Abstract
The correct duplication and transfer of genetic material to daughter cells is the major event of cell division. Dysfunction of DNA replication or chromosome segregation presents challenges in cancer initiation and development as well as opportunities for cancer treatment. Cyclic GMP-AMP synthase (cGAS) of the innate immune system detects cytoplasmic DNA and mediates downstream immune responses through the molecule stimulator of interferon genes (STING). However, how cytosolic DNA sensor cGAS participates in guaranteeing accurate cell division and preventing tumorigenesis is still unclear. Recent evidence indicates malfunction of cGAS/STING pathway in cancer progression. Cell cycle-targeted therapy synergizes with immunotherapy via cGAS/STING activation, leading to promising therapeutic benefit. Here, we review the interactions between cell cycle regulation and cGAS/STING signaling, thus enabling us to understand the role of cGAS/STING in cancer initiation, development, and treatment.
Keywords: cell cycle, cGAS/STING, tumorigenesis, immunotherapy
Graphical abstract

In this review, Long et al. provide a review that the cGAS/STING interacts with cell cycle and participates in cancer initiation, development, and treatment. Although recent evidence indicates malfunction of cGAS/STING signaling in cancer progression, cell cycle-targeted therapy can be combined with immunotherapy to enhance anti-tumor response by triggering cGAS/STING pathway.
Introduction
Maintenance of genome stability is essential for normal cellular homeostasis and proliferation, which depends on the precise control of DNA replication, chromosome segregation, DNA repair, as well as genome surveillance.1,2 Highly conserved DNA repair and cell cycle checkpoint signaling allow cells to deal with different types of DNA damage or other cell cycle defects. Many human tumors arise from DNA damage, or chromosome instability, while most therapeutic modalities used to treat cancers target DNA, including radiation therapy and chemotherapy.2,3
Cyclic GMP-AMP synthase (cGAS) and the downstream effector stimulator of interferon genes (STING) have been identified to be critical for the host innate immunity. cGAS is sensing cytoplasmic DNA derived from defective cell cycle progression, such as DNA damage and genomic instability.4,5 Subsequent studies reveal that cGAS provides additional anti-tumor roles by detecting DNA damage in cancer cells treated with classic cell cycle-targeted therapy. In this review, we focus on how cGAS/STING signaling supervises cell cycle progression and contributes to cancer therapy.
Cell cycle control and checkpoints
Cell cycle is a high demanding process that encompasses an ordered series of events to guarantee the correct duplication and segregation of the genome. The cell cycle checkpoints are the critical regulators in the processes of cell proliferation and growth as well as cell division after DNA duplication has finished, resulting in generation of two genetically identical daughter cells.6
Cell cycle progression includes two key events: DNA replication (S phase), when the nuclear genome is duplicated, and mitosis (M phase), when chromosomes are condensed, sorted, and equally distributed to daughter cells. Between the M and S phases are G1 phase and G2 phase, respectively.6 Cells can also enter quiescence (G0 phase), a state of replicative dormancy.7 The ataxia telangiectasia mutated protein kinase (ATM)/ATM and Rad3-related protein kinase (ATR)-checkpoint kinase 1 (CHK1)/checkpoint kinase 2 (CHK2)-controlled network response to genotoxic stress can transiently arrest cell cycle progression in G1, S, or G2 phase.8, 9 Numbers of studies demonstrate that the vulnerable stages of DNA synthesis and chromosome segregation are monitored and protected by a range of surveillance mechanism termed cell cycle checkpoints to detect possible defects. Once defects are sensed, checkpoints could halt cell cycle progression until it ensures these defects are repaired and the earlier process is completed. These regulatory checkpoints mainly include the DNA damage checkpoint, the DNA replication checkpoint, and the spindle assembly checkpoint (Figure 1).10
Figure 1.
Cell cycle is monitored by checkpoints
DNA damage checkpoint, DNA replication checkpoint, and spindle assembly checkpoint guarantee the correct duplication and segregation of the genome in the cell cycle.
The DNA damage checkpoint detects alterations of DNA molecules and protects cells from endogenous as well as exogenous attacks, including products of intracellular metabolism, chemicals, free radicals, or ionizing radiation.11 After defects are sensed, DNA damage response (DDR) signaling pathway is activated, followed by CDK inhibition and cell cycle arrest.3,8 The DNA damage checkpoint will next allow time for repair, preventing DNA defects transmission to the daughter cells. If excessive DNA damage exists or genetic deficiency appears in the checkpoint or DNA repair system, the defects will be incurable. Ultimately, cells may enter transient quiescence, senescence, and even undergo apoptotic cell death.12 Genetic alterations may also accumulate and alternatively induce cell transformation and oncogenesis.3
The DNA replication checkpoint primarily responds to improper DNA replication and maintains high fidelity by stabilizing replication forks, which ultimately prevents cell cycle progression.13 Replication stress, caused by damaged template DNA, collisions with transcription complexes, complexity of DNA sequences, or other sources, including oncogene activation, can recruit and activate the ATR-mediated pathway in order to ensure stalled forks remain stable, prevent fork collapse, promote repair, and restart damaged forks. When forks collapse or stop permanently, DNA synthesis is unable to resume.14,15
The spindle assembly checkpoint modulates CDK1 activity and spies on whether each chromosome is attached to the mitotic spindle through their kinetochores to guarantee accurate chromosome segregation.16 This primary role of the spindle assembly checkpoint produces a robust response in the presence of even a single unattached kinetochore.17 A defective spindle assembly checkpoint deregulates CDK1 activity, provokes unequal inheritance of the genetic information and survival of a viable population of cells from autonomous lethality, and allows them to mis-segregate a small number of chromosomes during one or multiple divisions, which eventually exhibits chromosomal instability. Accumulating numerous chromosomal aberrations may facilitate tumor initiation or progression.18
cGAS and cell cycle dysfunction
Cell cycle dysfunction leads to DNA defects
During the interphase and mitosis of the cell cycle, alternation of the internal heredity and external environment lead to the change of the genetic information. Interior factors include at least two aspects. First, reactive oxygen species derived from normal or abnormal cellular metabolism cause the formation of oxidized DNA bases and DNA breaks. Other cellular metabolites, such as aldehydes derived from lipid peroxidation or reactive radical species generated from hormone metabolites, could also cause DNA damage directly or indirectly. Second, DNA could be altered, such as dNTP mis-incorporation during DNA synthesis, damaged DNA induced by carbonyl stress, DNA base modification by alkylation, interconversion between DNA bases following deamination, or DNA base loss caused by depurination.11 All these incorrectly paired or incorporated nucleotides, which escape from detection and proofreading, become sources of mutations in the next round of replication and eventually lead to compromised fidelity.
Exogenous DNA damage is induced by physical or chemical sources. Physical factors include ionizing radiation caused by nature radiation and ultraviolet (UV) light from sunlight.19 Radiotherapy used in clinical cancer treatment mostly targets DNA. Toxic and chemical agents, including alkylating agents, aromatic amines, polycyclic aromatic hydrocarbon, and other reactive electrophiles, are also responsible for multiple DNA lesions and cause defects in the genome.11,19 Most chemotherapeutics that we currently apply to treat cancers are expected to suspend cell cycle progress or cause cells to exit from the cell cycle and end with programmed cell death.20 For example, paclitaxel (Taxol), which is used for the treatment of various types of malignancies, including ovarian, breast, and lung cancers, induces mitotic arrest, mostly due to activation of the spindle assembly checkpoint. Ultimately, it leads to multipolar divisions and chromosome mis-segregation, resulting in micronuclei formation.21, 22, 23
cGAS senses cytosol DNA during cell cycle progression
Indeed, besides checkpoints, there exist other mechanisms to guarantee genetic integrity. In normal cells, the defects derived from cell cycle progression could overcome the supervision of cell cycle checkpoints and cause DNA damage or chromosome instability, which eventually gain the possibility for oncogenesis. In cancer cells, the cell cycle checkpoints as well as other elements of the DDR pathway could also be found to protect tumor cells from different stress and consequently, to cause more damage to the whole monitoring system and further promote tumor progression.3,24 For example, CHK1 limits oncogene-induced replicative stress, promotes transformation, and contributes to cell survival.25 Given the critical significance of DNA replication and chromosome segregation for the maintenance of genomic integrity, how the signaling of DNA damage and chromosome instability cross-talks with human innate immune system, and how innate immune system guarantees the cell cycle accurate division, need to be taken into consideration for study of cancer initiation and development.
The innate immune system is the first line of defense against infection by pathogens, such as viruses and bacteria, which is triggered through the presence of nucleic acids in the cytoplasm by a series of pattern recognition receptors (PRRs). Several different PRRs have been identified to date, which bind to pathogen- or host-derived DNA, and initiate defensive immune response mostly in a cell-type-specific fashion.26 cGAS is a PRR that responds to endogenous DNA, which is released into the cytosol during pathogen invasion or self-DNA damage.27
The DNA-sensing function of cGAS depends on its localization. The N-terminal domain of cGAS determines nucleo-cytoplasmic distribution, centromere association, and activation of nuclear-localized cGAS.28 Nuclear localization sequence (NLS; 295DVIMKRKRGGS305) in cGAS mediates a classic importin-dependent nuclear translocation mechanism. In addition, suppression of B-lymphoid tyrosine kinase inhibits Tyr215 phosphorylation of cGAS and promotes its nuclear translocation.29 Cytosolic DNA-sensing function of cGAS relies on its presence within the cytoplasm by a nuclear export signal (NES; 169LEKLKL174). NES mutation or blockage of the exportin (CRM1) increases the sequestration of cGAS within the nucleus, which is thought to prevent cGAS from accessing cytosolic DNA.30 Indeed, cGAS is localized in the cytoplasm during the interphase, but enters the nucleus and associates with chromatin DNA during mitosis in proliferating cells.31 cGAS shows affinity with nucleosomes, which competitively inhibit DNA-dependent cGAS activation during normal mitosis.32 Nuclear histones linking to chromatin are reported to be essential in suppressing the immunogenicity of self-DNA, while chromatin without linker histone stimulates cGAS more efficiently.33 In certain types of cells, endogenous cGAS is predominantly a nuclear protein, and tethered tightly via a salt-resistant interaction.34 cGAS is also phosphorylated by Aurora kinase B or CDK1 and tethered to mitotic chromosomes without oligomerization, which prevents its autoimmune activation.35,36 Interestingly, acetylation of cGAS on either Lys384, Lys394, or Lys414 blocks cGAS activation and also inhibits autoimmunity.37 Thus, in normal cells, DNA is strictly separated from cytoplasm and cGAS is inactivated, while, in tumor cells, accumulation of DNA in cytoplasm promotes cGAS activation.
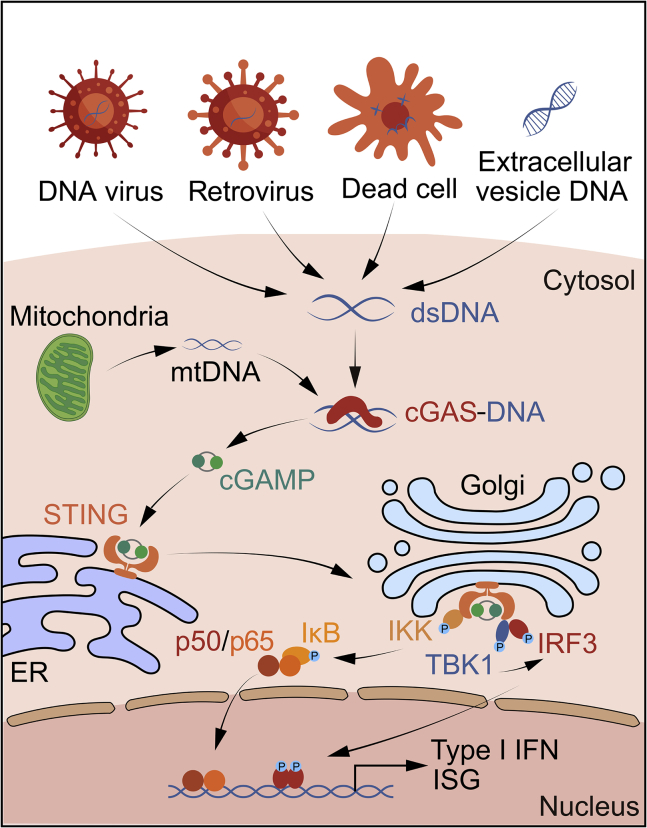
After binding to cytosolic DNA, cGAS catalyzes the formation of the cyclic GMP-AMP (cGAMP).38 cGAMP is a second messenger that binds to the adapter protein STING on the endoplasmic reticulum (ER) membrane, and then STING translocates from the ER to the Golgi apparatus, and next activates TANK-binding kinase 1 (TBK1) and IκB kinase (IKK). Subsequently, as the major effectors of innate immunity, interferon regulatory factor 3 (IRF3) and nuclear factor κB (NF-κB) are activated to induce the production of type I interferons (IFNs) and other cytokines, triggering immune responses (Figure 2).5,39,40
Figure 2.
cGAS/STING pathway is triggered by sensing of DNA in the cytosol
cGAS senses endogenous chromosomal fragments, micronuclei, and mitochondria DNA. cGAS directly binds to cytosolic DNA and subsequently catalyzes the production of cGAMP. STING is stimulated by cGAMP at the ER, and then translocates to the Golgi apparatus. STING recruits and activates TBK1, which further phosphorylates IRF3 and upregulates the expression of type I IFNs. Besides, STING activates NF-κB pathway by binding to IKK, which collaborates with TBK1-IRF3 signaling to induce type I IFN expression.
Cytosolic DNA and the RNA:DNA hybrids will trigger cGAS activity without sequence specificity, whereas RNA does not induce cGAMP formation.41,42 The DNA ligands may be derived from nucleus or mitochondria, and, on DNA binding, cGAS is activated through conformational transitions, leading to formation of a catalytically competent and accessible nucleotide-binding pocket for generation of c[G(2′,5′)pA(3′,5′)p].43 In addition, cGAS activation also relies on the formation of liquidlike droplets by DNA binding to cGAS.44 cGAS-DNA phase separation may form an environment to suppress cytosolic exonuclease, such as TREX1, reduce DNA degradation, and improve cGAS-mediated efficient sensing of immunostimulatory DNA.45 Of importance, long DNA activates cGAS more efficiently than short DNA, revealing that cGAS is activated by DNA in a length-dependent manner.46
Replication stress and chromosome mis-segregation activate cGAS
Replication stress can increase the accumulation of cytosolic DNA and activate cGAS/STING signaling. For example, the endonuclease MUS81, which suppresses chromosomal instability arising from stalled replication forks by cleaving DNA structures, promotes to the generation of cytosolic DNA, and ultimately activates STING.47 Deficiency of RNase H2, which is necessary for removing ribonucleotides incorporated in genome during DNA replication, may generate the nucleic acid ligands that are sensed by cGAS.48,49 Ablation of STING rescues RNase H2-mutant mice from autoinflammatory phenotypes.50 Depletion of SAMHD1, a deoxynucleoside triphosphate triphosphohydrolase required for replication fork progression, causes DNA fragments released from stalled forks, leading to cGAS/STING activation.51 New data recently revealed that depletion of the MutLα subunit MLH1, resulting in loss of MutLα-specific regulation of exonuclease 1 during DNA repair, induces unrestrained DNA excision, and eventually generates chromosomal abnormalities, activating the cGAS/STING pathway.52 Inhibition of KDM4A, a histone H3 lysine 9 trimethylation demethylase, induces DNA replication stress, which activates intrinsic cGAS/STING signaling in cancer cells.53 Other DNA-sensing proteins, such as G3BP1, have been identified to be associated with cGAS, and facilitate cGAS to bind to DNA by promoting the formation of cGAS complexes.54
Additionally, molecules coordinating in cell mitosis also display impacts on the cGAS/STING pathway. Barrier-to-autointegration factor 1 (BAF), a chromatin-binding protein essential for nuclear membrane reformation, competitively suppresses cGAS for DNA binding upon nuclear envelope rupture, and restricts the formation of DNA-cGAS complexes. BAF-deficient cells show repetitive nuclear envelope rupture events, which accumulate cGAS within discrete intranuclear foci.55 STAG2 is an important component of the cohesion complex that coordinates sister chromatid separation during cell division. Loss of STAG2 results in spontaneous genomic DNA damage and activation of the cGAS/STING pathways, which raises the possibility that maintaining the intact structures of chromatin contributes to inhibition of cGAS-mediated self-DNA sensing.56
Chromosome mis-segregation during mitosis leads to lagging chromosomes that can become separated and encased in nuclear envelopes, regarded as micronuclei.57,58 The formation of micronuclei appears to be associated with the cell cycle, and the micronucleus membrane is easy to break down. When micronuclei rupture, immunostimulatory DNA coming from micronuclei is recognized by cGAS, triggering an intrinsic immune surveillance.49,59 Nuclease TREX1 can limit cGAS activation at micronuclei by degrading micronuclear DNA on micronuclei envelope rupture.60 Of note, one study shows that micronuclei-localized cGAS mainly originates from nucleus-bound cGAS, suggesting the collapse of the micronuclei membrane may not be essential for cGAS activation.61 Another previous study reports that cGAS knockdown with induction of mitotic arrest leads to increased micronuclei formation and chromosome mis-segregation. In cGAS knockdown cells, micronuclei formation is decreased by CDK1 inhibition or p21 overexpression, and precocious G2/M transition is abrogated, indicating that the cell cycle defect is the main mechanism to induce chromosome instability when cGAS/STING signaling is disrupted.62 Interestingly, errors during chromosome segregation generate micronuclei and spill genomic DNA into the cytosol, leading to the activation of the cGAS/STING and promoting STING-dependent metastasis in breast cancer cells. Hence, chromosomal mis-segregation could also adopt chronic activation of innate immune responses through cytosolic DNA to accelerate cancer progression.63
cGAS and tumorigenesis
Cellular senescence is a state that persuades defective cells to enter into permanent cell cycle arrest under cellular stress, which is mostly attributed to accumulation of DNA damage and activates the p53 or p16 pathways. Senescent cells secrete inflammatory cytokines, growth factors, and proteases, referred to the senescence-associated secretory phenotype (SASP). The cGAS/STING pathway induced by the cytoplasmic DNA promotes SASP both in primary human cells and in mice through upregulating a set of IFN-stimulated genes (ISGs), which have been identified with multiple functions.31,64, 65, 66 cGAS or STING knockout cells display a decreased level of SASP when treated with irradiation or CDK4 inhibitor.66 Mice deficient in cGAS or STING also show impaired cellular senescence, production of ISGs, and immuno-surveillance upon oncogene activation or ionizing radiation.64,66 Importantly, senescence is predominant in premalignant cells and critical to suppress tumorigenesis.67 Premalignant hepatocytes transduced by oncogenic NrasG12V show cellular senescence and then are cleared by immune cells.68 However, absence of cGAS or STING in primary human cells and in mice leads to defect in SASP and immune cell infiltration and impairs clearance of the malignant cells, eventually promoting tumor development.31,64,66 Indeed, cGAS/STING could induce short-term inflammation to restrain activated oncogenes.64 Thus, the cGAS/STING pathway protects against aberrant cell survival by reinforcing cell cycle arrest or recruiting immune cells to clear the senescent cells. Accordingly, cGAS signaling deficiency is likely to promote tumorigenesis (Figure 3).
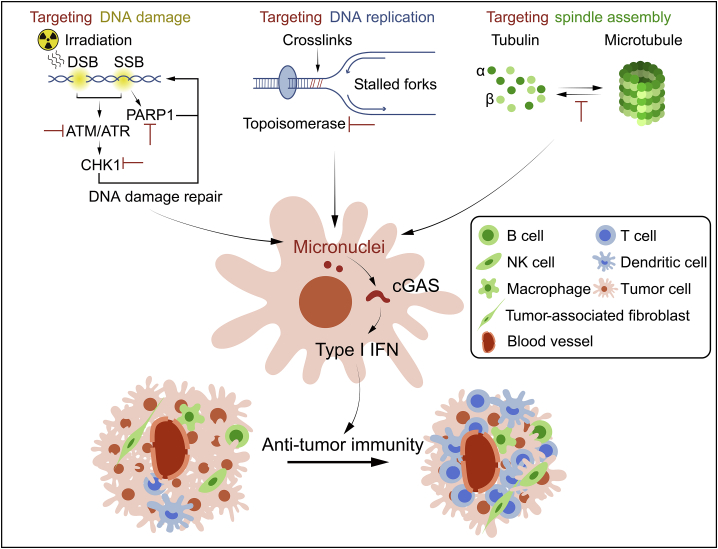
Figure 3.
Cell cycle defects promote DNA damage or chromosome mis-segregation, subsequently forming cytoplasmic chromatin fragments or micronuclei and activating cGAS pathways
Cytoplasmic chromatin fragments or micronuclei in the cytosol activate cGAS, stimulate the expression of type I IFNs as well as other cytokines, and promote SASP, leading to enhanced senescence and elimination of aberrant cells. However, cGAS deficiency is likely to promote tumorigenesis in premalignant cells.
Loss of cGAS in untransformed and cancer cells causes uncontrolled DNA replication and genomic instability. cGAS deficiency accelerates fork replication but compromises fork stability, revealing that cGAS is a decelerator of DNA replication forks to control replication dynamics.69 cGAS knockdown along with induction of mitotic arrest increases micronuclei formation and chromosome mis-segregation. Knockdown of STING, TBK1, or IRF3 also induces micronuclei formation. Meanwhile, p21 is decreased and precocious G2/M transition has occurred, giving evidence that the cGAS/STING/TBK1/IRF3 axis is essential for preventing chromosomal instability.62 Moreover, cGAS or STING knockout mouse embryonic fibroblasts (MEFs) are more proliferative with lower expression of p16, suggesting that the cGAS/STING pathway regulates cell cycle progression.66 STING also plays a pivotal role in the maintenance of cellular homeostasis through regulation of the cell cycle. STING depletion increases cell proliferation and leads to premature activation of CDK1, early onset to S and M phase, and enhanced chromosome instability after ionizing radiation.70 Deregulation of STING signaling impedes DDRs, prevents key cytokines including type I IFNs production in colorectal carcinoma, and correlates with tumorigenesis.71 Mice lacking STING are more susceptible to tumorigenesis induced by colon inflammation.72
Crucially, cGAS has been found to be associated with nucleosomes, replication forks, and centromeres,28,33,69,73,74 which raises a very interesting question as to whether nuclear cGAS has a cGAMP enzymatic function. Indeed, nuclear cGAS synthesizes cGAMP to promote innate immune activation of dendritic cells (DCs), although cGAMP is at a low level.28 Moreover, cGAS inhibits homologous recombination (HR) depending on its nuclear localization. cGAS causes compaction of template DNA into a higher-ordered state resistant to RAD51-mediated DNA strand invasion. This accelerates genome instability and micronucleus generation, and promotes cell death under stress.61 Besides, nuclear cGAS is recruited to DNA double-strand breaks (DSBs) and interacts with PARP1, which impedes the formation of the PARP1-Timeless complex, leading to suppression of HR. Furthermore, cGAS knockdown inhibits DNA damage and restrains the growth of Lewis lung carcinoma cells, revealing a role of nuclear cGAS in tumor promotion.29 Similarly, STING is involved in facilitating tumor initiation in some cancer types. The carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) activates STING to induce inflammation and ultimately promotes tumorigenesis, while STING-deficient mice are resistant to DMBA-induced skin carcinogenesis.75 cGAMP generated from cancer cells is transferred to astrocytes to activate the STING pathway, inducing inflammation and metastasis by gap junctions.76 All of the above evidence demonstrates that the subcellular distribution or function of cGAS/STING varies in a cell-type-specific manner.
cGAS and cancer therapy
Although cGAS/STING could be linked to tumorigenesis, activation of the cGAS/STING is associated with DNA damage and CDK inhibition, most of which is cell cycle dependent.77 Moreover, intrinsic expression of cGAS in cancer cells also promotes infiltration by CD8+ T cells and responds better to genotoxic treatment and immunotherapy, determining tumor immunogenicity.78 Activation of intrinsic cGAS/STING collaborates with programmed cell death protein-1 (PD-1) antibody and inhibits squamous cell carcinoma growth and metastasis by recruiting and activating CD8+ T cells, and efficiently eliminates cancer stem cells.53 Accordingly, loss of cGAS or STING in tumor cells with defective MLH1 represses tumor infiltration of T cells and endows resistance to checkpoint blockade. In the clinic, cGAS/STING downregulation is correlated with poor prognosis of MLH1-defective cancers.79 Low expression of cGAS is also correlated with poor survival of lung adenocarcinoma patients.31 Given that cGAS/STING activation underpins a fundamental immune response against cytosolic DNA derived from cancer therapy, it could be coordinated with checkpoint immunotherapy to gain better treatment responses.
Targeting DNA damage pathway and cGAS/STING
cGAS/STING signaling provides a connection between radiotherapy and activation of anti-tumor immunity. Several previous studies have shown that DNA damage induced by radiation causes micronuclei formation, activates the cGAS/STING pathway, and potentiates anti-tumor immunity in cancer cells.49,59,64,66,80 Injection of irradiated cancer cells in wild-type mice intensified the anti-tumor immune responses when combined with immune checkpoint inhibitor, while STING-deficient mice do not show benefits.59 Thus, cancer cells may develop resistance to therapy when cGAS/STING is deficient. Importantly, STING is essential for DCs to response to irradiated cancer cells and produce type I IFNs.80 Radiotherapy and cGAMP or STING ligands display distinct CD8+ T cell responses and tumor repression.80,81 However, radiation can also turn on a negative feedback loop by suppression of the immune system. For example, radiation is found to upregulate programmed death-ligand 1 (PD-L1) expression. Radiotherapy with PD-L1 antibody administration achieves a favorable therapeutic effect by reversing T cell suppression.82
The benefit from the anti-cancer agents used in the clinic is to block the cell cycle and induce cell death. Accumulating preclinical evidence has revealed that the DNA damage induced by chemotherapy or targeted therapy treatment generates chromosomal fragments and then leads to the formation of micronuclei, which are recognized by the innate immune system.83, 84, 85 Numerous agents could activate cGAS/STING, stimulate the expression of type I IFNs in cancer cells, and promote anti-tumor immunity, which is critical to obtaining therapeutic benefits.
ATM and ATR coordinate in DNA replication and repair, and play an initial role in sensing the DNA damage and activating the DDR pathway.9 The ATM inhibitor KU-60019 induces cytoplasmic DNA accumulation, STING-dependent production and secretion of proinflammatory cytokines in microglial cells.86 A recent report shows that, in AT-rich interaction domain 1A (ARID1A)-deficient tumors, KU-60019 increases the number of stalled replication forks, reduces DNA repair capacity, and leads to the accumulation of cytosolic DNA, which ultimately stimulates STING signaling. In addition, the combination of ATM inhibitors and PD-L1 antibody shows enhancement of the therapeutic efficacy in the mice bearing ARID1A-deficient tumors.87 Inhibition of ATR by its inhibitor VE-821 could induce premature mitotic entry and enhance genomic instability when combining with poly-(ADP-ribose) polymerase (PARP) inhibition in HR-deficient cancer cells. VE-821 also mediates synergistic cytotoxicity with the PARP inhibitor olaparib by increasing the numbers of cGAS-positive micronuclei and elevating levels of cGAS/STING-associated inflammatory signaling.84 The ATR inhibitor BAY1895433 represses ATR-CHK1 signaling, leading to PD-L1 destabilization, and resulting in a cGAS/STING-initiated apoptosis in castration-resistant prostate cancer. The combination of BAY1895433 with anti-PD-L1 therapy obtains a synergistic T cell-dependent therapeutic response.88 Another example is a triple therapy (ATR inhibitor AZD6738 plus radiotherapy plus anti-PD-L1) in tumor-bearing mice. A better immunophenotype is obtained relying on the activation of cGAS/STING signaling, and subsequently the therapeutic efficacy is enhanced.89
PARP1 is the enzyme that binds to damaged DNA and is critical to mediating the DDR pathway, modulating chromatin structure, and maintaining genomic stability.90 PARP inhibitors, which are approved for the treatment of BRCA-mutated breast or ovarian cancer, are believed to act through synthetic lethality with mutations in genes of DNA repair pathways.91,92 Another contribution the PARP inhibitor offered to its cytotoxic effects is the trapping of the DNA-PARP complexes. Through binding to the NAD+-binding pocket of PARP, PARP inhibitors induce an allosteric conformational change of PARP, which persistently stabilizes its reversible association to DNA.93 A recent finding also identifies that PARP1 trapping controls the immunomodulatory functions of PARP inhibitors, triggering cGAS/STING signaling and the downstream immune response.94 PARP inhibitors, including talazoparib and olaparib, induce production of cytosolic dsDNA and activate the cGAS/STING pathway in mouse models and multiple tumors. Talazoparib treatment induces the formation of cytosolic dsDNA and enhances γH2AX expression, which next activates cGAS/STING signaling and upregulates the expression of IFNs and ISGs.94 Olaparib improves the number of micronuclei in BRCA2-deficient cells and elicits a cGAS/STING-associated inflammatory response.84 Indeed, Olaparib activates the cGAS/STING signaling and further increases CD8+ T cell infiltration, while the anti-tumor effect is decreased after CD8+ T cell depletion or STING knockout in cancer cells.95 The anti-tumor benefits of PARP inhibitors could be achieved in ERCC1-deficient non-small cell lung cancer, BRCA-deficient triple-negative breast cancer, as well as ovarian cancer regardless of BRCA status.95, 96, 97 Meanwhile, ATR inhibition promotes formation of micronuclei in olaparib-treated BRCA2-deficient cancer cells. Olaparib induces synergistic cytotoxicity with ATR inhibition through inflammatory cytokine production.84 Combination of olaparib and the ATR inhibitor AZD6738 potentiates cell death in ATM-deficient cells. In xenograft and patient-derived xenograft (PDX) mouse models with ATM loss, olaparib and AZD6738 show synergistic effects as well.98 Mechanically, PARP inhibition-induced DNA damage can be repaired faithfully by HR, rendering cells deficient in HR repair or harboring mutations in DDR pathways (such as ATM/ATR deficiency) highly sensitive to PARP inhibitor. In the same way, ATM-deficient cells, which are likely dependent on ATR for HR, are also hypersensitive to ATR inhibitor.
To date, the PARP inhibitors olaparib, rucaparib, niraparib, and talazoparib have already been applied for the treatment of multiple types of cancer both in monotherapy and in combinations with other drugs.99 The newly identified immunomodulatory function of PARP inhibitors provides novel insights to improve the survival of cancer patients. PARP inhibitors also upregulate the expression of PD-L1 in the treated cells.83 Hence, PARP inhibitors offer a potential choice to make combinations with PD-L1 antibody in cancer therapy.83,97 In the future, combination regimens could be conceived for clinical improvement.
CHK1 monitors DNA damage during DNA replication, which recognizes a broad range of DNA abnormality. CHK1 also regulates replication origin activation and acts on S-phase progression.100,101 The CHK1 inhibitor prexasertib activates the STING pathway in both small cell lung cancer cells and immunocompetent small cell lung cancer in vivo model, and increases the level of chemokines to promote activation of cytotoxic T lymphocytes.83 Similarly, in a clinical trial of recurrent BRCA wild-type high-grade serous ovarian cancer, prexasertib monotherapy has already had a beneficial effect and possible enhances innate and adaptive immunity.102 It has been shown that PD-L1 expression is upregulated in response to DSBs in cancer cells, which requires ATM/ATR/CHK1 kinases.103 Prexasertib with anti-PD-L1 therapy blockade indeed reveals a significant anti-tumor effect in a small cell lung cancer mice model through a CD8+ T cell-dependent mechanism.83
Targeting DNA replication pathway and cGAS/STING
Topoisomerase I relaxes DNA supercoiling generated by transcription or replication, while topoisomerase II regulates DNA under- or overwinding, and relieves the knots and tangles of DNA during replication.104,105 Targeting topoisomerase causes replication fork collision, DSBs, and eventually cell death. The topoisomerase I inhibitor topotecan suppresses tumor growth in breast-tumor-bearing mice and promotes infiltration of DCs and CD8+ T cells. However, anti-tumor immune response triggered by topotecan is abrogated in STING-deficient mice. In addition, exosomes released from topotecan-treated cancer cells contain DNA to activate DCs via the STING pathway.106 The same benefit is observed during the use of the DNA topoisomerase II inhibitor teniposide. Teniposide induces NF-κB and the type I IFN pathway activation via STING signaling, which in turn potentiates DC-mediated antigen presentation to T cells. In multiple types of mouse tumor models, teniposide boosts the anti-tumor efficacy of anti-PD-1 therapy, suggesting the contribution of triggering tumor immunogenicity.107
Antimetabolites interfere with DNA replication by halting replication forks. Hydroxyurea, a typical antimetabolite, is applied to inhibit ribonucleoside diphosphate reductase and arrest replication forks.108 Exposure to hydroxyurea increases the production of chromosome aberration, oxidative stress, and micronucleus formation.109 Clinical evidence proves that increased micronucleus production after hydroxyurea treatment may be related to individual treatment sensitivity in children with sickle cell anemia.110 Besides, hydroxyurea-induced DNA damage upregulates expression of chemokines in a cGAS/STING-dependent manner in breast cancer cells.111
The mechanism of crosslinking agents is to form covalent adducts on cellular DNA. They induce interstrand cross-links that greatly distort the DNA structure and block replication fork progression.112 Typical crosslinking agents, such as cisplatin and mitomycin C, are found to induce DNA damage to accumulate cytosolic DNA and activate an innate immune response in breast cancer cells.111,113 After treating with cisplatin, glioma fibroblasts cells contain accumulating numbers of micronuclei that harbor damaged DNA and survive checkpoint adaptation.114 Cisplatin has also been reported to modify tumor immunogenicity in an ovarian cancer mouse model and increase antigen presentation and T cell infiltration, as well as upregulating PD-L1 expression. Chronic cisplatin treatment increases the level of cGAS and STING in platinum-resistant derivative 2F8cis cells, and acute exposure to cisplatin enhances the expression of cGAS and STING in both platinum-sensitive and -resistant cells. Importantly, anti-PD-L1 therapy also achieves benefits in a platinum-sensitive model as well as in a platinum-resistant model when combined with cisplatin.115
Targeting spindle assembly pathway and cGAS/STING
Microtubule-targeting agents (MTAs) represented by paclitaxel are found to interfere with chromosome segregation.22 A recent study reveals that paclitaxel treatment recruits cGAS/STING activation in response to micronuclei formation, and triggers proapoptotic secretome relying on induction of type I IFN and TNFα in organotypic cultures of primary human breast tumors or patient-derived xenografts, which indicates the potential of MTAs to improve the anti-tumor immunity.85 In another study, paclitaxel induces slow accumulation of cGAS-dependent IRF3 phosphorylation. The cGAS/STING/IRF3 axis promotes mitochondrial outer membrane permeabilization and accelerates cell death. Meanwhile, cGAS expression makes breast cancer cells and mouse xenograft tumors responsive to paclitaxel.32 The microtubule destabilizer eribulin, another MTA, could induce the cGAS/STING-dependent expression of ISGs in triple-negative breast cancer cells by cytoplasmic accumulation of mitochondrial DNA.116 Moreover, baseline cGAS expression may act as a potential biomarker to predict treatment response of combining MTAs and immune checkpoint inhibitors in triple-negative breast cancer patients.117 A STING agonist, 5,6-dimethylxanthenone-4-acetic acid (DMXAA; ASA404) efficiently synergizes with chemotherapy in an in vivo mice tumor model, especially with paclitaxel.118 DMXAA stimulates the STING pathway by inducing type I IFN secretion and eliciting the activation of DCs. In tumor-bearing mice, the DMXAA causes tumor regression at distant tumor sites, relying on the activation of the STING pathway and recruiting CD8+ T cells.119 In the context of non-small cell lung cancer, addition of DMXAA to the standard chemotherapy, including paclitaxel, improves the median survival in both squamous and non-squamous populations.120 The underlying mechanism of the interaction may be cytokines induced by DMXAA being cytotoxic to highly aneuploid tumor cells induced by MTAs. However, due to the small size of this trial, larger definitive trials should be confirmed. The latest evidence proves that DMXAA does not bind to human STING but only to its mouse counterparts, which may explain its limited activity.121, 122, 123 The STING agonist ADU-S100 (ML RR-S2 CDA, MIW815), potently activating five human STING alleles, optimizes for CD8+ T cell-mediated anti-tumor immunity, which generates a survival benefit when combined with checkpoint inhibitors.119,124 However, whether it could have syngeneic effects with chemotherapy including paclitaxel needs to be further studied.
Together with multiple tumor models treatments, this reveals that the combination of PD-1, PD-L1, cytotoxic T lymphocyte–associated protein 4 (CTLA4) antibodies, or other immune checkpoint inhibitors with cell cycle-targeted therapy shows synergistic effects,83,97,115,125 which may become the new treatment strategies for clinical application (Figure 4).
Figure 4.
Classic cancer therapy facilitates anti-tumor immunity by cGAS/STING activation
Targeting DNA damage, DNA replication, and spindle assembly pathway promotes the generation of cytoplasmic chromatin fragments or micronuclei, leading to cGAS/STING activation, which induces the production of type I IFNs as well as other cytokines to enhance anti-tumor immunity.
Conclusions
In the past, classic cell cycle regulation and the innate immune system seemed to be parallel. However, accumulating evidence shows that the innate immune system plays an essential role in guaranteeing accurate cell division, which depends on clearance of the damaged DNA or genetic deficiency. Besides, cell cycle regulation and cancer therapy show cross-talk with immune pathways. The cGAS/STING pathway itself promotes senescence in premalignant cells, and acute activation of the cGAS/STING mediates the interaction between the cell cycle and immune stimulation by sensing DNA damage or genome instability, contributing to anti-tumor immune responses. Classic cell cycle-related therapy can be combined with immunotherapy to facilitate anti-tumor immunity. Strategies of promoting DNA damage and genome instability can also be designed with the immunostimulatory effect.
Besides intrinsic function in tumor cells, cGAS/STING in the stromal compartments also contributes to anti-tumor immunity. cGAS could mediate DC sensing of irradiated tumor cells, while STING controls radiation-mediated type I IFN production, and triggers immune response in DCs.80 Tumor-derived cGAMP elicits the activation of the STING pathway and IFN production in immune cells within the tumor microenvironment, which in turn activates natural killer (NK) cells and induces anti-tumor immunity, while STING deficient mice fail to obtain optimal NK cell anti-tumor responses.126 The hematopoietic compartment induces acute serum cytokine response by STING signaling and is required for tumor-specific T cell activation.124 Furthermore, deficient cGAS/STING in CD8+ T cells interferes with anti-tumor immunity. Mechanistically, intrinsic cGAS/STING promotes the maintenance of stem cell-like CD8+ T cells.127 Accordingly, harnessing the inherent cGAS/STING pathway in stromal cells provides insight into the development of cancer immunotherapy.
Therefore, although the pro-tumorigenesis of cGAS/STING should be deeply considered, revealing the cross-talk between cell cycle regulation and the immunomodulatory system still increases the possibility of combining classic therapy with immunotherapy and will provide new insights in advancing cancer therapeutic approaches.
Acknowledgments
This study was supported by the Guangdong Basic and Applied Basic Research Foundation (no. 2019A1515011185 to Z.-J.L.), and Science and Technology Planning Project of Guangzhou (no. 202002030461 to Z.-J.L.).
Author contributions
Z.-J.L., J.-D.W., and J.-Q.X. wrote the manuscript. Q.L. and Z.-J.L. provided essential advice. X.-X.L. and J.-Q.X. drew the figures. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Zi-Jie Long, Email: longzij@mail.sysu.edu.cn.
Quentin Liu, Email: liuq9@mail.sysu.edu.cn.
References
- 1.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hustedt N., Durocher D. The control of DNA repair by the cell cycle. Nat. Cell Biol. 2016;19:1–9. doi: 10.1038/ncb3452. [DOI] [PubMed] [Google Scholar]
- 3.Kastan M.B., Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 4.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norbury C., Nurse P. Animal cell cycles and their control. Annu. Rev. Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 7.Pardee A.B. A restriction point for control of normal animal cell proliferation. Proc. Natl. Acad. Sci. U S A. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 9.Helt C.E., Cliby W.A., Keng P.C., Bambara R.A., O'Reilly M.A. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 2005;280:1186–1192. doi: 10.1074/jbc.M410873200. [DOI] [PubMed] [Google Scholar]
- 10.Elledge S.J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 11.De Bont R., van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B.B., Elledge S.J. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 13.Dungrawala H., Rose K.L., Bhat K.P., Mohni K.N., Glick G.G., Couch F.B., Cortez D. The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Mol. Cell. 2015;59:998–1010. doi: 10.1016/j.molcel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petermann E., Helleday T. Pathways of mammalian replication fork restart. Nat. Rev. Mol. Cell Biol. 2010;11:683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- 15.Zeman M.K., Cimprich K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musacchio A., Salmon E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 17.Rieder C.L., Cole R.W., Khodjakov A., Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee N., Walker G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghelli Luserna di Rora A., Iacobucci I., Martinelli G. The cell cycle checkpoint inhibitors in the treatment of leukemias. J. Hematol. Oncol. 2017;10:77. doi: 10.1186/s13045-017-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods C.M., Zhu J., McQueney P.A., Bollag D., Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol. Med. 1995;1:506–526. [PMC free article] [PubMed] [Google Scholar]
- 22.Long B.H., Fairchild C.R. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res. 1994;54:4355–4361. [PubMed] [Google Scholar]
- 23.Jordan M.A., Wendell K., Gardiner S., Derry W.B., Copp H., Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 24.Luo J., Solimini N.L., Elledge S.J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Contreras A.J., Gutierrez-Martinez P., Specks J., Rodrigo-Perez S., Fernandez-Capetillo O. An extra allele of Chk1 limits oncogene-induced replicative stress and promotes transformation. J. Exp. Med. 2012;209:455–461. doi: 10.1084/jem.20112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Cai X., Chiu Y.H., Chen Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 28.Gentili M., Lahaye X., Nadalin F., Nader G.P.F., Puig Lombardi E., Herve S., De Silva N.S., Rookhuizen D.C., Zueva E., Goudot C., et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 2019;26:2377–2393.e13. doi: 10.1016/j.celrep.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Zhang H., Wu X., Ma D., Wu J., Wang L., Jiang Y., Fei Y., Zhu C., Tan R., et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563:131–136. doi: 10.1038/s41586-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun H., Huang Y., Mei S., Xu F., Liu X., Zhao F., Yin L., Zhang D., Wei L., Wu C., et al. A nuclear export signal is required for cGAS to sense cytosolic DNA. Cell Rep. 2021;34:108586. doi: 10.1016/j.celrep.2020.108586. [DOI] [PubMed] [Google Scholar]
- 31.Yang H., Wang H., Ren J., Chen Q., Chen Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. U S A. 2017;114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zierhut C., Yamaguchi N., Paredes M., Luo J.D., Carroll T., Funabiki H. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell. 2019;178:302–315.e23. doi: 10.1016/j.cell.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uggenti C., Lepelley A., Depp M., Badrock A.P., Rodero M.P., El-Daher M.T., Rice G.I., Dhir S., Wheeler A.P., Dhir A., et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat. Genet. 2020;52:1364–1372. doi: 10.1038/s41588-020-00737-3. [DOI] [PubMed] [Google Scholar]
- 34.Volkman H.E., Cambier S., Gray E.E., Stetson D.B. Tight nuclear tethering of cGAS is essential for preventing autoreactivity. Elife. 2019;8:e47491. doi: 10.7554/eLife.47491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T., Huang T., Du M., Chen X., Du F., Ren J., Chen Z.J. Phosphorylation and chromatin tethering prevent cGAS activation during mitosis. Science. 2021;371:eabc5386. doi: 10.1126/science.abc5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L., Hu M.M., Bian L.J., Liu Y., Chen Q., Shu H.B. Phosphorylation of cGAS by CDK1 impairs self-DNA sensing in mitosis. Cell Discov. 2020;6:26. doi: 10.1038/s41421-020-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai J., Huang Y.J., He X., Zhao M., Wang X., Liu Z.S., Xue W., Cai H., Zhan X.Y., Huang S.Y., et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell. 2019;176:1447–1460.e14. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.M., Maniatis T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Civril F., Deimling T., de Oliveira Mann C.C., Ablasser A., Moldt M., Witte G., Hornung V., Hopfner K.P. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mankan A.K., Schmidt T., Chauhan D., Goldeck M., Honing K., Gaidt M., Kubarenko A.V., Andreeva L., Hopfner K.P., Hornung V. Cytosolic RNA:DNA hybrids activate the cGAS-STING axis. EMBO J. 2014;33:2937–2946. doi: 10.15252/embj.201488726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao P., Ascano M., Wu Y., Barchet W., Gaffney B.L., Zillinger T., Serganov A.A., Liu Y., Jones R.A., Hartmann G., et al. Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du M., Chen Z.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou W., Mohr L., Maciejowski J., Kranzusch P.J. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol. Cell. 2021;81:739–755.e7. doi: 10.1016/j.molcel.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luecke S., Holleufer A., Christensen M.H., Jønsson K.L., Boni G.A., Sørensen L.K., Johannsen M., Jakobsen M.R., Hartmann R., Paludan S.R. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18:1707–1715. doi: 10.15252/embr.201744017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho S.S.W., Zhang W.Y.L., Tan N.Y.J., Khatoo M., Suter M.A., Tripathi S., Cheung F.S.G., Lim W.K., Tan P.H., Ngeow J., et al. The DNA structure-specific endonuclease MUS81 mediates DNA sensor STING-dependent host rejection of prostate cancer cells. Immunity. 2016;44:1177–1189. doi: 10.1016/j.immuni.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Mackenzie K.J., Carroll P., Lettice L., Tarnauskaite Z., Reddy K., Dix F., Revuelta A., Abbondati E., Rigby R.E., Rabe B., et al. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J. 2016;35:831–844. doi: 10.15252/embj.201593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackenzie K.J., Carroll P., Martin C.A., Murina O., Fluteau A., Simpson D.J., Olova N., Sutcliffe H., Rainger J.K., Leitch A., et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461–465. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pokatayev V., Hasin N., Chon H., Cerritelli S.M., Sakhuja K., Ward J.M., Morris H.D., Yan N., Crouch R.J. RNase H2 catalytic core Aicardi-Goutieres syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J. Exp. Med. 2016;213:329–336. doi: 10.1084/jem.20151464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coquel F., Silva M.J., Techer H., Zadorozhny K., Sharma S., Nieminuszczy J., Mettling C., Dardillac E., Barthe A., Schmitz A.L., et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature. 2018;557:57–61. doi: 10.1038/s41586-018-0050-1. [DOI] [PubMed] [Google Scholar]
- 52.Guan J., Lu C., Jin Q., Lu H., Chen X., Tian L., Zhang Y., Ortega J., Zhang J., Siteni S., et al. MLH1 deficiency-triggered DNA hyperexcision by exonuclease 1 activates the cGAS-STING pathway. Cancer Cell. 2021;39:109–121.e5. doi: 10.1016/j.ccell.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W., Liu W., Jia L., Chen D., Chang I., Lake M., Bentolila L.A., Wang C.Y. Targeting KDM4A epigenetically activates tumor-cell-intrinsic immunity by inducing DNA replication stress. Mol. Cell. 2021;81:2148–2165.e9. doi: 10.1016/j.molcel.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z.S., Cai H., Xue W., Wang M., Xia T., Li W.J., Xing J.Q., Zhao M., Huang Y.J., Chen S., et al. G3BP1 promotes DNA binding and activation of cGAS. Nat. Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guey B., Wischnewski M., Decout A., Makasheva K., Kaynak M., Sakar M.S., Fierz B., Ablasser A. BAF restricts cGAS on nuclear DNA to prevent innate immune activation. Science. 2020;369:823–828. doi: 10.1126/science.aaw6421. [DOI] [PubMed] [Google Scholar]
- 56.Ding S., Diep J., Feng N., Ren L., Li B., Ooi Y.S., Wang X., Brulois K.F., Yasukawa L.L., Li X., et al. STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection. Nat. Commun. 2018;9:1485. doi: 10.1038/s41467-018-03782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenech M., Morley A.A. Measurement of micronuclei in lymphocytes. Mutat. Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 58.Hatch E.M., Fischer A.H., Deerinck T.J., Hetzer M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harding S.M., Benci J.L., Irianto J., Discher D.E., Minn A.J., Greenberg R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–470. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohr L., Toufektchan E., von Morgen P., Chu K., Kapoor A., Maciejowski J. ER-directed TREX1 limits cGAS activation at micronuclei. Mol. Cell. 2021;81:724–738.e9. doi: 10.1016/j.molcel.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang H., Xue X., Panda S., Kawale A., Hooy R.M., Liang F., Sohn J., Sung P., Gekara N.O. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. EMBO J. 2019;38:e102718. doi: 10.15252/embj.2019102718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basit A., Cho M.G., Kim E.Y., Kwon D., Kang S.J., Lee J.H. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp. Mol. Med. 2020;52:643–657. doi: 10.1038/s12276-020-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakhoum S.F., Ngo B., Laughney A.M., Cavallo J.A., Murphy C.J., Ly P., Shah P., Sriram R.K., Watkins T.B.K., Taunk N.K., et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J., Simithy J., Lan Y., Lin Y., Zhou Z., et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li T., Chen Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018;215:1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gluck S., Guey B., Gulen M.F., Wolter K., Kang T.W., Schmacke N.A., Bridgeman A., Rehwinkel J., Zender L., Ablasser A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017;19:1061–1070. doi: 10.1038/ncb3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collado M., Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang T.W., Yevsa T., Woller N., Hoenicke L., Wuestefeld T., Dauch D., Hohmeyer A., Gereke M., Rudalska R., Potapova A., et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 69.Chen H., Chen H., Zhang J., Wang Y., Simoneau A., Yang H., Levine A.S., Zou L., Chen Z., Lan L. cGAS suppresses genomic instability as a decelerator of replication forks. Sci. Adv. 2020;6:eabb8941. doi: 10.1126/sciadv.abb8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranoa D.R.E., Widau R.C., Mallon S., Parekh A.D., Nicolae C.M., Huang X., Bolt M.J., Arina A., Parry R., Kron S.J., et al. STING promotes homeostasis via regulation of cell proliferation and chromosomal stability. Cancer Res. 2019;79:1465–1479. doi: 10.1158/0008-5472.CAN-18-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia T., Konno H., Ahn J., Barber G.N. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–297. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Q., Man S.M., Gurung P., Liu Z., Vogel P., Lamkanfi M., Kanneganti T.D. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kujirai T., Zierhut C., Takizawa Y., Kim R., Negishi L., Uruma N., Hirai S., Funabiki H., Kurumizaka H. Structural basis for the inhibition of cGAS by nucleosomes. Science. 2020;370:455–458. doi: 10.1126/science.abd0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pathare G.R., Decout A., Gluck S., Cavadini S., Makasheva K., Hovius R., Kempf G., Weiss J., Kozicka Z., Guey B., et al. Structural mechanism of cGAS inhibition by the nucleosome. Nature. 2020;587:668–672. doi: 10.1038/s41586-020-2750-6. [DOI] [PubMed] [Google Scholar]
- 75.Ahn J., Xia T., Konno H., Konno K., Ruiz P., Barber G.N. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Q., Boire A., Jin X., Valiente M., Er E.E., Lopez-Soto A., Jacob L., Patwa R., Shah H., Xu K., et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yum S., Li M., Chen Z.J. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 2020;30:639–648. doi: 10.1038/s41422-020-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schadt L., Sparano C., Schweiger N.A., Silina K., Cecconi V., Lucchiari G., Yagita H., Guggisberg E., Saba S., Nascakova Z., et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 2019;29:1236–1248.e7. doi: 10.1016/j.celrep.2019.09.065. [DOI] [PubMed] [Google Scholar]
- 79.Lu C., Guan J., Lu S., Jin Q., Rousseau B., Lu T., Stephens D., Zhang H., Zhu J., Yang M., et al. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell. 2021;39:96–108.e6. doi: 10.1016/j.ccell.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baird J.R., Friedman D., Cottam B., Dubensky T.W., Jr., Kanne D.B., Bambina S., Bahjat K., Crittenden M.R., Gough M.J. Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 2016;76:50–61. doi: 10.1158/0008-5472.CAN-14-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R., Fu Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sen T., Rodriguez B.L., Chen L., Corte C.M.D., Morikawa N., Fujimoto J., Cristea S., Nguyen T., Diao L., Li L., et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646–661. doi: 10.1158/2159-8290.CD-18-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schoonen P.M., Kok Y.P., Wierenga E., Bakker B., Foijer F., Spierings D.C.J., van Vugt M. Premature mitotic entry induced by ATR inhibition potentiates olaparib inhibition-mediated genomic instability, inflammatory signaling, and cytotoxicity in BRCA2-deficient cancer cells. Mol. Oncol. 2019;13:2422–2440. doi: 10.1002/1878-0261.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lohard S., Bourgeois N., Maillet L., Gautier F., Fetiveau A., Lasla H., Nguyen F., Vuillier C., Dumont A., Moreau-Aubry A., et al. STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat. Commun. 2020;11:259. doi: 10.1038/s41467-019-13689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song X., Ma F., Herrup K. Accumulation of cytoplasmic DNA due to ATM deficiency activates the microglial viral response system with neurotoxic consequences. J. Neurosci. 2019;39:6378–6394. doi: 10.1523/JNEUROSCI.0774-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L., Yang L., Wang C., Zhao W., Ju Z., Zhang W., Shen J., Peng Y., An C., Luu Y.T., et al. Inhibition of the ATM/Chk2 axis promotes cGAS/STING signaling in ARID1A-deficient tumors. J. Clin. Invest. 2020;130:5951–5966. doi: 10.1172/JCI130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Z., Pilie P.G., Geng C., Manyam G.C., Yang G., Park S., Wang D., Peng S., Wu C., Peng G., et al. ATR inhibition induces CDK1-SPOP signaling and enhances anti-PD-L1 cytotoxicity in prostate cancer. Clin. Cancer Res. 2021;27:4898–4909. doi: 10.1158/1078-0432.CCR-21-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheng H., Huang Y., Xiao Y., Zhu Z., Shen M., Zhou P., Guo Z., Wang J., Wang H., Dai W., et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J. Immunother. Cancer. 2020;8:e000340. doi: 10.1136/jitc-2019-000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCabe N., Turner N.C., Lord C.J., Kluzek K., Bialkowska A., Swift S., Giavara S., O'Connor M.J., Tutt A.N., Zdzienicka M.Z., et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 92.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34:360–394. doi: 10.1101/gad.334516.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murai J., Huang S.Y., Das B.B., Renaud A., Zhang Y., Doroshow J.H., Ji J., Takeda S., Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim C., Wang X.D., Yu Y. PARP1 inhibitors trigger innate immunity via PARP1 trapping-induced DNA damage response. Elife. 2020;9:e60637. doi: 10.7554/eLife.60637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pantelidou C., Sonzogni O., De Oliveria Taveira M., Mehta A.K., Kothari A., Wang D., Visal T., Li M.K., Pinto J., Castrillon J.A., et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722–737. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chabanon R.M., Muirhead G., Krastev D.B., Adam J., Morel D., Garrido M., Lamb A., Henon C., Dorvault N., Rouanne M., et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Invest. 2019;129:1211–1228. doi: 10.1172/JCI123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen J., Zhao W., Ju Z., Wang L., Peng Y., Labrie M., Yap T.A., Mills G.B., Peng G. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res. 2019;79:311–319. doi: 10.1158/0008-5472.CAN-18-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lloyd R.L., Wijnhoven P.W.G., Ramos-Montoya A., Wilson Z., Illuzzi G., Falenta K., Jones G.N., James N., Chabbert C.D., Stott J., et al. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene. 2020;39:4869–4883. doi: 10.1038/s41388-020-1328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LaFargue C.J., Dal Molin G.Z., Sood A.K., Coleman R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:e15–e28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Syljuasen R.G., Sorensen C.S., Hansen L.T., Fugger K., Lundin C., Johansson F., Helleday T., Sehested M., Lukas J., Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol. Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maya-Mendoza A., Petermann E., Gillespie D.A., Caldecott K.W., Jackson D.A. Chk1 regulates the density of active replication origins during the vertebrate S phase. EMBO J. 2007;26:2719–2731. doi: 10.1038/sj.emboj.7601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lampert E.J., Cimino-Mathews A., Lee J.S., Nair J., Lee M.J., Yuno A., An D., Trepel J.B., Ruppin E., Lee J.M. Clinical outcomes of prexasertib monotherapy in recurrent BRCA wild-type high-grade serous ovarian cancer involve innate and adaptive immune responses. J. Immunother. Cancer. 2020;8:e000516. doi: 10.1136/jitc-2019-000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato H., Niimi A., Yasuhara T., Permata T.B.M., Hagiwara Y., Isono M., Nuryadi E., Sekine R., Oike T., Kakoti S., et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 105.Deweese J.E., Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitai Y., Kawasaki T., Sueyoshi T., Kobiyama K., Ishii K.J., Zou J., Akira S., Matsuda T., Kawai T. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J. Immunol. 2017;198:1649–1659. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z., Chen J., Hu J., Zhang H., Xu F., He W., Wang X., Li M., Lu W., Zeng G., et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J. Clin. Invest. 2019;129:4850–4862. doi: 10.1172/JCI127471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koc A., Wheeler L.J., Mathews C.K., Merrill G.F. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J. Biol. Chem. 2004;279:223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- 109.Ahmad M.F., Ansari M.O., Jameel S., Wani A.L., Parveen N., Siddique H.R., Shadab G. Protective role of nimbolide against chemotherapeutic drug hydroxyurea induced genetic and oxidative damage in an animal model. Environ. Toxicol. Pharmacol. 2018;60:91–99. doi: 10.1016/j.etap.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 110.Flanagan J.M., Howard T.A., Mortier N., Avlasevich S.L., Smeltzer M.P., Wu S., Dertinger S.D., Ware R.E. Assessment of genotoxicity associated with hydroxyurea therapy in children with sickle cell anemia. Mutat. Res. 2010;698:38–42. doi: 10.1016/j.mrgentox.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parkes E.E., Walker S.M., Taggart L.E., McCabe N., Knight L.A., Wilkinson R., McCloskey K.D., Buckley N.E., Savage K.I., Salto-Tellez M., et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J. Natl. Cancer Inst. 2017;109:djw199. doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rycenga H.B., Long D.T. The evolving role of DNA inter-strand crosslinks in chemotherapy. Curr. Opin. Pharmacol. 2018;41:20–26. doi: 10.1016/j.coph.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Erdal E., Haider S., Rehwinkel J., Harris A.L., McHugh P.J. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31:353–369. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lewis C.W., Golsteyn R.M. Cancer cells that survive checkpoint adaptation contain micronuclei that harbor damaged DNA. Cell Cycle. 2016;15:3131–3145. doi: 10.1080/15384101.2016.1231287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grabosch S., Bulatovic M., Zeng F., Ma T., Zhang L., Ross M., Brozick J., Fang Y., Tseng G., Kim E., et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38:2380–2393. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fermaintt C.S., Takahashi-Ruiz L., Liang H., Mooberry S.L., Risinger A.L. Eribulin activates the cGAS-STING pathway via the cytoplasmic accumulation of mitochondrial DNA. Mol. Pharmacol. 2021;100:309–318. doi: 10.1124/molpharm.121.000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu Y., Manasrah B.K., McGregor S.M., Lera R.F., Norman R.X., Tucker J.B., Scribano C.M., Yan R.E., Humayun M., Wisinski K.B., et al. Paclitaxel induces micronucleation and activates pro-inflammatory cGAS-STING signaling in triple-negative breast cancer. Mol. Cancer Ther. 2021;20:2553–2567. doi: 10.1158/1535-7163.MCT-21-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siim B.G., Lee A.E., Shalal-Zwain S., Pruijn F.B., McKeage M.J., Wilson W.R. Marked potentiation of the antitumour activity of chemotherapeutic drugs by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Cancer Chemother. Pharmacol. 2003;51:43–52. doi: 10.1007/s00280-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 119.Corrales L., Glickman L.H., McWhirter S.M., Kanne D.B., Sivick K.E., Katibah G.E., Woo S.R., Lemmens E., Banda T., Leong J.J., et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McKeage M.J., Jameson M.B., Investigators A.S.S.G. Comparative outcomes of squamous and non-squamous non-small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA) - retrospective analysis of pooled data. J. Thorac. Dis. 2010;2:199–204. doi: 10.3978/j.issn.2072-1439.2010.02.04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conlon J., Burdette D.L., Sharma S., Bhat N., Thompson M., Jiang Z., Rathinam V.A., Monks B., Jin T., Xiao T.S., et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prantner D., Perkins D.J., Lai W., Williams M.S., Sharma S., Fitzgerald K.A., Vogel S.N. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 2012;287:39776–39788. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim S., Li L., Maliga Z., Yin Q., Wu H., Mitchison T.J. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem. Biol. 2013;8:1396–1401. doi: 10.1021/cb400264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sivick K.E., Desbien A.L., Glickman L.H., Reiner G.L., Corrales L., Surh N.H., Hudson T.E., Vu U.T., Francica B.J., Banda T., et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep. 2018;25:3074–3085.e5. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 125.Nolan E., Savas P., Policheni A.N., Darcy P.K., Vaillant F., Mintoff C.P., Dushyanthen S., Mansour M., Pang J.B., Fox S.B., et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci. Transl. Med. 2017;9:eaal4922. doi: 10.1126/scitranslmed.aal4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marcus A., Mao A.J., Lensink-Vasan M., Wang L., Vance R.E., Raulet D.H. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49:754–763.e4. doi: 10.1016/j.immuni.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li W., Lu L., Lu J., Wang X., Yang C., Jin J., Wu L., Hong X., Li F., Cao D., et al. cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 2020;12:eaay9013. doi: 10.1126/scitranslmed.aay9013. [DOI] [PubMed] [Google Scholar]