Abstract
Objective
To evaluate the association between regulatory drug safety advisories and changes in drug utilisation.
Design
We conducted controlled, interrupted times series analyses with administrative prescription claims data to estimate changes in drug utilisation following advisories. We used random-effects meta-analysis with inverse-variance weighting to estimate the average postadvisory change in drug utilisation across advisories.
Study population
We included advisories issued in Canada, Denmark, the UK and the USA during 2009–2015, mainly concerning drugs in common use in primary care. We excluded advisories related to over-the-counter drugs, drug-drug interactions, vaccines, drugs used primarily in hospital and advisories with co-interventions within ±6 months.
Main outcome measures
Change in drug utilisation, defined as actual versus predicted percentage change in the number of prescriptions (for advisories without dose-related advice), or in the number of defined daily doses (for dose-related advisories), per 100 000 population.
Results
Among advisories without dose-related advice (n=20), the average change in drug utilisation was −5.83% (95% CI −10.93 to –0.73; p=0.03). Advisories with dose-related advice (n=4) were not associated with a statistically significant change in drug utilisation (−1.93%; 95% CI −17.10 to 13.23; p=0.80). In a post hoc subgroup analysis of advisories without dose-related advice, we observed no statistically significant difference between the change in drug utilisation following advisories with explicit prescribing advice, such as a recommendation to consider the risk of a drug when prescribing, and the change in drug utilisation following advisories without such advice.
Conclusions
Among safety advisories issued on a wide range of drugs during 2009–2015 in 4 countries (Canada, Denmark, the UK and the USA), the association of advisories with changes in drug utilisation was variable, and the average association was modest.
Keywords: health policy, health services research, medication safety, pharmacoepidemiology
Introduction
Medicines are essential in providing effective healthcare and are also associated with risk of harm.1–4 Among epidemiological studies quantifying adverse drug reactions (ADRs) in a European setting, a median of 3.6% of hospital admissions were due to an ADR, and a median of 10.1% of patients experienced an ADR during a hospital admission.1 Studies of drug safety in Canada and Europe indicate that close to one in five drugs was associated with a serious postmarket safety issue.3 4 Similarly, a cohort study of drugs approved by the US Food and Drug Administration (FDA) found that 32% had a postmarket safety issue.2
When new evidence of harm emerges during the postmarket period, regulators may issue drug safety advisories to warn health professionals and the public of harm and to promote safer use. Advisories may take the form of Direct Healthcare Professional Communications (DHPCs, which are letters or emails sent to individual health professionals), alerts (safety information posted to a regulator’s website and addressed to a broad audience rather than individual clinicians), investigations (statements on ongoing reviews or analyses, early monitoring reviews or detailed investigation reports) or bulletins (articles in a regulator’s newsletter or drug safety bulletin).5
Systematic reviews suggest advisories issued by regulators may influence clinical practice.6–9 Weatherburn et al found that regulatory risk communications in the UK with a recommendation to change practice based on a change or restriction in indication were associated with a 34% change in the rate of prescribing in the intended direction, while risk communications to ‘be aware’ of new information about a drug’s risk were associated with an 11% change in prescribing.9 These findings suggest prescribing changes may differ in relation to how information about drug risk is communicated in an advisory. However, it is difficult to know the average impact of drug safety advisories on drug prescribing from existing systematic reviews, due to the inconsistent methodological quality of studies of advisories,7 8 10 11 the literature’s focus on a limited number of drug classes7–10 and publication bias.9 10
This study aimed to estimate the average impact of drug safety advisories on drug utilisation with data from Australia, Canada, Denmark, the UK and the USA. A secondary aim was to evaluate whether the inclusion of prescribing advice in an advisory was associated with a greater postadvisory change in drug utilisation. Prescribing advice was defined as explicit advice regarding a prescribing decision, such as a change in indication or a recommendation to take the risk of a drug into account when considering treatment options.
Methods
Study design
We selected drug safety advisories for inclusion from among those issued in Australia, Canada, Denmark, the UK and the USA during 2009–2015 inclusive. We used interrupted time series analysis to estimate the change in drug utilisation following each advisory, adjusted by the change in drug utilisation in a concurrent or historical control12 13 (see box 1 for the criteria used in selection of advisories and controls, and the ‘Statistical analysis’ section for details on the interrupted time analysis). After performing time series analyses to estimate the change in drug utilisation following each advisory, we used random-effects meta-analysis to estimate the average postadvisory change in drug utilisation across advisories.14 We stratified our analyses based on whether an advisory contained dose-related advice, which was defined as advice that revised the recommended or maximum dose of a drug or warned about risk associated with higher doses.
Box 1. Criteria for selection of drug safety advisories and controls for analysis.
Inclusion criteria for advisories:
Safety alerts posted on a regulator’s website or Direct Healthcare Professional Communications.
Advisory related to a drug on the market for ≥24 months preceding an index advisory and ≥12 months following an advisory in at least one country, and the drug was on the market for ≥36 months in at least one country without the advisory (to serve as a control).
If advisories for different topics were issued for the same drug during 2009–2015, we only included an advisory on the first topic meeting other inclusion criteria to limit analysis to one advisory per drug.
Exclusion criteria for advisories:
Advisory related to an ‘all-clear’ statement (ie, no problem was ultimately identified), drugs available over-the-counter in ≥1 country, drug-drug interactions, drugs marketed in only one of the countries or vaccines.
Advisory was only an announcement that a safety concern was under investigation or an article in the regulatory agency’s drug safety bulletin.
Advisory was for a drug class or multiple drugs, or drugs used primarily in hospitals.
Advisories for drugs with lowest utilisation (based on data from US IBM MarketScan Research Databases) were excluded, but additional drugs not meeting this criterion were considered for inclusion to ensure a sufficient number of newer drugs were included (ie, drugs on the market for <6 years prior to the advisory).
Advisory had co-intervention(s) within ±6 months of an advisory (such as an additional advisory for the same drug coinciding with a marked change in drug utilisation).
Advisory was for a drug that had unstable use in the 24 months prior to the advisory (eg, a new drug might have an initial low rate of use followed a steep rise in use, rather than a consistent trend), based on visual inspection of preadvisory data.
For each advisory, we selected one control from among possible controls as follows:
We required use of the advisory drug to be stable during the 24-month preadvisory period in the control country (or historical control period), based on visual inspection, and we required the ratio of the preadvisory median monthly drug utilisation rates to be minimally comparable in the control and index country (ie, not exceeding a ratio of 10:1).
We preferred a control country in which we expected drug use was less likely to be affected by the advisory in the index country (to avoid controls with a spillover effect) (online supplemental table S3), based on a priori expectations (due to the population size, geographic proximity and interaction of medical cultures of countries) and an empirical analysis of changes in drug utilisation following a small subset of advisories.
We preferred a concurrent control over a historical control. If no suitable concurrent controls were available, we used data from the 36 months prior to an advisory as a historical control period.
If the above criteria were met by multiple possible controls, we preferred the control in which preadvisory drug utilisation rate was most similar to that in the index country.
bmjqs-2021-013910supp001.pdf (917.4KB, pdf)
Data sources
Data sources for selection of advisories
We previously created a database of advisories issued during 2007–2016 by the Australian Therapeutic Goods Administration, Health Canada, the UK Medicines and Healthcare products Regulatory Agency and the US FDA,5 and a similar database of DHPCs issued during 2007–2018 in Denmark.15 We used these databases and dates of drug approval and withdrawal collected from regulators’ websites to select advisories and controls to include in the study. We included advisories from Canada, Denmark, the UK and the USA in the study, but no Australian advisories met our inclusion criteria. We still used Australian drug utilisation data in the study, because Australia served as a control in several cases for studying the impact of advisories from other countries.
Data sources for measuring drug utilisation
To assess changes in drug utilisation, we used administrative health data from the National Prescription Drug Utilization Information System accessed through the Canadian Institute for Health Information, the Clinical Practice Research Datalink (CPRD) Gold database with approval granted by the Independent Scientific Advisory Committee of the Medicines and Healthcare products Regulatory Agency (protocol 20_000191) and US IBM MarketScan Research Databases accessed through IBM Watson Health.16–18 In Denmark, the Danish National Prescription Registry was accessed through the Research Service Unit of Statistics Denmark (FSEID-00004357/DST-project no. 707524), and approval for processing of personal health data was obtained through the UCHP (ref. no.: 514-0301/19-3000).19 Aggregate data by month on prescription drugs dispensed through the Pharmaceutical Benefits Scheme in Australia were publicly available online20 (for further detail on these databases, see online supplemental table S1). These data sources primarily captured drugs prescribed (CPRD) or dispensed (other databases) in a community setting rather than in hospital. Prescribing and dispensing are collectively referred to in this paper as ‘utilisation’.
Study population
The study included data from residents with public or private drug coverage in Australia, Canada, Denmark, the UK and the USA (online supplemental table S1). In Australia and Denmark, the study population included all residents. In Canada, the study population included residents of the provinces of British Columbia and Saskatchewan (which had better capture of prescription drug dispensations than other provinces), excluding the small proportion of residents with federal drug coverage. (Data from these provinces comprised approximately 15% of the Canadian population.) In the UK, the study population included patients whose general practitioners participated in the CPRD (comprising 9% of the UK population). The US study population included persons <65 years with private drug plans, and persons ≥65 years with Medicare coverage and supplemental private plans, collected by the US IBM MarketScan Research Databases (comprising 12% of the US population). If an advisory only applied to a specific demographic group, we restricted the analysis by age or sex. Similarly, if an advisory applied only to a specific drug form or route of administration (eg, oral), we restricted analysis to the relevant form of the drug.
Selection of advisories and controls
We applied several criteria to select advisories for inclusion from among those issued in Australia, Canada, the UK and the USA from January 2009 to December 2015 (box 1 shows selection criteria for advisories and controls, and online supplemental table S2 describes the rationale for the selection criteria). Subsequently, we identified Danish advisories that covered the same topics, in order to expand the number of jurisdictions available for analyses (eg, there was a UK advisory on clopidogrel and acquired haemophilia, and an advisory issued on this topic in Denmark). For each advisory topic (eg, all advisories on clopidogrel and acquired haemophilia), we designated the advisory from the country that issued the first advisory as the index advisory. We also identified a suitable control specific to that index advisory. A control was selected from among the five countries in the study, which was either a concurrent control (a country that did not issue a similar advisory within 12 months of the index advisory) or a historical control (data from the 36 months prior to an advisory from the same country, or a different country if necessary). When selecting concurrent controls, we preferred a control country in which we expected drug use was less likely to be affected by the advisory in the index country (to avoid controls with a spillover effect) (online supplemental table S3).
Outcomes
While all advisories included in the study highlighted drug risks and might influence whether a drug is prescribed, advisories with dose-related advice might also influence the dose prescribed. For advisories without dose-related advice, we used the monthly number of prescriptions written or dispensed per 100 000 population as the drug utilisation outcome measure. For advisories with dose-related advice, we used the monthly number of defined daily doses (DDDs)21 prescribed or dispensed per 100 000 population as the drug utilisation outcome measure, to capture changes in the dosage level as well as changes in the number of prescriptions. The number of DDDs was calculated as product of medication strength and quantity, divided by WHO DDD (an assumed average maintenance dose per day).21
Statistical analysis
We used interrupted time series analysis12 13 to estimate the change in drug utilisation for each index advisory and control during a postadvisory period. For each advisory, the crude change in drug utilisation was calculated as the difference between the actual and predicted postadvisory change in drug utilisation. We estimated the adjusted change in drug utilisation by adjusting the crude estimate by the change in drug utilisation in a concurrent control (a country in our study that did not issue an advisory during the same time period) or a historical control (if no suitable concurrent control was available). Each time series analysis used 24 months of data prior to an advisory, a transition period of 1 month during which an advisory was issued and an 11-month postadvisory period (or analogous periods during the 36 months prior to an advisory for historical controls). We estimated models with a linear time trend to adjust for secular trends, adjusted for seasonality22 and autocorrelation23 as necessary, using SAS V.9.4.
We calculated both the absolute difference and the percentage difference between the monthly actual and predicted drug utilisation rates during the postadvisory period for each index advisory and control. We used bootstrapping resampling methods with 5000 iterations to estimate percentile-based 95% CIs for the absolute and percentage differences.24 25 We estimated the adjusted percentage change in drug utilisation by taking the difference between the percentage change following the index advisory and the percentage change in the control, and calculating a 95% CI.26
We conducted random-effects meta-analyses with inverse-variance weighting to estimate the average association of advisories with percentage change in drug utilisation,14 stratified by advisories with and without dose-related advice. We used random-effects rather than fixed-effects models, because we anticipated the effects of advisories would be heterogeneous due to differences in the drugs targeted, content of advisories and populations studied.14 The random-effects estimates in our models represent the average intervention effect for the advisories included in each analysis, calculated as a weighted average where the weight was the inverse of the variance of the estimated effect of each advisory.14 Meta-analyses were performed with RevMan V.5.4.
Post hoc subgroup analysis of advisories with versus without prescribing advice
We conducted a post hoc subgroup analysis to investigate whether postadvisory changes in drug utilisation varied according to whether the advisory contained advice to change prescribing. This analysis compared advisories with versus without prescribing advice relevant to an immediate prescribing decision and not restricted to a small subgroup of patients. A member of the study team (RLM) classified the advisories without dose-related advice into subgroups for this analysis. We did not apply the same analysis to dose-related advisories, as they all by definition contained prescribing advice (regarding dose). First, advisories were classified according to whether they contained explicit prescribing advice relevant to an immediate prescribing decision. For example, this could include a recommendation to consider the risk of a drug when prescribing or describe a change in indication, but advice to consider discontinuation after a patient experienced an adverse effect was not considered ‘relevant to an immediate prescribing decision’. Second, advisories deemed to contain prescribing advice at the first step were assessed according to whether the advice was restricted to a small subgroup, which was defined as under 2% of patients receiving a medication. We excluded prescribing advice focused on changing practice after a patient experienced an adverse effect or targeting a small subgroup of patients, because we believed it was less likely to have a measurable impact on prescribing. A meta-analysis was conducted, and Cochran’s Q test was used to test for subgroup difference. In addition, we conducted a descriptive analysis of physician perspectives on prescribing advice in drug safety advisories, based on assessments of the advisories by a general practitioner who agreed to assist the study for this purpose (JAL) and an emergency department physician from our research team (JL).
Patient and public involvement
Neither patients nor member of the public were involved in the design, conduct, reporting or dissemination plans for this study.
Results
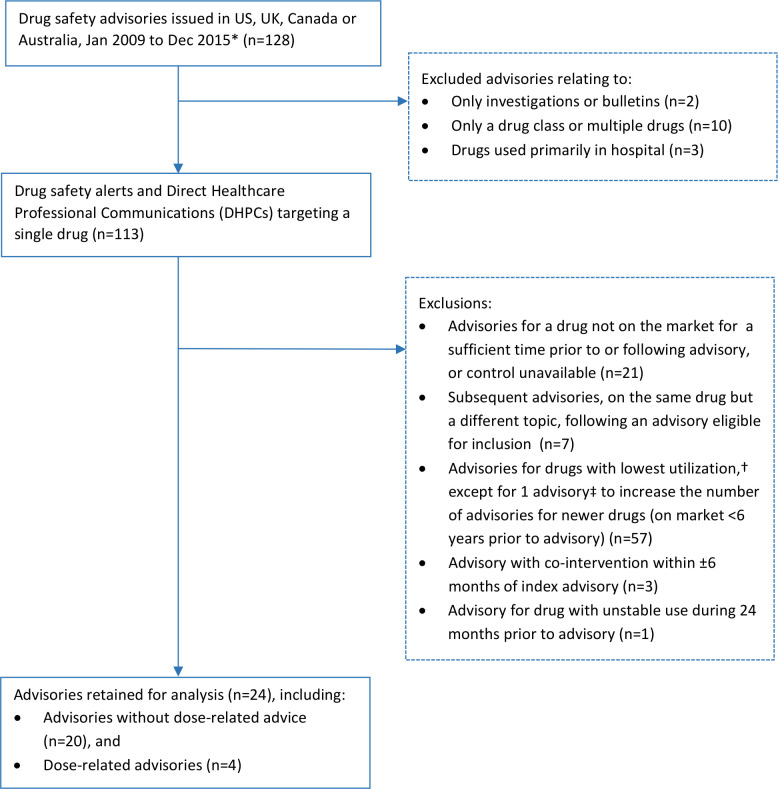
We screened 128 advisories from Australia, Canada, the USA and the UK to identify advisories for inclusion in the study (counting multiple advisories on the same topic only once) (figure 1). Following exclusions, we retained 24 advisories for analysis,27–50 including 20 advisories without dose-related advice and 4 with dose-related advice. Half of the index advisories were issued in the USA (12), while the remainder were issued in Canada (3), Denmark (3) and the UK (6) (table 1). No Australian advisories qualified as an index advisory. Safety alerts (17) served more frequently as index advisories compared with DHPCs (7). The 24 drugs featured in the advisories represent 19 different drug classes (according to the WHO Anatomical Therapeutic Chemical, level 3) (online supplemental table S4),21 and included 2 drugs (febuxostat and fingolimod) that entered the market within 6 years prior to the advisories studied.
Figure 1.
Selection of drug safety advisories for inclusion. *Excluding advisories relating to all-clear statements, drugs available over-the-counter in ≥1 country, drug-drug interactions, drugs marketed in only one of the countries and vaccines. Multiple advisories on the same topic were counted only once. Danish advisories were included in analysis, but not in the process of selection of advisories to include. †Based on data from US IBM MarketScan Research Databases. ‡Advisory for fingolimod and progressive multifocal leucoencephalopathy. Created by the authors.
Table 1.
Advisory characteristics
| Advisory (drug-risk group) | Index country | Control | Advisory date | Advisory type |
| (a) Advisories without dose-related advice* | ||||
| Aripiprazole-impulse control disorders30 | CA | DK | 2 November 2015 | Alert |
| Azithromycin-cardiac arrhythmias43 | USA | USA† | 12 March 2013 | Alert |
| Clopidogrel-acquired haemophilia38 | DK | AU | 28 August 2013 | DHPC |
| Febuxostat-epidermal and dermal conditions27 | UK | USA† | 6 May 2012 | DHPC |
| Finasteride-breast cancer male34 | UK | CA | 1 December 2009 | Alert |
| Fingolimod-PML40 | USA | CA | 29 August 2013 | Alert |
| Insulin-glargine-neoplasm malignant50 | USA | DK | 1 July 2009 | Alert |
| Isotretinoin-epidermal and dermal conditions32 | CA | DK | 11 February 2010 | DHPC |
| Ketoconazole-adrenal gland disorders‡41 | USA | USA† | 26 July 2013 | Alert |
| Leflunomide methotrexate-hepatotoxicity49 | USA | AU | 13 July 2010 | Alert |
| Methylphenidate-sexual dysfunction39 | USA | USA† | 17 December 2013 | Alert |
| Mycophenolate-aplasia pure red cell33 | UK | USA†§ | 2 June 2009 | DHPC |
| Nitrofurantoin-lack of effect36 | UK | AU¶ | 1 August 2013 | Alert |
| Olmesartan-malabsorption42 | USA | AU¶ | 3 July 2013 | Alert |
| Ondansetron-cardiac arrhythmias45 | USA | AU¶ | 15 September 2011 | Alert |
| Pioglitazone-bladder cancer48 | USA | USA† | 15 June 2011 | Alert |
| Quetiapine-metabolic syndrome29 | UK | UK† | 23 December 2011 | DHPC |
| Tacrolimus-neoplasm malignant‡28 | DK | CA | 1 May 2012 | DHPC |
| Testosterone-cardiovascular disorder31 | CA | UK | 15 July 2014 | Alert |
| Topiramate-congenital anomaly37 | DK | CA | 1 March 2011 | DHPC |
| (b) Advisories with dose-related advice* | ||||
| Citalopram escitalopram-cardiac arrhythmias46 | USA | USA† | 24 August 2011 | Alert |
| Fluconazole-congenital anomaly47 | USA | USA† | 3 August 2011 | Alert |
| Hydroxyzine-cardiac arrhythmias35 | UK | CA | 29 April 2015 | Alert |
| Zolpidem-cognitive impairment44 | USA | USA† | 10 January 2013 | Alert |
Created by the authors.
*Dose-related advice was defined as advice that revised the recommended or maximum dose of a drug or warned about risk associated with higher doses.
†Historical control.
‡Advisory applied to a specific route of administration (oral for ketoconazole and topical for tacrolimus), so analysis was restricted to relevant forms of the drug.
§A historical control from the UK was unavailable due to a lack of sufficient preadvisory data, so a US historical control was used.
¶Restricted to drug use of concessional beneficiaries (eg, seniors and individuals with a low household income), due to better data capture in this population for these drugs.
AU, Australia; CA, Canada; DHPC, Direct Healthcare Professional Communication; DK, Denmark; PML, progressive multifocal leucoencephalopathy.
The majority of controls (14) were concurrent controls (another country that did not issue a concurrent advisory on the same topic) rather than historical controls (10) (table 1). Each of the five countries served as a control for some advisories: Australia (5), Canada (5), Denmark (3), the UK (2) and the USA (9).
Interrupted time series analysis of changes in drug utilisation
Changes in drug utilisation following advisories without dose-related advice
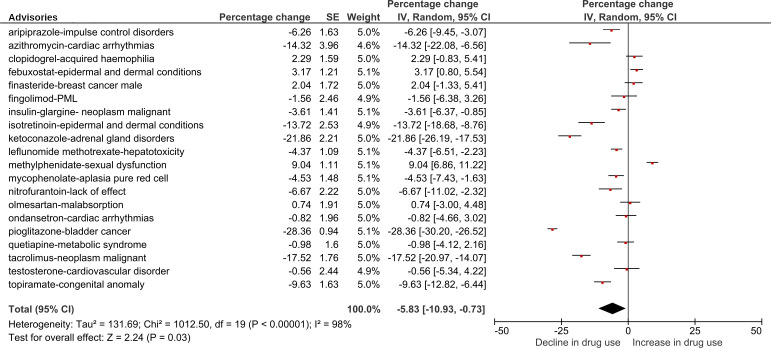
Among advisories without dose-related advice (n=20), the crude actual versus predicted change in the number of prescriptions per 100 000 population following the index advisories (unadjusted by the change in controls) ranged from a decrease of 29.2% following the pioglitazone-bladder cancer advisory to an increase of 5.5% following the methylphenidate-sexual dysfunction advisory (table 2). (Actual vs predicted change in drug utilisation among controls is reported in online supplemental table S5.) Adjusted analyses of actual versus predicted change in prescription rates following advisories without dose-related advice indicated that 8 of 20 advisories (40%) were followed by a decline in the prescription rate of >5%, and 5 (25%) were followed by a decline of >10% (figure 2).
Table 2.
Crude actual versus predicted change in drug utilisation in the 11 months following the month of each index advisory
| Advisory category | Advisory (drug-risk group) | Index country | Absolute change, prescription or DDD rate (95% CI)*† | Percentage change, % (95% CI)* |
| (a) Advisories without dose-related advice‡ | Aripiprazole-impulse control disorders | CA | −43.9 (−62.0 to –25.4) | −3.7 (−5.2 to −2.1) |
| Azithromycin-cardiac arrhythmias | USA | −246 (−323 to –164) | −16.5 (−21.7 to −11.0) | |
| Clopidogrel-acquired haemophilia | DK | 11 (1 to 22) | 2.2 (0.2 to 4.1) | |
| Febuxostat-epidermal and dermal conditions | UK | −0.5 (−0.6 to –0.3) | −5.7 (−7.6 to −3.7) | |
| Finasteride-breast cancer male | UK | 0.8 (−13.5 to 14.7) | 0.2 (−2.9 to 3.1) | |
| Fingolimod-PML | USA | −0.2 (−0.4 to –0.1) | −2.9 (−4.9 to −0.9) | |
| Insulin-glargine-neoplasm malignant | USA | −9.2 (−14.3 to –4.1) | −2.8 (−4.4 to −1.2) | |
| Isotretinoin-epidermal and dermal conditions | CA | −18.7 (−23.5 to –13.9) | −7.9 (−9.9 to −5.9) | |
| Ketoconazole-adrenal gland disorders | USA | −4.4 (−5.0 to –3.8) | −26.2 (−29.8 to −22.5) | |
| Leflunomide-hepatotoxicity | USA | −1.6 (−1.9 to –1.2) | −7.7 (−9.4 to −5.9) | |
| Methylphenidate-sexual dysfunction | USA | 29.2 (21.0 to 37.4) | 5.5 (4.0 to 7.1) | |
| Mycophenolate-aplasia pure red cell | UK | −1.1 (−1.9 to –0.3) | −3.7 (−6.3 to −1.0) | |
| Nitrofurantoin-lack of effect | UK | −10.7 (−20.7 to –0.5) | −2.8 (−5.5 to −0.1) | |
| Olmesartan-malabsorption | USA | 14.8 (9.8 to 19.8) | 4.6 (3.1 to 6.2) | |
| Ondansetron-cardiac arrhythmias | USA | −4.5 (−11.5 to 2.4) | −1.5 (−3.8 to 0.8) | |
| Pioglitazone-bladder cancer | USA | −107.7 (−112.7 to –102.6) | −29.2 (−30.5 to −27.8) | |
| Quetiapine-metabolic syndrome | UK | −7.6 (−17.5 to 2.2) | −1.8 (−4.2 to 0.5) | |
| Tacrolimus-neoplasm malignant | DK | −6.7 (−7.3 to –6.1) | −18.9 (−20.7 to −17.2) | |
| Testosterone-cardiovascular disorder | CA | −16.9 (−46.8 to 11.8) | −2.3 (−6.2 to 1.6) | |
| Topiramate-congenital anomaly | DK | −3.3 (−6.6, to –0.3) | −2.6 (−5.2 to −0.2) | |
| (b) Advisories with dose-related advice‡ | Citalopram-cardiac arrhythmias | USA | −286 (−1039 to 494) | −0.5 (−1.9 to 0.9) |
| Fluconazole-congenital anomaly | USA | −197 (−276 to –116) | −7.4 (−10.3 to −4.4) | |
| Hydroxyzine-cardiac arrhythmias | UK | −193 (−227 to –159) | −15.2 (−17.9 to −12.5) | |
| Zolpidem-cognitive impairment | USA | 8319 (7617 to 9029) | 19.5 (17.9 to 21.2) |
Created by the authors.
*Unadjusted by change in controls.
†In part (a), the units are monthly prescriptions written or dispensed per 100 000 population, and in part (b) the units are monthly DDDs prescribed or dispensed per 100 000 population.
‡Dose-related advice was defined as advice that revised the recommended or maximum dose of a drug or warned about risk associated with higher doses.
CA, Canada; DDD, defined daily dose; DK, Denmark; PML, progressive multifocal leucoencephalopathy.
Figure 2.
Actual versus predicted percentage change in the rate of prescriptions following drug safety advisories without dose-related advice,* adjusted by change in controls without an advisory. *Actual versus predicted percentage change in the number of prescriptions written or dispensed per 100 000 population during an 11-month period following the month a drug advisory was issued. Created by the authors. IV, inverse variance; PML, progressive multifocal leucoencephalopathy.
Changes in drug utilisation following dose-related advisories
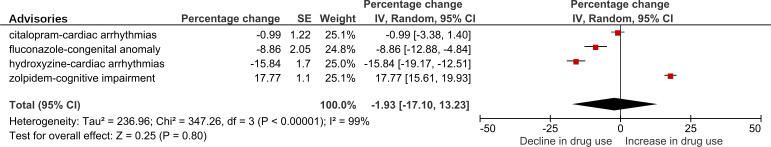
Among dose-related advisories (n=4), the crude actual versus predicted change in the number of DDDs per 100 000 population following the index advisories ranged from a decrease of 15.2% following the hydroxyzine-cardiac arrhythmias advisory to an increase of 19.5% following the zolpidem-cognitive impairment advisory.
Adjusted analyses of actual versus predicted change in the rates of DDDs following dose-related advisories indicated that two of four advisories were followed by a decrease in drug utilisation of >5% (the fluconazole-congenital anomaly and hydroxyzine-cardiac arrhythmias advisories), and one of four advisories was followed by a decrease of >10% (the hydroxyzine-cardiac arrhythmias advisory) (figure 3). In contrast, the zolpidem-cognitive impairment advisory was associated, in the controlled analysis, with an increase in the rate of DDDs dispensed of 17.77% (95% CI 15.61 to 19.93). A post hoc descriptive sensitivity analysis indicated that the zolpidem advisory was followed by a shift towards prescribing lower strengths of the drug (consistent with advice in the advisory), but that the average quantity of medication dispensed rose, apparently explaining the increased rate of DDDs dispensed (online supplemental figure S4).
Figure 3.
Actual versus predicted percentage change in the rate of defined daily doses following dose-related drug safety advisories,* adjusted by change in controls without an advisory. *Actual versus predicted percentage change in the number of defined daily doses prescribed or dispensed per 100 000 population during an 11-month period following the month a drug advisory was issued. Dose-related advisories are those with dose-related advice. Created by the authors. IV, inverse variance.
Meta-analysis of changes in drug utilisation
Average change in drug utilisation following advisories without dose-related advice
Among advisories without dose-related advice, random-effects meta-analysis yielded a crude average change in the number of prescriptions per 100 000 population of −6.03% (95% CI −10.35 to –1.70) (online supplemental figure S1). The actual versus predicted percentage change in drug utilisation following advisories without dose-related advice, adjusted by the change in controls, was heterogeneous (I2=98%) (figure 2). The adjusted average change in the number of prescriptions per 100 000 population following advisories without dose-related advice was −5.83% (95% CI −10.93 to –0.73) (figure 2). In a post hoc sensitivity analysis, the average change in the number of prescriptions per 100 000 population among controls was −0.43% (95% CI −2.11 to 1.26) (online supplemental figure S3).
Average change in drug utilisation following dose-related advisories
Among dose-related advisories, the crude average change in the number of DDDs per 100 000 population was −0.85% (95% CI −15.43 to 13.74) (online supplemental figure S2). The actual versus predicted per cent change in drug utilisation following dose-related advisories, adjusted by the change in controls, varied widely (I2=99%) (figure 3). Analysis of the adjusted average change in drug utilisation following dose-related advisories indicated that dose-related advisories were not associated with a statistically significant change in the number of DDDs per 100 000 population (−1.93%; 95% CI −17.10 to 13.23).
Post hoc subgroup analysis of advisories with versus without prescribing advice
Among 20 advisories without dose-related advice, 5 contained explicit prescribing advice relevant to an immediate prescribing decision and not restricted to a small subgroup as defined above (online supplemental table S6). Several other advisories also contained prescribing advice, but this advice either only applied to patients who had experienced an adverse effect (five advisories) or it was restricted to a small subgroup (two advisories) (online supplemental tables S7 and S8). In our post hoc subgroup analysis, the actual versus predicted percentage change in drug utilisation was −11.13% (95% CI −17.31 to −4.96) following advisories with prescribing advice relevant to immediate prescribing decisions and not limited to a small subgroup and −4.04% (95% CI −10.50 to 2.41) following advisories without such advice (online supplemental figure S5). However, Cochran’s Q test for difference between these subgroups was not statistically significant (p=0.12). A descriptive analysis of assessments of these advisories by two physician reviewers is reported in online supplemental box S1.
Discussion
Summary of findings
Overall, the association of drug safety advisories with changes in drug utilisation was modest but highly variable. Advisories without dose-related advice were associated with a modest, statistically significant decrease in the rate of utilisation. Among a small sample of dose-related advisories, the average association between advisories and DDDs used was not statistically significant. One of the dose-related advisories, concerning zolpidem and cognitive impairment, was associated with an increase in the rate of DDDs dispensed. The presence of explicit prescribing advice relevant to an immediate prescribing decision did not explain the heterogeneity in our meta-analysis of advisories without dose-related advice. Potential sources of the heterogeneity of effects in our analyses include other differences among advisories and populations in the study.
Comparison with other studies
Our finding that advisories have widely varied impacts was consistent with previous systematic reviews of studies of regulatory safety advisories.6 7 9 However, the modest association of advisories with changes in drug utilisation in our study differed from a systematic review by Weatherburn et al, which reported that UK regulatory risk communications were associated with changes in targeted prescribing of 11%–34%.9 This difference between the studies likely relates to differences in selection of risk communications. Many of the studies in systematic review by Weatherburn et al focused on only 4 classes of medication, suggesting that they do not reflect the diversity of drugs which are the subject of regulatory advisories, compared with the 19 classes in our study. In addition, their systematic review focused on published studies and its authors raised the possibility that the published literature could be subject to publication bias. Consequently, the more modest association of advisories with changes in drug utilisation in our study may provide a more realistic assessment of the average effect of advisories.
Weatherburn et al found that risk communications with a recommendation to change practice based on a change or restriction in indication were associated with a larger change in prescribing than those without an explicit recommendation to change practice,9 whereas we did not find a statistically significant difference between advisories with and without prescribing advice, although our exploratory analysis suggested a similar direction of effect. Again, the findings of our study may differ from those of Weatherburn et al due to differences in the risk communications included for analysis. The sample of risk communications by Weatherburn et al with a recommendation to change practice contained multiple risk communications related to major changes or restrictions in indication, such as regulatory communication to restrict the use of selective serotonin reuptake inhibitors among youth.51 In contrast, our sample did not contain advisories relating to major changes in indication with the exception of an advisory limiting use of ketoconazole.41
Varied impact of advisories on drug utilisation
The varied impact of advisories on drug utilisation might relate to several factors. Advisories may differ in content in various ways, including the severity of risks reported, identification of patients at risk, changes to labelling and strength of evidence. Advisories may be sent directly to individual healthcare professionals or communicated as an alert on a regulator’s website. Other factors may differ as well, such as the availability of alternative therapies, the extent of media coverage, repetition of messages in the healthcare community or changes to reimbursement of drugs. It is important to enhance our understanding of factors related to advisories that contribute to changes in drug utilisation, such as advisory content, mode of communication or other considerations.
Strengths and weaknesses of study
Strengths of this study included evaluating advisories related to a wide range of drug classes and applying rigorous methods to estimate the association of advisories with changes in drug utilisation. We selected advisories based on prespecified criteria and used data extracted from administrative health databases rather than from published studies, so our analyses were not subject to publication bias. This study also has limitations. Our data sources for analysing drug utilisation captured drugs prescribed in the UK and drugs dispensed in the other countries included in the study, so our analyses of UK advisories may more closely reflect prescribing behaviour while analyses of advisories in other countries may reflect both prescribing decisions and patient decisions regarding whether to fill a prescription. Neither measure precisely reflects drug use, because even filled prescriptions may not be used by the patient. Our analysis of dose-related advisories was inconclusive, due to a lack of statistical power. In addition, although we used a controlled interrupted time series design to adjust for time-varying confounders, we cannot conclude that our findings were unaffected by factors such as drug promotion, market entry of new drugs or changes to drug reimbursement. It is possible that the choice of controls influenced the estimated postadvisory changes in drug utilisation for some individual advisories. However, it is unlikely that the choice of controls biased our estimate of the average change in drug utilisation following advisories without dose-related advice, as a sensitivity analysis did not find a statistically significant change in drug utilisation among controls.
Our study had certain limitations in scope and generalisability. We limited the scope of our study to drug utilisation outcomes, which omitted important outcomes such as impacts on health monitoring and health outcomes. Our findings may not generalise to all types of drug safety advisories, such as those pertaining to vaccines (which were excluded because we lacked access to reliable data on vaccine use). In addition, this study focused on drugs prescribed or dispensed in a community setting in selected countries, and it is uncertain whether the findings apply in other care settings or countries. Further research is required to investigate direct and contextual factors that contribute to the effectiveness of drug safety advisories. It would also be valuable for future research to investigate the impact of drug safety advisories on patient health outcomes.6 9
Conclusions
Among drug safety advisories issued during 2009–2015 by regulators in Canada, Denmark, the UK and the USA, the association of advisories with changes in drug utilisation was variable and the average association was modest. Future research should investigate factors related to drug safety advisories that contribute to changes in prescribing.
Acknowledgments
We would like to thank Josh A. Levin, MD, (General Practitioner, Victoria, British Columbia) for reviewing the drug safety advisories included in our comparison of advisories with and without prescribing advice, and Ellen Reynolds, MPA, (Research Project Manager, Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia) for her role in project management for this study.
Footnotes
Contributors: CD and RLM take responsibility for the integrity of the data and the accuracy of the data analysis. RLM is the guarantor, accepts full responsibility for the finished work, had access to the data, and controlled the decision to publish. Conception and design: CD, S-AP, BM, AK-C, RL, DM, CH, MLDB, LP, ER, RLM, IS, JL, LB and PCS. Acquisition, analysis or interpretation of data: CD, S-AP, BM, AK-C, RL, DM, CH, MLDB, LP, ER, RLM, IS, JL, LB, PCS, AS, DG, RO-A and LTP. Drafting of the manuscript: RLM. Critical revision of the manuscript: CD, S-AP, BM, AK-C, RL, DM, CH, MLDB, LP, ER, RLM, IS, JL, LB, PCS, AS, DG, RO-A and LTP. Statistical analysis: CD and RLM. Obtaining funding: CD, S-AP, BM, AK, RL, DM, CH, MLDB, LP, ER, IS, JL, LB, PCS, AS and DG. Supervision: CD and BM. Final approval of version to be published: CD, S-AP, BM, AK-C, RL, DM, CH, MLDB, LP, ER, RLM, IS, JL, LB, PCS, AS, DG, RO-A and LPT. All authors agreed to be accountable to all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was funded by grants from the Canadian Institutes of Health Research (PJT-153275) and the Australian Government National Health and Medical Research Council (1122332).
Competing interests: MLDB declares PhD grants from Novo Nordisk, Lundbeck, Ferring Pharmaceuticals and LEO Pharma to the Copenhagen Centre for Regulatory Science; CH declares grants or contracts from Novo Nordisk A/S and H. Lundbeck A/S paid to her institution; BM is acting as an expert witness for Health Canada on a legal case and anticipates future payment for doing so; S-AP declares the Centre for Big Data Research in Health received funding for postmarket surveillance research, unrelated to the current study; LP has received a Michael Smith Foundation for Health Research Reach Grant; AS declares grants or contracts from Arnold Ventures and the FDA paid to his institution, consulting fees from West Health and payment for expert testimony from the ACLU; IS has received a CIHR Canadian Network for Observational Drug Effect Studies grant and a Drug Evaluation Alliance of Nova Scotia grant, and payment for serving as a member of the Patented Medicine Prices Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. We are unable to share data used for this study due to a lack of data permissions for this purpose.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf 2015;38:437–53. 10.1007/s40264-015-0281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Downing NS, Shah ND, Aminawung JA, et al. Postmarket safety events among novel therapeutics Approved by the US food and drug administration between 2001 and 2010. JAMA 2017;317:1854–63. 10.1001/jama.2017.5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lexchin J. New drugs and safety: what happened to new active substances Approved in Canada between 1995 and 2010? Arch Intern Med 2012;172:1680–1. 10.1001/archinternmed.2012.4444 [DOI] [PubMed] [Google Scholar]
- 4. Mol PGM, Arnardottir AH, Motola D, et al. Post-approval safety issues with innovative drugs: a European cohort study. Drug Saf 2013;36:1105–15. 10.1007/s40264-013-0094-y [DOI] [PubMed] [Google Scholar]
- 5. Perry LT, Bhasale A, Fabbri A, et al. A descriptive analysis of medicines safety advisories issued by national medicines regulators in Australia, Canada, the United Kingdom and the United States - 2007 to 2016. Pharmacoepidemiol Drug Saf 2020;29:1054–63. 10.1002/pds.5072 [DOI] [PubMed] [Google Scholar]
- 6. Dusetzina SB, Higashi AS, Dorsey ER, et al. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care 2012;50:466–78. 10.1097/MLR.0b013e318245a160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Georgi U, Lämmel J, Datzmann T, et al. Do drug-related safety warnings have the expected impact on drug therapy? A systematic review. Pharmacoepidemiol Drug Saf 2020;29:229–51. 10.1002/pds.4968 [DOI] [PubMed] [Google Scholar]
- 8. Piening S, Haaijer-Ruskamp FM, de Vries JTN, et al. Impact of safety-related regulatory action on clinical practice: a systematic review. Drug Saf 2012;35:373–85. 10.2165/11599100-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9. Weatherburn CJ, Guthrie B, Dreischulte T, et al. Impact of medicines regulatory risk communications in the UK on prescribing and clinical outcomes: systematic review, time series analysis and meta-analysis. Br J Clin Pharmacol 2020;86:698–710. 10.1111/bcp.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goedecke T, Morales DR, Pacurariu A, et al. Measuring the impact of medicines regulatory interventions - Systematic review and methodological considerations. Br J Clin Pharmacol 2018;84:419–33. 10.1111/bcp.13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gridchyna I, Cloutier A-M, Nkeng L, et al. Methodological gaps in the assessment of risk minimization interventions: a systematic review. Pharmacoepidemiol Drug Saf 2014;23:572–9. 10.1002/pds.3596 [DOI] [PubMed] [Google Scholar]
- 12. Briesacher BA, Soumerai SB, Zhang F, et al. A critical review of methods to evaluate the impact of FDA regulatory actions. Pharmacoepidemiol Drug Saf 2013;22:986–94. 10.1002/pds.3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13:S38–44. 10.1016/j.acap.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 14. Deeks JJ, Higgins JPT, Altman DG, Group obotCSM . Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for systematic reviews of interventions: cochrane, 2020. [Google Scholar]
- 15. Højer M-MG, De Bruin ML, Boskovic A, et al. Are monitoring instructions provided in direct healthcare professional communications (DHPCs) of sufficient quality? A retrospective analysis of DHPCs sent out between 2007 and 2018. BMJ Open 2020;10:e036498. 10.1136/bmjopen-2019-036498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The CIHI data quality assessment framework, 2009. Available: www.cihi.ca/sites/default/files/data_quality_framework_2009_en_0.pdf [Accessed 24 Nov 2020].
- 17. Kontopantelis E, Stevens RJ, Helms PJ, et al. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross-sectional population study. BMJ Open 2018;8:e020738. 10.1136/bmjopen-2017-020738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansen L. IBM MarketScan research databases for life sciences researchers. Armonk, NY: IBM Watson Health, 2018. [Google Scholar]
- 19. Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017;46:798–798f. 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Department of Health . Pharmaceutical benefits scheme: PBS and RPBS section 85 date of supply data. Available: https://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop [Accessed 18 Dec 2018].
- 21. ATC/DDD Index 2020 . Norwegian Institute of public health, 2020. Available: https://www.whocc.no/atc_ddd_index/ [Accessed 13 Nov 2020].
- 22. Barnett AG, Dobson AJ. Analysing seasonal health data. statistics for biology and health. Berlin: Springer-Verlag, 2010. [Google Scholar]
- 23. The AUTOREG Procedure . SAS/ETS(R) 93 User’s Guide [Internet]. Cary, NC: SAS Institute Inc, 2011. Available: https://support.sas.com/documentation/cdl/en/etsug/63939/HTML/default/viewer.htm#etsug_autoreg_sect005.htm [Accessed 13 Nov 2020].
- 24. Efron B. The jackknife, the bootstrap and other resampling plans. Philadelphia, PA: Society for Industrial and Applied Mathematics, 1982. [Google Scholar]
- 25. Efron B, Tibshirani R. An introduction to the bootstrap. Raton, FL: Chapman & Hall/CRC, 1994. [Google Scholar]
- 26. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. A. Menarini Pharma UK . Direct healthcare professional communication on the association of the risk of serious hypersensitivity reactions, including Stevens-Johnson syndrome and acute anaphylactic reaction/shock, with Adenuric (febuxostat). Available: https://webarchive.nationalarchives.gov.uk/20141206113928/http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con152835.pdf [Accessed 13 Nov 2020].
- 28. Astellas Pharma . Vigtige anbefalinger for hensigtsmæssig brug af Protopic (tacrolimus) (0,03 % og 0,1%) salve for at reducere 0risici [Important recommendations for the appropriate use of Protopic (tacrolimus) (0.03% and 0.1%) ointment to reduce risks], May 1, 2012. Accessed March 10, 2017 via information request to Danish Medicines Agency (DKMA).
- 29. AstraZeneca UK. Re: quetiapine and quetiapine prolonged release summary product characteristics update, 2011. Available: http://webarchive.nationalarchives.gov.uk/20150113113600/http://www.MHRA.gov.uk/home/groups/pl-p/documents/websiteresources/con140610.pdf [Accessed 13 Nov 2020].
- 30. Health Canada . Safety information for antipsychotic drug Abilify and risk of certain impulse-control behaviours, 2015. Available: https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2015/55668a-eng.php [Accessed 13 Nov 2020].
- 31. Health Canada . Information Update - Possible cardiovascular problems associated with testosterone products, 2014. Available: https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/40587a-eng.php [Accessed 13 Nov 2020].
- 32. Hoffmann-La Roche . Association of Accutane Roche (isotretinoin) with cases of severe skin reactions, 2010. Available: https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2010/14584a-eng.php [Accessed 13 Nov 2020].
- 33. Hoffmann-La Roche . Direct healthcare professional communication on the association of CellCept (mycophenolate mofetil) with pure red cell aplasia, 2009. Available: http://webarchive.nationalarchives.gov.uk/20141205150130/http://www.MHRA.gov.uk/home/groups/pl-p/documents/websiteresources/con049433.pdf [Accessed 13 Nov 2020].
- 34. Medicines and Healthcare products Regulatory Agency . Finasteride: potential risk of male breast cancer, 2009. Available: https://www.gov.uk/drug-safety-update/finasteride-potential-risk-of-male-breast-cancer [Accessed 13 Nov 2020].
- 35. Medicines and Healthcare products Regulatory Agency . Hydroxyzine (Atarax, Ucerax): risk of QT interval prolongation and torsade de pointes, 2015. Available: https://www.gov.uk/drug-safety-update/hydroxyzine-atarax-ucerax-risk-of-qt-interval-prolongation-and-torsade-de-pointes [Accessed 13 Nov 2020].
- 36. Medicines and Healthcare products Regulatory Agency . Nitrofurantoin: reminder on precautions for use, especially renal impairment in (elderly) patients. Drug Safety Update 2013;7:A3. [Google Scholar]
- 37. Ortho-McNeil Neurologics . Topamax (topiramate) change to pregnancy category D, March 1, 2011. accessed March 10, 2017 via information Request to Danish medicines Agency (DKMA).
- 38. Sanofi-aventis Denmark . Direkte sikkerhedsinformation til sundhedspersonale om sammenhængen mellem brugen af clopidogrel og erhvervet hæmofili [Direct safety information for healthcare professionals on the association between clopidogrel use and acquired haemophilia], 2013. Available: https://laegemiddelstyrelsen.dk/en/search/~/media/E9D1FAB54FA34B4FAC43291E966111F7.ashx [Accessed 13 Nov 2020].
- 39. United States Food and Drug Administration . FDA warns of rare risk of long-lasting erections in males taking methylphenidate ADHD medications and has Approved label changes, 2013. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-rare-risk-long-lasting-erections-males-taking [Accessed 13 Nov 2020].
- 40. United States Food and Drug Administration . FDA investigating rare brain infection in patient taking Gilenya (fingolimod), 2013. Available: http://wayback.archive-it.org/7993/20170112031628/http:/www.fda.gov/Drugs/DrugSafety/ucm366529.htm [Accessed 13 Nov 2020].
- 41. United States Food and Drug Administration . FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems, 2013. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-limits-usage-nizoral-ketoconazole-oral-tablets-due-potentially [Accessed 13 Nov 2020].
- 42. United States Food and Drug Administration . FDA approves label changes to include intestinal problems (sprue-like enteropathy) linked to blood pressure medicine olmesartan medoxomil, 2013. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-include-intestinal-problems-sprue [Accessed 13 Nov 2020].
- 43. United States Food and Drug Administration . Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms, 2013. Available: https://www.fda.gov/Drugs/DrugSafety/ucm341822.htm [Accessed 13 Nov 2020].
- 44. United States Food and Drug Administration . Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien Cr, Edluar, and Zolpimist), 2013. Available: http://wayback.archive-it.org/7993/20170111080036/http:/www.fda.gov/Drugs/DrugSafety/ucm334033.htm [Accessed 13 Nov 2020].
- 45. United States Food and Drug Administration . Abnormal heart rhythms may be associated with use of Zofran (ondansetron), 2011. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-abnormal-heart-rhythms-may-be-associated-use-zofran-ondansetron [Accessed 13 Nov 2020].
- 46. United States Food and Drug Administration . Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide), 2011. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-abnormal-heart-rhythms-associated-high-doses-celexa-citalopram [Accessed 13 Nov 2020].
- 47. United States Food and Drug Administration . Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants, 2011. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be [Accessed 13 Nov 2020].
- 48. United States Food and Drug Administration . Update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer, 2011. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-update-ongoing-safety-review-actos-pioglitazone-and-increased-risk [Accessed 13 Nov 2020].
- 49. United States Food and Drug Administration . New boxed warning for severe liver injury with arthritis drug Arava (leflunomide), 2010. Available: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-new-boxed-warning-severe-liver-injury-arthritis-drug-arava-leflunomide [Accessed 13 Nov 2020].
- 50. United States Food and Drug Administration . Early communication about safety of Lantus (insulin glargine), 2009. Available: http://wayback.archive-it.org/7993/20170112033108/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169722.htm [Accessed 13 Nov 2020].
- 51. Wheeler BW, Gunnell D, Metcalfe C, et al. The population impact on incidence of suicide and non-fatal self harm of regulatory action against the use of selective serotonin reuptake inhibitors in under 18S in the United Kingdom: ecological study. BMJ 2008;336:542–5. 10.1136/bmj.39462.375613.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2021-013910supp001.pdf (917.4KB, pdf)
Data Availability Statement
No data are available. We are unable to share data used for this study due to a lack of data permissions for this purpose.