The central nervous system (CNS) is largely unable to generate new neurons to compensate for the loss of neurons caused by disease or injury. One potentially promising approach to replace CNS neurons is to convert resident astrocytes into neurons in situ by gene therapy. Conversion of astrocytes to neurons in vitro by overexpression of the transcription factor Pax6 was first reported nearly 20 years ago.1 Since then, multiple groups have reported astrocyte-to-neuron conversion in vitro and in vivo following various genetic manipulations.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Recently, overexpression of NeuroD112, 13, 14, 15, 16, 17 and knockdown of the RNA splicing factor Ptbp118,19 were assessed for their ability to convert astrocytes to neurons in vivo in multiple CNS areas, with therapeutic effects in mouse models of disease, ischemia, and injury. However, key findings from this work were not readily reproduced,20 prompting responses from the original authors21,22 and generating controversy around astrocyte-to-neuron conversion. Here we report limited, brain-area-specific astrocyte-to-neuron conversion in mice and compare our findings with recent reports.
We initially set out to test astrocyte-to-neuron conversion across the mouse CNS using systemic delivery of the blood-brain-barrier-penetrating AAV-PHP.eB capsid23 and a shortened human GFAP promoter (hGFAP)24 to restrict expression to astrocytes. We achieved improved broad expression in astrocytes with self-complementary AAV genomes25 (Figures 1A–1C; Figure S1B) and confirmed that miR-124 targeting sites (miR-124-TSs)26 in the 3′ untranslated region (UTR) helped restrict expression to astrocytes (Figures S1A and S1B). However, the expression level was generally low, and we noted trace levels of off-target expression in neurons (Figures 1C and S1B) as well as scattered expression in the liver (data not shown). These results underscore the difficulty of targeting astrocytes specifically by systemic AAV injection.
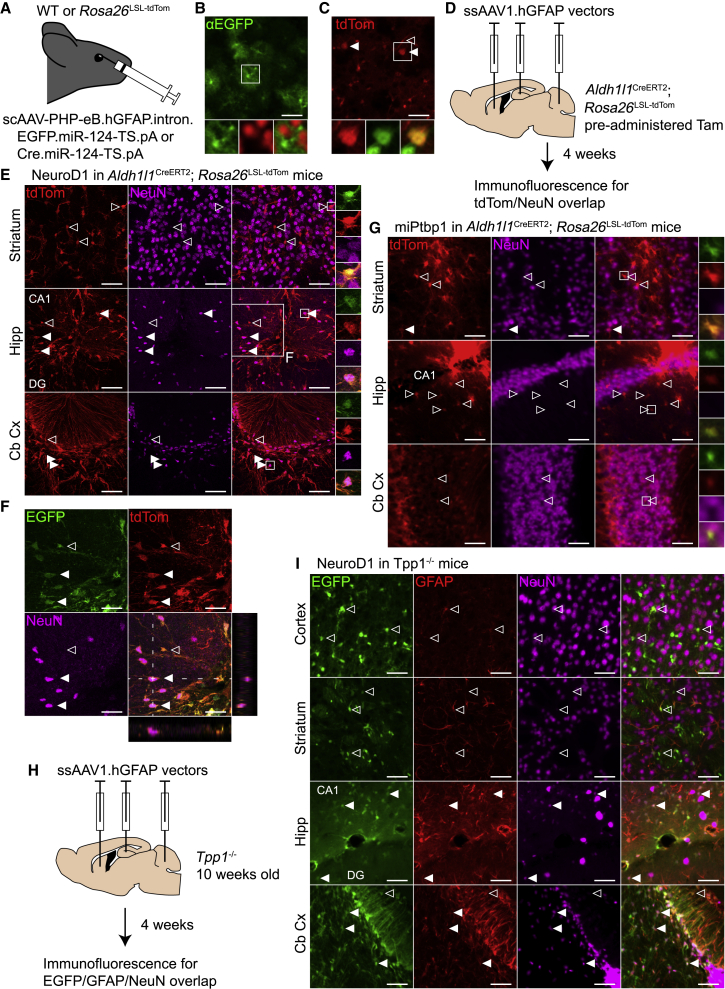
Figure 1.
Overexpression of NeuroD1 induces NeuN expression in astrocytes in the mouse hippocampus and cerebellar cortex
(A) Optimized AAVs expressing EGFP (3.16 × 1011 vg) or Cre (1.00 × 1011 vg) from a hGFAP promoter were injected retro-orbitally into wild-type (WT) or Rosa26LSL-tdTom mice, respectively. (B) Widespread but faint EGFP immunofluorescence (green, main and bottom left) did not co-localize with NeuN immunofluorescence (red, bottom center) in the primary visual cortex after AAV administration in WT mice (n = 2). Scale bar, 50 μm. (C) tdTom fluorescence (red, main and bottom left) and NeuN immunofluorescence (green, bottom center) in primary visual cortex following AAV administration in Rosa26LSL-tdTom mice (n = 2). Solid arrowheads, tdTom- and NeuN-positive neurons; open arrowhead, tdTom-positive endothelial cell. Scale bar, 50 μm. (D) ssAAV1 vectors with conversion factors or controls were infused into the striatum, hippocampus, and cerebellum in Aldh1l1CreERT2; Rosa26LSL-tdTom mice at a dose of 1 × 109 vg per site. Mice were perfused after 4 weeks for histology. (E) tdTom and NeuN immunofluorescence following NeuroD1 overexpression in the striatum, the molecular layers of the DG and CA1 in the hippocampus, and the cerebellar cortex (n = 2). All cells marked with arrowheads are tdTom+ and EGFP+. Closed arrowheads represent transduced, fate-mapped astrocytes that express NeuN. Scale bars, 50 μm. (F) Magnification of the hippocampus in (E), showing co-localization of EGFP, dTom, and NeuN, with orthogonal views through a NeuN+ cell. Scale bars, 100 μm. (G) Fluorescence images following miPtbp1 expression with the same channels and brain areas as in (E). Only a low, background level of tdTom/NeuN co-localization was observed (n = 1). Scale bars, 50 μm. (H) 10-week-old Tpp1−/− mice were infused with the same viruses as the Aldh1l1CreERT2; Rosa26LSL-tdTom mice, with an added infusion in the cerebral cortex above the hippocampus. Mice were perfused after 4 weeks for histology. (I) Immunofluorescence images from Tpp1−/− mice (n = 3) treated with NeuroD1. Shown are EGFP (green, left), Gfap immunofluorescence, and NeuN immunofluorescence in the cerebral cortex, striatum, hippocampus, and cerebellar cortex. Scale bars, 50 μm. In all panels, transduced cells that do and do not colocalize with NeuN are labeled with solid and open arrowheads, respectively. Tam, tamoxifen; Hipp, hippocampus; Cb Cx, cerebellar cortex; DG, dentate gyrus. See also Figure S1.
We instead decided to permanently label astrocytes with tdTomato (tdTom) in Aldh1l1CreERT2; Rosa26LSL-tdTom mice27 and deliver AAV1 vectors expressing conversion factors directly into the brain parenchyma. Tamoxifen administration in these mice led to tdTom expression in the vast majority of astrocytes, with only rare expression in neurons (data not shown). To test NeuroD1-mediated conversion, we designed expression cassettes with hGFAP promoters driving NeuroD1.P2A.EGFP or EGFP alone. Both cassettes included the 3′UTR miR-124-TS. We also designed an artificial microRNA28 targeting Ptbp1 (miPtbp1) and confirmed its activity in vitro (Figure S1C). We then cloned miPtbp1 or a control microRNA29 (miControl) into the 3′UTR of a hGFAP-EGFP transgene. Four weeks after tamoxifen administration, we infused AAV1 vectors with each of these four transgenes unilaterally into the striatum, hippocampus, and cerebellum at 1 × 109 vector genomes (vg) per injection site (Figure 1D). We perfused the mice after 4 weeks and collected their brains for histology.
We next performed immunofluorescence for the neuronal marker NeuN to identify fate-mapped tdTom-positive cells that had converted to neuron-like cells. In mice treated with NeuroD1, we observed extensive tdTom/NeuN overlap in the hippocampus and cerebellar cortex but not in the striatum (Figures 1E and 1F). In the hippocampus, viral expression was largely confined to the molecular layers of the dentate gyrus (DG) and cornu ammonis 1 (CA1), and apparent conversion was entirely confined to these regions (Figure S1D). Concerningly, conversion in the cerebellar cortex was localized to regions with a damaged granule cell layer (Figure S1D). In the hippocampus and cerebellar cortex, conversion was always within a few hundred micrometers of the needle track. However, despite the proximity to the needle track, NeuN staining in the far-red channel in converted cells was not due to autofluorescence, which was punctate and faint (data not shown). In addition, the hGFAP promoter remained active in induced NeuN-positive cells, unlike in mature neurons, because we noted continued EGFP expression (Figures 1E and 1F). In contrast to NeuroD1, we observed no evidence of astrocyte-to-neuron conversion in mice treated with the EGFP control virus (Figure S1E), miPtbp1 virus (Figure 1G; Figure S1D), or miControl virus (data not shown).
Although the NeuroD1 results were encouraging, apparent astrocyte-to-neuron conversion occurred in a narrow region close to the injection site. It has been proposed that reactive astrocytes may be more readily converted into neurons than nonreactive astrocytes.30 Therefore, we decided to test astrocyte-to-neuron conversion in Tpp1−/− mice,31 a model of late infantile neuronal ceroid lipofuscinosis. These mice develop progressive astrogliosis beginning by 9 weeks of age and some neurodegeneration by the end stage at 21 weeks.32 As expected, we observed elevated levels of the reactive astrocyte marker Gfap by 11 weeks of age that increased by 14 weeks (Figure S1F). We next infused the cerebral cortex, striatum, hippocampus, and cerebellum of 10-week-old Tpp1−/− mice with 1 × 109 vg of the same viruses as above (Figure 1H). To identify converted neurons, we performed NeuN immunofluorescence and identified transduced astrocytes by EGFP expression. In NeuroD1-treated Tpp1−/− mice, we detected no EGFP/NeuN overlap in the cerebral cortex (Figure 1I). In brain areas tested previously in Aldh1l1CreERT2; Rosa26LSL-tdTom mice, results were comparable in Tpp1−/− mice; we observed no NeuN/EGFP overlap in the striatum but significant overlap in the molecular layers of the hippocampus and in the cerebellar cortex (Figure 1I). As above, cerebellar regions with apparent conversion were marked by a damaged granule cell layer, and we noted particularly strong Gfap expression in these areas (Figures 1I). We again saw no evidence of astrocyte-to-neuron conversion in Tpp1−/− mice injected with EGFP control (Figure S1G) or miPtbp1 viruses (data not shown). Therefore, the astrogliosis in Tpp1−/− mice did not enhance astrocyte-to-neuron conversion compared with wild-type mice.
In summary, we observed evidence of NeuroD1-mediated astrocyte-to-neuron conversion in the mouse hippocampus and cerebellar cortex but not in the cerebral cortex or striatum. Where conversion was observed, it was limited spatially to within a few hundred micrometers of the injection site. This may be due to a localized dose effect or damage and inflammation along the needle track. In addition, converted cells were likely not fully mature neurons by 4 weeks after injection because the hGFAP promoter remained highly active in these cells. We did not follow converted cells for a longer time period because of the limited scope of conversion. In contrast to NeuroD1 overexpression, delivery of an artificial microRNA targeting Ptbp1 did not lead to astrocyte-to-neuron conversion in any brain area, although we did not quantify Ptbp1 knockdown in vivo. One caveat regarding our results is the small scale of this pilot study. However, the results were consistent across multiple animals in two mouse lines, including Aldh1l1CreERT2 mice, which enable robust fate mapping of astrocytes. We also note that Aldh1l1 and Gfap, the genes on which our Cre and AAV reporter systems are based, are expressed in neural stem cells in the subgranular zone (SGZ), which normally generate granule cells in the DG granule cell layer. Thus, it is possible that neural stem cells, rather than astrocytes, gave rise to the apparent converted neurons in the hippocampus. This possibility is made less likely by the distance between the SGZ and the converted neurons, the farthest of which were across the hippocampal fissure in CA1, and the relatively short time frame of the experiments.
Published results on astrocyte-to-neuron conversion using NeuroD1 overexpression vary widely.12, 13, 14,16,17,30 In a recent publication, Wang et al.20 propose that the Neurod1 DNA sequence upregulates GFAP promoter activity in neurons and that this can lead to spurious reports of astrocyte-to-neuron conversion. When they instead fate mapped astrocytes in Aldh1l1CreERT2; Rosa26LSL-tdTom mice, as we did above, they reported no NeuroD1-mediated conversion in the cerebral cortex and striatum. This is consistent with our results showing a lack of conversion in these brain areas but some conversion in the hippocampus and cerebellar cortex. However, Xian et al.22 do report conversion in the cerebral cortex and striatum in these mice. We cannot rule out the possibility of efficient conversion in the cerebral cortex and striatum with alternative viral vectors, a higher dose, or a longer time period after injection. Similar to NeuroD1-mediated conversion, widely varying efficiencies of astrocyte-to-neuron conversion by Ptbp1 knockdown have been reported.18, 19, 20,33 The degree of in vivo knockdown of Ptbp1 is difficult to compare between these studies and may be critical to achieve efficient conversion. Overall, we are concerned by how difficult it is to reproduce efficient astrocyte-to-neuron conversion. Additional work is clearly needed to verify the results of in vivo conversion experiments and establish the precise conditions that permit conversion.
Although astrocyte-to-neuron conversion may be possible, important questions remain unanswered regarding its therapeutic application. For us, NeuN expression was only induced in astrocytes very close to the injection site. Conversion on this scale is unlikely to have a therapeutic effect in the mouse brain and even less likely in the much larger human brain. Therefore, the route of administration and dose will have to be carefully considered to optimize conversion while minimizing toxicity. Systemic delivery of AAVs may be an option for broader conversion, but this would likely require a high dose of AAVs and lead to unintended expression in the periphery, as noted above for AAV-PHP.eB in mice. If more widespread conversion is achievable, long-term studies using rigorous methods will be needed to determine whether induced NeuN-positive cells develop into mature neurons that can integrate into neural circuits and rescue disease phenotypes. Although these are major challenges, it may be premature to abandon astrocyte-to-neuron conversion without further investigation.
Acknowledgments
We thank Kasey Brida, Laurence Busque, and Mary Doan for AAV vector preparation.
The project was (in part) supported by award T32NS007413 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent the official views of the NINDS of the National Institutes of Health.
Author contributions
D.L. designed the research, performed experiments, and analyzed data. D.L. and A.M.M. designed the artificial microRNA targeting Ptbp1. Y.H.C. and A.M.M. designed the ITR block sequence. B.L.D. designed and supervised the research. D.L. and B.L.D. wrote the manuscript with input from all authors.
Declaration of interests
B.L.D. is on the SAB and/or receives sponsored research support for the laboratory from Homology Medicines, Saliogen Therapeutics, Patch Bio, Moment Bio, Panorama Medicines, Resilience, Spirovant Sciences, Novartis Institute for Biomedical Research, Roche, and Sanofi.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.01.028.
Supplemental information
References
- 1.Heins N., Malatesta P., Cecconi F., Nakafuku M., Tucker K.L., Hack M.A., Chapouton P., Barde Y.A., Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 2.Buffo A., Vosko M.R., Ertürk D., Hamann G.F., Jucker M., Rowitch D., Götz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl. Acad. Sci. U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berninger B., Costa M.R., Koch U., Schroeder T., Sutor B., Grothe B., Götz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich C., Blum R., Gascón S., Masserdotti G., Tripathi P., Sánchez R., Tiedt S., Schroeder T., Götz M., Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grande A., Sumiyoshi K., López-Juárez A., Howard J., Sakthivel B., Aronow B., Campbell K., Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu W., Zang T., Zou Y., Fang S., Smith D.K., Bachoo R., Zhang C.L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torper O., Pfisterer U., Wolf D.A., Pereira M., Lau S., Jakobsson J., Björklund A., Grealish S., Parmar M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Miao Q., Yuan J., Han S., Zhang P., Li S., Rao Z., Zhao W., Ye Q., Geng J., Zhang X., Cheng L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aravantinou-Fatorou K., Ortega F., Chroni-Tzartou D., Antoniou N., Poulopoulou C., Politis P.K., Berninger B., Matsas R., Thomaidou D. CEND1 and NEUROGENIN2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursors and differentiated neurons. Stem Cell Rep. 2015;5:405–418. doi: 10.1016/j.stemcr.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berninger B., Jessberger S. Engineering of adult neurogenesis and Gliogenesis. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei W., Li W., Ge L., Chen G. Non-engineered and engineered adult neurogenesis in mammalian brains. Front. Neurosci. 2019;13:131. doi: 10.3389/fnins.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z., Zhang L., Wu Z., Chen Y., Wang F., Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y.C., Ma N.X., Pei Z.F., Wu Z., Do-Monte F.H., Keefe S., Yellin E., Chen M.S., Yin J.C., Lee G., et al. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol. Ther. 2020;28:217–234. doi: 10.1016/j.ymthe.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., Parry M., Hou X.Y., Liu M.H., Wang H., Cain R., Pei Z.F., Chen Y.C., Guo Z.Y., et al. Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease. Nat. Commun. 2020;11:1105. doi: 10.1038/s41467-020-14855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge L.J., Yang F.H., Li W., Wang T., Lin Y., Feng J., Chen N.H., Jiang M., Wang J.H., Hu X.T., et al. In vivo neuroregeneration to treat ischemic Stroke through NeuroD1 AAV-based gene therapy in adult non-human primates. Front. Cell Dev. Biol. 2020;8:590008. doi: 10.3389/fcell.2020.590008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puls B., Ding Y., Zhang F., Pan M., Lei Z., Pei Z., Jiang M., Bai Y., Forsyth C., Metzger M., et al. Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front. Cell Dev. Biol. 2020;8:591883. doi: 10.3389/fcell.2020.591883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y., Wu Q., Gao M., Ryu E., Pei Z., Kissinger S.T., Chen Y., Rao A.K., Xiang Z., Wang T., et al. Restoration of visual function and cortical connectivity after ischemic injury through NeuroD1-mediated gene therapy. Front. Cell Dev. Biol. 2021;9:720078. doi: 10.3389/fcell.2021.720078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian H., Kang X., Hu J., Zhang D., Liang Z., Meng F., Zhang X., Xue Y., Maimon R., Dowdy S.F., et al. Reversing a model of Parkinson's disease with in situ converted nigral neurons. Nature. 2020;582:550–556. doi: 10.1038/s41586-020-2388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H., Su J., Hu X., Zhou C., Li H., Chen Z., Xiao Q., Wang B., Wu W., Sun Y., et al. Glia-to-Neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell. 2020;181:590–603.e16. doi: 10.1016/j.cell.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Wang L.L., Serrano C., Zhong X., Ma S., Zou Y., Zhang C.L. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell. 2021;184:5465–5481.e16. doi: 10.1016/j.cell.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G. In vivo confusion over in vivo conversion. Mol. Ther. 2021;29:3097–3098. doi: 10.1016/j.ymthe.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Z., Xu L., Liu M., Wang Q., Li W., Lei W., Chen G. Lineage tracing of direct astrocyte-to-neuron conversion in the mouse cortex. Neural Regen. Res. 2021;16:750–756. doi: 10.4103/1673-5374.295925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.L., Sánchez-Guardado L., Lois C., Mazmanian S.K., Deverman B.E., et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y., Messing A., Su M., Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- 25.McCarty D.M., Fu H., Monahan P.E., Toulson C.E., Naik P., Samulski R.J. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 26.Taschenberger G., Tereshchenko J., Kügler S. A MicroRNA124 target sequence restores astrocyte specificity of gfaABC1D-driven transgene expression in AAV-mediated gene transfer. Mol. Ther. Nucleic Acids. 2017;8:13–25. doi: 10.1016/j.omtn.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan R., Lu T.Y., Chai H., Xu J., Huang B.S., Golshani P., Coppola G., Khakh B.S. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride J.L., Boudreau R.L., Harper S.Q., Staber P.D., Monteys A.M., Martins I., Gilmore B.L., Burstein H., Peluso R.W., Polisky B., et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc. Natl. Acad. Sci. U S A. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudreau R.L., Spengler R.M., Davidson B.L. Rational design of therapeutic siRNAs: minimizing off-targeting potential to improve the safety of RNAi therapy for Huntington's disease. Mol. Ther. 2011;19:2169–2177. doi: 10.1038/mt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brulet R., Matsuda T., Zhang L., Miranda C., Giacca M., Kaspar B.K., Nakashima K., Hsieh J. NEUROD1 Instructs neuronal conversion in non-reactive astrocytes. Stem Cell Rep. 2017;8:1506–1515. doi: 10.1016/j.stemcr.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleat D.E., Wiseman J.A., El-Banna M., Kim K.H., Mao Q., Price S., Macauley S.L., Sidman R.L., Shen M.M., Zhao Q., et al. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J. Neurosci. 2004;24:9117–9126. doi: 10.1523/JNEUROSCI.2729-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang M., Cooper J.D., Sleat D.E., Cheng S.H., Dodge J.C., Passini M.A., Lobel P., Davidson B.L. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 33.Maimon R., Chillon-Marinas C., Snethlage C.E., Singhal S.M., McAlonis-Downes M., Ling K., Rigo F., Bennett C.F., Da Cruz S., Hnasko T.S., et al. Therapeutically viable generation of neurons with antisense oligonucleotide suppression of PTB. Nat. Neurosci. 2021;24:1089–1099. doi: 10.1038/s41593-021-00864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.