Abstract
Background
Pseudocirrhosis is defined by radiologic changes of the liver parenchyma secondary to metastatic disease and/or cancer treatments, and portends a high rate of morbidity and mortality from sequelae of portal hypertension. Transjugular intrahepatic portosystemic shunt (TIPS) is an effective treatment for portal hypertension; however, TIPS is relatively contraindicated in the setting of hepatic metastases. The study aims to determine the technical efficacy and clinical outcomes of patients undergoing TIPS for symptomatic pseudocirrhosis.
Methods
Retrospective analysis of patients with hepatic malignancy who underwent TIPS between 2008 and 2020 at a single tertiary care center. Patients with imaging findings of pseudocirrhosis and without history of primary liver malignancy or confounding causes of cirrhosis were included. West Haven scores assessing hepatic encephalopathy were obtained from chart review. Technical success was defined as successful TIPS creation with reduction in the portosystemic gradient (PSG). Clinical success was defined as resolution of variceal bleeding and/or ascites.
Results
Nine patients (4 female/5 male), average (± SD) age 61.2±9.5 years with metastatic pseudocirrhosis were included for analysis. Primary malignancy was colorectal adenocarcinoma (n=5), neuroendocrine tumor (n=3), and malignant endothelial hemangioendothelioma (n=1). Average Model for End Stage Liver Disease (MELD-Na) score was 15.7±3.7. Technical success was 8/9 (89%) with average PSG reduced from 23.5±11.0 to 6.5±2.8 mmHg (P=0.001). Clinical success was 6/9 (67%). Two patients required TIPS revision after initial clinical success. Mild-moderate HE occurred in 6/9 patients post TIPS (67%), with a highest West Haven score of 2. Time from TIPS to death for acute variceal bleeding and ascites was 4.9±4.2 and 12±16.5 months, respectively. Cause of death was disease progression (n=5), variceal bleeding (n=1), or unavailable (n=2).
Conclusions
TIPS in the setting of malignant pseudocirrhosis can be created safely with similar clinical outcomes to TIPS performed for benign disease. Rates of low-grade hepatic encephalopathy may be higher amongst patients undergoing TIPS for pseudocirrhosis.
Keywords: Pseudocirrhosis, transjugular intrahepatic portosystemic shunt (TIPS), ascites, variceal bleeding
Introduction
Pseudocirrhosis is defined by micro- and macroscopic changes of the liver parenchyma secondary to metastatic disease and/or systemic therapies in a pattern which mimics the imaging findings of cirrhosis (1,2). Radiologic findings associated with this diagnosis include diffuse nodularity of the hepatic contour, multifocal capsular retraction, segmental volume loss, and/or caudate lobe enlargement (2-5). Pseudocirrhosis differs from cirrhosis at the cellular level by the absence of bridging fibrosis (2,6-8). The two primary hypothesizes for etiology are parenchymal changes caused by nodular regenerative hyperplasia and/or tumor deposits causing sinusoidal obstruction and obstructive portal hypertension (2,9).
Pseudocirrhosis is most commonly described in the breast cancer population where 50–75% of patients with hepatic metastases will develop pseudocirrhosis (1,5,10,11). It carries a high rate of morbidity and mortality related to sequelae of portal hypertension (1,3,12). In patients with metastatic disease to the liver and imaging findings consistent with pseudocirrhosis, the prevalence of clinically significant ascites ranges from 35–68% and 9–23% for esophageal varices (1-3,11). If radiologic evidence is considered, the prevalence of ascites reaches as high as 81.3% (3).
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective treatment for portal hypertension and its complications, including refractory ascites, variceal hemorrhage, and hepatic hydrothorax. According to the American Association for the Study of the Liver (AASLD) and Society of Interventional Radiology (SIR) Guidelines, extensive primary or metastatic malignancy is a relative contraindication to TIPS due to a perceived risk of complications such as shunt occlusion and hemorrhage (13,14). Multiple studies have reported the utility of TIPS in the setting of cirrhosis with concomitant hepatocellular carcinoma (HCC) (14-21). Minimal data exists on TIPS for the treatment of pseudocirrhosis secondary to extensive non-HCC hepatic malignancy. Case studies of 1–3 patients have reported successful use of TIPS for the treatment of symptomatic pseudocirrhosis secondary to metastatic breast cancer (3,7,22,23). In addition, Wallace et al. used TIPS for the treatment of two patients with portal hypertension from non-HCC and non-breast diffuse hepatic malignancy (24). The objective of this study was to determine the efficacy and clinical outcomes of patients undergoing TIPS for the palliative treatment of symptomatic malignant non-HCC pseudocirrhosis. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-501/rc).
Methods
Clinical Subjects
Clinical data were retrospectively collected from all patients with hepatic malignancy who underwent TIPS between 2008 and 2020, identified using an IR quality assurance database. Imaging findings were reviewed to confirm the presence of radiologic pseudocirrhosis. Exclusion criteria included patients <18 years of age, history of HCC, or medical history of any confounding causes of cirrhosis, including viral hepatitis or Budd-Chiari syndrome. Patient records were reviewed for demographic information, medical history, underlying malignancy, treatment regimens, TIPS procedural details, procedural complications, and clinical outcomes. West Haven (WH) scores assessing the degree of hepatic encephalopathy (HE) were obtained directly from chart review. If a WH score was not explicitly documented, the score was determined based on the description of symptoms provided in any available clinical documents. If there were indeterminate features (e.g., grade 2 versus grade 3), the higher score was used. If the patient was intubated for symptoms related to HE, they were automatically graded as a 4. All available patient data were used for the follow-up period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Hospital of the University of Pennsylvania (No. 811929) and individual consent for this retrospective analysis was waived.
TIPS procedural technique
All patients presenting for TIPS underwent clinical and imaging assessment with computerized tomography (CT) or magnetic resonance imaging (MRI). Sedation was provided based on individual patient assessment, ranging from moderate sedation to general anesthesia. Percutaneous access was obtained through the right internal jugular vein. A portal venous access set (Rosch-Uchida Transjugular Liver Access Set (Cook Medical, Bloomington, IN, USA), Ring set (Cook Medical, Bloomington, IN, USA), or Cope set (Cook Medical, Bloomington, IN, USA) was used to cannulate the portal vein. A 5 Fr pigtail maker catheter was then placed into the portal vein and digital subtraction angiography (DSA) was performed. Right atrial and portal pressures were measured. The intrahepatic tract was dilated and an expanded polytetrafluoroethylene (e-PTFE) covered stent (Viatorr; W.L. Gore and Associates, Flagstaff, AZ, USA) was placed from the portal vein into the right hepatic vein. An e-PTFE controlled expansion stent was used in one patient in the cohort (Viatorr with Controlled Expansion; W.L. Gore and Associates, Flagstaff, AZ, USA). The stent graft subsequently underwent balloon angioplasty. In cases in which the stent graft did not fully cover the TIPS tract to the level of the hepatic veins, an additional stent was placed (Zilver (Cook Medical, Bloomington, IN, USA) or Fluency (Becton Dickinson, Franklin Lakes, NJ, USA). The 5 Fr pigtail catheter was then replaced into the portal vein. Post-TIPS DSA was performed and right atrial and portal venous pressures were measured. The catheter and sheaths were then removed, and hemostasis achieved with manual compression.
Technical success was defined as successful creation of the TIPS with reduction in the portosystemic gradient (PSG). Clinical success was defined as technically successful TIPS creation and resolution of the clinical indication for which the procedure was performed. Adverse events were classified in accordance with the SIR guidelines and the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (25,26).
Statistical analysis
Statistical analysis was performed with SPSS Statistical Software (Version 27, IBM, Armonk, NY, USA) to compare PSG pre and post TIPS creation. Shapiro Wilk normality tests were performed on the data and a paired t-test was used. A P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Nine patients (4F/5M), average (± SD) age 61.2±9.5 years, underwent TIPS for the treatment of symptomatic pseudocirrhosis (Figure 1). Underlying primary malignancy consisted of metastatic colorectal adenocarcinoma (n=5), neuroendocrine tumor (n=3), and malignant hepatic endothelial hemangioendothelioma (n=1) (Table 1). Additional sites of metastatic disease were seen in the lung (n=3), bone (n=3), peritoneum (n=2), and spleen (n=1). Average number of treatments for the primary disease consisted of 3.4±2.9 medicinal/radiation regimens and 2.9±2.1 liver-directed therapies (Table 1). Prior to TIPS, the patient with hepatic endothelial hemangioendothelioma underwent liver transplant, which then developed recurrent disease.
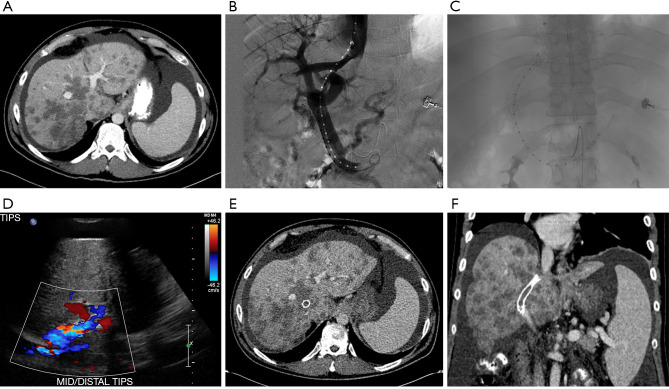
Figure 1.
Patient with colorectal cancer complicated by extensive metastatic disease to the liver. (A) Computerized tomography (CT) is notable for evidence of radiologic pseudocirrhosis including multifocal capsular retraction, segmental volume loss and caudate hypertrophy, as well as diffuse ascites; (B,C) transjugular intrahepatic portosystemic shunt (TIPS) placement; (D) post-TIPS ultrasound with color doppler showing appropriate flow through the TIPS stent; (E) post-TIPS axial CT image notable for appropriate placement of the stent extending through the liver and multiple hepatic metastases; (F) post-TIPS coronal CT image notable for appropriate placement of the stent extending through the liver and multiple hepatic metastases.
Table 1. Patient characteristics.
| Subject | Primary tumor | Liver intervention and chemotherapy regimens | TIPS indication | MELD Na | Clinical outcome | Follow up (months) and survival |
|---|---|---|---|---|---|---|
| 1 | Colorectal | Hepatic lobectomy; cyberknife; FOLFOX, avastin, FOLFIRI, modified FOLFOX; partial PVT | AVB | 18 | No further bleeding | 9.8, death |
| 2 | Colorectal | Wedge resection; RFA, TACE; FOLFOX, avastin, FOLFIRINOX, 5-FU, capecitabine, FOLFIRI/cetuximab | AVB | 11 | No further bleeding | 2.62, death |
| 3 | Colorectal | TARE, FOLFOX, Avastin, 5FU, LV, FOLFIRI/cetuximab, irinotecan, capecitabine | Ascites | 14 | No further LVP; TIPS occlusion requiring revision ×2 | 38.6, death |
| 4 | Colorectal | FOLFOX, 5FU, leucovorin | Ascites | 21 | No further LVP | 0.3, death |
| 5 | Colorectal | FOLFOX, avastin; FU/LV | Ascites | 13 | TIPS occlusion requiring revision; multiple LVPs required | 2.4, death |
| 6 | NET | TACE; PRRT | Ascites | 11 | No further LVP | 17.5, death |
| 7 | NET | Wedge resection; splenic vein to portosystemic shunt | AVB | 16 | Technically unsuccessful; pancreatectomy/splenectomy and Sugiura | 2.3, death |
| 8 | NET | Aspira catheter | Ascites | 20 | Aspira catheter removal | 1.2, death |
| 9 | Epithelioid hemangioendothelioma | Avastin; OLT | Ascites | 17 | TIPS occlusion requiring revision; 1 post TIPS LVP required | 59.5, alive, asymptomatic |
AVB, acute variceal bleeding; FOLFOX, 5-fluorouracil, oxaliplatin, leucovorin, FOLFIRI, 5-fluorouracil, leucovorin, irinotecan; LVP, large volume paracentesis; NET, neuroendocrine tumor; OLT, orthotopic liver transplant; PRRT, peptide receptor radionuclide therapy; PVT, portal vein thrombosis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TIPS, transjugular intrahepatic portosystemic shunt.
Liver disease
At the time of TIPS creation, average MELD and MELD-Na were 10.4±3.0 and 15.7±3.7, respectively. MELD-Na ranged from 11–21. Childs-Pugh score ranged from B7-B9. Evidence of symptomatic portal hypertension pre-TIPS consisted of ascites (n=3), varices (n=3), ascites and varices (n=2), and ascites with hepatic hydrothorax (n=1). Location of varices consisted of esophageal (n=1), esophageal varices with portal gastropathy (n=2), and esophageal/gastric varices with portal gastropathy (n=2). One patient underwent banding prior to TIPS. Of the three patients who underwent TIPS for the treatment of acute variceal bleeding (AVB), only one had known varcies prior to the sentinel bleed, which had not been treated. At the diagnosis of AVB, one patient underwent unsuccessful banding of 12 esophageal and gastric varices prior to TIPS. One patient had a history of mild HE (WH grade 1) prior to TIPS for which they had been prescribed lactulose. In patients with ascites, time from first large volume paracentesis to TIPS was 5.6±2.9 months. Among three patients with available fluid data, all had a serum ascites-albumin gradient (SAAG) >1.1 (range, 1.4–2.3). Among four patients with available fluid cytology, all were negative for malignancy. One patient had a tunneled peritoneal drainage catheter placed prior to TIPS. No other durable therapies for ascites were used.
Liver lesion sampling via biopsy or hepatectomy occurred in 7/9 patients. Of the five biopsies describing background liver parenchyma, none had pathology consistent with bridging fibrosis or nodular regenerative hyperplasia. One sample noted a hyalinized nodule. The patient with hepatic endothelial hemangioendothelioma was noted to have liver tissue notable for angiomatosis and hobnail growth pattern of the vascular channel cells, as well as circumscribed vascular lesions with open well-formed vascular channels. Additionally noted were small foci of rare vacuolated endothelial cells in a myxoid stroma.
TIPS
Mean (± SD) contrast dose was 68.9±26.6 mL and fluoroscopy time was 46.4±22.1 min. Viatorr PTFE-covered stents (W.L. Gore and Associates, Flagstaff, AZ, USA) with diameters of 8 (n=1) and 10 mm (n=8) with length ranging from 8–10 cm were used. Three patients had additional stents placed for tract elongation.
Technical success was 8/9 (89%). Average PSG was statistically significantly reduced from 23.5±11.0 to 6.5±2.8 mmHg (P=0.001). The patient who failed TIPS creation had metastatic pancreatic neuroendocrine tumor and presented for acute variceal bleeding, with prior MR showing significant portal vein narrowing. Three days prior to TIPS, the patient had undergone trans-splenic portal vein stenting which was complicated by venous rupture treated with stenting and balloon tamponade. During TIPS, significant thrombus was identified with the splenic and portal venous stents requiring mechanical thrombectomy [AngioJet (Boston Scientific, Marlborough, MA, USA)] and anticoagulation. Minimal flow was achieved through the portal and splenic venous stents and no PSGs were measured.
No immediate procedural adverse events occurred in any patient. Two patients experienced mild adverse events within the hospital stay after TIPS creation. One patient developed Grade 2 pulmonary edema requiring diuresis and one patient developed Grade 2 acute kidney injury which resolved with fluids as well as an episode of Grade 1 hematochezia which resolved without intervention.
Clinical outcomes
Clinical success was documented in 6/9 patients (67%). By primary TIPS indication, clinical success was 2/3 (67%) for acute variceal bleeding and 4/6 (67%) for ascites. Among the clinical failures, the patient who had a technically unsuccessful TIPS in the setting of acute bleeding varices underwent distal pancreatectomy/splenectomy the next day. Two months later he underwent a Sugiura procedure and expired shortly after. One patient treated for ascites was transferred to a skilled nursing facility and died five days after TIPS, without report of worsening of symptoms or complications related to the procedure. Cause of death is unknown. The third patient required multiple LVPs after TIPS and was found to have a shunt occlusion on imaging. The patient underwent revision 1.9 months after TIPS creation, with thrombectomy, balloon angioplasty, stent placement, and variceal embolization. One further paracentesis was performed 2 weeks after revision and the patient died shortly thereafter.
Two patients required TIPS revision after initial clinical success (Figure 2A). One had consistent improvement in symptoms and required no further LVPs. Follow up imaging showed occlusion of the TIPS and the patient was treated with anticoagulation and two TIPS revisions with the first occurring 2.1 years after initial TIPS placement. One patient required one further LVP 6 months after TIPS over a total follow up year of 5 years. This patient developed symptomatic TIPS occlusion requiring revision with balloon angioplasty 3.9 years post-TIPS.
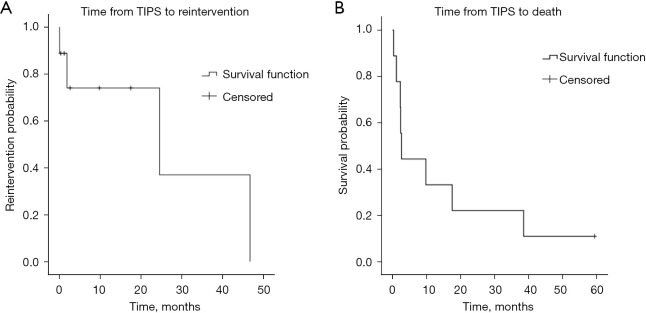
Figure 2.
Kaplan-Meier survival curves depicting (A) time from transjugular intrahepatic portosystemic shunt (TIPS) to reintervention (inclusive of surgical treatment or TIPS revision) and (B) time from TIPS to death.
Mild-moderate HE occurred in 6/9 patients (67%). The highest post-procedure HE score was 2. All patients had HE controllable with medical therapy. Three patients were treated with lactulose and three treated with a combination of lactulose and rifaximin. No patients required intubation for HE or TIPS reduction/occlusion.
Eight of the nine patients have died since TIPS creation. Average (median; 1st quartile, 3rd quartile) time from TIPS to death was 9.3±13.2 months (2.5; 2.0, 11.7 months) (Figure 2B). Time from TIPS to death for patients with acute variceal bleeding was 4.9±4.2 months (2.5; 2.5, 6.2 months). Time from TIPS to death for patients with refractory ascites was 12±16.5 months (2.4; 1.1, 17.5 months). Cause of death was disease progression (n=5), complications secondary to continued variceal bleeding following surgery (n=1), or unavailable (n=2).
Discussion
Cirrhosis-like parenchymal changes of the liver secondary to non-HCC malignancy, termed pseudocirrhosis, portends high rates of morbidity and mortality when compared to similar patient cohorts without findings of pseudocirrhosis (1,27). These patients can develop symptoms of portal hypertension ranging from ascites in 54–68% of patients and esophageal varices in 9–23% of patients (1-3). As life expectancies improve for malignant disease, more patients may develop symptomatic pseudocirrhosis requiring durable therapy. TIPS is a proven treatment for symptomatic cirrhosis-induced portal hypertension with variceal bleeding control in over 90% of patients as well as superior rates of ascites control and 1-year survival rates compared to LVP and albumin (13,28-30). Hepatic malignancy is considered a relative contraindication for TIPS in current guidelines due to concerns surrounding patient prognosis, device failure, and procedural complications (13,14). Our study showed technical success rates of 8/9 (89%) of patients, with the singular failure due primarily to extrahepatic malignant portal vein occlusion, which is consistent with reported rates in traditional liver cirrhosis cohorts (14).
There were no major complications related to TIPS insertion, in particular there was no peri-procedural TIPS-related hemorrhage. Three patients had TIPS occlusion that required revision at 1.9 months, 2.1 years, and 3.9 years post-insertion. Hepatic encephalopathy developed or progressed in 67% (6/9) of our patients. All were controlled with medical therapy and the highest WH score was a 2. Within the cirrhotic population, HE typically develops in 50% of patients (30). Our increased rates of HE may be due to the small cohort size available, however, they mirror results from Wallace et al. who reported similarly elevated rates of HE (44%) and HE requiring shunt reduction (17%) (24). Given the retrospective nature of this study, a conservative approach was taken to include any documentation of HE or any symptoms attributed to possible HE, which could cause overestimation. It is possible that patients with hepatic malignancy and pseudocirrhosis may prove to be more susceptible to HE after TIPS, warranting further evaluation.
Our results are consistent with high technical success and low complication rates of previous studies examining TIPS in the setting of hepatic and extrahepatic malignancy. Wallace et al. performed TIPS on 38 patients, directly through tumor in 9/38, with a technical success rate of 97% (24). Three studies with a total of five patients have specifically reported the outcomes of TIPS in the setting of pseudocirrhosis secondary to breast cancer (7,22,23). Indication for TIPS across the studies was ascites (n=4) and acute variceal bleeding (n=1). Technical success was reported in all patients. Of the patients undergoing TIPS for ascites, all had improvement of ascites with two patients later suffering from malignant pleural effusion and one expiring within five days of treatment (22,23). The patient with acute variceal bleeding died shortly after TIPS from massive variceal hemorrhage and diffuse pulmonary alveolar damage.
In the presence of hepatic malignancy and pseudocirrhosis, it is important to balance the desired benefit of TIPS with the patient’s prognosis and quality of life. Clinical success was achieved in 6/9 (67%) of patients. TIPS achieved a durable benefit until death at a mean of 9.4 months later at a cost of further procedures to revise the TIPS in 2 of those patients. Compared to data of benign liver disease, patients presenting with acute variceal bleeding had a shorter mean time to death compared to patients with refractory ascites, 4.9 vs. 12 months, respectively, and similar MELD scores (Table 1) (31). These differences may be secondary to pathologic differences of the liver, longer time to treatment, treatment effects, the critically ill nature of patients presenting with AVB, or small sample size. Improving cancer patients’ quality of life by mitigating large volume ascites and the need for frequent hospital visits for paracentesis, and/or hospitalizations for variceal bleeding, is a substantial palliative contribution that is technically feasible and safe.
This study was limited by retrospectively collected data, a small number of available patients, and lack of direct control group comparison. Follow-up data was limited to available recorded information and short follow up periods for some patients.
Conclusions
TIPS for the treatment non-HCC malignant symptomatic pseudocirrhosis can be created safely with few peri-procedural complications and similar clinical outcomes to TIPS performed for benign disease. Durable therapy for the treatment of symptomatic pseudocirrhosis can serve as a palliative treatment for cancer patients. Rates of low-grade hepatic encephalopathy may be higher amongst patients undergoing TIPS for pseudocirrhosis compared to TIPS for the treatment of cirrhosis induced portal hypertension.
Acknowledgments
The abstract was presented at the Society of Interventional Oncology Annual Meeting in June 2018 in Boston, Massachusetts, United States of America.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Hospital of the University of Pennsylvania (No. 811929) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-501/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-501/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-501/coif). TWIC has received royalties from Teleflex and Merit, and serves as a consultant for Teleflex, Becton Dickinson, Boston Scientific, Forge Medical, and B. Braun. No conflicts directly relate to the content of this manuscript. The other authors have no conflicts of interest to declare.
References
- 1.Oliai C, Douek ML, Rhoane C, et al. Clinical features of pseudocirrhosis in metastatic breast cancer. Breast Cancer Res Treat 2019;177:409-17. 10.1007/s10549-019-05311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young ST, Paulson EK, Washington K, et al. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol 1994;163:1385-8. 10.2214/ajr.163.6.7992734 [DOI] [PubMed] [Google Scholar]
- 3.Engelman D, Moreau M, Lepida A, et al. Metastatic breast cancer and pseudocirrhosis: an unknown clinical entity. ESMO Open 2020;5:e000695. 10.1136/esmoopen-2020-000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010;194:993-9. 10.2214/AJR.09.3409 [DOI] [PubMed] [Google Scholar]
- 5.Qayyum A, Lee GK, Yeh BM, et al. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging 2007;31:6-10. 10.1016/j.clinimag.2006.09.028 [DOI] [PubMed] [Google Scholar]
- 6.Jüngst C, Krämer J, Schneider G, et al. Subacute liver failure by pseudocirrhotic metastatic breast cancer infiltration. Ann Hepatol 2013;12:834-6. 10.1016/S1665-2681(19)31329-8 [DOI] [PubMed] [Google Scholar]
- 7.Sass DA, Clark K, Grzybicki D, et al. Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: a case of "pseudocirrhosis". Dig Dis Sci 2007;52:749-52. 10.1007/s10620-006-9332-9 [DOI] [PubMed] [Google Scholar]
- 8.Jeong WK, Choi SY, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol 2013;19:190-4. 10.3350/cmh.2013.19.2.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler M, Tang I, Gach MW, et al. Recurrent metastatic breast cancer presenting with portal hypertension and pseudocirrhosis. BMJ Case Rep 2019;12:231044. 10.1136/bcr-2019-231044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fennessy FM, Mortele KJ, Kluckert T, et al. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol 2004;182:651-5. 10.2214/ajr.182.3.1820651 [DOI] [PubMed] [Google Scholar]
- 11.Gopalakrishnan D, Shajihan A, Purysko AS, et al. Pseudocirrhosis in Breast Cancer - Experience From an Academic Cancer Center. Front Oncol 2021;11:679163. 10.3389/fonc.2021.679163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adike A, Karlin N, Menias C, et al. Pseudocirrhosis: A Case Series and Literature Review. Case Rep Gastroenterol 2016;10:381-91. 10.1159/000448066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases . The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology 2010;51:306. 10.1002/hep.23383 [DOI] [PubMed] [Google Scholar]
- 14.Dariushnia SR, Haskal ZJ, Midia M, et al. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J Vasc Interv Radiol 2016;27:1-7. 10.1016/j.jvir.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 15.Bettinger D, Knüppel E, Euringer W, et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther 2015;41:126-36. 10.1111/apt.12994 [DOI] [PubMed] [Google Scholar]
- 16.Qiu B, Li K, Dong X, et al. Transjugular Intrahepatic Portosystemic Shunt for Portal Hypertension in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol 2017;40:1372-82. 10.1007/s00270-017-1655-8 [DOI] [PubMed] [Google Scholar]
- 17.Qiu B, Zhao MF, Yue ZD, et al. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol 2015;21:12439-47. 10.3748/wjg.v21.i43.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace M, Swaim M. Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J Vasc Interv Radiol 2003;14:501-7. 10.1097/01.RVI.0000064846.87207.AB [DOI] [PubMed] [Google Scholar]
- 19.Zhao JB, Feng C, Zhu QH, et al. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2014;20:1602-7. 10.3748/wjg.v20.i6.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsauo J, Tie J, Xue H, et al. Transjugular Intrahepatic Portosystemic Shunt Creation for the Prevention of Gastric Variceal Rebleeding in Patients with Hepatocellular Carcinoma: A Multicenter Retrospective Study. J Vasc Interv Radiol 2021;32:963-9. 10.1016/j.jvir.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Luo SH, Chu JG, Huang H, et al. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases 2019;7:1599-610. 10.12998/wjcc.v7.i13.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier C, Tisman G, Kleinman R, et al. Clinical evidence for overcoming capecitabine resistance in a woman with breast cancer terminating in radiologically occult micronodular pseudo-cirrhosis with portal hypertension: a case report. J Med Case Rep 2010;4:112. 10.1186/1752-1947-4-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geeroms B, De Hertogh G, Vanslembrouck R, et al. Transjugular Intrahepatic Portosystemic Shunt for the Treatment of Portal Hypertension-Induced Refractory Ascites Due to Metastatic Carcinomatous Liver Disease. J Vasc Interv Radiol 2018;29:1713-6. 10.1016/j.jvir.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 24.Wallace MJ, Madoff DC, Ahrar K, et al. Transjugular intrahepatic portosystemic shunts: experience in the oncology setting. Cancer 2004;101:337-45. 10.1002/cncr.20367 [DOI] [PubMed] [Google Scholar]
- 25.Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2017;28:1432-1437.e3. 10.1016/j.jvir.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 26.Services USDoHaH. Common Terminology Criteria for Adverse Events Version 5.02017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60
- 27.Kashyap R, Reddy R, Voona MK. Pseudocirrhosis of the Liver in Setting of Metastatic Carcinoma Breast: An Ominous Sign to be Remembered. Indian J Nucl Med 2018;33:86-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azoulay D, Castaing D, Majno P, et al. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol 2001;35:590-7. 10.1016/S0168-8278(01)00185-4 [DOI] [PubMed] [Google Scholar]
- 29.Bucsics T, Hoffman S, Grünberger J, et al. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int 2018;38:1036-44. 10.1111/liv.13615 [DOI] [PubMed] [Google Scholar]
- 30.Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol 2014;20:16996-7010. 10.3748/wjg.v20.i45.16996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Membreno F, Baez AL, Pandula R, et al. Differences in long-term survival after transjugular intrahepatic portosystemic shunt for refractory ascites and variceal bleed. J Gastroenterol Hepatol 2005;20:474-81. 10.1111/j.1440-1746.2005.03601.x [DOI] [PubMed] [Google Scholar]