Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Pseudococcus cryptus Hempel (Hemiptera: Pseudococcidae), the citriculus mealybug, for the EU. P. cryptus originates from Southeast Asia but is now established in East Africa, the Middle East and South America. The pest is not currently known to occur in the EU (there was a record once, in 2006, in a zoo/botanical garden from southern Spain). P. cryptus is not listed in Commission Implementing Regulation (EU) 2019/2072. It is polyphagous, feeding on plants in more than 90 genera in 51 families, and exhibits a preference for citrus (Citrus spp.) and palms (especially Cocos nucifera, Elaeis guineensis and Areca catechu). It is an important pest of citrus in Japan and parts of the Middle East, although in Israel, it is controlled by natural enemies. It is sexually reproductive, has six overlapping generations each year in Israel, and each female lays up to approximately 150 eggs, depending on temperature and host species. The main natural dispersal stage is the first instar, which crawls over the host plant or may be dispersed further by wind and animals. Plants for planting, fruits, vegetables and cut flowers provide potential pathways for entry into the EU. Climatic conditions in EU member states around the Mediterranean Sea where there is host plant availability, especially citrus, are conducive for establishment. The introduction of P. cryptus is expected to have an economic impact in the EU through reduction in yield and quality of important crops (mainly citrus) and damage to various ornamental plants. Phytosanitary measures are available to reduce the likelihood of entry and further spread. P. cryptus meets the criteria that are within the remit of EFSA to assess for this species to be regarded as a potential Union quarantine pest.
Keywords: citriculus mealybug, cryptic mealybug, Hemiptera, Pseudococcidae, pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Pseudococcus cryptus is one of a number of pests listed in Annex 1 to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/ 2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was initiated following the commodity risk assessment of avocado (Persea americana Mill.) scions and grafted plants from Israel performed by EFSA (EFSA PLH Panel, 2021), in which P. cryptus was identified as a relevant non‐regulated EU pest which could potentially enter the EU on P. americana.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on P. cryptus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for P. cryptus which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for P. cryptus, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section I of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
| Identity of the pest ( Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory ( Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed. |
| Pest potential for entry, establishment and spread in the EU territory ( Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways for entry and spread. |
| Potential for consequences in the EU territory ( Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
|
Available measures (risk reduction options) ( Section 3.6 ) |
Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation ( Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel’s conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes , the identity of the pest is established and Pseudococcus cryptus Hempel is the accepted name.
The citriculus mealybug, also known as cryptic mealybug, Pseudococcus cryptus Hempel is an insect within the order Hemiptera, family Pseudococcidae. This species was initially described by Hempel in 1918 from specimens collected on roots of a coffee (Coffea arabica) tree in Brazil (García Morales et al., 2016). The same species was subsequently described as Pseudococcus citriculus by Green in 1922 from specimens collected on Citrus sp. in Sri Lanka (García Morales et al., 2016). Junior synonyms of the species are Planococcus cryptus (Hempel) and Dysmicoccus cryptus (Hempel) (EPPO, online).
The EPPO code 1 for this species is DYSMCR (EPPO, online).
3.1.2. Biology of the pest
Sexually reproductive females lay their eggs in ovisacs (Peri and Kapranas, 2012) (Figure 1). Kim et al. (2008) reported P. cryptus to be ovoviviparous (eggs develop and hatch within the maternal body, or hatch immediately after being released) but the evidence suggests that it is not, as the eggs are reported to take up to 2.9 days to hatch. The total number of eggs laid by a female ranged from 59 to 152 depending on temperature and host plant species (Kim et al., 2008; Holat et al., 2014). The first instar nymphs known as crawlers are mobile and disperse over the host plant, and potentially between host plants if they are touching, in search of suitable feeding sites. The later female nymphal instars resemble the adult female but are smaller. The male nymph, at the end of the second instar, secretes a lose cottony wax cocoon and moults inside to become a prepupa, a pupa and finally emerges as a winged adult (Peri and Kapranas, 2012). The egg stage lasts from 1 to 2.9 days while the immature development lasts from 17.4 to 54.9 days depending on temperature and host (Kim et al., 2008; Holat et al., 2014). The thermal requirement for the egg and first‐instar stages was 189.6 Degree Day (DD) above a threshold of 8.7°C, for the second instar 84.7 DD above a threshold of 12.8°C and for the third instar 69.8 DD (above 13.1°C). The thermal requirement for total development (eggs and all nymphal stages) was 316.6 DD (above 12.1°C) (Kim et al., 2008). Adult female longevity ranged from 28.6 to 80.4 days (Kim et al., 2008; Holat et al., 2014). Adult males lived for only 1–2 days and searched for females to mate. P. cryptus can infest all parts of its host plants, including the shallow roots, although it is much more common on the aerial parts.
Figure 1.
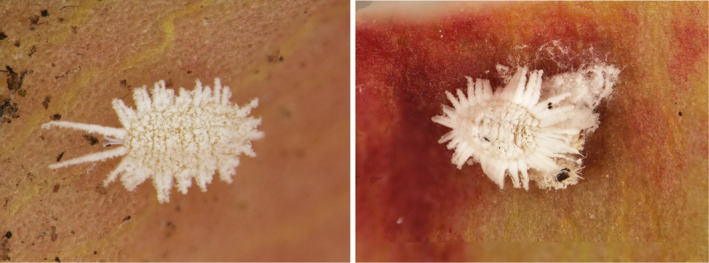
Pseudococcus cryptus, nymph (left) and adult female with ovisac (right) (source: Chris Malumphy)
In Israel, six generations per year are reported (Blumberg et al., 1999). In Turkey, population density of P. cryptus in citrus groves increased from March to July and declined in the beginning of August. All the developmental stages of P. cryptus existed together in the colonies throughout the year (Telli and Yiğit, 2019). However, Peri and Kapranas (2012) reported that P. cryptus overwintered mainly as second‐instar nymphs. In Japan, females lay their eggs at the end of May and first‐generation nymphs appeared in early June (Itioka and Inoue, 1996). According to Franco et al. (2004), in Israel, the highest population density of P. cryptus on citrus occurred in spring, when there is major new foliage growth.
Important features of the life‐history strategy of P. cryptus are summarised in Table 2.
Table 2.
Important features of the life‐history strategy of Pseudococcus cryptus
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Eggs are laid in ovisacs. The total number of eggs laid is affected by the host plant and ranged from 59 to 152 in different citrus species at 25°C (Holat et al., 2014). On satsuma mandarin leaves (Kim et al., 2008), the total number of eggs laid varied from 83 to 111 at temperatures ranging from 16°C to 28°C. | The egg stage lasts from 1 day at 28°C to 2.4 days at 16°C on satsuma mandarin leaves (Kim et al., 2008); or from 1.3 days to 2.9 days at 25°C on different citrus species (Holat et al., 2014). Arai (1996) stated that the egg stage lasts from 1.5 to 2.3 days at temperatures between 22.5°C and 27.5°C on citrus leaves. | |
| 1st instar nymph | First‐instar nymphs are known as crawlers. P. cryptus can infest all parts of the trees, including the shallow roots, but are mainly found on leaves and twigs. | Crawlers are mobile and they disperse over the host plant in search of a suitable feeding site. |
| Later instar nymphs | Later nymphal instars resemble the adult female but are smaller. There are three immature instars in the female and four in the male. | Nymphal development is affected by both temperature and host plant. The nymphal development lasts from 16.4 days at 28°C to 52.5 days at 16°C on satsuma mandarin leaves (Kim et al., 2008), while it requires from 21.3 days to 23.3 days at 25°C on various citrus species (Holat et al., 2014). The lower and upper threshold developmental temperatures for all the immatures stages were estimated at 12.1°C and 30.7°C, respectively (Kim et al., 2008). |
| The female adult is oval and covered by powdery white wax except at the intersegmental lines. The male adults are winged. All the developmental stages (eggs, nymphs and adults) exist together in colonies throughout the year in Turkey. | Adult females live for 31.3 days at 32°C to 80.4 days at 16°C (Kim et al., 2008), while at 25°C female longevity range from 28.6 days to 32.2 days on different citrus species (Holat et al., 2014). Adult males live for 1–2 days. They lack functional mouthparts and cannot feed. |
3.1.3. Host range/Species affected
P. cryptus is polyphagous, feeding on plants assigned to more than 90 genera from 51 plant families (Appendix A provides a full host list). Although it has a broad range of hosts, it is most frequently found on, and causes damage to, citrus (Citrus spp.) and palms (especially Areca catechu, Cocos nucifera and Elaeis guineensis) (Kanagaratnam et al., 1981; Fernando and Kanagaratnam, 1987; Blumberg et al., 1999; Kim et al., 2008; Mohan et al., 2016). It is an important pest of citrus (Citrus spp.) in Japan, Israel and Turkey (Arai, 1996; Blumberg et al., 1999; Arai, 2002; Yiğit and Telli, 2013). P. cryptus has also been recorded feeding on some solanaceous crops [potato (Solanum tuberosum), tomato (Solanum lycopersicum) and aubergine (Solanum melongena)], strawberry (Fragaria sp.), avocado (Persea americana), grapes (Vitis vinifera), bananas (Musa 2 ) and soybeans (Glycine max), that are economically important in the EU, but there appears to be no significant impact on these hosts.
3.1.4. Intraspecific diversity
No intraspecific diversity is reported for this species.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes. There are detection, and morphological and molecular identification methods available.
Detection
P. cryptus can infest all parts of its host plants, including the shallow roots, although it is more common on the aerial parts. When population levels are high, it tends to form dense colonies covering the leaves, twigs and trunks (Blumberg et al., 1999), and infestations can be easily detected by visual examination. However, when the density is low, it is difficult to be detected by visual observation, since it tends to inhabit the sheltered parts of the trees (Arai, 2002). Yellow sticky traps baited with sex pheromones are effective for monitoring adult males (Arai, 2002; Song et al., 2012). The sex pheromone of the citrus mealybug is [(1R,3R)‐3‐isopropenyl‐2,2‐ dimethylcyclobutyl] methyl 3‐methyl‐3‐butenoate (Nakahata et al., 2003).
Symptoms
The main symptoms of P. cryptus infestation (Blumberg et al., 1999) are:
large quantities of honeydew
black sooty mould growing on the honeydew and smothering parts of the plant
unsightly appearance of the fruit
wilting and general debilitation of the plant
fruit, leaf and flower drop
Identification
The identification of P. cryptus requires microscopic examination of slide‐mounted adult females and verification of the presence of key morphological characteristics. Detailed morphological descriptions, illustrations and keys of P. cryptus and other species of the genus Pseudococcus can be found in Kwon et al. (2002), Watson and Kubiriba (2005), Sirisena et al. (2013), Ellenrieder and Watson (2016), Granara de Willink and González (2018) and Pacheco da Silva et al. (2020).
Molecular techniques for species identification are also available (Pacheco da Silva et al., 2014; Kaydan et al., 2015; Ren et al., 2018) and there are a number of accessions in Genbank based on the cytochrome c oxidase I (COI) sequence.
Description (full description available in Kwon et al., 2002).
Some of the main morphological characteristics of P. cryptus are:
Eggs pale‐yellow in ovisacs (Peri and Kapranas, 2012)
Second and third nymphal instars are similar to the adult female but smaller.
The female adult of P. cryptus is up to 3–3.5 mm long, its body is oval shaped, it is pale yellow or green yellow and slightly rounded in lateral view covered by white wax except at the intersegmental lines (Peri and Kapranas, 2012). Legs are yellowish brown. Body margin with 17 pairs of long, slender marginal wax filaments (Kwon et al., 2002; Sirisena et al., 2013). The male adult is winged, about 1 mm in length (Peri and Kapranas, 2012).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
The origin of P. cryptus is believed to be Southeast Asia but it is now found in East Africa, the Middle East and South America (Figure 2) (Blumberg et al., 1999; Peri and Kapranas, 2012; García Morales et al., 2016). There is also a single record from Europe (see Section 3.2.2). For a detailed list of countries where P. cryptus has been recorded, see Appendix B.
Figure 2.
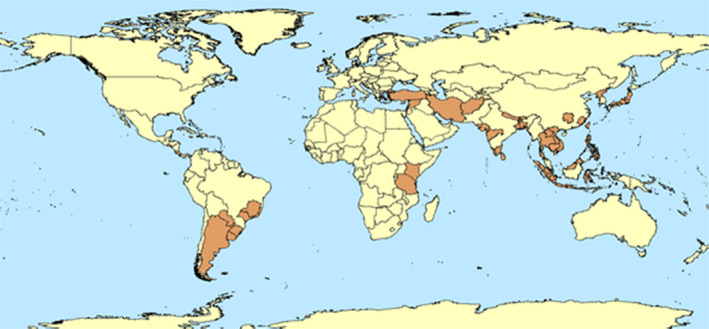
Global distribution of Pseudococcus cryptus (data source: García Morales et al., 2016)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
No, P. cryptus is not known to occur in the EU.
There is a record for Spain, based on a single finding in 2006 on Viburnum tinus in a zoo and botanical garden in Jerez (Sánchez‐García and Ben‐Dov, 2010 ). When approached, the Spanish NPPO reported that there had been no official findings and the status is ‘Absent, pest not recorded’.
It has also been intercepted in USA ports between 1995 and 2012 in commodities from France (Miller et al., 2014). However, there are no records of P. cryptus being found in France and this has probably resulted from produce being imported to France from areas where the mealybug occurs and re‐exported to the USA. Recent comprehensive checklists (Foldi and Germain, 2018) of Coccoidea of France do not mention P. cryptus.
3.3. Regulatory status
There is evidence that P. cryptus is the vector of Areca palm velarivirus 1 (APV1) which is considered the causal agent for yellow leaf disease, one of the most destructive diseases of betel palm (Areca catechu) (Zhang et al., 2021). APV1 is not an EU quarantine pest listed in Commission Implementing Regulation (EU) 2019/2072.
3.3.1. Commission Implementing Regulation 2019/2072
Pseudococcus cryptus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031. However, the insect is included in the list of pests that are regulated by the Commission Implementing Regulation (EU) 2021/1936 with regard to Ficus carica and Persea americana plants for planting, originating in Israel.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
According to the Commission Implementing Regulation (EU) 2019/2072, Annex VI, introduction of several P. cryptus hosts into the EU from certain third countries is prohibited (Table 3).
Table 3.
List of plants, plant products and other objects that are P. cryptus hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 9. | Plants for planting of […][…] and Fragaria L., other than seeds |
ex 0602 10 90 ex 0602 90 30 |
Third countries, other than: Albania, Algeria, Andorra, Armenia, Australia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canada, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, New Zealand, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey, Ukraine, and United States other than Hawaii. |
| 10. | Plants of Vitis L., other than fruits |
0602 10 10 0602 20 10 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Switzerland |
| 11. | Plants of Citrus L., Fortunella Swingle, Poncirus Raf. and their hybrids, other than fruits and seed |
ex 0602 10 90 ex 0602 20 20 0602 20 30 ex 0602 20 80 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
All third countries |
| 13. |
Plants of Phoenix spp. other than fruit and seeds |
ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
Algeria, Morocco |
| 18. |
Plants for planting of Solanaceae other than seeds and the plants covered by entries 15, 16 or 17 |
ex 0602 90 30 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than: Albania, Algeria, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey and Ukraine |
Some countries where the pest is present are exempt from prohibitions e.g. in Sections 9 (Israel, Syria, Turkey and USA (Hawaii)) and 18 (Israel, Syria, Turkey).
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways
Comment on plants for planting as a pathway
Yes. The pest is able to enter into the EU territory with plants for planting, fruits, vegetables and cut flowers as main pathways.
Plants for planting, fruits, vegetables and cut flowers are the main potential pathways for entry of P. cryptus. It can be associated with soil, however, because mealybugs are soft bodied, very delicate and easily damaged and cannot exist for long in the absence of living plant material, soil is not considered as a credible pathway (Table 4).
Table 4.
Potential pathways for Pseudococcus cryptus into the EU 27
|
Pathways Description (e.g. host/intended use/source) |
Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Plants for planting | Eggs, nymphs and adults |
A list of plants for planting hosts of P. cryptus are prohibited to import from third countries (Regulation 2019/2072, Annex VI), (Table 3). Although, some countries are exempt, e.g. Fragaria plants for planting from Turkey (Table 3). The growing medium attached to or associated with plants, intended to sustain the vitality of the plants, are regulated in Regulation 2019/2072, Annex VII. Plants for planting from third countries require a phytosanitary certificate (Regulation 2019/2072, Annex XI, Part A). |
| Fruits, vegetables and cut flowers | Eggs, nymphs and adults |
Fruits, vegetables and cut flowers from third countries require a phytosanitary certificate to import into the EU (2019/2072, Annex XI, Part A). According to Regulation 2019/2072, Annex XI, Part C there is a list of plants which a phytosanitary certificate is not required for their introduction into the Union territory. P. cryptus infests fruits of Musa spp. that are included in that list. |
Annual imports of some P. cryptus hosts from countries where the pest is known to occur are provided in Appendix C.
The import of some host plants of P. cryptus (Fragaria, Vitis, Citrus, Phoenix and Solanaceae) for planting from third countries is not allowed although there are exceptions (Regulation 2019/2072, Annex VI), while there are many other hosts that can be imported to the EU with a phytosanitary certificate.
Vegetables, cut flowers and most fruits that are imported into the EU must have a phytosanitary certificate. However, fruits of pineapple (Ananas comosus), banana (Musa) and coconut (Cocos nucifera) which can be hosts for P. cryptus are exempt by Regulation 2019/2072, Annex XI, Part C.
EU legislation (2019/2072) prohibits the import of soil from third countries so that pathway can be considered as closed. All the pathways for the introduction of P. cryptus into the EU, except soil, remain open.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As at 20/10/2021 (search date), there were no records of interception of P. cryptus in the Europhyt and TRACES databases. During the same period, P. cryptus was intercepted on 20 occasions in England, mostly frequently on coconut, rambutan (Nephelium lappaceum), mangosteen (Garcinia mangostana) and citrus imported from Bangladesh, Sri Lanka and Thailand.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes. In the EU countries of Southern Europe, the climate is suitable and there are many hosts available to support establishment.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker et al., 2000; Baker 2002). Availability of hosts is considered in 3.4.2.1. Climatic factors are considered in 3.4.2.2.
3.4.2.1. EU distribution of main host plants
P. cryptus is polyphagous and the main cultivated hosts in the EU 27 between 2016 and 2020 are shown in Table 5. Among others, citrus, avocados, bananas, grapes and soybean are economically important crops in the EU.
Table 5.
Crop area of Pseudococcus cryptus hosts in EU 27 in 1,000 ha (Eurostat accessed on 9/11/2021)
| Crop | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Grapes | 3,136.04 | 3,133.21 | 3,135.02 | 3,155,20 | 31,56.21 |
| Soybean | 831.18 | 962.39 | 955.40 | 907.91 | 947.67 |
| Citrus | 519.01 | 502.84 | 508.99 | 512.83 | 519.98 |
| Bananas | 20.30 | 18.91 | 17.94 | 18.27 | 19.62 |
| Avocados | 12.24 | 12.72 | 13.22 | 17.50 | 17.29 |
3.4.2.2. Climatic conditions affecting establishment
P. cryptus occurs mainly in countries with tropical and subtropical climates in Asia and south America. It has also been recorded in tropical areas of Africa and in Turkey, Israel and Spain (one occasion). Figure 3 shows the EU climates that occur in the countries in which P. cryptus has been recorded. The southern EU, where Köppen–Geiger climate zones (Kottek et al., 2006) Csa and Csb occur are considered most suitable for establishment. There is uncertainty as to whether the mealybug could establish in central Europe, and it is unlikely that it could establish in Northern Europe. Nevertheless, there is a possibility that P. cryptus could occur in glasshouses and on indoor plantings in cooler areas.
Figure 3.
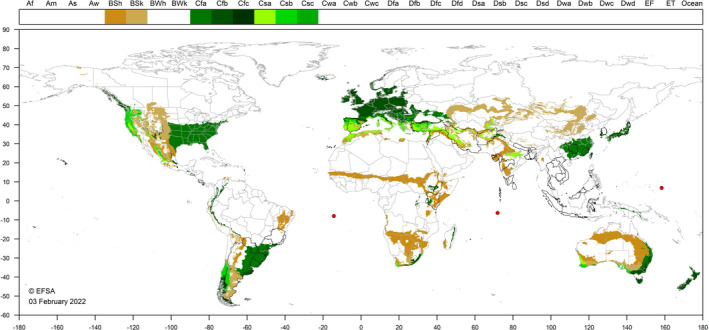
World distribution of Köppen–Geiger climate types that occur in the EU and which occur in countries where Pseudococcus cryptus has been reported (excluding Dfb and Dfc which are considered too cold)
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
Local spread is primarily by the first instars moving over the plant or being carried by wind or animals; long distance dispersal may occur with trade.
Comment on plants for planting as a mechanism of spread
Plants for planting would be the main means of distributing the pest over long distances in short periods of time.
Natural spread by the first instars crawling or being carried by wind, other animals or machinery will occur locally and relatively slowly. Later nymphal female instars and adult females can crawl over short distances on the host. The introduction of this pest to new territories over long distance is possible through the movement of infested plants for planting, and trade of infested fruits, vegetables, cut flowers or other plant products.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, if P. cryptus established in the EU, it would probably have an economic impact, especially on citrus, although the magnitude of impacts is uncertain.
P. cryptus can infest all parts of the host but it is found mainly on leaves and twigs (Blumberg et al., 1999; Franco et al., 2004). The insect feeds on plant sap which causes chlorosis, slows development and causes premature leaf and fruit drop. P. cryptus individuals egest honeydew that is colonised by sooty moulds, which contaminates plant surfaces, reducing photosynthesis and lowering the aesthetic value of ornamental plants (Peri and Kapranas, 2012).
P. cryptus is a major pest of citrus causing significant yield losses in parts of Asia and Mideastern Mediterranean (Arai, 1996; Arai, 2002; Blumberg et al., 1999; Yiğit and Telli, 2013). Citrus fruit become unmarketable due to being covered with sooty mould (Telli and Yiğit, 2019). In Korea, high populations frequently cause substantial yield losses, especially in organic citrus orchards (Kim et al., 2008). In the Near East, it is a major citrus pest infesting foliage and stems, damaging red grapefruit, pomelo and easy‐peeling citrus varieties (Peri and Kapranas, 2012). P. cryptus is a serious citrus pest in Japan and outbreaks often occur in greenhouse cultivation (Arai, 2002). In Turkey, P. cryptus and the citrus mealybug (Planococcus citri) are the main mealybug species causing losses in yield and quality in citrus orchards (Telli and Yiğit, 2019). P. cryptus was a significant citrus pest in Israel, after its introduction in 1937 (Blumberg et al., 1999), but it has been successfully controlled by the importation of the encyrtid Clausenia purpurea Ishii parasitoid (Bodenheimer, 1951). In many countries, P. cryptus is not a serious pest, possibly due to the climate being less favourable and natural enemies reducing its populations. The current management regimes for mealybug pests of citrus in the EU may reduce the magnitude of impact by P. cryptus, and organic production might be more vulnerable.
It is also damaging to palms (Cocos nucifera, Elaeis guineensis and Areca catechu) (Kanagaratnam et al., 1981; Fernando and Kanagaratnam, 1987; Mohan et al., 2016), and due to its polyphagous nature, impacts on palms in the EU may be expected.
According to Yiğit and Telli (2013) and Blumberg et al. (1999), there are many natural enemies of P. cryptus, recorded worldwide.
P. cryptus is associated with the Areca palm velarivirus 1 (APV1), considered the causal agent for yellow leaf disease and one of the most destructive diseases of betel palm (Zhang et al., 2021).
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts?
Yes. Although the existing phytosanitary measures identified in Section 3.3.2 do not specifically target P. cryptus, they mitigate the likelihood of its entry into the EU (see also Section 3.6.1).
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2). Additional potential risk reduction options are listed in Table 6 (Section 3.6.1.1) and supporting measures in Table 7 (Section 3.6.1.2).
Table 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Special requirements summary (with hyperlink to information sheet if available) | Potential control measure summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | As a pest with low mobility, a risk reduction option could be to source plants from a pest free area, or pest free place of production or pest free production site. | Entry/Spread |
| Growing plants in isolation | Plants could be grown in insect‐proof structures. | Entry/Spread |
| Chemical treatments on crops including reproductive material | Potential although the effective insecticides against P. cryptus are limited (Franco et al., 2004). In the past organophosphate insecticides were used (Blumberg et al., 1999; Franco et al., 2004) which are now not registered for use. | Entry/Establishment/Spread/Impact |
| Physical treatments on consignments or during processing | Washing, brushing and other mechanical cleaning methods can be used to reduce the prevalence of the pest in the consignments to be exported or to be planted. | Entry/Spread |
| Heat and cold treatments | Used to mitigate likelihood of infestation of pests susceptible to thermal treatments. | Entry/Spread |
| Controlled atmosphere |
Used to mitigate likelihood of infestation of pests susceptible to modified atmosphere (usually applied during transport) hence to mitigate entry. Controlled atmosphere storage can be used in commodities such as fresh and dried fruits, flowers and vegetables. |
Entry/Spread |
| Biological control and behavioural manipulation | The parasitoid Clausenia purpurea Ishii regulate the population of P. cryptus in Israel (see Section 3.5) | Impact |
| Limits on soil with plants | Used to mitigate likelihood of entry or spread of P. cryptus eggs, nymphs and adults in soil. | Entry/Spread |
Table 7.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Supporting measure | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping | Consignments of fresh plant material from countries where P. cryptus occurs should be inspected thoroughly for the presence of P. cryptus. | Entry/Spread |
| Phytosanitary certificate and plant passport | Used to attest which of the above requirements have been applied. | Entry/Spread |
3.6.1.1. Additional potential risk reduction options
Potential additional risk reduction measures are listed in Table 6
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
P. cryptus is widely distributed and polyphagous, making the inspections of all consignments containing hosts from countries where the pest occurs difficult.
P. cryptus is small and cryptic, and not easily detectable at low population densities.
Non‐systemic insecticides are not effective against P. cryptus, due in part to the natural wax coating covering the various life stages of the insect.
3.7. Uncertainty
The main sources of uncertainty regarding the establishment and impact potential of P. cryptus within the EU include:
The suitability of the climate of EU countries in central Europe.
The magnitude of potential economic impact. In many areas, e.g. Israel, P. cryptus causes little damage, due to natural enemies and the introduced parasitoid Clausenia purpurea reducing mealybug populations to low levels.
How quickly, natural enemies such as C. purpurea will follow the spread of P. cryptus into and within the EU. C. purpurea has been reported in Italy, but it is not as widespread as P. cryptus.
The efficiency of natural enemies being able to follow establishment in the EU to efficiently control the pest.
4. Conclusions
P. cryptus satisfies all of the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. Table 8 provides a summary of the PLH Panel conclusions.
Table 8.
The Panel’s conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel’s conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest ( Section 3.1 ) | The identity of the pest is established. Detailed morphological descriptions and illustrations exist. | None |
| Absence/presence of the pest in the EU ( Section 3.2 ) | P. cryptus is not known to occur in the EU. | |
| Pest potential for entry, establishment and spread in the EU ( Section 3.4 ) |
P. cryptus is able to enter into, become established and spread within the EU territory. The main pathways are: – plants for planting – fruits, vegetables and cut flowers |
None |
| Potential for consequences in the EU ( Section 3.5 ) | The introduction of the pest could cause yield and quality losses on several crops, especially citrus, and reduce the aesthetic value of ornamental plants. | The magnitude of impacts is uncertain. |
| Available measures ( Section 3.6 ) | There are measures available to prevent the entry, establishment and spread of P. cryptus within the EU. Risk reduction options include inspections, chemical and physical treatments on consignments of fresh plant material from infested countries and the production of plants for import in the EU in pest free areas. | None |
| Conclusion ( Section 4 ) | The criteria assessed by EFSA for consideration as a potential Union quarantine pest are met. | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate: | Establishment, impact and effectiveness of natural enemies. | |
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018)
- Degree day
Degree days (DD) are a measurement of heat units over time, often calculated from the average daily temperature above a threshold. For example, above a threshold temperature of 10oC, a 24‐hour period with an average temperature of 16oC would represent 6 DD
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy and Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2018)
Appendix A – Pseudococcus cryptus host plants/species affected
| Host status | Host name | Plant family | Common name | Reference |
|---|---|---|---|---|
| Cultivated hosts | Aglaonema | Araceae | Chinese evergreens | García Morales et al. (2016) |
| Ananas comosus | Bromeliaceae | Pineapple | García Morales et al. (2016) | |
| Annona muricata | Annonaceae | Prickly custard apple | García Morales et al. (2016) | |
| Annona squamosa | Annonaceae | Cuban sugar apple, custard apple, sugar apple, sweetsop | García Morales et al. (2016) | |
| Areca | Arecaceae | García Morales et al. (2016) | ||
| Areca catechu | Arecaceae | Reca palm, areca nut palm, betel palm, betel nut palm, Indian nut, Pinang palm, catechu | García Morales et al. (2016) | |
| Artocarpus | Moraceae | García Morales et al. (2016) | ||
| Artocarpus altilis | Moraceae | Breadfruit | García Morales et al. (2016) | |
| Artocarpus odoratissimus | Moraceae | Marang, madang, timadang, teraptarap, kiran, green pedalai, johey oak | García Morales et al. (2016) | |
| Aucuba japonica | Garryaceae | Potted laurel, Japanese laurel, Japanese aucuba, gold dust plant | García Morales et al. (2016) | |
| Averrhoa carambola | Oxalidaceae | Carambola | García Morales et al. (2016) | |
| Bambusa | Poaceae | Bamboo | García Morales et al. (2016) | |
| Bauhinia purpurea | Fabaceae | Orchid tree, purple bauhinia, camel's foot, butterfly tree, Hawaiian orchid tree | García Morales et al. (2016) | |
| Calophyllum inophyllum | Calophyllaceae | Tamanu, mastwood, beach calophyllum, beauty leaf | García Morales et al. (2016) | |
| Citrus aurantiifolia | Rutaceae | Lime, Key lime, West Indian lime | García Morales et al. (2016) | |
| Citrus aurantium | Rutaceae | Bitter orange, Seville orange, sour orange, bigarade orange, marmalade orange | García Morales et al. (2016) | |
| Citrus junos | Rutaceae | Yuzu | García Morales et al. (2016) | |
| Citrus limon | Rutaceae | Lemon, true lemon tree | García Morales et al. (2016) | |
| Citrus maxima | Rutaceae | Pummelo, pomelo | García Morales et al. (2016) | |
| Citrus paradisi | Rutaceae | Grapefruit | García Morales et al. (2016) | |
| Citrus reticulata | Rutaceae | Mandarin orange, mandarin, mandarine | García Morales et al. (2016) | |
| Citrus sinensis | Rutaceae | Navel orange, orange, sweet orange, Valencia orange | García Morales et al. (2016) | |
| Citrus unshiu | Rutaceae | Miyagawa mandrin, unshu mikan, cold hardy mandarin, satsuma mandarin, satsuma orange, naartjie, tangerine | García Morales et al. (2016) | |
| Citrus | Rutaceae | García Morales et al. (2016) | ||
| Cocos nucifera | Arecaceae | Coconut Palm | García Morales et al. (2016) | |
| Coffea arabica | Rubiaceae | Coffee (arabica), coffee tree | García Morales et al. (2016) | |
| Coffea liberica | Rubiaceae | Liberian coffee tree | García Morales et al. (2016) | |
| Crinum asiaticum | Amaryllidaceae | Poison bulb, giant crinum lily, grand crinum lily, spider lily | García Morales et al. (2016) | |
| Croton | Euphorbiaceae | Rushfoil, croton | García Morales et al. (2016) | |
| Cyrtostachys renda | Arecaceae | Red sealing wax palm, lipstick palm | García Morales et al. (2016) | |
| Dahlia | Asteraceae | García Morales et al. (2016) | ||
| Dendrobium | Orchidaceae | García Morales et al. (2016) | ||
| Diospyros kaki | Ebenaceae | Oriental persimmon, Chinese persimmon, Japanese persimmon, kaki persimmon | García Morales et al. (2016) | |
| Elaeis guineensis | Arecaceae | Oil palm, African oil palm, macaw‐fat | García Morales et al. (2016) | |
| Eriobotrya japonica | Rosaceae | Japanese medlar, Japanese plum, loquat | García Morales et al. (2016) | |
| Eugenia | Myrtaceae | García Morales et al. (2016) | ||
| Ficus concinna | Moraceae | García Morales et al. (2016) | ||
| Fragaria vesca | Rosaceae | Wild strawberry | Yiğit and Telli (2013) | |
| Garcinia kydia | Clusiaceae | García Morales et al. (2016) | ||
| Garcinia mangostana | Clusiaceae | Purple mangosteen | García Morales et al. (2016) | |
| Gardenia | Rubiaceae | García Morales et al. (2016) | ||
| Glycine max | Fabaceae | Soybean, soy bean, soya bean | García Morales et al. (2016) | |
| Heliconia | Heliconiaceae | Lobster‐claws, toucan beak, wild plantain, false bird‐of‐paradise | García Morales et al. (2016) | |
| Hevea brasiliensis | Euphorbiaceae | Brazilian rubber tree, para rubber, para rubber tree | García Morales et al. (2016) | |
| Hibiscus tiliaceus | Malvaceae | Coast hibiscus, hau tree, linden hibiscus, mahoe, mahoe tree, wild cotton tree | García Morales et al. (2016) | |
| Ixora | Rubiaceae | West Indian jasmine | García Morales et al. (2016) | |
| Jasminum | Oleaceae | Jasmine | García Morales et al. (2016) | |
| Juglans regia | Juglandaceae | Walnut | Yiğit and Telli, (2013) | |
| Laurus nobilis | Lauraceae | Sweet bay | Yiğit and Telli, (2013) | |
| Litchi chinensis | Sapindaceae | Lychee, lichi, leechee | García Morales et al. (2016) | |
| Mangifera indica | Anacardiaceae | Mango | García Morales et al. (2016) | |
| Monstera deliciosa | Araceae | Ceriman | Ellenrieder and Watson (2016) | |
| Moringa oleifera | Moringaceae | Moringa, drumstick tree, horseradish tree, ben oil tree, benzolive tree | García Morales et al. (2016) | |
| Morus | Moraceae | Mulberry tree | García Morales et al. (2016) | |
| Morus alba | Moraceae | White mulberry | Yiğit and Telli (2013) | |
| Musa acuminata | Musaceae | Wild banana | García Morales et al. (2016) | |
| Musa paradisiaca | Musaceae | Plantain | García Morales et al. (2016) | |
| Musa | Musaceae | Banana | García Morales et al. (2016) | |
| Myristica fragrans | Myristicaceae | Nutmeg | García Morales et al. (2016) | |
| Nephelium lappaceum | Sapindaceae | Rambutan | García Morales et al. (2016) | |
| Nerium oleander | Apocynaceae | Oleander, nerium | García Morales et al. (2016) | |
| Pandanus | Pandanaceae | Pandan, screw palm, screw pine | García Morales et al. (2016) | |
| Pandanus tectorius | Pandanaceae | Screw pine | Ellenrieder and Watson (2016) | |
| Paphiopedilum bellatulum | Orchidaceae | Egg‐in‐a‐nest orchid | García Morales et al. (2016) | |
| Papilionanthe teres | Orchidaceae | García Morales et al. (2016) | ||
| Parthenocissus tricuspidata | Vitaceae | Boston ivy, grape ivy, Japanese ivy | García Morales et al. (2016) | |
| Passiflora foetida | Passifloraceae | Love‐in‐a‐mist, stinking passion flower, wild water lemon | García Morales et al. (2016) | |
| Persea americana | Lauraceae | Avocado, avocado pear, alligator pear, holly ghost pear | García Morales et al. (2016) | |
| Phalaenopsis amabilis | Orchidaceae | Moon orchid, moth orchid | García Morales et al. (2016) | |
| Phoenix dactylifera | Arecaceae | Date, date palm | García Morales et al. (2016) | |
| Piper betle | Piperaceae | Piper betle | García Morales et al. (2016) | |
| Psidium guajava | Myrtaceae | Apple guava, Brazilian guava, common guava, Guinea guava, lemon guava, pear guava, tropical guava, yellow guava | García Morales et al. (2016) | |
| Punica granatum | Lythraceae | Pomegranate | García Morales et al. (2016) | |
| Solanum melongena | Solanaceae | Aubergine | Yiğit and Telli (2013) | |
|
Solanum lycopersicum |
Solanaceae | Tomato | Yiğit and Telli (2013) | |
| Solanum tuberosum | Solanaceae | Potato | Yiğit and Telli (2013) | |
| Spathoglottis | Orchidaceae | Purple orchids | García Morales et al. (2016) | |
| Spathoglottis plicata | Orchidaceae | Fernland orchid, large purple orchid, Philippine orchid | García Morales et al. (2016) | |
| Syzygium malaccense | Myrtaceae | Long fruited rose‐apple, mountain apple, Otaheite‐apple, pomerac, rose apple, wax jambu | García Morales et al. (2016) | |
| Tamarindus indica | Fabaceae | Tamarind | García Morales et al. (2016) | |
| Viburnum tinus | Adoxaceae | Laurustinus, laurustiner, Laurestine | García Morales et al. (2016) | |
| Vitis vinifera | Vitaceae | Grape, common grape vine, wine grape | García Morales et al. (2016) | |
| Zingiber officinale | Zingiberaceae | Ginger, common ginger, | Ellenrieder and Watson (2016) | |
| Wild weed hosts | Albizia saman | Fabaceae | Rain tree | Ellenrieder and Watson (2016) |
| Amorphophallus | Araceae | García Morales et al. (2016) | ||
| Arum | Araceae | Yiğit and Telli (2013) | ||
| Avicennia germinans | Acanthaceae | Black mangrove | García Morales et al. (2016) | |
| Avicennia officinalis | Acanthaceae | Indian mangrove | García Morales et al. (2016) | |
| Callerya nieuwenhuisii | Fabaceae | García Morales et al. (2016) | ||
| Coelogyne pulverula | Orchidaceae | García Morales et al. (2016) | ||
| Convolvulus arvensis | Convolvulaceae | Bindweed | Yiğit & Telli, 2013 | |
| Dillenia indica | Dilleniaceae | Elephant apple | García Morales et al. (2016) | |
| Erythrina | Fabaceae | Coral tree, flame tree | García Morales et al. (2016) | |
| Finlaysonia | Apocynaceae | García Morales et al. (2016) | ||
| Gliricidia | Fabaceae | García Morales et al. (2016) | ||
| Lansium parasiticum | Meliaceae | Langsat, lanzones, longkong | García Morales et al. (2016) | |
| Lithocarpus | Fagaceae | Stone oaks | García Morales et al. (2016) | |
| Malva sylvestris | Malvaceae | Common mallow | Yiğit & Telli, 2013 | |
| Melastoma malabathricum | Melastomataceae | Malabar melastome, Indian rhododendron, Singapore rhododendron, planter's rhododendron, senduduk | García Morales et al. (2016) | |
| Metroxylon | Arecaceae | García Morales et al. (2016) | ||
| Neonauclea | Rubiaceae | García Morales et al. (2016) | ||
| Ocotea atirrensis | Lauraceae | García Morales et al. (2016) | ||
| Osbornia octodonta | Myrtaceae | García Morales et al. (2016) | ||
| Pandanus samoensis | Pandanaceae | García Morales et al. (2016) | ||
| Piper majusculum | Piperaceae | García Morales et al. (2016) | ||
| Plumeria | Apocynaceae | Frangipani | García Morales et al. (2016) | |
| Rhizophora apiculata | Rhizophoraceae | True mangrove | García Morales et al. (2016) | |
| Ryparosa fasciculata | Achariaceae | García Morales et al. (2016) | ||
| Saintpaulia inconspicua | Gesneriaceae | Yiğit and Telli, (2013) | ||
| Selaginella | Selaginellaceae | Spike mosses, lesser clubmosses | García Morales et al. (2016) | |
| Sterculia sp. | Malvaceae | Ellenrieder and Watson (2016) | ||
| Strychnos vanprukii | Loganiaceae | García Morales et al. (2016) | ||
| Tetracera | Dilleniaceae | García Morales et al. (2016) |
Appendix B – Distribution of Pseudococcus cryptus
Distribution records based on CABI (CABI, 2021), García Morales et al. (2016) and other references.
| Region | Country | Sub‐national (e.g. State) | Status | Reference |
|---|---|---|---|---|
| Central America | Costa Rica | Present, no details | García Morales et al. (2016) | |
| El Salvador | Present, no details | CABI (2021) | ||
| Caribbean | Guadeloupe | Present, no details | CABI (2021) | |
| Virgin Islands (US) | Present, no details | CABI (2021) | ||
| South America | Brazil | Present, no details | CABI (2021) | |
| Brazil | Sao Paulo | Present, no details | CABI (2021) | |
| Brazil | Espirito Santo | Present, no details | García Morales et al. (2016) | |
| Brazil | Minas Gerais | Present, no details | García Morales et al. (2016) | |
| Brazil | Rio Grande do Sul | Present, no details | García Morales et al. (2016) | |
| Brazil | Rio de Janeiro | Present, no details | García Morales et al. (2016) | |
| Argentina | Present, no details | CABI (2021) | ||
| Paraguay | Present, no details | CABI (2021) | ||
| Uruguay | Present, no details | García Morales et al. (2016) | ||
| EU (27) | Spain | Present, no details | García Morales et al. (2016) | |
| Africa | Ascension Island | Present, no details | García Morales et al. (2016) | |
| Kenya | Present, no details | García Morales et al. (2016) | ||
| Mauritius | Present, no details | García Morales et al. (2016) | ||
| Tanzania | Zanzibar | Present, no details | García Morales et al. (2016) | |
| Asia | Afghanistan | Present, no details | García Morales et al. (2016) | |
| Andaman Islands | Present, no details | García Morales et al. (2016) | ||
| Bangladesh | Present, no details | García Morales et al. (2016) | ||
| Bhutan | Present, no details | García Morales et al. (2016) | ||
| British Indian Ocean Territory | Present, no details | García Morales et al. (2016) | ||
| Brunei | Present, no details | García Morales et al. (2016) | ||
| China | Present, no details | CABI (2021) | ||
| China | Fujian | Present, no details | García Morales et al. (2016) | |
| China | Hunan | Present, no details | García Morales et al. (2016) | |
| Hong Kong | Present, no details | CABI (2021) | ||
| India | Present, no details | CABI (2021) | ||
| India | Gujarat | Present, no details | García Morales et al. (2016) | |
| India | Kerala | Present, no details | García Morales et al. (2016) | |
| India | Maharashtra | Present, no details | García Morales et al. (2016) | |
| India | Sikkim | Present, no details | García Morales et al. (2016) | |
| India | Tamil Nadu | Present, no details | CABI (2021) | |
| India | West Bengal | Present, no details | García Morales et al. (2016) | |
| Indonesia | Present, no details | García Morales et al. (2016) | ||
| Indonesia | Java | Present, no details | García Morales et al. (2016) | |
| Indonesia | Lombok | Present, no details | García Morales et al. (2016) | |
| Indonesia | Sulawesi | Present, no details | García Morales et al. (2016) | |
| Indonesia | Sumatra | Present, no details | García Morales et al. (2016) | |
| Iran | Present, no details | García Morales et al. (2016) | ||
| Israel | Present, no details | CABI (2021) | ||
| Japan | Present, no details | CABI (2021) | ||
| Japan | Honshu | Present, no details | García Morales et al. (2016) | |
| Cambodia | Present, widespread | García Morales et al. (2016) | ||
| Laos | Present, no details | García Morales et al. (2016) | ||
| Malaysia | Present, no details | García Morales et al. (2016) | ||
| Malaysia | Sabah | Present, no details | García Morales et al. (2016) | |
| Malaysia | Sarawak | Present, no details | García Morales et al. (2016) | |
| Maldives | Present, no details | García Morales et al. (2016) | ||
| Nepal | Present, no details | García Morales et al. (2016) | ||
| Philippines | Present, no details | CABI (2021) | ||
| Philippines | Luzon | Present, no details | García Morales et al. (2016) | |
| Philippines | Mindanao | Present, no details | García Morales et al. (2016) | |
| Philippines | Mindoro | Present, no details | García Morales et al. (2016) | |
| Singapore | Present, no details | García Morales et al. (2016) | ||
| South Korea | Present, no details | García Morales et al. (2016) | ||
| Sri Lanka | Present, no details | CABI (2021) | ||
| Syria | Present, no details | Malausa et al. (2016) | ||
| Taiwan | Present, no details | García Morales et al. (2016) | ||
| Thailand | Present, no details | CABI (2021) | ||
| Turkey | Present, no details | Yiğit and Telli (2013), Holat et al. (2014) | ||
| Vietnam | Present, no details | CABI (2021) | ||
| Oceania | USA | Hawaii | Present, no details | García Morales et al. (2016) |
| American Samoa | Present, no details | García Morales et al. (2016) | ||
| Federated States of Micronesia | Present, no details | García Morales et al. (2016) | ||
| Federated States of Micronesia | Ponape Island | Present, no details | García Morales et al. (2016) | |
| Palau | Present, no details | García Morales et al. (2016) | ||
| Western Samoa | Present, no details | García Morales et al. (2016) |
Appendix C – Import data
Table C.1: Fresh or dried citrus (CN code: 0805) imported in 100 kg into the EU (27) from regions where Pseudococcus cryptus is known to occur (Source: Eurostat accessed on 12 November 2021)
| Country | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Afghanistan | 0,01 | 7,00 | |||
| Argentina | 2412706,76 | 1913772,23 | 2242298,89 | 1585087,09 | 1403569,93 |
| Bangladesh | 227,61 | 229,58 | 159,67 | 322,42 | 1183,66 |
| Brazil | 864863,09 | 903432,95 | 900907,24 | 822134,46 | 902354,68 |
| Brunei | 0,00 | ||||
| China | 827840,57 | 1084857,27 | 1024163,15 | 1108595,22 | 1098691,70 |
| Costa Rica | 4700,31 | 921,32 | 704,93 | 231,20 | 461,60 |
| Hong Kong | 0,00 | 2,27 | 1,00 | ||
| Indonesia | 566,73 | 555,70 | 779,35 | 836,73 | 864,54 |
| India | 246,80 | 1,00 | 449,63 | 88,51 | 254,95 |
| Israel | 799118,49 | 969403,62 | 824601,66 | 812738,57 | 878713,15 |
| Iran | 1533,22 | 1218,52 | 1208,01 | 2174,22 | 1882,74 |
| Japan | 352,58 | 417,44 | 270,73 | 319,24 | 162,50 |
| Kenya | 0,00 | 8,80 | 34,56 | ||
| Cambodia | 0,02 | 0,01 | 2,76 | 2,84 | |
| Laos | 51,94 | 2,10 | 20,23 | ||
| Mauritius | 213,74 | 0,00 | 14,00 | 7,35 | |
| Malaysia | 4,18 | 39,02 | 83,45 | 7,71 | |
| Nepal | 1170,00 | ||||
| Paraguay | 0,00 | 6,00 | |||
| Philippines | 0,00 | 0,20 | 7,71 | 0,10 | |
| South Korea | 12,70 | 0,01 | 21,09 | 15,00 | |
| Taiwan | 157,49 | 0,00 | 0,01 | ||
| Thailand | 426,42 | 1283,13 | 659,74 | 624,93 | 194,87 |
| Uruguay | 379726,08 | 369933,66 | 374356,50 | 402778,68 | 334468,29 |
| Vietnam | 28649,46 | 46738,17 | 70934,07 | 73964,35 | 63730,13 |
| United States | 301229,06 | 231210,47 | 185706,99 | 177755,45 | 148845,72 |
| El Salvador | 36,83 | 35,77 | 4,76 |
Table C.2: Fresh or dried bananas (CN code: 0803) imported in 100 kg into the EU (27) from regions where Pseudococcus cryptus is known to occur (Source: Eurostat accessed on 12 November 2021)
| Country | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Argentina | 240,00 | ||||
| Bangladesh | 174,66 | 79,85 | 72,75 | 38,05 | 35,64 |
| Brazil | 149108,03 | 26855,08 | 59677,31 | 104909,74 | 98434,39 |
| China | 252,64 | 188,73 | 390,56 | 545,74 | 854,93 |
| Costa Rica | 9662138,79 | 9663219,69 | 10125330,57 | 9405488,40 | 10342372,80 |
| Indonesia | 0,01 | 37,27 | 14,72 | 64,17 | |
| India | 515,19 | 445,99 | 571,13 | 607,74 | 1418,91 |
| Israel | 2,10 | 0,75 | |||
| Iran | 0,09 | 2,86 | 12,33 | ||
| Kenya | 1,90 | 0,72 | 6,15 | 11,23 | 14,95 |
| Cambodia | 17,46 | 45,59 | 35,02 | 42,28 | 26,91 |
| Laos | 81,44 | 65,75 | 69,83 | 45,51 | 20,40 |
| Malaysia | 8,02 | ||||
| Philippines | 2480,90 | 11415,47 | 1674,92 | 2160,35 | 1240,80 |
| Thailand | 550,44 | 674,34 | 603,32 | 526,15 | 334,58 |
| Vietnam | 276,26 | 178,84 | 190,96 | 210,11 | 142,71 |
| United States | 7,00 | 6,37 | 1,54 | 6,32 | 10,37 |
Table C.3: Fresh or dried guavas, mangoes and mangosteens (CN code: 08045000) imported in 100 kg into the EU (27) from regions where Pseudococcus cryptus is known to occur (Source: Eurostat accessed on 12 November 2021)
| Country | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Argentina | 14,40 | ||||
| Bangladesh | 438,53 | 256,66 | 331,27 | 310,73 | 323,91 |
| Brazil | 1025325,37 | 1158717,06 | 1241860,63 | 1437569,20 | 1576540,49 |
| China | 38,95 | 51,87 | 180,81 | 78,23 | 104,34 |
| Costa Rica | 17281,13 | 19119,58 | 18368,68 | 12830,62 | 14950,59 |
| Hong Kong | 6,56 | ||||
| Indonesia | 1981,20 | 2004,36 | 2926,64 | 2386,27 | 1406,94 |
| India | 5989,34 | 8148,87 | 9470,36 | 9315,51 | 7347,61 |
| Israel | 143726,08 | 140551,30 | 108353,48 | 121875,16 | 98185,83 |
| Iran | 15,65 | 12,12 | 3,00 | 9,10 | 1,56 |
| Japan | 0,66 | 0,01 | |||
| Kenya | 232,06 | 4,08 | 65,09 | 10,30 | 66,53 |
| Cambodia | 883,47 | 2098,02 | 2164,17 | 1533,79 | 904,49 |
| Laos | 753,34 | 620,36 | 603,14 | 806,50 | 525,32 |
| Malaysia | 289,86 | 197,22 | 170,64 | 72,72 | 44,57 |
| Philippines | 1028,05 | 519,88 | 795,56 | 368,97 | 128,10 |
| Singapore | 1,20 | 0,23 | 0,15 | ||
| Taiwan | 3,48 | 17,34 | 0,92 | ||
| Thailand | 6460,81 | 7401,80 | 6911,89 | 6743,91 | 5260,84 |
| Vietnam | 794,89 | 950,37 | 1346,64 | 1546,69 | 965,32 |
| United States | 78874,11 | 45478,21 | 54660,34 | 82580,54 | 82852,22 |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Malumphy C, Antonatos S, Kertesz V, Maiorano A, Papachristos D and MacLeod A, 2022. Scientific Opinion on the pest categorisation of Pseudococcus cryptus . EFSA Journal 2022;20(3):7145, 29 pp. 10.2903/j.efsa.2022.7145
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00705
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to acknowledge the contribution of Caterina Campese and Oresteia Sfyra to this opinion.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Courtesy of Chris Malumphy
Adopted: 27 January 2022
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed, the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger & Roy, 2015; EPPO, 2019).
Due to the long history of hybridisation, banana taxonomists agree that no single scientific name can be given to all edible bananas. Instead, it is accepted by banana taxonomists that banana cultivars should be referred to using the genus Musa followed by a code denoting the genome group and ploidy level, followed by the subgroup name (if any) then by the popular name of the cultivar e.g. Musa AAA ‘Dwarf Cavendish’. For brevity in this opinion, we use the term Musa and cultivar name without further details when referring to commercial bananas and plantains.
References
- Arai T, 1996. Temperature‐dependent developmental rate of three mealybug species, Pseudococcus citriculus (Green), Planococcus citri (Risso), and Planococcus kraunhiae (Kuwana) (Homoptera: Pseudococcidae) on citrus. Japanese Journal of Applied Entomology and Zoology, 40, 25–34, (in Japanese with English summary). [Google Scholar]
- Arai T, 2002. Attractiveness of sex pheromone of Pseudococcus cryptus Hempel (Homoptera: Pseudococcidae) to adult males in a citrus orchard. Applied Entomology and Zoology, 37, 69–72. [Google Scholar]
- Baker RH, Sansford CE, Jarvis CH, Cannon RJ, MacLeod A and Walters KF, 2000. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agriculture, Ecosystems & Environment, 82, 57-71. [Google Scholar]
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In: Hallman GJ and Schwalbe CP (eds.). Invasive Arthropods in Agriculture: Problems and Solutions. Science Publishers Inc, Enfield, USA. pp. 207–241.
- Blumberg D, Ben‐Dov Y and Mendel Z, 1999. The citriculus mealybug, Pseudococcus cryptus Hempel, and its natural enemies in Israel: history and present situation. Entomologica, 33, 233–242. [Google Scholar]
- Bodenheimer FS, 1951. Citrus Entomology in the Middle East, Junk Publ, The Hague. 663 pp. [Google Scholar]
- CABI (Centre for Agriculture and Biosciences International) , 2021. Available online: www.cabi.org [Accessed: 10 November 2021].
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertész V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Zappalà L, Gómez P, Lucchi A, Urek G, Tramontini S, Mosbach‐Schulz O, de la Peña E and Yuen J, 2021. Commodity risk assessment of Persea americana from Israel. EFSA Journal 2021;19(2):6354, 50 pp. 10.2903/j.efsa.2021.6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martınez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenrieder NV and Watson G, 2016. A new mealybug in the genus Pseudococcus Westwood (Hemiptera: Coccomorpha: Pseudococcidae) from North America, with a key to species of Pseudococcus from the New World. Zootaxa, 4105, 65–87. [DOI] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 10 November 2021].
- EPPO , 2019. EPPO codes. Available online: https://www.EPPO.int/RESOURCES/eppo_databases/eppo_codes
- FAO (Food and Agriculture Organization of the United Nations) , 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations) , 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/
- Fernando LCP and Kanagaratnam P, 1987. New records of some pests of the coconut inflorescence and developing fruit and their natural enemies in Sri Lanka. Cocos, 5, 39–42. [Google Scholar]
- Foldi I and Germain J‐F, 2018. Liste des Cochenilles de France (Hemiptera, Coccomorpha) [Checklist of the scale insects of France (Hemiptera, Coccomorpha)]. Bulletin De La Societe Entomologique De France, 123, 7–18. [Google Scholar]
- Franco JC, Suma P, Borges da Silva E, Blumberg D and Mendel Z, 2004. Management strategies of mealybug pests of citrus in Mediterranean countries. Phytoparasitica, 32, 507–522. [Google Scholar]
- García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, 2016. ScaleNet: a literature‐based model of scale insect biology and systematics. Database. 10.1093/database/bav118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granara de Willink MC and González P, 2018. Revisión taxonómica de Pseudococcus Westwood (Hemiptera: Pseudococcidae) de Centro y Sud América con descripciones de especies nuevas [Taxonomic revision of Pseudococcus Westwood (Hemiptera: Pseudococcidae) from Central and South America with descriptions of new species. Insecta Mundi, 0673, 1–117. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Holat D, Kaydan MB and Muştu M, 2014. Investigations on some biological characters of Pseudococcus cryptus (Hempel) (Hemiptera: Pseudococcidae) on four citrus species. Acta Zoologica Bulgarica, Suppl, 6, 35–40. [Google Scholar]
- Itioka T and Inoue T, 1996. The role of predators and attendant ants in the regulation and persistence of a population of the citrus mealybug Pseudococcus citriculus in a Satsuma orange orchard. Applied Entomology and Zoology, 31, 195–202. [Google Scholar]
- Kanagaratnam P, Pinto JLJG and Sinnathamby SV, 1981. Some minor pests of coconut: new record for Sri Lanka. Ceylon Cocon. Q, 32, 93–95. [Google Scholar]
- Kaydan MB, Kozar F and Hodgson C, 2015. A review of the phylogeny of Palaearctic mealybugs (Hemiptera: Coccomorpha: Pseudococcidae). Arthropod Systematics & Phylogeny, 73, 175–195. [Google Scholar]
- Kim SC, Song J and Kim D, 2008. Effect of temperature on the development and fecundity of the Cryptic Mealybug, Pseudococcus cryptus, in the laboratory. Journal of Asia Pacific Entomology, 11, 149–153. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐ Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Kwon G, Lee S, Han M and Goh H, 2002. The genus Pseudococcus (Westwood) (Sternorrhyncha: Pseudococcidae) of Korea. Journal of Asia‐Pasific Entomology, 5, 145–154. [Google Scholar]
- Malausa T, Delaunay M, Fleisch A, Groussier‐Bout G, Warot S, Crochard D, Guerrieri E, Delvare G, Pellizzari G, Kaydan B, Al‐Khateeb N, Germain J‐F, Brancaccio L, Le Goff I, Bessac M, Ris N and Kreiter P, 2016. Investigating biological control agents for controlling invasive populations of the mealybug Pseudococcus comstocki in France. PLoS One, 11, e0157965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Rung A, Parikh G, Venable G, Redford AJ, Evans GA and Gill RJ, 2014. Scale Insects, Edition 2. USDA APHIS Identification Technology Program (ITP). Fort Collins, CO. Available online: https://idtools.org/id/scales/ [Accessed: 9 November 2021].
- Mohan C, Rajan P and Josephrajkumar A, 2016. Plantation Crops. In: Mani M and Shivaraju C (eds.). Mealybugs and their Management in Agricultural and Horticultural crops, Springer, New Delhi. [Google Scholar]
- Nakahata T, Itagaki N, Arai T, Sugie H and Kuwahara S, 2003. Synthesis of the sep Pheromone of the Citrus Mealybug, Pseudococcus cryptus . Bioscience, Biotechnology, and Biochemistry, 67, 2627–2631. [DOI] [PubMed] [Google Scholar]
- Pacheco da Silva VC, Bertin A, Blin A, Germain JF, Bernardi D, Rignol G, Botton M and Malausa T, 2014. Molecular and morphological identification of mealybug species (Hemiptera: Pseudococcidae) in Brazilian vineyards. PLoS One, 9, e103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco da Silva VC, Kaydan MB and Basso C, 2020. Pseudococcidae (Hemiptera: Coccomorpha) in Uruguay: morphological identification and molecular characterization, with descriptions of two new species. Zootaxa, 4894, zootaxa‐4894. [DOI] [PubMed] [Google Scholar]
- Peri E and Kapranas A, 2012. Pseudococcidae and Monophlebidae. In: Vacante V and Gerson U “Integrated Control of Citrus Pests in the Mediterranean Region”. Bentham Science Publishers, 172–182 pp.
- Ren JM, Ashfaq M, Hu XN, Ma J, Liang F, Hebert PD, Lin L, Germain JF and Ahmed MZ, 2018. Barcode index numbers expedite quarantine inspections and aid the interception of nonindigenous mealybugs (Pseudococcidae). Biological Invasions, 20, 449–460. [Google Scholar]
- Sánchez‐García I and Ben‐Dov Y, 2010. New records and data on scale insects (Hemiptera: Coccoidea) from southern Spain. Boletín De La Sociedad Entomológica Aragonesa (S.E.A.), 46, 319–320. [Google Scholar]
- Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD and Karsch‐Mizrachi I, 2020. Genbank. Nucleic Acids Research, 48, Database issue, 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed]
- Sirisena UGAI, Watson GW, Hemachandra KS and Wijayagunasekara HNP, 2013. Mealybugs (Hemiptera: Pseudococcidae) species on Economically Important Fruit Crops in Sri Lanka. Tropical Agricultural Research, 25, 69–82. [Google Scholar]
- Song JH, Choi KS, Hong SY and Lee SC, 2012. Seasonal phenology of the cryptic Mealybug, Pseudococcus cryptus (Homoptera: Pseudococcidae) based on attraction of adult males to a sex pheromone trap. Korean Journal of Applied Entomology, 51, 207–213. [Google Scholar]
- Telli S and Yiğit A, 2019. Population fluctuations of the citriculus mealybug, Pseudococcus cryptus Hempel (Hemiptera: Pseudococcidae), in citrus orchards of Samandağ, Hatay, Turkey. Journal of Plant Diseases and Protection, 126, 421–426. [Google Scholar]
- Toy SJ and Newfield MJ, 2010. The accidental introduction of invasive animals as hitchhikers through inanimate pathways: a New Zealand perspective. Revue Scientifique Et Technique (International Office of Epizootics), 29, 123–133. [DOI] [PubMed] [Google Scholar]
- Watson GW and Kubiriba J, 2005. Identification of mealybugs (Hemiptera: Pseudococcidae) on banana and plantain in Africa. African Entomology, 13, 35–47. [Google Scholar]
- Yiğit A and Telli S, 2013. Distribution, host plants and natural enemies of Pseudococcus cryptus Hempel (Hemiptera: Pseudococcidae), injurious to citrus plantations in Hatay. Turkish Journal of Entomology, 37, 359–373. [Google Scholar]
- Zhang H, Zhao X, Cao X, Khan LU, Zhao R, Wang H and Huang X, 2021. Transmission of areca palm velarivirus 1 (APV1) by mealybugs causes yellow leaf disease (YLD) in betel palm (Areca catechu). Phytopathology, 10.1094/PHYTO-06-21-0261-R [DOI] [PubMed] [Google Scholar]


