Abstract
Purpose
Inflammasomes are multiprotein complexes that detect danger-associated signals and trigger an immunostimulatory form of cell death called pyroptosis. NLRP1 is an innate immune receptor that assembles into an inflammasome, but the primary cell types in which NLRP1 is functional have not yet been fully established. Mutations in NLRP1 are associated with diseases of barrier epithelial tissues, including skin lesions and corneal intraepithelial dyskeratosis, suggesting that NLRP1 functions within the eye. Here, we investigated the expression and activity of the NLRP1 inflammasome in primary human corneal epithelial (pHCE) cells.
Methods
The small molecule Val-boroPro (VbP) activates the NLRP1 inflammasome. Proteasome (bortezomib, MG132) and caspase-1 (VX-765, Z-VAD-FMK) inhibitors block NLRP1 activation and downstream pyroptosis, respectively. Here, we treated pHCE cells with VbP alone or in combination proteasome inhibitors and caspase-1 inhibitors. We assessed NLRP1 expression and hallmarks of pyroptosis, including lytic cell rupture, cytokine processing and release, and gasdermin D (GSDMD) processing.
Results
VbP triggered pyroptosis in pHCE cells, as determined by cytokine secretion, GSDMD processing, and lactate dehydrogenase (LDH) release. Proteasome and caspase-1 inhibitors completely blocked this pyroptotic cell death. In contrast, other primary ocular epithelial cells did not undergo NLRP1-dependent pyroptosis.
Conclusions
Our findings demonstrate that NLRP1 forms a functional inflammasome in pHCE cells. Importantly, these data reveal that NLRP1 is a key innate immune sensor of the corneal epithelium, and moreover indicate how aberrant inflammasome activation causes corneal damage. Blockade of NLRP1 signaling may benefit patients with hyperactive NLRP1 mutations and warrants further investigation.
Keywords: NLRP1, cornea, cytokines, inflammasome, innate immunity
Innate immunity is an ancient form of host defense that protects against invading pathogens. This system relies on the host's ability to sense conserved microbial features and rapidly mount a protective immune response.1 The cornea is the outermost layer of the eye and plays a critical role in protecting deeper ocular structures.2 As such, defense against ocular infection and other external hazards requires an intact host defense system within the cornea. It is well established that the cornea engages innate immune responses with pattern recognition receptors (PRRs), including toll-like receptors on the cell surface which detect extracellular danger.3
A number of cytosolic PRRs, including the NOD-like receptors (NLRs), sense intracellular danger. Upon activation, some NLRs assemble into multiple protein complexes called inflammasomes.4,5 These signaling platforms recruit and activate the cysteine protease caspase-1 (CASP1), which in turn cleaves the pro-inflammatory cytokines pro-interleukin-1β and -18 (proIL-1β/18) as well as the pore-forming protein gasdermin D (GSDMD).6–8 The released N-terminal fragment of GSDMD oligomerizes at the cell membrane, forms pores that release matured cytokines, and eventually induces cell lysis.9 This programmed form of inflammatory cell death, termed pyroptosis, leads to the rapid recruitment of immune cells to the perceived site of “danger.” The role of specific inflammasomes in ocular defense has not been definitively established. However, their dysregulation has been postulated to contribute to several autoinflammatory disorders, including those with ocular pathologies.10–17
Nucleotide-binding domain and leucine-rich repeat pyrin-domain containing protein 1 (NLRP1) was the first NLR protein discovered to form an inflammasome.18 Human NLRP1 is thought to be primarily expressed in air exposed barrier tissues, including the skin, airway, and eyes.19 Consistent with this expression pattern, NLRP1 has been linked to autoinflammatory diseases in these tissues.19–23 Soler and colleagues first described an NLRP1 missense mutation (M77T) in two related patients with corneal epithelial dyskeratosis.20 These corneal opacifications were accompanied by palmoplantar hyperkeratosis and laryngeal dyskeratosis.20 Zhong and colleagues then reported two related syndromes resulting from four germline mutations in NLRP1 (A54T, A66V, M77T, and F787_R843del).19 These conditions, termed multiple self-healing palmoplantar carcinoma (MSPC) and familial keratosis lichenoides chronica (FKLC), had predominant ocular, dermatologic, and rheumatic features.19 Concurrently, Grandemange and colleagues described a novel condition, NLRP1-associated autoinflammation with arthritis and dyskeratosis (NAIAD),21 in three patients from two unrelated families. These patients presented with diffuse skin dyskeratosis, autoinflammation, and arthritis, and were found to have NLRP1 missense mutations (R726W and P1214R). More recently, three additional missense mutations in NLRP1 (A59P, T755N, and P1214L) have been linked with corneal dyskeratosis,22 juvenile-onset recurrent respiratory papillomatosis (JRRP),23 and liver cirrhosis,24 respectively.
Several recent reports have at least partially characterized the molecular mechanisms for NLRP1 activation.19,25–32 Briefly, NLRP1 undergoes autoproteolysis between its ZU5 and UPA domains to generate inhibitory N-terminal (NT) and inflammatory C-terminal (CT) fragments that remain associated and restrained (Supplementary Fig. S1A).33–35 Under resting conditions, the proteasome slowly degrades NLRP1-NT, thereby releasing NLRP1-CT from autoinhibition. Low levels of NLRP1-CT are next captured and sequestered as part of a ternary complex formed with dipeptidyl peptidase 8 or 9 (DPP8/9) and a full-length copy of NLRP1.26,27 However, stimuli that either increase the rate of NLRP1-NT degradation and/or destabilize the sequestration complex can free enough NLRP1-CT to assemble into an inflammasome (see Supplementary Fig. S1B).28,29,36 Potent DPP8/9 inhibitors, including Val-boroPro (VbP), activate NLRP1 via both mechanisms.26,31,37
Patient derived NLRP1 mutations occur in both the NLRP1-NT and NLRP1-CT fragments (see Supplementary Fig. S1A). It is currently thought that the NLRP1-NT mutations produce an unstable protein that accelerates the rate of NT degradation.38 These mutations can manifest as autosomal dominant, recessive, or incompletely penetrant depending on the location of the mutation.19,21–23,26 NLRP1-CT mutations, on the other hand, reduce DPP9 binding, eliminating the buffering capacity for NLRP1-CT.26 These mutations present as autosomal dominant for disease. Thus, pathogenic NLRP1 mutations lower the activation threshold and enable spurious inflammasome formation in cells that express NLRP1.
NLRP1 activation was recently shown to induce pyroptosis in primary keratinocytes19 and bronchial epithelial cells,25 providing a molecular basis for disease in these tissues. Despite corneal symptoms associating with NLRP1-related diseases, it is unknown whether corneal cells themselves undergo NLRP1-dependent pyroptosis and directly cause corneal injury. Here, we investigated the activity of the NLRP1 inflammasome in primary human corneal epithelial (pHCE) cells. We show that NLRP1 assembles into a functional inflammasome, which processes pro-inflammatory cytokines and executes pyroptosis, in pHCE cells. We also demonstrate NLRP1 is not ubiquitously expressed in all ocular epithelial cells. We finally reveal that inhibitors of NLRP1 signaling including CASP1 inhibitors and proteasome inhibitors block NLRP1-dependent pyroptosis in pHCE cells. Together, this work provides a molecular framework for how hyperactive NLRP1 signaling directly induces corneal damage.
Materials and Methods
Cell Culture
The pHCE cells were purchased from Cell Applications (San Diego, CA, USA) and Millipore Sigma (Burlington, MA, USA) and maintained in Corneal Epithelial Cell Growth Medium (Cell Applications). Primary human retinal pigment epithelial cells (pHRPE), primary human lens epithelial cells (pHLE), and primary human iris pigmen epithelial cells (pHIPE) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and maintained on dishes pretreated with poly-L-lysine (2 µg/mL) to promote adhesion in epithelial cell medium (EpiCM) supplemented with epithelial cell growth supplement, 0.2% fetal bovine serum, and penicillin/streptomycin (ScienCell). N/TERT1 cells were a gift from the Rheinwald Laboratory,39 and maintained in Keratinocyte serum free medium (Thermo Scientific, Waltham, MA, USA) supplemented with penicillin/streptomycin, bovine pituitary extract (25 µg/mL), and epidermal growth factor (0.2 ng/mL). THP-1 cells were purchased from ATCC (Manassas, VA, USA) and maintained in RPMI (Thermo Scientific) supplemented with 10% fetal bovine serum. Primary T cells were purchased from HemaCare (Northridge, CA, USA) and Cellero (Lowell, MA, USA) and cultured in RPMI with 10% fetal bovine serum (FBS). All cells were grown at 37°C in a 5% CO2 atmosphere incubator and regularly tested for mycoplasma using the MycoAlert Mycoplasma Detection Kit (Lonza, Basel, Switzerland).
Cell Stimulations
In general, pHCE cells were seeded in 12-well or 24-well plates at 5000 cells per cm2 in 1 mL or 750 µL of corneal epithelial cell growth medium, respectively. Media was changed every other day until cells were 60% to 90% confluent. Cells were treated with the indicated concentration of VbP (Cayman Chemical, Ann Arbor, MI, USA), CQ31 (Rao et al. under review), bortezomib (Millipore Sigma), MG132 (Millipore Sigma), Z-VAD-FMK (Enzo Life Science, Farmingdale, NY, USA), or VX765 (Cayman) for the indicated amount of time. For NLRP3 experiments, cells were primed overnight with 1 µg/mL of LPS (InvivoGen, San Diego, CA, USA) followed by treatment with 10 µM nigericin (Cayman) for 6 hours. Adherent cells were seeded in 6-well dishes 3 days prior to priming, whereas suspension cells were seeded 1 day prior to priming. For poly(I:C) experiments, cells were transfected for 6 hours with 50 µL of Opti-MEM solution containing 1 µL Fugene HD (Promega, Madison, WI, USA) and either 10 µM VbP or 200 ng poly(I:C) HMW (InvivoGen, San Diego, CA, USA).
Cytotoxicity Measurements
Cell culture medium was harvested from samples at the indicated time along with a media only, and Triton X-100 (Millipore Sigma) lysis control. The quantity of lactate dehydrogenase (LDH) was measured using the CyQUANT LDH Cytotoxicity Assay Kit (Thermo Scientific) according to the manufacturer's instructions. Absorbance values were measured at 490 nm with a reference at 680 nm by spectrophotometer (BioTek Instruments, Cytation 5, Winooski, VT, USA).
ELISA Measurements
IL-1β (R&D Systems, Minneapolis, MN, USA; Catalog #DLB50) and IL-18 (Abcam, Cambridge, UK; Catalog #ab215539) were measured using the ELISA method from cell culture medium according to the manufacturer's specifications. Absorbance values were measured at 450 nm with a reference at 570 nm by spectrophotometer (BioTek).
Luminex Cytokine Assay
The pHCE cells were seeded in 6-well plates at a density of 7500 cells per cm2 and allowed to adhere. Primary human T cells (2,000,000 cells in 500 µL) were seeded in 24-well plates. Cells were treated with DMSO or VbP (10 µM) for 18 hours at which time cell culture media was harvested for analysis using the Inflammation 20-Plex Human ProcartaPlex Panel (Thermo Scientific) per the manufacturer's specifications. Data were collected on a Luminex FLEXMAP 3D system (Luminex, Austin, TX, USA).
Widefield Microscopy
Cells were treated with propidium iodine (1 µg/mL; Cayman) and Hoechst dye (1 µg/mL; Thermo Scientific) for 30 minutes prior to stimulation with DMSO, VbP (10 µM) and/or bortezomib (10 µM). Live-cell imaging was performed on a Zeiss Axio Observer equipped with a 10 × 0.3 NA air Zeiss Plan-NEOFLUAR DIC I objective (Zeiss, Oberkochen, Germany) in a chamber maintained at 37°C with 5% CO2. Selected positions were imaged with DAPI and TRITC filters. Data were exported as raw .czi files and visualized in ImageJ.
Immunoblotting
Cells were pelleted by centrifugation and lysed in PBS with HALT protease inhibitor cocktail (Thermo Scientific). Protein concentration was measured by DC assay (BIO-RAD, Hercules, CA, USA). Normalized protein samples were boiled in 2X loading dye (LI-COR, Lincoln, NE, USA). Supernatants were concentrated with 3 kD amicon filters (Millipore Sigma), and boiled in 2X dye. Samples ran on NuPAGE 4% to 12%, Bis-Tris, 1.0 mm, Midi Protein Gel (Thermo Scientific) for 45 to 60 minutes at 175 V. Gels were transferred to nitrocellulose. Membranes were blocked with Intercept Blocking Buffer (LI-COR) prior to incubating with primary antibody overnight (Supplementary Table S1). Blots were washed 3 times prior to incubating with secondary antibody for 60 minutes at ambient room temperature. Blots were washed three times with Tris-buffered saline-Tween 20 (TBST) buffer, rinsed with water and imaged via Odyssey CLx (LI-COR).
Statistical Analyses
All results were analyzed using GraphPad Prism (San Diego, CA, USA; version 9.1.0) using the unpaired t-tests, 1-way ANOVA with Dunnett multiple comparisons correction, or 2-way ANOVA with Tukey's multiple comparisons correction. P values ≤ 0.05 were considered statistically significant.
Results
DPP8/9 Inhibitors Induce Pyroptosis in pHCE Cells
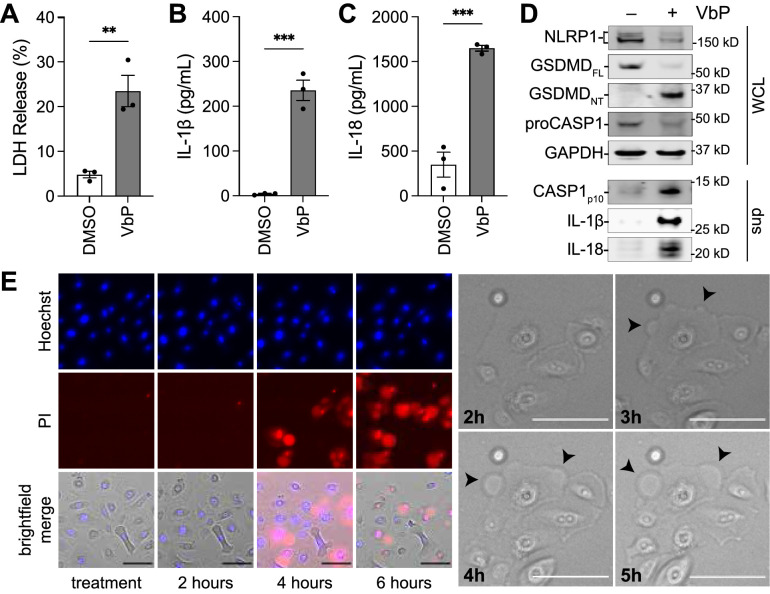
Based on the genetic disorders described above, we hypothesized that corneal epithelial cells express a functional NLRP1 inflammasome, and that hyperactive NLRP1 signaling causes corneal damage. We have previously reported that VbP, a potent inhibitor of DPP8/9, activates the related NLRP1 and CARD8 inflammasomes.28,30,40,41 To determine if NLRP1 and/or CARD8 are functional in pHCE cells, we treated these cells with VbP for 24 hours before assessing for pyroptotic cell death. We found that VbP-treated pHCE cells released significantly more LDH and inflammatory cytokines into the media relative to DMSO-treated control cells (Figs. 1A–C). Consistent with inflammasome activation, VbP also induced cleavage of GSDMD into its active pore-forming 30 kD fragment, as well as processing of CASP1, IL-1β, and IL-18 (Fig. 1D). Moreover, time-lapse microscopy of pHCE cells treated with VbP displayed cell swelling, membrane blebbing, and rapid propidium iodine uptake, characteristic features of pyroptosis (Fig. 1E). VbP induced pyroptosis was dose-dependent with a half-maximal inhibitor concentration (IC50) of 60 nM in these cells (Supplementary Fig. S2). Collectively, these data demonstrate that VbP induces pyroptosis in pHCE cells.
Figure 1.
VbP induces pyroptosis in pHCE cells. The pHCE cells were treated with DMSO or the DPP8/9 inhibitor VbP (10 µM) for 24 hours. Supernatants were evaluated for the release of LDH (A) and bioactive cytokines (B, C). Cell lysates and concentrated supernatants were assessed by immunoblotting (D). (E) Cells were treated with VbP (10 µM) and imaged by time-lapse microscopy. Representative images of propidium iodine (PI; red), and nuclei (Hoechst; blue) merged with brightfield (left panel); and zoom-in of pyroptotic cell, arrow heads reveal pyroptotic “blebs” (right panel). Scale bars 100 µm. Data are mean ± SEM from n = 3 donors A–C. ***P < 0.001, **P < 0.01 by unpaired t-test; WCL = whole cell lysate, sup = supernatant, GSDMDFL = full length GSDMD, GSDMDNT = N-terminal GSDMD p30 fragment resulting from caspase-1 cleavage.
VbP Induces NLRP1-Dependent Pyroptosis in pHCE Cells
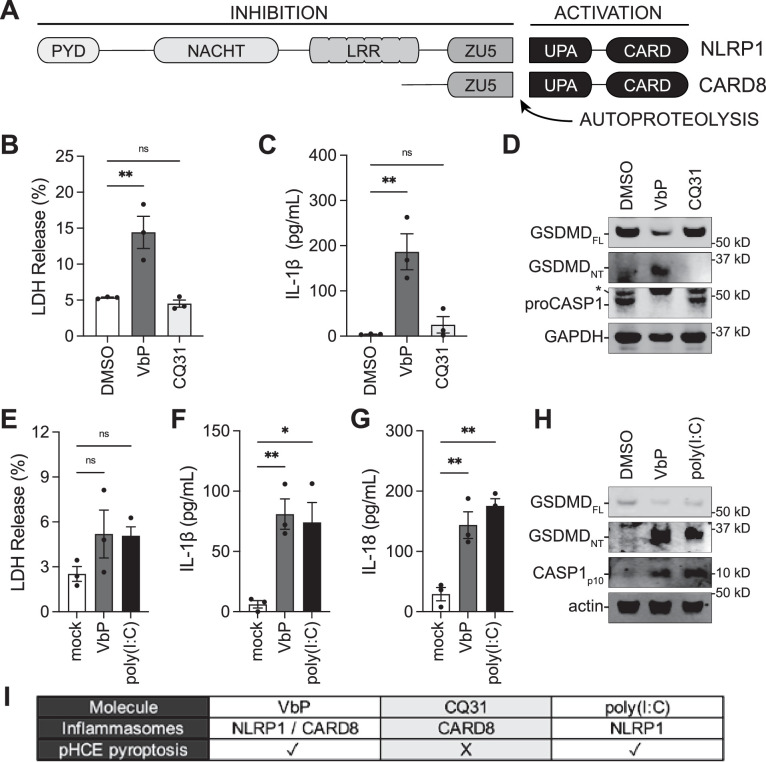
VbP activates both the NLRP1 and CARD8 inflammasomes.30,32,40,41 These related pattern-recognition receptors have similar domain organizations and activation mechanisms (Fig. 2A),26,28,29,38,42 but have at least four differences that are relevant to this study. First, CARD8 is highly expressed in hematopoietic cells,41,43,44 whereas NLRP1 is highly expressed in barrier tissues.38 Second, the CARD8 inflammasome, unlike the NLRP1 inflammasome, does not efficiently process pro-IL-1β and 18.36,45 Third, the long double stranded RNA mimetic poly(I:C) was recently reported to activate NLRP1, but not CARD8.46 Fourth, a small molecule we recently identified called CQ31 selectively activates the CARD8 inflammasome without simultaneously activating the NLRP1 inflammasome (Rao SD, Chen Q, Wang Q, et al. M24B aminopeptidase inhibitors selectively activate the CARD8 inflammasome. Nat Chem Biol. 2022;doi:10.1038/s41589-021-00964-7). Although we reasoned that VbP activated the NLRP1 inflammasome in pHCE cells based on our expression and cytokine data (see Fig. 1), it remained possible that CARD8 was also functional in corneal epithelial cells. To test whether CARD8 contributed to the observed phenotype, we treated pHCE cells with VbP or CQ31. Consistent with our previous results, VbP induced marked cell death, IL-1β release, and GSDMD processing at 18 hours (Figs. 2B–D). However, CQ31 did not induce any observable pyroptosis in these cells (see Figs. 2B–D). These data indicate that pHCE cells do not express a functional CARD8 inflammasome. Moreover, consistent with NLRP1 activation, we found that poly(I:C), like VbP, caused significant release of IL-1β and IL-18 as well as processing of CASP1 and GSDMD (Figs. 2E–H). It should be noted that we stimulated pHCE cells with poly(I:C) for 6 hours to avoid NLRP1-independent effects over longer intervals,46 and neither VbP nor poly(I:C) induced significant LDH release at this shorter time point (Fig. 2E). Collectively, these data confirm that pHCE cells express a functional NLRP1, but not CARD8, inflammasome (Fig. 2I).
Figure 2.
The pHCE cells undergo NLRP1 dependent pyroptosis. (A) Schematic of the related NLRP1 and CARD8 proteins with their subdomains and sites of autoprocessing labeled. Potent DPP8/9 inhibitors, including VbP, activate both NLRP1 and CARD8. (B–D) pHCE cells were treated with VbP (10 µM) and the selective CARD8 activator CQ31 (10 µM) for 18 hours before LDH B and cytokine C release were measured, and cellular contents assessed by immunoblotting D. Asterisk (*) indicates nonspecific band. (E–H) The pHCE cells were transfected with the NLRP1-selective activator, poly(I:C) (200 ng) or VbP (10 µM) for 6 hours. Pyroptosis was monitored by cytokine secretion to F, immunoblotting of cellular lysates G, and LDH release H. (I) Summary of NLRP1 versus CARD8 dependence in pHCE cells. Data are mean ± SEM from n = 3 donors. **P < 0.01, *P < 0.05, ns = not significant by 1-way ANOVA with Dunnett multiple comparison correction. FL = full length; NT = N-terminal.
Cytokine Profile for NLRP1 and CARD8 Dependent Pyroptosis
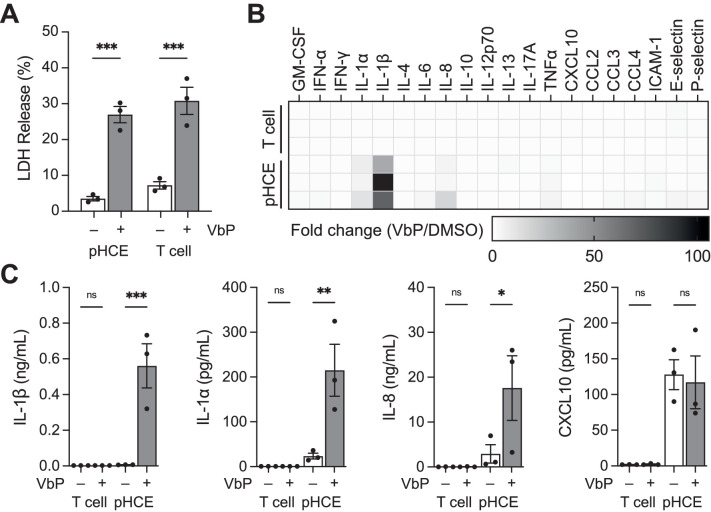
We next wanted to determine the full complement of cytokines released during NLRP1-dependent pyroptosis in pHCE cells and to compare those to the cytokines released during CARD8-dependent pyroptosis. Here, we treated pHCE cells and primary resting CD8-positive T cells with VbP for 24 hours. We selected T cells as a control because they are the best characterized primary human cell type that forms a functional CARD8 inflammasome in response to VbP.43,44,47 Importantly, VbP only induces CARD8-dependent cell death in resting, but not active T cells.43,44 Thus, all experiments were conducted in the absence of TCR/CD28 stimulation. As anticipated, both cell types released LDH, confirming pyroptotic cell death (Fig. 3A). However, analysis of cell culture media for cytokines with the Luminex coupled Inflammation 20-Plex Human ProcartaPlex Panel revealed stark differences (Fig. 3B). VbP induced IL-1β, IL-1α, and IL-8 release from pHCE cells (Fig. 3C), but did not stimulate any cytokine secretion from T cells. These data demonstrate that the NLRP1 inflammasome induces pro-inflammatory cytokine maturation and secretion, whereas the CARD8 inflammasome does not, at least in these cell types.
Figure 3.
Cytokine profile of NLRP1 and CARD8-dependent pyroptosis. (A–C) The pHCE cells (NLRP1-dependent) and primary resting T cells (CARD8-dependent) were treated with VbP (10 µM) for 24 hours. Pyroptotic cell death was confirmed by the release of intracellular LDH A. Cell culture media was analyzed for cytokines using the Luminex Inflammation 20-Plex Human ProcartaPlex Panel B. C Quantification of select cytokines from Luminex data shown in B. Data are means ± SEM from n = 3 donors for each cell type (A, C) and mean of n = 2 technical replicates from each donor B. ***P < 0.001, **P < 0.01, *P < 0.05, ns = not significant by 2-way ANOVA with Tukey's multiple comparison correction.
NLRP1 is Spatially Confined to the Cornea
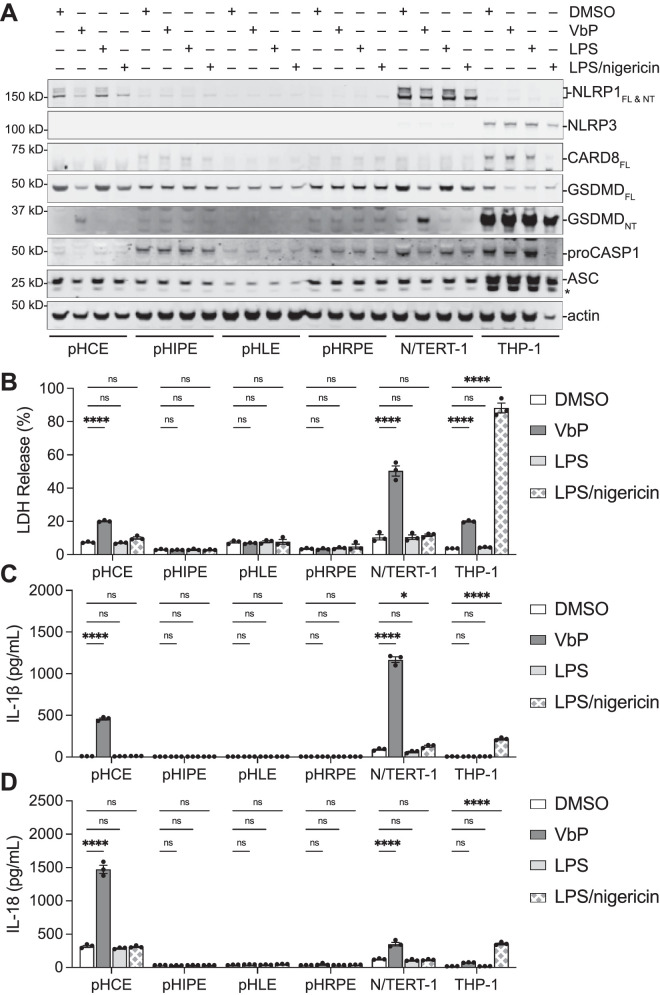
NLRP1 mRNA has previously been detected in several ocular tissues, including retina, choroid, sclera, cornea, and optic nerve by RT-qPCR.20 The NLRP1 protein levels, however, have not been extensively studied in these cell types. We next examined a panel of primary human ocular cells consisting of cornea epithelium, iris pigmented epithelium (pHIPE), lens epithelium (pHLE), and retinal pigmented epithelium (pHRPE) for inflammasome activity. Immortalized N/TERT-1 keratinocytes (NLRP1-dependent) and THP-1 monocytes (CARD8-dependent) were included as controls. We first immunoblotted for inflammasome forming proteins (Fig. 4A). As expected, we observed NLRP1 expression in pHCE and N/TERT-1 cells, as well as NLRP3 and CARD8 expression in THP-1 cells. However, the other primary ocular epithelial cells had no discernable NLRP1, NLRP3, or CARD8 expression even following LPS priming. Consistent with the expression profiles, VbP induced LDH, IL-1β, and IL-18 release in pHCE and N/TERT-1 cells, which are NLRP1-dependent (Figs. 4B–D). Likewise, VbP caused LDH release but no cytokine release in THP-1 cells, which are CARD8-dependent. None of the other primary eye cells responded to VbP stimulation, suggesting that NLRP1 is not functional in these cell types.
Figure 4.
NLRP1-dependent pyroptosis is unique to cornea epithelial cells compared to other ocular epithelial cells. (A–D) Cells were treated with DMSO, VbP (10 µM, 24 hours), LPS (1 µg/mL, 16 hours), or LPS and nigericin (10 µM, 6 hours). Cellular contents were evaluated by immunoblotting lysates A. Pyroptosis was monitored by cell death B and cytokine release C and D. An asterisk (*) indicates nonspecific band. Data are mean ± SEM from n = 3 independent biological replicates from individual donors. ****P < 0.0001, *P < 0.05, ns = not significant by 2-way ANOVA with Tukey's multiple comparison correction. FL = full length; NT = N-terminal.
We next tested whether a functional NLRP3 inflammasome could form in cells primed with LPS and stimulated with nigericin.48 As previously reported, the combination of LPS and nigericin triggered cell death and cytokine release in THP-1 cells. In contrast, LPS plus nigericin did not induce pyroptosis in any of the ocular epithelial cells or in N/TERT-1 keratinocytes, consistent with the lack of NLRP3 expression. Together, these data reveal that NLRP1 expression and activation is unique to the cornea and provide a rationale for why NLRP1-mediated inflammatory diseases do not extend into other ocular tissues.
Pharmacologic Modulation of NLRP1 Pyroptosis in Human Corneal Epithelial Cells
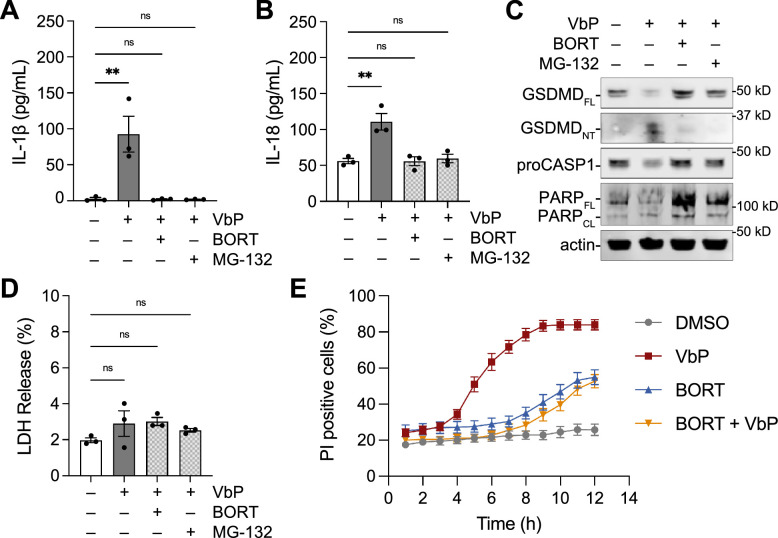
Having established that pHCEs undergo NLRP1-dependent pyroptosis, we next explored whether this pathway was amenable to pharmacological modulation. We first asked if proteasome inhibitors blocked NLRP1-dependent pyroptosis in pHCE cells. Because proteasome inhibition can cause toxicity (apoptosis) over long durations, we co-treated pHCE cells with VbP and one of two proteasome inhibitors, MG-132 or bortezomib, for only 6 hours. At this timepoint, proteasome inhibition ablated GSDMD processing and cytokine secretion, reducing IL-1β and IL-18 levels to that of untreated controls (Fig. 5). Similarly, bortezomib rescued poly(I:C) induced cytokine release (Supplementary Fig. S3). Notably, neither proteasome inhibitor killed pHCE cells in 6 hours as measured by poly (ADP-ribose) polymerase (PARP) cleavage, LDH release or PI uptake (Fig. 5C–E). Thus, proteasome function is necessary for activation of the NLRP1 inflammasome in pHCE cells.
Figure 5.
Proteasome inhibitors block NLRP1-dependent pyroptosis in pHCE cells. (A–D) The pHCE cells were treated with VbP (10 µM) and the indicated proteasome inhibitor (10 µM) for 6 hours. Supernatants were analyzed for cytokine release A and B, LDH release D, and lysates were immunoblotted C. Data are mean ± SEM from n = 3 donors. **P < 0.0001, ns = not significant by 1-way ANOVA with Dunnett multiple comparison correction. (E) The pHCE cells were treated with VbP (10 µM) and/or bortezomib (10 µM). Propidium iodine (PI) uptake was monitored by microscopy for 12 hours. The percentage of PI positive cells are graphed over time. Data are mean ± SEM from n = 10 fields of view from one donor. BORT = bortezomib; FL = full length; NT = N-terminal; CL = cleaved.
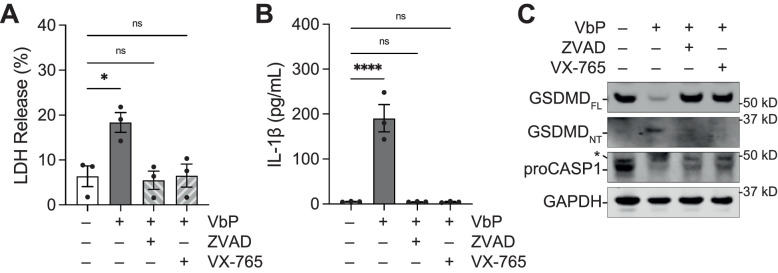
We next asked if caspase-1 inhibition could prevent NLRP1 driven pyroptosis in human corneal epithelial cells. We treated pHCE cells with VbP alone or in combination with the pan caspase inhibitor, Z-VAD-FMK, or the selective caspase-1 selective inhibitor VX-765 for 18 hours. Caspase-1 inhibition significantly reduced LDH release (Fig. 6A), IL-1β secretion (Fig. 6B), and GSDMD processing (Fig. 6C). Similarly, Z-VAD-FMK was able to rescue poly(I:C) induced cytokine release (see Supplementary Fig. S3). Thus, caspase-1 is an essential downstream effector of the NLRP1 inflammasome in pHCEs. Collectively, these data illustrate that the NLRP1 signaling is amenable to pharmacologic blockade in corneal epithelial cells.
Figure 6.
Caspase-1 inhibition protects against pyroptosis in pHCE cells. (A) The pHCE cells were treated with VbP (10 µM) and either the pan-caspase inhibitor Z-VAD-FMK (100 µM) or CASP1 specific inhibitor VX765 (50 µM) for 18 hours. Supernatants were analyzed for LDH release (B) and IL-1β (C). Lysates were inspected for GSDMD processing (D). Data are mean ± SEM from n = 3 donors. ****P < 0.0001, *P < 0.05, ns = not significant by 1-way ANOVA with Dunnett multiple comparison correction. ZVAD = Z-VAD-FMK; FL = full length; NT = N-terminal.
Discussion
Inflammasomes are pro-inflammatory signaling platforms that execute pyroptosis and are implicated in multiple human diseases, including ocular pathologies.17 Over the past decade, mutations in NLRP1 have been associated with at least four allelic Mendelian diseases – MSPC, FKLC, NAIAD, and JRRP.19–21,23 Patients with these conditions suffer from severe inflammation of their barrier tissues, including skin, airway, and cornea. Molecular studies have shown that keratinocytes and airway epithelial cells are capable of NLRP1-dependent pyroptosis.19,25 In the present study, we now show that NLRP1 activation induces pyroptosis in pHCE cells.
Our data indicate that NLRP1 inflammasome activation resulting from two distinct triggers, VbP and dsRNA, is associated with caspase-1 cleavage, lytic cell rupture, and the release of pro-inflammatory cytokines in pHCE cells. Importantly, this constellation of events occurred in the absence of a priming signal (e.g. LPS, PMA, or IFN-gamma), indicating that the NLRP1 inflammasome is inherently prepared for activation in the cornea. We speculate that the NLRP1-induced inflammatory milieu is sufficient to drive corneal inflammation and is likely the molecular basis for intraepithelial corneal dyskeratosis in patients with NLRP1 mutations.
There are currently no therapeutics for the treatment of NLRP1 inflammasomopathies. Here, we demonstrate that targeting the proteasome and caspase-1 greatly reduce pyroptotic markers in pHCE cells. However, although bortezomib is a US Food and Drug Administration (FDA)-approved compound for the treatment of multiple myeloma, it is highly toxic and unlikely to be a viable ocular drug.49 Moreover, caspase-1 inhibitors have thus far been limited by poor specificity and lack of efficacy with none yet advancing past phase II clinical trials.50,51 Nevertheless, we speculate other proteins that modulate the NLRP1 pathway are yet to be discovered, and that these proteins, or even potentially NLRP1 itself, may ultimately become targets for drugs to treat these inflammasomopathies.
Other inflammasomes, particularly NLRP3, have been implicated in inflammatory conditions of the ocular surface.15,16 For example, it was recently reported that patients with dry eye have increased levels of processed GSDMD in their tears.16 Given our data that NLRP1 is the primary inflammasome expressed in the corneal epithelium, perhaps NLRP1 may also contribute to dry eye or other inflammatory conditions of the anterior chamber.
Overall, this study elucidates the molecular mechanism of NLRP1-induced corneal damage and positions inflammasome modulators as potential therapeutic strategies for these orphan diseases. Furthermore, this work elevates the role of NLRP1 as an inflammasome of the barrier tissues, including the corneal epithelium. Additional research is necessary to clarify the evolutionary purpose of this enigmatic inflammasome and to elucidate its role in pathogen defense in the cornea.
Supplementary Material
Acknowledgments
The authors thank Sahana Rao, Daniel Ball, Jeffrey Hsiao, Cornelius Taabazuing, L. Robert Hollingsworth, Maria Passarelli, Kyle Kovacs, and Christopher Starr for insightful discussions.
Supported by National Institutes of Health (R01 AI137168 to D.A.B., T32 GM007739-Andersen, F30 CA243444 to A.R.G., and the Memorial Sloan Kettering Cancer Center Core Grants P30 CA008748). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure: A.R. Griswold, None; H.-C. Huang, None; D.A. Bachovchin, None
References
- 1. Janeway CA, Medzhitov R.. Innate immune recognition. Annu Rev Immunol. 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 2. Niederkorn JY. Cornea: Window to Ocular Immunology. Curr Immunol Rev. 2011; 7: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar A, Yu FS.. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006; 6: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broz P, Dixit VM.. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016; 16: 407–420. [DOI] [PubMed] [Google Scholar]
- 5. Lamkanfi M, Dixit VM.. Mechanisms and Functions of Inflammasomes. Cell. 2014; 157: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 6. Ding J, Wang K, Liu W, et al.. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016; 535: 111–116. [DOI] [PubMed] [Google Scholar]
- 7. Kayagaki N, Stowe IB, Lee BL, et al.. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015; 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 8. Shi J, Zhao Y, Wang K, et al.. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015; 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 9. Xia SY, Zhang ZB, Magupalli VG, et al.. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021; 593: 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chi W, Li F, Chen H, et al.. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc Natl Acad Sci USA. 2014; 111: 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Luo C, Cai J, et al.. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 2011; 52: 8442–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng WA, Thein T, Kinnunen K, et al.. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iyer SS, Pulskens WP, Sadler JJ, et al.. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009; 106: 20388–20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng QX, Ren YP, Reinach PS, et al.. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014; 125: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Niu LL, Zhang SJ, Wu JH, Chen L, Wang Y.. Upregulation of NLRP3 Inflammasome in the Tears and Ocular Surface of Dry Eye Patients. PLoS One. 2015; 10: e0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Dai YQ, Yang YJ, Xu JJ.. Calcitriol Alleviates Hyperosmotic Stress-Induced Corneal Epithelial Cell Damage via Inhibiting the NLRP3-ASC-Caspase-1-GSDMD Pyroptosis Pathway in Dry Eye Disease. J Inflamm Res. 2021; 14: 2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yerramothu P, Vijay AK, Willcox MDP.. Inflammasomes, the eye and anti-inflammasome therapy. Eye. 2018; 32: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinon F, Burns K, Tschopp J.. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10: 417–426. [DOI] [PubMed] [Google Scholar]
- 19. Zhong FL, Mamai O, Sborgi L, et al.. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016; 167: 187–202.e117. [DOI] [PubMed] [Google Scholar]
- 20. Soler VJ, Tran-Viet KN, Galiacy SD, et al.. Whole exome sequencing identifies a mutation for a novel form of corneal intraepithelial dyskeratosis. J Med Genet. 2013; 50: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grandemange S, Sanchez E, Louis-Plence P, et al.. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis. 2017; 76: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 22. Herlin T, Jorgensen SE, Host C, et al.. Autoinflammatory disease with corneal and mucosal dyskeratosis caused by a novel NLRP1 variant. Rheumatology. 2020; 59: 2334–2339. [DOI] [PubMed] [Google Scholar]
- 23. Drutman SB, Haerynck F, Zhong FL, et al.. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc Natl Acad Sci USA. 2019; 116: 19055–19063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasudo H, Ando T, Maehara A, et al.. A Possible Association Between a Nucleotide-Binding Domain LRR-Containing Protein Family PYD-Containing Protein 1 Mutation and an Autoinflammatory Disease Involving Liver Cirrhosis. Hepatology. 2021; 74: 2296–2299. [DOI] [PubMed] [Google Scholar]
- 25. Robinson KS, Teo DET, Tan KS, et al.. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science. 2020; 370: eaay2002. [DOI] [PubMed] [Google Scholar]
- 26. Hollingsworth LR, Sharif H, Griswold AR, et al.. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 2021; 592: 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang M, Zhang X, Toh GA, et al.. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. 2021; 592: 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chui AJ, Okondo MC, Rao SD, et al.. N-terminal degradation activates the NLRP1B inflammasome. Science. 2019; 364: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE.. Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science. 2019; 364: eaau1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okondo MC, Rao SD, Taabazuing CY, et al.. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol. 2018; 25: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong FL, Robinson K, Teo DET, et al.. Human DPP9 represses NLRP1 inflammasome and protects against auto-inflammatory diseases via both peptidase activity and FIIND domain binding. J Biol Chem. 2018; 293: 18864–18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okondo MC, Johnson DC, Sridharan R, et al.. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017; 13: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC.. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS One. 2011; 6: e27396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frew BC, Joag VR, Mogridge J.. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012; 8: e1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finger JN, Lich JD, Dare LC, et al.. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012; 287: 25030–25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robert Hollingsworth L, David L, Li Y, et al.. Mechanism of filament formation in UPA-promoted CARD8 and NLRP1 inflammasomes. Nat Commun. 2021; 12: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Griswold AR, Ball DP, Bhattacharjee A, et al.. DPP9's Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation. ACS Chem Biol. 2019; 14: 2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taabazuing CY, Griswold AR, Bachovchin DA.. The NLRP1 and CARD8 inflammasomes. Immunol Rev. 2020; 297: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickson MA, Hahn WC, Ino Y, et al.. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000; 20: 1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gai K, Okondo MC, Rao SD, et al.. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis. 2019; 10: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson DC, Taabazuing CY, Okondo MC, et al.. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med. 2018; 24: 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharif H, Hollingsworth LR, Griswold AR, et al.. Dipeptidyl peptidase 9 sets a threshold for CARD8 inflammasome formation by sequestering its active C-terminal fragment. Immunity. 2021; 54: 1392–1404.e1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Linder A, Bauernfried S, Cheng Y, et al.. CARD8 inflammasome activation triggers pyroptosis in human T cells. EMBO J. 2020; 39: e105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson DC, Okondo MC, Orth EL, et al.. DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes. Cell Death Dis. 2020; 11: 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ball DP, Taabazuing CY, Griswold AR, et al.. Caspase-1 interdomain linker cleavage is required for pyroptosis. Life Sci Alliance. 2020; 3: e202000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V.. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021; 371: eabd0811. [DOI] [PubMed] [Google Scholar]
- 47. Linder A, Hornung V.. Inflammasomes in T cells. J Mol Biol. 2021; 434: 167275. [DOI] [PubMed] [Google Scholar]
- 48. Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G.. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013; 38: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kane RC, Bross PF, Farrell AT, Pazdur R.. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003; 8: 508–513. [DOI] [PubMed] [Google Scholar]
- 50. Dhani S, Zhao Y, Zhivotovsky B.. A long way to go: caspase inhibitors in clinical use. Cell Death Dis. 2021; 12: 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS.. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013; 103: 2–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.