Abstract
Parasitic diseases are receiving increasing attention in developed countries in part because of their importance in travelers, immigrants, and immunocompromised persons. The main purpose of this review is to educate laboratorians, the primary readership, and health care workers, the secondary readership, about the potential hazards of handling specimens that contain viable parasites and about the diseases that can result. This is accomplished partly through discussion of the occupationally acquired cases of parasitic infections that have been reported, focusing for each case on the type of accident that resulted in infection, the length of the incubation period, the clinical manifestations that developed, and the means by which infection was detected. The article focuses on the cases of infection with the protozoa that cause leishmaniasis, malaria, toxoplasmosis, Chagas' disease (American trypanosomiasis), and African trypanosomiasis. Data about 164 such cases are discussed, as are data about cases caused by intestinal protozoa and by helminths. Of the 105 case-patients infected with blood and tissue protozoa who either recalled an accident or for whom the likely route of transmission could be presumed, 47 (44.8%) had percutaneous exposure via a contaminated needle or other sharp object. Some accidents were directly linked to poor laboratory practices (e.g., recapping a needle or working barehanded). To decrease the likelihood of accidental exposures, persons who could be exposed to pathogenic parasites must be thoroughly instructed in safety precautions before they begin to work and through ongoing training programs. Protocols should be provided for handling specimens that could contain viable organisms, using protective clothing and equipment, dealing with spills of infectious organisms, and responding to accidents. Special care should be exercised when using needles and other sharp objects.
Parasitic diseases are receiving increasing attention in developed countries, in part because of their importance in travelers, immigrants, and immunocompromised persons. Renewed clinical interest in parasitic diseases and the intellectual challenges posed by these diseases have stimulated laboratory research. Persons working in research and clinical laboratories, as well as health care workers providing patient care, are at risk of becoming infected with parasites through accidental exposures, which may or may not be recognized when they occur.
Even persons who realize they have had a laboratory accident often do not know whether they truly were exposed to organisms and what the inoculum size was. Even persons who are experts on parasitic diseases often do not know what clinical manifestations to expect when natural modes of transmission are bypassed, how to monitor for infection after accidental exposures, and whether to begin presumptive antimicrobial therapy before infection is documented. Because of such uncertainties and the potential severity of some parasitic diseases even in immunocompetent persons, the first reactions to laboratory accidents often are confusion and anxiety.
The main purpose of this review is to educate laboratorians, the primary readership, and health care workers, the secondary readership, about the potential hazards of handling specimens that contain viable parasites and about the diseases that can result. Table 1 provides information about parasites that have caused or could cause laboratory-acquired infections, and Table 2 lists factors that influence whether infection and disease develop after an exposure. Ideally, accurate counts of both accidental exposures and the resultant cases of infection would be available for the United States and other countries, as would information about the magnitude of the risks per person-hour or person-year of relevant work and of the risks associated with different types and severities of accidents. Unfortunately, exposures and infections often are unrecognized, and even if they are recognized, they often go unreported; risk data, with few exceptions (Table 3), are unavailable.
TABLE 1.
Parasites to which laboratory workers could be exposeda
| Parasite | Routes of exposurea | Infectious stage(s) | Protective measures | Diagnostic testingb | Common clinical manifestations of infectionc |
|---|---|---|---|---|---|
| Blood and tissue protozoa | |||||
| Acanthamoeba spp. | Wound, eye (aerosol?) (needle?) | Trophozoite, cyst | Gloves, mask, gown, class 2 BSCa, wound and needle precautions | Brain biopsy, culture, corneal scraping (serology?) | Headache, neurologic impairment, skin abscess, pneumonitis, keratitis, conjunctivitis |
| Babesia spp. | Needle, wound, vector | Intraerythrocytic stages, sporozoite | Gloves, wound and needle precautions | Blood smear, serology, animal inoculation | Fever, chills, fatigue, anemia |
| Balamuthia mandrillaris | Wound (aerosol?) (needle?) | Trophozoite, cyst | Gloves, mask, gown, class 2 BSC, wound and needle precautions | Brain biopsy, culture (serology?) | Headache, neurologic impairment, skin abscess (pneumonitis?) |
| Leishmania spp. | Needle, wound, transmucosal, vector | Amastigote, promastigote | Gloves; wound, mucous membrane,d and needle precautions | Cutaneous: lesion scraping, biopsy and impression smear, culture, animal inoculation Visceral: serology, biopsy, culture, animal inoculation Mucosal: serology, biopsy, culture, animal inoculation | Cutaneous: nodules/ulcers Visceral: fever (early), hepatosplenomegaly and pancytopenia (late) Mucosal: naso-oropharyngeal mucosal lesions |
| Naegleria fowleri | Transmucosal (nasopharynx), aerosol (needle?) | Trophozoite (flagellate?) (cyst?) | Gloves, mask, gown, class 2 BSC, wound and needle precautions | CSF exam and culture | Headache, stiff neck, coma, neurologic impairment (including sense of smell) |
| Plasmodium spp. | Needle, wound, vector | Intraerythrocytic stages, sporozoite | Gloves, wound and needle precautions | Blood smear, serology, culture, animal inoculation | Fever, chills, fatigue, anemia |
| Sarcocystis spp. | Oral | Sarcocyst; oocyst or sporocyst | Gloves, hand washing | Stool exam, muscle or cardiac biopsy | Gastrointestinal symptoms, eosinophilic myositis |
| Toxoplasma gondii | Oral, needle, wound, transmucosal (aerosol?) | Oocyst, tachyzoite, bradyzoite | Gloves, hand washing; wound, mucous membrane, and needle precautions | Serology, animal inoculation, tissue cell culture | Adenopathy, fever, malaise, rash |
| Trypanosoma cruzi (American trypanosomiasis) | Needle, wound, transmucosal, vector (aerosol?) | Trypomastigote | Gloves; wound, mucous membrane, and needle precautions | Blood smear, culture, biopsy, animal inoculation, xenodiagnosis, serology | Swelling and/or redness at inoculation site, fever, rash, adenopathy, electrocardiographic changes |
| Trypanosoma brucei rhodesiense and gambiense (African trypanosomiasis) | Needle, wound, transmucosal, vector (aerosol?) | Trypomastigote | Gloves; wound, mucous membrane, and needle precautions | Blood smear, CSF exam, culture, biopsy, animal inoculation, serology | Swelling and/or redness at inoculation site, fever, rash, adenopathy, headache, fatigue, neurologic signs |
| Intestinal protozoae | |||||
| Cryptosporidium parvum | Oral, transmucosal (aerosol?e) | Oocyst (sporozoite) | Gloves, hand washing, mucous membrane precautions | Stool exams with concentration and special stains, immunodiagnostic test for antigen in stool | Symptoms of gastroenteritis |
| Cyclospora cayetanensis | Orale | Oocyst (sporozoite) | Gloves, mask, hand washing | UV fluorescence microscopy, stool exams with concentration and special stains | Symptoms of gastroenteritis |
| Entamoeba histolytica | Orale | Cyst | Gloves, mask, hand washing | Stool exams with concentration, immunodiagnostic test for antigen in stool, serology (for invasive disease) | Symptoms of gastroenteritis (stools may be bloody) |
| Giardia lamblia | Oral (aerosol?e) | Cyst | Gloves, mask, hand washing | Stool exams with concentration, immunodiagnostic test for antigen in stool | Symptoms of gastroenteritis |
| Isospora belli | Orale | Oocyst (sporozoite) | Gloves, mask, hand washing | UV fluorescence microscopy, stool exams with concentration and special stains | Symptoms of gastroenteritis |
| Other protozoa | |||||
| Microsporidian spp.f | Eye (aerosol?), transmucosal, oral (wound?) (needle?) | Spore | Gloves, mask, gown, hand washing, class 2 BSC, wound and needle precautions | Microscopic exam and culture of corneal scraping, skin biopsy specimen, feces, urine, sputum, bronchoalveolar lavage, muscle biopsy specimen, CSF | Keratoconjunctivitis, skin ulceration, diarrhea, cystitis, pneumonitis |
| Helminthsg | |||||
| Ascaris lumbricoides | Oral | Egg | Gloves, mask, hand washing | Stool exam | Cough, fever, pneumonitis; abdominal cramps, diarrhea or constipationh |
| Enterobius vermicularis | Oral | Egg | Gloves, mask, hand washing, nail cleaning | Scotch tape test | Perianal pruritus |
| Fasciola hepatica | Oral | Metacercaria | Gloves, mask, hand washing | Exam of stool or bile for eggs, serology | Right upper quadrant pain, biliary colic, obstructive jaundice, elevated transaminase levels |
| Hookworm | Percutaneousi | Larva | Gloves, gown, hand washing | Stool exam | Animal speciesj: cutaneous larva migrans or creeping eruption (skin) Human species: diarrhea, abdominal pain, anemiah |
| Hymenolepis nana | Oral | Egg | Gloves, mask, hand washing | Stool exam | Abdominal pain, diarrhea |
| Schistosoma spp. | Percutaneousi | Cercaria | Gloves, gown, hand washing | Stool exam, serology | Acute schistosomiasis: dermatitis, fever, cough, hepatosplenomegaly, adenopathy |
| Strongyloides stercoralis | Percutaneousi | Larva | Gloves, gown, hand washing | Stool exam (motile larvae may be seen in wet preparations), serology | Cough and chest pain followed by abdominal pain and crampingh |
| Taenia solium | Oral | Egg, cysticercus | Gloves, hand washing | Cysticercosis: serology, brain scan, soft tissue X ray Worm: stool exam | Cysticercosis: neurologic symptoms Worm: usually asymptomatic but may cause vague abdominal symptoms |
| Trichinella spiralis | Oral | Larva | Gloves, mask, hand washing | Serology, muscle biopsy | Abdominal and muscle painh |
| Trichuris trichiura | Oral | Egg | Gloves, mask, hand washing | Stool exam | Abdominal pain, tenesmush |
The parasites listed here should be handled in accordance with BSL-2 standards. Laminar-flow biological safety cabinets (class 2 BSCs), other physical containment devices, and/or personal protective equipment (e.g., face shield) should be used whenever procedures with a high potential for creating aerosols or droplets are conducted. See the text for discussion of additional parasites. See the text and other tables for more details about routes of exposure. In this table, the “needle” route signifies parenteral transmission (i.e., percutaneous transmission, via a contaminated sharp such as a needle) and the “wound” route signifies contamination (e.g., via a spill or splash) of a preexisting abrasion, cut, or break in the skin.
PCR and other molecular techniques could also be useful for detecting infection with some of the listed parasites.
The clinical manifestations can be highly variable, depending in part on such factors as the species of the parasite, the size of the inoculum, and the stage of the infection. The listed manifestations are by no means all-inclusive and do not necessarily include some of the more serious manifestations of illness (e.g., cerebral malaria and myocarditis and encephalitis from toxoplasmosis).
Use of a class 2 BSC provides optimal protection against exposure of the mucous membranes of the eyes, nose, and mouth.
The possibility of becoming infected from swallowing inhaled infectious aerosols or droplets has been raised for C. parvum (N. Hojlyng, W. Holten-Andersen, and S. Jepsen, Letter, Lancet ii:271–272, 1987) and G. lamblia (154). The same principle could apply to the other intestinal protozoa. C. parvum oocysts can bypass the gastrointestinal tract and establish a pulmonary infection directly.
Some species of microsporidia have been recognized to be pathogens in immunocompromised persons, especially patients with AIDS, and occasionally have been found to cause disease in persons with normal immune systems (27). Laboratorians could be exposed to microsporidian spores from clinical specimens or cultures; several species belonging to four genera are now culturable. Although no laboratory-acquired infections with microsporidia have been reported to date, the risk for such infections could increase as research on microsporidia increases.
Eosinophilia is common for those helminthic infections with an invasive tissue stage.
Symptoms are unusual unless the infecting inoculum is heavy, which would be unlikely in most laboratory-acquired infections.
Parasite can penetrate intact skin.
Cutaneous larva migrans usually is caused by animal hookworms, typically Ancylostoma spp., and sometimes by animal and human Strongyloides spp. and other species.
TABLE 2.
Factors that affect whether infection and disease result from accidental exposures to parasites
| Factors related to the accident |
| Route and characteristics of the exposure (e.g., depth of penetra tion of a needle)a |
| Inoculum size |
| Factors related to the parasite |
| Pathogenicity, virulence, and viability of the species and isolate |
| Infectious dose |
| Factors related to the laboratorian |
| Immune status in general and with respect to the particular parasite |
| Status of barriers (e.g., whether exposed skin was intact) |
| Actions taken after the accident (e.g., wound care, presumptive antimicrobial therapy) |
Sometimes even seemingly inconsequential exposures result in infection.
TABLE 3.
Available data about rates of laboratory accidents and infections with specific parasitesa
| Toxoplasma gondii |
| Laboratory A in the United Kingdomb |
| Rate of recognized laboratory accidents per person-hour of relevant work: one accident per 9,300 person-hours (three accidents in 27,750 person-hours of “performing the dye test or demonstrating viable T. gondii”) |
| Total number of probable laboratory-acquired infections: one, which occurred in someone who had been symptomatic but had not noted an accident and whose case was detected through a serosurvey |
| Laboratory B in the United States |
| Number of person-years of work: ∼48 person-years (average of two to three persons working at a time, over a 19-year period; not limited to hours of relevant work) |
| Rate of recognized laboratory accidents per person-year: one accident per 12 person-years (four accidents in 48 person-years) |
| Rate of infections per person-year: one infection per 24 person-years (two symptomatic seroconversions in 48 person-years; testing done at baseline and after accidental exposures) |
| Trypanosoma cruzi |
| State of São Paulo, Brazilc |
| Number of person-years of work: 126.5 person-years over a period of ∼17 years, including 91.5 person-years of relatively high-risk work (e.g., working with needles, preparing viable parasites, working with tissue cultures with large numbers of parasites) by 21 persons |
| Rate of recognized laboratory accidents per high-risk person-year: one accident per 15 person-years (six accidents in 91.5 person-years) |
| Rate of infections per high-risk person-year: one infection per 46 person-years (two infections in 91.5 person-years) |
| Schistosomiasis |
| Laboratory C |
| Rate of infections: four asymptomatic seroconversions, without recognized accidents, among ∼20 persons, during the period from the late 1970s through mid-1999 (number of person-years of work not available); two of the four persons had positive stool specimens |
| Collective data from an unspecified number of laboratories that included “over 100 persons handling millions of cercariae for over 20 years”d Number of symptomatic infections: none Number of asymptomatic seroconversions: two |
See the text for additional details. The extent to which these data are representative of research laboratories and laboratorians that work with these parasites is unknown.
Data taken from reference 132.
Data taken from M. Rabinovitch and R. de Cassia Ruiz, personal communication.
Data taken from reference 58.
Even so, much can be learned from the cases of laboratory-acquired parasitic infections that have been reported; 199 cases are tallied in Table 4. Although most of the cases discussed here occurred in laboratory workers, occupationally acquired infections in health care workers are included as well because they illustrate some of the same principles. However, because the article focuses on the risks encountered by laboratorians, the term “laboratory-acquired cases” is generally used in this review. Although the possibility of natural infection could not be ruled out for some of the cases, no cases known to have been naturally acquired or to have resulted from intentional, experimental infection were included. The 115 parasitic cases enumerated in 1976 by Pike (137) in his review of 3,921 laboratory-associated infections of all types are listed in a separate column in Table 4 but are not discussed in the text or included in the case tallies. Pike did not provide any references for or details about the individual cases he counted, which precluded both evaluation of the merit of the cases and elimination of double counting between his cases and the cases described here.
TABLE 4.
Numbers of reported cases of laboratory-acquired parasitic infections
| Parasitea | No. of cases counted in this article (n = 199)b | No. of cases counted by Pike (137) (n = 115)c |
|---|---|---|
| Blood and tissue protozoa | ||
| Trypanosoma cruzi | 65 | |
| Toxoplasma gondii | 47 | 28 |
| Plasmodium spp. | 34 | 18 |
| Leishmania spp. | 12 | 4 |
| Trypanosoma brucei subspp. | 6 | |
| Trypanosoma spp.d | 17 | |
| Intestinal protozoa | ||
| Cryptosporidium parvum | 16 | |
| Isospora belli | 3 | 5e |
| Giardia lamblia | 2 | 2 |
| Entamoeba histolytica | 23 | |
| Helminths | ||
| Schistosoma spp. | 8–10 | 1 |
| Strongyloides spp. | 4f | 2g |
| Ancylostoma spp. | 1f | |
| Ascaris lumbricoides | 8 | |
| Enterobius vermicularis | 1 | |
| Fasciola hepatica | 1 possible case | 1 |
| Hookworm | 2g |
Under each subheading (e.g., Blood and tissue protozoa), the relevant parasites are ordered in descending frequency according to the numbers of cases counted in this article.
Some asymptomatic cases of Toxoplasma gondii and Trypanosoma cruzi infection were included, as were some cases in health care workers infected with Cryptosporidium parvum, Giardia lamblia, and Plasmodium spp. Cases of C. parvum infection in persons exposed to naturally infected animals were not counted.
The cases counted in Pike's article, which was published in 1976 (137), are listed in a separate column from the cases enumerated in this article. Pike did not provide any details or references for any of the individual cases. Therefore, the strength of the evidence for the cases could not be evaluated and potential double counting with the cases discussed here could not be eliminated. Pike counted a total of 115 cases; besides the 112 cases counted in the table, Pike counted one case of Sarcocystis infection (not listed in the table because of uncertainty about its plausibility [see the text]), one case of Chilomastix (not a pathogen) infection, and one case of infection with a Leukocytozoon sp. (not known to infect humans). Reportedly, Pike's list includes four intentional infections, but he did not specify which cases these were. Pike did not include species names; the species listed in the table presumably were the causative organisms.
Pike did not clarify whether the patients were infected with Trypanosoma cruzi, T. brucei rhodesiense, or T. brucei gambiense.
Pike classified these cases as cases of coccidiosis. Presumably, the etiologic agent was Isospora belli.
Cutaneous larva migrans (creeping eruption or “ground itch”).
Pike did not clarify whether these were cases of cutaneous larva migrans or of intestinal infection.
The case discussions focus on the type of exposure, if recognized, that resulted in infection; the length of the incubation period; the clinical manifestations that developed, especially those that were severe or were noted before infection was detected; and the laboratory methods used to document infection. Persons who have had accidental exposures typically find such information useful, despite its anecdotal nature and the possibility that the cases of infection that have been reported may not be representative of all that have occurred.
The cases described here were ascertained through such means as literature review, requests for the antiparasitic drugs available from the Centers for Disease Control and Prevention (CDC) Drug Service, telephone consultations provided by CDC personnel after laboratory accidents, and personal communications. Persons who provided information about unpublished cases are acknowledged at the end of the article or cited in personal communications; some persons asked to remain anonymous.
After accidental exposures to parasites, the exposed persons should be monitored for clinical and laboratory evidence of infection. Whether clinical manifestations or positive laboratory tests are noted first depends on such factors as the virulence of the parasite, which may have diminished during repeated passage in laboratory animals; the person's degree of self-awareness; the frequency of physical examination; and the type of laboratory testing. Although parasitic infections usually are diagnosed by conventional microbiologic methods, laboratorians in research settings often have access to investigational molecular methods, such as PCR, which may facilitate early diagnosis.
Persons working with organisms that can cause systemic infection detectable by serologic testing (Table 5) should have serum obtained at the time of employment, periodically thereafter (e.g., semiannually) to screen for asymptomatic infection, after laboratory accidents (i.e., immediately after the accident and periodically thereafter), and if clinical manifestations suggestive of parasitic infection develop. The specimens obtained at the time of employment and immediately after an accident are useful for comparison with subsequent post-accident specimens, particularly if the latter test positive. Freezing multiple aliquots of baseline specimens helps minimize repeated freezing and thawing of individual specimens, which could negatively influence the outcome of some tests. The time to seropositivity depends on such factors as the etiologic agent, the test, and the frequency of testing.
TABLE 5.
| Disease | Antibody test(s)c | Antigen test(s)cd |
|---|---|---|
| Amebiasis | EIA | EIA, Rapidd |
| Babesiosis | IFA, IB | |
| Chagas' disease | EIA, IFA | |
| Cryptosporidiosis | EIA, DFA, IFA, Rapidd | |
| Cysticercosis | EIA, IB | |
| Echinococcosis | EIA, IB | |
| Fascioliasis | EIA | |
| Filariasis | EIA | EIA, Rapid |
| Giardiasis | EIA, DFA, IFA, Rapidd | |
| Leishmaniasis | IFA, EIA | |
| Malaria | IFA | Rapid |
| Schistosomiasis | EIA, IB | |
| Strongyloidiasis | EIA | |
| Toxoplasmosis | EIA, IFA | |
| Trichinellosis | BF, EIA |
The word “available” signifies availability of the test through commercial laboratories or at reference laboratories (e.g., at the CDC). The list is not all-inclusive; additional tests (e.g., radioimmunoprecipitation assay for antibody to Trypanosoma cruzi) may be available through research laboratories. Inclusion of a test in the list does not imply that it is endorsed by CDC or that it has been well evaluated.
This table is adapted from reference 175 with permission from the publisher. If possible, serum specimens that may be tested repeatedly (e.g., preemployment specimens, which are useful for comparison with post-accident specimens) should be divided into aliquots to minimize repeated freezing and thawing of individual specimens, which could negatively influence the outcome of some serologic tests.
Some of the antibody tests and all of the antigen tests are available as commercial kits. Abbreviations, in alphabetical order: BF, bentonite flocculation; DFA, direct fluorescent-antibody assay; EIA, enzyme immunoassay; IB, immunoblot; IFA, indirect fluorescent-antibody assay; IHA, indirect hemagglutination; LA, latex agglutination; Rapid, rapid immunochromatographic diagnostic test.
The antigen tests listed for amebiasis, cryptosporidiosis, and giardiasis detect antigen in stool.
Additional information about the diagnostic evaluation and clinical management of persons with parasitic infections can be obtained from other reference materials (1, 114, 160, 175) and by consultation with staff of the CDC Division of Parasitic Diseases at (770) 488-7760. Questions about the availability of antiparasitic drugs can be directed to the CDC Drug Service at (404) 639-3670 during working hours and (404) 639-2888 otherwise. Table 6 lists factors to consider when deciding whether to treat presumptively, before infection is confirmed.
TABLE 6.
Considerations when deciding whether to provide presumptive antimicrobial therapy after accidental exposures to parasites, before documenting infectiona
| Factors related to the accidental exposure |
| What is the likelihood that the exposure will result in infection and disease (Table 2), keeping in mind that sometimes even seemingly inconsequential exposures do so? |
| Factors related to the infection |
| Could infection, if it develops, be severe (e.g., be life threatening or cause substantial morbidity)? Are severe manifestations likely to develop quickly, or are the initial manifestations of illness likely to be relatively mild? |
| Are sensitive techniques available for detecting infection? If not, what could be the consequences of not detecting the infection or of detecting it late (e.g., development of chronic infection or more morbidity)? |
| Factors related to the laboratorian |
| Is the person likely to comply with monitoring for clinical and laboratory evidence of infection? |
| Is the person immunocompromised, or does the person have characteristics (e.g., pregnancy or advanced age) or medical disorders (e.g., diabetes or heart disease) that could affect the course of infection, influence how well symptoms (e.g., fever) would be tolerated, or increase the risk for side effects from the therapy? Is the person allergic to or otherwise intolerant of the relevant drugs? |
| Factors related to the therapy |
| The threshold for administering presumptive therapy is generally low if highly effective, minimally toxic, and easily administrable therapy is readily available. Decisions about whether to treat presumptively are more difficult if the therapy is of moderate or uncertain efficacy, could cause substantial toxicity, or is difficult to administer. Consider the following: |
| Efficacy |
| How effective is the therapy likely to be for treating infection caused by the species and strain of interest? |
| Is the therapy active during the early stages of infection (e.g., during the hepatic stage of infection with Plasmodium spp.)? Is there evidence to suggest that early treatment improves outcome? Could a presumptive course of therapy that is shorter than the typical therapeutic course suppress infection and potentially result in delayed onset of clinical manifestations or in chronic infection? |
| How quickly does the therapy act? If treatment is not started until after infection is documented, could the person become very sick before the therapy becomes effective? |
| Toxicity |
| How toxic is the therapy in general, and is the person at hand at increased risk for particular toxicities? |
| Drug availability |
| Is the drug readily available? If not, how quickly can it be obtained? |
| Ease of administration |
| How is the drug administered (e.g., orally, intramuscularly, intravenously)? |
| What is the duration of a typical course of therapy? Could the course be shortened if therapy is begun presumptively, soon after the exposure? |
Decisions about instituting presumptive therapy should be individualized. Although the answers to many of the questions in this list may not be known with certainty, the questions should prompt consideration of the listed factors. Irrespective of whether presumptive therapy is given, laboratorians with accidental exposure to parasites should be monitored for clinical and laboratory evidence of infection.
Some of the accidents that resulted in laboratory-acquired infection were directly linked to poor laboratory practices (e.g., recapping a needle or working barehanded) (Table 7). Clearly, preventing laboratory accidents is preferable to managing their consequences. To minimize the risk for accidental exposures, laboratorians working with parasites should use the containment conditions known as biosafety level 2 (BSL-2) (www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm) (36), which are based on standard microbiological practices and incorporate personal protective equipment and biological safety cabinets when appropriate. Animal BSL-2 containment conditions specify practices for working safely with BSL-2 agents in the animal arena. Within the context of a parasitology laboratory, following Universal (Standard) Precautions when dealing with human specimens entails consistently using BSL-2 facilities and practices. The Occupational Safety and Health Administration (www.osha.gov) Bloodborne Pathogens Standard (29 CFR 1910.1030) (www.osha-slc.gov/OshStd_data/1910_1030.html) regulates occupational exposure to blood-borne pathogens. NCCLS, an organization that develops voluntary consensus standards, has standards for various laboratory issues and practices (www.nccls.org/genlab.htm), such as verification of training for laboratory personnel. Requirements for interstate shipment of etiologic agents have been delineated (www.cdc.gov/od/ohs/biosfty/shipregs.htm). Additional information about biosafety issues can be obtained from other reference materials (36, 64, 155).
TABLE 7.
Examples of practices and occurrences that have resulted in laboratory-acquired parasitic infections
| Parenteral transmission |
| Recapped a needle |
| Removed a needle from a syringe |
| Set aside a clogged needle with the point facing up |
| Dropped a syringe |
| Broke a capillary hematocrit tube while pressing it into clay sealant |
| Obtained blood from a restless person |
| Injured by an animal that kicked a syringe or suddenly moved during inoculation |
| Bite |
| Bitten by an infected animal |
| Other skin exposure |
| Worked barehanded |
| Wore short sleeves |
| Vector-borne transmission |
| Bitten by an escaped mosquito or after laying an arm on a cage of mosquitoes |
| Ingestion |
| Pipetted by mouth |
| Sprayed with droplets of inoculum by a coughing or regurgitating animal |
| Miscellaneous |
| Worked too fast |
| Assumed that a certain species or strain was not infectious to humans |
| Assumed that organisms were no longer viable |
| Used a defective syringe or tubing |
| Worked unsupervised |
This article, like its previous iterations (82, 85, 86), is intended as a reference document, with the expectation that readers will focus on the sections relevant to their work. The blood and tissue protozoa are the focus of the article and are discussed first because of the risk they pose to laboratorians. Discussion of intestinal protozoa and of helminths follows.
INFECTIONS WITH PROTOZOA
Blood and Tissue Protozoa
Summary data.
This section focuses on the protozoa that cause leishmaniasis, malaria, toxoplasmosis, Chagas' disease, and African trypanosomiasis. Summary data about 164 laboratory-acquired cases of infection with the protozoa that cause these diseases are provided in the text and in a figure and tables: some tables focus on individual parasites, and other tables (Tables 8 to 10) and the figure (Fig. 1) facilitate comparisons among the parasites.
TABLE 8.
Numbers of reported cases of laboratory-acquired parasitic infections caused by blood and tissue protozoa, by decade of occurrence (if known) or publicationa
| Decade | No. of cases of infection with:
|
Total no. (% of 164; % of 121b) | ||||
|---|---|---|---|---|---|---|
| Leishmania spp. (n = 12) | Plasmodium spp. (n = 34) | Toxoplasma gondii(n = 47) | Trypanosoma cruzi(n = 65) | Trypanosoma brucei subspp. (n = 6) | ||
| 1920s | 0 | 1 | 0 | 0 | 0 | 1 (0.6; 0.8) |
| 1930s | 1 | 0 | 0 | 1 | 0 | 2 (1.2; 1.7) |
| 1940s | 1 | 0 | 4 | 0 | 0 | 5 (3.0; 4.1) |
| 1950s | 0 | 4 | 18 | 0 | 0 | 22 (13.4; 18.2) |
| 1960s | 0 | 7 | 9 | 7 | 0 | 23 (14.0; 19.0) |
| 1970s | 0 | 8 | 7 | 3 | 1 | 19 (11.6; 15.7) |
| 1980s | 7 | 9 | 6 | 4 | 2 | 28 (17.1; 23.1) |
| 1990s | 3 | 4 | 3 | 8 | 3 | 21 (12.8; 17.4) |
| Unknown | 0 | 1 | 0 | 42c | 0 | 43 (26.2; NAd) |
The data represent cases, not rates, and do not account for the numbers of laboratorians at risk during the various periods. For 29 (24.0%) of the 121 cases for which the decade is provided in the table, the data are based on the decade of publication because the decade of occurrence was not known or specified. A total of 164 cases are included in the table.
Percentages are also provided using the number of cases with available data as the denominator.
Brener did not provide data for most of the cases that he tallied in his articles (22; Z. Brener, Letter, Trans. R. Soc. Trop. Med. Hyg. 81:527, 1987).
NA, not applicable.
TABLE 10.
Numbers of reported cases of laboratory-acquired infections caused by blood and tissue protozoa, by known or likely route of exposurea
| Route of exposure | No. of cases of infection with:
|
Total no. (% of 164; % of 125b) | ||||
|---|---|---|---|---|---|---|
| Leishmania spp. (n = 12) | Plasmodium spp. (n = 34) | Toxoplasma gondii(n = 47) | Trypanosoma cruzi(n = 65) | Trypanosoma brucei subspp. (n = 6) | ||
| Parenteralc | 7 | 10 | 14 | 11 | 5 | 47 (28.7; 37.6) |
| No available information | 1 | 38 | 39 (23.8; NAi) | |||
| Vector-borne transmission | 19 | 2 | 21 (12.8; 16.8) | |||
| No accident recognizedd | 1 | 12 | 7 | 20 (12.2; 16.0) | ||
| Mucous membrane exposuree | 1 | 8 | 3 | 12 (7.3; 9.6) | ||
| Other skin exposure (e.g., via a spill or splash)f | ||||||
| Nonintact skindf | 1 | 5 | 1 | 2 | 1 | 10 (6.1; 8.0) |
| Skin, other | 1g | 1 (0.6; 0.8) | ||||
| Ingestion (presumptive mode) | 9 | 9 (5.5; 7.2) | ||||
| Bite (not necessarily the source of infection)h | 2 | 1 | 1 | 4 (2.4; 3.2) | ||
| Aerosol transmission?d | 1 | 1 (0.6; 0.8) | ||||
The routes of exposure are listed by descending frequency (see last column). If there was uncertainty about the nature of the exposure (e.g., no accident was recognized) but evidence suggested that one route of transmission was most likely, this route usually was presumed, for the purposes of this table, to have been the mode of transmission. However, the threshold for doing this was subjective because the information available about the cases varied in quantity and quality. Similarly, the distinction between “no accident recognized” and “no available information” was not always clear in the case reports. See text and the tables on the individual parasites for caveats about the various cases.
Percentages are also provided using the number of cases with available data as the denominator. Cases without a recognized accident were kept in the denominator.
Parenteral exposures involved a needle or other sharp object (e.g., glass coverslip, Pasteur pipette, broken capillary hematocrit tube) that punctured, scratched, or grazed the skin.
Some of the laboratorians who did not recall a discrete accident may have had subtle exposures, such as contamination of unrecognized microabrasions or exposure through aerosolization or droplet spread.
With the exception of the case described in footnote g, the exposure was assumed to have been mucosal if the person's face was splashed.
This category includes a hodgepodge of nonparenteral skin exposures. Sometimes the report specified that the person had preexisting skin abrasions, cuts, or breaks (i.e., nonintact skin), whereas other times this was a presumption (e.g., someone who worked barehanded and did not recall parenteral exposures or someone who developed a chagoma at the site of a cuticle was assumed to have had transmission across nonintact skin).
The laboratorian apparently got infected murine blood on his face when a centrifuge tube broke (see the text); whether this represented skin or mucosal contact or transmission by aerosol or droplets is unclear.
All of the case-patients who were bitten by animals are counted here to highlight the importance of this type of injury, even though contamination of the bite wound rather than the bite itself may have been the route of transmission in some of these cases.
NA, not applicable.
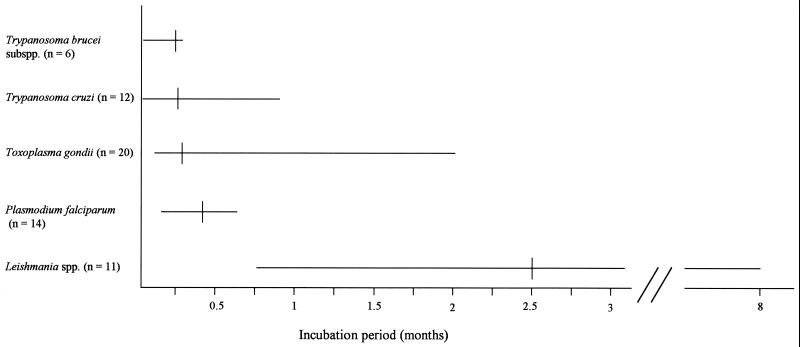
FIG. 1.
Incubation period (i.e., period from accidental exposure until first symptom or clinical sign of infection) for the clinically evident laboratory-acquired cases of infection with various blood and tissue protozoa. The ends of the lines designate the extremes of the ranges, and the short vertical lines designate the medians. The parasites are ordered from the lowest to the highest median incubation period. Factors that presumably affected the data include the virulence of the particular strain of the parasite, the extent to which the laboratorian correctly identified the time of exposure and was attentive to the earliest clinical manifestations of infection, and the frequency of physical examination after the accidental exposure. For malaria, only non-vector-borne cases are included. For toxoplasmosis, only cases related to exposure to tissue stages of the parasite (rather than to oocysts) are included.
The median age of the case-patients, for the 61 with available data, was 30 years (range, 19 to 71 years). Over half of the 94 whose sex was known were men (57 [60.6%]). Clearly, these age and sex data would be more meaningful if data were also available for the population at risk. The case-patients included students, house staff, technicians, principal investigators, an emeritus researcher, and ancillary staff (i.e., a secretary and someone who collected dirty glassware); they ranged from new employees to persons with decades of experience. The work settings included insectaries, animal facilities, research laboratories (e.g., in universities, public health agencies, and pharmaceutical companies), clinical laboratories, hospital wards, and autopsy suites.
The years when the case reports were published range from 1924 through 1999, and the reports were published in six different languages. Comparable proportions of the cases for which data were available occurred or were reported in each decade from the 1950s through the 1990s (Table 8, last column). However, for individual diseases, there was more variability from decade to decade (Table 8, first five columns). The case-patients worked in at least 26 countries; of the 123 case-patients for whom data were available, 57 (46.3%) worked in the United States and the others worked in various other regions of the world (Table 9). These data should be interpreted with caution because they do not consider the variability by time and place in the numbers of laboratories and laboratorians doing relevant work and in the likelihood that a case was reported.
TABLE 9.
Numbers of reported cases of laboratory-acquired parasitic infections caused by blood and tissue protozoa, by country or region of the world where the case occurreda
| Geographic area | No. of cases of infection with:
|
Total no. (% of 164; % of 123b) | ||||
|---|---|---|---|---|---|---|
| Leishmania spp. (n = 12) | Plasmodium spp. (n = 34) | Toxoplasma gondii(n = 47) | Trypanosoma cruzi(n = 65) | Trypanosoma brucei subspp. (n = 6) | ||
| United States | 6 | 20 | 23 | 8 | 0 | 57 (34.8; 46.3) |
| Europe | 1 | 12 | 20 | 3 | 5 | 41 (25.0; 33.3) |
| Latin America | 3 | 0 | 0 | 15 | 0 | 18 (11.0; 14.6) |
| Asia | 1 | 1 | 1 | 0 | 0 | 3 (1.8; 2.4) |
| Australia/New Zealand | 0 | 1 | 1 | 0 | 0 | 2 (1.2; 1.6) |
| Canada | 1 | 0 | 0 | 0 | 0 | 1 (0.6; 0.8) |
| Africa | 0 | 0 | 0 | 0 | 1 | 1 (0.6; 0.8) |
| Unknown | 0 | 0 | 2 | 39c | 0 | 41 (25.0; NAd) |
| Subtotals | ||||||
| United States | 6 | 20 | 23 | 8 | 0 | 57 (34.8; 46.3) |
| Other areas | 6 | 14 | 22 | 18 | 6 | 66 (40.2; 53.7) |
| Unknown | 0 | 0 | 2 | 39c | 0 | 41 (25.0; NA) |
The data represent cases, not rates, and do not account for the numbers of laboratorians at risk in the various regions. Therefore, they may simply reflect the amount of research done, in the regions, on particular parasitic diseases. The geographic areas are listed by descending frequency (see last column). A total of 164 cases are included in the table.
Percentages are also provided using the number of cases with available data as the denominator.
Brener did not provide data for most of the cases that he tallied in his reviews (22; Brener, Letter).
NA, not applicable.
Because protozoa, in contrast to most helminths, multiply in the human host, even a small inoculum can cause illness. Thus, as described below, some case-patients either did not recall an accident or initially considered it trivial and remembered and reported it only after they became ill. For example, the laboratorian may have simply been grazed by a needle and may not have been able to find the wound thereafter. Even more of the exposures were unrecognized than is apparent from the data in the table about the route of transmission (Table 10) because, for some cases, the most likely route of transmission could be identified (e.g., ingestion of Toxoplasma gondii oocysts) even though a specific accident had not been recognized. Of the 105 case-patients who either recalled an accident or for whom the likely route of transmission could be presumed, 47 (44.8%) had percutaneous exposure via a contaminated sharp (i.e., a needle or other sharp object), which is referred to here as parenteral transmission. Accidental puncture with a needle while working with animals was particularly common. Of note, under experimental conditions that simulated a needlestick injury (specifically, with a 22-gauge needle attached to a syringe containing 2 ml of blood), the mean inoculum was 1.40 μl (range, 0 to 6.13 μl; 20 replicates) (V. M. Napoli and J. E. McGowan, Letter, J. Infect. Dis. 155:828).
The infections that resulted from the accidental exposures ranged in severity from asymptomatic (two cases of Trypanosoma cruzi infection and nine cases of Toxoplasma gondii infection that were detected through serologic testing) to fatal (one case of Chagas' disease and one case of toxoplasmosis). The incubation periods for the symptomatic cases with available data ranged from 1 day to 8 months. The comparative data about incubation period (Fig. 1) show that the symptoms and signs of infection, for all diseases except leishmaniasis, typically developed no more than 2 weeks and sometimes within a few days of the exposure. Only two persons with malaria (14.3% of 14 with available data) became symptomatic more than 2 weeks (specifically 15 and 17 days) after the exposure; only two persons with toxoplasmosis (10.0% of 20) became symptomatic more than 2 weeks (specifically, 2 months) after the exposure; and only two persons with Chagas' disease (16.7% of 12) became symptomatic more than 2 weeks (specifically, 16 to 18 days and 24 days) after the exposure. In contrast, persons with leishmaniasis typically did not develop clinical manifestations of infection until months (although sometimes only weeks) after the exposure. These data should be helpful when deciding how intensely and how long to monitor for infection and whether to begin presumptive antimicrobial therapy before documenting infection. However, just as many accidental exposures seemed trivial when they occurred, the first clinical manifestations of infection in the reported cases often were mild or nonspecific and thus were initially overlooked or attributed to other etiologies (e.g., influenza), which resulted in delays in diagnosis and treatment. This highlights the importance of taking all accidental exposures seriously, reporting them to local authorities (e.g., supervisor and safety officer), and closely monitoring for clinical and laboratory evidence of infection.
The various blood and tissue protozoa of interest are discussed below, in alphabetical order.
Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri.
Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri are free-living amebae that cause life-threatening infection of the central nervous system (CNS) (116). Infection with Naegleria fowleri typically is acquired by swimming in fresh water. The parasite invades the CNS through the nasal mucosa and the cribriform plate and causes primary amebic meningoencephalitis, a disease that typically is rapidly fatal. Acanthamoeba spp. and Balamuthia mandrillaris cause more subacute or chronic infection. Both cause granulomatous amebic encephalitis, which may result from hematogenous dissemination in the context of pulmonary or skin lesions; and Acanthamoeba spp. cause keratitis in persons who wear contact lenses or have corneal abrasions. Relatively few laboratorians work with these parasites, and no laboratory-acquired infections have been reported. However, the possibility of becoming infected by inhaling infectious aerosols or droplets or through exposures to the mucous membranes (e.g., splashes) or perhaps through accidental needlestick injuries or pre-existing microabrasions of the skin should be considered. Immunocompromised persons, in particular, should be counseled about the risks associated with working with these parasites and given the option of not doing so and of not working in a laboratory in which others do so. Infections with these parasites are difficult to treat regardless of the host immune status (1).
Babesia spp.
In nature, Babesia spp. are transmitted by the bite of infected Ixodes ticks. Transmission by blood transfusion also occurs (51, 157). Most of the reported tick-borne cases in Europe have been caused by B. divergens. In the United States, the primary etiologic agent is B. microti; some reported cases have been caused by the WA1-type and related parasites (135) and by the B. divergens-like MO1 agent (87). The risk for severe illness is highest in immunocompromised, elderly, and asplenic persons.
Although no cases of laboratory-acquired babesiosis have been reported, such cases could be acquired through contact with infected ticks or blood from infected persons or animals. Because ticks can be controlled more easily than mosquitoes in the laboratory, the risk of becoming infected through contact with ticks is relatively low.
If babesiosis is suspected, Giemsa-stained blood smears should be examined for intraerythrocytic parasites. Animal inoculation and PCR can be used to detect subpatent parasitemia (i.e., parasitemia too low to be detectable on a blood smear). The type of animal to inoculate depends in part on the species of Babesia; hamsters (Mesocricetus auratus) and jirds (Mongolian gerbils [Meriones unguiculatus]) are typically used for B. microti. Serologic testing can also be useful and traditionally has been done by using an indirect fluorescent-antibody assay (IFA). A combination of two antimicrobial agents should be used for treatment—either the traditional combination of clindamycin and quinine or the recently proposed combination of atovaquone and azithromycin (1, 104).
Leishmania spp. (i) General.
Leishmaniasis is caused by species of the genus Leishmania, which are transmitted in nature by the bite of infected female phlebotomine sand flies (80). Transmission can also occur congenitally and by blood transfusion (157). The promastigote form of the parasite is found in the vector, and the amastigote form is found in macrophages in mammalian hosts. The major clinical syndromes are visceral leishmaniasis, which affects internal organs (e.g., spleen and bone marrow) and is life-threatening; cutaneous leishmaniasis, which causes skin lesions that can persist for months, sometimes years; and mucosal leishmaniasis, a sequela of New World (American) cutaneous leishmaniasis that involves the naso-oropharyngeal mucosa and can result in considerable morbidity (80).
In laboratory settings, leishmaniasis could be acquired through inadvertent contact with an infected sand fly; containment measures for infected flies should be strictly followed. Transmission could also occur through contact with cultured parasites or specimens from infected persons or animals (e.g., through accidental needlestick injuries or via preexisting microabrasions of the skin). Blood specimens should be handled with care, even though fewer parasites generally are found in the bloodstream than in infected tissues.
(ii) Laboratory-acquired cases.
(a) Summary data. Twelve cases of laboratory-acquired leishmaniasis caused by six different species have been reported (38, 48, 50, 60, 66, 85, 86, 100, 150, 162; R. N. Sampaio, L. M. P. de Lima, A. Vexenat, C. C. Cuba, A. C. Barreto, and P. D. Marsden, Letter, Trans. R. Soc. Trop. Med. Hyg. 77:274, 1983) (Table 11). Although most of the infected persons developed cutaneous leishmaniasis, sometimes with associated local lymphadenopathy, one person developed visceral leishmaniasis and one developed mucosal leishmaniasis as a sequela of cutaneous leishmaniasis. The first-reported case of laboratory-acquired leishmaniasis occurred in 1930 (38), the second occurred in 1948 (162), and the other 10 occurred or were published in the 1980s and 1990s. Half of the reported cases occurred in the United States, one-quarter occurred in South America, and one-quarter occurred elsewhere. Over half (7 [58.3%]) of the 12 cases were known to be associated with parenteral exposures; one person did not recall an accidental exposure. The median incubation period for the 11 cases with available data was 2 to 3 months (range, 3 weeks to 8 months), which is longer than for the reported cases caused by the other blood and tissue protozoa (Fig. 1). Decreased virulence of some of the strains from repeated passage in laboratory animals could have accounted in part for some of the long incubation periods.
TABLE 11.
Characteristics of the reported cases of laboratory-acquired infection with Leishmania spp.a
| Characteristic | No. (%) of cases (n = 12) |
|---|---|
| Species | |
| L. donovani | 4 (33.3) |
| L. (Viannia) braziliensis | 3 (25.0) |
| L. tropica | 2 (16.7) |
| L. (V.) guyanensis | 1 (8.3) |
| L. mexicana | 1 (8.3) |
| L. amazonensis | 1 (8.3) |
| Decade of occurrence (if known) or publication | |
| 1930s | 1 (8.3) |
| 1940s | 1 (8.3) |
| 1950s | 0 |
| 1960s | 0 |
| 1970s | 0 |
| 1980s | 7 (58.3) |
| 1990s | 3 (25.0) |
| Country or region of occurrence | |
| United States | 6 (50.0) |
| Latin America | 3 (25.0) |
| Canada | 1 (8.3) |
| Europe | 1 (8.3) |
| Asia | 1 (8.3) |
| Route of exposure | |
| Parenteral | 7 (58.3) |
| Biteb | 2 (16.7) |
| Nonintact skin | 1 (8.3) |
| Mucous membrane?c | 1 (8.3) |
| No accident recognized | 1 (8.3) |
| Clinical manifestations | |
| Symptomatic cases | 12 (100) |
| Severe cases | 2 (16.7)d |
| Fatal cases | 0 |
The median incubation period was 2 to 3 months (range, 3 weeks to 8 months) for infections due to all exposures (11 cases with available data) and 8 weeks (range, 3 weeks to 6 months) for the subset of infections due to parenteral exposures (n = 7).
For at least one of the cases, contamination of the bite wound rather than the bite itself was thought to have been the route of transmission (50).
The laboratorian had repeatedly contaminated his fingers and oral mucosa (apparently during mouth pipetting) with blood from infected squirrels and once had swallowed infected blood (38). For the purposes of this table, he is classified as having had a mucosal exposure.
The severe cases included one with mucosal leishmaniasis and one with visceral leishmaniasis.
(b) Description of four cases caused by Leishmania donovani.
Of the four laboratorians known to have become infected with L. donovani (38, 60, 66, 162), an organism that typically causes visceral leishmaniasis in infected humans but can cause cutaneous leishmaniasis, only one (38) developed clinical manifestations indicative of visceral involvement (e.g., fever, splenomegaly, and leukopenia). His case, which occurred in China in 1930, was the first documented case of laboratory-acquired leishmaniasis, although the remote possibility of vector-borne transmission could not be excluded. The affected laboratorian, who published his own case report, “hope[d] that the report of [his] case [would] at least serve as a warning to laboratory workers to safeguard themselves in handling Leishmania donovani” (38).
Apparently during mouth pipetting, “while making blood counts,” the Chinese researcher accidentally swallowed blood from an infected squirrel; he “sucked” an estimated 30 to 40 μl of blood into his mouth but probably swallowed much less than that. He added: “Through neglect of precautions contamination of the mouth cavity with infected blood subsequently occurred on many occasions. As it was often necessary to stop the hemorrhage from the infected squirrels following punctures of the superficial veins for blood counts, the fingers of the right hand were not infrequently contaminated due to the fact that the cotton sponges used to check the bleeding was [sic] often soaked with the infected blood. The fingers in turn contaminated the rubber tubes of the blood-counting pipettes” (38). The incubation period for his illness could not be determined with certainty because he had repeatedly had mucosal and skin contact and it was unclear whether his initial symptoms (i.e., lassitude and loose stools), which developed about 3.5 months after he swallowed the blood, or only his later symptoms were attributable to leishmaniasis; the incubation period was a minimum of several months. Alternative diagnoses that were considered included influenza and brucellosis. Ultimately, cultures of blood and liver were positive for L. donovani.
One of the other three infected laboratorians, a woman with “mild gait and sensory deficits secondary to multiple sclerosis,” punctured the palm of her right hand, on the thenar eminence, with a needle containing L. donovani amastigotes (5 × 108 amastigotes/ml) in a suspension of splenic tissue from a hamster (66). The strain (Humera; L82) had been passaged in hamsters for 14 years. Three weeks after the exposure, she developed intermittent erythema, swelling, and joint pain and stiffness in her entire thumb distal to the inoculation site. A nodule was noted at the inoculation site by week 7, and regional lymphadenopathy was detected by week 8. Organisms were noted histologically and in a culture of a biopsy specimen of skin. Serologic testing by complement fixation was negative at weeks 1 and 4 but positive at week 9. Microscopic examination and culture of bone marrow and peripheral-blood buffy coat were negative, and she did not have clinical or laboratory evidence of systemic infection.
While recapping a needle, a physician accidentally inoculated himself with amastigotes from a hamster infected with a strain of L. donovani (MHOM/SU/00/S3) that had been maintained in laboratory animals for >30 years (60). He noticed a nodule at the inoculation site 6 months later but did not develop lymphadenopathy or systemic symptoms. The prolonged incubation period might have been attributable to “reduced virulence of the isolate” (60). Organisms were noted histologically and in a culture of a biopsy specimen of skin, and his lymphocytes had an accentuated response to leishmanial antigen.
A technician working with laboratory animals infected with L. donovani developed a swollen finger and epitrochlear and axillary lymphadenopathy (162). His fingers had been bitten several times “within the few months” before the clinical manifestations first developed. Whether he became infected through subsequent contamination of the bite wounds is unknown. Culture of a biopsy specimen from a lymph node was positive, and amastigotes were noted in an impression smear of the specimen. No parasites were found in smears or in a culture of bone marrow. Serologic testing by complement fixation was repeatedly negative; the formal-gel test was weakly positive.
(c) Description of three cases caused by Leishmania (Viannia) braziliensis.
Of the three laboratorians known to have become infected with L. (V.) braziliensis, one was a student who, when unsupervised, passaged suspensions of amastigotes in hamsters barehanded (Sampaio et al., Letter). He did not recall a recent accident, but “spillage had occurred” (Sampaio et al., Letter). He ultimately developed an ulcerative lesion on one of his fingers. Leishmaniasis was diagnosed by demonstrating amastigotes in an impression smear of a biopsy specimen from the lesion and by inoculating a hamster with biopsy material. In addition, leishmanin skin testing and serologic testing by IFA were positive.
A student bitten by a hamster she was inoculating with L. (V.) braziliensis amastigotes from infected hamsters subsequently developed leishmaniasis (50). The bite wound was thought to have become contaminated with the inoculum, but the details of the exposure were not specified in the report. Two months after the bite, a papular lesion that had developed at the site at an unspecified time evolved into an ulcerative nodule and ascending lymphangitis was noted. She was initially thought to have erysipelas and was later thought to have sporotrichosis. She ultimately developed numerous papular lesions, and leishmaniasis finally was diagnosed, based on histopathology, 10 months after the accident.
A laboratorian became infected with L. (V.) braziliensis (L1794 MHOM/VE/84[VE3]) by accidentally puncturing her thumb with a needle that “pierced its plastic hood” after she inoculated a hamster with an infected macerate containing ∼2,000 amastigotes/μl (48). The inoculum was thought “to be low by experimental standards [but] likely high when compared with natural infections” (48). Eight weeks later, she developed an ulcerative lesion at the site. Although PCR of a blood specimen was positive then, leishmanin skin testing and serologic testing by IFA and Western blot analysis were not positive until week 18 (negative at weeks 8, 11, and 16). During week 18, amastigotes were detected in a biopsy specimen from the lesion.
(d) Description of two cases caused by Leishmania tropica.
Of the two laboratorians known to have become infected with L. tropica, one was a graduate student who had a needlestick injury while passaging amastigotes (NIH strain 173) in mice (150). He noticed an erythematous, tender nodule at the inoculation site after 4 weeks, which ulcerated 2 weeks later. A lymphoproliferative response to leishmanial antigen became detectable during week 5. No organisms were demonstrated histologically or in a culture of a biopsy specimen of skin obtained during week 12.
The other laboratorian became infected by accidental self-inoculation while injecting an animal and developed a nodule at the inoculation site 3 weeks later (85, 86). The diagnosis was confirmed parasitologically, and seroconversion was demonstrated by IFA.
(e) Description of one case caused by Leishmania (Viannia) guyanensis.
A graduate student in parasitology became infected with L. (V.) guyanensis by accidentally inoculating herself while preparing to inject mice with an organism isolated from a patient 8 years earlier (85, 86). She noted itching at the inoculation site 3 months after the exposure, and an ulcerative skin lesion developed over the next 2 months. A culture of a biopsy specimen was positive.
(f) Description of one case caused by Leishmania mexicana.
A technician receiving immunosuppressive therapy for systemic lupus erythematosus became infected with L. mexicana (100). She had accidentally cut her finger and dressed the wound. Several hours later, the dressing was soaked with ∼8 × 107 amastigote culture forms when she unintentionally opened a test tube during disposal. A papule developed at the site 8 months later and ulcerated 3 months thereafter. Leishmaniasis was diagnosed by histopathology, culture, and PCR. Serologic testing by IFA was negative.
(g) Description of one case caused by Leishmania amazonensis.
A laboratorian infected with L. amazonensis (Maria strain) ultimately developed mucosal leishmaniasis as a sequela of cutaneous leishmaniasis (85, 86). Initially, she developed a local erythematous nodule within 3 months of scratching herself with a needle that contained amastigotes. Culture of a biopsy specimen from the lesion was positive. She was treated with what now would be considered an inadequate course of the pentavalent antimonial compound sodium stibogluconate. The lesion regressed but recurred, and she was treated again with the drug and also with heat. Although the local lesion healed, she developed mucosal leishmaniasis several years later.
(iii) Post-accident management.
Laboratorians who have had accidental exposures to Leishmania spp. should be monitored for clinical and laboratory evidence of infection. Skin lesions that develop near the site of exposure should be evaluated (Table 12) (80). Periodic serologic testing should be done, especially if the organism to which the laboratorian was exposed can cause visceral infection. In addition to a baseline specimen at the time of employment, serum should be collected immediately after the accident, at least monthly for 8 to 12 months or until seroconversion is noted, and whenever clinical manifestations suggestive of leishmaniasis are noted. If seroconversion is noted or clinical illness suggestive of visceral infection develops, further evaluation (e.g., examination of bone marrow) may be indicated.
TABLE 12.
Practical guide for evaluating skin lesions that develop after accidental exposures to Leishmania spp.a
| General comments |
| To increase sensitivity, use several techniques and obtain multiple specimens per technique. Even under optimal circumstances, the maximum overall sensitivity of this approach, using conventional parasitologic methods, may be only ∼70–75% and is even lower with chronic lesions and mucosal disease. |
| After cleansing the skin with 70% ethanol, inject anesthetic (i.e., 1% lidocaine with epinephrine 1:100,000) through intact skin into the dermis under the area to be sampled. High concentrations of anesthetic could inhibit parasite growth in culture, as could residual iodine if iodine is used to cleanse the skin. Before obtaining dermal scrapings and biopsy specimens, debride eschar from the relevant portions of the lesions and apply pressure with sterile gauze to achieve hemostasis and to avoid making bloody smears. |
| Obtain needle aspirates for leishmanial culture. |
| Obtain 3–5 aspirates from different lesions or different portions of a lesion. Draw up ∼0.1 ml of preservative-free sterile 0.9% saline into a 1.0–3.0-ml syringe. For ulcerative lesions, insert the needle through intact skin into the dermis of the active border. Use a 23- to 27-gauge needle; use small-gauge needles for facial lesions. Repeatedly move the needle back and forth under the skin, tangentially to the ulcer, simultaneously rotating the syringe and applying gentle suction, until pink-tinged tissue fluid is noted in the hub of the needle. If no aspirate is obtained, inject 0.05–0.1 ml of saline under the skin and resume suction. |
| Discharge each aspirate into a separate tube of culture medium (e.g., Novy-MacNeal-Nicolle medium). Thin smears of aspirates typically are suboptimal unless a cytospin preparation is used. |
| Obtain biopsy specimens for cultures and histopathology. |
| Obtain one or two full-thickness punch biopsy specimens at the active border of one or more lesions, with some of the specimen from nonulcerated tissue. |
| Divide the specimen into three portions, or obtain multiple biopsy specimens: |
| Use one portion for leishmanial culture and, if appropriate, for bacterial, mycobacterial, and fungal cultures. |
| Use one portion for impression smears (i.e., touch preparations; see below). |
| Use one portion for histologic examination of tissue stained with hematoxylin and eosin; Giemsa; and, if appropriate, special stains to exclude mycobacterial, fungal, and other infectious etiologies. Although histopathology generally is the least sensitive technique for diagnosing cutaneous leishmaniasis (sensitivity, <20% in some studies), it is useful for excluding other diagnoses. Amastigotes are more easily recognizable in touch preparations and in thin smears of tissue scrapings (see below). |
| PCR, monoclonal antibody analyses, and animal inoculation can also be done. |
| Make tissue impression smears. |
| Grasp the biopsy specimen with forceps. Gently blot the cut surface onto a clean paper towel or gauze to remove excess blood. Gently press the blotted surface, with a rolling or circular motion, onto a glass slide. Repeat in a parallel row down the slide. Air dry the slide, fix in methanol, and stain with Giemsa. |
| Obtain dermal scrapings for thin smears. |
| Obtain 3–5 dermal scrapings from different lesions or different portions of a lesion (e.g., beneath the necrotic lip of the lesion). If aspirates and biopsy specimens for culture will also be obtained from these lesions, obtain the dermal scrapings last to minimize the risk of contaminating the sites. Some practitioners use the slit-skin smear technique and first make an incision before obtaining dermal scrapings. For this technique, pinch the skin to exclude blood and use a scalpel blade to incise a slit, several millimeters long and deep, through intact skin into the dermis. For ulcerative lesions, start the incision in the active border and proceed radially out across several millimeters of intact skin. |
| Obtain tissue fluid and flecks of tissue by scraping the dermis (e.g., beneath the necrotic lip of the lesion or along the walls of the incision) with a sharp instrument (e.g., a scalpel blade or stainless steel spatula). After obtaining as much tissue as possible, make as thin a smear as possible. Air dry the slide, fix in methanol, and stain with Giemsa. Although dermal scrapings can also be cultured, the risk for contamination is high. |
| Examine slides by light microscopy. |
| Slides should be examined under oil immersion for amastigotes, the tissue stage of the leishmanial parasite. Amastigotes are obligate intracellular organisms. However, on slides (e.g., thin smears of dermal scrapings), amastigotes may also be found extracellularly. Amastigotes are round-to-oval structures, 2–4 μm long, with 2 prominent internal organelles (i.e., a nucleus and a kinetoplast, which is a rod-shaped, specialized mitochondrial structure with extranuclear DNA). When stained with Giemsa, the cytoplasm of the amastigote typically is pale blue and the nucleus and kinetoplast are pinkish red or violet blue. |
This table is adapted from reference 80 with permission from the publisher. For questions about the diagnosis and treatment of leishmaniasis, call the CDC Division of Parasitic Diseases at (770) 488-7775 or (770) 488-7760. CDC can provide culture medium and perform isoenzyme analysis to identify the causative species.
The options for antileishmanial therapy have been reviewed (16, 80, 84). The issue of whether to treat presumptively, especially if the laboratorian is exposed to a species that can cause visceral infection, is complicated by the fact that the most highly effective therapies for leishmaniasis are administered parenterally (80). If highly effective, well-tolerated, orally administrable therapy becomes available (e.g., the drug miltefosine [81]), the option of presumptive therapy might become more attractive for such cases.
Plasmodium spp.
(i) General.
Malaria is transmitted in nature by the bite of infected female anopheline mosquitoes. Congenital transmission and transmission by blood transfusion also occur (157). In nature, human infection usually is caused by Plasmodium falciparum, P. vivax, P. ovale, and P. malariae.
A common means by which laboratorians have become infected is through inadvertent, unrecognized contact with a rogue mosquito that escaped from a mosquito colony. Strict containment measures should be followed for infected mosquitoes. Light traps should be operative 24 h per day, at various levels (e.g., high and low), in rooms where escaped mosquitoes could be present. Laboratorians who dissect mosquitoes could become infected through subcutaneous injection of sporozoites. Another means of transmission to laboratorians and health care workers is through contact with infected blood from persons or animals or with cultured parasites, thus bypassing the hepatic stage of the parasite's life cycle.
(ii) Laboratory-acquired cases.
(a) Summary data. Thirty-four cases of malaria in laboratorians and health care workers have been reported (14, 21, 26, 33, 35, 39, 46, 61, 70, 85, 86, 90, 95, 107, 109, 123, 136, 153, 173; G. Börsch, J. Odendahl, G. Sabin, and D. Ricken, Letter, Lancet ii:1212, 1982; J. C. Burne, Letter, Lancet ii:936, 1970; N. J. Cannon, S. P. Walker, and W. E. Dismukes, Letter, JAMA 222:1425, 1972; G. Carosi, A. Maccabruni, F. Castelli, and P. Viale, Letter, Trans. R. Soc. Trop. Med. Hyg. 80:667–668, 1986; F. L. M. Haworth and G. C. Cook, Letter, Lancet 346:1361, 1995; W. Kociecka and B. Skoryna, Letter, Lancet ii:220, 1987) (Table 13), including six cases that were not published previously. The 34 cases were caused by three Plasmodium spp. Two persons infected with P. falciparum developed manifestations suggestive of cerebral malaria, a severe complication of malaria. The earliest documented laboratory-acquired case was reported in 1924 (year of occurrence not specified) (90), and additional cases occurred in each decade from the 1950s through the 1990s. Over half (20 [58.8%]) of the reported cases occurred in the United States. In contrast to the other parasitic diseases, a substantial proportion of the reported cases of malaria (19 [55.9%]) were vector borne. Parenteral transmission was common as well (10 cases [29.4%]). The median incubation period for the 14 nonvector-borne cases with available data was 12.5 days (range, 4 to 17 days) (Fig. 1). The incubation periods were comparable for patients with parenteral exposures and those with exposures across nonintact skin.
TABLE 13.
Characteristics of the reported cases of laboratory-acquired infection with Plasmodium spp.a
| Characteristic | No. (%) of cases (n = 34) |
|---|---|
| Species | |
| P. falciparum | 15 (44.1) |
| P. cynomolgi | 10 (29.4) |
| P. vivax | 9 (26.5) |
| Decade of occurrence (if known) or publication | |
| 1920s | 1 (2.9) |
| 1930s | 0 |
| 1940s | 0 |
| 1950s | 4 (11.8) |
| 1960s | 7 (20.6) |
| 1970s | 8 (23.5) |
| 1980s | 9 (26.5) |
| 1990s | 4 (11.8) |
| Unknown | 1 (2.9) |
| Country or region of occurrence | |
| United States | 20 (58.8) |
| Europe | 12 (35.3) |
| New Zealand | 1 (2.9) |
| Asia | 1 (2.9) |
| Route of exposure | |
| Vector-borne transmission | 19 (55.9) |
| Parenteral | 10 (29.4) |
| Nonintact skin | 5 (14.7) |
| Clinical manifestations | |
| Symptomatic cases | 34 (100) |
| Severe cases | 2 (5.9)b |
| Fatal cases | 0 |
The median incubation period was 12.5 days (range, 4 to 17 days) for all infections due to non-vector-related exposures (14 cases with available data), 12 days (range, 7 to 17 days) for the subset of infections due to parenteral exposures (n = 9), and 13 days (range, 4 to 17 days) for the subset of infections due to exposures via nonintact skin (n = 5).
Two persons infected with P. falciparum developed manifestations suggestive of cerebral malaria.
(b) Description of 19 vector-borne cases.
At least 19 laboratory-acquired mosquito-borne (sporozoite-induced) cases have been reported, including at least 10 cases (one previously unpublished) of P. cynomolgi infection (39, 46, 61, 70, 85, 86, 123, 153), 5 cases (two previously unpublished) of P. vivax infection (85, 86), and 4 cases (two previously unpublished) of P. falciparum infection (35, 85, 86, 173). One of the P. vivax cases was in a secretary who laid her arm on a cage containing infected mosquitoes, not realizing that it did. The incubation period was reported for only one of the vector-borne cases (i.e., a case of P. cynomolgi infection) and was 10 days. In 55 volunteers with experimentally induced infection with P. cynomolgi (24 with sporozoite-induced infection and 31 with blood-induced infection), the mean prepatent period until blood smear positivity was 19 days (range, 15 to 37 days) (42).
Six of the laboratory-acquired vector-borne cases of P. cynomolgi infection occurred in 1960 and were the first such cases recognized. This organism, which naturally infects Asian monkeys, was isolated in 1957 and brought to the United States in 1960 for study (70). As expressed by some investigators (39), “up to 1960, the attitude among malariologists generally was: ‘Monkey malaria is for monkeys, and human malaria is for humans.’” In other words, “… it was thought [that] ‘man could not be infected with monkey malaria’” (39). Therefore, some investigators “paid scant attention to the occasional mosquito that escaped into the room” (39). In studies of rhesus monkeys intravenously inoculated with sporozoites of the B strain of P. cynomolgi, the infectious dose was 10 sporozoites (43).
(c) Description of 15 non-vector-borne cases.
Fifteen cases acquired through accidental contact with infected blood have been reported. Eleven cases were caused by P. falciparum, and four were caused by P. vivax. Most of the cases occurred among persons providing patient care or working in clinical laboratories rather than among researchers. Of the 15 cases, 10 (66.7%) were associated with parenteral exposures (14, 26, 33, 90, 95, 107, 109; Cannon et al., Letter; Carosi et al., Letter; Haworth and Cook, Letter). The median incubation period for the nine such cases with available data was 12 days (range, 7 to 17 days). The 10 parenterally acquired cases are briefly described below.
The following seven cases were caused by P. falciparum.
(i) A research laboratorian working with parasites that had been in continuous culture for almost 4 years became ill 17 days after suffering a minor puncture wound when he broke a capillary hematocrit tube while pressing it into clay sealant (95).
(ii) An assistant who pricked his finger during an autopsy became ill 15 days later (90). He developed symptoms suggestive of cerebral malaria but was initially thought to have postinfluenza encephalitis. His parasitemia was very high, with every second or third erythrocyte infected. His case, which was reported in 1924 (year of occurrence not specified), was the earliest reported case of laboratory-acquired malaria.
(iii) A senior house officer in pathology became ill 14 days after accidentally stabbing his finger while preparing a blood smear (14).
(iv) A medical student who had a needlestick injury while obtaining blood from a patient became ill 8 days later (Cannon et al., Letter).
(v) A medical student who had a needlestick injury after drawing arterial blood became ill 8 days later (33).
(vi) A nurse who had a needlestick injury while obtaining blood from a restless patient became ill 1 week later (Carosi et al., Letter).
(vii) A health care assistant who had a needlestick injury while resuscitating a patient became ill 7 days later (Haworth and Cook, Letter).
The following three cases were caused by P. vivax.
(i) A house physician who pricked her finger with a needle while doing a venipuncture became ill 12 days later (109).
(ii) A nurse who had a needlestick injury when placing an intravenous catheter became ill 14 days later (CDC, unpublished data).
(iii) A nurse who pricked her finger with a contaminated needle while giving an injection became ill after an unspecified incubation period (26).
Of the 15 non-vector-borne cases, 5 (33.3%) were associated with nonparenteral exposures (21, 136; Börsch et al., Letter; Burne, Letter; Kociecka and Skoryna, Letter). The median incubation period was 13 days (range, 4 to 17 days). These cases are briefly described below.
The following four cases were caused by P. falciparum.
(i) A student in a medical biology department who had skin excoriations became ill 4 days after handling infected blood. At the time of diagnosis, his parasitemia was 5%. Several days later, he developed oliguria and cerebral malaria, with altered mental status and hallucinations (136).
(ii) A senior house officer became ill about 10 days after admitting a patient with malaria (the admission included obtaining blood specimens, making blood smears, and placing an intravenous catheter) shortly after cutting one of his fingers “to the quick” while trimming his nails (Burne, Letter).
(iii) A nurse who had a 3-mm-long cut on a finger that was contaminated with a patient's blood during venipuncture became ill 17 days later (Kociecka and Skoryna, Letter). Her status deteriorated during 4 weeks of treatment for “sepsis of unknown origin.” The possibility of malaria was not considered until she mentioned what had happened during the venipuncture. Her parasitemia was 22%, and her hemoglobin level was 7.4 g/dl.
(iv) A nurse who had sores on her fingers that were contaminated with several drops of a patient's blood became ill 14 days after the patient was hospitalized (the date of the exposure was not specified) (21).
The following case was caused by P. vivax.
(i) A nurse with “several small scratches on her fingertips (caused by peeling potatoes)” became ill 13 days after performing a venipuncture barehanded (Börsch et al., Letter).
In addition, nosocomial patient-to-patient transmission (e.g., through contamination of a multidose heparin container) has been reported (1a, 3, 25, 37, 49, 107, 127, 170; P. P. Mortimer, Letter, Lancet 349:574, 1997). This type of transmission is beyond the scope of this review, as is malaria associated with blood transfusions, and is not discussed here.
(iii) Post-accident management.
The possibility of malaria should be considered in persons with unexplained febrile illness who might have been exposed to malaria parasites. Giemsa-stained blood smears should be examined for intraerythrocytic parasites. PCR and serologic testing by IFA can also be useful. Persons infected with P. cynomolgi typically have low-level or subpatent parasitemias; the diagnosis can be confirmed by performing PCR or by injecting the person's blood into a monkey and then monitoring the monkey for parasitemia.
When prescribing treatment for confirmed cases of malaria, the identity of the infecting species and its drug susceptibilities must be considered (1). Generally, therapy is not given unless infection is documented. However, presumptive therapy may be indicated in special circumstances (e.g., for persons who could have difficulty tolerating a febrile illness) (Table 6).
Sarcocystis spp.
Various Sarcocystis spp. can infect humans. Humans are the definitive host (i.e., the host for the sexual stage) for S. hominis and S. suihominis, for which the intermediate hosts (i.e., the hosts for the asexual stage) are cattle and swine, respectively. Persons working with raw beef or pork should guard against accidental ingestion of sarcocysts (i.e., the asexual stage) via contaminated fingers. Persons infected with these species can be asymptomatic or have various gastrointestinal symptoms. Infection is diagnosed by finding oocysts or sporocysts in the stool. Humans sometimes serve as intermediate hosts for other Sarcocystis spp.; sarcocysts with unknown life cycles and unknown carnivorous definitive hosts have been found in biopsy specimens of human skeletal and cardiac muscle (8, 13, 55), sometimes in association with eosinophilic myositis (8). No specific therapy has been identified for treating human sarcocystosis.
Whether laboratorians could become infected through accidental parenteral inoculation of Sarcocystis spp. is unknown. Although cell culture-derived merozoites of the classical Sarcocystis spp. of domestic animals do not induce disease when inoculated into other animals, culture-derived merozoites of S. neurona (an equine species) cause encephalitis after parenteral inoculation into immunosuppressed mice (54, 115).
Toxoplasma gondii. (i) General.
Toxoplasma gondii, the etiologic agent of toxoplasmosis, is transmitted in nature to persons who ingest tissue cysts in undercooked meat or oocysts from feline feces that have had time to sporulate and thus to become infectious; waterborne transmission of oocysts can also occur. The possibility of transmission through swallowing inhaled oocysts has been suggested (163). Congenital transmission and transmission by blood transfusion also occur (157). If symptomatic, Toxoplasma infection can range in severity from a syndrome of fever and lymphadenopathy to diffuse involvement of internal organs (e.g., myocarditis and encephalitis).
Laboratorians can become infected through ingestion of sporulated oocysts from feline fecal specimens or through skin or mucosal contact with either tachyzoites or bradyzoites in human or animal tissue or culture. All Toxoplasma isolates should be considered pathogenic for humans even if they are avirulent for mice (53). Procedures for separating oocysts from feline feces and for infecting mice have been described; fecal flotations should be performed before oocysts sporulate and thus become infectious (53). Instruments and glassware that are contaminated with oocysts should be sterilized because oocysts are not readily killed by exposure to chemicals or the environment (53). Immunocompromised persons and T. gondii-seronegative women who are pregnant or might become pregnant should be counseled about the risks associated with T. gondii infection (e.g., CNS infection and congenital infection) and given the option of not working with live T. gondii and of not working in a laboratory in which others do so.
(ii) Risk for laboratory accidents and infection.
The magnitude of the risk associated with laboratory work with T. gondii was assessed in a small case-control study in the United Kingdom (132). Comparable prevalences of antibody to T. gondii were found in the three groups of 16 persons each that were studied. Two seropositive persons were identified in laboratory A (Table 3), among “medical laboratory scientific officers with experience of working in the toxoplasma reference unit”; one of the two persons was seropositive before beginning this work. Among groups of age- and sex-matched controls from a routine microbiology laboratory and the general population, zero and three seropositive persons were identified, respectively.
Among the staff working with T. gondii in laboratory A, three reported having had accidental exposures to suspensions of viable organisms (i.e., needlestick injury, spillage onto skin, and splash into an eye), for a rate of three accidents per 27,750 person-hours of relevant work (i.e., working with viable parasites or performing the Sabin Feldman dye test, a serologic test that uses live tachyzoites) or one accident per 9,300 person-hours. Two of the three persons were treated with presumptive antimicrobial therapy, and none of the accidents resulted in seroconversion. On the other hand, one case of infection that was associated with seroconversion and probably was laboratory acquired and related to ingestion of oocysts was identified in the study in a person without a recognized accident (see below) (132).
In laboratory B in the United States (Table 3), in a 19-year period from 1980 to 1999, ∼30 to 40 persons worked directly with T. gondii. On average, two to three persons worked in the laboratory at a time (range, one to five), which translates into ∼48 person-years of work (not limited to hours of relevant work). Serologic testing was done at the time of employment and after accidental exposures. Only one person was already seropositive at the time of employment. Four persons had recognized laboratory accidents: three had percutaneous needlestick injuries, and one squirted a Toxoplasma-containing solution into one of his eyes. None of the four persons chose to be treated presumptively, and seroconversion was documented in two of the four, both of whom had needlestick injuries; their cases are described below. The four accidents occurred among the last five persons who joined the laboratory; three had accidents within a few months of starting work, in the context of increased turnover among the staff.
Some risk data regarding work with oocysts is provided below.
(iii) Laboratory-acquired cases.
(a) Summary data. Forty-seven laboratory-acquired cases of Toxoplasma infection have been reported (11, 17, 24, 62, 67, 71, 77, 78, 91, 96, 121, 124, 129, 132, 133, 141, 144, 149, 156, 161, 164, 169, 172, 178, 180) (Table 14), including three cases that were not published previously. In addition, a possible case of toxoplasmosis that might have been laboratory acquired has been described (167) and a laboratory-acquired case was mentioned, without details, in an article (177). Laboratory-acquired cases have occurred in every decade from the 1940s through the 1990s; the highest proportion of the reported cases (38.3%) occurred or were published in the 1950s. About half (23 [51.1%]) of the 45 reported cases with available data occurred in the United States. A substantial minority (8 [23.5%]) of the 34 cases for which the mode of transmission was known or suspected probably were attributable to ingestion of oocysts. Parenteral exposures, mucosal exposures, and unrecognized exposures also were quite common.
TABLE 14.
Characteristics of the reported cases of laboratory-acquired infection with Toxoplasma gondiia
| Characteristic | No. (%) of cases (n = 47) |
|---|---|
| Decade of occurrence (if known) or publication | |
| 1940s | 4 (8.5) |
| 1950s | 18 (38.3) |
| 1960s | 9 (19.1) |
| 1970s | 7 (14.9) |
| 1980s | 6 (12.8) |
| 1990s | 3 (6.4) |
| Country or region of occurrence | |
| United States | 23 (48.9) |
| Europe | 20 (42.6)b |
| Unknown | 2 (4.3) |
| Australia | 1 (2.1) |
| Asia | 1 (2.1) |
| Route of exposurec | |
| Parenteral | 14 (29.8) |
| No accident recognizedd | 12 (25.5) |
| Ingestion (presumptive route)e | 9 (19.1) |
| Mucous membrane | 8 (17.0) |
| Nonintact skin | 1 (2.1) |
| Bite (see the text) | 1 (2.1) |
| Aerosol transmission? (no evidence provided) | 1 (2.1) |
| No available information (during autopsy) | 1 (2.1) |
| Clinical manifestations | |
| Asymptomatic cases | 9 (19.1) |
| Symptomatic casesf | 38 (80.9) |
| Severe cases | 4 (8.5)g |
| Fatal cases | 1 (2.1) |
The median incubation period was 8.5 days (range, 3 days to 2 months) for all infections related to exposure to tissue stages of the parasite (20 cases with available data), 8 days (range, 3 to 13 days) for the subset of infections due to parenteral exposures (n = 11), and 7 days (range, 3 days to 2 months) for the subset of infections due to mucosal exposures (n = 7).
For five case-patients, Europe was the presumptive region of occurrence based on the little available information.
If there was uncertainty about the nature of the exposure or no accident was recognized but evidence suggested that one route of transmission was most likely, the most likely route usually was presumed, for the purposes of this table, to have been the mode of transmission. However, the threshold for doing this was somewhat subjective. Similarly, the distinction between “no accident recognized” and “no available information” was not always clear in the case reports. See the text for caveats about the cases.
See footnote c. At least three persons who did not recall an accident had performed the dye test and therefore might have gotten tachyzoites on their skin. The person whose case was fatal (156) had not reported an accident and is assumed, for purposes of this table, not to have recalled a specific accident.
Eight persons are thought to have ingested oocysts, and one person “often pipetted toxoplasma exudate” and might have become infected per os (161).
The presence of lymphadenopathy was classified as being symptomatic.
Four persons had encephalitis, two of whom also had myocarditis; one person with both conditions died (156).
The median incubation period for the 20 cases with available data, all of which were related to exposure to tissue stages of the parasite, was 8.5 days (range, 3 days to 2 months); with the exception of 2 cases with incubation periods of 2 months, all cases had incubation periods of ≤13 days (Table 14). Incubation periods among persons with parenteral exposures were comparable to those among persons with mucosal exposures. At one end of the spectrum, nine infected persons (19.1%) were asymptomatic; their cases were detected by serologic testing. At the other end of the spectrum, four infected persons (8.5%) developed encephalitis, two of whom also developed myocarditis. One of the two persons who developed both conditions died (141, 156).
(b) Description of eight cases attributed to ingestion of oocysts.
Eight cases documented by seroconversion and circumstantially attributed to ingestion of oocysts have been reported (121, 132). Seven of these cases were documented in several laboratories in the late 1960s and early 1970s, before oocysts were recognized to be extraordinarily hardy. The infected persons, who had worked mainly with the M-7741 strain, were essentially asymptomatic, although one person had midcervical lymphadenopathy and two persons had mild, nonspecific symptoms (i.e., flulike symptoms or fatigue and malaise). Before seropositivity was documented, the laboratorians had worked with tissue stages of the organism for 51 person-years (average, 10 years; range, 1 to 30 years) and with oocysts for 16 person-years (average, 2.3 person-years). Seven other laboratorians who had worked for 75 person-months but had not handled infectious oocysts had not become infected.
The eighth case was documented in laboratory A, in the study described above (Table 3) (132). The case was reported in 1992, but the year when it occurred was not specified. The laboratorian, who had been extracting oocysts from the feces of a cat infected with the RH strain of T. gondii, developed malaise, mild fever, and lymphadenopathy. Presumably, his hands had occasionally been contaminated with oocysts, which he then ingested.
(c) Rawal's review of 18 cases.
In 1959, Rawal described his own case of infection and reviewed 17 others (141), some of which had been described previously (17, 24, 91, 149, 156, 161, 164, 169, 172, 178). The probable mode of transmission was unknown for 10 of the 18 cases, including the author's. He suspected that organisms had contaminated his skin, particularly when he performed the dye test, which uses live tachyzoites. Four of the 18 persons had needlestick injuries. For example, one person pricked his finger on a clogged needle he had “set … aside point uppermost” (17). Three persons splashed infective material onto their faces or into their eyes. One person might have become infected by the bite of an infected rabbit (149); T. gondii, which can invade susceptible tissue cells throughout the body, has been isolated from rabbit and murine saliva (93). One person who did not recall an accident “often pipetted toxoplasma exudate” and might have become infected per os (161). He became ill just 18 days after starting work in the laboratory. One infected person did not yet have antibody detectable by the dye test ∼1 month after the onset of symptoms but did when next tested ∼3 weeks later (24).
The most commonly reported clinical manifestations in Rawal's case series were fever, headache, malaise, rash, and lymphadenopathy; two persons were asymptomatic. Three persons developed signs of encephalitis, two of whom also developed myocarditis. One person, who had not reported a laboratory accident, developed both encephalitis and myocarditis and died (141, 156). This person's case, which occurred in 1951, was described more fully in a separate case report (156). Its authors commented that “the handling of toxoplasma in the laboratory [had] not previously been regarded as hazardous.” Six days before the patient died, she was admitted to a psychiatric hospital with a history of having had delusions and hallucinations intermittently for 3 days, flulike symptoms and poor coordination 4 days before admission, and several months of fatigue, somnolence, and “lack of desire to do things” (156). When admitted, she was febrile, had a maculopapular rash, and was delirious. She “spoke frequently to imaginary characters in the room and indicated that she was going to die from toxoplasmosis” (156). She progressively became sicker and was transferred to a medical service in another hospital 4 days after admission to the psychiatric hospital. Although the diagnosis of toxoplasmosis was suspected after she became “seriously ill” (156), confirmatory laboratory results apparently did not become available until after she had died, and optimal therapy for toxoplasmosis had not yet been identified when this case occurred.
(d) Description of 21 other cases.
Four laboratory-acquired cases were described in a single report published in 1970 (144). The four affected persons worked in the same laboratory, and their cases, which are described below, were diagnosed by the dye test and IFA. Since 1962, three other persons in that laboratory had had accidents (i.e., a needlestick injury, a bite from an infected rabbit, and a cut with a coverslip containing infected tissue culture cells) that had not resulted in infection; one of the three persons had been treated presumptively with sulfadiazine and pyrimethamine, starting on the day of the accident. The number of persons who worked in the laboratory from 1962 to 1970 was not specified.
Of the four persons in the laboratory who became infected, two recalled accidents (i.e., a needle scratch or puncture with the RH strain) and two did not. The person who scratched herself with a contaminated needle developed cervical and supraclavicular lymphadenopathy, which were noted 10 days after the accident. On the same day, the first post-accident dye test titer was 1:4,096. The person who punctured herself with a needle began presumptive therapy with sulfadiazine and pyrimethamine immediately and remained asymptomatic but had a rise in her dye test titer, from 1:256 (the titer when multiple preexposure specimens were tested) to 1:4,096 (the titer from ∼1 month postexposure until at least 1 year thereafter).
One of the two persons in the laboratory who became infected but did not recall a specific accident was a medical student who worked with the RH strain in tissue culture and mice and who developed marked malaise and prolonged lymphadenopathy of unknown etiology. “Although familiar with the adenopathy caused by toxoplasma, [he] at no time considered this as a possible cause of his disease and did not inform [the laboratory director] of his illness” (144). Serologic testing was done after the student mentioned that he worked in a particular laboratory, whose director was then called. The other person who did not recall a specific accident was asymptomatic. Her case was detected through the laboratory's routine serologic monitoring program, which entailed testing at baseline and at least yearly thereafter. Her job included performing the dye test, and she was thought to have become infected while preparing the test. The possibility that she became infected outside the laboratory could not be excluded.
A researcher developed toxoplasmosis after piercing his thumb with a needle previously used for intraperitoneal inoculation of mice with a swine strain that had been passaged in mice for 26 months and had become highly pathogenic to mice (180). He was intermittently febrile on days 13 to 29 after the exposure and started therapy on day 30. He also had “slight respiratory involvement, malaise, and occasional profuse nighttime sweating” (180). Seroconversion was noted, from a negative IFA titer before and soon after the accident to titers of 1:64 on day 15 and 1:256 on day 34.
A technician who scratched a finger on her left hand with a contaminated needle (RH strain) became infected (67). The inoculum probably did not exceed 0.02 ml or from 1 to 100 mouse 50% lethal doses. She developed transient epigastric cramping on day 4 and fever, chills, and headache on day 5. On day 7, she was evaluated by a physician, who thought she had influenza. On day 8, she noted tenderness in her left axilla and a tender, erythematous lesion at the inoculation site, which prompted her to recall and report her accident. When she was hospitalized on day 9, an upper body rash and bilateral axillary and cervical lymphadenopathy were noted; the lesion on her finger was 3 by 3 mm, with a purulent center. The dye test was positive, and T. gondii was isolated from blood obtained on day 9.
A researcher who scratched a finger on his left hand with a needle while inoculating mice with peritoneal exudate from infected animals (RH strain) developed parasitologically confirmed toxoplasmosis (96). His wound was superficial and did not bleed spontaneously. The accident occurred just 21 days after he started work in the laboratory. He developed generalized myalgia on day 6 after the accident, malaise and headache on day 7, left axillary “swelling” on day 8, and fever on day 9. Ultimately, he also developed a petechial rash on his chest, cervical and inguinal lymphadenopathy, a pulmonary infiltrate, anemia, and lymphocytosis, with some atypical lymphocytes. T. gondii was recovered by animal inoculation from blood obtained on day 9 (the day of hospitalization) and lymph node tissue excised on day 15; the dye test was negative on day 9 but positive on day 11.
Two cases, which were not published previously, were related to needlestick injuries in the same laboratory (laboratory B; see above and Table 3). A technician working with a concentrated solution of T. gondii (RH strain) from murine peritoneal exudate stuck one of her fingers with a needle while recapping it. Approximately 7 to 10 days later, she awoke with a severe headache, stiff neck, and perhaps fever and was hospitalized to rule out meningitis. She developed ipsilateral axillary lymphadenopathy and Toxoplasma-specific antibody. Another technician in the same laboratory stuck herself with a needle while injecting 100 mice with T. gondii (C56 strain); she attributed the accident to working too fast. She noted malaise and fatigue on day 13 after the exposure, and seroconversion was detected 1 month postinoculation (the previous blood sample was from day 1). She had also had a needlestick injury about 2 years earlier, without subsequent seroconversion.
A technician who stuck one of her fingers with a contaminated needle developed headache, fever, and lymphadenopathy at an unspecified time thereafter (78). Antibody was detectable by IFA and solid-phase indirect hemadsorption when serologic testing was first done 1 week postexposure and was detectable later by complement fixation and indirect hemagglutination.
The same group of investigators reported two other cases, one in a medical assistant who accidentally injected parasites (BK strain) into her thumb (77). Three days later, her hand was painful and regional lymphadenopathy was noted. Antibody became detectable on day 14. The other person, a laboratory assistant, became infected through squirting a mixture of saline and tachyzoites (BK strain) from a syringe with a defective piston into his left eye (77). On day 4, his left ear was tender. On day 9, he developed edema of the left eye and the left side of his face. Mandibular lymphadenopathy was noted on day 11 and seropositivity on day 15.
Two cases confirmed by multiple serologic techniques, including the dye test, were described in one report (124). One of the two laboratorians stuck his hand with a needle containing infected murine exudate (R strain). He began presumptive therapy with a sulfa drug the next day and remained asymptomatic, but seroconversion occurred. The other person accidentally sprayed infected murine peritoneal exudate (BK strain) into his right eye. For 5 days, beginning on day 9 after the exposure, he complained of malaise, headache, and myalgia. Fever was noted on day 11, and lymphadenopathy at the right angle of his jaw was noted on day 17.
Three other persons became infected through splash-related exposures to their eyes. The first person, a laboratory assistant aspirating peritoneal exudate from an infected mouse (RH strain), splashed a small amount of exudate onto the right side of her face; the accident was attributed to using a defective syringe (62). Although she thought she had not gotten any exudate into her eye, the route of exposure was thought to have been through the conjunctiva. On day 9, her right eye was bloodshot and she had a headache, earache, sore throat, and painful ipsilateral cervical adenopathy. On day 12, she became febrile. She also developed malaise. Atypical mononuclear cells were detected 3.5 weeks after the exposure and peaked in number 1 week later. Seroconversion was noted in the dye test at 2 weeks, when the first post-exposure testing was done, and hemagglutinating antibody was detectable 2 weeks later. The second splash-related case, which was not published previously, occurred in an investigator who splashed infective material into his left eye while harvesting tissue cultures. He was passing the cells through a 25-gauge needle, to disrupt them and thus to release the Toxoplasma, and the needle might have become clogged. On day 7, he developed fever, conjunctivitis, and tender preauricular and cervical lymphadenopathy. He dreamed that night about the accident and then realized that the symptoms were attributable to the exposure. Serologic testing at an unspecified time demonstrated a high titer of Toxoplasma-specific immunoglobulin M (IgM). The last reported splash-related case was in a woman who splashed her eye while manipulating a Toxoplasma suspension (17; A. Franceschetti and F. Bamatter, First Latin Congr. Ophthalmol., p. 344, 1953). Reportedly, she developed “relapsing meningoencephalitis.”
A laboratorian who accidentally spilled ascitic fluid from infected mice onto small scratches on his left hand developed fever and left axillary lymphadenopathy 10 days later (133). Seroconversion was noted by enzyme immunoassay (EIA) for Toxoplasma-specific IgG, IgM, and IgA on day 40, when the first postexposure testing was done.
An animal technician who had worked with the RH strain developed a case of toxoplasmosis diagnosed by the dye test (11). He was thought to have become infected by inhaling aerosolized organisms, but no evidence to support this mode of transmission and no details about his work were provided in the report. His clinical manifestations included fever, rigors, vomiting, headache, generalized aching, tiredness, lethargy, dysphagia, a macular rash, lymphadenopathy (axillary, inguinal, and cervical), and hepatosplenomegaly.
A pathologist who supervised the autopsy of someone with cerebral toxoplasmosis became acutely ill 2 months later with parasitologically confirmed toxoplasmosis (129). Exposures during the autopsy were not detailed in the report. The pathologist's clinical manifestations included fever, chills, severe malaise, profound weakness, lethargy, lymphadenopathy, and hepatosplenomegaly. The adenopathy initially was in the anterior and posterior cervical areas and later became generalized. Infection was documented by serologic testing by the dye test and complement fixation and by intraperitoneal inoculation of a mouse with an emulsified lymph node.
A laboratorian developed fever, headache, conjunctivitis, a maculopapular rash on his face, and antibody to Toxoplasma (titer of 1:32 by complement fixation) (71). The persons who reported the case presented it as having been laboratory acquired. However, they did not provide any details about the nature of his work or state whether he recalled an accidental exposure. (For the purposes of this review, he is assumed not to have recalled an accident.) A complicating factor is that serologic testing for Q fever, a rickettsial disease caused by Coxiella burnetii, also was low-grade positive; rash is uncommon with Q fever.
(iv) Post-accident management.
The diagnosis of toxoplasmosis can be confirmed serologically or parasitologically, the latter by mouse inoculation or tissue cell culture; molecular methods also can be used. The most widely used screening methods for detecting Toxoplasma-specific IgG are IFA and EIA (174). A single test result demonstrating elevated levels of Toxoplasma-specific IgG can reflect a previous infection and therefore is of little value for diagnosing acute infection. If acute infection is suspected and the screening test for IgG is positive, the laboratorian's baseline specimens should be tested for IgG; testing by an IgM capture EIA can be helpful as well. Although a high titer of Toxoplasma-specific IgM suggests that the acute infection occurred within the past several months, IgM can persist for 18 months or more (174).
For naturally acquired Toxoplasma infection, typical practice is to treat persons who have organ involvement or persistent, severe symptoms with pyrimethamine and either sulfadiazine or trisulfapyrimidines, in conjunction with folinic acid, for at least 3 to 4 weeks (1). However, for persons who have had accidental exposures, administration of a 2-week course of presumptive therapy with these drugs (or alternative regimens for sulfa-intolerant persons [1]) should be considered while documentation of infection is in progress, because the risk for morbidity from toxoplasmosis is considered greater than the risks associated with therapy. Even laboratorians who receive presumptive therapy should be monitored serologically for several months after the exposure or until seroconversion is noted (i.e., they should be tested immediately after the exposure, weekly for at least 1 month, and at least monthly thereafter). As noted above, seroconversion can occur despite presumptive therapy. In other words, although presumptive therapy typically prevents disease or at least substantial morbidity, it does not necessarily prevent infection.
Trypanosoma cruzi. (i) General.
Trypanosoma cruzi, the etiologic agent of Chagas' disease (American trypanosomiasis), a disease endemic in Latin America, is transmitted by triatomine bugs when bug feces containing infective metacyclic trypomastigotes contaminate a wound (e.g., the bug's bite wound) or mucous membranes. Congenital transmission and transmission by blood transfusion also occur (157). After the parasite invades host cells, it replicates as the amastigote stage and differentiates into trypomastigotes, which are released when infected host cells rupture. Circulating trypomastigotes can invade other host cells or be taken up by the vector. In humans, the acute phase of infection lasts for weeks to months and often is asymptomatic. However, it can be associated with mild, nonspecific clinical manifestations or involve life-threatening myocarditis or meningoencephalitis. Years later, ∼10 to 30% of infected persons develop cardiac or gastrointestinal manifestations of chronic Chagas' disease.
Laboratorians can become infected through exposure to the feces of infected triatomine bugs, by handling cultures or blood specimens from infected persons or animals, and possibly by inhaling aerosolized organisms (179). Although the predominant stage of the parasite in axenic cultures usually is the epimastigote stage, trypomastigotes (the infectious stage) are found as well; the proportion of the organisms that are trypomastigotes depends on such factors as the strain of the parasite and the age of the culture. T. cruzi can infect persons through needlestick injuries or preexisting microabrasions of the skin or by crossing intact mucous membranes; mice have been experimentally infected by applying parasites to the conjunctiva or oral mucosa (98). Safety precautions for work with T. cruzi have been outlined (22, 72, 92).
(ii) Laboratory-acquired cases.
(a) Summary data. Sixty-five cases of laboratory-acquired T. cruzi infection have been reported (2, 6, 9, 22, 34, 45, 73, 79, 85, 86, 88, 120, 138, 165, 171; Z. Brener, Letter, Trans. R. Soc. Trop. Med. Hyg. 81:527, 1987), eight of which were not published previously (Table 15). For 37 (56.9%) of these cases, no information is available other than that they occurred (22; Brener, Letter), and limited information is available about some of the other cases. The earliest reported case occurred in 1938, and subsequent reported cases occurred starting in the 1960s. Slightly over half (15 [57.7%]) of the 26 cases with available data occurred in South America, which presumably reflects the amount of research on Chagas' disease done there. Of the 20 cases for which the route of transmission was known or suspected, 11 (55.0%) were attributed to parenteral exposures. The median incubation period for the 12 cases with available data was 7.5 days (range, 1 to 24 days). For the subset of seven cases with parenteral exposures, the median was 12 days (range, 5 to 24 days). Of the 26 infected persons with available data, 2 (7.7%) were asymptomatic and 9 (34.6%) had signs of cardiac or neurologic involvement; one patient who developed myocarditis died (Brener, Letter).
TABLE 15.
Characteristics of the reported cases of laboratory-acquired infection with Trypanosoma cruzia
| Characteristic | No. (%) of cases (n = 65)b |
|---|---|
| Decade of occurrence (if known) or publication | |
| 1930s | 1 (1.5; 4.3) |
| 1940s | 0 |
| 1950s | 0 |
| 1960s | 7 (10.8; 30.4) |
| 1970s | 3 (4.6; 13.0) |
| 1980s | 4 (6.2; 17.4) |
| 1990s | 8 (12.3; 34.8) |
| Unknown | 42 (64.6; NAg) |
| Country or region of occurrence | |
| Unknown | 39 (60.0; NA) |
| Latin America | 15 (23.1; 57.7) |
| United States | 8 (12.3; 30.8) |
| Europe | 3 (4.6; 11.5) |
| Route of exposure | |
| No available information | 38 (58.5; NA) |
| Parenteral | 11 (16.9; 40.7) |
| No accident recognized | 7 (10.8; 25.9) |
| Mucous membrane | 3 (4.6; 11.1) |
| Nonintact skin (includes cuticle) | 2 (3.1; 7.4) |
| Vector-borne transmission | 2 (3.1; 7.4)c |
| Bite | 1 (1.5; 3.7)d |
| Skin, other | 1 (1.5; 3.7)e |
| Clinical manifestations | |
| Asymptomatic cases | 2 (3.1; 7.7) |
| Symptomatic cases | 24 (36.9; 92.3) |
| Unknown clinical status | 39 (60.0; NA) |
| Severe cases | 9 (13.8; 34.6)f |
| Fatal cases | 1 (1.5; 3.8) |
The median incubation period was 7.5 days (range, 1 to 24 days) for infections due to all exposures (12 cases with available data) and 12 days (range, 5 to 24 days) for the subset of infections due to parenteral exposures (n = 7).
Percentages are also provided using the number of cases with available data as the denominator. These numbers are 23 for decade of occurrence, 26 for country or region of occurrence, 27 for route of exposure, and 26 for clinical manifestations.
For both cases, infection was attributed to exposure to metacyclic trypomastigotes from infected triatomine bugs (22). Whether the laboratorians were exposed to the bugs per se was not specified. The case of a laboratorian who had ocular mucosal contact with triatomine feces (79) was classified as a case of mucosal transmission; no details were provided in the case report about whether the laboratorian had contact with the bug per se or only its feces.
The laboratorian was bitten by an uninfected mouse (see the text). Presumably, the wound became contaminated.
The laboratorian apparently got infected murine blood on his face when a centrifuge tube broke (see the text); whether this represented skin or mucosal contact or transmission by aerosol or droplets is unclear.
Nine persons had signs of cardiac or neurologic involvement, one of whom died (Brener, Letter).
NA, not applicable.
(b) Data about eight cases from the State of São Paulo, Brazil, and incidence data about accidents and infection.
Some data are available for the State of São Paulo, Brazil, regarding the numbers of documented laboratory accidents and cases of T. cruzi infection (M. A. Shikanai-Yasuda and E. S. Umezawa, personal communication). As of 1999, an unknown number of persons in at least 15 institutions worked with T. cruzi. Eight laboratory-acquired cases of infection, which are discussed below, were documented from 1987 to 1998 in six institutions; presumably others occurred but were not reported. Of the eight reported cases, two were asymptomatic and two others were in persons who did not recall specific accidents.
In addition, 37 other persons in seven Brazilian institutions are known to have had laboratory accidents from 1984 to 1999 that did not result in infection; 22 (59.5%) of these 37 accidents occurred from 1997 to 1999. How many of the 37 persons were presumptively treated with benznidazole after their accidents is unknown. However, as discussed below, typical practice in Brazil is to treat for 10 days, sometimes longer, after needlestick injuries and other relatively high-risk accidents.
Incidence data for accidents and cases of infection are available for one of the laboratories in the State of São Paulo (M. Rabinovitch and R. de Cassia Ruiz, personal communication) (Table 3). The data are for a period of ∼17 years during the 1980s and 1990s, with 126.5 person-years of observation, including 91.5 person-years for 21 persons doing relatively high-risk work (e.g., working with needles, preparing viable parasites, and working with tissue cultures containing large numbers of parasites). Four accidents that did not result in infection and two that did were documented, all of which were included in the tallies of accidents and cases in the two previous paragraphs and occurred among the 21 persons doing relatively high-risk work. The persons who did not develop demonstrable infection had been treated presumptively. The two cases of infection occurred in persons working with the CL strain (Shikanai-Yasuda and Umezawa, personal communication). One of these persons apparently had conjunctival exposure because of defective (perforated) tubing attached to a syringe. Clinical manifestations, which occurred after an unknown incubation period, included fever, petechiae, a pericardial effusion, and peripheral edema; parasites were noted in a blood specimen. Dengue fever and leukemia also had been considered as possible diagnoses. The other infected person did not recall a specific accident. Clinical manifestations included fever, arthritis, congestive heart failure, and reversible facial paralysis; parasites were noted in a smear of a bone marrow aspirate. Leukemia also had been considered as a possible diagnosis.
Another laboratory in the State of São Paulo contributed five laboratory accidents, all with needles, to the above tallies (E. A. Almeida, M. E. Guariento, J. da S. Wanderley, and V. L. C. C. Rodrigues, Abstr. Rev. Soc. Bras. Med. Trop., vol. 27 [Suppl. II], abstr. 11, p. 145–146, 1994). All of the accidents occurred in 1993 and 1994, and one involved the principal investigator. The laboratorians who had the accidents were experienced, and their accidents were attributed to not being careful. Two of the five exposed persons became infected (Y strain), both asymptomatically. One of the two infected persons had a positive blood smear on day 15 postexposure, and the other had a negative smear on day 10 but was positive for T. cruzi-specific IgG and IgM on day 30. The three exposed persons who did not develop demonstrable infection had been treated presumptively.
All of the other four documented laboratory-acquired cases in the State of São Paulo (of the total of eight mentioned above) occurred among persons working with the Y strain (Shikanai-Yasuda and Umezawa, personal communication). One of the four persons did not recall a specific accident, one apparently got infected murine blood on his face when a centrifuge tube broke (whether this represented skin or mucosal contact or transmission by aerosol or droplets is unclear), one had a needlestick accident, and one cut his hand with a contaminated Pasteur pipette (107 trypomastigotes/ml). All four persons developed febrile illnesses and, at a minimum, had serologic evidence of infection; three also had parasitologic confirmation.
The case that involved the contaminated pipette is particularly noteworthy (M. A. Shikanai-Yasuda, E. S. Umezawa, J. E. Tolezano, and L. Matsubara, Abstr. Rev. Soc. Bras. Med Trop., vol. 26 [Suppl. II], abstr. 119, p. 127, 1993). The laboratorian developed acute Chagas' disease 14 days after his accident, despite having received a 10-day course of presumptive therapy with benznidazole (8.5 mg/kg/day). His clinical manifestations included fever, headache, mild hepatosplenomegaly, and lymphocytosis. Xenodiagnosis and mouse inoculation were done with blood collected on day 22 after the accident; both tests became positive 20 days later. He ultimately received a second course of therapy with benznidazole, this time for 80 days rather than 10.
(c) Brener's references to more than 50 laboratory-acquired cases.
In a published letter prompted by a laboratory-acquired case described below (88, 165), Brener reported being aware of >50 laboratory-acquired cases of Chagas' disease, including a fatal case in an untreated person with “unusually severe myocarditis” (details not provided) (Brener, Letter); Brener did not specify the exact number of cases (for the purposes of this article, it is assumed to have been 51). When he wrote an earlier publication (22), he had been aware of 45 of these cases, which included 8 previously published cases (9, 45, 73, 120, 138, 171) that are described below. Brener provided few details about the 45 cases as a whole or individually. He noted that they were distributed among 11 countries in North, Central, and South America and in Europe. Of these cases, 16 had been acquired in university laboratories, 14 had been acquired in nonacademic research laboratories, 12 had been acquired in pharmaceutical industries, and 3 had been acquired in public health laboratories. The most frequent type of accident apparently was “accidental puncture with the needle used to infect animals” (22). Infected blood was the source of infection in 15 of the 20 cases with a known source. Two persons were infected with tissue culture-derived trypomastigotes, two persons were infected with metacyclic trypomastigotes from infected triatomine bugs, and one person had pipetted and swallowed flagellates from acellular culture medium.
A biochemist whose case Brener described became infected while inoculating mice; a syringe containing infective blood (Y strain; 800,000 trypomastigotes in 0.4 ml of blood) “dropped from his hands and capriciously fell on his foot in an upright position” (22). Fever, malaise, and crural lymphadenopathy were noted 12 days later. On day 16, a chagoma (the inflammatory primary skin lesion) was noted at the inoculation site and trypomastigotes were found “by fresh blood examination.” Later, xenodiagnosis was positive as well.
(d) Description of 15 cases, including 9 cited by Brener.
Of the 15 cases described in this section, 6 were associated with needlestick injuries. A technician became infected when he stuck his left thumb with a needle contaminated with blood from a mouse infected with the CL strain. He had been trying to remove the needle from a syringe “in a manner prohibited” by the guidelines of the laboratory (88). His case of acute Chagas' disease had several interesting features (88, 165). He was well until 24 days after the exposure, when he developed fever and chills. He ultimately had high fever (up to 42°C), with relative bradycardia, considering the degree of fever. He developed a chagoma between the first and second metacarpals of the dorsum of his left hand (i.e., proximal to rather than at the inoculation site), which initially was a confusing feature. He had multiple negative smears of “concentrated blood” and relatively late seroconversion. Mouse inoculation and serologic testing by EIA and IFA simultaneously yielded positive results nearly 5 weeks after the accident; the mouse had been inoculated with the patient's blood 1 week earlier, when specific antibody was not yet detected. Serum neuraminidase activity was detected on day 12, peaked on day 24, and had become undetectable when specific antibodies were first demonstrable (165). Other clinical manifestations in this case included headache, malaise, lethargy, easy fatigability, anorexia, a generalized rash that initially was maculopapular and later consisted of erythematous blotches, left axillary lymphadenopathy, and T-cell lymphopenia (598 cells/μl, with a normal helper/suppressor cell ratio).
Three cases, all of which were diagnosed by examination of blood, were described in one report (138). Two of three infected persons recalled specific laboratory accidents (i.e., superficial needlestick injuries with the Tulahuen strain). One of them had a needlestick injury when a mouse he was inoculating suddenly moved. He became febrile on day 5 and developed swelling and redness at the inoculation site on day 8. The other person who recalled an accident was wearing short sleeves and was scratched on an uncovered part of her left forearm by a contaminated needle used for an inoculation. She noted a lesion at the site on day 5 and fever, chills, malaise, and left axillary pain on day 6. Both persons who recalled accidents developed manifestations suggestive of meningoencephalitis, which were particularly marked in the woman; she also developed manifestations suggestive of myocarditis. Other clinical manifestations in the three persons included generalized maculopapular rash, splenomegaly, and facial edema.
Other cases resulting from accidents with needles have been reported. One of these cases was briefly mentioned in an article about cases of acute Chagas' disease (6); the first author of that article subsequently provided more details about the case in a personal communication. In December, the laboratorian accidentally inoculated himself by needlestick with an axenic culture. In January, he had persistent fever and myalgia (incubation period unspecified), despite analgesic therapy. In February, he was hospitalized, but the cause of his illness was not determined. In March, he reported his accident and was evaluated for T. cruzi infection. Additional clinical manifestations included myocarditis, a pericardial effusion, and arrhythmias. No organisms were demonstrated by microscopic examination of blood, hemoculture, xenodiagnosis, or mouse inoculation. However, serologic testing by EIA, IFA, and direct agglutination was positive, and amastigotes were detected by histopathologic examination of an endomyocardial biopsy specimen.
A graduate student injecting mice with trypomastigotes (Brazil strain) was grazed on his abdominal skin by a contaminated needle after a mouse kicked the syringe he was using (85, 86). The wound was so superficial that it did not bleed and could not be found later that day. He noted an erythematous local skin lesion 10 days later, which gradually expanded to 5 to 7 cm. He was hospitalized 8 days thereafter, when he was febrile and had a headache. Although examinations of blood, buffy coat, and an impression smear of a biopsy specimen of skin were negative for T. cruzi and IFA testing was negative, xenodiagnosis, which was performed weeks later, was positive.
A research veterinarian became infected by accidentally puncturing a finger on his left hand with a needle being used to inject mice with T. cruzi (a Brazil strain) (2, 73). The inoculum was estimated to be 1,500 organisms. At 16 to 18 days later, he developed swelling and discoloration of the finger, tender unilateral epitrochlear and axillary lymphadenopathy, fever, shaking chills, and malaise. When he was hospitalized on day 19, his examination was also notable for an erythematous, blotchy, indurated rash on his upper body. Serum specimens from days 7, 19, 40, 72, 100, 128, and 159 were tested for total IgM and IgG concentrations and, by IFA, for T. cruzi-specific IgM and IgG (73). The results of all four types of tests showed elevated concentrations on day 40 and on all testing days thereafter, with some decrease in some of the levels on day 100 and thereafter. Whereas direct examination of blood on day 20 was negative for organisms, T. cruzi was isolated by hemoculture, mouse inoculation, and xenodiagnosis (2).
Accidents that did not involve needles have also been reported. A researcher emeritus who had been bleeding mice infected with T. cruzi recalled being bitten on his left index finger by a control mouse (85, 86). Whether he bled infected mice near the time of the bite and thus contaminated the wound is unclear. On the next day, he noted fatigue (which he also had had, along with headache, the day before the bite), anorexia, fever, and chills, and his finger became red, swollen, and tender. On day 2, a nontender left axillary lymph node was noted. On day 3, the lesion on his finger was lanced, releasing a small amount of serosanguineous material, the bacterial culture of which was positive for Enterobacter cloacae. Amastigotes were detected in a biopsy specimen obtained from the lymph node on day 13.
A microbiologist who spilled a solution of trypomastigotes (Tulahuen strain) onto slightly abraded skin on his left hand developed Chagas' disease (9). He was hospitalized 1 week later, with a 4- to 5-day history of headaches, low backache, anorexia, fever, chills, and fatigue. When hospitalized, he was drowsy and intermittently delerious and had photophobia, fever, sinus tachycardia, palatal petechiae, and lymphadenopathy in his left upper body. On day 4 of hospitalization (1.5 weeks after the accident), he developed a maculopapular rash on his trunk, arms, and thighs. He never developed a chagoma. Two days later, a systolic murmur, a pericardial friction rub, cardiomegaly, and T-wave changes on an electrocardiogram were noted. Testing for T. cruzi was done daily, starting on day 2 of hospitalization; on day 11, trypomastigotes were seen in direct smears of the patient's blood and in blood from a mouse inoculated with his blood on day 5. His course might have been affected by concomitant bacteremia and steroid therapy initiated on day 5.
A medical technician in a pharmaceutical company who operated barehanded in the peritoneal cavity of a mouse infected with the Tulahuen strain developed erythema along a cuticle 2 days later and fever and myalgia 2 days thereafter (34). When hospitalized 12 days after the incident, he had splenomegaly and generalized lymphadenopathy. Electrocardiographic findings were consistent with myocarditis, and trypomastigotes were noted in blood smears.
A xenodiagnosis-confirmed case resulted from ocular mucosal contact with triatomine feces (79). Thirteen days after the exposure, the investigator developed pain and redness of the internal angle of her exposed eye. The next day, she developed ipsilateral palpebral edema, dacryocystitis, and increased tearing; generalized malaise; and fever. Other manifestations in the ensuing days included headache, myalgia, edema of the ipsilateral cheek, lymphadenopathy, and splenomegaly. This case, which occurred in 1938, was the earliest documented case of unintentional, laboratory-acquired Chagas' disease.
Four infected persons, in addition to three mentioned above, did not recall specific laboratory accidents. However, for one of these persons, a laboratorian who worked barehanded with the blood of infected mice and infected triatomine feces, the presumptive date of infection is known (120). On that day, he developed a bruise on his hand, but the exact circumstances of what caused the bruise are unknown. Four days later, he noted local erythema and swelling. Subsequent clinical manifestations included anorexia; fatigue; myalgia; headache; fever with relative bradycardia; a rash on his trunk, extremities, and face; conjunctivitis; left axillary lymphadenopathy; and splenomegaly. The diagnosis was confirmed by mouse inoculation (done on day 22) and xenodiagnosis (day 25); seropositivity was demonstrated by complement fixation (day 22).
The other persons who did not recall accidents did not know when they were likely to have become infected. One of these persons was a technician who had worked for >20 years with the Tulahuen strain. She developed a chagoma on her thumb and subsequently developed weakness, headache, fever, night sweats, regional lymphadenopathy, transient pedal edema, intermittent tachycardia, and nonspecific T-wave changes on an electrocardiogram (171). Hemocultures obtained 7 days after the chagoma was noted were positive for T. cruzi, and seroconversion was noted later by complement fixation and hemagglutination. Another person who became infected was a nonscientist whose job included collecting the glassware used for culturing T. cruzi (45). The worker was evaluated for unexplained fever and was fortuitously discovered, through a positive hemoculture, to be infected with T. cruzi. Another person who became infected was a technician working with the Brazil strain (e.g., maintaining cultures and working with animals) who developed symptoms suggestive of a viral illness (85, 86). Trypomastigotes were found when wet mounts of buffy coat were examined, and an enlarged epitrochlear lymph node was subsequently noted. The diagnosis of Chagas' disease was supported serologically and by PCR.
(iii) Post-accident management.
(a) Presumptive therapy. Experts in the field generally recommend that laboratorians who have had moderate- to high-risk accidents with T. cruzi be treated presumptively rather than only if infection is documented (22, 63; Brener, Letter). Whereas persons with documented infection are treated for up to several months, the duration of presumptive therapy typically is shorter. Specifically, for benznidazole, a regimen of 7 to 10 mg/kg/day for 10 days has been recommended (63). The rationale for presumptive therapy is twofold: (i) Chagas' disease can be life-threatening, in both the acute and chronic stages of the disease; and (ii) therapy is more effective the earlier it is started. Although this rationale is strong, the efficacy and optimal duration of drug regimens for presumptive therapy have not been established, for obvious reasons, in controlled clinical trials. As described above, a clinically evident case of acute Chagas' disease has been documented in someone who received short-course presumptive therapy. Another potential concern is that such therapy could suppress parasitemia and mask indicators of inadequately treated infection. However, recommending that long-course therapy be used presumptively also is problematic because therapy can be associated with substantial toxicity of various types (e.g., hematologic, dermatologic, and neurologic); persons with laboratory-confirmed infections might be more willing than persons being treated presumptively to continue therapy despite its toxicity.
(b) Monitoring for infection.
After accidental exposures to T. cruzi, laboratorians should be monitored for clinical and laboratory evidence of infection, irrespective of whether presumptive therapy is given. An example of a monitoring protocol is provided in Table 16, and details about examining blood for T. cruzi are provided in Table 17. For persons treated presumptively, adaptation of the protocol to include more intensive monitoring after the end of therapy should be considered because of the possibility that the therapy will be suppressive (i.e., early test results could be negative and later results could be positive).
TABLE 16.
Clinical and laboratory monitoring for Trypanosoma cruzi infection after accidental exposures
| General comments |
| Monitoring should be done irrespective of whether the person is treated presumptively, before infection is documented. |
| For persons treated presumptively with short-course therapy (see the text), this protocol should be adapted to include more intensive monitoring after therapy, because therapy could be suppressive (i.e., early test results could be negative and later results could be positive). |
| Monitor clinically |
| Any rash, swelling, or erythema that develops near the site of exposure should be evaluated. Temperature should be monitored daily for 4 weeks, and febrile illnesses that develop during the next 6 months should be evaluated. |
| Monitor for development of antibody to the parasite |
| A suggested approach is to test serum weekly for 8 weeks or until seroconversion is noted, monthly for the next 4 months, and whenever clinical manifestations suggestive of Chagas' disease are noted. Preemployment serum and/or serum obtained immediately after the exposure should be tested in parallel with subsequent specimens, especially if the latter specimens are positive. |
| Monitor for parasitemia |
| A suggested approach is to monitor blood for parasitemia at least twice weekly for at least 4 weeks and whenever manifestations suggestive of Chagas' disease are noted. See Table 17 for details about examination of whole blood and buffy coat for motile trypomastigotes. PCR, an investigational technique, may facilitate early detection of infection (97, 99). Other conventional means of parasitologic confirmation of infection include tissue examination, hemoculture, animal inoculation, and xenodiagnosis. |
TABLE 17.
Practical guide for detection of circulating Trypanosoma cruzi trypomastigotes by light microscopya
| Obtain whole, anticoagulated blood by venipuncture or fingerstick. Process and examine the blood while it is fresh. Use sterile technique if specimens will also be cultured or inoculated into animals. |
| Prepare both whole blood and buffy coat for examination. |
| If the blood was obtained by venipuncture, remove ∼1 ml of whole blood from the tube, before centrifugation, and place it in a small vial so that whole blood can be examined as described below. Centrifuge the rest of the blood to separate the erythrocyte, leukocyte (buffy coat), and plasma layers. Pass a pipette through the plasma to the buffy coat layer. Carefully remove the buffy coat and place it in a small vial for examination as described below. |
| If the blood was obtained by fingerstick, fill at least two microhematocrit tubes with blood. Leave one tube uncentrifuged, so that whole blood can be examined. Centrifuge the other tube to separate the various layers of cells. Break the tube just above the buffy coat layer, remove the buffy coat, and place it in a small vial for examination as described below. |
| Prepare multiple slides for examination. To facilitate semiquantitative analysis (see below), if 12-mm-diameter circular coverslips are used, dot 1.5-μl aliquots of blood and separate aliquots of buffy coat onto slides and place a coverslip over each dot; if 22- by 22-mm square coverslips are used, use 6.4-μl aliquots. |
| Examine slides of both whole blood and buffy coat under high power by light microscopy, preferably phase-contrast, looking for motile trypomastigotes (length, ∼15–25 μm), which often are first manifest by the resultant movement of the other cells on the slide. Stain positive slides with Giemsa. |
| Specimens of whole blood can be examined more quickly than specimens of buffy coat because erythrocytes are homogeneous in size and color whereas the leukocytes and debris in the buffy coat are translucent and heterogeneous in size. On the other hand, trypomastigotes are present in higher concentrations in buffy coat than in whole blood. Therefore, both whole blood and buffy coat should be examined. |
| If these recommendations about the sizes of aliquots and coverslips are followed, examining 200 high-power fields (magnification, ×400) of whole blood is the equivalent of examining 0.48 μl of blood, and finding on average 1 parasite per high-power field indicates that the specimen contains ∼400,000 parasites/ml. |
| Residual buffy coat and whole blood can be used in hemoculture, PCR (97, 99), and animal inoculation. |
L. V. Kirchhoff was instrumental in the development of this table. Appropriate precautions should be used when handling specimens; see the text and Table 1.
Trypanosoma brucei rhodesiense and T. brucei gambiense. (i) General.
Trypanosoma brucei rhodesiense and T. brucei gambiense, the etiologic agents of East and West African trypanosomiasis, respectively, are transmitted in sub-Saharan Africa by tsetse flies. Congenital transmission has been documented occasionally, and transmission by blood transfusion could occur. Unlike the American trypanosome, T. cruzi, African trypanosomes multiply in the bloodstream of their mammalian hosts. East African trypanosomiasis typically follows a more acute course than the West African disease and is characterized by early invasion of the CNS. Cases of laboratory-acquired African trypanosomiasis can result from contact with blood or tissue from infected persons or animals.
(ii) Laboratory-acquired cases.
(a) Summary data. Six laboratory-acquired cases have been reported (76, 142, 146; A. O. Emeribe, Letter, Lancet i:470–471, 1988); four (two previously unpublished) caused by T. brucei gambiense and two (one previously unpublished) caused by T. brucei rhodesiense (Table 18). The earliest reported case occurred in the 1970s. All but one of the cases occurred in Europe, and all but one case resulted from parenteral exposures. The median incubation period for the six cases was 7 days (range, 1 to 8 days).
TABLE 18.
Characteristics of the reported cases of laboratory-acquired infection with Trypanosoma brucei subspp.a
| Characteristic | No (%) of cases (n = 6) |
|---|---|
| Subspecies | |
| T. brucei gambiense | 4 (66.7) |
| T. brucei rhodesiense | 2 (33.3) |
| Decade of occurrence (if known) or publication | |
| 1970s | 1 (16.7) |
| 1980s | 2 (33.3) |
| 1990s | 3 (50.0) |
| Country or region of occurrence | |
| Europe | 5 (83.3) |
| Africa | 1 (16.7) |
| Route of exposure | |
| Parenteral | 5 (83.3) |
| Nonintact skin | 1 (16.7)b |
| Clinical manifestations | |
| Symptomatic cases | 6 (100) |
| Fatal cases | 0 |
The median incubation period was 7 days (range, 1 to 8 days) for infections due to all exposures and 7 days (range, 1 to 8 days) for the subset of infections due to parenteral exposures (five of the six infections were due to parenteral exposures).
(b) Description of four cases caused by T. brucei gambiense.
One of the four persons known to have become infected with T. brucei gambiense was a technician who scratched his arm with a contaminated needle (strain Gboko/80/Hom/NITR.Kad.) “during pre-experimental passaging of Wistar rats with … parasites” (Emeribe, Letter). He was thought to have been exposed to a “tiny inoculum, part of which must have been washed out with soap and water” (Emeribe, Letter). When evaluated 1 week later, he had a large chancre (the inflammatory primary skin lesion) at the inoculation site, fever, headache, anorexia, and fatigue. Whether he first noted the “large chancre” earlier than 1 week postexposure was not specified. “Numerous trypanosomes” were found in blood smears. The case report did not include cerebrospinal fluid (CSF) findings.
A technician who stuck her thumb after inoculating mice became infected with a strain of T. brucei gambiense (FEO ITMAP-1893) that had been isolated from a patient 31 years earlier and maintained through passage in mice (142). She became febrile (39°C) 8 days later and developed erythema, warmth, and swelling of the thenar region of her hand 2 days thereafter; an axillary lymph node and splenomegaly were noted the next day. Laboratory abnormalities included leukopenia and thrombocytopenia. Trypanosomes were isolated from the chancre and were detected in a blood specimen passed through a DEAE-cellulose column. Seroconversion was noted by IFA 18 days after the accident. The cell count and protein level in CSF were normal, and a mouse inoculated with CSF did not become infected.
While manipulating a mouse infected with T. brucei gambiense (cloned antigenic variant LiTat 1.3, serodeme LiTAR 1), a technician stuck his left hand with a contaminated needle (A. Van Gompel, personal communication). Seven days later, he had fever, headache, and erythematous swelling at the inoculation site. On day 10, the site was still swollen, with bluish-red induration; he also had a red lymphangitic streak and swelling of the ipsilateral epitrochlear lymph node. On day 11, trypanosomes were noted in a Giemsa-stained thin blood smear and aminotransferase levels were three to five times the upper limit of normal. He was hospitalized on day 12 and was found to have 5 lymphocytes/μl in his CSF.
Another technician had a similar laboratory accident: he stuck his left fifth finger with a needle while manipulating a mouse infected with the same strain of T. brucei gambiense as in the previous case (Van Gompel, personal communication). He had chills on the next 2 days. On day 3 after the exposure, he had fever (39 to 40°C), headache, sore throat, and dark (concentrated) urine. On day 4, he developed nausea and vomiting and became agitated and profoundly fatigued. Upon evaluation, six trypanosomes were noted in 50 microscopic fields of a Giemsa-stained thick blood smear; his CSF was normal, including a negative culture for trypanosomes. He had leukopenia and thrombocytopenia, and his aminotransferase levels were normal but rose to two to three times the upper limit of normal on day 5.
(c) Description of two cases caused by T. brucei rhodesiense.
One of the two persons known to have become infected with T. brucei rhodesiense was a medical student doing a summer research project that involved infecting mice and rats with stabilates of various serotypes (i.e., variable antigen types); separating trypanosomes from animal blood by column chromatography on DEAE-cellulose, which resulted in a concentrated suspension of organisms (about 108/ml); and inoculating the parasites into chickens (76, 146). His role in the direct work with live animals was supportive (e.g., he restrained chickens that were being inoculated).
The trypanosomes were derived from a stock (BUSOGA/60/EATRO/3) isolated 14 years earlier from tsetse files in Uganda. The stock had mistakenly been thought to be T. brucei brucei and therefore not infectious for humans. The laboratory had stabilates of 12 different serotypes (ETat 1 to 12), only 1 of which (ETat 10) was infective for humans. The student had used several serotypes in his experiments. Retrospective serologic investigations after his case was diagnosed showed that he was infected with ETat 10 (76). He had worked with this serotype 8 and 5 days before he became ill. The relevant exposure may have occurred 5 days before he became ill, when he exsanguinated infected rodents that had been sacrificed and separated trypanosomes from their blood. Although he did not recall a discrete accident, the route of transmission could have been via the abrasions he had gotten on his hands while restraining chickens.
The student developed an erythematous, swollen area on one of his fingers. Other clinical manifestations included arthralgia, hyperalgesia of thigh and calf muscles, fever, rigors, fatigue, vomiting, diarrhea, tinnitus, headache, confusion, disorientation, generalized rash, cervical lymphadenopathy, and splenomegaly. One trypanosome was found in 400 microscopic fields of a Giemsa-stained thick blood smear (magnification not specified). Later, his serum IgM level increased markedly. The report did not include CSF findings.
The other case was in a technician who cut his left hand with a glass coverslip contaminated with T. brucei rhodesiense (cloned antigenic variant ETat 1.10, serodeme ETat 1 [Van Gompel, personal communication]). On days 7 to 11 after the exposure, he noted chills and fever (39 to 40°C), myalgia, and a painful ipsilateral axillary lymph node, which became more swollen and painful in the ensuing days. On day 11, a motile trypanosome was noted on examination of 40 microscopic fields of a wet mount of blood (magnification, ×400). On examination after he was hospitalized, he was febrile (38.1°C) and had a positive Giemsa-stained thick blood smear (one trypanosome in 25 microscopic fields; magnification, ×1,000). His CSF was normal, including a negative culture for trypanosomes. His leukocyte count was normal, but he had mild thrombocytopenia.
(iii) Post-accident management.
The diagnosis of African trypanosomiasis is parasitologically confirmed by detection of trypanosomes in peripheral blood, CSF, or an aspirate of a chancre, lymph node, or bone marrow. The ease of finding trypanosomes in various tissues and fluids depends on the infecting subspecies (T. brucei gambiense or T. brucei rhodesiense) and the stage of infection (hemolymphatic or CNS). Whereas T. brucei rhodesiense typically is relatively easy to find on a blood smear (at least in vector-borne cases), T. brucei gambiense is more difficult to detect. Concentration methods that facilitate detection include microhematocrit centrifugation followed by examination of buffy coat, as is done for T. cruzi (Table 17), and the miniature anion-exchange centrifugation technique using DEAE-cellulose (111). Animal inoculation (for T. brucei rhodesiense) and in vitro cultivation can be used to isolate the parasite. The sensitivity of the card agglutination test for trypanosomiasis (Institute of Tropical Medicine, Antwerp, Belgium) is high in most but not all areas where Gambian trypanosomiasis is endemic (112). PCR is an investigational technique for detecting parasite DNA (97).
The hemolymphatic stage of infection is treated with suramin or pentamidine isethionate (1). Because pentamidine is better tolerated than suramin but has somewhat lower efficacy against T. brucei rhodesiense, it is typically used to treat T. brucei gambiense infection and suramin is used to treat T. brucei rhodesiense infection. Infections with either subspecies that have spread to the CNS are treated with the arsenical melarsoprol. Difluoromethylornithine, which is available from the World Health Organization, is effective for treating both the hemolymphatic and CNS stages of T. brucei gambiense infection.
General issues to consider when deciding whether to treat presumptively, before documenting infection, are listed in Table 6. Specific issues to consider after laboratory accidents with African trypanosomes include the ease of diagnosis (diagnosis is easier with T. brucei rhodesiense than T. brucei gambiense) and the tolerability of the therapy (pentamidine, which is used for the hemolymphatic stage of T. brucei gambiense infection, is better tolerated than suramin, which is used for the hemolymphatic stage of T. brucei rhodesiense infection).
Intestinal Protozoa
(i) General.
Intestinal protozoa of potential concern to laboratorians include Entamoeba histolytica, Giardia lamblia, and the coccidian parasites Cryptosporidium parvum (and potentially some non-parvum Cryptosporidium spp.), Isospora belli, and Cyclospora cayetanensis. (See above concerning Sarcocystis spp.) Fecally excreted Isospora and Cyclospora oocysts require an extrinsic maturation period to become infectious (83), whereas E. histolytica cysts, Giardia cysts, and Cryptosporidium oocysts are infectious when excreted. Because protozoa multiply in the host, ingestion of even a small inoculum can cause illness (130, 145).
Laboratory personnel should observe routine precautions for work with stool specimens and fecally contaminated material, including careful hand washing after handling specimens. Even preserved specimens should be handled with care because parasites in inadequately preserved specimens could still be viable. Commercially available iodine-containing disinfectants are effective against E. histolytica and G. lamblia when used as directed, as are high concentrations of chlorine (1 cup of full-strength commercial bleach [∼5% chlorine] per gallon of water [1:16, vol/vol]). Environmental contamination with Cryptosporidium oocysts is problematic, especially for persons working with infected calves; during the peak period of shedding (approximately days 5 to 12 after exposure), infected calves shed billions of oocysts per day (M. Arrowood, personal communication). Although Cryptosporidium oocysts are inactivated by freezing (e.g., 20°C for 24 h) and moist heat (55°C for 15 to 20 min or 73°C for 1 min) (4), they are highly resistant to chemical disinfection (19, 29, 134), as are Isospora and Cyclospora oocysts. Solutions known to kill Cryptosporidium oocysts include 5% ammonia and 10% Formol saline (29), both of which are noxious, and 3% hydrogen peroxide (19); the contact times required to kill the parasite vary. Although these solutions probably also kill Isospora and Cyclospora oocysts, insufficient data are available to state this definitively. Although even full-strength bleach does not kill Cryptosporidium oocysts in a reasonable time, cleaners containing bleach may be useful for decontaminating surfaces (i.e., removing rather than killing contaminants). Contaminated skin should be thoroughly washed; no disinfectant effective against Cryptosporidium, Isospora, and Cyclospora oocysts is safe for use on skin. Contaminated clothing and equipment should be autoclaved.
(ii) Laboratory-acquired cases.
(a) Summary data. Relatively few cases of laboratory-acquired infections with intestinal protozoa have been reported (Table 4), probably in part because of the comparative ease with which such infection can be diagnosed and treated and because the illness is gastrointestinal rather than systemic. The cases described below include 2 cases of giardiasis, 3 cases of isosporiasis, and 16 cases of cryptosporidiosis and include some occupationally acquired cases among health care workers.
(b) Description of two cases caused by Giardia lamblia and three cases caused by Isospora belli.
A worker who “checked in several hundred stool survey specimens, stamping numbers and dates on report cards, many of which had been contaminated from leaky containers,” became infected with G. lamblia (44). The parasite was detected in the person's stool “after typical incubation period and course of disease” (44).
A “debilitating bout” of giardiasis thought to have represented patient-to-staff transmission has been reported (154). The case was in an orthopedic surgeon who had two preschool-age patients with giardiasis. One of these patients was a 1-year-old child who had her plastic cast adjusted on March 9 and removed on April 16; both times, the cast was noticeably stained with moist and dry feces. The physician became ill in early May and later had a positive stool specimen. Given that he typically washed his hands before and after changing casts but only rarely wore a mask, the authors of the case report speculated that he might have inhaled and then swallowed plaster dust contaminated with Giardia cysts (average length, 11 to 12 μm).
A laboratory technician who examined numerous stool specimens from a patient infected with I. belli became ill ∼1 week after the first specimens were examined, and I. belli was detected in his stool specimens (118). Two researchers who were feeding a rabbit a capsule containing about 400 Isospora oocysts were sprayed on their faces with droplets of infectious material when the rabbit regurgitated the material and vigorously shook its head; the researchers became ill 11 and 12 days later (44, 75). A case of isosporiasis that might have been laboratory acquired has been described (94) but is not included in the case counts (Table 4).
(c) Description of 16 cases caused by Cryptosporidium parvum.
Although cryptosporidiosis is a well-recognized occupational hazard for persons exposed to naturally infected calves and other animals (5, 47, 103, 105, 108, 143 [these cases were not included in the case counts]), cases of cryptosporidiosis have also been reported among persons exposed to experimentally infected animals (18, 139; N. Hojlyng, W. Holten-Andersen, and S. Jepsen, Letter, Lancet ii:271–272, 1987). Five veterinary students who had direct (four) or indirect (one) contact with experimentally infected calves became ill 6 to 7 days later and had diarrhea for a median of 5 days (range, 1 to 13 days) (139); one student was hospitalized. In addition, oocysts were found in a stool specimen from an infected student's spouse. In another instance, a researcher developed gastrointestinal symptoms 5 days after a rabbit, which was infected with oocysts through a gastric tube, coughed droplets of inoculum onto his face as he was removing the tube (18). The researcher's stool, which was first obtained for testing the day after he became ill, was positive for oocysts. A veterinary scientist developed flulike symptoms 7 days after smelling for gastric odor to check the position of a gastric tube in an infected calf; she was unaware of other exposures to C. parvum (Hojlyng et al., Letter). She developed gastrointestinal symptoms 10 days after this exposure, and oocysts were found in a stool specimen on day 16 (presumably the first specimen tested). Although airborne transmission of this small organism (average dimensions, 4.5 by 5 μm) is plausible, aerosolization of oocysts from the rumen of a calf is speculative.
At least nine cases of occupational transmission of C. parvum from human patients to health care workers have been reported and were included in the case counts (Table 4). The infected staff, who were symptomatic and had positive stool specimens, with one exception as noted below, included a nurse caring for an infected bone marrow transplant recipient (52), a nurse doing night duty on a ward where an infected 13-month-old boy was a patient (12), a nurse caring for infected patients before and after renal transplantation (148), and five nurses caring for an infected patient with AIDS (32, 69, 131). The exception with respect to parasitologic confirmation was a case in a symptomatic, stool-negative intern (and other staff) with serologic evidence of Cryptosporidium infection after exposure to an infected patient (101); the negative stool was from day 17 of his illness.
Nosocomial patient-to-patient transmission of C. parvum in hospitals has also been reported (7, 32, 65, 106, 117, 126, 128, 140, 148, 152, 176); the reports have varied with respect to the strength of the evidence that infection was hospital acquired. Patient-to-patient transmission of infection is beyond the scope of this article, and nosocomially acquired cases were not included in the case counts (Table 4). Suffice it to say that this type of transmission could be direct person-to-person transmission, perhaps via health care workers, or indirect, via contaminated medical devices, the environment, food, or water.
(iii) Post-accident management.
Infections with intestinal protozoa are diagnosed by examining stool specimens. Because organisms can be shed intermittently and in small numbers, multiple stools obtained on different days should be examined. Stools should be preserved in 10% formalin and in polyvinyl alcohol or alternative fixatives; a concentration technique should be used for examining stools, as well as a permanent stain such as trichrome. Detection of C. parvum is facilitated by special stains (e.g., acid-fast stain). Cryptosporidium, Isospora, and Cyclospora oocysts, all of which are acid fast, are distinguishable by size and shape; the last two demonstrate autofluorescence in UV fluorescence microscopy (56, 83). Immunodiagnostic tests for detecting antigen in stool are available for E. histolytica, G. lamblia, and C. parvum. PCR is an investigational technique for detecting various intestinal protozoa.
Highly effective treatment regimens are available for infections with all of these protozoa except C. parvum (1). Although the available drugs for treating cryptosporidiosis (e.g., paromomycin) are not optimally effective, the value of treatment before the onset of symptoms has not been assessed. Asymptomatic persons excreting only E. histolytica cysts should be treated with one of the so-called luminal agents (i.e., iodoquinol, paromomycin, or diloxanide furoate). Symptomatic amebiasis should be treated with metronidazole or tinidazole, followed by a luminal agent. Giardiasis is treated with metronidazole, tinidazole, or quinacrine, and isosporiasis and cyclosporiasis are treated with trimethoprim-sulfamethoxazole.
INFECTIONS WITH HELMINTHS
General Information and Laboratory-Acquired Cases
Few laboratory-acquired helminthic infections have been reported (Table 4). The scarcity of such reports might reflect in part the fact that helminthic infections generally are less likely than protozoan infections to be acquired in the laboratory. Even if laboratorians became infected by ingesting infective eggs or through penetration of skin by infective larvae, they typically would have low worm burdens and few, if any, symptoms because most helminths do not multiply in humans. Treatment regimens for persons with documented infection are provided elsewhere (1).
Flukes (trematodes) and most tapeworms (cestodes) require further larval development in a nonhuman host. One possible laboratory-acquired case of fascioliasis and at least eight cases of schistosomiasis are described below. Because the eggs of most intestinal nematodes (e.g., Ascaris lumbricoides and Trichuris trichiura) require an extrinsic maturation period of days to weeks to become infective, persons in diagnostic laboratories are unlikely to become infected with these organisms if the stool specimens were obtained recently. On the other hand, even preserved specimens should be handled with care because some helminth eggs can develop and remain viable in cold formalin (68). Laboratorians working with Ascaris spp. should be aware that allergic reactions can develop and can include respiratory, dermatologic, and gastrointestinal symptoms (40, 41, 110, 158, 166).
The eggs of Enterobius vermicularis (pinworm) and Hymenolepis nana (dwarf tapeworm), neither of which requires an intermediate host, are unusual in that they are infectious immediately or shortly after excretion in feces; H. nana eggs can be found in human and rodent feces. Therefore, staff who work in diagnostic laboratories or with rodents could become infected by ingesting these organisms if routine precautions, such as the use of gloves and careful hand washing, are neglected. Similarly, laboratory personnel exposed to mature filariform larvae of Strongyloides stercoralis, which can penetrate intact skin, could become infected. Although the larvae shed in stool typically are noninfective rhabditiform larvae, a few infective filariform larvae could be present. Hyperinfected persons can shed large numbers of larvae in respiratory secretions as well as in the stool, some of which might be infectious. Cases of cutaneous larva migrans (creeping eruption or “ground itch”) caused by skin contact with Strongyloides spp. (four cases) (113, 147) or Ancylostoma spp. (one case) (159) have been described. The latter case was in an animal caretaker who fed and cared for a cat infected with Ancylostoma braziliense and Ancylostoma caninum.
Laboratory personnel could also become infected by ingesting eggs of Taenia solium (pork tapeworm). Humans can serve as both the intermediate host and the definitive host of this parasite. Ingestion of eggs from a tapeworm carrier can result in the development of larval cysts (i.e., cysticercosis) in the brain and elsewhere.
Human infection with the cestode Echinococcus granulosus requires ingestion of eggs from the feces of infected dogs or other canids that can act as the definitive hosts of this cestode. Therefore, infection could be acquired by persons in veterinary diagnostic laboratories or research laboratories.
Trichinella spiralis, the etiologic agent of trichinosis, is the only tissue nematode that poses substantial risk to laboratory personnel. Preparations of fresh tissue and even specimens digested with pepsin hydrochloride can contain encysted Trichinella larvae that are infective if ingested. Because most infected laboratorians would have ingested few organisms, serologic testing would be more sensitive than muscle biopsy for establishing the diagnosis. Filarial infections, which also are caused by tissue nematodes, could be acquired by laboratory personnel working with infected arthropods.
Laboratory-Acquired Cases of Fascioliasis and Schistosomiasis
Because flukes require development in an intermediate host, the presence of their eggs in feces from mammals does not pose a risk to personnel in diagnostic laboratories. However, persons in research laboratories who handle snails that are competent intermediate hosts should exercise caution. Laboratorians working with aquaria for snail intermediate hosts could become infected by ingesting Fasciola metacercariae, which encyst on aquatic grasses or plants, or through skin penetration by schistosome cercariae, which swim freely; dissecting or crushing infected schistosome-infected snails could also result in exposure to droplets that contain cercariae. Therefore, laboratorians doing such work should wear gloves. In addition, persons at risk for exposure to schistosome cercariae should minimize the amount of uncovered skin by wearing a long-sleeved gown or coat and shoes rather than sandals.
One possible laboratory-acquired case of fascioliasis and at least eight cases of schistosomiasis in at least six persons have been reported. A technician who worked with Fasciola hepatica in a veterinary laboratory developed clinical manifestations consistent with fascioliasis (i.e., lassitude, fever, weight loss, slight tenderness at the right costal margin, and eosinophilia) (15; C. R. Ashton and O. D. Beresford, Letter, Br. Med. J. 2:121, 1974). Although he was thought to have become infected through his work, the nature of his work was not described. The conclusion that he was infected rested on serologic testing by a double-diffusion precipitin test; the finding that multiple stool specimens were negative for Fasciola was attributed to testing early in the invasive stage of infection.
A laboratory assistant working with snails (Biomphalaria pfeifferi) from an area where Schistosoma mansoni infection is endemic developed schistosomiasis (168). She had stopped wearing gloves 3 weeks after beginning this work because she thought the snails were no longer infectious. On day 31 after she began to work barehanded, she developed what later was thought probably to have been a mild case of Katayama fever, which lasted 5 days and was manifested by fever, headache, and fatigue. On day 54, eosinophilia was noted when her report of 3 days of “digestive complaints” prompted examination of her blood. Serologic testing by EIA was negative on days 54 and 82, weakly positive on day 101, and strongly positive on day 234. Stool specimens were negative for eggs on day 94 and positive on days 101 and 103.
Several asymptomatic cases of S. mansoni infection have been detected in a laboratory (laboratory C in Table 3) whose staff have worked daily with S. mansoni-infected snails and antigen preparations and have been monitored twice yearly by serologic testing. If seroconversion is documented, stool is tested. During the period from the late 1970s through mid-1999, seroconversion was noted in 4 of ∼20 persons. None of the four recalled a discrete laboratory accident, and all had followed standard precautions. Two of the four had positive stool specimens (<40 eggs/g; negative after treatment).
Several cases of schistosomiasis in persons working with cercariae were briefly mentioned in two reports (57, 58). In one of the reports, an investigator noted that he had been infected three times with S. mansoni (57). In the other report, a researcher who had solicited information about laboratory accidents from other investigators commented that “no lab infections were reported for over 100 people handling millions of cercariae for over 20 years, though two technicians became seropositive without developing symptoms, probably through torn gloves” (58). It is unclear whether these two cases were two of the four mentioned in the previous paragraph, although not all of the details match. No information was provided about whether and how staff in the various laboratories were monitored for infection.
CONCLUSION
Many of the key details about the laboratory-acquired cases of parasitic infections described here are summarized in tables, a figure, and various summary sections in the text. Clearly, preventing laboratory accidents is preferable to managing their consequences, which, if infection results, can range from asymptomatic infection detected through periodic serologic testing, to nonspecific clinical manifestations that are initially overlooked or mistakenly attributed to some other cause, to life-threatening disease. Two fatal cases of laboratory-acquired parasitic infections have been reported: one in a person with myocarditis caused by acute Chagas' disease (Brener, Letter) and the other in a person with myocarditis and encephalitis caused by toxoplasmosis (156).
Congenital transmission also is a potential risk for some protozoan parasites; women of childbearing age should exercise caution. Although parasitic diseases generally are treatable, some infections are difficult to treat because of antimicrobial resistance, drug-related toxicity, advanced disease (e.g., mucosal leishmaniasis, cerebral malaria, chronic Chagas' disease, and the CNS stage of African trypanosomiasis), or host factors, such as immunosuppression. Despite therapy, some parasites (e.g., Toxoplasma gondii) can persist for years in the body and can reactivate if the host becomes immunocompromised. Laboratorians working with parasites should also be aware that they may simultaneously be at risk for nonparasitic hazards, such as infections with viruses and bacteria.
To decrease the likelihood of accidental exposures, persons who could be exposed to pathogenic parasites must be thoroughly instructed in safety precautions before they begin to work and through ongoing training programs. Protocols should be provided for handling specimens that could contain viable organisms, using protective laboratory clothing and equipment, dealing with spills of infectious organisms, and responding to accidents. Laboratorians who work with parasites should follow parasite-specific and general laboratory precautions (e.g., wear gloves, wash hands frequently and adequately, use mechanical pipettors, adequately restrain animals that will be bled or inoculated, do not recap needles, restrict the use of sharps, use needleless systems or devices with safety features that reduce the risk for percutaneous injuries, decontaminate work surfaces, and use biological safety cabinets when appropriate). The fact that some infected laboratorians have not recalled a discrete accident suggests that subtle exposures (e.g., contamination of unrecognized microabrasions and exposure through aerosolization or droplet spread) can result in infection. The occurrence of cases of laboratory-acquired infection with species that were not previously known to be infective for humans (e.g., Plasmodium cynomolgi) or to be extraordinarily hardy in the environment (e.g., Toxoplasma gondii oocysts) highlights the need for special vigilance when working with organisms that have not been fully characterized in such regards.
ACKNOWLEDGMENTS
The following persons contributed to this article in various ways: Nestor Añez, Michael J. Arrowood, William E. Collins, J. P. Dubey, Mark L. Eberhard, Paul J. Edelson, Gregory A. Filice, Warren D. Johnson, Louis V. Kirchhoff, Anne C. Moore, Theodore E. Nash, Franklin A. Neva, Monica Parise, Malcolm R. Powell, Jack S. Remington, Scott W. Sorensen, Francis J. Steurer, Herbert B. Tanowitz, Govinda S. Visvesvara, Murray Wittner, and Marianna Wilson. Some persons who provided information about unpublished cases of parasitic diseases asked to remain anonymous. I thank Dennis D. Juranek for his many helpful suggestions.
REFERENCES
- 1.Abramowicz M, editor. Drugs for parasitic infections. Med Lett Drugs Ther. 2000;2000(March):1–12. . [Online.] [Google Scholar]
- 1a.Abulrahi H A, Bohlega E T, Fontaine R E, Al-Seghayer S M, Al-Ruwais A A. Plasmodium falciparum malaria transmitted in hospital through heparin locks. Lancet. 1997;349:23–25. doi: 10.1016/s0140-6736(96)03508-8. [DOI] [PubMed] [Google Scholar]
- 2.Allain D S, Kagan I G. Isolation of Trypanosoma cruzi in an acutely infected patient. J Parasitol. 1974;60:526–527. [PubMed] [Google Scholar]
- 3.Al-Saigul A M, Fontaine R E, Haddad Q. Nosocomial malaria from contamination of a multidose heparin container with blood. Infect Control Hosp Epidemiol. 2000;21:329–330. doi: 10.1086/501765. [DOI] [PubMed] [Google Scholar]
- 4.Anderson B C. Moist heat inactivation of Cryptosporidium sp. Am J Public Health. 1985;75:1433–1434. doi: 10.2105/ajph.75.12.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson B C, Donndelinger T, Wilkins R M, Smith J. Cryptosporidiosis in a veterinary student. J Am Vet Med Assoc. 1982;180:408–409. [PubMed] [Google Scholar]
- 6.Añez N, Carrasco H, Parada H, Crisante G, Rojas A, Gonzalez N, Ramirez J L, Guevara P, Rivero C, Borges R, Scorza J V. Acute Chagas' disease in western Venezuela: a clinical, seroparasitologic, and epidemiologic study. Am J Trop Med Hyg. 1999;60:215–222. doi: 10.4269/ajtmh.1999.60.215. [DOI] [PubMed] [Google Scholar]
- 7.Arikan S, Ergüven S, Aryön Y, Günalp A. Cryptosporidiosis in immunocompromised patients in a Turkish university hospital. Acta Microbiol Immunol Hung. 1999;46:33–40. doi: 10.1556/AMicr.46.1999.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Arness M K, Brown J D, Dubey J P, Neafie R C, Granstrom D E. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am J Trop Med Hyg. 1999;61:548–553. doi: 10.4269/ajtmh.1999.61.548. [DOI] [PubMed] [Google Scholar]
- 9.Aronson P R. Septicemia from concomitant infection with Trypanosoma cruzi and Neisseria perflava. First case of laboratory-acquired Chagas' disease in the United States. Ann Intern Med. 1962;57:994–1000. [Google Scholar]
- 10.Reference deleted.
- 11.Baker C C, Farthing C P, Ratnesar P. Toxoplasmosis, an innocuous disease? J Infect. 1984;8:67–69. doi: 10.1016/s0163-4453(84)93435-2. [DOI] [PubMed] [Google Scholar]
- 12.Baxby D, Hart C A, Taylor C. Human cryptosporidiosis: a possible case of hospital cross infection. Br Med J. 1983;287:1760–1761. doi: 10.1136/bmj.287.6407.1760-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaver P C, Gadgil R K, Morera P. Sarcocystis in man: a review and report of five cases. Am J Trop Med Hyg. 1979;28:819–844. [PubMed] [Google Scholar]
- 14.Bending M R, Maurice P D L. Malaria: a laboratory risk. Postgrad Med J. 1980;56:344–345. doi: 10.1136/pgmj.56.655.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beresford O D. A case of fascioliasis in man. Vet Rec. 1976;98:15. doi: 10.1136/vr.98.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 17.Beverley J K A, Skipper E, Marshall S C. Acquired toxoplasmosis: with a report of a case of laboratory infection. Br Med J. 1955;1:577–578. doi: 10.1136/bmj.1.4913.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blagburn B L, Current W L. Accidental infection of a researcher with human Cryptosporidium. J Infect Dis. 1983;148:772–773. doi: 10.1093/infdis/148.4.772. [DOI] [PubMed] [Google Scholar]
- 19.Blewett D A. Disinfection and oocysts. In: Angus K W, Blewett D A, editors. Proceedings of the 1st International Workshop on Cryptosporidiosis. Edinburgh, Scotland: Moredun Research Institute; 1988. pp. 107–115. [Google Scholar]
- 20.Reference deleted.
- 21.Bouree P, Fouquet E. Paludisme: contamination directe interhumaine. Nouv Presse Med. 1978;7:1865. [PubMed] [Google Scholar]
- 22.Brener Z. Laboratory-acquired Chagas' disease: an endemic disease among parasitologists? In: Morel C M, editor. Genes and antigens of parasites: a laboratory manual. 2nd ed. Rio de Janeiro, Brazil: Fundação Oswaldo Cruz; 1984. pp. 3–9. [Google Scholar]
- 23.Reference deleted.
- 24.Brown J, Jacobs L. Adult toxoplasmosis: report of a case due to laboratory infection. Ann Intern Med. 1956;44:565–572. doi: 10.7326/0003-4819-44-3-565. [DOI] [PubMed] [Google Scholar]
- 25.Bruce-Chwatt L J. Blood transfusion and tropical disease. Trop Dis Bull. 1972;69:825–862. [PubMed] [Google Scholar]
- 26.Bruce-Chwatt L J. Imported malaria: an uninvited guest. Br Med Bull. 1982;38:179–185. doi: 10.1093/oxfordjournals.bmb.a071756. [DOI] [PubMed] [Google Scholar]
- 27.Bryan R T, Schwartz D A. Epidemiology of microsporidiosis. In: Wittner M, editor. The microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 502–516. [Google Scholar]
- 28.Reference deleted.
- 29.Campbell I, Tzipori S, Hutchison G, Angus K W. Effect of disinfectants on survival of Cryptosporidium oocysts. Vet Rec. 1982;111:414–415. doi: 10.1136/vr.111.18.414. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Reference deleted.
- 32.Casemore D P, Gardner C A, O'Mahony C. Cryptosporidial infection, with special reference to nosocomial transmission of Cryptosporidium parvum: a review. Folia Parasitol. 1994;41:17–21. [PubMed] [Google Scholar]
- 33.Center for Disease Control. Malaria surveillance 1971 annual report. DHEW publication (HSM) 72-8152. Atlanta, Ga: Center for Disease Control; 1972. [Google Scholar]
- 34.Centers for Disease Control. Chagas' disease—Michigan. Morb Mortal Wkly Rep. 1980;29:147–148. [Google Scholar]
- 35.Centers for Disease Control. Malaria surveillance annual summary 1982. Atlanta, Ga: Centers for Disease Control; 1984. [Google Scholar]
- 36.Centers for Disease Control and Prevention and National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 4th ed. Washington, D.C.: Public Health Service. U.S. Government Printing Office; 1999. [Google Scholar]
- 37.Chen K-T, Chen C-J, Chang P-Y, Morse D L. A nosocomial outbreak of malaria associated with contaminated catheters and contrast medium of a computed tomographic scanner. Infect Control Hosp Epidemiol. 1999;20:22–25. doi: 10.1086/501557. [DOI] [PubMed] [Google Scholar]
- 38.Chung H-L. An early case of kala-azar, possibly an oral infection in the laboratory. Natl Med J China. 1931;17:617–621. [Google Scholar]
- 39.Coatney G R, Collins W E, Warren M, Contacos P G. The primate malarias. U.S. Washington, D.C.: Government Printing Office; 1971. [Google Scholar]
- 40.Coles G C. Gastro-intestinal allergy to nematodes. Trans R Soc Trop Med Hyg. 1975;69:362–363. doi: 10.1016/0035-9203(75)90137-6. [DOI] [PubMed] [Google Scholar]
- 41.Coles G C. Allergy and immunopathology of ascariasis. In: Crompton D W T, Nesheim M C, Pawlowski Z S, editors. Ascariasis and its public health significance. London, United Kingdom: Taylor and Francis; 1985. pp. 167–184. [Google Scholar]
- 42.Collins W E. Simian malaria. In: Steele J H, Jacobs L, Arambulo P, editors. Parasitic zoonoses. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1982. pp. 141–150. [Google Scholar]
- 43.Contacos P G, Collins W E. Malarial relapse mechanism. Trans R Soc Trop Med Hyg. 1973;67:617–618. doi: 10.1016/0035-9203(73)90104-1. [DOI] [PubMed] [Google Scholar]
- 44.Cook E B M. Safety in the public health laboratory. Public Health Rep. 1961;76:51–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Coudert J, Despeignes J, Battesti M R, Michel-Brun J. Un cas de maladie de Chagas par contamination accidentelle de laboratoire avec T. cruzi. Soc Pathol Exot. 1964;57:208–213. [PubMed] [Google Scholar]
- 46.Cross J H, Hsu-Kuo M-Y, Lien J C. Accidental human infection with Plasmodium cynomolgi bastianellii. Southeast Asian J Trop Med Public Health. 1973;4:481–483. [PubMed] [Google Scholar]
- 47.Current W L, Reese N C, Ernst J V, Bailey W S, Heyman M B, Weinstein W M. Human cryptosporidiosis in immunocompetent and immunodeficient persons: studies of an outbreak and experimental transmission. N Engl J Med. 1983;308:1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- 48.Delgado O, Guevara P, Silva S, Belfort E, Ramirez J L. Follow-up of a human accidental infection by Leishmania (Viannia) braziliensis using conventional immunologic techniques and polymerase chain reaction. Am J Trop Med Hyg. 1996;55:267–272. doi: 10.4269/ajtmh.1996.55.267. [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira C G, Freire F F., Jr An epidemic of inoculated malaria. Bol Clin Hosp Civis Lisb. 1948;12:375–404. [Google Scholar]
- 50.Dillon N L, Stolf H O, Yoshida E L A, Marques M E A. Leishmaniose cutânea acidental. Rev Inst Med Trop São Paulo. 1993;35:385–387. [PubMed] [Google Scholar]
- 51.Dobroszycki J, Herwaldt B L, Boctor F, Miller J R, Linden J, Eberhard M L, Yoon J J, Ali N M, Tanowitz H B, Graham F, Weiss L M, Wittner M. A cluster of transfusion-associated babesiosis cases traced to a single asymptomatic donor. JAMA. 1999;281:927–930. doi: 10.1001/jama.281.10.927. [DOI] [PubMed] [Google Scholar]
- 52.Dryjanski J, Gold J W M, Ritchie M T, Kurtz R C, Lim S L, Armstrong D. Cryptosporidiosis: case report in a health team worker. Am J Med. 1986;80:751–752. doi: 10.1016/0002-9343(86)90839-9. [DOI] [PubMed] [Google Scholar]
- 53.Dubey J P, Beattie C P. Toxoplasmosis of animals and man. Boca Raton, Fla: CRC Press, Inc.; 1988. [Google Scholar]
- 54.Dubey J P, Lindsay D S. Isolation in immunodeficient mice of Sarcocystis neurona from opossum (Didelphis virginiana) faeces, and its differentiation from Sarcocystis falcatula. Int J Parasitol. 1998;28:1823–1828. doi: 10.1016/s0020-7519(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 55.Dubey J P, Speer C A, Fayer R. Sarcocystosis of animals and man. Boca Raton, Fla: CRC Press, Inc.; 1989. [Google Scholar]
- 56.Eberhard M L, Pieniazek N J, Arrowood M J. Laboratory diagnosis of Cyclospora infections. Arch Pathol Lab Med. 1997;121:792–797. [PubMed] [Google Scholar]
- 57.Elsevier. Schistosomiasis: symptoms of mild infections? Parasitol Today. 1998;14:8. [Google Scholar]
- 58.Elsevier. Accidental infections. Parasitol Today. 1998;14:55. [Google Scholar]
- 59.Reference deleted.
- 60.Evans T G, Pearson R D. Clinical and immunological responses following accidental inoculation of Leishmania donovani. Trans R Soc Trop Med Hyg. 1988;82:854–856. doi: 10.1016/0035-9203(88)90016-8. [DOI] [PubMed] [Google Scholar]
- 61.Eyles D E, Coatney G R, Getz M E. Vivax-type malaria parasite of macaques transmissible to man. Science. 1960;131:1812–1813. doi: 10.1126/science.131.3416.1812. [DOI] [PubMed] [Google Scholar]
- 62.Field P R, Moyle G G, Parnell P M. The accidental infection of a laboratory worker with Toxoplasma gondii. Med J Aust. 1972;2:196–198. doi: 10.5694/j.1326-5377.1972.tb47232.x. [DOI] [PubMed] [Google Scholar]
- 63.Filho A A F, Luquetti A O, Prata A, Rassi A, Gontijo E D, Ferreira H D, Cancado J R, Coura J R, Andrade S G, Macedo V, Neto V A, de Oliveira W, Jr, Brener Z. Etiological treatment for Chagas disease. Parasitol Today. 1997;13:127–128. [Google Scholar]
- 64.Fleming D O, Hunt D L, editors. Biological safety: principles and practices. 3rd ed. Washington, D.C.: ASM Press; 2000. [Google Scholar]
- 65.Foot A B M, Oakhill A, Mott M G. Cryptosporidiosis and acute leukaemia. Arch Dis Child. 1990;65:236–237. doi: 10.1136/adc.65.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freedman D O, MacLean J D, Viloria J B. A case of laboratory acquired Leishmania donovani infection: evidence for primary lymphatic dissemination. Trans R Soc Trop Med Hyg. 1987;81:118–119. doi: 10.1016/0035-9203(87)90301-4. [DOI] [PubMed] [Google Scholar]
- 67.Frenkel J K, Weber R W, Lunde M N. Acute toxoplasmosis: effective treatment with pyrimethamine, sulfadiazine, leucovorin calcium, and yeast. JAMA. 1960;173:1471–1476. doi: 10.1001/jama.1960.03020310059017. [DOI] [PubMed] [Google Scholar]
- 68.Garcia L S. Diagnostic medical parasitology. 4th ed. Washington, D.C.: ASM Press; 2001. [Google Scholar]
- 69.Gardner C. An outbreak of hospital-acquired cryptosporidiosis. Br J Nurs. 1994;3:152. doi: 10.12968/bjon.1994.3.4.152. ,154–158. [DOI] [PubMed] [Google Scholar]
- 70.Garnham P C C. Malaria in mammals excluding man. Adv Parasitol. 1967;5:139–204. doi: 10.1016/s0065-308x(08)60377-2. [DOI] [PubMed] [Google Scholar]
- 71.Giroud P, Le Gac P, Roger F, Gaillard J-A. La toxoplasmose de l'adulte. Sem Hop Paris. 1953;29:4036–4039. [PubMed] [Google Scholar]
- 72.Gutteridge W E, Cover B, Cooke A J D. Safety precautions for work with Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1974;68:161. [Google Scholar]
- 73.Hanson W L, Devlin R F, Roberson E L. Immunoglobulin levels in a laboratory-acquired case of human Chagas' disease. J Parasitol. 1974;60:532–533. [PubMed] [Google Scholar]
- 74.Reference deleted.
- 75.Henderson H E, Gillepsie G W, Kaplan P, Steber M. The human Isospora. Am J Hyg. 1963;78:302–309. doi: 10.1093/oxfordjournals.aje.a120349. [DOI] [PubMed] [Google Scholar]
- 76.Herbert W J, Parratt D, Van Meirvenne N, Lennox B. An accidental laboratory infection with trypanosomes of a defined stock. II. Studies on the serological response of the patient and the identity of the infecting organism. J Infect. 1980;2:113–124. doi: 10.1016/s0163-4453(80)91109-3. [DOI] [PubMed] [Google Scholar]
- 77.Hermentin K, Hassl A, Picher O, Aspöck H. Comparison of different serotests for specific Toxoplasma IgM-antibodies (ISAGA, SPIHA, IFAT) and detection of circulating antigen in two cases of laboratory acquired Toxoplasma infection. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1989;270:534–541. doi: 10.1016/s0176-6724(89)80025-2. [DOI] [PubMed] [Google Scholar]
- 78.Hermentin K, Picher O, Aspöck H, Auer H, Hassl A. A solid-phase indirect haemadsorption assay (SPIHA) for detection of immunoglobulin M antibodies to Toxoplasma gondii: application to diagnosis of acute acquired toxoplasmosis. Zentbl Bakteriol Mikrobiol Hyg 1 Abt Orig A. 1983;255:380–391. [PubMed] [Google Scholar]
- 79.Herr A, Brumpt L. Un cas aigu de maladie de Chagas contractée accidentellement au contact de triatomes Mexicains: observation et courbe fébrile. Bull Soc Pathol Exot. 1939;32:565–571. [Google Scholar]
- 80.Herwaldt B L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 81.Herwaldt B L. Miltefosine—the long-awaited therapy for visceral leishmaniasis? N Engl J Med. 1999;341:1840–1842. doi: 10.1056/NEJM199912093412411. [DOI] [PubMed] [Google Scholar]
- 82.Herwaldt B L. Protozoa and helminths. In: Fleming D O, Hunt D L, editors. Biological safety: principles and practices. 3rd ed. Washington, D.C.: ASM Press; 2000. pp. 89–110. [Google Scholar]
- 83.Herwaldt B L. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- 84.Herwaldt B L, Berman J D. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 85.Herwaldt B L, Juranek D D. Laboratory-acquired malaria, leishmaniasis, trypanosomiasis, and toxoplasmosis. Am J Trop Med Hyg. 1993;48:313–323. doi: 10.4269/ajtmh.1993.48.313. [DOI] [PubMed] [Google Scholar]
- 86.Herwaldt B L, Juranek D D. Protozoa and helminths. In: Fleming D O, Richardson J H, Tulis J I, Vesley D, editors. Laboratory safety: principles and practices. 2nd ed. Washington, D.C.: ASM Press; 1995. pp. 77–91. [Google Scholar]
- 87.Herwaldt B L, Persing D H, Précigout E A, Goff W L, Mathiesen D A, Taylor P W, Eberhard M L, Gorenflot A F. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 88.Hofflin J M, Sadler R H, Araujo F G, Page W E, Remington J S. Laboratory-acquired Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:437–440. doi: 10.1016/0035-9203(87)90162-3. [DOI] [PubMed] [Google Scholar]
- 89.Reference deleted.
- 90.Holm K. Ueber einen Fall von Infektion mit Malaria tropica an der Leiche. Klin Wochenschr. 1924;3:1633–1634. [Google Scholar]
- 91.Hörmann J. Laborinfekt mit Toxoplasma gondii (Beitrag zum klinischen Bild der akuten Erwachsenentoxoplasmose) Z Gesamte Inn Med Grenzgeb. 1955;18:150–152. [PubMed] [Google Scholar]
- 92.Hudson L, Grover F, Gutteridge W E, Klein R A, Peters W, Neal R A, Miles M A, Williams J E, Scott M T, Nourish R, Ager B P. Suggested guidelines for work with live Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1983;77:416–419. doi: 10.1016/0035-9203(83)90176-1. [DOI] [PubMed] [Google Scholar]
- 93.Jacobs L. The interrelation of toxoplasmosis in swine, cattle, dogs, and man. Public Health Rep. 1957;72:872–882. [PMC free article] [PubMed] [Google Scholar]
- 94.Jeffery G M. Human coccidiosis in South Carolina. J Parasitol. 1956;42:491–495. [PubMed] [Google Scholar]
- 95.Jensen J B, Capps T C, Carlin J M. Clinical drug-resistant falciparum malaria acquired from cultured parasites. Am J Trop Med Hyg. 1981;30:523–525. doi: 10.4269/ajtmh.1981.30.523. [DOI] [PubMed] [Google Scholar]
- 96.Kayhoe D E, Jacobs L, Beye H K, McCullough N B. Acquired toxoplasmosis: observations on two parasitologically proved cases treated with pyrimethamine and triple sulfonamides. N Engl J Med. 1957;257:1247–1254. doi: 10.1056/NEJM195712262572601. [DOI] [PubMed] [Google Scholar]
- 97.Kirchhoff L V, Donelson J E. PCR detection of Trypanosoma cruzi, African trypanosomes, and Leishmania species. In: Persing D H, Tenover F C, Smith T F, White T J, editors. Diagnostic molecular microbiology—principles and applications. Washington, D.C.: ASM Press; 1993. pp. 443–455. [Google Scholar]
- 98.Kirchhoff L V, Hoft D F. Immunization and challenge of mice with insect-derived metacyclic trypomastigotes of Trypanosoma cruzi. Parasite Immunol. 1990;12:65–74. doi: 10.1111/j.1365-3024.1990.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 99.Kirchhoff L V, Votava J R, Ochs D E, Moser D R. Comparison of PCR and microscopic methods for detecting Trypanosoma cruzi. J Clin Microbiol. 1996;34:1171–1175. doi: 10.1128/jcm.34.5.1171-1175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Knobloch J, Demar M. Accidental Leishmania mexicana infection in an immunosuppressed laboratory technician. Trop Med Intern Health. 1997;2:1152–1155. doi: 10.1046/j.1365-3156.1997.d01-216.x. [DOI] [PubMed] [Google Scholar]
- 101.Koch K L, Phillips D J, Aber R C, Current W L. Cryptosporidiosis in hospital personnel: evidence for person-to-person transmission. Ann Intern Med. 1985;102:593–596. doi: 10.7326/0003-4819-102-5-593. [DOI] [PubMed] [Google Scholar]
- 102.Reference deleted.
- 103.Konkle D M, Nelson K M, Lunn D P. Nosocomial transmission of Cryptosporidium in a veterinary hospital. J Vet Intern Med. 1997;11:340–343. doi: 10.1111/j.1939-1676.1997.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 104.Krause P J, Lepore T, Sikand V K, Gadbaw J, Burke G, Telford S R, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 105.Lengerich E J, Addiss D G, Marx J J, Ungar B L P, Juranek D D. Increased exposure to cryptosporidia among dairy farmers in Wisconsin. J Infect Dis. 1993;167:1252–1255. doi: 10.1093/infdis/167.5.1252. [DOI] [PubMed] [Google Scholar]
- 106.Lettau L A. Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases. I. Introduction/enteric parasites. Infect Control Hosp Epidemiol. 1991;12:59–65. doi: 10.1086/646239. [DOI] [PubMed] [Google Scholar]
- 107.Lettau L A. Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases. II. Blood and tissue parasites. Infect Control Hosp Epidemiol. 1991;12:111–121. doi: 10.1086/646297. [DOI] [PubMed] [Google Scholar]
- 108.Levine J F, Levy M G, Walker R L, Crittenden S. Cryptosporidiosis in veterinary students. J Am Vet Med Assoc. 1988;193:1413–1414. [PubMed] [Google Scholar]
- 109.Lewis J. Iatrogenic malaria. N Z Med J. 1971;71:88–89. [PubMed] [Google Scholar]
- 110.Lloyd Jones T, Kingscote A A. Observations on Ascaris sensitivity in man. Am J Hyg. 1935;22:406–413. [Google Scholar]
- 111.Lumsden W H R, Kimber C D, Evans D A, Doig S J. Trypanosoma brucei: miniature anion-exchange centrifugation technique for detection of low parasitemias: adaptation for field use. Trans R Soc Trop Med Hyg. 1979;73:312–317. doi: 10.1016/0035-9203(79)90092-0. [DOI] [PubMed] [Google Scholar]
- 112.Magnus E, Vervoort T, van Meirvenne N. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. b. gambiense trypanosomiasis. Ann Soc Belge Med Trop. 1978;58:169–176. [PubMed] [Google Scholar]
- 113.Maligin S A. A case of cutaneous form of strongyloidiasis caused by larvae of S. ransomi, S. westeri and S. papillosus. Med Parazitol (Moscow) 1958;27:446–447. [PubMed] [Google Scholar]
- 114.Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone, Inc.; 2000. [Google Scholar]
- 115.Marsh A E, Barr B C, Lakritz J, Nordhausen R, Madigan J E, Conrad P A. Experimental infection of nude mice as a model for Sarcocystis neurona-associated encephalitis. Parasitol Res. 1997;83:706–711. doi: 10.1007/s004360050323. [DOI] [PubMed] [Google Scholar]
- 116.Martinez A J, Visvesvara G S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martino P, Gentile G, Caprioli A, Baldassarri L, Donelli G, Arcese W, Fenu S, Micozzi A, Venditti M, Mandelli F. Hospital-acquired cryptosporidiosis in a bone marrow transplantation unit. J Infect Dis. 1988;158:647–648. doi: 10.1093/infdis/158.3.647. [DOI] [PubMed] [Google Scholar]
- 118.McCracken A W. Natural and laboratory-acquired infection by Isospora belli. South Med J. 1972;65:800. doi: 10.1097/00007611-197207000-00005. [DOI] [PubMed] [Google Scholar]
- 119.Reference deleted.
- 120.Melzer H, Kollert W. Ein Beitrag zur Klinik und Therapie der Chagas-Krankheit. Dtsch Med Wochenschr. 1963;88:368–377. doi: 10.1055/s-0028-1111957. [DOI] [PubMed] [Google Scholar]
- 121.Miller N L, Frenkel J K, Dubey J P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J Parasitol. 1972;58:928–937. [PubMed] [Google Scholar]
- 122.Reference deleted.
- 123.Most H. Plasmodium cynomolgi malaria: accidental human infection. Am J Trop Med Hyg. 1973;22:157–158. doi: 10.4269/ajtmh.1973.22.157. [DOI] [PubMed] [Google Scholar]
- 124.Müller W A, Wachtel D, Färber I. Beziehungen zwischen indirekter Immunfluoreszenzreaktion, Serofarbtest und Komplementbindungsreaktion auf Toxoplasmose mit Titerverlaufsuntersuchungen bei zwei Laborinfektionen. Dtsch Gesundheitswes. 1972;27:82–85. [PubMed] [Google Scholar]
- 125.Reference deleted.
- 126.Navarrete S, Stetler H C, Avila C, Aranda J A G, Santos-Preciado J I. An outbreak of Cryptosporidium diarrhea in a pediatric hospital. Pediatr Infect Dis J. 1991;10:248–250. doi: 10.1097/00006454-199103000-00016. [DOI] [PubMed] [Google Scholar]
- 127.Navarro P, Betancurt A, Paublini H, Medina I, Núñez M J, Domínguez M. Falciparum malaria as a hospital-acquired infection. Bol Sanit Panam. 1987;102:476–482. [PubMed] [Google Scholar]
- 128.Neill M A, Rice S K, Ahmad N V, Flanigan T P. Cryptosporidiosis: an unrecognized cause of diarrhea in elderly hospitalized patients. Clin Infect Dis. 1996;22:168–170. doi: 10.1093/clinids/22.1.168. [DOI] [PubMed] [Google Scholar]
- 129.Neu H C. Toxoplasmosis transmitted at autopsy. JAMA. 1967;202:284–285. [PubMed] [Google Scholar]
- 130.Okhuysen P C, Chappell C L, Crabb J H, Sterling C R, DuPont H L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 131.O'Mahony C, Gardner A, Casemore D P. Hospital-acquired cryptosporidiosis. P.H.L.S. communicable disease report review no. 2. London, United Kingdom: Public Health Laboratory Service; 1992. pp. R18–R19. [PubMed] [Google Scholar]
- 132.Parker S L, Holliman R E. Toxoplasmosis and laboratory workers: a case-control assessment of risk. Med Lab Sci. 1992;49:103–106. [PubMed] [Google Scholar]
- 133.Partanen P, Turunen H J, Paasivuo R T A, Leinikki P O. Immunoblot analysis of Toxoplasma gondii antigens by human immunoglobulins G, M, and A antibodies at different stages of infection. J Clin Microbiol. 1984;20:133–135. doi: 10.1128/jcm.20.1.133-135.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pavlásek I. Effect of disinfectants in infectiousness of oocysts of Cryptosporidium sp. Cesk Epidemiol Microbiol Immunol. 1984;33:97–101. [PubMed] [Google Scholar]
- 135.Persing D H, Herwaldt B L, Glaser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Phillip D F, Conrad P A. Infection with a Babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 136.Petithory J, Lebeau G. Contamination probable de laboratoire par Plasmodium falciparum. Bull Soc Pathol Exot Fil. 1977;70:371–375. [PubMed] [Google Scholar]
- 137.Pike R M. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab Sci. 1976;13:105–114. [PubMed] [Google Scholar]
- 138.Pizzi T, Niedmann G, Jarpa A. Comunicación de tres casos de enfermedad de Chagas aguda producidos por infecciones accidentales de laboratorio. Bol Chil Parasitol. 1963;18:32–36. [PubMed] [Google Scholar]
- 139.Pohjola S, Oksanen H, Jokipii L, Jokipii A M M. Outbreak of cryptosporidiosis among veterinary students. Scand J Infect Dis. 1986;18:173–178. doi: 10.3109/00365548609032325. [DOI] [PubMed] [Google Scholar]
- 140.Ravn P, Lundgren J D, Kjaeldgaard P, Holten-Anderson W, Hojlyng N, Nielsen J O, Gaub J. Nosocomial outbreak of cryptosporidiosis in AIDS patients. Br Med J. 1991;302:277–280. doi: 10.1136/bmj.302.6771.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rawal B D. Laboratory infection with Toxoplasma. J Clin Pathol. 1959;12:59–61. doi: 10.1136/jcp.12.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Receveur M C, Vincendeau P. Laboratory-acquired Gambian trypanosomiasis. N Engl J Med. 1993;329:209–210. doi: 10.1056/NEJM199307153290317. [DOI] [PubMed] [Google Scholar]
- 143.Reif J S, Wimmer L, Smith J A, Dargatz D A, Cheney J M. Human cryptosporidiosis associated with an epizootic in calves. Am J Public Health. 1989;79:1528–1530. doi: 10.2105/ajph.79.11.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Remington J S, Gentry L O. Acquired toxoplasmosis: infection versus disease. Ann N Y Acad Sci. 1970;174:1006–1017. doi: 10.1111/j.1749-6632.1970.tb45622.x. [DOI] [PubMed] [Google Scholar]
- 145.Rendtorff R C. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am J Hyg. 1954;59:209–220. doi: 10.1093/oxfordjournals.aje.a119634. [DOI] [PubMed] [Google Scholar]
- 146.Robertson D H H, Pickens S, Lawson J H, Lennox B. An accidental laboratory infection with African trypanosomes of a defined stock. I. The clinical course of the infection. J Infect. 1980;2:105–112. doi: 10.1016/s0163-4453(80)91084-1. [DOI] [PubMed] [Google Scholar]
- 147.Roeckel I E, Lyons E T. Cutaneous larva migrans, an occupational disease. Ann Clin Lab Sci. 1977;7:405–410. [PubMed] [Google Scholar]
- 148.Roncoroni A J, Gomez M A, Mera J, Cagnoni P, Michel M D. Cryptosporidium infection in renal transplant patients. J Infect Dis. 1989;160:559. doi: 10.1093/infdis/160.3.559. [DOI] [PubMed] [Google Scholar]
- 149.Sabin A B, Eichenwald H, Feldman H A, Jacobs L. Present status of clinical manifestations of toxoplasmosis in man: indications and provisions for routine serologic diagnosis. JAMA. 1952;150:1063–1069. doi: 10.1001/jama.1952.03680110003002. [DOI] [PubMed] [Google Scholar]
- 150.Sadick M D, Locksley R M, Raff H V. Development of cellular immunity in cutaneous leishmaniasis due to Leishmania tropica. J Infect Dis. 1984;150:135–138. doi: 10.1093/infdis/150.1.135. [DOI] [PubMed] [Google Scholar]
- 151.Reference deleted.
- 152.Sarabia-Arce S, Salazar-Lindo E, Gilman R H, Naranjo J, Miranda E. Case-control study of Cryptosporidium parvum infection in Peruvian children hospitalized for diarrhea: possible association with malnutrition and nosocomial infection. Pediatr Infect Dis J. 1990;9:627–631. [PubMed] [Google Scholar]
- 153.Schmidt L H, Greenland R, Genther C S. The transmission of Plasmodium cynomolgi to man. Am J Trop Med Hyg. 1961;10:679–688. doi: 10.4269/ajtmh.1961.10.679. [DOI] [PubMed] [Google Scholar]
- 154.Schuman S H, Arnold A T, Rowe J R. Giardiasis by inhalation? Lancet. 1982;i:53. doi: 10.1016/s0140-6736(82)92603-4. [DOI] [PubMed] [Google Scholar]
- 155.Sewell D L. Laboratory-associated infections and biosafety. Clin Microbiol Rev. 1995;8:389–405. doi: 10.1128/cmr.8.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sexton R C, Eyles D E, Dillman R E. Adult toxoplasmosis. Am J Med. 1953;14:366–377. doi: 10.1016/0002-9343(53)90047-3. [DOI] [PubMed] [Google Scholar]
- 157.Shulman I A. Parasitic infections and their impact on blood donor selection and testing. Arch Pathol Lab Med. 1994;118:366–370. [PubMed] [Google Scholar]
- 158.Sprent J F A. On the toxic and allergic manifestations produced by the tissues and fluids of Ascaris. I. Effect of different tissues. J Infect Dis. 1949;84:221–229. doi: 10.1093/infdis/84.3.221. [DOI] [PubMed] [Google Scholar]
- 159.Stone O J, Levy A. Creeping eruption in an animal caretaker. Lab Anim Care. 1967;17:479–482. [PubMed] [Google Scholar]
- 160.Strickland G T, editor. Hunter's tropical medicine and emerging infectious diseases. 8th ed. Philadelphia, Pa: W. B. Saunders Co.; 2000. [Google Scholar]
- 161.Ström J. Toxoplasmosis due to laboratory infection in two adults. Acta Med Scand. 1951;139:244–252. [PubMed] [Google Scholar]
- 162.Terry L L, Lewis J L, Sessoms S M. Laboratory infection with Leishmania donovani: a case report. Am J Trop Med. 1950;30:643–649. doi: 10.4269/ajtmh.1950.s1-30.643. [DOI] [PubMed] [Google Scholar]
- 163.Teutsch S M, Juranek D D, Sulzer A, Dubey J P, Sikes R K. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979;300:695–699. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- 164.Thalhammer O. Zwei bemerkenswerte Fälle frischer Toxoplasmainfektion. Oesterr Z Kinderheilkd. 1954;10:316–321. [PubMed] [Google Scholar]
- 165.Titto E H, Araujo F G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988;46:157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]
- 166.Turner K J, Fisher E H, McWilliam A S. Homology between roundworm and hookworm antigens detected by human IgE antibodies. Aust J Exp Biol Med Sci. 1980;58:249–257. doi: 10.1038/icb.1980.25. [DOI] [PubMed] [Google Scholar]
- 167.Umdenstock R, Mandoul R, Pestre-Alexandre M. Laboratory accident caused by the bite of a mouse infected with Toxoplasma. Auto-observation. Bull Soc Pathol Exot. 1965;58:207–209. [PubMed] [Google Scholar]
- 168.Van Gompel A, Van den Enden E, Van den Ende J, Geerts S. Laboratory infection with Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1993;87:554. doi: 10.1016/0035-9203(93)90086-6. [DOI] [PubMed] [Google Scholar]
- 169.Van Soestbergen A A. A laboratory infection with Toxoplasma gondii. Ned T Geneesk. 1957;101:1649–1651. [PubMed] [Google Scholar]
- 170.Varma A J. Malaria acquired by accidental inoculation. Can Med Assoc J. 1982;126:1419–1420. [PMC free article] [PubMed] [Google Scholar]
- 171.Western K A, Schultz M G, Farrar W E, Kagan I G. Laboratory acquired Chagas' disease treated with Bay [sic] 2502. Bol Chil Parasitol. 1969;24:94. [Google Scholar]
- 172.Wettingfeld R F, Rowe J, Eyles D E. Treatment of toxoplasmosis with pyrimethamine (Daraprim) and triple sulfonamide. Ann Intern Med. 1956;44:557–564. doi: 10.7326/0003-4819-44-3-557. [DOI] [PubMed] [Google Scholar]
- 173.Williams J L, Innis B T, Burkot T R, Hayes D E, Schneider I. Falciparum malaria: accidental transmission to man by mosquitoes after infection with culture-derived gametocytes. Am J Trop Med Hyg. 1983;32:657–659. doi: 10.4269/ajtmh.1983.32.657. [DOI] [PubMed] [Google Scholar]
- 174.Wilson M, McAuley J M. Toxoplasma. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 1374–1382. [Google Scholar]
- 175.Wilson, M., P. M. Schantz, T. Nutman, and V. C. W. Tsang. Clinical immunoparasitology. In N. R. Rose, E. Conway. de Macario, J. D. Folds, H. C. Lane, and R. M. Nakamura (ed.), Manual of clinical laboratory immunology, 6th ed., in press. ASM Press, Washington, D.C.
- 176.Wittenberg D F, Miller N M, van den Ende J. Spiramycin is not effective in treating Cryptosporidium diarrhea in infants: results of a double-blind randomized trial. J Infect Dis. 1989;159:131–132. doi: 10.1093/infdis/159.1.131. [DOI] [PubMed] [Google Scholar]
- 177.Woodison G, Balfour A H, Smith J E. Sequential reactivity of serum against cyst antigens in Toxoplasma infection. J Clin Pathol. 1993;46:548–550. doi: 10.1136/jcp.46.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wright W H. A summary of the newer knowledge of toxoplasmosis. Am J Clin Pathol. 1957;28:1–17. doi: 10.1093/ajcp/28.1.1. [DOI] [PubMed] [Google Scholar]
- 179.Zeledón R. Epidemiology, modes of transmission and reservoir hosts of Chagas' disease. Ciba Found Symp. 1974;20:51–85. [Google Scholar]
- 180.Zimmermann W J. Prevalence of Toxoplasma gondii antibodies among veterinary college staff and students, Iowa State University. Public Health Rep. 1976;91:526–532. [PMC free article] [PubMed] [Google Scholar]