Abstract
Background
Pneumonia from SARS-CoV-2 is difficult to distinguish from other viral and bacterial etiologies. Broad-spectrum antimicrobials are frequently prescribed to patients hospitalized with COVID-19 which potentially acts as a catalyst for the development of antimicrobial resistance (AMR).
Objectives
We conducted a systematic review and meta-analysis during the first 18 months of the pandemic to quantify the prevalence and types of resistant co-infecting organisms in patients with COVID-19 and explore differences across hospital and geographic settings.
Methods
We searched MEDLINE, Embase, Web of Science (BioSIS), and Scopus from November 1, 2019 to May 28, 2021 to identify relevant articles pertaining to resistant co-infections in patients with laboratory confirmed SARS-CoV-2. Patient- and study-level analyses were conducted. We calculated pooled prevalence estimates of co-infection with resistant bacterial or fungal organisms using random effects models. Stratified meta-analysis by hospital and geographic setting was also performed to elucidate any differences.
Results
Of 1331 articles identified, 38 met inclusion criteria. A total of 1959 unique isolates were identified with 29% (569) resistant organisms identified. Co-infection with resistant bacterial or fungal organisms ranged from 0.2 to 100% among included studies. Pooled prevalence of co-infection with resistant bacterial and fungal organisms was 24% (95% CI 8–40%; n = 25 studies: I2 = 99%) and 0.3% (95% CI 0.1–0.6%; n = 8 studies: I2 = 78%), respectively. Among multi-drug resistant organisms, methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and multi-drug resistant Candida auris were most commonly reported. Stratified analyses found higher proportions of AMR outside of Europe and in ICU settings, though these results were not statistically significant. Patient-level analysis demonstrated > 50% (n = 58) mortality, whereby all but 6 patients were infected with a resistant organism.
Conclusions
During the first 18 months of the pandemic, AMR prevalence was high in COVID-19 patients and varied by hospital and geography although there was substantial heterogeneity. Given the variation in patient populations within these studies, clinical settings, practice patterns, and definitions of AMR, further research is warranted to quantify AMR in COVID-19 patients to improve surveillance programs, infection prevention and control practices and antimicrobial stewardship programs globally.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-022-01085-z.
Keywords: Antimicrobial resistance, COVID-19, SARS-CoV-2
Background
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has been one of the most significant challenges of our time and has overwhelmed healthcare systems worldwide [1]. Simultaneously, the rise in multi-drug resistant infections continues to threaten global heath through significant morbidity, mortality and global economic loss. Following the O’Neill review and recommendations in 2016 to respond to the antimicrobial resistance (AMR) crisis [1], important progress has been made. However, patient admissions to hospitals have contributed to and continue to increase the risk of health-care-associated infections and the transmission of multidrug-resistant (MDR) organisms. Recent evidence suggests that as a consequence of the coronavirus disease 2019 (COVID-19) pandemic [2], an increasing number of patients admitted to hospitals have been prescribed empirical antimicrobial therapy which may not always be appropriate [3–6], potentially increasing the number of resistant infections globally.
While treatment of COVID-19 with antimicrobials is ineffective, there are several reasons why antimicrobial prescribing may exist [7, 8]: patients may present with symptoms similar to that of bacterial or other viral pneumonias, there may be suspected or confirmed co-infections [4], and protocols and existing healthcare frameworks might suggest the use of antimicrobials [9]. While antimicrobial therapy in COVID-19 patients may be reasonable if bacterial or fungal infection is suspected, consideration for AMR and antimicrobial stewardship focused on supporting the selection of optimal empirical therapies and appropriate de-escalation or discontinuation of antimicrobials when bacterial co-infection is present or absent is important [7].
A growing body of evidence suggests AMR may be increasing following antimicrobial prescribing in COVID-19 patients10–13, but a quantification of the prevalence of AMR and relative proportions of associated pathogens within a systematic review has not been published to date. Understanding the emergence of AMR in COVID-19 patients is essential. There is clear evidence to suggest that excess antimicrobial use in humans leads to antimicrobial resistant microbes that negatively impact humans, and AMR is described as one of the top ten greatest threats to global public health, food security, and development [11]. An important knowledge gap exists regarding the prevalence, and characteristics of bacterial and fungal co-infections, including potential AMR in patients with COVID-19.
We conducted a systematic review and meta-analysis of the published literature to address the specific research question: “What is the prevalence of AMR in co-infected COVID-19 patients?” Our objective was to identify and characterize the available literature by (1) reviewing COVID-19 patients with co-infections, (2) assessing the healthcare settings and geography (3) documenting antimicrobial therapies prescribed including antibacterials and antifungals if available, and (4) estimating the proportion of resistant organisms reported in the literature.
Methods
Search strategy and selection criteria
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for reporting [see Additional file 1] [13]. The study protocol was registered with the PROSPERO database for systematic reviews: CRD42021227564.
Searches of MEDLINE, Embase, Web of Science (BioSIS), and Scopus were completed for literature published from November 1, 2019 to May 28, 2021. These searches were restricted to human studies and publications in the English language. A separate search of medRxiv for unpublished manuscripts was also conducted to ensure the search was comprehensive. The search strategy was designed to capture original research articles with a focus on human studies involving confirmed COVID-19 patients with co-infections that reported drug-resistant organisms. Reference lists from all articles included in the review were reviewed to identify additional studies. The complete search strategy is presented in Additional file 2.
Results from each database search were uploaded into Covidence [14], an online software platform for systematic reviews, which removed all duplicate articles. Title and abstract screening were divided among three authors (RMK, DAJ, DCJ) and conducted independently to identify potential articles for inclusion, with each article requiring approval from at least two authors prior to moving to full-text review. Eligibility conflicts were resolved by a fourth author (SLL) who was not involved in the initial screening. Manuscripts were excluded if a duplicate was missed by Covidence, lack of resistant organisms were reported, publication in a language other than English, evaluation in non-COVID-19 patient populations, inappropriate study design (as listed below), inappropriate comparators or inappropriate outcomes such as antibiotic prescribing. The same process was applied for full-text screening. Studies, including case reports, cohort studies, case series, case–control studies, and conference proceedings, were included if antimicrobial resistant organisms were documented among human patients with confirmed COVID-19 requiring hospital care, accompanied by either a laboratory confirmed co-infection, or an existing co-organism isolated from a site thought to be associated with infection as stated by the study authors. Editorials, commentaries, in vitro studies (non-clinical isolates, animal studies, mechanistic studies), reviews, and studies in which none of the patients had COVID-19 were excluded.
Data extraction was performed by the same three authors involved in study selection, and information was recorded relating to study details (author, geographic location, study design, sample size), demographics (healthcare setting, age, sex), clinical parameters (disease presentation, mechanical ventilation, comorbidities, antimicrobials used; type, length) and microbiology (organisms identified, method of identification, antimicrobial susceptibility testing method, antimicrobial resistance, definition of resistance), as available. Patient-level data were also collected if provided. Upon independent completion of the initial data extraction, articles were again divided among the three authors (RMK, DAJ, DCJ) and reviewed a second time to ensure accuracy and comprehensiveness of the extraction.
Given the lack of a standardized definition for co-infection or secondary infection, superinfection or colonization across studies, the authors’ reporting of any type of co-infection or secondary infection was used. For purposes of our study co-infection was defined as simultaneous infection with a virus, bacterial or fungal organism in addition to SARS-CoV-2 either at the time of presentation or during the course of hospitalization. Similarly, given the lack of standardized definitions for AMR across all articles, we defined antimicrobial resistance as per authors’ discretion whereby microbiological investigations provided evidence of resistance, and whether or not specific guidelines or interpretative criteria such as Clinical & Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST) were used.
Data analysis
The studies included in the review were assessed for risk of bias using the Joanna Briggs Institute (JBI) Critical Appraisal Tools (specifically the case report, case series, cross-sectional, and cohort study checklists) (see Additional file 3) [15]. Select questions from the QUADAS-2 Tool [16], specifically, Domain 3: reference standard, were also used to assess the diagnostic methods for organism identification across all studies (see Additional file 4). These assessments were performed by three authors (RMK, DAJ, DCJ), with each article assessed by two authors for completion. Issues encountered while conducting the assessments due to unspecified study designs were resolved by discussion among the authors conducting the assessments, and the most suitable checklist was chosen for these studies.
Descriptive statistics including means and ranges were reported for continuous outcomes. Dichotomous outcomes were reported as frequencies and proportions calculated on GraphPad Prism v8.2 (La Jolla, California, USA). Study-level analysis was reported either narratively (number of co-infecting organisms, number of co-infections, number of resistant co-infections, microbiological identification and resistance reporting methods) or with a formal meta-analysis where appropriate. Using the ‘metaprop’ command within Stata 16 statistical software (College Station, TX: StataCorp LLC), we calculated pooled prevalence estimates (with corresponding 95% confidence intervals (CIs)) of co-infection with resistant bacterial and fungal organisms separately using random effects models. We visualized all pooled estimates with forest plots and assessed between-study heterogeneity using the I2 statistic, which indicates the percentage of variation among the studies that occurs as a result of heterogeneity rather than chance. Variation across all studies was categorised as low (I2 < 25%), moderate (I2 between 50 and 75%), high (I2 > 75%), or no statistical heterogeneity (I2 = 0%). Given the variation in geographic and hospital settings among included studies, we also conducted stratified meta-analysis by the following variables: ICU vs. non-ICU settings, COVID-specific ICU vs. regular ICU or other hospital setting, North America vs. Europe vs. Other, Europe vs. Asia vs. Other and Italy vs. Europe (excluding Italy) vs. Other.
Patient-level analysis was reported narratively (age, sex, number of co-infecting organisms, number of co-infections, number of resistant co-infections, proportion of patients receiving antibiotic therapy, outcome) using descriptive statistics. Case reports, studies evaluating effectiveness of COVID-19 therapies such as tocilizumab, and those reporting MDR rates where patient-level data could not be interpreted were excluded (n = 12) for the narrative reporting.
Role of the funding source
The Antimicrobial Resistance—One Health Consortium is funded through the Major Innovation Fund Program of the Ministry of Jobs, Economy, and Innovation (JEI), Government of Alberta, Canada.
Results
The search strategy identified 1331 records from MEDLINE (n = 330), Embase (n = 296), Web of Science (n = 96), Scopus (n = 585), and an additional 24 records through medRxiv (Fig. 1). After removal of duplicates, 1049 articles remained for title and abstract screening. Seventy-five articles were eligible for full-text screening of which 38 met inclusion criteria (Fig. 1). Thirty-seven articles were excluded due to missed duplicates (missed by Covidence) during the initial automated de-duplication process (n = 11), lack of resistant organisms reported (n = 10), inappropriate study design as listed in the Methods (n = 10), inappropriate comparator (n = 2), inappropriate outcomes such as antibiotic prescribing (n = 2), wrong language (n = 1) and non-COVID-19 patients (n = 1). Geographical origin of the 38 studies (Table 1) was as follows: Belgium (n = 2), China (n = 1), Egypt (n = 1), France (n = 3), Greece (n = 1), India (n = 2), Italy (n = 11), Iran (n = 3), Mexico (n = 1), Saudi Arabia (n = 1), Spain (n = 4), Switzerland (n = 1), Qatar (n = 1), United Kingdom (n = 2), and United States (n = 4). Twenty-seven (71%) studies enrolled patients from the intensive care unit (ICU), whereas 6 (16%) studies enrolled patients from COVID-specific care units, and 5 (13%) studies had an unspecified setting. The following study designs were identified: retrospective cohort (n = 8), case series (n = 5), case report (n = 3), cross-sectional (n = 3), prospective observational (n = 1), prospective cohort (n = 3), retrospective observational (n = 12), and 3 case–control studies. Sample sizes ranged from 1 to 4267 patients. Twelve studies contained patient-level data for 112 individuals (Table 1). Patient characteristics in studies meeting inclusion criteria are presented in Table 1. Risk of bias (Additional file 3) revealed the overall quality of the included studies. Majority of the studies were poor with limited reporting of microbiological detail including sample type, microbiological investigations, antimicrobial susceptibility testing methods, and definitions of resistance.
Fig. 1.
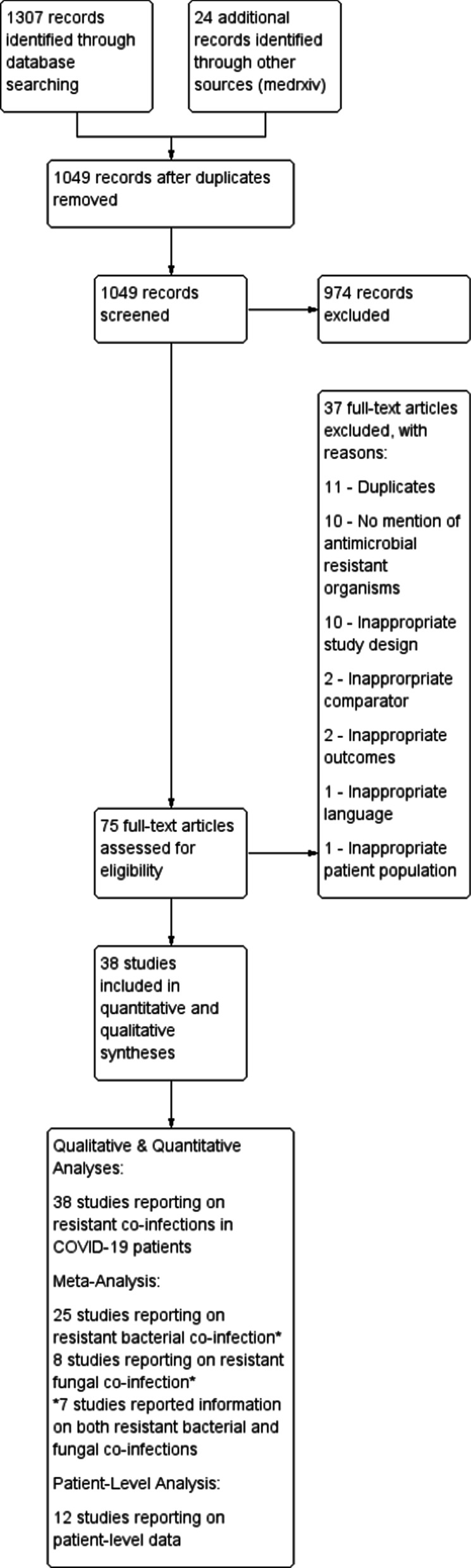
PRISMA flow diagram
Table 1.
Summary of study characteristics of the 38 included studies
| First Author | Location | Hospital setting | Study type | Microbiological detection and/or identification | Antimicrobial susceptibility testing method |
|---|---|---|---|---|---|
| Chowdhary [31] | New Delhi, India | ICU | Retro-spective Cohort | MALDI-ToF MS | CLSI Broth Microdilution Method M27-A3/S4 |
| Bogossian [44] | Brussels, Belgium | ICU | Retro-spective Case Control | Routine surveillance swabs: chromID® CARBA SMART agar, MacConkey agar containing ceftazidime chromID® VRE agar, MALDI-ToF MS |
EUCAST: VITEK 2 and disk diffusion Carbapenemases OXA-48, KPC, NDM, VIM and IMP; VanA, VanB were detected via PCR analysis or Coris Resist-5 O.O.K.N.V. antigenic. MDR Pseudomonas spp. and Acinetobacter spp. were defined as recommended considering antimicrobial resistance phenotype |
| Ramadan [33] | Assiut, Egypt | Tertiary hospitals: Alrahji Liver Hospital and Assiut University Hospital | Prospective Cohort | MALDI-ToF MS | Detection of antibiotic resistance genes by Monoplex PCR Technique (mecA, NDM-1, KPC, TEM, CTX-M, SHV) |
| Amarsy [45] | Nantes, France | ICU | Retro-spective Cohort | Blood cultures and respiratory cultures | Detection of resistance genes by Illumina WGS |
| Perez [19] | New Jersey, USA | ICU, Medical-surgical unit, progressive care unit | Retro-spective Cohort | Clinical specimens, colonization screening |
CRAB definition: Detection using RT-PCR for carbapenemase genes |
| Salehi [22] | Tehran, Iran | Three tertiary care training hospitals | Cross-Sectional | Budding yeasts and pseudohyphae in KOH 10% preparation and culture | CLSI M60 and M59 supplements |
| Cataldo [46] | Rome, Italy | ICU | Retro-spective Cohort | Blood cultures | NA |
| Posteraro [47] | Basel, Switzerland | COVID care unit | Case Report | MALDI-ToF MS | Sensititre YeastOne® method confirmed by the CLSI M27-A3 reference method |
| Nori [3] | New York, City, NY, USA | ICU | Retro-spective Observa-tional | Respiratory cultures, blood cultures | NA |
| Mahmoudi [48] | Hamedan, Iran | Nahavand Hospitals | Cross-Sectional | Blood and endotracheal aspirate samples | CLSI |
| Li [22] | Wuhan, China | Hospital (designated for COVID patients) | Retro-spective Cross-Sectional | Qualified sputum, endotracheal aspirate, bronchoalveolar lavage fluid, blood samples, or qualified urine | CLSI |
| Contou [18] | Argenteuil, France | COVID ICU | Retro-spective Cross-Sectional | Blood cultures, cultures of the respiratory tract secretions | Panel RP2 plus (Film Array Biomerieux®), Panel Pneumonia Plus (Film Array Biomerieux®) |
| Mo [22] | Brooklyn, New York | Community Teaching Hospital | Case Series (Retro-spective Observa-tional) | NA | NA |
| Garcia-Menino [8] | Oviedo, Spain | ICU | Case Series (Retro-spective Observa-tional) | MALDI TOF/MS - | Microscan System (BeckmanCoulter, Brea, CA, USA); results interpreted according to EUCAST |
| Sharifipour [20] | Qom, Iran, | ICU | Prospective Cohort | Samples were cultured on Blood Agar, Chocolate Agar, Eosin Methylene Blue (EMB), and MacConkey Agar | CLSI |
| Walpole [50] | United Kingdom | ICU | Case Report | Sputum sample | NA |
| Razazi [10] | France | ICU | Retro-spective Cohort | Bacterial co-infection at ICU admission evidenced by detection of bacteria in sputum or blood samples, in the absence of other sources of infection, or by a positive pneumococcal or L. pneumophila serotype 1 urinary antigen test | Susceptibility profiles of recovered microorganisms were recorded |
| Guisado-Gil [17] | Seville, Spain | ICU | Retro-spective Cohort | Blood cultures obtained > 48 h after admission | EUCAST. MDR categorization according to the Germany Society for Hygiene and Microbiology |
| Montrucchio [51] | Turin, Italy | ICU | Case Series (Retro-spective Observa-tional) | MALDI-ToF MS | EUCAST: Microscan WalkAway plus System, MASTDISCS® Combi Carba plus disk system |
| Mady [23] | Riyadh, Saudi Arabia | ICU | Case Series (Retro-spective Observa-tional) | Blood and respiratory cultures | Not described |
| Tiri [52] | Terni, Italy | ICU | Retro-spective Observa-tional Cohort | MALDI-ToF MS | VITEK2; Immunochromatography for OXA-48-like, OXA-163, KPC, NDM, VIM |
| Kokkoris [53] | Athens, Greece | ICU | Case Series (Retro-spective Observatio-nal Cohort) | Blood specimen | NA |
| Perrotta [54] | FG, Italy | ICU | Case Report | NA | NA |
| Baiou [55] | Doha, Qatar | ICU | Retro-spective Case–Control | MALDI-ToF MS | BD Phoenix according to CLSI standards |
| Segrelles-Calvo [56] | Madrid, Spain | ICU, RICU | Prospective Observa-tional Cohort | Aspergillus galactomannan antigen on BAL | Not described |
| Martinez-Guerra [57] | Mexico City, Mexico | COVID-19 dedicated facility | Prospective Cohort | MALDI-Tof MS | VITEK2; AmpC producers considered with known chromosomal AmpC Beta-Lactamases, ESBL considered in those resistant to 3rd generation cephalosporins and monobactams, CRE considered with resistance to carbapenems in VITEK and confirmed with modified CIM test, MDR P. aeruginosa considered in isolates with resistance to at least one agent in three or more antibiotic categories |
| Karruli [58] | Naples, Italy | ICU | Retro-spective Observa-tional Cohort | Microbiological sampling of blood, urine, and airways | MDR defined according to Magiorakos et al. [59] criteria |
| Gomez-Simmonds [60] | New York City, USA | ICU | Retro-spective Observational Cohort | Surveillance using MicroScan | Xpert Carba-R, BMD, E-test, WGS |
| Cultrera [61] | Ferrara, Italy | ICU | Retro-spective Observational Cohort | MALDI-ToF MS | VITEK2 |
| Khurana [62] | New Delhi, India | COVID-19 dedicated facility | Retro-spective Observational Cohort | VITEK2 and BioFire FilmArray Respiratory Panel | VITEK2 AST card interpreted by CLSI guidelines |
| Posteraro [47] | Rome, Italy | ICU | Retro-spective Observa-tional Cohort | Positive blood culture using BacT/ALERT VIRTUO and MALDI Biotyper | VITEK2 and Sensititre YeastOne® following EUCAST breakpoints |
| Pascale [63] | Bologna, Italy | ICU and non-ICU settings | Cross-Sectional | Active surveillance of blood and respiratory cultures | CRE defined as per EUCAST criteria; WGS |
| Baskaran [64] | England | ICU | Multicentre Retro–spective Observa-tional Cohort | Standard culture, respiratory viral PCR and urinary antigen tests | NA |
| Moretti [65] | Brussels, Belgium | ICU | Retro-spective Observational Cohort | Endotracheal aspiration or BAL with > 105 and > 104 CFU/mL | Not described. MDR or extreme-drug resistant (XDR) based on European Center of Disease Prevention and Control (ECDC) |
| Grasselli [66] | Genoa, Italy | ICU | Retro-spective Observa-tional Cohort | Routine microbiological surveillance: perineal swabs, nasal swabs, tracheal aspirate, urine culture | Not described |
| Magnasco [67] | Genoa, Italy | ICU | Retro-spective Observa-tional Cross-Sectional | Blood, respiratory and urinary samples using VITEK MS | VITEK2; Sensititre YeastOne® Panel (antifungal) |
| Bentivegna [68] | Rome, Italy | COVID-19 Depart-ment | Case–Control | NA | NA |
| Suarez-de-la-Rica [69] | Madrid, Spain | CCU | Retro-spective Observational Cohort | Conventional culture | Not described |
Abbreviations: Bronchoalveolar Lavage (BAL), Clinical and Laboratory Standards Institute (CLSI), Critical Care Unit (CCU), European Committee on Antimicrobial Susceptibility Testing (EUCAST), intensive care unit (ICU), multi-drug resistant (MDR), matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), polymerase chain reaction (PCR), whole-genome sequencing (WGS), carbapenem resistant Acinetobacter baumannii (CRAB), respiratory intensive care unit (RICU), real-time polymerase chain reaction (RT-PCR), oropharyngeal candidiasis (OPC)
Twelve (32%) articles documented the use of matrix-associated laser desorption ionization time of flight mass spectrometry (MALDI-ToF MS) as a method of organism identification, whereas 26 (68%) articles did not report microbiological investigations beyond specimen type (e.g., blood culture, respiratory culture etc.) and basic culturing (blood agar plate, chocolate agar plate etc.). Three studies had no description of microbiological investigations whatsoever (Table 1). Antimicrobial resistance definitions varied across studies, with the majority of articles not explicitly defining resistance, hence the authors’ final interpretation of resistance per isolate was used. One study reported multi-drug resistant categorization according to the Germany Society for Hygiene and Microbiology [17]. Details of antimicrobial susceptibility testing varied across studies, with 13 (34%) articles documenting use of standardized protocols. Seven articles followed CLSI criteria whereas 6 articles followed the EUCAST interpretive criteria. Moreover, reporting of resistance mechanisms were poor, with many reporting both acquired and intrinsic resistance as resistance (Table 2).
Table 2.
Proportion of COVID-19 patients with resistant co-infections
| First author | Disease presentation | Patients screened (No.) | SARS-CoV-2 patients (No.) | Number of patients with co-infections (%)* | Number of patients with resistant co-infections (%)* |
|---|---|---|---|---|---|
| Chowdhary [31] | BSI | 596 | 596 | 15 (2.5%) | 10 (1.7%) |
| Bogossian [44] | COVID-19 | 75 | 69 | 24 (34%) | 23 (33%) |
| Ramadan [33] | COVID-19 | 260 | 260 | 28 (11%) | 28 (11%) |
| Amarsy [45] | BSI, Respiratory Distress | 96 | 95 | 4 (45%) | 4 (4%) |
| Perez [19] | VAP, VAP with bacteremia, bacteremia, bone or soft tissue infections | 34 | 17 | 17 (100%) | 17 (100%) |
| Salehi [22] | OPC | 1059 | 1059 | 53 (5.0%) | 2 (0.2%) |
| Cataldo [46] | BSI | 57 | 57 | 28 (49%) | 9 (16%) |
| Posteraro [47] | 79 M with complicated T2DM who had fever, necrotic and ulcerative lesions on the amputated leg stump, BSI | 1 | 1 | 1 (100%) | 1 (100%) |
| Nori [3] | COVID-19 and subsequent positive microbiological results (within 30 days) | 4267 | 4267 | 152 (3.6%) | 25 (0.6%) † |
| Mahmoudi [48] | COVID-19 patients assessed for bacterial infections | 340 | 340 | 43 (13%) | 31 (72%) |
| Li [49] | Lung, BSI and UTI | 1495 | 1495 | 102 (6.8%) | 102 (6.8%)‡ |
| Contou [18] | All microbiological investigations performed within the first 48 h of ICU admission were reviewed | 92 | 92 | 26 (28%) | 7 (8%)§ |
| Mo [22] | COVID-19 patients who received Tocilizumab | 617 | 38 | 15 (39%) | 11 (29%) |
| Garcia-Menino [8] | Suspected or confirmed COVID-19 Patients | 62 | 62 | 7 (11%) | 7 (11%) |
| Sharifipour [20] | COVID-19 Patients | 19 | 19 | 19 (100%) | 17 (89%) |
| Walpole [50] | 33 M with fever for 3 days, abdominal pain for 1 day and one episode of vomiting | 1 | 1 | 1 (100%) | 1 (100%) |
| Razazi [10] | Viral ARDS | 3821 | 90 | NA | 21 (23%) |
| Guisado-Gil [17] | Hospital-acquired Candidemia and MDR BSI | 282 | 282 | NA¶ | NA¶ |
| Montrucchio [51] | COVID patients screened for carbapenemase-producing K. pneumoniae | 35 | 7 | 6 (86%) | 6 (86%) |
| Mady [23] | COVID-19 patients with ARDS receiving tocilizumab | 61 | 61 | 12 (20%) | 3 (5%) |
| Tiri [52] | Patients admitted to ICU screened using rectal swabs or clinical cultures for CRE | 62 | 62 | 17 (27%) | 17 (27%) |
| Perrotta [54] | 57 M admitted to hematology with K. pneumoniae NDM sepsis | 1 | 1 | 1 (100%) | 1 (100%) |
| Baiou [55] | Critical COVID-19 Patients | 1231 | 234 | 78 (6%) | NA |
| Segrelles-Calvo [56] | Adult patients admitted to ICU or RICU | 215 | 215 | 7 (3%) | NA |
| Martinez-Guerra [57] | Severe COVID-19 patients | 794 | 794 | 74 (11%) | 127 (20%)†† |
| Karruli [58] | Critically Ill COVID-19 Patients | 32 | 32 | NA/32 | 16 (50%) |
| Gomez-Simmonds [60] | Secondary CPE infections in COVID-19 patients | 3152 | 3152 | NA | 13 (0.4%) |
| Cultrera [61] | COVID-19 patients admitted in ICU and non-COVID-19 ICU settings | NA | NA | 28 | NA |
| Khurana [62] | Severely ill COVID-19 patients | 1179 | 1179 | 151 (13%) | 105 (9%) |
| Posteraro [70] | BSI in COVID-19 patients | 293 | 293 | 46 (16%) | 12 (5%) |
| Pascale [63] | > 18 years admitted to ICU | 1151 | 1151 | NA/1151 | 23 (1.8%) |
| Baskaran [64] | COVID-19 patients in ICU | 579 | 254 | 83 (33%) | NA/254 |
| Moretti [65] | Patients with COVID-19 and VAP | 39 | 39 | 21 (54%) | 67%# |
| Grasselli [66] | Patients with COVID-19 pneumonia | 813 | 813 | 359 (44%) | 38%** |
| Magnasco [67] | Patients with severe COVID-19 | 118 | 118 | NA/118 | 14 (12%) |
| Bentivegna [68] | Patients in COVID-19 Departments | NA | NA | NA/NA | 150/NA |
| Suarez-de-la-Rica [69] | Mechanically ventilated critically ill COVID-19 patients | 107 | 107 | 46 (43%) | 17 (16%) |
| Kokkoris [42] | BSI in COVID-19 patients | 50 | 50 | 27 (54%) | 17 (34%)‡‡ |
| TOTAL | 23,086 | 16,602 |
Abbreviations: BSI (blood stream infection), VAP (ventilator-associated pneumonia), OPC (oropharyngeal candidiasis), MDR (multi-drug resistant), T2DM (type II diabetes mellitus), UTI (urinary tract infection), ARDS (acute respiratory distress syndrome), CRE (carbapenem-resistant Enterobacteriaceae), RICU (Respiratory Intermediate Care Unit)
*Denominator: Patients with SARS-CoV-2
†24 organisms resistant; no patient level data provided
‡159 organisms detected; no patient level data provided; however, all resistant
§7 organisms resistant to 3rd generation cephalosporins and amoxicillin-clavulanate, no patient level data
¶Rates of MDR BSIs
#67% of 27 organisms isolated were MDR, 1 was XDR
**272/723 microbiologically confirmed hospital acquired infections were MDR
††19.3% (127/656) episodes of hospital-acquired infections demonstrated resistance
‡‡34% (17/50) were reported as extensively drug-resistant, pan-drug resistant or resistant
In total, 16,602 (72%) of 23,086 patients had laboratory-confirmed SARS-CoV-2 infection. The proportion of co-infection with either bacterial or fungal organisms in those with confirmed SARS-CoV-2 ranged from 2.5 to 100% across the 35 studies with exclusion of the single case reports. (Table 2). There were no reports of parasitic co-infections. Moreover, 1 case of viral co-infection was captured by our search strategy. One study evaluated viral co-infections using the Cepheid Xpert Xpress Flu/RSV, Panel Pneumonia Plus Film away and Panel RP2 plus Film array and found no cases of viral co-infection in 68 patients, whereas another detected metapneumovirus using the Biofire Film Array [18]. Two cohort studies [19, 20] reported co-infection prevalence of 100%. In contrast, 8 studies [3, 10, 21, 22] (with sample sizes greater than 1000) reported prevalence of co-infection from 3.6 to 13%.
The range of those co-infected with a resistant organism was 0.2 to 100%, with the 4 cohort studies [19, 20] contributing to the higher limit as previously mentioned (Table 2). Studies with larger sample sizes (> 1000) had resistant co-infection estimates ranging from 0.2 to 9%. In 15 studies where blood stream infections, acute respiratory distress syndrome or ventilator-associated pneumonia was reported, total resistant co-infections ranged from 1.7 to 100%. Seven studies reported both bacterial and fungal infections in COVID-19 patients. Notably, one study evaluated 61 patients who received tocilizumab, of whom 3 (5.0%) had resistant bacterial co-infections [23].
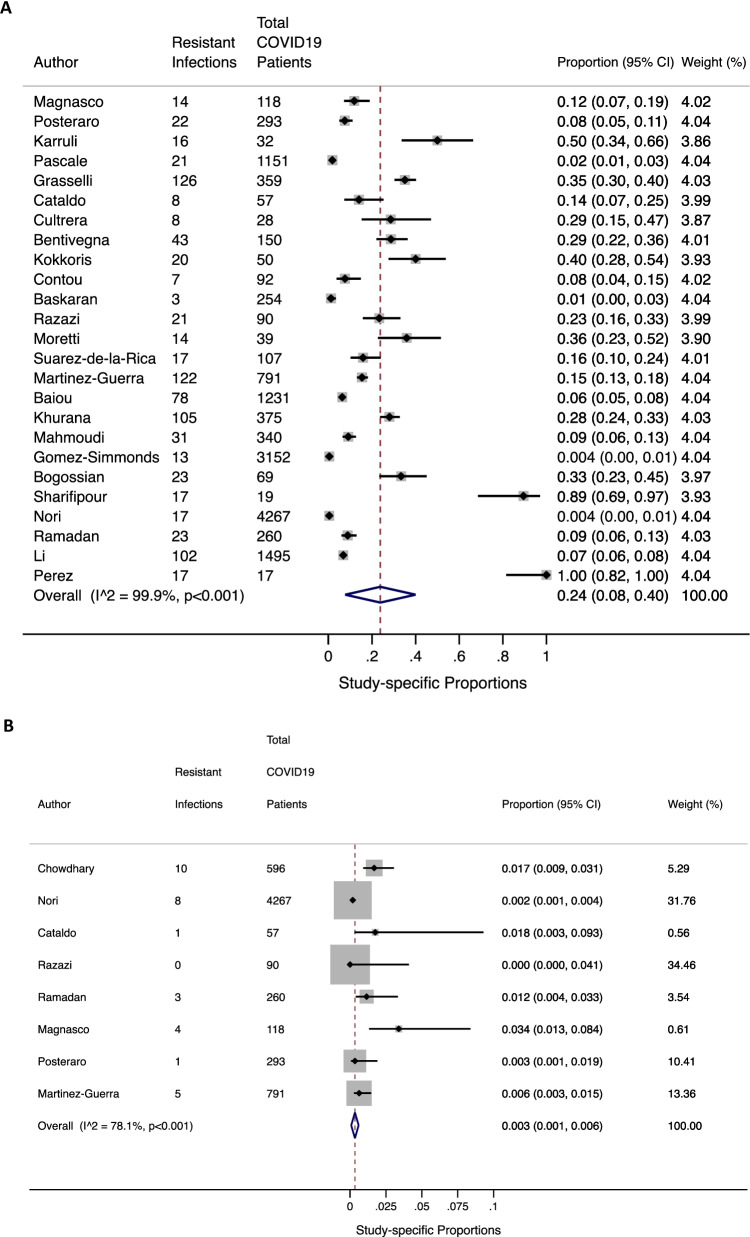
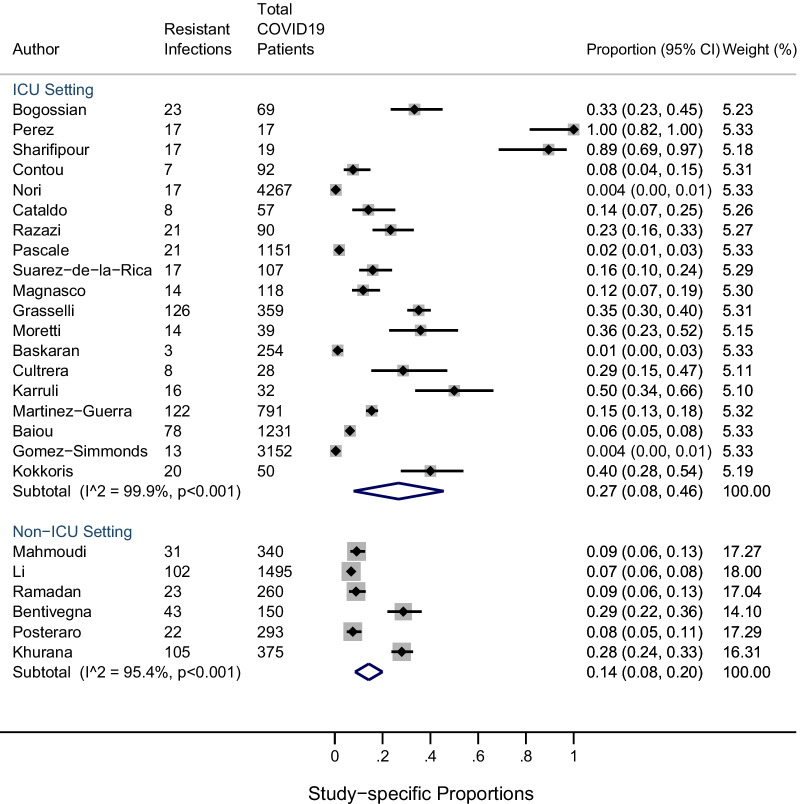
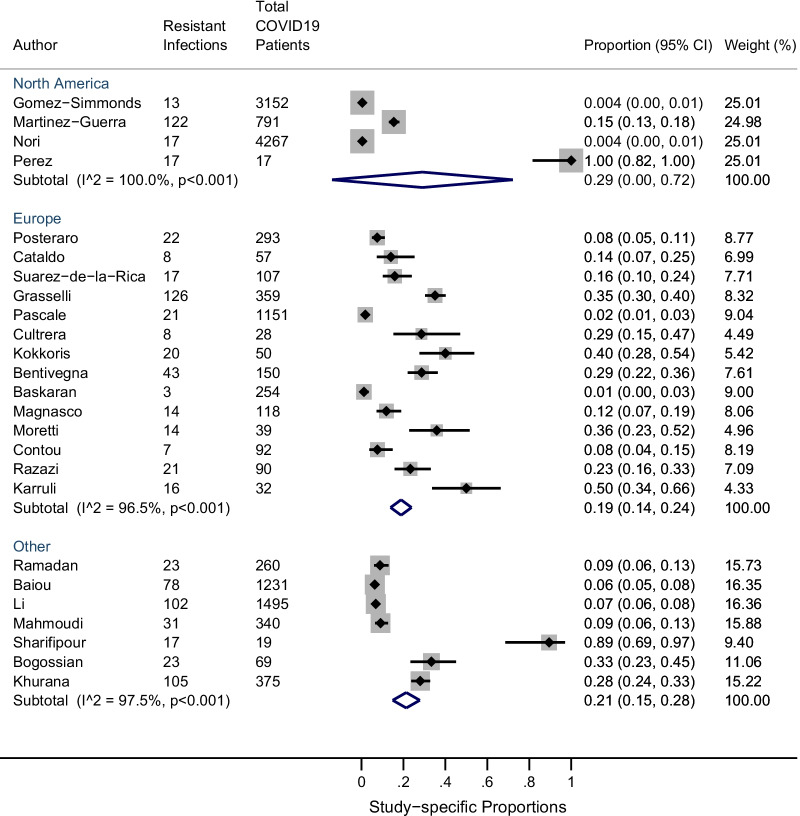
The pooled prevalence of co-infection with resistant bacterial and fungal organisms was 24% (95% CI 8–40%; n = 25 studies: I2 = 99%) and 0.3% (95% CI 0.1–0.6%; n = 8 studies: I2 = 78%) respectively (Fig. 2sA and B) . Between-study heterogeneity across bacterial and fungal resistant co-infections was high. Stratified meta-analysis by ICU setting among resistant bacterial infections revealed that the overall proportion of resistant infections amongst COVID19 patients was higher in the ICU setting (n = 19) [0.27 (95% CI 0.08, 0.46)] compared to the non-ICU settings (n = 6) [0.14 (95% CI 0.08, 0.20)], although not significant (Fig. 3). Furthermore, comparison between regular ICU or hospital settings (n = 22) to COVID-specific ICUs (n = 3) showed a similar trend ([0.25 (95% CI 0.07, 0.42)] vs. 0.19 [0.14 (95% CI 0.07, 0.22)]) (see Additional file 5). Moreover, a stratified analysis was performed by geography whereby the prevalence of resistant bacterial infections in studies conducted outside Europe [0.19 (95% CI 0.14, 0.24)] was higher, particularly in Asia [0.21 (95% CI 0.15, 0.28)] and more prominent in North America [0.29 (95% CI: 0.00, 0.72)] although not significant (Fig. 4, Additional files 6–7). Again, statistical heterogeneity remained high across all stratified analyses.
Fig. 2.
A Studies reporting resistant bacterial infections (n = 25); B studies reporting resistant fungal infections (n =85)
Fig. 3.
Proportion of resistant infections among ICU and non-ICU settings
Fig. 4.
Proportion of resistant infections among North America, Europe and other geographical settings
There were 1959 unique organisms identified across 387 studies where data were available, with 569 (29%) organisms identified as resistant to one or more antimicrobials (Table 3). The most common Gram-negative organisms resistant to at least one antimicrobial (regardless of intrinsic resistance) were Klebsiella pneumoniae (n = 169), Acinetobacter baumannii (n = 148), Pseudomonas aeruginosa (n = 65), Escherichia coli (n = 43), Enterobacter cloacae (n = 29), Stenotrophomonas maltophilia (n = 24) and Serratia marcescens (n = 17). Wide-spread resistance mechanisms were documented including β-lactamases, carbapenemases, and extended spectrum β-lactamases (ESBLs). The most common resistant Gram-positive organisms included: methicillin-resistant Staphylococcus aureus (MRSA) (n = 132), coagulase-negative staphylococci (n = 30) and vancomycin-resistant Enterococcus (VRE) spp. (n = 10). More specifically, isolates of E. faecium were documented with high levels of vancomycin resistance. Resistance to at least one antifungal agent was also documented, including Candida auris (n = 10), C. albicans (n = 3) and unidentified Candida spp. (n = 5).
Table 3.
Co-infecting organisms and resistance profiles
| Organism | Number | Proportion Resistant | Resistance Phenotype |
|---|---|---|---|
| Gram-positives | |||
| Staphylococcus aureus | 2 | 0 (0%) | – |
| Methicillin-sensitive Staphylococcus aureus (MSSA) | 104 | 0 (0%) | – |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 132 | 132 (100%) | Methicillin resistance |
| Coagulase-negative staphylococci (CNS) | 99 | 30 | Unknown resistance (24) |
| Vancomycin-resistant enterococci (VRE), unspecified | 4 | 4 (100%) | Vancomycin resistance |
| Enterococcus faecalis | 29 | 1 (3%) | High-level aminoglycoside resistance |
| Enterococcus faecium | 33 | 6 (18%) | Vancomycin resistance (3); high-level aminoglycoside resistance (3), ampicillin resistance (1) |
| Enterococcus casseliflavus/Enterococcus gallinarum | 2 | 1 (50%)* | Vancomycin resistance (1 – E. gallinarum)* |
| Enterococcus spp. | 12 | 0 (0%) | – |
| Streptococcus pneumoniae | 15 | 3 (20%) | Amoxicillin, amoxicillin/clavulanic acid, cefoxitin, gentamicin, erythromycin, clindamycin, piperacillin/tazobactam, trimethoprim/sulfamethoxazole (2); amikacin, ciprofloxacin, levofloxacin, cefotaxime, ceftriaxone, ceftazidime, cefepime (1), unknown resistance (1) |
| Streptococcus spp. | 4 | 0 (0%) | – |
| Clostridium difficile | 7 | 0 (0%) | – |
| Gram-negatives | |||
| Acinetobacter baumannii | 218 | 148 (68%) | Wide-spread resistance except to colistin (98); 26 isolates harbored OXA-23, 2 harbored NDM; Carbapenem resistance (4); extensively resistant (5), pan-drug resistant (3), unknown resistance (19) |
| Klebsiella pneumoniae | 274 | 169 (62%) | 26 (carbapenem-producing KPC), OXA-48 (7), ESBL (13), NDM (1), multi-drug resistant (23), Carbapenem resistance (13), KPC-2 (1), KPC-3 (1), unknown resistance (25), VIM (1) |
| Klebsiella oxytoca | 5 | 2 (40%) | ESBL (2) |
| Klebsiella aerogenes | 4 | 0 (0%) | – |
| Klebsiella spp. | 9 | 0 (0%) | – |
| Pseudomonas spp. | 4 | 0 (0%) | – |
| Pseudomonas aeruginosa | 203 | 65 (25%) | Unknown resistance (34), piperacillin/tazobactam (4), carbapenems (18), MDR (6), cephalosporin resistance (1), XDR (1) |
| Serratia marcescens | 18 | 17 (94%) | Resistance to amoxicillin, amoxicillin/-clavulanic acid, 1st and 2nd generation cephalosporins (including AmpC B-lactamase) with low level of resistance to amikacin, multi-drug resistant (7) |
| Escherichia coli | 118 | 43 (36%) | ESBL (2), AmpC resistance (1), multi-drug resistant (12), unknown resistance (18) |
| Stenotrophomonas maltophilia | 60 | 24 (40%) | Multidrug resistant (24) |
| Proteus mirabilis | 5 | 0 (0%) | – |
| Proteus putida | 1 | 0 (0%) | – |
| Haemophilus influenzae | 7 | 0 (0%) | – |
| Moraxella catarrhalis | 1 | 0 (0%) | – |
| Enterobacteriaceae, unspecified | 5 | 0 (0%) | – |
| Yersinia enterocolitica | 1 | 1 (100%) | Amoxicillin and amoxicillin/-clavulanic acid resistance |
| Enterobacter spp. | 8 | 5 (63%) | Imipenem resistance |
| Enterobacter aerogenes | 8 | 8 (100%) | Carbapenem-resistant Enterobacteriaceae (1), ESBL (1), AmpC B lactamase (6), unknown resistance (1) |
| Enterobacter cloacae | 68 | 29 (43%) | AmpC B lactamase (7), multi-drug resistant (18), NDM-1 (2) |
| Elizabethkingia meningoseptica | 1 | 1 (100%) | Multi-drug resistant (1) |
| Chryseobacterium gleum | 1 | 1 (100%) | Multi-drug resistant (1) |
| Citrobacter koseri | 1 | 0 (0%) | – |
| Mycoplasma pneumoniae | 2 | 0 (0%) | – |
| Enterobacterales | 113 | 34 (30%) | Cephalosporin resistance (2); Carbapenem resistance (3), unknown resistance (29) |
| Bacteroides fragilis | 2 | 0 (0%) | – |
| Viral organisms | |||
| Metapneumovirus | 1 | 0 (0%) | – |
| Fungal organisms | |||
| Candida auris | 11 | 10 (91%) | Fluconazole resistance (10), Voriconazole non-susceptible (3); overall 3 multi-azole resistant (fluconazole + voriconazole), 7 multi-drug resistant including 3 to 3 classes of drugs (azoles, amphotericin B and 5-flucytosine) and 4 resistant to 2 classes of drugs (azoles + 5-flucytosine and azoles + amphotericin B) |
| Candida albicans | 88 | 3 (3%) | Fluconazole and voriconazole resistance (3); caspofungin intermediate (2) |
| Candida dubliniensis | 6 | 2 (33%) | Fluconazole resistance (1), caspofungin resistance (1) |
| Candida parapsilosis | 25 | 1 (4%) | Fluconazole resistance |
| Candida glabrata | 15 | 1 (7%) | Pan-echinocandin resistance (1), caspofungin intermediate (7) |
| Candida spp. | 22 | 5 (23%) | Azole resistance (5); Echinocandin resistance (1) |
| Pichia kudriavzevii | 1 | 1 (100%) | Caspofungin and fluconazole resistance |
| Aspergillus fumigatus | 3 | 0 (0%) | – |
| Aspergillus flavus | 7 | 0 (0%) | – |
| Aspergillus niger | 2 | 0 (0%) | – |
| Other | 73 | 20 (27%) | Unknown resistance (9), ESBL (11) |
*Intrinsic resistance
Clinical data for 112 patients were available from 12 studies, of which sex and age were documented for 72. Fifty-two (72%) patients were male with a median age of 65 years (range 25–86) (see Additional file 8). Sixty-five (80%) had co-morbidities present, with all but 3 patients receiving antimicrobials prior to identification and susceptibility testing of co-infecting organisms for which data were available. Sixteen (45%) patients received tocilizumab, 13 (20%) patients received a combination of steroids and tocilizumab and 37 (56%) received a combination of other drugs. Sixty of 67 (90%) patients were receiving mechanical ventilation with a median duration of 34 days (range 24–46 days). All but 20 patients (colonization) had a co-infecting organism as the cause of their disease presentation in addition to COVID-19. The most commonly identified organisms were: A. baumannii (n = 38), K. pneumoniae (n = 29), C. auris (n = 11), P. aeruginosa (n = 7), MRSA (n = 3), Aspergillus fumigatus (n = 3), A. flavus (n = 2), A. niger (n = 2), E. cloacae complex (n = 2) and MSSA (n = 2). S. maltophilia, K. oxytoca, Y. enterocolitica, P. aeruginosa and C. glabrata were documented in 1 patient each. Mixed infections were documented in 5 patients: P. aeruginosa, C. auris; P. aeruginosa, C. auris, VRE; A. baumannii, K. pneumoniae in 2 patients; MRSA and C. albicans. All organisms acquired resistance to at least one antimicrobial except for 10 cases pertaining to (1) MSSA, (2) mixed infection with A. baumannii and K. pneumoniae, (3), K. pneumoniae, (4) A. fumigatus (n = 3), (5) A. flavus (n = 2), and (6) A. niger (n = 2). Overall, mortality was documented in 58 (52%) patients with all but 6 infected with resistant organisms.
Discussion
In this systematic review and meta-analysis, we analyzed data from over 16,000 patients with microbiologically confirmed COVID-19 admitted to hospitals between November 2019 and June 2021. While the prevalence of co-infections was highly variable based on sampling and setting within each of the included studies, we estimated a pooled prevalence of co-infection with resistant bacterial and fungal organisms of 24% and 0.3% respectively. Further, of the 1959 unique isolates identified within the included studies, 569 (29%) were deemed resistant. Despite the large body of literature describing the potential effects of the COVID-19 pandemic on AMR, this is the first study to summarize data surrounding AMR which may have major implications for current and future antimicrobial stewardship as well highlighting gaps in methods of organism identification and reporting of resistance. The concern for AMR during the first full year of the COVID-19 pandemic appears to be low based on our findings. However, with very few reports and poor-quality data, further research is warranted to better understand the landscape of AMR during COVID-19. Additionally, as the pandemic is still ongoing there will be a need to re-assess these findings as further evidence emerges.
The risk of co-infection in patients with influenza has been well documented, with estimates ranging from 2 to 65% [24]. Our study identified a SARS-CoV-2 co-infection congruent with previously reported systematic reviews and large multi-center studies assessing antimicrobial usage that also captured the prevalence of bacterial co-infection [6, 25]. The true prevalence of AMR is currently lacking in literature, and even prior to influenza or the recent COVID-19 pandemic, it has not been well described. A few studies have documented the prevalence of MRSA co-infections in patients with influenza, ranging from 20 to 48%; however other resistant organisms were not frequently reported [26–28]. Furthermore, reports of co-infections with antimicrobial-resistant Gram-negative organsims during influenza season ranged from 2.2% for carbapenems and up to 21% for fluoroquinolones, not necessarily from patients co-infected with influenza [29]. Moreover, inferences of antimicrobial usage could serve as a strong predictor for AMR [24]. In studies evaluating influenza-associated co-infections, antimicrobial usage ranged from 20 to 50% [6, 25]. During the initial stages of the COVID-19 pandemic, up to 60% of patients were prescribed antimicrobials [6, 25]. At the same time, a number of social distancing and public health measures, coupled with increased public adherence to mandates, reduction in travel and increased hand hygiene may have contributed to a decrease in spread [30]. Although our study did not explicitly capture antimicrobial usage or social and public health measures in place at the time of study, patient-level analysis revealed 95% of patients were prescribed antimicrobials prior or during admission to the hospital. Given the difficulty differentiating viral pneumonia from bacterial pneumonia, it is challenging to avoid unnecessary usage of antimicrobials until confirmation of SARS-CoV-2 is obtained. Given this, it is imperative to quantify true rates of AMR to inform the use of appropriate empiric therapies and to understand the types of resistant co-infections that occur in patients with COVID-19.
A large number of carbapenem-resistant A. baumannii (CRAB) and multi-drug resistant C. auris was identified from some studies highlighting the urgent need for the development of newer and more robust antimicrobial agents [19, 31]. In addition, large numbers of Klebsiella pneumoniae (n = 169), MRSA (n = 132) and MDR Pseudomonas spp. (n = 65) infections were noted. A majority of COVID-19 patients received azithromycin– a macrolide with known increasing resistance to both Gram-positive and Gram-negative infections. Globally, macrolides are one of the top 5 antimicrobial classes dispensed by pharmacies, with known increases in resistance. One study has suggested an increase in erythromycin resistance in S. aureus (26 vs. 43%), which may be associated with high azithromycin use [32]. However, despite our study not being able to specifically capture resistance to azithromycin, there is the possibility of increases in macrolide resistance as a result the initial empiric therapy used during this pandemic.
A number of studies documented blood stream infections and ventilator-associated pneumonia co-infections; however, it was hard to tease out the differences in co-infecting organisms between these different populations. A few studies have reported respiratory co-infections with Haemophilus influenzae and S. aureus; however, our analysis only found one case of H. influenzae co-infection with very low rates of Streptococcus pneumoniae co-infections [33]. Conversely, a large number of S. aureus bacteremia and candidemia were reported, the latter of which may have been a result of prolonged antimicrobial usage.
A large number of studies were conducted in ICU settings, which numerically reported higher rates of AMR compared to non-ICU settings. Although driven by two cohort studies, the likelihood of AMR to be detected in much greater proportions in ICU settings is not uncommon. Pre-COVID-19, patients admitted to ICU settings are at an increased risk of acquiring infections, with a number of studies citing nosocomial infections in 20–50% of ICU admissions [34–36]. Given the COVID-19 pandemic, where a priori patients are given a combination of antimicrobial and immunosuppressive agents, it is unsurprising to find higher co-infection rates, and in particular, those of resistant nature, especially in patients who have been mechanically ventilated for long periods of time. Moreover, there are geographical differences that increase the risk of acquiring AMR infections, particularly in areas of low- and middle-income countries, poor clean water and sanitation facilities, high movement of livestock and food products as well as lack of routine surveillance in these areas that contribute to the overall inflation of AMR [37]. Our study also demonstrated slightly higher rates of AMR in settings outside of Europe, particularly Asia and some settings in North America. Surveillance programs, robust testing using standardized protocols and reporting; and importantly multimodal strategies focusing on the stringent use of antibiotics in combination with infection, prevention and control practices could enhance antimicrobial stewardship in certain settings, ultimately reducing mortality and morbidity, especially in patients with COVID-19. Recent studies have suggested the use of these multimodal strategies can be very effective to limit the epidemic spread of resistant microorganisms [38, 39].
Identifying clinical and sociodemographic factors that increase a patients’ risk of developing such co-infections have been established and include: healthcare settings, socioeconomic status, prior antibiotic usage, and length of stay in a hospital setting. A priori identifying patients who are higher risk of developing MDR or XDR infections may improve overall prognosis and outcomes, especially in the context of SARS-CoV-2. Future studies that are prospective in nature with well-designed microbiological investigations would enhance our current understanding of AMR during COVID-19 and what is forthcoming.
Our study has several limitations, the largest being the heterogenous reporting of clinically significant isolates causing co-infection versus secondary infection and clinically insignificant isolates found in colonization and contamination. It is also unclear if identified co-infections were the cause of mortality as opposed to other causes of death such as immune dysregulation and cytokine storm. In addition, given the high risk of bias observed within our included studies, our findings may prove contrary if more rigorous studies with larger sample sizes were available or conducted in the future.
Furthermore, the number of true resistant co-infections is largely underestimated due to asynchronous sampling across studies and sites (or lack of), inappropriate sampling due to administration of antibiotics prior to specimen collection as well as lack of appropriate AST in a number of studies beyond basic culturing [40–42]. Moreover, given the likelihood of COVID-19 upon initial examination, delay in other microbiological investigations in combination with empiric antimicrobial therapy mask the true prevalence of co-infections, let alone resistant co-infections. Taken together, our data, and many others, highlight a small subset of the true burden of co-infections and resistant co-infections, which ultimately impact our understanding of disease progression regarding the timing of therapeutic failure due to resistance [43]. Prospective population-based studies incorporating robust initial and follow-up screening protocols can identify key drivers of resistance in co-infected COVID-19 patients, as well as robust microbiological methods including WGS that may pick up heteroresistance may add to our understanding of the effects in which impact AMR, as well as rates.
More importantly, the varying procedures regarding microbiological identification and antimicrobial susceptibility testing in addition to a lack of standardized AMR definition proved difficult when interpreting results. Studies where standardized procedures, such as CLSI or EUCAST, are applied would make data interpretation much more feasible and allow for potential stratification by patient, geography, or clinical factors in future studies. In our analysis, less than 50% of studies used well-defined interpretive criteria and guidelines such as EUCAST or CLSI. To add, some studies reported highly resistant organisms without resistance profiles, driving the overall rate of AMR down given lack of detail. To add, there is a lack of representation from many low- and middle-income countries (LMICs) and smaller studies which introduce publication and selection bias in our analysis that may differ from articles captured from larger centres across Africa, Asia, Europe and North America. Lastly, meta-analyses were conducted using random effects models in light of known clinical heterogeneity by patient and geographic factors. Thus, the pooled estimates should be interpreted with caution while also highlighting the need for more scientific rigour when it comes to reporting AMR, to truly tease out any differences that may translate to effective clinical and laboratory management, as well as public health policy.
Conclusions
Overall, microbiologically confirmed AMR during the first 18 months of the COVID-19 pandemic was relatively high among patients with bacterial co-infections. The most common resistance documented was in CRAB, MRSA, Klebsiella pneumoniae, and Pseudomonas aeruginosa, although some C. auris isolates were also identified. Despite no demonstrative differences across hospital ICU settings and geography, further high-quality research is warranted to truly capture the prevalence of AMR during COVID-19 and beyond.
Supplementary Information
Additional file 5. Proportion of resistant infections among regular ICU, or other hospital setting and COVID-specific ICU settings
Additional file 6. Proportion of resistant infections among Europe, Asia and other geographical settings
Additional file 7. Proportion of resistant infections among Italy, Europe (excluding Italy), and other geographical settings
Acknowledgements
The authors thank Sandy Campbell from the John W. Scott Health Sciences Library at the University of Alberta, Edmonton, Alberta, and Dr. Susanne King-Jones from the Alberta Health Services, Knowledge Resource Service at the Royal Alexandra Hospital, Edmonton, Alberta, for assisting with the search strategy.
Abbreviations
- AMR
Antimicrobial resistance
- ARDS
Acute respiratory distress syndrome
- BAL
Bronchoalveolar lavage
- BSI
Blood stream infection
- CCU
Critical care unit
- CLSI
Clinical & Laboratory Standards Institute
- COVID-19
Coronavirus disease 2019
- CRAB
Carbapenem-resistant Acinetobacter baumannii
- CRE
Carbapenem-resistant Enterobacteriaceae
- ESBLs
Extended spectrum β-lactamases
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- ICU
Intensive care unit
- JBI
Joanna Briggs Institute
- MALDI-ToF MS
Matrix-associated laser desorption ionization time of flight mass spectrometry
- MDR
Multi-drug resistant
- MRSA
Methicillin-resistant Staphylococcus aureus
- OPC
Oropharyngeal candidiasis
- PCR
Polymerase chain reaction
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RICU
Respiratory intensive care unit
- RT-PCR
Real-time polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- T2DM
Type II diabetes mellitus
- UTI
Urinary tract infection
- VAP
Ventilator-associated pneumonia
- VRE
Vancomycin-resistant Enterococcus spp
- WGS
Whole-genome sequencing
Authors' contributions
RMK designed the literature search and created the figures. RMK, DAJ and DCJ wrote the first draft of the manuscript. RMK, DAJ, DCJ and SLL screened and reviewed articles, and extracted and analyzed data. RMK and PER performed the statistical analysis. HWB designed the study and was responsible for overall supervision. TCD, ERM, JZC, JMC and GTT critically reviewed and revised the manuscript and approved the final draft. All authors read and approved the final manuscript.
Funding
The Antimicrobial Resistance—One Health Consortium is funded through the Major Innovation Fund Program of the Ministry of Jobs, Economy, and Innovation (JEI), Government of Alberta, Canada.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global Action Plan on AMR. https://apps.who.int/iris/handle/10665/193736. Accessed 20 Sept 2021.
- 2.O' Neill J. The review on antimicrobial resistance: tackling drug-resistant infections globally: final report and recommendations: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. Accessed 20 September 2021.
- 3.Impact of COVID-19 on people’s livelihoods, their health and our food systems. https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people's-livelihoods-their-health-and-our-food-systems. Accessed 20 September 2021.
- 4.Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman H, Nguyen P. First case of COVID-19 complicated with Burkholderia cepacia pneumonia and bacteremia. Chest. 2020;158:A544. [Google Scholar]
- 6.Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect. 2021;27:9–11. doi: 10.1016/j.cmi.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieswerda E, De Boer M, Bonten M, et al. Recommendations for antibacterial therapy in adults with COVID-19—An evidence based guideline. Clin Microbiol Infect. 2021;1:61–66. doi: 10.1016/j.cmi.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Meniño I, Forcelledo L, Rosete Y, García-Prieto E, Escudero D, Fernández J. Spread of OXA-48-producing Klebsiella pneumoniae among COVID-19-infected patients: the storm after the storm. J Infect Public Health. 2021;14:50–52. doi: 10.1016/j.jiph.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei R, Goodarzi P, Asadi M, et al. Bacterial co-infections with SARS-CoV -2. IUBMB Life. 2020;72:2097–2111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razazi K, Arrestier R, Haudebourg AF, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care. 2020;24:699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Council of Canadian Academies. When antibiotics fail: The expert panel on the potential socio-economic impacts of antimicrobial resistance in Canada. http://www.deslibris.ca/ID/10102747 Accessed 20 September, 2021).
- 13.World Health Organization. Antibiotic resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. Accessed 20 September, 2021.
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed]
- 15.Covidence. Covidence - Better systematic review management. https://www.covidence.org/ Accessed 20 September, 2021.
- 16.Aromataris E, Munn Z. JBI manual for evidence synthesis. JBI. 2020 doi: 10.46658/JBIMES-20-01. [DOI] [Google Scholar]
- 17.Whiting PF. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Guisado-Gil A, Infante-Domínguez C, Peñalva G, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics. 2020;9:816. doi: 10.3390/antibiotics9110816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez S, Innes GK, Walters MS, et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions—New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Wang J, Yang Y, et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9:153. doi: 10.1186/s13756-020-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63:771–778. doi: 10.1111/myc.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mady A, Aletreby W, Abdulrahman B, et al. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multifaceted critical illness: A retrospective case series. Ann Med Surg. 2020;60:417–424. doi: 10.1016/j.amsu.2020.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardo CDO, Gonzalez-Chica D, Stocks N. Influenza-like illness and antimicrobial prescribing in Australian general practice from 2015 to 2017: a national longitudinal study using the MedicineInsight dataset. BMJ Open. 2019;9:e026396. doi: 10.1136/bmjopen-2018-026396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2:e354–e365. doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Ling L, Wong SH, et al. Outcomes of respiratory viral-bacterial co-infection in adult hospitalized patients. EClinicalMedicine. 2021;37:100955. doi: 10.1016/j.eclinm.2021.100955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah NS, Greenberg JA, McNulty MC, et al. Bacterial and viral co-infections complicating severe influenza: incidence and impact among 507 U.S. patients, 2013–14. J Clin Virol. 2016;80:12–19. doi: 10.1016/j.jcv.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V, Yu KC, Kabler H et al. Antibiotic resistance patterns and association with the influenza season in the United States: A multicenter evaluation reveals surprising associations between influenza season and resistance in Gram-negative pathogens. Open For Infect Dis. 2022; ofac039. 10.1093/ofid/ofac039. [DOI] [PMC free article] [PubMed]
- 31.van Duin D, Barlow G, Nathwani D. The impact of the COVID-19 pandemic on antimicrobial resistance: a debate. J Antimicrob Resist. 2020; 2: dlaa053. [DOI] [PMC free article] [PubMed]
- 32.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill Coronavirus Disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Jácome LE, Fernández-Rodríguez D, Franco-Cendejas R, Camacho-Ortiz A. Increment antimicrobial resistance during the COVID-19 pandemic: results from the Invifar Network. Microb Drug Resist. 2021 doi: 10.1089/mdr.2021.0231. [DOI] [PubMed] [Google Scholar]
- 34.Ramadan HK-A, Mahmoud MA, Aburahma MZ, et al. Predictors of severity and co-infection resistance profile in COVID-19 patients: first report from Upper Egypt. Infect Drug Resist. 2020; 13: 3409–22. [DOI] [PMC free article] [PubMed]
- 35.Vincent J-L, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe: results of the European prevalence of infection in intensive care (EPIC) study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 36.Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 37.Hanberger H, Garcia-Rodriguez JA, Gobernado M, et al. Antibiotic susceptibility among aerobic Gram-negative bacilli in intensive care units in 5 European countries. JAMA. 1999;281:67–71. doi: 10.1001/jama.281.1.67. [DOI] [PubMed] [Google Scholar]
- 38.Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019;26:taz036. doi: 10.1093/jtm/taz036. [DOI] [PubMed] [Google Scholar]
- 39.Al-Maani A, Al Wahaibi A, Al-Zadjali N, et al. The impact of the hand hygiene role model project on improving healthcare workers’ compliance: A quasi-experimental observational study. J Infect Public Health. 2022;15:324–330. doi: 10.1016/j.jiph.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Gallouche M, Terrisse H, Larrat S, et al. Effect of a multimodal strategy for prevention of nosocomial influenza: a retrospective study at Grenoble Alpes University Hospital from 2014 to 2019. Antimicrob Resist Infect Control. 2022;11:31. doi: 10.1186/s13756-021-01046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang C-Y, Chan K-G. Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J Infect. 2020;81:e29–30. doi: 10.1016/j.jinf.2020.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clancy CJ, Nguyen MH. COVID19, superinfections and antimicrobial development: What can we expect? Clin Infect Dis. 2021;71:2736–43. [DOI] [PMC free article] [PubMed]
- 45.Bogossian EG, Taccone FS, Izzi A, et al. The acquisition of multidrug-resistant bacteria in patients admitted to COVID-19 intensive care units: A monocentric retrospective case control study. Microorganisms. 2020;8:1821. doi: 10.3390/microorganisms8111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amarsy R, Pean de Ponfilly G r, Benmansour H a, Jacquier H, Cambau E e, Mégarbane B. Serratia marcescens outbreak in the intensive care unit during the COVID-19 pandemic: A paradoxical risk? Médecine Mal Infect. 2020; 50: 750–1. [DOI] [PMC free article] [PubMed]
- 47.Cataldo MA, Tetaj N, Selleri M, et al. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming “collateral effect”. J Glob Antimicrob Resist. 2020;23:290–291. doi: 10.1016/j.jgar.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Posteraro B, Torelli R, Vella A, et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J Fungi. 2020;6:163. doi: 10.3390/jof6030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmoudi H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hyg Infect Control. 2020;15:Doc35. [DOI] [PMC free article] [PubMed]
- 50.Mo Y, Adarkwah O, Zeibeq J, Pinelis E, Orsini J, Gasperino J. Treatment with tocilizumab for patients with COVID-19 infections: A case-series study. J Clin Pharmacol. 2021;61:406–411. doi: 10.1002/jcph.1787. [DOI] [PubMed] [Google Scholar]
- 51.Walpole SC, McHugh R, Samuel J, Schmid ML. COVID-19 presenting as severe, persistent abdominal pain and causing late respiratory compromise in a 33-year-old man. BMJ Case Rep. 2020;13:e236030. doi: 10.1136/bcr-2020-236030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montrucchio G, Corcione S, Sales G, Curtoni A, De Rosa FG, Brazzi L. Carbapenem-resistant Klebsiella pneumoniae in ICU-admitted COVID-19 patients: keep an eye on the ball. J Glob Antimicrob Resist. 2020;23:398–400. doi: 10.1016/j.jgar.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiri B, Sensi E, Marsiliani V, et al. Antimicrobial stewardship program, COVID-19, and infection control: spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J Clin Med. 2020;9:2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokkoris S, Papachatzakis I, Gavrielatou E, et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect. 2021;107:95–97. doi: 10.1016/j.jhin.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrotta F, Perrini MP. Successful treatment of Klebsiella pneumoniae NDM sepsis and intestinal decolonization with ceftazidime/avibactam plus aztreonam combination in a patient with TTP complicated by SARS-CoV-2 nosocomial infection. Medicina (Mex) 2021;57:424. doi: 10.3390/medicina57050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baiou A, Elbuzidi AA, Bakdach D, et al. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J Hosp Infect. 2021;110:165–171. doi: 10.1016/j.jhin.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses. 2021;64:144–151. doi: 10.1111/myc.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez-Guerra BA, Gonzalez-Lara MF. Antimicrobial resistance patterns and antibiotic use during hospital conversion in the COVID-19 pandemic. Antibiotics (Basel) 2021;10:182. doi: 10.3390/antibiotics10020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karruli A, Boccia F, Gagliardi M, et al. Multidrug-resistant infections and outcome of critically ill patients with Coronavirus Disease 2019: a single center experience. Microb Drug Resist. 2021 doi: 10.1089/mdr.2020.0489. [DOI] [PubMed] [Google Scholar]
- 60.Pulia MS, Wolf I, Schwei RJ et al. Antibiotic prescribing pattenrs for coronavirus diseas 2019 (COVID-19) in two emergency departments with rapid procalcitonin. Infect Control Hosp Epidemiol. 2020;1–3. [DOI] [PMC free article] [PubMed]
- 61.Gomez-Simmonds A, Annavajhala MK, McConville TH, et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J Antimicrob Chemother. 2021;76:380–384. doi: 10.1093/jac/dkaa466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cultrera R, Barozzi A, Libanore M, et al. Co-infections in critically ill patients with or without COVID-19: a comparison of clinical microbial culture findings. Int J Environ Res Public Health. 2021;18:4358. doi: 10.3390/ijerph18084358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khurana S, Singh P, Sharad N, et al. Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: Implication on antimicrobial resistance. Indian J Med Microbiol. 2021;39:147–153. doi: 10.1016/j.ijmmb.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pascale R, Bussini L, Gaibani P, et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: a multicenter before-and-after cross-sectional study. Infect Control Hosp Epidemiol 2021: 1–6. [DOI] [PMC free article] [PubMed]
- 65.Baskaran V, Lawrence H, Lansbury LE, et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England: Read the story behind the paper on the Microbe Post here. J Med Microbiol. 2021 doi: 10.1099/jmm.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moretti M, Van Laethem J, Minini A, Pierard D, Malbrain MLNG. Ventilator-associated bacterial pneumonia in coronavirus 2019 disease, a retrospective monocentric cohort study. J Infect Chemother. 2021;27:826–833. doi: 10.1016/j.jiac.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magnasco L, Mikulska M, Giacobbe DR, et al. Spread of carbapenem-resistant Gram-negatives and Candida auris during the COVID-19 pandemic in critically ill patients: One step back in antimicrobial stewardship? Microorganisms. 2021;9:95. doi: 10.3390/microorganisms9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentivegna E, Luciani M, Arcari L, Santino I, Simmaco M, Martelletti P. Reduction of multidrug-resistant (MDR) bacterial infections during the COVID-19 pandemic: A retrospective study. Int J Environ Res Public Health. 2021;18:1003. doi: 10.3390/ijerph18031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suarez de la Rica A, Serrano P, de-la-Olivia R, Sahcez-Diaz P, Molinero P, Falces-Rmero I, Ferrado C, Rello J, Maseda E. Secondary infections in mechanically ventilated patients with COVID-10: An overlooked matter? Rev Esp Quimioter. 2021; 34: 330–336. [DOI] [PMC free article] [PubMed]
- 71.Posteraro B, De Angelis G, Menchinelli G, et al. Risk factors for mortality in adult COVID-19 patients who develop bloodstream infections mostly caused by antimicrobial-resistant organisms: analysis at a large teaching hospital in Italy. J Clin Med. 2021;10:1752. doi: 10.3390/jcm10081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 5. Proportion of resistant infections among regular ICU, or other hospital setting and COVID-specific ICU settings
Additional file 6. Proportion of resistant infections among Europe, Asia and other geographical settings
Additional file 7. Proportion of resistant infections among Italy, Europe (excluding Italy), and other geographical settings
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.