Abstract
The current SARS-CoV-2 pandemic diffused worldwide has encouraged the rapid development of vaccines to counter the spread of the virus. At present in Italy, 75.01% of the population completed the vaccination course (AIFA.gov.it) and very few adverse events have been recorded by now. Side-effects related to a theoretical over-reaction of the immune system in response to vaccines administration have been described, and the possibility that an autoimmune or a hyperinflammatory condition may occur was recently observed. Herein, we report four cases of hyperinflammatory syndrome with features indicative of Adult-onset Still’s disease (AOSD) and macrophage activation syndrome (MAS), occurred after anti-SARS-CoV-2 vaccine injection and seen at our Unit between March and May 2021. Since interleukin (IL)-1 is one of the pivotal cytokines involved in AOSD pathogenesis, the inhibition of IL-1 is crucial in ameliorating the clinical symptoms of those patients. Moreover, it has been highlighted the central role of IL-1 as a hallmark of the hyperinflammatory status elicited by SARS-CoV-2 infection. In this case series, we successfully employed the IL-1 receptor antagonist anakinra to curb the cytokine release likely unleashed by the vaccine stimulation in potentially predisposed subjects. We also made a literature search to detect other patients with hyperinflammation temporally related to vaccines injection who benefited from IL-1 inhibition, while other AOSD/MAS-like described syndromes improved with other immunomodulatory strategies.
Keywords: Hyperinflammation, COVID-19, vaccines, adult-onset-Still’s disease, macrophage activation syndrome, interleukin-1, anakinra
Impact Statement
The vaccination campaign against SARS-CoV-2 infection diffused worldwide is one of the most extensive public health operations of the recent decades. For this reason, given the broad scale diffusion of anti-SARS-CoV-2 vaccines, it is possible that, in predisposed subjects, some side effects may be observed. Cases of “hyperinflammatory syndrome” characterized by an excess of pro-inflammatory cytokines elicited by the shot, arrived at our attention during the last months, and others have been reported in the literature by now. Based on the pathophysiology and on the clinical/bio-humoral similarity with adult-onset Still’s disease, the treatment approach with the anti-interleukin-1 drug anakinra was successfully employed, with a remarkable improvement on the hyperinflammatory condition.
Introduction
With the ongoing intensive campaign for vaccination against severe acute respiratory syndrome (SARS)-coronavirus disease 2 (CoV-2), rare and novel vaccine-associated, systemic inflammatory and immune-mediated adverse events are increasingly being reported. However, it is uncertain whether vaccination itself could exacerbate or unleash pre-existing inflammatory rheumatic musculoskeletal diseases. Herein, we report detection and management of four patients recently admitted to our Unit for severe systemic inflammatory symptoms occurred after SARS-CoV-2 vaccination (Table 1). Moreover, we carried out a literature review to identify other inflammatory conditions that temporally may be related to vaccines administration and other cases which benefited from interleukin (IL)-1 blockade.
Table 1.
Clinical features of patients with hyperinflammatory syndrome treated with anakinra: four cases from our Unit and three from the literature.
| 1 | 2 | 3 | 4 | Leone F et al. Lancet Rheumatol, 2021 1 | Baicus C et al. Rom J. Intern Med, 2021 2 | Salzman MB et al. Emerg Infect Dis, 2021 3 | |
|---|---|---|---|---|---|---|---|
| Gender, age | Male, 65 | Female, 57 | Female, 53 | Female, 50 | Male, 36 | Male, 22 | Male, 18 |
| Previous diseases | Psoriatic arthritis | Adult-onset Still’s disease | Psoriasis Hashimoto’s thyroiditis Vitiligo |
Psoriasis Non-Hodgkin’s lymphoma (2008) Hypothyroidism |
None | None | Asthma |
| SARS-CoV2 vaccine | ChAdOx1 (AstraZeneca) | BNT162b2 (Pfizer-BioNTech) | BNT162b2 (Pfizer-BioNTech) | BNT162b2 (Pfizer-BioNTech) | ChAdOx1 (AstraZeneca) | BNT162b2 (Pfizer-BioNTech) | BNT162b2 (Pfizer-BioNTech) |
| Body temperature (°C) | 39.0 | 37.5 | 39.5 | 39.0 | >39 | na | 40 |
| Time from vaccination to symptoms onset (days) | 1 (first dose) | 18 (first dose) | 10 (second dose) | 3 (second dose) | 1 (first dose) | 13 (first dose) | 18 (first dose) |
| Previous SARS-CoV-2 infection | No | No | No | No | No | No | Yes |
| Duration of fever (days) until therapy | 18 | 15 | 29 | 32 | 10 | 14 | 15 |
| CRP (mg/L) | 480 | 354.7 | 51.5 | 208 | 188 | High increase | 185.5 |
| Ferritin (µg/L) | 1550 | 813 | 223.400 | 6.000 | 946 | 9.834 | 3.002 |
| WBC (*106 mmc) | 15.5 | 24.6 | 8.34 | na | 30.830 | High increase | 7.000 |
| Neutrophils (*106 mmc) | 13.2 | 22.2 | 3.31 | na | 26.500 | High increase | 6280 |
| Autoantibodies | No | No | ANA 1:160 fine speckled | ANA 1:160 | No | No | No |
| Liver dysfunction | No (ALP, GGT, bilirubin) | Yes (LDH) | Yes (GOT, GPT, LDH, TGL) | Yes (GOT, GPT) | Yes (GOT, GPT) | Yes | Yes (GOT/GPT) |
| Splenomegaly | Yes | No | Yes | No | No | No | No |
| Lymphadenopathy | Yes | No | Yes | No | No | No | No |
| Skin rash | Yes | No | Yes | Yes | Yes | Yes | No |
| Arthritis/arthralgias | Yes | Yes | Yes | Yes | No | Yes | No |
| Treatment | Prednisone 75 mg daily i.v. anakinra 100 mg BD |
Prednisone 25 mg daily s.c. anakinra 100 mg daily |
Dexamethasone 10 mg BD i.v. anakinra 200 mg BD |
Prednisone 25 mg daily s.c. anakinra 100 mg daily Cyclosporine 150 mg/day After a flare i.v. anakinra 200 mg BD |
Methylprednisolone 0.75 mg/kg and then s.c. anakinra 100 mg/day | Dexamethasone i.v. 16 mg/day, IVIg 1–2 g/kg, 3 g pulses methylprednisolone, then s.c. anakinra 100 mg every other day/2/3 days | IVIg 100 mg, methylprednisolone 1 g/day for 3 days, s.c. anakinra 100 mg/day for 3 days |
ALP: alkaline phosphatase; BD: bis-in-die; CRP: C-reactive protein; GGT: gamma-glutamyl transpeptidase; GPT: glutamic pyruvic transaminase; GOT: glutamic oxaloacetic transaminase; IVIg: intravenous immunoglobulin; i.v.: intravenous; LDH: lactate dehydrogenase; s.c.: subcutaneous; WBC: white blood cell count; na: not available.
Case series
All the patients arrived at our attention between March and May 2021. They presented with a triad of unremitting fever, joint pain, and high inflammatory markers. All of them received the anti SARS-CoV-2 vaccine (one patient ChAdOx1 DNA viral-vector vaccine and three patients BNT162b2 mRNA vaccine) prior the development of the symptoms.
Case 1
A 60-year-old man with a remote history of psoriatic arthritis, received the first dose of ChAdOx1 (AstraZeneca) vaccine in May 2021. The day after he started to develop fever, cervical pain, and stiffness in his shoulders. After 10 days of hyperpyrexia and diffuse arthralgias, with apparently no signs of infection, he arrived at our attention. The laboratory work-up revealed neutrophilic leukocytosis, high C-reactive protein (CRP), and a moderate increase in ferritin. At physical examination, lymphadenopathy, splenomegaly, and joint stiffness were noticed. The patient had also elevated interleukin-1α (2166 ng/L, range = 0.0–3.9), IL-1β (10.1 ng/L, range = 0.0–5.0), IL-6 (66.5 ng/L, range = 3.0–7.0), and serum soluble interleukin-2 receptor (sIL-2r) was 2495 kU/L (range = 223–710). The main serological analysis excluded infections, while a positron emission tomography/computed tomography (PET/CT) excluded areas or pathological hyper-uptake which could be attributed to hematological malignancies. Based on the exclusion of mimickers, the diagnosis of adult-onset Still’s disease (AOSD) was made (Yamaguchi’s criteria) 4 and the patient was treated with 1 mg/kg of oral prednisone and IL-1 inhibition with intravenous (i.v.) anakinra (100 mg every 8 h); clinical improvement began in less than 24 h; the patient was discharged in good disease control with subcutaneous (s.c.) anakinra 100 mg/day and oral prednisone.
Case 2
The second patient is a woman with AOSD who developed a macrophage activation syndrome (MAS) in 2015; she had discontinued s.c. anakinra 1 year prior to receive SARS-CoV-2 immunization, due to a persistent AOSD remission for 5 years. Fifteen days after the first BNT162b2 (Pfizer-BioNTech) injection, she started to complain diffuse arthralgias, pharyngodynia, and low-grade non-remitting fever. When she arrived at our attention, we observed synovitis of the second and third right metacarpophalangeal joint and shoulders at ultrasonography (US); in addition, laboratory work-up showed high transaminases, a marked neutrophilic leukocytosis, hyperferritinemia, and elevated CRP. She promptly regained remission with moderate-dose of oral prednisone and s.c. anakinra (100 mg/day), which was re-started with no further disease relapses, even after the second dose of mRNA vaccine, that she regularly received 1 month after the acute phase.
Case 3
A 53-year-old woman with a previous history of cutaneous psoriasis and hypothyroidism, presented with arthralgias, pharyngodynia, skin rash, and low-grade fever after her first Pfizer-BioNTech shot. Her general practitioner treated her with low-dose oral prednisone with a significant improvement on her symptoms. However, a few days after the second dose, she deteriorated rapidly with hyperpyrexia and a polyarthritis worsening. She was hospitalized and treated with i.v. methylprednisolone; however, her conditions did not improve within 10 days, and thus, she was admitted at our Department. On admission she presented with objective small joints polyarthritis and she complained also abdominal and chest pain which were compatible with serositis; at work-up, neutrophilic leukocytosis, elevated ferritin, and CRP were detected. Her disease course was later complicated by secondary hemophagocytic lymphohistiocytosis (s-HLH), providing a dramatic increase of ferritin, respiratory insufficiency, and an initial systolic myocardial dysfunction. She was treated successfully with i.v. dexamethasone (8 mg bid) and high-dose i.v. anakinra (100 mg every 6 h). After 3 months, no disease relapses were observed, her bio-humoral work-up was unremarkable and she continued s.c. anakinra 100 mg twice a day without adverse events until the last follow-up.
Case 4
A 50-year-old woman with a previous history of psoriatic arthritis and non-Hodgkin’s lymphoma, on remission since 2008 and treated with chemo- and radiotherapy, arrived at our attention in April 2021. Three days after the second Pfizer-BioNTech shot, she started to complained elevated fever (TC 39°C), diffuse arthralgias, and a pink salmon rash on arms and legs. She was admitted to the Emergency Room and her laboratory exams revealed leukocytosis (white blood cells (WBCs) = 12.500 mmc/L), low platelet count (99.000 mmc/L), a slight increase in transaminases (glutamic oxaloacetic transaminase (GOT) = 58 U/L and glutamic pyruvic transaminase (GPT) = 41 U/L), and CRP was 38 mg/L. Chest X-ray and echocardiogram were normal. During the hospitalization, she started to develop daily spiking fever (until 40°C), and she complained a worsening of her skin rash on chest and legs. Serological exams excluded main viral and bacterial infections, while the autoimmunity panel was unremarkable (antinuclear antibodies 1/160); however, high ferritin (>6000 ng/L) and a low percentage of glycosylated ferritin (<20%) were observed. Based on these data and on the clinical features, the diagnosis of AOSD was made. She was treated with high doses i.v. methylprednisolone (80 mg/day) and oral cyclosporine 150 mg/day; however, due to the persistence of fever, arthralgias and an initial pulmonary involvement, s.c. anakinra (100 mg/day) was added. At discharge, she switched to oral prednisone (starting from 50 mg/day) and cyclosporine was slowly tapered (given also the previous hematological history) with a progressive improvement of her conditions. However, after 4 months along with prednisone tapering, she started to complain again fever (TC max 39°C) accompanied by a diffuse urticarial rash on arms and chest; laboratory biomarkers showed high ferritin (nadir = 36.646 µg/L), CRP 110 mg/L, a marked increase in transaminases (GOT = 181 U/L and GPT = 226 U/L), triglycerides (3.02 mmol/L), and a low platelets count (59.000 mmc/L); all these signs and symptoms were compatible with an initial MAS. For this reason, she was hospitalized again and treated with i.v. anakinra (100 mg every 6 h) and i.v. dexamethasone (6 mg bid) with a gradually response on fever and systemic inflammation. At discharge, she continued oral prednisone (from 25 mg/day) and s.c. anakinra 100 mg twice a day with benefit until the last follow-up.
Cases reported in the literature
At the best of our knowledge, a few other cases of AOSD-like syndromes occurred after anti SARS-CoV-2 vaccination and benefited from anakinra administration.
One case was reported in Italy in a 36-year-old male who developed AOSD after the first shot of ChAdOx1 vaccine. 1 One day after the injection, he started to complain hyperpyrexia (>39°), sore throat, chest pain, and an evanescent cutaneous rash. He was initially treated with non-steroid anti-inflammatory drugs (NSAIDs) and antibiotics without improvement. Due to the persistence of fever, chest pain, and the new onset of tachycardia, he was admitted to the hospital. The serological work-up for infections was unremarkable, thus given the persistent leukocytosis and in the suspicion of an inflammatory disorder, he started methylprednisolone 0.75 mg/kg/day with improvement of symptoms; however, after 3 weeks of glucocorticoids tapering, all the inflammatory symptoms reappeared including hyperpyrexia and chest pain, and laboratory data showed high increase in CRP, erythrocyte sedimentation rate (ESR), ferritin, IL-6, and sIL-2r (see Table 1). In addition, due to an initial myocardial involvement with a transitory increase in troponin, he was treated with s.c. anakinra 100 mg/day resulting in a bio-humoral and clinical sustained improvement until discharge and until the follow-up visit after 1 month. 1
Another case of hyperinflammation described as an AOSD-like form (Yamaguchi’s criteria fulfilled) was reported in Romania in a 22-year-old male with a past unremarkable history. 2 Thirteen days after receiving the first shot of BNT162b2 vaccine, he started to complain fever associated with sore throat, myalgias, maculopapular rash and elevated inflammatory markers; because of an acute chest pain and an initial myocardial dysfunction (high troponin, ST elevation at electrocardiogram (ECG), and left ventricular hypokinesia), he was hospitalized. After the exclusion of infections, major neoplasia and autoimmune conditions he started broad spectrum antibiotics without amelioration; thus, i.v. dexamethasone at 16 mg/day was started, but soon after tapering glucocorticoids, the hyperinflammatory symptoms reappeared and i.v. immunoglobulins (1 g/kg) were started without benefit. Because of high ferritin levels, the persistence of inflammatory symptoms, the new onset of arthralgias in the large joints, and despite the high dose of glucocorticoids, he started s.c. anakinra 100 mg every other day and then every 2 and 3 days (due to supply issues) with a progressive improvement of inflammatory markers, leading to a general decrease in glucocorticoids daily intake. 2
Six cases of multisystem autoinflammatory syndrome of adults (MIS-A), which draws MAS features, were described in Southern California. 3 Among those six, an 18-year-old Asian-American man, presented at the emergency department with fever, headache, vomiting, diarrhea, and abdominal pain; he had SARS-CoV-2 infection 43 days before and got the first shot of BNT162b2 vaccine 19 days before the symptoms onset. Because of elevated inflammatory markers, thrombocytopenia and the absence of infections at blood and urine cultures, he was initially treated with methylprednisolone and intravenous immunoglobulin (IVIg) and then with s.c. anakinra (100 mg/day for 3 days) achieving soon the symptoms remission. 3 Table 1 depicts the features of our patients and of the patients described in the literature and treated with anakinra by now.
Cases of hyperinflammation (AOSD/MAS-like) not treated with IL-1 inhibitors
Other two cases of AOSD-like forms with typical clinical (Yamaguchi’s criteria) and laboratory features are described in the literature; nonetheless in one case, the patient, a 37-year-old Japanese woman with a former diagnosis of AOSD in remission since 13 years, developed a disease relapse after the second dose of BNT162b2 vaccine and was successfully treated with s.c. tocilizumab (162 mg/2 weeks); 5 another patient from the United States instead developed AOSD following mRNA-1273 vaccine and required prednisone 1 mg/kg/day with an immediate resolution of fever, arthralgias, and skin rash within 3 days. 6
The MAS-like condition named MIS-A, which was observed in the Asian-American patient above described, was detected also in other three adult vaccinated subjects (with Pfizer-BioNTech vaccine) with a previous history of COVID-19 infection; however, they were not treated with the IL-1 inhibitor, but they ameliorated with i.v. glucocorticoids and IVIg. Similarly, a case of cytokine-release syndrome (CRS) was described in a patient with colorectal cancer on long-standing anti-PD-1 monotherapy, after receiving BNT162b2 vaccine and was treated successfully with i.v. methylprednisolone.4,7
Overall, new onsets or flares of immune-mediated diseases (IMDs) temporally attributed to SARS-CoV-2 vaccination were identified in a multicenter cohort study involving 27 subjects; however, most of the patients had an already pre-existing autoimmune/rheumatic condition prior vaccination, and their manifestations generally responded quickly to therapy. Most of the outlined patients developed arthritis, followed by connective-tissue diseases; one case of polymyalgia was observed but no cases of AOSD, MAS, or MIS-A were recordered in this cohort. 8 Other cohorts reported patients with temporal arteritis-like form, recurrent pericarditis, and a uveitis relapse in a patient with Behçet’s disease; all of them were successfully treated with NSAIDs or glucocorticoids. 9
By now, other non-rheumatological inflammatory conditions are reported in the literature as probably unleashed by anti-SARS-CoV-2 vaccines, such as subacute thyroiditis, autoimmune hepatitis, and acute demyelinating of central nervous system (optic nerve, brain, and/or spinal cord).8,10–12 Taken together those evidences suggest that a temporal association between vaccine injection and hyperinflammation onset is plausible, however, a coincidental association cannot be excluded, although we observed an unusual cluster of AOSD-like new-onset presentations. In addition, there is not an established time frame for which the association between vaccines and inflammatory symptoms is assured. In our cases, the range of time from the vaccine injection to symptoms onset was within 10 days for three patients, and after 18 days for another one, while in other reports, the gap lasted between 1 and 43 days.2,4
Discussion
Vaccines against SARS-CoV-2 may elicit innate immunity activation. BNT162b2 vaccination induces modest innate immune responses after primary immunization mainly sustained by interferon-gamma (IFN-γ), which increase strikingly after the secondary immunization. 13 It is possible that some people could develop uncontrolled inflammation after receiving vaccines to prevent COVID-19. 14 In such persons, SARS-CoV-2 vaccines might precipitate or exacerbate subclinical or unrecognized inflammatory diseases. Indeed, a genetic susceptibility with recognized HLA associations or polymorphisms on IL-18 has been widely described; 15 therefore, it is conceivable that an external trigger such as the vaccination shot could induce an exaggerate inflammatory response in predisposed individuals. In our cohort, three out of four patients received vaccine with mRNA technology and lipid nanoparticle (LNP) delivery system. One patient received ChAdOx1 shot which contains DNA delivered with non-replicating recombinant adenovirus vector system. 16 Since mRNA may serve as both immunogen and adjuvant, once intracellular sensors are activated by single stranded (ssRNA) and double stranded RNA (dsRNA), the innate immune response is elicited. In addition, the LNP carrier, once engulfed by dendritic cells, lead to the adaptive immune response expansion. Adenovirus vaccine contains adjuvant properties and engages other types of toll-like receptors. However, both the vaccines induce the final production of IFN-γ.17,18 It is possible that in genetically predisposed subjects, and/or if environmental and epigenetic factors interfere, the immune response generated by vaccines is skewed into a pro-inflammatory direction, with an excessive production of pro-inflammatory cytokines, which provokes the symptoms described in our patients (Figure 1). However, it is not excluded that also autoantibodies might be produced, leading to autoimmune conditions as recently reported. 8
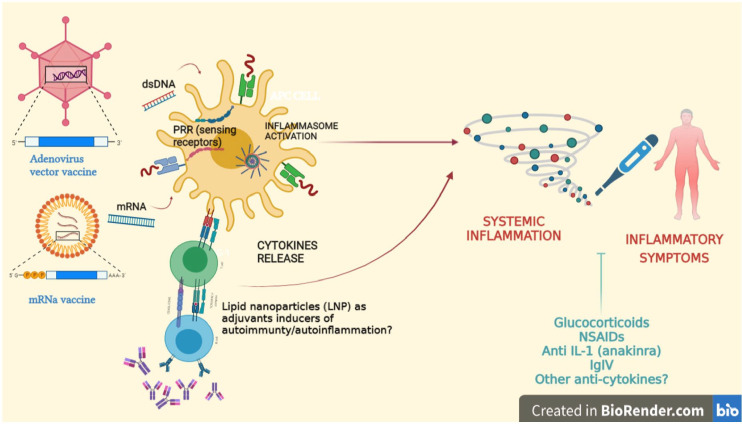
Figure 1.
The interactions between anti SARS-COV-2 DNA/mRNA vaccines and the immune system are depicted. The two vaccines can activate the innate immune response through Toll-like receptors (TLR), sensing receptors, and the inflammasome. In patients with a genetic predisposition and/or exposed to environmental triggers, an imbalance between pro- and anti-inflammatory cytokines may lead to the onset of inflammatory symptoms provoked by a sort of cytokine storm. Therapies available to contrast the cytokine storm are mentioned. Moreover, the expansion of the adaptive immune response induced by lipid nanoparticles may be responsible for the production of autoantibodies which may lead to autoimmune disorders. (A color version of this figure is available in the online journal.)
Made with BioRender.com.
IL-1: interleukin-1; IVIg: intravenous immunoglobulin; DNA: deoxyribonucleic acid; LNP: lipid nanoparticle; mRNA: microribonucleic acid; NSAIDs: non-steroidal anti-inflammatory drugs; PRR: pattern recognition receptor.
It is well-described the mechanism by which LNPs (e.g. liposomes and polymer-based nanoparticles) can induce the inflammasome activation. 19 However, it is unclear whether other components of the inflammasome or different inflammasome sensors such as NLR Family CARD Domain Containing 4 (NLRC4), NLR family pyrin domain containing 3/6/7 (NLRP3/6/7), or Absent-In-Melanoma 2 (AIM2) are involved in the final production of IL-1 according to the type of external stimulus. In our cohort, two patients had a pre-existent immunological disorder, since one patient had psoriatic arthritis, and the other one was affected with AOSD, but it is unclear why the patient with AOSD responded promptly to anakinra, while “naϊve” patients for inflammatory disorders required i.v. glucocorticoids and anakinra and were hospitalized for a longer time. In addition, although the prevalence of SARS-CoV-2 infection among patients with rheumatic musculoskeletal diseases was similar to the general population in our region, 20 we could not exclude prior immunization from COVID-19 and over-reaction after SARS-CoV-2 vaccination.
The extensive diagnostic work-up in our patients did not reveal infection (including SARS-CoV-2), malignancy, any other rheumatic disease, or alternative explanation other than vaccination for provoking such a severe systemic inflammatory response. Interestingly, all the patients fulfilled one or more criteria set for AOSD or MAS and they all showed a very rapid clinical and biochemical improvement in a matter of hours or days after starting the anti-IL-1 drug. It is interesting that the detection of very high levels of IL-1β in one patient and the dramatic response to IL-1 blockade suggest an IL-1 mediated inflammatory response 21 induced by SARS-CoV-2 vaccines, similar to the cytokine storm described both in AOSD 22 and in SARS-CoV-2 infection. 23 Anakinra, the IL-1 receptor antagonist, blocks activity of the pro-inflammatory cytokines IL-1α and IL-1β and is commonly used in the treatment of many autoinflammatory disorders (e.g. AOSD, systemic-onset Juvenile Idiopathic Arthritis (soJIA), and Familial Mediterranean Fever) at the daily dose of 100 mg subcutaneously (adult patients). 24 In our cohort, three patients received i.v. anakinra (100 mg every 6–8 h) to curb the massive cytokine release typical of secondary HLH, while the other was treated with s.c. anakinra 100 mg/day or bid. The rationale of using i.v. anakinra to treat the hyperinflammatory condition presented by our patients comes from the excellent results obtained during the SARS-CoV-2 pandemic in which i.v. anakinra was proved to be safe and to reduce both mortality and the need for invasive mechanical ventilation in hospitalized COVID-19.25,26 Of note, this is the first case series in which patients developing hyperinflammation after vaccination were treated with i.v. anakinra, since the other reports present in the literature describe subjects who received the canonical s.c. route of administration.
In conclusion, larger studies are required to define the close relationship between inflammatory manifestations and vaccination; however, anti SARS-CoV-2 vaccine remains safe and effective and the benefits of vaccinations far outweigh the risk of immune side effects even in patients with pre-existing autoimmune or autoinflammatory disorders. 27
Conclusions
Of note, one should be aware of the possible occurrence of hyperinflammation with AOSD-like presentations in patients receiving anti-SARS-CoV-2 vaccines; the causes of the onset of inflammatory symptoms are unclear yet; however a genetic predisposition may be one of the key factors involved in the pathogenesis of this hyperinflammatory condition. In addition, other unclear points regard the different immunogenicity of DNA and mRNA vaccines, especially in subjects with prior immunological impairment. Considering that some of these patients may evolve to life-threatening conditions, a prompt recognition of symptoms, an accurate anamnesis about immunization, possible prior COVID-19 infection, and the past rheumatological/immunological history are required to start as soon as possible the correct immune-modulating treatment. 28
Footnotes
Authors’ Contributions: S.B. and A.G. provided the conception of the study, literature search, and interpretation of data, drafting the article, revised it critically for important intellectual content. P.G. provided draft the article, and revised it critically for important intellectual content. A.D. provided interpretation of data, and revised it critically for important intellectual content. P.S. provided the conception of the study and interpretation of data, drafting the article, revised it critically for important intellectual content. All authors revised the article critically for important intellectual content and gave final approval of the version to be submitted. S.B. and A.G. contributed equally to this paper.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sara Bindoli  https://orcid.org/0000-0002-9409-3329
https://orcid.org/0000-0002-9409-3329
Andrea Doria  https://orcid.org/0000-0003-0548-4983
https://orcid.org/0000-0003-0548-4983
References
- 1. Leone F, Cerasuolo PG, Bosello SL, Verardi L, Fiori E, Cocciolillo F, Merlino B, Zoli A, D’Agostino MA. Adult-onset Still’s disease following COVID-19 vaccination. Lancet Rheumatol 2021;3:e678–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baicus C, Delcea C, Pinte L, Dan GA. Hyper-inflammation after COVID-19 mRNA vaccination: at the crossroads of multisystem inflammatory disease and adult-onset Still’s disease. Does terminology matter? Rom J Intern Med. Epub ahead of print 6 September 2021. DOI: 10.2478/rjim-2021-0035 [DOI] [PubMed] [Google Scholar]
- 3. Salzman MB, Huang CW, O’Brien CM, Castillo RD. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis 2021;27:1944–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, Kashiwazaki S, Tanimoto K, Matsumoto Y, Ota T. Preliminary criteria for classification of adult Still’s disease. J Rheumatol 1992;19:424–30 [PubMed] [Google Scholar]
- 5. Yamamoto S, Nishimura K, Yo K, Waki D, Murabe H, Yokota T. Flare-up of adult-onset Still’s disease after receiving a second dose of BNT162b2 COVID-19 mRNA vaccine. Clin Exp Rheumatol 2021;39:139–40 [DOI] [PubMed] [Google Scholar]
- 6. Magliulo D, Narayan S, Ue F, Boulougoura A, Badlissi F. Adult-onset Still’s disease after mRNA COVID-19 vaccine. Lancet Rheumatol 2021;3:e680–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, Carlyle E, Edmonds K, Del Rosario L, Shon J, Haynes WA, Ward B, Shum B, Gordon W, Gerard CL, Xie W, Joharatnam-Hogan N, Young K, Pickering L, Furness AJS, Larkin J, Harvey R, Kassiotis G, Gandhi S, Crick COVID-19 Consortium, Swanton C, Fribbens C, Wilkinson KA, Wilkinson RJ, Lau DK, Banerjee S, Starling N, Chau I, CAPTURE Consortium, Turajlic S. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 2021;27:1362–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, Brodavka M, Cohen Y, Abu-Much A, Abu Elhija M, Bridgewood C, Langevitz P, McLorinan J, Bragazzi NL, Marzo-Ortega H, Lidar M, Calabrese C, Calabrese L, Vital E, Shoenfeld Y, Amital H, McGonagle D. Immune-mediated disease flares or new-onset disease in 27 subjects following MRNA/DNA SARS-CoV-2 vaccination. Vaccines 2021;9:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishay Y, Kenig A, Tsemach-Toren T, Amer R, Rubin L, Hershkovitz Y, Kharouf F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int Immunopharmacol 2021;99:107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khayat-Khoei M, Bhattacharyya S, Katz J, Harrison D, Tauhid S, Bruso P, Houtchens MK, Edwards KR, Bakshi R. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol 2021:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab 2021;106:2600–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casualty. J Hepatol 2021;75:728–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arunachalam PS, Scott MKD, Hagan T, Li C, Feng Y, Wimmers F, Grigoryan L, Trisal M, Edara VV, Lai L, Chang SE, Feng A, Dhingra S, Shah M, Lee AS, Chinthrajah S, Sindher SB, Mallajosyula V, Gao F, Sigal N, Kowli S, Gupta S, Pellegrini K, Tharp G, Maysel-Auslender S, Hamilton S, Aoued H, Hrusovsky K, Roskey M, Bosinger SE, Maecker HT, Boyd SD, Davis MM, Utz PJ, Suthar MS, Khatri P, Nadeau KC, Pulendran B. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021;596:410–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014;14:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun 2018;93:24–36 [DOI] [PubMed] [Google Scholar]
- 16. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021;21:195–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sayedahmed EE, Elkashif A, Alhashimi M, Sambhara S, Mittal SK. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines 2020;8:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma B, McLeland CB, Potter TM, Stern ST, Adiseshaiah PP. Assessing NLRP3 inflammasome activation by nanoparticles. Methods Mol Biol 2018;1682:135–47 [DOI] [PubMed] [Google Scholar]
- 20. Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, Cozzi G, Depascale R, Zanatta E, Gasparotto M, Benvenuti F, Bindoli S, Gatto M, Felicetti M, Ortolan A, Campaniello D, Larosa M, Lorenzin M, Ramonda R, Sfriso P, Schiavon F, Iaccarino L, Doria A. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun 2020;112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galozzi P, Bindoli S, Doria A, Sfriso P. The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun Rev 2021;20:102785. [DOI] [PubMed] [Google Scholar]
- 22. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bindoli S, Felicetti M, Sfriso P, Doria A. The amount of cytokine-release defines different shades of SARS-CoV2 infection. Exp Biol Med (Maywood) 2020;245:970–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cavalli G, Farina N, Campochiaro C, Baldissera E, Dagna L. Current treatment options and safety considerations when treating adult-onset Still’s disease. Expert Opin Drug Saf 2020;19:1549–58 [DOI] [PubMed] [Google Scholar]
- 25. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:e325–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasin L, Cavalli G, Navalesi P, Sella N, Landoni G, Yavorovskiy AG, Likhvantsev VV, Zangrillo A, Dagna L, Monti G. Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies. Eur J Intern Med 2021;86:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, Calabrese C, Gravallese EM, Harpaz R, Kroger A, Sadun RE, Turner AS, Williams EA, Mikuls TR. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cavalli G, Larcher A, Tomelleri A, Dagna L. Interleukin-1 and interleukin-6 inhibition in patients with COVID-19 and hyperinflammation. The Lancet Rheumatol 2021;3:e248–9 [DOI] [PMC free article] [PubMed] [Google Scholar]