Abstract
Introduction
Bronchiectasis is an increasingly common chronic inflammatory airway disease. We evaluated secondary safety outcomes in a comparative effectiveness study of chronic inhaled corticosteroids (ICS) and macrolide monotherapy in bronchiectasis patients.
Methods
We conducted a retrospective study using US Medicare Parts A, B and D (but not C) 2006–2014 datasets. Among those with a pulmonologist-associated bronchiectasis claim (ICD-9-CM 494.0 or 494.1), without cystic fibrosis, we identified the first new use of either chronic (>28 days) ICS or macrolide monotherapy. For each drug exposure, we calculated crude incidence rates of the secondary safety outcomes: arrhythmia, myocardial infarction, sensorineural hearing loss, hip fracture and opportunistic infections. We calculated a propensity score (PS) for ICS use using demographic, clinical and utilisation characteristics and compared risks of macrolides versus ICS for each outcome using PS decile-adjusted Cox regression models.
Results
Of 285 043 Medicare patients with bronchiectasis, we identified 6500 (2%) macrolide and 83 589 (29%) ICS new users. Key covariates were balanced across exposure groups within decile. Myocardial infarction, hip fracture and opportunistic infection were not significantly associated with treatment. Macrolides were associated with a decreased risk of arrhythmia (adjusted hazard ratio (aHR) 0.87, 95% CI 0.80–0.94) and an increased risk of sensorineural hearing loss (aHR 1.38, 95% CI 1.56–1.22) compared to ICS.
Conclusions
Macrolides were not associated with an elevated risk of acute cardiac events compared to ICS. The increased risk of hearing loss in macrolide users compared to ICS users in older bronchiectasis patients should be balanced against known benefits of macrolides.
Short abstract
Comparison of risks of cardiac outcomes (arrhythmia and myocardial infarction), hearing loss, opportunistic infections and hip fracture between macrolide and ICS users with bronchiectasis using a robust propensity-score adjusted new-user methodology https://bit.ly/3KIVp0O
Introduction
Bronchiectasis is a chronic inflammatory airway disease associated with chronic cough and acute infectious exacerbations. Current treatment strategies focus on airway clearance and anti-inflammatory medications including oral or inhaled corticosteroids (ICS) and oral or inhaled antibiotics. Very little data exist to guide treatment decisions, though several expert-based guidelines that describe pharmacotherapy options have been published [1–3]. These guidelines recommend the chronic use of macrolides to prevent exacerbations in the population of patients with a history of exacerbations. However, they do not recommend ICS to treat bronchiectasis due to a lack of evidence supporting their use unless asthma or other indication for use of ICS is present.
In three small placebo-controlled randomised trials, 6 or 12 months of azithromycin [4, 5] and 12 months of erythromycin [6] decreased the rate of exacerbations in bronchiectasis patients. However, the risks of chronic antibiotic therapy for older patients with bronchiectasis are not clear. Azithromycin use has been linked to sudden cardiac death in population-based studies [7], and it is known to cause QT prolongation with potential for causing other cardiac arrhythmias, particularly when used with other drugs that also cause QT prolongation [8]. Several meta-analyses of cardiovascular risks of macrolides in comparison to other medication classes have produced inconsistent results [9–12]. Further, macrolides have also been associated with ototoxicity [13, 14].
We have previously reported an increased risk of hospitalised respiratory infection associated with ICS compared to macrolide monotherapy in US Centers for Medicare and Medicaid Services (Medicare) enrollees [15]. Oral corticosteroids have been shown to increase the risk of opportunistic infections due to mycobacteria, fungi and other pathogens in patients with rheumatoid arthritis [16]. In addition, long-term ICS use has been associated with bone loss in asthmatic and COPD patients, and bone loss is a known risk of systemic oral corticosteroids [17, 18]. If there is a risk of either opportunistic infections or bone loss with ICS use beyond acute use, it would likely be attenuated compared to systemic oral corticosteroids.
Despite the reported efficacy of chronic macrolide monotherapy, ICS are still commonly prescribed [19]. Accordingly, we sought to provide evidence of relative risks and benefits of ICS and macrolide monotherapy in older Medicare enrollees with bronchiectasis. We evaluated the comparative risks of acute cardiac events (arrhythmia and myocardial infarction) and sensorineural hearing loss that may be increased with macrolide use, and hip fracture and opportunistic infection that may be increased with ICS use.
Methods
Study design
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI), which specifically supports comparative effectiveness studies with an active comparator that is commonly used in practice. Our previously reported primary analysis was a comparison of ICS and azithromycin where we hypothesised that ICS increases the risk of respiratory infections compared to macrolide monotherapy [15]. As part of the study design, we considered other potential risks of each therapy as secondary safety outcomes, identified by our literature review and patient and clinical stakeholder input. These analyses are presented in the current manuscript.
Cohort assembly
As previously reported [15], we obtained data from the Center for Medicare and Medicaid Services (Medicare) enrollees in Part A (hospital), B (outpatient) and D (prescription drug coverage) (but not C, Medicare Advantage) during 2006–2014. The cohort of patients was defined as those given a bronchiectasis ICD-9-CM code (494.0 or 494.1) from a pulmonologist, excluding those with a cystic fibrosis diagnosis. Within this cohort, we identified new users of chronic ICS or macrolide (erythromycin or azithromycin) monotherapy, defined as the first ≥28-day prescription for either medication group after a 12-month clean period. Macrolide monotherapy was restricted to those with no potential nontuberculous mycobacteria (NTM)-related therapy (ethambutol, a rifamycin or a fluoroquinolone). Patients who initiated both medication groups within the first 28 days were excluded.
Exposure and follow-up
The treatment episode began on the date of first prescription of either drug exposure group and ended at the occurrence of the outcome of interest, loss of medical and pharmacy coverage, death, the end of 2014 or end of drug exposure plus a 30-day extension. Patients were censored at the time of prescription if they added or switched to the comparison drug class.
Outcomes
The safety outcomes described in this analysis included secondary outcomes identified by our clinical experts and patient advisory panel. We evaluated the comparative risks of arrhythmia, myocardial infarction, hip fracture, opportunistic infection (excluding NTM and respiratory infections) and sensorineural hearing loss. Table 1 details the algorithms for each of the outcomes. Non-opportunistic respiratory infections in those with bronchiectasis are typically bacterial or viral. The previous publication evaluated the differential risk of respiratory infections between the ICS and macrolide users, so they were not included in the present analysis [15].
TABLE 1.
Outcome definitions for acute exacerbation, arrhythmia, myocardial infarction, hip fracture, opportunistic infection and sensorineural hearing loss
| Outcome | Algorithm/ICD-9-CM principal diagnostic codes | Reference |
| Arrhythmia | ICD-9-CM 427.x (principal diagnosis) | Tamariz 2012 [44] |
| Myocardial infarction | ICD-9-CM 410.x1 | Kiyota 2004 [45] |
| Hip fracture | ICD-9-CM 820.0x or 820.20 or 820.21 or 820.22 or 820.8 or 821.0x | Narongroeknawin 2012 [46] |
| Opportunistic infection | Any of the following diagnoses: | |
| Tuberculosis | ICD-9-CM 010-018 and pharmacy records indicating prescription for pyrazinamide prescribed on same day within ±90 days of first code date | Schneeweiss 2007 [47] |
| Histoplasmosis | ICD-9-CM 115 with cumulative supply of >30 days of any of the following three drugs: fluconazole, itraconazole or voriconazole given within ±90 days of first code date | Schneeweiss 2007 [47] |
| Blastomycosis | ICD-9-CM 116.0 with cumulative supply of >30 days of any of the following three drugs: fluconazole, itraconazole or voriconazole given within ±90 days of first code date | Schneeweiss 2007 [47] |
| Coccidioidomycosis | ICD-9-CM 114 with cumulative supply of >30 days of any of the following three drugs: fluconazole, itraconazole or voriconazole given within ±90 days of first code date | Schneeweiss 2007 [47] |
| Cryptococcosis | ICD-9-CM 117.5 with cumulative supply of >30 days of any of the following three drugs: fluconazole, itraconazole or voriconazole given within ±90 days of first code date | Schneeweiss 2007 [47] |
| Endemic mycosis (nonspecific outcome of all endemic fungi above) | ICD-9-CM 484.7 with cumulative supply of >30 days of any of the following three drugs: fluconazole, itraconazole or voriconazole given within ±90 days of first code date | Schneeweiss 2007 [47] |
| Nocardiosis/actinomycosis | ICD-9-CM 039 | Schneeweiss 2007 [47] |
| Listeriosis | ICD-9-CM 027.0 | Schneeweiss 2007 [47] |
| Toxoplasmosis | ICD-9-CM 130 | Schneeweiss 2007 [47] |
| Pneumocystis | ICD-9-CM 136.3 | Schneeweiss 2007 [47] |
| Legionellosis | ICD-9-CM 482.84 | Schneeweiss 2007 [47] |
| Salmonellosis | ICD-9-CM 003.1, 003.2x | Schneeweiss 2007 [47] |
| Aspergillosis | ICD-9-CM 117.3 or 484.6 with any outpatient prescription for voriconazole, itraconazole, posaconazole given within ±90 days of first code date | Schneeweiss 2007 [47] Baddley 2014 [36] |
| Zoster | ICD-9-CM 053 (outpatient or inpatient code) with any outpatient prescription for acyclovir, famciclovir or valacyclovir given within ±90 days of first code date | Jumaan 2005 [48] Mullooly 2005 [49] |
| Sensorineural hearing loss | ICD-9-CM 389.10 or 389.11 or 389.12 or 389.14 or 389.18 or 389.20 or 389.22 | Not validated |
ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification.
Study covariates
Baseline covariates listed in table 2 included demographics at therapy start (age, sex, race/ethnicity, region of residence, rural or urban residence, nursing home residence, median area income, calendar year), any history of lung or immunosuppressive comorbidities (allergic bronchopulmonary aspergillosis, α-1 antitrypsin deficiency, asthma, COPD or emphysema, lung cancer, NTM history, primary ciliary dyskinesia, primary immune deficiency, Pseudomonas spp. infection, silicosis), Charlson comorbidity index (prior 12 months), and markers of disease severity and healthcare utilisation during the prior 12 months (number of office visits, physician encounters, pulmonologist visits, inpatient admissions, hospitalised respiratory infections, acute respiratory infections, distinct medication classes, nebuliser prescription and home oxygen prescription). Mean prednisone equivalent dose categories for the prior 6 months were categorised as none, low (<2.5 mg·day−1), medium-low (2.5–5 mg·day−1), medium-high (5–10 mg·day−1) and high (10+ mg·day−1).
TABLE 2.
Characteristics of US Medicare bronchiectasis new users of inhaled corticosteroids or macrolide monotherapy
| Variable | Inhaled steroid therapy | Macrolide monotherapy |
| Subjects n | 83 589 | 6500 |
| Demographics at therapy start | ||
| Age (mean±sd) | 74.42±10.18 | 74.82±10.11 |
| Year of therapy start | ||
| 2006 | 7326 (8.8) | 429 (6.6) |
| 2007 | 6374 (7.6) | 339 (5.2) |
| 2008 | 6753 (8.1) | 483 (7.4) |
| 2009 | 7949 (9.5) | 523 (8.0) |
| 2010 | 8260 (9.9) | 570 (8.8) |
| 2011 | 8976 (10.7) | 782 (12.0) |
| 2012 | 10 721 (12.8) | 978 (15.0) |
| 2013 | 15 991 (19.1) | 1275 (19.6) |
| 2014 | 11 239 (13.4) | 1121 (17.2) |
| Female sex | 56 583 (67.7) | 4750 (73.1) |
| Race/ethnicity | ||
| American Indian or Alaska native | 362 (0.4) | 17 (0.3) |
| Asian/Pacific Islander | 3353 (4.0) | 160 (2.5) |
| Black or African-American | 5338 (6.4) | 236 (3.6) |
| Hispanic | 5188 (6.2) | 203 (3.1) |
| White (non-Hispanic) | 68 508 (82.0) | 5820 (89.5) |
| Other/unknown | 840 (1.0) | 64 (1.0) |
| Region | ||
| Midwest | 17 129 (20.5) | 1612 (24.8) |
| Northeast | 18 629 (22.3) | 992 (15.3) |
| South | 33 289 (39.8) | 2897 (44.6) |
| West | 14 542 (17.4) | 999 (15.4) |
| Metropolitan residence | 65 261 (78.1) | 4798 (73.8) |
| Median household income USD (mean±sd) | 59 210±25 500 | 59 490±25 220 |
| Nursing home residence | 8408 (10.1) | 470 (7.2) |
| Comorbidities (any history) | ||
| Allergic bronchopulmonary aspergillosis | 854 (1.0) | 64 (1.0) |
| α-1 antitrypsin deficiency | 292 (0.3) | 43 (0.7) |
| Asthma | 33 480 (40.1) | 1795 (27.6) |
| COPD/emphysema | 70 548 (84.4) | 5050 (77.7) |
| Interstitial lung disease | 5526 (6.6) | 507 (7.8) |
| Lung cancer | 3497 (4.2) | 196 (3.0) |
| NTM history# | 3164 (3.8) | 1307 (20.1) |
| Primary ciliary dyskinesia | 141 (0.2) | 23 (0.4) |
| Primary immune deficiency | 3857 (4.6) | 466 (7.2) |
| Pseudomonas infection | 5123 (6.1) | 810 (12.5) |
| Silicosis | 103 (0.1) | 6 (0.1) |
| Charlson comorbidity index (prior 12 months) | ||
| 0 | 20 514 (24.5) | 1534 (23.6) |
| 1 | 33 959 (40.6) | 3119 (48.0) |
| 2+ | 29 116 (34.8) | 1847 (28.4) |
| Medication and healthcare utilisation (prior 12 months) | ||
| Office visit (outpatient) | 81 073 (97.0) | 6365 (97.9) |
| Physician encounters | ||
| 0–7 | 24 345 (29.1) | 1482 (22.8) |
| 8–12 | 20 027 (24.0) | 1526 (23.5) |
| 13–19 | 20 549 (24.6) | 1771 (27.2) |
| 20+ | 18 668 (22.3) | 1721 (26.5) |
| Pulmonologist encounters | ||
| 0 | 19 013 (22.7) | 692 (10.6) |
| 1 | 15 157 (18.1) | 941 (14.5) |
| 2 | 15 437 (18.5) | 1032 (15.9) |
| 3 | 11 025 (13.2) | 1005 (15.5) |
| 4 | 7232 (8.7) | 724 (11.1) |
| 5+ | 15 725 (18.8) | 2106 (32.4) |
| Inpatient admissions | ||
| 1 | 17 943 (21.5) | 1299 (20.0) |
| 2+ | 15 851 (19.0) | 1093 (16.8) |
| 1+ hospitalised respiratory infections | 9583 (11.5) | 885 (13.6) |
| Number of acute respiratory infections | ||
| 0 | 40 746 (48.7) | 2478 (38.1) |
| 1 | 20 193 (24.2) | 1384 (21.3) |
| 2–3 | 15 993 (19.1) | 1499 (23.1) |
| 4+ | 6657 (8.0) | 1139 (17.5) |
| Distinct medication classes | ||
| 1–8 | 24 768 (29.6) | 2169 (33.4) |
| 9–12 | 18 871 (22.6) | 1540 (23.7) |
| 13–17 | 19 182 (22.9) | 1478 (22.7) |
| 18+ | 20 768 (24.8) | 1313 (20.2) |
| Mean prednisone equivalent dose category | ||
| No oral corticosteroid | 50 227 (60.1) | 4077 (62.7) |
| Low (<2.5 mg·day−1) | 25 572 (30.6) | 1614 (24.8) |
| Medium–low (2.5–5 mg·day−1) | 4226 (5.1) | 358 (5.5) |
| Medium–high (5–10 mg·day−1) | 2739 (3.3) | 343 (5.3) |
| High (10+ mg·day−1) | 825 (1.0) | 108 (1.7) |
| Nebuliser | 31 502 (37.7) | 2681 (41.2) |
| Home oxygen | 26 810 (32.1) | 1934 (29.8) |
Data are presented as n (%) unless otherwise indicated. Bold indicates standardised mean difference >0.10 (considered significant). NTM: nontuberculous mycobacteria. #: no distinction is made between active and past infections, given lack of culture results. Adapted from Henkle et al. [15].
Statistical analysis
We included baseline covariates in a propensity-score (PS) logistic regression model to estimate the probability a patient receives therapy with ICS (versus macrolide therapy). The two groups were compared with differences in standardised mean difference (SMD) >0.1 considered significant. Visual inspection showed substantial overlap in the distribution of predicted probabilities, and non-overlapping regions between exposure groups were trimmed [20]. We assessed the association between exposure and outcomes using Cox proportional hazard regression models, adjusted for PS decile category and oral corticosteroid use category, a pre-specified potential confounder [21]. We defined a priori that a >25% increase (hazard ratio (HR) of 1.25) or decrease (HR of 0.80) is a clinically meaningful risk of a given outcome. Sensitivity analysis consisted of the following alternate outcome algorithms: excluding patients with a history of arrhythmia or sensorineural hearing loss from their respective analyses, stratifying hip fractures by prior osteoporosis diagnosis, and including tinnitus and unilateral hearing loss in the sensorineural hearing loss algorithm. Additional sensitivity analyses were conducted for arrhythmia: 1) adjusting for drugs associated with QT prolongation taken at treatment start and 2) stratified long-acting β-agonists (LABAs)/ICS and ICS alone. All analyses were done in SAS 9.4 (SAS Institute, Cary, NC, USA). The Oregon Health and Science University and University of Alabama at Birmingham institutional review boards approved the study protocol.
Results
Out of 285 043 eligible bronchiectasis patients, we identified 83 589 (29.3%) chronic ICS and 6500 (2.3%) chronic macrolide monotherapy new users. Most (58 179; 69.6%) ICS users were taking ICS in combination with LABAs. Patients were a mean age of 74 years in both groups (SMD 0.04); 73% female and 89% White taking macrolide monotherapy; and 67% female and 82% White taking ICS (SMD 0.12 and 0.23, respectively). After trimming tails on the PS, there were 83 495 patients remaining in the ICS group and 6498 in the macrolide monotherapy group. Grouping by ICS dose category, 20 623 (24.7%) started on high doses, 11 925 (14.3%) on medium doses and 51 020 (61.1%) on low doses. The median duration of drug exposure was similar across outcomes (60–73 days for ICS, 95–109 days for macrolide monotherapy) (table 3). Overall 92% of macrolide use was azithromycin, increasing from 81% in 2006 to 91% in 2010 and stabilising at 95% in 2013–2014. Patients in each exposure group differed, but these characteristics were balanced within PS deciles, with the exception of NTM history, which was included as a covariate in the final model [15]. Each of the outcomes is described below, with table 4 reporting crude incidence rates by exposure cohort and figure 1 showing unadjusted and adjusted hazard ratios (aHRs).
TABLE 3.
Median, quartile 1 (Q1) and quartile 3 (Q3) duration of exposure (days prescribed +30 days) by treatment exposure group for each included outcome
| Outcome | Treatment exposure | Median | Q1 | Q3 |
| Arrhythmia | Inhaled corticosteroids | 60 | 60 | 123 |
| Macrolide monotherapy | 95 | 60 | 201 | |
| Myocardial infarction | Inhaled corticosteroids | 73 | 60 | 143 |
| Macrolide monotherapy | 109 | 60 | 228 | |
| Hip fracture | Inhaled corticosteroids | 72 | 60 | 143 |
| Macrolide monotherapy | 109 | 60 | 228 | |
| Opportunistic infection | Inhaled corticosteroids | 69 | 60 | 142 |
| Macrolide monotherapy | 107 | 60 | 220 | |
| Sensorineural hearing loss | Inhaled corticosteroids | 63 | 60 | 138 |
| Macrolide monotherapy | 101 | 60 | 208 |
TABLE 4.
Incident rates of arrhythmia, myocardial infarction, hip fracture, opportunistic infection and sensorineural hearing loss among new users of inhaled corticosteroids and macrolide monotherapy with bronchiectasis
| Outcome | Inhaled corticosteroid | Macrolide monotherapy | ||||
| Events | Patient-years | Incidence rate # | Events | Patient-years | Incidence rate # | |
| Arrhythmia | 9508 | 31 395 | 30.3 (29.7–30.9) | 728 | 2855 | 25.5 (23.7–27.4) |
| Myocardial infarction | 892 | 34 688 | 2.6 (2.4–2.8) | 81 | 3171 | 2.6 (2.0–3.2) |
| Hip fracture | 919 | 34 661 | 2.7 (2.5–2.8) | 77 | 3173 | 2.4 (1.9–3.0) |
| Opportunistic infection | 1585 | 34 200 | 4.6 (4.4–4.9) | 180 | 3113 | 5.8 (5.0–6.7) |
| Sensorineural hearing loss | 2574 | 33 491 | 7.7 (7.4–8.0) | 347 | 2993 | 11.6 (10.4–12.9) |
#: incidence rate per 100 patient-years (95% confidence interval).
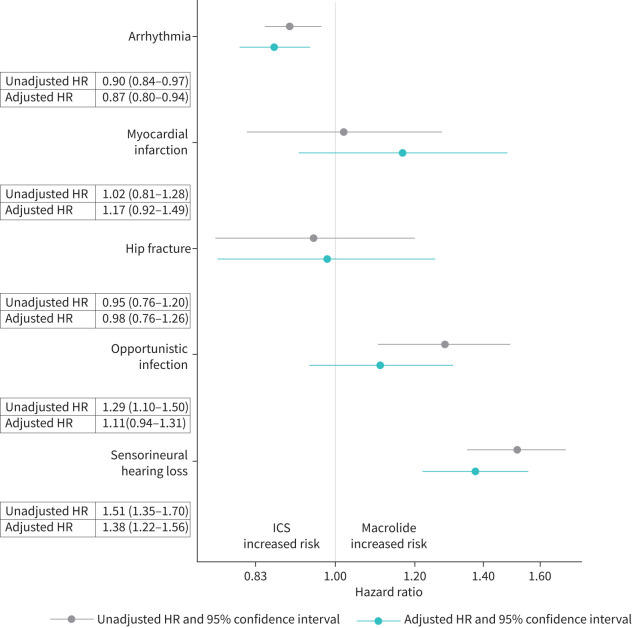
FIGURE 1.
Forest plot of unadjusted and adjusted secondary safety outcomes hazard ratios comparing new users of inhaled corticosteroids with macrolide monotherapy for bronchiectasis. Adjusted hazard ratio (HR) included propensity score decile, oral corticosteroid dose category and nontuberculous mycobacteria (NTM) history.
Arrhythmia and myocardial infarction
Arrhythmia was the most common evaluated outcome, with a crude incidence of 25.5 (95% CI 23.7–27.4) per 100 person-years in the macrolide monotherapy cohort and 30.3 (95% CI 29.7–30.9) per 100 person-years in the ICS cohort. The majority of arrhythmia diagnoses (78%) were atrial fibrillation (ICD-9-CM 427.31). The crude incidence rates for myocardial infarction were 10-fold less than for arrhythmia. Macrolide use was not statistically associated with a higher risk of myocardial infarction (aHR 1.17, 95% CI 0.92–1.49), but was associated with a statistically decreased risk of arrhythmia (aHR 0.87, 95% CI 0.80–0.94) compared to ICS. The hazard ratio was similar when patients with a history of arrhythmia were excluded (aHR 0.79, 95% CI 0.69–0.89). The aHR of arrhythmia was unchanged after adjusting for the use of drugs that may prolong QT interval.
Hip fracture
Hip fracture occurred with a crude incidence of 2.4 (95% CI 1.9–3.0) per 100 person-years in the macrolide monotherapy cohort and 2.7 (95% CI 2.5–2.8) per 100 person-years in the ICS cohort. There was no difference in hip fracture (aHR 0.98, 95% CI 0.76–1.26) between the two exposure groups. Stratification by osteoporosis produced similar adjusted HRs in each comparison group (aHR 0.94, 95% CI 0.69–1.30 with a history of osteoporosis, aHR 1.03, 95% CI 0.67–1.59 with no history of osteoporosis).
Opportunistic infection
Opportunistic infection occurred with a crude incidence rate of around 5 per 100 person-years in each cohort. The most common diagnoses were zoster (51%), candidiasis (16%) and aspergillosis (10%). Although the unadjusted HR suggested an increase in risk (HR 1.29, 95% CI 1.10–1.50), after adjustment there was no difference in opportunistic infection (aHR 1.11, 95% CI 0.94–1.08) comparing chronic macrolide monotherapy to ICS use.
Sensorineural hearing loss
The crude incidence rate of sensorineural hearing loss was 11.6 (95% CI 10.4–12.9) and 7.7 (95% CI 7.4–8.0) per 100 person-years in the macrolide monotherapy and ICS cohorts, respectively. The aHR suggested an increased hazard for macrolide monotherapy compared to ICS, 1.38 (95% CI 1.22–1.56). Sensitivity analyses that excluded patients with a history of hearing loss (aHR 1.33, 95% CI 1.16–1.54) and expanded the definition to include unilateral hearing loss and tinnitus (aHR 1.35, 95% CI 1.22–1.52) produced similar results.
Discussion
Overall, adverse safety outcomes occurred at rates ranging from 2.5/100 person-years (myocardial infarction and hip fracture) to just over 25/100 person-years (arrhythmia) in older bronchiectasis patients treated with macrolides and ICS. In our study, chronic macrolide use among Medicare patients was associated with a decreased risk of arrhythmia, and not associated with a statistically significant increased risk of myocardial infarction when compared to ICS. However, chronic macrolide monotherapy was associated with a statistically significant, moderately increased risk of hearing loss compared to ICS. Other safety outcomes were similar in the PS-adjusted analysis.
Our study evaluated two cardiac outcomes, arrhythmia and myocardial infarction, which are potential risks of azithromycin. Macrolides can also induce QT prolongation, particularly when used with drugs that cause QT prolongation or in patients with conditions associated with QT prolongation [8]. QT prolongation is associated with torsade de pointes and sudden cardiac death [22]. In 2012, near the end of our observation period, Ray et al. [7] linked azithromycin use to sudden cardiac death during the first 1–5 days of use in a population-based study. However, multiple subsequent studies of varying cardiac outcomes 1–90 days after azithromycin initiation have produced conflicting results, with risks confirmed for some high-risk populations [23–26]. Two meta-analyses found an increased risk of sudden cardiovascular disease and ventricular tachyarrhythmias [9] and a small but statistically significant increase in myocardial infarction, though less likely for azithromycin than erythromycin [10]. However, another meta-analysis found no long-term cardiovascular risk assessed >30 days to >3 years [12]. A fourth meta-analysis identified a possible increased risk of cardiac death in adults aged >48 years, but not overall, in those using macrolides compared to non-macrolide [11]. Most relevant to our analysis, there are some prior data on safety of long-term use of azithromycin in patients with other chronic lung disease diagnoses. A prospective, randomised, placebo-controlled trial of 250 mg azithromycin daily for 12 months evaluated efficacy and safety in 1142 COPD patients [27]. There was no observed increase in risk of QTc prolongation in the azithromycin group, although patients with risk factors for QTc prolongation were excluded, as is common in randomised clinical trials.
In our study, macrolides were associated with a lower risk of arrhythmia than ICS, even after excluding those with a history of arrhythmia. One possible explanation is that the LABAs the majority of ICS patients were taking could increase the risk of arrhythmia, although a placebo-controlled, randomised trial in high-risk COPD patients found similar rates comparing placebo, LABA alone, ICS alone and ICS/LABA combination [28]. Similarly we did not see a difference in aHR stratifying by ICS/LABA and ICS alone. Findings of a non-elevated risk for macrolides are consistent with a population-based retrospective cohort study in Canada, where macrolide and non-macrolide antibiotics had a similar 30-day risk of ventricular arrhythmia (PS-matched RR 1.06, 95% CI 0.83–1.36) in adults ≥65 years old [29]. Age, smoking, male sex and pulmonary diseases are risk factors for atrial fibrillation, the most common arrhythmia diagnosis [30]. Further, atrial fibrillation has been associated with high-dose oral or parenteral dosing (≥7.5 mg of prednisone equivalent), although not low dose or ICS in patients with and without asthma/COPD [31]. As previously published, our Medicare population taking ICS was more likely to be male, to have COPD or asthma, and have a higher Charlson morbidity index [15]. It is possible, despite adjustment for PS and oral steroids, that unmeasured risk factors were not balanced between the two exposure groups or that there was residual confounding. Regardless, Albert et al. [32] suggest that clinical history and ECG prior to starting chronic therapy for lung disease would be cost-effective and reassuring to the patient and physician.
Bone loss that increases the risk of hip fracture has long been associated with oral corticosteroid use [18]. As recently described in patients with chronic airway diseases, inhaled corticosteroid use is associated with fracture risk with longer duration and higher doses [33–35]. Our findings in a Medicare population with a median 60 days of ICS use and 61% with low dose are consistent with observed minimal or no risk with shorter duration and lower doses [33]. However, the relatively short duration of exposure may have meant it was not possible to detect an increased risk if it occurs after longer use. One longitudinal study observed this risk only with very long duration (>4 years) and high doses (>1000 µg in fluticasone equivalents) (RR 1.10 95% CI 1.02–1.19) [35]. However, a PS-matched study in Taiwan reported a hip fracture aHR of 1.54 (95% CI 1.31–1.81) in adults age ≥65 years comparing ICS users to non-users, and a clear dose response over 250 µg fluticasone equivalents [34].
Our included bronchiectasis population had a relatively high incidence of opportunistic infections, 10-fold higher than non-viral rates of 1.8–3/1000 reported in younger adults on treatment for rheumatoid arthritis [36]; even accounting for the fact that half in our study were due to herpes zoster diagnoses. This may be influenced by low herpes zoster vaccine uptake (estimated 14% of adults over 65 between 2007 and 2013 in a Marketscan database study) in this high-risk population [37]. In our study, the crude risk of opportunistic infections was higher in those taking macrolides, but not statistically different comparing those using ICS and macrolides after adjusting for PS, oral corticosteroid use and NTM history. This finding is not readily understood. Some of these infections likely reflect the underlying lung disease which could put them at higher risk for pulmonary infections (e.g. endemic coccidioidomycosis and aspergillosis). To mitigate against misclassifying fungal colonisation (e.g. Candida or Aspergillus) as disease, we required a code plus evidence of >30 days of anti-fungal treatment. Our definitions for herpes zoster also included anti-viral treatment, to increase the likelihood that we identified active cases.
A systematic review identified 41 cases of audiometrically confirmed sensorineural hearing loss associated with oral azithromycin in the literature [14]. Two of these were prospective studies of patients treated for NTM disease, where azithromycin is given in lower doses (e.g. 250 mg daily) similar to macrolide monotherapy [38, 39]. The previously described randomised controlled trial of azithromycin in COPD patients showed a small but significantly higher proportion of patients with audiogram-confirmed hearing decrement in patients receiving azithromycin (25% versus 20% in the placebo group, p=0.04) [27]. Patients had no hearing abnormalities at baseline, and the conclusion was that there was no hearing impairment because hearing loss improved upon repeat-testing regardless of whether the study drug was discontinued or not. In contrast, our study provides population-based evidence of a moderately increased risk of sensorineural hearing loss due to macrolides with an active treatment comparison, and a crude incidence of around 10/100 patient-years in the older Medicare population with bronchiectasis. Physicians should consider evaluating patients who are initiating chronic macrolide therapy for baseline hearing loss, in order to facilitate diagnosis of macrolide-associated ototoxicity, if it could impact treatment decisions.
Strengths of the study include the fact that it is a large population-based study of older bronchiectasis patients. In our clinical experience in the USA, the majority of patients with bronchiectasis are cared for outside of specialty clinics. ICS are used routinely without evidence of efficacy, and macrolides are used routinely without sufficient evidence of risks [19]. Further, there are no expert guidelines in the USA for bronchiectasis management, and our data support the need for such guidelines. Our study fills a gap in knowledge that is unlikely to be answered with any other data. The new-user comparative effectiveness design has been used extensively in other clinical settings of chronic disease (e.g. rheumatoid arthritis, COPD, cancer) to improve the validity of findings [20, 40, 41]. We compare two active treatments with PS decile adjustment to account for differences in the two treatment populations that could bias the analyses. As a result we are comparing the medications in patients with similar characteristics. We included important patient and clinical characteristics in the PS, and confirmed that key demographics and underlying diagnoses are balanced across PS deciles.
One of the main limitations of claims data is the lack of confirmation of diagnoses and misclassification of outcomes. We used validated outcome measures, with the exception of sensorineural hearing loss, which was defined by our expert study advisory committee. Second, our patient population probably included a mix of primary and secondary bronchiectasis. However, we believe the evidence supports that most are primary bronchiectasis with concomitant (or erroneous) diagnosis of COPD. First, the demographics are majority female (70%) and older (mean age 74 years), suggestive of primary bronchiectasis [42, 43]. Further, we required that codes be given by a pulmonologist, so we have more confidence in the diagnosis. Last, the bronchiectasis diagnostic claim had to be associated with a clinical visit raising the likelihood that it was clinically relevant (if not, COPD or asthma would have been used as the code associated with the visit). Bronchiectasis is not normally a diagnosis used as a general code for billing, unlike COPD. Our comparison of two drugs with very different side-effect profiles could also be considered a weakness, if there is residual unmeasured confounding from prescribing practices or patients are monitored differently. Outcomes that are recognised side-effects, such as hearing loss, could be more closely monitored in patients taking macrolides, which would inflate the observed risk due to detection bias. However, we saw the opposite for arrhythmia, which could be affected by a decrease in the use of macrolides in high-risk patients due to awareness of cardiac risks. In addition, we did not control for ototoxic concomitant medications in the hearing loss model. Last, our population was older, so the results may not be generalisable to a younger population of bronchiectasis patients who are likely to have lower rates of adverse events such as cardiac abnormalities or hearing loss.
Our results suggest that the risk of hearing loss is increased in older patients taking macrolides for >30 days and that macrolides were associated with a lower risk of arrhythmia. Older patients who are on chronic macrolide treatment should be monitored for hearing loss where appropriate, including a baseline evaluation. Further study of potential long-term risks, particularly the development of antibiotic-resistant microorganisms, and benefits of macrolide monotherapy in this older, high-risk population is recommended.
Acknowledgements
The authors would like to recognise the contributions of the Megan Wardrop at OHSU-PSU School of Public Health, Lang Chen at the University of Alabama at Birmingham, David Griffith at University of Texas Health Science Center Northeast, and key stakeholders including Alexandra Quittner at Nicklaus Children's Health Institute, Elisha Malanga, Delia Prieto and Gretchen McCreary at the COPD Foundation, Amy Leitman at NTM Info and Research, and patients from the project's Patient Advisory Panel.
Provenance: Submitted article, peer reviewed.
Author contributions: E. Henkle had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. E. Henkle drafted the manuscript. All other co-authors contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Conflict of interest: C.L. Daley and K.L. Winthrop have received grant support and served on advisory boards for Insmed. All other authors have nothing to declare.
Support statement: Research reported in this manuscript was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1503-29191). The statements and conclusions in this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its Board of Governors or Methodology Committee. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65: Suppl. 1, i1–58. doi: 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Garcia MA, Maiz L, Olveira C, et al. . Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol (Engl Ed) 2018; 54: 88–98. doi: 10.1016/j.arbr.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Polverino E, Goeminne PC, McDonnell MJ, et al. . European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 4.Altenburg J, de Graaff CS, Stienstra Y, et al. . Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309: 1251–1259. doi: 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 5.Wong C, Jayaram L, Karalus N, et al. . Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380: 660–667. doi: 10.1016/S0140-6736(12)60953-2 [DOI] [PubMed] [Google Scholar]
- 6.Serisier DJ, Martin ML, McGuckin MA, et al. . Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; 309: 1260–1267. doi: 10.1001/jama.2013.2290 [DOI] [PubMed] [Google Scholar]
- 7.Ray WA, Murray KT, Hall K, et al. . Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366: 1881–1890. doi: 10.1056/NEJMoa1003833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zareba W. Drug induced QT prolongation. Cardiol J 2007; 14: 523–533. [PubMed] [Google Scholar]
- 9.Cheng YJ, Nie XY, Chen XM, et al. . The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 2015; 66: 2173–2184. doi: 10.1016/j.jacc.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 10.Gorelik E, Masarwa R, Perlman A, et al. . Systematic review, meta-analysis, and network meta-analysis of the cardiovascular safety of macrolides. Antimicrob Agents Chemother 2018; 62: e00438-18. doi: 10.1128/AAC.00438-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Wang M, Liu G, et al. . Association of macrolides with overall mortality and cardiac death among patients with various infections: a meta-analysis. Eur J Intern Med 2016; 28: 32–37. doi: 10.1016/j.ejim.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 12.Wong AYS, Chan EW, Anand S, et al. . Managing cardiovascular risk of macrolides: systematic review and meta-analysis. Drug Saf 2017; 40: 663–677. doi: 10.1007/s40264-017-0533-2 [DOI] [PubMed] [Google Scholar]
- 13.Olivier KN, Shaw PA, Glaser TS, et al. . Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 2014; 11: 30–35. doi: 10.1513/AnnalsATS.201307-231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda AK, Prince AA, Chen JX, et al. . Macrolide-associated sensorineural hearing loss: a systematic review. Laryngoscope 2018; 128: 228–236. doi: 10.1002/lary.26799 [DOI] [PubMed] [Google Scholar]
- 15.Henkle E, Curtis JR, Chen L, et al. . Comparative risks of chronic inhaled corticosteroids and macrolides for bronchiectasis. Eur Respir J 2019; 54: 1801896. [DOI] [PubMed] [Google Scholar]
- 16.Jick SS, Lieberman ES, Rahman MU, et al. . Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum 2006; 55: 19–26. doi: 10.1002/art.21705 [DOI] [PubMed] [Google Scholar]
- 17.Richy F, Bousquet J, Ehrlich GE, et al. . Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int 2003; 14: 179–190. doi: 10.1007/s00198-003-1398-z [DOI] [PubMed] [Google Scholar]
- 18.van Staa TP, Leufkens HG, Abenhaim L, et al. . Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology 2000; 39: 1383–1389. doi: 10.1093/rheumatology/39.12.1383 [DOI] [PubMed] [Google Scholar]
- 19.Henkle E, Aksamit TR, Barker AF, et al. . Pharmacotherapy for non-cystic fibrosis bronchiectasis: results from an NTM Info & Research Patient Survey and the Bronchiectasis and NTM Research Registry. Chest 2017; 152: 1120–1127. doi: 10.1016/j.chest.2017.04.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158: 915–920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 21.Cox D. Regression models and life tables (with discussion). J R Stat Soc Ser B 1972; 34: 187–220. [Google Scholar]
- 22.Drew BJ, Ackerman MJ, Funk M, et al. . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121: 1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013; 368: 1704–1712. doi: 10.1056/NEJMoa1300799 [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EM, Halm EA, Pugh MJ, et al. . Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 2014; 311: 2199–2208. doi: 10.1001/jama.2014.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaroff JG, Cheetham TC, Palmetto N, et al. . Association of azithromycin use with cardiovascular mortality. JAMA Netw Open 2020; 3: e208199. doi: 10.1001/jamanetworkopen.2020.8199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polgreen LA, Riedle BN, Cavanaugh JE, et al. . Estimated cardiac risk associated with macrolides and fluoroquinolones decreases substantially when adjusting for patient characteristics and comorbidities. J Am Heart Assoc 2018; 7: e008074. doi: 10.1161/JAHA.117.008074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert RK, Connett J, Bailey WC, et al. . Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365: 689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brook RD, Anderson JA, Calverley PM, et al. . Cardiovascular outcomes with an inhaled beta2-agonist/corticosteroid in patients with COPD at high cardiovascular risk. Heart 2017; 103: 1536–1542. doi: 10.1136/heartjnl-2016-310897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trac MH, McArthur E, Jandoc R, et al. . Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 2016; 188: E120–E129. doi: 10.1503/cmaj.150901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin EJ, Levy D, Vaziri SM, et al. . Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994; 271: 840–844. doi: 10.1001/jama.1994.03510350050036 [DOI] [PubMed] [Google Scholar]
- 31.van der Hooft CS, Heeringa J, Brusselle GG, et al. . Corticosteroids and the risk of atrial fibrillation. Arch Intern Med 2006; 166: 1016–1020. doi: 10.1001/archinte.166.9.1016 [DOI] [PubMed] [Google Scholar]
- 32.Albert RK, Schuller JL, Network CCR. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 2014; 189: 1173–1180. doi: 10.1164/rccm.201402-0385CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon JY, Sin DD. Inhaled corticosteroids and fractures in chronic obstructive pulmonary disease: current understanding and recommendations. Curr Opin Pulm Med 2019; 25: 165–172. doi: 10.1097/MCP.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 34.Tsai CH, Liao LY, Lin CL, et al. . Inhaled corticosteroids and the risks of low-energy fractures in patients with chronic airway diseases: a propensity score matched study. Clin Respir J 2018; 12: 1830–1837. doi: 10.1111/crj.12744 [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez AV, Coulombe J, Ernst P, et al. . Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest 2018; 153: 321–328. doi: 10.1016/j.chest.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 36.Baddley JW, Winthrop KL, Chen L, et al. . Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann Rheum Dis 2014; 73: 1942–1948. doi: 10.1136/annrheumdis-2013-203407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Johnson K, Newransky C, et al. . Herpes zoster vaccine coverage in older adults in the U.S., 2007–2013. Am J Prev Med 2017; 52: e17–e23. doi: 10.1016/j.amepre.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 38.Brown BA, Griffith DE, Girard W, et al. . Relationship of adverse events to serum drug levels in patients receiving high-dose azithromycin for mycobacterial lung disease. Clin Infect Dis 1997; 24: 958–964. doi: 10.1093/clinids/24.5.958 [DOI] [PubMed] [Google Scholar]
- 39.Griffith DE, Brown BA, Girard WM, et al. . Azithromycin activity against Mycobacterium avium complex lung disease in patients who were not infected with human immunodeficiency virus. Clin Infect Dis 1996; 23: 983–989. doi: 10.1093/clinids/23.5.983 [DOI] [PubMed] [Google Scholar]
- 40.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 2015; 11: 437–441. doi: 10.1038/nrrheum.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson ES, Bartman BA, Briesacher BA, et al. . The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf 2013; 22: 1–6. doi: 10.1002/pds.3334 [DOI] [PubMed] [Google Scholar]
- 42.Aksamit TR, O'Donnell AE, Barker A, et al. . Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henkle E, Chan B, Curtis JR, et al. . Characteristics and health-care utilization history of patients with bronchiectasis in US Medicare enrollees with prescription drug plans, 2006 to 2014. Chest 2018; 154: 1311–1320. doi: 10.1016/j.chest.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 44.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol Drug Saf 2012; 21: Suppl. 1, 148–153. doi: 10.1002/pds.2340 [DOI] [PubMed] [Google Scholar]
- 45.Kiyota Y, Schneeweiss S, Glynn RJ, et al. . Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004; 148: 99–104. doi: 10.1016/j.ahj.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 46.Narongroeknawin P, Patkar NM, Shakoory B, et al. . Validation of diagnostic codes for subtrochanteric, diaphyseal, and atypical femoral fractures using administrative claims data. J Clin Densitom 2012; 15: 92–102. doi: 10.1016/j.jocd.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneeweiss S, Robicsek A, Scranton R, et al. . Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol 2007; 60: 397–409. doi: 10.1016/j.jclinepi.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 48.Jumaan AO, Yu O, Jackson LA, et al. . Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis 2005; 191: 2002–2007. doi: 10.1086/430325 [DOI] [PubMed] [Google Scholar]
- 49.Mullooly JP, Riedlinger K, Chun C, et al. . Incidence of herpes zoster, 1997–2002. Epidemiol Infect 2005; 133: 245–253. doi: 10.1017/S095026880400281X [DOI] [PMC free article] [PubMed] [Google Scholar]