Abstract
Background
Stroke is a devastating disease, including intracerebral haemorrhage (ICH) and ischaemic stroke. Emerging evidences indicate that systemic inflammatory cascades after stroke contribute to brain damage. However, the direct effects and features of systemic inflammation on brain injury, especially comparing between ischaemic and haemorrhagic stroke, are still obscure.
Methods
Pertussis toxin (PT) was used to build a pro-inflammatory milieu after ICH and ischaemic stroke in mouse model. The neurodeficits, stroke lesion, immune response and blood–brain barrier (BBB) destruction were assessed.
Results
In ICH mouse model, PT-induced systemic inflammation exacerbated neurological deficits, and enlarged haemorrhage lesion and perihaematomal oedema. We also found promoted leucocyte infiltration and inflammatory cytokine release into the brain after PT treatment. Moreover, the integrity of the BBB was further disrupted after receiving PT. Furthermore, we demonstrated that PT enhanced brain inflammation and aggravated stroke severity in middle cerebral artery occlusion mouse model.
Conclusions
Our results suggest that PT increases inflammatory response that exacerbates brain injury after ICH or ischaemic stroke in mouse model.
Keywords: haemorrhage
Background
Stroke is an acute and severe disease resulting in long-term motor and cognitive neurological deficits, with the characteristics of high morbidity, mortality, disability rate and recurrence rate, including intracerebral haemorrhage (ICH) and ischaemic stroke.1 2 At present, although progress has been made in understanding the molecular and cellular pathways leading to brain injury after stroke, the current clinical treatments remain poorly effective. ICH accounts for about 10% to 15% of all strokes. The damage from ICH includes the primary tissue injury due to the mechanical effects of the haemorrhage and also the development of perihaematomal oedema (PHO), which induces a severe secondary injury and/or destruction of the adjacent tissue, in addition to an impairment in the integrity of the blood–brain barrier (BBB).3 4 Ischaemic stroke constitutes 85% of all strokes. In ischaemic brain injury, the neuronal cell death induced by deprivation of glucose and oxygen orchestrates a secondary immune response, which participates in the progression of neuronal damage, BBB disruption and vasogenic oedema after ischaemia/reperfusion (I/R).5 6 Although the primary injury mechanisms differ between acute ischaemic stroke and ICH, recent evidence has shown that inflammation contributes to the development and progression of oedema, and thus subsequent neurological deterioration in ischaemic or haemorrhagic stroke. On the other hand, the inflammation after ischaemic stroke or ICH also plays a key role in the tissue reconstruction and brain recovery.7 8 So whether the direct inflammatory response after stroke is deleterious or beneficial remains a matter of controversies.
Pertussis toxin (PT), an exotoxin produced by Bordetella pertussis, is essential for B. pertussis infection.9 Many lines of evidence show that PT plays an important role in neurological complications of whooping cough.10 In stroke model models, PT has been proved to reduce neuronal calcium influx, thus minimising neuronal damage and protecting cell viability after ischaemic stroke.11 However, PT administration also concomitantly induces high frequencies of neuroantigen-specific IFN-γ-producing and IL-17-producing T cells that boost inflammatory response in autoimmune disease model, such as experimental autoimmune encephalomyelitis.12 Therefore, the use of PT offers an opportunity to investigate the features and direct effect of systematic inflammation on brain injury following ischaemic stroke and haemorrhagic stroke. In this study, we adopted the ICH model by injection of collagenase and used a mouse model of transient cerebral ischaemia and reperfusion. We administered PT to mice and quantified neurological function and brain pathology. Our results provide direct evidence that PT-induced inflammation exacerbated brain injury in haemorrhagic or ischaemic stroke.
Materials and methods
Animals
Male C57BL/6 mice were purchased from Charles River (Wilmington, DE, USA). Age-matched male littermates 8 to 10 weeks old, 20–25 g body weight, were used in this study. All mice were randomly assigned to each experiment. All mice were kept in pathogen-free conditions and housed under a 12-hour inverted light–dark cycle with access to food and water ad libitum. Reporting of this study complied with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines.13 14
PT administration
Pertussis toxin (List Biological Laboratories, Campbell, CA) was dissolved in distilled-deionised water followed by dilution with phosphate-buffered saline (PBS) under sterile conditions. Also, PT was administered intraperitoneally at a dose of 250 ng or 500 ng (1 ng/µL) per mouse immediately after surgery. The same volume of PBS was given in the vehicle group.
ICH and middle cerebral artery occlusion (MCAO) induction, neuroimaging and behaviour testing
Adult male mice 10 to 12 weeks old were subjected to transient 60 min intraluminal occlusion of MCA or injected collagenase in intra-striatal as previously described.15 16 The modified Neurological Severity Score (mNSS) test and the rota-rod test were performed to evaluate motor, sensory, reflex and balance. Details of MCAO and ICH procedures, MRI scan, reactive oxygen species (ROS) generation and behaviour testing are provided in online supplemental experimental procedures.
svn-2021-000987supp001.pdf (150.7KB, pdf)
Cortical cerebral blood flow (CBF) measurements and immunostaining
Cortical CBF was monitored by a laser speckle technique, as previously described.17 The immunostaining was performed as we previously described.18 More details could be found in online supplemental experimental procedures.
Flow cytometry and evaluation of BBB permeability
Flow cytometry was performed to analyse immune cell infiltration of brain.19 In addition, the BBB permeability was assessed by Evans Blue (EB) dye. More details are given in online supplemental experimental procedures.
Cytokine array
Inflammatory cytokines in brain tissues were analysed by Proteome Profiler Mouse XL Cytokine Array. Brain homogenates were prepared from ICH mice treated with or without PT at day 3 after ICH. After the total protein concentration was adjusted to 1 mg/mL, cytokine levels in these samples were detected using a Mouse XL Cytokine Array Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis
Details on statistical analysis are given in online supplemental experimental procedures.
Results
PT augments neurodeficits and brain edema after ICH
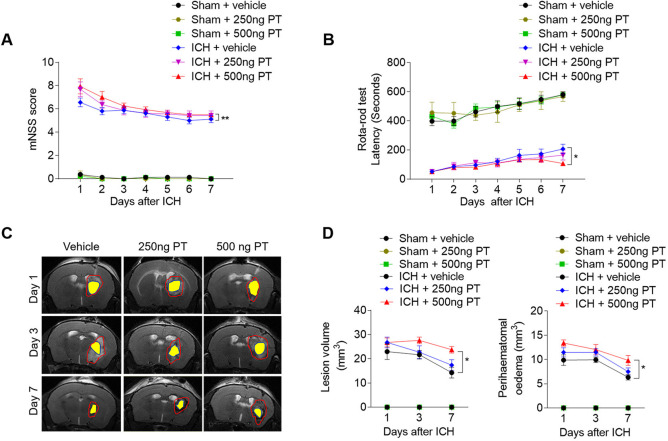
Mice were treated with PT (250 ng or 500 ng) or vehicle (PBS) by intraperitoneal injection immediately after ICH. To determine the impact of PT on brain injury after ICH, we examined neurological deficits, lesion volume and PHO in ICH mice receiving PT or vehicle. After ICH induction from day 1 to day 7, PT administration at a dose of 500 ng aggravated neurodeficits, and enlarged lesion size and PHO as compared with ICH group (figure 1A–D). In addition, PT injection does not affect the baseline of the neurodeficits score in sham group (figure 1A, B). These results indicate that 500 ng PT administration exacerbates collagenase-induced haemorrhagic brain injury. Thus, we adopted 500 ng dose of PT for the following investigation.
Figure 1.
Pertussis toxin (PT) increases stroke severity in intracerebral haemorrhage (ICH) mice. ICH was induced by injection of 0.0375 U collagenase. After surgeries, mice were treated with PT immediately by intraperitoneal injection at a dose of 250 ng or 500 ng or vehicle. Mice were subjected to neurological assessment and MRI scanning until day 7 after ICH. (A, B) Cumulative data illustrate the neurological assessments of ICH mice and sham mice receiving PT or vehicle from day 1 to day 7 after surgery, including modified Neurological Severity Score (mNSS) (A) and rota-rod test (B). n=8 mice per group from three independent experiments. Data were expressed as mean±SEM; *p<0.05; **p<0.01. (C) Representative MR images show lesion area and haematoma area in ICH mice receiving PT or vehicle. Red lines delineate lesion area, yellow shadows represent haematoma area and perihaematomal oedema volume was calculated by subtracting the haematoma volume from lesion volume. (D) Cumulative data show the lesion volume and perihaematomal oedema volume of ICH mice receiving PT or vehicle. n=4 mice per group from two independent experiments. Data were expressed as mean±SEM; *p<0.05.
PT enhances brain inflammation after ICH
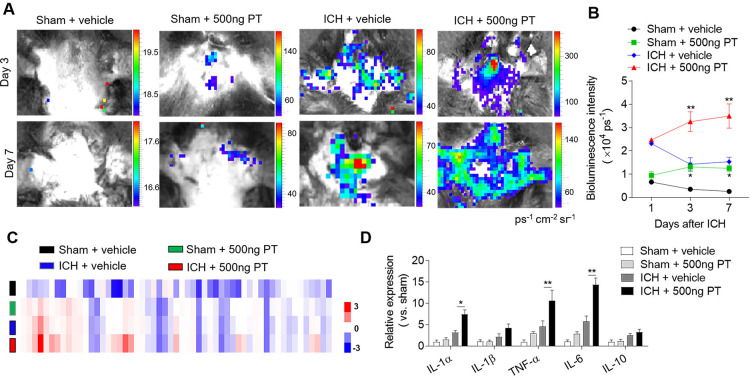
Reactive oxygen species (ROS) induce oxidative stress, activate alternative death pathways and play an important role in the progression of inflammation. To examine the impact of PT on brain inflammation after ICH, we quantified the ROS production using in vivo bioluminescence at day 1, day 3 and day 7 after ICH. As shown in figure 2, PT administration induced the production of ROS even in sham mice, suggesting the pro-inflammatory effect of PT. ROS levels were significantly higher in ICH mice treated with PT relative to vehicle at day 3 and day 7 after ICH (figure 2A, B). We next assessed the release of inflammatory factors in the brain homogenates of ICH mice after PT administration using a Proteome Profiler Mouse XL Cytokine Array. At 24 hours after ICH surgery, we found that PT treatment significantly upregulated the expression of pro-inflammatory cytokines including IL-1, TNF-α and IL-6 (figure 2C, D). These data show that PT exacerbates ICH-induced brain inflammation.
Figure 2.
Pertussis toxin (PT) enhances brain inflammation after intracerebral haemorrhage (ICH). ICH in mice was induced using 0.0375 U collagenase injection. PT treatment was given immediately after ICH by intraperitoneal injection, at a dose of 500 ng. (A, B) At day 1, day 3 and day 7 after ICH, representative bioluminescence images and quantification analysis show reactive oxygen species generation in sham and ICH mice receiving PT or vehicle. n=3 mice per group from two independent experiments. (C) Brain tissues were obtained from ICH mice receiving PT or vehicle. Sham-operated mice receiving PT or vehicle were used as control. Brain homogenates were analysed by a Mouse XL Cytokines Array kit. Heat map and cluster analysis show the expression of inflammatory factors in brain homogenates from sham and ICH mice with indicated treatments. A heat map was generated and the relative pixel intensity of spots signal is indicated by the representative colour code (red, upregulated; blue, downregulated). (D) Bar graphs show the top significantly dysregulated factors. n=6 mice per group. The data were calculated as mean±SEM; *p<0.05; **p<0.01.
PT promotes brain-infiltrating leucocytes after ICH
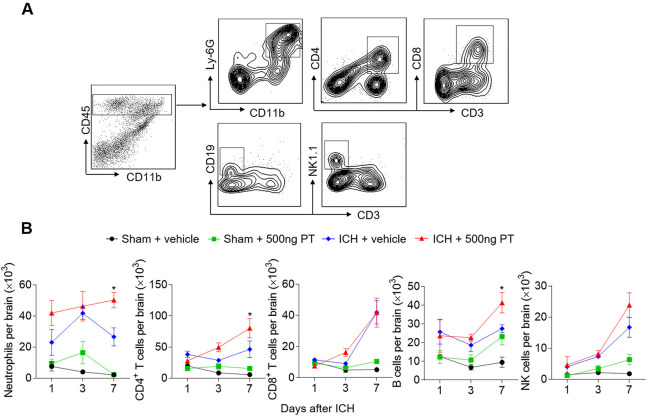
Infiltrating leucocytes are a prominent contributor to brain inflammation and brain damage. To determine the impact of PT on leucocyte infiltration, we gated and quantified the numbers of brain-infiltrating leucocytes in ICH mice and sham mice treated with PT or vehicle (figure 3). PT treatment did not significantly influence immune cell infiltration in sham mice from day 1 to day 7 (figure 3B). We found infiltrated immune cells after ICH induction, including neutrophils, T cells, B cells and NK cells. Moreover, at day 7 after ICH, brain-infiltrating neutrophils, CD4+ T cells and B cells were further increased in ICH mice treated with 500 ng PT as compared with vehicle (figure 3B). These data show that PT promotes ICH induced leucocyte infiltration in the brain.
Figure 3.
Pertussis toxin (PT) promotes brain-infiltrating leucocytes after intracerebral haemorrhage (ICH). ICH was induced by 0.0375 U collagenase injection. At day 1, day 3 or day 7 after ICH, single cell suspensions were isolated from brain tissues of ICH mice treated with PT or vehicle. (A) Gating strategy of brain-infiltrating immune cells including neutrophils (CD45highCD11b+Ly6G+), CD4+ T cells (CD45highCD3+CD4+), CD8+ T cells (CD45highCD3+CD8+), B cells (CD45highCD3−CD19+), and NK cells (CD45highCD3−NK1.1+). (B) Quantification of lymphocytes, monocytes and neutrophils in the brain of sham and ICH mice receiving PT or vehicle at indicated time points. Day 1 after ICH, n=3 mice per group from two independent experiments; day 3 and day 7 after ICH, n=5 mice per group from three independent experiments. The data were calculated as mean±SEM; *p<0.05.
PT aggravates haemorrhagic stroke injury through of BBB breakdown
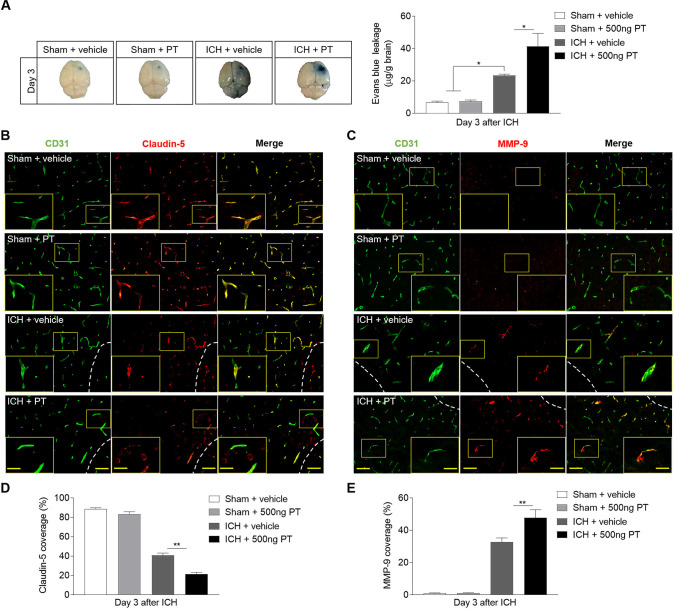
BBB dysfunction after ICH play a key role in vasogenic brain oedema, PHO expansion and system immune cell invasion. Meanwhile matrix metalloproteinase-9 (MMP-9) contributes to the further disruption of BBB under inflammatory conditions.4 The BBB breakdown is characterised by extravasation of large circulating molecules, such as EB, which binds tightly to serum albumin. PT displayed aggravated BBB disruption on ICH, as documented by increased EB leakage (figure 4A). The tight junction (TJ) proteins between adjacent endothelial cells are responsible for the extremely low paracellular permeability the BBB. During stroke, the BBB integrity is damaged due to the degradation of these proteins.20 To examine the impact and possible mechanisms of PT on the integrity of the BBB after ICH, we assessed the levels of TJ protein claudin-5 and MMP-9 by immunostaining (figure 4B–E). As indicated, ICH mice subjected to PT treatment demonstrated reduced claudin-5 coverage compared with ICH mice treated with vehicle (figure 4B, D). Also, we found augmented expression of MMP-9 in ICH mice treated with PT as compared with vehicle (figure 4C, E). However, no significant effects of PT were observed on EB extravasation and expression levels of claudin-5 or MMP-9 on sham mice (figure 4A-E). These data suggest that PT treatment aggravated the TJ breakdown, resulting in the increased disruption of BBB after ICH. Also, the loss of claudin-5 caused by PT was mainly related to the degraded effect of increased MMP-9. Meanwhile, the BBB destruction caused by PT may contribute to enhanced inflammation and worsen brain injury after ICH.
Figure 4.
Pertussis toxin (PT) increases the permeability of blood–brain barrier after intracerebral haemorrhage (ICH). ICH was induced by 0.0375 U collagenase injection. (A) Representative dorsal surfaces and quantification analysis show the Evans Blue leakage of brain at day 3 after surgeries. n=6 mice per group from three independent experiments. (B, C) Representative immunostaining for CD31 (green) and claudin-5 (red) and MMP-9 (red) in ipsilateral hemisphere from ICH and sham mice treated with 500 ng PT or vehicle at day 3 after onset. White lines delineate lesion area. Scale bar: 50 µm, insert: 25 µm. (D, E) Quantification of claudin-5 and MMP-9 coverage in sham mice and peri-lesion area of ICH mice receiving PT or vehicle at the indicated time. n=9 sections from 3 mice each group. Data were expressed as mean±SEM; *p<0.05; **p<0.01.
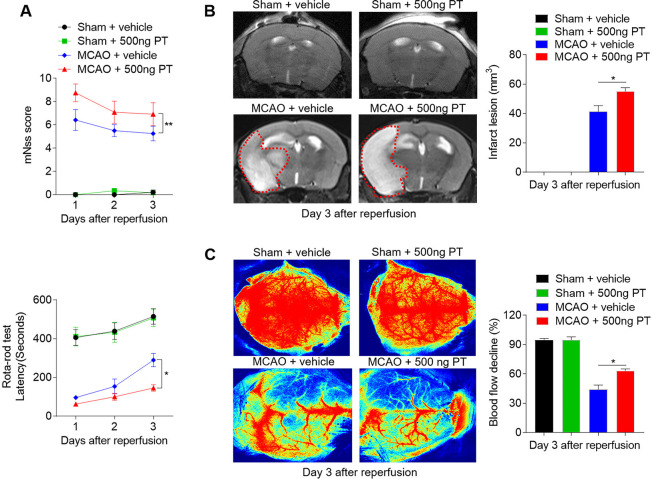
PT worsens neurological deficits and enlarges infarct lesion after ischaemic stroke in mice
A prominent inflammatory response occurs following haemorrhagic and also ischaemic stroke, thereby exacerbating secondary injury.21 To detect the influences of PT on ischaemic brain injury, we assessed neurological deficits and ischaemic lesion in MCAO mice treated with 500 ng PT or vehicle. From day 1 to day 3 after MCAO and reperfusion, the mice treated with PT exhibited higher mNSS and shorter latency in the rota-rod test compared with controls, as well as enlarged infarct lesion (figure 5A–C). In contrast, PT treatment did not significantly alter neurological function in sham control (figure 5A). These data suggest that PT also exacerbates acute ischaemic brain injury.
Figure 5.
Pertussis toxin (PT) exacerbates neurological deficits and brain infarction after cerebral ischaemia and reperfusion. Mice were treated with 500 ng PT or vehicle immediately by intraperitoneal injection after 60 min of middle cerebral artery occlusion (MCAO) and reperfusion. Neurological deficits, lesion volume and the cerebral blood flow (CBF) were evaluated in MCAO mice. (A) Summarised results show the modified Neurological Severity Scores and the rota-rod test latency of MCAO mice and sham mice receiving PT or vehicle at the indicated times. n=6 mice per group from three independent experiments. (B) Representative MR images show infarct area (outlined in red) in MCAO mice and sham mice receiving PT or vehicle. Bar graphs show the infarct lesion at day 3 after ischaemia/reperfusion. n=4 mice per group. (C) Images of CBF in MCAO mice that received PT or vehicle treatment. Quantification of reduced blood flow in the ipsilateral hemisphere. The data were calculated as mean±SEM; *p<0.05, **p<0.01.
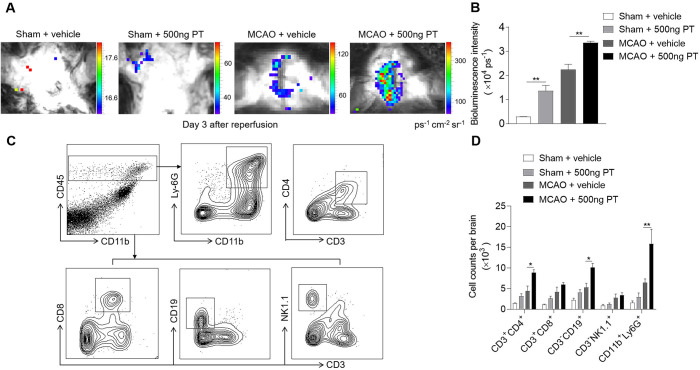
PT augments brain inflammation and enhances leucocyte infiltration after brain ischaemia
To understand the impact of PT on brain inflammation after brain ischaemia, we quantified the ROS levels using in vivo bioluminescence at day 3 after MCAO and reperfusion. In sham mice, ROS level was higher in PT group as compared with vehicle. Also, we found dramatically increased ROS signals in MCAO mice treated with PT relative to vehicle at day 3 after ischaemic stroke (figure 6A, B). These data demonstrate the pro-inflammatory effect of PT in ischaemic brain.
Figure 6.
Pertussis toxin (PT) augments brain inflammation after brain ischaemia and reperfusion. (A, B) Visualisation of reactive oxygen species generation, in vivo bioluminescence imaging and quantification of signal strength in sham and middle cerebral artery occlusion (MCAO) mice given PT or vehicle at day 3 after ischaemia and reperfusion. n=3 mice per group from two independent experiments. (C) Counts of central nervous system–infiltrating immune cell subsets were measured using flow cytometry on day 3 after reperfusion. Representative flow cytometry plots show the gating strategy of leucocyte subpopulations isolated from the brain. (D) Summarised results show the cell counts of the indicated subsets in the brain of sham and MCAO mice receiving PT or vehicle. n=4 mice per group. The data were calculated as mean±SEM; *p<0.05, **p<0.01.
Recruitment of leucocytes occurs in successive waves in the early phase of cerebral ischaemia, which significantly influence the pathogenesis of ischaemic brain injury.1 Thus, we tested whether PT treatment can impact immune cell infiltration. Flow cytometric results indicated that PT increased the brain infiltration of leucocytes, especially neutrophils, CD4+ T cells and B cells in MCAO mice on day 3 after ischaemia (figure 6C, D). These results suggest that PT expands inflammatory milieu of the ischaemic brain during the acute stage.
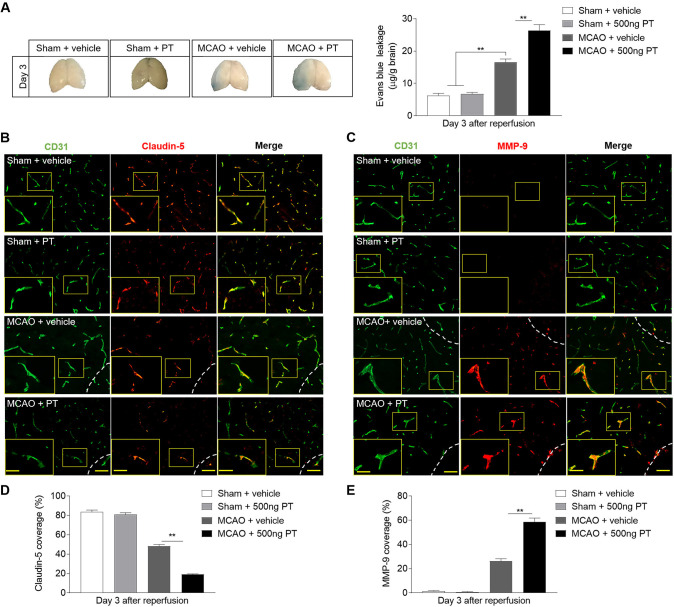
PT aggravates ischaemic stroke injury through BBB destruction
The initial BBB breakdown occurs 3 hours after I/R by inflammatory mediator upregulation and MMP activation, which facilitates injury progression. Therefore, EB leakage, and claudin-5 and MMP-9 staining were adopted to identify the effect and the potential mechanism of PT on the permeability of BBB. As shown in figure 7, the MCAO mice treated with PT showed a significant increased extent of EB dye extravasation at day 3 after brain ischaemia (figure 7A), and which was relevant to the capacity of PT to increase claudin-5 degradation and MMP-9 expression (figure 7B–E). Also, in sham control, no significant differences were found (figure 7A–E). These results allow us to speculate that MMP-9 opens the BBB by degrading the TJ protein claudin-5. These data support that the further destruction of BBB caused by PT may promote immune cell migration and exacerbate brain injury after cerebral ischaemia.
Figure 7.
Pertussis toxin (PT) enhances the permeability of blood–brain barrier after ischaemia. (A) Photographs of the dorsal surfaces of brain display Evans Blue extravasation at day 3 after ischaemia and reperfusion, and quantification of Evans Blue dye leakage at day 3 after surgery in the indicated groups. (B, C) Representative images show staining of CD31 (green) and claudin-5 (red) and MMP-9 (red) in brain sections from middle cerebral artery occlusion (MCAO) and sham mice at day 3 after ischaemia and reperfusion. Infarct areas are outlined in white dotted line. Scale bar: 50 µm, insert: 25 µm. (D, E) Summarised results show the claudin-5 and MMP-9 positive areas covered to the total endothelial surface area (CD31) in the sham mice and peri-infarct of MCAO mice at day 3 after MCAO. n=3 mice in each group from three independent experiments. The data were calculated as mean±SEM; **p<0.01.
Discussion
This study provides evidence that PT-induced inflammation exacerbates brain injury after ICH or ischaemic stroke. As documented here, PT aggravates neurological deficits, lesion size and brain PHO after ICH. PT increased ROS generation, and enhanced BBB permeability, leucocyte infiltration and inflammatory cytokine production in the brain, which may contribute to the aggravated brain injury after ICH. In MCAO model, PT augments brain inflammation and exacerbates stroke severity in a similar way. These results suggest that enhanced inflammatory response by PT exacerbates brain injury after ICH or ischaemic stroke.
Mounting evidence have shown that systemic inflammatory response plays a critical role in the secondary injury process of stroke, and the extent of neuronal damage seemed to correlate with the degree of immune response.22 23 For example, the inhibition of vascular adhesion protein-1 attenuated adhesion and transmigration of circulating immune cells to the site of local injury and ameliorated brain injury during acute phase in ICH and ischaemic stroke.24 25 The sphingosine-1-phosphate receptor agonist, siponimod or fingolimod, confirmed a neuroprotective effect in ischaemic or haemorrhagic stroke experimentally and clinically, which reduced brain oedema and improved neurological outcome by preventing the egress of peripheral lymphocytes from peripheral stores.26 27 Despite these studies, there is lack of direct evidence about the effect of systemic inflammation on brain injury in the early stage of stroke. In line with previous studies, we show that PT-induced systemic inflammation directly exacerbates brain injury in ICH and MCAO mouse model. These results suggest a detrimental impact of systemic inflammatory response at least in the early phase of stroke.
Primary brain injury occurs immediately after the onset of stroke and is often irreversible. In ICH, primary brain injury is caused by the tissue disruption due to the parenchymal blood accumulation and the mechanical effect damage associated with the mass effect.28 In ischaemic stroke, the arterial occlusion results in the death of neural cells, engendering an ischaemic core, surrounded by a hypoperfused region termed the penumbra.1 7 Despite the different primary injury mechanisms, the release of damage-associated molecular patterns defines a common pathway that triggers the innate and adaptive immune response in the brain, which induces the secondary brain injury in stroke.29 PT mainly contributes to the secondary injury by increasing permeability of BBB and infiltration of the inflammatory cells to the central nervous system. Therefore, PT has a similar effect to brain injury in ICH and ischaemic stroke, which was proved by our results.
MMP-9 is a member of the MMP family and belongs to the group of gelatinases.30 Various studies in human and mice brains show that the protein level of MMP-9 is increased both in ischaemic and haemorrhagic stroke,31 32 which is consistent with our results. At the acute phase, these changes in MMP-9 protein level result in aberrant proteolysis that contributes to BBB dysfunction.33 Also, Toft-Hansen and colleagues proved that the parenchymal brain infiltration induced by PT was associated with changes in expression of MMP genes in an animal model of multiple sclerosis.34 Combining with our findings, these reveal that MMP-9 mediates PT-induced BBB destruction in ischaemic and haemorrhagic stroke.
Inflammatory cells and inflammatory mediators have a major role in the pathology of secondary brain damage by exacerbating BBB damage, microvascular failure, brain oedema, oxidative stress and by directly inducing neuronal cell death.3 8 In our study, we found that PT increased the infiltration of systemic immune cells, specifically neutrophils, CD4+ T cells and B cells, to the brain parenchyma. In addition, with the increase of systemic immune cells accumulating in the brain, there was a marked increase in pro-inflammatory cytokines and an increase in ROS levels. These events in turn led to oedema expansion and worsened the clinical outcome during the early phase of stroke.
Our study has several limitations to be resolved in the future. In this study, we show the direct effect of PT on brain injury, and compared between haemorrhagic or ischaemic stroke. Future work should extend to immune regulation and mechanism study after stroke. Moreover, it is still unclear whether and how PT may impact brain recovery after haemorrhagic or ischaemic stroke. Few studies address the effect of inflammatory response on long-term outcome during late stage of stroke. Hirudin, which inhibits the conversion from fibrinogen to fibrin, reduced leucocyte infiltration, modulated microglia phenotype and improved long-term outcome from day 7 to day 28 after ICH.35 Also, lymphocyte infiltration persists during late-stage cerebral ischaemia, which probably plays some part in the resolution phase.36 Therefore, future studies are required to determine the potential impact of PT-induced inflammatory response during stroke recovery.
Conclusions
In summary, our data reveal that PT increases inflammatory response and exacerbates brain injury after ICH and brain ischaemia in a mouse model. This study will advance our understanding of brain inflammatory features after haemorrhagic or ischaemic stroke, as well as pave the way for immune intervention to benefit patients who had a stroke.
Acknowledgments
We thank H Zhao, Q Chen and S Wen for technical assistance.
Footnotes
MZ and YF contributed equally.
Contributors: W-NJ formulated the study concept and designed the studies. YF, YL, YZ, JF, HL and JC performed the studies, executed the experiments and interpreted the results. YF, YL and YX assisted to edit the revised manuscript. W-NJ, YF and YL wrote and edited the manuscript.
Funding: This study was supported in part by the National Science Foundation of China (81971094, 81771274) and the Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data generated or analysed during this study are included in this published article and its supplemental information files, and are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All animal experiments were approved by the Committee on the Ethics of Animal Experiments of Tianjin Neurological Institute and Tianjin Medical University (Tianjin, China).
References
- 1. Jian Z, Liu R, Zhu X, et al. The involvement and therapy target of immune cells after ischemic stroke. Front Immunol 2019;10:2167. 10.3389/fimmu.2019.02167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sternberg Z, Schaller B. Central noradrenergic agonists in the treatment of ischemic stroke—an overview. Transl Stroke Res 2020;11:165–84. 10.1007/s12975-019-00718-7 [DOI] [PubMed] [Google Scholar]
- 3. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 2012;11:720–31. 10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci 2014;8:388. 10.3389/fncel.2014.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dokalis N, Prinz M. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Semin Immunopathol 2019;41:699–709. 10.1007/s00281-019-00764-1 [DOI] [PubMed] [Google Scholar]
- 6. Dong X, Gao J, Zhang CY, et al. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano 2019;13:1272–83. 10.1021/acsnano.8b06572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X, Xuan W, Zhu Z-Y, et al. The evolving role of neuro-immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther 2018;24:1100–14. 10.1111/cns.13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zera KA, Buckwalter MS. The local and peripheral immune responses to stroke: implications for therapeutic development. Neurotherapeutics 2020;17:414–35. 10.1007/s13311-020-00844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen AW, Wagner EK, Laber JR, et al. A cocktail of humanized anti-pertussis toxin antibodies limits disease in murine and baboon models of whooping cough. Sci Transl Med 2015;7:316ra195. 10.1126/scitranslmed.aad0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grant CC, McKay EJ, Simpson A, et al. Pertussis encephalopathy with high cerebrospinal fluid antibody titers to pertussis toxin and filamentous hemagglutinin. Pediatrics 1998;102:986–9. 10.1542/peds.102.4.986 [DOI] [PubMed] [Google Scholar]
- 11. Zhou F, Liu R, Han P, et al. Pertussis toxin ameliorates microglial activation associated with ischemic stroke. Front Cell Neurosci 2020;14:152. 10.3389/fncel.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofstetter HH, Forsthuber TG. Kinetics of IL-17- and interferon-gamma-producing PLPp-specific CD4 T cells in EAE induced by coinjection of PLPp/IFA with pertussis toxin in SJL mice. Neurosci Lett 2010;476:150–5. 10.1016/j.neulet.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 13. Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin W-N, Yang X, Li Z, et al. Non-invasive tracking of CD4+ T cells with a paramagnetic and fluorescent nanoparticle in brain ischemia. J Cereb Blood Flow Metab 2016;36:1464–76. 10.1177/0271678X15611137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Shi K, Li Z, et al. Organ- and cell-specific immune responses are associated with the outcomes of intracerebral hemorrhage. Faseb J 2018;32:220–9. 10.1096/fj.201700324r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Y, Liu H, Zhang H, et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci 2017;37:4692–704. 10.1523/JNEUROSCI.3233-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin W-N, Shi SX-Y, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab 2017;37:2224–36. 10.1177/0271678X17694185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gan Y, Liu Q, Wu W, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A 2014;111:2704–9. 10.1073/pnas.1315943111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang X, Andjelkovic AV, Zhu L, et al. Blood–brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018;163-164:144–71. 10.1016/j.pneurobio.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol 2009;22:294–301. 10.1097/WCO.0b013e32832b4db3 [DOI] [PubMed] [Google Scholar]
- 22. Saand AR, Yu F, Chen J, et al. Systemic inflammation in hemorrhagic strokes – a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab 2019;39:959–88. 10.1177/0271678X19841443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tapia-Pérez JH, Karagianis D, Zilke R, et al. Assessment of systemic cellular inflammatory response after spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2016;150:72–9. 10.1016/j.clineuro.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 24. Sun P, Hernandez-Guillamón M, Campos-Martorell M, et al. Simvastatin blocks soluble SSAO/VAP-1 release in experimental models of cerebral ischemia: possible benefits for stroke-induced inflammation control. Biochim Biophys Acta Mol Basis Dis 2018;1864:542–53. 10.1016/j.bbadis.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 25. Watcharotayangul J, Mao L, Xu H, et al. Post-ischemic vascular adhesion protein-1 inhibition provides neuroprotection in a rat temporary middle cerebral artery occlusion model. J Neurochem 2012;123:116–24. 10.1111/j.1471-4159.2012.07950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bobinger T, Manaenko A, Burkardt P, et al. Siponimod (BAF-312) attenuates perihemorrhagic edema and improves survival in experimental intracerebral hemorrhage. Stroke 2019;50:3246–54. 10.1161/STROKEAHA.119.027134 [DOI] [PubMed] [Google Scholar]
- 27. Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol 2014;71:1092–101. 10.1001/jamaneurol.2014.1065 [DOI] [PubMed] [Google Scholar]
- 28. Tschoe C, Bushnell CD, Duncan PW, et al. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 2020;22:29–46. 10.5853/jos.2019.02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu Y, Liu Q, Anrather J, et al. Immune interventions in stroke. Nat Rev Neurol 2015;11:524–35. 10.1038/nrneurol.2015.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–39. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 31. Luo S, Li H, Mo Z, et al. Connectivity map identifies luteolin as a treatment option of ischemic stroke by inhibiting MMP9 and activation of the PI3K/Akt signaling pathway. Exp Mol Med 2019;51:1–11. 10.1038/s12276-019-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 2006;37:1399–406. 10.1161/01.STR.0000223001.06264.af [DOI] [PubMed] [Google Scholar]
- 33. Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood–brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab 2016;36:1481–507. 10.1177/0271678X16655551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toft-Hansen H, Buist R, Sun X-J, et al. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol 2006;177:7242–9. 10.4049/jimmunol.177.10.7242 [DOI] [PubMed] [Google Scholar]
- 35. Li X, Zhu Z, Gao S, et al. Inhibition of fibrin formation reduces neuroinflammation and improves long-term outcome after intracerebral hemorrhage. Int Immunopharmacol 2019;72:473–8. 10.1016/j.intimp.2019.04.029 [DOI] [PubMed] [Google Scholar]
- 36. Shi K, Tian D-C, Li Z-G, et al. Global brain inflammation in stroke. Lancet Neurol 2019;18:1058–66. 10.1016/S1474-4422(19)30078-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2021-000987supp001.pdf (150.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data generated or analysed during this study are included in this published article and its supplemental information files, and are available from the corresponding author on reasonable request.