Abstract
Osteosarcoma (OS) is the most common primary malignant bone tumor, which usually occurs in children and adolescents. It is generally a high-grade malignancy presenting with extreme metastases to the lungs or other bones. The etiology of the disease is multifaceted and still remains obscure. A combination of surgery and chemotherapy has played a major role in the treatment of OS over the past three decades, and consequently, the overall survival rates for the disease have remained unchanged. Therefore, there is an urgent need to employ new comprehensive analyses and technologies to develop significantly more informative classification systems, with the aim of developing more effective and less toxic therapies for OS patients. This review discusses the existing knowledge of OS therapy and potential methods to develop novel therapeutic agents for the disease.
Keywords: Osteosarcoma, sarcoma, targeted therapy
Impact Statement
Osteosarcoma is not entirely curable. The main treatment of the disease is surgery combined with aggressive chemotherapy, which has not changed in almost 30 years. Thus, patients’ overall survival also has not significantly improved. Currently, there are no drugs undergoing clinical trials for osteosarcoma. In the review, we have offered potential techniques to develop novel therapies for osteosarcoma patients.
Introduction
Sarcomas are rare malignant tumors derived from mesenchymal tissues. 1 They have been divided into two major categories; soft-tissue sarcoma (muscle, fat, blood vessels, peripheral nerves, and fibrous connective tissues) that comprises less than 10% of all adult solid tumors and bone sarcoma as the name refers a malignancy arising in bone which comprise 15% of all pediatric tumors. 2 The primary approach to treat most sarcoma malignancies is surgery. However, patients with metastatic tumors have been treated with chemotherapy. Notably, for the pediatric sarcomas, treatment has evolved to include surgery followed by chemotherapy and/or radiotherapy.2,3 There is a clear need for a novel, less toxic, and more effective treatment for sarcoma malignancies, especially osteosarcoma (OS) which is the most common pediatric sarcoma type. In this review, we aim to summarize the current therapeutic strategies for OS and suggest several techniques, which may be used to develop potentially novel therapeutics.
Background of OS
OS, can be also called as osteogenic sarcoma, is the most common primary bone malignancy among children, adolescents, and young adults. 4 Osteogenic sarcomas are commonly locally aggressive tumor types and frequently produce early systemic metastases to the lungs. 5 They usually occur near the metaphysis of the long bones such as distal femur and proximal tibia.4,6 The molecular pathogenesis of the OS initiation and progression remains one of the major unsolved questions of the disease pathophysiology.
According to the World Health Organization (WHO), histological classification of OS can be divided into several categories: conventional, telangiectatic, parosteal, periosteal, high-grade surface, low-grade central, and small cell. 7 The conventional OS is the most common one among other classifications, which represents approximately 80% of all cases and can be subdivided into osteoblastic, chondroblastic, and fibroblastic types depending on the predominant characteristics of the tumor cells.5,7 The symptoms of OS usually are intermittent pain, tenderness, and swelling near the affected bone. 8 Diagnosis and tracking progression are achieved by a combination of imaging (X-ray, magnetic resonance imaging scan, positron emission tomography, and computed tomography scan) and histology assessing the characteristic appearance of tumor cells forming osteoid. 9
Current therapeutic strategies
In cancer, therapeutic approaches and strategies are usually based on several factors including tumor stage, age of patient, general condition, patient’s quality of life, and life expectancy. Currently, there are three major therapeutic options are available for patients of OS: surgery, chemotherapy, and radiation therapy. 10
The main aim of surgical amputation and limb-salvage in OS therapy is a complete tumor removal with a wide-ranging margin of non-cancerous normal tissue in order to avoid local reoccurrence, and improve overall survival. 10 Surgery alone creates surgical stress, which causes ischemia–reperfusion injury, activation of sympathetic nervous system, endocrine and metabolic changes, acute and chronic inflammation, and immune suppression within the body, and consequently promotes tumor metastasis.11,12 That is preciously why current management of OS includes surgery followed by chemotherapy and radiotherapy with the aim of reducing overall tumor size as well as eliminating micrometastases.8,13
Chemotherapy has been the most common treatment for OS patients since the 1970s. 14 The main chemotherapy regimens applied for the disease are high-dose methotrexate (HDMTX) with leucovorin rescue, doxorubicin, cisplatin, and ifosfamide with or without etoposide. 10
Methotrexate (MTX) is a folate antimetabolite that has been used to treat neoplastic diseases, psoriasis, and rheumatoid arthritis for a long time. 15 It inhibits the production of pyrimidine and purine nucleotides, and thymidylic acid by binding dihydrofolate reductase to block proliferation of cancer cells. 16 Despite its efficacy, HDMTX has serious life-threatening side effects including renal failure, mucositis, hepatotoxicity, pulmonary toxicity, and neurotoxicity.16–18 Notably, the standard chemotherapy dose of 8–12 g/m2 HDMTX is extremely higher than the absolute lethal dose of 2–4 mg/kg.19–21 Although, leucovorin, folinic acid, is widely used to decrease the toxic effects of HDMTX, therapy is still very harmful to the patients. 19
Doxorubicin (DOX), also known as Adriamycin, is an anthracycline drug extracted from a bacterium species of Streptomyces peucetius var. caesius in the 1970s and is widely used in various cancer types, such as lung carcinoma gastric adenocarcinoma, breast cancer, ovarian cancer, thyroid carcinoma, non-Hodgkin’s/Hodgkin’s lymphoma, multiple myeloma, soft-tissue sarcomas, and pediatric cancers.22–24 Its cumulative dose is from 240 to 480 mg/m2; dose per cycle is from 60 to 90 m2, and its mechanism of action is through intercalation into DNA double helix and disruption of topoisomerase-II-mediated DNA repair and inhibits the synthesis of DNA and RNA.5,10,24 Despite its good effects on patients survival, like many cytotoxic drugs, DOX also has serious effects for patients such as cardiomyopathy, symptomatic cardiac toxicity, transient electrocardiographic abnormalities, alopecia, and myelosuppression.16,25
Cisplatin, (SP-4-2)-diamminedichloridoplatinum(II), is widely used and was the first metal-platinum-based chemotherapeutic drugs for the treatment of almost all cancers including testicular, cervical, ovarian, head and neck, blood, bladder, lung, cervical cancer, melanoma, lymphomas, sarcomas, and others.26–28 Its cumulative dose is from 480 to 600 mg/m2; dose per cycle is from 100 to 120 mg/m2. 5 Cisplatin binds to the N7 reactive center on purine residues of malignant cells’ DNA resulting in inhibition of DNA synthesis, DNA damage, and blocking their cell division activities, further promoting apoptotic cell death.26,27 However, the drug also has extreme side effects such as acute and chronic renal failure, peripheral neuropathy, ototoxicity, hypomagnesemia, gastrointestinal disorders, and hemorrhage.16,27
Ifosfamide, (IFO), a bifunctional alkylating agent, is a member of the nitrogen mustard family and has been used to treat several tumor types including lymphoblastic leukemia, soft-tissue sarcoma, and OS.29,30 Its common cumulative dose starts from 480 to 600 mg/m2; dose per cycle is from 100 to 120 mg/m2. 5 The drug’s mechanism of action is through the cross-linking of DNA strands, inhibition of DNA synthesis, and protein translation. 16 IFO also has dramatic side effects such as hemorrhagic cystitis, acute kidney injury, Fanconi’s syndrome, interstitial nephritis, glomerular disease, and encephalopathy.16,29
New therapeutic approaches
As we discussed the current therapy techniques for OS and unfortunately the results are extremely disappointing, leading to a five-year overall survival rate of 65–70%. 31 The outcomes for OS patients have not significantly improved or changed for over 30 years and this lack of new treatment strategies is reflected by the failure to improve survival rates. There is an urgent need for more effective and less toxic treatment for OS patients. Predictive biomarkers and prognostic markers are needed for use in the development of new treatments. In this section, we summarize the potential ways to develop new therapeutic approaches for the disease (Table 1).
Table 1.
Comparison of different widely used methods for drug development. (A color version of this table is available in the online journal.)
| ASO (single-stranded DNA) | siRNA (double-stranded RNA) | Synthetic mRNA | Peptides and proteins | Protacs | Small molecules | |
|---|---|---|---|---|---|---|

|

|

|

|
|
|
|
| Molecular weight | 6–10 kDa | 14–18 kDa | 200–400 kDa | 1.5–70 kDa | Up to 1.5 kDa | 200–700 Da |
| Physicochemical properties | High solubility Polyanionic |
High solubility Polyanionic |
High solubility Polyanionic |
High solubility Variable charge state |
Variable solubility Variable charge state |
Variable solubility Variable charge state |
| General characteristics | Improved binding affinity Excellent nuclease resistance Does not support RNase H Rapid renal clearance Poor uptake in cell nucleus |
High degree of specificity Non-infections Low toxicity Membrane impermeability Rapid renal clearance |
High efficacy and rapid uptake in cell Natural degradation Non-infectious Low severity of side effects Low attainment costs |
Low tissue distribution Broad range of targets High chemical and biological diversify Stability dependent on Plasma-protein binding Short half-life and rapid renal clearance |
Can target proteins without an active binding site Can target mutated proteins Low toxicity Heterogeneous distribution, dependent on chemistry Rapid hepatic and renal clearance |
Distribution dependent on chemistry Can target proteins on cell surface and inside a cell Heat and metabolic stability High permeability Administered orally Hepatic and renal clearance |
| Route of administration | Intravenous or subcutaneous | Intravenous or subcutaneous | Intravenous, subcutaneous, or intramuscular | Intravenous or subcutaneous | Intravenous, subcutaneous or oral | Oral |
| Examples | Name: Nusinersen Target: SMN2 exon 7 Approved indications – year: Spinal Muscular Atrophy, 2016 Corporation: Ionis Pharma Biogen |
Name: Patisiran Target: Transthyretin Approved indications – year: Hereditary transthyretin-mediated amyloidosis, 2018 Corporation: Alnylam Pharmaceuticals |
Name: Pegaptanib Target: VEGF Approved indications – year: Age-related macular degeneration, 2004 Corporation: NeXstar Pharmaceuticals |
Name: Setmelanotide Target: MC4R Approved indications – year: Chronic weight management (Obesity), 2020 Corporation: Rhythm Pharmaceuticals |
Name: Alectinib Target: TKI Approved indications – year: Non-Small Cell Lung Cancer, 2017 Corporation: Chugai Pharmaceutical |
Name: Axitinib Target: VEGFR-1/2/3 Approved indications – year: Renal cell carcinoma – 2012 Corporation: Pfizer |
ASO: antisense oligonucleotide; siRNA: small interfering RNA. (A color version of this table is available in the online journal.)
Antisense oligonucleotides
In 1978, Zamecnik and Stephenson reported that antisense oligonucleotides (ASOs) can obstruct the replication of Rous sarcoma virus in vitro. 32 Twenty years later, the first oligonucleotide agent, Novartis Pharmaceutical’s Vitravene (Fomivirsen), was approved by the US Food and Drug Administration (FDA) to treat cytomegalovirus retinitis afflicting HIV patients. 33 Since then, ASOs have gained popularity as therapeutics for a wide range of inherited and acquired diseases. They are short, single-stranded, synthetic RNA or DNA molecules with an average length of 8–50 nucleotides that can specifically anneal to a complementary target via Watson–Crick base pairing. ASOs are able to alter RNA, reduce, and adjust protein expression through several different mechanisms.34–36 The molecular weight of ASOs is usually between 6 and 10 kDa. 37 ASOs are able to localize in both the cytoplasm and nucleus with the aim of reaching cytoplasmic and/or nuclear targets. 38 Hence, the molecules have chemical modification for protecting them against the action of nucleases as well as allowing them to easily travel through the plasma membrane without the requirement for vectorization. 39 Phosphorodiamidate morpholino oligomer, third generation, is the most commonly used oligo, has high binding affinity and neutral charge. However, its main disadvantages are rapid renal clearance and poor uptake into the cell nucleus. 37
Oligonucleotide therapeutics have been investigated as cancer treatments for decades with a high potential and promising in vitro outcomes. Currently, there are no approved ASOs in oncology yet and most therapeutics for cancer are still in clinical Phase I or II. 40 Disappointingly, none of the current ASOs therapeutics in clinical trials for oncology are targeting OS or any sarcoma types. 41
Small interfering RNAs
The first introduction of double-stranded RNA that can trigger gene silencing of the complementary mRNA sequences was reported by Fire and Mello and the term “RNA interference” (RNAi) was coined. 42 Recent studies have highlighted that RNAi is a fundamental pathway found in eukaryotic cells. In the cytoplasm of mammalian cells, the Dicer enzyme initiates RNA silencing by breaking down long double-stranded RNA to create small interfering RNA (siRNA) of approximately 21–23 nucleotides long.42,43 Theoretically, siRNA can silence any disease-related transcript in a sequence-specific manner, manufacturing a promising therapeutic modality. 44 The advantages of siRNAs have certain over small molecules and monoclonal antibodies are siRNAs carry out their function through complete Watson–Crick base pairing with messenger RNA (mRNA) as opposed to the requirement of small molecules and monoclonal antibodies to recognize the complex spatial conformation of their target proteins.44–46 Although siRNA technique has widely promising prospects in drug development, it has a few disadvantages including being negatively charged, membrane-impermeable, activation of the immune system and can be unstable in the systemic circulation. 47
The first FDA approved siRNA drug, 20 years after RNAi was first discovered, is Patisiran used to treat hereditary transthyretin amyloidosis (hATTR). 48 The second, Givosiran, was approved to treat acute hepatic porphyria in 2019. 49 In 2020, Lumasiran was approved to treat primary hyperoxaluria type I. 50 Presently, there are seven siRNA drugs in late stages of Phase III in clinical trials some of which are very close to obtaining FDA approval. There are also 11 siRNA drugs in early stages of Phases I and II to treat different cancer types and again none of the drugs are targeted to treat OS or any sarcoma types. 51
Synthetic mRNA
mRNAs are single-stranded RNA molecule that are encoded in genomic DNA of a gene. Furthermore, the mRNA transcripts of genes carry the genetics information to be translated into proteins. 52 The therapeutics field of mRNA vaccine had already been developing rapidly and on top of this due to COVID-19, the field has received an enormous amount of attention. The mRNA molecule is non-infectious, and non-integrating platform, and there is no possibility of risk of infection or insertional mutagenesis. Furthermore, mRNA is degraded by usual-normal mechanism of cellular processes and its half-life in vivo can be potentially regulated with several different modifications and delivery methods. The mRNA vaccines generally have high efficiency through the use of several modifications which make mRNA more stable as well as extensively translatable. 53 In addition, the method has a remarkably low severity of reactions and side effects as well as low attainment costs; therefore, it is easier to be part of preclinical and clinical trials against wide range of diseases including cancer. 54
Currently, three core pharmaceutical and biotechnology companies that focus on developing mRNA therapeutic agents: Moderna, Inc. (founded: 2010, Boston, MA, USA), CureVac (founded: 2000, Tubingen, Germany), and BioNTech (founded: 2008, Mainz, Germany).51,53,54 These companies all have an incredibly diversified portfolio of gene therapy products in the pipeline that cover metabolic disorders, cardiovascular diseases, and immune modulators for applications in immunotherapy on cancer.51,54 Not surprisingly, there are many mRNA therapeutics in cancer that are undergoing clinical trials; sadly, none of the drugs were designed to target OS or any sarcoma types.
Peptides and proteins
Peptides and proteins both have limitless potential as therapeutic agents. Contemporary, the market for peptide and protein drugs is estimated to be higher than US$40 billion per annum. 55 This market is still continuously growing and is expected to comprise an even greater proportion of the market in the future. Currently, there are already more than 100 approved peptide-based therapeutic agents on the market, and the most of the drugs are shorter than 20 amino acids and molecular weight of 1.5–70 kDa.55–57 Peptides and proteins could be particularly selective due to the various points of contact they have with their potential target; consequently, higher selectivity results in reduction of toxicity as well as after affects. 55 Peptides can be easily designed to target a wide range of molecules, and as such they hold endless possibilities in a range of fields including immunology, infectious disease, endocrinology, and oncology. 55
Mifamurtide (L-MTP-PE), a macrophage activator, was designed to treat children and adolescents with non-metastatic OS.58,59 In a Phase III clinical trial in approximately 800 newly diagnosed OS patients, mifamurtide was combined with other OS antineoplastic agents (doxorubicin and methotrexate), with or without cisplatin and ifosfamide. Six years after the treatment, 78% of patients were still alive with no evidence of cancer. However, after Phase III trial, the drug was rejected by the FDA panel in 2007 (US National Library of Medicine, ClinicalTrials.gov, access date 08 October 2021). Interestingly, mifamurtide has been licensed by the European Medicines Agency (EMA) since 2009 and it was approved in the 27 European member states (European Medicines Agency, ema.europa.eu, access date 8 October 2021).
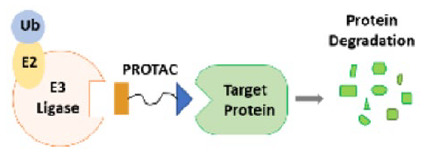
Proteolysis targeting chimeras
Proteolysis targeting chimeras (PROTACs), also called a bivalent chemical protein degrader, is a method which able to ubiquitinate the unwanted proteins by the ubiquitin–proteasome system (UPS). 60 The UPS is responsible for controlling nearly all basic cellular processes including cell cycle progression, cell signaling, apoptosis, immune responses, cell metabolism, protein quality control, and eliminating denatured, mutated, or structurally abnormal proteins in cells.61,62 Fundamentally, PROTAC uses the cell’s protein destruction system to remove unwanted “big garbage” proteins from cells by proteolysis. Consequently, it is possible to target various unwanted or abnormal proteins using the technology such as transcription factors, biological catalysts, cytoskeletal, and regularity proteins. Many studies highlighted that degrading protein is more efficient than translational blocking or inhibiting the function of a protein for anticancer activities.63,64
The first oral PROTAC drugs, ARV-110 and ARV-471, have indicated promising results in clinical trials for the treatments of prostate and breast cancer, respectively. 65 The outcome encourages scientists and creates a greater enthusiasm for PROTACs drug development.
Small molecules
Virtually almost all traditional drugs, as well as >90% of therapeutics already marketed are small molecule drugs.66,67 They are mainly referring to chemically synthesized or organic compounds with a low molecular weight, between 200 and 700 Da. 68 Small molecules are able to affect the function of many proteins and their interactions with other proteins by forming complexes with their targets. 67
The first small molecule cancer drug, tyrosine kinase-inhibitor imatinib, was approved by FDA panel in 2001. Since then, numerous small molecule targeted drugs have been developed for the treatment of different cancer types. The histone deacetylase inhibitors (HDACi) are, rapidly developing, new class of cytostatic agents that inhibit the proliferation of cancer cells by inducing tumor cell cycle arrest, cell differentiation, and cell death, reduction of angiogenesis and modulation of immune response. 69 Growing evidence from both in vitro and in vivo studies has highlighted that HDACi exert their antitumor activity in OS, sensitize tumor cells to radiation, promote cell death, and increase natural killer cell–mediated (innate immunity) cytotoxicity.70,71
By December 2020, 89 small molecule targeted antitumor drugs have been approved by the FDA. One of the 89 small molecule drugs, Tazverik (Tazemetostat-EPZ-6438), was designed to treat metastatic or locally advanced epithelioid sarcoma. 72 The small molecule is also in current clinical trials testing for other sarcoma and cancer types including soft-tissue sarcoma (clinical trial ID: NCT04705818), synovial sarcoma (clinical trial ID: NCT02875548), and follicular lymphoma (clinical trial ID: NCT04762160). 73 Notably, the HDACi agent Abexinostat (PCI24781) is in clinical trials (Phase II) for sarcoma and lymphoma (clinical trial ID: NCT00724984). 69 Currently, no small molecule drug has been approved by FDA or undergoing any clinical trials for OS.
Conclusions
This review has discussed the current treatment for OS and possible ways to develop novel therapeutic agents for the disease with the aim of less toxic and more effective treatment. Due to the complex and unknown mechanism of OS, researchers could not establish effective therapeutic treatments based on underlying disease mechanisms. In fact, the disease still does not have an accurate prognostic biomarker. Therefore, there is, certainly, a need to employ new comprehensive investigation of technologies and methods to develop significantly more informative classification systems and to identify and develop novel therapeutic agents. Genomic analysis of OS samples can help to identify the molecular profiles predicting the outcomes and drug response. This profile can help to develop a classification for a genomics-driven management of the OS and eventually change the outcome of this malignancy. A simple question: what are we waiting for?
Footnotes
Authors’ contributions: E.R. wrote the manuscript. A.L.P. and S.K. reviewed and edited the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Abbie Basson Sarcoma Foundation (Sock it to Sarcoma!).
ORCID iDs: Emel Rothzerg  https://orcid.org/0000-0003-1957-930X
https://orcid.org/0000-0003-1957-930X
Abigail L Pfaff  https://orcid.org/0000-0002-2231-9800
https://orcid.org/0000-0002-2231-9800
Sulev Koks  https://orcid.org/0000-0001-6087-6643
https://orcid.org/0000-0001-6087-6643
References
- 1. Lee DY, Staddon AP, Shabason JE, Sebro R. Phase I and phase II clinical trials in sarcoma: implications for drug discovery and development. Cancer Med 2019;8:585–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Constantinidou A, Pollack S, Loggers E, Rodler E, Jones RL. The evolution of systemic therapy in sarcoma. Expert Rev Anticancer Ther 2013;13:211–23 [DOI] [PubMed] [Google Scholar]
- 3. Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer 2003;3:685–94 [DOI] [PubMed] [Google Scholar]
- 4. Rothzerg E, Ho XD, Xu J, Wood D, Martson A, Maasalu K, Koks S. Alternative splicing of leptin receptor overlapping transcript in osteosarcoma. Exp Biol Med 2020;245:1437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev 2014;40:523–32 [DOI] [PubMed] [Google Scholar]
- 6. Zhang P, Li J. Down-regulation of circular RNA hsa_circ_0007534 suppresses cell growth by regulating miR-219a-5p/SOX5 axis in osteosarcoma. J Bone Oncol 2021;27:100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yarmish G, Klein MJ, Landa J, Lefkowitz RA, Hwang S. Imaging characteristics of primary osteosarcoma: nonconventional subtypes. Radiographics 2010;30:1653–72 [DOI] [PubMed] [Google Scholar]
- 8. Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther 2017;4:25–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther 2016;3:221–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ando K, Heymann MF, Stresing V, Mori K, Redini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers 2013;5:591–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, Lau B, Li Y, Zhao X, Wei Y, Zhou S. Surgical stress and cancer progression: the twisted tango. Mol Cancer 2019;18:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg 2010;110:1636–43 [DOI] [PubMed] [Google Scholar]
- 13. Carrle D, Bielack SS. Current strategies of chemotherapy in osteosarcoma. Int Orthop 2006;30:445–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018;18:39–50 [DOI] [PubMed] [Google Scholar]
- 15. Padmanabhan S, Tripathi DN, Vikram A, Ramarao P, Jena GB. Methotrexate-induced cytotoxicity and genotoxicity in germ cells of mice: intervention of folic and folinic acid. Mutat Res 2009;673:43–52 [DOI] [PubMed] [Google Scholar]
- 16. Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar-Graney K, Shmookler B, Malawer MM. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician 2002;65:1123–32 [PubMed] [Google Scholar]
- 17. Janeway KA, Grier HE. Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol 2010;11:670–8 [DOI] [PubMed] [Google Scholar]
- 18. Crews KR, Liu T, Rodriguez-Galindo C, Tan M, Meyer WH, Panetta JC, Link MP, Daw NC. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 2004;100:1724–33 [DOI] [PubMed] [Google Scholar]
- 19. Xu M, Xu SF, Yu XC. Clinical analysis of osteosarcoma patients treated with high-dose methotrexate-free neoadjuvant chemotherapy. Curr Oncol 2014;21:e678–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graf N, Winkler K, Betlemovic M, Fuchs N, Bode U. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 1994;12:1443–51 [DOI] [PubMed] [Google Scholar]
- 21. Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: let the questions surcease – time for final acceptance. J Clin Oncol 2008;26:4365–6 [DOI] [PubMed] [Google Scholar]
- 22. Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Reprinted from Biotechnology and Bioengineering, Vol. XI, Issue 6, Pages 1101–1110 (1969). Biotechnol Bioeng 2000;67:704–13 [DOI] [PubMed] [Google Scholar]
- 23. Cortes-Funes H, Coronado C. Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol 2007;7:56–60 [DOI] [PubMed] [Google Scholar]
- 24. Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 2011;21:440–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Longhi A, Ferrari S, Bacci G, Specchia S. Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anticancer Drugs 2007;18:737–44 [DOI] [PubMed] [Google Scholar]
- 26. Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S. Advances in our understanding of the molecular mechanisms of action of cisplatin in CANCER THERAPY. J Exp Pharmacol 2021;13:303–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014;740:364–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szentmihalyi K, Blazovics A, May Z, Mohai M, Jr, Sule K, Albert M, Szenasi G, Sebesteny A, Mathe C. Metal element alteration in the lung by cisplatin and CV247 administration. Biomed Pharmacother 2020;128:110307. [DOI] [PubMed] [Google Scholar]
- 29. Sprangers B, Lapman S. The growing pains of ifosfamide. Clin Kidney J 2020;13:500–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarbay H, Demir UF, Yilmaz G, Atay AA, Malbora B. Ifosfamide induced encephalopathy in a child with osteosarcoma. J Oncol Pharm Pract 2021;27:1302–6 [DOI] [PubMed] [Google Scholar]
- 31. Rothzerg E, Ingley E, Mullin B, Xue W, Wood D, Xu J. The hippo in the room: targeting the hippo signalling pathway for osteosarcoma therapies. J Cell Physiol 2021;236:1606–15 [DOI] [PubMed] [Google Scholar]
- 32. Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci USA 1978;75:285–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther 2017;25:1069–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 2018;14:9–21 [DOI] [PubMed] [Google Scholar]
- 35. Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov 2002;1:503–14 [DOI] [PubMed] [Google Scholar]
- 36. Muntoni F, Wood MJ. Targeting RNA to treat neuromuscular disease. Nat Rev Drug Discov 2011;10:621–37 [DOI] [PubMed] [Google Scholar]
- 37. Evers MM, Toonen LJ, van Roon-Mom WM. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev 2015;87:90–103 [DOI] [PubMed] [Google Scholar]
- 38. Potaczek DP, Garn H, Unger SD, Renz H. Antisense molecules: a new class of drugs. J Allergy Clin Immunol 2016;137:1334–46 [DOI] [PubMed] [Google Scholar]
- 39. Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 2020;19:673–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wan J, Bauman JA, Graziewicz MA, Sazani P, Kole R. Oligonucleotide therapeutics in cancer. Cancer Treat Res 2013;158:213–33 [DOI] [PubMed] [Google Scholar]
- 41. Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov 2021;20:427–53 [DOI] [PubMed] [Google Scholar]
- 42. Singh A, Trivedi P, Jain NK. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol 2018;46:274–83 [DOI] [PubMed] [Google Scholar]
- 43. Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev 2009;61:850–62 [DOI] [PubMed] [Google Scholar]
- 44. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013;12:967–77 [DOI] [PubMed] [Google Scholar]
- 45. Ahmadzada T, Reid G, McKenzie DR. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys Rev 2018;10:69–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scherman D, Rousseau A, Bigey P, Escriou V. Genetic pharmacology: progresses in siRNA delivery and therapeutic applications. Gene Ther 2017;24:151–6 [DOI] [PubMed] [Google Scholar]
- 47. Sajid MI, Moazzam M, Kato S, Yeseom Cho K, Tiwari RK. Overcoming barriers for siRNA therapeutics: from bench to bedside. Pharmaceuticals 2020;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoy SM. Patisiran: first global approval. Drugs 2018;78:1625–31 [DOI] [PubMed] [Google Scholar]
- 49. Scott LJ. Givosiran: first approval. Drugs 2020;80:335–9 [DOI] [PubMed] [Google Scholar]
- 50. Scott LJ, Keam SJ. Lumasiran: first approval. Drugs 2021;81:277–82 [DOI] [PubMed] [Google Scholar]
- 51. Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The limitless future of RNA therapeutics. Front Bioeng Biotechnol 2021;9:628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990;247:1465–8 [DOI] [PubMed] [Google Scholar]
- 53. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sahin U, Kariko K, Tureci O. mRNA-based therapeutics – developing a new class of drugs. Nat Rev Drug Discov 2014;13:759–80 [DOI] [PubMed] [Google Scholar]
- 55. Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013;4:1443–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. D’Hondt M, Bracke N, Taevernier L, Gevaert B, Verbeke F, Wynendaele E, De Spiegeleer B. Related impurities in peptide medicines. J Pharm Biomed Anal 2014;101:2–30 [DOI] [PubMed] [Google Scholar]
- 57. Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des 2013;81:136–47 [DOI] [PubMed] [Google Scholar]
- 58. Kager L, Potschger U, Bielack S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther Clin Risk Manag 2010;6:279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Punzo F, Bellini G, Tortora C, Pinto DD, Argenziano M, Pota E, Paola AD, Martino MD, Rossi F. Mifamurtide and TAM-like macrophages: effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget 2020;11:687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zou Y, Ma D, Wang Y. The PROTAC technology in drug development. Cell Biochem Funct 2019;37:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benanti JA. Coordination of cell growth and division by the ubiquitin-proteasome system. Semin Cell Dev Biol 2012;23:492–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci 2009;85:12–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paiva SL, Crews CM. Targeted protein degradation: elements of PROTAC design. Curr Opin Chem Biol 2019;50:111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun X, Gao H, Yang Y, He M, Wu Y, Song Y, Tong Y, Rao Y. PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther 2019;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qi SM, Dong J, Xu ZY, Cheng XD, Zhang WD, Qin JJ. PROTAC: an effective targeted protein degradation strategy for cancer therapy. Front Pharmacol 2021;12:692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov 2002;1:727–30 [DOI] [PubMed] [Google Scholar]
- 67. Gurevich EV, Gurevich VV. Therapeutic potential of small molecules and engineered proteins. Handb Exp Pharmacol 2014;219:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rao MS, Gupta R, Liguori MJ, Hu M, Huang X, Mantena SR, Mittelstadt SW, Blomme EAG, Van Vleet TR. Novel computational approach to predict off-target interactions for small molecules. Front Big Data 2019;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Suraweera A, O’Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol 2018;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wirries A, Jabari S, Jansen EP, Roth S, Figueroa-Juarez E, Wissniowski TT, Neureiter D, Klieser E, Lechler P, Ruchholtz S, Bartsch DK, Boese CK, Di Fazio P. Panobinostat mediated cell death: a novel therapeutic approach for osteosarcoma. Oncotarget 2018;9:32997–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chaiyawat P, Pruksakorn D, Phanphaisarn A, Teeyakasem P, Klangjorhor J, Settakorn J. Expression patterns of class I histone deacetylases in osteosarcoma: a novel prognostic marker with potential therapeutic implications. Mod Pathol 2018;31:264–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoy SM. Tazemetostat: first approval. Drugs 2020;80:513–21 [DOI] [PubMed] [Google Scholar]
- 73. Nogueira T, Souza M. New FDA oncology small molecule drugs approvals in 2020: mechanism of action and clinical applications. Bioorg Med Chem 2021;46:116340. [DOI] [PubMed] [Google Scholar]


