Abstract
Background
Gastrointestinal symptoms have been reported in patients with COVID-19. Several clinical investigations suggested that gastrointestinal symptoms were associated with disease severity of COVID-19. However, the relevance of gastrointestinal symptoms and mortality of COVID-19 remains largely unknown. We aim to investigate the relationship between gastrointestinal symptoms and COVID-19 mortality.
Methods
We searched the PubMed, Embase, Web of science and Cochrane for studies published between Dec 1, 2019 and May 1, 2021, that had data on gastrointestinal symptoms in COVID-19 patients. Additional literatures were obtained by screening the citations of included studies and recent reviews. Only studies that reported the mortality of COVID-19 patients with/without gastrointestinal symptoms were included. Raw data were pooled to calculate OR (Odds Ratio). The mortality was compared between patients with and without gastrointestinal symptoms, as well as between patients with and without individual symptoms (diarrhea, nausea/vomiting, abdominal pain).
Results
Fifty-three literatures with 55,245 COVID-19 patients (4955 non-survivors and 50,290 survivors) were included. The presence of GI symptoms was not associated with the mortality of COVID-19 patients (OR=0.88; 95% CI 0.71–1.09; P=0.23). As for individual symptoms, diarrhea (OR=1.01; 95% CI 0.72–1.41; P=0.96), nausea/vomiting (OR=1.16; 95% CI 0.78–1.71; P=0.46) and abdominal pain (OR=1.55; 95% CI 0.68–3.54; P=0.3) also showed non-relevance with the death of COVID-19 patients.
Conclusions
Gastrointestinal symptoms are not associated with higher mortality of COVID-19 patients. The prognostic value of gastrointestinal symptoms in COVID-19 requires further investigation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-022-02132-0.
Keywords: Gastrointestinal symptom, COVID-19, Mortality, Prognosis
Background
The occurrence and rapid spread of novel coronavirus (SARS-CoV-2)-infected pneumonia (COVID-19) since December, 2019, has brought troublesome challenges to worldwide public health [1]. Globally, as of February 25, 2022, there have been 430,257,564 confirmed cases of COVID-19, including 5,922,049 deaths, reported to the WHO. In response to the alarming levels of its spread, severity and death threat of COVID-19, the WHO issued a statement of Public Health Emergency of International Concern on January 30, 2020 and further declared COVID-19 a pandemic on March 11, 2020 [2].
The most frequent symptoms in COVID-19 patients are respiratory manifestations. However, emerging studies have found that gastrointestinal (GI) symptoms including diarrhea, nausea/vomiting and abdominal pain, are also commonly observed in patients with COVID-19, with a prevalence of up to 31.9% [3, 4].
As the major receptor of SARS-CoV-2, angiotensin-converting enzyme 2, is also expressed in the gastrointestinal tract [5]. Early evidence has identified gastrointestinal infection of SARS-CoV-2 via immunofluorescent [6]. Intriguingly, several case-control studies and meta-analysis suggested that COVID-19 patients with GI symptoms might be at a higher risk of clinical deterioration [7, 8]. Physicians are also anxious to find out whether GI symptoms in patients with COVID-19 indicate a higher probability of death. In the first few months of COVID-19 pandemic, Mao et al. performed a meta-analysis and found that COVID-19 patients with GI symptoms tended to have higher prevalence of death (OR (odds ratio) = 1.21) but without statistical significance (P = 0.52) [8]. The question remains controversial due to the limited number of studies and population at that time. Now with the numerous emerging publications reporting the characteristics and outcomes of COVID-19 patients, there is a pressing need to determine the role of GI symptoms in the prognosis of COVID-19. Hence, this meta-analysis is conducted to investigate the relationship between GI symptoms and the mortality of COVID-19 patients.
Methods
Search strategy and selection criteria
We searched PubMed, Embase, Web of Science and Cochrane databases on May 1, 2021 for articles published from Dec 1, 2019, using the keywords combination of “COVID-19”, “SARS-CoV-2”, “2019 novel coronavirus”, “2019-nCoV”, “coronavirus disease 2019”, “coronavirus disease-19”, “severe acute respiratory syndrome coronavirus” and “novel Coronavirus 2019” for COVID-19, and “gastrointestinal”, “vomiting”, “vomit”, “nausea”, “diarrhoea”, “diarrhea”, “appetite”, “anorexia”, “abdominal”, “abdomen”, “digestive” and “alimentary” for GI symptoms. The reference lists of relevant reviews, meta-analysis and included literatures were also screened manually to identify additional articles that might be missed in the database search. Search records were managed with EndNote (version X7) for excluding duplicates and further literature screening. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. The protocol of this meta-analysis has been registered with the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42020197032).
The eligibility for inclusion of literatures were determined by three authors (YW, YmL and YZ) independently, and dissonance were discussed with another author (YL) and subsequently resolved via consensus. Articles reporting the mortality of COVID-19 patients with and without GI symptoms respectively were considered eligible for inclusion. Preprint studies without peer-review were excluded due to potential misinformation. The following literatures were excluded at title and abstract screening: reviews, meta-analysis, guidelines, case reports, letter, comment, editorial, protocol, clinical research with less than 20 patients, basic research and non-relevant literatures. Then full-text review was performed to exclude articles without needed data and those written in languages other than English.
Data extraction and definitions
Three authors (YW, YmL and YZ) independently extracted the data, and dissonance were resolved with another author (YL) by discussion and consensus. The following variables were extracted: first author, study location, number of patients, basic characteristics of study population, mortality of COVID-19 patients with and without GI symptoms, respectively (Additional file 1).
For studies only reporting individual symptoms such as diarrhea, nausea, vomiting and abdominal pain, “GI symptom” was defined as the most common one of these digestive symptoms. For studies reporting either nausea or vomiting but not nausea/vomiting, “nausea/vomiting” was defined as the more frequent one of the two symptoms.
Assessment of study quality
For included studies, Newcastle-Ottawa Scale (NOS) was used for the assessment of quality. NOS is a quality assessment tool for observational studies that has been endorsed by the Cochrane Collaboration [9, 10]. The studies were considered as high quality if they scored > 6 points, moderate quality if they scored 5 or 6 points, and poor quality if they scored < 5 points.
Data synthesis and statistical analysis
To ensure the accuracy of the results, analysis were performed by two authors (YW and YmL) independently. Dissonance was resolved by discussion. To evaluate the risk of mortality associated with GI symptoms, OR with 95% confidence intervals (CI) were calculated by the Cochrane Review Manager program (RevMan 5.3, Denmark) following the Mantel-Haenszel method. The heterogeneity of included literatures was detected by I2 statistic.
Subgroup analysis was performed according to the study location, severity of disease, patient age and population size. Funnel-plot and Egger’s test were used to investigate the possibility of publication bias. P < 0.05 for Egger’s test was considered significant bias. If publication bias was indicated, trim-and-fill method was used for adjusting OR. A sensitivity analysis was also performed by omitting each study using the meta package in R, version 4.0.2.
Results
Search results and study characteristics
The study selection process is depicted in Fig. 1. A total of 4,873 records were initially identified. After removal of duplicates, 3,756 remained. After screening by titles/abstracts and full-text review, 53 studies [11–63] were finally included for data analysis.
Fig. 1.
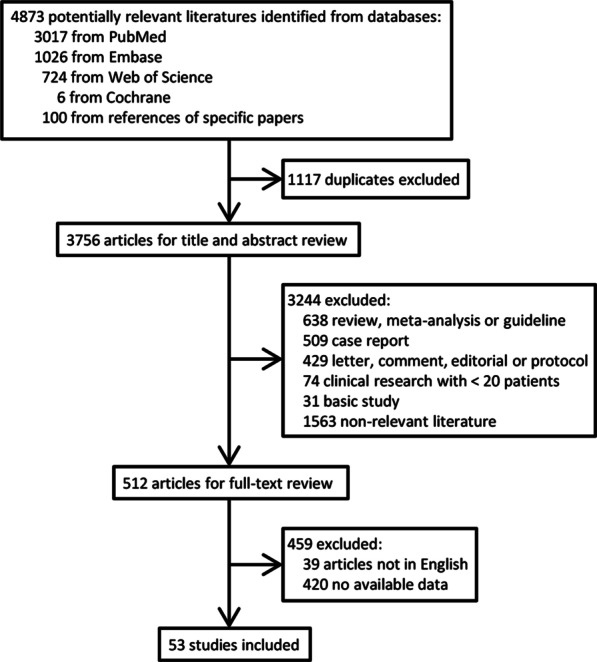
Flow chart showing the flow of study selection
The characteristics of the included studies are shown in Table 1. Of the 53 included studies with a total of 55,245 patients, 21 were carried out in China, 12 in USA and 20 in other countries. One and four studies included pediatric and geriatric patients, respectively. Seven studies investigated COVID-19 combined with other disease history including chronic liver disease, cancer, kidney transplantation and interstitial lung disease. Six studies included critically ill patients. All papers were considered high quality with NOS score > 6.
Table 1.
Characteristics of the studies included for meta-analysis
| No. of study | First author | Study location | No. of patients | Age (year) | Male | Special patient population | NOS score |
|---|---|---|---|---|---|---|---|
| 1 | Alizadehsani R et al. | Iran | 123 | 45 | 62 | None | 8 |
| 2 | An P et al. | China | 205 | 54 | 122 | None | 8 |
| 3 | Atalla E et al. | USA | 111 | 87 | 23 | Older patients | 7 |
| 4 | Caillard S et al. | France | 243 | 62 | 162 | Kidney transplant recipients | 7 |
| 5 | Chadalavada P et al. | USA | 84 | 60 | 52 | None | 8 |
| 6 | Chen R et al. | China | 1077 | 59 | 532 | None | 9 |
| 7 | Chen T et al. | China | 274 | 62 | 171 | Critically ill patients | 7 |
| 8 | Comoglu Ş et al. | Turkey | 1086 | 48 | 563 | None | 8 |
| 9 | Crespo M et al. | Spain | 414 | 62 | 265 | Kidney transplant recipients | 7 |
| 10 | Doganci S et al. | Turkey | 397 | 57 | 200 | None | 8 |
| 11 | Du H et al. | China | 182 | 6 | 120 | Pediatric patients | 7 |
| 12 | Elimian K et al. | Nigeria | 3215 | 36 | 2293 | None | 8 |
| 13 | Ferm S et al. | USA | 877 | 59 | 534 | None | 8 |
| 14 | Gayam V et al. | USA | 408 | 67 | 231 | African-Americans | 7 |
| 15 | Ghoshal U et al. | India | 252 | 40 | 204 | None | 8 |
| 16 | Hajifathalian K et al. | USA | 1059 | 61 | 611 | None | 8 |
| 17 | Huang H et al. | China | 49 | 37 | 23 | Patients with pre-existing ILD | 7 |
| 18 | Jiang Y et al. | China | 281 | 70 | 143 | Older severe patients | 7 |
| 19 | Jin X et al. | China | 651 | 46 | 331 | None | 9 |
| 20 | Kang M et al. | Korea | 118 | 59 | 52 | None | 8 |
| 21 | Kim D et al. | USA | 867 | 57 | 473 | Patients with chronic liver disease | 7 |
| 22 | Lanthier N et al. | Belgium | 50 | 88 | NA | Geriatric patients | 7 |
| 23 | Laszkowska M et al. | USA | 2804 | 66 | 1565 | None | 8 |
| 24 | Leal T et al. | Portugal | 201 | 71 | 113 | Symptomatic patients | 7 |
| 25 | Liang J et al. | China | 109 | 65 | 57 | Patients with cancer | 7 |
| 26 | Liu J et al. | China | 29,393 | 47 | 15,501 | None | 8 |
| 27 | Livanos A et al. | USA | 634 | 61 | 369 | None | 8 |
| 28 | Luo S et al. | China | 1411 | 54 | 895 | None | 8 |
| 29 | Ma X et al. | China | 467 | 44 | 289 | None | 8 |
| 30 | Montazeri M et al. | Iran | 611 | 56 | 377 | None | 8 |
| 31 | Moura D et al. | Brazil | 400 | 56 | 225 | None | 8 |
| 32 | Nobel Y et al. | USA | 278 | NA | 145 | None | 7 |
| 33 | Pan L et al. | China | 204 | 53 | 107 | None | 9 |
| 34 | Peng X et al. | China | 49 | 63 | 17 | Critically ill patients | 7 |
| 35 | Ramachandran P et al. | USA | 150 | 57 | 83 | None | 8 |
| 36 | Redd W et al. | USA | 318 | 63 | 174 | None | 9 |
| 37 | Renelus B et al. | USA | 734 | 68 | 379 | None | 8 |
| 38 | Russell B et al. | UK | 156 | 65 | 90 | Patients with cancer | 7 |
| 39 | Schettino M et al. | Italy | 190 | 65 | 127 | None | 8 |
| 40 | Shang H et al. | China | 564 | 59 | 286 | None | 8 |
| 41 | Soares R et al. | Brazil | 1152 | NA | 494 | None | 8 |
| 42 | Sulaiman T et al. | Iraq | 140 | 45 | 100 | None | 8 |
| 43 | Tsibouris P et al. | Greece | 61 | 70 | 34 | None | 8 |
| 44 | Vena A et al. | Italy | 275 | 71 | 183 | None | 8 |
| 45 | Villanego F et al. | Spain | 1011 | 60 | 635 | Kidney transplant recipients | 7 |
| 46 | Vrillon A et al. | France | 52 | 90 | 34 | Older adults | 7 |
| 47 | Wan, Y et al. | China | 230 | 48 | 129 | None | 8 |
| 48 | Wang Z et al. | China | 59 | 67 | 38 | Critically ill patients | 7 |
| 49 | Yang X et al. | China | 52 | 60 | 35 | Critically ill adults | 7 |
| 50 | Zhang J et al. | China | 663 | 56 | 321 | None | 7 |
| 51 | Zhang L et al. | China | 409 | 65 | 234 | Severe COVID-19 patients | 7 |
| 52 | Zhou F et al. | China | 191 | 56 | 72 | None | 7 |
| 53 | Zhou Z et al. | China | 254 | 50 | 115 | None | 9 |
NOS, Newcastle-Ottawa Scale; ILD, interstitial lung disease; COVID-19, corona virus disease 2019
Clinical features and outcomes of COVID-19 patients are listed in Table 2. Of the 55,245 patients, 4,955 non-survivors were reported. A total of 8,535 patients had GI symptoms. Individual GI symptoms included diarrhea (1,341 reported in 10,983 patients), nausea/vomiting (525 reported in 7,175 patients) and abdominal pain (92 reported in 5,012 patients). The cumulative incidences of GI symptom, diarrhea, nausea/vomiting and abdominal pain in COVID-19 patients were 25%, 16%, 7.5% and 3.6%, respectively.
Table 2.
Clinical outcomes and manifestations of the patients included for meta-analysis
| No. of study | Author | No. of patients | No. of death | No. of GI symptom | No. of diarrhea | No. of nausea/vomiting | No. of abdominal pain |
|---|---|---|---|---|---|---|---|
| 1 | Alizadehsani R et al. | 123 | 15 (12.2%) | 11 (8.9%) | NA | NA | NA |
| 2 | An P et al. | 205 | 6 (2.9%) | 79 (38.5%) | NA | NA | NA |
| 3 | Atalla E et al. | 111 | 48 (43.2%) | 8 (7.2%) | 8 (7.2%) | 2(1.8%) | NA |
| 4 | Caillard S et al. | 243 | 43 (17.7%) | 96 (39.5%) | 96 (39.5%) | NA | NA |
| 5 | Chadalavada P et al. | 84 | 11 (13.1%) | 44 (52.4%) | NA | NA | NA |
| 6 | Chen R et al. | 1077 | 85 (7.9%) | 359 (33.3%) | NA | NA | NA |
| 7 | Chen T et al. | 274 | 113 (41.2%) | 77 (28.1%) | 77 (28.1%) | 24(8.8%) | 19(6.9%) |
| 8 | Comoglu Ş et al. | 1086 | 38 (3.5%) | 78 (7.2%) | 78 (7.2%) | NA | NA |
| 9 | Crespo M et al. | 414 | 109 (26.3%) | 152 (36.7%) | NA | NA | NA |
| 10 | Doganci S et al. | 397 | 34 (8.6%) | 292 (73.6%) | NA | NA | NA |
| 11 | Du H et al. | 182 | 1 (0.5%) | 20 (11.0%) | 9 (4.9%) | 7(3.8%) | 7(3.8%) |
| 12 | Elimian K et al. | 3215 | 295 (9.2%) | 132 (4.1%) | 132 (4.1%) | 103(3.2%) | 20(0.6%) |
| 13 | Ferm S et al. | 877 | 208 (23.7%) | 219 (25.0%) | NA | NA | NA |
| 14 | Gayam V et al. | 408 | 132 (32.4%) | 111 (27.2%) | NA | NA | NA |
| 15 | Ghoshal U et al. | 252 | 5 (2.0%) | 26 (10.3%) | NA | NA | NA |
| 16 | Hajifathalian K et al. | 1059 | 147 (13.9%) | 349 (33.0%) | NA | NA | NA |
| 17 | Huang H et al. | 49 | 9 (18.4%) | 3 (6.1%) | 3 (6.1%) | 1 (2.0%) | NA |
| 18 | Jiang Y et al. | 281 | 114 (40.6%) | 33 (11.7%) | 33 (11.7%) | 13 (4.6%) | NA |
| 19 | Jin X et al. | 651 | 1 (0.2%) | 74(11.4%) | NA | NA | NA |
| 20 | Kang M et al. | 118 | 6 (5.1%) | 54 (45.8%) | 54(45.8%) | NA | NA |
| 21 | Kim D et al. | 867 | 121 (14.0%) | 181 (20.9%) | 181 (20.9%) | 175 (20.2%) | NA |
| 22 | Lanthier N et al. | 50 | 26 (52.0%) | 15 (30.0%) | 12 (24.0%) | 3 (6.0%) | 3 (6.0%) |
| 23 | Laszkowska M et al. | 2804 | 542 (19.3%) | 1084 (38.7%) | NA | NA | NA |
| 24 | Leal T et al. | 201 | 55 (27.4%) | 60 (29.9%) | NA | NA | NA |
| 25 | Liang J et al. | 109 | 23 (21.1%) | 26 (23.9%) | 26 (23.9%) | 10 (9.2%) | 5 (4.6%) |
| 26 | Liu J et al. | 29,393 | 711 (2.4%) | 2289 (7.8%) | NA | NA | NA |
| 27 | Livanos A et al. | 634 | 151 (23.8%) | 299 (47.2%) | NA | NA | NA |
| 28 | Luo S et al. | 1411 | 66 (4.7%) | 183 (13.0%) | NA | NA | NA |
| 29 | Ma X et al. | 467 | 16 (3.4%) | 25 (5.4%) | 25(5.4%) | NA | NA |
| 30 | Montazeri M et al. | 611 | 104 (17.0%) | 155 (25.4%) | NA | NA | NA |
| 31 | Moura D et al. | 400 | 89 (22.3%) | 133 (33.3%) | NA | NA | NA |
| 32 | Nobel Y et al. | 278 | 9 (3.2%) | 97 (34.9%) | 56 (20.1%) | 63 (22.7%) | NA |
| 33 | Pan L et al. | 204 | 36 (17.6%) | 103 (50.5%) | NA | NA | NA |
| 34 | Peng X et al. | 49 | 16 (32.7%) | 22 (44.9%) | 11 (22.4%) | 15 (30.6%) | 3(6.1%) |
| 35 | Ramachandran P et al. | 150 | 58 (38.7%) | 31 (20.7%) | NA | NA | NA |
| 36 | Redd W et al. | 318 | 32 (10.1%) | 195 (61.3%) | NA | NA | NA |
| 37 | Renelus B et al. | 734 | 237 (32.3%) | 231 (31.5%) | NA | NA | NA |
| 38 | Russell B et al. | 156 | 34 (21.8%) | 25 (16.0%) | NA | NA | NA |
| 39 | Schettino M et al. | 190 | 41 (21.6%) | 138 (72.6%) | NA | NA | NA |
| 40 | Shang H et al. | 564 | 51 (9.0%) | 157 (27.8%) | 157 (27.8%) | NA | NA |
| 41 | Soares R et al. | 1152 | 456 (39.6%) | 126 (10.9%) | 126 (10.9%) | NA | NA |
| 42 | Sulaiman T et al. | 140 | 12 (8.6%) | 78 (55.7%) | NA | NA | NA |
| 43 | Tsibouris P et al. | 61 | 16 (26.2%) | 11 (18.0%) | 11 (18.0%) | 4 (6.6%) | 2 (3.3%) |
| 44 | Vena A et al. | 275 | 120 (43.6%) | 14 (5.1%) | 14(5.1%) | 11(4.0%) | NA |
| 45 | Villanego F et al. | 1011 | 220 (21.8%) | 323 (31.9%) | NA | NA | NA |
| 46 | Vrillon A et al. | 52 | 17 (32.7%) | 17 (32.7%) | NA | NA | NA |
| 47 | Wan, Y et al. | 230 | 6 (2.6%) | 49 (21.3%) | 49 (21.3%) | NA | NA |
| 48 | Wang Z et al. | 59 | 41 (69.5%) | 22 (37.3%) | 22 (37.3%) | 4 (6.8%) | NA |
| 49 | Yang X et al. | 52 | 32 (61.5%) | 2 (3.8%) | NA | 2 (3.8%) | NA |
| 50 | Zhang J et al. | 663 | 25 (3.8%) | 61 (9.2%) | 61 (9.2%) | 31 (4.7%) | 5 (0.8%) |
| 51 | Zhang L et al. | 409 | 102 (24.9%) | 91 (22.2%) | 91 (22.2%) | 50 (12.2%) | 28 (6.8%) |
| 52 | Zhou F et al. | 191 | 54 (28.3%) | 9 (4.7%) | 9 (4.7%) | 7 (3.7%) | NA |
| 53 | Zhou Z et al. | 254 | 16 (6.3%) | 66 (26.0%) | NA | NA | NA |
| Cumulative incidence | 25% | 16% | 7.5% | 3.6% | |||
GI, gastrointestinal; NA, not available
Association of GI symptoms with the mortality of COVID-19
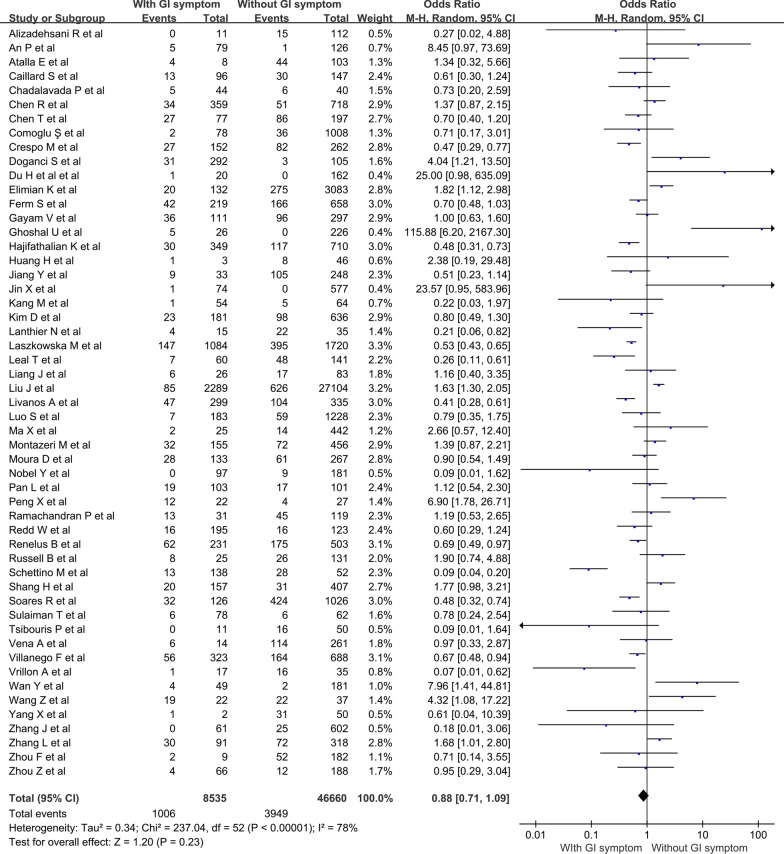
As shown in Fig. 2, presence of GI symptom was found to have no significant association with the mortality of COVID-19 (OR = 0.88; 95% CI 0.71–1.09; P = 0.23). There was substantial heterogeneity among the 53 studies included (I2 = 78%, P < 0.001).
Fig. 2.
Forest plots showing pooled odds ratio of gastrointestinal symptoms associated with the mortality of COVID-19
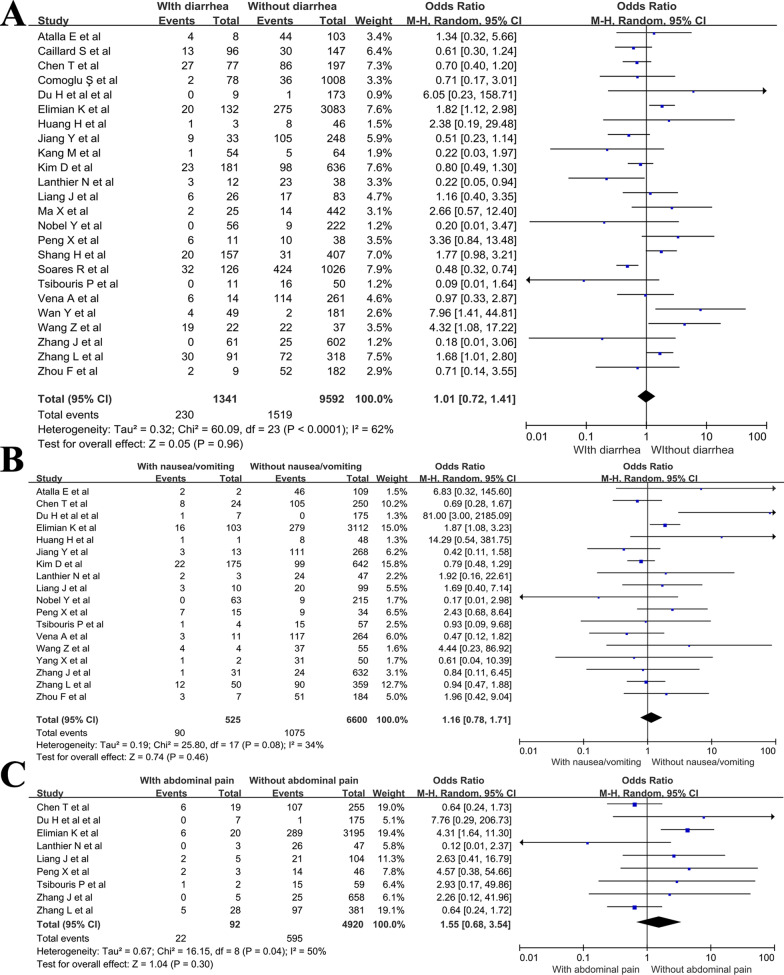
For individual GI symptoms, there were 24 studies reporting on diarrhea (Fig. 3a), 18 on nausea/vomiting (Fig. 3b), and 9 on abdominal pain (Fig. 3c). The pooled OR of diarrhea was 1.01 (95% CI 0.72–1.41; P = 0.96), of nausea/vomiting was 1.16 (95% CI 0.78–1.71; P = 0.46), and of abdominal pain was 1.55 (95% CI 0.68–3.54; P = 0.3). No substantial heterogeneity was found in the studies included for the analysis of nausea/vomiting and abdominal pain (I2 = 34% and 50%, respectively). While moderate heterogeneity was observed for diarrhea (I2 = 62%).
Fig. 3.
Forest plots showing pooled odds ratio of (A) diarrhea, (B) nausea/vomiting and (C) abdominal pain associated with the mortality of COVID-19
Subgroup Analysis
Since substantial heterogeneity was observed for GI symptom, we performed subgroup analysis to explore the source of heterogeneity. As shown in Table 3, as for studies conducted in different locations, the heterogeneity was moderate in the subgroups of Asia and America (I2 = 54.7% and 42.7%, respectively). The heterogeneity remained significant in the subgroups of Europe and other continents (I2 = 77% and 87.8%, respectively). Notably, data from Asian studies indicated that GI symptom was a significant risk factor for the death of COVID-19 patients (OR = 1.43, P = 0.01). On the contrary, American and European studies showed that GI symptom was associated with a lower mortality risk (OR = 0.64 and 0.4, respectively; P < 0.01 for both). The study location seemed to be a major source of heterogeneity. Meanwhile heterogeneity remained substantial in the other subgroups in terms of disease severity and population size, and GI symptom had no significant relevance with mortality in these subgroups. Nevertheless, in subgroup analysis Asian literatures indicate GI symptom is a significant risk factor for the mortality of COVID-19 (OR > 1, P < 0.05). Meanwhile European and American studies suggest that GI symptom is a significant protective factor (OR < 1, P < 0.05). The possible explanation for these contradictory observations has always been controversial. In an European research Crespo et al. found that patients with gastrointestinal COVID-19 phenotype recovered more frequently [19]. Several studies from the USA described that COVID-19 patients with GI symptoms were younger, with less comorbidity [33, 37]. Therefore, we further reviewed the literatures in our analysis. Among the literatures that had data on the age of patients with/without GI symptoms, all of the European and American literatures (n = 8 of 8, 100%) [15, 19, 33, 34, 37, 45, 46, 49] reported that patients with GI symptoms were younger than those without. And OR values were < 1 in 7 [15, 19, 33, 34, 37, 46, 49] of these 8 literatures. On the other hand, 7 [12, 16, 18, 25, 29, 36, 57] of 13 Asian studies [12, 16, 18, 25, 29, 30, 36, 38, 40, 43, 50, 52, 57] reported that patients with GI symptoms were older, and OR values were > 1 in 6[12, 16, 25, 29, 36, 57] of these 7 studies. It turns out that the studies carried out in different locations vary in the characteristics of included patients, especially in the age of patients with/without GI symptoms. Given that old age is an important risk factor for the death of COVID-19 patients [14], the discordance in the findings in different study locations may be due to the differences in the age of included patients.
Table 3.
Subgroup analysis based on study location, type of participants and population size
| Subgroups | No. of studies | No. of patients | OR and P value for mortality of different symptoms | ||||
|---|---|---|---|---|---|---|---|
| GI symptom | I 2 for GI symptom | Diarrhea | Nausea/vomiting | Abdominal pain | |||
| 1. Study location | |||||||
| 1.1 Asia | 28 | 39,501 (72%) | 1.43, P=0.01 | 54.7% | 1.32, P=0.21 | 1.3, P=0.36 | 1.07, P=0.85 |
| Sub-subgroups for studies in Asia: | |||||||
| 1.1.1 GI group older than non-GI group | 7 | 32,894 | 2.43, P<0.01 | 66.9% | |||
| 1.1.2 GI group younger than non-GI group | 6 | 3048 | 1.2, P=0.29 | 15.8% | |||
| 1.2 America | 12 | 8324 (15%) | 0.64, P<0.01 | 42.7% | 0.81, P=0.37 | 0.84, P=0.8 | NA |
| Sub-subgroups for studies in America: | |||||||
| 1.2.1 GI group older than non-GI group | NA | NA | NA | NA | |||
| 1.2.2 GI group younger than non-GI group | 5 | 3990 | 0.55, P<0.01 | 30.6% | |||
| 1.3 Europe | 10 | 2653 (5%) | 0.4, P<0.01 | 77% | 0.51, P=0.07 | 0.7, P=0.51 | 0.61, P=0.76 |
| Sub-subgroups for studies in Europe: | |||||||
| 1.3.1 GI group older than non-GI group | NA | NA | NA | NA | |||
| 1.3.2 GI group younger than non-GI group | 3 | 805 | 0.23, P<0.01 | 84% | |||
| 1.4 Other | 3 | 4767(8%) | 0.92, P=0.83 | 87.8% | 0.93, P=0.92 | NA | NA |
| 2. Only include critically ill patients? | |||||||
| 3.1 Yes | 6 | 1124(2%) | 1.42, P=0.36 | 74.6% | 1.3, P=0.45 | 0.92, P=0.71 | 0.75, P=0.45 |
| 3.2 No | 47 | 54,121 (98%) | 0.83, P=0.1 | 78.3% | 0.92, P=0.69 | 1.37, P=0.28 | 2.67, P=0.05 |
| 3. Population size | |||||||
| 4.1 <500 | 36 | 7436 (13%) | 0.93, P=0.66 | 72.1% | 1.05, P=0.82 | 1.19, P=0.51 | 1.04, P=0.92 |
| 4.2 >=500 | 17 | 47,809 (87%) | 0.84, P=0.25 | 85.7% | 0.94, P=0.83 | 1.16, P=0.68 | NA |
| 4. Average age of GI group and non-GI group | |||||||
| 4.1 GI group older than non-GI group | 8 | 33,294 (60%) | 1.89, P=0.02 | 69% | |||
| 4.2 GI group younger than non-GI group | 14 | 7843 (14%) | 0.61, P=0.01 | 80% | |||
| 4.3 Unknown | 31 | 14,058 (26%) | 0.89, P=0.36 | 64% | |||
OR, odds ratio; GI, gastrointestinal; NA, not available
To demonstrate the above finding, we explored the age related sub-analysis by study region. As shown in Table 3, the studies in Asia, America and Europe were divided into subgroups based on the age difference of included patients. Consistent with the age distribution, GI symptom was found to be a significant risk factor for mortality (OR > 1 and P < 0.05) in the subgroup that GI group was older than non-GI group (Table 3, subgroup 1.1.1). Meanwhile, GI symptom was a significant protective factor (OR < 1 and P < 0.05) in the subgroups that GI group was younger than non-GI group (Table 3, subgroup 1.2.2 and subgroup 1.3.2). Since the number of studies in each subgroup was quite small, we also performed subgroup analysis based on the age difference of included patients irrespective of the study region. As shown in Table 3, three additional subgroups were determined: [1] subgroup 4.1 included the studies in which the patients in GI group were older than those in non-GI group; [2] subgroup 4.2 included the studies in which the patients in GI group were younger than those in non-GI group; [3] subgroup 4.3 included the studies without available information on the age of patients in GI and non-GI group. Interestingly, in subgroup 4.1, GI symptom was a significant risk factor for mortality (OR = 1.89, P = 0.02). On the contrary, in subgroup 4.2, GI symptom was a significant protective factor for mortality (OR = 0.61, P = 0.01). This finding further supports our deduction that the difference in the age of GI and non-GI groups leads to the discordance in the findings in different study locations. The forest plots of these additional subgroup analysis are available in the Supplementary Material (Additional file 2: Figs. S1 to S6).
As for individual symptoms including diarrhea, nausea/vomiting and abdominal pain, none of these symptoms showed significant correlation with mortality (P of OR > 0.05 for all subgroups).
Age stratification analysis
To further explore the relationship of GI symptom with mortality in different age groups, we performed additional age stratification analysis. As shown in Table 4, we stratified the studies into 5 groups according to the average age of the study population: 0–39, 40–49, 50–59, 60–69 and 70~. We expected that with the increased population age, GI symptom might be a risk factor from mortality. However, the actual results were contrary to our expectation: in younger populations (0–39, 40–49 and 50–59), GI symptom seemed to be a risk factor (OR > 1) while in older populations (60–69 and 70~) GI symptom showed a significant protective effect (OR < 1 and P < 0.05). The forest plots are available in Additional file 2. To clarify this finding, we reviewed the included studies again. We found that the average age of GI group was older than non-GI group in most studies (83.3%) with younger populations (40–49); meanwhile the average age of GI group was younger than non-GI group in all studies (100%) with older populations (60–69 and 70~). Overall, we supposed that the potential patients selection bias in the age of patients with/without GI symptom led to the discordance in the results.
Table 4.
Age stratification analysis
| Age stratification | No. of studies | OR and 95% CI of GI symptom for mortality | P value of OR | No. of studies that average age: GI group >non GI group | No. of studies that average age: GI group <non-GI group |
|---|---|---|---|---|---|
| 0–39 | 3 | 2.36 [0.88; 6.33] | 0.088 | NA | NA |
| 40–49 | 8 | 2.22 [0.96; 5.11] | 0.061 | 5 (83.3%) | 1 (16.7%) |
| 50–59 | 15 | 1.09 [0.85; 1.40] | 0.517 | 3 (33.3%) | 6 (66.7%) |
| 60–69 | 18 | 0.71 [0.54; 0.95] | 0.02 | 0 (0%) | 6 (100%) |
| 70~ | 7 | 0.40 [0.21; 0.76] | 0.006 | 0 | 1 (100%) |
OR, odds ratio; CI, confidence interval; GI, gastrointestinal; NA, data are not available because the studies in the subgroups did not report the age information of patients with/without GI symptoms
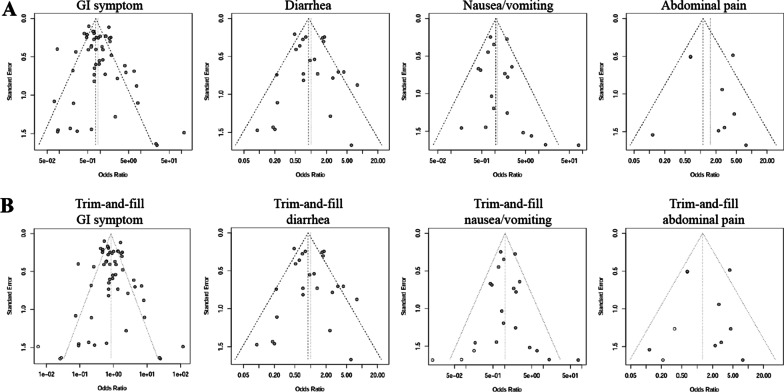
Publication bias analysis
The funnel plots (Fig. 4 a) were found to be slightly asymmetric for GI symptom and nausea/vomiting. As shown in Table 5, Egger’s regression test also revealed publication bias for both factors (P = 0.05 and 0.04, respectively). Thus we performed trim-and-fill method to estimate missing studies (Fig. 4b) so as to make pooled OR more reliable. The P values of Egger’s test were > 0.05 after trim-and-fill adjustment (Table 5), indicating that the publication bias was reduced. After adjustment for presumed un-published reports after trim-and-fill analysis (Table 5), GI symptoms and individual symptoms remained uncorrelated with the death risk of COVID-19 (OR close to 1, and P > 0.05 for all).
Fig. 4.
Funnel plots for evaluation of publication bias. A shows the funnel plots of gastrointestinal symptoms, diarrhea, nausea/vomiting and abdominal pain. B shows the funnel plots after trim-and-fill method
Table 5.
Publication bias analysis
| No. of included literatures | OR | P value for OR | P value of Egger’s test | |
|---|---|---|---|---|
| Original data | ||||
| GI symptom | 53 | 0.88 | 0.23 | 0.05 |
| Diarrhea | 24 | 1.01 | 0.96 | 0.55 |
| Nausea/vomiting | 18 | 1.16 | 0.46 | 0.04 |
| Abdominal pain | 9 | 1.55 | 0.3 | 0.61 |
| After trim-and-fill | ||||
| GI symptom | 56 | 0.84 | 0.11 | 0.82 |
| Diarrhea | 24 | 1.01 | 0.96 | 0.55 |
| Nausea/vomiting | 21 | 1.02 | 0.91 | 0.9 |
| Abdominal pain | 11 | 1.28 | 0.51 | 0.94 |
OR, odds ratio; GI, gastrointestinal
Sensitivity analysis
As depicted in Table 6, the results of GI symptom showed good stability with all OR estimates (ranging from 0.86 to 0.92) within the 95% CI of pooled OR. The OR estimates of diarrhea, nausea/vomiting and abdominal pain were also stable when omitting one study at a time. All of the estimates showed no statistical significance, which were also in accordance with the major conclusion that the relationship of GI symptoms and mortality was not significant.
Table 6.
Sensitivity analysis
| OR and P value for mortality of different symptoms | ||||
|---|---|---|---|---|
| Study omitted | GI symptom | Diarrhea | Nausea/vomiting | Abdominal pain |
| Omitting Alizadehsani R et al. | 0.88, P=0.25 | NA | NA | NA |
| Omitting An P et al. | 0.86, P=0.17 | NA | NA | NA |
| Omitting Atalla E et al. | 0.87, P=0.22 | 1, P=1 | 1.13, P=0.55 | NA |
| Omitting Caillard S et al. | 0.89, P=0.28 | 1.04, P=0.81 | NA | NA |
| Omitting Chadalavada P et al. | 0.88, P=0.25 | NA | NA | NA |
| Omitting Chen R et al. | 0.87, P=0.2 | NA | NA | NA |
| Omitting Chen T et al. | 0.89, P=0.27 | 1.04, P=0.83 | 1.23, P=0.33 | 1.91, P=0.17 |
| Omitting Comoglu Ş et al. | 0.88, P=0.25 | 1.02, P=0.9 | NA | NA |
| Omitting Crespo M et al. | 0.9, P=0.31 | NA | NA | NA |
| Omitting Doganci S et al. | 0.86, P=0.15 | NA | NA | NA |
| Omitting Du H et al. et al | 0.87, P=0.18 | 0.99, P=0.96 | 1.08, P=0.63 | 1.42, P=0.42 |
| Omitting Elimian K et al. | 0.86, P=0.16 | 0.96, P=0.82 | 1.05, P=0.82 | 1.05, P=0.9 |
| Omitting Ferm S et al. | 0.88, P=0.28 | NA | NA | NA |
| Omitting Gayam V et al. | 0.88, P=0.23 | NA | NA | NA |
| Omitting Ghoshal U et al. | 0.86, P=0.15 | NA | NA | NA |
| Omitting Hajifathalian K et al. | 0.9, P=0.32 | NA | NA | NA |
| Omitting Huang H et al. | 0.87, P=0.21 | 1, P=0.98 | 1.12, P=0.57 | NA |
| Omitting Jiang Y et al. | 0.89, P=0.29 | 1.05, P=0.77 | 1.23, P=0.3 | NA |
| Omitting Jin X et al. | 0.87, P=0.18 | NA | NA | NA |
| Omitting Kang M et al. | 0.89, P=0.27 | 1.04, P=0.83 | NA | NA |
| Omitting Kim D et al. | 0.88, P=0.26 | 1.03, P=0.88 | 1.25, P=0.32 | NA |
| Omitting Lanthier N et al. | 0.9, P=0.31 | 1.06, P=0.72 | 1.15, P=0.5 | 1.77, P=0.16 |
| Omitting Laszkowska M et al. | 0.9, P=0.32 | NA | NA | NA |
| Omitting Leal T et al. | 0.9, P=0.34 | NA | NA | NA |
| Omitting Liang J et al. | 0.87, P=0.22 | 1, P=0.99 | 1.14, P=0.54 | 1.46, P=0.42 |
| Omitting Liu J et al. | 0.86, P=0.14 | NA | NA | NA |
| Omitting Livanos A et al. | 0.9, P=0.33 | NA | NA | NA |
| Omitting Luo S et al. | 0.88, P=0.25 | NA | NA | NA |
| Omitting Ma X et al. | 0.87, P=0.19 | 0.98, P=0.9 | NA | NA |
| Omitting Montazeri M et al. | 0.87, P=0.19 | NA | NA | NA |
| Omitting Moura D et al. | 0.88, P=0.24 | NA | NA | NA |
| Omitting Nobel Y et al. | 0.89, P=0.27 | 1.03, P=0.87 | 1.19, P=0.37 | NA |
| Omitting Pan L et al. | 0.87, P=0.22 | NA | NA | NA |
| Omitting Peng X et al. | 0.85, P=0.13 | 0.97, P=0.84 | 1.1, P=0.64 | 1.42, P=0.44 |
| Omitting Ramachandran P et al. | 0.87, P=0.21 | NA | NA | NA |
| Omitting Redd W et al. | 0.89, P=0.28 | NA | NA | NA |
| Omitting Renelus B et al. | 0.89, P=0.29 | NA | NA | NA |
| Omitting Russell B et al. | 0.86, P=0.18 | NA | NA | NA |
| Omitting Schettino M et al. | 0.92, P=0.43 | NA | NA | NA |
| Omitting Shang H et al. | 0.86, P=0.17 | 0.97, P=0.85 | NA | NA |
| Omitting Soares R et al. | 0.9, P=0.32 | 1.08, P=0.66 | NA | NA |
| Omitting Sulaiman T et al. | 0.88, P=0.24 | NA | NA | NA |
| Omitting Tsibouris P et al. | 0.89, P=0.27 | 1.04, P=0.83 | 1.17, P=0.45 | 1.49, P=0.38 |
| Omitting Vena A et al. | 0.88, P=0.23 | 1.01, P=0.95 | 1.23, P=0.32 | NA |
| Omitting Villanego F et al. | 0.89, P=0.29 | NA | NA | NA |
| Omitting Vrillon A et al. | 0.89, P=0.3 | NA | NA | NA |
| Omitting Wan Y et al. | 0.86, P=0.15 | 0.96, P=0.78 | NA | NA |
| Omitting Wang Z et al. | 0.86, P=0.16 | 0.96, P=0.8 | 1.13, P=0.53 | NA |
| Omitting Yang X et al. | 0.88, P=0.24 | NA | 1.18, P=0.43 | NA |
| Omitting Zhang J et al. | 0.89, P=0.26 | 1.03, P=0.86 | 1.18, P=0.43 | 1.52, P=0.35 |
| Omitting Zhang L et al. | 0.86, P=0.17 | 0.97, P=0.86 | 1.21, P=0.4 | 1.91, P=0.17 |
| Omitting Zhou F et al. | 0.88, P=0.24 | 1.02, P=0.91 | 1.13, P=0.56 | NA |
| Omitting Zhou Z et al. | 0.88, P=0.23 | NA | NA | NA |
OR, odds ratio; GI, gastrointestinal; NA, not available
Discussion
Several previous literatures have revealed that the GI symptoms might be associated with the prognosis with COVID-19 [7]. However, in the current meta-analysis, neither GI symptoms nor individual symptoms including diarrhea, nausea/vomiting and abdominal pain shows a significant relevance with the mortality of COVID-19 patients. Besides, the present data suggest that older age might be a significant predictor of poor prognosis in COVID-19 patients with GI symptoms. Based on the current available data, there is no convincing evidence that GI symptoms may be associated with higher risk of mortality in COVID-19 patients.
The prognosis index of COVID-19 includes several aspects such as admission to intensive care unit, low pulse oxygen saturation, development of acute respiratory distress syndrome and death of disease. A large number of previous studies investigated the relationship of GI symptoms and the severity of COVID-19, but not mortality [64–66]. This is mainly due to the limited death cases in the first few months of disease outbreak. GI symptoms have been found common in COVID-19 patients in numerous studies [67], and are considered to indicate the involvement of digestive system by virus [68]. Xiao et al. identified the infection of SARS-CoV-2 in the cytoplasm of gastric, duodenal, and rectum glandular epithelial cell by immunofluorescent staining of gastrointestinal tissues from hospitalized patients infected with SARS-CoV2 [6]. There have been views that GI symptoms might indicate a more invasive pattern of virus [7, 8, 69]. Quite a few clinical researches have observed the GI symptoms as a risk factor for disease severity of COVID-19. Jin et al. [29] found that for patients with GI symptoms (n = 74), 22.97% developed severe/critical type of disease; while for patients without GI symptoms (n = 577), only 8.14% were severe/critical type (P < 0.001). The meta-analysis by Mao et al. also found GI symptoms a significant risk factor for disease severity (OR = 3.97; 95% CI 1.49–10.62; P = 0.006) [8]. They included 4 studies to explore the influence of GI symptoms on mortality. Although they yielded an OR of 1.21, it was without statistical significance (95% CI 0.68–2.16; P = 0.52). The limited number of included studies and death cases (n = 29) might restrict the statistical power. However, with more abundant patients who met the endpoint in out meta-analysis, the correlation of GI symptoms in COVID-19 patients and mortality is still non-significant.
Despite the points of view highlighting the importance of GI symptoms in COVID-19, there exist arguments. In another meta-analysis by Wang et al., no significant differences were detected in the prevalence of diarrhea (OR = 1.24; 95% CI 0.90 to 1.72; P = 0.19) and nausea/vomiting (OR = 1.24; 95% CI 0.57 to 2.69; P = 0.58) between non-severe and severe COVID-19 patients [70]. They held the view that GI symptoms were not associated with the COVID-19 progression, and SARS-CoV-2-induced liver injury deserved more attention [70]. Nobel et al. proposed that gastrointestinal symptoms were associated with a more indolent form of COVID-19 based on their clinical observation [42]. Although the digestive system can be involved, most of the symptoms are mild and can be improved by supportive treatments, thus might have less impact upon disease severity. On the other hands, the respiratory tract is more commonly involved in COVID-19 and most patients died of respiratory failure. The gastrointestinal involvement might not be a prominent factor compared with other underlying diseases or respiratory failure.
There are several strengths of this meta-analysis. To the best of our knowledge, up to now this is a relatively large meta-analysis on the specific influence of GI symptoms on the mortality of COVID-19. We have included a large number of literatures, with patient population above fifty thousand and 4,955 non-survivors among them, spanning five continents. We have also excluded studies with small sample size (< 20), and most studies included in calculating the pooled OR estimates had more than 100 patients. Besides, the publication bias has been adjusted and the outcome remains the same, which make the conclusion more reliable.
Old age have been found to be independently associated with mortality in quite a few investigations [14]. As is known old age is related with increased incidence of comorbidities, cognitive impairment, dependence, and frailty. The immuno-senescence in the elderly might also lead to a different reaction against infections. The recent reports and the present meta-analysis have emphasized the differences in mortality for patients of a certain age exhibiting GI symptoms. It has been reported that adults over 60 years of age account for 96% of deaths caused by COVID-19 [71] A significant portion of COVID-19 patients have digestive symptoms, mostly at presentation. Therefore, GI symptoms should also be taken into account so as to maintain a high level of suspicion to reach an early diagnosis and set up infection control measures to improve the prognosis of elderly patients with COVID-19.
This meta-analysis has two potential limitations. As mentioned, there might exist potential patients selection bias in the age of patients with/without GI symptoms in different countries. This might lead to the discordance in the results of different study subgroups. On the other hand, currently there are no studies designed to prospectively compare the mortality of COVID-19 patients with/without GI symptoms, thus we have to include retrospective reports, which might limit the quality of evidence. Future prospective observational studies are needed to further clarify the role of GI symptoms in COVID-19.
Conclusions
In summary, we have shown in this meta-analysis that the presence of GI symptoms is not associated with the risk of mortality in COVID-19 patients. The prognostic value of GI symptoms in COVID-19 might not be as significant as other factors such as age, concomitant underlying diseases and respiratory manifestations. Further investigations are needed to clarify the role of gastrointestinal involvement in the disease course of COVID-19, and to explore its therapeutic implications.
Supplementary Information
Additional 1. Lists all the extracted data which were used to generate all the results of this study.
Additional 2. Contains the supplementary forest plots and the corresponding figure legends.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
corona virus disease 2019
- GI
gastrointestinal
- OR
odds ratio
- NOS
Newcastle-Ottawa Scale
- CI
confidence interval
Authors’ contributions
YW, YmL, YZ and YL collected the literatures and extracted the data. YW and YmL did the data analysis and drafted the manuscript. YlL designed the study and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (Grant Numbers 81873549 and 82070539). The sponsors have no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The dataset generated and analysed during the current study is available in the Additional file 1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Wang and Yimin Li contributed equally.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 3.Remes-Troche JM, Ramos-de-la-Medina A, Manriquez-Reyes M, Martinez-Perez-Maldonado L, Lara EL, Solis-Gonzalez MA. Initial Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 112 Patients From Veracruz in Southeastern Mexico. Gastroenterology. 2020;159(3):1179–81. doi: 10.1053/j.gastro.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer S, et al. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159(2):775–77. doi: 10.1053/j.gastro.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135–40. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–33. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng T, Yang C, Wang HY, Chen X, Yu L, Wu ZL, et al. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. 2020;92(11):2735–41. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–78. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1. 0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000.
- 11.Alizadehsani R, Sani ZA, Behjati M, Roshanzamir Z, Hussain S, Abedini N, et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J Med Virol. 2021;93(4):2307–20. doi: 10.1002/jmv.26699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An P, Chen H, Ren H, Su J, Ji M, Kang J, et al. Gastrointestinal Symptoms Onset in COVID-19 Patients in Wuhan, China. Dig Dis Sci. 2021;66(10):3578–87. doi: 10.1007/s10620-020-06693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atalla E, Zhang R, Shehadeh F, Mylona EK, Tsikala-Vafea M, Kalagara S, et al. Clinical Presentation, Course, and Risk Factors Associated with Mortality in a Severe Outbreak of COVID-19 in Rhode Island, USA, April-June 2020. Pathogens. 2020;10(1):8. doi: 10.3390/pathogens10010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–58. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadalavada P, Padbidri V, Garg R, Alomari M, Babar A, Kewan T, et al. Transaminases are Potential Biomarkers of Disease Severity in COVID-19 Patients: A Single-Center Experience. Cureus. 2020;12(11):e11555. doi: 10.7759/cureus.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Yu YL, Li W, Liu Y, Lu JX, Chen F, et al. Gastrointestinal Symptoms Associated With Unfavorable Prognosis of COVID-19 Patients: A Retrospective Study. Front Med (Lausanne) 2020;7:608259. doi: 10.3389/fmed.2020.608259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comoglu Ş, Öztürk S, Kant A, Arslan M, Karakoc HN, Yilmaz G. Evaluation of Diarrhea in Patients with COVID-19. Dig Dis. 2021;39(6):622–25. doi: 10.1159/000515521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crespo M, Mazuecos A, Rodrigo E, Gavela E, Villanego F, Sánchez-Alvarez E, et al. Respiratory and Gastrointestinal COVID-19 Phenotypes in Kidney Transplant Recipients. Transplantation. 2020;104(11):2225–33. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 20.Doganci S, Ince ME, Ors N, Yildirim AK, Sir E, Karabacak K, et al. A new COVID-19 prediction scoring model for in-hospital mortality: experiences from Turkey, single center retrospective cohort analysis. Eur Rev Med Pharmacol Sci. 2020;24(19):10247–57. doi: 10.26355/eurrev_202010_23249. [DOI] [PubMed] [Google Scholar]
- 21.Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021;76(2):510–32. doi: 10.1111/all.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elimian KO, Ochu CL, Ebhodaghe B, Myles P, Crawford EE, Igumbor E, et al. Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: retrospective cohort study. BMJ Open. 2020;10(12):e044079. doi: 10.1136/bmjopen-2020-044079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferm S, Fisher C, Pakala T, Tong M, Shah D, Schwarzbaum D, et al. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 patients in Queens, NY. Clin Gastroenterol Hepatol. 2020;18(10):2378–79. doi: 10.1016/j.cgh.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayam V, Chobufo MD, Merghani MA, Lamichhane S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2021;93(2):812–19. doi: 10.1002/jmv.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghoshal UC, Ghoshal U, Mathur A, Singh RK, Nath A, Garg A, et al. The Spectrum of Gastrointestinal Symptoms in Patients With Coronavirus Disease-19: Predictors, Relationship With Disease Severity, and Outcome. Clin Transl Gastroenterol. 2020;11(12):e00259. doi: 10.14309/ctg.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, et al. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159(3):1137–40. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Zhang M, Chen C, Zhang H, Wei Y, Tian J, et al. Clinical Characteristics of COVID-19 in patients with pre-existing ILD: A retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92(11):2742–50. doi: 10.1002/jmv.26174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Abudurexiti S, An MM, Cao D, Wei J, Gong P. Risk factors associated with 28-day all-cause mortality in older severe COVID-19 patients in Wuhan, China: a retrospective observational study. Sci Rep. 2020;10(1):22369. doi: 10.1038/s41598-020-79508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–09. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang MK, Kim KO, Kim MC, Cho JH, Kim SB, Park JG, et al. Clinical characteristics of coronavirus disease 2019 patients with diarrhea in Daegu. Korean J Intern Med. 2020;35(6):1261–69. doi: 10.3904/kjim.2020.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19(7):1469–79. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanthier N, Mahiat C, Henrard S, Stärkel P, Gilard I, De Brauwer I, et al. Gastro-intestinal symptoms are associated with a lower in-hospital mortality rate in frail older patients hospitalized for COVID-19. Acta Gastroenterol Belg. 2021;84(1):135–36. doi: 10.51821/84.1.824. [DOI] [PubMed] [Google Scholar]
- 33.Laszkowska M, Faye AS, Kim J, Truong H, Silver ER, Ingram M, et al. Disease Course and Outcomes of COVID-19 Among Hospitalized Patients With Gastrointestinal Manifestations. Clin Gastroenterol Hepatol. 2021;19(7):1402–09. doi: 10.1016/j.cgh.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal T, Costa E, Arroja B, Goncalves R, Alves J. Gastrointestinal manifestations of COVID-19: results from a European centre. Eur J Gastroenterol Hepatol. 2021;33(5):691–94. doi: 10.1097/MEG.0000000000002152. [DOI] [PubMed] [Google Scholar]
- 35.Liang J, Jin G, Liu T, Wen J, Li G, Chen L, et al. Clinical characteristics and risk factors for mortality in cancer patients with COVID-19. Front Med. 2021;15(2):264–74. doi: 10.1007/s11684-021-0845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Tao L, Liu X, Yao H, Yu S, Wang Q, et al. GI symptoms and fever increase the risk of severe illness and death in patients with COVID-19. Gut. 2021;70(2):442–44. doi: 10.1136/gutjnl-2020-321751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livanos AE, Jha D, Cossarini F, Gonzalez-Reiche AS, Tokuyama M, Aydillo T, et al. Intestinal Host Response to SARS-CoV-2 Infection and COVID-19 Outcomes in Patients With Gastrointestinal Symptoms. Gastroenterology. 2021;160(7):2435–50. doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo S, Deng Z, Zhang X, Pan Z, Xu H. Clinical characteristics and outcomes of 2019 novel coronavirus disease patients presenting with initial gastrointestinal symptoms in Wuhan, China: A retrospective cohort study. J Gastroenterol Hepatol. 2021;36(3):694–99. doi: 10.1111/jgh.15199. [DOI] [PubMed] [Google Scholar]
- 39.Ma X, Li A, Jiao M, Shi Q, An X, Feng Y, et al. Characteristic of 523 COVID-19 in Henan Province and a Death Prediction Model. Front Public Health. 2020;8:475. doi: 10.3389/fpubh.2020.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montazeri M, Maghbouli N, Jamali R, Sharifi A, Pazoki M, Salimzadeh A, et al. Clinical Characteristics of COVID-19 Patients with Gastrointestinal Symptoms. Arch Iran Med. 2021;24(2):131–38. doi: 10.34172/aim.2021.21. [DOI] [PubMed] [Google Scholar]
- 41.Moura DTH. Gastrointestinal Manifestations and Associated Health Outcomes of COVID-19: A Brazilian Experience From the Largest South American Public Hospital. Clin (Sao Paulo) 2020;75:e2271. doi: 10.6061/clinics/2020/e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. J Gastroenterol. 2020;159(1):373–5. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–73. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng X, Chen Y, Deng L, Liu Q, Li Q, Xiong J, et al. Clinical features of critically ill patients infected with SARS-CoV-2 outside Wuhan with and without diabetes. Int J Diabetes Dev Ctries. 2020;5:1–9. doi: 10.1007/s13410-020-00888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramachandran P, Onukogu I, Ghanta S, Gajendran M, Perisetti A, Goyal H, et al. Gastrointestinal Symptoms and Outcomes in Hospitalized Coronavirus Disease 2019 Patients. Dig Dis. 2020;38(5):373–79. doi: 10.1159/000509774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients with SARS-CoV-2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020;159(2):765–67. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renelus BD, Khoury N, Chandrasekaran K, Bekele E, Briggs WM, Jamorabo DS. Hospitalized coronavirus disease-2019 (COVID-19) patients with gastrointestinal symptoms have improved survival to discharge. Dig Liver Dis. 2020;52(12):1403–06. doi: 10.1016/j.dld.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell B, Moss C, Papa S, Irshad S, Ross P, Spicer J, et al. Factors Affecting COVID-19 Outcomes in Cancer Patients: A First Report From Guy’s Cancer Center in London. Front Oncol. 2020;10:1279. doi: 10.3389/fonc.2020.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schettino M, Pellegrini L, Picascia D, Saibeni S, Bezzio C, Bini F, et al. Clinical Characteristics of COVID-19 Patients With Gastrointestinal Symptoms in Northern Italy: A Single-Center Cohort Study. Am J Gastroenterol. 2021;116(2):306–10. doi: 10.14309/ajg.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 50.Shang H, Bai T, Chen Y, Huang C, Zhang S, Yang P, et al. Outcomes and implications of diarrhea in patients with SARS-CoV-2 infection. Scand J Gastroenterol. 2020;55(9):1049–56. doi: 10.1080/00365521.2020.1800078. [DOI] [PubMed] [Google Scholar]
- 51.Soares RCM, Mattos LR, Raposo LM. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184–90. doi: 10.4269/ajtmh.20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulaiman T, Algharawi AA, Idrees M, Alzaidy RH, Faris K, Cullingford G, et al. The prevalence of gastrointestinal symptoms among patients with COVID-19 and the effect on the severity of the disease. JGH Open. 2020;4(6):1162–66. doi: 10.1002/jgh3.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsibouris P, Ekmektzoglou K, Agorogianni A, Kalantzis C, Theofanopoulou A, Toumbelis K, et al. Gastrointestinal involvement in COVID-19 patients: a retrospective study from a Greek COVID-19 referral hospital. Ann Gastroenterol. 2020;33(5):465–72. doi: 10.20524/aog.2020.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vena A, Giacobbe DR, Di Biagio A, Mikulska M, Taramasso L, De Maria A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537–44. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villanego F, Mazuecos A, Pérez-Flores IM, Moreso F, Andrés A, Jiménez-Martín C, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: Analysis of the Spanish Registry. Am J Transplant. 2021;21(7):2573–82. doi: 10.1111/ajt.16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vrillon A, Hourregue C, Azuar J, Grosset L, Boutelier A, Tan S, et al. COVID-19 in Older Adults: A Series of 76 Patients Aged 85 Years and Older with COVID-19. J Am Geriatr Soc. 2020;68(12):2735–43. doi: 10.1111/jgs.16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5(6):534–35. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang ZH, Shu C, Ran X, Xie CH, Zhang L. Critically Ill Patients with Coronavirus Disease 2019 in a Designated ICU: Clinical Features and Predictors for Mortality. Risk Manag Healthc Policy. 2020;13:833–45. doi: 10.2147/RMHP.S263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–72. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Han C, Zhang S, Duan C, Shang H, Bai T, et al. Diarrhea and altered inflammatory cytokine pattern in severe coronavirus disease 2019: Impact on disease course and in-hospital mortality. J Gastroenterol Hepatol. 2021;36(2):421–29. doi: 10.1111/jgh.15166. [DOI] [PubMed] [Google Scholar]
- 62.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158(8):2294–97. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–39. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–73. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. [DOI] [PMC free article] [PubMed]
- 67.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–37. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21(1):3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44(5):653–61. doi: 10.1016/j.clinre.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casas-Deza D, Bernal-Monterde V, Aranda-Alonso AN, Montil-Miguel E, Julian-Gomara AB, Letona-Gimenez L, et al. Age-related mortality in 61,993 confirmed COVID-19 cases over three epidemic waves in Aragon, Spain. Implications for vaccination programmes. PLoS ONE. 2021;16(12):e0261061. doi: 10.1371/journal.pone.0261061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional 1. Lists all the extracted data which were used to generate all the results of this study.
Additional 2. Contains the supplementary forest plots and the corresponding figure legends.
Data Availability Statement
The dataset generated and analysed during the current study is available in the Additional file 1.