Abstract
Background:
In Denmark the boundaries between cannabis as an illicit drug and licit medicine have shifted rapidly in recent years, affecting also policy. However, the vast majority of Danes, who use cannabis as medicine (CaM) continue to rely on the unregulated market for supply. This study explores patterns of use and motives for use of CaM in Denmark.
Methods:
An anonymous online survey was made available to a convenience sample of users of CaM from July 14, 2018 to November 1, 2018. Participants were recruited through patient organisations, social and public media, and the illegal open cannabis market.
Results:
Of the final sample (n = 3,021), a majority were women (62.6%) and the mean age was 49 years. Most had no prescription for CaM (90.9%), a majority had no or limited previous experience with recreational cannabis use (63.9%), and had used CaM for two years or less (65.0%). The most common form of intake was oil (56.8%) followed by smoke (24.0%). CBD oil (65.0%) was used more than hash, pot or skunk (36.2%). Most frequent conditions treated were chronic pain (32.0%), sleep disturbances (27.5%), stress (23.7%), osteoarthritis (22.7%), anxiety (19.6%), and depression (19.6%). Overall, users experienced CaM to be effective in managing somatic and mental health conditions and reported relatively few side-effects. CBD oil only users were more likely to be women, older, have limited recreational experience and have initiated use recently.
Conclusions:
A new user group has emerged in Denmark that, for the most part, use illegally sourced CaM to treat a broad range of somatic and mental health conditions, often with experienced effect and relatively low level of side-effects. The prevalent use of low-potency CBD oil indicates an interest in effects beyond the high normally associated with cannabis use. More clinical research into the effects and side-effects of CaM is needed to draw the boundaries of the medical utility of cannabis.
Keywords: cannabis, CBD, medical cannabis, medicinal cannabis, motives for use, user patterns
The first documented use of cannabis as medicine (CaM) dates back almost 5,000 years (Touw, 1981) and at the turn of the 19th century, cannabis was an integral part of medicine in Europe and the United States, where commercial preparations of cannabis tinctures, pills, and extracts were used mainly as analgesic, anti-inflammatory, and anti-spastic drugs (Pisanti & Bifulco, 2017). As recreational cannabis use increased in the latter half of the 20th century, cannabis was prohibited, and the plant’s medical use was all but forgotten (Zuardi, 2006). In the new millennium, the boundaries between cannabis as an illicit “drug” and licit “medicine” have shifted once again, and it has been argued that we are witnessing a re-medicalisation of cannabis (O’Brien, 2013; Pedersen & Sandberg, 2013; Taylor, 2008), which for a large part is driven by three forces: (1) The discovery of the endogenous cannabinoid system (eCS) in the 1980s; (2) The pharmaceutical industry and its interest in the eCS, which led to the development and licensing of cannabis-based medicines; (3) The user demand for safe access to medical cannabis (Taylor, 2008). In recent years, the user demand for medical cannabis has intensified (Hurley, 2018; Pacula & Sevigny, 2014) and cannabis policy has undergone rapid changes in various jurisdictions around the world, with more than 30 states in the US (National Conference of State Legislatures, 2020) and several European countries (Abuhasira et al., 2018) adopting medical cannabis laws.
In Denmark, the user demand has been a substantial factor in the public health debate, and in shaping the current cannabis policy. For years, patient organisations have advocated for access to medical cannabis (i.e., legal, prescribed cannabis) (Færch, 2014), which have been supported by Danish celebrities and politicians, who have been vocal about their medicinal cannabis use (i.e., no prescription, illicitly sourced cannabis) for their somatic and mental health conditions (Elabdi & Ehrbahn, 2017; Jørgensen, 2017). That the public debate on CaM has received widespread attention in recent years is underlined by a content analysis of Danish newspapers that found that articles on CaM increased from approximately 2% in 2012 to 16% in 2016 (Houborg & Enghoff, 2018), and a national survey in 2017 that found that 80% of Danes supported a legalisation of medical cannabis (Blackman, 2017).
In November 2016, a large majority of the Danish parliament agreed to initiate a four-year medical cannabis pilot programme (MCPP) from January 2018. The purpose of the MCPP was to establish a “safe framework for the use of medical cannabis within the healthcare system (…) providing a legal alternative for some of the patients who are already self-medicating with cannabis” (Ministry of Health, 2016, p. 2). As instructed by the political parties, The Danish Medicines Agency recommended four patient groups, where there existed some documentation that medical cannabis may have an effect (Lægemiddelstyrelsen, 2019a, our translation) to be included in the MCPP: treatment-resistant patients with multiple sclerosis, spinal cord injury, chronic pain (with a neuralgic component) or side-effects from chemotherapy (Lægemiddelstyrelsen, 2019a). Thus, the MCPP marked a paradigm shift in Denmark, as it gave selected patient groups access to the cannabis flower as medicine, and from January 2018 to July 2019, 2,133 patients have been included in the trial (Danish Health Data Authority, 2019b). However, several factors indicate that the vast majority of cannabis consumption with a medical purpose still occurs outside the safe and legal framework that the MCPP intended to create. A national poll estimated already in 2016 that at least 50,000 Danes use CaM, and there has been a considerable growth in web-shops selling low-potency cannabis oil (Damløv, 2016). Moreover, there are several Danish social media support groups offering guidance in medicinal use of cannabis oil. Furthermore, cannabis oil has for some time been a standard commodity at the largest open illegal cannabis market, Christiania, in the capital of Denmark (Færch, 2013), and the Danish police and customs have reported an increase in confiscations of cannabis oil (Sørensen & Skaaning, 2017). Users of cannabis oil are in a legal grey area, as sale and possession of a cannabis product with less than 0.2% tetrahydrocannabinol (THC) became legal in 2018, unless the cannabis product is regarded as a drug by the Danish Medicines Agency (Lægemiddelstyrelsen, 2018). The Danish Medicines Agency estimates that most cannabis oil sold online, containing less than 0.2% THC, are in fact drugs, and therefore illegal to sell (but not illegal to possess) (Lægemiddelstyrelsen, 2019b).
In order to evaluate the public health effects of the current use of CaM and to qualify future cannabis policy, two areas are important to investigate: patterns of use and motives for use. Patterns of use are important, due to the complexity of the cannabis plant and the various forms of intake, resulting in diverse effects of using CaM. To date, 120 cannabinoids and 445 non-cannabinoids have been identified (Bonn-Miller et al., 2018), of which the primary focus have been the cannabinoids tetrahydrocannabinol (THC) and cannabidiol (CBD). A growing body of evidence suggest that THC and CBD display opposing neural, cognitive, and behavioural effects (Colizzi & Bhattacharyya, 2017; Rømer Thomsen et al., 2017), and that CBD may have a superior safety profile (Bergamaschi et al., 2011; Iffland & Grotenhermen, 2017) and limited abuse potential (Schoedel et al., 2018) compared to THC. Nonetheless, pre-clinical and clinical studies have found therapeutic effects of THC (National Academies of Sciences Engineering and Medicine, 2017) and THC may be more effective when combined with CBD (Boggs et al., 2018; Russo, 2011) and other components of the cannabis plant (McPartland & Russo, 2014). Moreover, the effects of cannabis use depend on mode of intake (Newmeyer et al., 2017); while inhalation has a rapid onset, onset is delayed when ingesting (30–60 minutes), which may increase the risk of overdosing (Grotenhermen, 2001), but hold fewer health risks compared to smoking cannabis (Russell et al., 2018).
An equally important area to investigate is motives for use, as it is relevant to know which conditions and symptoms drive some users to disregard advice from the medical authorities in Denmark and consult an illegal market for medicine. Users of CaM risk stigmatisation (Satterlund et al., 2015), criminalisation (in cases where the cannabis used contains more than 0.2% THC), and hazards of interacting with an illegal market, so it is important to know what motivates them to take these risks. Motives for both medicinal and medical cannabis use have been explored on a larger scale in the United Kingdom (Ware et al., 2005), Australia (Lintzeris et al., 2018), Norway (Pedersen & Sandberg, 2013), Canada (Lucas et al., 2019; Walsh et al., 2013), and cross-nationally (Corroon & Phillips, 2018; Hazekamp et al., 2013; Sexton et al., 2016). A recent literature review found that pain was the most common motive for use of CaM, and that users of CaM frequently report symptom relief of pain conditions, sleep disturbances and anxiety symptoms (Park & Wu, 2017).
The current body of scientific literature on the health effects of cannabis and cannabinoids has set the parameters for the legal use of medical cannabis in Denmark, but we lack knowledge about the parameters set by both the medical and medicinal users in practice. This is important, as user perspectives are instrumental in elucidating perspectives on cannabis use unseen by society at large (Dahl, 2004; Hakkarainen et al., 2015). To our knowledge, the use of CaM in Denmark has, until now, only been explored in selected samples, i.e., small-scale cannabis cultivators (Dahl & Frank, 2011; Hakkarainen et al., 2015) and specific patient groups (Gustavsen et al., 2019).
It is of particular public health interest to study the use of low-potency CBD oil with a medical motive. In recent years, there has been a dramatic increase in the availability of CBD-based cannabis products globally, while regulatory control is lacking (Hazekamp, 2018; Manthey, 2019). However, to our knowledge, there are no systematic studies on users of CBD products in Europe. The trend in use of low-potency CBD oil is important to study, because it seeks to exclude the main component, THC, that causes the psychotropic effects of cannabis (Colizzi & Bhattacharyya, 2017; McPartland & Russo, 2014). The availability of these relatively novel cannabis products that seek to exclude effects conventionally associated with cannabis use (the “high”), is likely to attract novice users who would have otherwise refrained from using cannabis products (Manthey, 2019).
The aim of this study was to characterise the users of CaM in Denmark, and to map patterns and motives related to the use of CaM. An additional aim was to explore user characteristics of those who use low-potency CBD oil only.
Methods
Design
The present study is part of a larger study on the use of CaM in Denmark. A novel survey was developed inspired by previous surveys on medicinal and medical cannabis use (Grotenhermen & Schnelle, 2003; Hazekamp et al., 2013; Reiman et al., 2017; Reinarman et al., 2011; Sexton et al., 2016; Ware et al., 2005; Webb & Webb, 2014), and was tested and revised following nine pilot interviews with users of CaM as well as input from researchers and selected others with insight into the use of CaM in Denmark. The questionnaire consisted of 42 structured questions and 21 possible follow-up questions, answered in a Yes/No format, multiple-choice response, and rating scales. All questions included the possibility to answer “Do not know” or “Do not wish to answer”, except for questions on conditions treated and evaluation of effect of use. Questions used for the current study involved six key domains: sociodemographics, motivation for use, duration and frequency of use, method of administration, evaluation of experienced effect, and adverse effects. The questionnaire took approximately 15 minutes to complete, and was available in Danish only. Data were collected through Survey-Xact. IP addresses of the respondents were not saved, or available to the researchers, as the respondents’ fear of loss of anonymity was considered a greater issue than the possibility of repeated participation (Barratt et al., 2017).
Sampling and recruitment
The survey was made available online to a self-selected convenience sample of users of CaM from July 14, 2018 to November 1, 2018. Inclusion criteria were age 18 years or older and being a current or former user of CaM (it was possible to answer on behalf of someone else – next of kin). Participants were recruited online and via flyers and posters containing survey information, a survey link and QR code. Recruitment material was disseminated on Facebook groups for medicinal and recreational cannabis use, through patient organisations, in selected doctors’ offices and hospitals, at the illegal open drug market, Christiania, at the first Cannabis Expo in Denmark held in Copenhagen, and via headshops selling cannabis-related items. Additionally, the survey was made available to users of Smokeboddy (an app where users monitor potential police presence on the illegal open drug market in Christiania) and the survey was reported on by the national media, The Danish Broadcasting Corporation. Previous studies have found Facebook to be a valuable recruitment tool in hard-to-reach populations (Weiner et al., 2017), and this study made great use of the platform, since many Facebook administrators agreed to share information on the survey with group members, increasing the reach and trustworthiness of the survey among potential respondents. In order to engage prospective participants (Miller & Sonderlund, 2010), the primary researcher was available on the platform to answer questions related to the study throughout the recruitment period.
Measures
Patterns of use
Respondents were presented with a list of cannabis products, and could choose more than one. Respondents were presented with a list of forms of most frequent intake, and could choose only one. All users were asked to indicate their daily dose of cannabis, either as drops of oil or grams of plant matter. Users of CBD oil and THC oil were asked to indicate the strength of the oil as a percentage. Further, we asked users to indicate the level of “recreational (non-medicinal)” use of cannabis before their use of CaM.
Motives for use
Respondents were presented with a list of 52 somatic and mental health conditions, and asked to indicate conditions for which they used CaM. The 52 conditions were categorised as either somatic conditions (n = 37) or psychiatric conditions (n = 13) (see Appendix 1) based on ICD-10 classifications, except for “sleep disturbances” and “stress”, which were kept as independent categories as they are relatively large and difficult to classify. For every chosen condition, respondents were asked to indicate the purpose of their use by choosing one or more of three listed purposes (“symptom relief”, “cure”, and “managing side-effects of other treatment”). Respondents were asked if they had used CaM with the purpose of replacing a prescribed drug. The experienced effect of using CaM was evaluated on the symptoms of each of the chosen conditions on a nine-point Likert scale (–4 = very large aggravation and +4 = very large improvement). Respondents were asked which side-effects they had experienced when using CaM, and were presented with a list of 18 potential side-effects. Further, respondents were presented with a list of 17 potential symptoms, and asked to indicate on which symptoms they had experienced an effect of CaM. Respondents who indicated that CaM had an effect on relief from pain rated their experienced pain with and without the use of CaM on a pain visual analogue scale, ranging from (0 = no pain to 10 = worst pain imaginable) (VAS; Jensen et al., 2003). Respondents who indicated that CaM had an effect on relief from sleep disturbances were ask to report the increase in number of hours slept. To evaluate the overall effect of using CaM on daily life, respondents were asked to evaluate three statements on a five-point Likert scale (0 = Strongly disagree to 5 = Strongly agree).
Data analysis
Statistical analyses were conducted using Stata SE/15. Means with standard deviations and simple proportions were used to describe respondent characteristics, patterns of use and motives for use. The overall experienced effect of CaM was calculated by dividing the total experienced effect by the total number of conditions reported. Shapiro-Wilks tests were used to assess normality before choosing a test for comparison of means. As none of the variables were normally distributed, the Wilcoxon signed-rank test was used to assess the difference between means related to experienced effect of CaM on sleep and pain. We coded a dummy variable on CBD oil only use, distinguishing between those who exclusively used CBD oil and those who used other forms of cannabis, with CBD oil only coded as 1. This variable was used as the dependent variable in the logistic regression analysis. Odds ratios (ORs) were used to estimate the strength of association.
Ethics
All data collected were anonymous. Participants could withdraw from the survey at any time before completion without any data being included in the results. Participants who agreed to be contacted at a later stage for participation in a qualitative interview were asked to list their contact information. Data were stored on secure servers, and procedures for data handling and storage were approved by the Danish Data Protection Agency. Since the data used for this study were collected and stored for monitoring, no ethics evaluation was needed under Danish law.
Results
Sample characteristics
A total of 4,570 respondents opened the survey, and 3,140 answered all questions. Of these, 119 respondents were excluded: 59 were under the age of 18 years, seven respondents had inconsistencies in answers, and 53 were identified as duplicates, leaving a total number of respondents of 3,021 (see Table 1).
Table 1.
Sample characteristics.
| N = 3,021 | |
|---|---|
| n (%) | |
| Gender | |
| Male | 1,097 (36.3) |
| Female | 1,891 (62.6) |
| Other | 6 (0.2) |
| Missing | 27 (0.9) |
| Age (years) | |
| Mean | 49 (SD 13.8) |
| 18–24 | 138 (4.6) |
| 25–34 | 356 (11.8) |
| 35–44 | 583 (19.3) |
| 45–54 | 831 (27.6) |
| 55–64 | 710 (23.6) |
| 65–74 | 328 (10.9) |
| > 75 | 68 (2.3) |
| Missing | 7 (0.2) |
| Current employment | |
| Full-time employment | 767 (25.4) |
| Part-time employment | 114 (3.8) |
| Student | 132 (4.4) |
| Unemployed | 52 (1.7) |
| Retired (pension and early retirement) | 430 (14.2) |
| Stay-at-home | 25 (0.8) |
| Disability pension (due to reduced working capacity) | 651 (21.6) |
| Sick leave | 94 (3.1) |
| Reduced employment (due to reduced working capacity) | 548 (18.1) |
| Other | 163 (5.4) |
| Missing | 45 (1.5) |
| Education | |
| None | 45 (1.5) |
| 9th grade | 346 (11.5) |
| 9th–11th | 150 (5) |
| High school | 295 (9.8) |
| Vocational secondary education | 541 (17.9) |
| Short-cycle higher education | 349 (11.6) |
| Medium-cycle higher education | 855 (28.3) |
| Long-cycle higher education | 251 (8.3) |
| Other | 112 (3.7) |
| Missing | 77 (2.6) |
| Region | |
| Capital | 713 (23.6) |
| Central Jutland | 652 (21.6) |
| Zealand | 593 (19.6) |
| Southern Denmark | 572 (18.9) |
| Northern Jutland | 367 (12.2) |
| Missing | 124 (4.1) |
More than half of the sample were women (62.6%), and the age range was 18–99 years (Mean (M) 49, standard deviation (SD) 13.8), with most respondents aged between 45 and 64 years (51.2%). Current employment status was mixed, and the most prevalent categories were full-time employment (25.4%), disability pension (21.6%), reduced employment (18.1%), and retirement (14.2%). More than a quarter of respondents had a medium-cycle higher education (28.3%), followed by vocational secondary education (17.9%). All five regions in Denmark were represented relative to the total sample size.
Patterns of use
The most common type of cannabis used was CBD oil (65%), followed by “hash, pot or skunk” (36.2%) and THC oil (25.3%) (see Table 2). About one third of the sample (37.8%) reported using CBD oil only, and more than half of the sample (56.8%) reported oil as their most frequent form of intake, followed by “smoked (with tobacco)” (20.0%). The total proportion of the sample who reported inhalation as the most frequent form of intake (either smoke or vapour) was 27.4%. The majority (75.4%) used cannabis 6–7 days a week. A majority of the sample (64.8%) had used CaM for 2 years or less, 18.1% had used CaM for 2–5 years and 16.5% for more than 5 years. Almost half (48.1%) had no previous recreational (non-medicinal) experience before initiating use of CaM, and 15.8% had limited experience with recreational cannabis use (less than five times). Most of the respondents (90.9%), did not have a prescription, whereas 8.4% did. Of those with a prescription for CaM, 37.8% supplemented their use with cannabis from the illegal market. Of those without a prescription, 72.2% had not asked their doctor for a prescription for CaM (see Table 2). The majority (87.5%) were current users of CaM, 3.8% answered on behalf of a family member (not able to answer due to illness or old age) and 8.7% were previous users of CaM.
Table 2.
Patterns of use.
| (N = 3,021) | |
|---|---|
| Types of cannabis | n (%) |
| THC oil | 765 (25.3) |
| CBD oil | 1,965 (65.0) |
| Hash, pot, skunk | 1,092 (36.2) |
| Cannabis-based therapy (prescription: Sativex, Marinol, Nabilone) | 173 (6.0) |
| Whole-plant trial (prescription Bedrocan, Bediol) | 56 (1.8) |
| Other | 232 (7.7) |
| Missing | 32 (1.1) |
| CBD oil only users | 1,141 (37.8) |
| Most frequent form of intake | |
| Smoked (with tobacco) | 603 (20.0) |
| Smoked (no tobacco) | 120 (4.0) |
| Vaporised | 103 (3.4) |
| Oil | 1,717 (56.8) |
| Rectal | 15 (0.5) |
| Tea | 41 (1.4) |
| “Edibles” (cannabis in food) | 47 (1.6) |
| Topical (cream) | 50 (1.7) |
| Capsules | 129 (4.3) |
| Other | 175 (6.3) |
| Missing | 21 (0.7) |
| Frequency of use | |
| 6–7 days a week | 2,277 (75.4) |
| 3–5 days a week | 386 (12.8) |
| 1–2 days a week | 170 (5.6) |
| A few times a month | 96 (3.2) |
| Very rarely | 54 (1.8) |
| Missing | 38 (1.3) |
| Duration of use | |
| < 6 months | 578 (19.1) |
| 6 months–1 year | 628 (20.8) |
| 1–2 years | 752 (24.9) |
| 2–5 years | 547 (18.1) |
| 6–10 years | 227 (7.5) |
| 11–20 years | 130 (4.3) |
| < 20 years | 141 (4.7) |
| Missing | 18 (0.6) |
| Previous recreational experience | |
| None (never tried before medicinal use) | 1,452 (48.1) |
| Little (lifetime use < 5 times) | 477 (15.8) |
| Some (occasionally < 10 times/year) | 319 (10.6) |
| High degree (periodically several times/month) | 355 (11.8) |
| Very high degree (almost daily for several years) | 352 (11.7) |
| Missing | 66 (2.2) |
| Status of use | |
| Current user | 2,642 (87.5) |
| Previous user | 264 (8.7) |
| Answering on behalf of someone else | 115 (3.8) |
| Prescription user | |
| No | 2,745 (90.9) |
| Yes | 158 (5.2) |
| Yes, and I supplement with unregulated cannabis | 96 (3.2) |
| Missing | 22 (0.7) |
| Asked doctor for prescription for medical cannabis (n = 2,745) | |
| Yes | 748 (27.3) |
| No | 1,982 (72.2) |
| Missing | 15 (0.5) |
| Mean percent of oil | – |
| THC oil (n = 377) | 37.5% (SD 30.0) |
| CBD oil (n = 1,518) | 13.8% (SD 14.4) |
| Mean daily dose | |
| THC oil (n = 518) | 6.5 drops (SD 8.4) |
| CBD oil (n = 1,528) | 8.3 drops (SD 9.0) |
| Hash (n = 409) | 1.3 g (SD 1.3) |
| Pot (n = 189) | 1.4 g (SD 2.6) |
| Skunk (n = 248) | 1.4 g (SD 1.7) |
Note. THC = Tetrahydrocannabinol; CBD = Cannabidiol.
Of the THC oil users, 49.3% indicated the percentage compared to 77.3% of the CBD oil users. The mean for THC oil was 37.5% THC (SD 30) and 13.8% (SD 14.4) for CBD oil. The mean daily dose indicated by THC users was 6.5 (SD 8.4) drops/day and 8.3 (SD 9) drops/day for CBD oil users. Users of plant material (hash, pot, skunk) reported means ranging from 1.3–1.4 grams per day; hash (M 1.3, SD 1.3), pot (M 1.4, SD 2.6) and skunk (M 1.4, SD 1.7) (see Table 2).
Motives for use
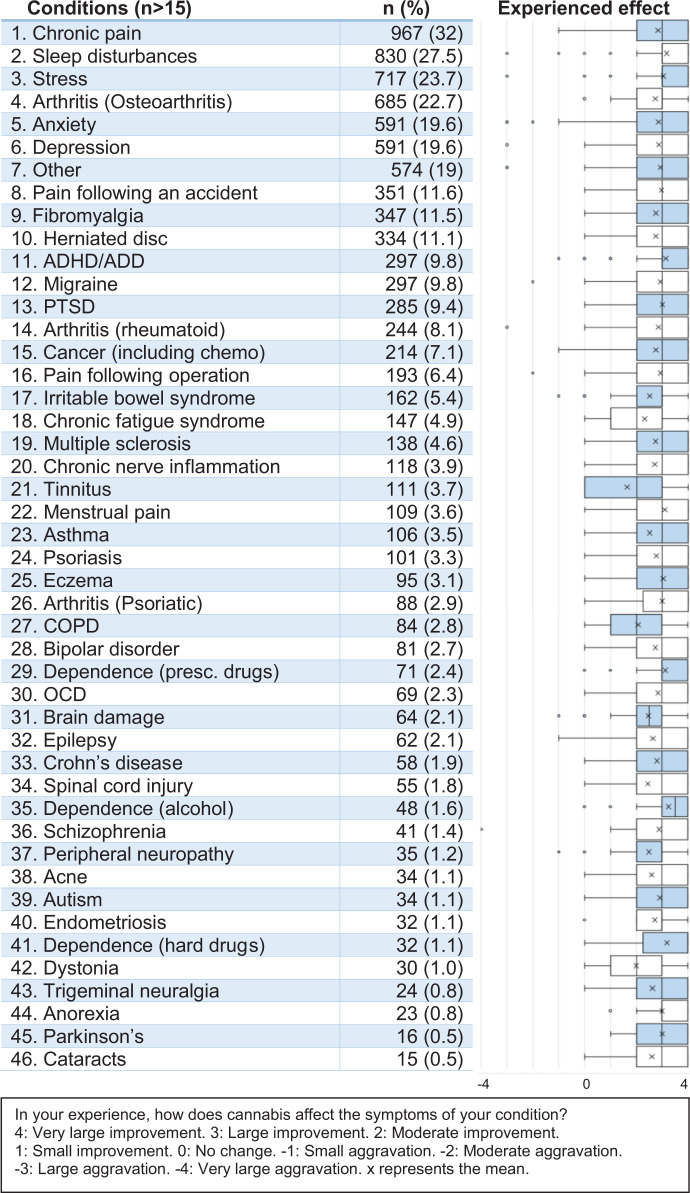
The most frequent conditions treated with CaM were chronic pain (32.0%), sleep disturbances (27.5%), and stress (23.7%) (see Figure 1). 1 Arthritis was also listed as a frequent condition, as 22.7% treated osteoarthritis, 8.1% treated rheumatoid arthritis and 3.3% treated psoriatic arthritis. Mental health conditions, such as anxiety (19.6%), depression (19.6%), ADHD/ADD (9.8%) and PTSD (9.4%) were also frequent motives for use of CaM. Pain-related conditions such as fibromyalgia (11.5%), herniated disc (11.1%) and migraine (9.8%) were also frequently reported, and CaM was also used in the treatment of pain related to an accident (11.6%) and in the treatment of post-operative pain (6.4%). Further, 7.1% indicated the use of CaM in relation to cancer or cancer-related treatment (e.g., chemotherapy).
Figure 1.
Motives for use: Conditions treated with CaM and experienced effect.
A large majority indicated treating a somatic condition with CaM (79.3%) and more than a third used CaM in the treatment of a mental health condition (36.7%) (see Table 3). The most prevalent purpose for use of CaM was “symptom relief” (81.2%), followed by “cure” (22.7%), and “managing side-effects of other treatment” (6.1%). A majority (52.8%) had used CaM with the purpose of replacing a prescribed drug. In total, the respondents reported using CaM for 9,653 conditions, with a mean of just above three conditions per respondent (M 3.2, SD 2.49) (see Table 3). The reported mean effect of using CaM for each condition is indicated in Figure 1. The reported mean effect across all 9,653 conditions was 2.82 (SD 0.96), resulting in an overall effect of just below “Large improvement (3)” (see Figure 1). Seven respondents (0.23%) reported an overall negative effect on symptoms, and 67 (2.2%) reported an overall null effect on symptoms. The highest mean effect was reported on alcohol dependence (M 3.25, SD 0.96), dependence on hard drugs (M 3.19, SD 1.12) and sleep disturbances (M 3.16, SD 1.03) (see Figure 1). The lowest mean effects were reported for tinnitus (M 1.64, SD 1.34), dystonia (M 2.0, SD 1.20) and chronic obstructive pulmonary disease (COPD) (M 2.07, SD 1.10), meaning the lowest mean effect was above “Small improvement (1)”.
Table 3.
Motives for use: Purpose, symptoms, side-effects and means.
| N = 3,021 | |
|---|---|
| Purpose for use for all conditions (n = 9,653) | n (%) |
| To relieve symptoms of the condition | 7,839 (81.2) |
| To cure the condition | 2,188 (22.7) |
| To manage side-effects of other treatment | 588 (6.1) |
| Missing | 100 (1.0) |
| Conditions treated with CaM | |
| Somatic condition | 2,394 (79.3) |
| Mental health condition | 1,109 (36.7) |
| Sleep disturbances | 830 (27.5) |
| Stress | 717 (23.7) |
| Used CaM with the purpose of replacing a prescribed drug | |
| Yes | 1,596 (52.8) |
| No | 1,348 (44.6) |
| Missing | 77 (2.5) |
| Symptoms | |
| Pain | 2,067 (68.4) |
| Sleep disturbances | 1,804 (59.7) |
| Inner turmoil | 1,531 (50.7) |
| Muscle tension | 1,373 (45.5) |
| Stress | 1,204 (39.9) |
| Racing thoughts | 1,106 (36.6) |
| Thoughts of worry | 1,050 (34.8) |
| Depressed mood | 964 (31.9) |
| Irritability | 894 (29.6) |
| Lack of appetite | 801 (26.5) |
| Nausea | 704 (23.3) |
| Hyperactivity | 427 (14.1) |
| Other | 423 (14.0) |
| Nightmares | 363 (12.0) |
| Vomiting | 252 (8.3) |
| Essential tremor | 237 (7.9) |
| Spasticity | 217 (7.2) |
| Tics | 146 (4.8) |
| No effect on any symptoms | 101 (3.3) |
| Missing | 48 (1.6) |
| Side-effects | n (%) |
| No side-effects of cannabis | 1,581 (52.3) |
| Dryness of mouth | 671 (22.2) |
| Being high | 488 (16.2) |
| Red eyes | 388 (12.8) |
| Other | 281 (9.3) |
| Increased tiredness | 263 (8.7) |
| Memory impairment | 254 (8.4) |
| Diarrhoea | 124 (4.1) |
| Palpitations/elevated pulse | 117 (3.9) |
| Sweating | 116 (3.8) |
| Dizziness | 102 (3.4) |
| Headache | 90 (3.0) |
| Heartburn | 83 (2.8) |
| Anxiety | 49 (1.6) |
| Thoughts of worry | 45 (1.5) |
| Constipation | 42 (1.4) |
| Muscle fatigue | 38 (1.3) |
| Dry skin | 34 (1.1) |
| Irritability | 32 (1.1) |
| Passing psychosis | 19 (0.6) |
| Missing | 58 (1.9) |
| Means | Mean (SD) |
| Conditions treated | 3.20 (2.49) |
| Experienced effect across conditions | 2.82 (0.96) |
| Symptoms | 5.23 (3.78) |
| Side-effects | 1.09 (1.60) |
Note. CaM = cannabis as medicine.
Almost all respondents (95.1%) chose at least one symptom on which they had experienced an effect of CaM, with a mean of 5.23 (SD 3.78) symptoms chosen. In all, 3.3% indicated no effect on any symptoms (see Table 3). Pain (68.4%) was the most common symptom, followed by sleep disturbances (59.7%), and inner turmoil (50.7%). A majority (52.3%) indicated no side-effects of CaM, and 45.8% chose at least one side-effect, with a mean of 1.09 (SD 1.60) side-effects chosen. The most common side-effect was “dryness of mouth” (22.2%), followed by “being high” (16.2%).
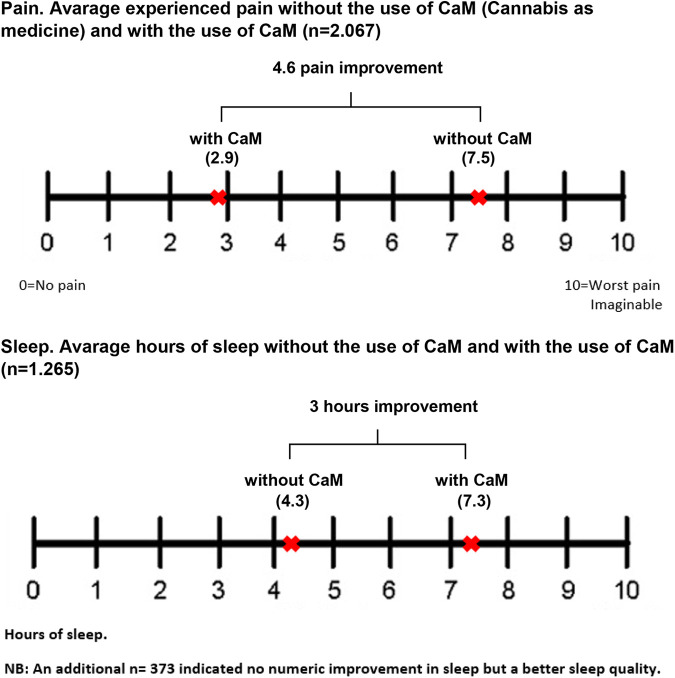
Among respondents reporting “pain” as a symptom relieved by use of CaM (68.4%), the average VAS pain score was 7.5 (SD 1.78) without the use of CaM, and 2.9 (SD 1.76) with the use of CaM. The average improvement of 4.6 in the VAS pain score (see Figure 2) was significant (p < .001). Among respondents reporting “sleep disturbances” as a symptom relieved by use of CaM (59.7%), 70.1% indicated a mean of 4.3 (SD 1.71) hours of sleep without the use of CaM, and a mean of 7.3 (SD 1.23) hours of sleep with the use of CaM. The average improvement was 3 hours, and the difference was significant (p < .001) (see Figure 2). An additional 20.7% reported no improvement in hours slept, but an improvement in sleep quality.
Figure 2.
Motives for use: Pain and sleep.
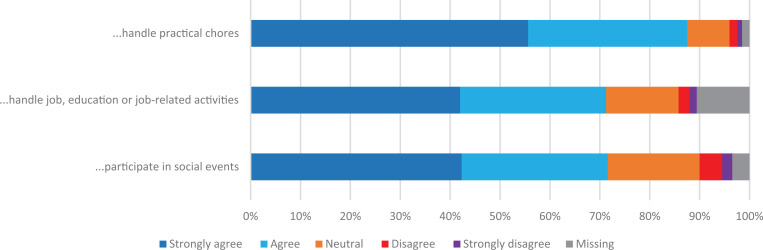
A total of 87.5% agreed/strongly agreed that their use of CaM made it easier to handle practical chores in their daily life, 71.3%, agreed/strongly agreed that CaM made it easier to handle job, education or job-related activities, and 71.6% agreed/strongly agreed that CaM made it easier for them to participate in social events (see Figure 3).
Figure 3.
Effect of CaM on daily life: How do these statements apply to your situation: “CaM makes it easier for me to…”.
Compared to other CaM users, CBD oil only users were 3.36 times more likely to be women (CI: 1.95–4.01, p < 0.001) and there was a trend of an association between increased age and increased odds of using CBD oil only (see Table 4). There were few significant differences between CBD oil only users and other CaM users with respect to current employment and level of education. Using CBD oil only was negatively associated with duration of use and level of previous experience with recreational use (see Table 4).
Table 4.
Odds ratios (ORs) and 95% confidence intervals (Cis) of using CBD oil only (n = 1,141).
| Total sample N = 3,021 | CBD oil only users (n = 1,141) | OR of CBD oil only use | |
|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | |
| Gender | |||
| Male | 1,097 (36.3) | 220 (19.3) | 1.00 (reference) |
| Female | 1,891 (62.6) | 909 (79.6) | 3.36*** (1.95–4.01) |
| Age (years) | |||
| 18–24 | 138 (4.6) | 23 (2.0) | 1.00 (reference) |
| 25–34 | 356 (11.8) | 67 (5.9) | 1.14 (0.66–1.99) |
| 35–44 | 583 (19.3) | 181 (15.9) | 2.07** (1.24–3.46) |
| 45–54 | 831 (27.6) | 343 (30.1) | 2.88*** (1.75–4.74) |
| 55–64 | 710 (23.6) | 319 (28.0) | 3.28*** (1.99–5.43) |
| 65–74 | 328 (10.9) | 169 (14.8) | 4.55*** (2.68–7.72) |
| > 75 | 68 (2.3) | 38 (3.3) | 5.35*** (2.67–10.72) |
| Current employmenta,b | |||
| Disability pension (due to reduced working capacity) | 651 (21.6) | 220 (19.3) | 0.53*** (0.41–0.67) |
| Other | 163 (5.4) | 38 (3.3) | 0.42*** (0.27–0.64) |
| Educationa,b | |||
| Short-cycle higher education | 349 (11.6) | 167 (14.6) | 2.37* (1.03–5.48) |
| Duration of usea | |||
| < 6 months | 578 (19.1) | 352 (31.0) | 1.00 (reference) |
| 6 months–1 year | 628 (20.8) | 338 (29.7) | 0.75* (0.59–0.95) |
| 1–2 years | 752 (24.9) | 335 (29.5) | 0.52*** (0.42–0.66) |
| 2–5 years | 547 (18.1) | 101 (8.9) | 0.16*** (0.12–0.22) |
| 6–10 years | 227 (7.5) | 8 (0.7) | 0.04*** (0.02–0.08) |
| 11–20 years | 130 (4.3) | 1 (0.1) | 0.01*** (0.00–0.06) |
| < 20 years | 141 (4.7) | 2 (0.2) | 0.01*** (0.00–0.05) |
| Previous recreational experiencea | |||
| None (never tried before medicinal use) | 1,452 (48.1) | 839 (73.5) | 1.00 (reference) |
| Little (lifetime use < 5 times) | 477 (15.8) | 191 (16.7) | 0.55*** (0.45–0.69) |
| Some (occasionally < 10 times/year) | 319 (10.6) | 44 (3.9) | 0.15*** (0.11–0.21) |
| High degree (periodically several times/month) | 355 (11.8) | 24 (2.1) | 0.08*** (0.05–0.12) |
| Very high degree (almost daily for several years) | 352 (11.7) | 23 (2.0) | 0.08*** (0.05–0.12) |
a Controlled for age and gender. bOnly significant results reported.
*p < 0.5. ** = p < .01. *** = p < .001.
Discussion
To our knowledge, this is the first study mapping the use of CaM in Denmark. The findings indicate that use of CaM may be a growing trend among Danes, and that the majority of the current users of CaM in Denmark remain outside the “safe framework” established by the MCPP. The study shows that users of CaM are far from homogenous when it comes to demographics, patterns of use and motives for use, and that the unregulated use of CaM is associated with treatment of somatic and mental health conditions not included in the MCPP.
Most of the users of CaM in our study were women and a majority were 45 year or older. Nearly half of the users had a medium-cycle higher or a vocational secondary education, and employment status was diverse and nearly evenly distributed between full-time employment, disability pension, reduced employment, and retirement. Most of the users of CaM had initiated use within the last two years and a majority had little to no previous experience with recreational cannabis use. Furthermore, few had a prescription for CaM and more than one third of those with a prescription supplemented their use with cannabis from the unregulated market. Oil was the most frequent form of intake and CBD oil was the most frequent form of cannabis used. CaM was used for a variety of somatic and mental health conditions, of which the most prevalent were chronic pain, sleep disturbances, stress, osteoarthritis, anxiety and depression. Most users experienced substantial symptom relief from using CaM, limited or no side-effects and a positive impact on daily life function. CBD oil only users were more likely to be women, older, have initiated use recently and have no recreational experience.
The study resembles previous studies in terms of recruitment strategies and findings on motives for use, but differs from some studies in terms of demographics and patterns of use. Two studies (Lintzeris et al., 2018; Sexton et al., 2016) reported a majority of male users, a mean age in the late 30s, and more than 80% reporting inhalation as the most common form of intake. The difference in samples and study findings is likely to reflect the rapid change in the use of CaM, due to the increasing popularity of medicinal use of CBD oil (see also Hazekamp, 2018; Manthey, 2019). Of note, a recent survey on the use of CBD products in the US found that the majority of medicinal CBD users were women, and that nearly half were over 55 years of age (Corroon & Phillips, 2018). These findings match the findings from our study, where the odds of using CBD oil only were higher among women and increased with age.
Patterns of use
The average daily dose among users of “hash, pot or skunk” in our study is almost double the average dose used by medical cannabis patients in the Netherlands (de Hoop et al., 2018). This discrepancy may be related to differences in setting related to use, as Dutch medical cannabis patients have access to medical guidance, while the Danish CaM users self-medicate. The preference for CBD oil found in our study indicates that the majority of the medicinal cannabis users in Denmark are interested in effects beyond the “high”, as CBD does not induce the euphoric effects most often associated with cannabis use (Colizzi & Bhattacharyya, 2017; McPartland & Russo, 2014). It is worth noting that the preference for low-potency CBD oil among the Danish medicinal cannabis users found in this study differs significantly from a recent development on the illegal cannabis market in Denmark. In analyses of hashish seized by police in Denmark we found a three-fold increase in THC concentration from 2000 (mean: 8.3%) to 2017 (mean: 25.3%) (Rømer Thomsen et al., 2019) while CBD levels remained stable (mean around 6%); a development that is also found elsewhere in Europe (Freeman et al., 2019). From a public health perspective, it could be considered positive that most medicinal users in our study prefer CBD oil and bypass smoked plant material like hashish, as CBD oil may be less harmful, both in terms of cannabinoid composition (Bergamaschi et al., 2011; Englund et al., 2017) and mode of intake (Russell et al., 2018). Conversely, it has been suggested that the popularity of CBD products may have adverse net public health outcomes, as it may expose a subgroup of the population that otherwise would have remained cannabis novices (Manthey, 2019). This may indeed be the case in our study, where CBD oil only users were more likely to have been cannabis novices before onset of CaM use. However, the risks associated with use of CBD products may not be related to the effects of CBD, but to the composition and quality of the available CBD products (Hazekamp, 2018). A recent examination of CBD oils available in Denmark by the Department of Forensic Medicine in Odense, revealed that 38% of CBD oils tested contained between 0.2% and 1.2% THC, despite being advertised as below 0.2% THC (Eriksen & Christoffersen, 2020) and similar labelling inaccuracies of CBD products have also been found in the US (Bonn-Miller et al., 2017) and Europe (Hazekamp, 2018; International Cannabis and Cannabinoids Institute, 2018). This is problematic as it increases the risk of exposure to undesired euphoric effects among users and could potentially leave the users unknowingly in violation of the Euphoric Substances Act, which prohibits the consumption of cannabis with more than 0.2% THC (Lægemiddelstyrelsen, 2018). The challenges related to product quality do not only concern CBD oils, but all types of cannabis use, as the products may contain hazardous contaminants related to production (fungi, bacteria, heavy metals, growth enhancers or pesticides) or marketing (lead or glass beads to increase weight or psychoactive substances to increase effect) (Lenton et al., 2018; National Academies of Sciences Engineering and Medicine, 2017). The risks associated with product quality and marketing are relevant in the public health assessment of the unregulated use of CaM, as they may be particularly problematic in clinical populations (Ruchlemer et al., 2015).
Motives for use
The heterogeneity among the CaM users in the current study is reflected in the great variety in motives for use, as they involved a broad range of somatic and mental health conditions, of which chronic pain, sleep disturbances, stress, osteoarthritis, anxiety, and depression were most prevalent; a finding which resembles other studies (Corroon & Phillips, 2018; Lintzeris et al., 2018; Lucas et al., 2019; Park & Wu, 2017; Reinarman et al., 2011; Sexton et al., 2016; Walsh et al., 2013; Ware et al., 2005). The fact that sleep disturbances and stress were among the most prevalent conditions treated with CaM, may indicate a tendency to manage these conditions without formal medical advice and assistance, or that these conditions are more likely to be underdiagnosed, as they are often co-morbidities of other somatic or mental health conditions (Cranford et al., 2017; Melkevik et al., 2018). The reported effects of CaM on pain and sleep found in this study were substantial, and while it can be argued that pain is a subjective experience (Koyama et al., 2005), the improvement in number of hours slept provides a more tangible and objective result. An improvement in sleep may mediate an improvement in somatic and mental health conditions. A plethora of studies have found that sleep disturbances are associated with an array of disease risk (Laposky et al., 2016) and mental health disorders (Sutton, 2014), such as anxiety and depression (Alvaro et al., 2013). Indeed, there is an intimate and bidirectional relationship between sleep and emotion (Kahn et al., 2013), and a positive change in sleep patterns improves positive affect and overall wellbeing (Ong et al., 2017). However, research on cannabinoids and sleep is in its infancy, and more research is needed regarding the long-term effects on sleep patterns (Babson et al., 2017).
The use of CaM in treatment of mental health conditions has raised concerns due to the limited scientific support for such use, for example regarding anxiety and depression (Black et al., 2019; Turna et al., 2017) and a substantial body of evidence has linked the use of cannabis with a worsening of some mental health issues and increased incidence of certain psychiatric conditions (National Academies of Sciences Engineering and Medicine, 2017). However, the current evidence is primarily based on observational and epidemiological studies on non-medical use of cannabis and may not be generalisable to the clinical implications of medical cannabis use in treatment of mental health disorders (Walsh et al., 2017). Importantly, research shows that the clinical implications of medical cannabis use are related to patterns of use, due to the opposing effects of THC and CBD (Boggs et al., 2018; Englund et al., 2017; Rømer Thomsen et al., 2017), and that CBD may hold potential for the treatment of mental health conditions (Khan et al., 2020). Consequently, more research is needed into the safety and effects of subtypes of cannabis.
Medicinal use of CaM and the MCPP
The current re-medicalisation of cannabis has prompted Danish politicians to push the boundaries between cannabis as an illicit drug and as a licit medicine, with the initiation of the MCPP. However, substantial discrepancies between the MCPP and the unregulated use of CaM in Denmark remain. For one, it is striking that the only consistently available product in the MCPP has been the cannabis flower, with suggested intake as a tea or in a vaporiser (Danish Health Data Authority, 2019a; Lægemiddelstyrelsen, 2019d), whereas findings from our study and trends in the medical cannabis programme in the Netherlands (de Hoop et al., 2018) suggest that cannabis oil may in fact be the most preferred form of CaM. It is also striking that the most prevalent conditions reported in our study (chronic pain, sleep disturbances, stress, arthritis, anxiety, and depression) differ from the recommended conditions in the MCPP, except for chronic pain. It could be argued that the need for safe and legal access to medical cannabis is relevant for other patient groups than the patients who are currently included in the MCPP. Conversely, it could be argued that even the current inclusion of chronic pain patients in the MCPP presents a considerable problem, as the current evidence does not allow a full recommendation of using CaM in treatment of chronic pain (Hoffman, 2018; Stockings et al., 2018).
Aside from the limitations of the MCPP in terms of available products and eligible conditions that can be treated, other factors may also contribute to the high rate of medicinal use found in our study, including: limitations on access to the MCPP, fear of stigma, and cost of medical cannabis. For one, access to the MCPP may have been limited, as some Danish GPs may be unwilling to prescribe medical cannabis to their patients. The Danish College of General Practitioners have critiqued the MCPP and advised their members against prescribing medical cannabis to patients, due to the lack of high-quality documentation (Bro, 2018) and several doctors’ offices have issued official statements to their patients declaring that they do not prescribe medical cannabis (Hjort, 2018). Moreover, fear of stigma has been shown to have a profound impact on treatment-seeking behaviour of potential medical cannabis patients (Satterlund et al., 2015) and fear of stigma may also represent a barrier to the MCPP, as findings from our survey showed that a large majority (72.2%) had not asked their GP for a prescription for medical cannabis. Lastly, we cannot rule out that the high prevalence of users of CaM outside the MCPP is a consequence of the cost of the legal cannabis products that, despite government reimbursements, remain many times higher than that of illegally sourced products (Videbæk & Bergmann, 2019). Unfortunately, we cannot compare the experienced effect among users of whole-plant cannabis in the MCPP to the experienced effect reported in our study, as only adverse events of medical cannabis use have been reported by medical doctors (Dahlin, 2017; Lægemiddelstyrelsen, 2019c), of which there have been relatively few (Lægemiddelstyrelsen, 2020).
Our study indicates that the pressure on politicians to formulate medical cannabis policy remains largely unresolved. Firstly, the majority of users of CaM remain outside the MCPP and therefore they continue to rely on an unregulated illegal market. Secondly, as use of CaM appears to be a growing trend among Danes and as many users find CaM effective in managing their conditions, the demand for safe access to medical cannabis is likely to increase. More clinical research on the effects and side-effects of whole-plant cannabis is needed, as this would allow for a more qualified drawing of boundaries of the medical utility of cannabis.
Strengths and limitations
The strengths of the study include the sample size, the wide distribution on age, geography, and the depth of exploration of patterns and motives for use. The method of using anonymous internet-based surveys holds an advantage in studying sensitive topics in so-called “hidden populations” (Barratt, Potter, et al., 2015), where it is impossible to establish who constitutes the population, and where strong privacy issues are at stake, due to illegal behaviour and risk of stigmatisation (Heckathorn, 1997).
However, some limitations must be noted. Firstly, we wish to state that this is not a study on the efficacy of CaM, as this should be assessed through double-blind placebo-controlled trials (D’Souza & Ranganathan, 2015). Furthermore, the self-selected convenience sample is limited by selection bias, and may not be representative of the population of users of CaM in Denmark (Barratt, Ferris, & Lenton, 2015). The sample may weigh towards successful users of CaM, as those who have found cannabis either ineffective or experienced adverse effects may have disengaged from the topic. Moreover, the sample may weight towards users with internet access, a familiarity with online surveys, and the cognitive abilities to answer such surveys, and represent a part of the population that is more actively engaged in the topic of CaM on social media.
We should also note that the data in this study may be subject to self-reporting biases such as recall bias or social desirability bias (Althubaiti, 2016). Although all questions were framed with respect to their sensitivity in wording, in order to reduce social desirability bias (Näher & Krumpal, 2012), respondents may still exaggerate effects of cannabis or underreport adverse effects, as they may perceive themselves to be stakeholders in the outcomes of potential survey findings. Although duplicates were excluded from the analyses, we cannot rule out multiple responses from the same person. Also, although the reported use of dose would have been more precise in mg/k, we made a trade-off between precision and presumed knowledge of the respondents. Likewise, the response categories concerning use of THC oil and CBD oil may cover a wide variety of products. In the interest of mapping this new area in Denmark, we included previous users and answers on behalf of someone next of kin, which may have decreased the validity of the findings. Despite these limitations, the study provides a valuable insight in into the unregulated use of CaM in Denmark.
Acknowledgements
The authors would like to express their appreciation to all respondents of the survey, and to those who helped distribute the survey. A special thanks to Professor Vibeke Asmussen Frank for comments on both survey and manuscript.
Appendix 1
Somatic conditions (37): acne, AIDS, Alzheimer’s, asthma, Chron’s disorder, herniated disc, dystonia, eczema, epilepsy, endometriosis, fibromyalgia, osteoarthritis, rheumatoid arthritis, psoriatic arthritis, cataracts, hepatitis, brain damage, COPD, cancer, CFS (chronic fatigue), chronic nerve inflammation, chronic pain, migraine, menstrual pain, multiple sclerosis, neurodermatitis, IBS, lupus (SLE), end-of-life (palliative care), Parkinson’s, peripheral neuropathy, psoriasis, spinal cord injury, pain following operation, pain following an accident, tinnitus, trigeminal neuralgia
Psychiatric conditions (13): ADHD, anxiety, dependence (alcohol), dependence (hard drugs), dependence (prescription drugs), anorexia, autism, bipolar disorder, depression, OCD, PTSD, schizophrenia, Tourette’s/tics
Potential comorbidities (2): sleep disturbances, stress
Note
Conditions with n < 15 indications [AIDS, Alzheimer’s, hepatitis, neurodermatitis, Lupus (SLE), end-of-life (palliative care) Tourette’s] were excluded from the figures.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The funding source was a PhD project at Aarhus University. The funding source was not involved in the project.
ORCID iD: Sinikka L. Kvamme  https://orcid.org/0000-0002-8376-2013
https://orcid.org/0000-0002-8376-2013
Contributor Information
Sinikka L. Kvamme, Aarhus University, Aarhus C, Denmark
Michael M. Pedersen, Aarhus University, Aarhus C, Denmark
Sagi Alagem-Iversen, Consultant, Aarhus N, Denmark.
Birgitte Thylstrup, Aarhus University, Copenhagen S, Denmark.
References
- Abuhasira R., Shbiro L., Landschaft Y. (2018). Medical use of cannabis and cannabinoids containing products: Regulations in Europe and North America. European Journal of Internal Medicine, 49, 2–6. 10.1016/j.ejim.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Althubaiti A. (2016). Information bias in health research: Definition, pitfalls, and adjustment methods. Journal of Multidisciplinary Healthcare, 9, 211. http://doi:10.2147/JMDH.S104807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro P. K., Roberts R. M., Harris J. K. (2013). A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36(7), 1059–1068. http://10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson K. A., Sottile J., Morabito D. (2017). Cannabis, cannabinoids, and sleep: A review of the literature. Current Psychiatry Reports, 19(4), 23. 10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- Barratt M. J., Ferris J. A., Lenton S. (2015). Hidden populations, online purposive sampling, and external validity: Taking off the blindfold. Field Methods, 27(1), 3–21. 10.1177/1525822X14526838 [DOI] [Google Scholar]
- Barratt M. J., Ferris J. A., Zahnow R., Palamar J. J., Maier L. J., Winstock A. R. (2017). Moving on from representativeness: Testing the utility of the Global Drug Survey. Substance Abuse: Research and Treatment, 11, 1178221817716391. 10.1177/1178221817716391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt M. J., Potter G. R., Wouters M., Wilkins C., Werse B., Perälä J., Pedersen M. M., Nguyen H., Malm A., Lenton S., Korf D., Klein A., Heyde J., Hakkarainen P., Frank V. A., Decorte T., Bouchard M., Blok T. (2015). Lessons from conducting trans-national internet-mediated participatory research with hidden populations of cannabis cultivators. International Journal of Drug Policy, 26(3), 238–249. 10.1016/j.drugpo.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Bergamaschi M. M., Queiroz R. H. C., Zuardi A. W., Crippa A. S. (2011). Safety and side effects of cannabidiol, a Cannabis sativa constituent. Current drug safety, 6(4), 237–249. https://doi:10.2174/157488611798280924 [DOI] [PubMed] [Google Scholar]
- Black N., Stockings E., Campbell G., Tran L. T., Zagic D., Hall W. D., Farrell M., Degenhardt L. (2019). Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. The Lancet Psychiatry, 6(12), 995–1010. 10.1016/S2215-0366(19)30401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. (2017, 26 February). Stor opbakning: Så mange danskere vil gøre medicinsk cannabis lovligt [Great support: This many Danes want to make medical cannabis legal]. Avisen.dk. https://www.avisen.dk/stor-opbakning-saa-mange-danskere-vil-goere-medicins_431326.aspx
- Boggs D. L., Nguyen J. D., Morgenson D., Taffe M. A., Ranganathan M. (2018). Clinical and preclinical evidence for functional interactions of cannabidiol and delta(9)-tetrahydrocannabinol. Neuropsychopharmacology, 43(1), 142–154. 10.1038/npp.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller M. O., ElSohly M. A., Loflin M. J., Chandra S., Vandrey R. (2018). Cannabis and cannabinoid drug development: Evaluating botanical versus single molecule approaches. International Review of Psychiatry, 30(3), 277–284. 10.1080/09540261.2018.1474730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller M. O., Loflin M. J., Thomas B. F., Marcu J. P., Hyke T., Vandrey R. (2017). Labeling accuracy of cannabidiol extracts sold online. JAMA, 318(17), 1708–1709. http://doi:10.1001/jama.2017.11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro N. (2018, 5 January). DSAM støtter praktiserende læger mod medicinsk cannabis [The Danish College of General Practitioners support GPs against medical cannabis]. MS tidsskrift. https://mstidsskrift.dk/behandling/144-dsam-stotter-praktiserende-laeger-mod-medicinsk-cannabis.html [Google Scholar]
- Colizzi M., Bhattacharyya S. (2017). Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Current Addiction Reports, 4(2), 62–74. 10.1007/s40429-017-0142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J., Phillips J. A. (2018). A cross-sectional study of cannabidiol users. Cannabis and Cannabinoid Research, 3(1), 152–161. 10.1089/can.2018.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford J. A., Arnedt J. T., Conroy D. A., Bohnert K. M., Bourque C., Blow F. C., Ilgen M. (2017). Prevalence and correlates of sleep-related problems in adults receiving medical cannabis for chronic pain. Drug and Alcohol Dependence, 180, 227–233. 10.1016/j.drugalcdep.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza D. C., Ranganathan M. (2015). Medical marijuana: Is the cart before the horse? JAMA, 313(24), 2431–2432. http://doi:10.1001/jama.2015.6407 [DOI] [PubMed] [Google Scholar]
- Dahl H. V. (2004). Ilde hørt? Den larmende tavshed om etnografisk rusmiddelforskning [Uncomfortable truths? The alarming silence of ethnographic intoxicant research]. In Asmussen V., Jöhncke S. (Eds.), Brugerperspektiver (pp. 39–71). Aarhus Universitetsforlag. [Google Scholar]
- Dahl H. V., Frank V. A. (2011). Medical marijuana: Exploring the concept in relation to small scale cannabis growers in Denmark. In Decorte T., Potter G., Bouchard M. (Eds.), World wide weed: Global trends in cannabis cultivation and its control (pp. 116–141). Ashgate Publishing, Ltd. [Google Scholar]
- Dahlin U. (2017, 6 September). Forsøgsordning med medicinsk cannabis opsamler ikke erfaringer om effekten [The medical cannabis pilot programme does not gather experience on effect]. Information. https://www.information.dk/indland/2017/09/forsoegsordning-medicinsk-cannabis-opsamler-erfaringer-effekten [Google Scholar]
- Damløv L. (2016, 11 October). Cannabis-sælgere på nettet vildleder syge mennesker [Cannabis sellers on the internet mislead sick people]. Danmarks Radio. https://www.dr.dk/nyheder/indland/cannabis-saelgere-paa-nettet-vildleder-syge-mennesker [Google Scholar]
- Danish Health Data Authority. (2019. a). Medicinforbrug - Udbredelse af forsøgsordningen med medicinsk cannabis [Drug consumption: Prevalence of the medical cannabis pilot programme]. Sundhedsdatastyrelsen. https://sundhedsdatastyrelsen.dk/da/tal-og-analyser/analyser-og-rapporter/laegemidler/emnespecifikke-analyser/analyser-om-medicinsk-cannabis. [Google Scholar]
- Danish Health Data Authority. (2019. b). Medicinforbrug Monitorering: Cannabisprodukter – monitorering af brugen 2. kvartal 2019, Sundhedsdatastyrelsen [Drug consumption Monitoring: Cannabisproducts - Monitoring of use in Q2 2019, The Danish Health Data Authorit]. Sundhedsdatastyrelsen. https://sundhedsdatastyrelsen.dk/da/tal-og-analyser/analyser-og-rapporter/laegemidler/emnespecifikke-analyser/analyser-om-medicinsk-cannabis [Google Scholar]
- de Hoop B., Heerdink E. R., Hazekamp A. (2018). Medicinal cannabis on prescription in the Netherlands: Statistics for 2003–2016. Cannabis and Cannabinoid Research, 3(1), 54–55. 10.1089/can.2017.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabdi F., Ehrbahn J. (2017, 28 October). Manu Sareen: »Jeg klarede ministertiden på cannabis-olie« [Manu Sareen: “I made it through my time as minister on cannabis-oil”]. Politiken. https://politiken.dk/indland/art6180403/%C2%BBJeg-klarede-ministertiden-p%C3%A5-cannabis-olie%C2%AB [Google Scholar]
- Englund A., Freeman T. P., Murray R. M., McGuire P. (2017). Can we make cannabis safer? The Lancet Psychiatry, 4(8), 643–648. 10.1016/S2215-0366(17)30075-5 [DOI] [PubMed] [Google Scholar]
- Eriksen T., Christoffersen D. J. (2020). Have you also ingested cannabis without knowing it [Har du også indtaget cannabis uden at vide det]. Videnskab. https://videnskab.dk/forskerzonen/krop-sundhed/har-du-ogsaa-indtaget-cannabis-uden-at-vide-det [Google Scholar]
- Færch M. (2013, 22 October). Pusherstreet er blevet et alternativt lægehus [Pusherstreet has become an alternative doctors’ office]. Information. https://www.information.dk/indland/2013/10/pusherstreet-blevet-alternativt-laegehus
- Færch M. (2014, 19 March). Hækkerup: “Jeg vil ikke tilbage i diskussionen om fri hash eller ej” [Hækkerup: “I do not want to go back to the discussion about legal hash”]. Information. https://www.information.dk/indland/2014/03/haekkerup-tilbage-diskussionen-fri-hash-ej
- Freeman T., Groshkova T., Cunningham A., Sedefov R., Griffiths P., Lynskey M. T. (2019). Increasing potency and price of cannabis in Europe, 2006–16. Addiction, 114(6), 1015–1023. 10.1111/add.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. (2001). Harm reduction associated with inhalation and oral administration of cannabis and THC. Journal of Cannabis Therapeutics, 1(3–4), 133–152. 10.1300/J175v01n03_09 [DOI] [Google Scholar]
- Grotenhermen F., Schnelle M. (2003). Survey on the medical use of cannabis and THC in Germany. Journal of Cannabis Therapeutics, 3(2), 17–40. http://www.cannabis-med.org/data/pdf/2003-02-2.pdf [Google Scholar]
- Gustavsen S., Søndergaard H., Andresen S., Magyari M., Sørensen P., Sellebjerg F., Oturai A. (2019). Illegal cannabis use is common among Danes with multiple sclerosis. Multiple Sclerosis and Related Disorders, 33, 5–12. 10.1016/j.msard.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Hakkarainen P., Frank V. A., Barratt M. J., Dahl H. V., Decorte T., Karjalainen K., Lenton S., Potter G., Werse B. (2015). Growing medicine: Small-scale cannabis cultivation for medical purposes in six different countries. International Journal of Drug Policy, 26(3), 250–256. 10.1016/j.drugpo.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Hazekamp A. (2018). The trouble with CBD oil. Medical Cannabis and Cannabinoids, 1(1), 65–72. 10.1159/000489287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A., Ware M. A., Muller-Vahl K. R., Abrams D., Grotenhermen F. (2013). The medicinal use of cannabis and cannabinoids: An international cross-sectional survey on administration forms. Journal of Psychoactive Drugs, 45(3), 199–210. 10.1080/02791072.2013.805976 [DOI] [PubMed] [Google Scholar]
- Heckathorn D. D. (1997). Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems, 44(2), 174–199. 10.2307/3096941 [DOI] [Google Scholar]
- Hjort A. (2018, 8 January). Lægehus i Jylland nægter at udlevere medicinsk cannabis [Doctors’ office in Jutland refuses to supply medical cannabis]. Tv2 nyhederne. https://nyheder.tv2.dk/2018-01-08-laegehus-i-jylland-naegter-at-udlevere-medicinsk-cannabis
- Hoffman T. (2018, 15 November). Ny forskning udstiller tyndt grundlag for dansk forsøgsordning med medicinsk cannabis [New research exposes the weak basis for the Danish medical cannabis pilot programme]. Videnskab.dk. https://videnskab.dk/krop-sundhed/ny-forskning-udstiller-tyndt-grundlag-for-dansk-forsoegsordning-med-medicinsk-cannabis
- Houborg E., Enghoff O. (2018). Cannabis in Danish newspapers. Tidsskrift for Forskning i Sygdom og Samfund, 15(28), 173–204. 10.7146/tfss.v15i28.107265 [DOI] [Google Scholar]
- Hurley R. (2018). Cannabis, cannabis everywhere: UK to review medical cannabis policy as Canada plans imminent legalisation for all uses. BMJ: British Medical Journal (Online), 361. 10.1136/bmj.k2695 [DOI]
- Iffland K., Grotenhermen F. (2017). An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis and Cannabinoid Research, 2(1), 139–154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Cannabis and Cannabinoids Institute. (2018, 16 February). Warning for consumers of CBD and cannabis oils sold on the EU market. ICCI Science. https://www.icci.science/en/article/news/warning-for-consumers-of-cbd-and-cannabis-oils-sold-on-the-eu-market/ [Google Scholar]
- Jensen M. P., Chen C., Brugger A. M. (2003). Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. The Journal of Pain, 4(7), 407–414. 10.1016/S1526-5900(03)00716-8 [DOI] [PubMed] [Google Scholar]
- Jørgensen A. S. (2222222222). Pernille Vermund: Cannabisolie gav min mor en sidste god sommer [Pernille Vermund: Cannabis oil gave my mother one last good summer]. Danmarks Radio. https://www.dr.dk/nyheder/indland/pernille-vermund-cannabisolie-gav-min-mor-en-sidste-god-sommer [Google Scholar]
- Kahn M., Sheppes G., Sadeh A. (2013). Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology, 89(2), 218–228. 10.1016/j.ijpsycho.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Khan R., Naveed S., Mian N., Fida A., Raafey M. A., Aedma K. K. (2020). The therapeutic role of Cannabidiol in mental health: A systematic review. Journal of Cannabis Research, 2(1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., McHaffie J. G., Laurienti P. J., Coghill R. C. (2005). The subjective experience of pain: Where expectations become reality. Proceedings of the National Academy of Sciences, 102(36), 12950–12955. 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky A. D., Van Cauter E., Diez-Roux A. V. (2016). Reducing health disparities: The role of sleep deficiency and sleep disorders. Sleep Medicine, 18, 3–6. 10.1016/j.sleep.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lægemiddelstyrelsen. (2018). Change of the THC limit as of 1 July 2018. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/citizens/change-of-the-thc-limit-as-of-1-july-2018/ [Google Scholar]
- Lægemiddelstyrelsen. (2019. a). Medicinal cannabis pilot programme. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/citizens/medicinal-cannabis-pilot-programme/ [Google Scholar]
- Lægemiddelstyrelsen. (2019. b). Questions and answers on medicinal cannabis. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/citizens/questions-and-answers-on-medicinal-cannabis/ [Google Scholar]
- Lægemiddelstyrelsen. (2019. c). Side effects and safety of medicinal cannabis. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis/citizens/side-effects-and-safety-of-medicinal-cannabis/ [Google Scholar]
- Lægemiddelstyrelsen. (2019. d). Supply of cannabis oil from Stenocare affected in Denmark. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/en/news/2019/supply-of-cannabis-oil-from-stenocare-affected-in-denmark/ [Google Scholar]
- Lægemiddelstyrelsen. (2020). Bivikningsindberetninger om cannabisslutprodukter og forbrug under forsøgsordningen [Side-effect reports on cannabis products and use during the pilot programme]. Laegemiddelstyrelsen. https://laegemiddelstyrelsen.dk/da/nyheder/2020/ny-rapport-om-indberettede-formodede-bivirkninger-ved-medicinsk-cannabis/∼/media/E8FE11D274F148A28167F23CFB9C8522.ashx [Google Scholar]
- Lenton S., Frank V. A., Barratt M. J., Potter G. R., Decorte T. (2018). Growing practices and the use of potentially harmful chemical additives among a sample of small-scale cannabis growers in three countries. Drug and Alcohol Dependence, 192, 250–256. 10.1016/j.drugalcdep.2018.07.040 [DOI] [PubMed] [Google Scholar]
- Lintzeris N., Driels J., Elias N., Arnold J. C., McGregor I. S., Allsop D. J. (2018). Medicinal cannabis in Australia, 2016: The Cannabis as Medicine Survey (CAMS-16). The Medical Journal of Australia, 209(5), 211–216. 10.5694/mja17.01247 [DOI] [PubMed] [Google Scholar]
- Lucas P., Baron E. P., Jikomes N. (2019). Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances: Results from a cross-sectional survey of authorized patients. Harm Reduction Journal, 16(1), 9. 10.1186/s12954-019-0278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey J. (2019). Cannabis use in Europe: Current trends and public health concerns. International Journal of Drug Policy, 68, 93–96. 10.1016/j.drugpo.2019.03.006 [DOI] [PubMed] [Google Scholar]
- McPartland J. M., Russo E. B. (2014). Non-phytocannabinoid constituents of cannabis and herbal synergy. In Pertwee R. G. (Ed.), Handbook of cannabis (pp. 280–295). Oxford University Press. [Google Scholar]
- Melkevik O., Clausen T., Pedersen J., Garde A. H., Holtermann A., Rugulies R. (2018). Comorbid symptoms of depression and musculoskeletal pain and risk of long term sickness absence. BMC public health, 18(1), 981. 10.1186/s12889-018-5740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. G., Sonderlund A. L. (2010). Using the internet to research hidden populations of illicit drug users: A review. Addiction, 105(9), 1557–1567. 10.1111/j.1360-0443.2010.02992.x [DOI] [PubMed] [Google Scholar]
- Ministry of Health. (2016, 8 November). Aftale om forsøgsordning med medicinsk cannabis [Agreement on medical cannabis pilot programme]. Sundhedsministeriet. http://sundhedsministeriet.dk/Aktuelt/Nyheder/Medicin/2016/November/∼/media/Filer%20-%20dokumenter/Aftale-om-medicinsk-cannabis/Politisk-aftale-om-forsogsordning-med-medicinsk-cannabis.ashx [Google Scholar]
- National Academies of Sciences Engineering and Medicine. (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. The National Academies Press. 10.17226/24625 [DOI] [PubMed] [Google Scholar]
- Näher A.-F., Krumpal I. (2012). Asking sensitive questions: The impact of forgiving wording and question context on social desirability bias. Quality & Quantity, 46(5), 1601–1616. 10.1007/s11135-011-9469-2 [DOI] [Google Scholar]
- National Conference of State Legislatures. (2020). State medical marijuana laws. National Conference of State Legislatures. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx [Google Scholar]
- Newmeyer M. N., Swortwood M. J., Taylor M. E., Abulseoud O. A., Woodward T. H., Huestis M. A. (2017). Evaluation of divided attention psychophysical task performance and effects on pupil sizes following smoked, vaporized and oral cannabis administration. Journal of Applied Toxicology, 37(8), 922–932. 10.1002/jat.3440 [DOI] [PubMed] [Google Scholar]
- O’Brien P. K. (2013). Medical marijuana and social control: Escaping criminalization and embracing medicalization. Deviant Behavior, 34(6), 423–443. 10.1080/01639625.2012.735608 [DOI] [Google Scholar]
- Ong A. D., Kim S., Young S., Steptoe A. (2017). Positive affect and sleep: A systematic review. Sleep Medicine Reviews, 35, 21–32. 10.1016/j.smrv.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Pacula R. L., Sevigny E. L. (2014). Marijuana liberalizations policies: Why we can’t learn much from policy still in motion. Journal of Policy Analysis and Management: The Journal of the Association for Public Policy Analysis and Management, 33(1), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. Y., Wu L. T. (2017). Prevalence, reasons, perceived effects, and correlates of medical marijuana use: A review. Drug and Alcohol Dependence, 177, 1–13. http://10.1016/j.drugalcdep.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W., Sandberg S. (2013). The medicalisation of revolt: A sociological analysis of medical cannabis users. Sociology of Health & Illness, 35(1), 17–32. 10.1111/j.1467-9566.2012.01476.x [DOI] [PubMed] [Google Scholar]
- Pisanti S., Bifulco M. (2017). Modern history of medical cannabis: From widespread use to prohibitionism and back. Trends in Pharmacological Sciences, 38(3), 195–198. 10.1016/j.tips.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Reiman A., Welty M., Solomon P. (2017). Cannabis as a substitute for opioid-based pain medication: Patient self-report. Cannabis and Cannabinoid Research, 2(1), 160–166. 10.1089/can.2017.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinarman C., Nunberg H., Lanthier F., Heddleston T. (2011). Who are medical marijuana patients? Population characteristics from nine California assessment clinics. Journal of Psychoactive Drugs, 43(2), 128–135. 10.1080/02791072.2011.587700 [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K., Callesen M. B., Ewing S. W. F. (2017). Recommendation to reconsider examining cannabis subtypes together due to opposing effects on brain, cognition and behavior. Neuroscience & Biobehavioral Reviews, 80, 156–158. 10.1016/j.neubiorev.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rømer Thomsen K., Lindholst C., Thylstrup B., Kvamme S., Reitzel L. A., Worm-Leonhard M., Englund A., Freeman T. P., Hesse M. (2019). Changes in the composition of cannabis from 2000–2017 in Denmark: Analysis of confiscated samples of cannabis resin. Experimental and Clinical Psychopharmacology, 27(4), 402–411. 10.1037/pha0000303 [DOI] [PubMed] [Google Scholar]
- Ruchlemer R., Amit-Kohn M., Raveh D., Hanuš L. (2015). Inhaled medicinal cannabis and the immunocompromised patient. Supportive Care in Cancer, 23(3), 819–822. 10.1007/s00520-014-2429-3 [DOI] [PubMed] [Google Scholar]
- Russell C., Rueda S., Room R., Tyndall M., Fischer B. (2018). Routes of administration for cannabis use–basic prevalence and related health outcomes: A scoping review and synthesis. International Journal of Drug Policy, 52, 87–96. 10.1016/j.drugpo.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Russo E. B. (2011). Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344–1364. 10.1111/j.1476-5381.2011.01238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlund T. D., Lee J. P., Moore R. S. (2015). Stigma among California’s medical marijuana patients. Journal of Psychoactive Drugs, 47(1), 10–17. 10.1080/02791072.2014.991858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel K. A., Szeto I., Setnik B., Sellers E. M., Levy-Cooperman N., Mills C., Etges T., Sommerville K. (2018). Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy & Behavior, 88, 162–171. 10.1016/j.yebeh.2018.07.027 [DOI] [PubMed] [Google Scholar]
- Sexton M., Cuttler C., Finnell J. S., Mischley L. K. (2016). A cross-sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis and Cannabinoid Research, 1(1), 131–138. https://DOI:10.1089/can.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen L. D., Skaaning J. (2017, 27 June). Politi og SKAT beslaglægger mere cannabisolie [Police and customs seize more cannabis oil]. Danmarks Radio. https://www.dr.dk/nyheder/indland/politi-og-skat-beslaglaegger-mere-cannabisolie [Google Scholar]
- Stockings E., Campbell G., Hall W. D., Nielsen S., Zagic D., Rahman R., Murnion B., Farrell M., Weier M., Degenhardt L. (2018). Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain, 159(10), 1932–1954. http://doi:10.1097/j.pain.0000000000001293 [DOI] [PubMed] [Google Scholar]
- Sutton E. L. (2014). Psychiatric disorders and sleep issues. Medical Clinics, 98(5), 1123–1143. 10.1016/j.mcna.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Taylor S. (2008). Medicalizing cannabis – Science, medicine and policy, 1950–2004: An overview of a work in progress. Drugs: Education, Prevention and Policy, 15(5), 462–474. 10.1080/09687630802114038 [DOI] [Google Scholar]
- Touw M. (1981). The religious and medicinal uses of Cannabis in China, India and Tibet. Journal of Psychoactive Drugs, 13(1), 23–34. 10.1080/02791072.1981.10471447 [DOI] [PubMed] [Google Scholar]
- Turna J., Patterson B., Van Ameringen M. (2017). Is cannabis treatment for anxiety, mood, and related disorders ready for prime time? Depression & Anxiety, 34(11), 1006–1017. 10.1002/da.22664 [DOI] [PubMed] [Google Scholar]
- Videbæk K., Bergmann M. (2019, 23 December). Pris på over 30.000 kroner for medicinsk cannabis sender Per ud på det illegale marked [A price of more than 30,000 kr for medical cannabis leads Per to the illegal market]. Danmarks Radio. https://www.dr.dk/nyheder/regionale/nordjylland/pris-paa-over-30000-kroner-medicinsk-cannabis-sender-ud-paa-det [Google Scholar]
- Walsh Z., Callaway R., Belle-Isle L., Capler R., Kay R., Lucas P., Holtzman S. (2013). Cannabis for therapeutic purposes: Patient characteristics, access, and reasons for use. International Journal of Drug Policy, 24(6), 511–516. http://10.1016/j.drugpo.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Walsh Z., Gonzalez R., Crosby K., Thiessen M. S., Carroll C., Bonn-Miller M. O. (2017). Medical cannabis and mental health: A guided systematic review. Clinical Psychology Review, 51, 15–29. 10.1016/j.cpr.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Ware M., Adams H., Guy G. (2005). The medicinal use of cannabis in the UK: Results of a nationwide survey. International Journal of Clinical Practice, 59(3), 291–295. 10.1111/j.1742-1241.2004.00271.x [DOI] [PubMed] [Google Scholar]
- Webb C. W., Webb S. M. (2014). Therapeutic benefits of cannabis: A patient survey. Hawai’i Journal of Medicine & Public Health, 73(4), 109. [PMC free article] [PubMed] [Google Scholar]
- Weiner M. D., Puniello O. T., Siracusa P. C., Crowley J. E. (2017). Recruiting hard-to-reach populations: The utility of Facebook for recruiting qualitative in-depth interviewees. Survey Practice, 10(4), 1–13. 10.7282/T3P2722C [DOI] [Google Scholar]
- Zuardi A. W. (2006). History of cannabis as a medicine: A review. Revista Brasileira de Psiquiatría, 28(2), 153–157. 10.1590/S1516-44462006000200015 [DOI] [PubMed] [Google Scholar]