Abstract
Background
Recurrence is a major risk factor affecting the postoperative survival of patients with hepatocellular carcinoma (HCC), especially those with high preoperative serum γ-glutamyl transpeptidase (GGT) levels. This study had the aim of developing a personalized predictive tool to accurately determine the risk of postoperative recurrence of hepatitis B-virus (HBV)-related HCC in patients with high preoperative serum GGT levels.
Methods
Patients who underwent curative liver resection of HBV-related HCC and had high preoperative GGT levels were consecutively enrolled between 2008 and 2011. Prognostic indicators for recurrence were determined using Cox regression analysis. A nomogram was then developed and assessed by integrating the independent risk factors into the model.
Results
A total of 603 eligible patients were included. The final nomogram for predicting HCC recurrence in patients with high preoperative GGT levels consisted of five independent prognostic factors: α-fetoprotein (AFP), HBV-DNA, satellite nodules, microvascular invasion, and tumor grade. The C-index of the nomogram for predicting recurrence was 0.759, and validation showed high accuracy and discriminatory.
Conclusions
The predictive nomogram developed and validated in this study performs well in predicting postoperative recurrence of HBV-related HCC in patients with high preoperative GGT levels. It can provide personalized assessments to inform the development of surveillance strategies and allows patients with a high risk of recurrence to be selected for further adjuvant treatment.
Keywords: Hepatocellular carcinoma (HCC), γ-glutamyl transpeptidase (GGT), hepatitis B-virus (HBV), recurrence, nomogram
Introduction
As the fifth most common and third most lethal malignancy worldwide, hepatocellular carcinoma (HCC) accounts for approximately 80% to 90% of primary liver cancers (1,2). Hepatitis B virus (HBV) infection is the primary cause of HCC globally. The incidence of HCC is especially high in Asia, where HBV infection is epidemic (3).
Hepatectomy is the first-choice treatment for early-stage HCC (1). Although surgical technologies and perioperative management have seen improvements in recent years, the overall survival (OS) of patients with HCC following resection has shown only a slight increase due to the high rate of recurrence (4). Postoperative recurrence, including intrahepatic metastases (early recurrence <2 years) and de novo tumors (late recurrence ≥2 years), complicates 70% of surgical cases (1,4). HCC recurrence after resection should therefore be regarded as a major risk factor that affects patient survival (5).
To improve the long-term outcomes of patients with HCC who undergo curative resection, a variety of biochemical indexes, such as α-fetoprotein (AFP), des-γ-carboxyprothrombin (DCP), and γ-glutamyl transpeptidase (GGT), have been assessed as potential prognostic predictors (6-10). In previous studies, many clinical indicators that reflect the body’s inflammatory reactions and nutritional status have also been found. These indicators, which include the neutrophil-to-lymphocyte ratio (11,12), platelet-to-lymphocyte ratio (11,13), lymphocyte-to-monocyte ratio (11,14), and C-reactive protein-albumin-lymphocyte index (15), also serve as valuable biomarkers for predicting postoperative recurrence of HCC.
Among these indicators, GGT has attracted considerable attention and interest due to its obvious advantage in predicting the postoperative prognosis of HCC (8,10,16). As a critical enzyme, GGT is present in the cell membrane and is involved in glutathione metabolism via catalyzing the hydrolysis of glutathione, delivering γ-glutamyl residues to multiple acceptor molecules, and producing cysteine products to maintain intracellular homeostasis of oxidative stress (17,18). Previous studies have discovered that GGT is abnormally expressed in several human tumors (19), and that it plays an important role in tumor formation, and cell proliferation and apoptosis (20).
In the last few years, the attention of researchers has increasingly been drawn to the close relationship of GGT levels with the recurrence rate and poor prognosis of HCC (16,21). The latest studies have revealed that increased GGT levels were associated with a decreased survival rate following liver resection (21-23). Furthermore, patients with HBV-related HCC who have high preoperative serum GGT levels tend to relapse early, resulting in unsatisfactory long-term survival.
In order to overcome the major challenge of the high postoperative recurrence rate of HBV-related HCC in patients with high preoperative GGT levels, those who are at high risk of recurrence after hepatectomy should be identified as soon as possible, as this could help to determine subsequent management strategies. Several well-known prognostic staging systems exist for HCC, including the TNM staging system (24), the Cancer of the Liver Italian Program (CLIP) score (25), and the Barcelona Clinical Liver Cancer (BCLC) staging system (26), and can aid in dividing HCC patients into different groups with diverse outcomes. However, as these tools were not developed specifically for prognostic prediction (27), their ability to predict recurrence-free survival (RFS) is controversial, and they are not suitable for predicting HCC recurrence in specific populations with high risk of recurrence. Notably, patients with high heterogeneity may be graded at the same stage but receive different prognoses. Therefore, a novel model that can predetermine the risk of HBV-related HCC recurrence after radical resection is warranted, especially for patients with high preoperative serum GGT levels.
To address this need, the present study set out to develop and validate an accurate model for predicting the risk of postoperative recurrence in HBV-related HCC patients who undergo curative resection and have high preoperative serum GGT levels.
We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-450/rc).
Methods
Patients and clinicopathologic data
A total of 1,856 patients with HCC who underwent curative hepatectomy at the Eastern Hepatobiliary Surgery Hospital (EHBH), the Second Military Medical University of China, between 2008 and 2011 were included in this study.
The inclusion criteria for patients were as follows: (I) the pathological diagnosis was HCC only (i.e., not combined HCC or cholangiocarcinoma); (II) hepatitis B surface antigen (HBsAg) positivity; (III) complete clinicopathologic and follow-up data; and (IV) underwent curative hepatectomy for the first time and had not received any other treatments before the operation. The exclusion criteria were as follows: (I) patients with recurrent HCC; (II) patients with extrahepatic metastasis or macroscopic major portal or hepatic vein tumor thrombus; and (III) recurrence occurred within 1 month after the hepatectomy.
A total of 1,234 patients with HCC with HBsAg positivity were finally enrolled into this retrospective study. According to their preoperative serum GGT levels, patients were grouped into the GGT-high (n=603) and GGT-low (n=631) groups. The 603 patients in the GGT-high group were randomly divided into a training cohort (n=393) and a validation cohort (n=210).
Conventional clinicopathologic variables, including age, sex, total bilirubin (TBil), albumin, alanine aminotransferase (ALT), glutamate aspartate aminotransferase (AST), Child-Pugh score, AFP, HBV-DNA load, TNM stage, BCLC stage, CLIP score, anatomical or non-anatomical hepatectomy, surgical margin, tumor number, tumor diameter, tumor distribution, satellite nodules, microvascular invasion, tumor grade, and liver cirrhosis, are recorded in Table S1.
This study was approved by the Clinical Research Ethics Committee of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2014-03-019). All procedures involving human participants in this study were conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was obtained from each study participant.
Follow-up procedure and study endpoints
The comprehensive medical histories of all patients were recorded on admission and during follow-up, and the baseline characteristics of all patients were documented before the hepatectomy. After surgery, all patients were periodically followed up at 3-month intervals for the first 2 years, at 6-month intervals during the subsequent 3 years, and annually thereafter. For patients whose examination findings suggested recurrence, computed tomography (CT) and/or magnetic resonance imaging (MRI) were performed to confirm whether intrahepatic recurrence and/or distant metastasis was present. Recurrence was diagnosed depending on representative imaging findings in the CT and/or MRI scans. RFS was defined as the time interval between the date of surgery and the date of initial diagnosis of recurrence. If recurrence was confirmed, the RFS was calculated as the time interval from the date of surgery to the date recurrence was first suspected. For patients who showed no evidence of recurrence, the last follow-up date was obtained from the medical record.
Definitions
Serum GGT levels were measured using enzyme-coupled assay kits (Shanghai Kehua Bio Engineering Co., Ltd, Shanghai, China). Blood samples were taken 1 day before the operation. The cutoff value of the GGT level customarily used at the Eastern Hepatobiliary Surgery Hospital is 60 U/L (21,28). Using a cutoff of 2,000 IU/mL, HBV-DNA load was defined as high (≥2,000 IU/mL) or low (<2,000 IU/mL), and 400 ng/mL was considered as the cutoff for high and low AFP levels (29,30).
Statistical analysis
SPSS software (Version 25, IBM Corp., Armonk, NY, USA) was used for statistical analysis. Chi-square tests were used for categorical data. P<0.05 was considered to indicate statistical significance. Survival curves were depicted using the Kaplan-Meier method, and the log-rank test was used for data analysis. The patients in the GGT-high group were divided into training and validation cohorts using a random number table. Multivariate Cox regression analyses were used to assess the relationships of relevant variables with patient survival. Independent risk factors that were shown to be significantly associated with survival in the univariate Cox models (P<0.05) were included in the multivariate Cox regression model through backward selection.
Based on the results of the multivariate analysis, with the endpoints of 2-, 3-, and 5-year RFS, a nomogram model was built using the rms package in R version 3.5.1 (http://www.r-project.org/), which enabled survival probability estimates to be obtained. The concordance index (C-index) was used to evaluate the performance of the nomogram. To compare the nomogram-predicted survival probability with the actual survival probability estimated using the Kaplan–Meier method, calibration curves were plotted at 2, 3, and 5 years (1,000 bootstrap resamples). The nomogram was compared with the conventional HCC staging systems (BCLC staging system, TNM staging system, and CLIP score) using the rcorrp.cens package in Hmisc in R. The validity of the four models (the nomogram and the three conventional HCC staging systems) was assessed using the C-index, with a larger C-index reflecting a more accurate prognostic stratification performance. To ascertain the clinical effectiveness of the nomogram, decision curve analysis (DCA) was applied to evaluate the net benefit of the nomogram, and BCLC-, TNM-, and CLIP-assisted decisions across different ranges of threshold probabilities compared with the net benefit of treat-all or treat-none strategies.
Results
Patients with high preoperative GGT have a poorer postoperative prognosis
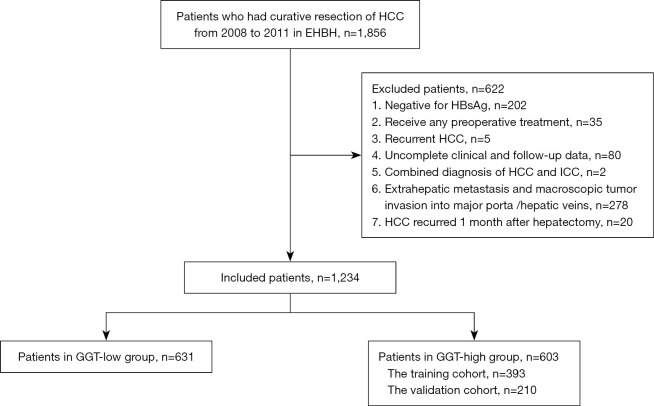
In total, 1,234 patients with HBV-related HCC with HBsAg positivity who underwent curative hepatic resection at EHBH were enrolled. Based on their preoperative serum GGT levels, the patients were divided into the GGT-high (n=603) and GGT-low (n=631) groups (Figure 1). Baseline characteristics of the patients are summarized in Table S1.
Figure 1.
Selection process for the study population. HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; GGT, γ-glutamyl transpeptidase.
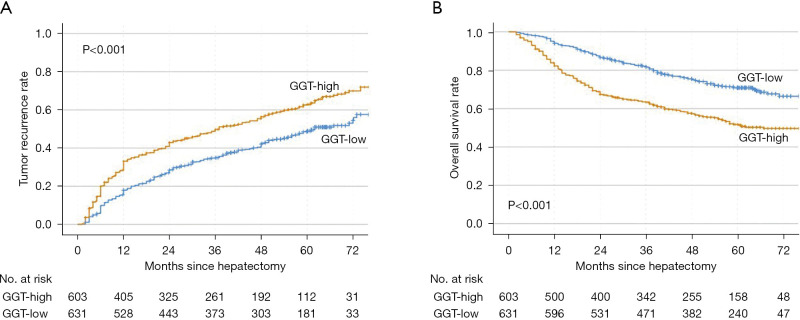
The median follow-up times in the GGT-high and GGT-low groups were 25 (range, 2–83) months and 42 (range, 2–83) months, respectively. In the GGT-high group, 363 patients experienced recurrence, and the 1-, 2-, 3-, 4-, and 5-year recurrence rates were 28%, 41%, 49%, 55%, and 62%, respectively. In the GGT-low group, 289 patients experienced recurrence, and the 1-, 2-, 3-, 4-, and 5-year recurrence rates were 15%, 27%, 35%, 41%, and 49%, respectively. In both the early and the late postoperative periods, the GGT-high group had significantly higher rates of recurrence than the GGT-low group (Figure 2A). Patients with low GGT levels also had a higher OS rate than those with high levels (Figure 2B). Moreover, in the univariate and multivariate Cox regression analyses, having higher GGT levels was determined to be an independent risk factor for postoperative recurrence in patients with HBV-related HCC (Table S2).
Figure 2.
Kaplan-Meier curves of patients with hepatitis B virus-related hepatocellular carcinoma with hepatitis B surface antigen positivity. Compared to the GGT-low group, the GGT-high group showed poor prognosis in tumor recurrence (A) and OS (B). GGT, γ-glutamyl transpeptidase; OS, overall survival.
Clinicopathologic characteristics of patients with high preoperative GGT
To establish a predictive model that could evaluate the risk of postoperative recurrence of HBV-related HCC in patients with high preoperative serum GGT levels, the GGT-high group was randomly divided into a training cohort (n=393) and a validation cohort (n=210) (Figure 1). Detailed baseline characteristics of the two cohorts are presented in Table 1.
Table 1. Baseline characteristics of all patients in training and validation cohorts.
| Characteristics | Training cohort, n=393 (%) | Validation cohort n=210 (%) | P value |
|---|---|---|---|
| Age, >50 years | 213 (54.2) | 110 (52.4) | 0.670 |
| Gender, Male | 345 (87.8) | 187 (89.0) | 0.647 |
| HBV-DNA, >2,000 IU/mL | 242 (61.6) | 126 (60.0) | 0.705 |
| TBil, >17.1 μmol/L | 70 (17.8) | 34 (16.2) | 0.616 |
| Albumin, <3.5 g/dL | 135 (34.4) | 80 (38.1) | 0.360 |
| ALT, >44 U/L | 177 (45.1) | 77 (36.7) | 0.047 |
| AST, >40 U/L | 218 (55.5) | 114 (54.3) | 0.780 |
| Child-Pugh, B | 8 (2.0) | 7 (3.3) | 0.330 |
| AFP, >400 ng/mL | 248 (63.1) | 144 (68.6) | 0.449 |
| Nonanatomical hepatectomy, yes | 160 (40.7) | 85 (40.5) | 0.955 |
| Surgical margin, <1 cm^ | 130 (33.1) | 71 (33.8) | 0.856 |
| Tumor number, >1^ | 54 (13.7) | 22 (10.5) | 0.250 |
| Tumor diameter, >5 cm^ | 197 (50.1) | 108 (51.4) | 0.681 |
| Bilateral tumor distribution^ | 84 (21.4) | 45 (21.4) | 0.988 |
| Satellite nodules^ | 129 (32.8) | 61 (29.0) | 0.342 |
| Microvascular invasion^ | 123 (31.3) | 83 (39.5) | 0.683 |
| Edmondson-Steiner grade, III-IV | 271 (69.0) | 152 (72.4) | 0.162 |
| Cirrhosis^ | 289 (73.5) | 163 (77.6) | 0.270 |
| TNM stage: I-II, IIIA-IIIB | 306 (77.9), 87 (22.1) | 186 (88.6), 24 (11.4) | 0.001 |
| BCLC stage: 0, A-B | 15 (3.8), 378 (96.2) | 11 (5.2), 199 (94.8) | 0.413 |
| CLIP score: 0-1, 2-3 | 291 (74.0), 102 (26.0) | 101 (48.1), 109 (51.9) | 0.324 |
^, Post-operative parameter. TBil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, α-fetoprotein; TNM, tumor-node-metastasis staging system; CLIP, Cancer of the Liver Italian Program score; BCLC, Barcelona Clinic Liver Cancer staging system.
The tumor burden of the two cohorts was comparable. In the training cohort, the majority of participants were male (87.8%), and those over the age of 50 accounted for 54.2% of cases. Only 13.7% of patients had multiple tumor nodules at the time of resection, and about half of patients (50.1%) had a tumor of >5 cm in diameter, in addition, the liver function of most patients was classified as Child-Pugh A (98.0%) in the training cohort.
Identification of independent prognostic factors for RFS in the training cohort
In the training cohort, the median follow-up time was 30 (range, 2–85) months, and the median RFS was 28 (range, 2–81) months. The postoperative 2-, 3-, and 5-year recurrence rates were 46%, 55%, and 70%, respectively. In the univariate analysis, AST (P=0.026), AFP (P<0.001), HBV-DNA load (P=0.046), tumor number (P<0.001), tumor size (P<0.001), satellite nodules (P<0.001), microvascular invasion (P<0.001), and tumor grade (P<0.001) were identified as significant predictors of RFS (Table 2). In the multivariate analysis, only AFP [P<0.001, hazard ratio (HR) =1.720, 95% confidence interval (CI): 1.270–2.330], HBV-DNA load (P=0.017, HR =1.400, 95% CI: 1.060–1.840), satellite nodules (P=0.018, HR =1.390, 95% CI: 1.060–1.840), microvascular invasion (P<0.001, HR =2.140, 95% CI: 1.600–2.850), and tumor grade (P<0.001, HR =4.210, 95% CI: 2.830–6.270) retained significance as prognostic indicators of RFS (Table 2).
Table 2. Independent prognostic factors predicting RFS in training cohort.
| Characteristics | Univariate | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age, >50 years | 1.000 | 0.780–1.290 | 0.995 | NA | NA | NA | |
| Gender, Male | 0.820 | 0.540–1.250 | 0.353 | NA | NA | NA | |
| HBV-DNA, >2,000 IU/mL | 1.310 | 1.000–1.700 | 0.046 | 1.400 | 1.060–1.840 | 0.017 | |
| TBil, >17.1 μmol/L | 0.940 | 0.670–1.310 | 0.698 | NA | NA | NA | |
| Albumin, <3.5 g/dL | 1.200 | 0.920–1.550 | 0.176 | NA | NA | NA | |
| ALT, >44 U/L | 1.040 | 0.810–1.340 | 0.759 | NA | NA | NA | |
| AST, >40 U/L | 1.340 | 1.040–1.720 | 0.026 | 0.990 | 0.750–1.290 | 0.916 | |
| Child-Pugh, B | 1.040 | 0.390–2.780 | 0.945 | NA | NA | NA | |
| AFP, >400 ng/mL | 2.320 | 1.750–3.080 | <0.001 | 1.720 | 1.270–2.330 | <0.001 | |
| Nonanatomical hepatectomy | 1.002 | 0.885–1.125 | 0.251 | NA | NA | NA | |
| Surgical margin, <1 cm^ | 1.011 | 0.899–1.094 | 0.136 | NA | NA | NA | |
| Tumor number, >1^ | 2.210 | 1.610–3.050 | <0.001 | 1.160 | 0.830–1.630 | 0.386 | |
| Tumor diameter, >5 cm^ | 2.220 | 1.710–2.870 | <0.001 | 1.100 | 0.830–1.450 | 0.517 | |
| Bilateral tumor distribution^ | 1.105 | 0.856–1.356 | 0.469 | NA | NA | NA | |
| Satellite nodules^ | 1.960 | 1.510–2.540 | <0.001 | 1.390 | 1.060–1.840 | 0.018 | |
| Microvascular invasion | 3.710 | 2.860–4.820 | <0.001 | 2.140 | 1.600–2.850 | <0.001 | |
| Edmondson-Steiner grade, III-IV | 5.530 | 3.820–8.030 | <0.001 | 4.210 | 2.830–6.270 | <0.001 | |
| Cirrhosis^ | 0.860 | 0.650–1.140 | 0.293 | NA | NA | NA | |
^, Post-operative parameter. RFS, recurrence-free survival; TBil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, α-fetoprotein.
Construction of the prognostic nomogram for RFS
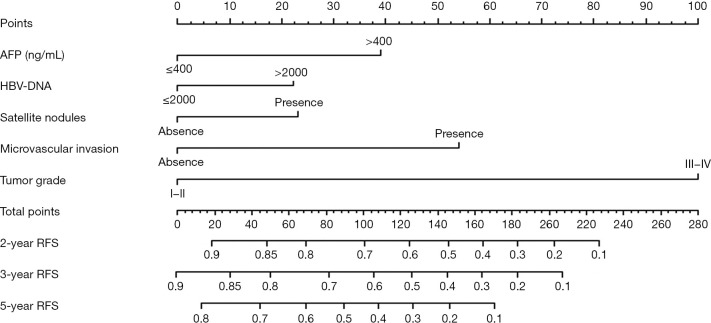
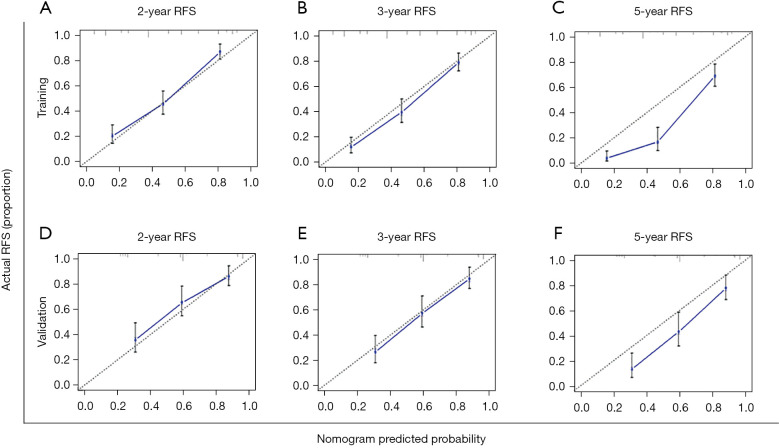
The five significant predictors identified through the multivariate analysis in the training cohort were used to establish a nomogram for predicting RFS (Figure 3). The discrimination ability of this nomogram was measured using the C-index and calibration curves. The C-index of the RFS nomogram was 0.759 (95% CI: 0.731–0.787) (Table 3). The calibration plots demonstrated good consistency between the nomogram-predicted probabilities and those actually observed for 2- and 3-year RFS (Figure 4A-4C).
Figure 3.
Prognostic nomogram for predicting recurrence-free survival in patients with hepatitis B virus-related hepatocellular carcinoma with high preoperative serum GGT levels. The nomogram contains 10 rows. The variables, including AFP, HBV-DNA, satellite nodules, microvascular invasion, and tumor pathological grade, are presented on rows 2 to 6, and each variable is given a score identified by the scale on row 1. The total points of an individual patient are calculated by adding the score of all five variables and is located on the total point axis (row 7). The estimated 2-, 3-, or 5-year probabilities of RFS of the individual patient will be acquired from the nomogram (row 8 to 10) according to their total points. RFS, recurrence-free survival; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; GGT, γ-glutamyl transpeptidase.
Table 3. Ranking of staging systems via the C-index.
| Model | Training cohort | Validation cohort | |||
|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | ||
| Nomogram | 0.759 | 0.731–0.787 | 0.736 | 0.691–0.781 | |
| BCLC stage | 0.584 | 0.550–0.617 | 0.570 | 0.524–0.617 | |
| TNM stage | 0.594 | 0.558–0.631 | 0.583 | 0.533–0.633 | |
| CLIP score | 0.575 | 0.538–0.610 | 0.611 | 0.562–0.659 | |
TNM, tumor-node-metastasis staging system; CLIP, Cancer of the Liver Italian Program score; BCLC, Barcelona Clinic Liver Cancer staging system.
Figure 4.
Calibration curve for the nomogram. The 2- (A), 3- (B), and 5- (C) year RFS rates in the training cohort; the 2- (D), 3- (E), and 5- (F) year RFS rates in the validation cohort. The x-axis represents nomogram-predicted survival; the y-axis represents actual survival. RFS, recurrence-free survival.
Validation of the RFS nomogram
To validate the predictive ability of the constructed RFS nomogram, the probabilities of outcomes were predicted for the validation cohort. In the validation cohort, the median follow-up time was 57 (range, 2–85) months, and the median RFS was 55 (range, 2–83) months. The 2-, 3-, and 5-year RFS rates were 36%, 42%, and 54%, respectively. The C-index in the validation cohort was 0.736 (95% CI: 0.691–0.781) (Table 3), and the calibration plots fitted well between the actual and predicted probabilities for 2-, 3-, and 5-year RFS (Figure 4D-4F).
Comparison of accuracy for predicting RFS between the nomogram and conventional staging systems
As previous research has revealed the important roles of liver function, performance status, and tumor burden and stage in HCC prognosis, we next compared the predictive performance of our model with those of three conventionally applied HCC staging systems—TNM stage, BCLC stage, and CLIP score—to ascertain the accuracy and validity of our model. The discriminatory ability of each prognostic system was compared using Harrell’s C-index (Table 3). Among the four models, our nomogram had the highest discriminatory capacity for predicting RFS in the training cohort, with the highest C-index (0.759, 95% CI: 0.731–0.787). Consistent with this, our nomogram also had the highest C-index (0.736, 95% CI: 0.691–0.781) for predicting RFS in the validation cohort.
Evaluating the clinical utility of our model through DCA
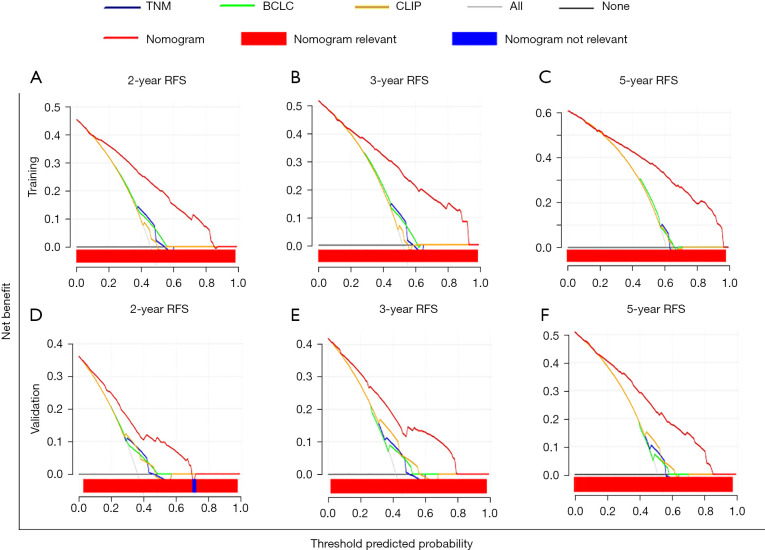
Because our nomogram exhibited superior predictive power to the conventional staging systems in terms of C-index, we next sought to ascertain the clinical effectiveness of our model by comparing it with the three competing models (TNM stage, BCLC stage, and CLIP score) through DCA. In both the training cohort and the validation cohort, the proposed model showed greater net benefit and a wider threshold probability range than the three conventional models, and it gave a better performance in predicting 2-, 3-, and 5-year RFS in patients with HBV-related HCC with high GGT (Figure 5A-5F).
Figure 5.
Decision curve analysis of the nomogram. DCA graphically illustrating the clinical utility of the nomogram model. The x-axis represents threshold probability and the y-axis represents net benefit. The gray lines indicate that all patients are assumed to have postoperative recurrence, and the black lines indicate that no patient is assumed to have postoperative recurrence. With a broad range for predicting postoperative recurrence, the nomogram has greater net benefit than the conventional staging systems and treat-all or treat-none strategies for patients with HCC. DCA, decision curve analysis; RFS, recurrence-free survival; TNM, tumor-node-metastasis staging system; CLIP, Cancer of the Liver Italian Program score; BCLC, Barcelona Clinic Liver Cancer staging system.
Discussion
At present, surgical management is still the first-line treatment for patients with HCC without local or distant metastasis. However, the high postsurgical recurrence rate is the major risk factor threatening the long-term prognosis of HCC. Previous research has revealed that tumor characteristics (including tumor size, multiplicity, and differentiation), liver function, and laboratory indicators (such as AFP and DCP) are all prominent indicators for postoperative recurrence of HCC (6,31,32). Having high preoperative GGT levels was also found to be an independent risk factor for postoperative recurrence after curative resection for HCC (9). Although the conventional staging systems are widely used at present, the clinical and pathologic variables affecting prognosis vary and the existing systems cannot sufficiently predict the prognosis of patients at high risk of recurrence. For these high-risk populations, constructing an individualized model that can predict the risk of recurrence is urgently warranted.
Nomograms are visualizations of quantized risk variables and are useful in aiding clinician and patient understanding of the short- and long-term prognoses of specific high-risk HCC populations. A previous study created a novel prognostic nomogram to predict RFS in patients with HBV-related HCC undergoing hepatectomy (C-index =0.712) and reported that the risk factors for predicting HCC recurrence were alkaline phosphatase, albumin, protein induced by vitamin K absence or antagonism-II, multiple tumors, tumor hemorrhage, portal vein tumor thrombosis, intrahepatic metastasis, and a tumor-free resection margin (33). Liao et al. (34) reported that the C-index value of their RFS nomogram was 0.736, with the independent prognostic factors being AFP, tumor number, tumor size, HBV load-related peritumoral inflammatory score, and HBV load-related albumin–bilirubin score. In our study, patients with homogeneous HBV-related HCC and high preoperative serum GGT levels, all of whom underwent curative resection, were enrolled for analysis. First, we identified the independent risk factors for postoperative recurrence in the training cohort, and then built a nomogram to predict the probability of 2-, 3-, and 5-year recurrence after curative resection in patients with HBV-related HCC with high GGT levels. Because our nomogram was specifically established for the high-GGT population of patients with HBV-related HCC, the independent prognostic factors in our study were somewhat different from those identified in previous studies on HBV-related HCC (33,34). At the same time, the previous studies classified these factors into two categories—preoperative serum indicators and postoperative pathological features—which was similar to our current work.
Our model was validated in the validation cohort and showed high discrimination power, with C-indexes of 0.759 and 0.736 in the training and validation cohorts, respectively. In subsequent research, the predictive accuracy and clinical utility of our RFS nomogram was assessed using a calibration curve and DCA. The results showed that our model exhibited higher predictive accuracy and had a greater net benefit than three conventionally used staging systems: the TNM staging system, BCLC staging system, and CLIP score.
Although the above staging systems are all commonly used in HCC, they are controversial to some extent (27,35-37). The TNM system, for instance, has limited applicability in clinical practice, because it does not take into account tumor differentiation (38,39). Furthermore, previous studies that used the BCLC staging system and CLIP score uncovered limitations in differentiating and predicting the prognosis of patients with advanced-stage HCC (40-42). Nomogram models have been demonstrated to offer higher accuracy and greater personalization than the conventional staging systems for predicting the prognosis of some cancers (43,44). In this study, a nomogram which integrates independent prognostic factors including pathological features and serum biomarkers demonstrated better accuracy and discriminatory capacity than the other three staging systems for predicting HCC prognosis in both the training and validation cohorts, suggesting that it is a feasible individualized prognostic model.
Among the five independent factors included in our nomogram, serum AFP and HBV-DNA load are preoperative clinical characteristics of patients, while satellite nodules, microvascular invasion, and tumor grade constitute postoperative pathologic features. AFP has long been regarded as the most potent prognostic predictor for patients with HCC, and thus, it is the most widely used marker in HCC prognostic stratification (32,45). HBV-DNA load is a critical risk factor for HCC recurrence after hepatectomy. Furthermore, the persistent inflammatory microenvironment of the liver caused by chronic viral infection can result in the occurrence of de novo tumors, which elevates the possibility of postoperative recurrence (46). Hepatitis B spliced protein can enhance the invasion and motility of HCC cells (47), and HBV infection status is also closely related to the vascular dissemination and microvascular invasion of HCC cells, which increases the risk of recurrence and patient death after HCC surgery (48). Previous studies have shown that antiviral treatment reduces the postoperative recurrence of HCC in patients who have high preoperative HBV-DNA levels (30,49). And enlarging the surgical margin during surgery increases the likelihood of removing microvascular invasion (50). The occurrence of satellite lesions is closely correlated with long-term survival following a diagnosis of HCC (31). Tumor grade was also found to be a powerful indicator of HCC recurrence. Therefore, serum AFP, HBV-DNA load, satellite nodules, microvascular invasion, and tumor grade should be treated with more attention in patients with HBV-related HCC who have high preoperative serum GGT levels. Our nomogram model, which integrates these factors, can be applied to guide routine follow-up. Patients whose nomogram score shows a high risk of recurrence should be monitored closely and undergo more precise examinations, such as MRI or CT scans, and personalized therapy. Also, follow-up intervals should be shortened, regardless of whether there are concerns about the most recent postoperative examination results.
HCC recurrence complicates 70% of cases of hepatic resection at 5 years and can occur in the form of intrahepatic metastasis (early recurrence, <24 months) or de novo tumors (late recurrence, >24 months) (4,51). The treatment strategies for recurrent HCC mainly include local therapy (repeated hepatectomy, ablative, and transcatheter arterial chemoembolization) and systemic therapy (targeted drugs, immune checkpoint inhibitors, and systemic chemotherapy) (51). The clinician can choose the appropriate treatment according to the indication. However, the clinical curative effect of these strategies for early and late recurrence of HCC is not ideal, and monitoring and early treatment of HCC recurrence are still key to prolonging survival. Our nomogram can predict the individual probability of 2-, 3-, and 5-year HCC recurrence, and clinicians can make follow-up protocols and treatment strategies based on the early (2-year) and late (3-year and 5-year) RFS predicted by the nomogram.
This study has several limitations which need to be considered. First, cross-validation and further exploration of this nomogram should be performed in more institutions. Second, due to the retrospective nature of the study, our results need to be validated in future research using prospective cohorts with more etiologic factors being assessed. Finally, it remains uncertain whether this nomogram can be applied to patients with non-HBV-related HCC or those with HBV-related HCC who have received other treatment preoperatively.
Conclusions
In conclusion, we designed and validated a predictive model for postoperative recurrence of HBV-related HCC which specifically targets patients with high GGT levels. The model can accurately discriminate populations with higher risk of recurrence following surgery, and its application would thus allow clinicians to offer more stringent surveillance strategies and further adjuvant treatments.
Acknowledgments
Funding: The study was funded by the National Natural Science Foundation of China (No. 81972704).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Clinical Research Ethics Committee of the Eastern Hepatobiliary Surgery Hospital. All procedures in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was signed by all study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-450/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-450/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-450/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-450/coif). All authors report that the work was funded by the National Natural Science Foundation of China (No. 81972704). The authors have no other conflicts of interest to declare.
(English Language Editors: B. Meiser and J. Reynolds)
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. 10.1016/S0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- 2.Gansler T, Ganz PA, Grant M, et al. Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin 2010;60:345-50. 10.3322/caac.20088 [DOI] [PubMed] [Google Scholar]
- 3.de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015;62:1190-200. 10.1002/hep.27969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marasco G, Colecchia A, Colli A, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol 2019;70:440-8. 10.1016/j.jhep.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Chang KV, Chen JD, Wu WT, et al. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018;7:90-103. 10.1159/000484950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaibori M, Matsui Y, Yanagida H, et al. Positive status of alpha-fetoprotein and des-gamma-carboxy prothrombin: important prognostic factor for recurrent hepatocellular carcinoma. World J Surg 2004;28:702-7. 10.1007/s00268-004-7205-y [DOI] [PubMed] [Google Scholar]
- 7.Sawada T, Kubota K, Kita J, et al. Clinical outcome of hepatectomy for hepatocellular carcinomas≤2 cm. World J Surg 2011;35:377-85. 10.1007/s00268-010-0851-3 [DOI] [PubMed] [Google Scholar]
- 8.Fukuda S, Itamoto T, Amano H, et al. Clinicopathologic features of hepatocellular carcinoma patients with compensated cirrhosis surviving more than 10 years after curative hepatectomy. World J Surg 2007;31:345-52. 10.1007/s00268-006-0513-7 [DOI] [PubMed] [Google Scholar]
- 9.Wu SJ, Lin YX, Ye H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg 2016;36:143-51. 10.1016/j.ijsu.2016.10.033 [DOI] [PubMed] [Google Scholar]
- 10.Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg 2001;88:515-22. 10.1046/j.1365-2168.2001.01732.x [DOI] [PubMed] [Google Scholar]
- 11.Li P, Huang W, Wang F, et al. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage I/II hepatocellular carcinoma. Biosci Rep 2018;38:BSR20180464. 10.1042/BSR20180464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura Y, Sugiura T, Ito T, et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg 2016;103:891-8. 10.1002/bjs.10123 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol 2015;36:2263-9. 10.1007/s13277-014-2833-9 [DOI] [PubMed] [Google Scholar]
- 14.Wu SJ, Lin YX, Ye H, et al. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. J Surg Oncol 2016;114:202-10. 10.1002/jso.24297 [DOI] [PubMed] [Google Scholar]
- 15.Iida H, Tani M, Komeda K, et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB (Oxford) 2021. [Epub ahead of print]. [DOI] [PubMed]
- 16.Whitfield JB. Serum gamma-glutamyltransferase and risk of disease. Clin Chem 2007;53:1-2. 10.1373/clinchem.2006.080911 [DOI] [PubMed] [Google Scholar]
- 17.Xia J, Song P, Sun Z, et al. Advances of diagnostic and mechanistic studies of γ-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther 2016;10:181-7. 10.5582/ddt.2016.01052 [DOI] [PubMed] [Google Scholar]
- 18.Stark AA, Porat N, Volohonsky G, et al. The role of gamma-glutamyl transpeptidase in the biosynthesis of glutathione. Biofactors 2003;17:139-49. 10.1002/biof.5520170114 [DOI] [PubMed] [Google Scholar]
- 19.Hanigan MH, Frierson HF, Jr, Swanson PE, et al. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum Pathol 1999;30:300-5. 10.1016/S0046-8177(99)90009-6 [DOI] [PubMed] [Google Scholar]
- 20.Corti A, Franzini M, Paolicchi A, et al. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res 2010;30:1169-81. [PubMed] [Google Scholar]
- 21.Ma H, Zhang L, Tang B, et al. γ-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol 2014;21:3084-9. 10.1245/s10434-014-3724-4 [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa H, Osaki Y, Iguchi E, et al. Radiofrequency ablation for hepatocellular carcinoma: the relationship between a new grading system for the ablative margin and clinical outcomes. J Gastroenterol 2013;48:951-65. 10.1007/s00535-012-0690-0 [DOI] [PubMed] [Google Scholar]
- 23.Hung TH, Tsai CC, Lin CC, et al. Is transarterial chemoembolization beneficial for patients with diffuse infiltrative hepatocellular carcinoma? Hepatol Int 2013;7:676-82. 10.1007/s12072-012-9392-1 [DOI] [PubMed] [Google Scholar]
- 24.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760-9. 10.1002/cncr.10384 [DOI] [PubMed] [Google Scholar]
- 25.Toyoda H, Hiraoka A, Tada T, et al. Characteristics and Prognosis of Hepatocellular Carcinoma in Japanese Patients Undergoing Dialysis. Ther Apher Dial 2017;21:465-72. 10.1111/1744-9987.12563 [DOI] [PubMed] [Google Scholar]
- 26.Labgaa I, Demartines N, Melloul E. Surgical Resection Versus Transarterial Chemoembolization for Intermediate Stage Hepatocellular Carcinoma (BCLC-B): An Unsolved Question. Hepatology 2019;69:923. 10.1002/hep.30338 [DOI] [PubMed] [Google Scholar]
- 27.Faria SC, Szklaruk J, Kaseb AO, et al. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging 2014;39:1070-87. 10.1007/s00261-014-0130-0 [DOI] [PubMed] [Google Scholar]
- 28.Dong ZR, Zhang PF, Wang CH, et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: a retrospective analysis. Am J Cancer Res 2014;5:450-7. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Guo X, Dong W, et al. Postoperative adjuvant TACE-associated nomogram for predicting the prognosis of resectable Hepatocellular Carcinoma with portal vein Tumor Thrombus after Liver Resection. Int J Biol Sci 2020;16:3210-20. 10.7150/ijbs.46896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Lei Z, Xia Y, et al. Association of Preoperative Antiviral Treatment With Incidences of Microvascular Invasion and Early Tumor Recurrence in Hepatitis B Virus-Related Hepatocellular Carcinoma. JAMA Surg 2018;153:e182721. 10.1001/jamasurg.2018.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol 2001;19:3037-44. 10.1200/JCO.2001.19.12.3037 [DOI] [PubMed] [Google Scholar]
- 32.Rich N, Singal AG. Hepatocellular carcinoma tumour markers: current role and expectations. Best Pract Res Clin Gastroenterol 2014;28:843-53. 10.1016/j.bpg.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Kwon CHD, Joh JW, et al. Nomograms in Hepatectomy Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma. J Gastrointest Surg 2019;23:1559-67. 10.1007/s11605-018-04074-z [DOI] [PubMed] [Google Scholar]
- 34.Liao R, Du CY, Gong JP, et al. HBV-DNA Load-Related Peritumoral Inflammation and ALBI Scores Predict HBV Associated Hepatocellular Carcinoma Prognosis after Curative Resection. J Oncol 2018;2018:9289421. 10.1155/2018/9289421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa H, Osaki Y. Non-B, non-C hepatocellular carcinoma (Review). Int J Oncol 2013;43:1333-42. 10.3892/ijo.2013.2061 [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto M, Tashiro H, Kobayashi T, et al. Clinical characteristics and prognosis of non-B, non-C hepatocellular carcinoma: The impact of patient sex on disease-free survival - A retrospective cohort study. Int J Surg 2017;39:206-13. 10.1016/j.ijsu.2017.01.110 [DOI] [PubMed] [Google Scholar]
- 37.Omichi K, Shindoh J, Yamamoto S, et al. Postoperative Outcomes for Patients with Non-B Non-C Hepatocellular Carcinoma: A Subgroup Analysis of Patients with a History of Hepatitis B Infection. Ann Surg Oncol 2015;22 Suppl 3:S1034-40. 10.1245/s10434-015-4845-0 [DOI] [PubMed] [Google Scholar]
- 38.Okamura Y, Sugiura T, Ito T, et al. The Impact of the Hepatitis B Core Antibody Status on Recurrence in Patients with Non-B Non-C Hepatocellular Carcinoma after Curative Surgery. Dig Surg 2018;35:243-51. 10.1159/000479340 [DOI] [PubMed] [Google Scholar]
- 39.Minagawa M, Ikai I, Matsuyama Y, et al. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg 2007;245:909-22. 10.1097/01.sla.0000254368.65878.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207-15. 10.1007/s005350300038 [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. 10.1093/jnci/djn134 [DOI] [PubMed] [Google Scholar]
- 42.Gan W, Huang JL, Zhang MX, et al. New nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resection. J Surg Oncol 2018;117:1540-7. 10.1002/jso.25046 [DOI] [PubMed] [Google Scholar]
- 43.Touijer K, Scardino PT. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer 2009;115:3107-11. 10.1002/cncr.24352 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. 10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 45.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009;29:502-10. 10.1111/j.1478-3231.2008.01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890-7. 10.1016/j.jhep.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 47.Chen WN, Chen JY, Jiao BY, et al. Interaction of the hepatitis B spliced protein with cathepsin B promotes hepatoma cell migration and invasion. J Virol 2012;86:13533-41. 10.1128/JVI.02095-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang P, Qiu J, Li J, et al. Nomograms for Pre- and Postoperative Prediction of Long-term Survival for Patients Who Underwent Hepatectomy for Multiple Hepatocellular Carcinomas. Ann Surg 2016;263:778-86. 10.1097/SLA.0000000000001339 [DOI] [PubMed] [Google Scholar]
- 49.Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015;261:56-66. 10.1097/SLA.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 50.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. 10.1053/j.gastro.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]