Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Zaprionus indianus (Diptera: Drosophilidae), the African fig fly for the territory of the EU. This species successfully colonised the Indian subcontinent more than four decades ago, and more recently South and North America. Within the EU, the pest occurs in Cyprus, Malta, Portugal (Madeira) and Spain (Canary Islands and Andalusia). Z. indianus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072. The larvae of this fly feed on more than 80 plant species both cultivated and non‐cultivated. Females produce around 60–70 eggs. Egg laying mostly occurs in decaying fruit or fruit with injuries or mechanical damage. However, Z. indianus can oviposit on undamaged healthy fruit such as figs, strawberries and guavas which provide a potential pathway for entry into the EU. Lower temperature thresholds are around 9–10°C. Optimum development occurs at 28°C. The number of generations per year varies from 12 to 16. Climatic conditions in many EU member states and host plant availability in those areas are conducive for establishment. The introduction of Z. indianus is expected to have an economic impact in the EU especially on fig and strawberry production. Damage caused by other fruit flies (Drosophilidae and Tephritidae) could be increased by mixed infestations. Phytosanitary measures are available to reduce the likelihood of entry and further spread. Z. indianus satisfies all of the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: African fig fly, Drosophilidae, pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Zaprionus indianus is one of a number of pests listed in Annex 1 to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/ 2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This categorisation was initiated by reports of interceptions and/or outbreaks of the pest notified by the Member States to the European Commission.
2. Data and methodologies
2.1. Data
2.1.1. Information on pest status from NPPOs
In the context of the current mandate, EFSA is preparing pest categorisations for new/emerging pests that are not yet regulated in the EU. When official pest status is not available in the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), EFSA consults the NPPOs of the relevant MS. To obtain information on the official pest status for Z. indianus, EFSA has consulted the NPPOs of France, Malta, Portugal and Spain. The results of this consultation are presented in Section 3.2.2
2.1.2. Literature search
A literature search on Z. indianus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.3. Database search
Pest information, on host(s), distribution and official status, was retrieved from EPPO Global Database, the CABI databases and scientific literature databases as referred above in Section 2.1.2.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for Z. indianus which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for Z. indianus, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
| Identity of the pest ( Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory ( Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed. |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways for entry and spread. |
| Potential for consequences in the EU territory ( Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
| Available measures ( Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impact? |
| Conclusion of pest categorisation ( Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel’s conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of the species is established and Zaprionus indianus Gupta is the accepted name.
Zaprionus indianus Gupta, 1970 is an insect within the order Diptera, family Drosophilidae (Figure 1). It is commonly known as the African fig fly (Gupta, 1970). The species is distributed throughout the entire Afrotropical region (Vilela et al., 2001).
Figure 1.

Zaprionus indianus adult (Source: Fera Science Ltd, UK)
The genus Zaprionus is divided into two subgenera that are distinguished by their geographic origin: the subgenus Anaprionus which contains 10 species from the Oriental biogeographic region and the subgenus Zaprionus sensu stricto including 49 essentially Afrotropical species, among which is Z. indianus (Yassin and David, 2010; Commar et al., 2012).
Tsacas (1985) reviewed all of the problems concerning the nomenclature of Z. indianus and ponted out that synonymous species names include Z. inermis (Séguy, 1983), Z. paravittiger (Goodbole and Vaidya, 1972) and Z. collarti (Tsacas, 1980).
The EPPO code 1 (Griessinger and Roy, 2015; EPPO, 2019) for this species is: ZAPRIN. (EPPO, online).
3.1.2. Biology of the pest
The larvae of Z. indianus feed on more than 80 plant species both cultivated and non‐cultivated and the species was considered ecologically diverse by Yassin and David (2010) and the most polyphagous drosophilid in the Afrotropical fauna (Commar et al., 2012; Pfeiffer et al., 2019). The species has the tendency to attack and feed only on decaying fruit (Joshi et al., 2014); this is related to the inability of females to oviposit on ripe fruit without prior injuries or mechanical damage caused by other insects like Drosophila suzukii Matsumura (Bernardi et al., 2017). The co‐occurrence of these species has been reported in the United States in D. suzukii traps in vineyards (Van Timmeren & Isaacs, 2014; Joshi et al., 2014), in guava crops in Mexico (Lasa & Tadeo, 2015) and, in ripe strawberry fruit in southern Brazil (Bernardi et al., 2017). Z. indianus females produce around 60–70 eggs on average during their life (Fartyal et al., 2014). Like many drosophilids, Z. indianus adults are frugivorous and mycophagous (Gottschalk et al., 2009) and they are expected to be attracted to fermenting food materials, such as fruit. Z. indianus feeds on the bacteria and yeast found in decomposing fruits, principally on the yeast Candida tropicalis (Commar et al., 2012). However, the species is able to attack also unripe healthy fruit of species with a natural opening such as an ostiole, found in figs (Commar et al., 2012). In Brazil, it was reported attacking figs, where females oviposit in and around the ostiole and damage is caused by larvae when they penetrate the fruit tissue (Vilela et al., 1999). Bernardi et al. (2017), under laboratory conditions, observed Z. indianus laying eggs near the achenes in ripe strawberries, and the larvae were then able to enter and develop in the berries; however, its attacks were more successful, in terms of number of eggs laid and adults developed, if the berries were injured by D. suzukii or by mechanical means. Z. indianus could also infest healthy guavas but only over‐ripened (Fartyal et al., 2014), as well as Malpighia emarginata (Barbados cherry) and Dimocarpus longan (longan) ripe fruits (Lasa and Tadeo, 2015).
Amoudi et al. (1991) and Nava et al. (2007) studied the effect of temperature on the fly life cycle. The lower temperature development threshold (TT) and thermal constant (K) values for the egg, larval and pupal stages were 9.7°C and 10.5 degree days (DD); 9.2°C and 148.6 DD, and 10.7°C and 66.25 DD, respectively, for a total thermal constant of 262.2 DD for the egg‐adult biological cycle (Nava et al., 2007). When temperature increased from 25°C to 30°C, the mean larval and pupal duration decreased as well as adult longevity, but an increase in mean generation life span (including mean egg incubation period, mean larval and pupal duration periods plus the mean preoviposition period) was observed (i.e. 22.4 days at 25°C and 29.4 days at 30°C). The reduction in mean adult longevity between 25 and 30°C was associated with a significant reduction in mean oviposition period, from 35.8 d to 1.3 days, and in mean fecundity from 112.5 eggs to 1.2 eggs. Besides, all eggs laid at 30°C failed to hatch (Amoudi et al., 1991). Temperatures near 28°C are the thermal optimum, allowing shorter development time and high viability (Nava et al., 2007). At 18°C, the cycle may extend for up to 1 month (Coutihno‐Silva et al., 2017). The number of generations per year varies from 12 to 16 (Karan et al., 2000; Setta and Carareto, 2005; Nava et al., 2007). Field and laboratory studies on Indian populations showed that Z. indianus overwinters in the egg stage and to a small extent as pupae (Alawamleh et al., 2016).
3.1.3. Host range/Species affected
Z. indianus feeds on more than 80 plant species (Lachaise and Tsacas, 1983; van der Linde et al., 2006); this polyphagy has contributed to its ability to invade new areas (Commar et al., 2012). While it can attack healthy unripe fruit of species with a natural opening such as figs (Commar et al., 2012), most hosts are fruits that have been injured or have fallen. Although Z. indianus has been reported emerging from grape, whether it causes injury, or rather exacerbates injury from other pests, namely D. suzukii, needs to be clarified (Pfeiffer et al., 2019). In Brazil, it adapted to Solanum lycocarpum attacking fruits throughout the fruit development period (Leão and Tldon, 2004). This plant has edible berries and is the most abundant native fleshy fruited plant in the region of Cerrado (a vast tropical savanna ecoregion in Brazil). Leão and Tidon (2004) found that African fig fly predominated in fresh fruit of that host, but declined markedly in damaged fruit, rising again in severely over‐ripe fruit. Z. indianus is generally regarded as unable to attack intact fruit (Renkema et al., 2018). Joshi et al. (2014) noted that the African fig fly could become an economic pest in smooth‐skinned fruit that are harvested close to ripeness, such as nectarines and grapes. A complete list of hosts is provided in Appendix A.
3.1.4. Intraspecific diversity
Z. indianus is considered a cryptic species of the indianus complex together with Zaprionus africanus Yassin & David, Z. gabonicus Yassin & David and Z. megalorchis Chassagnard & Tsacas (Yassin et al., 2008; Yassin and David, 2010).
Geographic genetic variation was investigated in Indian, African and South American populations and revealed latitudinal clines of size except for the introduced American populations (David et al., 2006). A study of wing shape plasticity revealed a progressive elongation of the wing with decreasing temperature (Loh et al., 2008).
Based on studies of populations from Asia, Africa and South America, using mitochondrial DNA (mtDNA) haplotypes of CO‐I and CO‐II genes, there are two phylogenetic lineages (phylads); phylad I includes some of the African populations and phylad II includes the Atlantic populations, including South and North America, Madeira, Islands of the Pacific Ocean, Middle‐East and India. mtDNA was also able to define a distinct phylogeographical pattern, showing the presence of two independent geographical radiation within the cosmopolitan populations of Z. indianus: the older to the East, the younger to the West (Yassin et al., 2008).
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, there are detection and identification methods for Z. indianus.
Detection
Several different baits have been tested to attract Z. indianus. A combination of red wine and vinegar, actively fermenting grape juice, a blend of apple cider vinegar and beer (Epsky et al., 2014, 2015; Renkema et al., 2018) were the most successful. Fig juice diluted in water placed in a clear plastic bottle proved also effective (Pasini and Link, 2011) as well as orange traps with brown circles baited with apple cider vinegar (Lasa et al., 2020).
Symptoms
Z. indianus larvae having access and feeding on the interior fruit flesh cause the fruit to become soft and unmarketable.
Identification
The drosophilid genus Zaprionus Coquillett, 1902 is characterised by the presence of longitudinal white stripes on the frons and the mesonotum (Yassin, 2010). Z. indianus is easily identified because of its unique longitudinal black and white stripes that can be observed with the unaided eye (Pfeiffer et al., 2019).
Eggs are small, white and oblong with four filaments. There are three larval stages. Larvae have white, cylindrical bodies (3.5 mm long when fully grown), tapered anteriorly with posterior spiracles. Pupae are spindle‐shaped, reddish brown with two anterior stigmata (EPPO, online; van der Linde et al., 2006; Nava et al., 2007).
Adults are small (between 2.5 and 3.0 mm in length) with a reddish‐brown head and thorax, yellow abdomen and red eyes. The dorsal region of the head and thorax has two longitudinal silvery‐white stripes, between which run narrow black stripes. The black and white stripes on Z. indianus are of equal size with the stripe width maintained over the full length of the head to the thorax (van der Linde, 2010).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Z. indianus is an afrotropical species which is now considered to be almost cosmopolitan. It invaded India, where it was first described in 1970, and America starting from the 1990s, with a first published record in 1999 in Brazil which was followed by a rapid expansion in South America. In North America, it was first reported in Chiapas (Mexico) in 2002 and in Florida (USA) in 2005. Its presence has been reported in some countries around the Mediterranean Basin (Yassin et al., 2008; Da Mata et al., 2010; EPPO, online). Figure 2 shows the global distribution of Z. indianus.
Figure 2.
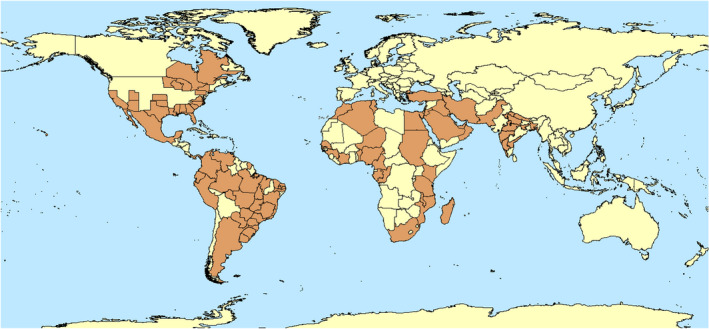
Global distribution of Zaprionus indianus (Data source: EPPO Global Database accessed on 20 November 2021)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
Yes, Z. indianus is present in the EU but not widely distributed.
Z. indianus is present in Cyprus with restricted distribution, in Malta, in Portugal (only Madeira) and in Spain (Canary Islands and Andalusia) (EPPO, online). In France, this insect was captured in 2016 in traps (Kremmer et al., 2017). At that time, its establishment was not expected. Indeed, since 2016, the species has not been caught in the perimeter of the surveillance. Its current status in France is ‘absent, pest no longer present’. In Spain, the official pest status is ‘present, restricted distribution’. Its presence in the Canary Islands and Andalusia (provinces of Malaga, Huelva and Granada) has been confirmed by the Spanish NPPO. There is no national or regional measure applied or planned to be applied other than surveillance. In Malta, the presence of the pest has been confirmed by the NPPO; no surveys are carried out for the pest. In Portugal, the pest is present only in Madeira, but no detailed information is available; in mainland Portugal, there have been no reports of presence and no surveys are carried out.
Yassin and David (2010) mention Austria and Italy in the list of countries where the pest occurs, but EPPO considers these records unreliable as they are not confirmed by other sources (EPPO, online).
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
Z. indianus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031, or in any emergency plant health legislation.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
Whilst some host species are prohibited (Table 2), these prohibitions do not apply to fruits. Since Z. indianus completes development only on fruit, these prohibitions do not prevent the likelihood of pest entry.
Table 2.
List of plants, plant products and other objects that are Zaprionus indianus hosts whose introduction into the Union from certain third countries is prohibited. (source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 8. | Plants for planting of Chaenomeles Ldl.,[…]Prunus L., Pyrus L. and Rosa L., other than dormant plants free from leaves, flowers and fruits |
see 2019/2072 Annex VI for details |
Third countries other than: specified European third countries (see 2019/2072 Annex VI for details) |
| 9. |
Plants for planting of [...], Prunus L. and [...], other than seeds |
Third countries, other than: specified European third countries, specified countries bordering the Mediterranean Sea, specified Eurasian countries, Australia, Canada, New Zealand, specified parts of Russia, United States other than Hawaii (see 2019/2072 Annex VI for details) |
|
|
10. |
Plants of Vitis L>, other than fruits |
Third countries other than Switzerland |
|
| 11. |
Plants of Citrus L. [...] and their hybrids, other than fruits and seeds |
All third countries |
|
| 13. |
Plants of Phoenix spp. other than fruit and seeds |
Algeria, Morocco |
|
| 18. | Plants for planting of Solanaceae other than seeds […] |
Third countries other than: specified European third countries (see 2019/2072 Annex VI for details) |
|
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways
Yes, Z. indianus could enter the EU territory.
Comment on plants for planting as a pathway
Plants for planting are not a pathway, unless such plants are bearing fruits (unlikely).
Pathways are presented in Table 3. The main pathway is fruit.
Table 3.
Potential pathways for Zaprionus indianus into the EU 27
| Pathways | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) ) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Fruit | Eggs, larvae, pupae | A phytosanitary certificate is required to import fresh fruits into the EU (2019/2072, Annex XI, Part A) unless exempt by being listed in 2019/2072 Annex XI, Part C. A few Z. indianus fruit hosts (Musa L., dates) are in Annex XI, Part C; hence, their introduction does not require a phytosanitary certificate. However, no specific requirements are set for Z. indianus. As not all, but only a proportion of imported consignments are liable to be physically inspected, this requirement does not preclude the entry of Z. indianus in fruit. |
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As of 04 October 2021, there was one record of interception of Z. indianus in Austria on Prunus persica fruits from Egypt (in 2018) and two records of an outbreak in Cyprus on Ficus carica plants (in 2016 and 2017) in the Europhyt and TRACES databases.
Z. indianus has been intercepted in England and Wales on ripe guava, mango, cherry, blueberry and Indian jujube fruit (often damaged or infested by tephritid larvae), imported most frequently from India, Iran and Sri Lanka. Zaprionus sp. has been reared from damaged apple imported from Brazil (species not confirmed as only female adults emerged).
EU 27 statistics showing imports of fresh produce hosts for which Z. indianus is a primary pest from areas where the pest is present are shown in Table 4.
Table 4.
Annual EU 27 imports of selected* hosts of Zaprionus indianus from Africa, Asia, Canada, USA and Latin America 2016–2020 (hundreds of kg) (Eurostat – Accessed 21 October 2021)
| Commodity | HS Code | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Guavas 1 | 0804 5000 | 2,410,060 | 2,691,703 | 3,090,655 | 3,193,710 | 3,378,588 |
| Figs | 0804 2010 | 13,489 | 14,648 | 16,364 | 15,977 | 17,802 |
| Strawberries | 0810 1000 | 223,612 | 286,323 | 261,453 | 301,723 | 283,847 |
Guavas, mangoes.
Selected based on the fact that Z. indianus is a primary pest.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes. Given the known invasive nature of Z. indianus, it appears that the pest can transfer to new hosts when introduced to new areas. Indeed, as Z. indianus has established in some parts of the EU (see Section 3.2.2), it could probably establish in most of the warmer southern EU MSs; Scandinavian and Baltic EU MSs are mostly unsuitable for establishment.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker et al., 2000, Baker, 2002). Availability of hosts is considered in Section 3.4.2.1. Climatic factors are considered in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
The harvested area in the EU 27 between 2016 and 2020 of the hosts directly damaged by the pest (figs and strawberries) is shown in Tables 5 and 6. Appendix B provides an extensive list of hosts and plants affected.
Table 5.
Harvested area of figs in EU 27, 2016–2020 (thousand ha). (Source: Eurostat, Code: F2100 X 1,000 ha) (Accessed on 20/10/21)
| MS/Year | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| EU 27 | 23.74 | 24.63 | 24.99 | 25.59 | 27.21 |
| Spain | 12.61 | 13.56 | 13.98 | 14.60 | 15.72 |
| Portugal | 4.10 | 4.13 | 4.13 | 3.81 | 3.81 |
| Greece | 3.79 | 3.82 | 3.77 | 3.99 | 4.40 |
| Italy | 2.39 | 2.26 | 2.23 | 2.15 | 2.06 |
| France | 0.38 | 0.40 | 0.44 | 0.44 | 0.44 |
| Croatia | 0.35 | 0.27 | 0.27 | 0.42 | 0.57 |
| Cyprus | 0.10 | 0.16 | 0.14 | 0.16 | 0.17 |
| Slovenia | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 |
| Bulgaria | 0 | 0 | 0 | 0.01 | 0.03 |
Table 6.
Harvested area of strawberries in EU 27, 2016–2020 (thousand ha). (Source: Eurostat, Code: S0000) (Accessed on 20/10/21)
| MS/Year | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| EU 27 | 103.78 | 103.76 | 106.42 | 100.93 | 84.14 |
| Poland | 50.78 | 49.84 | 49.18 | 49.90 | 33.20 |
| Germany | 14.30 | 14.16 | 14.00 | 13.20 | 12.86 |
| Spain | 6.87 | 6.82 | 7.03 | 7.26 | 7.35 |
| Finland | 6.30 | 6.89 | 10.16 | 4.40 | 4.44 |
| Italy | 4.88 | 4.86 | 4.72 | 4.74 | 4.62 |
| France | 3.34 | 3.37 | 3.35 | 3.35 | 3.33 |
| Romania | 2.72 | 3.25 | 3.27 | 3.30 | 3.29 |
| Sweden | 2.01 | 1.97 | 2.07 | 1.96 | 2.08 |
| Belgium | 1.90 | 1.98 | 1.97 | 1.97 | 1.60 |
| Netherlands | 1.72 | 1.69 | 1.62 | 1.64 | 1.52 |
| Greece | 1.49 | 1.47 | 1.47 | 1.61 | 1.72 |
| Denmark | 1.17 | 1.16 | 1.15 | 1.11 | 1.07 |
| Austria | 1.14 | 1.14 | 1.21 | 1.19 | 1.18 |
| Hungary | 0.79 | 0.79 | 0.73 | 0.73 | 0.88 |
| Lithuania | 0.78 | 0.84 | 0.83 | 0.88 | 0.94 |
| Czechia | 0.71 | 0.69 | 0.71 | 0.68 | 0.46 |
| Bulgaria | 0.68 | 0.66 | 0.73 | 0.71 | 0.74 |
| Latvia | 0.50 | 0.50 | 0.50 | 0.49 | 0.50 |
| Estonia | 0.44 | 0.53 | 0.62 | 0.63 | 0.66 |
| Portugal | 0.39 | 0.31 | 0.32 | 0.32 | 0.81 |
| Croatia | 0.37 | 0.37 | 0.25 | 0.25 | 0.30 |
| Ireland | 0.19 | 0.19 | 0.19 | 0.18 | 0.18 |
| Slovakia | 0.17 | 0.12 | 0.17 | 0.27 | 0.21 |
| Slovenia | 0.11 | 0.11 | 0.12 | 0.11 | 0.14 |
| Cyprus | 0.04 | 0.06 | 0.05 | 0.05 | 0.05 |
| Luxembourg | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
3.4.2.2. Climatic conditions affecting establishment
Z. indianus has great adaptability to a range of environmental conditions with the ability to establish in different ecological niches in new areas of invasion, with different temperature regimes (da Mata et al., 2010). This makes this species a fully invasive one (Lavagnino et al., 2020). Several morphological traits have shown variation with latitude and elevation, suggesting genetic adaptation to different environmental conditions (Karan et al., 2000).
Z. indianus is known to occur in countries where BSh (hot semi‐arid), BSk (cold semi‐arid) Cfa (humid subtropical), Cfb (oceanic), Cfc (oceanic‐subpolar), Csa (hot‐summer Mediterranean), Csb (warm‐summer Mediterranean) and Csc (cold‐summer Mediterranean) Köppen‐Geiger climatic zones (Kottek et al., 2006) occur, these climate types also occur in the EU (Figure 3). We assume, based on literature data that the subarctic climate, though, is not suitable for the development of this pest. As a consequence, climatic conditions would not limit the ability of Z. indianus to establish in the EU, with Scandinavian and Baltic EU MSs being mostly unsuitable and warmer southern MSs mostly suitable.
Figure 3.
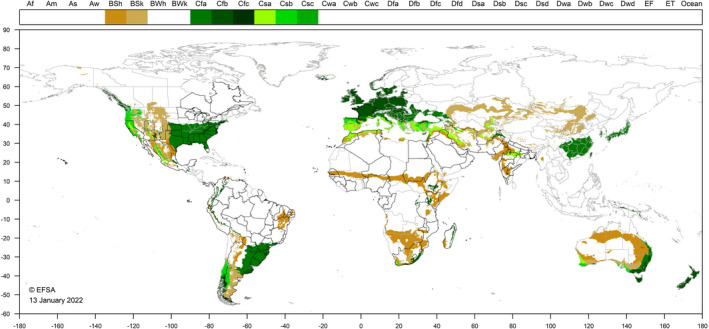
Occurrence of BSh, BSk, Cfa, Cfb, Cfc, Csa, Csb and Csc Köppen‐Geiger climate types in the world
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
Z. indianus adults are able to fly, and long‐distance dispersal is possible on air currents as well as with trade. Indeed, transportation of commercial infested fruits may greatly contribute to spread.
Comment on plants for planting as a mechanism of spread
Plants for planting are not a pathway for spread, unless such plants were bearing fruits (unlikely).
Z. indianus is known to be a highly vagile fly, capable of spreading from Brazil to the USA in less than 10 years (van der Linde et al., 2006). Data on esterase loci polymorphisms in Brazilian populations show that Z. indianus spread throughout the country, probably together with the transportation of commercial fruits by way of the two main Brazilian freeways (Galego and Carareto, 2010; Yassin et al., 2009). Aided by international trade and commerce, Z. indianus has been introduced to a wide variety of localities outside of its native range including North and South America, Europe and Asia (Westphal et al., 2008; Hulme, 2009).
Studies have been carried out in Brazil to better understand the invasion history of Z. indianus. It is hypothesised that it first arrived in Sao Paulo state with air transport of fruit from Africa. It then further spreads within the country by natural means and more importantly by road transportation of commercial fruit (EPPO, online).
Wind‐assisted long‐distance dispersal has been demonstrated in a closely related species, Drosophila melanogaster (Leitch et al., 2021).
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes. Although no specific report of damage by Z. indianus in the EU is known, considering the damage caused to figs in South America, the species establishment and spread could thus have significant economic consequences in Europe where about 60,000 tons of figs are produced per year.
The capacity of Z. indianus to damage crops directly has been observed on figs, which is an important crop around the Mediterranean Basin. Its interactions with other drosophilids or true fruit flies (Tephritidae, like Ceratitis capitata (Wiedemann)) might enhance the negative impacts of the latter on cultivated fruit crops (EPPO, online). In Brazil, Z. indianus was responsible for 50% of fig losses because it can feed on this fruit while it is still on the tree (Dettler et al., 2021). Heavy losses were observed on figs in Jordan, but they were not quantified.
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impact?
Yes, a phytosanitary certificate is required to import fresh fruits and nuts into the EU (see Sections 3.3.2 and 3.4.2). Fruits could be further sourced from areas free of Z. indianus (see Section 3.6.1).
3.6.1. Identification of potential additional measures
Phytosanitary measures are currently applied to fruits. Therefore, this entry pathway can be considered as open and regulated.
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 7.
Table 7.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Control measure/Risk reduction option (Blue underline = Zenodo doc) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Used to mitigate likelihood of infestation by specified pest at origin, hence, to mitigate entry | Entry/Spread |
| Biological control and behavioural manipulation |
pest control such as: a) Biological control Various hymenopteran parasitoids have been documented parasitising Z. indianus in Brazil, though with low levels of parasitisation (2–4%) (Marchiori et al., 2003, Marchiori and Silva, 2003, Silva et al., 2004). Bacterial isolates are considered promising for the formulation of toxic baits (Geisler et al., 2019). b) Mass trapping Several different baits have been tested to attract Z. indianus (Epsky et al., 2014, 2015; Renkema et al., 2018), as well as different traps (Pasini and Link, 2011; Renkema et al., 2018; Lasa et al., 2020). |
Entry/Spread/Impact |
| Chemical treatments on crops including reproductive material | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. Organophosphates, pyrethroids and spinosyns have been effective when applied by foliar spraying. A possible alternative to foliar spraying is the use of toxic baits or low‐volume, reduced‐risk sprays in conjunction with feeding attractants (Andreazza et al., 2016). | Entry/Establishment/Spread/Impact |
| Chemical treatments on consignments or during processing |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage. Possible treatments are:
Used to mitigate likelihood of infestation of pests susceptible to chemical treatments |
Entry/Spread |
| Physical treatments on consignments or during processing |
Used to mitigate likelihood of infestation of pests susceptible to physical treatments Washing, brushing and other mechanical cleaning methods can be used to reduce the prevalence of the pest in the consignments to be exported or to be planted. |
Entry/Spread |
| Waste management | Care of disposal of contaminated fruit may be necessary through waste management, (e.g. deep burial, composting) in authorised facilities and official restriction on the movement of waste. | Entry/Establishment |
| Heat and cold treatments | Several fresh fruit commodities can be managed using hot water treatments, others such as guava, could undergo cold treatment (Lin et al., 2020). | Entry/Spread |
|
Specific requirements for mode and timing of transport of commodities to prevent escape of the pest and/or contamination.
Used to mitigate likelihood of entry of pests that could otherwise infest material post‐production |
Entry/Spread | |
| Controlled atmosphere |
Treatment of plants by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure). Used to mitigate likelihood of infestation of pests susceptible to modified atmosphere (usually applied during transport) hence to mitigate entry. Controlled atmosphere storage can be used in commodities such as fresh and dried fruits, flowers and vegetables. |
Entry/Spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 8.
Table 8.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Supporting measure (Blue underline = Zenodo doc) | RRO Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5). The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques. Several different baits have been tested to attract Z. indianus (Epsky et al., 2014, 2015; Renkema et al., 2018), as well as different traps (Pasini and Link, 2011; Renkema et al., 2018; Lasa et al., 2020). Used to mitigate likelihood of infestation by specified pest at origin |
Entry/Spread |
|
Phytosanitary certificate and plant passport |
An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) a) export certificate (import) b) plant passport (EU internal trade) Used to attest which of the above requirements have been applied |
Entry |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
The species develops only inside fruits. Therefore, it might be difficult to detect and identify unless fruit are cut open.
The species is difficult to identify and could be confused with other non‐regulated fruit flies
Wide range of potential hosts (high volume to inspect)
3.7. Uncertainty
The main uncertainties refer to (1) the lack of information about potential additional hosts that can be attacked without previous injury; (2) whether the capacity to infest strawberries will be maintained under field conditions; (3) extending host range which could include new plant species in newly colonised areas. These uncertainties, though, do not affect the conclusion of this categorisation.
4. Conclusions
Z. indianus satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. Table 9 shows the summary of the PLH Panel conclusions.
Table 9.
The Panel’s conclusions on the pest categorisation criteria derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel’s conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest ( Section 3.1 ) | The identity of Z. indianus has been established. | None |
| Absence/presence of the pest in the EU ( Section 3.2 ) | Z. indianus is present in the EU but not widely distributed in Cyprus, Malta, Portugal and Spain. | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | Z. indianus could enter into, establish in, and spread within the EU territory. The main pathway is host fruit. | None |
| Potential for consequences in the EU ( Section 3.5 ) | Although no specific report of damage by Z. indianus in the EU is known, it can contribute to damage caused by other pests and directly harm figs. | None |
| Available measures ( Section 3.6 ) | There are measures available to prevent the likelihood of entry into the EU (i.e. import of fruit and nuts is subject to certification). | None |
|
Conclusion ( Section 4 ) |
Z. indianus satisfies all of the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. | |
|
Aspects of assessment to focus on/scenarios to address in future if appropriate: |
||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018)
- Degree day
Degree days (DD) are a measurement of heat units over time, often calculated from the average daily temperature above a threshold. For example, above a threshold temperature of 10oC, a 24‐hour period with an average temperature of 16oC would represent 6 DD
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy and Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2018)
Appendix A – Zaprionus indianus host plants/species affected (fruit)
Source: EPPO Global Database (EPPO, online) and CABI Crop Protection Compendium (CABI CPC, online) [Accessed on 20 October 2021].
| Host status | Host name | Plant family | Common name | Reference |
|---|---|---|---|---|
| Cultivated host | Actinidia chinensis | Actinidiaceae | Chinese gooseberry, golden kiwifruit | EPPO |
| Aleurites moluccanus | Euphorbiaceae | Candle nut, Indian walnut | EPPO | |
| Anacardium occidentale | Anacardiaceae | Cashew, cashew apple | EPPO | |
| Annona glabra | Annonaceae | Pond apple, alligator apple | EPPO | |
| Averrhoa carambola | Oxalidaceae | Star fruit, caramba | EPPO | |
| Butia capitata | Arecaceae | Jelly palm, butia palm | CABI CPC | |
| Campomanesia aromatica | Myrtaceae | Strawberry guava, wild guava | EPPO | |
| Capsicum frutescens | Solanaceae | Chilli, bird chilli | EPPO | |
| Carissa macrocarpa | Apocynaceae | Carissa, natal palm | EPPO | |
| Citrus | Rutaceae | – | EPPO | |
| Citrus sinensis | Rutaceae | Sweet orange | EPPO | |
| Dimocarpus longan | Sapindaceae | Dragon's eye, longan | EPPO | |
| Diospyros kaki | Ebenaceae | Chinese date plum, Chinese persimmon | EPPO | |
| Eriobotrya japonica | Rosaceae |
Japanese medlar, loquat |
EPPO | |
| Fragaria x ananassa | Rosaceae | Strawberry | EPPO | |
| Ficus carica | Moraceae | Common fig | EPPO | |
| Genipa americana | Rubiaceae | Genip, marmelade box | EPPO | |
| Malpighia glabra | Malpighiaceae | Barbados cherry | EPPO | |
| Mangifera indica | Anacardiaceae | Mango | EPPO | |
| Musa | Musaceae | Banana | EPPO | |
| Myrciaria cauliflora | Myrtaceae | Jaboticaba, Brazilian grape | CABI CPC | |
| Olea europaea | Oleaceae | Common olive, olive | EPPO | |
| Olea europaea subsp. europaea | Oleaceae | European olive | CABI CPC | |
| Persea americana | Lauraceae | Avocado | EPPO | |
| Phoenix dactylifera | Arecaceae | Date‐palm, common date palm | EPPO | |
| Prunus armeniaca | Rosaceae | Apricot | EPPO | |
| Prunus cerasus | Rosaceae | Sour cherry, amarello cherry | EPPO | |
| Prunus persica | Rosaceae | Peach | EPPO | |
| Prunus persica var. nucipersica | Rosaceae | Nectarine | EPPO | |
| Psidium guajava | Myrtaceae | Yellow guava, guava | EPPO | |
| Punica granatum | Lythraceae | Pomegranate | EPPO | |
| Rubus idaeus | Rosaceae | European red raspberry | EPPO | |
| Solanum lycopersicum | Solanaceae | Tomato | EPPO | |
| Spondias tuberosa | Anacardiaceae | Imbu | EPPO | |
| Syzygium jambos | Myrtaceae | Malabar plum | EPPO | |
| Vaccinium | Ericaceae | – | EPPO | |
| Vitis vinifera | Vitaceae | Grape vine | EPPO | |
| Ziziphus jujuba | Rhamnaceae | Chinese date, common jujube | EPPO | |
| Ziziphus spina‐christi | Rhamnaceae | Christ's thorn jujube | EPPO |
Appendix B – Distribution of Zaprionus indianus
Distribution records based on EPPO Global Database (EPPO, online).
| Region | Country | Subnational (e.g. State) | Status |
|---|---|---|---|
| Africa | Algeria | Present, restricted distribution | |
| Benin | Present, no details | ||
| Cameroon | Present, no details | ||
| Cape Verde | Present, no details | ||
| Comoros | Present, no details | ||
| Congo | Present, no details | ||
| Cote d'Ivoire | Present, no details | ||
| Egypt | Present, no details | ||
| Gabon | Present, no details | ||
| Guinea | Present, no details | ||
| Kenya | Present, no details | ||
| Madagascar | Present, widespread | ||
| Malawi | Present, no details | ||
| Mauritius | Present, no details | ||
| Mayotte | Present, no details | ||
| Morocco | Present, no details | ||
| Mozambique | Present, no details | ||
| Niger | Present, no details | ||
| Nigeria | Present, no details | ||
| Reunion | Present, no details | ||
| Saint Helena | Present, no details | ||
| Sao Tome & Principe | Present, no details | ||
| Senegal | Present, no details | ||
| Seychelles | Present, no details | ||
| South Africa | Present, no details | ||
| Sudan | Present, no details | ||
| Tanzania | Present, no details | ||
| Tunisia | Present, restricted distribution | ||
|
America |
Argentina | Present, no details | |
|
Brazil |
Present, no details | ||
| Amazonas | Present, no details | ||
| Bahia | Present, no details | ||
| Ceara | Present, no details | ||
| Distrito Federal | Present, no details | ||
| Goias | Present, no details | ||
| Maranhao | Present, no details | ||
| Mato Grosso | Present, no details | ||
| Mato Grosso do Sul | Present, no details | ||
| Minas Gerais | Present, no details | ||
| Para | Present, no details | ||
| Paraiba | Present, no details | ||
| Parana | Present, no details | ||
| Pernambuco | Present, no details | ||
| Rio de Janeiro | Present, no details | ||
| Rio Grande do Norte | Present, no details | ||
| Rio Grande do Sul | Present, no details | ||
| Rondonia | Present, no details | ||
| Santa Catarina | Present, no details | ||
| Sao Paulo | Present, no details | ||
| Tocantins | Present, no details | ||
|
Canada |
Present, few occurrences | ||
| Ontario | Present, few occurrences | ||
| Québec | Present, few occurrences | ||
| Cayman Islands | Present, no details | ||
| Colombia | Present, widespread | ||
| Dominican Republic | Absent, unreliable record | ||
| Ecuador | Present, no details | ||
| French Guiana | Present, no details | ||
| Martinique | Present, no details | ||
| Mexico | Present, no details | ||
| Panama | Present, no details | ||
| Paraguay | Present, no details | ||
| Peru | Present, no details | ||
|
United States of America |
Present, restricted distribution | ||
| Alabama | Present, no details | ||
| Arizona | Present, no details | ||
| California | Present, no details | ||
| Connecticut | Present, no details | ||
| Florida | Present, no details | ||
| Georgia | Present, no details | ||
| Hawaii | Present, widespread | ||
| Kansas | Present, no details | ||
| Louisiana | Present, no details | ||
| Michigan | Present, no details | ||
| Minnesota | Present, few occurrences | ||
| Mississippi | Present, no details | ||
| United States of America | New York | Present, no details | |
| United States of America | North Carolina | Present, no details | |
| United States of America | Oklahoma | Present, no details | |
| United States of America | Pennsylvania | Present, no details | |
| United States of America | South Carolina | Present, no details | |
| United States of America | Texas | Present, no details | |
| United States of America | Virginia | Present, no details | |
| United States of America | Wisconsin | Present, no details | |
| Uruguay | Present, no details | ||
| Venezuela | Present, no details | ||
|
Asia |
Bangladesh | Present, restricted distribution | |
|
India |
Present, no details | ||
| Andhra Pradesh | Present, no details | ||
| Chandigarh | Present, no details | ||
| Delhi | Present, no details | ||
| Haryana | Present, no details | ||
| Jharkand | Present, no details | ||
| Karnataka | Present, no details | ||
| Kerala | Present, no details | ||
| Madhya Pradesh | Present, no details | ||
| Maharashtra | Present, no details | ||
| Uttarakhand | Present, no details | ||
| Uttar Pradesh | Present, no details | ||
| Iran | Present, no details | ||
| Iraq | Present, no details | ||
| Israel | Present, no details | ||
| Jordan | Present, no details | ||
| Lebanon | Present, no details | ||
| Nepal | Present, no details | ||
| Oman | Present, no details | ||
| Pakistan | Present, no details | ||
| Saudi Arabia | Present, no details | ||
| Turkey | (Özbek Çatal et al., 2019) | ||
| United Arab Emirates | Present, no details | ||
|
Europe |
Austria | Absent, unreliable record | |
| Cyprus | Present, restricted distribution | ||
| France | Absent, pest no longer present | ||
| Italy | Absent, unreliable record | ||
| Malta | Present, no details | ||
|
Portugal |
Madeira |
Present, restricted distribution Present, no details |
|
|
Spain |
Canary Islands |
Present, restricted distribution (Andalusia) Present, no details |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Grégoire J‐C, Malumphy C, Kertesz V, Maiorano A and MacLeod A, 2022. Pest categorisation of Zaprionus indianus . EFSA Journal 2022;20(3):7144, 24 pp. 10.2903/j.efsa.2022.7144
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00380
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to acknowledge the contribution of Caterina Campese and Oresteia Sfyra to this opinion.
Figure 1: © Fera Science Ltd, UK
Adopted: 27 January 2022
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger & Roy, 2015; EPPO, 2019).
References
- Alawamleh A, Katbeh‐Bader A, Hassan N, Al‐Jboory I and D'Onghia AM, 2016. Biological studies on the African fig fly, Zaprionus indianus Gupta (Diptera: Drosophilidae). Poljoprivreda I Sumarstvo, 62, 65. [Google Scholar]
- Amoudi MA, Diab FM and Abou‐Fannah SS, 1991. Zaprionus indiana (Diptera: Drosophilidae) in Saudi Arabia and the effect of temperature on the life cycle. Journal of King Saud University, 3, 111–121. [Google Scholar]
- Andreazza F, Haddi K, Oliveira EE and Ferreira JAM, 2016. Drosophila suzukii (Diptera: Drosophilidae) arrives at Minas Gerais State, a main strawberry production region in Brazil. Florida Entomologist, 99, 796–798. [Google Scholar]
- Baker RH, Sansford CE, Jarvis CH, Cannon RJ, MacLeod A, and Walters KF, 2000. The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agriculture, Ecosystems & Environment, 82, 57–71. [Google Scholar]
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In: Hallman GJ and Schwalbe CP (eds.). Invasive Arthropods in Agriculture: Problems and Solutions. Science Publishers Inc, Enfield, USA. pp. 207–241.
- Bernardi D, Andreazza F, Botton M, Baronio CA and Nava DE, 2017. Susceptibility and interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in damaging strawberry. Neotropical Entomology, 46, 1–7. [DOI] [PubMed] [Google Scholar]
- Commar LS, Galego LGDC, Ceron CR and Carareto CMA, 2012. Taxonomic and evolutionary analysis of Zaprionus indianus and its colonization of Palearctic and Neotropical regions. Genetics and Molecular Biology, 35, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho‐Silva RD, Montes MA, Oliveira GF, de Carvalho‐Neto FG, Rohde C and Garcia ACL, 2017. Effects of seasonality on drosophilids (Insecta, Diptera) in the northern part of the Atlantic Forest, Brazil. Bulletin of Entomological Research, 107, 634–644. [DOI] [PubMed] [Google Scholar]
- Da Mata RA, Tidon R, Côrtes LG, De Marco P and Diniz‐Filho JAF, 2010. Invasive and flexible: niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biological Invasions, 12, 1231–1241. [Google Scholar]
- David JR, Araripe LO, Bitner‐Mathe BC, Capy P, Goni B, Klaczko LB, Legout H, Martins MB, Vouidibio J, Yassin A and Moreteau B, 2006. Sexual dimorphism of body size and sternopleural bristle number: a comparison of geographic populations of an invasive cosmopolitan drosophilid. Genetica, 128, 109–122. [DOI] [PubMed] [Google Scholar]
- Dettler MA, Barrientos GN, Martinez E, Ansa MA, Santadino MV, Coviella CE and Virgala MBR, 2021. Nivel de infestación a campo de Zaprionus indianus Gupta y Drosophila suzukii (Matsumura)(Diptera: Drosophilidae) en Ficus carica L.(Rosales: Moraceae) y Rubus idaeus L.(Rosales: Rosaceae) en el noreste de la provincia de Buenos Aires. Revista de la Sociedad Entomológica Argentina, 80, 43–47.
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Grégoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertész V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martınez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int
- EPPO (European and Mediterranean Plant Protection Organization) , 2019. EPPO codes. Available online: https://www.eppo.int/resources/eppo_databases/eppo_codes
- Epsky ND, Gill MA, Cha DH and Landolt PJ, 2014. Trapping the African fig fly (Diptera: Drosophilidae) with combinations of vinegar and wine. Florida Entomologist, 97, 85–89. [Google Scholar]
- Epsky ND, Gill MA and Mangan RL, 2015. Grape juice as a bait for Anastrepha suspensa (Diptera: Tephritidae) and Zaprionus indianus (Diptera: Drosophilidae). Journal of Economic Entomology, 108, 2065–2073. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) , 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/
- Fartyal RS, Sarswat M, Lhamo N, Sati PC and Asha L, 2014. Records of Zaprionus indianus and Drosophila suzukii indicus as invasive fruit pests from mid valley region of Garhwal Uttarakhand, India. Drosophila Information Service, 97, 119–123. [Google Scholar]
- Galego LGDC and Carareto CMA, 2010. Scenario of the spread of the invasive species Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) in Brazil. Genetics and Molecular Biology, 33, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FCS, Cunha NDS, Martins LN, Oliveira DDC, Stupp P, de Oliveira IG, Leite FPL, Garcia FRM and Bernardi D, 2019. Toxicity of Bacterial Isolates on Adults of Zaprionus indianus (Diptera: Drosophilidae) and Parasitoids Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). Journal of Economic Entomology, 112, 2817–2823. [DOI] [PubMed] [Google Scholar]
- Gottschalk MS, Bizzo L, Döge JS, Profes MS, Hofmann PR and Valente VL, 2009. Drosophilidae (Diptera) associated to fungi: differential use of resources in anthropic and Atlantic Rain Forest areas. Iheringia. Série Zoologia, 99, 442–448. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Hulme PE, 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. Journal of Applied Ecology, 46, 10–18. [Google Scholar]
- Joshi NK, Biddinger DJ, Demchak K and Deppen A, 2014. First report of Zaprionus indianus (Diptera: Drosophilidae) in commercial fruits and vegetables in Pennsylvania. Journal of Insect Science, 14, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan D, Dubey S, Moreteau B, Parkash R and David JR, 2000. Geographical clines for quantitative traits in natural populations of a tropical drosophilid: Zaprionus indianus . Genetica, 108, 91–100. [DOI] [PubMed] [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of the Köppen_Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. 10.1127/0941-2948/2006/0130 [DOI] [Google Scholar]
- Kremmer L, David J, Borowiec N, Thaon M, Ris N, Poirie M and Gatti JL, 2017. The African fig fly Zaprionus indianus: a new invasive pest in France. Bulletin of Insectology, 70, 57–62. [Google Scholar]
- Lachaise D and Tsacas L, 1983. Breeding sites in tropical African drosophilids. pp. 221–332. In: Ashburner M, Carson HL and Thompson JN (eds.). The Genetics and Biology of Drosophila. London, Academic Press. 382 pp.
- Lasa R, Gschaedler‐Mathis AC, Bello G and Williams T, 2020. Laboratory evaluation of trap color and vinegar, yeast and fruit juice lure combinations for monitoring of Zaprionus indianus (Diptera: Drosophilidae). International Journal of Pest Management, 66, 279–287. [Google Scholar]
- Lasa R and Tadeo E, 2015. Invasive drosophilid pests Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Veracruz. Mexico. Florida Entomologist, 98, 987–988. [Google Scholar]
- Lavagnino NJ, Imberti M, Ortiz VE, Flaibani N and Fanara JJ, 2020. Contrasting levels of genotype by environment interaction for life history and morphological traits in invasive populations of Zaprionus indianus (Diptera: Drosophilidae). Insect Science, 27, 1090–1100. [DOI] [PubMed] [Google Scholar]
- Leão BFD and Tldon R, 2004. January. Newly invading species exploiting native host‐plants: the case of the African Zaprionus indianus (Gupta) in the Brazilian Cerrado (Diptera, Drosophilidae). Annales de la Société entomologique de France (Vol. 40, No. 3–4, pp. 285–290). Taylor & Francis Group. [Google Scholar]
- Leitch KJ, Ponce FV, Dickson WB, van Breugel F and Dickinson MH, 2021. The long‐distance flight behavior of Drosophila supports an agent‐based model for wind‐assisted dispersal in insects. Proceedings of the National Academy of Sciences, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KW, Lin HL, Shiesh CC, Hsu YL, Lin CH, Chen SC and Yeh WB, 2020. Cold treatment for guava fruits infested with oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Applied Entomology and Zoology, 55, 37–44. [Google Scholar]
- van der Linde K, 2010. Zaprionus indianus: species identification and taxonomic position. Drosophila Information Service, 93, 95. [Google Scholar]
- van der Linde K, Steck GJ, Hibbard K, Birdsley JS, Alonso LM and Houle D, 2006. First records of Zaprionus indianus (Diptera: Drosophilidae), a pest species on commercial fruits from Panama and the United States of America. Florida Entomologist, 89, 402–404. [Google Scholar]
- Loh R, David JR, Debat V and Bitner‐Mathé BC, 2008. Adaptation to different climates results in divergent phenotypic plasticity of wing size and shape in an invasive drosophilid. Journal of Genetics, 87, 209. [DOI] [PubMed] [Google Scholar]
- Marchiori CH, Rodrigues Caldas E and Almeida GSK, 2003. Parasitoids collected from artificial bovine dung pats exposed for different periods of time in Itumbiara, Goiás, Brazil. Acta Sci. Biol. Sci., pp. 9–13.
- Marchiori CH and Silva CG, 2003. First occurrence of parasitoid Spalangia endius (Walker) (Hymenoptera: Pteromalidae) in pupae of Zaprionus indianus Gupta (Diptera: Drosophilidae) in Brazil. Brazilian Journal of Biology, 63, 361–362. [DOI] [PubMed] [Google Scholar]
- Nava DE, Nascimento AM, Stein CP, Haddad ML, Bento JM and Parra JR, 2007. Biology, thermal requirements, and estimation of the number of generations of Zaprionus indianus (Diptera: Drosophilidae) for the main fig producing regions of Brazil. Florida Entomologist, 90, 495–501. [Google Scholar]
- Pasini MPB and Link D, 2011. Efficiency of different traps to capture Zaprionus indianus (Diptera: Drosophilidae) in fig orchard in Santa Maria county, Rio Grande do Sul state, Brazil. International Research Journal of Agricultural Science and Soil Science, 1, 349–354. [Google Scholar]
- Pfeiffer DG, Shrader ME, Wahls JC, Willbrand BN, Sandum I, van der Linde K, Laub CA, Mays RS and Day ER, 2019. African fig fly (Diptera: Drosophilidae): biology, expansion of geographic range, and its potential status as a soft fruit pest. Journal of Integrated Pest Management, 10, 20. [Google Scholar]
- Renkema JM, Iglesias LE, Bonneau P and Liburd OE, 2018. Trapping system comparisons for and factors affecting populations of Drosophila suzukii and Zaprionus indianus in winter‐grown strawberry. Pest Management Science, 74, 2076–2088. [DOI] [PubMed] [Google Scholar]
- Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD and Karsch‐Mizrachi I, 2020. Genbank. Nucleic Acids Research, 48, Database issue, 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed]
- Setta ND and Carareto C, 2005. Fitness components of a recently‐established population of Zaprionus indianus (Diptera, Drosophilidae) in Brazil. Iheringia. Série Zoologia, 95, 47–51. [Google Scholar]
- Silva JC, Loreto EL and Clark JB, 2004. Factors that affect the horizontal transfer of transposable elements. Current Issues in Molecular Biology, 6 , 57–72. [PubMed] [Google Scholar]
- Toy SJ and Newfield MJ, 2010. The accidental introduction of invasive animals as hitchhikers through inanimate pathways: a New Zealand perspective. Revue Scientifique Et Technique (International Office of Epizootics), 29, 123–133. [DOI] [PubMed] [Google Scholar]
- Tsacas L, 1985. Zaprionus indianus Gupta, 1970 nouveau nom pour le plus commun des Zaprionus africains (Diptera, Drosophilidae). In Annales de la Société entomologique de France (Vol. 21, No. 3, pp. 343–344).
- Van Timmeren S and Isaacs R, 2014. Drosophila suzukii in Michigan vineyards, and the first report of Zaprionus indianus from this region. Journal of Applied Entomology, 138, 519–527. [Google Scholar]
- Vilela CR, Teixeira EP and Stein CP, 1999. Nova praga nos figos: Zaprionus indianus Gupta, 1970. Informativo Da Sociedade Entomológica do Brasil, 24, 2. [Google Scholar]
- Vilela CR, Teixeira EP and Stein CP, 2001. Mosca‐africana‐do‐figo, Zaprionus indianus (Diptera: Drosophilidae), Histórico e impacto das pragas introduzidas no Brasil. Holos, Ribeirao Preto, Brazil. pp. 48–52. [Google Scholar]
- Westphal MI, Browne M, MacKinnon K and Noble I, 2008. The link between international trade and the global distribution of invasive alien species. Biological Invasions, 10, 391–398. [Google Scholar]
- Yassin A, Borai F, Capy P, David JR, Elias E, Riad SA, Shalaby HG, Serour S and Abou‐Youssef AY, 2009. Evolutionary Genetics of Zaprionus. II. Mitochondrial DNA and chromosomal variation of the invasive drosophilid Zaprionus indianus in Egypt: full‐length research article. Mitochondrial DNA, 20, 34–40. [DOI] [PubMed] [Google Scholar]
- Yassin A, Capy P, Madi‐ravazzi L, Ogereau D and David JR, 2008. DNA barcode discovers two cryptic species and two geographical radiations in the invasive drosophilid Zaprionus indianus . Molecular Ecology Resources, 8, 491–501. [DOI] [PubMed] [Google Scholar]
- Yassin A and David JR, 2010. Revision of the Afrotropical species of Zaprionus (Diptera, Drosophilidae), with descriptions of two new species and notes on internal reproductive structures and immature stages. ZooKeys, 51, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]


