bovine listeriosis. J Am Vet Med Assoc. 1982;180:395–398. [PubMed] [Google Scholar]
- 535.Redline R W, Lu C Y. Role of local immuno-suppression in murine foetoplacental listeriosis. J Clin Investig. 1987;79:1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Redline R W, Lu C Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988;140:3947–3955. [PubMed] [Google Scholar]
- 537.Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch B M, Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch B M, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Reiter W D, Palm P, Yeats S. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 1989;17:1907–1914. doi: 10.1093/nar/17.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Renzoni A, Cossart P, Dramsi S. PrfA, the transcriptional activator of virulence genes, is upregulated during interaction of Listeria monocytogenes with mammalian cells and in eukaryotic cell extracts. Mol Microbiol. 1999;34:552–561. doi: 10.1046/j.1365-2958.1999.01621.x. [DOI] [PubMed] [Google Scholar]
- 541.Renzoni A, Klarsfeld A, Dramsi S, Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect Immun. 1997;65:1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Riedo F X, Pinner R W, de Lourdes Tosca M, Cartter M L, Graves L M, Reeves M W, Weaver R E, Plikaytis B D, Broome C V. A point-source foodborne listeriosis outbreak: documented incubation period and possible mild illness. J Infect Dis. 1994;170:693–696. doi: 10.1093/infdis/170.3.693. [DOI] [PubMed] [Google Scholar]
- 543.Ripio M-T, Brehm K, Lara M, Suarez M, Vazquez-Boland J-A. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179:7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Ripio M-T, Dominquez-Bernal G, Lara M, Suárez M, Vázquez-Boland J-A. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol. 1997;179:1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Ripio M-T, Dominquez-Bernal G, Suárez M, Brehm K, Berche P, Vázquez-Boland J-A. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res Microbiol. 1996;147:311–384. doi: 10.1016/0923-2508(96)84712-7. [DOI] [PubMed] [Google Scholar]
- 546.Ripio M-T, Geoffroy C, Domínguez G, Alouf J E, Vázquez-Boland J A. The sulphydryl-activated cytolysin and a sphingomyelinase C are the major membrane-damaging factors involved in cooperative (CAMP-like) haemolysis of Listeria spp. Res Microbiol. 1995;146:303–313. doi: 10.1016/0923-2508(96)81053-9. [DOI] [PubMed] [Google Scholar]
- 547.Reference deleted.
- 548.Ripio M-T, Vazquez-Boland J-A, Vega Y, Nair S, Berche P. Evidence for expressional crosstalk between the central virulence regulator PrfA and the stress response mediator ClpC in Listeria monocytogenes. FEMS Microbiol Lett. 1998;158:45–50. doi: 10.1111/j.1574-6968.1998.tb12798.x. [DOI] [PubMed] [Google Scholar]
- 549.Robbins J R, Barth A I, Marquis H, de Hostos E L, Nelson W J, Theriot J A. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J Cell Biol. 1999;146:1333–1350. doi: 10.1083/jcb.146.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Rocourt J. Listeria monocytogenes: the state of the art. Dairy Food Environ Sanitation. 1994;14:70–82. [Google Scholar]
- 551.Rocourt J. Risk factors for listeriosis. Food Control. 1996;7:195–202. [Google Scholar]
- 552.Rocourt J. The genus Listeria and Listeria monocytogenes: phylogenetic position, taxonomy, and identification. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 1–20. [Google Scholar]
- 553.Rocourt J, Alonso J M, Seeliger H P R. Virulence comparée des cinq groupes génomiques de Listeria monocytogenes (sensu lato) Ann Microbiol. 1983;134A:359–364. [PubMed] [Google Scholar]
- 554.Rocourt J, Brosch R. Human listeriosis 1990. Document WHO/HPP/FOS/92.4. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 555.Rocourt J, Grimont P A D. Listeria welshimeri sp. nov. and sp. Listeria seeligeri sp. nov. Int J Syst Bacteriol. 1983;33:866–869. [Google Scholar]
- 556.Rocourt J, Hof H, Schrettenbrunner A, Malinverni R, Bille J. Acute purulent Listeria seeligeri meningitis in an immunocompetent adult. Schweiz Med Wochenschr. 1986;116:248–251. [PubMed] [Google Scholar]
- 557.Rocourt J, Jacquet C. Epidémiologie des infections humaines àListeria monocytogenes en 1994: certitudes et interrogations. Ann Inst Pasteur/Actualités. 1994;5:168–174. [Google Scholar]
- 558.Rocourt J, Jacquet C. Surveillance épidémiologique de la listériose humaine en France: rôle du centre national de référence. Med Mal Infect. 1995;25:177–183. [Google Scholar]
- 559.Rocourt J, Seeliger H P R. Distribution des espèces du genre Listeria. Zentbl Bakteriol Hyg A. 1985;259:317–330. [PubMed] [Google Scholar]
- 560.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 561.Rogers H W, Unanue E R. Neutrophils are involved in acute, non-specific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner M W. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 563.Rosen H, Gordon S, North R J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Rosenblatt J, Agnew B J, Abe H, Barnburg J R, Mitchison T J. Xenopus actin-depolimerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails. J Cell Biol. 1997;136:1323–1332. doi: 10.1083/jcb.136.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Reference deleted.
- 566.Rossjohn J, Feil S C, McKinstry W J, Tweten R K, Parker M W. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 567.Rouquette C, Bolla J M, Berche P. An iron-dependent mutant of Listeria monocytogenes of attenuated virulence. FEMS Microbiol Lett. 1995;133:77–83. doi: 10.1111/j.1574-6968.1995.tb07864.x. [DOI] [PubMed] [Google Scholar]
- 568.Rouquette C, de Chastellier C, Nair S, Berche P. The ClpC ATPase of Listeria monocytogenes is a general stress protein required for virulence and promoting early bacterial escape from the phagosome of macrophages. Mol Microbiol. 1998;27:1235–1245. doi: 10.1046/j.1365-2958.1998.00775.x. [DOI] [PubMed] [Google Scholar]
- 569.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J-A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 570.Ruhland G J, Hellwig M, Wanner G, Fiedler F. Cell-surface location of Listeria-specific protein p60 -detection of Listeria cells by indirect immunofluorescence. J Gen Microbiol. 1993;139:609–616. doi: 10.1099/00221287-139-3-609. [DOI] [PubMed] [Google Scholar]
- 571.Rutberg B. Antitermination of transcription of catabolic operons. Mol Microbiol. 1997;23:413–421. doi: 10.1046/j.1365-2958.1997.d01-1867.x. [DOI] [PubMed] [Google Scholar]
- 572.Ryser E T. Foodborne listeriosis. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 299–358. [Google Scholar]
- 573.Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. [Google Scholar]
- 574.Safley S A, Cluff C W, Marshall N E, Ziegler H K. Role of listeriolysin O in the T lymphocyte response to infection with Listeria monocytogenes. J Immunol. 1991;146:3604–3616. [PubMed] [Google Scholar]
- 575.Sage A E, Vasil M L. Osmoprotectant-dependent expression of plcH, encoding the hemolytic phospholipase C, is subject to novel catabolite repression control in Pseudomonas aeruginosa PAO1. J Bacteriol. 1997;179:4874–4881. doi: 10.1128/jb.179.15.4874-4881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J-J. Protein phosphorylation and regulation of carbon metabolism in gram-negative versus gram-positive bacteria. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 576a.Saklani-Jusforgues H, Fontan E, Goossens P L. Effect of acid-adaptation on Listeria monocytogenes survival and translocation in a murine intragastric infection model. FEMS Microbiol Lett. 2000;193:155–159. doi: 10.1111/j.1574-6968.2000.tb09418.x. [DOI] [PubMed] [Google Scholar]
- 577.Salamina G, Dalle Donne E, Nicolini A, Poda G, Cesarone D, Bucci M, Fini R, Maldini M, Schuchat A, Swaminathan B, Bibb W, Rocourt J, Binkin N, Salmaso S. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes. Epidemiol Infect. 1996;117:429–436. doi: 10.1017/s0950268800059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Salem M L, Matsuzaki G, Madkour G A, Nomoto K. Beta-estradiol-induced decrease in IL-12 and TNF-α expression suppresses macrophage functions in the course of Listeria monocytogenes infection in mice. Int J Immunopharmacol. 1999;21:481–497. doi: 10.1016/s0192-0561(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 579.Sallen B A, Rajoharison S, Desverenne S, Quinn F, Mabilat C. Comparative analysis of 16S and 23S rRNA sequences of Listeria species. Int J Syst Bacteriol. 1996;46:669–674. doi: 10.1099/00207713-46-3-669. [DOI] [PubMed] [Google Scholar]
- 580.Samuelsson S, Rothgardt N P, Carvajal A, Fredriksen W. Human listeriosis in Denmark 1981–1987, including an outbreak November 1985–March 1987. J Infect. 1990;20:251–259. doi: 10.1016/0163-4453(90)91244-8. [DOI] [PubMed] [Google Scholar]
- 581.Sanders M C, Theriot J A. Tails from the hall of infection: actin-based motility of pathogens. Trends Microbiol. 1996;4:211–213. doi: 10.1016/0966-842X(96)30017-6. [DOI] [PubMed] [Google Scholar]
- 581a.Santiago N I, Zipf A, Bhunia A K. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl Environ Microbiol. 1999;65:2765–2769. doi: 10.1128/aem.65.6.2765-2769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Sarrut S, Alison F. Etude du placenta dans 21 cas de listériose congénitale. Arch Fr Pediatr. 1967;24:285–302. [PubMed] [Google Scholar]
- 583.Saunders F K, Mitchell T J, Walker J A, Andrew P W, Boulnois G J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989;57:2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Sawyer R T, Drevets D A, Campbell P A, Potter T A. Internalin A can mediate phagocytosis of Listeria monocytogenes by mouse macrophage cell lines. J Leukocyte Biol. 1996;60:603–610. doi: 10.1002/jlb.60.5.603. [DOI] [PubMed] [Google Scholar]
- 585.Schirmer E C, Glover J R, Singer M A, Lindquist S. HSP-100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- 586.Schlech W F., III An animal model of foodborne Listeria monocytogenes virulence: effect of alterations of local and systemic immunity on invasive infection. Clin Investig Med. 1993;16:219–225. [PubMed] [Google Scholar]
- 586a.Schlech W F, III, Chase D P, Badley A. A model of food-borne infection Listeria monocytogenes infection in the Sprague-Dawley rat using gastric inoculation: development and effect of gastric acidity on infective dose. Int J Food Microbiol. 1993;18:15–24. doi: 10.1016/0168-1605(93)90003-y. [DOI] [PubMed] [Google Scholar]
- 587.Schlech W F, III, Lavigne P M, Bortolussi R A, Allen A C, Haldane E V, Wort A J, Hightower A W, Johnson S E, King S H, Nicholls E S, Broome C V. Epidemic listeriosis—evidence for transmission by food. N Engl J Med. 1983;308:203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 588.Schlüter D, Chahoud S, Lassmann H, Schumann A, Huf H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. J Neuropathol Exp Neurol. 1996;55:14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 589.Schlüter D, Domann E, Buck C, Hain T, Hof H, Chakraborty T, Deckert-Schlüter M. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect Immun. 1998;66:5930–5938. doi: 10.1128/iai.66.12.5930-5938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 591.Schönberg A, Teufel P, Weise E. Serovars of Listeria monocytogenes and Listeria innocua from food. Acta Microbiol Hung. 1989;36:249–253. [PubMed] [Google Scholar]
- 592.Schuchat A, Deaver K A, Wenger J D, Plikaytis B D, Mascola L, Pinner R W, Reingold A L, Broome C V the Listeria Study Group. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. J Am Med Assoc. 1992;267:2041–2045. [PubMed] [Google Scholar]
- 593.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active surveillance team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 594.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Schüller S, Kügler S, Goebel W. Suppression of major histocompatibility complex class I and class II gene expression in Listeria monocytogenes-infected murine macrophages. FEMS Immunol Med Microbiol. 1998;20:289–299. doi: 10.1111/j.1574-695X.1998.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 596.Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukocyte Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 597.Schwan W R, Demuth A, Kuhn M, Goebel W. Phosphatidylinositiol-specific phospholipase C from Listeria monocytogenes contributes to intracellular survival and growth of Listeria innocua. Infect Immun. 1994;62:4795–4803. doi: 10.1128/iai.62.11.4795-4803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Schwan W R, Goebel W. Host cell responses to Listeria monocytogenes infection include differential transcription of host stress genes involved in signal transduction. Proc Natl Acad Sci USA. 1994;91:6428–6432. doi: 10.1073/pnas.91.14.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Schwartz B, Hexter D, Broome C V, Hightower A W, Hirschhorn R B, Porter J D, Hayes P S, Bibb W F, Lorber B, Faris D G. Investigation of an outbreak of listeriosis: new hypotheses for the etiology of epidemic Listeria monocytogenes infections. J Infect Dis. 1989;159:680–685. doi: 10.1093/infdis/159.4.680. [DOI] [PubMed] [Google Scholar]
- 600.Schwarzer N, Nost R, Seybold J, Parida S K, Fuhrmann O, Krüll M, Schmidt R, Newton R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Two distinct phospholipases C of Listeria monocytogenes induce ceramide generation, nuclear factor-kappa B activation, and E-selectin expression in human endothelial cells. J Immunol. 1998;161:3010–3018. [PubMed] [Google Scholar]
- 601.Sechi A S, Wehland J, Small J V. The isolated comet tail pseudopodium of Listeria monocytogenes: a tail of two actin filament populations, long and axial and short and random. J Cell Biol. 1997;137:155–167. doi: 10.1083/jcb.137.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Seeliger H P R. Listeriosis. New York, N.Y: Karger; 1961. [Google Scholar]
- 603.Seeliger H P R. New outlook on the epidemiology and epizootiology of listeriosis. Acta Microbiol Acad Sci Hung. 1972;19:273–286. [PubMed] [Google Scholar]
- 604.Seeliger H P R. Listeriosis—history and actual developments. Infection. 1988;16:S80–S84. doi: 10.1007/BF01639726. [DOI] [PubMed] [Google Scholar]
- 605.Seeliger H P R, Jones D. Genus Listeria. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1235–1245. [Google Scholar]
- 606.Seeliger H P R, Rocourt J, Schrettenbrunner A, Grimont P A D, Jones D. Listeria ivanovii sp. nov. Int J Syst Bacteriol. 1984;34:336–337. [Google Scholar]
- 607.Seeliger H P R, Schrettenbrunner A, Pongratz G, Hof H. Zur Sonderstellung stark hämolysierender Stäme der Gattung Listeria. Zentbl Bakteriol Mikrobiol Hyg 1 Abt Orig A. 1982;252:176–190. [PubMed] [Google Scholar]
- 608.Sekino-Suzuki N, Nakamura M, Mitsui K I, Ohno-Iwashita Y. Contribution of individual triptophane residues to the structure and activity of theta-toxin (perfringolysin O), a cholesterol-binding cytolysin. Eur J Biochem. 1996;241:941–947. doi: 10.1111/j.1432-1033.1996.00941.x. [DOI] [PubMed] [Google Scholar]
- 609.Sekiya K, Satoh R, Danbara H, Futaesaku Y. A ring-shaped structure with a crown formed by streptolysin O on the erythrocyte membrane. J Bacteriol. 1993;175:5953–5961. doi: 10.1128/jb.175.18.5953-5961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Sergeant E S G, Love S C J, McInnes A. Abortions in sheep due to Listeria ivanovii. Aust Vet J. 1991;68:39. doi: 10.1111/j.1751-0813.1991.tb09846.x. [DOI] [PubMed] [Google Scholar]
- 611.Shatursky O, Heuck A P, Shepard L A, Rossjohn J, Parker M W, Johnson A E, Tweten R K. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 612.Sheehan B, Klarsfeld A, Ebright R, Cossart P. A single substitution in the putative helix-turn-helix motiv of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol Microbiol. 1996;20:785–797. doi: 10.1111/j.1365-2958.1996.tb02517.x. [DOI] [PubMed] [Google Scholar]
- 613.Sheehan B, Klarsfeld A, Msadek T, Cossart P. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J Bacteriol. 1995;177:6469–6476. doi: 10.1128/jb.177.22.6469-6476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Shen H, Tato C M, Fan X. Listeria monocytogenes as a probe to study cell-mediated immunity. Curr Opin Immunol. 1998;10:450–458. doi: 10.1016/s0952-7915(98)80120-9. [DOI] [PubMed] [Google Scholar]
- 615a.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 616.Shiloh M U, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide sythase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 617.Sibelius U, Chakraborty T, Krögel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatydilinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 618.Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, Seeger W, Grimminger F. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Sibelius U, Schulz E-C, Rose F, Hattar K, Jacobs T, Weiss S, Chakraborty T, Seeger W, Grimminger F. Role of Listeria monocytogenes exotoxins listeriolysin and phosphatydilinositol-specific phospholipase C in activation of human neutrophils. Infect Immun. 1999;67:1125–1130. doi: 10.1128/iai.67.3.1125-1130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Siddique I H, Fong Lin M S I-, Chung R A. Purification and characterization of hemolysin produced by Listeria monocytogenes. Am J Vet Res. 1974;35:289–296. [PubMed] [Google Scholar]
- 621.Siddique I H, McKenzie B E, Sapp W J, Rich P. Light and electron microscopic study of the livers of pregnant mice infected with Listeria monocytogenes. Am J Vet Res. 1978;39:887–892. [PubMed] [Google Scholar]
- 622.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 623.Siebers A, Finlay B B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 624.Sijts A J A M, Neisig A, Neefjes J, Pamer E G. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J Immunol. 1996;156:685–692. [PubMed] [Google Scholar]
- 625.Sirard J-C, Fayolle C, de Chastellier C, Mock M, Leclerc C, Berche P. Intracytoplasmic delivery of listeriolysin O by a vaccinal strain of Bacillus anthracis induces CD8-mediated protection against Listeria monocytogenes. J Immunol. 1997;159:4435–4443. [PubMed] [Google Scholar]
- 626.Skalka B, Smola J, Elischerová K. Routine test for in vitro of pathogenic and apathogenic Listeria monocytogenes strains. J Clin Microbiol. 1982;15:503–507. doi: 10.1128/jcm.15.3.503-507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626a.Skoble J, Portnoy D A, Welch M D. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J Cell Biol. 2000;150:527–538. doi: 10.1083/jcb.150.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Skogberg K, Syrjanen J, Jahkola M, Renkonen O V, Paavonen J, Ahonen J, Kontiainen S, Ruutu P, Valtonen V. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clin Infect Dis. 1992;14:815–821. doi: 10.1093/clinids/14.4.815. [DOI] [PubMed] [Google Scholar]
- 628.Slutsker L, Schuchat A. Listeriosis in humans. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 75–95. [Google Scholar]
- 629.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Smith G A, Portnoy D A. How the Listeria monocytogenes ActA protein converts actin polymerization into a motile force. Trends Microbiol. 1997;5:272–276. doi: 10.1016/S0966-842X(97)01048-2. [DOI] [PubMed] [Google Scholar]
- 631.Smith G A, Portnoy D A, Theriot J A. Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol Microbiol. 1995;17:945–951. doi: 10.1111/j.1365-2958.1995.mmi_17050945.x. [DOI] [PubMed] [Google Scholar]
- 632.Smith G A, Theriot J A, Portnoy D A. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Smyth J, Duncan J L. Thiol-activated (oxygen labile) cytolysins. In: Jeljaszewicz J, Wadström T, editors. Bacterial toxins and cell membranes. London, England: Academic Press Ltd.; 1978. pp. 129–183. [Google Scholar]
- 634.Sokolovic Z, Fuchs A, Wuenscher M, Goebel W. Synthesis of species-specific stress proteins by virulent strains of Listeria monocytogenes. Infect Immun. 1990;58:3582–3587. doi: 10.1128/iai.58.11.3582-3587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Sokolovic Z, Riedel J, Wuenscher M, Goebel W. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol Microbiol. 1993;8:219–227. doi: 10.1111/j.1365-2958.1993.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 636.Southwick F S, Purich D L. Dynamic remodeling of the actin cytoskeleton: lessons learned from Listeria locomotion. BioEssays. 1994;16:885–891. doi: 10.1002/bies.950161206. [DOI] [PubMed] [Google Scholar]
- 637.Spreng S, Dietrich G, Niewiesk S, ter Meulen V, Gentschev I, Goebel W. Novel bacterial systems for the delivery of recombinant protein or DNA. FEMS Immunol Med Microbiol. 2000;27:299–304. doi: 10.1111/j.1574-695X.2000.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 638.Squires C, Squires C L. The Clp proteins: proteolysis regulators and molecular chaerones ? J Bacteriol. 1992;174:1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Stelma G N, Reyes A L, Peeler J T, Francis D W, Hunt J M, Spaulding P L, Johnson C H, Lovett J. Pathogenicity test for Listeria monocytogenes using immunocompromised mice. J Clin Microbiol. 1987;25:2085–2089. doi: 10.1128/jcm.25.11.2085-2089.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Reference deleted.
- 641.Sun A N, Camilli A, Portnoy D A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990;58:3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 643.Swartz M A, Welch D F, Narayanan R P, Greenfield R A. Catalase-negative Listeria monocytogenes causing menigitis in an adult: clinical and laboratory features. Am J Clin Pathol. 1991;96:130–133. doi: 10.1093/ajcp/96.1.130. [DOI] [PubMed] [Google Scholar]
- 644.Sword C P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966;92:536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Synnott M B, Morse D L, Hall S M. Neonatal meningitis in England and Wales: a review of routine national data. Arch Dis Child. 1994;71:F75–80. doi: 10.1136/fn.71.2.f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Szalay G, Hess J, Kaufmann S H E. Restricted replication of Listeria monocytogenes in a gamma interferon-activated murine hepatocyte line. Infect Immun. 1995;63:3187–3195. doi: 10.1128/iai.63.8.3187-3195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Tang P, Rosenshine I, Cossart P, Finlay B B. Listeriolysin O activates mitogen-activated protein kinase in eukaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647a.Tang P, Rosenshine I, Tang P, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Tappero J W, Schuchat A, Deaver K A, Mascola L, Wenger J D the Listeriosis Study Group. Reduction in the incidence of human listeriosis in the United States. JAMA. 1995;273:1118–1122. doi: 10.1001/jama.1995.03520380054035. [DOI] [PubMed] [Google Scholar]
- 650.Temm-Grove C, Jokush B M, Röhde M, Niebuhr K, Chakraborty T, Wehland J. Exploitation of microfilament proteins by Listeria monocytogenes: microvillus-like composition of comet tails and vectorial spreading in polarized epithelial cells. J Cell Sci. 1994;107:2951–2960. doi: 10.1242/jcs.107.10.2951. [DOI] [PubMed] [Google Scholar]
- 651.Reference deleted.
- 652.Theriot J A. The cell biology of infection by intracellular pathogens. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- 653.Theriot J A, Rosenblatt J, Portnoy D A, Goldschmidt-Clermont P J, Mitchison T J. Involvement of profilin in the actin-based motility of Listeria monocytogenes in cells and in cell-free extracts. Cell. 1994;76:505–517. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 654.Theriot J A, Mitchison T J, Tilney L G, Portnoy D A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 655.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Tilney L G, Tilney M S. The wily ways of a parasite, induction of actin assembly by Listeria. Trends Microbiol. 1993;1:25–31. doi: 10.1016/0966-842x(93)90021-i. [DOI] [PubMed] [Google Scholar]
- 657.Titball R W, Hunter S E C, Martin K L, Morris B C, Shuttleworth A D, Rubidge T, Anderson D W, Kelly D C. Molecular cloning and nucleotide sequence of the alpha-toxin (phospholipase C) of Clostridium perfringens. Infect Immun. 1989;57:367–376. doi: 10.1128/iai.57.2.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Tsolis R M, Bäumler A J, Heffron F. Role of Salmonella typhymurium Mn-superoxide dismutase (SodA) in protection against early killing by J774 macrophages. Infect Immun. 1995;63:1739–1744. doi: 10.1128/iai.63.5.1739-1744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 661.Urbaneck D. Zur Neuropathogenese der Listeriose. Histologische Untersuchungen am N. trigeminus bei spontanen zerebraler Form der Listeriose erkrankten Schafen. Arch Exp Vetmed. 1962;20:23–48. [Google Scholar]
- 662.Valenti P, Greco R, Pitari G, Rossi P, Ajello M, Melino G, Antonini G. Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: protective effect of lactoferrin. Exp Cell Res. 1999;250:197–202. doi: 10.1006/excr.1999.4500. [DOI] [PubMed] [Google Scholar]
- 663.Van Langendonck N, Bottreau E, Bailly S, Tabouret M, Marly J, Pardon P, Velge P. Tissue culture assays using Caco-2 cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J Appl Microbiol. 1998;85:337–346. doi: 10.1046/j.1365-2672.1998.00515.x. [DOI] [PubMed] [Google Scholar]
- 664.Van Langendonck N, Velge P, Bottreau E. Host cell protein tyrosine kinases are activated during the entry of Listeria monocytogenes: possible role of pp60c-src family protein kinases. FEMS Microbiol Lett. 1998;162:169–176. doi: 10.1111/j.1574-6968.1998.tb12995.x. [DOI] [PubMed] [Google Scholar]
- 665.Reference deleted.
- 666.Vázquez-Boland J A, Domínguez L, Blanco M, Rocourt J, Fernández-Garayzábal J F, Gutiérrez C B, Tascón R I, Rodríguez-Ferri E F. Epidemiologic investigation of a silage-associated epizootic of ovine listeric encephalitis, using a new Listeria-selective enumeration medium and phage typing. Am J Vet Res. 1992;53:368–371. [PubMed] [Google Scholar]
- 667.Vázquez-Boland J A, Domínguez L, Fernández J F, Rodríguez-Ferri E F, Briones V, Blanco M, Suárez G. Revision of the validity of CAMP tests for Listeria identification. Proposal of an alternative method for the determination of haemolytic activity by Listeria strains. Acta Microbiol Hung. 1990;37:201–206. [PubMed] [Google Scholar]
- 668.Vázquez-Boland J A, Domínguez L, Fernández-Garayzábal J F, Suárez G. Listeria monocytogenes cAMP reaction. Clin Microbiol Rev. 1992;5:343. doi: 10.1128/cmr.5.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Vázquez-Boland J-A, Dominguez L, Rodriguez-Ferri E-F, Suarez G. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinas C and a thiol-activated toxin (ivanolysin O) Infect Immun. 1989;57:3928–3935. doi: 10.1128/iai.57.12.3928-3935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Vázquez-Boland J-A, Dominguez L, Rodriguez-Ferri E-F, Fernandez-Garayzabal J-F, Suarez G. Preliminary evidence that different domains are involved in cytolytic activity and receptor (cholesterol) binding in listeriolysin O, the Listeria monocytogenes thiol-activated toxin. FEMS Microbiol Lett. 1989;65:95–100. doi: 10.1016/0378-1097(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 670a.Vázquez-Boland J A, Domínguez-Bernal G, González-Zorn B, Kreft J, Goebel W. Pathogenicity islands and virulence evolution in Listeria. Microb Infect. 2001;3:571–584. doi: 10.1016/s1286-4579(01)01413-7. [DOI] [PubMed] [Google Scholar]
- 671.Vázquez-Boland J A, Gamallo J A, Ripio M T, Domínguez-Bernal G, Lara M, Vega Y, Mainar R C, Suárez M. Listeriosis animal: aspectos epidemiológicos y diagnósticos, implicaciones en salud pública, y situación en Espafia. Med Vet. 1996;13:333–344. [Google Scholar]
- 672.Vázquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Vega Y, Dickneite C, Ripio M-T, Böckmann R, González-Zorn B, Novella S, Domínguez-Bernal G, Goebel W, Vázquez-Boland J-A. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly 145Ser) mutation increases binding affinity for target DNA. J Bacteriol. 1998;180:6655–6660. doi: 10.1128/jb.180.24.6655-6660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Reference deleted.
- 675.Velge P, Bottreau E, Kaeffer B, Yurdusev N, Pardon P, van Langendonck N. Protein tyrosine kinase inhibitors block the entries of Listeria monocytogenes and Listeria ivanovii into epithelial cells. Microb Pathog. 1994;17:37–50. doi: 10.1006/mpat.1994.1050. [DOI] [PubMed] [Google Scholar]
- 676.Vicente M F, Baquero F, Pérez-Díaz J C. Cloning and expression of the Listeria monocytogenes haemolysin in Escherichia coli. FEMS Microbiol Lett. 1985;30:77–79. [Google Scholar]
- 677.Villanueva M S, Sijts A J A M, Pamer E. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–5233. [PubMed] [Google Scholar]
- 678.Vines A, Reeves M W, Hunter S, Swaminathan B. Restriction fragment length polymorphism in four virulence-associated genes of Listeria monocytogenes. Res Microbiol. 1992;143:281–294. doi: 10.1016/0923-2508(92)90020-o. [DOI] [PubMed] [Google Scholar]
- 679.von Both U, Otten S, Darbouche A, Domann E, Chakraborty T. Physical and genetic map of the Listeria monocytogenes EGD serotype 1/2a chromosome. FEMS Microbiol Lett. 1999;175:281–289. doi: 10.1111/j.1574-6968.1999.tb13632.x. [DOI] [PubMed] [Google Scholar]
- 680.Wadsworth S J, Goldfine H. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun. 1999;67:1770–1778. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Reference deleted.
- 682.Watkins J, Sleath K P. Isolation and enumeration of Listeria monocytogenes from sewage, sewage sludge and river water. J Appl Bacteriol. 1981;50:1–9. doi: 10.1111/j.1365-2672.1981.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 683.Watson B B, Lavizzo J C. Extracellular antigens from Listeria monocytogenes. II. cytotoxicity of hemolytic and lipolytic antigens of Listeria for cultured mouse macrophages. Infect Immun. 1973;7:753–758. doi: 10.1128/iai.7.5.753-758.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Wawrzynow A, Banecki B, Zylicz M. The Clp ATPases define a novel class of molecular chaperones. Mol Microbiol. 1996;21:895–899. doi: 10.1046/j.1365-2958.1996.421404.x. [DOI] [PubMed] [Google Scholar]
- 685.Weiglein L, Goebel W, Troppmair J, Rapp U R, Demuth A, Kuhn M. Listeria monocytogenes infection of HeLa cells results in listeriolysin O-mediated transient activation of the Raf-MEK-MAP kinase pathway. FEMS Microbiol Lett. 1997;148:189–195. doi: 10.1111/j.1574-6968.1997.tb10287.x. [DOI] [PubMed] [Google Scholar]
- 686.Weinberg E D. Pregnanacy-associated depression of cell-mediated immunity. Rev Infect Dis. 1984;6:814–830. doi: 10.1093/clinids/6.6.814. [DOI] [PubMed] [Google Scholar]
- 687.Weis J, Seeliger H P R. Incidence of Listeria monocytogenes in nature. Appl Microbiol. 1975;30:29–32. doi: 10.1128/am.30.1.29-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Weiskirch L M, Paterson Y. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol Rev. 1997;158:159–169. doi: 10.1111/j.1600-065x.1997.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 689.Welch M D, DePace A H, Verma S, Iwamatsu A, Mitchison T J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Welch M D, Iwamatsu A, Mitchison T J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 691.Welch M D, Rosenblatt J, Skoble J, Portnoy D A, Mitchison T J. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- 692.Welshimer H J, Donker-Voet J. Listeria monocytogenes in nature. Appl Microbiol. 1971;21:516–519. doi: 10.1128/am.21.3.516-519.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Wesley I V. Listeriosis in animals. In: Ryser E T, Marth E H, editors. Listeria, listeriosis, and food safety. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1999. pp. 39–73. [Google Scholar]
- 694.West S E H, Sample A K, Runyen-Janecky L J. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol. 1994;176:7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Wiedmann M, Arvik T J, Hurley R J, Boor K J. General stress transcription factor ςB and ist role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Wiedmann M, Bruce J L, Keating C, Johnson A E, McDonough P L, Batt C A. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect Immun. 1997;65:2707–2716. doi: 10.1128/iai.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Williams A E, Blakemore W F. Pathogenesis of meningitis caused by Streptococcus suis type 2. J Infect Dis. 1990;162:474–481. doi: 10.1093/infdis/162.2.474. [DOI] [PubMed] [Google Scholar]
- 698.Williams J R, Thayyullathil C, Freitag N E. Sequence variations within PrfA DNA binding sites and effects on Listeria monocytogenes virulence gene expression. J Bacteriol. 2000;182:837–841. doi: 10.1128/jb.182.3.837-841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Wilson S L, Drevets D A. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis. 1998;178:1658–1666. doi: 10.1086/314490. [DOI] [PubMed] [Google Scholar]
- 700.Winter D, Lechler T, Li R. Activation of the Arp2/3 complex by Bee 1p, a WASP-family protein. Curr Biol. 1999;9:501–504. doi: 10.1016/s0960-9822(99)80218-8. [DOI] [PubMed] [Google Scholar]
- 701.Wood S, Maroushek N, Czuprinski C J. Multiplication of Listeria monocytogenes in a murine hepatocyte cell line. Infect Immun. 1993;61:3068–3072. doi: 10.1128/iai.61.7.3068-3072.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Wuenscher M D, Köhler S, Bubert A, Gerike U, Goebel W. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993;175:3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Yap A S, Niessen C M, Gumbiner B M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Yarar D, To W, Abo A, Welch M D. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr Biol. 1999;9:555–558. doi: 10.1016/s0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- 705.Yoder M D, Keen N T, Jurnak F. New domain motif: the structure of the pectate lyase C, a secreted plant virulence factor. Science. 1993;260:1503–1507. doi: 10.1126/science.8502994. [DOI] [PubMed] [Google Scholar]
- 706.Yoshikai Y. The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense. Immunol Res. 2000;20:219–235. doi: 10.1007/BF02790405. [DOI] [PubMed] [Google Scholar]
- 707.Yoshikawa H, Kawamura I, Fujita M, Tsukada H, Arawaka M, Mitsuyama M. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect Immun. 1993;61:1334–1339. doi: 10.1128/iai.61.4.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Zalevsky J, Grigorova I, Mullins R D. Activation of the Arp2/3 complex by the Listeria ActA protein: ActA binds two actin monomers and three subunits of the Arp2/3 complex. J Biol Chem. 2001;276:3468–3475. doi: 10.1074/jbc.M006407200. [DOI] [PubMed] [Google Scholar]
- 709.Zhan Y, Cheers C. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect Immun. 1995;63:720–723. doi: 10.1128/iai.63.2.720-723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Zimmermann P, David G. The syndecans, tuners of transmembrane signalling. FASEB J. 1999;13:S91–S100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]