Abstract
Background
Given the expanding clinical use of poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors (PARPis), there is a significant need for optimal strategies with which to treat patients whose cancer progresses while using a PARPi. However, the treatment consensus after PARPi has not been established. The aim of the Korean Gynecologic Oncology Group (KGOG) 3056/NIRVANA-R trial is to investigate the efficacy of niraparib in combination with bevacizumab as a maintenance therapy in platinum-sensitive ovarian cancer patients who were previously treated with a PARPi.
Methods
The KGOG 3056/NIRVANA-R is a multi-centre, investigator-initiated, single-arm, phase II trial of patients with platinum-sensitive recurrent ovarian cancer recruited from seven KGOG sites. This study included patients with platinum-sensitive recurrent epithelial ovarian cancer who received at least 2 previous courses of platinum-containing therapy and had been treated with a PARPi. Mucinous histology type was excluded. Patients who had responded to the last platinum regimen (either complete or partial response) were eligible to participate in this study. Forty-four patients will be recruited. All enrolled patients are treated with niraparib and bevacizumab for maintenance therapy until disease progression, unacceptable toxicity, or withdrawal of patient consent. The primary endpoint of the study is 6-month progression-free survival rate. Accrual is expected to be completed in 2022, followed by presentation of results in 2023.
Trial Registration
ClinicalTrials.gov Identifier: NCT04734665
Keywords: Ovarian Neoplasms, Poly(ADP-ribose) Polymerase Inhibitors, Bevacizumab, Retreatment, Recurrence
INTRODUCTION
Worldwide, there is an estimated 313,959 patients with ovarian cancer, and 207,252 deaths related to the disease are recorded annually [1]. Most patients receive platinum-based chemotherapy, but despite aggressive treatment, about 80% of patients at an advanced stage experience recurrence that is ultimately fatal. Maintenance therapy that continues beyond chemotherapy has become an important area of interest to prevent recurrence and extend disease control.
Bevacizumab, a vascular endothelial growth factor A inhibitor that can be added to chemotherapy or used in maintenance therapy, has emerged as the first targeted agent to be approved for the treatment of epithelial ovarian cancer (EOC) [2]. In addition, the United States Food and Drug Association has approved the use of poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors (PARPis) for maintenance of recurrent EOC based on the NOVA, SOLO2, and ARIEL3 trials [3,4,5]. These studies demonstrated improvement of progression-free survival (PFS) in patients with recurrent EOC who exhibited a complete or partial response after platinum-based chemotherapy. Recently, the survival benefits of maintenance therapy with a PARPi in newly diagnosed EOC patients have been validated by the SOLO1, PRIMA, and VELIA trials [6,7,8]. Olaparib, the first PARPi approved for EOC, lowered the risk of disease progression or death by 70% in EOC patients with a BRCA1/2 mutation according to the SOLO1 trial [6], and niraparib lowered the same risk by 67% in homologous-recombination deficient (HRD) EOC patients [7]. The clinical use of PARPis in EOC is increasing, and more patients are expected to be offered PARPis for front-line or second-line therapy.
However, PARPis are unable to prevent all recurrences of EOC. Patients with deleterious BRCA1/2 mutation or genomic alterations resulting in HRD are susceptible to PARPis [9,10,11], while these drugs have little to no efficacy in BRCA1/2 wild-type or platinum-resistant patients. As more patients receive PARPi as a maintenance therapy, the number of recurrent EOC patients who have previously been exposed to a PARPi will increase in the near future. Therefore, the need for a treatment strategy to treat PARPi resistance is increasing. Platinum-based chemotherapy is one option to consider for platinum-sensitive recurrent EOC that has been previously treated with a PARPi. However, data regarding re-challenging patients with a PARPi are limited, and it remains unknown whether PARPi re-treatment is beneficial as a maintenance therapy. To address this issue, clinical trials, such as A Study to Examine Olaparib Maintenance Retreatment in Patients With Epithelial Ovarian Cancer (OReO study, NCT03106987), are actively underway [12].
It is known that most common PARPi resistance mechanism comes from homologous recombination (HR) restoration [13]. In particular, somatic reversion mutation have clinically relevant evidence in BRCA1/2-mutated ovarian cancer [14]. And recent studies have provided preclinical evidence of epigenetic reversion, and reacquisition of DNA end resection to restore HR capacity [15]. In addition to HR restoration, stabilization of replication forks, diminished trapping of PARP-1 and drug efflux mechanism were suggested as other resistance mechanisms of PAPRi [16,17]. However, further studies are required to reveal clinical relevant evidence, except somatic reversion. In this situation where resistant mechanisms are not clearly identified, it is not easy to proposed the next-treatment options for PARPi resistant patients.
In the KGOG 3056/NIRVANA-R, bevacizumab is added as an alternative to overcoming PARPi resistance on the basis that anti-angiogenic agent can induce DNA repair defect caused by hypoxic damage [18,19]. Through NIRVANA-R, we will investigate the efficacy of niraparib and bevacizumab combination maintenance therapy for platinum-sensitive recurrent EOC patients previously treated with a PARPi.
METHODS
Trial design
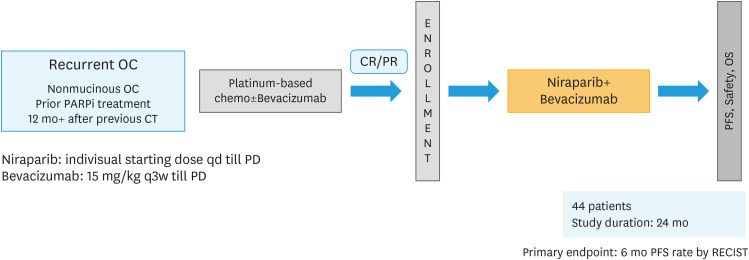
The KGOG 3056/NIRVANA-R trial is a multi-centre, investigator-initiated, single-arm phase II trial of niraparib with addition of bevacizumab in patients with platinum-sensitive recurrent EOC who have received at least 2 previous courses of platinum-containing therapy and were previously treated with a PARPi. Patients who have responded to the last platinum regimen (complete or partial response) are offered niraparib and bevacizumab as a maintenance treatment. This treatment is continued unless there is unacceptable toxicity or disease progression where the patient is no longer deriving any clinical benefit. Fig. 1 shows the trial schema. The treatment used in this trial is described in Table 1. The trial treatment is started on Day 1 of each cycle on an outpatient basis. The starting dose of niraparib is determined individually (200 or 300 mg once daily). Bevacizumab (15 mg/kg) is administered as an intravenous infusion for 30 minutes every three weeks. This study was approved by the national and local research ethics committees, and written informed consent was obtained from all patients. Sevene sites planned to recruit patients for the KGOG 3056/NIRVANA-R trial in Korea.
Fig. 1. Trial schema.
CR, complete remission; OC, ovarian cancer; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial remission; RECIST, Response Evaluation Criteria In Solid Tumors.
Table 1. Trial treatment.
| Drug | Dose/potency | Dose frequency | Route of administration | Regimen/treatment period |
|---|---|---|---|---|
| Niraparib | 200 mg or 300 mg* | QD | Oral | During each cycle |
| Bevacizumab | 15 mg/kg | Q3W | IV infusion | Q3W; Day 1 of each 3-week cycle |
IV, intravenous; Q3W, every 3 weeks; QD, daily.
*The recommended starting dosage of niraparib is 200 mg QD. For patients who weigh ≥77 kg and have a baseline platelet count ≥150,000/μL, the recommended starting dosage is 300 mg QD.
Participants
Inclusion/exclusion criteria for enrolment are outlined in Table 2. Briefly, EOC patients who recurred despite PARPi maintenance treatment are eligible. Previous use of bevacizumab use is not considered. There is no upper limit on the number of previous coursed of platinum-based chemotherapy, but two or more prior courses of platinum-based chemotherapy is necessary. Importantly, platinum-sensitivity should be guaranteed. All patients were required to provide informed consent to participate in this study. A participant must have adequate organ function; all screening laboratory tests should be performed within 10 days prior to the start of the study treatment.
Table 2. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| ► Age ≥20 years | ► Histology: mucinous, germ line, or borderline tumor |
| ► Histology: High-grade, predominantly serous, clear cell or low-grade serous ovarian cancer, primary peritoneal cancer, or fallopian tube cancer | ► History of non-infectious pneumonitis that required treatment with steroids |
| ► ≥Two previous courses of platinum-based chemotherapy | ► Participant who either has MDS/AML or has features suggestive of MDS/AML. |
| ► Platinum sensitivity following the penultimate platinum course (more than 12 months between penultimate platinum regimen and progression of disease) | ► Known additional malignancy that is progressing or has required active treatment within the past 2 years. |
| ► Complete or partial response to the last platinum regimen | ► Drainage of ascites during last 2 cycles of last chemotherapy. |
| ► Within 8 weeks of completing the last platinum regimen | ► Palliative radiotherapy within 1 week encompassing >20% of the bone marrow. |
| ► Prior treatment with a PARPi | ► Persistent >grade 2 toxicities from prior cancer therapy. |
| ► Available core or excisional biopsy of the tumor | ► Symptomatic uncontrolled brain or leptomeningeal metastases. |
| ► ECOG performance status 0–1 | ► Known hypersensitivity to the components of niraparib |
| ► Informed consent | ► Known active hepatic disease (i.e., Hepatitis B or C). |
AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; MDS, myelodysplastic syndrome; PARPi, poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitor.
Endpoints
The primary endpoint is a 6-month PFS rate. Analyses thereof will be performed when all enrolled patients have completed 6 months of follow-up and/or when investigator-assessed response assessments have been completed for all patients. PFS is defined as the time from the start of treatment until disease progression or death from any cause.
The secondary endpoints are toxicity, compliance, investigator-assessed response (RECIST v1.1 and/or Gynecologic Cancer InterGroup [GCIG] CA-125 criteria), overall survival (time frame: up to 1 year), time to progression (time frame: up to 1 year), time until the first subsequent treatment or death (time frame: date of first documented subsequent treatment or date of death, assessed for up to 72 months), time until the second subsequent treatment (time frame: date of the first documented and the second subsequent treatments assessed for up to 72 months), and PFS2 (time from the start of treatment until second progression or death). Overall survival (OS) is defined as the time from the first treatment until death from any cause. Patients without an event will be censored at the date they were last seen in a clinic or known to be alive. Additional updated efficacy and safety analyses could be conducted at later times if deemed appropriate.
Statistical methods
The rate of patients in a progression-free state at 6 months is expected to be about 50% without maintenance (based on the current standard of care and results from the GOG-213 and SOLO2 trials), and the hazard ratio of adding maintenance therapy of niraparib and bevacizumab was assumed to be 0.5, which is equivalent to 70.7% of the PFS rate. When applying the same expected efficacy (hazard ratio=0.5) as the PAOLA-1 study (NCT02477644), the null hypothesis for this study was a 6-month PFS rate of 50%, and an alternative hypothesis of interest was a 6-month PFS rate of 70%. Using Simon’s 2-stage optimal design at a one-sided, 5% level of significance, with 80% power, a minimum of 39 patients is required for this study. In the first stage, 22 patients will be enrolled and followed for 6 months. If 10 or more progressions are observed, the trial will be terminated. If not, the trial will continue to the second stage until 39 patients have been studied. If the total number of progressions is ≤15, the null hypothesis will be rejected. If we assume a 10% follow-up loss, the sample size should include at least 44 patients.
We will perform survival analyses using Kaplan-Meier plots and Cox regression analyses to produce hazard ratios, along with a log-rank test on the modified intent-to-treat approach (patients should receive at least one treatment dose). Safety analyses are based on the safety population (participants treated with at least one dose of the study drug). Adverse events are graded according to Common Terminology Criteria for Adverse Events, version 5.0.
DISCUSSION
Currently, PARPis are changing clinical practice for treating EOC patients. Many recent clinical trials have demonstrated a survival benefit for PARPi maintenance therapy from a later-line to a front-line setting. For this reason, PARPi maintenance therapy is being recommended to all patients with EOC who have received standard care.
In addition to PARPis, another treatment option is bevacizumab. In the ICON7 and GOG-218 trials, bevacizumab was incorporated into chemotherapy and continued as a maintenance therapy; it showed benefits to PFS in first-line treatment of ovarian cancer [20,21]. After the ICON7 and GOG-218 trials, bevacizumab incorporation could be considered a first-line ovarian cancer therapy, especially in wild-type BRCA1/2 patients.
More currently, in two randomized phase II studies, the combination of PARPi and anti-angiogenic agent produced a better outcome than PARPi alone, even in BRCA wild type or HRD-negative patients [22,23]. In the first biomarker driven phase II umbrella study (KGOG 3045/AMBITION) conducted in patients with platinum-resistant recurrent ovarian cancer [24], olaparib plus cediranib showed promising objective response rate (50%; 95% confidence interval [CI]=24.7–75.4], unpublished data). In addition, the synergistic effects of olaparib and bevacizumab in front-line setting was also demonstrated in the PAOLA-1 randomized phase III study (hazard ratio=0.59; 95% CI=0.49–0.72; p<0.001) [25].
As PARPis become more widely used and standard-of-care therapy in EOC patients, new clinical challenges are emerging. The issues are focused on strategies to maximize the effect of PARPis to prevent recurrence and how to treat relapsed patients that have been exposed to PARPi. To prevent recurrence, clinical trials regarding combination of PARPi and anti-PD-1/L1 for front-line (DUO-O [26], KEYLINK-001 [27], and ATHENA [28]) and second-line maintenance therapy (OPEB-01) [29] are actively ongoing. And future treatment options could become more diverse depending on results of these studies.
PARPi and anti-angiogenic agent combination therapy is a promising option to treat relapsed patients exposed to a PARPi. The synergistic efficacy of dual maintenance therapy (anti-angiogenic agent plus PARPi) in EOC patients was approved in many previous studies (NRG-GY004 [NCT02446600] and OVARIO trial [NCT03326193]), but there were few data in patients after PARPi progression.
In the phase II trial of cediranib plus olaparib for EOC patients after PARP inhibition progression (EVOLVE study, [NCT02681237]), dual maintenance therapy after disease progression on a PARPi was evaluated and showed various activities according to the PARPi resistance mechanism [30]. Further study will be required to reach a consensus on the best treatment strategy for such patients. To help with this, the KGOG 3056/NIRVANA-R trial is seeking to demonstrate the efficacy of niraparib and bevacizumab as a maintenance therapy to increase the survival rate of patients with platinum-sensitive recurrent EOC who were previously treated with a PARPi.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.J., L.M.C., L.J.K., J.D.H., K.S.I., C.M.C., K.B.G., L.J.Y.
- Data curation: L.J.Y.
- Formal analysis: L.J.Y.
- Funding acquisition: L.J.Y.
- Investigation: P.J., L.M.C., K.S.I., L.J.Y.
- Methodology: P.J., K.S.I., C.M.C., L.J.Y.
- Project administration: P.J., L.M.C., J.D.H., K.S.I., C.M.C., L.J.Y.
- Resources: K.B.G., L.J.Y.
- Software: K.B.G., L.J.Y.
- Supervision: P.J., L.M.C., L.J.K., J.D.H., K.S.I., K.B.G., L.J.Y.
- Validation: L.J.Y.
- Visualization: C.M.C., K.B.G., L.J.Y.
- Writing - original draft: P.J., L.M.C., L.J.K., J.D.H., K.S.I., C.M.C., L.J.Y.
- Writing - review & editing: P.J., L.M.C., L.J.K., J.D.H., K.B.G., L.J.Y.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Monk BJ, Minion LE, Coleman RL. Anti-angiogenic agents in ovarian cancer: past, present, and future. Ann Oncol. 2016;27(Suppl 1):i33–i39. doi: 10.1093/annonc/mdw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 7.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58. doi: 10.1016/j.ejca.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay A, Plummer ER, Elattar A, Soohoo S, Uzir B, Quinn JE, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675–5682. doi: 10.1158/0008-5472.CAN-12-0324. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–1154. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Colombo N, Glasspool R, Asselain B, Huzarski T, Korach J, et al. OReO/ENGOT Ov-38: a phase IIIb trial of olaparib maintenance retreatment in patients with epithelial ovarian cancer. Ann Oncol. 2017;28:v351–v352. [Google Scholar]
- 13.Noordermeer SM, van Attikum H. PARP inhibitor resistance: a tug-of-war in BRCA-mutated cells. Trends Cell Biol. 2019;29:820–834. doi: 10.1016/j.tcb.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7:984–998. doi: 10.1158/2159-8290.CD-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunting SF, Callén E, Wong N, Chen HT, Polato F, Gunn A, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao H, Ji F, Helleday T, Ying S. Mechanisms for stalled replication fork stabilization: new targets for synthetic lethality strategies in cancer treatments. EMBO Rep. 2018;19:e46263. doi: 10.15252/embr.201846263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaspers JE, Sol W, Kersbergen A, Schlicker A, Guyader C, Xu G, et al. BRCA2-deficient sarcomatoid mammary tumors exhibit multidrug resistance. Cancer Res. 2015;75:732–741. doi: 10.1158/0008-5472.CAN-14-0839. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan AR, Gueble SE, Liu Y, Oeck S, Kim H, Yun Z, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51 . Sci Transl Med. 2019;11:eaav4508. doi: 10.1126/scitranslmed.aav4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan N, Bristow RG. “Contextual” synthetic lethality and/or loss of heterozygosity: tumor hypoxia and modification of DNA repair. Clin Cancer Res. 2010;16:4553–4560. doi: 10.1158/1078-0432.CCR-10-0527. [DOI] [PubMed] [Google Scholar]
- 20.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 21.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 22.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–1419. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Yi JY, Kim HS, Lim J, Kim S, Nam BH, et al. An umbrella study of biomarker-driven targeted therapy in patients with platinum-resistant recurrent ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG 3045), AMBITION. Jpn J Clin Oncol. 2019;49:789–792. doi: 10.1093/jjco/hyz085. [DOI] [PubMed] [Google Scholar]
- 25.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 26.Harter P, Bidziński M, Colombo N, Floquet A, Pérez MJ, Kim JW, et al. DUO-O: a randomized phase III trial of durvalumab (durva) in combination with chemotherapy and bevacizumab (bev), followed by maintenance durva, bev and olaparib (olap), in newly diagnosed advanced ovarian cancer patients. J Clin Oncol. 2019;37(15_suppl):TPS5598 [Google Scholar]
- 27.Vergote I, Sehouli J, Salutari V, Zola P, Madry R, Wenham RM, et al. ENGOT-OV43/KEYLYNK-001: a phase III, randomized, double-blind, active- and placebo-controlled study of pembrolizumab plus chemotherapy with olaparib maintenance for first-line treatment of BRCA-nonmutated advanced epithelial ovarian cancer. J Clin Oncol. 2019;37(15_suppl):TPS5603 [Google Scholar]
- 28.Westin SN, Kristeleit RS, Coleman RL, Fujiwara K, Oza AM, O’Malley DM, et al. Abstract CT158: ATHENA (GOG-3020/ENGOT-ov45): a randomized, double-blind, placebo-controlled, Phase III study of rucaparib + nivolumab following front-line platinum-based chemotherapy in ovarian cancer. Cancer Res. 2019;79:CT158. [Google Scholar]
- 29.Lee YJ, Lim MC, Kim BG, Ngoi NY, Choi CH, Park SY, et al. A single-arm phase II study of olaparib maintenance with pembrolizumab and bevacizumab in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer (OPEB-01) J Gynecol Oncol. 2021;32:e31. doi: 10.3802/jgo.2021.32.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lheureux S, Oaknin A, Garg S, Bruce JP, Madariaga A, Dhani NC, et al. EVOLVE: a multicenter open-label single-arm clinical and translational phase II trial of cediranib plus olaparib for ovarian cancer after PARP inhibition progression. Clin Cancer Res. 2020;26:4206–4215. doi: 10.1158/1078-0432.CCR-19-4121. [DOI] [PubMed] [Google Scholar]