Abstract
Objective:
To describe interventions that target patient, provider, and system barriers to sedative-hypnotic (SH) deprescribing in the community and suggest strategies for healthcare teams.
Data Sources:
Ovid MEDLINE ALL and EMBASE Classic + EMBASE (March 10, 2021).
Study Selection and Data Extraction:
English-language studies in primary care settings.
Data Synthesis:
20 studies were themed as patient-related and prescriber inertia, physician skills and awareness, and health system constraints. Patient education strategies reduced SH dose for 10% to 62% of participants, leading to discontinuation in 13% to 80% of participants. Policy interventions reduced targeted medication use by 10% to 50%.
Relevance to Patient Care and Clinical Practice:
Patient engagement and empowerment successfully convince patients to deprescribe chronic SHs. Quality improvement strategies should also consider interventions directed at prescribers, including education and training, drug utilization reviews, or computer alerts indicating a potentially inappropriate prescription by medication, age, dose, or disease. Educational interventions were effective when they facilitated patient engagement and provided information on the harms and limited evidence supporting chronic use as well as the effectiveness of alternatives. Decision support tools were less effective than prescriber education with patient engagement, although they can be readily incorporated in the workflow through prescribing software.
Conclusions:
Several strategies with demonstrated efficacy in reducing SH use in community practice were identified. Education regarding SH risks, how to taper, and potential alternatives are essential details to provide to clinicians, patients, and families. The strategies presented can guide community healthcare teams toward reducing the community burden of SH use.
Keywords: sedatives, hypnotics, primary care, prescription, deprescribing
Introduction
In community practice, sedatives and hypnotics (SH), including certain benzodiazepines and nonbenzodiazepine GABA-receptor agonists, as well as drugs with sedating adverse effects, are frequently prescribed, especially for insomnia.1-4 Systematic reviews and clinical practice guidelines for insomnia suggest that these prescriptions should primarily be of short duration because of insufficient evidence to support extended use, evidence of harm, and availability of safe and effective nonpharmacological alternatives (eg, cognitive behavioral therapy for insomnia [CBTi]).5-10 However, chronic SH utilization in the community is high, with reported rates between 3% and 17%.1-3,11-13
SH exposure is associated with serious adverse events, including falls, cognitive impairment, and increased mortality, and extended use of some classes may be associated with drug dependence (eg, benzodiazepines).14-17 Professional societies include SH on their list of potentially inappropriate or high-risk medications for insomnia management for older adults and endorse reduction of SH use to minimize the risk of adverse events.18-20 Recent observational data suggest that prescribing practices have shifted from traditional benzodiazepines to newer Z-drugs and other sedating medication classes (eg, off-label use of quetiapine and trazodone) because these newer medications are falsely perceived as safer alternatives.1,2,21-25
Intervention studies and systematic reviews have shown the plausibility of reducing SH utilization with strategies such as patient education, prescribing effective nonpharmacological alternatives, and tapering or deprescribing in various patient care settings. There are specific patient- and health care provider–related barriers to applying these interventions in the outpatient setting.26-28 Patient-related barriers include being unaware of medication-related harms, developing psychological dependence, and being unable to find effective alternatives. 26 The prescriber-patient relationship can also create barriers such as when prescribers perceive that patients are expectant of a SH prescription and ambivalent or reluctant to taper or stop. 27 Prescriber inertia is often a result of health care system constraints, such as difficulty finding the time to assess the appropriateness of medications not directly related to the presenting complaint.27,28 Furthermore, health care systems and regulatory policies may permit multiple months to be dispensed at once, thus limiting the opportunity to review SH prescription use. Prescriber self-efficacy can be a barrier if prescribers are unaware of the evidence or clinical tools to guide tapering, deprescribing, and substitution with nondrug therapy. 27 Finally, access to effective nonpharmacological therapies such as CBTi may be limited depending on the resources available to the patient or provider.27,28 The primary objective of this narrative review is to describe interventions that target patient, health care provider, and system barriers to reduce SH use for adults in primary care. Our secondary objectives are to describe the impact of these identified interventions on reported outcomes and provide strategies for quality improvement (QI) teams to use these interventions to reduce SH use in primary care.
Methods
Data Sources
With the assistance of a Medical Information Specialist, we conducted an electronic search of Ovid MEDLINE ALL, EMBASE Classic + EMBASE (inception to June 15, 2021) to identify studies that investigated interventions to reduce SH use.
Study Selection and Data Extraction
The search strategy was previously published; articles were sorted into those that applied to the inpatient setting and those for the outpatient setting (the focus of this article). 29 We sought studies that tested an intervention in adult participants to limit SH use (either reduced initiation of new prescriptions, drug tapering, or deprescribing) in the community, outpatient, or primary care setting. We included studies that also addressed other drugs classes (eg, proton pump inhibitors, antihypertensives) if SH results were individually reported. We excluded studies that (1) were set specifically in a hospital, long-term care, or psychiatric setting; (2) focused on pediatric populations; (3) focused specifically on alcohol withdrawal or seizure management; or (4) were not published in English. We extracted (TM, CT, HJC, EG, FK, AV) the details of the studies using a standardized case report form. Findings were summarized (LB, JT) as 3 broad themes based on the barriers addressed: (1) patient-related barriers and prescriber inertia; (2) physician skills and awareness; and (3) health system constraints.
Results
We (LB, CS) screened the 5286 citations identified from the electronic search, of which 22 citations met inclusion criteria: 20 studies and 2 citations were long-term follow-up of the included studies (Appendix A, available online).
Data Synthesis
Included studies were themed and are presented as patient-related and prescriber inertia (Table 1),30-40 physician skills and awareness (Table 2),41-47 and health-system constraints (Table 3).48-52 Among the identified studies, we noted considerable heterogeneity in study design, sample size, population assessed, and outcomes reported. Of the 20 studies, 12 (60%) were randomized controlled or cluster randomized trials. The majority of studies focused specifically on benzodiazepine use; however, more recent studies included a broad range of SH drugs or targeted multiple drug classes, including SH. Although many of the interventions were shown to successfully reduce SH use in the community setting, the majority of interventions (1) were tested in a single study or with a limited sample size or duration of exposure, (2) were studied in a specific setting or demographic that may limit generalizability of findings, (3) did not examine clinical outcomes to allow understanding of the full impact of the intervention, and (4) may have included other indications for SH beyond insomnia.
Table 1.
Studies Evaluating Patient Education to Overcome Patient Barriers to Reduce SH Use in Community-Dwelling Adults.
| Study description | Intervention | Results | Number needed to treat |
|---|---|---|---|
| Subcategory: patient education delivered by physician | |||
| Morgan et al (2002)
30
• Design: prospective pre-post intervention • Duration: 1 year preintervention, 1 year postintervention • Setting: 2 general medical practices in the United Kingdom, n = 242 patients • Target: BZD |
• Educational letters sent to patients: ° explaining the problems associated with long-term BZD use and encouraged patients to gradually reduce their BZD intake ° containing sleep hygiene advice ° signed by patient’s physician |
• 31% Of patients discussed drug use with their
physician • 17% Reduced BZD use, and 5% discontinued prescriptions • Mean DDD decreased from 336.6 in the preintervention to 283.0 in the postintervention group (P < 0.001) • Patients discussing the letter with their physician (P = 0.008) and those with shorter durations of BZD use (P = 0.03) were more likely to reduce their usage |
N/A |
| Baillargeon et al (2003)
31
• Design: RCT (randomization at patient level) • Duration: baseline, 3, and 12 months • Setting: community dwelling, Québec city, Canada • Target: BZD |
• Intervention 1: gradual dose reduction during a weekly
physician consultation for 8 weeks plus 90-minute sessions
of group cognitive behavioral therapy for insomnia weekly
for 8 weeks • Intervention 2: gradual dose reduction during a weekly physician consultation for 8 weeks |
• Intervention group 1: 77%, 67%, 70% complete cessation at
0, 3, 12 months post–intervention
completion • Intervention group 2: 38%, 34%, 24% complete cessation at 0, 3, 12 months post–intervention completion (P < 0.05) at all time points |
NNT for cessation at 12 months: • 2.2 For intervention 1 compared to intervention 2 |
| Heather et al (2004)
32
• Design: RCT (randomization at patient level) • Duration: 6 months preintervention, 6 months intervention • Setting: UK general practice • Target: BZD |
Intervention 1: Patients were sent a letter from their GP
inviting them for a medication review • GPs received training and written guidelines for patient communication and BZD tapering • Guidelines were attached to the notes of eligible patients • Patients provided with self-help booklets • Intervention 2: Patients received an educational letter signed by their GP • Control: Usual care |
• Reduction in BZD use for both interventions compared to
control at 6 months ° Intervention 1: 22% reduction ° Intervention 2: 24% reduction ° Control: 16% reduction • Both interventions produced a significant reduction compared to control (P = 0.042 and P = 0.012, respectively) • There was no significant difference between intervention 1 and 2 for dose reduction (P = 0.47) • Proportion of patients achieving ≥25% reduction in BZD use at 6 months ° Intervention 1: 37% ° Intervention 2: 41% ° Control: 24% • Intervention 2 was significantly different to control (P = 0.011), whereas intervention 1 was not (P = 0.076) • Cessation of BZD use at 6 months ° Intervention 1: 10.5% ° Intervention 2: 10.2% ° Control: 6.7% • There was no significant difference between intervention 1 or 2 and control for cessation (P = 0.37) |
NNT for reduction in BZD use at 6
months: • Intervention 1: 16.7 • Intervention 2: 12.5 • NNT for ≥25% dose reduction at 6 months • Intervention 1: 26.3 • Intervention 2: 28.6 NNT for cessation at 6 months • Intervention 1: 26.3 • Intervention 2: 28.6 |
| Gorgels et al (2005),
33
De Gier et al (2010)
34
• Design: prospective controlled intervention study • Duration: 21-month follow-up • Setting: 30 primary care practices in Netherlands, n = 3833 patients • Target: BZD |
• Intervention: patients received letter from GP with advice
to gradually discontinue (discontinuation letter); followed
at 3 months with a written invitation to meet with GP to
evaluate actual use (evaluation
consultation) • Control: usual care |
• At 4-6 months, reduction in BZDs was higher at 24% in
intervention vs 5% in control group (difference in PDD =
14.2; 95% CI = 10.6-17.8) • At 21 months, results were sustained with difference in PDD = 12.5% (95% CI = 8.2-16.8) • Percentage of participants without a prescription (ie, cessation): ° At 6 months: 24% in intervention vs 12% in control group (RR = 2.1; 95% CI = 1.8-2.4) ° End of the study: 13% in intervention vs 5% in control group (RR = 2.6; 95% CI = 2.0-3.4) • No additional physician workload reported • 10-Year follow-up data (for a subset of patients) showed that 73% remained abstinent or showed minimal use over 10 years; 59% were completely BZD free during the 10th-year follow-up data collection |
NNT for cessation at 6 months: • 8.3 NNT for cessation at 21 months • 12.5 |
| Vicens et al (2014)
35
• Design: cluster RCT (randomization at practitioner level) • Duration: baseline and 12 months postintervention • Setting: 3 regions within Spain • Target: BZD |
Patient education components: • Intervention 1: structured intervention with written instructions and follow up visits • Intervention 2: structured intervention with written instructions • Control: usual care GP Education components: All GPs received baseline education and training, with intervention GPs receiving an additional 3 hours |
• Intervention 1: 45% discontinuation rate, RR = 3.01 (95%
CI = 2.03-4.46) • Intervention 2: 45% discontinuation rate, RR = 3.00 (95% CI = 2.04-4.40) • Control: 15% discontinuation rate • No significant difference between intervention 1 and 2 (RR = 1.0; 95% CI = 0.78-1.28) • Most frequently reported withdrawal symptoms were insomnia, anxiety, and irritability |
NNT for cessation at 12 months • Intervention 1: 3.3 • Intervention 2: 3.3 |
| Vicens et al (2016)
36
• Design: cluster RCT (randomization at practitioner level) • Duration: baseline and 36 months postintervention • Setting: 3 regions within Spain • Target: BZD |
Patient education components: • Intervention 1: structured intervention with follow-up visits • Intervention 2: structured intervention with written instructions • Control: usual care GP Education components: all GPs received baseline education and training, with intervention GPs receiving an additional 3 hours |
• Intervention 1: 41% discontinuation rate, RR = 1.59 (95%
CI = 1.15-2.19) • Intervention 2: 39% discontinuation rate, RR = 1.51 (95% CI = 1.10-2.05) • Control: 26% discontinuation rate • No significant difference between intervention 1 and 2 (P value not provided) |
NNT for cessation at 36 months • Intervention 1: 6.7 • Intervention 2: 7.7 |
| Subcategory: patient education delivered by nonphysician | |||
| Oude Voshaar et al (2003)
37
• Design: RCT (randomization at patient level) • Duration: 3 months • Setting: 30 general practice, Netherlands, 180 patients • Target: BZD |
• Intervention 1: tapering off chronic BZDs (by converting
to diazepam; 25% weekly reductions)
alone • Intervention 2: tapering off chronic BZDs plus CBT (5 weekly 2-hour sessions) • Control: usual care |
• For patients who completed the study: tapering off led to significantly higher proportion of successful discontinuations than usual care (62% taper only vs 58% taper + CBT vs 21% usual care, P = 0.002) | NNT for cessation at 3 months • Intervention 1: 2.4 • Intervention 2: 2.7 |
| Lopez-Peig et al (2012)
38
• Design: pre-post pseudo-experimental • Duration: baseline compared to 6 and 12 months post intervention • Setting: GP practices in Spain • Target: chronic BZD |
• Nurses provided patient education on gradual dose
reduction to chronic users • Substitution with hydralazine or valerian allowed |
• 80% Cessation of BZDs at 6 months and 64% cessation
maintained at 12 months • An improvement in all parameters of the Goldberg scale for depression and anxiety (P < 0.05) and in the mental component of SF-12 at 3.3 points (P = 0.024), as well as in most components of the MOS scale, was observed in the group that had discontinued BZD. No significant differences in these scales before and after the intervention were observed in the group that had not discontinued |
N/A |
| Tannenbaum et al (2014)
39
• Design: cluster RCT (randomization at the community pharmacy level) • Duration: 6 months, n = 303 • Setting: 30 community pharmacies in Québec, Canada • Target: BZD |
• Direct to consumer education using patient education tool:
“Eliminating Medications Through Patient Ownership of End
Results
(EMPOWER)” • Intervention: ° Self-assessment on risks of BZDs ° Evidence of BZD-induced harms, drug interactions ° Peer champion stories to augment self-efficacy ° Suggestions of equally or more effective therapies for insomnia and/or anxiety ° Stepwise tapering protocol (21-week visual chart) ° Encourage recipients to discuss deprescribing with their physician/pharmacists • Control: usual care |
• 86% Of participants completed 6 months of
follow-up • 27% Of intervention group completed cessation of BZD use at 6 months vs 5% of controls; OR = 8.1 (95% CI = 3.5%-18.5%) • Dose reduction of BZD occurred in another 11% (95% CI = 6%-16%) • NNT for any discontinuation or dose reduction = 3.7 • In multivariate subanalyses, age greater than 80 years, sex, duration of use, indication for use, dose, previous attempt to taper, and concomitant polypharmacy (≥10 prescriptions per day) were not associated with BZD therapy discontinuation |
NNT for cessation at 6 months • 4.5 |
| Martin et al (2018)
40
• Design: pragmatic cohort RCT (randomization at the community pharmacy level) • Duration: 6 months • Setting: 69 community pharmacies in Québec, Canada • Target: SH, NSAIDs, antihistamines, glyburide |
• Intervention group: Pharmacists received lists of their
patients aged ≥65 years who were chronic users of target
medications ° Pharmacists provided patients with an educational brochure and physicians with an evidence-based pharmaceutical opinion • Control group: usual care |
• 43% Of interventions ceased a target medication vs 12% of
control group at 6 months (risk difference = 31% [95% CI =
23%-38%]); NNT to discontinue = 3 • 43.2% vs 9.0% Ceased an SH (risk difference = 34% [95% CI = 25-43]) |
NNT for cessation at 6 months • 3.0 |
Abbreviations: BZD, benzodiazepine; CBT, cognitive behavioral therapy; DDD, defined daily dose; GP, general practitioner; N/A, not applicable; NNT, number needed to treat; MOS, medical outcome study; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio; PPD, prescribed daily dose; RCT, randomized controlled trial; RR, risk ratio; RRR, relative risk ratio; SF-12, Short-Form 12; SH, sedative-hypnotic.
Table 2.
Studies Evaluating Prescriber-Focused Interventions to Overcome Prescriber Barriers to Reduce SH Use in Community-Dwelling Adults.
| Study description | Intervention | Results | NNT |
|---|---|---|---|
| Subcategory: education | |||
| Smith and Tett (2010)
41
• Design: controlled pre-post • Duration: 6-month intervention • Setting: Australian general practice • Target: BZD |
Education components: • GPs, pharmacists, and nurses in aged care facilities received 3 emails with educational facts related to BZDs (side effects, indications, nonpharmacological sleep management) • Consumers filling prescriptions for BZDs received a bookmark containing facts on BZDs and a website address with further information |
• No difference in BZD use between before or after education (P > 0.05) | N/A |
| Avdagic et al (2018)
42
• Design: retrospective cohort study • Duration: 12-month, 24-month assessment periods • Setting: Community Behavioral Health Services, San Francisco • Target: BZD, nonbenzodiazepine hypnotics (Z-drugs) |
Multimodal intervention: • Psychiatrists, therapists, pharmacists, case managers, other health care providers provided education, coordination of care, guideline development, safe prescribing of SHs in 3 time periods: 1. Preintervention period: October 2013 to December 2013 2. 12-month assessment period: October 2014 to December 2014 3. 24-month assessment period: October 2015 to December 2015 |
• Number of chronic SH prescriptions decreased from
preintervention period (1764 [15.3%]) to 12-month assessment
period (1634 [14.9%]) to 24-month assessment period (1018
[9.8%]) • Difference not statistically significant between preintervention and 12-month assessment period (absolute decrease 0.4%, P = 0.32) • Significant decrease between preintervention and 24-month assessment period (absolute decrease 5.5%, P < 0.0001) and between 12-month and 24-month assessment period (absolute decrease 5.1%, P < 0.0001) |
N/A |
| Subcategory: audit and feedback | |||
| Zwar et al (2000)
43
• Design: RCT (randomization at the practitioner level) • Duration: 2 follow-up surveys • Setting: GP practices in New South Wales, Australia, n = 157 primary care physicians • Target: BZD |
• Intervention group: ° 20-Minute education on BZD prescribing ° Academic detailing by trained GP ° Management guidelines detailed to GP suggested reassessing need for SH followed by gradual withdrawal ° Provided information on anxiety, insomnia, exercise and a patient aid to managing BZD withdrawal • Control group: educational session on unrelated topic |
• Overall BZD prescriptions decreased from 2.3 to 1.7/100
encounters in the intervention group vs 2.2 to 1.6/100
encounters in controls • A statistically significant change was observed over time (P = 0.042); however, there was no difference between intervention and control groups (P = 0.99) |
N/A |
| Pimlott et al (2003)
44
• Design: RCT • Duration: 6 months • Setting: community setting of primary care GPs in Ontario, Canada. Identified primary care physician who wrote at least 10 prescriptions for target drugs in 2-month period • Targeted: BZD |
• Intervention group: physician-level feedback on
prescribing patterns and educational bulletins about BZD
prescribing among older adults • Control group: physician-level feedback on prescribing patterns and education on antihypertensive treatment among older adults • Intervention was mailed to providers every 2 months for 6 months. Feedback presented as bar graphs comparing the prescriber with peers and with a hypothetical best practice |
• No significant differences between experimental and
control group physicians or baseline BZD prescribing
patterns • Small reduction in the proportion of long-acting BZDs prescribed by intervention group (19.6 vs 20.9; P = 0.036) • No significant impact on the proportion of seniors who received long-term BZD therapy or the proportion who were prescribed BZDs in combination with other psychoactive medications |
N/A |
| Subcategory: electronic prescriber alerts (ie, pop-ups) | |||
| Smith et al (2006)
45
• Design: interrupted time series • Duration: 39 months • Setting: group model HMO in US Pacific Northwest • Target: potentially inappropriate drugs for the elderly |
• Decision support alerted clinicians to preferred alternative medications when they ordered certain nonpreferred medications (including long-acting BZDs) for all patients | • 22% Relative decrease in prescribing of target medication
compared to baseline (21.9% vs 16.8%; P
< 0.01) • No change observed in monthly initial prescribing of nonpreferred BZDs • Decrease in target medications was only observed in elderly patients, primarily driven by tertiary tricyclic agents |
N/A |
| Simon et al (2006)
46
• Design: cluster RCT (randomization at the clinic level) • Duration: 42 months, n = 239 clinicians; 50 924 patients • Setting: 15 clinics of a HMO in Oregon and Washington, USA • Target: tertiary tricyclic amine antidepressants, long-acting BZD, propoxyphene This is a continuation of Smith et al, 2006 above |
• Intervention group: 7 practices (113 clinicians, 24 119
patients) were randomly assigned to receive age-specific
alerts for target medications plus academic detailing
intervention (interactive educational program delivering
evidence-based information) • Control group: 8 practices (126 clinicians, 26 805 patients) received age-specific alerts for target medications alone • Two alert types were studied with a time series analysis: 1. computerized drug-specific alerts (preintervention) based on ordering of a target medication (eg, tertiary tricyclic amine antidepressants, long-acting BZD, propoxyphene) and 2. age-specific alerts (postintervention) occurred when a targeted medication was newly prescribed for patients 65 years and older • Both alerts suggested an alternative medication |
• Age-specific alerts sustained, but did not change, the
effect of non–age-specific alerts observed by Smith et al
46
• There was no additional effect resulting from academic detailing (P = 0.52 for level change) • Age-specific alerts led to fewer false-positive alerts for clinicians |
N/A |
| Fortuna et al (2009)
47
• Design: Cluster RCT (randomized at the practice level) • Duration: 12-month baseline and 12-month follow-up • Setting: 14 outpatient, internal medicine practice sites within Harvard Vanguard Medical Associates, USA • Target: new SH prescriptions on the market |
Practice sites randomized to: 1. Computerized prescription alerts: a new prescription for a study SH (Ambien CR, Lunesta, Sonata, and Rozerem) triggered an alert that recommended an alternative medication and prompted the prescriber to continue to an order set with decision support: • alternate drug, co-payment information, patient educational materials about insomnia and sleep hygiene 2. Alert and education • 45-minute sessions led by experienced internist • incorporated principles of academic detailing • emphasized nonpharmacological therapies • informational packet distributed to those who did not attend session 3. Usual care Alert stating co-payment tier of the medication (1, 2, 3 corresponding to the out-of-pocket cost of the medication) |
• 89 Providers received at least 1 alert; 245 alerts
activated during the study period • 23.3% of prescriptions for study SH that activated an alert were changed to a generic equivalent • Computerized alert groups experienced preintervention levels of study SH prescribing in both the alert-only (adjusted RR = 0.97; 95% CI = 0.82-1.14) and alert plus education groups (RR = 0.98; 95% CI = 0.83-1.17) • Usual care group experienced an increase in prescribing (RR = 1.31; 95% CI = 1.08-1.60) • Compared to usual care, both the alert groups had lower RR of prescribing study SH (RRR = 0.74; 95% CI = 0.58-0.97) • The prescribing of study SH was similar in the alert group and alert plus education group (RRR = 1.02; 95% CI = 0.80-1.29) |
N/A |
Abbreviations: BZD, benzodiazepine; GP, general practitioner; HMO, health maintenance organization; N/A, not applicable; NNT, number needed to treat; RCT, randomized controlled trial; RR, risk ratio; RRR, relative risk ratio; SH, sedative-hypnotic.
Table 3.
Studies Evaluating Interventions to Overcome Health System Constraints to Reduce SH Use by Community-Dwelling Adults.
| Study description | Intervention | Results | NNT |
|---|---|---|---|
| Subcategory: government level | |||
| Jørgensen (2007)
48
• Design: uncontrolled pre-post • Duration: 15 months • Setting: 10 Danish medical practices with 13 medical practitioners and 18 500 patients • Target: BZD, Z-drugs |
• New government restrictions for BZDs, hypnotics,
anxiolytics and Z-drugs. Limit maximum supply of a single
prescription for up to 30-day supply, and only following
consultation • Telephone prescriptions were not permitted • Public awareness campaign (local press, newspapers, educational posters, meetings, staff guides, patient guides) |
• Postintervention, Z-drug prescriptions were reduced by
50.5%, BZD hypnotics by 46.5%, and BZD anxiolytics by 41.7%
(no difference between drug classes [P =
0.30]) • During first 3 months, only 4.3 additional consultations per week per 1000 patients were required, which then reduced to 2.1 |
N/A |
| Hooper et al (2009)
49
• Design: before-and-after • Duration: 8.5 years • Setting: registered medical practitioners in 3 major regions of Tasmania, Australia • Targeted: BZD (alprazolam) |
• Pharmacies required to report all alprazolam prescriptions
to the government monitoring agency
monthly • Application for authorization if prescribing for ≥4 weeks to patients coprescribed an opiate • Physicians were notified to not prescribe alprazolam to patients currently receiving BZD and/or opioids from another medical practitioner. Patients on methadone or buprenorphine were required to have approval to receive an alprazolam prescription • Educational sessions (n = 3) on evidence-based interventions for panic disorder provided by psychiatrists, pharmacists, and addiction medicine specialists. Education topics: diagnosis of panic disorder, evidence for BZD use in panic disorder, issue of BZD abuse among opiate users, and forthcoming regulatory changes |
• 26% GPs attended educational sessions • Alprazolam prescriptions decreased from 19 228 in the preintervention period to 16 261 in the postintervention period (relative reduction 15.4%) • In the rest of Australia, prescriptions for alprazolam increased by 1.3% in this same period • The number of individuals receiving both alprazolam and opioid prescriptions declined linearly in this time period [patients = −6.3 (month) + 246.1; R2 = 0.59; t(11) = 4.0 (P < 0.01)] |
N/A |
| Schaffer et al (2016)
50
and Lloyd et al (2017)
51
• Design: interrupted time series analysis • Duration: 6 years • Setting: 10% sample of Australian prescription data • Target: alprazolam |
• In 2014, alprazolam was selectively “up-scheduled” to be a
controlled drug in an attempt to curb
prescribing • Pharmacies had to submit a monthly report to the government for all alprazolam prescriptions dispensed • Prescribers had to apply for authority to prescribe if prescribing for ≥4 weeks |
• Alprazolam use reduced by ~33% (95% CI = −36.3% to −30.1%)
vs the 12 months prior to change in
schedule • Unintended consequences: (1) switching to another BZD increased 214%; (2) BZD overdoses increased from 380 in 2013 to 453 in 2015 |
N/A |
| Subcategory: local level | |||
| Larkin et al (2017)
52
• Design: controlled pre-post • Duration: 6 years • Setting: 38 AMCs in 5 US states • Target: multiple drugs |
• AMCs with pharmaceutical detailing policies (includes any
policies addressing gifts, access to AMC staff and
enforcement) compared to AMCs without
policies • Multiple drug classes were studied |
• Mean change in SH prescriptions (predominantly BZDs and Z-drugs) following detailing policy was decrease of 10.5% (95% CI = −18.87 to −2.16; P = 0.01) compared to an increase of 4.7% (95% CI = 0.81-8.50) in the control group | N/A |
Abbreviations: AMC, academic medical centers; BZD, benzodiazepine; GP, general practitioner; N/A, not applicable; NNT, number needed to treat; SH, sedative-hypnotics; Z-drugs, zopiclone, zolpidem.
Evidence Identified
Studies Overcoming Patient-Related Barriers and Prescriber Inertia
Patients need to be informed of the risks associated with chronic SH use in order to be engaged in shared decision-making. Identified studies showed that patient education reduced the SH dose for 10% to 62% of participants and led to SH discontinuation in 13% to 80% of participants (Table 1).30-40 All identified trials examined strategies to reduce use, taper, or discontinue existing SH use rather than deter SH initiation. Patient education interventions included a doctor’s letter or educational brochure explaining adverse consequences of chronic SH therapy and encouraging the patient to gradually taper their dose (with complete cessation if possible) on their own and an invitation to see their doctor for further discussion or CBT to address insomnia. Empowering patients to engage in conversations with their prescriber about SH use can overcome prescriber inertia by reducing patient expectations for prescribing and reviewing the necessity of the medication and how it could be stopped.40,53
Gorgels et al 33 conducted a prospective controlled intervention at 30 Dutch primary care sites with 3833 participants. Benzodiazepine users received a letter from their primary care doctor with advice to gradually discontinue therapy followed by an invitation at 3 months for consultation, whereas the control group received usual care. At 21 months, 26% of the intervention group reduced their benzodiazepine consumption versus 9% in the usual care group (prescribed daily dosages difference: 12.5%; 95% CI = 8.2%-16.8%). The authors demonstrated a sustained effect of their intervention in a subset of patients at 10 years. 34
Vicens et al 35 evaluated the efficacy of 2 structured interventions that enabled discontinuation of long-term benzodiazepines. In this multicenter, 3-arm cluster-controlled study with randomization at the physician level, general practitioners were allocated (1:1:1) to usual care, or a patient education intervention with or without follow-up visits. The intervention consisted of structured educational patient interviews, where physicians provided information about risks of SH use, a self-help leaflet, and a written tailored gradual dose reduction plan. In the arm including follow-up, physicians scheduled appointments with the patient every 2 to 3 weeks. At 12 months, 45% of the patients in both intervention groups had discontinued benzodiazepine compared with 15% in the control group (RR = 3.01, 95% CI = 2.03-4.45, for intervention with writing and RR = 3.0, 95% CI = 2.04-4.40, for intervention with follow-up visits). The results were sustained at 36 months. The most frequently reported withdrawal symptoms were insomnia, anxiety, and irritability. Sleep satisfaction was reported as significantly higher in the intervention group with follow-up.
Nurses and pharmacists can also provide patient education and empowerment regarding deprescribing. Lopez-Peig et al 38 evaluated a nurse-led benzodiazepine withdrawal program using a before-after study design in 2 primary care centers (n = 51 participants). Nurses provided patient education, and SH doses were reduced every 2 to 4 weeks by approximately 25%. Alternative drugs valerian or hydroxyzine were permitted for sleep to support benzodiazepine tapering. At 6 months, 80% of the patients had discontinued benzodiazepine use and 64% maintained discontinuation at 1 year. Those who successfully discontinued benzodiazepine therapy reported improvements in depression and anxiety without reduction in sleep quality. A pharmacy-based patient education program was examined in 2 randomized controlled trials.39,40 Tannenbaum et al 39 conducted a cluster randomized trial to compare the effect of a direct-to-consumer education tool versus usual care via 30 community pharmacies in Québec. Pharmacies were randomly allocated to the intervention, which involved mailing an 8-page brochure to chronic benzodiazepine users aged 65 years and older either immediately (intervention) or after a 6-month wait period (control). The brochure included education about harms, peer support stories, a tapering protocol, and alternatives. At 6 months, 27% of the intervention group had discontinued therapy versus 5% of the control group (risk difference = 23%; 95% CI = 14%-32%), and 11% had reduced their dose (95% CI = 6%-16%). Lack of physician support was identified as a deterrent to deprescribing. To address this barrier, a second randomized trial that added an evidence-based pharmaceutical opinion sent to physicians, at the same time as pharmacists mailed the education brochure to patients, sought to better foster communication around deprescribing. 40 The study enrolled 489 participants from 69 community pharmacies who were prescribed 1 of 4 Beers Criteria medications (SH, nonsteroidal anti-inflammatories, glyburide, or first-generation antihistamine). SH discontinuation was 43.2% for the intervention group versus 9.0% for the control group (risk difference = 34%; 95% CI = 25%-43%); 38% of individuals who attempted to taper SH reported withdrawal symptoms.
CBT has been investigated as an add-on to patient education to improve deprescribing rates and sustainability of discontinuing SH use. Voshaar et al 37 conducted a trial to evaluate the efficacy and feasibility of tapering off long-term benzodiazepine use in patients who did not stop benzodiazepines within 3 months of receiving a letter. This study randomized 180 participants from 30 Dutch general practices to either medication tapering with weekly follow-up visits, tapering with weekly visits plus CBT, or usual care. The CBT delivered included relaxation training and cognitive restructuring aimed at reducing impact of any perceived withdrawal, without stimulus control, sleep restriction, or other modalities aimed specifically at insomnia treatment. Both intervention groups showed similar reductions in the proportion of patients who successfully discontinued benzodiazepines at the 3-month evaluation. Benzodiazepine dosage was the only independent predictor of successful discontinuation (OR = 4.5, 95% CI = 2.0-10.2, for daily doses < 10 mg diazepam equivalent). Although delivering CBT for insomnia may be limited in some outpatient settings, research supports the use of digital CBT for insomnia, which some patients may find suitable and easy to access. 54
Studies Increasing Prescriber Awareness
Electronic alerts and audit-and-feedback (ie, process of measuring and reporting individual performance to desired standard or peers) have also been studied to increase prescriber awareness to reduce SH use (Table 2).41-47 One RCT explored audit-and-feedback on benzodiazepine prescriptions for 374 primary care physicians. Physicians were randomized to the intervention group that received physician-level feedback on prescribing patterns and education, whereas the control group received similar information on antihypertensives. The study found a 0.7% decrease from baseline for long-acting benzodiazepines in the intervention group and a 1.1% increase in the control group (P = 0.036). 44
Clinical decision support aids, such as guidelines, recommendations, or indications/contraindications, can be incorporated into electronic prescribing systems as computerized alerts or pop-ups. Smith et al 45 examined the effect of clinical decision support on use of potentially contraindicated medications in older adults. In this interrupted time series analysis, there was a 22% relative decrease in the prescribing of target medications (21.9% vs 16.8%; P < 0.01) following alert implementation, with reduction primarily driven by reduced tricyclic antidepressant use. In a follow-up cluster-randomized study, the investigators examined the effect of replacing medication-specific prescribing alerts with age-specific alerts compared to alerts with academic detailing. 46 The addition of academic detailing did not enhance the effect of the alerts.
Fortuna et al 47 demonstrated the greatest impact of alerts on prescribing in a cluster-randomized controlled trial in 14 outpatient internal medicine sites. The objective of this study was to reduce prescribing of heavily marketed new SHs (zolpidem, eszopiclone, zaleplon, or ramelteon). The sites were randomized to receive computer alerts with and without prescriber education or usual care for new prescriptions only. Targeted SH prescriptions dropped in the alert group (relative risk reduction [RRR] = 0.74; 95% CI = 0.57-0.96) and the alert plus education group (RRR = 0.74; 95% CI = 0.58-0.97) compared to the usual care group. Overall, clinicians reported that the alerts did not interfere with their workflow and that the alerts provided information perceived to be beneficial. The impact on patients or clinical outcomes were not examined.
Studies Investigating Health System Policy Interventions
Policy interventions to reduce SH prescribing can be implemented at the local, state, or national health systems level (Table 3). Policy interventions we identified involved prescribing restrictions such as reducing the number of days supplied, rescheduling, prohibiting telephone orders, and local policies that restricted detailing visits by pharmaceutical company representatives to prescribers. Some policies were supported with prescriber and public education. Implementation of these policies produced a 10% to 50% reduction in targeted medication use.48-52 The greatest reduction in SH use was reported by Jørgensen 48 who conducted a pre-post study following new government restrictions that broadly targeted multiple SH classes (benzodiazepines, hypnotics, anxiolytics, and Z-drugs) to limit prescribing to a 1-month supply at a time following consultation. The new restrictions were publicly advertised, targeting both patients and staff. The 15-month pre-post study involved 10 medical practices, targeting 18 500 patients. Postintervention Z-drug use was reduced by 50.5%, benzodiazepine-hypnotics by 46.5%, and benzodiazepine-anxiolytics by 41.7%, with no difference between the various SH classes. Hooper et al 49 conducted an 8.5-year before-and-after regional study specifically targeting alprazolam. Physicians were notified to not prescribe alprazolam to patients who were receiving benzodiazepines and/or opioids from other medical practitioners. Pharmacists were required to submit a monthly report of alprazolam prescriptions dispensed to a government monitoring agency. Physicians required authorization to prescribe for more than 4 weeks to patients also prescribed an opioid. Supporting education for general practitioners was provided of whom 26% attended the educational sessions. There was a 15.4% relative reduction in alprazolam prescriptions following the intervention; in regions without the policy, alprazolam use increased by 1.3%. In 2014, the alprazolam intervention was upscaled nationally, and alprazolam was rescheduled from a prescription medication to a controlled drug. Schaffer et al 50 found that alprazolam use reduced by 33.3% (95% CI = 36.3%-30.1%) compared to 12 months prior to the policy change, but alternative benzodiazepine use increased by 214%. Poison control center data after the policy change showed that benzodiazepine overdoses also increased. 51 These studies suggest that balancing measures should be factored into policy interventions and safer pharmacological and nonpharmacological alternatives should be considered.
Relevance to Patient Care and Clinical Practice
QI initiatives need to address patient and physician barriers while considering the context and environment in which the intervention will be applied as well as the resources available (see Table 4 for specific resources). The outpatient setting is expansive, and local ecology factors as well as province/state rules or policy should be considered. We emphasize an approach that can be applied within a microsystem (eg, individual prescriber or primary care clinic) and upscaled over time (Figure 1). Regardless of the scale of the intervention(s) applied, it is clear that patient engagement is valuable. A common thread between the most effective interventions was facilitation of communication/relationship development between patients and health care providers. Based on our interpretation of the literature reviewed and the harm associated with chronic SH use, we propose the following implementation strategies to guide QI initiatives in primary care settings.
Table 4.
Patient and Health Care Provider Resources to Facilitate Sedative-Hypnotic Reduction.
| Patient educational brochure with written tapering protocol:
https://www.deprescribingnetwork.ca/patient-handouts
Sedative hypnotic deprescribing whiteboard videos for physicians: https://www.deprescribingnetwork.ca/video-benzodiazepines Letter to patients inviting them to review their sedative hypnotic use: https://www.deprescribingnetwork.ca/patient-letter Cognitive behavioral therapy for insomnia resources: https://mysleepwell.ca/cbti/ and https://www.criugm.qc.ca/fichier/pdf/Sleep_brochure.pdf Evidence-based deprescribing algorithms: https://deprescribing.org/wp-content/uploads/2019/03/deprescribing_algorithms2019_BZRA_vf-locked.pdf Sedative hypnotic reduction toolkit for primary care: https://choosingwiselycanada.org/perspective/toolkit-benzos-primary-care/ Sedative hypnotic reduction toolkit for hospitals: https://choosingwiselycanada.org/perspective/benzos-hospital-toolkit/ |
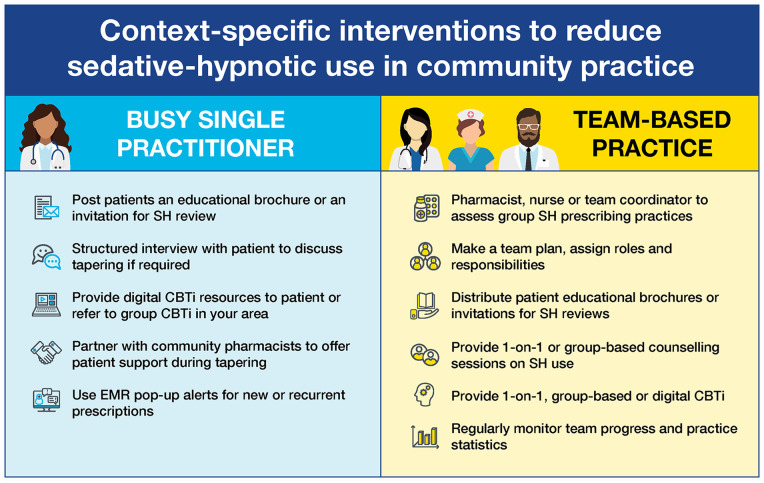
Figure 1.
Context-specific interventions to reduce SH use in community practice.
Abbreviations: CBTi, cognitive behavioral therapy for insomnia; EMR, electronic medical record; SH, sedative-hypnotic.
Strategy 1: Interventions to Reduce Patient Barriers
Patient engagement and empowerment by the QI team will be crucial to convincing patients to reduce or discontinue chronic SH use because patients or caregivers are ultimately in control of their medication (eg, continue to fill repeats or seek out an alternative prescriber). Patients’ attitudes, beliefs, and experiences will influence their willingness to deprescribe.55,56 Primary care clinics can send information to their patients via letters or brochures and invite them to participate in a medication review process with members of their health care team. A possible explanation for the observed success of education targeting patients to reduce SH is that these interventions simultaneously addressed multiple barriers: patients received the information directly from a known influencer; patients were encouraged to discuss their SH use with their prescriber, thus overcoming prescribing inertia; and both patients and prescribers increased their knowledge of the benefit to harm ratio associated with SH use and the potential alternatives for managing insomnia. 57 Community pharmacists are readily accessible to the public, uniquely positioned to easily identify chronic SH use, and are poised to easily distribute patient and provider educational materials.
Strategy 2: Interventions to Overcome Prescriber Barriers
The QI teams’ strategy should also consider interventions directed at prescribers given the demonstrated effectiveness reported in the literature. These can include education and training, drug utilization reviews, and computer alerts indicating a potentially inappropriate prescription by medication, age, dose, or disease. Educational interventions were effective when they facilitated patient engagement and provided information on the limited evidence supporting chronic SH use and the associated harms, as well as the effectiveness of alternatives. Despite the ease with which they can be incorporated into prescribing software, decision support tools were less effective than prescriber education with patient engagement. Monitoring and tracking practice-wide rates of SH use provides benchmarks and enables goal setting. Most electronic health record software permit this function. We also note that an interdisciplinary team approach involving pharmacists and nurses can support drug utilization reviews and share the responsibility of educating patients and prescribers about SH harms and alternative therapies. Depending on the local context of the community practice setting and resources available, some or all of these interventions can be implemented.
Strategy 3: Interventions to Overcome Health System Barriers
Although policy interventions such as prescribing restrictions can have a large immediate impact on SH use, these interventions require tremendous state/province, community, and stakeholder engagement to be successfully implemented. These interventions are beyond the scope of QI teams, but policy interventions can be successfully implemented at a local level. In particular, enabling a multidisciplinary approach and implementing restrictive pharmaceutical detailing policies can effectively reduce SH use.
Conclusions
It is imperative to overcome barriers to reducing both initiation and continued SH use in the community to reduce unnecessary harm. We identified several intervention strategies with demonstrated efficacy in reducing SH use in community practice: engaging and educating prescribers may be effective; however, better results are achieved by providing patients with educational materials that highlight SH risks, how to taper therapy, and potential alternatives. More substantial reductions were observed when providing prescribers and patients with educational materials with or without engaging patients in CBT for insomnia. The strategies presented can guide multidisciplinary QI teams toward reducing the community burden of SH use.
Supplemental Material
Supplemental material, sj-pdf-1-aop-10.1177_10600280211033022 for Addressing Barriers to Reducing Prescribing and Implementing Deprescribing of Sedative-Hypnotics in Primary Care by Lisa Burry, Justin Turner, Timothy Morgenthaler, Cara Tannenbaum, Hyung J. Cho, Evelyn Gathecha, Flora Kisuule, Abi Vijenthira and Christine Soong in Annals of Pharmacotherapy
Acknowledgments
CS, LB, HJC, EG, FK, AV, and TM are members of the High Value Practice Academic Alliance. The High Value Practice Academic Alliance is a consortium of academic medical centers in the United States and Canada working to advance high value health care through collaborative quality improvement, research, and education. For additional information, please visit http://www.hvpaa.org. We thank Patrician J. Erwin for her assistance in the development of the literature search.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lisa Burry  https://orcid.org/0000-0002-6545-3890
https://orcid.org/0000-0002-6545-3890
Justin Turner  https://orcid.org/0000-0003-0613-108X
https://orcid.org/0000-0003-0613-108X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011;101:1429-1433. doi: 10.2105/AJPH.2010.300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson CT, Frei C, Downes N, McTaggart SA, Akram G. Benzodiazepine and z-hypnotic prescribing for older people in primary care: a cross-sectional population-based study. Br J Gen Pract. 2016;66:e410-e415. doi: 10.3399/bjgp16X685213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertisch S, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343-349. doi: 10.5665/sleep.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halme AS, Beland SG, Preville M, Tannenbaum C. Uncovering the source of new benzodiazepine prescriptions in community-dwelling older adults. Int J Geriatr Psychiatry. 2013;28:248-255. doi: 10.1002/gps.3818 [DOI] [PubMed] [Google Scholar]
- 5. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 6. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350. doi: 10.1007/s11606-007-0251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sys J, Van Cleynenbreugel S, Deschodt M, Van der Linden L, Tournoy J. Efficacy and safety of non-benzodiazepine and non-Z-drug hypnotic medication for insomnia in older people: a systematic literature review. Eur J Clin Pharmacol. 2020;76:363-381. doi: 10.1007/s00228-019-02812-z [DOI] [PubMed] [Google Scholar]
- 9. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8:e63773. doi: 10.1371/journal.pone.0063773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204. doi: 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- 11. Weymann D, Gladstone EJ, Smolina K, Morgan SG. Long-term sedative use among community-dwelling adults: a population-based analysis. CMAJ Open. 2017;5:E52-E60. doi: 10.9778/cmajo.20160056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sgnaolin V, Engroff P, Andrade CP, et al. Patterns of chronic benzodiazepine use in the elderly. Arch Clin Psychiatry. 2016;43:79-82. doi: 10.1590/0101-60830000000089 [DOI] [Google Scholar]
- 13. Jackson G, Gerard C, Minko N, Parsotam N. Variation in benzodiazepine and antipsychotic use in people aged 65 years and over in New Zealand. N Z Med J. 2014;127:67-78. [PubMed] [Google Scholar]
- 14. Seppala LJ, Wermelink AMAT, de Vries M, et al. ; EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19:371.e11-e17. doi: 10.1016/j.jamda.2017.12.098 [DOI] [PubMed] [Google Scholar]
- 15. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi: 10.1136/bmj.i90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: results from two large cohort studies in France and UK. Eur Neuropsychopharmacol. 2015;25:1566-1577. doi: 10.1016/j.euroneuro.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 17. Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63-71. doi: 10.2174/157488606775252674 [DOI] [PubMed] [Google Scholar]
- 18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246. doi: 10.111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 19. Canadian Geriatrics Society. Five things physicians and patients should question. Accessed July 6, 2021. http://www.choosingwiselycanada.org/recommendations/geriatrics/
- 20. O’Mahony D, O’Sullivan DSB, O’Connor M, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213-218. doi: 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chong Y, Fryar CD, Gu Q. Prescription sleep aid use among adults: United States, 2005–2010. NCHS Data Brief. 2013;(127):1-8. [PubMed] [Google Scholar]
- 22. Siriwardena AN, Qureshi Z, Gibson S, Collier S, Latham M. GPs’ attitudes to benzodiazepine and “Z-drug” prescribing: a barrier to implementation of evidence and guidance on hypnotics. Br J Gen Pract. 2006;56:964-967. [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffman F. Perceptions of German GPs on benefits and risks of benzodiazepines and Z-drugs. Swiss Med Wkly. 2007;143:w13745. doi: 10.4414/smw.2013.13745 [DOI] [PubMed] [Google Scholar]
- 24. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zopidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1-6. doi: 10.1002/jhm.1985 [DOI] [PubMed] [Google Scholar]
- 25. Brandt J, Leong C. Benzodiazepines and Z-Drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17:493-507. doi: 10.1007/s40268-017-0207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30:793-807. doi: 10.1007/s40266-013-0106-8 [DOI] [PubMed] [Google Scholar]
- 27. Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4:e006544. doi: 10.1136/bmjopen-2014-006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anthieren S, Pasteels I, Habraken H, Steinberg P, Declercg T, Christiaens T. Barriers to non-pharmacologic treatments for stress, anxiety, and insomnia: family physicians’ attitudes toward benzodiazepine prescribing. Can Fam Physician. 2010;56:e398-e406. [PMC free article] [PubMed] [Google Scholar]
- 29. Soong C, Burry L, Cho HJ, et al. An implementation guide to promote sleep and reduce sedative-hypnotic initiation for noncritically ill inpatients. JAMA Intern Med. 2019;179:965-972. doi: 10.1001/jamainternmed.2019.1196 [DOI] [PubMed] [Google Scholar]
- 30. Morgan JD, Wright DJ, Chrystyn H. Pharmacoeconomic evaluation of a patient education letter aimed at reducing long-term prescribing of benzodiazepines. Pharm World Sci. 2002;24:231-235. doi: 10.1023/a:1021587209529 [DOI] [PubMed] [Google Scholar]
- 31. Baillargeon L, Landreville P, Verreault R, Beauchemin JP, Grégoire JP, Morin CM. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial. CMAJ. 2003;169:1015-1020. [PMC free article] [PubMed] [Google Scholar]
- 32. Heather N, Bowie A, Ashton H, et al. Randomised controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12:141-154. doi: 10.1080/1606635310001634528 [DOI] [Google Scholar]
- 33. Gorgels WJ, Oude Voshaar RE, Mol AJ, et al. Discontinuation of long-term benzodiazepine use by sending a letter to users in family practice: a prospective controlled intervention study. Drug Alcohol Depend. 2005;78:49-56. doi: 10.1016/j.drugalcdep.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 34. De Gier N, Gorgels W, Lucassen P, Voshaar RO, Mulder J, Zitman F. Discontinuation of long-term benzodiazepine use: 10-year follow-up. Fam Pract. 2010;28:253-259. doi: 10.1093/fampra/cmq113 [DOI] [PubMed] [Google Scholar]
- 35. Vicens C, Bejarano F, Sempere E, et al. Comparative efficacy of two interventions to discontinue long-term benzodiazepine use: cluster randomised controlled trial in primary care. Br J Psychiatry. 2014;204:471-479. doi: 10.1192/bjp.bp.113.134650 [DOI] [PubMed] [Google Scholar]
- 36. Vicens C, Sempere E, Bejarano F, et al. Efficacy of two interventions on the discontinuation of benzodiazepines in long-term users: 36-month follow-up of a cluster randomised trial in primary care. Br J Gen Pract. 2016;66:e85-e91. doi: 10.3399/bjgp16X683485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Voshaar RCO, Gorgels WJMJ, Mol AJJ, et al. Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: three-condition, randomised controlled trial. Br J Psychiatry. 2003;182:498-504. [DOI] [PubMed] [Google Scholar]
- 38. Lopez-Peig C, Mundet X, Casabella B, del Val JL, Lacasta D, Diogene E. Analysis of benzodiazepine withdrawal program managed by primary care nurses in Spain. BMC Res Notes. 2012;5:684. doi: 10.1186/1756-0500-5-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174:890-898. doi: 10.1001/jamainternmed.2014.949 [DOI] [PubMed] [Google Scholar]
- 40. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE Randomized Clinical Trial. JAMA. 2018;320: 1889-1898. doi: 10.1001/jama.2018.16131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith AJ, Tett SE. An intervention to improve benzodiazepine use-a new approach. Fam Pract. 2010;27:320-327. doi: 10.1186/1472-6963-10-321 [DOI] [PubMed] [Google Scholar]
- 42. Avdagic K, Geier M, Coralic Z, Finley P. Evaluation of the impact of a multimodel intervention on prescribing patterns of sedative-hypnotics in a behavioral health system. Prim Care Companion CNS Disord. 2018;20:18m02269. doi: 10.4088/PCC.18m02269 [DOI] [PubMed] [Google Scholar]
- 43. Zwar NA, Wolk J, Gordon JJ, Sanson-Fisher RW. Benzodiazepine prescribing by GP registrars: a trial of educational outreach. Aust Fam Physician. 2000;29:1104-1107. [PubMed] [Google Scholar]
- 44. Pimlott NJG, Hux JE, Wilson LM, Kahan M, Li C, Rosser WW. Educating physicians to reduce benzodiazepine use by elderly patients: a randomized controlled trial. CMAJ. 2003;168:835-839. [PMC free article] [PubMed] [Google Scholar]
- 45. Smith DH, Perrin N, Feldstein A, et al. The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Arch Intern Med. 2006;166:1098-1104. doi: 10.1007/s11606-009-1013-x [DOI] [PubMed] [Google Scholar]
- 46. Simon SR, Smith DH, Feldstein AC, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc. 2006;54:963-968. doi: 10.1111/j.1532-5415.2006.00734.x [DOI] [PubMed] [Google Scholar]
- 47. Fortuna RJ, Zhang F, Ross-Degnan D, et al. Reducing the prescribing of heavily marketed medications: a randomized controlled trial. J Gen Intern Med. 2009;24:897-903. doi: 10.1007/s11606-009-1013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jørgensen VRK. An approach to reduce benzodiazepine and cyclopyrrolone use in general practice: a study based on a Danish population. CNS Drugs. 2007;21:947-955. doi: 10.2165/00023210-200721110-00006 [DOI] [PubMed] [Google Scholar]
- 49. Hooper S, Bruno R, Sharpe M, Tahmindjis A. Alprazolam prescribing in Tasmania: a two-fold intervention designed to reduce inappropriate prescribing and concomitant opiate prescription. Australas Psychiatry. 2009;17:300-305. doi: 10.1080/10398560902998626 [DOI] [PubMed] [Google Scholar]
- 50. Schaffer AL, Buckley NA, Cairns R, Pearson SA. Interrupted time series analysis of the effect of rescheduling alprazolam in Australia: taking control of prescription drug use. JAMA Intern Med. 2016;176:1223-1225. doi: 10.1001/jamainternmed.2016.2992 [DOI] [PubMed] [Google Scholar]
- 51. Lloyd B, Dwyer J, Bugeja L, Jamieson A. Alprazolam in fatal overdose following regulatory rescheduling: a response to Deacon et al. Int J Drug Policy. 2017;39:138-139. doi: 10.1016/j.drugpo.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 52. Larkin I, Ang D, Steinhart J, et al. Association between academic medical center pharmaceutical detailing policies and physician prescribing. JAMA. 2017;317:1785-1795. doi: 10.1001/jama.2017.4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turner JP, Richard C, Lussier M-T, et al. Deprescribing conversations: a closer look at prescriber–patient communication. Ther Adv Drug Saf. 2018;9:687-698. doi: 10.1177/2042098618804490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luik AI, Kyle SD, Espie CA. Digital cognitive behavioral therapy (dCBT) for insomnia: a state-of-the science review. Curr Sleep Med Rep. 2017;3:48-56. doi: 10.1007/s40675-017-0065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reeve E, Wiese MD, Hendrix I, Roberts M, Shakib S. People’s attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61:1508-1514. doi: 10.1111/jgs.12418 [DOI] [PubMed] [Google Scholar]
- 56. Reeve E, Low LF, Hilmer SN. Beliefs and attitudes of older adults and carers about deprescribing of medications. Br J Gen Pract. 2016;66:e552-e560. doi: 10.3399/bjgp16X685669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92:81-87. doi: 10.1016/j.pec.2013.02.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-aop-10.1177_10600280211033022 for Addressing Barriers to Reducing Prescribing and Implementing Deprescribing of Sedative-Hypnotics in Primary Care by Lisa Burry, Justin Turner, Timothy Morgenthaler, Cara Tannenbaum, Hyung J. Cho, Evelyn Gathecha, Flora Kisuule, Abi Vijenthira and Christine Soong in Annals of Pharmacotherapy