Abstract
Platinum-based drugs cisplatin, carboplatin, and oxaliplatin are widely used for chemotherapeutic eradication of cancer. However, the side effects of platinum drugs, such as lack of selectivity, high systemic toxicity, and drug resistance, seriously limit their clinical application. With advancements in nanotechnology and chemical synthesis, Pt-based anti-cancer drugs have made great progress in cancer therapy in recent years. Many strategies relied on the anti-cancer mechanism similar to cisplatin and achieved some success by modifying existing platinum drugs. Pt-based nanodrugs, such as platinum nanoclusters, have novel anti-cancer mechanisms and great potential in tumor-targeted therapy and have shown promising results in clinical application. In this review, we systematically explored the development of first-line platinum chemotherapy drugs in the clinic and their anti-cancer mechanisms. We also summarize the progress of Pt-based anti-cancer drug application in cancer therapy, emphasizing their modification to enhance the anti-tumor effect. Finally, we address challenges faced by platinum chemotherapy drugs, especially Pt nanocluster-based nanodrugs, in cancer treatment. The new platinum drugs and their targeted modifications undoubtedly provide a promising prospect for improving the current anti-cancer treatments.
Keywords: Platinum-based drugs, Cancer therapy, Anti-cancer mechanism, Systemic toxicity, Platinum nanoclusters
Introduction
Chemotherapy is an effective method of anti-tumor treatment 1-4. Before the 1960s, all drugs used to treat cancer were pure organic compounds 5. In the late 1960s, a simple coordination compound with anti-cancer properties, known as cisplatin, was accidentally discovered, and its cytostatic property to inhibit bacterial growth was detected. This discovery opened up a new possibility for cancer chemotherapy 6, 7.
The platinum-based anti-cancer drugs, including cisplatin 8, carboplatin 9, and oxaliplatin 10, with manifest therapeutic effects and well-defined mechanisms of action, are widely used in the clinic. As the first generation of the platinum anti-cancer drug, cisplatin has evident therapeutic effects on many malignant tumors, such as breast, ovarian, and colorectal 11, 12. However, cisplatin is a non-specific chemotherapeutic drug, causing systemic toxicity besides killing tumor cells 13. Thus, platinum anti-cancer drugs have serious undesirable effects, including dose-limiting toxicity, especially nephrotoxicity, neurotoxicity, ototoxicity, and myelosuppression 14, and long-term use of cisplatin causes serious damage to normal tissues 15. Due to the considerable therapeutic effect of cisplatin and other first-line clinical platinum drugs on tumor tissues, various strategies have been employed to reduce the damage to normal tissues, such as liposome encapsulation 16, 17, drug delivery by nanomaterial carriers 18, 19, and bioconjunction targeting highly expressed protein moieties on tumors 20, 21.
Many reformed and new anti-cancer platinum drugs have been formulated and reported recently. Among the diverse anti-cancer platinum drugs, multifunctional high-performance platinum nanocluster-based (Pt NC-based) nanodrugs, fabricated using different biocompatible materials leading to flexible designs, have attracted much attention for cancer-specific therapy and drug delivery 22, 23. Compared with traditional first-line clinical platinum drugs, Pt NC-based nanodrugs exhibited high stability, good water dispersibility and solubility in vitro, and low systemic toxicity and biocompatibility in vivo 24-26. The unique advantages of Pt NC-based nanodrugs for controllable fabrication, biosafety, and anti-tumor activity provide a broad prospect for their anti-cancer applications 27.
In this review, we summarize recent scientific advances in Pt-based drugs designed for anti-cancer treatment and address other relevant issues: (1) investigation of molecular mechanisms of platinum drugs in clinical use; (2) summary of the strategies developed to avoid systemic toxicity and improve bioavailability of platinum drugs; (3) development of novel Pt NC-based nanodrugs designed for anti-cancer treatment; (4) challenges of platinum chemotherapy drugs, especially Pt NC-based nanodrugs, for the anti-cancer treatment.
Development of First-line Pt Drugs
Cisplatin, carboplatin, and oxaliplatin are clinically approved worldwide and are the first choice for malignant tumor treatment 28. As shown in Table 1, cisplatin is the first generation of Pt-based anti-cancer drugs, discovered in the late 1960s and approved for cancer treatment in 1978 29. Cisplatin has a therapeutic effect on many malignant tumors, such as breast, ovarian, and colorectal cancers. However, besides killing tumor cells, it is a non-specific therapeutic drug causing systemic toxicity 30 and serious damage to normal tissues by long-term use 31. Therefore, based on the first-generation platinum drug cisplatin, the second-generation platinum chemotherapy drug carboplatin was developed, which took more than 10 years to reach the clinic. Compared with cisplatin, carboplatin exhibits a lower hydration rate due to the bidentate cyclobutane dicarboxylic acid ligands 32, 33 and has high biosafety with greatly reduced systemic toxicity, including hepatotoxicity, nephrotoxicity, neurotoxicity, and ototoxicity 34. Because of its lower toxicity, carboplatin can be used as high-dose chemotherapy for aggressive tumors. Nevertheless, Pt drug resistance is the main concern in platinum chemotherapy 35. Cisplatin and carboplatin eventually produce drug resistance during treatment. Therefore, the third generation of platinum clinical drug oxaliplatin was developed. The mechanism of action of oxaliplatin is similar to cisplatin, without producing cross-resistance with cisplatin or carboplatin 36, 37. Therefore, oxaliplatin and cisplatin can achieve a complementary effect in clinical anti-cancer treatment and have been widely used 38-40. Although, much effort has been devoted to developing new platinum-based anti-cancer drugs, none has reached worldwide clinical application.
Table 1.
Timeline of major milestones in three generations of first-line Pt drug clinical application
| Generation | Pt Drug | Molecular Structure | Market Time | Listed Country |
|---|---|---|---|---|
| First | Cisplatin |
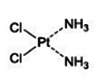
|
1978 | Japan/Italy |
| Second | Carboplatin |
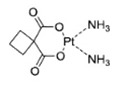
|
1986 | America |
| Third | Oxaliplatin |
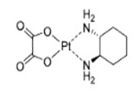
|
1996 | France |
Molecular Mechanism of Cisplatin
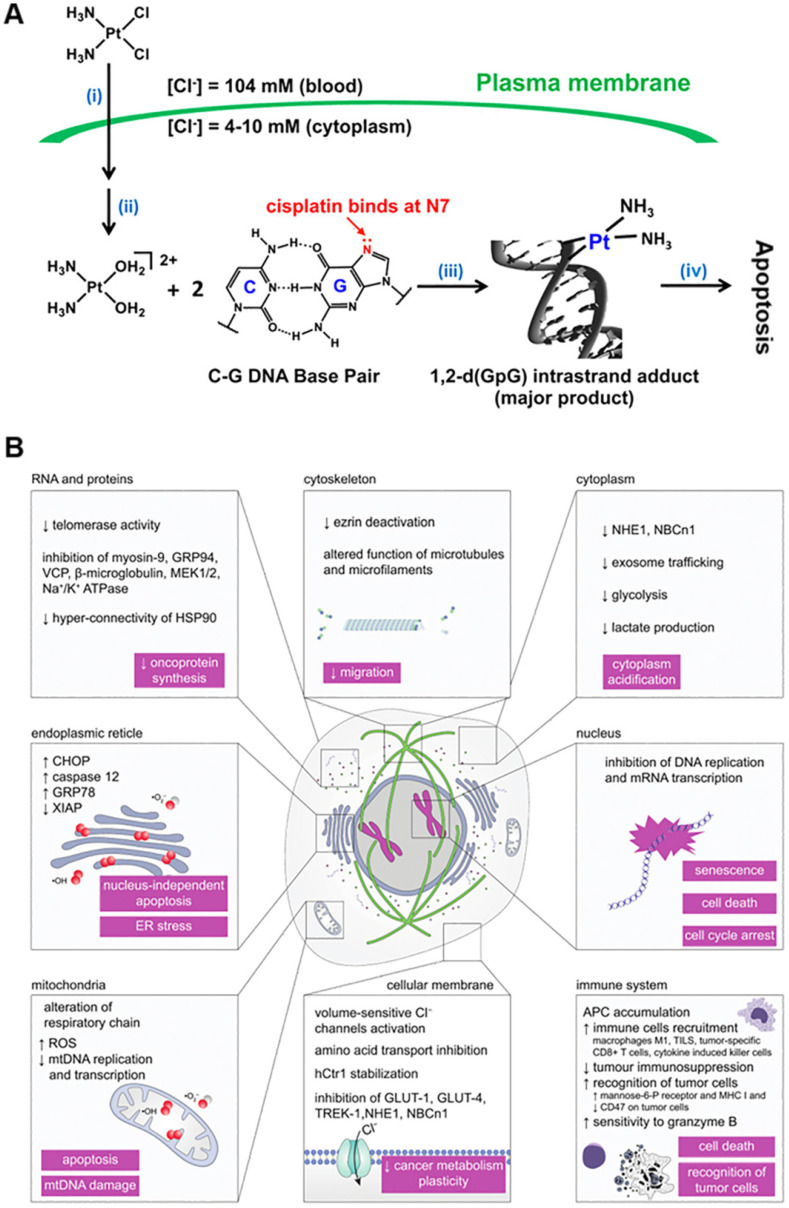
As Pt-based anti-cancer treatment, cisplatin, carboplatin, oxaliplatin, and other drugs are widely used in the clinic with obvious therapeutic effects and a clear mechanism of action 41-43. Cisplatin is generally believed to be first transported into tumor cells through copper transporter 1 (CTR1). After entering the tumor cell, the platinum complex undergoes the activation step of chloro-ligand(s) replacement, generally by water molecules or other small molecules containing sulfhydryl groups. This replacement is triggered by the significantly lower intracellular chloride ion concentration (about 4 mM) compared to the extracellular matrix (about 100 mM), promoting transformation to cationic hydrate, such as cis-[Pt(NH3)2Cl(OH2)]+ and cis-[Pt(NH3)2(OH2)2]2+ 44. Due to the chelation of the leaving ligand, carboplatin and oxaliplatin are more stable to aquation. More importantly, the rate of hydration and reaction with ammonia for transplatin is much faster than cisplatin. Following a 4-h incubation with red blood cells, transplatin reacts with 70% of the glutathione, whereas cisplatin reacts with only 35% 45. The high reactivity of transplatin results in rapid deactivation of the complex before reaching its target, likely contributing to its lack of anti-cancer activity. After a series of chemical reactions in the cytoplasm, platinum binds to DNA by forming intra- and inter-stranded crosslinks, changing the DNA structure and causing DNA damage (Figure 1A) 46, 47. The most nucleophilic DNA site is the N7 position of guanine, which is exposed in the major groove and is preferentially platinated. This DNA damage can prevent the cell cycle and induce apoptosis in rapidly proliferating tumor cells 48, 49.
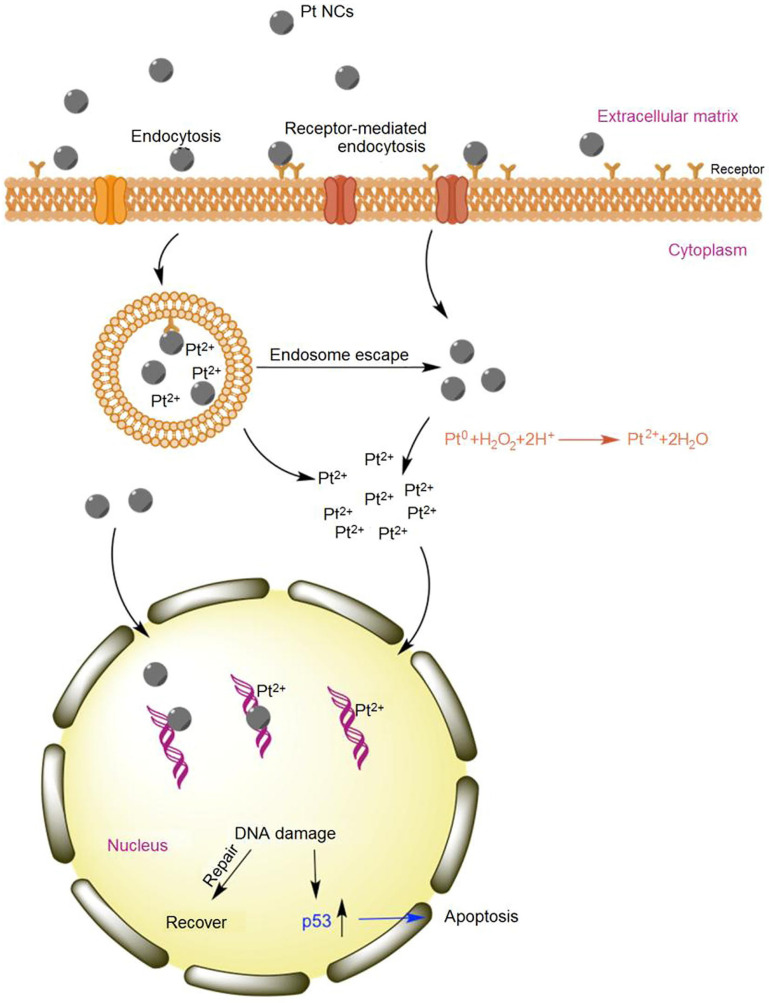
Figure 1.
Summary of the action mechanism of cisplatin. (A) Mechanism of action of cisplatin comprising (i) cellular uptake, (ii) aquation/activation, (iii) DNA platination, and (iv) cellular processing leading to apoptosis. Adapted with permission from ref 44, copyright 2016 American Chemical Society. (B) Alternative effects of cisplatin. Other interesting mechanisms such as acidification of the cytoplasm, ER stress, disruption of RNA transcription, inhibition of important oncogenic proteins, and decrease in metabolic plasticity of cancer cells as well as changes in their mechanobiology. Adapted with permission from ref 46, copyright 2019 Royal Society of Chemistry.
It is generally accepted that the principal mechanism of cisplatin anti-cancer action is platinum binding to DNA by forming intra-stranded and inter-stranded crosslinks 50, 51. However, some literature reported that probably only 1-10% of intracellular cisplatin might eventually enter the nucleus and react with DNA, resulting in cell cycle arrest and apoptosis in rapidly proliferating tumor cells. In this context, other novel action mechanisms, such as acidification of the cytoplasm 52, estrogen receptor (ER) stress 53, disruption of RNA transcription 54, inhibition of key oncogenic proteins, and decrease in metabolic plasticity of cancer cells and changes in their mechanobiology 55, have also been discovered (Figure 1B). The discovery of action mechanisms that may be affected by cisplatin may provide us with an important clue to design new anti-cancer treatment strategies by finding new potential therapeutic intervention targets.
Strategies to Improve Anti-cancer Efficiency and Reduce Systemic Toxicity of Pt Drugs
Numerous studies have addressed the limitations of first-line platinum chemotherapy drugs due to potential toxicity and side effects and developed strategies to improve anti-cancer efficiency while reducing systemic toxicity 56. Abundant evidence has demonstrated that chemical modification of first-line platinum chemotherapy drugs to achieve targeted therapy is an effective method to effectively improve drug utilization and reduce side effects 16-18. In this section, we summarize these strategies, describing including bioconjunction targeting moiety, nanomaterials as drug carriers, and glutathione-scavenging Pt drugs.
Bioconjunction Targeting Moiety
As stated earlier, the efficacy of first-line Pt drugs is limited due to the occurrence of severe side effects (nephrotoxicity, ototoxicity, peripheral neurotoxicity, and vomiting) together with the ability of cancer cells to limit drug accumulation 56. For Pt-based anti-cancer drugs, Pt(II) complexes are commonly used to treat malignant tumors. Photoactive Pt(IV) complexes are promising prodrug Pt(II) candidates activated by reduction in cancer cells and are being developed to lessen the side effects and improve pharmacological properties. Upon entering the cancer cells, the Pt(IV) center is reduced to Pt(II) and released 57. Under physiological conditions, the photosensitive Pt(IV) prodrugs retain their +4 valence state in the circulation system and are selectively converted to biologically active +2 valence state via mild ultraviolet light (UVA) irradiation after they reach the tumor 58, 59.
Extensive research indicated that the killing effect of Pt-based drugs on cancer cells could be improved by integrating the cancer cell-targeting moiety into Pt(IV) prodrugs. Notably, peptide-based drug delivery systems can enhance drug targeting properties and significantly reduce side effects 60, 61 due to their bioactivity and low immune response of peptides specifically expressed on tumor cell membranes 62-65. Peptide sequences with specific recognition characteristics of overexpressed proteins or other receptors can be introduced into Pt(IV) prodrugs to achieve the targeted function 66-68. More importantly, polypeptide sequences could be designed to perform different targeting functions. In recent years, our research group has made significant progress in functional targeted peptide design and exploring the biological effects of these peptides 69-71. In summary, different functional polypeptide-modified targeted platforms were developed to enhance effective utilization and reduce the side effects of Pt-based drugs.
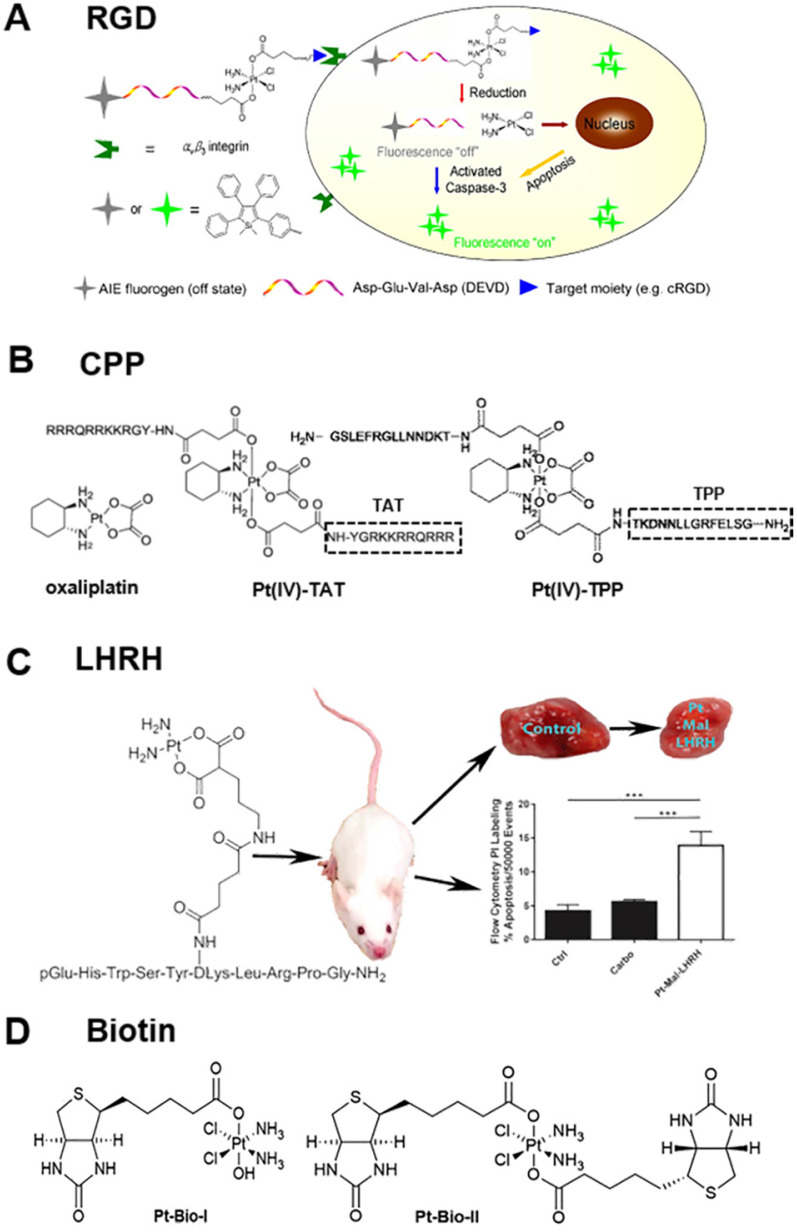
Integrins mediate cell-cell adhesion events and are overexpressed on tumor cell membranes with a key role in cancer progression 72, 73. The tripeptide arginine-glycine-aspartic (abbreviated as RGD) recognizes integrin αvβ3 overexpressed on tumor cells 66, 74-76. Yuan et al. reported the synthesis and biological evaluation of a chemotherapeutic Pt(IV) prodrug whose two axial positions were functionalized with the cyclic tripeptide cRGD for targeting integrin αvβ3-overexpressing cancer cells, an apoptosis sensor composed of tetraphenylsilole (TPS) fluorophore with aggregation-induced emission (AIE) characteristics, and a caspase-3 enzyme-specific Asp-Glu-Val-Asp (DEVD) peptide. The targeted Pt(IV) prodrug could selectively bind to integrin αvβ3 overexpressed on cancer cells to facilitate cellular uptake. Furthermore, the Pt(IV) prodrug was reduced to active Pt(II) drug in cells, releasing the apoptosis sensor TPS-DEVD. The reduced Pt(II) drug could induce cell apoptosis and activate the caspase-3 enzyme to cleave the DEVD peptide sequence. Due to the free rotation of the phenylene rings, TPS-DEVD was nonemissive in aqueous media. The specific cleavage of DEVD by caspase-3 generated the hydrophobic TPS residue, which aggregated, resulting in restriction of intramolecular rotations of the phenyl rings and ultimately leading to fluorescence enhancement (Figure 2A) 77.
Figure 2.
Summary of the bio-conjunction targeting moiety to improve the anti-cancer efficiency of Pt drugs, (A) RGD peptide 77, (B) CPP peptide 81, (C) LHRH peptide 9, and (D) biotin 84. Adapted with permission from ref 77, copyright 2014 American Chemical Society, ref 81, copyright 2017 Royal Society of Chemistry, ref 9, copyright 2017 American Chemical Society and ref 84, copyright 2017 Royal Society of Chemistry, respectively.
Similarly, Gandioso et al. reported an anti-cancer agent based on the conjugation of a photoactivatable Pt(IV) prodrug to a cyclic RGD-containing peptide 20. Upon visible light irradiation, phototoxicity was induced preferentially in SK-MEL-28 melanoma cancer cells overexpressing αvβ3 integrin compared to control DU-145 human prostate carcinoma cells. The fact that the Pt-cyclo(RGDfK) conjugate (where f represents D-amino acids, and the others are L-amino acids) can also be internalized by αvβ5 integrin opens up the door to delivering promising anti-cancer metallodrugs to tumors overexpressing αvβ5 integrin or to tumors coexpressing αvβ3 and αvβ5 integrins. The multi-integrin targeting approach would provide new metal-based anti-cancer strategies and benefit a broader range of patients by increasing the types of tumors which can be targeted.
Furthermore, cell-penetrating peptides (CPP), such as HIV-1 TAT, which can penetrate proteins and oligoarginine, are valuable tools for transporting therapeutic macromolecules into tumor cells 78-80. As displayed in Figure 2B, McKeon et al. reported a novel Pt(IV) tumor-penetrating peptide (TPP) conjugate, which constitutes the first example of metallodrugs to target the membrane-bound heat shock protein 70-positive (memHSP70+) phenotype in cancer cells. The conjugates exhibited superior cytotoxicity as compared to oxaliplatin alone in Pt-resistant colorectal cancer cells with relatively high memHSP70+ expression. Substitution of TPP in Pt(IV) peptide conjugates with scrambled peptide (ScP) essentially abolished the observed cytotoxicity 81.
The luteinizing hormone-releasing hormone (LHRH) peptide also acts as a targeting moiety, whose receptors are overexpressed in several types of cancer cells, such as breast, ovarian, prostate, lung, and liver 9, 82, 83. The LHRH grafted with Pt drugs enabled selective accumulation and distribution of Pt drugs in tumor cells. Calderon et al. reported a new targeting chemotherapeutic agent, Pt-Mal-LHRH, synthesized by linking activated cisplatin to LHRH (Figure 2C) 9. They found that Pt-Mal-LHRH significantly enhanced cellular cytotoxicity in 4T1 cells compared to the normal 3T3 cell line. Both in vitro and in vivo data suggested that Pt-Mal-LHRH elicits tumor-targeted drug delivery with increased potency, efficacy, and a possible reduction in chemotherapeutic side effects allowing the use of a high dose of chemotherapy in patients compared to other platinum drugs. Also, in vitro scratch assay data demonstrated a reduction in migration of tumor cells. Importantly, in vivo metastasis was investigated since the major cause of mortality in breast cancer patients is metastasis to distant sites, including the lungs. The in vivo data supported the in vitro data, as a significant decrease was observed in tumor volume and lung tumor colonization by Pt-Mal-LHRH treatment. Thus, the Pt-Mal-LHRH conjugate was selective for tumors overexpressing LHRH receptors while avoiding systemic distribution.
Interestingly, conjugating non-functional small molecules with platinum drugs improves the targeting ability of Pt drugs. For example, Muhammad et al. reported the design and biological properties of two Pt(IV) complexes, Pt-Bio-I and Pt-Bio-II, carrying one or two biotin moieties in the axial positions of the Pt center (Figure 2D) 84. Tethering biotin moieties to the Pt(IV) prodrug remarkably increased cellular uptake of Pt in breast cancer cells but lowered its accumulation in breast epithelial cells. The mono-biotinylated Pt(IV) complex was more active than the di-biotinylated one in reactivity and cytotoxicity. Compared with cisplatin, Pt-Bio-I showed much stronger inhibition against cisplatin-insensitive MDA-MB-231 and MCF-7 cancer cells. Considering its low toxicity towards mammary epithelial cells, Pt-Bio-I may be superior over cisplatin in breast cancer therapy. Interestingly, Pt(IV) complexes with one hydroxyl ligand in the axial position appear to be beneficial for their interaction with DNA and cytotoxicity to cancer cells.
Nanomaterials as Drug Carriers
Gold Nanoclusters (GNCs) as Drug Carriers
Nanocarrier-based platinum drug delivery systems are promising alternatives to avoid the disadvantages of conventional platinum drugs 22, 85, 86. In recent years, there has been much interest in GNCs as drug transport carriers due to their high photostability 87, water solubility, and biocompatibility 25, 88. Compared with traditional nanoparticles, the particle size of GNCs is usually less than 2 nm with increased blood circulation time. Pt drugs can be loaded efficiently in GNCs with increased accumulation in tumor tissues through the enhanced permeation and retention (EPR) effect, resulting in improved therapeutic efficacy and reduced systemic toxicity. Furthermore, ultrasmall GNCs are usually filtered out of the body through effective renal clearance, indicating good metabolism and biocompatibility 89-91.
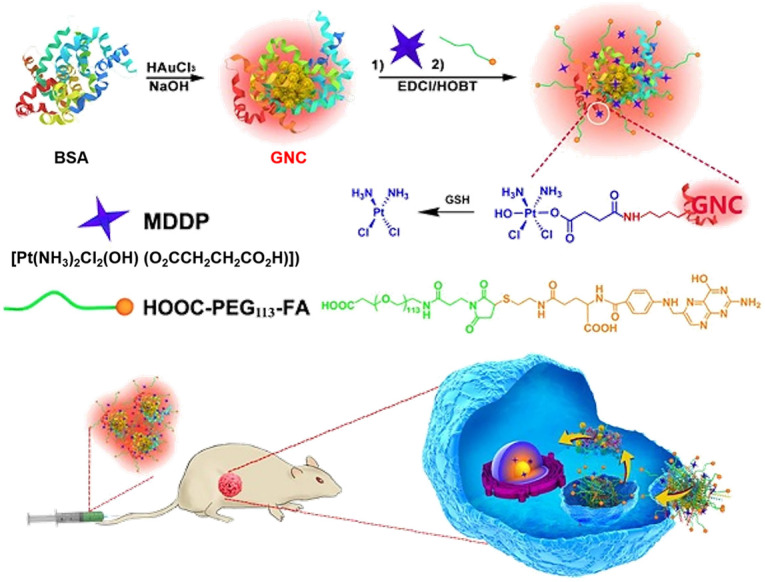
Utilizing the unique biological effects of GNCs, Zhou et al. employed BSA-protected GNCs as a dual-functional nanoplatform for drug delivery and fluorescence imaging of the tumor (Figure 3) 18. The GNCs were first conjugated with reduction-sensitive cisplatin coupled with a prodrug (cis,cis,trans[Pt(NH3)2Cl2(OH)(O2CCH2CH2CO2H)]) (MDDP), and then functionalized with a targeting ligand folic acid (FA) 92, which can target folate receptor α (FR-α) overexpressed on the surface of cancer cells 93. Using the highly aggressive 4T1 breast cancer cell line and its orthotopic tumor model, the investigators demonstrated selective accumulation of the prodrug and FA dual-conjugated GNCs inside the cancer cells and the tumor. The nanoparticles could efficiently inhibit the growth of the primary tumor and suppress metastasis of cancer cells to the lung. These data demonstrated the good potential of the GNC-based theranostic nanoplatform for fluorescence tumor imaging and cancer therapy and the advantages of GNCs as a drug delivery platform. First, when BSA is used for nanoparticle synthesis, GNCs are biocompatible and biodegradable. For clinical translation, BSA can be replaced with human serum protein (HSA) without changing the physicochemical properties of the nanoparticles. Second, the prodrug and FA dual-conjugated GNC nanoparticles selectively accumulated in the orthotopic 4T1 tumor model, displayed high fluorescence signals for nanoparticle tracking and tumor imaging 94 and efficiently inhibited primary tumor growth and suppressed metastasis of cancer cells to the lung. Moreover, the hydrodynamic diameter of FA-GNC-Pt was ~10 nanometers, allowing escape from the reticuloendothelial system (RES) in the liver and fast kidney clearance, avoiding liver accumulation and minimizing side effects.
Figure 3.
Schematic illustration of GNC-based theranostic nanoplatform for tumor-targeted chemotherapy and fluorescence imaging. Adapted with permission from ref 18, copyright 2016 Ivyspring International Publisher.
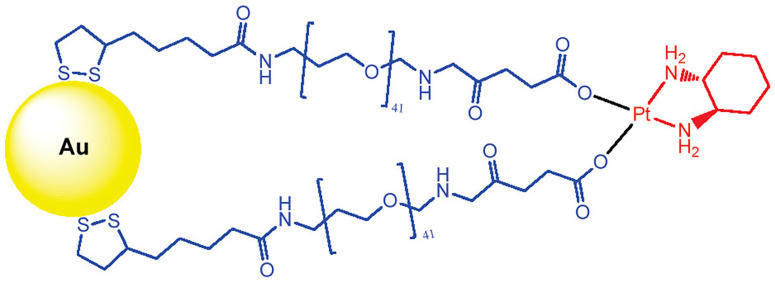
Brown et al. tethered the active component of the anti-cancer drug oxaliplatin to gold nanoparticles (AuNPs) for improved drug delivery 95. Poly(ethylene glycol) (PEG)-modified AuNPs have been used to functionalize cisplatin or oxaliplatin. For example, the active component of oxaliplatin was tethered to AuNPs that were functionalized with a thiolated PEG monolayer capped with a carboxylate group. The platinum-tethered NPs demonstrated comparable or significantly higher cytotoxicity than oxaliplatin against the A549 epithelial lung cancer and several colon cancer cell lines. In particular, the nanoparticles showed an unusual ability to penetrate the nucleus in lung cancer cells. The platinum-tethered nanoparticles demonstrated as good as, or significantly better, cytotoxicity than oxaliplatin alone in all cell lines (HCT116, HCT15, HT29, and RKO) and an unusual ability to penetrate the nucleus in lung cancer cells (Figure 4).
Figure 4.
Chemical synthesis of the platinum-tethered gold nanoparticles. Adapted with permission from ref 95, copyright 2010 American Chemical Society.
Magnetic Iron Oxide Nanoparticles as Drug Carriers
Superparamagnetic iron oxide nanoparticles (SPIONs) are usually employed as targeted delivery regents due to their advantages of low toxicity, biocompatibility, biodegradability, and well water dispersion. SPIONs can bind to drugs and be directed to the tissues of interest or tumors using an external magnetic field 96, 97.
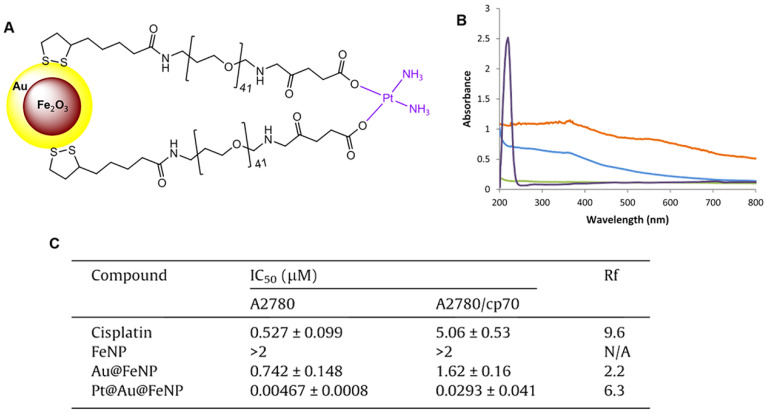
Wagstaff et al. tethered the active component of cisplatin to gold-coated iron oxide nanoparticles (Pt@Au@FeNPs) to improve their delivery to tumors and increase efficacy 98. The nanoparticle-based drug delivery system of Pt@Au@FeNPs was functionalized with thiolated polyethylene glycol (PEG) linkers to which the active component of the anti-cancer drug cisplatin, [Pt(NH3)2]2+ was attached via the terminal carboxylate groups (Figure 5A). The successful introduction of cisplatin was confirmed by UV-visible spectra (Figure 5B). The cytotoxicity of the Pt@Au@FeNPs was examined using in vitro growth inhibition assays in the human ovarian carcinoma cell line A2780 and its cisplatin-resistant derivative A2780/cp70. Pt@Au@FeNPs demonstrated activity at nanomolar concentrations and were 110-fold more active than cisplatin in A2780 cells, while iron oxide nanoparticles (FeNP) showed no cytotoxicity at concentrations up to 2 μM. However, in this study, the cisplatin Pt@Au@FeNPs, despite having activity at nanomolar concentrations, were cross-resistant with cisplatin in A2780/cp70 cells (Figure 5C).
Figure 5.
(A) Delivery system of gold-coated iron oxide nanoparticles functionalized with thiolated polyethylene glycol (PEG) linkers to which the active component of the anti-cancer drug cisplatin, [Pt(NH3)2]2+, is attached via the terminal carboxylate groups. (B) UV-Vis spectra of the four nanoparticles: FeNPs (blue), Au@FeNPs (orange), PEGylated Au@FeNPs (green), and Pt@Au@FeNPs (purple). (C) In vitro cytotoxicity of the nanoparticles in the human ovarian carcinoma cell line A2780 and its cisplatin-resistant sub-line A2780/cp70. Resistance factor (Rf) is defined as the IC50 of the complex in the resistant line divided by the IC50 of the complex in the sensitive line; any complex with an Rf less than 1 can overcome cisplatin resistance. Adapted with permission from ref 98, copyright 2012 Elsevier.
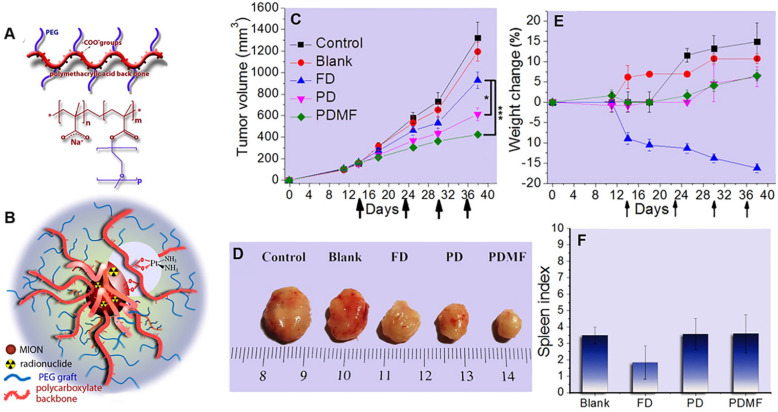
SPIONs are promising drug carriers because of the targeted delivery to tumors and increased efficacy through external magnets. Voulgari et al. synthesized a magnetic nanocarrier poly(methacrylic acid)-graft-poly(ethyleneglycol methacrylate) (p(MAA-g-EGMA)) by radical copolymerization of methoxy-PEG-methacrylate with methacrylic acid (Figure 6A). The cisplatin-loaded (PD) magnetic nanocarriers (Figure 6B) facilitated magnetically-triggered drug release and displayed in vitro anti-cancer activity comparable to free cisplatin at the same drug concentrations. In addition, they exhibited an enhanced anti-cancer effect in vivo on a cisplatin-resistant HT-29 tumor model in mice, particularly when a magnetic field was applied to the tumor area (Figure 6C-D). During the study period, a decrease in mouse weight was observed in the free cisplatin group but not in the p(MAA-g-EGMA)-treated group (Figure 6E), indicating a significant reduction of side-effects with the cisplatin-loaded magnetic nanocarriers. Moreover, spleen indices in the groups of mice injected with cisplatin-loaded magnetic nanocarriers (Figure 6F) were identical with the control group, suggesting a pronounced reduction of cisplatin systemic toxicity 99.
Figure 6.
(A) Chemical structure of copolymers poly(methacrylic acid)-graft-poly(ethyleneglycol methacrylate) (p(MAA-g-EGMA)). (B) Schematic structure of the studied magnetic drug delivery systems. Evolution with time of (C) Tumor volume and (E)% weight change of mice (n=4) after i.v. injections with: saline (Control), nanocarriers without the drug (Blank), aqueous cisplatin solution (FD), cisplatin-loaded nanocarriers (PD), and cisplatin-loaded nanocarriers in the presence of an external magnetic field in the tumor area (PDMF). Arrows in (a) and (b) represent tail vein injection events. (D) Pictures of the tumors taken at the end of the study period are shown for comparison. (F) Spleen index of mice sacrificed at the end of the in vivo experiment. Adapted with permission from ref 99, copyright 2016 Elsevier.
Other Nanomaterials as Drug Carriers
Besides SPIONs and GNCs, other nanomaterials, such as mesoporous silica, have multiple potential applications as drug carriers 100, 101. Mesoporous silica nanoparticles (MSNs) with large surface area and pore volume have an extraordinary ability to store drugs, and the controllable release of Pt drugs from the designed mesoporous structures is advantageous for the bioavailability of drugs 102. Also, organic nanoparticles, such as polymeric nanoparticles, exhibit great potential in drug delivery because of their unusual properties, including simple encapsulation, high capacity, controlled release, and low toxicity. The benefits of encapsulating Pt drugs in polymeric nanoparticles to reduce side effects without affecting drug efficacy have been demonstrated in tumor-bearing mice and preclinical cancer models 103.
Glutathione-Scavenging Pt Drugs
Glutathione (GSH) is one of the most abundant non-protein thiols in tumor cells, with its intracellular content of about 0.5-10 mM 37, 104, 105 and is the most important intracellular thiol compound which participates in cellular detoxification mechanisms 106, 107. Previous reports indicated that cancer cells could utilize endogenous GSH to chelate Pt drugs and produce inactive GSH-Pt adducts, which can be preferentially pumped out via membrane transport proteins and are non-toxic to cancer cells 12, 108, 109. In this context, GSH-scavenging Pt drugs have been reported.
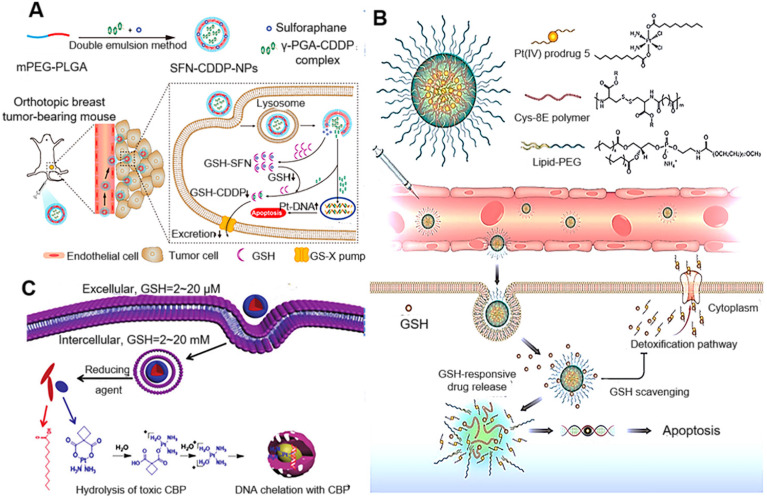
Sulforaphane (SFN) has been reported to deplete GSH by directly binding with GSH to form the GSH-SFN complex, which can be exported out of the cell. Recently, Xu et al. proposed that the therapeutic efficacy of SFN-CDDP-NPs could be significantly improved by SFN-mediated GSH depletion 110. As displayed in Figure 7A, the investigators designed an NP-enabled codelivery system consisting of a water-soluble poly(γ, L-glutamic acid)-CDDP (γ-PGA-CDDP) conjugate and SFN for breast cancer treatment. The therapeutic efficacy of SFN-CDDP-NPs was systematically investigated and compared with free drugs both in vitro and in vivo. After efficient internalization of SFN-CDDP-NPs by tumor cells, the rapidly released SFN could notably decrease the GSH content and thus significantly increase DNA-bound Pt, resulting in severe DNA damage and cellular apoptosis. Due to the improved chemosensitivity and preferential tumor accumulation, SFN-CDDP-NPs greatly inhibited orthotopic breast cancer progression with reduced toxic side effects.
Figure 7.
(A) Schematic diagram of the preparation of SFN-CDDP-NPs for improved anti-tumor therapy. Adapted with permission from ref 110, copyright 2020 American Chemical Society. (B) Illustration of the redox-responsive nanoplatform, composed of Pt(IV) prodrug 5, Cys-8E polymer, and lipid-PEG, for in vivo Pt delivery and treatment of cisplatin-resistant tumors. Adapted with permission from ref 111, copyright 2018 American Chemical Society. (C) Schematic illustration of phospholipid-mimic CBP-LA conjugates that self-assemble into micelle-like nanoparticles and the possible mechanism of their anti-cancer activity. Adapted with permission from ref 112, copyright 2018 Royal Society of Chemistry.
Ling et al. reported the synthesis of cysteine-based poly(disulfide amide) (Cys-PDSA) polymers (Cys-8E polymer) that readily react with GSH via disulfide-mediated reduction and their combination with a series of Pt(IV) prodrugs with tunable hydrophobicity. Optimized polymers rapidly disassembled and released Pt drugs in response to intracellular GSH while simultaneously consuming GSH to restore Pt sensitivity in cisplatin-resistant tumor cells 111. Moreover, in vivo efficacy and safety results showed that NPs effectively inhibited the growth of cisplatin-resistant xenograft tumors with an inhibition rate of 83.32% while alleviating serious side effects associated with cisplatin. GSH-scavenging polymeric NP technology reported herein could provide a unique strategy for improving the therapeutic efficacy of current Pt drugs (Figure 7B). Liang et al. also developed novel small-molecule-based nanodrugs of carboplatin-lauric acid nanoparticles (CBP-LA NPs) to reduce GSH-mediated platinum resistance and improve the anti-tumor efficiency of Pt(II) 112. The intracellular glutathione determination and the Pt-DNA adduct assay revealed that CBP-LA NPs could reduce intracellular GSH levels and improve the efficiency of platinum chelating with DNA to overcome GSH-mediated Pt(II) resistance (Figure 7C).
Pt Nanoclusters (Pt NCs) as a New Pt Drug for Cancer Therapy
Properties of Pt Nanoclusters
Pt NCs, similar to noble metal clusters such as GNCs, are relatively stable molecular aggregates composed of up to hundreds of metal atoms 113. Their physical size is normally between atoms and nanoparticles, close to the Fermi wavelength of a single electron. Pt NCs have attracted much attention in bioanalysis and biomedicine due to their special physicochemical properties, such as ultra-small size, precise structure, photoluminescence, X-ray absorption, low cytotoxicity, and good biocompatibility 114, 115. In particular, due to distinct molecular composition and a good biological safety profile, Pt NCs have great application prospects in anti-cancer treatment.
Application of Pt NC-based Drugs in Cancer Therapy
Although multiple studies of the Pt(II) complex and modified Pt(IV) prodrugs have attempted to improve the anti-cancer efficiency of Pt drugs, these strategies relied on anti-cancer mechanisms similar to cisplatin and were not very successful 116-118. In this section, we discuss Pt NCs as anti-cancer drugs with different mechanisms for cancer therapy.
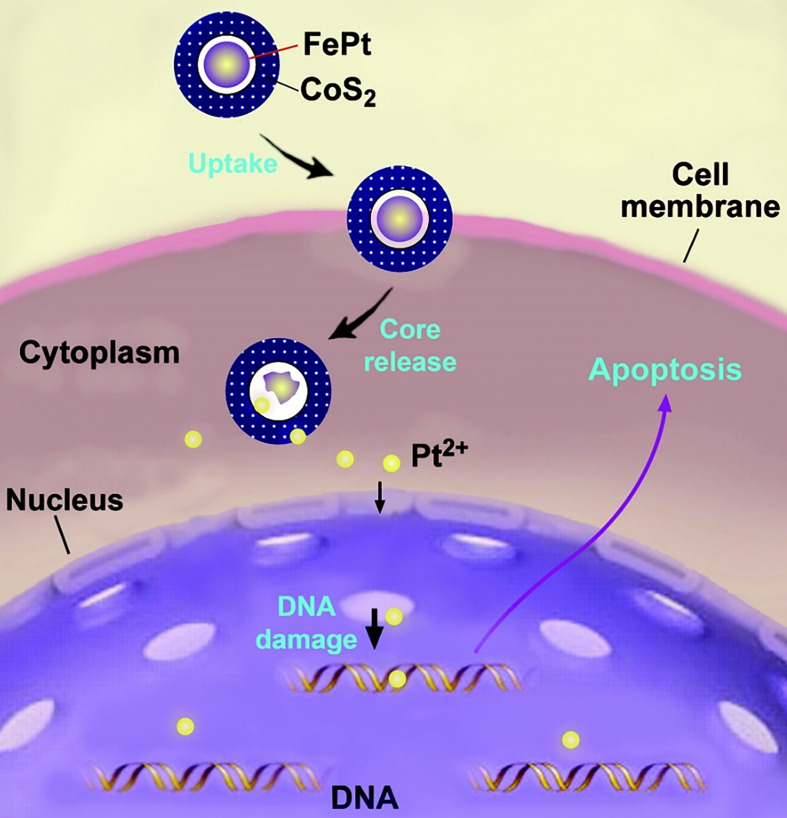
So far, the cytotoxicity mechanism of Pt NCs is still unclear because of the differences in size, shape, surface coatings, and purity of the particles 119. Nevertheless, it is generally accepted that the cytotoxicity of Pt NCs depends primarily on abundant Pt2+ ions leaching under low pH conditions, such as in cell endosomes, to induce DNA damage 32. As early as 2007, the inhibitory mechanism of Pt NCs on tumor cells was described. Gao et al. reported that the synthesized FePt@CoS2 yolk-shell nanoclusters exhibited an IC50 of 35.5 ng/mL (4.7 ng/mL of Pt) in HeLa cells that was much lower than cisplatin (230 ng/mL of Pt). The FePt nanoclusters were oxidized to generate Pt2+ and Fe3+ ions, especially in intracellular late lysosome with a low pH (pH < 5.5) environment, as hollow nanospheres were found in mitochondria of cancer cells, implying breakdown of the FePt core. Thus, after cellular uptake, FePt cores disintegrated to generate metal ions inside the acidic environment of secondary lysosomes. Subsequently, these metal ions could escape from endosomes and enter the cell nucleus to bind DNA, forming DNA-Pt adducts and eventually leading to tumor cell apoptosis (Figure 8). Furthermore, transmission electron microscopy (TEM) confirmed cellular uptake of FePt@CoS2 nanocrystals, and the magnetic properties analysis corroborated the release of FePt nanoparticles from yolk-shell nanostructures after cellular uptake 120.
Figure 8.
Illustration of a possible mechanism accounting for FePt@CoS2 yolk-shell nanocrystals killing HeLa cells. After cellular uptake, FePt nanoparticles were oxidized to generate Fe3+ (omitted for clarity) and Pt2+ ions (yellow). The Pt2+ ions enter into the nucleus (and mitochondria), bind to DNA, and lead to apoptosis of the HeLa cell. Adapted with permission from ref 120, copyright 2008 American Chemical Society.
Although the anti-tumor mechanism of Pt NCs remains obscure, it is believed that most Pt atoms are exposed on the ultrasmall <2 nm size nanocluster surface 36. These high surface-active Pt NCs are affected by intracellular acidic organelles like endosomes and lysosomes and then rapidly decompose to form oxidative states of Pt (Figure 9) that can attach to and change DNA structure, resulting in cancer cell apoptosis. In addition, ultrasmall Pt NCs can anchor onto the grooves of DNA double helix to further damage the DNA. Thus, eradicating cancer cells by Pt NC-based nanodrugs appears to be the synergistic effect of both Pt NCs and Pt ions causing DNA damage 32.
Figure 9.
Schematic of apoptosis mechanism of Pt NCs. Abundant oxidized Pt ions and Pt NCs coordinate the DNA damage activating the p53 pathway. Adapted with permission from ref 32, copyright 2017 Elsevier.
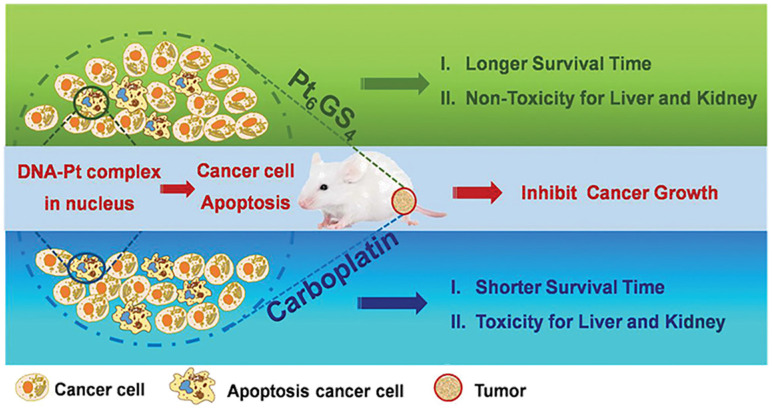
Recently, our research group explored a facile approach to develop an endogenous GSH-chelated Pt molecule containing multiple Pt atoms for efficient cancer treatment (Figure 10) 121. These polynuclear Pt NCs were identified by electrospray ionization mass spectra (ESI-MS) and density functional theory (DFT) study as Pt6GS4. High efficacy for anti-cancer treatment was achieved by Pt6GS4 both in vitro and in vivo when compared with traditional first-line carboplatin at the same dosage. The Pt6GS4 molecule could be readily taken up by aggressive triple-negative breast cancer (TNBC) cells. Subsequently, its metabolites entered nuclei to interact with DNA, and finally, the DNA-Pt complex triggered TNBC cell apoptosis via the p53 pathway. These data revealed that Pt6GS4 was comparable to carboplatin for cancer cell uptake, nuclear localization, and cancer cell proliferation inhibition. More significantly, compared with carboplatin, Pt6GS4 was non-toxic for the liver and kidneys, and Pt6GS4-treated mice lived longer. Our study opened a new avenue to explore polynuclear Pt compounds with accurate architecture for enhancing therapeutic effects and reducing systemic toxicity.
Figure 10.
Schematic illustration of GSH-chelated Pt molecule (Pt6GS4) as a potent anti-cancer agent. High efficacy for anti-cancer treatment and lower systemic toxicity were achieved by Pt6GS4 both in vitro and in vivo, compared to carboplatin at the same dosage. Adapted with permission from ref 121, copyright 2020 Wiley.
Targeting peptides have widely been used to synthesize Pt NCs 24, 113, 122, 123, improving the existing first-line platinum drugs in the clinic that inhibit rapid proliferation of tumor cells, and can also improve drug bioavailability. For example, Feng et al. synthesized mitochondria-targeting Pt NCs (CytcApt-Pt NCs) using cytochrome c aptamer (CytcApt) as a template. In vitro experiments showed that CytcApt-Pt NCs could kill 4T1 tumor cells in a pH-dependent manner but did not affect normal 293T cells. These results showed good therapeutic efficacy and excellent biosafety of CytcApt-Pt NCs, indicating their great potential for tumor treatment and reducing systemic toxicity 124.
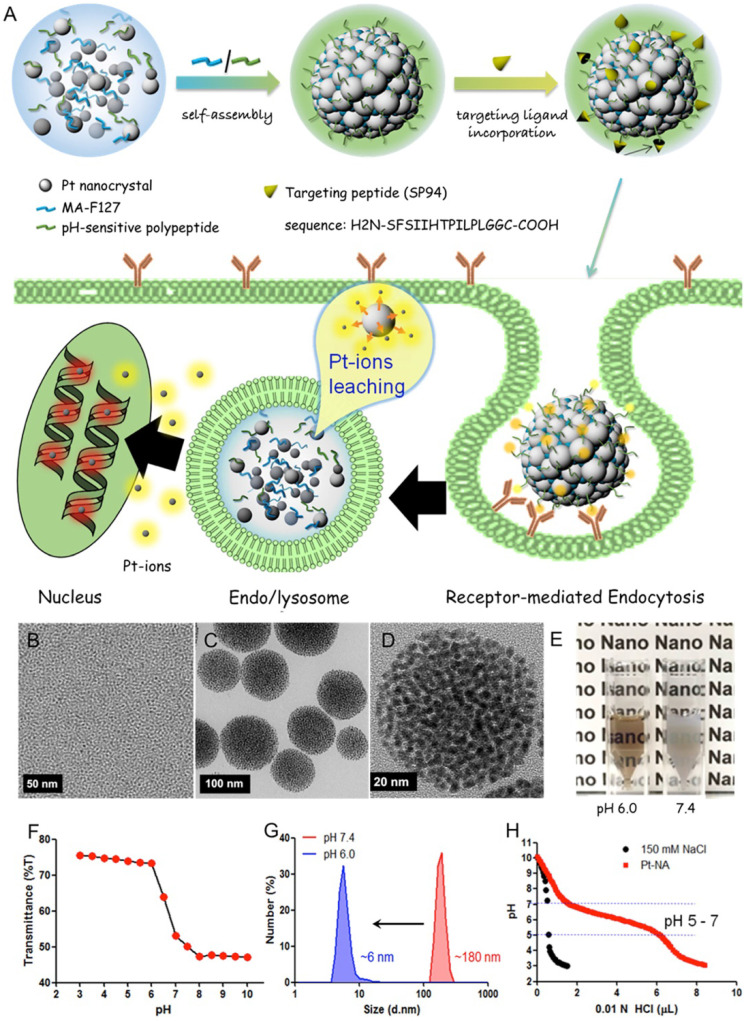
Xia et al. synthesized a Pt nanocluster assembly (Pt-NA) composed of assembled Pt NCs incorporating a pH-sensitive polymer and hepatocellular carcinoma (HCC)-targeting peptide (Figure 11) 125. Pt-NA was latent in peripheral blood, readily targeted disseminated HCC cancer stem-like cells (CSLCs), and disassembled into small Pt NCs in acidic subcellular compartments, eventually inducing DNA damage. Moreover, the study demonstrated the underlying mechanism of these effects at the molecular level as downregulation of many genes that are highly expressed in liver cancer patients. Thus, Pt-NA has a good potential in clinical HCC treatment 125.
Figure 11.
Design and characterization of HCC-targeted pH-sensitive Pt nanocluster assembly (Pt-NA). (A) Schematic representation of Pt-NA synthesis, targeted HCC uptake, and intracellular Pt ion release. (B) TEM image of the synthesized Pt NCs. (C) TEM image of Pt-NA. (D) High-resolution TEM image of Pt-NA. (E) Photographs of Pt-NA in pH 6.0 and 7.4. (F) The transmittance of a suspension of Pt-NA as a function of pH. (G) DLS size measurement of Pt-NA (0.1 mg mL-1) as a function of pH. (H) pH profile of Pt-NA by acid-base titration. Adapted with permission from ref 125, copyright 2016 American Chemical Society.
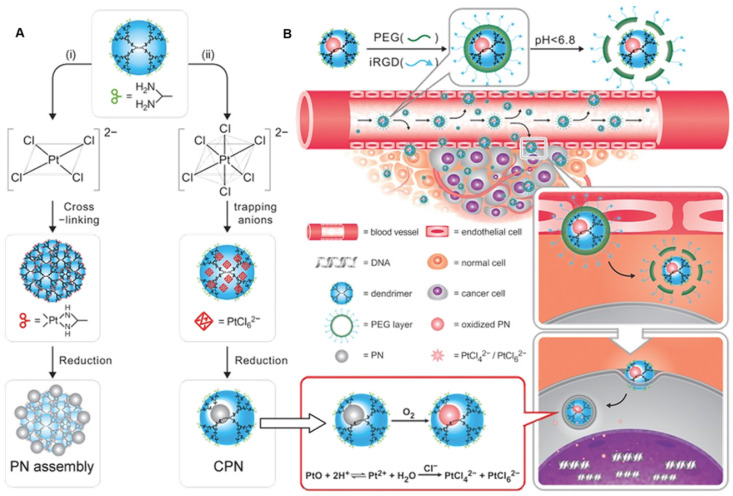
Another example is of a first-generation dendrimer-caged Pt nanocluster (CPN) with the size of an atomic level (0.93 ± 0.22 nm in diameter). CPN was endowed with targeting function by conjugating with the iRGD peptide (Figure 12A) 126. Especially, CPN could be easily oxidized, resulting in the loss of its intrinsic chemical inertness and its surface corrodibility for further dissolution in weakly acidic organelles, such as endosomes and lysosomes, to release toxic Pt ions for DNA cross-linking (Figure 12B). Employing subcutaneous breast cancer xenografts in mice, the therapeutic effect of CPN was examined by intratumoral injection in vivo. Results indicated that this chemotherapeutic had efficacy comparable to cisplatin.
Figure 12.
(A) Schematic representation of a novel strategy based on tuning anionic geometry for the formation of PN. (B) Schematic representation of the caged PN mixed with a tumor-penetrating peptide to target the tumor and kill malignant cells by shedding the outer PEG corona to exert tumor-inside activation. Adapted with permission from ref 126, copyright 2013 Wiley.
Conclusion and Perspectives
Traditional tumor chemotherapy employs chemo-drugs, which usually have strong side effects for normal cells and tissues 13. Pt-based anti-cancer drugs play a vital role in clinical cancer therapy with satisfactory efficacy. The first-line clinical platinum anti-cancer drugs represented by cisplatin are relatively old drugs that have a therapeutic effect on tumors with a known molecular mechanism. However, the side effects seriously limit the application of platinum anti-cancer drugs. Therefore, modified Pt-based drugs, which could improve anti-cancer efficiency and reduce systemic toxicity have been investigated. Pt NC-based nanodrugs have attracted much attention due to the inherent higher blood circulation time, EPR effect, and facile surface functionalization. Advances in nanotechnology and nanoscience have facilitated the development of Pt NCs, representing an important research orientation for exploring platinum drugs with the precise structure to improve therapeutic effect and reduce systemic toxicity 127.
The challenges accompanying these advances provide us with future directions and efforts for designing and constructing more effective Pt-based drugs for possible clinical applications:
(1) For cisplatin and other platinum anti-cancer drugs, systemic toxicity is still the most challenging problem. Platinum drugs are modified by many methods, such as linking target molecules and adding drug delivery carriers, with the ultimate goal of reducing their toxicity.
(2) Many new platinum nanodrugs, such as Pt NCs, have been developed. Pt NCs generate platinum ions in cells and induce irreversible DNA damage 32, 37, 125, 126. However, Pt NC-based drugs are still cytotoxic, and the possible harmful mechanisms are not entirely understood. Investigations on the role of Pt NCs size in cytotoxicity indicated that it could represent an important parameter affecting molecular mechanisms. Recent synchrotron radiation X-ray techniques may provide insights into nanomaterial biotransformation to address the anti-tumor mechanism of Pt NCs. It is crucial to study the dynamic process of Pt NCs metabolism in vivo and their interaction with biomolecules to treat malignant tumors and other diseases 128, 129, which would be critical for designing Pt NC-based nanodrugs and high efficiency.
(3) As a new type of platinum anti-cancer drug, the molecular composition of Pt NCs needs to be further improved. Mass spectrometric techniques, such as MALDI-TOF-MS and ESI-MS, would help characterize the molecular formula of clusters or the metal to ligand ratio in clusters, but the precise structural characterization methods of cluster molecules need to be further explored 130-133. We believe that nanotechnology would be immensely helpful in addressing these issues. Besides understanding the precise molecular composition of Pt NCs, we need to study the impact of Pt NCs on their anti-tumor function in the context of the configuration of cisplatin and transplatin, which is an area worthy of in-depth exploration. The application of nanotechnology in the field of Pt NC-based nanodrugs undoubtedly provides a promising prospect for improving the current anti-cancer treatment.
(4) Combining Pt-based nanodrugs with other therapeutic methods, such as synergistic chemo-electrodynamic therapy, can maximize the bio-function of Pt NCs and strengthen their anti-tumor effect 102, 134-136. In addition to individual Pt-based drugs, bimetallic composites, such as platinum complexes in combination with ruthenium, also showed excellent anti-cancer performance 57, 137. This represents a new strategy to overcome the deactivation pathways during Pt drug treatment and a good option as a promising anti-cancer agent.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21727817, U2067214, 11621505), and the Academic Promotion Program of Shandong First Medical University (2019LJ003).
Author contributions
All authors contributed to writing the manuscript and approved the final version.
Abbreviations
- CDDP
cisplatin
- CTR1
copper transporter 1
- ER
endoplasmic reticulum
- RGD
arginine-glycine-aspartic
- AIE
aggregation-induced emission
- CPP
cell-penetrating peptides
- TPP
tumor penetrating peptide
- LHRH
luteinizing hormone-releasing hormone
- GNC
gold nanoclusters
- Pt NCs
platinum nanoclusters
- MSNs)
mesoporous silica nanoparticles
- SPIONs)
superparamagnetic iron oxide nanoparticles
- FA
folic acid
- HSA
human serum protein
- RES
reticuloendothelial system
- AuNPs
gold nanoparticle
- PEG
polyethylene glycol
- GSH
Glutathione
- SFN
Sulforaphane
- CBP-LA NPs
carboplatin-lauric acid nanoparticles
- ESI-MS
electrospray ionization mass spectra
- DFT
density functional theory
- TNBC
triple-negative breast cancer
- BSA
bovine serum albumin protein
- HCC
hepatocellular carcinoma
- CPN
dendrimer-caged Pt nanoclusters
- PN
Pt nanoclusters
- Pt-NA
Pt nanocluster assembly
- MALDI-TOF-MS
matrix-assisted laser desorption/ionization-time of flight mass spectrometry
References
- 1.Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park GY, Wilson JJ, Song Y, Lippard SJ. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc Natl Acad Sci U S A. 2012;109:11987–11992. doi: 10.1073/pnas.1207670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian M, Fan R, Zhao S, Liu W. Targeting the thioredoxin system as a strategy for cancer therapy. J Med Chem. 2019;62:7309–7321. doi: 10.1021/acs.jmedchem.8b01595. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Li X, Han X, Liu R, Fang J. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci. 2017;38:794–808. doi: 10.1016/j.tips.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2020;21:37–50. doi: 10.1038/s41568-020-00308-y. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 7.Ohmichi M, Hayakawa J, Tasaka K, Kurachi H, Murata Y. Mechanisms of platinum drug resistance. Trends Pharmacol Sci. 2005;26:113–116. doi: 10.1016/j.tips.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Corte-Rodriguez M, Espina M, Sierra LM, Blanco E, Ames T, Montes-Bayon M. et al. Quantitative evaluation of cellular uptake, DNA incorporation and adduct formation in cisplatin sensitive and resistant cell lines: Comparison of different Pt-containing drugs. Biochem Pharmacol. 2015;98:69–77. doi: 10.1016/j.bcp.2015.08.112. [DOI] [PubMed] [Google Scholar]
- 9.Calderon LE, Keeling JK, Rollins J, Black CA, Collins K, Arnold N. et al. Pt-Mal-LHRH, a newly synthesized compound attenuating breast cancer tumor growth and metastasis by targeting overexpression of the LHRH receptor. Bioconjug Chem. 2017;28:461–470. doi: 10.1021/acs.bioconjchem.6b00610. [DOI] [PubMed] [Google Scholar]
- 10.Zayed A, Jones GD, Reid HJ, Shoeib T, Taylor SE, Thomas AL. et al. Speciation of oxaliplatin adducts with DNA nucleotides. Metallomics. 2011;3:991–1000. doi: 10.1039/c1mt00041a. [DOI] [PubMed] [Google Scholar]
- 11.Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32:1469–1486. doi: 10.1021/acs.chemrestox.9b00204. [DOI] [PubMed] [Google Scholar]
- 12.Garcia Sar D, Montes-Bayon M, Blanco Gonzalez E, Sierra Zapico LM, Sanz-Medel A. Reduction of cisplatin-induced nephrotoxicity in vivo by selenomethionine: the effect on cisplatin-DNA adducts. Chem Res Toxicol. 2011;24:896–904. doi: 10.1021/tx200085n. [DOI] [PubMed] [Google Scholar]
- 13.Wong. E, Giandomenico. CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 14.Wlodarczyk MT, Dragulska SA, Camacho-Vanegas O, Dottino PR, Jarzęcki AA, Martignetti JA. et al. Platinum(II) complex-nuclear localization sequence peptide hybrid for overcoming platinum resistance in cancer therapy. ACS Biomater. Sci. Eng. 2018;4:463–467. doi: 10.1021/acsbiomaterials.7b00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mjos KD, Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem Rev. 2014;114:4540–4563. doi: 10.1021/cr400460s. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Yang Y, Lin X, Ma W, Chen G, Li W. et al. Platinum(iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem Commun (Camb) 2018;54:5369–5372. doi: 10.1039/c8cc02791a. [DOI] [PubMed] [Google Scholar]
- 17.Alas M, Saghaeidehkordi A, Kaur K. Peptide-drug conjugates with different linkers for cancer therapy. J Med Chem. 2021;64:216–232. doi: 10.1021/acs.jmedchem.0c01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Feng B, Yu H, Wang D, Wang T, Liu J. et al. Cisplatin prodrug-conjugated gold nanocluster for fluorescence imaging and targeted therapy of the breast cancer. Theranostics. 2016;6:679–687. doi: 10.7150/thno.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turiel-Fernandez D, Gutierrez-Romero L, Corte-Rodriguez M, Bettmer J, Montes-Bayon M. Ultrasmall iron oxide nanoparticles cisplatin (IV) prodrug nanoconjugate: ICP-MS based strategies to evaluate the formation and drug delivery capabilities in single cells. Anal Chim Acta. 2021;1159:338356. doi: 10.1016/j.aca.2021.338356. [DOI] [PubMed] [Google Scholar]
- 20.Gandioso A, Shaili E, Massaguer A, Artigas G, Gonzalez-Canto A, Woods JA. et al. An integrin-targeted photoactivatable Pt(IV) complex as a selective anticancer pro-drug: synthesis and photoactivation studies. Chem Commun (Camb) 2015;51:9169–9172. doi: 10.1039/c5cc03180j. [DOI] [PubMed] [Google Scholar]
- 21.Palchoudhury S, Xu Y, Rushdi A, Bao Y. DNA interaction of Pt-attached iron oxide nanoparticles. IEEE T Magn. 2013;49:373–376. [Google Scholar]
- 22.Chen H, Gu Z, An H, Chen C, Chen J, Cui R. et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61:1503–1552. [Google Scholar]
- 23.Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S. et al. Diverse applications of nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Cui Y, Anderson CF, Zhang C, Li Y, Wang R. et al. Peptide-based nanoprobes for molecular imaging and disease diagnostics. Chem Soc Rev. 2018;47:3490–3529. doi: 10.1039/c7cs00793k. [DOI] [PubMed] [Google Scholar]
- 25.Spicer CD, Jumeaux C, Gupta B, Stevens MM. Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications. Chem Soc Rev. 2018;47:3574–3620. doi: 10.1039/c7cs00877e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu HW, Chen L, Xu C, Li Z, Zhang H, Zhang XB. et al. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem Soc Rev. 2018;47:7140–7180. doi: 10.1039/c7cs00862g. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Zhang B, Yao Q, Yang Y, Xie J, Yan N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coordin Chem Rev. 2016;322:1–29. [Google Scholar]
- 28.Wang K, Zhu C, He Y, Zhang Z, Zhou W, Muhammad N. et al. Restraining cancer cells by dual metabolic inhibition with a mitochondrion-targeted platinum(II) complex. Angew Chem Int Ed Engl. 2019;58:4638–4643. doi: 10.1002/anie.201900387. [DOI] [PubMed] [Google Scholar]
- 29.Rebecca A. Alderden, Matthew D. Hall, Hambley TW. The discovery and development of cisplatin. J Chem Educ. 2006;83:728–734. [Google Scholar]
- 30.Kuwata K, Nakamura I, Ide M, Sato H, Nishikawa S, Tanaka M. Comparison of changes in urinary and blood levels of biomarkers associated with proximal tubular injury in rat models. J Toxicol Pathol. 2015;28:151–164. doi: 10.1293/tox.2014-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazlitt RA, Min J, Zuo J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J Med Chem. 2018;61:5512–5524. doi: 10.1021/acs.jmedchem.7b01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Li F, Noor N, Ling D. Platinum drugs: from Pt(II) compounds, Pt(IV) prodrugs, to Pt nanocrystals/nanoclusters. Sci Bull. 2017;62:589–596. doi: 10.1016/j.scib.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol. 2016;77:1103–1124. doi: 10.1007/s00280-016-2976-z. [DOI] [PubMed] [Google Scholar]
- 34.Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol. 2016;102:37–46. doi: 10.1016/j.critrevonc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Kenny RG, Marmion CJ. Toward multi-targeted platinum and ruthenium drugs-a new paradigm in cancer drug treatment regimens? Chem Rev. 2019;119:1058–1137. doi: 10.1021/acs.chemrev.8b00271. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Li Z, Yu Z, Deng X, Xin Y. Recent advances in the synthesis, properties, and biological applications of platinum nanoclusters. J Nanomater. 2019;2019:1–31. [Google Scholar]
- 37.Browning RJ, Reardon PJT, Parhizkar M, Pedley RB, Edirisinghe M, Knowles JC. et al. Drug delivery strategies for platinum-based chemotherapy. ACS Nano. 2017;11:8560–8578. doi: 10.1021/acsnano.7b04092. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Q, Liu Y. Multifunctional platinum-based nanoparticles for biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1410. doi: 10.1002/wnan.1410. [DOI] [PubMed] [Google Scholar]
- 39.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–1071. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 40.Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyala L. In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother Pharmacol. 2001;48:398–406. doi: 10.1007/s002800100363. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Adebali O, Wu G, Selby CP, Chiou YY, Rashid N. et al. Cisplatin-DNA adduct repair of transcribed genes is controlled by two circadian programs in mouse tissues. Proc Natl Acad Sci U S A. 2018;115:E4777–E4785. doi: 10.1073/pnas.1804493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zayed A, Shoeib T, Taylor SE, Jones GDD, Thomas AL, Wood JP. et al. Determination of Pt-DNA adducts and the sub-cellular distribution of Pt in human cancer cell lines and the leukocytes of cancer patients, following mono- or combination treatments, by inductively-coupled plasma mass spectrometry. Int J Mass Spectrom. 2011;307:70–78. [Google Scholar]
- 43.Alessio E, Guo Z. Metal Anticancer complexes-activity, mechanism of action, future perspectives. Eur J Inorg Chem. 2017;2017:1539–1540. [Google Scholar]
- 44.Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev. 2016;116:3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnstone TC, Suntharalingam K, Lippard SJ. Third row transition metals for the treatment of cancer. Philos Trans A Math Phys Eng Sci. 2015;373:20140185–20140185. doi: 10.1098/rsta.2014.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raudenska M, Balvan J, Fojtu M, Gumulec J, Masarik M. Unexpected therapeutic effects of cisplatin. Metallomics. 2019;11:1182–1199. doi: 10.1039/c9mt00049f. [DOI] [PubMed] [Google Scholar]
- 47.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.P. J S, Mukherjee A, Chandrasekaran N. DNA damage and mitochondria-mediated apoptosis of A549 lung carcinoma cells induced by biosynthesised silver and platinum nanoparticles. RSC Adv. 2016;6:27775–27787. [Google Scholar]
- 49.Liu S, Wang Y. Mass spectrometry for the assessment of the occurrence and biological consequences of DNA adducts. Chem Soc Rev. 2015;44:7829–7854. doi: 10.1039/c5cs00316d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yimit A, Adebali O, Sancar A, Jiang Y. Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs. Nat Commun. 2019;10:309. doi: 10.1038/s41467-019-08290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu D, Yang C, Lok CN, Xing F, Lee PY, Fung YME. et al. Anti-tumor bis(N-heterocyclic carbene)platinum(II) complex engages asparagine synthetase as an anti-cancer target. Angew Chem Int Ed Engl. 2019;58:10914–10918. doi: 10.1002/anie.201904131. [DOI] [PubMed] [Google Scholar]
- 52.Liang ZD, Long Y, Chen HH, Savaraj N, Kuo MT. Regulation of the high-affinity copper transporter (hCtr1) expression by cisplatin and heavy metals. J Biol Inorg Chem. 2014;19:17–27. doi: 10.1007/s00775-013-1051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rashid HO, Yadav RK, Kim HR, Chae HJ. ER stress: Autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raudenska M, Kratochvilova M, Vicar T, Gumulec J, Balvan J, Polanska H. et al. Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci Rep. 2019;9:1660. doi: 10.1038/s41598-018-38199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Legin AA, Schintlmeister A, Jakupec MA, Galanski M, Lichtscheidl I, Wagner M. et al. NanoSIMS combined with fluorescence microscopy as a tool for subcellular imaging of isotopically labeled platinum-based anticancer drugs. Chem Sci. 2014;5:3135–3143. doi: 10.1039/c3sc53426j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eskandari A, Kundu A, Ghosh S, Suntharalingam K. A triangular platinum(II) multinuclear complex with cytotoxicity towards breast cancer stem cells. Angew Chem Int Ed Engl. 2019;58:12059–12064. doi: 10.1002/anie.201905389. [DOI] [PubMed] [Google Scholar]
- 57.Karges J, Yempala T, Tharaud M, Gibson D, Gasser G. A multi-action and multi-target Ru(II)-Pt(IV) conjugate combining cancer-activated chemotherapy and photodynamic therapy to overcome drug resistant cancers. Angew Chem Int Ed Engl. 2020;59:7069–7075. doi: 10.1002/anie.201916400. [DOI] [PubMed] [Google Scholar]
- 58.Du R, Xiao H, Guo G, Jiang B, Yan X, Li W. et al. Nanoparticle delivery of photosensitive Pt(IV) drugs for circumventing cisplatin cellular pathway and on-demand drug release. Colloid Surf B Biointerfaces. 2014;123:734–741. doi: 10.1016/j.colsurfb.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Xiao H, Noble GT, Stefanick JF, Qi R, Kiziltepe T, Jing X. et al. Photosensitive Pt(IV)-azide prodrug-loaded nanoparticles exhibit controlled drug release and enhanced efficacy in vivo. J Control Release. 2014;173:11–17. [PubMed] [Google Scholar]
- 60.Li C, Zhang N, Zhou J, Ding C, Jin Y, Cui X. et al. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6:178–188. doi: 10.1158/2326-6066.CIR-17-0035. [DOI] [PubMed] [Google Scholar]
- 61.Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM. et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54:11760–11764. doi: 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- 62.Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y. et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 63.de MRJF, de Medeiros RSS, Braghiroli MI, Galvao B, Neto JEB, Munhoz RR. et al. Expression of ERCC1, Bcl-2, Lin28a, and Ki-67 as biomarkers of response to first-line platinum-based chemotherapy in patients with high-grade extrapulmonary neuroendocrine carcinomas or small cell lung cancer. Ecancermedicalscience. 2017;11:767. doi: 10.3332/ecancer.2017.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C, Yao S, Xu C, Chang Y, Zong Y, Zhang K. et al. 3D imaging and quantification of the integrin at a single-cell base on a multisignal nanoprobe and synchrotron radiation soft X-ray tomography microscopy. Anal Chem. 2021;93:1237–1241. doi: 10.1021/acs.analchem.0c04662. [DOI] [PubMed] [Google Scholar]
- 65.Zhai J, Wang Y, Xu C, Zheng L, Wang M, Feng W. et al. Facile approach to observe and quantify the alpha(IIb)beta3 integrin on a single-cell. Anal Chem. 2015;87:2546–2549. doi: 10.1021/ac504639u. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Liu R, Shu Q, Yuan Q, Xing G, Gao X. Quantitative analysis of multiple proteins of different invasive tumor cell lines at the same single-cell level. Small. 2018;14:e1703684. doi: 10.1002/smll.201703684. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Zhang X, Gao F, Yuan Q, Zhang C, Yuan H. et al. Catalytic clusterbody for enhanced quantitative protein immunoblot. Anal Chem. 2021;93:10807–10815. doi: 10.1021/acs.analchem.1c00779. [DOI] [PubMed] [Google Scholar]
- 68.Chen L, X G, L G. Advances in analytic nanotechniques for the capture and detection of circulating tumor cells. Prog Biochem Biophys. 2021;48:35–53. [Google Scholar]
- 69.Yao Y, Lu C, Gao L, Cao K, Yuan H, Zhang X. et al. Gold cluster capped with a BCL-2 antagonistic peptide exerts synergistic antitumor activity in chronic lymphocytic leukemia cells. ACS Appl Mater Interfaces. 2021;13:21108–21118. doi: 10.1021/acsami.1c05550. [DOI] [PubMed] [Google Scholar]
- 70.Liu C, Zhang X, Han X, Fang Y, Liu X, Wang X. et al. Polypeptide-templated Au nanoclusters with red and blue fluorescence emissions for multimodal imaging of cell nuclei. ACS Appl Bio Mater. 2020;3:1934–1943. doi: 10.1021/acsabm.9b01078. [DOI] [PubMed] [Google Scholar]
- 71.Zhai J, Jia Y, Zhao L, Yuan Q, Gao F, Zhang X. et al. Turning on/off the anti-Tumor effect of the Au cluster via atomically controlling its molecular size. ACS Nano. 2018;12:4378–4386. doi: 10.1021/acsnano.8b00027. [DOI] [PubMed] [Google Scholar]
- 72.Zhai J, Zhao L, Zheng L, Gao F, Gao L, Liu R. et al. Peptide-Au cluster probe: precisely detecting epidermal growth factor receptor of three tumor cell lines at a single-cell level. ACS Omega. 2017;2:276–282. doi: 10.1021/acsomega.6b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su D, Gao L, Gao F, Zhang X, Gao X. Peptide and protein modified metal clusters for cancer diagnostics. Chem Sci. 2020;11:5614–5629. doi: 10.1039/d0sc01201g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gao L, Zhang Y, Zhao L, Niu W, Tang Y, Gao F et al. An artificial metalloenzyme for catalytic cancer-specific DNA cleavage and operando imaging, Sci Adv 2020; 6, eabb1421. [DOI] [PMC free article] [PubMed]
- 75.Hu C, Yang X, Liu R, Ruan S, Zhou Y, Xiao W. et al. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl Mater Interfaces. 2018;10:22571–22579. doi: 10.1021/acsami.8b04847. [DOI] [PubMed] [Google Scholar]
- 76.Nie X, Zhang J, Xu Q, Liu X, Li Y, Wu Y. et al. Targeting peptide iRGD-conjugated amphiphilic chitosan-co-PLA/DPPE drug delivery system for enhanced tumor therapy. J Mater Chem B. 2014;2:3232. doi: 10.1039/c3tb21744b. [DOI] [PubMed] [Google Scholar]
- 77.Yuan Y, Kwok RT, Tang BZ, Liu B. Targeted theranostic platinum(IV) prodrug with a built-in aggregation-induced emission light-up apoptosis sensor for noninvasive early evaluation of its therapeutic responses in situ. J Am Chem Soc. 2014;136:2546–2554. doi: 10.1021/ja411811w. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Cui Y, Zhao Y, Liu R, Sun Z, Li W. et al. Bifunctional peptides that precisely biomineralize Au clusters and specifically stain cell nuclei. Chem Commun (Camb) 2012;48:871–873. doi: 10.1039/c1cc15926g. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Cui Y, Liu R, Wei Y, Jiang X, Zhu H. et al. Blue two-photon fluorescence metal cluster probe precisely marking cell nuclei of two cell lines. Chem Commun (Camb) 2013;49:10724–10726. doi: 10.1039/c3cc46690f. [DOI] [PubMed] [Google Scholar]
- 80.Jiang W, Wang J, Yang J, He Z, Hou Z, Luo Y. et al. Acidity-triggered TAT-presenting nanocarriers augment tumor retention and nuclear translocation of drugs. Nano Res. 2018;11:5716–5734. [Google Scholar]
- 81.McKeon AM, Noonan J, Devocelle M, Murphy BM, Griffith DM. Platinum(iv) oxaliplatin-peptide conjugates targeting memHsp70+ phenotype in colorectal cancer cells. Chem Commun (Camb) 2017;53:11318–11321. doi: 10.1039/c7cc04764a. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Xu C, Chang Y, Zhao L, Zhang K, Zhao Y. et al. Ultrasmall superparamagnetic iron oxide nanoparticle for T2-weighted magnetic resonance imaging. ACS Appl Mater Interfaces. 2017;9:28959–28966. doi: 10.1021/acsami.7b10030. [DOI] [PubMed] [Google Scholar]
- 83.Gao F, Yang W, Xue J, Gao L, Liu R, Wang Y, Zhao Y, He X. et al. Ultrasmall [64Cu] Cu nanoclusters for targeting orthotopic lung tumors using accurate positron emission tomography imaging. ACS Nano. 2015;9:4976–4986. doi: 10.1021/nn507130k. [DOI] [PubMed] [Google Scholar]
- 84.Muhammad N, Sadia N, Zhu C, Luo C, Guo Z, Wang X. Biotin-tagged platinum(iv) complexes as targeted cytostatic agents against breast cancer cells. Chem Commun (Camb) 2017;53:9971–9974. doi: 10.1039/c7cc05311h. [DOI] [PubMed] [Google Scholar]
- 85.Lambert IH, Sorensen BH. Facilitating the Cellular Accumulation of Pt-Based Chemotherapeutic Drugs. Int J Mol Sci. 2018;19:2249. doi: 10.3390/ijms19082249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Wang X, Guo Z. Functionalization of platinum complexes for biomedical applications. Acc Chem Res. 2015;48:2622–2631. doi: 10.1021/acs.accounts.5b00203. [DOI] [PubMed] [Google Scholar]
- 87.Luo D, Wang X, Zeng S, Ramamurthy G, Burda C, Basilion JP. Targeted gold nanocluster-enhanced radiotherapy of prostate cancer. Small. 2019;15:e1900968. doi: 10.1002/smll.201900968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115:10410–10488. doi: 10.1021/acs.chemrev.5b00193. [DOI] [PubMed] [Google Scholar]
- 89.Yuan Q, Zhao Y, Cai P, He Z, Gao F, Zhang J. et al. Dose-dependent efficacy of gold clusters on rheumatoid arthritis therapy. ACS Omega. 2019;4:14092–14099. doi: 10.1021/acsomega.9b02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan Q, Gao F, Yao Y, Cai P, Zhang X, Yuan J. et al. Gold clusters prevent inflammation-induced bone erosion through inhibiting the activation of NF-κB pathway. Theranostics. 2019;9:1825–1836. doi: 10.7150/thno.31893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao F, Yuan Q, Cai P, Gao L, Zhao L, Liu M. et al. Au clusters treat rheumatoid arthritis with uniquely reversing cartilage/bone destruction. Adv Sci. 2019;6:1801671. doi: 10.1002/advs.201801671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, Li S, Zhang L, Chen X, Wang T, Zhang M. et al. Tunable fabrication of folic acid-Au@poly(acrylic acid)/mesoporous calcium phosphate Janus nanoparticles for CT imaging and active-targeted chemotherapy of cancer cells. Nanoscale. 2017;9:14322–14326. doi: 10.1039/c7nr05382g. [DOI] [PubMed] [Google Scholar]
- 93.Xu C, Wang Y, Zhang C, Jia Y, Luo Y, Gao X. AuGd integrated nanoprobes for optical/MRI/CT triple-modal in vivo tumor imaging. Nanoscale. 2017;9:4620–4628. doi: 10.1039/c7nr01064h. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Yan L, Liu J, Chen C, Zhao Y. Quantification of nanomaterial/nanomedicine trafficking in vivo. Anal Chem. 2018;90:589–614. doi: 10.1021/acs.analchem.7b04765. [DOI] [PubMed] [Google Scholar]
- 95.Sarah D. Brown, Paola Nativo, Jo-Ann Smith, David Stirling, Paul R. Edwards, Balaji Venugopal, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc. 2010;132:4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conesa JJ, Oton J, Chiappi M, Carazo JM, Pereiro E, Chichon FJ. et al. Intracellular nanoparticles mass quantification by near-edge absorption soft X-ray nanotomography. Sci Rep. 2016;6:22354. doi: 10.1038/srep22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian F, Chen G, Yi P, Zhang J, Li A, Zhang J. et al. Fates of Fe3O4 and Fe3O4@SiO2 nanoparticles in human mesenchymal stem cells assessed by synchrotron radiation-based techniques. Biomaterials. 2014;35:6412–6421. doi: 10.1016/j.biomaterials.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 98.Wagstaff AJ, Brown SD, Holden MR, Craig GE, Plumb JA, Brown RE. et al. Cisplatin drug delivery using gold-coated iron oxide nanoparticles for enhanced tumour targeting with external magnetic fields. Inorg Chim Acta. 2012;393:328–333. [Google Scholar]
- 99.Voulgari E, Bakandritsos A, Galtsidis S, Zoumpourlis V, Burke BP, Clemente GS. et al. Synthesis, characterization and in vivo evaluation of a magnetic cisplatin delivery nanosystem based on PMAA-graft-PEG copolymers. J Control Release. 2016;243:342–356. doi: 10.1016/j.jconrel.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, He C, Liu X, Chen Y, Zhao P, Chen C. et al. One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1-targeted combination cancer therapy. Theranostics. 2020;10:2918–2929. doi: 10.7150/thno.41077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park JS, Kinsella JM, Jandial DD, Howell SB, Sailor MJ. Cisplatin-loaded porous Si microparticles capped by electroless deposition of platinum. Small. 2011;7:2061–2069. doi: 10.1002/smll.201100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu T, Chen T, Cheng L, Li X, Han G, Liu Z. Mesoporous silica decorated with platinum nanoparticles for drug delivery and synergistic electrodynamic-chemotherapy. Nano Res. 2020;13:2209–2215. [Google Scholar]
- 103.Duan X, He C, Kron SJ, Lin W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:776–791. doi: 10.1002/wnan.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Djavanmard MP, Budinsky AC, Steiner B, Brix R, Kautzky M, Swoboda A. et al. 169 P-Glutatiflon (GSH) for protection of cisplatinum (CDDP) induced neuro- and nephrotoxicity. Eur J Cancer. 1996;32:S34. [Google Scholar]
- 105.Xu Y, Jiang N, Yu H. Effect of glutathione combined with cisplatin and oxaliplatin on the proliferation and apoptosis of lung carcinoma cell line. Toxicol Mech Methods. 2010;20:487–492. doi: 10.3109/15376516.2010.508081. [DOI] [PubMed] [Google Scholar]
- 106.Zamora A, Rodriguez V, Cutillas N, Yellol GS, Espinosa A, Samper KG. et al. New steroidal 7-azaindole platinum(II) antitumor complexes. J Inorg Biochem. 2013;128:48–56. doi: 10.1016/j.jinorgbio.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Kasherman Y, Sturup S, Gibson D. Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. J Med Chem. 2009;52:4319–4328. doi: 10.1021/jm900138u. [DOI] [PubMed] [Google Scholar]
- 108.Casini A, Reedijk J. Interactions of anticancer Pt compounds with proteins: an overlooked topic in medicinal inorganic chemistry? Chem Sci. 2012;3:3135. [Google Scholar]
- 109.Zhao L, Wang Z, Wu H, Xi Z, Liu Y. Glutathione selectively modulates the binding of platinum drugs to human copper chaperone Cox17. Biochem J. 2015;472:217–223. doi: 10.1042/BJ20150634. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y, Han X, Li Y, Min H, Zhao X, Zhang Y. et al. Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity. ACS Nano. 2019;13:13445–13455. doi: 10.1021/acsnano.9b07032. [DOI] [PubMed] [Google Scholar]
- 111.Ling X, Chen X, Riddell IA, Tao W, Wang J, Hollett G. et al. Glutathione-scavenging poly(disulfide amide) nanoparticles for the effective delivery of Pt(IV) prodrugs and reversal of cisplatin resistance. Nano Lett. 2018;18:4618–4625. doi: 10.1021/acs.nanolett.8b01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang S, Han L, Mu W, Jiang D, Hou T, Yin X. et al. Carboplatin-loaded SMNDs to reduce GSH-mediated platinum resistance for prostate cancer therapy. J Mater Chem B. 2018;6:7004–7014. doi: 10.1039/c8tb01721b. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Xia K, Wang L, Wu M, Sang X, Wan K. et al. Peptide-engineered fluorescent nanomaterials: Structure design, function tailoring, and biomedical applications. Small. 2021;17:e2005578. doi: 10.1002/smll.202005578. [DOI] [PubMed] [Google Scholar]
- 114.Ma Y, Huang J, Song S, Chen H, Zhang Z. Cancer-targeted nanotheranostics: recent advances and perspectives. Small. 2016;12:4936–4954. doi: 10.1002/smll.201600635. [DOI] [PubMed] [Google Scholar]
- 115.Wang W, Anderson CF, Wang Z, Wu W, Cui H, Liu CJ. Peptide-templated noble metal catalysts: syntheses and applications. Chem Sci. 2017;8:3310–3324. doi: 10.1039/c7sc00069c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Y, Liu X, Ma W, Xu Q, Chen G, Wang Y. et al. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265:120456. doi: 10.1016/j.biomaterials.2020.120456. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J, Zhao B, Chen S, Wang Y, Zhang Y, Wang Y. et al. Near-infrared light irradiation induced mild hyperthermia enhances glutathione depletion and DNA interstrand cross-link formation for efficient chemotherapy. ACS Nano. 2020;14:14831–14845. doi: 10.1021/acsnano.0c03781. [DOI] [PubMed] [Google Scholar]
- 118.Luo K, Guo W, Yu Y, Xu S, Zhou M, Xiang K. et al. Reduction-sensitive platinum (IV)-prodrug nano-sensitizer with an ultra-high drug loading for efficient chemo-radiotherapy of Pt-resistant cervical cancer in vivo. J Control Release. 2020;326:25–37. doi: 10.1016/j.jconrel.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 119.Pedone D, Moglianetti M, De Luca E, Bardi G, Pompa PP. Platinum nanoparticles in nanobiomedicine. Chem Soc Rev. 2017;46:4951–4975. doi: 10.1039/c7cs00152e. [DOI] [PubMed] [Google Scholar]
- 120.Gao J, Liang G, Zhang B, Kuang Y, Zhang X, Xu B. FePt@CoS2 yolk-shell nanocrystals as a potent agent to kill HeLa cells. J Am Chem Soc. 2006;129:1428–1433. doi: 10.1021/ja067785e. [DOI] [PubMed] [Google Scholar]
- 121.Zhang C, Gao L, Yuan Q, Zhao L, Niu W, Cai P. et al. Is GSH chelated Pt molecule inactive in anti-cancer treatment? A case study of Pt6GS4. Small. 2020;16:2002044. doi: 10.1002/smll.202002044. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y, Zhang C, Xu C, Wang X, Liu C, Waterhouse GIN. et al. Ultrasmall Au nanoclusters for biomedical and biosensing applications: A mini-review. Talanta. 2019;200:432–442. doi: 10.1016/j.talanta.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 123.Yang W, Guo W, Chang J, Zhang B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J Mater Chem B. 2017;5:401–417. doi: 10.1039/c6tb02308h. [DOI] [PubMed] [Google Scholar]
- 124.Feng B, Zhao D, Peng Y, Wang F. Cytochrome-c aptamer functionalized Pt nanoclusters for enhanced chemodynamic therapy. J Innov Opt Heal Sci. 2021;14:2141004. [Google Scholar]
- 125.Xia H, Li F, Hu X, Park W, Wang S, Jang Y. et al. pH-sensitive Pt nanocluster assembly overcomes cisplatin resistance and heterogeneous stemness of hepatocellular carcinoma. ACS Cent Sci. 2016;2:802–811. doi: 10.1021/acscentsci.6b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chien CT, Yan JY, Chiu WC, Wu TH, Liu CY, Lin SY. Caged Pt nanoclusters exhibiting corrodibility to exert tumor-inside activation for anticancer chemotherapeutics. Adv Mater. 2013;25:5067–5073. doi: 10.1002/adma.201302363. [DOI] [PubMed] [Google Scholar]
- 127.Zhu S, Li L, Gu Z, Chen C, Zhao Y. 15 years of Small: research trends in nanosafety. Small. 2020;16:e2000980. doi: 10.1002/smll.202000980. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Cai R, Chen C. The nano-bio interactions of nanomedicines: understanding the biochemical driving forces and redox reactions. Acc Chem Res. 2019;52:1507–1518. doi: 10.1021/acs.accounts.9b00126. [DOI] [PubMed] [Google Scholar]
- 129.Cao M, Cai R, Zhao L, Guo M, Wang L, Wang Y. et al. Molybdenum derived from nanomaterials incorporates into molybdenum enzymes and affects their activities in vivo. Nat Nanotechnol. 2021;16:708–716. doi: 10.1038/s41565-021-00856-w. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Z, Yao Y, Yuan Q, Lu C, Zhang X, Yuan J. et al. Gold clusters prevent breast cancer bone metastasis by suppressing tumor-induced osteoclastogenesis. Theranostics. 2020;10:4042–4055. doi: 10.7150/thno.42218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cui Y, Wang Y, Zhao L. Cysteine-Ag cluster hydrogel confirmed by experimental and numerical studies. Small. 2015;11:5118–5125. doi: 10.1002/smll.201501245. [DOI] [PubMed] [Google Scholar]
- 132.An D, Su J, Weber JK, Gao X, Zhou R, Li J. A peptide-coated gold nanocluster exhibits unique behavior in protein activity inhibition. J Am Chem Soc. 2015;137:8412–8418. doi: 10.1021/jacs.5b00888. [DOI] [PubMed] [Google Scholar]
- 133.Sun Z, Wang Y, Wei Y, Liu R, Zhu H, Cui Y. et al. Ag cluster-aptamer hybrid: specifically marking the nucleus of live cells. Chem Commun (Camb) 2011;47:11960–11962. doi: 10.1039/c1cc14652a. [DOI] [PubMed] [Google Scholar]
- 134.Chen T, Gu T, Cheng L, Li X, Han G, Liu Z. Porous Pt nanoparticles loaded with doxorubicin to enable synergistic Chemo-/Electrodynamic Therapy. Biomaterials. 2020;255:120202. doi: 10.1016/j.biomaterials.2020.120202. [DOI] [PubMed] [Google Scholar]
- 135.Gu T, Wang Y, Lu Y, Cheng L, Feng L, Zhang H. et al. Platinum Nanoparticles to Enable Electrodynamic Therapy for Effective Cancer Treatment. Adv Mater. 2019;31:1806803. doi: 10.1002/adma.201806803. [DOI] [PubMed] [Google Scholar]
- 136.Chen T, Chu Q, Li M, Han G, Li X. Fe3O4@Pt nanoparticles to enable combinational electrodynamic/chemodynamic therapy. J Nanobiotechnol. 2021;19:206. doi: 10.1186/s12951-021-00957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zeng X, Wang Y, Han J, Sun W, Butt HJ, Liang XJ. et al. Fighting against drug-resistant tumors using a dual-responsive Pt(IV)/Ru(II) bimetallic polymer. Adv Mater. 2020;32:2004766. doi: 10.1002/adma.202004766. [DOI] [PubMed] [Google Scholar]