Abstract
Objective
To compare the diagnostic accuracies of ultrasound and magnetic resonance imaging (MRI) for deep (≥50%) myometrial invasion (DMI) and cervical stromal invasion (CSI) in women with endometrial cancer.
Methods
This was a prospective study at a gynecology clinic for women with postmenopausal bleeding. Between October 2015–October 2018, consecutive women with suspected endometrial cancer based on ultrasound subjective pattern recognition were simultaneously assessed for DMI and CSI on ultrasound. Subsequently, they also underwent preoperative MRI. We compared the diagnostic accuracies of ultrasound and MRI in predicting DMI and CSI with the final histology as the gold standard.
Results
We included 51 women. The prevalence of DMI and CSI were 22/51 (43%) and 7/51 (14%), respectively. The majority of malignancies were of endometrioid histological subtype (38/51, 75%) and FIGO stage 1 or 2 (40/51, 78%). Ultrasound diagnosed more cases of DMI compared to MRI (19/22 vs. 17/22), however, the difference was not statistically significant. The sensitivities and specificities of ultrasound and MRI for DMI were 86% vs. 77% and 66% vs. 76%, respectively. For CSI, ultrasound and MRI correctly diagnosed the same number of cases (5/7, 71%); their respective false-positive rates were low, 0/44 (0%) and 1/44 (2%). Ultrasound and MRI had a moderate agreement for DMI (ƙ=0.49; 95% confidence interval [CI]=0.26–0.73), whereas the agreement for CSI was substantial (ƙ=0.69; 95% CI=0.36–1.00).
Conclusion
Endometrial cancer can be simultaneously diagnosed and staged at women’s initial ultrasound assessment. The accuracies of ultrasound for DMI and CSI are comparable to MRI.
Trial Registration
ISRCTN Identifier: ISRCTN24363390
Keywords: Endometrial Neoplasms, Neoplasm Staging, Ultrasound, Magnetic Resonance Imaging, Diagnostic Errors
Synopsis
For women with endometrial cancer, the assessment for myometrial and cervical stromal invasion on ultrasound is comparable to magnetic resonance imaging. Agreement between the two imaging modalities for cervical stromal invasion is substantial, whereas it is moderate for myometrial invasion.
INTRODUCTION
Endometrial cancer is the most common gynecological malignancy. Its incidence has risen globally over the past 30 years due to changes in lifestyle, socioeconomic factors and a growing epidemic of obesity [1]. Most women with endometrial cancer present early with symptoms of postmenopausal bleeding and the mainstay of treatment for endometrial cancer is surgery. However, the radicality of surgery and the need for adjuvant therapy depend on the risks of lymph node metastasis and disease recurrence. Endometrial cancers are considered to be “high-risk” of metastasis and recurrence if there are features of deep (≥50%) myometrial invasion (DMI), cervical stromal invasion (CSI), lymphovascular space invasion, poorly differentiated (grade 3) or non-endometrioid histological types [2].
The depth of myometrial invasion is clinically important because it is an independent predictor for lymph node metastasis in endometrial cancer. For example, lymph node metastasis is only found in 2% of women with a myometrial invasion of <50% and a well to moderately differentiated tumor, whereas it is 18% in women with all other “high-risk” endometrial cancers [3]. If lymph node metastasis is present at the time of diagnosis, the 5-year disease-free-survival drops from 90% to 54% [4].
The presence of CSI is also an important clinical finding because it is an indication for radical rather than simple hysterectomy and women will usually require adjuvant brachytherapy.
Magnetic resonance imaging (MRI) is the imaging test most commonly used to assess endometrial cancer for DMI and CSI preoperatively, though ultrasound is an acceptable alternative option [5]. The advantages of ultrasound over MRI are its wider availability, lower cost, shorter examination time and no requirement for intravenous contrast. The avoidance of intravenous contrast is particularly relevant in elderly patients with renal impairment (glomerular filtration rate <30 mL/min/1.73 m2), who are at an increased risk of nephrogenic systemic fibrosis with gadolinium-based contrast media [6]. Furthermore, in regions of limited resources, ultrasound is recommended over MRI for the preoperative staging of endometrial cancer [7].
When women present with postmenopausal bleeding, endometrial cancer can be accurately diagnosed on ultrasound by identifying the typical endometrial morphological features and vascular patterns, termed subjective pattern recognition, with a false positive rate of less than 10% [8]. This allows for malignancies to be simultaneously staged at women’s initial ultrasound examination. Previous studies have reported good diagnostic accuracy of ultrasound in staging endometrial cancer [9], however, few of them have compared ultrasound with MRI in the same cohort of women [10].
The primary aim of this study was to compare the diagnostic accuracies of ultrasound and MRI for the DMI in women with endometrial cancer. The secondary aim was to compare their respective diagnostic accuracies for CSI.
MATERIALS AND METHODS
This was a prospective study that was carried out between October 2015 and October 2018 in a dedicated Rapid Access clinic of a university teaching hospital. We included women with a history of postmenopausal bleeding or unscheduled vaginal bleeding while on hormone replacement therapy (HRT). Menopause was defined as amenorrhoea of at least 12-month duration in women who were over the age of 45. Only endometrial cancers with epithelial or mixed epithelial and mesenchymal histological types were included, i.e., endometrioid, mucinous, serous, clear cell, mixed, undifferentiated and carcinosarcoma.
All women underwent a transvaginal ultrasound examination during their initial visit. Ultrasound examinations were conducted with the same ultrasound equipment (Voluson E8; GE Healthcare Ultrasound, Milwaukee, WI, USA), equipped with a 4–9 MHz transvaginal probe. The scans were performed by a clinical research fellow who had received intensive training in early pregnancy and gynecology ultrasound before starting the study. He was supervised indirectly by consultant gynecologists who were all expert ultrasound examiners.
The ultrasound examination technique and terminologies used to describe the endometrial morphology were in keeping with the consensus statement by the International Endometrial Tumor Analysis (IETA) group [11]. Endometrial cancers were diagnosed on ultrasound based on subjective pattern recognition, where the endometrium appears heterogeneous or there is an irregular focal lesion. The endometrial-myometrial junction (EMJ) could be intact or it is interrupted, which is suggestive of myometrial invasion. On Doppler ultrasound, multiple vessels are crossing the EMJ with focal or multifocal origins.
Following the ultrasound diagnosis of endometrial cancer, women were assessed simultaneously for myometrial and CSI by the same operator. To estimate the depth of myometrial invasion, the outline of the EMJ was systemically assessed, scanning in the sagittal plane making sure that the whole of the uterus was covered and then in the transverse plane from the tip of the cervix to the top of the uterine fundus, looking for the point where the tumor appears to be invading most deeply into the myometrium (this may involve the anterior, posterior or lateral uterine wall, as well as the uterine fundus). The depth of tumoral invasion into the myometrium was then estimated subjectively as illustrated before [12,13]. All women were categorized into having either i) no myometrial invasion or <50% myometrial invasion of the entire myometrial thickness, or ii) ≥50% myometrial invasion.
The CSI was diagnosed when there was a tumor infiltrating and disrupting the regular outline of the cervical stroma. In some cases, the lower edge of an intracavitary tumor may reach the level of the internal cervical orifice but it does not invade into the cervical stroma; to exclude any invasion into the cervical stroma, the ultrasound probe is gently used to push on the cervix to see whether the tumor is sliding off the cervix. If there is a true CSI, there will be no sliding of the tumor [12].
All endometrial tumors were measured in the sagittal plane for their maximal diameters in 2 perpendicular planes (d1 and d2, respectively) and the transverse plane for its width (d3). The mean diameter of the tumor was calculated by d1+d2+d3/3.
After the ultrasound examination, women were offered endometrial sampling by Pipelle suction curette (Laboratoire CCD, Paris, France) or hysteroscopy. MRI scan was requested for women with a histologically confirmed malignancy, which included the T2-weighted imaging (T2WI), dynamic T1-weighted gadolinium sequences (DCE-MRI) and diffusion-weighted imaging (DWI-MRI) with an apparent diffusion coefficient map. On T2WI, endometrial cancer typically has an intermediate signal intensity and is hyperintense compared to the adjacent myometrium. In the absence of myometrial invasion, the EMJ will appear intact on T2WI and there is a clear sub-endometrial enhancement (SEE) on DCE-MRI. However, when the myometrial invasion is present, the EMJ may appear irregular on T2WI and there is a disruption of the SEE and peritumoral enhancement on DCE-MRI [14]. The depth of myometrial invasion in our study was assessed subjectively [15]. Women were categorized into i) no myometrial invasion or <50% myometrial invasion of the entire myometrial thickness, or ii) ≥50% myometrial thickness.
The CSI was diagnosed subjectively on MRI when there was an intermediate signal intensity tumor disrupting the low signal intensity fibrous cervical stroma on T2WI. On DCE-MRI, the normal enhancement of the cervical stroma may be replaced by a hypo-enhancing tumor. On DWI-MRI, the cervical stroma invasion may have a higher signal intensity on high b value compared to the lower signal intensity of normal cervical stroma.
MRI scans were interpreted by consultant radiologists with extensive subspecialty experience in gynecological oncology, who were blinded to the ultrasound findings of myometrial invasion and CSI. Similarly, all ultrasound examinations were performed before patients underwent MRI scans.
The pathologists who performed the histological examinations were blinded to the ultrasound findings of myometrial invasion and CSI. The International Federation of Gynaecology and Obstetrics (FIGO) classification system for endometrial cancer was used [16].
1. Statistical analysis
The primary outcome of this study was the diagnostic accuracy of preoperative ultrasound and MRI in detecting DMI in women with endometrial cancer. The secondary outcome was the respective accuracy of ultrasound and MRI for CSI.
In our final statistical analysis, we included only women who underwent both ultrasound and MRI examinations. We excluded women who did not undergo hysterectomy following the imaging tests. The diagnostic accuracies (sensitivity, specificity, positive and negative likelihood ratios, false positive and negative rates, and overall accuracy) of ultrasound and MRI were calculated with the final histology from hysterectomy as the gold standard.
For sample size calculation, we focused on the sensitivity of ultrasound and MRI for DMI because a high sensitivity is important to reduce the risk of women having incomplete surgical staging procedures due to false-negative results. According to Liu et al. [17], this study required a minimum of 49 women to undergo both the ultrasound and MRI examinations, to detect a difference of 10% in sensitivity, with a power of 80%, a significance level of 5% and the assumption that the expected percentage of a discrepancy between ultrasound and MRI is 5% [18].
Descriptive methods were used to describe the study population. Reliability between ultrasound and MRI in the preoperative staging of endometrial cancer was assessed using Cohen’s kappa (ƙ) statistic, in which a ƙ value of ≤0.2 represents a very poor agreement between the 2 imaging tests; 0.21–0.40 poor agreement; 0.41–0.60 moderate agreement; 0.61–0.80 good agreement; and 0.81–1.00 very good agreement [19]. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA).
2. Ethical approval and reporting
This study was granted ethical approval from the Central London REC2 committee (10/H0713/66) as well as local R&D sponsorship from University College Hospital (10/0316); ISRCTN registration number ISRCTN24363390. We followed the guidelines of the Standards for Reporting of Diagnostic Accuracy Studies (STARD) statement in the conduct and reporting of our research [20].
RESULTS
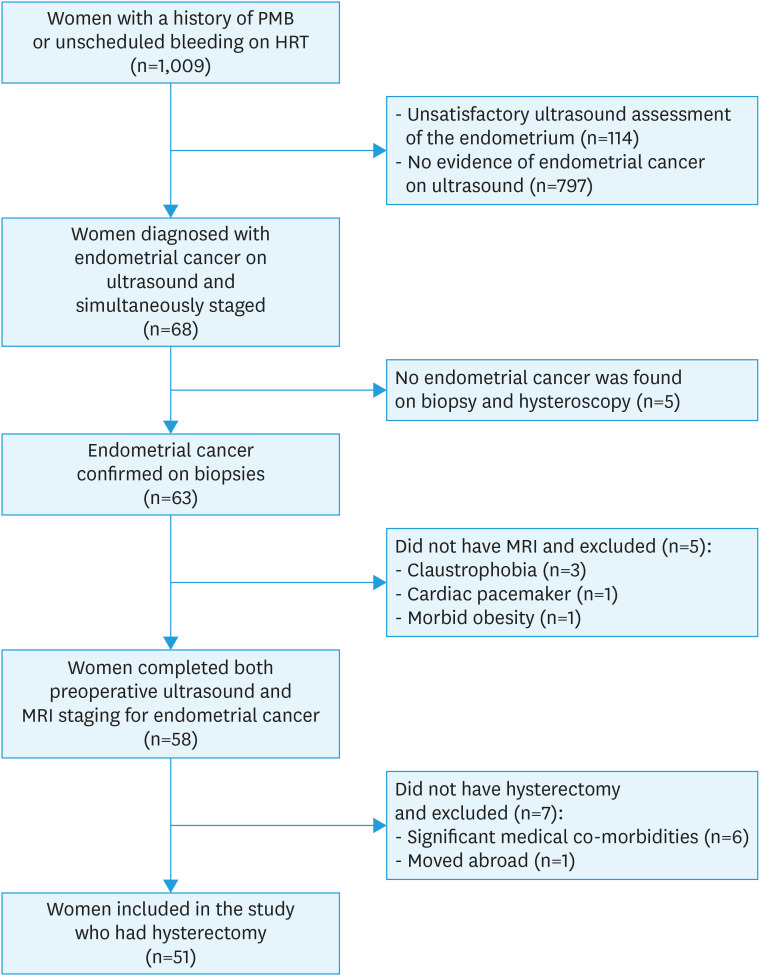
During the study period, 1,009 women underwent ultrasound examination for postmenopausal bleeding or unscheduled vaginal bleeding while on HRT; 144 were excluded as the endometrium could not be satisfactorily assessed (Fig. 1). In the remaining women, 68 were suspected with endometrial cancer on ultrasound and they were simultaneously assessed for the presence of DMI and CSI. Later, we excluded 5 women who had no evidence of malignancy on endometrial biopsy and hysteroscopy: 2 of them were diagnosed with benign endometrial polyps, 2 with proliferative endometrium and one with endometrial hyperplasia without atypia. A further 5 women were also excluded as they did not undergo MRI due to claustrophobia, presence of a cardiac pacemaker or morbid obesity. And finally, 7 more women were excluded as they did not undergo hysterectomy due to significant medical co-morbidities or patient moved abroad. Table 1 summarises the clinical characteristics of the remaining 51 women who were included in our study. The median time between the ultrasound and MRI scan was 20 days (interquartile range [IQR]=13–29), whereas the median time between ultrasound and hysterectomy for endometrial cancer was 37 days (IQR=27–50).
Fig. 1. Study flowchart.
HRT, hormone replacement therapy; MRI, magnetic resonance imaging; PMB, postmenopausal bleeding.
Table 1. Patient clinical characteristics and the final histological diagnoses (n=51).
| Characteristics | Value | ||
|---|---|---|---|
| Age | 66 (57–76) | ||
| Nulliparous | 15 (29) | ||
| Body mass index (kg/m2) | 28.8 (25.6–32.7) | ||
| Time since menopause | 12 (5–27) | ||
| Hypertension | 18 (35) | ||
| Diabetes mellitus | 5 (10) | ||
| Fibroids (total) | 14 (27) | ||
| Fibroids (submucosal) | 1 (2) | ||
| Adenomyosis | 5 (10) | ||
| Endometrial thickness (mm) | 16.5 (11.4–30) | ||
| Tumor mean diameter (mm) | 25 (18–41) | ||
| Final histological diagnoses | |||
| Endometrioid histological subtype | 38 (75) | ||
| Grade 1 | 19 (50) | ||
| Grade 2 | 18 (47) | ||
| Grade 3 | 1 (3) | ||
| Non-endometrioid histological subtype | 13 (25) | ||
| Carcinosarcoma | 5 (38) | ||
| Serous | 4 (31) | ||
| Neuroendocrine | 2 (15) | ||
| Clear cell | 1 (8) | ||
| Mixed serous/Endometrioid | 1 (8) | ||
| FIGO stage of endometrial cancer | |||
| Stage 1a | 25 (49) | ||
| Stage 1b | 11 (22) | ||
| Stage 2 | 4 (8) | ||
| Stage 3a | 2 (4) | ||
| Stage 3b | 3 (6) | ||
| Stage 3c1 | 4 (8) | ||
| Stage 3c2 | 0 (0) | ||
| Stage 4 | 2 (4) | ||
Values are presented as median (interquartile range) or number (%).
The majority of the endometrial cancers (38/51, 75%) included in our study were of endometrioid histological type and of which 37/38 (97%) were well to moderately differentiated (grade 1 or 2). The prevalence of DMI and CSI were 22/51 (43%) and 7/51 (14%), respectively. Most malignancies were diagnosed at FIGO stage 1 or 2 (40/51, 78%).
The diagnoses of ultrasound and MRI for DMI and CSI against the final histology are shown in Table 2; their respective diagnostic accuracies are summarised in Table 3.
Table 2. Ultrasound and MRI preoperative diagnoses of DMI and CSI against the final histology (n=51).
| Characteristics | Final histology | Total (n=51) | |||||
|---|---|---|---|---|---|---|---|
| DMI | CSI | ||||||
| Present (n=22) | Absent (n=29) | Present (n=7) | Absent (n=44) | ||||
| Ultrasound | |||||||
| DMI | |||||||
| Present | 19 | 10 | 29 | ||||
| Absent | 3 | 19 | 22 | ||||
| MRI | |||||||
| DMI | |||||||
| Present | 17 | 7 | 24 | ||||
| Absent | 5 | 22 | 27 | ||||
| Ultrasound | |||||||
| CSI | |||||||
| Present | 5 | 0 | 5 | ||||
| Absent | 2 | 44 | 46 | ||||
| MRI | |||||||
| CSI | |||||||
| Present | 5 | 1 | 6 | ||||
| Absent | 2 | 43 | 45 | ||||
CSI, cervical stromal invasion; DMI, deep myometrial invasion (≥50%); MRI, magnetic resonance imaging.
Table 3. Diagnostic accuracies of ultrasound and MRI for DMI and CSI in endometrial cancer (n=51).
| Characteristics | Sensitivity | Specificity | +LR | −LR | Accuracy | |
|---|---|---|---|---|---|---|
| Ultrasound | ||||||
| DMI | 86 (65–97) | 66 (46–82) | 2.5 (1.5–4.3) | 0.2 (0.1–0.6) | 75 (60–86) | |
| CSI | 71 (29–96) | 100 (92–100) | n/a | 0.3 (0.1–0.9) | 96 (87–100) | |
| MRI | ||||||
| DMI | 77 (55–92) | 76 (56–90) | 3.2 (1.6–6.3) | 0.3 (0.1–0.7) | 76 (63–87) | |
| CSI | 71 (29–96) | 98 (88–100) | 31.4 (4.3–231) | 0.3 (0.1–0.9) | 94 (84–99) | |
Values are presented as % (95% CI) or ratio (95% CI).
CI, confidence interval; CSI, cervical stromal invasion; DMI, deep myometrial invasion (≥50%); LR, likelihood ratio; MRI, magnetic resonance imaging; n/a, not available.
Ultrasound correctly identified more women with DMI compared to MRI (19/22, 86% vs. 17/22, 77%), however, the difference was not statistically significant. The respective false-positive rates were 10/29 (34%, 95% confidence interval [CI]=17–52) and 7/29 (24%, 95% CI=9–40). The proportion of women with DMI who were under-staged by ultrasound but correctly staged on MRI was 2/51; whereas in 4/51 women, it was the opposite.
Both ultrasound and MRI correctly identified the same number of women with CSI (5/7, 71%). The respective false-positive rates were both low, 0/44 (0%) and 1/44 (2%).
Ultrasound and MRI agreed on 38/51 (75%, 95% CI=63–87) diagnoses of DMI (Table 4). A ƙ statistic of 0.49 (95% CI=0.26–0.73) means that the agreement was moderate. For the assessment of CSI, ultrasound and MRI agreed on 48/51 (94%) diagnoses. A ƙ statistic of 0.69 (95% CI=0.36–1.00) means that the agreement was substantial.
Table 4. Agreement between ultrasound and MRI on the preoperative assessment of myometrial invasion and CSI with final histology as the reference standard (n=51).
| Ultrasound | MRI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MI | CSI | ||||||||
| Correctly staged (n=39) | Over-staged (n=7) | Under-staged (n=5) | Total (n=51) | Correctly staged (n=48) | Over-staged (n=1) | Under-staged (n=2) | Total (n=51) | ||
| MI | |||||||||
| Correctly staged | 32 | 2 | 4 | 38 | |||||
| Over-staged | 5 | 5 | 0 | 10 | |||||
| Under-staged | 2 | 0 | 1 | 3 | |||||
| CSI | |||||||||
| Correctly staged | 47 | 1 | 1 | 49 | |||||
| Over-staged | 0 | 0 | 0 | 0 | |||||
| Under-staged | 1 | 0 | 1 | 2 | |||||
CSI, cervical stromal invasion; MI, myometrial invasion; MRI, magnetic resonance imaging.
We carried out a subgroup analysis for women with “low-risk” endometrial cancers, i.e., low grade (grade 1 or 2 endometrioid) and clinically stage 1 tumors. This is because, from the gynecological oncologists’ perspective, the depth of myometrial invasion is most relevant in these women, who may not require more invasive surgeries such as lymphadenectomy or sentinel lymph node biopsy.
The results of our subgroup analysis are presented in Table S1, which shows that ultrasound detected more women with DMI (8/9, 89%) compared to MRI (6/9, 67%), however, the difference was not statistically significant (Table S2). There were only 2 women with CSI in the subgroup and therefore we did not carry out a comparison between ultrasound and MRI in the detection of CSI.
DISCUSSION
Our results show that ultrasound and MRI have a comparable diagnostic accuracy for DMI and CSI in women with endometrial cancer. Their agreement and reliability were higher in the diagnosis of CSI (κ=0.69) when comparing to DMI (κ=0.49). In the subgroup of “low-risk” endometrial cancers, ultrasound performed equally well in detecting DMI in comparison to the main cohort of women (89% vs. 86%).
We did not find evidence of selection bias in our study. Our prevalence of DMI (43%) and the proportion of low-grade endometrial cancers (73%) are in keeping with a previous prospective study where consecutively diagnosed endometrial cancers were included [21].
A previous meta-analysis, which included 560 women, reported that the diagnostic accuracy of ultrasound and MRI in detecting DMI were not significantly different, the pooled sensitivities were 75% and 83%, respectively [10]. In contrast to their findings, we found that the sensitivity of ultrasound for DMI was higher than MRI (86% vs. 77%), this may be because we only included endometrial cancers where the ultrasound examination was satisfactory. Among the 144 women with an unsatisfactory ultrasound assessment, 3 were diagnosed with endometrial cancer and their preoperative MRI correctly identified one of the 2 women with DMI.
Two recent prospective studies have compared the accuracies of ultrasound and MRI for DMI in women with “low-risk” (grade 1 or 2 endometrioid) endometrial cancer [22,23]. Cubo-Abert et al. [22] measured the depth of myometrial invasion on ultrasound objectively with the formula, (1−Minimal Distance between the Tumor and the Serosa/Depth of Healthy Myometrium)×100%, whereas, Gastón et al. [23] used both objective (Karlsson’s ratio) and subjective assessments. Cubo-Abert et al. [22] concluded that the sensitivities and specificities of ultrasound and MRI for DMI were not significantly different, 69% vs. 51% and 87% vs. 91%, respectively. On the contrary, Gastón et al. [23] reported that MRI has a higher specificity for DMI than ultrasound, but only when assessed subjectively, 87% vs. 74%, respectively (the corresponding sensitivities were 80% vs. 75%, respectively).
We found that both ultrasound and MRI had a high false-positive rate for DMI, which is in keeping with previous studies [10]. The most common cause for over-staging is the presence of a large polypoid tumor [24]. A large polypoid tumor may cause distension of the uterine cavity and results in thinning of the myometrium. And when the echogenicity of the tumor is similar to the myometrium, it can be very difficult to distinguish between the tumor and the surrounding myometrium [25]. Furthermore, over-staging of myometrial invasion is more common in tumors with a high colour score and in those with multiple vessels of multifocal origins crossing the EMJ [13]. Contrarily, under-staging of myometrial invasion is more likely in women with fibroids.
It has been reported that the presence of adenomyosis may also make it more difficult to assess the depth of myometrial invasion in women with endometrial cancer, as adenomyosis reduces the sonographic contrast between the tumor and the surrounding myometrium [26]. Also, there is an increased risk of DMI when endometrial cancer extends into pre-existing adenomyosis [27]. In our study, the prevalence of adenomyosis was 5/50 (10%), which is in keeping with previous studies (8%–16%) [22,28,29]. We did not find any cases of over-staging or under-staging in women with adenomyosis.
In this study, we assessed the depth of myometrial invasion and CSI on ultrasound subjectively as there is no consensus on whether a subjective assessment or objective measures is more accurate. In a recent multi-centre prospective study, IETA-4, the performance of subjective assessment was compared against objective measures (such as the tumor/uterine anterior-posterior diameter ratio (Karlsson’s ratio) and minimal tumor-free margin), in 1,275 measurable tumors [30]. They found that subjective assessment had similar sensitivity for DMI compared to objective measures, but importantly, subjective assessment had a significantly better specificity (76%) against Karlsson’s ratio (69%) or minimal tumor free margins (67%). In another prospective study of 210 women with endometrial cancer, subjective assessment also had better accuracy (76%) when compared to Karlsson’s ratio (68%) and Gordon’s ratio (67%) [31]. The author suggested that subjective assessment performed better because more ultrasound morphological features could be taken into account against the more rigid system of objective measures. The additional information available on subjective assessment may include the size of the tumor, the vascular patterns on Doppler examination and dynamic tests, such as the sliding sign of tumor against the uterine wall or endocervical canal; as it is known that “high-risk” tumors are more likely to be larger, have a non-uniform endometrial echogenicity, multifocal vessel pattern across the EMJ and a moderate/high colour score on Doppler studies [28].
Although we did not utilize 3-dimensional ultrasound (3D-US) in our preoperative staging of endometrial cancer, it has been reported that 3D-US may have some advantages over conventional 2-dimensional ultrasound (2D-US). These include the option of off-line analysis in any plane, such as the reconstruction of the coronal plane, more accurate measurement of the tumor volume, automated quantification of the vascular indices and 3D display of the tumor vascular tree [32]. The reported sensitivities and specificities of 3D-US in detecting DMI are 84%–89% and 86%–91%, respectively [33,34]. However, some studies have compared objective measures on 3D-US, such as 3D tumor volume and shortest myometrial tumor free distance to the serosa, against subjective assessment on 2D-US, and they did not find that 3D-US improved the accuracy of preoperative staging [35,36]. Furthermore, the addition of 3D-US also did not appear to improve the diagnostic accuracy of 2D-US in detecting CSI [32].
Previous studies on the accuracy of ultrasound for DMI or CSI in endometrial cancer were mostly carried out by expert operators [10,30]. This is supported by Eriksson et al. [37] who reported that the accuracy for CSI and interrater reliability were higher among expert operators compared to general gynecologists, though no difference was found regarding the assessment for DMI. In contrast, a recent prospective study by Dueholm et al. [38] showed that the specificity for DMI was significantly lower in non-expert operators (resident trainees in obstetrics and gynecology) compared to experts, 37% vs. 72%, respectively (the corresponding sensitivities were 96% and 81%, respectively). These findings could be due to non-experts being specifically instructed to classify all uncertain cases as suspected DMI.
The main strength of our study is that the diagnostic accuracy of ultrasound and MRI were tested in the same cohort of women, which is not commonly carried out in previous studies [10]. Secondly, this was the only study where ultrasound diagnosis and staging of endometrial cancer were carried out simultaneously at women’s initial assessment. As the ultrasound examination was carried out before any endometrial biopsies were taken, it avoided any potential iatrogenic disruption to the EMJ, which may affect the accuracy of ultrasound assessment. In some studies, following invasive diagnostic procedures, such as dilatation and curettage, no residual tumor is found in 2%–11% of final hysterectomy specimens [30,39].
The main limitation of this study is the inclusion of both “high-risk” and “low-risk” endometrial cancers. We attempted to perform subgroup analysis by including women with “low-risk” endometrial cancers only, however, due to the small sample size, our comparison of diagnostic accuracies between ultrasound and MRI was not adequately powered. Regrettably, this is also a common limitation in other studies [13,25,30,31,36]. Furthermore, the ultrasound and MRI examinations were carried out on different days, therefore the measurement and interpretation of myometrial invasion could be affected by organ motion of the uterus due to inflation and deflation of the bladder and rectum [40].
In conclusion, our study shows that endometrial cancer can be simultaneously diagnosed and assessed for myometrial and CSI at women’s initial ultrasound scan for postmenopausal bleeding. The diagnostic accuracy of ultrasound for DMI and CSI is comparable to MRI.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: W.M., N.J., J.D.
- Data curation: W.M., A.T., T.N.
- Formal analysis: W.M., A.T., T.N., J.D.
- Investigation: W.M., A.T., T.N.
- Methodology: W.M., N.J., J.D.
- Project administration: W.M.
- Supervision: J.D.
- Writing - original draft: W.M.
- Writing - review & editing: W.M., A.T., T.N., N.J., J.D.
SUPPLEMENTARY MATERIALS
Preoperative diagnosis of DMI by ultrasound and MRI in women with “low-risk” endometrial cancer against the final histology (n=31)
Diagnostic accuracies of ultrasound and MRI for DMI in women with “low-risk” endometrial cancer (n=31)
References
- 1.Zhang S, Gong TT, Liu FH, Jiang YT, Sun H, Ma XX, et al. Global, regional, and national burden of endometrial cancer, 1990-2017: results from the global burden of disease study, 2017. Front Oncol. 2019;9:1440. doi: 10.3389/fonc.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Luomaranta A, Leminen A, Loukovaara M. Magnetic resonance imaging in the assessment of high-risk features of endometrial carcinoma: a meta-analysis. Int J Gynecol Cancer. 2015;25:837–842. doi: 10.1097/IGC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 4.Lurain JR, Rice BL, Rademaker AW, Poggensee LE, Schink JC, Miller DS. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1991;78:63–69. [PubMed] [Google Scholar]
- 5.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen HS, Morcos SK, Almén T, Bellin MF, Bertolotto M, Bongartz G, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307–318. doi: 10.1007/s00330-012-2597-9. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro R, Fontes Cintra G, Barrozo A, Tieko Tsunoda A, Pupo Nogueira A, Andreazza Laporte G, et al. Brazilian Society of Surgical Oncology guidelines for surgical treatment of endometrial cancer in regions with limited resources. J Surg Oncol. 2020;121:730–742. doi: 10.1002/jso.25797. [DOI] [PubMed] [Google Scholar]
- 8.Dueholm M, Marinovskij E, Hansen ES, Møller C, Ørtoft G. Diagnostic methods for fast-track identification of endometrial cancer in women with postmenopausal bleeding and endometrial thickness greater than 5 mm. Menopause. 2015;22:616–626. doi: 10.1097/GME.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 9.Alcázar JL, Orozco R, Martinez-Astorquiza Corral T, Juez L, Utrilla-Layna J, Mínguez JA, et al. Transvaginal ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:405–413. doi: 10.1002/uog.14905. [DOI] [PubMed] [Google Scholar]
- 10.Alcázar JL, Gastón B, Navarro B, Salas R, Aranda J, Guerriero S. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: a systematic review and meta-analysis. J Gynecol Oncol. 2017;28:e86. doi: 10.3802/jgo.2017.28.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone FP, Timmerman D, Bourne T, Valentin L, Epstein E, Goldstein SR, et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol. 2010;35:103–112. doi: 10.1002/uog.7487. [DOI] [PubMed] [Google Scholar]
- 12.Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28:721–739. doi: 10.1016/j.bpobgyn.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Fischerova D, Frühauf F, Zikan M, Pinkavova I, Kocián R, Dundr P, et al. Factors affecting sonographic preoperative local staging of endometrial cancer. Ultrasound Obstet Gynecol. 2014;43:575–585. doi: 10.1002/uog.13248. [DOI] [PubMed] [Google Scholar]
- 14.Fujii S, Kido A, Baba T, Fujimoto K, Daido S, Matsumura N, et al. Subendometrial enhancement and peritumoral enhancement for assessing endometrial cancer on dynamic contrast enhanced MR imaging. Eur J Radiol. 2015;84:581–589. doi: 10.1016/j.ejrad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Nougaret S, Horta M, Sala E, Lakhman Y, Thomassin-Naggara I, Kido A, et al. Endometrial cancer MRI staging: updated guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2019;29:792–805. doi: 10.1007/s00330-018-5515-y. [DOI] [PubMed] [Google Scholar]
- 16.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Liu JP, Hsueh HM, Hsieh E, Chen JJ. Tests for equivalence or non-inferiority for paired binary data. Stat Med. 2002;21:231–245. doi: 10.1002/sim.1012. [DOI] [PubMed] [Google Scholar]
- 18.DelMaschio A, Vanzulli A, Sironi S, Spagnolo D, Belloni C, Garancini P, et al. Estimating the depth of myometrial involvement by endometrial carcinoma: efficacy of transvaginal sonography vs MR imaging. AJR Am J Roentgenol. 1993;160:533–538. doi: 10.2214/ajr.160.3.8430547. [DOI] [PubMed] [Google Scholar]
- 19.Kottner J, Audigé L, Brorson S, Donner A, Gajewski BJ, Hróbjartsson A, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799. doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savelli L, Ceccarini M, Ludovisi M, Fruscella E, De Iaco PA, Salizzoni E, et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet Gynecol. 2008;31:560–566. doi: 10.1002/uog.5295. [DOI] [PubMed] [Google Scholar]
- 22.Cubo-Abert M, Díaz-Feijoo B, Bradbury M, Rodríguez-Mías NL, Vera M, Pérez-Hoyos S, et al. Diagnostic performance of transvaginal ultrasound and magnetic resonance imaging for preoperative evaluation of low-grade endometrioid endometrial carcinoma: prospective comparative study. Ultrasound Obstet Gynecol. 2021;58:469–475. doi: 10.1002/uog.23607. [DOI] [PubMed] [Google Scholar]
- 23.Gastón B, Muruzábal JC, Lapeña S, Modroño A, Guarch R, García de Eulate I, et al. Transvaginal ultrasound versus magnetic resonance imaging for assessing myometrial infiltration in endometrioid low grade endometrial cancer: a prospective study. J Ultrasound Med. doi: 10.1002/jum.15708. 2021 Mar 29. [DOI] [PubMed] [Google Scholar]
- 24.Fleischer AC, Dudley BS, Entman SS, Baxter JW, Kalemeris GC, James AE., Jr Myometrial invasion by endometrial carcinoma: sonographic assessment. Radiology. 1987;162:307–310. doi: 10.1148/radiology.162.2.3541025. [DOI] [PubMed] [Google Scholar]
- 25.Savelli L, Testa AC, Mabrouk M, Zannoni L, Ludovisi M, Seracchioli R, et al. A prospective blinded comparison of the accuracy of transvaginal sonography and frozen section in the assessment of myometrial invasion in endometrial cancer. Gynecol Oncol. 2012;124:549–552. doi: 10.1016/j.ygyno.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Utsunomiya D, Notsute S, Hayashida Y, Lwakatare F, Katabuchi H, Okamura H, et al. Endometrial carcinoma in adenomyosis: assessment of myometrial invasion on T2-weighted spin-echo and gadolinium-enhanced T1-weighted images. AJR Am J Roentgenol. 2004;182:399–404. doi: 10.2214/ajr.182.2.1820399. [DOI] [PubMed] [Google Scholar]
- 27.Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes S, Bernardini M, Ackerman I, et al. Adenomyosis involved by endometrial adenocarcinoma is a significant risk factor for deep myometrial invasion. Ann Diagn Pathol. 2007;11:252–257. doi: 10.1016/j.anndiagpath.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Epstein E, Fischerova D, Valentin L, Testa AC, Franchi D, Sladkevicius P, et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: prospective multicenter study. Ultrasound Obstet Gynecol. 2018;51:818–828. doi: 10.1002/uog.18909. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Yang B, Zhang W, Gao X, Zhao C, Zhang X, et al. Clinicopathological characteristics and survival outcomes of patients with coexistence of adenomyosis and endometrial carcinoma. Int J Clin Exp Pathol. 2018;11:956–962. [PMC free article] [PubMed] [Google Scholar]
- 30.Verbakel JY, Mascilini F, Wynants L, Fischerova D, Testa AC, Franchi D, et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International Endometrial Tumor Analysis)-4 cohort. Ultrasound Obstet Gynecol. 2020;55:115–124. doi: 10.1002/uog.20374. [DOI] [PubMed] [Google Scholar]
- 31.Frühauf F, Zikan M, Semeradova I, Dundr P, Nemejcova K, Dusek L, et al. The diagnostic accuracy of ultrasound in assessment of myometrial invasion in endometrial cancer: subjective assessment versus objective techniques. BioMed Res Int. 2017;2017:1318203. doi: 10.1155/2017/1318203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green RW, Valentin L, Alcazar JL, Chiappa V, Erdodi B, Franchi D, et al. Endometrial cancer off-line staging using two-dimensional transvaginal ultrasound and three-dimensional volume contrast imaging: Intermethod agreement, interrater reliability and diagnostic accuracy. Gynecol Oncol. 2018;150:438–445. doi: 10.1016/j.ygyno.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Yildirim N, Saatli B, Kose S, Sancar C, Ulukus C, Koyuncuoglu M, et al. Predictability of myometrial, lower uterine segment and cervical invasion with 3D transvaginal ultrasonography and magnetic resonance imaging in endometrial cancer patients: a prospective cohort study. Med Ultrason. 2018;20:348–354. doi: 10.11152/mu-1493. [DOI] [PubMed] [Google Scholar]
- 34.Yang T, Tian S, Li Y, Tian X, Wang W, Zhao J, et al. Magnetic resonance imaging (MRI) and three-dimensional transvaginal ultrasonography scanning for preoperative assessment of high risk in women with endometrial cancer. Med Sci Monit. 2019;25:2024–2031. doi: 10.12659/MSM.915276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcázar JL, Galván R, Albela S, Martinez S, Pahisa J, Jurado M, et al. Assessing myometrial infiltration by endometrial cancer: uterine virtual navigation with three-dimensional US. Radiology. 2009;250:776–783. doi: 10.1148/radiol.2503080877. [DOI] [PubMed] [Google Scholar]
- 36.Mascilini F, Testa AC, Van Holsbeke C, Ameye L, Timmerman D, Epstein E. Evaluating myometrial and cervical invasion in women with endometrial cancer: comparing subjective assessment with objective measurement techniques. Ultrasound Obstet Gynecol. 2013;42:353–358. doi: 10.1002/uog.12499. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson LS, Lindqvist PG, Flöter Rådestad A, Dueholm M, Fischerova D, Franchi D, et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol. 2015;45:476–482. doi: 10.1002/uog.14645. [DOI] [PubMed] [Google Scholar]
- 38.Dueholm M, Hjorth IM, Dahl K, Marinovskij E, Ørtoft G. Preoperative prediction of high-risk endometrial cancer by expert and non-expert transvaginal ultrasonography, magnetic resonance imaging, and endometrial histology. Eur J Obstet Gynecol Reprod Biol. 2021;263:181–191. doi: 10.1016/j.ejogrb.2021.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Ørtoft G, Dueholm M, Mathiesen O, Hansen ES, Lundorf E, Møller C, et al. Preoperative staging of endometrial cancer using TVS, MRI, and hysteroscopy. Acta Obstet Gynecol Scand. 2013;92:536–545. doi: 10.1111/aogs.12103. [DOI] [PubMed] [Google Scholar]
- 40.Jadon R, Pembroke CA, Hanna CL, Palaniappan N, Evans M, Cleves AE, et al. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 2014;26:185–196. doi: 10.1016/j.clon.2013.11.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative diagnosis of DMI by ultrasound and MRI in women with “low-risk” endometrial cancer against the final histology (n=31)
Diagnostic accuracies of ultrasound and MRI for DMI in women with “low-risk” endometrial cancer (n=31)