Abstract
Background:
Recent studies have reported an increase in Inflammatory bowel disease (IBD) incidence in young children, highlighting the need to better understand risk factors for the development of IBD. Licensed for use in infants in 2006, the oral, live-attenuated rotavirus vaccine has biologic plausibility for instigating inflammation of the gut mucosa as a pathway to immune dysregulation.
Methods:
Over a ten-year period, we evaluated incidence of IBD within a cohort of children under the age of ten, enrolled in seven integrated healthcare delivery systems. We conducted a nested case-control study to evaluate the association between rotavirus vaccination and IBD using conditional logistic regression. Cases were confirmed via medical record review and matched to non-IBD controls on date of birth, sex, and study site.
Results:
Among 2.4 million children under the age of 10 years, 333 cases of IBD were identified with onset between 2007 and 2016. The crude incidence of IBD increased slightly over the study period (p-value for trend = 0.046). Of the 333 cases, 227 (68%) were born prior to 2007. Forty-two cases born in 2007 or later, with continuous enrollment since birth were included in the case-control study and matched to 210 controls. The adjusted odds ratio for any rotavirus vaccination in IBD cases, compared to matched controls, was 0.72 (95% confidence interval 0.19–2.65).
Conclusions:
Data from this large pediatric cohort demonstrate a small overall increase in IBD incidence in young children over a ten-year period. The data suggest that rotavirus vaccination is not associated with development of IBD.
Keywords: Inflammatory bowel disease, Pediatric, Incidence, Rotavirus vaccine
1. Introduction
Pediatric inflammatory bowel disease (IBD) is comprised of three categories of disease: ulcerative colitis (UC), Crohn’s disease (CD) and indeterminate colitis (IC) and represents 10–20% of incident IBD cases overall. [1] The colon is the most common site of disease for both UC and CD in young children, and differentiation of UC from colonic CD may be difficult, resulting in the third classification of indeterminate colitis. [2-3]
Multiple studies have reported an increase in pediatric IBD incidence within the last two decades. [2,4-9] As 10–25% of affected individuals have a first degree relative with IBD, [10-11] a genetic link to IBD is widely accepted. However, environmental risk factors for the disease are poorly understood. The perceived increase in pediatric IBD incidence has prompted research focusing on prenatal and perinatal exposures, hygienic practices, antibiotic use, and vaccination. [4,12-14] A 2016 white paper from the Centers for Disease Control and Prevention (CDC) listed pediatric IBD among twenty outcomes of high priority for studying the safety of the childhood immunization schedule within the Vaccine Safety Datalink (VSD). [15]
Within the current childhood immunization schedule, the live-attenuated oral rotavirus vaccine has biologic plausibility for instigating inflammation of the gut mucosa as a pathway to immune dysregulation. While no studies have identified an association between IBD and rotavirus, a previous study demonstrated that interaction between a murine norovirus and a Crohn’s disease susceptibility gene triggered Crohn’s-like intestinal pathology in mice. [16] Observational studies have also indicated an association between all-cause acute gastroenteritis episodes and the development of IBD. [17-18] However, data have not been consistent, as other studies have found no apparent increase in antibodies to rotavirus among Crohn’s disease patients. [19-20]
To better understand the trajectory of IBD diagnoses among children in the United States, we evaluated the incidence of pediatric IBD among children within the VSD over a ten-year period. To evaluate the association between rotavirus vaccines and the risk of pediatric IBD, we then conducted a nested case-control study within our large study cohort.
2. Materials and methods
2.1. Setting and study populations
The VSD is an established collaboration between CDC and eight integrated health systems, in which electronic health record (EHR) data are used to conduct observational studies of vaccine safety. [21] Data for the current study were obtained from seven VSD sites: the Colorado, Northern California, Northwest, Southern California, and Washington Kaiser Permanente regions, HealthPartners (Minnesota) and Marshfield Clinic (Wisconsin). The study protocol was approved by the institutional review boards of all participating VSD sites. All data were de-identified and presented in aggregate form. We neither contacted study participants nor required any active participation; all sites were granted waivers of informed consent.
The study population was limited to children under the age of 10, in concordance with the Paris classification group A1a. [22]
2.1.1. Population-based retrospective cohort
The population at risk for IBD was estimated from the number of children ages 0 through 9 years with at least 12 months of continuous enrollment between January 1, 2007 and December 31, 2016 in a VSD health system. We excluded children who had a diagnosis of IBD within the first 61 days of enrollment, based on the assumption that these would represent prevalent cases. We included only those children in the cohort with the first recorded IBD diagnosis occurring in 2007 or later. Among cases identified, we examined electronic medical records from 2003 through 2006 to ensure that cases were truly incident during the study period.
2.1.2. Nested case-control population
For the nested case-control study, we included only those children born on or after January 1st, 2007 so they would have opportunity for exposure to rotavirus vaccine, given the Advisory Committee on Immunization Practices (ACIP) recommendation for this vaccination in August of 2006. [23] We excluded those who received no routine childhood vaccinations from the study population, as this group would likely differ from the vaccinated group by health care utilization and other confounding variables. [24] Both cases and controls required continuous enrollment from birth until the index date (the date of first IBD diagnosis for cases or reference date for controls). The reference date of each control was the date of diagnosis of their matched case.
Controls were children with no evidence of IBD in their electronic medical records. Controls were matched without replacement, five to one to cases on date of birth (within one month), sex, and VSD site. We excluded potential controls with a diagnosis of ’unspecified abdominal pain’ (ICD-9 789.0, ICD-10 R10.9), ‘blood in stool’ (ICD-9 578.1, ICD-10K92.1), or ‘abnormal weight loss’ (ICD-9 783.21, ICD-10 R63.4) within one year of the reference date, as these conditions can be symptoms of undiagnosed IBD.
2.2. Ascertainment of IBD diagnoses
2.2.1. Retrospective cohort study of IBD incidence
We identified all children ages 0 through 9 years with International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes specific for Crohn’s disease (CD), ulcerative colitis (UC), and the related diagnoses below. These included all ICD-9 555 and 556 codes (i.e., 555.X, 556.X) and all ICD-10 K50 and K51 codes (K50.XX, K51.XX), and K52.3 (indeterminate colitis). We required ≥ 1 of these diagnoses from two separate inpatient, emergency, or outpatient encounters over the study period, with the first diagnosis occurring at age < 10 years, and the second occurring at any time during the study period.
Cases with two codes with the same prefix (indicating the same diagnosis group) were categorized as that diagnosis (i.e., either CD or UC). Cases with codes for both diagnoses (CD and UC) were categorized as indeterminate colitis. We accepted the earliest IBD diagnosis code entry date as a surrogate for disease onset.
2.2.2. Nested case-control study
For the case-control study, we confirmed all cases through manual chart review. During the chart review, a case of confirmed IBD was defined as one with ≥ 1 of the ICD code(s) above on > 1 occasion and either: (1) an endoscopy, pathology, or radiology report definitive of IBD; [4,25] or (2) ≥ 12 dispensations (or one year of administration) of an IBD-related drug. [26]
If IBD categorization (i.e., CD versus UC) was not confirmed during chart review, the case was reviewed by a clinician (EL). The clinical adjudicator reviewed all endoscopic, imaging, and pathology reports, relevant clinical notes, and the specialist’s rationale for the most recent diagnosis listed in the medical record; cases were subsequently categorized as either CD or UC.
2.3. Statistical analyses
Data were analyzed using SAS version 9.4.
2.3.1. Retrospective cohort study of IBD incidence
We calculated age- and sex-stratified incidence rates for each calendar year over the ten-year period from January 1, 2007 to December 31, 2016. In addition to calculating overall IBD incidence rates (which included indeterminate colitis), we calculated rates for CD and UC separately. The age strata included ages 0–4 and 5–9 years. [4,27]
Crude incidence rates were calculated by dividing the number of incident cases each calendar year by the number of eligible person-years. Person-time calculation began at the start of the study period, the child’s date of birth, or the child’s first health plan enrollment date, whichever occurred last. Person-time calculation ended at date of first diagnosis among cases, or else disenrollment, death, the child’s 10th birthday, or the end of the study period, whichever occurred first.
Using multivariable Poisson regression, we estimated the effect of study year, age group (0–4 and 5–9 years), and sex on IBD incidence.
2.3.2. Nested case-control study
Cases confirmed by medical record review and/or adjudication, and their matched controls, were included in the case control analysis. We electronically extracted rotavirus vaccination history in both cases and controls. We extracted all doses received and created two analytic exposure variables: (1) dichotomous ever/never exposed to rotavirus vaccination and (2) continuous number of rotavirus vaccinations (0, 1, 2, and ≥ 3 doses).
A conditional logistic regression model was used to estimate the association between rotavirus vaccination and risk of IBD. The following data elements were examined as covariates: healthcare utilization within the first year of life (number of visits as a continuous variable), and vaccination history (defined as total number of administered vaccine dosages of any type received prior to diagnosis/reference date, as a continuous variable). For healthcare utilization within the first year of life, each day with a scheduled healthcare visit counted as one day of use, and each day of hospitalization counted as one day of use.
Odds ratios and their 95% confidence intervals for the association between overall IBD, CD, and UC and any rotavirus vaccination were reported; p-values < 0.05 were considered statistically significant.
3. Results
3.1. Retrospective cohort study of IBD incidence
Approximately 2.4 million children met criteria for inclusion in the study population and contributed person-time to the analysis. From the electronic data, 333 cases of IBD in children younger than 10 years old were identified across the study period (Table 1). Of these, 82 cases were diagnosed at ages 0–4 years old, and 251 were diagnosed at 5–9 years old. There were 153 cases of CD and 142 cases of UC; age and sex strata are shown in Table 1. There were more male cases of IBD, with the most pronounced difference in proportions in the 0–4 age range. We identified 38 cases of indeterminate colitis during the study period (i.e., these children received ICD diagnosis codes for both CD and UC).
Table 1.
Crude incidence of inflammatory bowel disease among children < 10 years of age from seven Vaccine Safety Datalink sites, overall and by age group and sex – 2007–2016*.
| Sex | Age group | No. children** (col %) |
Person-years | No. IBD cases (col %) |
IBD incidence | No. CD cases (col %) |
CD incidence | No. UC cases (col %) |
UC incidence |
|---|---|---|---|---|---|---|---|---|---|
| F | 0–4 years | 744,135 | 2,030,387 | 37 | 1.8 | 12 | 0.6 | 20 | 1.0 |
| 5–9 years | 907,050 | 2,341,072 | 115 | 4.9 | 50 | 2.1 | 53 | 2.3 | |
| Total female | 1,183,015 (48.9) | 4,371,459 | 152 (45.6) | 3.5 | 62 (40.5) | 1.4 | 73 (51.4) | 1.7 | |
| M | 0–4 years | 781,606 | 2,133,470 | 45 | 2.1 | 14 | 0.7 | 25 | 1.2 |
| 5–9 years | 948,210 | 2,455,132 | 136 | 5.5 | 77 | 3.1 | 44 | 1.8 | |
| Total male | 1,238,138 (51.1) | 4,588,602 | 181 (54.4) | 3.9 | 91 (59.5) | 2.0 | 69 (48.6) | 1.5 | |
| Total | 0–4 years | 1,525,741 | 4,163,857 | 82 | 2.0 | 26 | 0.6 | 45 | 1.1 |
| 5–9 years | 1,855,260 | 4,796,203 | 251 | 5.2 | 127 | 2.7 | 97 | 2.0 | |
| Total population | 2,421,153 (100) | 8,960,060 | 333 (100) | 3.7 | 153 (100) | 1.7 | 142 (100) | 1.6 |
Abbreviations: IBD – inflammatory bowel disease, CD – Crohn’s disease, UC – ulcerative colitis, F – female, M – male
Incidence shown per 100,000 person-years; IBD columns include cases of CD, UC, and indeterminate colitis (n = 38); ages represent age at first IBD diagnosis.
Total female, total male, and total population columns represent numbers of unique individuals. Due to age progression from 0–4 to 5–9-year age groups, the age groups are not restricted to unique individuals and do not align with group totals.
Crude annual incidence rates for all IBD (i.e., including CD, UC, and indeterminate colitis) ranged from a low of 2.8 (95% CI, 0.5–8.4) per 100,000 person-years to a high of 4.9 (95% CI, 1.6–11.5) per 100,000 person-years (Fig. 1). Across the study period, the overall crude incidence per 100,000 person-years was 3.7 (95% CI, 1.0–9.8) for IBD, 1.7 (95% CI, 0.2–6.8) for CD, and 1.6 (95% CI, 0.1–6.6) for UC (Table 1). The crude incidence rates for IBD, UC, and CD among children aged 0–9 years by calendar year across the study period are shown in Fig. 1. While the overall change was small, the crude incidence of IBD significantly increased between 2007 and 2016 (p = 0.046). The overall crude incidence rate for CD also significantly increased over the study period (p = 0.012). Fig. 2 shows crude incidence rates of IBD by age groups (0–4 years and 5–9 years) and sex. Incidence rates were significantly higher among children in the 5–9 years age group than the 0–4 years age group (p < 0.0001).
Fig. 1.
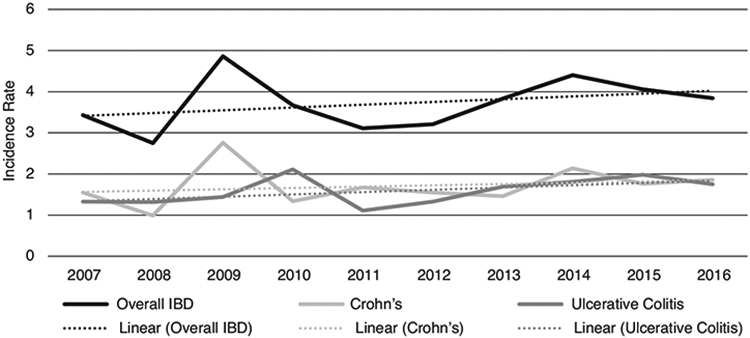
Abbreviations: IBD – inflammatory bowel disease, CD – Crohn’s disease, UC – ulcerative colitis. Crude incidence and linear trends of inflammatory bowel disease among children <10 years of age from six Vaccine Safety Datalink sites, 2007-2016. Incidence rates are shown per 100,000 person-years; “overall IBD” rate includes cases of CD, UC, and indeterminate colitis. Trend lines for overall IBD, CD, and UC represent the linear incidence trends within those categories across the study period. P-values for linear trends were 0.046, 0.012, and 0.281 for overall IBD, CD, and UC, respectively.
Fig. 2.
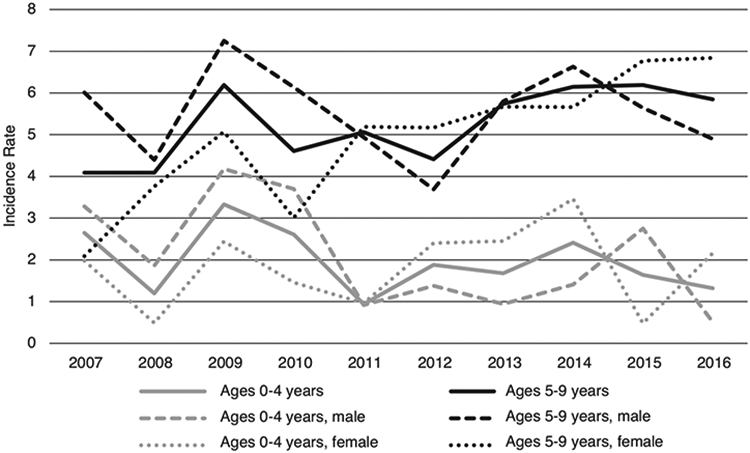
Crude incidence of inflammatory bowel disease among children <10 years of age from six Vaccine Safety Datalink sites, by age group and sex, 2007-2016. Incidence rates are shown per 100,000 person-years and include all cases of Crohn’s disease, ulcerative colitis, and indeterminate colitis; ages represent age at first IBD diagnosis.
3.2. Nested case-control study
Of the 333 IBD cases identified via electronic diagnoses in the retrospective cohort, 227 (68%) were born prior to January 1, 2007 and excluded from analysis. An additional 45 presumptive, electronic cases did not meet the continuous enrollment criterion (n = 40) or were completely unvaccinated (n = 5). This resulted in 61 eligible, presumptive IBD cases. Medical record review occurred for all 61 cases; 29 (48%) of these cases also required clinical adjudication. Following medical record review and/or adjudication, 19 (31%) cases did not meet IBD case definitions and were excluded from analysis; ten of the excluded cases were under the age of one at initial diagnosis (Fig. 3).
Fig. 3.
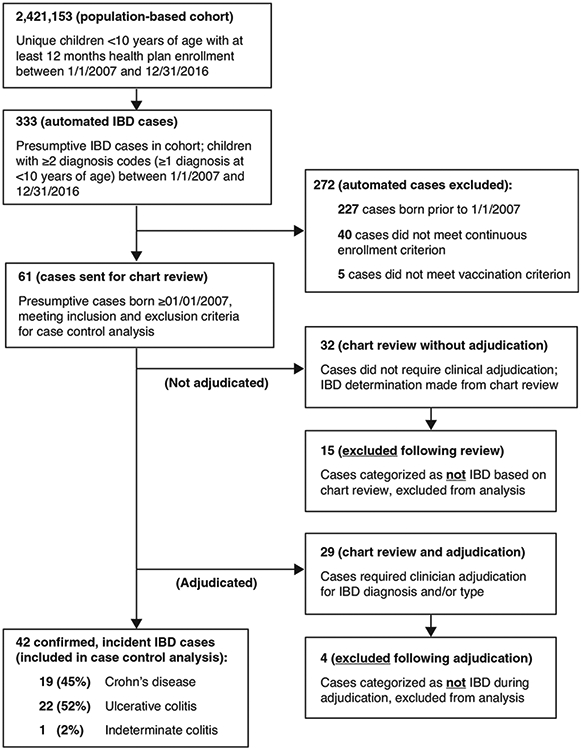
Identification and confirmation of inflammatory bowel disease cases from electronic medical records of approximately 2.4 million members of the Vaccine Safety Datalink network aged <10 years.
Forty-two incident, review-confirmed IBD cases met eligibility criteria and were included in the case-control analysis. Of these, 19 (45%) were CD, 22 (52%) were UC, and one (2%) case could not be classified (i.e., indeterminate colitis). Of the 42 cases, 22 (52%) were male and 20 (48%) were female; 25 (60%) of the cases were diagnosed in children ages 0–4 years and 17 (40%) were diagnosed in children ages 5–9 years (data not shown). There was a single case of IBD onset in a child under the age of one year old.
Table 2 shows the vaccine-related exposures and healthcare utilization covariates, comparing cases and controls. The rate of receiving any rotavirus vaccine for controls (90%) was slightly higher than that for cases (81%). Among vaccinated cases, 32% received two doses of rotavirus vaccine, and 62% received three or more doses. Among vaccinated controls, 30% received two doses of rotavirus vaccine, and 67% received three or more doses. The total number of vaccines of any type administered prior to the reference date did not differ between cases and controls (p = 0.797). As shown in Table 2, cases had higher healthcare utilization in the first year of life, as compared to controls (p = 0.046).
Table 2.
Nested case-control analysis exposures and covariates.
| Cases (n = 42) |
Controls (n = 210) |
|
|---|---|---|
| Received any rotavirus vaccine, no. (column %) | 34 (81%) | 189 (90%) |
| Received 1 dose rotavirus vaccine, no. (column %) | 2 (5%) | 6 (3%) |
| Received 2 doses rotavirus vaccine, no. (column %) | 11 (26%) | 57 (27%) |
| Received ≥ 3 doses rotavirus vaccine, no. (column %) | 21 (50%) | 126 (60%) |
| Number of health care visits in first year of life, mean (SD)* | 23.2 (32.5) | 12.6 (13.3) |
| Number of total vaccines received prior to reference date – any type, mean (SD) | 22.2 (6.1) | 22.3 (6.0) |
Number of days with a healthcare encounter in the first year of life; each day of a hospitalization included in total.
The adjusted odds ratio for the association between IBD and any rotavirus vaccine exposure was 0.72 (95% CI, 0.2–2.6). Odds ratios for the associations with CD and UC, specifically, were also less than one (Table 3).
Table 3.
Odds ratios of associations between ever vaccinated with rotavirus vaccine and risk of inflammatory bowel disease, by type of inflammatory bowel disease.
| Adjusted odds ratio* (95% confidence interval) |
No. discordant pairs |
|
|---|---|---|
| Inflammatory bowel disease** | 0.72 (0.2–2.6) | 47 |
| Crohn’s disease | 0.54 (0.1–2.7) | 29 |
| Ulcerative colitis | 1.0 (0.1–7.1) | 18 |
Models adjusted for health care utilization within the first year of life and total vaccination prior to diagnosis/reference date (any type); cases and controls matched 1:5 on study site, sex, and birth year.
Includes all cases of ulcerative colitis, Crohn’s disease, and indeterminate colitis.
4. Discussion
We found a small but statistically significant increase in the incidence of inflammatory bowel disease among children younger than 10 over a ten-year period, across the populations from seven integrated health systems in the United States. This was likely driven by the small but statistically significant increase in the incidence of Crohn’s disease, as the incidence of ulcerative colitis remained stable. In a nested case-control study of the same population, we did not identify an association between IBD and exposure to the live-attenuated oral rotavirus vaccine, though due to limited case numbers a small increase in risk may not have been detectable.
Our IBD incidence rates for children ages 0–9 are similar to those of other studies, despite geographic differences and variation in methodologies for identifying cases. [4,6,28-29] A study in northern France using a prospective, population-based registry and census data reported incidence rates for children ages 0–9 in 2009–2011 that were similar to ours for CD (1.2 (95% CI, 0.8–1.7), compared to our rate of 1.7 (95% CI, 0.2–6.8) cases per 100,000 person-years) and for UC (0.6 (95% CI, 0.3–1.0), compared to our rate of 1.6 (95% CI, 0.1–6.6) cases per 100,000 person-years).6 A study using a validated algorithm for identifying cases of pediatric IBD from administrative data in Ontario, Canada also had similar age-specific rates of IBD, UC, and CD for the year 2009 in their study compared to our rates for 2007–2016 study period.[29] Each of these studies also identified increases in IBD incidence among children younger than 10 years of age over their respective time periods—1988–2011 [6] and 1994–2009.[29] In these studies, the incidence rates rose for both UC and CD, whereas in our study, we saw only a statistically significant increase in CD incidence.
Our cohort study included nearly-two and a half million children under the age of 10 and used population-based denominators to identify trends in incidence of pediatric IBD over a ten-year period. Due to the resources required, all cases in the cohort study could not be manually reviewed; we identified IBD cases using ICD-9 or ICD-10 diagnosis codes given on two separate dates in the medical record during the study period. In contrast, some previous studies utilized IBD case registries as a source for their incidence population.[6,27] Because we did not confirm each case via chart review for the cohort study, our incidence rates are certainly an overestimate. Further, we do not know how case review and adjudication of those cases with codes for both CD and UC (i.e., those in the indeterminate colitis category) might have changed incidence estimates for each disease individually.
The possible association between live rotavirus vaccination and pediatric IBD has biological plausibility through the triggering of inflammation of the gut mucosa and is therefore worthy of examination.[15,30] Further, as parents seeking to limit the overall number of vaccinations their young children receive in a visit may eliminate or delay newer vaccines, such as the rotavirus vaccine, it is important to thoroughly examine vaccine safety to provide reassurance.[31,32] In our nested case-control study, drawn from a cohort of more than two million insured children, we found that administration of rotavirus vaccine was not associated with a diagnosis of IBD in children under ten; this finding persisted even when adjusting for health care utilization within the first year of life and for total vaccinations previously received. In our population, the rate of rotavirus vaccination was slightly higher among controls than cases, but overall vaccination rates (of any type) were similar across the groups. Cases of IBD had a significantly higher number of health care visits in the first year of life than did controls; from observations during medical record review, we theorize that this is due to many of the cases having multiple medical problems, some of them genetic, and therefore needing more intensive and frequent health care. The apparent increase in IBD incidence among children under the age of ten has not been fully explained and may be due to a shift in diagnosing patterns, or to environmental and socioeconomic factors, dietary habits, antibiotic consumption, or changing composition of the population. [28,33-34]
While robust, our study is not without limitations. In the cohort study, cases were counted if they had two diagnoses of IBD in their medical record, and not all cases were manually reviewed. Two previous studies utilized a validated algorithm based on the occurrence of endoscopy and number of hospitalizations and outpatient visits for IBD during the study period, [28-29] an approach that may prove promising as U.S. health systems increasingly integrate inpatient and outpatient medical records. Our experience in manual case confirmation indicates that selective chart review of cases under one year of age, those with codes for both CD and UC, and those with missing endoscopy or imaging results who have received less than 12 months of IBD-related medication may be the best use of resources. With regard to the case control study, due to methodologically necessary exclusions (e.g., restriction to children born after rotavirus vaccine licensure, requirement of continuous health plan enrollment from birth until the date of IBD diagnosis) and the rare nature of pediatric IBD, the overall number of eligible IBD cases in our analysis was low. This limited our ability to detect small associations between IBD and exposure to rotavirus vaccine. Power for the case-control study was determined post-hoc using the a-priori parameter of a two-tailed logistic regression, at 80% power and alpha level of 0.05. The minimum odds ratio detectable based on these assumptions was 3.367. We did not have sufficient power to examine rotavirus vaccine type (pentavalent versus monovalent). Additionally, given the sources of data for this study we were not able to control for all known associations with inflammatory bowel disease, such as genetic predisposition or environmental factors outside of vaccination. Finally, in both studies we used initial IBD diagnosis date as a surrogate for onset, as has been done previously. [4,28] As IBD is insidious in nature, symptom onset was likely earlier than diagnosis; the incidence estimates by year reported here represent year of initial diagnosis. Strengths of our study include the large study population, the detailed enrollment and diagnosis data available for person-time calculation, and detailed manual review, including clinician adjudication for case confirmation and categorization of IBD type (in the nested case-control study).
5. Conclusions
This large cohort study identified a small but statistically significant increase in the incidence of inflammatory bowel disease among children younger than 10 over a ten-year period, across the populations from seven integrated health systems in the United States. Though case numbers were limited, data from this large pediatric cohort suggest that rotavirus vaccination is not associated with development of IBD in children less than ten years of age.
Acknowledgements
The authors wish to acknowledge the contributions of Ning Smith (Kaiser Permanente Center for Health Research); Umesh Parashar (Centers for Disease Control and Prevention); Kate Burniece (Kaiser Permanente Colorado); Stacy Harsh and Mara Kalter (Kaiser Permanente Center for Health Research); and Bernie Dizon, Anna Lawless, Gina Lee, Kerresa Morrissette, Claire Park, Jose Pio, and Denison Ryan (Kaiser Permanente Southern California). This work was supported by the U.S. Centers for Disease Control and Prevention (Task Order 200-2012-53584-0006). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Liles received unrelated research funding from Merck, Pfizer, Epigenomics and Medial Solutions. Dr. Jackson received unrelated research funding from Pfizer. Dr. Jacobsen received unrelated research funding from Dynavax Technologies. Dr. Klein received unrelated research funding from Merck, GlaxoSmithKline, Pfizer, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur). Dr. Naleway received unrelated research funding from Pfizer.
References
- [1].Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58(2):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–39. [DOI] [PubMed] [Google Scholar]
- [3].Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): Analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146(1):35–40. [DOI] [PubMed] [Google Scholar]
- [4].Abramson O, Durant M, Mow W, Finley A, et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J Pediatr. 2010;157(2):233–239.e231. [DOI] [PubMed] [Google Scholar]
- [5].Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24(25):2741–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ghione S, Sarter H, Fumery M, et al. Dramatic increase in incidence of Ulcerative Colitis and Crohn’s Disease (1988–2011): A population-based study of French adolescents. Am J Gastroenterol. 2018;113(2):265–72. [DOI] [PubMed] [Google Scholar]
- [7].Jakobsen C, Paerregaard A, Munkholm P, et al. Pediatric inflammatory bowel disease: Increasing incidence, decreasing surgery rate, and compromised nutritional status: A prospective population-based cohort study 2007–2009. Inflamm Bowel Dis. 2011;17(12):2541–50. [DOI] [PubMed] [Google Scholar]
- [8].Lehtinen P, Ashorn M, Iltanen S, et al. Incidence trends of pediatric inflammatory bowel disease in Finland, 1987–2003, a nationwide study. Inflamm Bowel Dis. 2011;17(8):1778–83. [DOI] [PubMed] [Google Scholar]
- [9].Castro M, Papadatou B, Baldassare M, et al. Inflammatory bowel disease in children and adolescents in Italy: Data from the pediatric national IBD register (1996–2003). Inflamm Bowel Dis. 2008;14(9):1246–52. [DOI] [PubMed] [Google Scholar]
- [10].Orholm M, Munkholm P, Langholz E, Nielsen OH, Sorensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324(2):84–8. [DOI] [PubMed] [Google Scholar]
- [11].Bayless TM, Tokayer AZ, Polito JM 2nd, Quaskey SA, Mellits ED, Harris ML. Crohn’s disease: Concordance for site and clinical type in affected family members–potential hereditary influences. Gastroenterology 1996;111(3):573–9. [DOI] [PubMed] [Google Scholar]
- [12].Aujnarain A, Mack DR, Benchimol EI. The role of the environment in the development of pediatric inflammatory bowel disease. Curr Gastroenterol Rep 2013;15(6):326. [DOI] [PubMed] [Google Scholar]
- [13].Benchimol EI, Kaplan GG, Otley AR, et al. Rural and urban residence during early life is associated with risk of Inflammatory Bowel Disease: A population-based inception and birth cohort study. Am J Gastroenterol. 2017;112(9):1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baron S, Turck D, Leplat C, et al. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut 2005;54(3):357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Glanz JM, Newcomer SR, Jackson ML, et al. White Paper on studying the safety of the childhood immunization schedule in the Vaccine Safety Datalink. Vaccine. 2016;34(Suppl 1):A1–a29. [DOI] [PubMed] [Google Scholar]
- [16].Cadwell K, Patel KK, Maloney NS, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 2010;141(7):1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Porter CK, Tribble DR, Aliaga PA, Halvorson HA, Riddle MS. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology 2008;135(3):781–6. [DOI] [PubMed] [Google Scholar]
- [18].Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006;130(6):1588–94. [DOI] [PubMed] [Google Scholar]
- [19].Greenberg HB, Gebhard RL, McClain CJ, Soltis RD, Kapikian AZ. Antibodies to viral gastroenteritis viruses in Crohn’s disease. Gastroenterology 1979;76(2):349–50. [PubMed] [Google Scholar]
- [20].Whorwell PJ, Davidson IW, Beeken WL, Wright R. Search by immunofluorescence for antigens of Rotavirus, Pseudomonas maltophilia, and Mycobacterium kansasii in Crohn’s disease. Lancet 1978;2(8092 Pt 1):697–8. [DOI] [PubMed] [Google Scholar]
- [21].Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: A model for monitoring immunization safety. Pediatrics 2011;127(Suppl 1):S45–53. [DOI] [PubMed] [Google Scholar]
- [22].Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm Bowel Dis. 2011;17(6):1314–21. [DOI] [PubMed] [Google Scholar]
- [23].Parashar UD, Alexander JP, Glass RI. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-12):1–13. [PubMed] [Google Scholar]
- [24].Glanz JM, Newcomer SR, Narwaney KJ, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167(3):274–81. [DOI] [PubMed] [Google Scholar]
- [25].North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America, Bousvaros A, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: Report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–674. [DOI] [PubMed] [Google Scholar]
- [26].Herrinton LJ, Liu L, Lewis JD, Griffin PM, Allison J. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996–2002. Am J Gastroenterol. 2008;103(8):1998–2006. [DOI] [PubMed] [Google Scholar]
- [27].Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: A 12-year study. J Pediatr Gastroenterol Nutr. 2010;50(1):27–31. [DOI] [PubMed] [Google Scholar]
- [28].Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: Evidence from health administrative data. Gut 2009;58(11):1490–7. [DOI] [PubMed] [Google Scholar]
- [29].Benchimol EI, Mack DR, Nguyen GC, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014;147(4):803–e15. [DOI] [PubMed] [Google Scholar]
- [30].Grimaldi-Bensouda L, Guillemot D, Godeau B, et al. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J Intern Med. 2014;275(4):398–408. [DOI] [PubMed] [Google Scholar]
- [31].Boyle J, Berman L, Nowak GJ, Iachan R, Middleton D, Deng Y. An assessment of parents’ childhood immunization beliefs, intentions, and behaviors using a smartphone panel. Vaccine. 2020;38(10):2416–23. [DOI] [PubMed] [Google Scholar]
- [32].Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics 2011;128(5):848–56. [DOI] [PubMed] [Google Scholar]
- [33].Rogler G, Zeitz J, Biedermann L. The search for causative environmental factors in Inflammatory Bowel Disease. Dig Dis. 2016;34(Suppl 1):48–55. 10.1159/000447283. [DOI] [PubMed] [Google Scholar]
- [34].Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not Ulcerative Colitis: A meta-analysis. Am J Gastroenterol. 2014;109(11):1728–38. [DOI] [PubMed] [Google Scholar]


