Abstract
Objectives
Early detection of aminoglycoside-induced acute kidney injury (AKI) is crucial in intensive care unit (ICU) patients, but it is not adequately reflected by serum creatinine (SrCr) levels. This study proposed investigating the relationship between amikacin trough levels and the development of nephrotoxicity using both conventional markers and a new biomarker of renal function in critically ill elderly patients.
Methods
Thirty-three critically ill patients aged ≥65 years with normal SrCr who received once-daily amikacin were evaluated. Trough levels of amikacin, creatinine clearance (CrCL) and urinary neutrophil gelatinase-associated lipocalin (uNGAL) were measured during the 10-day study period. The patients were divided into three groups and were compared based on the trough levels on both day 3 and day 7: <3 µg/mL (low trough (LT)), 3–6 µg/mL (moderate trough (MT)) and >6 µg/mL (high trough (HT)).
Results
In the LT group, neither CrCL nor uNGAL levels significantly changed from baseline (p=0.364 and p=0.562, respectively). In the MT group, the CrCL level altered significantly over time from baseline (p=0.007), but the uNGAL level did not change significantly over the study period (p=0.916). In the HT group, both CrCL and uNGAL levels significantly changed from baseline during the study period (p=0.002 and p=0.046, respectively).
Conclusions
In critically ill elderly patients with MT, the mean uNGAL level changed at least 4 days earlier than the SrCr level. Instead, the trough level of amikacin demonstrated a potential value for predicting subclinical AKI for implementing necessary interventions. Amikacin trough levels <3 µg/mL in the once-daily dosing regimen appeared safe, even in geriatric patients. Further studies are needed to confirm this finding.
Keywords: critical care, acute kidney injury, geriatrics, drug monitoring, drug-related side effects and adverse reactions
Introduction
Aminoglycosides have been used extensively for a long time to treat Gram-negative infections in intensive care units (ICUs).1 However, this group of antimicrobial agents has been shown to disturb renal function. Nephrotoxicity induced by aminoglycosides occurs in approximately 10%–20% of patients treated with these medications, and usually presents as a non-oliguric acute kidney injury (AKI). The onset of renal damage is slow, and serum creatinine (SrCr) typically rises after 5–7 days of therapy. In addition to these disturbances, various electrolyte abnormalities are infrequently observed.2 3 Critically ill patients who experience aminoglycoside nephrotoxicity have higher mortality rates than patients without AKI.4 Moreover, AKI is independently associated with an increased risk of some morbidities, including an increase in the costs of healthcare and the length of ICU or hospital stay, developing chronic kidney disease, and end-stage renal disease.5 Although AKI is often a preventable and treatable disorder, patients are missed in early diagnosis and management.6
Different strategies have been proposed to reduce aminoglycoside nephrotoxicity, and the once-daily dosing strategy is the most prevalent approach. In patients receiving once-daily dosing of aminoglycoside therapy, high trough levels have been related to nephrotoxicity.7 Elevated trough levels exceeding 2–3 µg/mL for tobramycin, gentamicin or netilmicin and exceeding 5 µg/mL for amikacin have been reported as a risk of aminoglycoside nephrotoxicity.8 Although maintaining levels within the therapeutic ranges would not entirely prevent the occurrence of AKI, it may decrease the severity of this complication.9 Other major risk factors that have been shown to increase the likelihood of aminoglycoside nephrotoxicity include prolonged duration of therapy, advanced age, comorbid disease, volume depletion, and concomitant nephrotoxic drug administration.10 In the meantime, close monitoring of elderly patients is essential as they represent the majority of patients admitted to ICUs. This population often suffers from multiple comorbidities that make them more susceptible to AKI complications.11
The diagnosis and aetiological classification of AKI largely depend on detecting changes in conventional endogenous surrogate kidney function markers, specifically SrCr, blood urea nitrogen level and, less frequently, other urinary tests.12 Despite the prevalent use of these traditional markers by clinicians at the bedside, they are insensitive and non-specific for the diagnosis of AKI because no single test reflects real-time dynamic changes in glomerular filtration rate and genuine kidney injury.13 Conversely, the SrCr level only begins to rise when more than 50% of the renal function is lost, which means that the identification of AKI occurs after a delay that constrains effective early interventions.14 Several novel biomarkers including urinary neutrophil gelatinase-associated lipocalin (uNGAL) have been identified for the early detection of AKI, and these may more reliably predict changes in renal function.15 16
The present study investigated the relationships between amikacin trough levels and the development of nephrotoxicity by using both conventional and novel biomarkers of renal function in critically ill elderly patients.
Methods
This was a post hoc analysis of a study that has already been published, which aimed to compare higher-than-standard doses of amikacin in critically ill elderly patients.17 The details of the study design and rationale have been reported previously. Briefly, 33 older patients aged over 65 years who had a normal SrCr level (<1.2 mg/dL), as well as an indication for treatment with amikacin following sepsis, were randomly assigned to either standard-dose (15 mg/kg) or high-dose (25 mg/kg) amikacin therapy. Amikacin was given to all patients on the once-daily regimen via a 1-hour intravenous infusion. Both peak and trough levels of amikacin were measured at days 3 and 7 to calculate pharmacokinetic parameters including the elimination constant rate (Kel) and volume of distribution (Vd). Peaks and troughs were drawn 30 min after the end and before the initiation of infusion, respectively. Urine samples were collected via the patient’s Foley catheter on days 0, 3, 5, 7 and 10 of the study. Samples were collected in a 5 mL plain tube (without anticoagulant) and then centrifuged for 5 min to precipitate cells. The NGAL ELISA kit was used to detect uNGAL (NGAL ELISA Kit; BioVendor Research and Diagnostic Products, Brno, Czech Republic). Renal function was also monitored in all patients using SrCr, uNGAL and urine output for at least 10 days. CrCL was estimated using the Cockcroft–Gault equation.18 All the included patients were divided into three groups based on the amikacin trough levels: group 1 (LT) had low trough levels of <3 µg/mL, group 2 (MT) had moderate trough levels of 3–6 µg/mL and group 3 (HT) had high trough levels of >6 µg/mL on one of the measured levels on the third or seventh days. Typical paper ICU charts were reviewed for each patient to extract relevant demographic, laboratory and clinical data, including age, sex, body weight, height, acute physiology and chronic health evaluation (APACHE) II score, sequential organ failure assessment (SOFA) score and biochemistry tests.
All the continuous variables were presented as mean±standard, and categorical variables are presented as n (%). The Kolmogorov–Smirnov normality test was performed on all data. Repeated measures ANOVA was used for comparison of trends over time between the three groups for each studied variable. Moreover, one-way ANOVA (parametric) or Kruskal–Wallis H tests (non-parametric) were used to test for statistical differences (two-tailed) between two independent groups. Two-sided Chi-square/Fisher’s exact tests were used to assess the associations between the groups and the categorical variables. For making an unbiased comparison between three groups in handling missing data, intention-to-treat (ITT) analysis was used. All the statistical analyses were done using SPSS software (Version 25). P values ˂0.05 were considered statistically significant.
Results
The three amikacin trough level groups were distributed as LT (n=7, 21.2%), MT (n=16, 48.5%) and HT (n=10, 30.3%). Baseline data were compared, and no significant differences were found to exist between the groups as regards age, gender, body weight, APACHE II, SOFA score, CrCL baseline and uNGAL baseline. Therefore, the three groups were matched based on all the baseline variables. Baseline demographics and clinical parameters of the patients are shown in table 1.
Table 1.
Baseline demographics and clinical parameters of the patients
| Variable/group | LT (trough <3 µg/mL) |
MT (trough 3–6 µg/mL) |
HT (trough >6 µg/mL) |
P value |
| (n=7) | (n=16) | (n=10) | ||
| Male (n, (%)) | 4 (57.1) | 11 (68.7) | 5 (50.0) | 0.646 |
| Age (years) | 68.6±6.0 | 73.7±7.0 | 74.6±9.4 | 0.168 |
| IBW (kg) | 61.8±17.2 | 58.3±7.8 | 57.6±8.0 | 0.944 |
| APACHE II score | 14.4±6.1 | 16.5±3.3 | 16.6±4.9 | 0.619 |
| SOFA score | 5.6±2.6 | 5.7±1.1 | 4.5±1.9 | 0.244 |
| CrCL | 74.5±24.6 | 57.5±13.8 | 57.8±14.5 | 0.226 |
| uNGAL | 11.1±9.3 | 23.4±23.8 | 31.3±37.2 | 0.209 |
APACHE, acute physiology and chronic health evaluation; CrCL, creatinine clearance; HT, high trough; IBW, ideal body weight; LT, low trough; MT, moderate trough; SOFA, sequential organ failure assessment; uNGAL, urine neutrophil gelatinase-associated lipocalin.
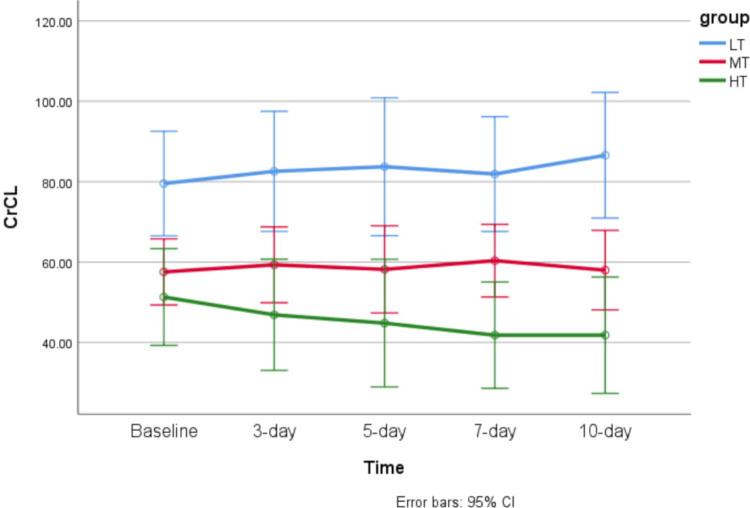
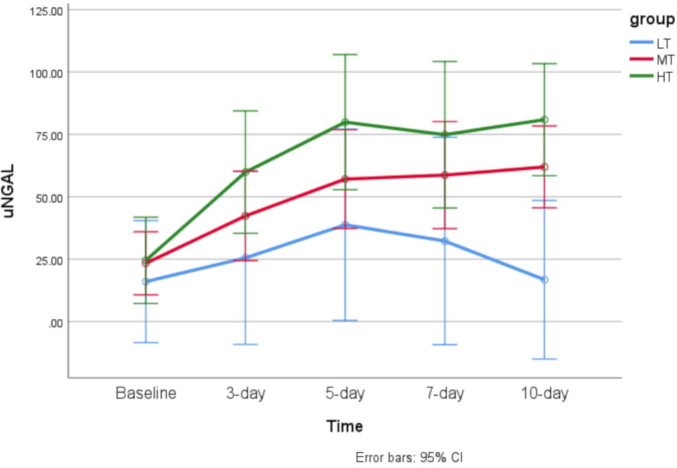
As presented in table 2, in the LT group, neither the CrCL nor uNGAL levels significantly changed from baseline during the 10-day study period (p=0.562 and p=0.364, respectively). In the MT group, the uNGAL level significantly rose over time from baseline during the 10-day study period (p=0.007). The CrCL level did not significantly alter during the study period (p=0.916). In the HT group, both the CrCL and uNGAL levels significantly changed from baseline during the 10-day study period (p=0.046 and p=0.002, respectively). Comparison of CrCL and uNGAL levels between the three groups at each time point indicated that the CrCL level was significantly different between the three groups on days 7 and 10 (day 3: p=0.079, day 5: p=0.070, day 7: p=0.002, day 10: p=0.012). Likewise, uNGAL levels were significantly different between the three groups on days 3, 5 and 10 (day 3: p=0.005, day 5: p=0.008, day 7: p=0.189, day 10: p=0.010).
Table 2.
Comparison of nephrotoxicity markers between the three groups during the 10-day study
| Parameter | Baseline | Day 3 | Day 5 | Day 7 | Day 10 | P value of linear trend time | ||||||
| uNGAL | CrCL | uNGAL | CrCL | uNGAL | CrCL | uNGAL | CrCL | uNGAL | CrCL | uNGAL | CrCL | |
| LT (n=7) |
11.1±8.6 | 74.5±22.7 | 18.8±24.8 | 78.9±23.5 | 27.8±31.7 | 78.8±30.8 | 34.0±37.7 | 81.9±23.7 | 16.7±14.4 | 86.5±28.0 | 0.364 | 0.562 |
| MT (n=16) |
23.3±23.1 | 57.4±13.4 | 41.2±29.9 | 59.5±15.6 | 56.4±36.2 | 58.7±15.9 | 59.5±39.4 | 60.5±15.0 | 61.9±32.2 | 57.9±14.9 | 0.007 | 0.916 |
| HT (n=10) |
31.3±35.2 | 57.8±13.8 | 70.2±36.8 | 55.0±15.0 | 86.6±30.5 | 48.3±13.4 | 74.8±26.7 | 42.4±5.8 | 80.8±28.0 | 45.2±12.4 | 0.002 | 0.046 |
| P value* | 0.209 | 0.226 | 0.005 | 0.079 | 0.008 | 0.070 | 0.189 | 0.002 | 0.010 | 0.012 | – | – |
All the continuous variables are presented as mean±SD. P value* is the p value for comparisons of the parameters between the three groups at each time point.
CrCL, creatinine clearance; HT, high trough; LT, low trough; MT, moderate trough; uNGAL, urine neutrophil gelatinase-associated lipocalin.
Linear trends of CrCL and uNGAL levels over time in the three groups are shown in figures 1 and 2, respectively.
Figure 1.
Error bar of time trend of creatinine clearance (CrCL) level over time in each of the studied groups. HT, high trough; LT, low trough; MT, moderate trough.
Figure 2.
Error bar of time trend of urine neutrophil gelatinase-associated lipocalin (uNGAL) level over time in each of the studied groups. HT, high trough; LT, low trough; MT, moderate trough.
Discussion
The results reveal that patients with low trough levels of amikacin (<3 µg/mL) might not experience renal side effects during the once-daily regimen. In the group whose mean trough level increased to more than 3 µg/mL, uNGAL began to rise from the fifth day of treatment, and in the group whose mean trough level was above 6 µg/mL, uNGAL rose more rapidly (from the third day). In the latter group, CrCL also began to decline with a 4-day delay (from the seventh day of treatment). The most remarkable study finding is that the patients in the MT group (amikacin trough level of 3–6 µg/mL) who had increased uNGAL levels in the absence of SrCr elevation do not fulfil AKI criteria. However, subclinical AKI may be the potential scenario.19 If the patients continue to be exposed to the toxic insult (in our study, a supra-therapeutic level of an aminoglycoside), the SrCr levels may alter with a significant delay. These results are in good agreement with the findings of a recent study by McWilliam et al,20 which demonstrated the potential validity of urinary renal biomarkers to identify aminoglycoside-induced nephrotoxicity in patients with cystic fibrosis. These authors concluded that kidney injury molecule-1 (KIM-1) might be considered to be a biomarker of subclinical AKI during tobramycin exposure.20 The results of an experimental study in rats suggested the potential use of urinary KIM-1 as a highly specific biomarker for the early diagnosis of gentamicin-induced proximal tubular injury.21
Moreover, several studies have validated novel biomarkers for detecting drug-induced subclinical AKI that may help in the earlier implementation of interventions to decrease the risk of complications.22–24 There is a paucity of data about using drug plasma levels for detection of early or subclinical stages of kidney injury.25 26 It has been reported that an amikacin trough level above 5 µg/mL would increase the risk of nephrotoxicity.27 However, this threshold may not be used as an indication to predict AKI promptly and probably cannot be employed to detect subclinical AKI, due to the use of SrCr as a gold standard for AKI definition. Moreover, SrCr is a late marker of aminoglycoside nephrotoxicity. These agents initially cause proximal tubular cell damage, which may not be adequately reflected by a conventional kidney marker such as SrCr.28
The present study's results specify that patients with MT are at high risk of a further decrease in renal function because of the escalating uNGAL level compared with baseline. Based on the detection of subclinical AKI in this group of patients by means of a novel biomarker, a new threshold of renal toxicity for amikacin trough levels has been suggested. Maintaining the amikacin trough level below the threshold of 3 µg/mL in the once-daily dose regimen can decrease drug nephrotoxicity substantially. Conversely, it can be stated that the new biomarkers (or at least uNGAL) can detect subclinical AKI and help clinicians to initiate preventive strategies in a timelier manner, even though the exact threshold of uNGAL levels that predict nephrotoxicity and other potential causes of its rise still need be determined. The fact that the patients in this study were elderly ought not to be ignored since older patients are more vulnerable to AKI development, even with lower trough levels of aminoglycosides.29 Furthermore, critically ill patients frequently have multiple conditions, for example, sepsis, shock and coadministration of other nephrotoxic medications that can potentiate the drug-induced nephrotoxicity.30
To the best of our knowledge, the current study is the first to evaluate the relationship between kidney biomarker levels and amikacin serum concentrations. Such an evaluation may lead to the discovery of a new threshold of nephrotoxicity for amikacin trough levels in geriatric critically ill patients. Further studies with larger sample sizes will, however, be needed to better understand the optimum use of aminoglycosides in elderly patients with sepsis.
Conclusions
We found that in critically ill elderly patients with amikacin trough levels of 3–6 µg/mL, a change in uNGAL levels was observed at least 4 days earlier than the SrCr level during a once-daily regimen. Owing to the fact that treatment of serious infections in ICUs is extremely vital, amikacin trough levels less than 3 µg/mL in a once-daily dosing regimen appear safe, even in geriatric patients. Conversely, the trough level of amikacin, where available, has potential value for use in cases of subclinical AKI to prevent more complications. In our view, new tools such as trough levels and novel kidney biomarkers (ie, uNGAL) may be beneficial in the early detection of aminoglycoside-induced AKI and improvement of the clinical outcomes in critically ill elderly patients. Further studies are, however, needed to confirm this finding.
What this paper adds.
What is already known on this subject
Aminoglycoside-associated acute kidney injury typically manifests as a rise in serum creatinine. However, creatinine elevation significantly delays the diagnosis of this complication in critically ill patients.
An elevated trough level has been shown to correlate with nephrotoxicity in older adults.
What this study adds
The present study confirms the trough level/nephrotoxicity correlation.
Additionally, the results reveal that urinary neutrophil gelatinase-associated lipocalin may be a useful, non-invasive biomarker for identifying aminoglycoside nephrotoxicity promptly in critically ill elderly patients.
Acknowledgments
The authors are grateful to all the patients who participated in this study.
Footnotes
Contributors: Conceptualisation: KS, HH, MM. Methodology: KS, HH, MM. Investigation: KS, HH, MM. Formal analysis and interpretation of results: KS, BS, FHF. Writing–original draft: KS, BS. Writing–review, editing and approval: KS, BS, FHF, HH, MM.
Funding: This research was supported by Tehran University of Medical Sciences.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The University Review Board/University Research Council of Tehran University of Medical Sciences reviewed and confirmed both research and ethical aspects of this study (Approval Number 91-03-33-19352).
References
- 1. Becker B, Cooper MA. Aminoglycoside antibiotics in the 21st century. ACS Chem Biol 2013;8:105–15. 10.1021/cb3005116 [DOI] [PubMed] [Google Scholar]
- 2. Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. J Pharm Pract 2014;27:573–7. 10.1177/0897190014546836 [DOI] [PubMed] [Google Scholar]
- 3. Paquette F, Bernier-Jean A, Brunette V, et al. Acute kidney injury and renal recovery with the use of aminoglycosides: a large retrospective study. Nephron 2015;131:153–60. 10.1159/000440867 [DOI] [PubMed] [Google Scholar]
- 4. Oliveira JFP, Silva CA, Barbieri CD, et al. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother 2009;53:2887–91. 10.1128/AAC.01430-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashemian SM, Jamaati H, Farzanegan Bidgoli B, et al. Outcome of acute kidney injury in critical care unit, based on AKI network. Tanaffos 2016;15:89. [PMC free article] [PubMed] [Google Scholar]
- 6. Potter DA, Wroe N, Redhead H, et al. Outcomes in patients with acute kidney injury reviewed by Critical Care Outreach: what is the role of the National Early Warning Score? J Intensive Care Soc 2017;18:300–9. 10.1177/1751143717715968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raveh D, Kopyt M, Hite Y, et al. Risk factors for nephrotoxicity in elderly patients receiving once-daily aminoglycosides. QJM 2002;95:291–7. 10.1093/qjmed/95.5.291 [DOI] [PubMed] [Google Scholar]
- 8. Beaucaire G. Does once-daily dosing prevent nephrotoxicity in all aminoglycosides equally? Clin Microbiol Infect 2000;6:355–60. 10.1046/j.1469-0691.2000.00105.x [DOI] [PubMed] [Google Scholar]
- 9. Plaut ME, Schentag JJ, Jusko WJ. Aminoglycoside nephrotoxicity: comparative assessment in critically ill patients. J Med 1979;10:257–66. [PubMed] [Google Scholar]
- 10. Gerlach AT, Stawicki SP, Cook CH, et al. Risk factors for aminoglycoside-associated nephrotoxicity in surgical intensive care unit patients. Int J Crit Illn Inj Sci 2011;1:17. 10.4103/2229-5151.79277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pongsittisak W, Phonsawang K, Jaturapisanukul S, et al. Acute kidney injury outcomes of elderly and nonelderly patients in the medical intensive care unit of a university hospital in a developing country. Crit Care Res Pract 2020;2020:1–7. 10.1155/2020/2391683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31–8. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shahrami B, Najmeddin F, Rouini MR, et al. Evaluation of amikacin pharmacokinetics in critically ill patients with intra-abdominal sepsis. Adv Pharm Bull 2020;10:114–8. 10.15171/apb.2020.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endre ZH, Westhuyzen J. Early detection of acute kidney injury: emerging new biomarkers. Nephrology 2008;13:91–8. 10.1111/j.1440-1797.2007.00905.x [DOI] [PubMed] [Google Scholar]
- 15. Oh D-J. A long journey for acute kidney injury biomarkers. Ren Fail 2020;42:154–65. 10.1080/0886022X.2020.1721300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beker BM, Corleto MG, Fieiras C, et al. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol 2018;50:705–13. 10.1007/s11255-017-1781-x [DOI] [PubMed] [Google Scholar]
- 17. Sadeghi K, Hamishehkar H, Najmeddin F, et al. High-dose amikacin for achieving serum target levels in critically ill elderly patients. Infect Drug Resist 2018;11:223–8. 10.2147/IDR.S150839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 19. Haase M, Kellum JA, Ronco C. Subclinical AKI--an emerging syndrome with important consequences. Nat Rev Nephrol 2012;8:735–9. 10.1038/nrneph.2012.197 [DOI] [PubMed] [Google Scholar]
- 20. McWilliam SJ, Antoine DJ, Jorgensen AL, et al. Urinary biomarkers of aminoglycoside-induced nephrotoxicity in cystic fibrosis: kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin. Sci Rep 2018;8:1–9. 10.1038/s41598-018-23466-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Udupa V, Prakash V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol Rep 2019;6:91–9. 10.1016/j.toxrep.2018.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tajima S, Yamamoto N, Masuda S. Clinical prospects of biomarkers for the early detection and/or prediction of organ injury associated with pharmacotherapy. Biochem Pharmacol 2019;170:113664. 10.1016/j.bcp.2019.113664 [DOI] [PubMed] [Google Scholar]
- 23. Tanase DM, Gosav EM, Radu S, et al. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int J Mol Sci 2019;20:5238. 10.3390/ijms20205238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al-Naimi MS, Rasheed HA, Hussien NR, et al. Nephrotoxicity: role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res 2019;10:95. 10.4103/japtr.JAPTR_336_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones TE, Peter JV, Field J. Aminoglycoside clearance is a good estimate of creatinine clearance in intensive care unit patients. Anaesth Intensive Care 2009;37:944–52. 10.1177/0310057X0903700611 [DOI] [PubMed] [Google Scholar]
- 26. Shahrami B, Najmeddin F, Ghaffari S, et al. Area under the curve-based dosing of vancomycin in critically ill patients using 6-hour urine creatinine clearance measurement. Crit Care Res Pract 2020;2020:1–6. 10.1155/2020/8831138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tod M, Lortholary O, Seytre D, et al. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob Agents Chemother 1998;42:849–56. 10.1128/AAC.42.4.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tzovaras V, Tsimihodimos V, Kostara C, et al. Aminoglycoside-Induced nephrotoxicity studied by proton magnetic resonance spectroscopy of urine. Nephrol Dial Transplant 2011;26:3219–24. 10.1093/ndt/gfr074 [DOI] [PubMed] [Google Scholar]
- 29. Bertino JS, Booker LA, Franck PA, et al. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 1993;167:173–9. 10.1093/infdis/167.1.173 [DOI] [PubMed] [Google Scholar]
- 30. Cartin-Ceba R, Kashiouris M, Plataki M, et al. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012;2012:1–15. 10.1155/2012/691013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.