Abstract
Metformin is one of the most prescribed drugs in the world giving potential health benefits beyond that of type 2 diabetes (T2DM). Emerging evidence suggests that it may have protective effects for retinal/posterior segment diseases including diabetic retinopathy (DR), age-related macular degeneration (AMD), inherited retinal degeneration such as retinitis pigmentosa (RP), primary open angle glaucoma (POAG), retinal vein occlusion (RVO), and uveitis. Metformin exerts potent anti-inflammatory, antiangiogenic, and antioxidative effects on the retina in response to pathologic stressors. In this review, we highlight the broad mechanism of action of metformin through key preclinical studies on animal models and cell lines used to simulate human retinal disease. We then explore the sparse but promising retrospective clinical data on metformin’s potential protective role in DR, AMD, POAG, and uveitis. Prospective clinical data is needed to clarify metformin’s role in management of posterior segment disorders. However, given metformin’s proven broad biochemical effects, favorable safety profile, relatively low cost, and promising data to date, it may represent a new therapeutic preventive and strategy for retinal diseases.
Keywords: Metformin, retina, ophthalmology, glaucoma, diabetes
Impact Statement
Metformin is the most commonly prescribed oral antihyperglycemic agent used in diabetes and has been demonstrated to have protective effects in many different diseases including stroke, cancer, dementia, glaucoma, and cardiovascular disease. Metformin’s impact on retinal diseases has been studied in both preclinical and clinical studies with evidence that there may be some favorable effect. This work is a comprehensive review of key studies as it relates to metformin and retinal diseases, and it may help provide a better understanding to help spark future studies to better elucidate the role of metformin in retinal diseases.
Introduction
Metformin is an orally administered biguanide hypoglycemic agent that has been used for over 60 years in the treatment of type 2 diabetes mellitus (T2DM). Metformin is derived from guanidine, the active moiety of the French lilac, or goat’s rue (Galega officinalis), a shrub that was used to treat diabetes in Europe during medieval times.1,2 Diabetes mellitus (DM) is the most common metabolic disease worldwide, affecting an estimated 463 million people in 2019 and projected to affect over 700 million people by 2045. 3 While the therapeutic effects of metformin in diabetes are not completely understood, its broad function is to inhibit hepatic gluconeogenesis with a consequential increase in insulin sensitivity. 4 In addition, it increases glucose uptake by skeletal muscle 5 and impacts lipid metabolism. 6 Emerging evidence, however, suggests that the primary site of action of metformin may be the human gastrointestinal (GI) tract 7 where metformin is able to coordinate complex gut–brain–liver crosstalk to increase glucose utilization, decrease glucose and lipid synthesis, and decrease levels of proinflammatory cytokines in the circulation. 8
In addition to the success of metformin in diabetes, several studies have shown potential health benefits in several systemic diseases. Regarding ocular diseases, metformin has shown promise in preclinical studies for the treatment of glaucoma, 9 uveitis, 10 diabetic retinopathy (DR), age-related macular degeneration (AMD), and inherited retinal dystrophies such as retinitis pigmentosa (RP). Both in vivo and in vitro animal studies have shown that metformin protects against oxidative stress and disorganization of tight junctions in retinal pigment epithelial cells and against inflammation and angiogenesis of retinal vascular endothelial cells in a dose-dependent manner.11,12 While prospective clinical data is lacking, retrospective studies show that metformin may be able to help reduce the risk of visual loss from both DR and age-related macular degeneration (AMD), two of the most common causes of visual loss worldwide.13–18 In this review, we will briefly discuss research regarding the mechanism of action, safety profile, and systemic benefits of metformin, as well as the major proposed mechanisms of metformin in retinal diseases and highlight its potential therapeutic role for the treatment of retinal conditions such as DR, AMD, RP, POAG, and uveitis.
Metformin’s mechanism of action, safety profile, and beneficial systemic effects
The mechanism of action of metformin is complex and still being elucidated. Originally, metformin was shown to activate AMP-dependent kinase (AMPK) in the liver, an essential enzyme that maintains cellular energy stores through its modulation of ATP production. 6 Newer evidence reveals that metformin acts in both an AMPK-dependent and AMPK-independent manner. In addition to its antihyperglycemic properties, metformin helps quench toxic reactive oxygen species (ROS) generated by mitochondria, promotes autophagy through lysosomal activation, reduces levels of proinflammatory cytokines, and inhibits cellular apoptosis. 19 This broad range of function likely explains the therapeutic potential of metformin in diseases that span many different organ systems.
The safety of metformin has been well-studied and proven to be favorable. In a large, randomized trial, the Diabetes Prevention Program (DPP), over 3000 subjects were followed over a 15-year period, and safety events closely monitored with no severe adverse events reported. 20 In regard to tolerability, GI upset was the most frequent side effect (28% vs 16% in placebo group), but these effects reduced over time and the rates of GI upset was equal between the groups with chronic use. 20 In addition, lactic acidosis is a serious known yet exceedingly rare side effect of metformin, but during this follow up time period of ~40,000 patient-years, no cases of lactic acidosis were reported. 21 Further research studies have suggested that the risk of lactic acidosis with metformin use has been overemphasized in literature, 22 and that only those with significant renal or hepatic impairment may be at an increased risk.20,23 As for systemic benefits of metformin use, a large systematic review and meta-analysis highlighted that not only is metformin associated with lower mortality, cancer risk, and cardiovascular disease in patients with diabetes, but also that metformin appears to extend life spans independent of its effect on diabetes. 22 Furthermore, recent studies have suggested that metformin may have beneficial effects on obesity, 24 gestational hypertension and preeclampsia, 25 colorectal cancer, breast cancer, 26 ovarian cancer, 27 neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, 28 cerebral ischemia, 29 and neurologic function after cardiac arrest. 30
Metformin in retinal disease
Preclinical trials
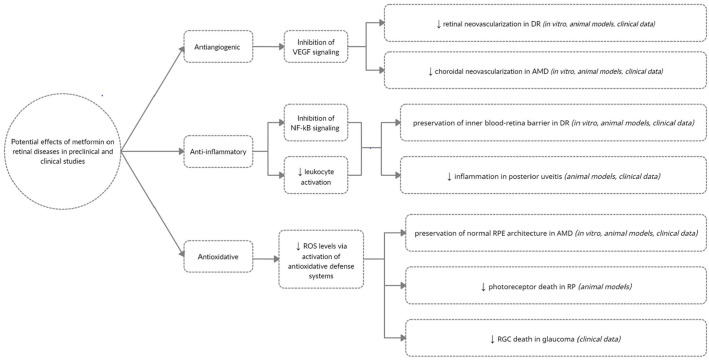
Numerous studies have examined the effect(s) of metformin on research animals and cell lines exposed to stressors that model human retinal disease. These studies show that metformin exerts potent anti-inflammatory, antioxidative, antiapoptotic, and antiangiogenic effects in both an AMPK-dependent and AMPK-independent fashion. For this review, we will focus on key preclinical studies that illustrate the range of functions of metformin and highlight retinal diseases where metformin holds a therapeutic potential (Table 1 and Figure 1).
Table 1.
Targets of metformin in retinal diseases in preclinical studies.
| Study | Model and study design | Molecular effect(s) of metformin | Overall effect(s) of metformin | Human disease correlate |
|---|---|---|---|---|
| Luodan et al. 31 | Rd1 mouse model. Intravitreal injections of metformin on P6, P9, and P12. | ↑alpha-crystallin, ↑BIRC3, ↑BIRC5. ↓ IL-4, ↓IL-10, ↓TGFB, ↓BAK1 | ↑photoreceptor survival. ↓microglia activation | RP |
| Athanasiou et al. 32 | P23H KI (RhoP23H/P23H) mouse model. Daily metformin treatment from P9 to P14. | ↑AMPK activation → localization of P23H mutant rhodopsin to the retinal outer segments | ↓ photoreceptor survival | RP |
| Han et al. 11 | Human retinal vascular endothelial cell line. Pretreatment with metformin. | ↓NFkB p65, ↓ICAM-1, ↓MCP-1, ↓IL-8. ↑pAMPK leading to ↓ICAM-1, ↓MCP-1. | ↓hRVEC proliferation | DR |
| Han et al. 11 | vldr–/– mouse model. Metformin treatment starting on P10 with 10 consecutive days of treatment. | N/A | ↓intraretinal neovascular bulbs | DR |
| Han et al. 11 | STZ-induced diabetic mouse model. Daily metformin starting on day 5 for 10 consecutive days. | N/A | ↓leukocyte adherence to retinal vessel walls | DR |
| Joe et al. 33 | OIR mouse model. Daily from P12 until P17 or P21. | ↓VEGF-R via UPS mediated degradation. | ↓neovascularization, ↑ avascular retina | ROP, ischemic retinopathies |
| Kalariya et al. 10 | EIU mouse model. Intraperitoneal metformin injection 12 h before or 2 h after LPS injection. | ↓inflammatory cells, ↓protein levels in AqH. ↓TNF-a, ↓MCP-1, ↓IL-1b, ↓MIP-1a, ↓IL-6, ↓leptin, ↓IL-8, ↓GRO/KC in AqH. ↑AMPK, ↓COX-2, ↓NF-kB on retina sections | ↓intraocular inflammation | uveitis |
| Kim et al. 34 | High fat diet (HFD) diabetic mouse model. Daily metformin two months after induction with HFD. | ↑ pAMPK, ↑pAKT. ↓ pERK, ↓NFkB p65, ↓IL-6, ↓G-CSF, ↓VEGF | No effect on degree of neovascularization, severity of retinopathy, or ERG responses. | DR |
| Kim et al. 35 | STZ induced diabetic mouse model. Daily metformin treatment for eight weeks after final STZ injection. | ↓OGT, ↓O-GlcNAc modification, ↓ChREBP, ↓TXNIP, ↓NF-kB, ↓PARP | ↓ganglion cell death | DR |
| Nahar et al. 36 | STZ-induced diabetic mouse model. Daily metformin two weeks after induction of diabetes. | ↓TNF-α, ↓ VEGF, ↑claudin-1, ↑GSH: MDA ratio | Preservation of overall retinal architecture. ↓DR severity score. | DR |
| Oubaha et al. 37 | OIR mouse model. Single intravitreal metformin injection on P12 | ↓IRE1a activation, ↓NF-kB, ↓IL-6, ↓cdkn1a and ↓cdkn2a gene expression. ↓number of senescent retinal cells on P14 and P17. | ↓neovascularization, ↑ restoration of normal retinal vasculature | ROP, ischemic retinopathies |
| Qu et al. 12 | Glyoxal treated ARPE-19 cell line. Pretreatment with metformin (glyoxol + metformin ARPE-19 cells) with variations in concentration and total duration of treatment. | ↑NO, ↑pAMPK, ↑Sirt1, ↑TXNIP. ↓ROS Increased levels of . ↑occludin and ZO-1 in RPE cells | ↑ ARPE-19 cells. Preservation of ARPE-19 cell tight junctions. | AMD |
| Qu et al. 12 | Sprague Dawley (SD) mice injected with sodium iodite. Simultaneous injection of metformin. | N/A | Preservation of outer blood-retinal layer and normal RPE architecture characterized by normal ZO-1 staining and less RPE pleomorphism | AMD |
| Xu et al. 38 | Chx10-Cre mouse model with retina-specific KO of either AMPKalpha1 or AMPKalpha2. Daily metformin for seven consecutive days followed by exposure to damaging light. | Activation of AMPK2alpha in AMPKalpha1 KO mice. | Increased photoreceptor survival in AMPKalpha1 KO mice but not AMPKalpha2 KO mice. | AMD, RP |
| Xu et al. 38 | Light damage (LD) albino mouse model. Daily metformin for seven consecutive days followed by exposure to damaging light | ↓ PARP-14. ↑SOD-2, ↑Nrf1, ↑Tfam, ↑PGC-1α, ↑COX-II, ↑NADH:NAD+ | Preservation of ONL in metformin treated mice exposed to LD. Preserved a-wave photopic ERG response. | AMD, RP |
| Xu et al. 38 | Rd10 mouse model. Daily metformin starting on day P13. | ↑ COX1-1, ↑ATP, ↑mitochondrial DNA | Prolonged survival of rods and cones in treated mice. Preservation of scotopic, photopic, and flicker ERG responses of treated mice at six weeks | RP |
| Xu et al. 38 | BALB/cJ albino mouse model injected with SI. Pretreatment with metformin prior to SI injection. | N/A | Preservation of RPE architecture on IHC with ZO-1 staining. Preserved photoreceptor and RPE function as detected by scotopic a and c waves on ERG. | AMD |
| Xu et al. 39 | PPARΔ KO mouse model. Daily metformin at six to eight weeks age for one consecutive week | ↑PPARΔ in WT mice | Reduction in light induced retinal toxicity based on retinal thickness on OCT and ERG responses in both KO and WT mice | AMD |
| Yi et al. 40 | STZ-induced diabetic mouse model. Two weeks after STZ treatment, daily metformin for 10 weeks. | ↓phosphorylation of VEGFR2. ↑VEGF120: VEGF164 | ↓vessel density in metformin treated mice | DR |
| Ying et al. 44 | Laser-induced choroidal neovascularization mouse model. Daily intraperitoneal injection of metformin one day prior to laser irradiation until day six. | ↓ALK1 expression | ↓size and vascularity of CNV lesions | wet AMD |
| Zhang et al. 41 | Alloxon-induced hyperglycemic mouse model. Two weeks after alloxan treatment, daily metformin for 10 weeks. | ↑miR-497A-5p, ↓VEGF-A | ↓vessel density in metformin treated mice | DR |
| Zhao et al. 42 | Human RPE cell line incubated with H2O2 to generate ROS. Pretreatment with metformin. | ↑AMPK mediated autophagosome formation | ↑survival of treated RPE cells | AMD |
IL: interleukin; BAK1: brassinosteroid insensitive-associated receptor kinase-1; RP: retinitis pigmentosa; AMPK: AMP-dependent kinase; NF-κB: nuclear factor kappa B; hRVEC: human retinal vascular endothelial cells; pAMPK: phosphorylated AMPK; ICAM-1: intercellular adhesion molecule-1; TNF-α: tumor necrosis factor alpha; MCP-1: monocyte chemoattractant protein-1; DR: diabetic retinopathy; STZ: streptozocin; OIR: oxygen-induced retinopathy; VEGF: vascular endothelial growth factor; UPS: ubiquitin-proteasome system; ROP: retinopathy of prematurity; EIU: endotoxin induced uveitis; LPS: lipopolysaccharide; COX-2: cyclooxygenase-2; HFD: high fat diet; G-CSF: granulocyte colony-stimulating factor; ERG: electroretinogram; OGT: O-linked N-acetylglucosaminyltransferase (O-GlcNAc) transferase; O-GlcNAc: O-linked N-acetylglucosaminyltransferase; TXNIP: thioredoxin-interacting protein; PARP: Poly(ADP-ribose) polymerase; IRE1a: inositol-requiring enzyme 1a; NO: nitrous oxide; ROS: reactive oxygen species; ZO-1: zonula occludens-1; AMD: age-related macular degeneration; SD: Sprague Dawley; KO: knockout; LD: light damage; SOD-2: superoxide dismutase-2; PGC-1α: peroxisome proliferator-activated receptor-γ coactivator; COX-II: cytochrome c oxidase subunit II; DNA: Deoxyribonucleic acid; WT: wild type; ALK1: activin A receptor like type 1.
Figure 1.
Potential effects of metformin on retinal diseases in preclinical and clinical studies.
Metformin inhibits pathologic neovascularization in response to retinal ischemia
Pinpointing the specific role of metformin in DR bolsters our overall understanding of the disease, helps identify specific biochemical pathways that can be individually targeted by future drugs, and provides a framework for identifying other retinal diseases for which metformin may have a therapeutic role. Metformin targets both the vascular and inflammatory pathologic components of DR and has been shown to exert antiangiogenic effects in DR by regulating vascular endothelial growth factor (VEGF) signaling through various mechanisms.
Yi et al. used a streptozocin (STZ)-induced diabetes mouse model to study the effects of metformin on the development of DR. Two weeks after induction of diabetes with STZ, one group of mice was administered metformin daily for 10 additional weeks, one group was administered saline, and the control group was not given STZ or metformin. At 10 weeks, STZ+ metformin mice had less severe DR than STZ mice, although the amount of avascular retina was similar in the two groups. At 12 weeks, retinal vessel density was significantly lower in the STZ+ metformin group compared with the STZ group, suggesting that metformin was inhibiting diabetic retinal neovascularization. Interestingly, total VEGF and VEGF-receptor (VEGFR) levels were similar between STZ and STZ+ metformin mice. However, metformin significantly decreased levels of phosphorylated VEGFR, the active form of VEGFR. In addition, STZ increased expression of VEGF164, the most potent of three VEGF isoforms. Metformin attenuated this response by increasing the levels of the less potent isoform, VEGF120. Collectively, these findings suggest that metformin may prevent neovascularization in DR by decreasing expression of VEGFR, inducing expression of the less active VEGF120 isoform of VEGF, as well as altering the ratio of VEGF164:VEGF120. 40
Zhang et al. showed in an alloxan-induced diabetic mouse model that metformin reduces the severity of DR via microRNA (miRNA)-mediated inhibition of VEGF-A protein translation. Two weeks after alloxan administration, mice were divided into two groups: one group was administered metformin daily for 10 weeks and the other administered normal saline. There were no statistically significant differences in fasting blood glucose or serum insulin levels, retinopathy scores, and percentage of avascular retina between the two groups at 10 weeks. However, retinal vessel density was significantly higher in the alloxan group compared with the alloxan + metformin group. Furthermore, both groups demonstrated similar levels of VEGF-A messenger RNA (mRNA) levels, but the alloxan group had significantly higher VEGF-A protein levels. The levels of miR-497A-5p, a micro-RNA that specifically targets and inhibits VEGF-A protein translation via antisense binding, were significantly increased in the metformin treated group. These findings suggest that metformin may inhibit neovascularization in DR by increasing miR-497A-5p levels, which decreases VEGF-A protein translation through antisense binding with VEGF-A mRNA. 41
Joe et al. studied oxygen-induced retinopathy (OIR) in a mouse model and in human umbilical vein endothelial cells (HUVECs) and found that metformin did not alter VEGF levels but instead decreased VEGF receptor 2 (VEGFR-2) levels by marking VEGFR-2 for degradation via the ubiquitin-proteasome system (UPS), thus preventing angiogenesis. Of the three VEGF receptors expressed by retinal tissue: VEGF receptor 1 (VEGFR-1), VEGF receptor 2 (VEGFR-2), and VEGF receptor 3 (VEGFR-3), VEGFR-2 is the predominantly expressed VEGF receptor involved in retinal angiogenesis. OIR is meant to replicate human retinopathy of prematurity (ROP), and the results of this study suggest that metformin may prevent neovascularization in pathologic states other than DR where retinal ischemia is present as well. 33
In the OIR mouse model, mice are exposed to hyperoxemic conditions from postnatal day seven (P7) until P12 to inhibit retinal vessel growth with subsequent return to ambient conditions. Pathological neovascularization begins on P14 and peaks on P17. Normal vasculature is restored by P21. 43 In an OIR mouse model, Oubaha et al. 37 showed that in response to hypoxia, retinal cells, most likely RGCs, enter a senescent state, otherwise known as the senescence-associated secretory phenotype (SASP), through the activation of the endoplasmic reticulum stress inositol-requiring enzyme 1a (IRE1a) pathway. The SASP is thought to both precede pathological neovascularization and paradoxically mediate it as senescent retinal cells release plasminogen activator inhibitor 1, IL-6, IL-8, and VEGF. In this study, a single intravitreal injection of metformin on P12 inhibited the SASP of retinal cells through downregulation of IRE1a, NF-kB, IL-6, and the specific senescence-associated genes Cdkn1a and Cdkn2a. Metformin also significantly reduced the extent of retinal neovascularization and restored normal vasculature by 50% (P < 0.0001) compared with untreated mice. Interestingly, administration of the anti-VEGF inhibitor aflibercept only inhibited pathological retinal neovascularization but had no significant effect on the senescence of cells and did not restore normal vasculature. Although metformin inhibited cells from entering a senescent state, this did not lead to an increase in apoptosis of RGCs or cells of the INL. 37 Cellular senescence may be the bridge between retinal hypoxia and eventual neovascularization, and a potential additional target of metformin.
Metformin inhibits inflammation and oxidative damage in DR and uveitis
In an STZ-induced diabetic mouse model, Kim et al. showed that metformin prevents retinal ganglion cell layer (GCL) death through its inhibition of O-linked N-acetylglucosaminyltransferase (O-GlcNAc) transferase (OGT), an enzyme that normally catalyzes O-Glc-NAc modification and activation of certain apoptotic proteins in high glucose environments. Specifically, O-Glc-NAc modification of carbohydrate response element binding protein (ChREBP), a regulator of glucose metabolism, activates thioredoxin-interacting protein (TXNIP), a key molecule in glucotoxicity-induced apoptosis. O-GLcNAC modification of nuclear factor kappa B (NF-κB), a transcription factor with wide immunologic and inflammatory functions, also promotes glucotoxicity. Poly(ADP-ribose) polymerase (PARP) is a nuclear enzyme activated in response to DNA injury and can induce cell death in hyperglycemic environments. The authors found that metformin significantly decreased levels of OGT, ChREBP, TXNIP, NF-kB, and PARP in retinas of diabetic mice and human retinal pigment epithelium (RPE) cells exposed to high glucose levels. Metformin also preserved the GCL in diabetic mice. Metformin may inhibit OGT and consequential N-Glc-NAc modification of key proteins that induce ganglion cell death under hyperglycemic conditions. 35
Nahar et al. demonstrated that metformin attenuated histological changes in mouse eyes in an STZ diabetic model in the cornea, lens, conjunctiva, sclera, iris, lens, ciliary, body, retina, and optic nerve. Furthermore, metformin helped restore imbalances in the levels of VEGF, tumor necrosis factor alpha (TNF-α), claudin-1, glutathione, and malondialdehyde caused by diabetes. Interestingly, histopathologic structural changes caused by diabetes impacted all retinal layers, and retinal architecture was substantially restored after metformin administration. 36
Han et al. found that metformin has key antiangiogenic and anti-inflammatory effects both in vitro in human retinal vascular endothelial cells (hRVECs) and in vivo in very low-density lipoprotein receptor (vldr) knockout (KO) mice (vldr–/–) and STZ induced diabetic mice, respectively. In hRVEC, metformin inhibited cellular proliferation, migration, and capillary formation by hRVEC in a dose-dependent manner, highlighting its potential antiangiogenic effects. The authors showed that metformin administration prior to the onset of intraretinal neovascularization (IRNV) in vldr–/–mice reduced IRNV by over 50% compared with vldr–/–mice receiving a control vehicle solution. 11 Furthermore, to study the anti-inflammatory effects of metformin, TNF-alpha was administered to hRVECs, which induced significant increases in the proinflammatory molecules NF-kB p65, intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8). Pretreatment with metformin significantly reduced production of all four of these molecules. This study also found that metformin inhibits the expression of ICAM-1 and MCP-1 via an AMPK-dependent mechanism and NFkB p65 and IL-8 via an AMPK-independent mechanism. Furthermore, in an STZ-induced diabetes mouse model, metformin reduced leukocyte adhesion to retinal vessel walls by half. Therefore, metformin may prevent leukocyte migration and adhesion to retinal vessel walls and subsequent breakdown of the inner blood-retinal layer through its inhibition of key chemokines and inflammatory mediators of the body. 11
Kalariya et al. also demonstrated the potent anti-inflammatory effects of metformin in an endotoxin-induced uveitis (EIU) mouse model. 10 Mice were injected subcutaneously with lipopolysaccharide (LPS) to induce uveitis. Mice treated with metformin had a statistically significant reduction in the number of inflammatory cells and total protein levels in the aqueous humor (AqH) compared with untreated mice injected with LPS. Metformin also lowered AqH levels of TNF-a, MCP-1, IL-1b, MIP-1a, IL-6, leptin, IL-8, and GRO/KC. Immunohistostaining of both ciliary body and retina sections showed that metformin reduced expression of cyclooxygenase-2 (COX-2) and phosphorylated p65(phospho-p65) in addition to increased expression of phosphorylated AMPK (pAMK). Phospho-p65 is one of the catalytic subunits of NF-kB and COX-2 activates prostanoids as a part of the inflammatory response by tissues. The authors hypothesized that metformin reduced the levels of phospho-p65 and COX-2 by increasing pAMPK though a direct relationship was not established. 10
Metformin does not slow the progression of DR in a high fat diet mouse model
Kim et al. studied mice fed a high fat diet (HFD) and found that metformin did not reverse obesity-induced (OID) retinal dysfunction as measured by electroretinogram (ERG) responses, oscillatory potential (OP) responses, and levels of retinal neovascularization. The HFD mouse model is a diet induced model which may more closely resemble the changes that occur in humans with diabetes compared with other models where genetic mutations are created. Mice were placed on an HFD regimen for two months, after which half of the mice received daily metformin. An additional control group of mice were fed a regular diet for the entire study. At one month, scotopic ERG recordings showed that HFD mice had similar a and b wave amplitudes compared with control mice but prolonged a and b wave implicit times. At two months, a and b wave amplitudes were decreased in addition to prolonged implicit times. HFD mice demonstrated reduced amplitude and increased latency in OP, but had not yet developed hyperglycemia, indicating that an HFD may start to affect retinal function even in the prediabetic state. 34
Mice receiving an HFD for six months had decreased phosphorylation and consequential deactivation of protein kinase B (AKT) and AMPK, and increased phosphorylation and subsequent activation of extracellular signal regulated kinase (ERK). With metformin administration, the levels of phosphorylated AKT (pAKT), pAMPK, and pERK were restored to similar levels to those of controls. HFD mice retinas had higher levels of NFkB p65 compared with control mice, but metformin administration significantly lowered levels of NFkB p65. Metformin also significantly reduced levels of the proinflammatory cytokines IL-6, granulocyte colony-stimulating factor (G-CSF), and VEGF when the vitreous of HFD and HFD+ metformin mice, respectively, were examined.
Despite the effect of metformin on these key pathologic mediators of DR, metformin did not attenuate the degree of DR and retinal dysfunction in HFD mice. At five months, HFD + metformin mice had similarly reduced scotopic a and b wave amplitudes and increased a and b wave implicit times, respectively, compared with HFD mice. At five months, HFD + metformin mice had worse OP responses (decreased amplitude and prolonged implicit time) compared with HFD mice. At six months both HFD and HFD+ metformin mice had similar levels of retinal neovascularization on fluorescein angiography (FA). Overall, the results of this study show that metformin does not protect against development of DR in an OID mouse model of retinal dysfunction. An important caveat of this study is that metformin treatment did not begin until two months after mice were fed an HFD. This was to better simulate real-world conditions in humans in which an HFD may be present prior to the diagnosis of diabetes. 34
Metformin protects RPE cells and photoreceptors from oxidative damage in AMD models
While pathophysiology of AMD is complex and poorly understood, research suggests it is an inflammatory process in which RPE cells are damaged by oxidative stress. Normally, these damaged RPE cells are removed through autophagy; however, in AMD the failure of autophagy may lead to the accumulation of toxic metabolic products and drusen resulting in RPE cell death and overlying photoreceptor loss. Zhao et al. hypothesized that metformin stimulates autophagy in RPE cells, thereby preventing the accumulation of damaged RPE cells and the subsequent development of AMD. In the Zhao et al. study, pretreatment of human RPE cells with metformin followed by incubation with hydrogen peroxide (H2O2) mitigated the accumulation of ROS in RPE cells and decreased RPE cell apoptosis. With the addition of an AMPK inhibitor, metformin was not protective. The findings of this study suggest that metformin may be beneficial in AMD via an AMPK-dependent stimulation of autophagy that removes damaged RPE cells. 42
In a similar study by Qu et al., a human RPE cell line ARPE-19 pretreated with metformin was protected from glyoxal-induced oxidative damage with decreased ROS levels, increased nitrous oxide (NO) levels, and decreased cellular apoptosis compared with untreated ARPE-19 cells exposed to glyoxal. Metformin increased levels of pAMPK in addition to the antioxidative molecules Sirtuin 1 (Sirt1), nuclear factor-erythroid factor 2-related factor 2 (Nrf2) and TXNIP. Metformin also increased levels of the RPE tight junction proteins occludin and zonula occludens-1 (ZO-1), suggesting that it may help maintain the outer blood-retinal layer. In rats exposed to sodium iodate and phosphate buffered solution (PBS) to induce oxidative stress, RPE cells demonstrated significant pleomorphism and the outer blood-retinal layer was compromised simulating RPE loss in advanced dry AMD with geographic atrophy. Metformin preserved the outer blood-retinal layer with normal ZO-1 staining and helped maintain an overall normal RPE architecture with fewer pleomorphic RPE cells. 12
Xu et al. showed that in albino mice exposed to toxic levels of white fluorescent light inducing oxidative damage, metformin protected against photoreceptor degeneration with preserved ERG photopic a-wave amplitudes and preserved photoreceptor nuclei on histological sections compared with untreated mice. qRT-PCR showed increases in the key antioxidative mitochondrial enzymes superoxide dismutase-2 (SOD-2), Nrf1, Tfam (transcription factor A, mitochondrial), peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, mitochondrial-encoded cytochrome c oxidase subunit II (COX-II), and restoration of NADH: NAD+ ratios. Metformin treatment also decreased expression of poly(ADP ribose) polymerase-14 (PARP-14), a DNA-damage response gene. Publicly available RNA sequencing of mice showed that mice express equal levels of AMPK alpha 1 (AMPKα1) AMPK alpha 2 (AMPKα2), two isoforms of the catalytic α-subunit of AMPK. Metformin protected retina-specific AMPKα1 KO mice from light damage (LD) but not AMPKα2 KO mice, suggesting that metformin exerts its protective effects through the activation of AMPKα2. In albino mice exposed to sodium iodate, metformin pretreatment preserved RPE architecture shown on immunohistochemistry with ZO-1 staining. Metformin also maintained scotopic ERG a wave and c wave amplitudes reflecting preserved function of photoreceptors and RPE cells, respectively. 38 These studies suggest that metformin holds great promise for AMD due to its potent antioxidative effects, likely mediated through upregulation of AMPK-dependent pathways but possibly also via AMPK-independent pathways.
In a laser-induced choroidal neovascularization mouse model meant to replicate human wet AMD, metformin-treated mice had smaller, less vascularized CNV lesions compared with mice receiving vehicle solution. 44 Metformin treatment also lowered levels of activin A receptor like type 1 (ALK1), a key mediator of angiogenesis expressed by endothelial cells. 44 Collectively, all of these studies suggest that metformin holds great promise for AMD due to its potent antioxidative and antiangiogenic effects, likely mediated through upregulation of AMPK-dependent pathways but possibly also via AMPK-independent pathways.
Metformin may play a neuroprotective role in RP
RP is a heterogeneous genetic disorder characterized progressive, irreversible loss of vision. Generally, rod photoreceptors are targeted early in the disease followed by cone photoreceptors. The rd1 mouse model is a useful surrogate for human RP in which rods start degenerating in a predictable manner on P8 and complete photoreceptor loss occurs by week four. 45 Luodan et al. used an RP rd1 mouse model to study the effects of intravitreally administered metformin on retinal degeneration. Overall, rd1 mice that received metformin demonstrated prolonged survival of photoreceptors, less microglia activation, and lower levels of inflammatory mediators compared with untreated rd1 mice cells with crystallins being a potential site of action of metformin. In addition, there was improvement in visual function of rd1 mice at P14, P18, and P22 with both ERG and light/dark transition testing. The main conclusion of this study is that metformin has both neuroprotective and immunoregulatory effects on rd1 mice. 31
Athanasiou et al. hypothesized that a reduction in the speed of translation of mutant rod opsin might result in improved trafficking of the protein and less severe RP. Protein translation is in part is regulated by AMPK, which controls both the elongation step of protein translation through its phosphorylation of elongation factor 2 kinase (EF2K) and through its modulation of mammalian target of rapamycin (mTOR) levels. Since metformin is known to activate AMPK, the authors investigated its potential therapeutic role in RP by improving the trafficking of misfolded opsin. In SK-N-SH cells (neuroblastoma cell line) and HEK293S cells (human embryonic kidney cell line) expressing mutant P23H-GFP rod opsin, metformin reduced both intracellular levels of mutant P23H-GFP rod opsin and increased cell surface expression of P23H-GFP rod opsin along the plasma membrane compared with untreated mutant cells leading to reduced cell death. Metformin did not alter cellular turnover of P23H-GFP rod opsin, suggesting that its effect was on trafficking of the misfolded protein. Metformin also increased P23H pigment formation, indicating that metformin may also functionally improve mutant rhodopsin. 32 Unfortunately, these findings did not translate into animal models, as P23H rod opsin was thermally unstable in the metformin group. However, metformin may still play an important role in retinal conditions where the inherent function of a mutant protein is not wholly compromised and improved trafficking of a mutant protein with some retained function may lead to less severe disease. 32
PPARΔ activation is not necessary for the protective effects of metformin
PPARs are a group of nuclear steroid hormones that regulate the expression of genes involved in fatty acid B-oxidation, cytochrome P450 enzymes and the overall cellular response in situations of oxidative stress. 46 Three subtypes exist – alpha, delta, and gamma. One of the postulated functions of metformin is reduction of oxidative stress through the activation of PPARs. Xu et al. showed that PPAR delta (PPARΔ) is not essential to the protective effects of metformin. RNA sequencing of mouse retinas showed that both PPARɑ and PPARΔ are expressed with PPARΔ expressed at four times higher levels than PPARɑ. In both wild type (WT) mice and Rho P23H/P23H mice, there was no significant difference in PPARΔ expression between the two groups, and levels of PPARΔ did not change in response to light-induced retinal damage. When WT mice were compared with mice with selective KO of the PPARΔgene, KO mice had normal retinal architecture and normal ONL thickness compared with WT mice measured on OCT at six weeks and at 12 months. To study the protective effects of metformin, WT and KO mice were injected with either subcutaneous metformin or PBS daily for one week at age six to eight weeks. Metformin protected both KO and WT mice from light induced retinal toxicity as evidenced by retinal thickness on OCT and ERG responses with no significant differences between KO and WT mice. Interestingly, WT mice receiving metformin did have higher levels of PPARΔ expression compared with WT mice receiving PBS injections. This study implies that PPARΔ is not the main mechanism by which metformin exerts its antioxidative effects on retinas. Furthermore, PPARΔ may either be non-essential or share redundant functions with other mediators of the oxidative stress response of the retina. 39
Clinical trials
Effects of metformin on the development of DR
Despite metformin’s status as the oldest oral treatment for diabetes, there is limited data from clinical trials regarding its specific role in the development and progression of DR. The Diabetes Prevention Program (DPP) was a three-year landmark clinical trial comparing lifestyle intervention, metformin, or placebo on the incidence of T2DM. In this study, metformin reduced the incidence of T2DM by 31% in patients with baseline impaired glucose intolerance (IGT) compared with similarly matched controls receiving a placebo at an average of 2.8-year follow-up. 47 The DPP Outcomes Study (DPPOS) followed the original participants of the DPP study for a mean of 15 years and studied not only the incidence of T2DM but also the development of aggregate microvascular complications (retinopathy, nephropathy, and neuropathy). 21 Metformin reduced the incidence of T2DM by 18% (HR 0.82, 0.72–0.93) compared with placebo, significantly less than the 31% reported in the original DPP. The cumulative incidence of T2DM at 15 years was 62% in the placebo group and 56% in the metformin group. Metformin was not protective against the development of aggregate microvascular complications compared with placebo (metformin vs placebo RR 1.05 (0.91–1.23); P = 0.50). However, a limitation of the study was that it was not powered to report on the incidence of DR. 21 Furthermore, there was a one-year gap between the termination of the DPP and the start of the DPPOS during which all groups were offered lifestyle intervention. Therefore, the baseline characteristics may have been biased between the DPP study and those enrolled in the DPPOS. 21
Data from a few large retrospective studies suggest that metformin is protective both against the development of DR in patients with diabetes 13 and in reducing the severity of DR among diabetics with underlying DR. 14 In a retrospective cohort study of 10,044 patients with T2DM, Fan et al. showed that the risk of non-proliferative DR (NPDR) (adjusted hazard ratio [aHR] 0.76; 95% CI = 0.68–0.87) and sight-threatening retinopathy (STDR) (aHR 0.29; 95% CI = 0.19–0.45) is reduced in metformin users compared with non-users. The overall risk of retinopathy in patients with T2DM was reduced by 24% with metformin administration. Patients receiving the highest doses of metformin (>1440 cumulative defined daily dose [DDD]) had the greatest protection against development of both NPDR and STDR, suggestive of a dose-dependent response of metformin therapy. However, metformin did not protect against the development of STDR among NPDR metformin users when compared with NPDR non-users (adjusted HR 0.54; 95% CI = 0.28–1.01). Overall, the protection of metformin against the development of DR was greater when it was prescribed within three months of diagnosis of T2DM and when adapted Diabetes Complications Severity Index (aDCSI) scores were low at time of diagnosis. A multivariate analysis demonstrated that those taking dipeptidyl peptidase 4 (DPP-4) inhibitors in addition to metformin further reduces the risk NPDR and STDR, whereas metformin in combination with a sulfonylurea was not protective against NPDR and STDR. Of note, the large database used in this study did not control for many important confounding variables such as body mass index (BMI), smoking status, and hemoglobin A1c which is a limitation of the study. Furthermore, metformin users with high aDCSI scores at the time of diagnosis were excluded due to lack of adequate controls, potentially leading to an overestimation of the protective effects of metformin. 13
In a retrospective cross-sectional study of 335 patients with T2DM for more than 15 years with DR, Li et al. demonstrated that long-term metformin use, defined as metformin use for at least 5 years, was associated with reduced severity of DR. In all, 48/193 metformin users (25%) developed SNPDR/PDR compared with 67/142 non-users (47%) (P < 0.001). The OR of developing severe NPDR or proliferative DR associated with metformin use was 0.37; 95% CI = 0.23–0.59; P < 0.001). This was found to be independent of sulfonylurea or insulin use. A lower percentage of metformin users underwent PRP (25%, 48/193 users) compared with non-users (43%, 61/142 non-users) (P < 0.001). However, rates of clinically significant macular edema (CSME) requiring focal/grid laser were similar between groups: 83/193 (43%) of metformin users and 69/142 (49%) of non-users. 14
In addition to metformin’s role in attenuating the development of DR, it may also play a role in preventing other retinal vascular diseases such as retinal vein occlusions (RVOs) in diabetic populations. A retrospective cohort study executed in Taiwan found that metformin use among diabetic patients was associated with a decreased risk of RVO development compared with non-diabetic controls (aHR of 1.61 in metformin users vs 2.37 in non-users). In this study, a random selection of 1 million patients was analyzed from the NHIRD, excluding patients with a previous history of diabetes or RVO. The final study cohort contained 907,277 patients, of whom 44,609 were diabetics and 862,668 who were not. The risk of a central RVO in diabetic non-metformin users was 3.7 times higher than control patients, which decreased to a 2.4 times higher risk with metformin treatment. The risk of branch RVO was stated to be similar between metformin users and controls, but specific values not reported. An important limitation of this study is that DM severity and stratification by glycemic control was not performed. It is possible that the cohort of metformin users had less severe underlying diabetes, thus leading to an overestimation of the protective effects of metformin on RVO.
Further research is needed to understand the role metformin might play in DR and other retinal diseases such as RVO. In particular, the timing and dose of metformin and the stage of DR at which it exerts its maximal effect are of paramount importance. An early Phase 1 trial (CORRECT) is investigating the effects of metformin alone versus insulin alone versus insulin/oral drug combination (including metformin) on the 5-year incidence of DR in patients with T2DM for <5 years and no evidence of DR at the time of enrollment. 48 ePREDICE is an ongoing 2-year international, randomized clinical trial looking at the incidence of microvascular complications, including DR, in prediabetic patients who all undergo lifestyle modification in addition to being randomized to placebo versus metformin alone versus linagliptin alone versus metformin + linagliptin. 49 The results of these two studies will hopefully help further elucidate the therapeutic potential of metformin in DR.
Metformin reduces the risk of macular degeneration
To date, six retrospective clinical studies have examined the potential role of metformin in AMD. Data from retrospective studies are mixed but overall promising.15–18,50,51
A large case–control study by our group revealed that metformin use was associated with reduced odds of developing age-related macular degeneration in a dose-dependent manner (OR, 0.93; 95% CI = 0.91–0.95), with low to moderate doses showing the greatest potential benefit. 312,404 patients with newly diagnosed AMD and 313,376 control patients matched on the following criteria: age, anemia, HTN, geography US region, and Charlson comorbidity index (CCI) score (0, 1, 2, or 3+) were identified from the IBM MarketScan Commercial and Medicare Supplemental Databases. The findings from this study suggested an inverse, dose-dependent relationship between metformin use and AMD with low and moderate doses being most protective. In a subgroup analysis of only diabetic patients, metformin had a similar protective effect in AMD development at all doses except for doses greater than 1080 g. Interestingly, metformin was protective in the absence of DR (OR, 0.93; 95% CI = 0.91–0.95; P < 0.0001), but in patients with DR (OR, 1.07; 95% CI = 1.01–1.15; P = 0.03). Collectively, these findings suggest that metformin may have a protective effect on reducing the risk of AMD where low to moderate doses are most protective. Among diabetics, metformin may be more beneficial the earlier it is prescribed, ideally before the development of DR. 15
Another case–control study conducted by Brown et al. using electronic health records from patients at the University of Florida found a more robust protective effect of metformin. In this study 1947 newly diagnosed AMD cases were matched in a 1:3 ratio to 5841 controls using propensity score matching (PSM) based on age, CCI score, hypertension, and anemia. Multivariable analysis showed that metformin use reduced odds of developing AMD (OR 0.58; 95% CI = 0.43–0.79). Variables accounted for in multivariable analysis were age; sex; race/ethnicity; CCI; BMI; diabetes; insurance status; ocular comorbidities such as retinal edema/ischemia/exudates/deposits, detached retina, macular cyst/hole/pseudohole, drusen (prior to diagnosis of AMD), or macular pucker; and the medication classes of DDP4 inhibitors, selective serotonin reuptake inhibitors (SSRIs) tricyclic antidepressants (TCAs), and statins. Smoking status was not included. DDP4 inhibitors, SSRIs, TCAs, and statins were not protective against the development of AMD. A diagnosis of diabetes was shown to be protective against AMD development (OR 0.32; 95% CI = 0.28–0.36; P < 0.001). In a subgroup analysis of patients with diabetes, metformin still demonstrated a protective effect on the development of AMD (OR 0.70; 95% CI = 0.49–0.98; P = 0.043). 18
Chen et al. conducted a retrospective cohort study to examine the potential benefits of metformin on AMD development in patients with a known diagnosis of T2DM. Using Taiwan’s NHIRD, 45,524 metformin users and 22,681 non-users were identified among T2DM patients. Considered confounders included age; gender; diagnosis of hypertension, hyperlipidemia, coronary artery disease, DR, and chronic kidney disease; insulin treatment; other antidiabetic oral drugs; antihypertensive drugs; and lipid-lowering drugs. Smoking status was not controlled. Overall, metformin users had a significantly lower risk of developing AMD compared with non-users upon multivariate analysis (HR 0.54; 95% CI = 0.50–0.58; P < 0.001). Among patients with DR, metformin increased the risk of AMD (HR 1.98; 95% CI = 1.78–2.20; P < 0.0001). After 1:1 propensity score matching between users and non-users to further reduce the effect of confounders, metformin was still protective against AMD development (HR 0.57; 95% CI = 0.52–0.61; P < 0.001). The protective effects of metformin increased with duration of treatment (>4 years), greater total treatment dose (>1400 g) and greater daily dose (>2.1 g/day). 17
Stewart et al. conducted a retrospective cross-sectional study using electronic health records of diabetic patients at the University of San Francisco and found that metformin was associated with a significantly reduced odds of developing both AMD (OR 0.70; 95% CI = 0.55–0.88; P = 0.003) and neovascular AMD only (OR 0.59; 95% CI = 0.46–0.77; P < 0.001). 3,120 diabetic patients 60 years or older were included in the study, of which 122 (3.9%) had dry AMD and 26 (0.8%) had wet AMD at their first ophthalmology encounter. Metformin was not protective among smokers and former smokers (OR 0.92; 95% CI = 0.64–1.31) but was among non-smokers or unknown smoking status (OR 0.56; 95% CI = 0.41–0.77; P = 0.046). 16
Lee et al. conducted a nested case–control study using the Korean National Health Service database and found that metformin was not protective against AMD development (adjusted OR 1.15 [CI = 0.91–1.45]). This study included patients 65 or older from a national Korean database with either a diagnosis of diabetes or cardiovascular diseases. Patients with a new diagnosis of AMD were included as cases, and each case was matched to 10 controls based on sex, age, cohort entry date, and follow-up duration. A total of 2330 cases were identified, matched to 23,278 controls. Even when categorizing patients based on treatment duration, metformin use did not demonstrate a protective effect (>300 days metformin use adjusted OR 0.93;95% CI = 0.68–1.26). 50
A recent retrospective cohort study by Eton et al. 51 found that metformin was not protective against the development of dry AMD (dAMD). The authors queried a national US medical insurance claims database between January 2002 and December 2016 to identify an initial cohort of 1,007,226 diabetic patients 55 or older with no previous diagnosis of AMD, no history of DR or treatment for DR, and no other underlying retinal diseases. The association of metformin with development of dAMD was determined using multivariable Cox hazard ratios. Two separate analyses were used to study the association of metformin with dry AMD. In one, current metformin use was compared with historical use with historical use defined as metformin use prior to the index date. A dose-dependent analysis was also performed using cumulative doses of metformin separated into categories (0–290,000 mg, 290,000–720,000 mg, 720,000–1,430,000 mg, and >1,430,000 mg). Current metformin use was associated with an increased risk of dAMD (aHR 1.08; 95% CI = 1.04–1.12; P < 0.001), whereas historical use of metformin was associated with a decreased risk of dAMD (aHR 0.95; 95% CI = 0.92–0.98; P = 0.002). The lowest cumulative dosage of metformin (0–290,000 mg) conferred a small protective effect (aHR 0.95; 95% CI = 0.91–0.99; P < 0.001), but metformin was not protective at the other cumulative dosage categories. The authors concluded that metformin is not protective against dAMD development among diabetic patients due to these conflicting results.
A recent meta-analysis of five of these previous studies found that metformin is not protective against AMD development (pooled adjusted OR 0.80; 95% CI = 0.54–1.05). 52 However, this finding should be interpreted with caution given the significant differences in study designs and patient populations between the retrospective studies. A Phase II randomized, single-blind clinical trial on the effects of metformin on the progression of geographic atrophy in non-diabetic AMD patients began in 2016 and is ongoing with estimated completion date of April 2022. 53 Metformin does hold promise for the treatment of AMD but prospective clinical trials are needed to better understand and quantify its effects accurately, especially regarding timing, duration, and dosage of therapy.
Metformin in glaucoma and uveitis
Glaucoma is the leading cause of irreversible blindness with almost 80 million people affected worldwide and open angle glaucoma (OAG) accounting for 74% of all glaucoma patients. 54 Although typically not classified as a retinal disease, glaucoma involves progressive destruction of RGC axons. 55 Metformin may inhibit inflammation, fibrosis, and mitochondrial dysfunction of RGC axons at the level of the lamina cribrosa though no preclinical studies exist to date. 56 In a recent retrospective cohort study involving 150,016 DM2 patients ⩾40 years-old with no prior diagnosis of OAG, Lin et al. found that a cumulative dose of >1110 g of metformin over a 2-year period resulted in a 25% reduction in the risk of developing OAG compared with non-users (HR = 0.75; 95% CI = 0.59–0.95; P = 0.02) with every 1 g increase in metformin associated with an additional 0.16% risk reduction (aHR = 0.99984; 95% CI = 0.99969–0.99999; P = 0.04). Interestingly, the effects of metformin were significant even when controlling for HbA1C levels, further bolstering the notion that metformin has far reaching effects beyond glycemic control. 9
Only one clinical study to date has investigated the role of metformin in uveitis. Subring et al. 57 conducted a retrospective cohort and case–control study, respectively, using a large US insurance database to look for an association between metformin and non-infectious uveitis (NIU) among newly diagnosed DMII patients. In the cohort arm of the study, which included 359,139 new metformin users and 162,847 new users of other diabetic medications, metformin use was not protective against the development of uveitis (HR = 1.19; 95% CI = 0.92–1.54; P = 0.19), including the subtypes of anterior, intermediate or posterior uveitis, though average follow-up was only 166 days and a maximum of 365 days. In the case–control arm, 502 NIU cases were matched to 10,040 similar controls. Metformin use at any point 2 years prior to the index date was not protective against NIU (adjusted odds ratio (aOR) = 0.64; 95% CI = 0.39–1.04; P = 0.07). However, longer duration of metformin use (445–729 days of use, aOR = 0.49; 95% CI = 0.27–0.90; P = 0.02) and greater cumulative dosage (165,001–390,000 mg aOR = 0.57; 95% CI = 0.34–0.98; >390,000 mg aOR = 0.44; 95% CI = 0.25–0.78; P = 0.001) were protective against NIU when compared with no metformin use. 57 This study further highlights the largely preventive, long-term effects of metformin in a variety of conditions.
Discussion
Metformin is one of the oldest and commonly prescribed medications worldwide, and a growing body of evidence shows it has far-reaching potential beyond its role in diabetes. Our aim in this review is to give an overview of the preclinical and clinical trials as it relates to metformin’s protective effects in diseases of the posterior segment.
It is difficult to conceptualize the vast array of biochemical pathways metformin acts upon. Rather than focus on metformin’s role in individual retinal diseases, it may be more useful to broadly organize metformin’s effects as belonging to one of three categories: anti-inflammatory, antiangiogenic, or antioxidative (Figure 1). While not complete, this framework highlights just how expansive of an effect metformin exerts. Furthermore, it may reveal how metformin might be considered a therapeutic option for additional retinal diseases beyond those discussed in this article.
The anti-inflammatory effects of metformin are mediated in large part through its downregulation of NF kB signaling and a decrease in levels of pro-inflammatory cytokines such as TNF-A, IL-4, IL-6, IL-8 IL-10, TGF-B in retinal tissue.10,11,31,34,35 Metformin also inhibits leukocyte proliferation, chemotaxis, and tissue extravasation by inhibiting levels of G-CSF, ICAM-1, and MCP-110,11,34 Metformin’s antiangiogenic effects include downregulation of VEGF levels,34,36,41 potentially via microRNA antisense binding; 41 alterations in the expression of VEGF isoforms so that less potent isoforms are expressed; 40 reduction in total VEGF-R levels; 33 and less phosphorylation and activation of existing VEGF-R. 40 Metformin is able to decrease levels of molecules that mediate oxidative stress such as ChREBP);34,58 PARP;35,59 restore GSH:MDH ratios so that greater GSH is available to quench ROS; 36 replenish NADH:NAD + ratios;12,38 and activate antioxidative defense enzymes such as COX-I, COX-II, SOD-2, PGC-1ɑ, and Tfam. 38 Metformin also inhibits pathological mediators of cellular senescence (i.e. IRE1a, SEMA3A), which may be what links the inflammatory and angiogenic components of ischemic retinal diseases. 37
Additional functions outside these broad categories include inhibition of apoptosis through the upregulation of antiapoptotic genes BIRC3 and BIRC5 and downregulation of pro-apoptotic genes such as brassinosteroid insensitive-associated receptor kinase-1 (BAK1) and TXNIP in response to environmental stressors;31,35 enhancement of proper cellular protein trafficking; 32 maintenance of intercellular junctions (i.e. claudin-1, occludin, ZO-1);12,36,38 enhancement of autophagy through downregulation of TXNIP; 12 and upregulation of AMPK-dependent autophagosome formation. 42
Metformin may affect the levels of these molecules partly via AMPK activation, including specific isoforms, 38 or through its modulation of other intracellular kinases (i.e. AKT, ERK); 34 inhibition of O-GlcNAc modification and activation of cytotoxic molecules; 35 and activation of nuclear hormone receptors including PPARΔ. 39
An important consideration moving forward is to identify exactly where along the course of a particular retinal disease metformin is able to exert its maximal effect. Many of the preclinical studies showing a benefit from metformin use involved either pretreatment of animals or cell lines with metformin prior to induction with a pathologic stressor or shortly thereafter. Interestingly, in an HFD mouse model for diabetes, metformin use did not result in less development or severity of DR despite causing profound changes at a biochemical level. 34 Perhaps this is because metformin treatment did not begin until mice were fed an HFD for two months, at which point irreversible damage had occurred along metformin effector pathways due to prolonged toxicity from obesity and an HFD. This suggests that earlier metformin use has a greater impact, perhaps even prior to the clinical manifestation of pathologic retinal disease and could be considered as a preventive measure. This is further bolstered by the results for the few clinical studies that exist for metformin. In the study by our group on protective effects of metformin on AMD, we found metformin was protective when patients had yet to develop DR. 15 Fan et al. 13 found that metformin protected against STDR among newly diagnosed diabetics who had no NPDR but did not protect against STDR in newly diagnosed diabetics with NPDR at the time of diagnosis.
Clinical trial data with metformin use is still relatively scarce. Recent studies have shown that the risk and severity of DR is reduced with metformin use, as well as the risk of developing AMD, POAG, and uveitis. Further studies are needed to confirm the results of these studies, as well as elucidate the effects of metformin in other retinal diseases. Unfortunately, inherent limitations of these studies exist as the development of these diseases takes several years, and many confounding variables such as medication use, smoking status, and presence of other systemic disease can lead to bias in studies if careful selection criteria and patient matching is not employed. Three prospective clinical trials are ongoing on the role of metformin outside of DR, one trial examining whether metformin can slow the progression of geographic atrophy among non-diabetic advanced dry AMD patients, 53 a second trial examining whether metformin can slow the progression of ABCA4 retinopathy, 60 and a third trial studying whether metformin can slow the rate of visual field loss among POAG patients. 61 The results of these studies will hopefully clarify the optimal dosage and timing of metformin therapy and help pinpoint clinical situations where metformin can be beneficial.
Ultimately, more data is needed in both preclinical and clinical trials to further demonstrate the entirety of the effects of metformin on the retina. Fortunately, the data from the studies presented above shows that metformin may be at least somewhat beneficial to the development of inflammatory and neovascular retinal disease such as DR and AMD, and perhaps even for other conditions such as RP, POAG, and uveitis. Metformin has a well-documented safety profile extending over the past 60 years. Therefore, metformin shows promise to become a potential novel strategy for prevention of development and management of retinal diseases, both in healthy and diabetic populations.
Footnotes
Authors’ Contributions: All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; all authors assisted in the writing of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Saira Khanna  https://orcid.org/0000-0001-7373-9429
https://orcid.org/0000-0001-7373-9429
References
- 1. White JR, Campbell RK, White JR. Overview of the medications used to treat type 2 diabetes. In: White JR, Campbell RK. (eds) Medications for the treatment of diabetes. Alexandria, VA: American Diabetes Association, 2008, pp.5–15 [Google Scholar]
- 2. White JR., Jr. A brief history of the development of diabetes medications. Diabetes Spectr 2014;27:82–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 4. LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev 2021;42:77–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galuska D, Zierath J, Thörne A, Sonnenfeld T, Wallberg-Henriksson H. Metformin increases insulin-stimulated glucose transport in insulin-resistant human skeletal muscle. Diabete Metab 1991;17:159–63 [PubMed] [Google Scholar]
- 6. Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002;51:2420–5 [DOI] [PubMed] [Google Scholar]
- 7. Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care 2016;39:198–205 [DOI] [PubMed] [Google Scholar]
- 8. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017;60:1577–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin H-C, Stein JD, Nan B, Childers D, Newman-Casey PA, Thompson DA, Richards JE. Association of geroprotective effects of metformin and risk of open-angle glaucoma in persons with diabetes mellitus. JAMA Ophthalmol 2015;133:915–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalariya NM, Shoeb M, Ansari NH, Srivastava SK, Ramana KV. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci 2012;53:3431–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han J, Li Y, Liu X, Zhou T, Sun H, Edwards P, Gao H, Yu F-S, Qiao X. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS ONE 2018;13:e0193031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, Xu G-T, Liu F, Zhang J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxid Med Cell Longev 2020;2020:1740943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan Y-P, Wu C-T, Lin J-L, Hsiung CA, Liu HY, Lai J-N, Yang C-C. Metformin treatment is associated with a decreased risk of nonproliferative diabetic retinopathy in patients with type 2 diabetes mellitus: a population-based cohort study. J Diabetes Res 2020;2020:9161039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Ryu C, Munie M, Noorulla S, Rana S, Edwards P, Gao H, Qiao X. Association of metformin treatment with reduced severity of diabetic retinopathy in type 2 diabetic patients. J Diabetes Res 2018;2018:2801450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blitzer AL, Ham SA, Colby KA, Skondra D. Association of metformin use with age-related macular degeneration: a case-control study. JAMA Ophthalmol 2021;139:302–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart JM, Lamy R, Wu F, Keenan JD. Relationship between oral metformin use and age-related macular degeneration. Ophthalmol Retina 2020;4:1118–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y-Y, Shen Y-C, Lai Y-J, Wang C-Y, Lin K-H, Feng S-C, Liang C-Y, Wei L-C, Chou P. Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J Ophthalmol 2019;2019:1649156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown EE, Ball JD, Chen Z, Khurshid GS, Prosperi M, Ash JD. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci 2019;60:1470–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 2015;15:196–205 [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2012;35:731–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev 2017;40:31–44 [DOI] [PubMed] [Google Scholar]
- 23. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou J, Massey S, Story D, Li L. Metformin: an old drug with new applications. Int J Mol Sci 2018;19:2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol 2017;217:282–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med 2014;2:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, Pannain S, Lengyel E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol 2012;119:61–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porceddu PF, Ishola IO, Contu L, Morelli M. Metformin prevented dopaminergic neurotoxicity induced by 3,4-methylenedioxymethamphetamine administration. Neurotox Res 2016;30:101–9 [DOI] [PubMed] [Google Scholar]
- 29. Jiang T, Yu J-T, Zhu X-C, Wang H-F, Tan M-S, Cao L, Zhang Q-Q, Gao L, Shi J-Q, Zhang Y-D, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 2014;171:3146–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z. Metformin improves neurologic outcome via AMP-activated protein kinase-mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc 2018;7:e008389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luodan A, Zou T, He J, Chen X, Sun D, Fan X, Xu H. Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front Mol Neurosci 2019;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Athanasiou D, Aguila M, Opefi CA, South K, Bellingham J, Bevilacqua D, Munro PM, Kanuga N, Mackenzie FE, Dubis AM, Georgiadis A, Graca AB, Pearson RA, Ali RR, Sakami S, Palczewski K, Sherman MY, Reeves PJ, Cheetham ME. Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration. Hum Mol Genet 2017;26:305–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joe SG, Yoon YH, Choi JA, Koh J-Y. Anti-angiogenic effect of metformin in mouse oxygen-induced retinopathy is mediated by reducing levels of the vascular endothelial growth factor receptor Flk-1. PLoS ONE 2015;10:e0119708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim AJ, Chang JY-A, Shi L, Chang RC-A, Ko ML, Ko GY-P. The effects of metformin on obesity-induced dysfunctional retinas. Invest Ophthalmol Vis Sci 2017;58:106–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim YS, Kim M, Choi MY, Lee DH, Roh GS, Kim HJ, Kang SS, Cho GJ, Kim S-J, Yoo J-M, Choi WS. Metformin protects against retinal cell death in diabetic mice. Biochem Biophys Res Commun 2017;492:397–403 [DOI] [PubMed] [Google Scholar]
- 36. Nahar N, Mohamed S, Mustapha NM, Lau S, Ishak NIM, Umran NS. Metformin attenuated histopathological ocular deteriorations in a streptozotocin-induced hyperglycemic rat model. Naunyn Schmiedebergs Arch Pharmacol 2021;394:457–67 [DOI] [PubMed] [Google Scholar]
- 37. Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain M-A, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, Mallette FA, Sapieha P. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 2016;8:362ra144 [DOI] [PubMed] [Google Scholar]
- 38. Xu L, Kong L, Wang J, Ash JD. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci USA 2018;115:10475–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu L, Brown EE, Santiago CP, Keuthan CJ, Lobanova E, Ash JD. Retinal homeostasis and metformin-induced protection are not affected by retina-specific Pparδ knockout. Redox Biol 2020;37:101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi Q-Y, Deng G, Chen N, Bai Z-S, Yuan J-S, Wu G-H, Wang Y-W, Wu S-J. Metformin inhibits development of diabetic retinopathy through inducing alternative splicing of VEGF-A. Am J Transl Res 2016;8:3947–54 [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Chen F, Wang L. Metformin inhibits development of diabetic retinopathy through microRNA-497a-5p. Am J Transl Res 2017;9:5558–66 [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Liu L, Jiang Y, Silva M, Zhen X, Zheng W. Protective effect of metformin against hydrogen peroxide-induced oxidative damage in human retinal pigment epithelial (RPE) cells by enhancing autophagy through activation of AMPK pathway. Oxid Med Cell Longev 2020;2020:2524174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–11 [PubMed] [Google Scholar]
- 44. Ying Y, Ueta T, Jiang S, Lin H, Wang Y, Vavvas D, Wen R, Chen Y-G, Luo Z. Metformin inhibits ALK1-mediated angiogenesis via activation of AMPK. Oncotarget 2017;8:32794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 1990;347:677–80 [DOI] [PubMed] [Google Scholar]
- 46. Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 1998;273:32833–41 [DOI] [PubMed] [Google Scholar]
- 47. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Comparison of diabetes retinopathy among type 2 diabetic patients treated with different regimens – full text view. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02587741 (accessed 3 October 2021)
- 49. Gabriel R, Boukichou Abdelkader N, Acosta T, Gilis-Januszewska A, Gómez-Huelgas R, Makrilakis K, Kamenov Z, Paulweber B, Satman I, Djordjevic P, Alkandari A, Mitrakou A, Lalic N, Colagiuri S, Lindström J, Egido J, Natali A, Pastor JC, Teuschl Y, Lind M, Silva L, López-Ridaura R, Tuomilehto J. e-PREDICE Consortium. Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. PLoS ONE 2020;15:e0231196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee H, Jeon HL, Park SJ, Shin JY. Effect of statins, metformin, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers on age-related macular degeneration. Yonsei Med J 2019;60:679–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eton EA, Wubben TJ, Besirli CG, Hua P, McGeehan B, VanderBeek BL. Association of metformin and development of dry age-related macular degeneration in a U.S. insurance claims database. Eur J Ophthalmol. Epub ahead of print 19 February 2021. DOI: 10.1177/1120672121997288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romdhoniyyah DF, Harding SP, Cheyne CP, Beare NAV. Metformin, a potential role in age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Ther 2021;10:245–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Metformin for the minimization of geographic atrophy progression in patients with AMD. ClinicalTrails.gov, https://clinicaltrials.gov/ct2/show/NCT02684578 (2016, accessed 22 September 2021)
- 54. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hurley DJ, Irnaten M, O’Brien C. Metformin and glaucoma-review of anti-fibrotic processes and bioenergetics. Cells 2021;10:2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Subring L, Yu Y, Han S, Searle G, Kempten JH, Hubbard RA, VanderBeek BL. Risk of non-infectious uveitis with metformin therapy in a large Healthcare Claims Database. Oculi Immune Inflame. Epub ahead of print 8 March 2021. DOI: 10.1080/09273948.2021.1872650 [DOI] [Google Scholar]
- 58. Ziizika K. The transcription factor carbohydrate-response element-binding protein (ChREBP): a possible link between metabolic disease and cancer. Biochip Biophys Act Mol Basis Dis 2017;1863:474–85 [DOI] [PubMed] [Google Scholar]
- 59. Berger NA, Bison VC, Boilers AH, Buckle A, Charge A, Clark RS, Curtin NJ, Cuzzocrea S, Dawson TM, Dawson VL, Haskó G, Liaudet L, Moroni F, Pacher P, Radermacher P, Salzman AL, Snyder SH, Soriano FG, Strosznajder RP, Sümegi B, Swanson RA, Szabo C. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol 2018;175:192–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oral metformin for treatment of ABCA4 retinopathy, https://clinicaltrials.gov/ct2/show/NCT04545736 (accessed 11 October 2021)
- 61. Case Medical Research. Effect of metformin on visual function in patients with glaucoma. Case Med Res. Epub ahead of print 7 November 2019. DOI: 10.31525/ct1-nct04155164 [DOI] [Google Scholar]