Introduction
For patients with locally advanced breast cancer (LABC) involving the skin surface with or without distant metastasis who are in an inoperable state or who refuse surgery, radiation therapy, as a palliative therapy for breast tumor control, bleeding prevention, and pain control, is considered to be the treatment option to improve the patients’ quality of life.1 However, in radiation therapy, the cell-killing effect for hypoxic cancer cells using photon or electron therapy, which is a low linear energy transfer (LET) radiation therapy, is weak, and patients who have LABC with a large tumor may have a large hypoxic component area.2 Therefore, the long-term local control of large LABC tumors exposed to the skin surface using low LET radiation therapy may be difficult.
In recent years, studies have shown that hydrogen peroxide has favorable radiosensitizing effects with low LET radiotherapeutic modality.3, 4, 5 The biological mechanism of hydrogen peroxide as a radiosensitizer is considered to be that irradiation in the presence of hydrogen peroxide induces reactive oxygen species formation, oxidative DNA damage, dysfunction of the mitochondrial membrane potential, and early apoptotic changes.6 In a previous clinical report, the radiosensitizing effect was obtained by placing a gauze bolus soaked in a hydrogen peroxide solution over the irradiated area of the tumor that involved the skin surface.5 Obtaining this radiosensitizing effect using a hydrogen peroxide–soaked bolus does not require any special technique, and it can be performed at any facility. However, to date, there have been limited reports on the radiosensitizing effects of a bolus soaked in a hydrogen peroxide solution.
Herein, we report a case of inoperable LABC involving the skin surface that was treated using x-ray radiation therapy and a gauze bolus soaked in a hydrogen peroxide solution, with favorable local effects and without severe toxic effects.
Case Presentation
Patient
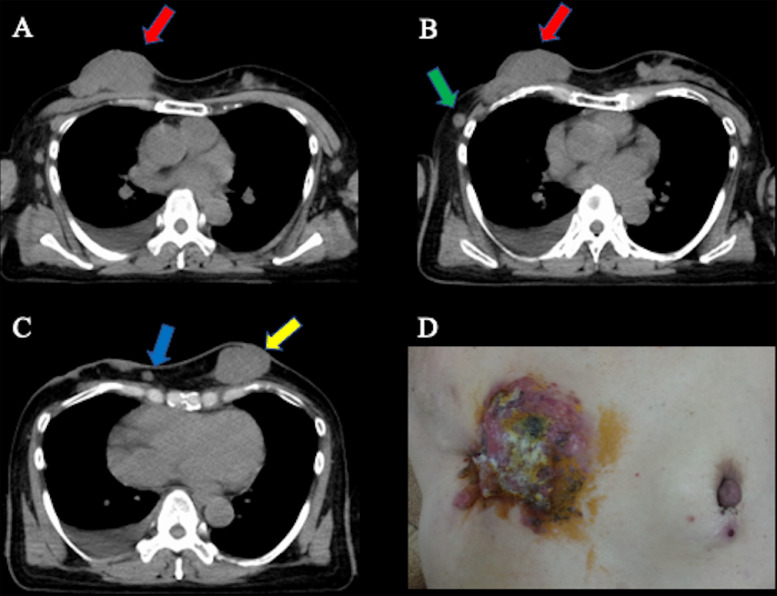
A 54-year-old female Japanese patient with primary LABC involving the skin surface, with ipsilateral and contralateral breast, liver, bone, and lymph node metastases, was referred to the Department of Radiation Oncology. The pathologic diagnosis was estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative invasive ductal carcinoma with a Ki-67 index of 25% to 37%. The clinical stage was T4bN2aM1 stage IV based on the eighth edition of the Union for International Cancer Control–American Joint Committee on Cancer TNM staging system.7 Before referral to our department, the patient had already received hormone therapy (including tamoxifen, letrozole, luteinizing hormone-releasing hormone [LH-RH] agonist, and fulvestrant), chemotherapy (epirubicin + cyclophosphamide, docetaxel, vinorelbine detartrate, tegafur–gimeracil–oteracil, and capecitabine), molecular target therapy (palbociclib), and zoledronic acid therapy. The patient refused surgery for breast tumor control. Figure 1A-C shows the computed tomography (CT) of the breast tumor (68 × 33 × 70 mm) and the multiple ipsilateral (12 × 10 × 12 mm and 11 × 7 × 9 mm) and contralateral (47 × 29 × 51 mm) breast metastases. Figure 1D shows the local findings of the LABC involving the skin surface. Radiation therapy was planned for breast tumor control. During the radiation therapy, we used a bolus to prevent a decrease in the surface dose in order to avoid the build-up characteristic of x-rays. The patient continued receiving the LH-RH agonist, fulvestrant, and zoledronic acid therapy during radiation therapy.
Fig. 1.
Computed tomography and the local finding of locally advanced breast cancer (LABC) involving the skin surface (68 × 33 × 70 mm) with ipsilateral (12 × 10 × 12 mm and 11 × 7 × 9 mm) and contralateral (47 × 29 × 51 mm) breast metastases. (A) Axial image. Red arrow shows the primary breast tumor. (B) Axial image. Red and green arrows show the primary breast tumor and ipsilateral breast metastasis, respectively. (C) Axial image. Blue and yellow arrows show the ipsilateral and contralateral breast metastases, respectively. (D) The local findings of LABC involving the skin surface.
Radiation therapy and hydrogen peroxide solution–soaked gauze bolus
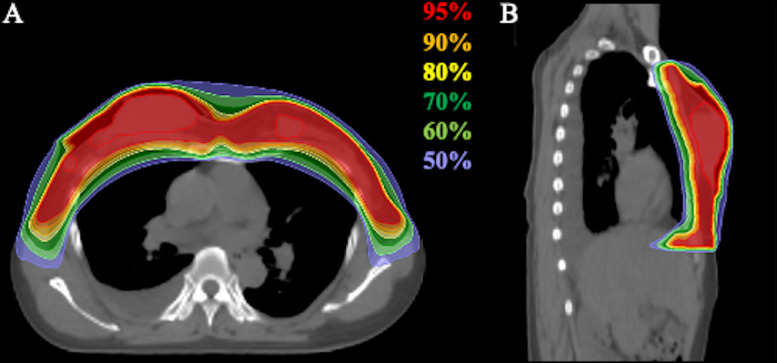
Before the radiation therapy, treatment planning CT was performed. The gross tumor volume was delineated on planning CT. The clinical target volume was the bilateral whole breast, with a margin of at least 5 mm around the gross tumor volume. The planning target volume was defined as the sum of the clinical target volume, internal margin, and setup margin. Radiation therapy was performed using an x-ray beam with helical tomotherapy (Accuray Inc, Sunnyvale, CA), and the administration dose for the bilateral whole-breast irradiation was 51 Gy in 17 fractions. Radiation therapy was performed 5 times per week. Figure 2 shows the dose distribution of the radiation therapy.
Fig. 2.
Dose distribution of radiation therapy with helical tomotherapy. (A) Axial image. (B) Sagittal image. The area within the red outline is the gross tumor volume. Highlighted are 95% (red), 90% (orange), 80% (yellow), 70% (dark green), 60% (light green), and 50% (light blue) isodose curves (100% was 51 Gy).
A water-soaked gauze bolus is often used in x-ray radiation therapy; however, a gauze bolus soaked in a hydrogen peroxide solution (Oxydol, which is 2.5%-3.5% hydrogen peroxide) was used in this patient for its radiosensitizing effect. At each time of irradiation, the surface of the tumor was covered with a bolus consisting of 5 sheets of 4-fold gauze, with a thickness of 5 mm, soaked in the hydrogen peroxide solution. We waited for at least 5 minutes after placing the bolus to allow the hydrogen peroxide solution to soak deeply into the tumor before treatment positioning.5
Megavoltage CT (MVCT) was performed before each fraction was acquired. The automatic fusion from MVCT to planning CT was performed based on the regions of interest. Next, manual fusion was performed to improve the registration accuracy by verifying the axial, coronal, and sagittal images of each fraction. Once the registration was approved, the couch was positioned according to the determined registration coordinates, and the treatment was started. The daily use of the pretreatment MVCT imaging for the patient setup verification allowed for the correction of the interfraction setup error.
Toxic effects were assessed using the Common Terminology Criteria for Adverse Events, version 4.0.8 The tumor response was assessed using the Response Evaluation Criteria in Solid Tumors, version 1.1.9 Before the initiation of therapy, informed consent for this treatment and publication of this case report was obtained from the patient.
Results
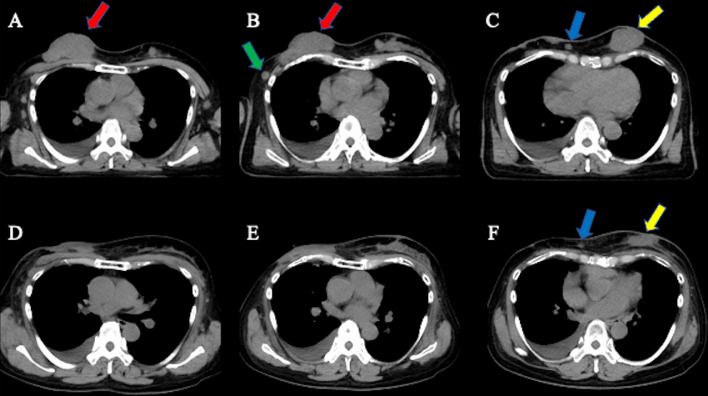
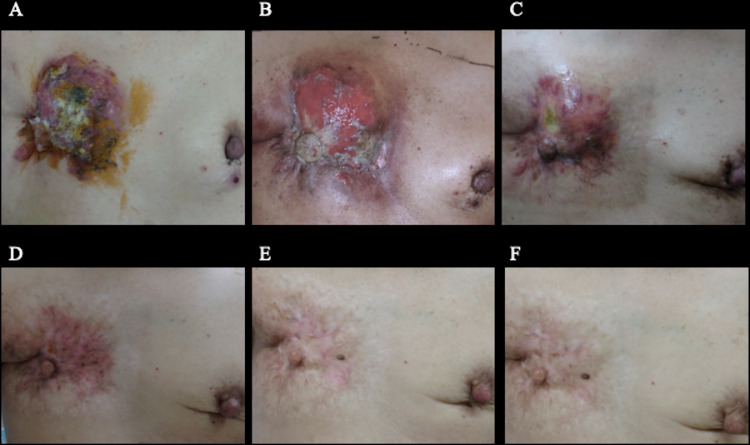
The patient completed radiation therapy using a hydrogen peroxide solution–soaked gauze bolus. Palliative radiation therapy for bone metastases was also performed. After completion of radiation therapy, the patient received palbociclib, an LH-RH agonist, fulvestrant, and zoledronic acid therapy. During radiation therapy, the patient experienced grade 3 acute radiation dermatitis (Fig 3B), which was treated with topical steroids. At 2 months after radiation therapy, the breast tumor was reduced in size, and the acute radiation dermatitis improved (Fig 3C). At 4 months after radiation therapy, the tumor showed a clinically complete response in the primary tumor and partial response in the contralateral breast metastasis (Fig 4B and 4C). Six months after radiation therapy, the site of self-destruction was epithelialized (Fig 3D). Figure 3E and 3F show the changes in the local findings at 12 and 19 months after radiation therapy, respectively. At 19 months after radiation therapy, the patient was alive without evidence of local recurrences or late toxic effects.
Fig. 3.
Treatment response and skin reactions regarding the local findings after radiation therapy. (A) Before the initiation of the radiation therapy. (B) At the end of radiation therapy. Grade 3 dermatitis is observed. (C) At 2 months after radiation therapy, the breast tumor has reduced in size, and the acute radiation dermatitis has improved. (D) At 6 months after radiation therapy, the site of self-destruction is epithelialized, and the primary tumor shows clinically complete response. (E) At 12 months after radiation therapy, no evidence of local recurrence and no late dermatitis are seen. (F) At 19 months after radiation therapy, no evidence of local recurrence and no late dermatitis are seen.
Fig. 4.
Treatment response of radiation therapy assessed by computed tomography (CT). (A) Axial CT image before radiation therapy. Red arrow shows the primary breast tumor. (B) Axial CT image before radiation therapy. Red and green arrows show the primary breast tumor and ipsilateral breast metastasis, respectively. (C) Axial CT image before radiation therapy. Blue and yellow arrows show the ipsilateral and contralateral breast metastases, respectively. (D) Axial CT image at 4 months after radiation therapy. The primary breast tumor shows a clinically complete response, referred to by the red arrow in part A. (E) Axial CT image at 4 months after radiation therapy. The primary breast tumor and ipsilateral breast metastasis show clinically complete response, referred to by red and green arrows in part B. (F) Axial CT image at 4 months after radiation therapy. The ipsilateral and contralateral breast metastases show a clinically partial response, referred to by blue and yellow arrows in part C.
Discussion
We observed a favorable clinical effect with x-ray radiation therapy using a gauze bolus soaked in a hydrogen peroxide solution for an inoperable primary LABC involving the skin surface. We considered that the bolus might have contributed to the favorable clinical results as a radiosensitizer, because hydrogen peroxide solution may show radiosensitizing and high local effects on tumors, with tolerable toxic effects.
With regard to the treatment of radioresistant tumors (eg, bone and soft-tissue sarcomas, melanomas, glioblastoma multiformes, and hypoxic tumors), although favorable therapeutic results have been reported with high-LET radiation such as carbon ion radiation therapy and boron neutron capture therapy,10, 11, 12, 13 these treatment modalities are not yet widely available, and low-LET radiation therapy such as photon and electron radiation therapy is the mainstream treatment used worldwide. Therefore, radiosensitizers (eg, metronidazole and misonidazole) have been developed to increase the radiotherapeutic effect in low-LET radiation therapy for radioresistant tumors. However, these radiosensitizers are not widely used owing to their uncertain effects and toxicity. Regarding the effects and toxicity of hydrogen peroxide solution as a radiosensitizer, favorable radiosensitizing effects with tolerable toxic effects were reported in 1 study with hydrogen peroxide solution–soaked gauze boluses, although in a small number of patients. Furthermore, in a limited number of studies, favorable radiosensitizing effects were reported with local injections, hydrogen peroxide, and sodium hyaluronate.3, 4, 5,14 In the current study, as in previous studies, although the evidence was at the level of a case report, we observed a favorable clinical effect, which might contribute to the increased use of gauze boluses soaked in a hydrogen peroxide solution. Furthermore, the use of such a bolus does not require any special technique and can be performed easily.
In the current study, we aimed to achieve long-term tumor control, and a higher dose administration was required than for conventional palliative irradiation. Additionally, the previous clinical report of radiation therapy with a hydrogen peroxide solution–soaked gauze bolus used the administration dose of 48 Gy in 12 fractions.5 This irradiation dose is approximately 67.2 Gy in biological effective dose 10 (BED10) (α/β = 10) and 112.0 Gy in BED3 (α/β = 3).15 In contrast, the administration dose of 51 Gy in 17 fractions is approximately 66.3 Gy in BED10 and 102.0 Gy in BED3. These doses of BED10 and BED3 mean that 51 Gy in 17 fractions might have had an almost equal anticancer effect with a lower possibility for toxic effects compared with the previously reported administration dose of 48 Gy in 12 fractions. Therefore, we selected this dose of 51 Gy in 17 fractions.
One of the key points in the use of radiosensitizers is the control of toxic effects. With regard to toxic effects, the patients developed grade 3 acute radiation dermatitis. However, high-dose administration to the skin was necessary because the tumor involved the skin surface, and dermatitis, which was considered to be within the tolerable range, could be controlled with the use of topical steroids. No late toxic effects were observed. Additionally, another previous study showed no severe toxic effects with the use of a hydrogen peroxide solution–soaked gauze bolus.5 Based on the findings of previous studies and the current case, a gauze bolus soaked in a hydrogen peroxide solution is considered to be a safe radiosensitizer.5
There are several treatment options for breast tumor control. Surgery is one of them; however, in the current case, the patient refused surgery. Another treatment option is Mohs chemosurgery, which has been used for palliative treatment in skin-involved LABC to improve malodor, bleeding, exudate, and pain, with favorable results for the improvement of the patient's quality of life.16 Although no study has compared radiation therapy and Mohs chemosurgery with respect to the efficacy of palliative treatment, patients who receive palliative treatment have different backgrounds (eg, treatment histories, age, performance statuses, life expectancies, and symptoms), and therefore, tailored palliative treatment should be performed. In the current case, the patient had multiple ipsilateral and contralateral breast metastases, and bilateral whole-breast irradiation was needed; therefore, we performed radiation therapy.
To date, because there have been no studies to our knowledge on the direct comparison of gauze boluses soaked in a hydrogen peroxide solution versus water-soaked gauze boluses, the extent of the radiosensitizing effect is unclear. Although the local effect was favorable in the current case, it is unclear how much the bolus contributed to the local effect. Additionally, it is also unclear how severe the toxic effects that may develop with the use of a hydrogen peroxide solution–soaked bolus will be, compared with the use of a water-soaked bolus. In the future, multicenter retrospective analyses or randomized controlled trials will be necessary to compare the efficacy and safety between the use of a gauze bolus soaked in a hydrogen peroxide solution and one soaked in water.
Conclusion
We report a case of LABC involving the skin surface treated with x-ray radiation therapy using a gauze bolus soaked in a hydrogen peroxide solution that showed favorable local effects. Although grade 3 acute radiation dermatitis was observed, it was considered tolerable. The use of a hydrogen peroxide solution–soaked gauze bolus as a radiosensitizer does not require any special technique and can be performed easily. On the other hand, there are insufficient data to support the benefits of using a bolus soaked in a hydrogen peroxide solution as a radiosensitizer; therefore, further clinical research is needed.
Acknowledgments
The authors thank A.I. and their colleagues at Hanyu General Hospital and Editage (www.editage.com) for English language editing.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Yee C, Alayed Y, Drost L, et al. Radiotherapy for patients with unresected locally advanced breast cancer. Ann Palliat Med. 2018;7:373–384. doi: 10.21037/apm.2018.05.13. [DOI] [PubMed] [Google Scholar]
- 2.Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: A target for selective cancer therapy. Cancer Sci. 2003;94:1021–1028. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimalasena S, Gothard L, Anbalagan S, et al. Intratumoral hydrogen peroxide with radiation therapy in locally advanced breast cancer: Results from a phase 1 clinical trial. Int J Radiat Oncol Biol Phys. 2020;108:1019–1029. doi: 10.1016/j.ijrobp.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama N, Ogawa Y, Yasuoka M, et al. Therapeutic response to a novel enzyme-targeting radiosensitization treatment (KORTUC II) for residual lesions in patients with stage IV primary breast cancer, following induction chemotherapy with epirubicin and cyclophosphamide or taxane. Oncol Lett. 2017;13:69–76. doi: 10.3892/ol.2016.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa Y, Ue H, Tsuzuki K, et al. New radiosensitization treatment (KORTUC I) using hydrogen peroxide solution-soaked gauze bolus for unresectable and superficially exposed neoplasms. Oncol Rep. 2008;19:1389–1394. [PubMed] [Google Scholar]
- 6.Ogawa Y, Takahashi T, Kobayashi T, et al. Apoptotic-resistance of the human osteosarcoma cell line HS-Os-1 to irradiation is converted to apoptotic-susceptibility by hydrogen peroxide: A potent role of hydrogen peroxide as a new radiosensitizer. Int J Mol Med. 2003;12:845–850. [PubMed] [Google Scholar]
- 7.Sobin LH, Gospodarowicz MK, Wittekind C, et al. 8th ed. Wiley-Blackwell; Oxford: 2017. International Union Against Cancer (UICC): TNM Classification of Malignant Tumours. [Google Scholar]
- 8.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed August 11, 2021.
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Shiba S, Okamoto M, Kiyohara H, et al. Impact of carbon ion radiotherapy on inoperable bone sarcoma. Cancers (Basel) 2021;13:1099. doi: 10.3390/cancers13051099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano T, Suzuki Y, Ohno T, et al. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res. 2006;12:2185–2190. doi: 10.1158/1078-0432.CCR-05-1907. [DOI] [PubMed] [Google Scholar]
- 12.Kageji T, Mizobuchi Y, Nagahiro S, Nakagawa Y, Kumada H. Clinical results of boron neutron capture therapy (BNCT) for glioblastoma. Appl Radiat Isot. 2011;69:1823–1825. doi: 10.1016/j.apradiso.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Hiratsuka J, Kamitani N, Tanaka R, et al. Long-term outcome of cutaneous melanoma patients treated with boron neutron capture therapy (BNCT) J Radiat Res. 2020;61:945–951. doi: 10.1093/jrr/rraa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa Y, Kubota K, Ue H, et al. Safety and effectiveness of a new enzyme-targeting radiosensitization treatment (KORTUC II) for intratumoral injection for low-LET radioresistant tumors. Int J Oncol. 2011;39:553–560. doi: 10.3892/ijo.2011.1069. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JF. Review: Total doses in fractionated radiotherapy—Implications of new radiobiological data. Int J Radiat Biol Relat Stud Phys Chem Med. 1984;46:103–120. doi: 10.1080/09553008414551181. [DOI] [PubMed] [Google Scholar]
- 16.Arima M, Saito K, Maeda T, Fukushima H, Iwata Y, Sugiura K. Clinical usefulness of a modified Mohs’ technique and topical application of zinc oxide powder for treating skin infiltration caused by unresectable malignant tumors. Palliat Med Rep. 2021;2:168–174. doi: 10.1089/pmr.2020.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]