Abstract
Development of standardized antifungal susceptibility testing methods has been the focus of intensive research for the last 15 years. Reference methods for yeasts (NCCLS M27-A) and molds (M38-P) are now available. The development of these methods provides researchers not only with standardized methods for testing but also with an understanding of the variables that affect interlaboratory reproducibility. With this knowledge, we have now moved into the phase of (i) demonstrating the clinical value (or lack thereof) of standardized methods, (ii) developing modifications to these reference methods that address specific problems, and (iii) developing reliable commercial test kits. Clinically relevant testing is now available for selected fungi and drugs: Candida spp. against fluconazole, itraconazole, flucytosine, and (perhaps) amphotericin B; Cryptococcus neoformans against (perhaps) fluconazole and amphotericin B; and Aspergillus spp. against (perhaps) itraconazole. Expanding the range of useful testing procedures is the current focus of research in this area.
Antifungal susceptibility testing remains an area of intense interest. Susceptibility testing can be used for drug discovery and epidemiology, but this review will focus on use of antifungal susceptibility testing to predict therapeutic outcome. With the demonstration that susceptibility of Candida spp. to azole antifungal agents (particularly fluconazole) generated correlations with clinical outcome for some forms of candidiasis that were qualitatively similar to that seen for antibacterial agents (182) and the steady introduction of new drugs of both preexisting and new classes (9), the interest in and need for clinically relevant susceptibility testing has increased. The need extends beyond testing Candida spp. With resistance demonstrated among such diverse fungi as Cryptococcus neoformans (1, 150, 167, 185, 227), Aspergillus fumigatus (40, 47, 96, 104, 130), Aspergillus terreus (207, 216), Histoplasma capsulatum (224), Pseudallescheria boydii (Scedosporium apiospermum) (219), and Trichosporon spp. (217, 218), it is clear that the need for meaningful susceptibility test results is as great for the fungi as it is for the bacteria.
Although antifungal susceptibility testing remains less well developed and utilized than antibacterial testing, the scientific support for its validity has benefited greatly by extrapolation from antibacterial testing. Knowledge of mechanisms of antifungal resistance has been valuable in identifying resistant isolates and using them to validate in vitro measurement systems (75, 130, 213, 225). The application of the concepts of pharmacodynamics (W. A. Craig, Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. S67, 1998) to antifungal susceptibility testing (4–7; 23, 52, 54, 81, 85, 99, 100, 113; D. A. Andes, M. van Ogtrop, T. Stamsted, and B. Conklin, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. F-88, 1998; E. J. Wolfe, M. E. Klepser, and M. A. Pfaller, Abstr. 34th Annu. Meet. Infect. Dis. Soc. Am., abstr. 76, 1996) has provided significant new insights into both the methods for and interpretation of antifungal susceptibility testing. In addition, novel techniques for susceptibility testing provide useful additional tools and insights. It is the purpose of this review to summarize developments in this area since the last major reviews on this topic (59, 67, 74, 160, 183). This paper is not an exhaustive review of all prior work in this area, and it will focus on the issues that are important to testing in the clinical laboratory with special emphasis on the well-studied National Committee for Clinical Laboratory Standards (NCCLS) methodologies. For additional background information, the interested reader is referred to these reviews for additional data.
METHODS FOR SUSCEPTIBILITY TESTING
NCCLS Broth-Based Methodology for Yeasts
Status of the reference method.
After passing through the stages of being a (P)roposed document (M27-P) in 1992 (134) and a (T)entative document (M27-T) in 1995 (136), the NCCLS M27 methodology for testing of yeasts became an (A)pproved level document (M27-A) in 1997 (135). The process by which this method was developed has been extensively reviewed (74, 160, 183). Publication of M27-A was the culmination of approximately 15 years of collaborative work. Although imperfect, this method has the great advantage of high interlaboratory reproducibility. Combined with the secondary knowledge generated during its development concerning factors that both add to and detract from reproducibility, M27 has become a valuable tool for all investigators in this field. The method specifies inoculum size and preparation, test medium, incubation time and temperature, and end-point reading for flucytosine, amphotericin B, fluconazole, ketoconazole, and itraconazole (Table 1). The reference method was initially defined solely as a macrodilution methodology, but this was extended with a microdilution method that was included beginning with the T-level revision of the document. The microdilution method has become the method of choice because of its less cumbersome nature. Microdilution plates may easily be prepared and frozen well in advance of use.
TABLE 1.
NCCLS conditions for antifungal susceptibility testing
| Characteristic | NCCLS M27-A (135) | NCCLS M38-P (133) |
|---|---|---|
| Suitability | Yeasts | Conidium- and spore-forming fungi |
| Inoculum | 0.5 × 103–2.5 × 103 CFU/ml | 0.4 × 104–5 × 104 CFU/ml |
| Inoculum standardization | Spectrophotometric, with reference to a 0.5 McFarland BaSO4 turbidity standard | Spectrophotometric, with specificatiion of a target optical density that varies by fungal genus |
| Test medium | RPMI 1640, buffered to pH 7.0 with 0.165 M MOPS | Same as M27-A |
| Format | Macrodilution or microdilution | Microdilution |
| Temp | 35°C | 35°C |
| Duration of incubation | 48 h (Candida spp.), 72 h (C. neoformans) | 24 h (Rhizopus spp.), 72 h (Pseudallescheria boydii), 48 h (most fungi, including Fusarium spp., Aspergillus spp., and Sporothrix schenckii) |
| End point | Optically clear well for amphotericin B, ∼80% reduction in growth (macrodilution testing with azoles), prominent decrease in turbidity (microdilution testing with flucytosine and the azole antifungals) | Prominent growth reduction from control (∼50%) for flucytosine, fluconazole, and azoles; optically clear well for amphotericin B; the T-level revision will propose the use of an optically clear end point for intraconazole and the newer azoles (e.g., voriconazole) |
| QC isolates and drugs | Two isolates of Candida against amphotericin B, flucytosine, fluconazole, voriconazole, ketoconazole, itraconazole, caspofungin (MK-0991), ravuconazole (BMS 207147), posaconazole (SCH 56592), and anidulafungin (LY303366, V-echinocandin) (14) | Two isolates of Aspergillus against amphotericin B and intraconazole |
The M27 method continues to be augmented. The M27-A document provides Quality Control (QC) limits at 48 h for amphotericin B, flucytosine, fluconazole, ketoconazole, and itraconazole. QC data were recently expanded to include 24-h QC limits as well for these previously mentioned agents (14). In addition, QC limits at 24 and 48 h for voriconazole (UK-109,496), caspofungin (MK-0991), ravuconazole (BMS 207147), posaconazole (SCH 56592), and anidulafungin (LY303366, V-echinocandin) have been provided (14). However, and as noted below, interpretive breakpoints have been established for fluconazole, itraconazole, and flucytosine against Candida spp. only after 48 h of incubation.
The M27-A methodology for Candida recommends an end-point reading at 48 h (Table 1). For most isolates, the difference between reading at 24 and 48 h is minimal and will not alter the interpretative category (i.e., does not change whether the isolate would be categorized as susceptible or resistant). However, recent work has begun to include 24-h readings because (i) MICs can often be read at 24 h and (ii) readings taken at 24 h may be more clinically relevant for some isolates. Isolates for which the earlier reading is important show a dramatic rise in drug MIC between 24 and 48 h due to significant trailing growth (partial inhibition of growth over an extended range of antifungal concentrations). Estimated as occurring in about 5% of isolates (12), this trailing growth can be so great as to make an isolate that appears susceptible after 24 h appear completely resistant at 48 h. Two independent in vivo investigations of this phenomenon that employed murine models of disseminated candidiasis (12, 181) have shown that isolates with this behavior should be categorized as susceptible rather than resistant. This concept has been corroborated by a demonstration that trailing growth can be eliminated by lowering the pH of the test medium to 5 or less (122) and by a clinical demonstration that oropharyngeal candidiasis due to such isolates responds to a low dose of fluconazole used to treat typical susceptible isolates (179).
The MIC end point is also of major importance, especially for isolates exhibiting trailing growth. The end point for azoles for both macro- and microdilution testing has been defined at the point at which there is prominent reduction in growth. For macrodilution testing, prominent reduction in growth was shown to be equivalent to the turbidity of a 1:5 dilution of the growth control (63). This end point could also be referred to as 80% reduction in growth relative to the growth control. However, when the microdilution format is utilized and read spectrophotometrically, the 80% rule is overly restrictive and 50% inhibition of growth by spectrophotometry best approximates the visual endpoint (55, 145, 156, 205).
Potentially useful modifications of the reference method.
Although not included in the reference NCCLS methodology, supplementation of the RPMI 1640 incubation medium with 18 g of glucose per liter (to change the final glucose concentration from 2 to 20 g/liter) has been shown repeatedly to enhance isolate growth without significantly altering the observed MICs of amphotericin B, flucytosine, ketoconazole and fluconazole (25, 38, 45, 66, 189, 190). Agitation of microdilution plates prior to reading is required for spectrophotometric readings and appears to simplify visual reading of end points (3). Agitation is also very useful for reading end points with Cryptococcus neoformans, since this yeast tends to form a button-like deposit in the microdilution wells (73).
Another modification that might be of value is reducing the molarity of the morpholinepropanesulfonic acid (MOPs) buffer. Tornatore et al. demonstrated that reduction of MOPS concentration from 0.165 to 0.0165 M lowered the frequency with which the NCCLS methodology overcalled resistance in a selected set of C. albicans isolates (211). Shortening the incubation period to 24 h (181) and reducing the medium pH below 5 (122) have also been proposed as methods to clarify trailing end points for Candida spp. In addition, continuous agitation of the microdilution plate may enhance the growth of C. neoformans (188). However, none of these modifications have achieved broad acceptance.
Commercial broth-based MIC systems.
Recently studied broth-based commercial systems include Candifast (International Microbio/Stago Group, Milan, Italy), Integral Systems Yeasts (Liofilchem Diagnostics, L'Aquila, Italy), and Fungitest (Bio-Rad SDP [formerly Sanofi Diagnostics Pasteur], Paris, France). These kits provide reagents for testing a limited number of drug concentrations selected as critical breakpoint values. Direct comparisons have found these methods to possess limited correlation with the M27-A reference method (42, 165, 196, 208, 226, 229). ATB Fungus (API-bioMerieux, Marcy l'Etoile, France), Mycostandard (Institut Pasteur, Paris, France), and Mycototal (Behring Diagnostic, Rueil-Malmaison, France) are similarly designed systems for which only limited data are available on correlation with the M27-A methodology (51, 84, 174, 196).
A commercial system that includes a colorimetric response based on the Alamar Blue redox marker is marketed as Sensititre Yeast One Colorimetric Antifungal Panel (Trek Diagnostic Systems, Inc., Westlake, Ohio). This kit tests a full range of drug concentrations, and rates of concordance of ≥85% with the reference method are generally obtained (43, 65, 112, 157, 165, 172), although one group noted some difficulties with testing of C. neoformans (112). The ASTY colorimetric panel (Kyokyuto Pharmaceutical Industrial, Ltd., Tokyo, Japan) demonstrated a similarly good correlation with the M27 reference method (153). Some of these kits are currently being evaluated by the U.S. Food and Drug Administration; however, to date none have received approval for marketing in the United States.
NCCLS Methodology for Molds
Direct adaptations of the M27-A methodology to Aspergillus spp., Chaetomium spp., Cladophialophora spp., Fusarium spp., Pseudallescheria boydii, Rhizopus arrhizus, and Sporothrix schenckii were shown to generate reproducible results (62, 170, 171). Following the principles established for testing yeasts, a proposed standard method entitled “Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi” has now been published as NCCLS M38-P (133). This method was developed using isolates of Aspergillus spp., Fusarium spp., Rhizopus spp., P. boydii, and S. schenckii. Recent data suggest that Bipolaris spp., Cladophialophora spp., Dactylaria spp., Paecilomyces spp., Scedosporium spp., Trichoderma spp., and Wangiella dermatitidis can be tested using this method (A. Espinel-Ingroff, V. Chatuvedi, A. Doney, A. Fothergill, and M. Rinaldi, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2, 2000). Other fungi have not been specifically studied, nor is there a methodology that addresses the issue of fungi which do not produce spores. The methods of inoculum preparation, choice of inoculum size, time of reading, and end-point selection have all been evaluated in a series of collaborative studies (60, 62). Nongerminated conidia are used since, at least for Aspergillus spp., similar results are obtained for germinated and nongerminated conidia (18, 56, 120). Different results might be obtained with other species (83), and this area may require further study. A significant difference from M27-A is the inoculum, which is approximately 10-fold higher than for yeasts and requires a different method of preparation (Table 1). Due to the differences in size and light-scattering properties of many kinds of spores that can be generated by these fungi, the M38 method specifies different optical density ranges for each genus. Careful preparation of the inoculum is important, since a concentration outside the specified range will result in an elevated MIC to most antifungal agents (72, 130).
The end-point definition is another area of difference for M27 and M38. In M27, azoles are read at a partial inhibition end point (defined as the lowest drug concentration producing a prominent reduction in growth). While this wording is used in the M38-P document for the azoles, more recent data suggests that reading the end point at 100% inhibition (no growth) better detects resistance of Aspergillus spp. to itraconazole and the newer triazoles (45; A. Espinel-Ingroff, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 154, 1999). This modification has been proposed for the T-level revision of the M38 document.
Etest
Etest (AB Biodisk, Solna, Sweden) is a proprietary, commercially available method for antimicrobial susceptibility testing. MICs are determined from the point of intersection of a growth inhibition zone with a calibrated strip impregnated with a gradient of antimicrobial concentration and placed on an agar plate lawned with the microbial isolate under test. This methodology has been adapted to a number of antifungal agents. Both nonuniform growth of the fungal lawn and the frequent presence of a feathered or trailing growth edge can make end-point determination difficult. However, with experience and standardized techniques, the correlation between this method and the reference method has been acceptable for most Candida spp. and the azole antifungal agents (30, 35, 64, 68, 155, 158, 165, 199, 221, 222). One study, however, did observe less than 50% concordance between the methods for C. tropicalis and C. glabrata with fluconazole (199), and another found a limited correlation for ketoconazole and C. lusitaniae (68). These results suggest that users of the Etest methodology should carefully validate their local procedures against the reference methodology. For many molds, including Aspergillus spp., good correlations with amphotericin B and itraconazole Etest and MICs by the M38 method have been demonstrated (159, 209). Etest has significant value for the determination of amphotericin B MICs and represents one of the more reliable ways to identify resistant isolates (see below). The choice of growth medium appears critical with this technique, with RPMI-based agars generally appearing most useful (e.g., this agar gave >96% correlation with the reference microdilution method in one study [159]). QC Etest limits for the two NCCLS M27 QC isolates (C. krusei ATCC 6258 and C. parapsilosis ATCC 22019) against amphotericin B, flucytosine, fluconazole, itraconazole, and ketoconazole have been proposed (155).
Other Agar-Based Testing Methods
Disk-based susceptibility testing is convenient and economical. Adaptations with good correlation with the reference broth methods have been shown for fluconazole (13, 125, 194). Flooding the surface of the plate with methylene blue (0.5 μg/ml) appears to improve zone edge definition and facilitates reading (165). A commercial disk system (Neo-Sensitabs; Rosco Diagnostica, Taastrup, Denmark) is available, but the results were not found to correlate well with the reference method (25, 202, 208, 226). Reports on the Diff Test (Diagnostics Pasteur, Paris, France) disk diffusion system and a spiral gradient method did not include comparisons with the reference method (51, 196).
Flow Cytometry and Use of Viability Dyes
Flow cytometry has been long recognized as a possible tool for antifungal susceptibility testing and has been developed for yeasts in studies that focused principally on Candida spp. (2, 69, 79, 146, 161–164). Staining (or lack of staining) with suitable dyes permits the rapid detection of damaged fungi. Recent work has further explored this possibility (80, 88, 97, 151, 175, 176, 223) and shown the potential for correlation of flow cytometry-based techniques with the reference method. One study suggested that flow cytometry might be especially useful for detection of amphotericin B resistance (69). In conceptually related studies, fluorescent viability dyes have been used to examine the nature of drug-induced damage to yeasts and moulds (50, 111) and to estimate MICs and minimal fungicidal concentrations (MFCs) for molds (105).
Direct Measurement of Azole Effect: Quantitation of Ergosterol Synthesis
Given that azole antifungal agents act by inhibition of ergosterol synthesis, direct measurement of alterations in ergosterol synthesis appears relevant. Arthington-Skaggs et al. have described a workable laboratory method for such testing (11) and have shown that it correlates well with the NCCLS M27-A methodology for Candida spp. Importantly, both in vitro (11) and in vivo (12) testing of isolates exhibiting trailing growth further corroborates the notion that these isolates should be classified as susceptible, rather than resistant.
Other Antifungal Susceptibility Testing Methods for Yeasts and Molds
Many alternative methods for antifungal susceptibility testing have been proposed. Shimokawa and Nakayama have shown that testing azoles against Candida spp. on acetate-supplemented media produces MICs that more closely approximate the critical concentration at which ergosterol biosynthesis is inhibited (200, 201). Incorporation of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] or XTT (2,3-bis(2-methoxy-4-nitro-5-dulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide) as a colorometric marker for redox potential has been found convenient for Candida spp. (33, 86, 91, 210, 231), C. neoformans (33, 86), and molds such as Aspergillus or Fusarium spp. (91, 92, 126; C. C. Chiou, N. Mavrogiorgos, and T. J. Walsh, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 940, 2000; T. J. Walsh, A. Field-Ridely, D. Sutton, V. Petraitis, R. Petraitiene, T. Sein, R. Schaufele, M. J. Rinaldi, M. Ghannoum, and C. A Lyman, Abstr. 14th Congr. Int. Soc. Hum. Anim. Mycol., abstr. 557, 2000). This approach generates MICs comparable to those in the NCCLS reference method and presents substantial opportunities for automation. Testing with XTT eliminates an extraction step that is needed with MTT-based assays (86, 210). Similarly, methods for measurement of intracellular ATP concentration (102) and consumption of glucose from the incubation medium (184) have yielded excellent correlation with the reference method. Measurement of medium capacitance provides a very early assessment of fungal growth and could permit the estimation of an MIC directly from the initial positive culture specimen (e.g., from the material in the blood culture bottle) without the need for subculture (29). Spectrophotometric measurement of incorporation of neutral red by viable cells was shown to correlate with an estimate of the MFC for Trichophyton spp. (71). Radiometric and direct microscopic methods have also been described in the past (183) but are cumbersome and little used in recent work.
Agar-based methods are attractive due to their simplicity. An agar dilution-based method incorporating a specific concentration of fluconazole (8 μg/ml), in combination with the chromogenic CHROMagar Candida medium, is a useful screening tool for fluconazole resistance (147, 148). This method, however, still requires formal testing of putatively resistant isolates. An agar dilution method based on semisolid agar has also been reported to render significant concordance with the reference method (169). Incubation under 20% CO2 has been suggested as a way to clarify the end point in an agar dilution method (232).
Testing of Dermatophytes
Dermatophytes grow slowly, and therefore agar-based methods have often been employed (24, 118, 214). Most studies, however, have focused on drug comparisons and employed methods that are not standardized or correlated with clinical outcomes. Thus, the clinical relevance of in vitro testing for the dermatophytes remains unclear. However, multiple efforts are under way to develop standardized methods for these fungi (95, 142; K. C. Hazen, A. E. Moon, and E. S. Atillasoy, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 204, 2000). Much of the initial work has focused on the selection of medium, with particular attention to choosing a medium that supports conidial formation in Trichophyton rubrum. Subsequent work investigating the inoculum and end point is under way.
Determination of MFCs rather than MICs
MFCs have the potential for being more relevant to clinical outcome, especially in the context of profoundly immunocompromised hosts or body sites (e.g., heart valves) where host defenses are at a disadvantage. Indeed, some work with MFCs has suggested that they may be more predictive than MICs even for general testing (137, 186, 217, 218). However, all of the issues of standardization that occur with MICs also apply to MFCs. And, new issues arise including questions of drug carryover, sample volume, and end point. Even the very definitions of the terms “microbicidal” and “microbistatic” come into question—a bactericidal antibacterial drug kills ≥99.9% of organisms, but should such a definition apply to fungi with sixfold-longer doubling times (e.g., 20 min versus 2 h)? Nevertheless, and extrapolating from standardized testing for measurement of bactericidal activity (132), Klepser et al. have approached MFCs by measuring time-kill curves for antifungal agents (98). Use of time-kill curves has been relevant to their work on antifungal pharmacodynamics (see below) and provides additional support for the validity of any chosen measurement. Methods that would reliably generate such a curve could be directly applied to the measurement of MFCs at any given time point. Klepser has proposed a standardized method (98) and made extensive use of this technique in a series of pharmacodynamic investigations (52, 54, 85, 99, 100, 110; Wolfe et al., 34th Annu. Meet. IDSA). Killing tests are being further investigated in a collaborative fashion, and these methods would appear to represent an excellent starting point for further research in this area.
INTERPRETATION OF RESULTS
General Principles
The ability to generate an MIC is of little value without the corresponding ability to interpret its clinical meaning. However, this process is far from straightforward because (i) MICs are not a physical measurement, (ii) host factors play a critical role in determining clinical outcome, (iii) susceptibility in vitro does not uniformly predict clinical success in vivo, and (iv) resistance in vitro will often, but not always, correlate with treatment failure (182). The initial publication of the M27 document included interpretive breakpoints for Candida spp. when tested against fluconazole, itraconazole, and flucytosine (Table 2). The data for each drug had different strengths and weaknesses for determining these interpretive breakpoints, as discussed below.
TABLE 2.
Meaningful test methods and conditions for antifungal susceptibility testing
| Organism | Drug | Methods | Interpretation | References |
|---|---|---|---|---|
| Candida spp. | Fluconazole | M27-A and methods that give concordant results | S, <8 μg/ml; S-DD, >8 μg/ml and ≤32 μg/ml; R, >32μg/ml; data are best supported for mucosal disease, but the general principles also appear to hold for invasive disease; adequate drug dose is critical as the MIC rises; C. krusei is assumed intrinsically resistant and should not be tested | 32, 108, 135, 182 |
| Itraconazole | M27-A and methods that give concordant results | S, ≤0.125 μg/ml; S-DD; >0.125 μg/ml and ≤0.5 μg/ml; R, >0.5 μg/ml, data apply only to mucosal disease; ensuring adequate absorption by use of the solution is often critical to drug efficacy | 135, 182 | |
| Ketoconazole | M27-A-like methods | No specific breakpoints proposed, but aggregate data suggest that isolates with an MIC of >0.125 μ/ml by M27-A are less likely to respond | 27, 123, 128, 191, 205 | |
| Flucytosine | M27-A-like methods | S, ≤4 μg/ml; I, >4 μg/ml and ≤16 μg/ml; R, >16 μg/ml; these breakpoints are based largely on historical data and animal models but appear rational based on the available clinical and pharmacokinetic data | 7, 70, 89, 135 | |
| Amphotericin B | M27-A-like methods that use AM3 broth, Etest, or MFC methods | Significant controversy and difficulty; agar-based methods appear to have the most potential, in general, and isolates with amphotericin B MICs of >0.5 μg/ml are probably resistant; specific breakpoints have NOT been proposed, and intralaboratory variation makes use of reference isolates with known resistance mandotory; fortunately, resistance appears uncommon among the four most common species (106) | 34, 137, 180, 221 | |
| C. neoformans | Fluconazole | Modified M27-A (YNB broth, higher inoculum) | No specific breakpoints proposed; Data are limited but consistent: MICs of ≥2–4 μg/ml by this method appear to predict a greater chance of failure; failure definitely appears more likely as the MIC reaches 16 μg/ml | 1, 93; Witt et al., abstr. a |
| Flucytosine | M27-A-like methods | No specific breakpoints proposed, but breakpoints similar to those for Candida spp. (see above) would appear rational | ||
| Amphotericin B | M27-A-like methods that use AM3 broth, Etest | Significant controversy and difficulty; agar-based methods have the most potential in general. Measurement of fungicidal concentrations may have value; no specific breakpoints proposed—testing should incorporate reference isolates with known resistance. | 117, 185, 186 | |
| Mold fungi | Any | Unknown | M38-like methods correlate for itraconazole and A. fumigatus; correlations for amphotericin B are ill-defined | See text |
M. D. Witt, L. A. Mortara, R. A. Larsen, R. J. Lewis, J. E. Edwards, and M. Ghannoun, Abstr. IXth Int. Coaf. AIDS, abstr. WS-B12-4, 1993.
A weakness that applies to all of the MIC-clinical outcome correlation data sets is the relative paucity of clinical outcome data for isolates for which the MICs are elevated. Such potentially resistant isolates are infrequently encountered in most early clinical trials, and this circumstance limits the strength of proposed breakpoints. A second limitation, unique to antifungal drugs, is that all (itraconazole) or most (fluconazole) of the data were derived from studies of mucosal candidiasis. There are fewer data for invasive candidiasis. Despite these limitations, the general level of clinical correlation achieved to date is similar to that seen with antibacterial agents.
While quality outcome data relating a range of drug doses to treatment results for infections due to isolates for which the MICs are elevated are not likely to be forthcoming, there are other means of providing additional support in this area. In particular, lessons can be learned from applying antifungal susceptibility testing to the field of time-kill studies and pharmacodynamics that may have direct clinical applications. This area has been well developed for antibacterial agents, and excellent reviews are available (36, 37, 131). In brief, pharmacodynamic analyses attempt to integrate both blood level and MIC into the prediction of clinical outcome. Although interrelated, one of three possible parameters tend to produce the best correlation for any given drug: (i) the proportion of the time that the drug's concentration exceeds its MIC for the organism, (ii) the ratio of the maximum concentration of the drug to its MIC for the organism (Cmax/MIC), and (iii) the ratio of the drug's area under the exposure curve to its MIC for the organism (AUC/MIC). Data from relevant pharmacodynamic studies will be introduced as each drug is discussed in turn, below.
On a more practical note, inter-run and interlaboratory variation is a definite limitation for all susceptibility testing methodologies. It must be clearly understood that a single measurement of a MIC is at best an estimate. Inter-run and interlaboratory variability consistently lead to the observation that repeated testing for any given isolate produces MIC that usually span no less than three twofold dilutions. This is often seen in collaborative studies used to generate QC limits (14). Even when highly experienced laboratories use common materials to perform testing, isolates which generate MICs only within a span of three twofold dilutions are the exception rather than the rule. And, if great care is not taken with all the technical details (177), it is unfortunately easy to generate erroneous results. Thus, prediction of a therapeutic outcome from an isolate for which the MIC is at the boundary between susceptible and resistant should always be undertaken with great care!
Support for the Clinical Relevance of NCCLS M27 MICs in Yeasts
Fluconazole and Candida spp.
The M27-A method includes interpretive breakpoints for Candida spp. tested against fluconazole (182). These breakpoints were based on an analysis of treatment outcomes in both mucosal and invasive disease and included the description of a novel end point named S-DD, (susceptibility is dose dependent). For fluconazole, the S-DD range includes MICs of 16 and 32 μg/ml. Isolates with fluconazole MICs above this range are classified as resistant (R), whereas those inhibited at lower concentrations are simply labeled susceptible (S). The S-DD breakpoint concept emphasizes the importance of attaining maximal fluconazole levels in blood and tissue for isolates with higher MICs. Maximal dose is defined in this context as being at least 400 mg/day in a 70-kg adult with normal renal function. The weaknesses of these data were that (i) two-thirds of the results were drawn from cases of mucosal candidiasis and (ii) the concept of dose-dependent susceptibility was most clearly proven only for mucosal candidiasis. An additional concern is that children have significantly different pharmacokinetic parameters for fluconazole than adults (20, 107, 195, 197), and none of the data have addressed in vitro-in vivo correlations in this population.
These breakpoints have been supported by additional clinical data on oropharyngeal candidiasis in recent publications. Using the Etest method, Dannaoui et al. reported that infection due to C. albicans isolates with a fluconazole MIC of ≥48 μg/ml was likely to be unresponsive to fluconazole at median doses of 100 to 200 mg/day (41). Using a non-M27 broth-based method, Cartledge et al. reported that infections due to isolates with fluconazole MICs of >8 μg/ml were unlikely to respond to 100 mg of fluconazole per day (27). Quereda reported that infections due to isolates with fluconazole MICs of ≥8 μg/mL (by M27-A, but in medium supplemented to 20 g of glucose per liter) were less likely to respond to 100 mg of fluconazole per day (173). Using doses of fluconazole that increased from 100 mg/day to as high as 800 mg/day as needed, Revankar et al. reported that clinical failure only occurred when the M27-A MIC was ≥64 μg/ml (178). This study clearly documented the need for increased doses of fluconazole as the fluconazole MIC rose. In addition, Revankar et al. observed that a longer duration of therapy was required to achieve control over infections due to less susceptible isolates.
In one of the two data sets available for nonmucosal candidiasis, Lee et al. reported outcome in 32 fluconazole-treated (400 mg/day) non-AIDS patients with deep-seated candidal infections (108). C. albicans was the most common species in this series, but all the major species were represented. Success rates were 79% (19 of 24 patients infected with S isolates), 66% (4 of 6 patients infected with S-DD isolates), and 0% (0 of 2 patients infected with R isolates). Clancy et al. reported on 34 candidal bloodstream infections treated during very early work with 100 to 200 mg of fluconazole per day. All the major Candida spp. were represented, and failure rates were 48, 86, and 100% for isolates testing in the S, S-DD, and R ranges, respectively (C. J. Clancy, C. A. Kauffman, A. Morris, M. L. Nguyen, D. C. Tanner, D. R. Snydman, V. L. Yu, and M. H. Nguyen, Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 98, 1998). This data set is particularly helpful in that it is one of the few where infections due to isolates for which the MICs were higher were treated with doses of fluconazole that were (by current standards) low. The doses used would not have been predicted to be adequate for isolates with fluconazole MICs in the S-DD range, and the response of these isolates would be expected to be similar to that of the R isolates. This data set thus provides helpful support for the importance of appropriate dosing in cases of invasive infection.
(i) Pharmacodynamic support for these ideas.
Pharmacodynamic issues have been most extensively studied for fluconazole. In vitro work suggested that the effect of fluconazole is concentration independent and is maximal when the concentration is two- to eightfold greater than the MIC (23, 99). Response might thus be expected to correlate best with time above the MIC (as for the β-lactam antibiotics) (99). However, and perhaps due to sub-MIC effects on growth, in vivo work has shown that the AUC/MIC ratio is the best predictor of response. The two independent studies in this area examined isolates with fluconazole MICs ranging from 0.5 to 32 μg/ml and concluded that the 50% effective dose ED50 for in vivo studies was achieved when the AUC/MIC ratio exceeded 12 to 24 (6, 113).
The consistency of the pharmacokinetics of fluconazole permits an instructive calculation to be done from these data. It is known that the 24-h AUC for healthy adults given fluconazole is almost exactly equal to the daily dose in milligrams. That is, a 70-kg adult given 400 mg of fluconazole will have an AUC of 400 mg · h/liter (114). The validity of this assumption for patient groups depends, of course, on the likelihood that they would have both normal renal function and approximately normal body habitus. The data set from which the M27 interpretive breakpoints for fluconazole were derived contains the dose and MIC for each patient. Body habitus and serum creatinine levels are not available. However, for patients with systemic infection, it is known that the study protocols all specified a fluconazole dose of 400 mg/day. Thus, any variation in dose is likely to have been due to issues of renal function or body habitus.
However, the patients receiving therapy for oropharyngeal disease were generally treated at 100 mg/day and were outpatients, who were likely to have had relatively normal renal function. Dose increases were therefore more likely to have occurred in instances of poor response. In this subset of patients, it is thus of interest to compare outcome with the dose/MIC ratio on the assumption that this approximates the AUC/MIC ratio (Table 3). The data show a clear relationship between the dose/MIC ratio and clinical outcome. Using 48-h MICs, a clear decrement in response is seen when the dose/MIC ratio falls below 25. Given the well-known tendency for MICs to rise 1 dilution between 24 and 48 h of incubation, it is not surprising that the critical ratio shifts a corresponding amount between 24 and 48 h. Looking at 48-h MICs and the critical AUC (dose)/MIC ratio of 25, this translates to needing 800 mg/day to treat an isolate with a fluconazole MIC of 32 μg/ml and 100 mg/day for an isolate with a fluconazole MIC of 4 μg/ml. The relationships between these values and the NCCLS interpretive categories S, S-DD, and R are apparent.
TABLE 3.
Dose/MIC ratios from the NCCLS M27 fluconazole correlation data seta
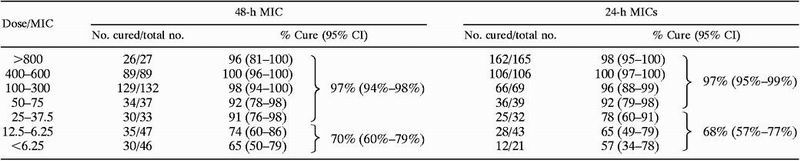 |
The original data set presented as the support for the NCCLS M27 breakpoints (182) was reanalyzed using dose/MIC as the grouping factor. MICs were obtained by the M27-A macrodilution methodology, and the analysis was limited to patients with oropharyngeal candidasis. The analysis is also shown for isolates wherein a 24-h MIC was reported using the M27-A methodology. For each percentage cure or aggregate percentage cure, a 95% confidence interval (CI) (233) is also shown in parentheses.
Also relevant to this discussion, Graninger et al. (78) describe increased rates of successful therapy of candidemia due to C. albicans when the dose was increased from 400 to 800 mg/day in two sequential cohorts of ∼30 patients each. All isolates had a similar low fluconazole MIC (MIC for 90% of isolates [MIC90] by an NCCLS M27-like method of 1.25 μg/ml), and other factors might have been in play. However, this increase in success might also have reflected the consequences of an increased AUC/MIC ratio, and these results are at least consistent with the data reported by Lee et al. (108) and Clancy et al. (36th Annu. Meet. IDSA).
Itraconazole and Candida spp.
For itraconazole, M27-A provided interpretive breakpoints only for mucosal candidal infections. Isolates with an itraconazole MIC of ≤0.125 μg/ml were classified as S, those with an itraconazole MIC of >0.5 μg/ml were classified as R, and those in between were classified as S-DD, where the implication is that susceptibility depends on drug delivery. Unlike the case with fluconazole, where its reliable absorption means that the administered dose is virtually always well delivered to the site of infection, itraconazole's bioavailability is unpredictable. The data supplied with the NCCLS itraconazole data set included drug levels in blood, and infections due to isolates in the S-DD range showed a trend toward a better response with higher drug levels (182). The critical level in blood was thought to be approximately 0.5 μg/ml on the basis of high-pressure liquid chromatography. The method of blood measurement is important, since bioactive itraconazole metabolites cause bioassay-based measurement systems to render higher levels in blood (90). This concept is consistent with observations that achieving approximately this level in blood as determined by high-pressure liquid chromatography predicts a greater likelihood of efficacy in the treatment of both experimental (16) and human (76; A. Glasmacher, C. Hahn, E. Molitor, T. Sauerbruch, G. Marklein, and I. G. H. Schmidt-Wolf, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 700, 2000).
Few supportive data have emerged with respect to itraconazole interpretive breakpoints for Candida species. Cartledge et al. reported that AIDS patients with oropharyngeal candidiasis treated with 200-mg itraconazole capsules (the least well absorbed formulation of the drug) had a greater rate of failure when the MIC was >2 μg/ml (27). In a supportive laboratory study, isolates with elevated fluconazole MICs were found to have elevated itraconazole MICs that were usually ≥0.5 μg/ml, in contrast to ≤0.125 μg/ml for isolates with lower fluconazole MICs (123).
In contrast to these results, a study of itraconazole oral solution as therapy for fluconazole-refractory oropharyngeal candidiasis in human immunodeficiency virus (HIV)-infected patients with oropharyngeal candidiasis found no meaningful correlation of itraconazole MICs by M27-A and outcome in a group of 74 patients (193). Itraconazole levels in blood were not monitored, but the use of the oral solution should have produced adequate levels in most patients. Lack of effective antiretroviral therapy in this group of patients may have limited the possible efficacy of any form of therapy, but the lack of correlation is otherwise unexplained. It is also possible that administration of oral itraconazole solution in these patients amounts, at least partially, to topical therapy for oral Candida infections, with the direct effects of contact between drug and mucosa complicating any relationship between levels in blood and mucosal disease. However, the itraconazole oral solution was also used in the experiments collecting the data that contributed to the NCCLS correlation data set (182). In aggregate, the data on correlation between therapeutic outcome and itraconazole MIC are somewhat confusing, and this area would benefit from additional work.
(i) Pharmacodynamic support for these ideas.
In vivo pharmacodynamic data are scant for itraconazole. Using time-kill studies, Burgess et al. found that an isolate of C. albicans with itraconazole MICs in the NCCLS M27-A susceptible range responded to itraconazole, with maximal response seen when the drug concentration was double the MIC (23). Interestingly, isolates with itraconazole MICs in the S-DD and R range both failed to respond to itraconazole (23). The authors conclude that isolates with itraconazole MICs in the S-DD range may be less responsive. Of interest, a dose-dependent pharmacodynamic relationship was observed in an experimental model of treatment of invasive aspergillosis with itraconazole (16). This area also requires further study.
Ketoconazole and Candida spp.
Interpretive criteria have not been proposed for ketoconazole MICs. Cartledge et al. reported two correlations of laboratory results with therapeutic outcome in a group of HIV-infected individuals with oropharyngeal candidiasis treated with ketoconazole at 200 mg twice a day. First, testing of relative growth inhibition by a fixed concentration of ketoconazole (0.05 μg/ml) in a broth-based system (104 CFU/ml of inoculum, pancreatic casein digest [CYG] broth, 72-h incubation at 37°C, spectrophotometric reading) was found to correlate with clinical outcome (26). Failure to achieve at least 25% reduction in growth relative to the growth control predicted a high likelihood of failure. In a study using the reference method to generate MICs, a ketoconazole MIC of >0.125 μg/ml correlated with increased clinical failure (27).
These broth-based observations have been supported by other work using the reference method (sometimes with the RPMI 1640 medium supplemented to 20 g of glucose per liter) (123, 128, 191, 205). Two of these reports provide clinical data on ketoconazole-treated patients with oropharyngeal candidiasis. One report found no clinical failures in a group of isolates with a ketoconazole MIC of <0.25 μg/ml (205), whereas results from the other study suggested that clinical resistance would be seen if the MIC was ≥0.06 μg/ml (191). The amount of data on patients infected with isolates for which the MICs were higher were limited in these reports, thus limiting the strength of these conclusions. However, in a supportive analysis, Milan et al. reported that isolates with ketoconazole MICs of ≥0.25 μg/ml often had elevated fluconazole MICs. Likewise, Martinez-Suarez and Rodriguez-Tudela reported that isolates with elevated fluconazole MICs usually had ketoconazole MICs of ≥0.06 μg/ml (123). The correlation with increased fluconazole MIC is relevant because the mechanisms of azole resistance are common across the azoles, and azole MICs tend to rise in parallel (225). Collectively, these observations support the idea that relevant resistance to ketoconazole begins at an MIC of ∼0.125 μg/ml. Pharmacodynamic data are not available.
Amphotericin B and Candida spp.
Establishing a clear correlation between the amphotericin B MIC and outcome has been difficult. In vitro and in vivo resistance clearly exists (21, 48, 127, 139, 141, 168, 198, 203, 204, 220) and in vivo-in vitro correlations are possible (143). After the M27 methodology was developed, it became clear that its ability to detect amphotericin B-resistant isolates was limited (180). Isolates with proven resistance would have a higher modal or geometric mean MIC on repeated testing, but the extent of MIC differences between resistant and susceptible isolates was too narrow to permit separation of resistant isolates by the type of single-run testing that is normally performed in a clinical laboratory setting. Modifying M27 by substitution of Antibiotic Medium 3 (also known as Penassay broth) supplemented with glucose to 20 g/liter and buffered to pH 7.0 with 0.1 M phosphate and reading the MIC after only 24 h partially alleviated this problem (116, 180, 221). Subsequent work with the colorimetric Alamar Blue marker found that it did not improve the detection of resistant isolates (115).
However, this research also revealed the importance of as yet unidentified technical factors. Looking first at the reports from one of our laboratories (J.H.R.), work with an initial (but rather old) lot of Antibiotic Medium 3 suggested that resistant isolates had an amphotericin B MIC of ≥1 μg/ml (180). Subsequent work with three newer lots yielded similar results, except that now resistant isolates had an amphotericin B MIC of ≥0.25 μg/ml (116). This procedure yielded comparable results in a report from another laboratory (106). However, results from a third laboratory found that this approach did not yield an acceptable correlation with clinical outcome when used to test a series of isolates from candidemic patients treated with amphotericin B. Rather, reading at 48 h and without glucose supplementation was preferred, and optimal results were actually obtained if an MFC was determined (M. H. Nguyen, C. J. Clancy, Y. C. Yu, V. L. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi, Program Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-81, 1997). The critical values were an MIC of ≥1 μg/ml or an MFC of >1 μg/ml. The cause(s) of these differences in results is unknown.
Agar-based testing generally seems to produce a broader separation of high and low amphotericin B MICs. Broad MIC ranges are obtained by Etest (34, 106, 152, 154, 221), and the three groups who reported conflicting data with the broth-based system have reported quite comparable agar-based testing results. By the Etest method, cutoff values for resistance of ≥0.5 μg/ml on Antibiotic Medium 3 agar (106), >1 μg/ml on Antibiotic Medium 3 agar (221), and ≥0.38 μg/ml on RPMI 1640 agar (34, 152) are suggested. The study with the higher breakpoint was from one of our laboratories (J.H.R.) and used an older lot of Antibiotic Medium 3 agar—most recent work with newer lots of AM3 found that lower breakpoints are more suitable for these newer lots (unpublished data).
In practice, none of these cutoff values should be treated as a confirmed interpretive breakpoint without significant additional testing. Use of adequate control isolates is critical for testing with any format, and well characterized amphotericin B-resistant Candida isolates have been deposited with the American Type Culture Collection (ATCC 200950, ATCC 200951, ATCC 200952, ATCC 200953, ATCC 200955, and ATCC 200956 are resistant, and ATCC 200954 is susceptible).
(i) Pharmacodynamic support for these ideas.
Available in vitro data demonstrate that the effect of amphotericin B is concentration dependent (23, 99). This pharmacodynamic pattern has also been shown for nystatin, a related polyene antifungal (85). The in vivo prediction of such a result is that the most relevant pharmacodynamic correlate should be the ratio of peak drug concentration to MIC, and precisely this in vivo result was recently shown for amphotericin B in a murine model of invasive candidiasis (5). These data can be combined in an effort to predict the likely clinical response for isolates with differing amphotericin B MICs. In the in vitro studies with this drug, peak/MIC ratios of ∼2 correlated with near-maximal activity. The pharmacology of amphotericin B is poorly understood, but peak levels of 1 to 2 μg/ml are typical (19, 82; I. Bekersky, R. M. Fielding, D. Dressler, D. N. Buell, and T. J. Walsh, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 85S, 2000; W. G. Powderly, G. G. Granich, G. P. Herzig, and D. J. Krogstod, Program Abstr. 27th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 233, 1987). Taking the lower end of the range of levels in blood as a conservative estimate of the achieved peak level, amphotericin B MICs of ∼0.5 μg/ml might thus represent the upper end of the treatable range, and this is consistent with estimates reported above. However, this estimate is made problematic by technical factors that influence amphotericin B MIC determinations, as discussed above. It is further unclear how to interpret the blood levels obtained with lipid-associated formulations of amphotericin B (82, 187).
Flucytosine, Candida spp., and C. neoformans.
When flucytosine is used as monotherapy for yeast infections, there is a high tendency for rapid development of resistance (70, 89). This agent has therefore been used almost always in combination with other antifungal agents, and there are no (and never will be any) extensive data on correlations of MIC with outcome of flucytosine monotherapy. Thus, the M27-A document (135) proposed interpretive breakpoints based on a combination of historical data and results from animal studies (49, 206). Isolates with a flucytosine MIC of ≤4 μg/ml are said to be susceptible, those with a flucytosine MIC of >16 μg/ml are said to be resistant, and those in between are said to be intermediate. MIC distributions for collections of yeasts are strongly bimodal, with relatively few isolates having an intermediate flucytosine MIC. These breakpoints are described primarily for Candida spp. but may well also apply to C. neoformans. As an example of this, Hospenthal and Bennett provided a recent review of their experience with flucytosine monotherapy for cryptococcal meningitis (89). In the patients in whom resistance developed, MICs rose from <5 to >320 μg/ml.
(i) Pharmacodynamic support for these ideas.
In a recent study, flucytosine was found to show concentration-independent activity in time-kill assays, with little additive effect beyond a concentration fourfold higher than the MIC (110). The usual doses of flucytosine (25 mg/kg every 6 h) produce levels of ∼40 to 60 μg/ml in blood (70), suggesting an upper end for the treatable MIC range of 10 to 15 μg/ml. In addition to validating the putative interpretive breakpoints, this suggests that lower levels of flucytosine in blood might be just as effective. Such lower levels would also presumably further reduce the rate of toxicity. Andes et al. generated in vivo data supporting this observation by using a murine model of disseminated candidiasis (7). They concluded that a dosing regimen in which the drug maintained levels greater than the MIC for 25 to 50% of the dosing interval would be adequate.
C. neoformans, fluconazole, and amphotericin B.
The M27 method includes details for testing C. neoformans but does not specify any interpretive breakpoints for agents against this fungus. Although an early report suggested that recurrent cryptococcal meningitis in HIV-infected patients was mostly a function of immune status (28), many subsequent reports have shown the potential for development of true microbiological resistance (1, 10, 39, 167, 185, 227). The basis of resistance is under investigation (150). From a practical standpoint, detection of susceptibility and resistance to both fluconazole and amphotericin B appears to require modification of the reference testing method.
For fluconazole, initial work suggested that testing in yeast nitrogen base (YNB) with a slightly larger than usual inoculum (104 CFU/ml) might yield more clinically meaningful MICs (73). This concept has been supported by three clinical data sets (1, 93, 227). In the first study (227), MICs for isolates from 86 HIV-infected and fluconazole-treated patients with cryptococcal meningitis were measured. Testing by the reference method (RPMI 1640 medium) showed little correlation with outcome. On the other hand, MICs obtained by the modified YNB-based method showed a good correlation with outcome. In the second study, this modified method was used to test isolates from a group of 120 HIV-infected and fluconazole-treated patients with cryptococcal meningitis. Although clinical data were limited to 35 of these patients, the YNB-based method again demonstrated an overall correlation with outcome. MICs obtained by the M27 reference method were, however, tightly clustered in a very narrow range and did not show any trend toward correlation with outcome. Unfortunately, neither of these studies provides adequate data for an estimation of interpretive breakpoints, but both suggest that failure rates begin to rise noticeably once the MIC determined by YNB-based method reaches 2 to 4 μg/ml. In the third study, the YNB-based modification of M27-A was employed exclusively, and clinical failure was uniformly seen when the MIC was ≥16 μg/mL (1).
As with Candida spp., consistent detection of amphotericin B resistance in C. neoformans has proven difficult. Again, testing on agar or use of Antibiotic Medium 3 broth appears to be helpful. Using a highly selected set of isolates from patients with cryptococcal meningitis and known responses to amphotericin B, Lozano-Chiu et al. demonstrated that the reference method did not reliably distinguish putatively amphotericin B-susceptible and -resistant isolates (117). As was the case with Candida spp., separation could be discerned by repeated testing, but results from any individual test run were too narrowly clustered to permit reliable differentiation. Use of Antibiotic Medium 3 broth provided good separation between resistant and susceptible isolates, but the best results were obtained with Etest on either RPMI 1640 or Antibiotic Medium 3 agar. Isolates from five patients with cryptococcal meningitis who failed to respond to initial therapy with amphotericin B were shown to be tolerant (i.e., loss of the usual fungicidal effect) to amphotericin B in a study by Rodero et al. (185). The same group subsequently reported that MFCs might yield the best correlation with therapeutic response (186); however, correlation was incomplete, and further work is clearly required.
Pharmacodynamic studies with C. neoformans have only recently begun to be reported (22, 53, 100, 110), and the behavior of the azoles and polyenes for C. neoformans appears similar to that for Candida spp. In vivo studies have not yet been reported.
Molds
Interpretation of mould MICs has long been known to be problematic. For example, Dixon and Polak found no relationship between agar dilution MICs of amphotericin B, flucytosine, ketoconazole, fluconazole, amorolfine, and terbinafine and therapeutic outcome in experimental infection with Cladophialophora bantiana, Ochroconis constricta, and Wangiella (Exophiala) dermatitidis (49). Likewise, an extensive study of nine mold isolates (two Aspergillus spp., three Fusarium spp., two Pseudallescheria boydii strains, and two Rhizopus arrhizus strains) found that amphotericin B and itraconazole MICs obtained by the NCCLS reference method provided few clues to the likelihood of in vivo response (144). Uncontrolled host response variables presumably confounded any potential in vivo-in vitro correlations. This exhaustive study also illustrated the limitations of in vivo models—interpretable in vivo systems could not be established for all organism-drug combinations.
Using a collection of carefully selected isolates of A. fumigatus, Denning and coworkers have made substantial progress in this area (45, 46, 96) and have recently reviewed the available information on antifungal drug resistance in Aspergillus (130). The most definitive work is in the area of detection of azole resistance. Two isolates (NPCF 7099 and NPCF 7100) were collected from patients who did not respond to therapy with itraconazole. These isolates were resistant to itraconazole in a murine model of invasive aspergillosis and had elevated itraconazole MICs (45, 46). The choice of assay system was critical in the detection of these elevated MICs. For microdilution testing, the recommended conditions were 0.165 M MOPS-buffered RMPI 1640 medium (pH 7.0) supplemented to 20 g/liter with glucose, an inoculum of 106 conidia/ml, incubation at 35°C, and MIC determination after 48 h as the lowest drug concentration that produced no more than trace growth (45). For agar dilution-based testing, the optimum system consisted of similarly buffered but glucose-supplemented RPMI 1640, an inoculum of 106 to 107 conidia/ml, incubation at 35°C, and end-point determination after 48 to 72 h as the lowest concentration yielding no growth. These conditions gave the best correlation with in vivo treatment outcome in the animal model.
Unfortunately, the observations just discussed did not, in these investigators' hands, extend to amphotericin B. An isolate of A. fumigatus (AF65; deposited in the National Collection of Pathogenic Fungi, Bristol, United Kingdom, as NCPF 7097) from a patient with pulmonary aspergillosis who responded incompletely to high-dose amphotericin B and an isolate of the intrinsically amphotericin B-resistant species A. terreus (Walsh et al., 14th Congr. ISHAM) were both unresponsive to amphotericin B in murine models of invasive aspergillosis (96, 215). Only the A. terreus isolate tested resistant in vitro. A wide range of media and test conditions (including Antibiotic Medium 3) failed to distinguish the resistant A. fumigatus isolate from a pair of isolates that were proven susceptible in vivo.
Since amphotericin B-resistant mutants of A. fumigatus can be generated and detected in vitro (119, 121) and the isolate of A. terreus did test resistant, the above difficulty in identifying the resistant isolate of A. fumigatus is surprising. Using a straightforward adaptation of the NCCLS M27 methodology, Lass-Flörl et al. demonstrated a good correlation between amphotericin B MIC, of ≥2 μg/ml and an increased likelihood of clinical failure in patients with hematological malignancies (104).
Considered as a whole, these studies with filamentous fungi provide a cautionary note regarding the interpretation of mold MICs. Underlying diseases and technical laboratory factors are always important, but they may be especially so for invasive mold infections. It would appear that testing of A. fumigatus isolates against azole antifungal agents has significant potential value, and the interested reader is referred to the detailed review by Moore et al. on this topic (130). Testing of amphotericin B is, as for the yeasts, problematic. The most useful information is often derived from complete identification of the fungus itself. As examples of the value of identification to the species level, isolates of Aspergillus terreus (104, 207; Walsh et al., 14th Congr. ISHAM), Scedosporium apiospermum (Pseudallescheria boydii) (57, 219), and Scedosporium prolificans (17) are usually resistant to amphotericin B.
Other Drugs
Using the M27-A methodology, Pelletier et al. reported emergence of resistance to clotrimazole in a prospectively monitored HIV-infected pediatric population (149). If the clotrimazole MIC for the infecting C. albicians isolate was ≥0.5 μg/ml, there was a significant risk (P < 0.001) of cross-resistance to other azoles and a risk of clinically overt failure of antifungal azole therapy.
The allylamine terbinafine is widely used for treatment of nail, skin, and hair infections caused by both dermatophyte and nondermatophyte fungi (44, 109, 212). Susceptibility testing of molds and yeasts against terbinafine has been reported with NCCLS M-27A, M-38P, and modifications of these methods (94, 101, 124, 126, 140, 192). The relevance of terbinafine susceptibility testing, however, remains uncertain. For example, one study found that the identity of the etiologic fungus and not the in vitro MIC was more likely to predict the outcome of therapy for tinea capitis (129).
A number of other antifungal agents are now entering the clinical arena. These include new azoles (ravuconazole, voriconazole, and posaconazole) and agents of the glucan synthesis inhibitor groups (caspofungin, FK463, and anidulafungin). QC data for yeasts with the M-27 method are now available for voriconazole, ravuconazole, posaconazole, caspofungin, and anidulafungin (14). Clinically relevant methods and interpretive breakpoints for these agents are unknown. The azole antifungal agents will presumably provide reasonable correlations with an M27-A like method. Whether M27-A will be a suitable method for the glucan synthesis inhibitors is not currently known, and available data suggest that new approaches be required for in vitro prediction of their in vivo activity (103; C. M. Douglas, J. C. Bowman, G. K. Abruzzo, A. M. Flattery, C. J. Gill, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, M. B. Kurtz, and H. Rosen, Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1683, 2000).
CONCLUSIONS
Antifungal susceptibility testing has evolved rapidly during the last decade and has now become a relevant clinical tool (Table 2). However, susceptibility testing is not an infallible answer to questions about treatment of fungal diseases. Routine susceptibility testing is appropriate for Candida isolates (especially non-albicans species) from deep sites (160) but not at present for other fungi or settings. Testing of Candida isolates from the oropharynges of patients who fail to respond to standard therapy can help distinguish microbiological failures from other causes (e.g., noncompliance or drug interactions). Importantly, knowledge of the species of Candida is a useful guide to the probable pattern of susceptibility, and identification can be obtained in less than 1 day with some of the newer identification systems. Testing of isolates of C. neoformans might be pursued cautiously, since there are strong suggestions of relevant correlations. Mold fungi should not at present be routinely tested for susceptibility. They should, however, be identified to the species level since this often gives very useful therapeutic clues.
REFERENCES
- 1.Aller A I, Martin-Mazuelos E, Lozano F, Gomez-Mateos J, Steele-Moore L, Holloway W J, Gutierrez M J, Recio F J, Espinel-Ingroff A. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob Agents Chemother. 2000;44:1544–1548. doi: 10.1128/aac.44.6.1544-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Barrientos A, Arroyo J, Canton R, Nombela C, Sanchez-Perez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000;13:167–195. doi: 10.1128/cmr.13.2.167-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anaissie E J, Paetznick V L, Ensign L G, Espinel-Ingroff A, Galgiani J N, Hitchcock C A, LaRocco M, Patterson T, Pfaller M A, Rex J H, Rinaldi M G. Microbroth antifungal susceptibility testing of Candida albicans and Cryptococcus neoformans with and without agitation: An eight-center collaborative study. Antimicrob Agents Chemother. 1996;40:2387–2391. doi: 10.1128/aac.40.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes D, Craig W A. Pharmacokinetics and pharmacodynamics of outpatient intravenous antimicrobial therapy. Infect Dis Clin North Am. 1998;12:849–860. doi: 10.1016/s0891-5520(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 5.Andes D, Stamsted T, Conklin R. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother. 2001;45:922–926. doi: 10.1128/AAC.45.3.922-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes D, van Ogtrop H. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother. 1999;43:2116–2120. doi: 10.1128/aac.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andes D, van Ogtrop M. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine disseminated candidiasis model. Antimicrob Agents Chemother. 2000;44:938–942. doi: 10.1128/aac.44.4.938-942.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Arikan S, Rex J H. New agents for treatment of systemic fungal infections. Emerg Drugs. 2000;5:135–160. doi: 10.1517/14728214.7.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Armengou A, Porcar C, Mascaro J, Garcia-Bragado F. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:1337–1338. doi: 10.1093/clinids/23.6.1337-a. [DOI] [PubMed] [Google Scholar]
- 11.Arthington-Skaggs B A, Jradi H, Desai T, Morrison C J. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1999;37:3332–3337. doi: 10.1128/jcm.37.10.3332-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthington-Skaggs B A, Warnock D W, Morrison C J. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 2000;44:2081–2085. doi: 10.1128/aac.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry A L, Brown S D. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J Clin Microbiol. 1996;34:2154–2157. doi: 10.1128/jcm.34.9.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry A L, Pfaller M A, Brown S D, Espinel-Ingroff A, Ghannoum M A, Knapp C, Rennie R P, Rex J H, Rinaldi M G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J Clin Microbiol. 2000;38:3457–3459. doi: 10.1128/jcm.38.9.3457-3459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Berenguer J, Ali N, Allende M C, Lee J W, Garrett K, Battaglia S, Rinaldi M G, Piscitelli S, Pizzo P A, Walsh T J. Itraconazole in experimental pulmonary aspergillosis: comparison with amphotericin B, interaction with cyclosporin A, and correlation between therapeutic response and itraconazole plasma concentrations. Antimicrob Agents Chemother. 1994;38:1303–1308. doi: 10.1128/aac.38.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berenguer J, Rodriguez-Tudela J L, Richard C, Alvarez M, Sanz M A, Gaztelurrutia L, Ayats J, Martinez-Suarez J V. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Scedosporium Prolificans Spanish Study Group. Medicine (Baltimore) 1997;76:256–65. doi: 10.1097/00005792-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Bezjak V. Standardization of a hyphal inoculum of aspergilli for amphotericin B susceptibility testing. J Clin Microbiol. 1985;21:509–512. doi: 10.1128/jcm.21.4.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindschadler D D, Bennet J E, Abernathy R S. A pharmacologic guide to the clinical use of amphotericin B. Br J Infect Dis. 1969;120:427–436. doi: 10.1093/infdis/120.4.427. [DOI] [PubMed] [Google Scholar]
- 20.Brammer K W, Coates P E. Pharmacokinetics of fluconazole in pediatric patients. Eur J Clin Microbiol Infect Dis. 1994;13:325–329. doi: 10.1007/BF01974613. [DOI] [PubMed] [Google Scholar]
- 21.Bryce E, Roberts F, Sekhon A, Coldman A. Yeast in blood cultures: evaluation of factors influencing outcome. Diagn Microbiol Infect Dis. 1992;15:233–237. doi: 10.1016/0732-8893(92)90118-d. [DOI] [PubMed] [Google Scholar]
- 22.Burgess D S, Hastings R W. A comparison of dynamic characteristics of fluconazole, itraconazole, and amphotericin B against Cryptococcus neoformans using time-kill methodology. Diagn Microbiol Infect Dis. 2000;38:87–93. doi: 10.1016/s0732-8893(00)00173-5. [DOI] [PubMed] [Google Scholar]
- 23.Burgess D S, Hastings R W, Summers K K, Hardin T C, Rinaldi M G. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn, Microbiol. Infect Dis. 2000;36:13–18. doi: 10.1016/s0732-8893(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 24.Butty P, Lebecq J-C, Mallie M, Bastide J-M. Evaluation of the susceptibility of dermatophytes to antifungal drugs: a new technique. J Med Vet Mycol. 1996;33:403–409. doi: 10.1080/02681219580000771. [DOI] [PubMed] [Google Scholar]
- 25.Canton E, Peman J, Carrillo-Munoz A, Orero A, Ubeda P, Viudes A, Gobernado M. Fluconazole susceptibilities of bloodstream Candida sp. isolates as determined by National Committee for Clinical Laboratory Standards method M27-A and two other methods. J Clin Microbiol. 1999;37:2197–2200. doi: 10.1128/jcm.37.7.2197-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartledge J D, Midgley J, Gazzard B G. Clinical response to ketoconazole of HIV-related oral candidosis is predicted by Odds' relative growth method of susceptibility testing. J Antimicrob Chemother. 1997;40:117–119. doi: 10.1093/jac/40.1.117. [DOI] [PubMed] [Google Scholar]
- 27.Cartledge J D, Midgley J, Petrou M, Shanson D, Gazzard B G. Unresponsive HIV-related oro-oesophageal candidosis—an evaluation of two new in-vitro azole susceptibility tests. J Antimicrob Chemother. 1997;40:517–523. doi: 10.1093/jac/40.4.517. [DOI] [PubMed] [Google Scholar]
- 28.Casadevall A, Spitzer E D, Webb D, Rinaldi M G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother. 1993;37:1383–1386. doi: 10.1128/aac.37.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H C, Chang J J, Huang A H, Chang T C. Evaluation of a capacitance method for direct antifungal susceptibility testing of yeasts in positive blood cultures. J Clin Microbiol. 2000;38:971–976. doi: 10.1128/jcm.38.3.971-976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S C, O'Donnell M L, Gordon S, Gilbert G L. Antifungal susceptibility testing using the E test: comparison with the broth macrodilution technique. J Antimicrob Chemother. 1996;37:265–273. doi: 10.1093/jac/37.2.265. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Reference deleted.
- 33.Clancy C J, Nguyen M H. Comparison of a photometric method with standardized methods of antifungal susceptibility testing of yeasts. J Clin Microbiol. 1997;35:2878–2882. doi: 10.1128/jcm.35.11.2878-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy C J, Nguyen M H. Correlation between in vitro susceptibility determined by E test and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob Agents Chemother. 1999;43:1289–1290. doi: 10.1128/aac.43.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig W A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Cuenca-Estrella M, Diaz-Guerra T M, Mellado E, Rodriguez-Tudela J L. Influence of glucose supplementation and inoculum size on growth kinetics and antifungal susceptibility testing of Candida spp. J Clin Microbiol. 2001;39:525–532. doi: 10.1128/JCM.39.2.525-532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Currie B P, Ghannoum M, Bessen L, Casadevall A. Decreased fluconazole susceptibility of a relapse Cryptococcus neoformans isolate after fluconazole treatment. Infect Dis Clin Pract. 1995;4:318–319. [Google Scholar]
- 40.Dannaoui E, Borel E, Persat F, Monier M F, Piens M A. In-vivo itraconazole resistance of Aspergillus fumigatus in systemic murine aspergillosis. J Med Microbiol. 1999;48:1087–1093. doi: 10.1099/00222615-48-12-1087. [DOI] [PubMed] [Google Scholar]
- 41.Dannaoui E, Colin S, Pichot J, Piens M A. Evaluation of the E test for fluconazole susceptibility testing of Candida albicans isolates from oropharyngeal candidiasis. Eur J Clin Microbiol Infect Dis. 1997;16:228–232. doi: 10.1007/BF01709586. [DOI] [PubMed] [Google Scholar]
- 42.Davey K G, Holmes A D, Johnson E M, Szekely A, Warnock D W. Comparative evaluation of FUNGITEST and broth microdilution methods for antifungal drug susceptibility testing of Candida species and Cryptococcus neoformans. J Clin Microbiol. 1998;36:926–930. doi: 10.1128/jcm.36.4.926-930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davey K G, Szekely A, Johnson E M, Warnock D W. Comparison of a new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J Antimicrob Chemother. 1998;42:439–444. doi: 10.1093/jac/42.4.439. [DOI] [PubMed] [Google Scholar]
- 44.De Backer M, De Vroey C, Lesaffre E, Scheys I, De Keyser P. Twelve weeks of continuous oral therapy for toenail onychomycosis caused by dermatophytes: a double-blind comparative trial of terbinafine 250 mg/day versus itraconazole 200 mg/day. J Am Acad Dermatol. 1998;38:S57–S63. doi: 10.1016/s0190-9622(98)70486-4. [DOI] [PubMed] [Google Scholar]
- 45.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 46.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denning D W, Warn P. Dose range evaluation of liposomal nystatin and comparisons with amphotericin B and amphotericin B lipid complex in temporarily neutropenic mice infected with an isolate of Aspergillus fumigatus with reduced susceptibility to amphotericin B. Antimicrob Agents Chemother. 1999;43:2592–2599. doi: 10.1128/aac.43.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dick J, Merz W, Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980;18:158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon D M, Polak A. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy. 1987;33:129–40. doi: 10.1159/000238485. [DOI] [PubMed] [Google Scholar]
- 50.Reference deleted.
- 51.Druetta A, Freydiere A, Guinet R, Gille Y. Evaluation of five commercial antifungal susceptibility testing systems. Eur J Clin Microbiol Infect Dis. 1993;12:336–342. doi: 10.1007/BF01964429. [DOI] [PubMed] [Google Scholar]
- 52.Ernst E J, Klepser M E, Ernst M E, Messer S A, Pfaller M A. In vitro pharmacodynamic properties of MK-099I determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 53.Ernst E J, Klepser M E, Pfaller M A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst M E, Klepser M E, Wolfe E J, Pfaller M A. Antifungal dynamics of LY 303366, an investigational echinocandin B analog, against Candida spp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 55.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole Posaconazole and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Espinel-Ingroff A. Germinated and nongerminated conidial suspensions for testing of susceptibilities of Aspergillus spp. to amphotericin B, itraconazole, posaconazole, ravuconazole, and voriconazole. Antimicrob Agents Chemother. 2001;45:605–607. doi: 10.1128/AAC.45.2.605-607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espinel-Ingroff A. In vitro activities of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Espinel-Ingroff A, Barchiesi F, Hazen K C, Martinez-Suarez J V, Scalise G. Standardization of antifungal susceptibility testing and clinical relevance. Med Mycol. 1998;36:68–78. [PubMed] [Google Scholar]
- 60.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Jr, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Espinel-Ingroff A, Kish C W, Kerkering T M, Fromtling R A, Bartizal K, Galgiani J N, Villareal K, Pfaller M A, Gerarden T, Rinaldi M G, Fothergill A. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992;30:3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espinel-Ingroff A, Pfaller M, Erwin M E, Jones R N. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using casitone agar and solidified RPMI 1640 medium with 2% glucose. J Clin Microbiol. 1996;34:848–852. doi: 10.1128/jcm.34.4.848-852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espinel-Ingroff A, Pfaller M, Messer S A, Knapp C C, Killian S, Norris H A, Ghannoum M A. Multicenter comparison of the Sensititre YeastOne Colorimetric Antifungal Panel with the National Committee for Clinical Laboratory Standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeasts and yeast-like organisms. J Clin Microbiol. 1999;37:591–595. doi: 10.1128/jcm.37.3.591-595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinel-Ingroff A, Rodriguez-Tudela J L, Suarez-Martinez J V. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards Reference Macrodilution Method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J Clin Microbiol. 1995;33:3154–3158. doi: 10.1128/jcm.33.12.3154-3158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espinel-Ingroff A, Warnock D W, Vazquez J A, Arthington-Skaggs B A. In vitro susceptibility methods and clinical implications of antifungal resistance. Med Mycol. 2000;38(Suppl. 1):293–304. [PubMed] [Google Scholar]
- 68.Favel A, Michel-Nguyen A, Chastin C, Trousson F, Penaud A, Regli P. In vitro susceptibility pattern of Candida lusitaniae and evaluation of the Etest method. J Antimicrob Chemother. 1997;39:591–596. doi: 10.1093/jac/39.5.591. [DOI] [PubMed] [Google Scholar]
- 69.Favel A, Peyron F, De Meo M, Michel-Nguyen A, Carriere J, Chastin C, Regli P. Amphotericin B susceptibility testing of Candida lusitaniae isolates by flow cytofluorometry: comparison with the Etest and the NCCLS broth macrodilution method. J Antimicrob Chemother. 1999;43:227–232. doi: 10.1093/jac/43.2.227. [DOI] [PubMed] [Google Scholar]
- 70.Francis P, Walsh T J. Evolving role of flucytosine in immunocompromised patients—new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis. 1992;15:1003–1018. doi: 10.1093/clind/15.6.1003. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda T, Naka W, Tajima S, Nishikawa T. Neutral red assay in minimum fungicidal concentrations of antifungal agents. J Med Vet Mycol. 1996;34:353–356. doi: 10.1080/02681219680000601. [DOI] [PubMed] [Google Scholar]
- 72.Gehrt A, Peter J, Pizzo P A, Walsh T J. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J Clin Microbiol. 1995;33:1302–1307. doi: 10.1128/jcm.33.5.1302-1307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of the correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghannoum M A, Rice L B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glasmacher A, Hahn C, Leutner C, Molitor E, Wardelmann E, Losem C, Sauerbruch T, Marklein G, Schmidt-Wolf I G H. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses. 1999;42:443–451. doi: 10.1046/j.1439-0507.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- 77.Reference deleted.
- 78.Graninger W, Presteril E, Schneeweiss B, Teleky B, Georgopoulos A. Treatment of Candida albicans fungaemia with fluconazole. J Infect. 1993;16:133–146. doi: 10.1016/0163-4453(93)92761-k. [DOI] [PubMed] [Google Scholar]
- 79.Green L, Petersen B, Steimel L, Haeber P, Current W. Rapid determination of antifungal activity by flow cytometry. J Clin Microbiol. 1994;32:1088–1091. doi: 10.1128/jcm.32.4.1088-1091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Green L J, Marder P, Mann L L, Chio L C, Current W L. LY303366 exhibits rapid and potent fungicidal activity in flow cytometric assays of yeast viability. Antimicrob Agents Chemother. 1999;43:830–835. doi: 10.1128/aac.43.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. Antifungal pharmacodynamics: concentration-effect relationships in vitro and in vivo. Pharmcotherapeutics, in press. [DOI] [PubMed]
- 82.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–499. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 83.Guarro J, Llop C, Aguilar C, Pujol I. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob Agents Chemother. 1997;41:2760–2762. doi: 10.1128/aac.41.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guinet R, Nerson D, Declosets F, Dupouy-Camet J, Kures L, Marjollet M, Poirot J L, Ros A, Texier-Maugein J, Volle P J. Collaborative evaluation in seven laboratories of a standardized micromethod for yeast susceptibility testing. J Clin Microbiol. 1988;26:2307–2312. doi: 10.1128/jcm.26.11.2307-2312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gunderson S M, Hoffman H, Ernst E J, Pfaller M A, Klepser M E. In vitro pharmacodynamic characteristics of nystatin including time-kill and postantifungal effect. Antimicrob Agents Chemother. 2000;44:2887–2890. doi: 10.1128/aac.44.10.2887-2890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawser S P, Norris H, Jessup C J, Ghannoum M A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the Standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reference deleted.
- 88.Hopfer R L, Sparks S D, Cox G M. The use of flow cytometry as a tool for monitoring filament formation of fungi. Med Mycol. 2001;39:103–107. doi: 10.1080/mmy.39.1.103.107. [DOI] [PubMed] [Google Scholar]
- 89.Hospenthal D R, Bennett J E. Flucytosine monotherapy for cryptococcosis. Clin Infect Dis. 1998;27:260–264. doi: 10.1086/514669. [DOI] [PubMed] [Google Scholar]
- 90.Hostetler J S, Heykants J, Clemons K V, Woestenborghs R, Hanson L H, Stevens D A. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother. 1993;37:2224–2227. doi: 10.1128/aac.37.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jahn B, Koch A, Schmidt A, Wanner G, Genringer H, Bhakdi S, Brakhage A A. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun. 1997;65:5110–5117. doi: 10.1128/iai.65.12.5110-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jahn B, Stuben A, Bhakdi S. Colormetric susceptibility testing for Aspergillus fumigatus: comparison of menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide and Alamar Blue tests. J Clin Microbiol. 1996;34:2039–2041. doi: 10.1128/jcm.34.8.2039-2041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jessup C J, Pfaller M A, Messer S A, Zhang J, Tumberland M, Mbidde E K, Ghannoum M A. Fluconazole susceptibility testing of Cryptococcus neoformans: comparison of two broth microdilution methods and clinical correlates among isolates from Ugandan AIDS patients. J Clin Microbiol. 1998;36:2874–2876. doi: 10.1128/jcm.36.10.2874-2876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jessup C J, Ryder N S, Ghannoum M A. An evaluation of the in vitro activity of terbinafine. Med Mycol. 2000;38:155–159. doi: 10.1080/mmy.38.2.155.159. [DOI] [PubMed] [Google Scholar]
- 95.Jessup C J, Warner J, Isham N, Hasan I, Ghannoum M A. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J Clin Microbiol. 2000;38:341–344. doi: 10.1128/jcm.38.1.341-344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson E M, Oakley K L, Radford S A, Moore C B, Warn P, Warnock D W, Denning D W. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J Antimicrob Chemother. 2000;45:85–93. doi: 10.1093/jac/45.1.85. [DOI] [PubMed] [Google Scholar]
- 97.Kirk S M, Callister S M, Lim L C L, Schell R F. Rapid susceptibility testing of Candida albicans by flow cytometry. J Clin Microbiol. 1997;35:358–363. doi: 10.1128/jcm.35.2.358-363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klepser M E, Ernst E J, Lewis R E, Ernst M E, Pfaller M A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klepser M E, Wolfe E J, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J Antimicrob Chemother. 1998;41:397–401. doi: 10.1093/jac/41.3.397. [DOI] [PubMed] [Google Scholar]
- 101.Korting H C, Ollert M, Abeck D The German Colloborative Dermatophyte Drug Susceptibility Study Group. Results of German multicenter study of antimicrobial susceptibilities of Trichophyton rubrum and Trichophyton mentagrophytes strains causing tinea unguium. Antimicrob Agents Chemother. 1995;39:1206–1208. doi: 10.1128/aac.39.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kretschmar M, Nichterlein T, Kuntz P, Hof H. Rapid detection of susceptibility to fluconazole in Candida species by a bioluminescence assay of intracellular ATP. Diagn Microbiol Infect Dis. 1996;25:117–121. doi: 10.1016/s0732-8893(96)00130-7. [DOI] [PubMed] [Google Scholar]
- 103.Kurtz M B, Abruzzo G, Flattery A, Bartizal K, Marrinan J A, Li W, Milligan J, Nollstadt K, Douglas C M. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect Immun. 1996;64:3244–3251. doi: 10.1128/iai.64.8.3244-3251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lass-Flörl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich M P, Niederwieser D. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 105.Lass-Florl C, Nagl M, Speth C, Ulmer H, Dierich M P, Wurzner R. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob Agents Chemother. 2001;45:124–128. doi: 10.1128/AAC.45.1.124-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Law D, Moore C B, Denning D W. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J Antimicrob Chemother. 1997;40:109–112. doi: 10.1093/jac/40.1.109. [DOI] [PubMed] [Google Scholar]
- 107.Lee J W, Seibel N I, Amantea M A, Whitcomb P, Pizzo P A, Walsh T J. Safety, tolerance, and pharmacokinetics of fluconazole in children with neoplastic diseases. J Pediatr. 1992;120:987–993. doi: 10.1016/s0022-3476(05)81975-4. [DOI] [PubMed] [Google Scholar]
- 108.Lee S C, Fung C P, Huang J S, Tsai C J, Chen K S, Chen H Y, Lee N, See L C, Shieh W B. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob Agents Chemother. 2000;44:2715–2718. doi: 10.1128/aac.44.10.2715-2718.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lesher J L., Jr Oral therapy of common superficial fungal infections of the skin. J Am Acad Dermatol. 1999;40:S31–S34. doi: 10.1016/s0190-9622(99)70395-6. [DOI] [PubMed] [Google Scholar]
- 110.Lewis R E, Klepser M E, Pfaller M A. In vitro pharmacodynamic characteristics of flucytosine determined by time-kill methods. Diagn Microbiol Infect Dis. 2000;36:101–105. doi: 10.1016/s0732-8893(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 111.Liao R S, Rennie R P, Talbot J A. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob Agents Chemother. 1999;43:1034–1041. doi: 10.1128/aac.43.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopez-Jodra O, Torres-Rodriguez J M, Mendez-Vasquez R, Ribas-Forcadell E, Morera-Lopez Y, Baro-Tomas T, Alia-Aponte C. In vitro susceptibility of Cryptococcus neoformans isolates to five antifungal drugs using a colorimetric system and the reference microbroth method. J Antimicrob Chemother. 2000;45:645–649. doi: 10.1093/jac/45.5.645. [DOI] [PubMed] [Google Scholar]
- 113.Louie A, Drusano G L, Banerjee P, Liu Q F, Liu W G, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Louie A, Liu Q F, Drusano G L, Liu W G, Mayers M, Anaissie E, Miller M H. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob Agents Chemother. 1998;42:1512–1514. doi: 10.1128/aac.42.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lozano-Chiu M, Lancaster M V, Rex J H. Evaluation of a colorimetric method for detecting amphotericin B-resistant Candida isolates. Diagn Microbiol Infect Dis. 1998;31:417–424. doi: 10.1016/s0732-8893(98)00031-5. [DOI] [PubMed] [Google Scholar]
- 116.Lozano-Chiu M, Nelson P W, Lancaster M, Pfaller M A, Rex J H. Lot-to-lot variability of antibiotic medium 3 when used for susceptibility testing of Candida isolates to amphotericin B. J Clin Microbiol. 1997;35:270–272. doi: 10.1128/jcm.35.1.270-272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performance of three different media assessed by using E-Test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Macura A B. In vitro susceptibility of dermatophytes to antifungal drugs: a comparison of two methods. Int J Dermatol. 1993;32:533–536. doi: 10.1111/j.1365-4362.1993.tb02844.x. [DOI] [PubMed] [Google Scholar]
- 119.Manavathu E K, Alangaden G J, Chandrasekar P H. In-vitro isolation and antifungal susceptibility of amphotericin B-resistant mutants of Aspergillus fumigatus. J Antimicrob Chemother. 1998;41:615–619. doi: 10.1093/jac/41.6.615. [DOI] [PubMed] [Google Scholar]
- 120.Manavathu E K, Cutright J, Chandrasekar P H. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J Clin Microbiol. 1999;37:858–861. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Manavathu E K, Vazquez J A, Chandrasekar P H. Reduced susceptibility in laboratory-selected mutants of Aspergillus fumigatus to itraconazole due to decreased intracellular accumulation of the antifungal agent. Int J Antimicrob Agents. 1999;12:213–219. doi: 10.1016/s0924-8579(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 122.Marr K A, Rustad T R, Rex J H, White T C. The trailing endpoint phenotype in antifungal susceptibility testing is pH-dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martinez-Suarez J V, Rodriguez-Tudela J L. Patterns of in vitro activity of itraconazole and imidazole antifungal agents against Candida albicans with decreased susceptibility to fluconazole from Spain. Antimicrob Agents Chemother. 1995;39:1512–1516. doi: 10.1128/aac.39.7.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McGinnis M R, Pasarell L. In vitro evaluation of terbinafine and itraconazole against dematiaceous fungi. Med Mycol. 1998;36:243–246. [PubMed] [Google Scholar]
- 125.Meis J, Petrou M, Bille J, Ellis D, Gibbs D. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Diagn Microbiol Infect Dis. 2000;36:215–223. doi: 10.1016/s0732-8893(99)00152-2. [DOI] [PubMed] [Google Scholar]
- 126.Meletiadis J, Meis J, Mouton J W, Donnelly J P, Verweij P E. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J Clin Microbiol. 2000;38:2949–2954. doi: 10.1128/jcm.38.8.2949-2954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merz W G, Sanford G R. Isolation and characterization of a polyene-resistant variant of Candida tropicalis. J Clin Microbiol. 1979;9:677–680. doi: 10.1128/jcm.9.6.677-680.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Milan E P, Burattini M N, Kallas E G, Fischmann O, Costa P R, Colombo A L. Azole resistance among oral Candida species isolates from AIDS patients under ketoconazole exposure. Diagn Microbiol Infect Dis. 1998;32:211–216. doi: 10.1016/s0732-8893(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 129.Mock M, Monod M, Baudraz-Rosselet F, Panizzon R G. Tinea capitis dermatophytes: susceptibility to antifungal drugs tested in vitro and in vivo. Dermatology. 1998;197:361–367. doi: 10.1159/000018032. [DOI] [PubMed] [Google Scholar]
- 130.Moore C B, Sayers N, Mosquera J, Slaven J, Denning D W. Antifungal drug resistance in Aspergillus. J Infect. 2000;41:203–220. doi: 10.1053/jinf.2000.0747. [DOI] [PubMed] [Google Scholar]
- 131.Mouton J W, van Ogtrop M L, Andes D, Craig W A. Use of pharmacodynamic indices to predict efficacy of combination therapy in vivo. Antimicrob Agents Chemother. 1999;43:2473–2478. doi: 10.1128/aac.43.10.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Tentative standard NCCLS document M26-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 133.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard. NCCLS document M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 134.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Proposed standard M27-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 135.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 136.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; tentative standard. NCCLS document M27-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 137.Nguyen M H, Clancy C J, Yu V L, Yu Y V, Morris A J, Snydman D R, Sutton D A, Rinaldi M G. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177:425–430. doi: 10.1086/514193. [DOI] [PubMed] [Google Scholar]
- 138.Reference deleted.
- 139.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: Emergence of non-C. albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 140.Niewerth M, Splanemann V, Korting H C, Ring J, Abeck D. Antimicrobial susceptibility testing of dermatophytes—comparison of the agar macrodilution and broth microdilution tests. Chemotherapy. 1998;44:31–35. doi: 10.1159/000007087. [DOI] [PubMed] [Google Scholar]
- 141.Nolte F S, Parkinson T, Falconer D J, Dix S, Williams J, Gilmore C, Geller R, Wingard J R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;44:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Norris H A, Elewski B E, Ghannoum M A. Optimal growth conditions for the determination of the antifungal susceptibility of three species of dermatophytes with the use of a microdilution method. J Am Acad Dermatol. 1999;40:S9–S13. doi: 10.1016/s0190-9622(99)70392-0. [DOI] [PubMed] [Google Scholar]
- 143.O'Day D M, Ray W A, Robinson R D, Head W S. Correlation of in vitro and in vivo susceptibility of Candida albicans to amphotericin B and natamycin. Investig Ophthalmol Visual Sci. 1987;28:596–603. [PubMed] [Google Scholar]
- 144.Odds F C, van Gerven F, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Odds F C, Vranckx L, Woestenborghs F. Antifungal susceptibility testing of yeasts: evaluation of technical variables for test automation. Antimicrob Agents Chemother. 1995;39:2051–2060. doi: 10.1128/aac.39.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O'Gorman M R, Hopfer R L. Amphotericin B susceptibility testing of Candida species by flow cytometry. Cytometry. 1991;12:743–747. doi: 10.1002/cyto.990120808. [DOI] [PubMed] [Google Scholar]
- 147.Patterson T F, Kirkpatrick W R, Revankar S G, McAtee R K, Fothergill A W, McCarthy D I, Rinaldi M G. Comparative evaluation of microdilution and chromogenic agar screening for determining fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1996;34:3237–3239. doi: 10.1128/jcm.34.12.3237-3239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patterson T F, Revankar S G, Kirkpatrick W R, Dib O, Fothergill A W, Redding S W, Sutton D A, Rinaldi M G. A simple method for detecting fluconazole resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–1797. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pelletier R, Peter J, Antin C, Gonzalez C, Wood L, Walsh T J. Emergence of resistance of Candida albicans to clotrimazole in human immunodeficiency virus-infected children: in vitro and clinical correlations. J Clin Microbiol. 2000;38:1563–1568. doi: 10.1128/jcm.38.4.1563-1568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perfect J R, Cox G M. Drug resistance in Cryptococcus neoformans. Drug Resist Update. 1999;2:259–269. doi: 10.1054/drup.1999.0090. [DOI] [PubMed] [Google Scholar]
- 151.Peyron F, Favel A, Guiraud-Dauriac H, El Mzibra J, Chastin C, Dumenil G, Regli P. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob Agents Chemother. 1997;41:1537–1540. doi: 10.1128/aac.41.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peyron F, Favel A, Michel-Nguyen A, Gilly M, Regli P, Bolmstrom A. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J Clin Microbiol. 2001;39:339–342. doi: 10.1128/JCM.39.1.339-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pfaller M A, Arikan S, Lozano-Chiu M, Chen Y S, Coffman S, Messer S A, Rennie R, Sand C, Heffner T, Rex J H, Wang J, Yamane N. Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J Clin Microbiol. 1998;36:2609–2612. doi: 10.1128/jcm.36.9.2609-2612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pfaller M A, Messer S A, Bolmstrom A. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn Microbiol Infect Dis. 1998;32:223–227. doi: 10.1016/s0732-8893(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 155.Pfaller M A, Messer S A, Bolmström A, Odds F C, Rex J H. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1996;34:1691–1693. doi: 10.1128/jcm.34.7.1691-1693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pfaller M A, Messer S A, Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D070. J Clin Microbiol. 1995;33:1094–1097. doi: 10.1128/jcm.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pfaller M A, Messer S A, Hollis R J, Espinel-Ingroff A, Ghannoum M A, Plavan H, Killian S B, Knapp C C. Multisite reproducibility of MIC results by the Sensititre (R) YeastOne colorimetric antifungal susceptibility panel. Diagn Microbiol Infect Dis. 1998;31:543–547. doi: 10.1016/s0732-8893(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 158.Pfaller M A, Messer S A, Houston A, Mills K, Bolmstrom A, Jones R N. Evaluation of the Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different agar media. J Clin Microbiol. 2000;38:3715–3717. doi: 10.1128/jcm.38.10.3715-3717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pfaller M A, Messer S A, Mills K, Bolmstrom A. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J Clin Microbiol. 2000;38:3359–3361. doi: 10.1128/jcm.38.9.3359-3361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 161.Pore R S. Amphotericin B synergy testing by the FCST. Curr Microbiol. 1992;24:171–177. [Google Scholar]
- 162.Pore R S. Antibiotic susceptibility testing by flow cytometry. J Antimicrob Chemother. 1994;34:613–627. doi: 10.1093/jac/34.5.613. [DOI] [PubMed] [Google Scholar]
- 163.Pore R S. Antibiotic susceptibility testing of Candida albicans by flow cytometry. Curr Microbiol. 1990;20:323–328. [Google Scholar]
- 164.Pore R S. Ketoconazole susceptibility of yeasts by the FCST method. Curr Microbiol. 1991;23:45–50. [Google Scholar]
- 165.Posteraro B, Romano L, Sanguinetti M, Masucci L, Morace G, Fadda G. Commercial systems for fluconazole susceptibility testing of yeasts: comparison with the broth microdilution method. Diagn Microbiol Infect Dis. 2000;38:29–36. doi: 10.1016/s0732-8893(00)00174-7. [DOI] [PubMed] [Google Scholar]
- 166.Reference deleted.
- 167.Powderly W G, Keath E J, Sokol-Anderson M, Robinson K, Kitz D, Little J R, Kobayashsi G. Amphotericin B-resistant Cryptococcus neoformans in a patient with AIDS. Infect Dis Clin Pract. 1992;1:314–316. [Google Scholar]
- 168.Powderly W G, Kobayashi G S, Herzig G P, Medoff G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med. 1988;84:826–832. doi: 10.1016/0002-9343(88)90059-9. [DOI] [PubMed] [Google Scholar]
- 169.Provine H, Hadley S. Preliminary evaluation of a semisolid agar antifungal susceptibility test for yeasts and molds. J Clin Microbiol. 2000;38:537–541. doi: 10.1128/jcm.38.2.537-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pujol I, Guarro J, Llop C, Soler L, Fernandez-Ballart J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pujol I, Guarro J, Sala J, Riba M D. Effects of incubation temperature, inoculum size, and time of reading on broth microdilution susceptibility test results for amphotericin B against Fusarium. Antimicrob Agents Chemother. 1997;41:808–811. doi: 10.1128/aac.41.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qian Q, Sinkeldam I, Roberts G D. Comparison of a colorimetric microdilution method and the NCCLS macrodilution method for antifungal susceptibility testing of yeasts. J Mycol Med. 1999;9:181–184. [Google Scholar]
- 173.Quereda C, Polanco A M, Giner C, Sanchez-Sousa A, Pereira E, Navas E, Fortun J, Guerrero A, Baquero F. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1996;15:30–37. doi: 10.1007/BF01586182. [DOI] [PubMed] [Google Scholar]
- 174.Quindos G, Salesa R, Carrillo-Munoz A J, Lipperheide V, Jaudenes L, San Millan R, Torres-Rodriguez J M, Ponton J. Multicenter evaluation of ATB fungus: a standardized micromethod for yeast susceptibility testing. Chemotherapy. 1994;40:245–251. doi: 10.1159/000239200. [DOI] [PubMed] [Google Scholar]
- 175.Ramani R, Chaturvedi V. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob Agents Chemother. 2000;44:2752–2758. doi: 10.1128/aac.44.10.2752-2758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ramani R, Ramani A, Wong S J. Rapid flow cytometric susceptibility testing of Candida albicans. J Clin Microbiol. 1997;35:2320–2324. doi: 10.1128/jcm.35.9.2320-2324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rambali, B., J. A. Fernandez, L. van Nuffel, F. Woestenborghs, L. Baert, D. L. Massart, and F. C. Odds. Susceptibility testing of pathogenic fungi with itraconazole: a process analysis of test variables. J Antimicrob. Chemother. in press. [DOI] [PubMed]
- 178.Revankar S G, Dib O P, Kirkpatrick W R, McAtee R K, Fothergill A W, Rinaldi M G, Redding S W, Patterson T F. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:960–963. doi: 10.1086/513950. [DOI] [PubMed] [Google Scholar]
- 179.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 183.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Riesselman M H, Hazen K C, Cutler J E. Determination of antifungal MICs by a rapid susceptibility assay. J Clin Microbiol. 2000;38:333–340. doi: 10.1128/jcm.38.1.333-340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rodero L, Cordoba S, Cahn P, Hochenfellner F, Davel G, Canteros C, Kaufman S, Guelfand L. In vitro susceptibility studies of Cryptococcus neoformans isolated from patients with no clinical response to amphotericin B therapy. J Antimicrob Chemother. 2000;45:239–242. doi: 10.1093/jac/45.2.239. [DOI] [PubMed] [Google Scholar]
- 186.Rodero L, Cordoba S, Cahn P, Soria M, Lucarini M, Davel G, Kaufman S, Canteros C, Guelfand L. Timed-kill curves for Cryptococcus neoformans isolated from patients with AIDS. Med Mycol. 2000;38:201–207. doi: 10.1080/mmy.38.3.201.207. [DOI] [PubMed] [Google Scholar]
- 187.Rodriguez L J, Rex J H, Anaissie E J. Update on invasive candidiasis. Adv Pharmacol. 1996;137:349–400. doi: 10.1016/s1054-3589(08)60955-2. [DOI] [PubMed] [Google Scholar]
- 188.Rodriguez-Tudela J L, Martin-Diez F, Cuenca-Estrella M, Rodero L, Carpintero Y, Gorgojo B. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27A medium, buffered yeast nitrogen base, and RPMI-2% glucose. Antimicrob Agents Chemother. 2000;44:400–404. doi: 10.1128/aac.44.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rodriguez-Tudela J L, Martinez-Suarez J V. Defining conditions for microbroth antifungal susceptibility tests: influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]
- 190.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rodriguez-Tudela J L, Martinez-Suarez J V, Dronda F, Chaves F, Valencia E. Correlation of in vitro susceptibility test results with in vivo response: a study of azole therapy in AIDS patients. J Antimicrob Chemother. 1995;35:793–804. doi: 10.1093/jac/35.6.793. [DOI] [PubMed] [Google Scholar]
- 192.Ryder N S, Wagner S, Leitner I. In vitro activities of terbinafine against cutaneous isolates of Candida albicans and other pathogenic yeasts. Antimicrob Agents Chemother. 1998;42:1057–1061. doi: 10.1128/aac.42.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saag M S, Fessel W J, Kaufman C A, Merrill K W, Ward D J, Moskovitz B L, Thomas C, Oleka N, Guarnieri J A, Lee J, Brenner-Gati L, Klausner M. Treatment of fluconazole-refractory oropharyngeal candidiasis with itraconazole oral solution in HIV-positive patients. AIDS Res Hum Retroviruses. 1999;15:1413–1417. doi: 10.1089/088922299309919. [DOI] [PubMed] [Google Scholar]
- 194.Sandven P. Detection of fluconazole-resistant Candida strains by a disc diffusion screening test. J Clin Microbiol. 1999;37:3856–3859. doi: 10.1128/jcm.37.12.3856-3859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saxen H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther. 1993;54:269–277. doi: 10.1038/clpt.1993.147. [DOI] [PubMed] [Google Scholar]
- 196.Schmalreck A F, Kottmann I, Reiser A, Ruffer U, Scharr E, Vanca E. An evalution of seven methods of in vitro susceptibility testing of clinical yeast isolates against fluconazole. Mycoses. 1995;38(Suppl. 1):55–63. doi: 10.1111/j.1439-0507.1995.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 197.Seay R E, Larson T A, Toscano J P, Bostrom B C, O'Leary M C, Uden D L. Pharmcokinetics of fluconazole in immune-compromised children with leukemia or other hematologic disease. Pharmacotherapy. 1995;15:52–58. [PubMed] [Google Scholar]
- 198.Seidenfeld S M, Cooper B H, Smith J W, Luby J P, Mackowiak P A. Amphotericin B tolerance: a characteristic of Candida parapsilosis not shared by other Candida species. J Infect Dis. 1983;147:116–119. doi: 10.1093/infdis/147.1.116. [DOI] [PubMed] [Google Scholar]
- 199.Sewell D L, Pfaller M A, Barry A L. Comparison of broth macrodilution, broth microdilution, and Etest antifungal susceptibility tests for fluconazole. J Clin Microbiol. 1994;32:2099–2102. doi: 10.1128/jcm.32.9.2099-2102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shimokawa O, Nakayama H. Acetate-mediated growth inhibition in sterol 14 alpha-demethylation-deficient cells of Candida albicans. Antimicrob Agents Chemother. 1999;43:100–105. doi: 10.1128/aac.43.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shimokawa O, Nakayama H. Estimation of minimum sterol 14 alpha-demethylation-inhibitory concentration of azoles in Candida yeasts using acetate-mediated growth inhibition: potential utility in susceptibility testing. J Clin Microbiol. 2000;38:2893–2896. doi: 10.1128/jcm.38.8.2893-2896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Soni L M, Burattini M N, Pignatari A C, Gompertz O F, Colombo A L. Comparative study of agar diffusion test and the NCCLS macrobroth method for in vitro susceptibility testing of Candida spp. Mycopathologia. 1999;145:131–135. doi: 10.1023/a:1007068826861. [DOI] [PubMed] [Google Scholar]
- 203.Sterling T R, Gasser R A, Jr, Ziegler A. Emergence of resistance to amphotericin B during therapy for Candida glabrata infection in an immunocompetent host. Clin Infect Dis. 1996;23:187–188. doi: 10.1093/clinids/23.1.187. [DOI] [PubMed] [Google Scholar]
- 204.Sterling T R, Merz W G. Resistance to amphoterricin B: emerging clinical and microbiological patterns. Drug Resis Updates. 1998;1:161–165. doi: 10.1016/s1368-7646(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 205.St. Germain G, Dion C, Espinel-Ingroff A, Ratelle J, De Repentigny L. Ketoconazole and itraconazole susceptibility of Candida albicans isolates from patients infected with HIV. J Antimicrob Chemother. 1995;36:109–118. doi: 10.1093/jac/36.1.109. [DOI] [PubMed] [Google Scholar]
- 206.Stiller R L, Bennett J E, Scholer H J, Wall M, Polak A, Stevens D A. Correlation of in vitro susceptibility test results with in vivo response: flucytosine therapy in a systemic candidiasis model. J Infect Dis. 1983;147:1070–1077. doi: 10.1093/infdis/147.6.1070. [DOI] [PubMed] [Google Scholar]
- 207.Sutton D A, Sanche S E, Revankar S G, Fothergill A W, Rinaldi M G. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J Clin Microbiol. 1999;37:2343–2345. doi: 10.1128/jcm.37.7.2343-2345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Swinne D, Raes-Wuytack C, Van Looveren K, Desmet P. Comparative evaluation of Fungitest (R), Neo-Sensitabs (R) and M27T-NCCLS broth microdilution methods for antifungal drug susceptibility testing of Candida species and Cryptococcus neoformans. Mycoses. 1999;42:231–237. doi: 10.1046/j.1439-0507.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 209.Szekely A, Johnson E M, Warnock D W. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J Clin Microbiol. 1999;37:1480–1483. doi: 10.1128/jcm.37.5.1480-1483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tellier R, Karjden M, Grigoriew G A, Campbell I. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–1625. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tornatore M A, Noskin G A, Hacek D M, Obias A A, Peterson L R. Effects of incubation time and buffer concentration on in vitro activities of antifungal agents against Candida albicans. J Clin Microbiol. 1997;35:1473–1476. doi: 10.1128/jcm.35.6.1473-1476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torres-Rodriguez J M, Madrenys-Brunet N, Siddat M, Lopez-Jodra O, Jimenez T. Aspergillus versicolor as cause of onychomycosis: report of 12 cases and susceptibility testing to antifungal drugs. J Eur Acad Dermatol Venereol. 1998;11:25–31. [PubMed] [Google Scholar]
- 213.Vanden Bossche H, Dromer F, Improvisi I, Lozano-Chiu M, Rex J H, Sanglard D. Antifungal drug resistance in pathogenic fungi. Med Mycol. 1998;36:119–128. [PubMed] [Google Scholar]
- 214.Venugopal P V, Venugopal T V. Disk diffusion susceptibility testing of dermatophytes with allylamines. Int J Dermatol. 1994;33:730–732. doi: 10.1111/j.1365-4362.1994.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 215.Verweij P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reference deleted.
- 217.Walsh T J, Lee J W, Melcher G P, Navarro E, Bacher J, Callender D, Reed K D, Wu T, Lopez-Berestein G, Pizzo P A. Experimental Trichosporon infection in persistently granulocytopenic rabbits: Implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J Infect Dis. 1992;166:121–133. doi: 10.1093/infdis/166.1.121. [DOI] [PubMed] [Google Scholar]
- 218.Walsh T J, Melcher G P, Rinaldi M G, Lecciones J, McGough D A, Kelly P, Lee J, Callender D, Rubin M, Pizzo P A. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol. 1990;28:1616–1622. doi: 10.1128/jcm.28.7.1616-1622.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Walsh T J, Peter J, McGough D A, Fothergill A W, Rinaldi M G, Pizzo P A. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob Agents Chemother. 1995;39:1361–1364. doi: 10.1128/aac.39.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Walsh T J, Salkin I F, Dixon D M, Hurd N J. Clinical, microbiological, and experimental animal studies of Candida lipolytica. J Clin Microbiol. 1989;27:927–931. doi: 10.1128/jcm.27.5.927-931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Warnock D W, Johnson E M, Rogers T R F. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. J Antimicrob Chemother. 1998;42:321–331. doi: 10.1093/jac/42.3.321. [DOI] [PubMed] [Google Scholar]
- 223.Wenisch C, Linnau K F, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol. 1996;35:5–10. doi: 10.1128/jcm.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wheat J, Marichal P, Vanden Bossche H, Monte A L, Connolly P. Hypothesis on the mechanisms of resistance to fluconazole in Histoplasma capsulatum. Antimicrob Agents Chemother. 1997;41:410–414. doi: 10.1128/aac.41.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Willinger B, Apfalter P, Hirschl A M, Makristathis A, Rotter M, Seibold M. Susceptibility testing of Candida species: comparison of NCCLS microdilution method with Fungitest (R) Diagn Microbiol Infect Dis. 2000;38:11–15. doi: 10.1016/s0732-8893(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 227.Witt M D, Lewis R J, Larsen R A, Milefchik E N, Leal M A E, Haubrich R H, Richie J A, Edwards J E, Jr, Ghannoum M A. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: The role of antifungal susceptibility testing. Clin Infect Dis. 1996;22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]
- 228.Reference deleted.
- 229.Witthuhn F, Toubas D, Beguinot I, Aubert D, Rouger C, Remy G, Pinon J M. Evaluation of the fungitest kit by using strains from human immunodeficiency virus-infected patients: study of azole drug susceptibility. J Clin Microbiol. 1999;37:864–866. doi: 10.1128/jcm.37.3.864-866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Reference deleted.
- 231.Yang H C, Mikami Y, Yazawa K, Taguchi H, Nishimura K, Miyaji M, Branchini M L M, Aoki F H, Yamamoto K. Colorimetric MTT assessment of antifungal activity of D0870 against fluconazole-resistant Candida albicans. Mycoses. 1998;41:477–480. doi: 10.1111/j.1439-0507.1998.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 232.Yoshida T, Jono K, Okonogi K. Modified agar dilution susceptibility testing method for determining in vitro activities of antifungal agents, including azole compounds. Antimicrob Agents Chemother. 1997;41:1349–1351. doi: 10.1128/aac.41.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zar J H. Biostatistical analysis. 2nd ed. Englewood Cliffs, N. J.: Prentice-Hall, Inc.; 1984. [Google Scholar]


