Abstract
Poly(ethylene glycol) or PEG has a long history of use in medicine. Many conventional formulations utilize PEG as either an active ingredient or an excipient. PEG found its use in biotechnology therapeutics as a tool to slow down drug clearance and shield protein therapeutics from undesirable immunogenicity. Nanotechnology field applies PEG to create stealth drug carriers with prolonged circulation time and decreased recognition and clearance by the mononuclear phagocytic cells (MPS). Most nanomedicines approved for clinical use and experimental nanotherapeutics contain PEG. Among the most recent successful examples are two mRNA-based COVID-19 vaccines that are delivered by PEGylated lipid nanoparticles. The breadth of PEG use in a wide variety of over the counter (OTC) medications as well as in drug products and vaccines stimulated research which uncovered that PEG is not as immunologically inert as it was initially expected. Herein, we review the current understanding of PEG’s immunological properties and discuss them in the context of synthesis, biodistribution, safety, efficacy, and characterization of PEGylated nanomedicines. We also review the current knowledge about immunological compatibility of other polymers that are being actively investigated as PEG alternatives.
Graphical Abstract
1. Introduction
1.1. Chemical structure and varieties of PEG
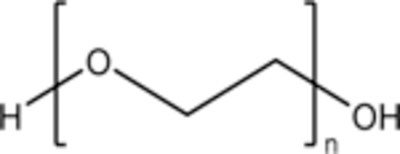
Polyethylene glycol (PEG) is produced from polymerization of ethylene oxide, ethylene glycol, or diethylene glycol in the presence of alkaline catalysts and the reaction is ended by neutralizing the catalyst when polymer reaches the desired molecular weight [1, 2]. The chemical structure of PEG is HO-[CH2-CH2-O]n-H, where n is the number of ethylene oxide units and the molecular weight is calculated by (44 g/mol)*n. PEG is hydrophilic and each ethylene glycol subunit surrounded by 2–3 water molecules [3, 4]. PEG is soluble in water, methanol, ethanol, acetonitrile, glycerin, glycols, benzene, and dichloromethane, and this property makes PEG useful in many formulations and products [1]. PEG can have different geometries, including linear, tube, branched, star, and comb [5]. Modifications can be made to PEG to allow for further customization. A methyl ether cap (mPEG) can be added to PEG to prevent hydrogen bonding at the cap end, which can restrict nonspecific interactions with proteins and with other PEG chains [2]. Some PEGylated nanomaterials will have many opportunities for multiple hydrogen bonds; a methoxy-PEG will have significantly less risk of non-covalent crosslinking in such an environment.
PEG is also very flexible and exhibits high chain mobility that results in a large number of polymer chain conformations, and a reduction in chain conformational freedom is thermodynamically unfavorable [6–8]. PEG can be used to form a shell around micelles [9]. PEG shells have a hydration sheath that sterically prevents biomacromolecules from penetrating the polymer layer and binds to the core by hydrophobic or electrostatic interactions [10–12]. PEG is a common product in drug formulations, pharmacological and food products [1, 2, 13, 14]. PEG units used in drug formulations and consumables generally range between molecular weights of 200–60,000 Da [2, 13, 15]. The molecular weight of PEG used in nanomedicines such as Doxil, and in recent lipid-nanoparticles mRNA-based COVID-19 vaccines is 2000 [16]; that of nanomedicine CYT6091 is 20,000 [17]. Conjugation of the end hydroxyl group on PEG to reactive groups on compounds to make formulations and larger PEG-matrices is also a common use for PEG, in the form of PEGylated nanoparticles or medicine that allows for improved circulation, sustained release, improved efficacy and/or dissolution of drug, or production of artificial PEG-environment [18–21]. Stealth characteristics of PEG require large amounts of PEG coating and may depend on the type nanoparticle to which it is conjugation (i.e., more for metals or polymers) [22, 23].
1.2. Hydrophilic and stealth properties of PEG
One of the early examples of using PEG to modify biomolecules include the conjugation of PEG-1900 and bovine albumin that resulted in changes in the protein’s physical and chemical properties, including greater solubility in a wider pH range, from 1–12; prevented interaction of ion exchangers with the protein, and allowed the PEGylated-albumin to remain in circulation longer than unconjugated albumin [24]. Intravenous (i.v.) and intramuscular (i.m.) injection of unconjugated albumin resulted in antibody production whereas reduced antibody levels were observed with PEGylated albumin [24]. This study suggested that PEGylation masked antigenic sites on albumin thereby inhibiting their recognition and an immune response.
Conjugation of PEG to a nanoparticle surface reduces opsonization (the binding of plasma proteins) and stalls clearance of PEGylated nanoparticles by the mononuclear phagocyte system [25–27]. As such, PEGylated nanoparticles have longer circulation time than their unPEGylated counterparts; this property contributes to an improved efficacy of the PEGylated nanoformulations through the increased circulation time and sustained drug release. For example, chitosan-nanoparticles formulated with longer PEG chain (2000 or 5000 molecular weight) or greater PEG surface density (43% and 75%) showed more sustainable drug release than control formulations [28, 29]. In another study, an increase of mPEG surface density on chitosan nanoparticles also increased cumulative drug release rates and prolonged circulation half-life [26].
1.3. Protective properties of PEG
PEG found several applications in organ transplantation and ischemia reperfusion areas. Some examples of PEG’s protective properties are reviewed in this section. High molecular weight PEG is used in organ preservation to provide protection and as a restoring agent that prevents apoptosis [30, 31]. Some studies suggested that PEG can direct tolerogenic action of donor antigen delivered with a transplanted organ[32]. PEG is also used in blood banks as potentiator that enhances agglutination for the detection of antibodies and antigens in patients’ blood[33].
Hepatocyte ischemia reperfusion injury (IRI) is inherent to the liver surgical procedures and transplantation. In this case, pretreatment with PEG is considered for organ protection during surgery planning. PEG with molecular weight 35 kD has also been shown to protect heart against reperfusion injury and steatotic liver against cold IRI[34]. Protective effects of PEG are dose dependent. For example, PEG-35kD at the 10 mg/kg dose reduced liver injury biomarkers, reduced elevated mitochondrial depolarization, caspase 3 and caspase 9 activity, and prevented decrease of F-actin/G-actin ratio, whereas 2 mg/kg did not [35]. Endothelial cells (EC) are more insulated with PEG treatment because PEG induces potent endothelial barrier enhancement, increases resistance compared to cells without PEG. Addition of PEG also decreased permeability of FITC-dextran from cells compared to cells without PEG for up to 24 hours [35]. Wu et al. showed that PEG attaches to the cell’s membrane and does not wash off presumably limiting cell’s permeability and increasing the resistance to opportunistic pathogen Pseudomonas aeruginosa. In contrast, removing PEG from media normalized the resistance[35]. PEG pretreatment was shown to protect against ionomycin-induced barrier disruption but only for a short time, suggesting that PEG does not form a barrier around EC cells. Addition of PEG after the barrier disruption led to faster restoration of barrier function with thrombin [35]. Staining for F-actin showed F-actin bundling and ruffling of lamellipodia at periphery of EC cells after PEG treatment, which correlates with barrier enhancement [35].
The addition of PEG for 5 minutes to a wound site was able to seal neuronal membranes and reduce biomarkers for oxidative stress, including decreased lipid peroxidation and protein carbonyl content as well as increase of glutathione levels, following acute spinal cord injury in guinea pigs at an injury site and surrounding area[36]. A second study by the same group showed that the addition of PEG-2000 to an injury site reduced calcium levels and higher mitochondrial function compared to the vehicle treatment group. They showed PEG-FITC is able to diffuse into cytoplasm of injured cells where it is presumably able to gather around organelles; the addition of PEG also reduced mitochondrial permeability and prevented apoptotic events like mitochondrial swelling[37].
1.4. PEG in consumer product, pharmaceutical and biological applications
PEG has been formulated into a variety of consumer products including foods, cosmetics, ointments, and medicine. PEG use in consumer goods is varied from surfactants to dispensing agent, solvents, and excipients. The use of PEG with a mean molecular weight 200–9,500 is FDA-approved for human consumption[38] and previous reviews have shown that PEG, with molecular weight of 200–10,000, in cosmetics and pharmaceuticals at the most commonly used concentrations and formulations is considered safe [13, 39]. Commonly, PEG is used for pharmaceutical and biomedical applications in the form of pill binders, tablet surface coating, parenteral liquid preparations, lubricants, ultrasound gel, ointment base, suppositories and organ preservatives[40]. An earlier study adding PEG 8000 to rat diet resulted in reduction of tumor instances after exposure to carcinogen azoxymethane. Both 2% and 5% PEG significantly decreased number of tumorogenic events, with almost none found in 5% PEG feed after 42 days of PEG-diet[41]. PEG at high concentrations can be used as laxative, though cases of renal insufficiency and toxicity have been reported[42, 43].
PEG is known to be a fusogen, i.e., an agent that is able to fuse cells by the cell membranes. The fusogenic activity is not known mechanistically but thought to be due to the PEG hydrophilic nature and may be due to dehydrating cell membranes to allow protein and lipid structural elements to resolve into each other and eliminating membrane breaches. PEG is a known membrane surfactant and may encourage membrane resealing by reducing membrane surface tension and enhancing membrane fluidity [44]. This property found a use of PEG in the hybridoma formation step of monoclonal antibody production technology[45].
The fusogenic activity of PEG is also used in vitro to improve a detection of some cellular organelles. For example, mitochondria go through continuous cycles of fusion and fission. They can vary from extensive interconnected networks to separate small populations, depending on cell’s energy need and nutrients available. The only direct measure of fusion and fission is the PEG fusion assay, which measures content mixing between separately labeled fluorescent mitochondria in two cells whose plasma membranes have been fused through addition of PEG [46]. However, when the number of fusion events is low, searching manually for events can add bias. Mitochondria fusion in this assay is assessed by applying different fluorescent labels to mitochondria and observing colocalization of the fluorescent signal; the negative control is double knockout and shows approximately ~1 % of colocalization whereas the positive control shows ~96% colocalization. PEG addition improves fusion rates and shows greater dye colocalization in HEK cells.
1.5. PEG in nanomedicine
PEG is the most common component of nanomedicines, largely due to its stealth properties discussed above in section 1.2. Herein, we review some examples of PEGylated nanomedicines and their improved performance in comparison to unformulated drugs or products formulated using unPEGylated carriers.
Anticancer drug, doxorubicin, conjugated to PEGylated nanotubes decreased tumor size in mouse. Release was measured over 42 days, with most being released over the first seven days and much lower following days[5]. The authors of this study concluded that PEG-nanotubes can release effectively over 5 days and that only 85–90% of doxorubicin were released. In vitro PEG-nanotubes carrier was not cytotoxic to MCF-7 and MDA-MB-231 cells whereas doxorubicin loaded PEG-nanotubes decreased cell viability; reduced tumor size was observed in vivo when doxorubicin loaded PEG-nanotubes were injected intratumorally and, to the lesser extent, after i.v. injection[5]. PEGylated mesoporous silica and polysilsesquioxane nanoparticles were shown to successfully deliver nucleic acids, functional nucleic acid nanoparticles, and small molecules to various cancer cells [47–49].
PEGylated chitosan nanoparticles are a promising carrier that allows for improved systemic circulation time and reduced uptake by macrophage, and improved solubility for drugs, proteins and genes[26, 50]. Formulation of chitosan with mPEG increased particle solubility in water[50]. Methotrexate (MTX), a commonly used drug for cancer treatments, by itself is not soluble in water and has poor permeability. Spherical mPEG-chitosan-MTX drugs with various mPEG MW (750, 2000, 5000) resulted in release of MTX at 0.5h point and sustained for 12h in vitro. Larger molecular weight mPEG resulted in reduced macrophage uptake.
Lipoplexes (liposome & DNA complexes) are more likely to be adsorbed into cell membrane by 100x with addition of 2–6% of PEG to transfection media [51]. Aggregation potential is molecular weight dependent, 8000–10000 most effective, below-not absorbed, above adsorbed into membrane and inhibit particle aggregation [51]. Lipofection rates increased with the addition of PEG when cationic lipid:plasmid ratio was 1:2 vs 2:1; increased concentration of PEG, however, led to increased toxicity from greater lipoplex uptake[51]. Addition of PEG also resulted in an increase in the lipoplex size following 2 hours of incubation.
PEG-PLA particles include a hydrophobic polylactide core for drugs and hydrophilic PEG shell for solubility, stabilization, and better circulation. Smaller size (10–100nm) of these particles allows for passive accumulation through leaky vasculature to the tumor site through enhanced permeability and retention effect[52]. PEG-PLA particles have been used in conjunction with photodynamic therapeutics, immune prep to stabilize immune system to treat illness using vaccine adjuvants that modulate immune response or the delivery system, proteins (cytokines and antibodies), nucleic acids (e.g., plasmid DNA, antisense oligonucleotides, siRNA). Despite many advantages, adverse neuro side effects were reported with some PEG-PLA nanoparticles; therefore, more safety studies are needed.
Liposomes with PEG of molecular weight of 2000 are considered gold standard for drug delivery [53]. Stefanick et al. found in vitro liposome uptake was higher when using a peptide-targeting liposome consisting of PEG350 with a shorter ethylene glycol peptide linker compared to a liposome consisting of PEG2000 with a longer peptide linker. In addition, the uptake plateaued around 2–3% peptide density. Their findings revealed that the linker length and peptide density, in addition to the PEG length of the liposome can contribute to drug uptake [54]. However, these in vitro findings need verification using in vivo models.
PEG is used in implanted hydrogels to improve their tunability, biocompatibility and non-fouling surface as well as in engineered tissue matrices [20, 55, 56]. Macromolecular PEG hydrogels inserted into the rodent body show signs of inflammation, cellular infiltration and recruitment of macrophages. However, immune responses to such hydrogels vary depending on composition and the type of PEG[20, 57]. For example, PEG-vinyl sulfone (PEG-VS) formulation hydrogel shows less immune response compared to PEG-acrylate (PEG-Ac) and PEG-maleimide (PEG-Mal) formulations [20]. Findings from semi-interpenetrating network of PEG and PEG-diacrylate with human mesenchymal stem cells showed intracellular deposition of proteoglycan and collagen around the plug[18, 58], suggesting cellular interaction of embedded cells with artificial extracellular matrix. Interestingly, Day et al., found instances of angiogenesis around inserted PEG-formulated plug-in tissue[20], further suggesting biological response with respect to PEG beyond immune response. Day et al., found increased blood vessels around implanted PEG hydrogel[20]. This finding raised to important questions: 1) does PEG component or the entire formulation influence angiogenesis? and 2) how do other host cells respond to PEG? For example, in one study presence of cell lining and infiltration of M1 and M2 macrophages was observed with PEG-Mal and PEG-Ac hydrogels. In contrast, PEG-VS showed little immune response with thinner collagen deposition and cell lining[20].
More recently, PEGylated nanoparticles and formulated compounds are becoming more common to circumvent issues of blood brain barrier permeability. In addition to treating central nervous system at injury site, a single dose of PEG (30% wt/wt) by intravenous injection is also able to mitigate the reduction of apparent diffusion coefficient associated with cytotoxic edema in the brain following acceleration induced brain injury in rats. This suggests that PEGylation may allow passage of compounds normally unable to pass through the blood brain barrier. Recent study with curcumin showed formulation with PEG polylactide-co-glycolide (PLGA) was better at reducing reactive oxygen species and apoptosis than curcumin itself following cerebral ischemia reperfusion in rats; a larger dose of curcumin was needed to achieve a comparable efficacy as free curcumin showed poor biodistribution to the brain [59].
1.6. PEGylated materials approved for clinical use
Many formulations approved for clinical use utilize PEG as either active ingredient or excipient or a component of a delivery vehicle. Some examples are summarized in Table 1 and reviewed below. Only immune-mediated and immune-related side-effects that have been either attributed to PEG or hypothesized to be related to PEG are mentioned; other toxicities and limitations of these products are not discussed.
Table 1. Examples of PEG-containing products approved for clinical use.
The table summarizes some examples of products in various areas including over the counter (OTC) medications, vaccines, macromolecular drugs (therapeutic recombinant proteins, nucleic acids, amino acids), and nanomedicines. Information about these products is reproduced from individual product package inserts found at https://www.accessdata.fda.gov/. mPEG = methoxyPEG; COVID-19 = coronavirus induced disease-19; SARS = severe acute respiratory syndrome; * - only immunotoxicities to which PEG may contribute are listed in the table; mechanisms are not always understood; anti-PEG antibodies and related hypersensitivity reactions were either confirmed for some of these products or suspected; non-PEG related immunotoxicities are not included; N = PEG is the active pharmaceutical ingredient (API); RP = recombinant protein API; SM= small molecule API; NP = nanoparticles; MW = molecular weight; AA = amino acid; s.c. = subcutaneous; i.m. = intramuscular. Some drugs contain multiple units of PEG and are indicated by the number of units x PEG type, for example 9xPEG-20000 refers to units of PEG-20000.
| Product | Indication | PEGylated entity | How supplied | Type of PEG-MW | Immunotoxicity* | |
|---|---|---|---|---|---|---|
| Moviprep | Laxative; preparation for colonoscopy | N | Powder for solution | PEG-3350 | Hypersensitivity | |
| Miralax | OTC laxative | N | Powder for solution | PEG-3350 | Hypersensitivity | |
| Movantik | Opioid-induced constipation | SM | Film-coated tablets | PEG-339 | Hypersensitivity | |
| Asclera | Varicose veins | SM | 0.5–1% solution | PEG-600 | Anaphylaxis | |
| Macugen | Macular degeneration | Aptamer | Solution for intravitreal injection | 2 x mPEG-20000 | Hypersensitivity | |
| Palynziq | Phenylketonuria | RP | Solution for subcutaneous injection | ~9 x mPEG-20000 | Hypersensitivity; anaphylaxis; complement consumption; immunogenicity | |
| Fulphila | Chemotherapy-associated infections | RP | Solution for injection | PEG-20000 | Anaphylaxis | |
| Revcovi | ADA-SCID | RP | Solution for i.m. injection | PEG-80000 | Immunogenicity | |
| Esperoct | Hemophilia A | RP | Lyophilized powder for reconstitution before injection | PEG-40000 | Anaphylaxis and anaphylactoid reactions | |
| Ziextenzo | Chemotherapy-associated infections | RP | Solution for injection | PEG-20000 | Hypersensitivity | |
| Pegintron | Hepatitis C, melanoma | RP | Lyophilized powder for reconstitution before injection | PEG-40000 | Hypersensitivity | |
| Pegasys | Hepatitis B and C | RP | Solution for s.c. injection | PEG-12000 | Hypersensitivity | |
| Krystexxa | Gout | RP | Concentrated solution for infusion following a dilution | 40 x PEG-10000 | Infusion reactions; anaphylaxis | |
| Jivi | Hemophilia A | RP | Lyophilized powder for reconstitution before injection | 2 x PEG-30000 | Hypersensitivity; immune response to PEG in children under the age of 6 | |
| Cimzia | Rheumatoid arthritis | RP | Lyophilized powder for reconstitution before s.c. injection | PEG-40000 | Anaphylaxis | |
| Doxil | Cancer | NP | Liposomal doxorubicin for infusion | mPEG-2000 | Hypersensitivity | |
| Onivyde | Cancer | NP | Liposomal Irinotecan for injfusion | mPEG-2000 | Hypersensitivity | |
| COVID-19 mRNA vaccine Pfizer | Prevention of COVID-19 | NP | PEGylated lipid nanoparticles for i.m. injection to deliver mRNA encoding SARS-CoV2 antigen (S protein) | PEG-2000 | Hypersensitivity; Anaphylaxis | |
| COVID-19 mRNA vaccine Moderna | Prevention of COVID-19 | NP | PEGylated lipid nanoparticles for i.m. injection to deliver mRNA encoding SARS-CoV2 antigen (S protein) | mPEG-2000 | Hypersensitivity; Anaphylaxis | |
Traditional formulations – over the counter Miralax and prescription Moviprep – are solutions of PEG3350 that are laxatives used for occasional relief of constipation and colon evacuation in preparation for colonoscopy, respectively. Side effects, among others, include vomiting, nausea, and headache. Hypersensitivity reactions to these formulations were attributed to their active ingredient PEG-3350 [60–63].
Macugen (pegaptanib) is a PEGylated oligonucleotide, an aptamer that acts as selective antagonist of the vascular endothelial growth factor (VEGF) [64]. This product is intended for intravitreal administration in patients with macular degeneration. Allergic reactions were reported in patients receiving this formulation [65].
Asclera (polidocanol) is intended for the treatment of varicose veins; it contains two components dodecyl alcohol and PEG. Injection site reactions reported for this formulation include thrombosis, hematoma, pruritis and warmth[66]. Severe anaphylaxis has also been reported [66]. Another small molecular drug formulation Movantik (naloxegol) is a PEGylated version of an opioid antagonist naloxone; it is provided in a tablet form, which also contains PEG as an excipient in the tablet coating. Anaphylaxis and hypersensitivity were reported among immunological side effects for this formulation [67].
Palinziq is a PEGylated phenylalanine metabolizing enzyme intended for the treatment of phenylketonuria; anaphylaxis and other hypersensitivity reactions were reported for this product[68]. Another biotechnology product, PEGylated interferon alfa-2a (Pegasys) is approved for treating hepatitis C in the US, EU and China and used worldwide[69]. It is also approved for hepatitis B in US, EU and China, and used for some T-cell lymphoma. This product contains 40 kg/mol of branched PEG. Among others, side effects include trouble breathing and irregular heartbeat[70]. PEGylated interferon alfa-2b (PegIntron) is used for hepatitis C and melanoma. Side effects include headache, nausea, pain at injection site, and fever, severe side effects include blood clots and irregular heartbeat. Approved for use in the US in 2001, it activates JAK-STAT pathway by binding to interferon-alpha receptor 1 and 2, followed by phosphorylation of Tyk2 protein. The product also activates IFNalpha response to induce apoptosis[71].
Doxil is a PEGylated liposome formulated to deliver anti-cancer drug doxorubicin, that is approved for therapy of ovarian cancer, multiple myeloma, and AIDS-related Kaposi’s sarcoma (https://www.rxlist.com/doxil-drug.htm#indications). One of the benefits of this liposomal formulation in comparison to doxorubicin hydrochloride is the reduction in the drug’s cardiotoxicity[72], that is attributed to the doxorubicin-mediated induction of inflammatory cytokines in cardiomyocytes[73]. Among undesirable side effects is the palmar plantar erythrodysesthesia, also known as hand and foot syndrome, that is an immune-mediated inflammatory reaction in the skin due to the liposomal drug accumulation by the skin-resident phagocytes, Langerhans cells[74].
Another PEGylated cancer nanomedicine, liposomal irinotecan or Onyvide, is approved for cancer therapy and has several advantages over the traditional formulation of irinotecan such as improved pharmacokinetics [75]. Clinical use of this formulation is associated with severe neutropenia and neutropenic sepsis; it may cause severe hypersensitivity reactions, including anaphylaxis [76].
Two lipid-based nanoparticles delivering mRNA encoding SARS-CoV2 antigen and authorized for the emergency use in the US and several other countries, Pfizer/BioNTec and Moderna, contain PEG and methoxy-PEG, respectively [77]. Anaphylaxis, myocarditis and multisystem inflammatory syndrome have been reported for these formulations; hypersensitivity to PEG were suggested among several potential mechanisms underlying these conditions [78–87].
1.7. PEG degradation and clearance
Despite a decrease in nanoparticles recognition by immune cells through PEGylation and a related to it extension of the circulation time, eventually PEGylated products breakdown and get removed by phagocytic cells. PEG is excreted through urine and feces mainly unchanged; part of the administered PEG is metabolized to oligomers, glycolic acid, hydroxyglycolic acids, diglycolic acids homologs, carbon dioxide and very few oxalic acid [13]. Metabolic pathways of PEG have been extensively discussed elsewhere [88]. The percent of PEG eliminated by degradation to carbon dioxide was shown to decrease with larger PEG size [89, 90]. Alcohol dehydrogenase was shown to initiate oxidation of PEG-6[91]. PEG can be degraded by sonication and degradation products may be toxic, where sonication for just 15 minutes may cause degradation of PEGylated particles[92]. Generally, biodegradation occurs through hydrolytic and proteolytic activities, as well as metabolic pathways that involve oxidative modification or degradation of molecules[93]. Unlike some other biomaterials, PEG is prone to (per)oxidation that can degrade its polymer chain[94–98], but not much is known about such degradation mechanism in biological setting. Surface tethered PEG to the cell membrane of cultured neurons showed rapid degradation after day 25 [96]. In vitro scaffolding consisting of polymers including PEG and poly(ε-caprolactone) was found to show greater rate of degradation in highly oxidative environments [98].
Reactive oxygen species (ROS), including those produced by the activated immune cells, contribute to biodegradability of synthetic biomaterial polymers. PEG was shown to degrade in the presence of hydrogen peroxide, with the degradation depending on the hydrogen peroxide concentration; the lower the concentration, the slower the degradation[93]. PEG may form hydroperoxides in oxygenated environment [99] and other byproducts such as formate esters and formaldehyde, which may be further oxidized to carboxylates following hepatic excretion [100]. Moreover, PEG formulated biomaterial induced exogenous and intracellular ROS production and can trigger apoptotic pathways[97]. Based on the fact that neutrophils produce more ROS than macrophages, Ulbricht et al., hypothesized that neutrophils are primarily responsible for degradation of polymers[93].
Cytoplasmic vacuolation of cells in various tissues has been observed in many in vivo studies utilizing repeated administration of PEG, PEG-containing and PEGylated drug products [101]. In general, such vacuolation depended on PEG molecular weight (more pronounced in low molecular weight PEG) and physical properties of the molecules (more pronounced in liner PEG), was not associated with adverse effects and was reversible[101]. Interestingly, PEG’s molecular weight also influenced the type of cells in which vacuolation was observed; specifically, larger PEGs, with molecular weight more than 30 kD, most frequently resulted in vacuolation of cells in parenchymal organs, whereas low molecular weight PEGs, 15–20 kD, did so in the renal tubular epithelium [102–104]. It is likely, that such difference is explained by the route and rate of clearance with smaller PEGs staying in the circulation for the shorter time and being more readily cleared by kidneys versus larger PEGs circulating longer and, thus, allowing more time for being captured by the phagocytic cells of parenchymal organs. Phagocytic cells, such as macrophages are commonly reported for vacuolation and described in the literature as “foamy macrophages”, “histiocytic macrophages” and “vacuolated macrophages”. Interestingly, some studies suggested inter-species differences in this process[103, 105]. For example, in rats vacuolation is primarily seen in the spleen whereas in dogs it is in the kidneys[105]. While it is unknown how such vacuolation, when observed in preclinical animal studies, is predictive of vacuolation in humans, it is worth mentioning because it suggests a potential inter-species variability in the PEG clearance by phagocytes.
PEGylation of nanoparticles decreases opsonization in a manner dependent on PEG molecular weight and density [106, 107]. However, PEGylation does not completely block protein binding. For example, a study with colloidal gold nanoparticles demonstrated that the quantity of plasma proteins bound to particle’s surface was less on PEGylated gold nanoparticles than on their non-PEGylated counterparts; however, the composition of the protein corona was essentially the same [106]. This explains why even PEGylated nanomaterials eventually end up in the phagocytic cells. Accelerated blood clearance of PEGylated proteins and liposomal formulations were attributed to the formation of antibodies specific to PEG [108–110], and will be discussed in further details later in this review.
1.8. Additional safety considerations and introduction to immunotoxicity
Acceptable World Health Organization (WHO) daily maximum PEG intake is 10 mg/kg. Generally, the LD50 for PEG is very high. Oral ingestion LD50 in rodents is 2000 mg/kg, though LD50 of PEG with fatty acids, soritan esters and PEG castor oils are between 1000–2000 mg/kg. Dermal uptake LD50 was around 10,000mg/kg, however, some systemic toxicity effects were reported related to PEG in burn victims given topical antimicrobial ointment [13, 111].
While earlier studies have shown that PEG is safe, more recent studies have shown signs of toxicity and activation of immune response in animal models and human subjects [39, 112]. For example, repeated i.v. injection of PEG-400 at high dose (8.45 g/kg) caused reversible renal toxicity, while a lower dose (6.34 g/kg) resulted in dry mouth and dry mucous membrane in animals [13, 113]. Gastrointestinal discomfort, histological damage, oxidative stress, increased free radical levels in colonic mucosal tissues and neutrophils infiltration of lamina propria have been observed in rats given PEG solution by oral gavage [114]. These findings led the authors to suggest a more careful use of PEG and PEGylated drugs and increasing the awareness of physicians about toxicities associated with the PEG use.
Having a better understanding of the immunological effects of PEG and PEGylated compounds is essential for several reasons. First, phagocytic cells of the immune system are at the forefront of clearance of PEG and PEGylated materials; therefore, toxicity to these cells may influence body’s general defense against infections and damaged or transformed host’s cells. Second, generation of the specific immune response to PEG in the form of antibodies contributes to hypersensitivity reactions (HSRs) to PEG and PEGylated products. Such HSRs include true allergy (IgE mediated, type I hypersensitivity), anaphylactoid reactions (complement-mediated immediate type hypersensitivity or complement-mediated pseudoallergy, CARPA), type II and type III hypersensitivity (IgM and IgG-mediated) reactions [115]. Third, neutralization and cross-reactivity of such antibodies may contribute to HSRs and altered PK of other products containing PEG or other structures similar to PEG.
The antibody-mediated immune reactions to PEGylated nanomedicines range from local inflammation to accelerated clearance to more severe reactions such as CARPA [115]. However, it is important to note that the presence of anti-PEG IgM and IgG per se is not predictive of the antibody-mediated toxicity. For example, a recent in vitro study revealed that the presence of anti-PEG antibodies in donors’ blood did not correlate with complement activation in response to PEGylated liposomes [112]. Therefore, the detection of such antibodies is helpful to identify patients at the increased risk for anti-PEG antibody mediated toxicities, and for the mechanistic understanding of toxicities when they occur.
2. Synthesis of PEGylated products
2.1. General information
The length of PEG chains is defined by the degree of polymerization during its synthesis. Preparation via anionic polymerization relies on nucleophilic attack by an alkoxide on ethylene oxide (EO) to open the ring and add a EO unit to the initiating alkoxide[116]. The new alkoxide formed can continue the chain growth though the attack of an additional EO as a living process until a Poisson distribution is reached. The degree of polymerization determines the length and weight of the PEG chains, allowing for the preparation of varying lengths of polymer Additionally, the use of functional initiators can provide access to additional conjugation strategies such as an azide-alkyne click[117]. The synthetic tunability makes the resulting PEG derivatives ideal spacers for diverse biomedical applications [118].
Chemical synthesis of PEG is cost-effective and can be scaled up with a possibility for the incorporation of a broad range of chemical modifications needed for attachment of PEG molecules into various biomedical platforms. Additionally, several functional PEG derivatives are commercially available, expanding the options for labs with synthetic limitations or concerns around use of ethylene oxide. The flexibility of PEG chains allows for conjugation to various macromolecules without causing their denaturation [119–121] and thus, maintaining the intended biological reactivity and function [122–124]. The terminal hydroxyl groups of PEG is the basis for many different synthetic routes for attachment of PEG to the macromolecules or to surfaces [125]. Branched PEG derivatives are structured to have multi-armed PEG chains attached to a surface whereas linear PEG provides a single chain. Table 2 depicts structural differences between common linear and branched PEG molecules. Different functionalization of PEGs are chosen for their chemical reactivity, the handle through which they attach to reactive features of a macromolecule, or chosen by their added features that are used to exhibit specific characteristics.
Table 2.
Some representative structural differences between common linear and branched PEG molecules.
|
General structure of polyethylene glycol |
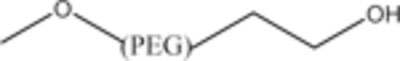
|
Methoxy Linear PEG (mPEG) |
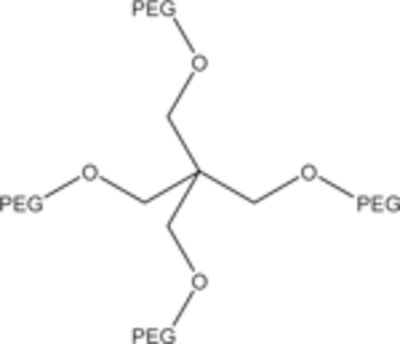
|
4-Arm branched PEG |
|
8-Arm branched PEG |
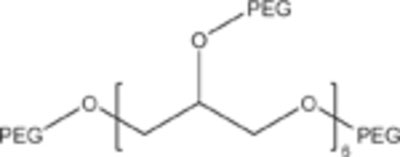
|
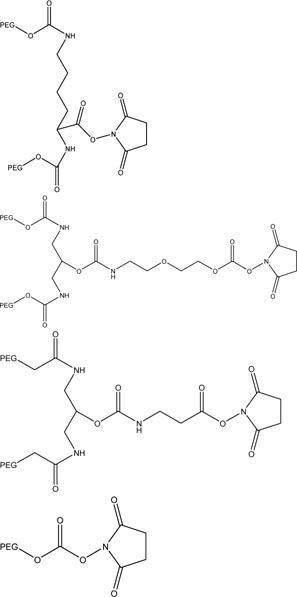
N-hydroxysuccinimide functionalized PEGs can be amine selective binding to specific regions of a protein. These PEGs can be used to conjugate to lysine residues of a protein[139]. |
|
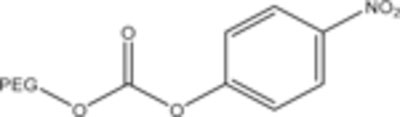
PEG-p-nitrophenyl carbonate bonds to amino groups of a protein. This is less reactive than hydroxysuccinimide and forms the same a carbamate linkage [333]. |
|
PEG aldehyde is used for conjugation to N-terminal α-amino groups of peptides and proteins [333]. |
|
PEG-o- pyridylsulfide is used to selectively bind to thiols [333]. |
|
PEG-chol readily binds to liposomes [147]. |
|
PEG hydrogen bonding with silane groups is used to adhere PEG to silica NPs |
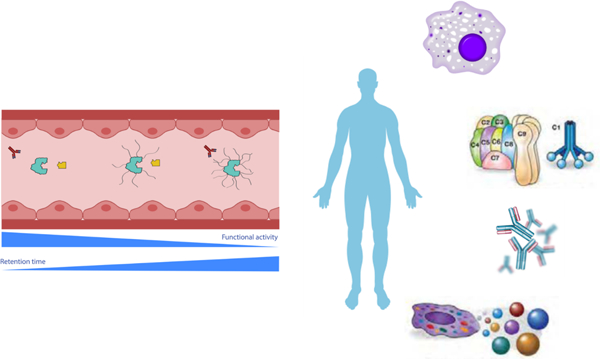
While linear PEG is known to better maintain the functionality of the bound macromolecule, the branched PEG becomes more efficient at stealth coating macromolecules and making them less vulnerable to opsonization, filtration, and other mechanisms of in vivo removal [125–131]. As schematically illustrated in Figure 1A, macromolecules decorated with branched PEG demonstrate lower clearance rates from blood circulation and when linear and branched PEG coated carbon nanotubes were injected in mice, the nanotubes with branched PEGylation exhibited significantly longer retention [132, 133]. Since the branched PEG is also larger in size when compared to their linear analogs it lowers the rates of kidney filtration and renal clearance while also providing a better protection for PEG-coated surfaces from reticuloendothelial action [134]. It has been shown that PEGs (up to molecular weight 20 kDa) is primarily excreted through the renal system, whereas higher molecular weight PEG chains are eliminated by fecal excretion [135].
Figure 1. Properties of poly(ethylene glycol) (PEG).
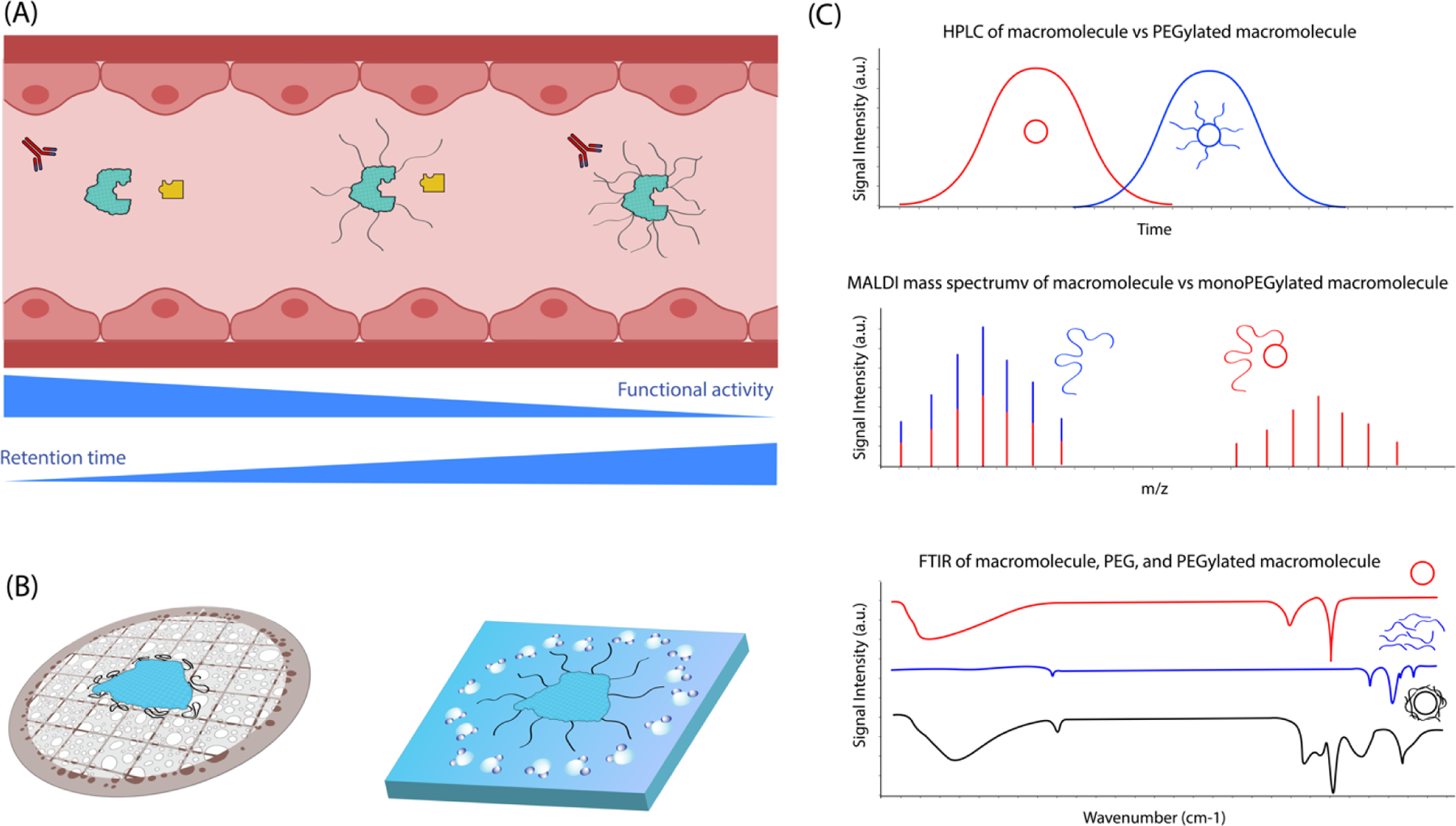
(A) PEG stealth coating reduces interaction with opsonic molecules but may affect the intended biological activities of coated macromolecules. (B) Morphology difference between dehydration and vitrification preparation methods for electron microscopy. (C) Schematic illustrations of some representative characterization data verifying the successful PEGylation of macromolecules.
2.2. PEGylated protein therapeutics
With the increasing interest in protein drug carriers, methods to prevent elimination of these structures from the body has also accrued interest [136]. PEGylation of proteins involves the covalent attachment between PEG and protein. PEG is commonly conjugated to proteins through the formation of an amide bond with lysine, although this strategy often results in the formation of heterogenous bioconjugates due to the possibility of reaction with numerous surface lysine groups [137]; in most cases PEG is linked to epsilon-amino groups of lysines [138]. A library of amine selective PEG functionalization strategies are known to selectively bind on engineered proteins where the location and number of PEG groups can be controlled [139]. The presence of PEG on the protein surface shields the latter from interaction with antibodies or other proteins involved with clearance of foreign proteins [140] and molecular simulations of PEGylated proteins (e.g., bovine serum albumin) show correlation between the molecular weight of used PEG and the shelf life of proteins [141]. Site specific PEGylation of proteins provides a vast control over molecular interactions occurring in vivo, in mice and rats models [139]. Additionally, site specific PEGylation minimizes the loss of function that is associated with the non-specific bioconjugation as is the case when the modification is made on a site responsible for interaction[142].
2.3. PEGylated drug delivery carriers
PEG chemistry is ideal for functionalization of inorganic nanomaterials as well. In preparation of PEGylated magnetic nanoparticles, oleic acid coated onto iron NP followed by addition of PEG to solution which assembles at room temperature [143]. Without chemical modification, PEG may also bind via hydrogen bonding by the polymer chain to surfaces such as silanol groups on silica[144, 145]. Oxidized graphene produced by Hummer’s method produces graphene nanoplatelets that readily bind to amphipathic PEG and chemotherapy drugs [146].
Encapsulation of insoluble drugs within liposomes has enabled the successful systemic delivery of various therapeutics despite their incompatibility with water [147–151]. Liposomes are nanosized particles that comprise of a lipid bilayer with hydrophilic outer surface to allow for solubility in water. An insoluble therapeutic cargo is loaded inside liposomes in order to be delivered to a specific site in the body. However, liposomes are quickly cleared from the body circulation through several different mechanisms. PEGylated liposomes have been highly successful in increasing the retention time of formulations within the body when compared to non-PEGylated analogs. This stealth coating considerably slows the in vivo clearance of the drug-loaded liposomes [152–158]. Lipids used for drug carrying liposomes are selected based on their ability to load the highest amount of drug while maintaining stability and retention time in circulation. Soy lecithin liposomes are a common choice for drug delivery systems for their high dispersion stability, good compatibility with insoluble payloads, and cost-effective production. However, they are more rapidly removed from circulation compared to liposomes of other lipid sources. PEG-cholesterol conjugates can be synthesized by a two-step reaction and used to PEGylate soy lecithin liposomes. This addition can greatly reduce the rate of elimination of lipid nanoparticles from the body [149].
3. Biodistribution of PEGylated products
Biodistribution of PEGylated materials was extensively studied with metallic nanoparticles. Herein, we will review some of literature examples. PEGylated gold nanoparticles (GNPs) following i.v. injection in rats were detectable in blood 48 hours after the injection; in contrast, unPEGylated, cetyl trimethyl ammonium bromide-GNP were quickly cleared and not detectable in blood after 15 minutes[159]. After the i.v. injection in rats, GNPs functionalized with greater molecular weight PEG stayed in circulation longer compared to their counterparts conjugated to the lower MW PEG [160]. In addition to prolonging circulation, PEGylation of GNPs also altered the time course but not the biodistribution when compared to untreated GNPs. Biodistribution panels revealed the i.v. injected GNPs with and without PEGylation accumulated mainly in spleen and liver in rats after 1 hour to 6 days [159–161]. Greater molecular weight PEG coating on GNPs also contributed to circulation and biodistribution differences; the majority of GNPs without PEGylation were found in liver and spleen, GNPs with PEG-coating of 750 Da showed significantly less GNPs, and GNPs with PEG-coating of 10 kDa showed even less gold in liver and spleen[160]. PEGylation of other nanoparticles showed similar biodistribution and circulation properties. PEGylated nanoparticles formulated to deliver methotrexate showed reduced cellular uptake in vitro and greater concentration in blood compared to free methotrexate when PEG molecular weight was 5000 vs. PEG 750 and PEG 2000. In addition, nanoparticles with greater surface density of mPEG were found in circulation and were not taken up by J774A macrophages in vitro, whereas lower surface density PEGylated nanoparticles were found in liver and spleen at 24 and 48h after i.v. injection [26]. In dogs, the analysis of PEG accumulation in organs by H-NMR revealed that amount of PEG in the renal cortex was 20 times higher than in the renal medulla and 120 times higher than in the spleen. This study also revealed a correlation between renal cortical cytoplasmic vacuoles and an increase in renal cortical PEG concentration, which was especially evident after repeated dosing. In the systemic circulation, assessed by measuring PEG concentration in plasma, PEG was detected on day 8; it was also found in urine on day 11, and peaked in renal tissues on day 15 [101, 105].
4. Immune Responses to PEG
4.1. Immunogenicity
One of the most remarkable phenomenon in the current knowledge of PEG immunogenicity is the generation of anti-PEG antibodies - IgM, IgG, IgE [61, 63, 108, 162–174]. Anti-PEG antibodies reduce circulation half-life of PEGylated drug product by 2–10-fold and increase hepatic and spleen uptake by 2–5 and 1–2-fold, respectively[175]. Besides accelerating the removal of PEGylated drugs and nanocarriers from blood, both in animal models and in clinical studies involving patients and healthy individuals, various types of anti-PEG antibodies were shown to contribute to the hypersensitivity reactions and premature drug release from PEGylated carriers [108, 162–174]. Due to consequences of such interactions for both the efficacy and safety of PEGylated drug products, a recent FDA guideline recommends screening for both anti-protein and anti-PEG antibodies[176].
Most of our current understanding of anti-PEG antibodies come from animal studies of PEGylated liposomes investigating the accelerated blood clearance (ABC) phenomenon. Studies in rabbits, mice, rats, and non-human primates revealed that PEG-antibody-dependent ABC phenomenon depends on many conditions and physicochemical properties of both PEG and PEGylated nanomaterials. For example, the available data demonstrated that cytotoxic drugs suppress [177, 178], whereas immunostimulatory payload, especially that are known to activate Toll-Like Receptors (TLRs) (e.g. siRNA, CpG DNA, endotoxin), enhances the ABC phenomenon [179–181]. Furthermore, it is independent of T-cells [177, 178], but requires functional antigen presenting cells [177, 182, 183]. Liposome composition, size, zeta potential, density of PEG coating, time interval between repeated administrations, dose and animal species further contribute to PEG-immunogenicity, and, related to it, ABC phenomenon [177, 182, 184–195]. Some studies demonstrated that anti-PEG antibody formed at low lipid dose (< 1µmol/kg), but not at the high dose [177, 185, 188]. In contrast, other studies reported antibody response after the injection of high (5–7, 10 and 25 µmol /kg) [183, 184, 190]. The most robust ABC was observed when the interval between the first and the subsequent doses was short (~1 week) [184, 196]. Longer (> 1 week) interval between the injections resulted either less pronounced clearance or did not influence it at all [190, 197]. Several studies demonstrated that role of particle size in the induction of anti-PEG antibody response. For example, administration of PEGylated liposomes (large particles) after PEGylated micelles (small particles) resulted in accelerated clearance of PEGylated liposomes [186]. Another study demonstrated the formation of anti-PEG antibodies in response to PEGylated liposomes with subsequent antibody mediated clearance of both PEGylated liposome and PEGylated micelles; interestingly, PEGylated micelles induced a low titer of anti-PEG antibody but the antibody did not influence the clearance of either PEG-liposomes or PEG-micelles [184]. The authors of this study suggested that anti-PEG antibody triggered by the administration of micelles are not capable of recognizing PEG on micelles, but they bind to the more defined epitope exposed on the larger surface of PEGylated liposomes [184]. Difference in methodologies between these studies may further explain the discrepancy in the observed results. Surface charge is another parameter that may contribute to the induction of the anti-PEG antibodies. For example, one study reported no difference in particle clearance when cationic, anionic and neutral liposomes were used in the initial dosing; the repeated administration of PEGylated, neutral liposomes, however, resulted in accelerated clearance [185]. Polymer terminal groups (e.g., thiol-, methoxy-, t-butoxy) are known to have different immunogenicity; for example, butoxy-PEG is more immunogenic than methoxy-PEG, which, in turn, is more immunogenic than hydroxy-PEG [198, 199]. It is therefore, expected that using PEG with different terminal groups may contribute to the differences in the anti-PEG antibody response to nanoparticles containing these polymers. Experience with PEGylated biotechnology products revealed that both PEG type and linkers used to attach PEG to a protein, or a nanoparticle surface contribute to PEG immunogenicity [199, 200]. While molecular weight of PEG did not influence clearance of PEGylated liposomes, an increase in the concentration of PEGylated lipid with accompanying increased density of the PEGylation attenuated accelerated clearance [188].
The number of studies reporting the presence of so-called natural or preexisting anti-PEG antibodies continues to increase [201–205]. Some studies demonstrated that these antibodies neutralize PEGylated therapeutic proteins [203, 204]. Others linked anti-PEG IgG and IgM to complement activation and anaphylactoid reactions along with the premature drug release and changes in drug’s efficacy [112, 163, 206–209]. Anti-PEG IgE were linked to the true allergy and anaphylaxis in response to PEG-based or PEGylated products [61, 63]. However, the titer of anti-PEG IgG and IgM does not directly correlate with complement activation by PEGylated liposomes [112]. While the clinical significance of anti-PEG antibodies is not completely understood, several studies suggested that screening for these antibodies may help to identify individuals who are at higher risk for developing anti-PEG antibody mediated toxicities, and suggested the usefulness of such tests for providing a mechanistic explanation of certain types of immunotoxicities such as anaphylaxis and anaphylactoid reactions should such toxicities occur [112, 210, 211].
How a hydrophilic molecule like PEG can induce an antibody-response? What is the source and the route of immunization that result in anti-PEG antibodies in the blood of healthy individuals? To answer these important questions, one has to look at the fundamental mechanisms of the antigen recognition by the immune system.
4.2. Immunological mechanisms
Two types of antigens have been described in the literature; they are thymus-dependent and thymus-independent, also known as TD (type 1) and TI (type 2), respectively (Figure 2 ). T-dependent antigens are typically proteins, that after the uptake by the antigen-presenting cells (particularly dendritic cells) are digested into peptides that are presented in the context with major histocompatibility complex type 2 to the T helper lymphocytes. The T helpers produce cytokines that improve antigen-presenting properties of APCs by upregulating the expression of co-stimulatory molecules and MHC on their surface; they also communicate in secondary lymphatic organs (spleen and lymph nodes) to follicular B cells, which, when activated, expand and differentiate into plasma cells producing antibodies specific to the peptide portions of the protein antigen. Over the course of the immune response to these antigens, several processes occur that lead to the antibody isotype switch, affinity maturation and formation of memory cells. As such, the antibodies to type 1 antigens are mature, highly specific and provide long-living protection. In contrast, many non-protein antigens (lipids, nucleic acids, polysaccharides, and other both natural and synthetic polymers) stimulate antibody response in the absence of T helpers, hence is the name for these antigens – thymus-independent. Type 2 antigens are not presented to T cells in the context of MHC. Typical property of these antigens is the multivalency characterized by the presence of repetitive structures which cross-link surface immunoglobulins on B cells leading to the B cell activation. The subsets of B cells responding to type 2 antigens are also different and include marginal zone B cells and B1 cells, which, following activation, differentiate into short-lived plasma cells producing IgM. Typical location of this response is the spleen (if the antigen is delivered systemically via blood), mucosal sites and peritoneal cavity (if the antigen is delivered locally), where macrophages produce supporting cytokines leading to, though less efficiently, the isotype switch towards non-IgM antibodies (IgG, and IgA). The isotype switch to type 2 antigens may be further directed by substances activating Toll-Like Receptors (TLRs) which trigger supporting cytokine responses in both macrophages and B lymphocytes. Natural or so-called pre-existing antibodies, which are detected in the blood of individuals without apparent exposure to pathogens, are often low-affinity and directed against phospholipids and carbohydrates. The available literature discussed in the previous section suggest that free PEG behaves as type 2 antigen. PEG conjugated to a protein carrier, such as recombinant therapeutic proteins and antibodies, behaves as a hapten and stimulates T helper responses with subsequent isotype switch and affinity maturation. The exposure of humans to PEG occurs during their first days of life via lotions, soaps, toothpaste, food packaging and, later, over-the-counter laxatives. Such exposure is responsible for the “hidden” immunization that explains the presence of PEG-specific antibodies in the blood of healthy individuals. Depending on the quantity (“dose”) of the PEG, the route of exposure and the surrounding microbiological environment that provides TLR agonists, these natural antibodies could be low or high affinity, mature or immature. Anti-PEG antibodies found in the blood of patients treated with PEGylated products further contributes to “immunization” of human population and the formation of anti-PEG IgM, IgG, IgE. It is currently unknown whether vaccination of millions of people around the globe with PEGylated lipid nanoparticles used in Pfizer/BioNtech and Moderna vaccines against SARS-CoV2 results in the induction of anti-PEG antibodies. While theoretical risk of induction of such antibodies exists, studies are needed to confirm it. If vaccines induce anti-PEG antibodies, it would be important to understand isotypes and allotypes of these antibodies, and their effects on safety and efficacy of PEGylated drug products and products containing PEG.
Figure 2. Types of antigens and their main characteristics.
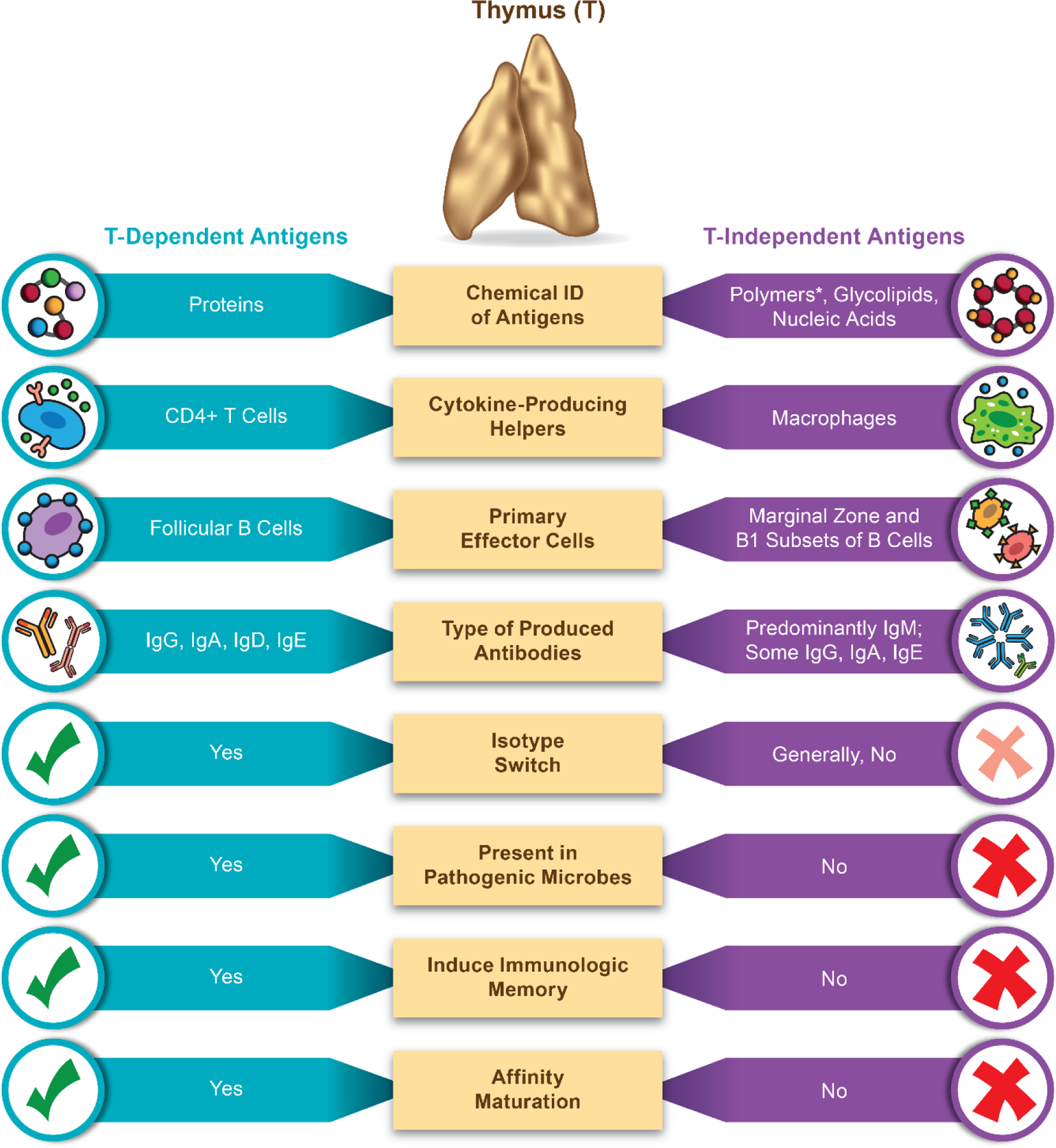
This infographics depicts the main properties and differences between the two types of antigens - thymus (T)-dependent and T-independent. Thymus is the organ of the immune system responsible for the maturation of T-cells. Antigens vary in their properties. Proteins are taken up and digested into short peptide by the antigen presenting cells (APCs) such as dendritic cells (DC); next these antigens are presented on major histocompatibility complex (MHC) to T-cells. Presentation via MHC class I results in the generation of the antigen-specific cytotoxic (CD8+) T-cells; that via MHC class II results in antigen-specific helper (CD4+) T -cells. These helper cells via cytokine secretion support antigen-specific clones of cytotoxic T-cells and follicular B-cells to expand, and B-cells to also differentiate into the antibody producing plasma cells. If the exposure to the antigen persists, T-dependent antibody response involves isotype switch and affinity maturation that leads to the formation of mature types of immunoglobulins IgG, IgA, IgD, IgE with high affinity and specificity to the antigen, as well as to the formation of memory cells. Some antigens, however, due to their chemical structures do not involve digestion and presentation in the context of the MHC to T-cells. Instead, these antigens directly activate B-cells via a cross-linking of the B-cell receptor. The result of this activation is the generation of immature antibody response in the form of IgM and lack of the immunological memory. Occasionally, in the presence of TLR agonists, isotype switch occurs and leads to the generation of some IgG, IgA and IgE. The subsets of B-cells and cells providing cytokine support to T-independent antigens are different. * - polymers include both naturally occurring non-proteinatious and synthetic polymers such as polysaccharides, polyethylene glycol, polivynilpirrolidone. Polymers linked to a protein carrier behave as hapten; in this case, the proteins are phagocytosed by B-cells, next activated B-cells produce cytokines that activate T-cells; both cell-types become activated and produce specific (antibody) response to the protein antigen and hapten (e.g., polymer coating of the protein).
4.3. Clinical examples of HSR triggered by anti-PEG antibodies
Various types of hypersensitivity reactions (HSRs) including both immediate and delayed responses have been described for PEGylated drug products. Herein, we review some examples of such reports. The risk of HSRs to Doxil, known as Caelyx in Europe, represents a major warning in the drug’s prescription label [112] and is, therefore, widely recognized for special attention. However, there are many other PEGylated nanomedicines that have more or less similar risk, including Oncaspar® [212, 213], Neulasta®[214], Macugen® [65], Mircera® [215], Palynziq® [216], Omontys® [217], Krystexxa® [218] and Revolixys® [219–222]. Some of these drugs have been withdrawn from the market, e.g., Omontys (https://www.drugs.com/history/omontys.html ), while commercial development of Revolixys was abandoned after Phase III trials [223]. Importantly, anti-PEG antibodies were implicated in the HSRs to Oncaspar® [203] and Revolixys® [218, 224–226].
4.4. Complement Activation
Addition of PEG to formulations produced many hypersensitive reactions that occurred with PEGs of various molecular weight [39]. CARPA have been demonstrated with PEGylated nanoparticles. These non-IgE-mediated type I HSR were very similar to those caused by a variety of i.v. administered nanomedicines without PEGylation, including liposomal and micellar drugs, radiocontrast agents, biologicals, enzyme therapies, iron compounds, even small molecules [227–230]. The mild to severe allergy symptoms arose shortly after the first treatment with PEGylated nanoparticles, although reactions starting later or after repeated treatments were also observed. In most cases the problem spontaneously resolved, but occasionally, the reaction could escalate into fatal anaphylaxis [231] .
The causal involvement of complement activation in these reactions has been demonstrated in several in vivo studies using rats, mice and pigs [232–242]. The evidence of causality included several facts. First, anaphylatoxin effects to those caused by PEGylated liposomes, namely, the essential identity of long-ago discovered hemodynamic and leukocyte stimulating effects of C3a and C5a [243–248] were similar to those described with PEGylated nanomedicines [232–237, 242, 249–254]. Second, the rise of C3a in mice and rats, and that of sC5b-9 in pigs coincided with the HSR [235, 255, 256]. Third, the efficacy of specific complement inhibitors in suppressing the liposome-induced hemodynamic changes and qualitative similarity of hemodynamic effects of i.v. injected human C5a in pigs to those observed in cardiac anaphylaxis in man have been reported [239, 257–260].
The role of anti-PEG antibody-triggered complement activation behind PEGylated liposome-induced HSRs was specifically tested in pigs [255] using PEGylated doxorubicin (Doxil)-mimicking empty liposomes called Doxebo. The study showed correlation of HSRs with anti-PEG IgM levels in pigs and paralleling rise of pulmonary hypertension and rise of plasma sC5b-9, thus providing direct evidence for CARPA. The fact that the reaction was due to complement activation triggered by anti-PEG IgM binding to the vesicles was shown by the rapid clearance of anti-PEG IgM from blood proceeding with the same kinetics as the rises of pulmonary arterial pressure and the plasma level of sC5b-9. Since IgM binding to antigens is a very strong, if not the strongest signal for classical pathway complement activation, the mechanism in this case was classical pathway activation. However, in the absence of pathway-specific complement activation biomarkers for pigs, the contribution of alternative pathway activation or feedback amplification of classical activation cannot be ruled out. A remarkable unexpected finding of the above study was the predominant rise of anti-PEG IgM within 2–3 days after i.v. immunization of the animals with Doxebo, peaking at 6–9 days and slowly declining without class switch over 6–9 weeks. This suggests T-independent type 2 immunogenicity, a potential mechanism of unexpected HSRs in people without known allergy to PEG. transient rises of anti-PEG IgM antibodies in man.
Another notable observation was the immensity of cardiopulmonary distress within 2–3 min only in seroconverted animals, corresponding to life-threatening anaphylaxis [255]. Thus, the model may enable studying the mechanism of PEGylated nanomedicine-induced anaphylaxis, in general, which came to the focus of interest recently, with the heightened frequency of anaphylactic reactions to mRNA vaccines against COVID-19 that contain PEGylated lipid components [261–265].
The clinical reality of potential, anti-PEG antibody mediated complement attack against PEGylated liposomes obtained striking visual evidence in a recent study by Chen, et al., who showed the formation of complement terminal complex (C5b-9) on the surface of PEGylated liposomal doxorubicin (Doxisome), entailing membrane damage and leakage of doxorubicin from the vesicles [206].
5. PEG alternatives and their immunological properties
The polymer-based nanoparticles have been intensely used as carriers for drugs and therapeutic nucleic acids, for example mRNA. One key advantage of polymeric systems is the possibility of modifying their chemical properties to adapt them to the active substance. The binding of cationic polymers and nucleic acids leads to the formation of polyplexes[266]. Different cationic polymers have been studied for RNA complexation, including polyethyleneimine (PEI), polyacrylates, poly(b-amino esters) (PBAEs) and poly(aspartamides) (PAsp). Interestingly, a lot of the polymeric nanoparticles also contained polyethylene glycols (PEGs) with different size range from 2 to 40 kDa among others.
Due to the increasing concerns about PEG-related hypersensitivity and accelerated clearance driven by PEG-specific antibodies, however, researchers started actively investigating other polymers in a search of a better, PEG-free alternative for both the polymeric drug delivery systems and for other nanomedicines that traditionally utilized PEG. Despite finding comparable physicochemical properties and pharmacology profiles of nanoparticles modified with PEG-alternatives, many of these studies ended up with disappointing results demonstrating that these alternatives are also not immunologically inert (Table 3).
Table 3. Examples of PEG alternatives and their immunological properties.
Common immunological responses to PEG alternatives include allergy, hypersensitivity reactions and immunogenicity. The information in the table is based on references [61, 267–276, 278–283, 286–288, 294–298]. HSR = hypersensitivity reaction; CARPA = complement activation related pseudoallergy; APTT = activated partial thromboplastin time; HIT = heparin-induced thrombocytopenia; PF4 = platelet factor 4; DC = dendritic cells;
| Polymer | Type of response | Mechanism |
|---|---|---|
| Poly(vinylpyrrolidone) | Anaphylaxis; inhibition of blood coagulation | IgE-mediated activation and degranulation of mast cells’ Prolongation of APTT; inhibition of coagulation factor VIII; |
| Poly(sorbate) | HSR; anaphylactoid reactions (CARPA); true anaphylaxis | Complement activation; IgE-mediated mast cell degranulation; basophil activation |
| Poly(glycerol) | Allergic contact dermatitis | unknown |
| Poly(docanol) | Allergic contact dermatitis, skin sensitization | unknown |
| Poly(oxazoline) | Inhibition of blood coagulation | Prolongation of APTT |
| Poly(2-methyl-2-oxazoline) (PMOXA) | Pseudoallergy; increased uptake by phagocytes | Complement activation; opsonization; induction of polymer-specific IgM |
| Poly(acrylamide) | Contact dermatitis to monomer components | unknown |
| Poly(N-acryloyl morpholine) | Skin sensitization by monomers | unknown |
| Poly(sialic) acid | Immunogenicity Monomers inhibit complement and phagocytosis Monomers enhance allergy |
Unknown Binding to complement factor H Mast cell degranulation |
| Pro, Ala and Ser (PAS) polypeptide | Potential immunogenicity | Tendency to aggregate |
| Poly(glutamic acid) | Late onset anaphylaxis Adjuvanticity | Polymer specific IgE DC maturation |
| Poly-(carboxybetaine) | Inflammation | Cytokine induction |
| Ala, Asp, Gly, Pro, Ser, and Thr polypeptide (XTEN) | Potential immunogenicity | Tendency to aggregate (though at a less degree than PAS) |
| Heparin | Inhibition of blood coagulation; immunogenicity; HIT; delayed anaphylaxis | Prolongation of APTT; inhibition of coagulation factors; type II antigen; formation of complex with the chemokine PF4 resulting in an irreversible aggregation and depletion of blood platelets |
| Hyaluronic acid | Delayed type hypersensitivity, anaphylaxis and vascular thrombosis | Unknown; in some cases development of these reactions were preceded by viral infections |
Anaphylaxis due to the IgE specific to polyvinylpyrrolidone (PVP) have been described[267]. PVP like many other synthetic non-proteinaceous polymers are known as type II antigens. Anaphylaxis to polysorbate[268] and immediate hypersensitivity to PEG 3350 due to PEG-specific IgE that also cross-react with polysorbate 80 have also been reported[61]. Skin sensitization and allergic contact dermatitis were reported for polyglycerols and polydocanol excipients in topically applied cosmetic products [269–271]. Poly(2-oxazoline) or POx did not activate human complement in vitro[272]; however, poly(2-methyl-2-oxazoline) (PMOXA) was shown to activate classical pathway of complement in human serum which was responsible for the opsonization and accelerated uptake of PMOXA-coated nanoparticles by human phagocytes[273]. Another study reported pseudoallergy and ABC of PMOXA-coated liposomes due to the formation of polymer-specific IgM in rats[274]. POx and PVP resulted in prolongation of human plasma coagulation in activated partial thromboplastic time (APTT) assay[272, 275, 276]; this property is similar to that of commercial blood thinning agents such as heparin. In the case of PVP, anticoagulant activity was attributed to the inhibition of coagulation factor VIII [275, 276].
Heparin is known for its ability to inhibit blood coagulation; this property results in therapeutic use of this polysaccharide for preventing blood clotting. However, beneficial anti-coagulant properties of heparin are often complicated with hemorrhage [277]. Heparin was also shown to be immunogenic in some individuals [278]. Clinical data shows that heparin and low molecular weight heparin can lead to the heparin-induced thrombocytopenia (HIT). This side effect is attributed to the formation of antibodies recognizing heparin in complex with a chemokine platelet factor (PF4), with subsequent irreversible aggregation and depletion of blood platelets [279]. Delayed type hypersensitivity, anaphylaxis and vascular thrombosis were reported in response to hyaluronic acid used in dermal filler applications [280–282]. In two of these studies, common viral infections (influenza and SARS-coV2) preceded the development of hypersensitivity to hyaluronic acid [280, 281].
Poly(carboxybetaine) investigated for providing hydration and anti-biofouling properties to nanoparticles was found to be proinflammatory; interestingly, poly(carboxybetaine)-coated nanoparticles were more pro-inflammatory and induced greater spectrum of cytokines than their PEGylated counterparts[283]. Despite being proinflammatory, poly(carboxybetaine) even after conjugation to a protein carrier was not immunogenic as estimated by the absence or very low levels of polymer-specific IgM and IgG [284].
Another polymer, poly(glutamic acid) or PGA, when compared to PEG as nanoparticle coating, demonstrated promising pharmacokinetics profile and favorable immunostimulatory properties [285]. However, several studies demonstrated that poly(glutamic acid) is responsible for the late onset anaphylaxis due to the presence of polymer specific IgE in individuals with allergies to fermented soybeans (natto) [286, 287]. Due to the use of PGA in cosmetics, food and dietary supplements, the authors of one of these studies suggested that patients with a known history of natto allergy should avoid PGA containing products [286]. This study raises similar safety question for PGA-functionalized nanomaterials in individuals allergic to fermented soybeans. Nanoparticles made of PGA possess intrinsic adjuvant properties and act by promoting the maturation of dendritic cells [288]. While this property is beneficial for vaccine applications, it may complicate the use of PGA nanoparticles as carriers for protein therapeutics and other agents prone to immunogenicity.
The information about immunological properties of other recently emerged PEG alternatives - poly(N-acryloyl morpholine), poly(sialic) acid, PASylation (addition of Proline, Alanine, Serine residues), poly(carboxybetaine) and XTENylation (addition of a peptide composed of Alanine, Asparagine, Glycine, Proline, Serine, and Threonine)– is limited. Polysialylation of therapeutic proteins led to the reduction of protein immunogenicity which was proportional to the degree of polysialylation [289]. Since sialic acid is known as a marker of self and is recognized by immunosuppressive and monocyte inhibitory glycan-binding receptors, sialic acid-modified nanoparticles are expected to avoid clearance by phagocytic cells. Indeed, a study by Kim et al., demonstrated lower uptake by phagocytes of sialic acid-modified gold nanoparticles in comparison to their PEGylated counterparts [290]. Sialic acid, through interaction with complement Factor H, was also shown to inhibit complement activation [291]. However, presence of sialic acid residues on IgEs of people with peanut allergy was responsible for triggering degranulation of mast cells; in contrast, IgEs of people without peanut allergy were not sialylated [292]. Sialic acid was recently identified as an antigen for a monoclonal antibody HIgM12 investigated for therapy of multiple sclerosis [293]; therefore, immunogenicity of this PEG alternative cannot be excluded and requires additional investigation.
Sensitization and development of contact dermatitis to acrylamide was reported in laboratory worker daily preparing polyacrylamide gels [294]; the mechanism of acrylamide sensitization and hypersensitivity reaction to polyacrylamide degradation byproducts remain unclear. Likewise, skin sensitization by monomers of another PEG alternative poly(N-acryloyl morpholine) has also been reported [295, 296]. In addition to establishing safety profiles of acrylamide-based polymers and their chemical derivatives, these limited data emphasize the importance of understanding the metabolism of these polymers and considering it in the context of safety data.
PASylation provided extended circulation time for modified peptides and proteins [297]. However, one potential drawback of PASylated proteins is their tendency to aggregate [298]. While a reduction in protein immunogenicity was reported after PASylation [297], aggregation is known to contribute to immunogenicity and may, therefore, require additional monitoring especially if long-term storage of PASylated products is needed. A similar approach is XTENylantion. Like PEG, this peptide is hydrophilic and helps solubilize hydrophobic substances. Similar to PASylation, XTENylated molecules can aggregate; however, its tendency to aggregate is less pronounced [298]. This emphasizes the importance of formulation for both PASylated and XTENylated products to prevent their aggregation and avoid immunogenicity. It is currently unknown how these peptides would behave in the context of nanoparticles containing immunogenic components such as some lipids, polymers, proteins and peptides.
Obviously, more research is needed to understand immunological compatibility of nanoparticles utilizing PEG alternatives.
6. Methods for characterization of PEG-based carriers
There are several common experimental techniques available for routine physicochemical characterization of PEGylated cargos and nanoparticles. These methods along with in vitro and in vivo methods relevant to HSR evaluation of PEGylated products are reviewed further below.
6.1. Fourier-transform infrared spectroscopy (FTIR)
FTIR is used to confirm the PEGylation of macromolecules by observing the bands of the macromolecule and the bands of PEG. PEG bands shift after particle assembly indicating hydrogen bonding to a surface [154, 299]. PEG-PLGA copolymerization can be confirmed as bands for terminal hydroxy groups, CH, CH2, CH3, and CO are easily recognizable for this copolymer [300].
6.2. Matrix Assisted laser desorption ionization-time of flight mass spectrometry (MALDITOF MS)
MALDI-TOF MS is used to determine the efficiency of drug loading reveal the stoichiometry of PEG-cargo conjugates, such as, for example, PEG-HCPT and PEG-oligos [301, 302]. Also, polydispersity of PEG conjugates can be determined from the analysis of mass spectra [303, 304].
6.3. High-performance liquid chromatography (HPLC)
The HPLC is used for the analysis of supernatant from drug loading solutions to determine the loading or encapsulation efficiency of a particular PEG-based drug delivery systems [305, 306]. Drug loading to liposomes is assessed by their rupture with methanol followed by quantification of the drugs concentration in solution [147]. For PEG-protein conjugation, HPLC can also be used to determine which protein fragments have been successfully PEGylated. After proteolysis of the PEGylated protein of interest, the solution is run through a HPLC column. Stability of PEGylated structures can be assessed by testing supernatant for the fragments of degraded structures. Sensitive structures are exposed to a range of pH, proteolysis agents, or other destabilizing factors and the solution is tested for free molecules from the original structure [307, 308].
6.4. Transmission electron microscopy (TEM)
TEM aids to determine the morphology of PEGylated nanoparticle-based drug delivery systems. However, due to the requirements of sample preparation, the particles are dehydrated and the resulting analysis depicts structures of PEG in a collapsed state as shown in Figure 1D. This method is reliable to determine the success of PEGylation but primarily reveals more about the size distribution or morphology of the macromolecule core of nanoparticles [309]. Structural characterization of the solvated state of PEG functionalization is not possible here.
6.5. Dynamic Light Scattering (DLS)
DLS provides information about a pegylated nanoparticle in its solvated form. The Use of DLS as a compliment to other electron microscopy techniques provides useful information regarding the solution stability and hydrodynamic diameter of a nanomaterial.[310] Drift in the median size over time may be an indication of sedimentation of the material or the formation of agglomerates. Either result may indicate the need to address the degree of PEG incorporation to a material.
6.6. Cryogenic electron microscopy (CryoEM)
Cryo-EM is a method that requires vitrification of samples and therefore maintains in-solution structure [311]. With the recent advancements with detector sensitivity during the resolution revolution, structure may be determined to the molecular and atomic scale [312]. With the ability to resolve ultrastructure of drug delivery systems, insight can be had to the pharmacological mechanism of action or the effect of functionalization on structure the of macromolecules. CryoEM has been used to characterize liposomes PEGylated with PEG-cholesterol structures. This process entails imaging of many lipid nanoparticles and image analysis to determine nanoparticle structure with advantage over DLS [311].
6.7. Nuclear Magnetic Resonance (NMR)
NMR is able to characterize PEGylated nanoparticle systems and reveal structural information at a molecular level of PEG polymers and certain macromolecules. 1H-NMR can easily determine common PEG copolymers such as PEG-PLGA as well as quantify amounts of the individual components within the polymers[300]. With both 1H-NMR and 13C-NMR, it is critical to understand how the environment of the PEG carbons or hydrogens change as the solvent, the temperature, and the state (solid or liquid) the groups are in. These variables can affect the resolution of peaks and chemical shifts [309].
6.8. Role of in vitro and in vivo models
Due to both quantitative and qualitative differences in the immune system, animals may not accurately mimic human responses. Differences in nanoparticle immune response from animal models has been broadly reviewed elsewhere [313, 314]. Nanoparticle clearance have been documented to occur through Kupffer cells in humans, non-human primates, dogs, rats and mice, whereas the pulmonary intravascular macrophages are involved nanoparticle clearance in sheep, calves, cats, goats and pigs [313]. Moreover, mice and pigs commonly used in basic research are not formally recognized as preclinical models, whereas the use of dogs and non-human primates, recognized for preclinical studies, raise many ethical concerns. Nevertheless, some models have been found very predictive and reproducible when it comes to the certain types of immunotoxicities; for example, the pig model for the detection of complement activation and anaphylactoid reactions to PEGylated drug products discussed below [314].
6.9. In vivo hemodynamic changes in pig model
Pigs’ response to reactogenic medicines including certain nanoparticle formulations is similar to that of humans; therefore, pigs are used as a model to screen novel formulations for the potential to cause infusion reactions. In this model, animals are pre-anesthetized intramuscularly with Calypsol and Xilazine. The anesthesia is maintained using isoflurane inhalation throughout the procedure during which test materials and controls are injected as a bolus or infusion. Following the injection, the cardiovascular parameters are continuously recorded. Animals’ blood is collected and used for ex vivo analysis of complement split products, cytokines, thromboxane and other pro-inflammatory mediators along with blood cell counts. Respiratory and skin reactions are also documented [315, 316].
6.10. Ex vivo detection of anti-PEG antibodies
Blood is collected from human donor volunteers or patients as well as from animals used as research models (e.g., mice, rats, dogs, pigs) into vacutainers containing anticoagulants to obtain plasma. Not anticoagulated blood is used to obtain serum. Plasma or sera specimens are stored frozen and analyzed by enzyme-linked immunosorbent assay (ELISA) for the presence of anti-PEG antibodies. In this assay, PEG is coated on 96-well plates, which after blocking are incubated with plasma or serum samples. When anti-PEG antibodies are present in the tested specimen, they are subsequently detected using secondary antibodies conjugated to the horse radish peroxidase enzyme and visualized using a TMB substrate. The intensity of the color produced during this reaction is proportional to the level of anti-PEG antibodies. The secondary antibodies are selected to allow identification of antibodies from different species and isotypes[164, 317]. Interestingly, Roffler et al., found that BSA and Tween 20 detergent commonly used in wash and blocking buffers for ELISA assays interfere with detection of anti-PEG antibodies and suggested that non-fat milk and CHAPS are more optimal for anti-PEG ELISAs[164]
Recently, a flow cytometry based method was developed for anti-PEG antibody quantification in plasma[166]. In this assay, TentaGel™ M OH beads are incubated with plasma; next the beads are incubated with secondary antibodies conjugated to a fluorescent dye, and the mean fluorescent intensity is recorded using flow cytometer after gating the beads in forward and side scatter plot. The brightness of the fluorescent signal is proportional to the level of anti-PEG antibodies[166].
6.11. In vitro analysis of complement activation
Several experimental approaches are available to understand the activation of the complement system. One such method – CH50 – relies on assessing the lysis of antibody sensitized sheep erythrocytes [318]. Since some nanomaterials can cause hemolysis, and sheep erythrocytes are not always available, the CH50 method is frequently replaced with ELISA detecting the presence of complement split products (C3a, iC3b,C4a, C5a) or terminal complex (sC5b-9) in plasma or sera[319]. Another alternative to CH50 is a liposome immunoassay (LIA) in which liposomes are used to mimic erythrocytes. Both ELISA and LIA compare well with CH50 assay [320]. When plasma is used for the complement studies, it is important to recognize that different commercial anti-coagulants may influence the assay performance [321]. While hirudin is often cited as the best anticoagulant for complement assays, it is not available in the US. Therefore, serum, citrate- and EDTA-anticoagulated plasma are also used. Complement studies utilizing citrate and EDTA anticoagulated plasma must include veronal buffer to supplement study samples with divalent ions such as calcium and magnesium that are needed for the complement activation [321].
7. Conclusions and Future directions
Rephrasing a piece from the famous play Hamlet by William Shakespeare brings us to the central theme of this review - “to PEGylate, or not to PEGylate, that is the question”. Before dropping PEG from formulations, it is important to consider several facts.
First, both purified (recombinant) anti-PEG antibodies and plasmas from individuals with high titers of anti-PEG IgG and IgM cross-react with other polymers containing C-C-O groups, such as polypropylene glycol (PPG), polytetramethylene ether glycol (PTMEG), poly-1,4-butylene adipate (PBA), and polyethylenimine (PEI) [172]. Therefore, using these polymers as PEG alternatives will most likely be associated with the same spectrum of biological responses, including but not limited to immunotoxicity and clearance, as those to PEGylated compounds. This is especially important to consider as the number of individuals with anti-PEG antibodies is expected to grow in the future due to the ongoing mass vaccination with PEGylated LNP-mRNA vaccines against SARS-CoV-2. As such, studies investigating both the incidence and the type of anti-PEG antibodies in general population along with cross-reactivity of these antibodies to other polymers used in medical applications and consumer products are urgently needed.
Second, many polymers were qualified as type 2 antigens and shown to induce polymer-specific antibodies. Some examples include polyvinylpyrrolidone and poly(2-methyl-2-oxazoline) [267, 322–325]. Depending on the isotype and posttranslational modifications, these antibodies contribute to various types of hypersensitivity reactions and particle clearance. Therefore, fundamental understanding of immunocompatibility of these polymers alone and in the context of pharmaceutical products needs to be considered on a case-by-case basis.
Third, immunological status of the host, the presence of adjuvants such as TLR agonists, the type of API delivered by a nanoformulation play a critical role in the process of immunogenicity of both the API and the nanoparticle carrier or its components [326–331]. Therefore, it is important to investigate immunogenicity of each polymeric PEG alternative both alone and in the context of the product (e.g., protein, nanoparticle carrier, nanotechnology-based formulation of an API), dose, dose regimen and route of administration. Studying the immunogenicity of polymer alone is insufficient and may generate misleading results.
Forth, allergic sensitization by monomer components of PEG alternatives has been described [296]. Therefore, it is important to understand metabolism of PEG alternatives and consider it in the context of product safety.
Finally, blood of individuals with certain types of food allergies (e.g., that to natto) contains IgE specific to certain PEG alternatives, such as poly(glutamic acid); these antibodies are responsible for the late onset anaphylaxis to foods and cosmetic products containing such polymers[286, 287]. This pre-existing immune response would create a safety concern for nanomedicines and other drug products containing these polymers. Therefore, it is important to understand the presence of PEG alternatives in common daily life products, estimate pre-existing immune response to these compounds and identify population with higher risk of developing immune-mediated side effects to these PEG alternatives.
In summary, there is no ideal PEG alternative. Common immune-mediated reactions include immunogenicity, allergy, and hypersensitivity reactions. To overcome current translational and clinical hurdles arising from PEG’s immunogenicity, it is imperative to conduct thorough studies that would improve fundamental knowledge about immunological properties of PEG alternatives. Since each nanoparticle formulation is unique and various formulation components contribute to immunological properties of the final product, the analysis of polymers must include both polymers alone and in the context of the whole product. Establishing a publicly available database summarizing research findings regarding the immunological and physicochemical properties of PEG and PEG alternatives in the context with various excipients, nanoparticle carriers, APIs, route of administration, dose and dose regimen would aid formulation scientists in selecting optimal polymers for their formulations. Such database would also boost bioinformatics field by allowing artificial intelligence researchers to create algorithms for matching formulation components to APIs and indications, and for creating formulations with optimal properties.
Currently available allergen panels used by clinical service lab such as LabCorp are broad but do not include polymers[332]. Establishing a panel of polymers containing both PEG and its alternatives for use in the clinically available skin prick test would boost the ability of physicians to identify patients predisposed to allergic reactions to medicinal products containing these polymers. This would also allow safer administration of both PEGylated and PEG-alternatives containing products and better management of adverse reactions should they occur.
Improving the education and knowledge sharing between physicians, nanotechnology researchers, immunologists, formulation, and regulatory scientists, and consolidating efforts is needed to advance the field.
Acknowledgments.
The study was funded in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract 75N91019D00024 (to M.A.D. and D.S.) and supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers R01GM120487 and R35GM139587 (to K.A.A.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. J.S. acknowledges the support by the European Union Horizon 2020 projects 825828 (Expert) and 952520 (Biosafety), and the Department of Nanobiotechnology and Regenerative Medicine, Faculty of Health, Miskolc University, Miskolc, Hungary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brady JD, T.; Lee PI; Li JX, Polymer Properties and Characterization, in: Qui YC, Y.; Zhang GGZ; Yu L; Mantri RV (Ed.) Developing Solid Oral Dosage Forms, Elsevier; 2017, pp. 181–223. [Google Scholar]
- [2].H. T., Polyethylene glycols (PEGs) and the pharmaceutical industry, Fine, Specialty & Performance Chemicals, (2002) 57–59. [Google Scholar]
- [3].Alexis F, Pridgen E, Molnar LK, Farokhzad OC, Factors affecting the clearance and biodistribution of polymeric nanoparticles, Mol Pharm, 5 (2008) 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roser M, Fischer D, Kissel T, Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats, Eur J Pharm Biopharm, 46 (1998) 255–263. [DOI] [PubMed] [Google Scholar]
- [5].Newland B, Taplan C, Pette D, Friedrichs J, Steinhart M, Wang W, Voit B, Seib FP, Werner C, Soft and flexible poly(ethylene glycol) nanotubes for local drug delivery, Nanoscale, 10 (2018) 8413–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Damodaran VB, Fee CJ, Ruckh T, Popat KC, Conformational studies of covalently grafted poly(ethylene glycol) on modified solid matrices using X-ray photoelectron spectroscopy, Langmuir, 26 (2010) 7299–7306. [DOI] [PubMed] [Google Scholar]
- [7].Sharma S, Johnson RW, Desai TA, XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors, Biosens Bioelectron, 20 (2004) 227–239. [DOI] [PubMed] [Google Scholar]
- [8].Vonarbourg A, Passirani C, Saulnier P, Benoit JP, Parameters influencing the stealthiness of colloidal drug delivery systems, Biomaterials, 27 (2006) 4356–4373. [DOI] [PubMed] [Google Scholar]
- [9].Akiba I, Terada N, Hashida S, Sakurai K, Sato T, Shiraishi K, Yokoyama M, Masunaga H, Ogawa H, Ito K, Yagi N, Encapsulation of a hydrophobic drug into a polymer-micelle core explored with synchrotron SAXS, Langmuir, 26 (2010) 7544–7551. [DOI] [PubMed] [Google Scholar]
- [10].Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D, Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times, Biochim Biophys Acta, 1070 (1991) 187–192. [DOI] [PubMed] [Google Scholar]
- [11].Needham D, McIntosh TJ, Lasic DD, Repulsive interactions and mechanical stability of polymer-grafted lipid membranes, Biochim Biophys Acta, 1108 (1992) 40–48. [DOI] [PubMed] [Google Scholar]
- [12].Jeon SI, Lee LH, Andrade JD, De Gennes PG, Protein-surface interactions in the presence of polyethylene oxide. I. Simpified theory, J. Colloid Interf.Scie, 142 (1991) 149–158. [Google Scholar]
- [13].Fruijtier-Pölloth C, Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products, Toxicology, 214 (2005) 1–38. [DOI] [PubMed] [Google Scholar]
- [14].Jang HJ, Shin CY, Kim KB, Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use, Toxicol Res, 31 (2015) 105–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baumann A, Piel I, Hucke F, Sandmann S, Hetzel T, Schwarz T, Pharmacokinetics, excretion, distribution, and metabolism of 60-kDa polyethylene glycol used in BAY 94–9027 in rats and its value for human prediction, Eur J Pharm Sci, 130 (2019) 11–20. [DOI] [PubMed] [Google Scholar]
- [16].Nordström R, Zhu L, Härmark J, Levi-Kalisman Y, Koren E, Barenholz Y, Levinton G, Shamrakov D, Quantitative Cryo-TEM Reveals New Structural Details of Doxil-Like PEGylated Liposomal Doxorubicin Formulation, Pharmaceutics, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L, Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery, Drug Deliv, 11 (2004) 169–183. [DOI] [PubMed] [Google Scholar]
- [18].Buxton AN, Zhu J, Marchant R, West JL, Yoo JU, Johnstone B, Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells, Tissue Eng, 13 (2007) 2549–2560. [DOI] [PubMed] [Google Scholar]
- [19].Cruise GM, Scharp DS, Hubbell JA, Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels, Biomaterials, 19 (1998) 1287–1294. [DOI] [PubMed] [Google Scholar]
- [20].Day JR, David A, Kim J, Farkash EA, Cascalho M, Milasinovic N, Shikanov A, The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomater, 67 (2018) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elisseeff JA, K.; Sims D; McIntosh W; Randolph M; Langer R, Transdermal photopolymerization for minimally invasive implantation, Proc. Natl. Acad. Sci. USA, 96 (1999) 3104–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walkey CD, Olsen JB, Guo H, Emili A, Chan WC, Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake, J Am Chem Soc, 134 (2012) 2139–2147. [DOI] [PubMed] [Google Scholar]
- [23].Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK, Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation, Mol Pharm, 11 (2014) 1250–1258. [DOI] [PubMed] [Google Scholar]
- [24].Abuchowsk AVE, T.; Palczuk NC; Davis FF, Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol, Journal of Biological Chemistry, 252 (1977) 3578–3581. [PubMed] [Google Scholar]
- [25].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv Drug Deliv Rev, 99 (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ait Bachir Z, Huang Y, He M, Huang L, Hou X, Chen R, Gao F, Effects of PEG surface density and chain length on the pharmacokinetics and biodistribution of methotrexate-loaded chitosan nanoparticles, Int J Nanomedicine, 13 (2018) 5657–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hou Z, Zhan C, Jiang Q, Hu Q, Li L, Chang D, Yang X, Wang Y, Li Y, Ye S, Xie L, Yi Y, Zhang Q, Both FA- and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution, Nanoscale Res Lett, 6 (2011) 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Papadimitriou SA, Achilias DS, Bikiaris DN, Chitosan-g-PEG nanoparticles ionically crosslinked with poly(glutamic acid) and tripolyphosphate as protein delivery systems, Int J Pharm, 430 (2012) 318–327. [DOI] [PubMed] [Google Scholar]
- [29].Yang C, Gao S, Dagnæs-Hansen F, Jakobsen M, Kjems J, Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo, ACS Appl Mater Interfaces, 9 (2017) 12203–12216. [DOI] [PubMed] [Google Scholar]
- [30].Valuckaite V, Seal J, Zaborina O, Tretiakova M, Testa G, Alverdy JC, High molecular weight polyethylene glycol (PEG 15–20) maintains mucosal microbial barrier function during intestinal graft preservation, J Surg Res, 183 (2013) 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].L.B. Inc., Compositions containing polyethylene glycol and uses thereof, 1999. [Google Scholar]
- [32].Tokunaga Y, Wicomb WN, Garcia-Kennedy R, Esquivel CO, Collins GM, The immunosuppressive effect of polyethylene glycol in a flush solution for rat liver transplantation, Transplantation, 54 (1992) 756–758. [PubMed] [Google Scholar]
- [33].Shirey RS, Boyd JS, Ness PM, Polyethylene glycol versus low-ionic-strength solution in pretransfusion testing: a blinded comparison study, Transfusion, 34 (1994) 368–370. [DOI] [PubMed] [Google Scholar]
- [34].Mack JE, Kerr JA, Vreugdenhil PK, Belzer FO, Southard JH, Effect of polyethylene glycol on lipid peroxidation in cold-stored rat hepatocytes, Cryobiology, 28 (1991) 1–7. [DOI] [PubMed] [Google Scholar]
- [35].Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC, Garcia JG, Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement, Microvasc Res, 77 (2009) 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Luo J, Shi R, Diffusive oxidative stress following acute spinal cord injury in guinea pigs and its inhibition by polyethylene glycol, Neurosci Lett, 359 (2004) 167–170. [DOI] [PubMed] [Google Scholar]
- [37].Luo J, Borgens R, Shi R, Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury, J Neurotrauma, 21 (2004) 994–1007. [DOI] [PubMed] [Google Scholar]
- [38].USFDA, Center for Food Safety and Applied Nutrition. Food additive status list. “Polyethylene glycol.” 2019. [Google Scholar]
- [39].Wenande E, Garvey LH, Immediate-type hypersensitivity to polyethylene glycols: a review, Clin Exp Allergy, 46 (2016) 907–922. [DOI] [PubMed] [Google Scholar]
- [40].21CFR, Code of Federal Regulations Title 21. accessdata.fda.gov. (n.d.). , 2021. [Google Scholar]
- [41].Parnaud G, Taché S, Peiffer G, Corpet DE, Polyethylene-glycol suppresses colon cancer and causes dose-dependent regression of azoxymethane-induced aberrant crypt foci in rats, Cancer Res, 59 (1999) 5143–5147. [PubMed] [Google Scholar]
- [42].Descamps C, Cabrera G, Depierreux M, Devière J, Acute renal insufficiency after colon cleansing, Endoscopy, 32 (2000) S11. [PubMed] [Google Scholar]
- [43].Erickson TB, Aks SE, Zabaneh R, Reid R, Acute renal toxicity after ingestion of Lava light liquid, Ann Emerg Med, 27 (1996) 781–784. [DOI] [PubMed] [Google Scholar]
- [44].Shi R, Polyethylene glycol repairs membrane damage and enhances functional recovery: a tissue engineering approach to spinal cord injury, Neurosci Bull, 29 (2013) 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Greenfield EA, Polyethylene Glycol Fusion for Hybridoma Production, Cold Spring Harb Protoc, 2018 (2018). [DOI] [PubMed] [Google Scholar]
- [46].Nascimento A, Lannigan J, Kashatus D, High-throughput detection and quantification of mitochondrial fusion through imaging flow cytometry, Cytometry A, 89 (2016) 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Juneja R, Lyles Z, Vadarevu H, Afonin KA, Vivero-Escoto JL, Multimodal Polysilsesquioxane Nanoparticles for Combinatorial Therapy and Gene Delivery in Triple-Negative Breast Cancer, ACS Appl Mater Interfaces, 11 (2019) 12308–12320. [DOI] [PubMed] [Google Scholar]
- [48].Rackley L, Stewart JM, Salotti J, Krokhotin A, Shah A, Halman JR, Juneja R, Smollett J, Lee L, Roark K, Viard M, Tarannum M, Vivero-Escoto J, Johnson PF, Dobrovolskaia MA, Dokholyan NV, Franco E, Afonin KA, RNA Fibers as Optimized Nanoscaffolds for siRNA Coordination and Reduced Immunological Recognition, Adv Funct Mater, 28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Juneja R, Vadarevu H, Halman J, Tarannum M, Rackley L, Dobbs J, Marquez J, Chandler M, Afonin KA, Vivero-Escoto J, Combination of Nucleic Acid and Mesoporous Silica Nanoparticles: Optimization and Therapeutic Performance In Vitro ACS Appl Mater Interfaces, 12 (2020) 38873–38886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chan P, Kurisawa M, Chung JE, Yang YY, Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery, Biomaterials, 28 (2007) 540–549. [DOI] [PubMed] [Google Scholar]
- [51].Ross PC, Hui SW, Polyethylene glycol enhances lipoplex-cell association and lipofection, Biochim Biophys Acta, 1421 (1999) 273–283. [DOI] [PubMed] [Google Scholar]
- [52].Wang J, Li S, Han Y, Guan J, Chung S, Wang C, Li D, Poly(Ethylene Glycol)-Polylactide Micelles for Cancer Therapy, Front Pharmacol, 9 (2018) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Saw PE, Park J, Lee E, Ahn S, Lee J, Kim H, Kim J, Choi M, Farokhzad OC, Jon S, Effect of PEG pairing on the efficiency of cancer-targeting liposomes, Theranostics, 5 (2015) 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B, A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptide-targeted liposomes, ACS Nano, 7 (2013) 2935–2947. [DOI] [PubMed] [Google Scholar]
- [55].Bryant SJ, Bender RJ, Durand KL, Anseth KS, Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production, Biotechnol Bioeng, 86 (2004) 747–755. [DOI] [PubMed] [Google Scholar]
- [56].Tibbitt MW, Anseth KS, Hydrogels as extracellular matrix mimics for 3D cell culture, Biotechnol Bioeng, 103 (2009) 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Browning MB, Cereceres SN, Luong PT, Cosgriff-Hernandez EM, Determination of the in vivo degradation mechanism of PEGDA hydrogels, J Biomed Mater Res A, 102 (2014) 4244–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R, Transdermal photopolymerization for minimally invasive implantation, Proc Natl Acad Sci U S A, 96 (1999) 3104–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mukherjee A, Sarkar S, Jana S, Swarnakar S, Das N, Neuro-protective role of nanocapsulated curcumin against cerebral ischemia-reperfusion induced oxidative injury, Brain Res, 1704 (2019) 164–173. [DOI] [PubMed] [Google Scholar]
- [60].Sari Gökay S, Çelik T, Yusuf Sari M, Ekinci F, Dinçer Yildizdaş R, Levent Yilmaz H, Urticaria as a Rare Side Effect of Polyethylene Glycol-3350 in a Child: Case Report, Acta Clin Croat, 57 (2018) 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stone CA Jr., Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, Hemler JA, Phillips EJ, Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized, J Allergy Clin Immunol Pract, 7 (2019) 1533–1540.e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang H, Henry WA, Chen LA, Khashab MA, Urticaria due to polyethylene glycol-3350 and electrolytes for oral solution in a patient with jejunal nodular lymphoid hyperplasia, Ann Gastroenterol, 28 (2015) 148–150. [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou ZH, Stone CA Jr., Jakubovic B, Phillips EJ, Sussman G, Park J, Hoang U, Kirshner SL, Levin R, Kozlowski S, Anti-PEG IgE in anaphylaxis associated with polyethylene glycol, J Allergy Clin Immunol Pract, 9 (2021) 1731–1733.e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].FDA, Macugen product insert, (2021). [Google Scholar]
- [65].Steffensmeier AC, Azar AE, Fuller JJ, Muller BA, Russell SR, Vitreous injections of pegaptanib sodium triggering allergic reactions, Am J Ophthalmol, 143 (2007) 512–513. [DOI] [PubMed] [Google Scholar]
- [66].FDA, Asclera package insert, 2021. [Google Scholar]
- [67].FDA, Movantik package insert, 2021. [Google Scholar]
- [68].FDA, Palinziq package insert, 2021. [Google Scholar]
- [69].WHO, WHO Model List of Essential Medicines. 21st List , 2019. [Google Scholar]
- [70].M.f.M.E.a. Research, Peginterferon ALFA-2A (Subcutaneous route) side effects Mayo Clinic., (2021). [Google Scholar]
- [71].Myler H, Hruska MW, Srinivasan S, Cooney E, Kong G, Dodge R, Krishna M, Zhu J, Felix T, Gleason C, Piccoli SP, DeSilva B, Anti-PEG antibody bioanalysis: a clinical case study with PEG-IFN-λ−1a and PEG-IFN-α2a in naive patients, Bioanalysis, 7 (2015) 1093–1106. [DOI] [PubMed] [Google Scholar]
- [72].Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D, Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis, Exp Hematol Oncol, 1 (2012) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bozza WP, Takeda K, Alterovitz WL, Chou CK, Shen RF, Zhang B, Anthracycline-Induced Cardiotoxicity: Molecular Insights Obtained from Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs), Aaps j, 23 (2021) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhu Y, Wang F, Zhao Y, Zheng X, Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia: a literature review of pharmaceutical and clinical aspects, Eur J Hosp Pharm, 28 (2020) 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang H, Onivyde for the therapy of multiple solid tumors, Onco Targets Ther, 9 (2016) 3001–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].FDA, Onyvide package insert, 2021. [Google Scholar]
- [77].Panigaj M, Dobrovolskaia MA, Afonin KA, 2021: an immunotherapy odyssey and the rise of nucleic acid nanotechnology, Nanomedicine (Lond), 16 (2021) 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine - United States, December 14–23, 2020, MMWR Morb Mortal Wkly Rep, 70 (2021) 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Caballero ML, Quirce S, Excipients as Potential Agents of Anaphylaxis in Vaccines: Analyzing the Formulations of Currently Authorized COVID-19 Vaccines, J Investig Allergol Clin Immunol, 31 (2021) 92–93. [DOI] [PubMed] [Google Scholar]
- [80].Castells MC, Phillips EJ, Maintaining Safety with SARS-CoV-2 Vaccines, N Engl J Med, 384 (2021) 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Garvey LH, Nasser S, Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit?, Br J Anaesth, 126 (2021) e106–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Selvaraj G, Kaliamurthi S, Peslherbe GH, Wei DQ, Are the Allergic Reactions of COVID-19 Vaccines Caused by mRNA Constructs or Nanocarriers? Immunological Insights, Interdiscip Sci, (2021) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shimabukuro T, Nair N, Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine, Jama, 325 (2021) 780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shimabukuro TT, Cole M, Su JR, Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021, Jama, 325 (2021) 1101–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sokolowska M, Eiwegger T, Ollert M, Torres MJ, Barber D, Del Giacco S, Jutel M, Nadeau KC, Palomares O, Rabin RL, Riggioni C, Vieths S, Agache I, Shamji MH, EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines, Allergy, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Salzman MB, Huang CW, O’Brien CM, Castillo RD, Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination, CDC, 27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Crist C, CDC Looks Into Post-COVID Vaccine Heart Inflammation, WebMD, (2021). [Google Scholar]
- [88].Kawai F, Biodegradation of Polyethers (Polyethylene Glycol, Polypropylene Glycol, Polytetramethylene glycol, and Others), Biopolymers Online [Google Scholar]
- [89].Mathews JM, Parker MK, Matthews HB, Metabolism and disposition of diethylene glycol in rat and dog, Drug Metab Dispos, 19 (1991) 1066–1070. [PubMed] [Google Scholar]
- [90].Nishiyama T, Iwata Y, Nakajima K, Mitsui T, In vivo per cutaneous absorption of polyoxyethylene lauryl ether surfactants in hairless mice, J. Soc. Cosmet. Chem , 34 (1983) 263–271. [Google Scholar]
- [91].Herold DA, Rodeheaver GT, Bellamy WT, Fitton LA, Bruns DE, Edlich RF, Toxicity of topical polyethylene glycol, Toxicol Appl Pharmacol, 65 (1982) 329–335. [DOI] [PubMed] [Google Scholar]
- [92].Murali VS, Wang R, Mikoryak CA, Pantano P, Draper R, Rapid detection of polyethylene glycol sonolysis upon functionalization of carbon nanomaterials, Exp Biol Med (Maywood), 240 (2015) 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ulbricht J, Jordan R, Luxenhofer R, On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s, Biomaterials, 35 (2014) 4848–4861. [DOI] [PubMed] [Google Scholar]
- [94].McGary JC, Degradation of poly (ethylene oxide), J. Polym. Sci,, 46 (1960) 51e57. [Google Scholar]
- [95].Han S, Kim C, Kwon D, Thermal/oxidative degradation and stabilization of polyethylene glycol, Polymer, 38 (1997) 317e323. [Google Scholar]
- [96].Branch DW, Wheeler BC, Brewer GJ, Leckband DE, Long-term stability of grafted polyethylene glycol surfaces for use with microstamped substrates in neuronal cell culture, Biomaterials, 22 (2001) 1035–1047. [DOI] [PubMed] [Google Scholar]
- [97].Sung HJ, Chandra P, Treiser MD, Liu E, Iovine CP, Moghe PV, Kohn J, Synthetic polymeric substrates as potent pro-oxidant versus anti-oxidant regulators of cytoskeletal remodeling and cell apoptosis, J Cell Physiol, 218 (2009) 549–557. [DOI] [PubMed] [Google Scholar]
- [98].Yu SS, Koblin RL, Zachman AL, Perrien DS, Hofmeister LH, Giorgio TD, Sung HJ, Physiologically relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds, Biomacromolecules, 12 (2011) 4357–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B, Weimer R, Mero A, Pasut G, Veronese FM, Polyoxazoline: chemistry, properties, and applications in drug delivery, Bioconjug Chem, 22 (2011) 976–986. [DOI] [PubMed] [Google Scholar]
- [100].Friman S, Egestad B, Sjövall J, Svanvik J, Hepatic excretion and metabolism of polyethylene glycols and mannitol in the cat, J Hepatol, 17 (1993) 48–55. [DOI] [PubMed] [Google Scholar]
- [101].Irizarry Rovira AR, Bennet BM, Bolon B, Braendli-Baiocco A, Chandra S, Fleurance R, Garman R, Hutto D, Lane J, Romeike A, Sargeant A, Zimmerman B, Scientific and Regulatory Policy Committee Points to Consider: Histopathologic Evaluation in Safety Assessment Studies for PEGylated Pharmaceutical Products, Toxicol Pathol, 46 (2018) 616–635. [DOI] [PubMed] [Google Scholar]
- [102].Bendele A, Seely J, Richey C, Sennello G, Shopp G, Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins, Toxicol Sci, 42 (1998) 152157. [DOI] [PubMed] [Google Scholar]
- [103].Ivens IA, Achanzar W, Baumann A, Brändli-Baiocco A, Cavagnaro J, Dempster M, Depelchin BO, Rovira AR, Dill-Morton L, Lane JH, Reipert BM, Salcedo T, Schweighardt B, Tsuruda LS, Turecek PL, Sims J, PEGylated Biopharmaceuticals: Current Experience and Considerations for Nonclinical Development, Toxicol Pathol, 43 (2015) 959–983. [DOI] [PubMed] [Google Scholar]
- [104].Turecek PL, Bossard MJ, Schoetens F, Ivens IA, PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs, J Pharm Sci, 105 (2016) 460–475. [DOI] [PubMed] [Google Scholar]
- [105].Forest T, Xu Q, Kuruvilla S, Vu H, Vlasakova K, Glaab WE, Hines C, Xun S, Magnetic Resonance and Ultrastructural Characterization of PEGylation-associated Vacuolation in Nonclinical Models, Toxicol Pathol, 45 (2017) 604–613. [DOI] [PubMed] [Google Scholar]
- [106].Dobrovolskaia MA, Neun BW, Man S, Ye X, Hansen M, Patri AK, Crist RM, McNeil SE, Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles, Nanomedicine, 10 (2014) 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, Neun BW, Hall JB, McNeil SE, Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles, Nanomedicine, 5 (2009) 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mima Y, Hashimoto Y, Shimizu T, Kiwada H, Ishida T, Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein, Mol Pharm, 12 (2015) 2429–2435. [DOI] [PubMed] [Google Scholar]
- [109].Suzuki T, Ichihara M, Hyodo K, Yamamoto E, Ishida T, Kiwada H, Ishihara H, Kikuchi H, Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs, Int J Pharm, 436 (2012) 636–643. [DOI] [PubMed] [Google Scholar]
- [110].Suzuki T, Ichihara M, Hyodo K, Yamamoto E, Ishida T, Kiwada H, Kikuchi H, Ishihara H, Influence of dose and animal species on accelerated blood clearance of PEGylated liposomal doxorubicin, Int J Pharm, 476 (2014) 205–212. [DOI] [PubMed] [Google Scholar]
- [111].Bruns DE, Herold DA, Rodeheaver GT, Edlich RF, Polyethylene glycol intoxication in burn patients, Burns Incl Therm Inj, 9 (1982) 49–52. [DOI] [PubMed] [Google Scholar]
- [112].Neun BW, Barenholz Y, Szebeni J, Dobrovolskaia MA, Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro, Molecules, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li BQ, Dong X, Fang SH, Gao JY, Yang GQ, Zhao H, Systemic toxicity and toxicokinetics of a high dose of polyethylene glycol 400 in dogs following intravenous injection, Drug Chem Toxicol, 34 (2011) 208–212. [DOI] [PubMed] [Google Scholar]
- [114].Coskun A, Uzunkoy A, Duzgun SA, Bozer M, Ozardali I, Vural H, Experimental sodium phosphate and polyethylene glycol induce colonic tissue damage and oxidative stress, Br J Surg, 88 (2001) 85–89. [DOI] [PubMed] [Google Scholar]
- [115].Szebeni J, Simberg D, González-Fernández Á, Barenholz Y, Dobrovolskaia MA, Roadmap and strategy for overcoming infusion reactions to nanomedicines, Nat Nanotechnol, 13 (2018) 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Herzberger J, Niederer K, Pohlit H, Seiwert J, Worm M, Wurm FR, Frey H, Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation, Chemical Reviews, 116 (2016) 2170–2243. [DOI] [PubMed] [Google Scholar]
- [117].Vojkovsky T, Sullivan B, Sill KN, Synthesis of heterobifunctional polyethylene glycols: Polymerization from functional initiators, Polymer, 105 (2016) 72–78. [Google Scholar]
- [118].Li Y, Xiao K, Luo J, Lee J, Pan S, Lam KS, A novel size-tunable nanocarrier system for targeted anticancer drug delivery, Journal of Controlled Release, 144 (2010) 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, Cosco D, Schiavon O, Shen H, Fresta M, Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy, Journal of Controlled Release, 199 (2015) 106–113. [DOI] [PubMed] [Google Scholar]
- [120].Wang M, Gustafsson OJR, Siddiqui G, Javed I, Kelly HG, Blin T, Yin H, Kent SJ, Creek DJ, Kempe K, Ke PC, Davis TP, Human plasma proteome association and cytotoxicity of nano-graphene oxide grafted with stealth polyethylene glycol and poly(2-ethyl-2-oxazoline), Nanoscale, 10 (2018) 10863–10875. [DOI] [PubMed] [Google Scholar]
- [121].Li M, Jiang S, Simon J, Paßlick D, Frey M-L, Wagner M, Mailänder V, Crespy D, Landfester K, Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime, Nano Letters, 21 (2021) 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ishida T, Iden DL, Allen TM, A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs, FEBS Letters, 460 (1999) 129–133. [DOI] [PubMed] [Google Scholar]
- [123].Amoozgar Z, Yeo Y, Recent advances in stealth coating of nanoparticle drug delivery systems, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 4 (2012) 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nakamura M, Hayashi K, Nakano M, Kanadani T, Miyamoto K, Kori T, Horikawa K, Identification of Polyethylene Glycol-Resistant Macrophages on Stealth Imaging in Vitro Using Fluorescent Organosilica Nanoparticles, ACS Nano, 9 (2015) 1058–1071. [DOI] [PubMed] [Google Scholar]
- [125].Veronese FM, Caliceti P, Schiavon O, Branched and Linear Poly(Ethylene Glycol): Influence of the Polymer Structure on Enzymological, Pharmacokinetic, and Immunological Properties of Protein Conjugates, Journal of Bioactive and Compatible Polymers, 12 (1997) 196–207. [Google Scholar]
- [126].Veronese FM, Pasut G, PEGylation, successful approach to drug delivery, Drug Discovery Today, 10 (2005) 1451–1458. [DOI] [PubMed] [Google Scholar]
- [127].Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung W-J, Porter JE, Ehrlich GK, Pan W, Xu Z-X, Modi MW, Farid A, Berthold W, Graves M, Rational Design of a Potent, Long-Lasting Form of Interferon: A 40 kDa Branched Polyethylene Glycol-Conjugated Interferon α−2a for the Treatment of Hepatitis C, Bioconjugate Chemistry, 12 (2001) 195–202. [DOI] [PubMed] [Google Scholar]
- [128].Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A, PEG — A versatile conjugating ligand for drugs and drug delivery systems, Journal of Controlled Release, 192 (2014) 67–81. [DOI] [PubMed] [Google Scholar]
- [129].Liu Z, Robinson JT, Sun X, Dai H, PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs, Journal of the American Chemical Society, 130 (2008) 10876–10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Greenwald RB, Choe YH, Mcguire J, Conover CD, Effective drug delivery by PEGylated drug conjugates, Advanced Drug Delivery Reviews, 55 (2003) 217–250. [DOI] [PubMed] [Google Scholar]
- [131].Monfardini C, Schiavon O, Caliceti P, Morpurgo M, Harris JM, Veronese FM, A BRANCHED MONOMETHOXYPOLY(ETHYLENE GLYCOL) FOR PROTEIN MODIFICATION, Bioconjugate Chemistry, 6 (1995) 62–69. [DOI] [PubMed] [Google Scholar]
- [132].Liu Z, Davis C, Cai W, He L, Chen X, Dai H, Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy, Proceedings of the National Academy of Sciences, 105 (2008) 1410–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai H, PEG Branched Polymer for Functionalization of Nanomaterials with Ultralong Blood Circulation, Journal of the American Chemical Society, 131 (2009) 4783–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Fee CJ, Size comparison between proteins PEGylated with branched and linear poly(ethylene glycol) molecules, Biotechnology and Bioengineering, 98 (2007) 725–731. [DOI] [PubMed] [Google Scholar]
- [135].Yamaoka T, Tabata Y, Ikada Y, Distribution and Tissue Uptake of Poly(ethylene glycol) with Different Molecular Weights after Intravenous Administration to Mice, Journal of Pharmaceutical Sciences, 83 (1994) 601–606. [DOI] [PubMed] [Google Scholar]
- [136].Hassanin IA, Elzoghby AO, Self-assembled non-covalent protein-drug nanoparticles: an emerging delivery platform for anti-cancer drugs, Expert Opinion on Drug Delivery, 17 (2020) 1437–1458. [DOI] [PubMed] [Google Scholar]
- [137].Zamani M, Rostamizadeh K, Kheiri Manjili H, Danafar H, In vitro and in vivo biocompatibility study of folate-lysine-PEG-PCL as nanocarrier for targeted breast cancer drug delivery, European Polymer Journal, 103 (2018) 260–270. [Google Scholar]
- [138].Dozier JK, Distefano MD, Site-Specific PEGylation of Therapeutic Proteins, Int J Mol Sci, 16 (2015) 25831–25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Basu A, Yang K, Wang M, Liu S, Chintala R, Palm T, Zhao H, Peng P, Wu D, Zhang Z, Hua J, Hsieh M-C, Zhou J, Petti G, Li X, Janjua A, Mendez M, Liu J, Longley C, Zhang Z, Mehlig M, Borowski V, Viswanathan M, Filpula D, Structure−Function Engineering of Interferon-β−1b for Improving Stability, Solubility, Potency, Immunogenicity, and Pharmacokinetic Properties by Site-Selective Mono-PEGylation, Bioconjugate Chemistry, 17 (2006) 618–630. [DOI] [PubMed] [Google Scholar]
- [140].Kontermann RE, Strategies for extended serum half-life of protein therapeutics, Current Opinion in Biotechnology, 22 (2011) 868–876. [DOI] [PubMed] [Google Scholar]
- [141].Munasinghe A, Mathavan A, Mathavan A, Lin P, Colina CM, Molecular Insight into the Protein–Polymer Interactions in N-Terminal PEGylated Bovine Serum Albumin, The Journal of Physical Chemistry B, 123 (2019) 5196–5205. [DOI] [PubMed] [Google Scholar]
- [142].Sadiki A, Vaidya SR, Abdollahi M, Bhardwaj G, Dolan ME, Turna H, Arora V, Sanjeev A, Robinson TD, Koid A, Amin A, Zhou ZS, Site-specific conjugation of native antibody, Antib Ther, 3 (2020) 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yallapu MM, Foy SP, Jain TK, Labhasetwar V, PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications, Pharmaceutical Research, 27 (2010) 2283–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Xiong Z, Zhao L, Wang F, Zhu J, Qin H, Wu RA, Zhang W, Zou H, Synthesis of branched PEG brushes hybrid hydrophilic magnetic nanoparticles for the selective enrichment of N-linked glycopeptides, Chemical Communications, 48 (2012) 8138. [DOI] [PubMed] [Google Scholar]
- [145].Roberts MJ, Milton Harris J, Attachment of Degradable Poly(Ethylene Glycol) to Proteins has the Potential to Increase Therapeutic Efficacy, Journal of Pharmaceutical Sciences, 87 (1998) 1440–1445. [DOI] [PubMed] [Google Scholar]
- [146].Chowdhury SM, Surhland C, Sanchez Z, Chaudhary P, Suresh Kumar MA, Lee S, Peña LA, Waring M, Sitharaman B, Naidu M, Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme, Nanomedicine: Nanotechnology, Biology and Medicine, 11 (2015) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gao D, Tang, Duan, Tong, Oleanolic acid liposomes with polyethylene glycol modification: promising antitumor drug delivery, International Journal of Nanomedicine, (2012) 3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Klibanov AL, Maruyama K, Torchilin VP, Huang L, Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes, FEBS Letters, 268 (1990) 235–237. [DOI] [PubMed] [Google Scholar]
- [149].Le NTT, Nguyen DTD, Nguyen NH, Nguyen CK, Nguyen DH, Methoxy polyethylene glycol–cholesterol modified soy lecithin liposomes for poorly water-soluble anticancer drug delivery, Journal of Applied Polymer Science, 138 (2021) 49858. [Google Scholar]
- [150].Lukyanov AN, Gao Z, Mazzola L, Torchilin VP, Polyethylene Glycol-Diacyllipid Micelles Demonstrate Increased Acculumation in Subcutaneous Tumors in Mice, Pharmaceutical Research, 19 (2002) 1424–1429. [DOI] [PubMed] [Google Scholar]
- [151].Allen TM, Liposomes, Drugs, 54 (1997) 8–14. [DOI] [PubMed] [Google Scholar]
- [152].Lu Y, Aimetti AA, Langer R, Gu Z, Bioresponsive materials, Nat. Rev. Mater, 2 (2017) 17. [Google Scholar]
- [153].Milla P, Dosio F, Cattel L, PEGylation of Proteins and Liposomes: a Powerful and Flexible Strategy to Improve the Drug Delivery, Current Drug Metabolism, 13 (2012) 105–119. [DOI] [PubMed] [Google Scholar]
- [154].Nguyen AK, Nguyen TH, Bao BQ, Bach LG, Nguyen DH, Efficient Self-Assembly of mPEG End-Capped Porous Silica as a Redox-Sensitive Nanocarrier for Controlled Doxorubicin Delivery, Int. J. Biomater, 2018 (2018) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Li J, Wu N, Wu J, Wan Y, Liu C, Effect of protein adsorption on cell uptake and blood clearance of methoxy poly(ethylene glycol)-poly(caprolactone) nanoparticles, Journal of Applied Polymer Science, 133 (2016) n/a-n/a. [Google Scholar]
- [156].Shan X, Mao J, Long M, Ahmed KS, Sun C, Qiu L, Chen J, Influence of polyethylene glycol molecular weight on the anticancer drug delivery of pH-sensitive polymeric micelle, Journal of Applied Polymer Science, 136 (2019) 47854. [Google Scholar]
- [157].Gu Z, Biswas A, Zhao M, Tang Y, Tailoring nanocarriers for intracellular protein delivery, Chemical Society Reviews, 40 (2011) 3638. [DOI] [PubMed] [Google Scholar]
- [158].Cadinoiu AN, Rata DM, Atanase LI, Daraba OM, Gherghel D, Vochita G, Popa M, Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma, Polymers, 11 (2019) 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lankveld DP, Rayavarapu RG, Krystek P, Oomen AG, Verharen HW, van Leeuwen TG, De Jong WH, Manohar S, Blood clearance and tissue distribution of PEGylated and non-PEGylated gold nanorods after intravenous administration in rats, Nanomedicine (Lond), 6 (2011) 339–349. [DOI] [PubMed] [Google Scholar]
- [160].Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, Kissel T, Parak WJ, Kreyling WG, Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection, Biomaterials, 31 (2010) 6574–6581. [DOI] [PubMed] [Google Scholar]
- [161].Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS, Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice, ACS Nano, 6 (2012) 10366–10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Chang CJ, Chen CH, Chen BM, Su YC, Chen YT, Hershfield MS, Lee MM, Cheng TL, Chen YT, Roffler SR, Wu JY, A genome-wide association study identifies a novel susceptibility locus for the immunogenicity of polyethylene glycol, Nat Commun, 8 (2017) 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Chang TC, Chen BM, Lin WW, Yu PH, Chiu YW, Chen YT, Wu JY, Cheng TL, Hwang DY, Roffler AS, Both IgM and IgG Antibodies Against Polyethylene Glycol Can Alter the Biological Activity of Methoxy Polyethylene Glycol-Epoetin Beta in Mice, Pharmaceutics, 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chen BM, Su YC, Chang CJ, Burnouf PA, Chuang KH, Chen CH, Cheng TL, Chen YT, Wu JY, Roffler SR, Measurement of Pre-Existing IgG and IgM Antibodies against Polyethylene Glycol in Healthy Individuals, Anal Chem, 88 (2016) 10661–10666. [DOI] [PubMed] [Google Scholar]
- [165].Chen E, Chen BM, Su YC, Chang YC, Cheng TL, Barenholz Y, Roffler SR, Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex, ACS nano, 14 (2020) 7808–7822. [DOI] [PubMed] [Google Scholar]
- [166].Fang JL, Beland FA, Tang Y, Roffler SR, Flow cytometry analysis of anti-polyethylene glycol antibodies in human plasma, Toxicol Rep, 8 (2021) 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Fix SM, Nyankima AG, McSweeney MD, Tsuruta JK, Lai SK, Dayton PA, Accelerated Clearance of Ultrasound Contrast Agents Containing Polyethylene Glycol is Associated with the Generation of Anti-Polyethylene Glycol Antibodies, Ultrasound Med Biol, 44 (2018) 1266–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Henry CE, Wang YY, Yang Q, Hoang T, Chattopadhyay S, Hoen T, Ensign LM, Nunn KL, Schroeder H, McCallen J, Moench T, Cone R, Roffler SR, Lai SK, Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus, Acta Biomater, 43 (2016) 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Khalil A, Würthwein G, Golitsch J, Hempel G, Fobker M, Gerss J, Möricke A, Zimmermann M, Smisek P, Zucchetti M, Nath C, Attarbaschi A, Von Stackelberg A, Gökbuget N, Rizzari C, Conter V, Schrappe M, Boos J, Lanvers-Kaminsky C, Pre-existing antibodies against polyethylene glycol reduce asparaginase activities on first administration of pegylated E. coli asparaginase in children with acute lymphocytic leukemia, Haematologica, Online ahead of print (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kozma GT, Shimizu T, Ishida T, Szebeni J, Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals, Adv Drug Deliv Rev, 154–155 (2020) 163–175. [DOI] [PubMed] [Google Scholar]
- [171].Lubich C, Allacher P, de la Rosa M, Bauer A, Prenninger T, Horling FM, Siekmann J, Oldenburg J, Scheiflinger F, Reipert BM, The Mystery of Antibodies Against Polyethylene Glycol (PEG) - What do we Know?, Pharm Res, 33 (2016) 2239–2249. [DOI] [PubMed] [Google Scholar]
- [172].McCallen J, Prybylski J, Yang Q, Lai SK, Cross-Reactivity of Select PEG-Binding Antibodies to Other Polymers Containing a C-C-O Backbone, ACS Biomater Sci Eng, 3 (2017) 1605–1615. [DOI] [PubMed] [Google Scholar]
- [173].Poppenborg SM, Wittmann J, Walther W, Brandenburg G, Krähmer R, Baumgart J, Leenders F, Impact of anti-PEG IgM antibodies on the pharmacokinetics of pegylated asparaginase preparations in mice, Eur J Pharm Sci, 91 (2016) 122–130. [DOI] [PubMed] [Google Scholar]
- [174].Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, Lai SK, Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population, Anal Chem, 88 (2016) 11804–11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yang Q, Lai SK, Anti-PEG immunity: emergence, characteristics, and unaddressed questions, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7 (2015) 655–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].USFDA, Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products, 2014. [Google Scholar]
- [177].Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H, Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes, J Control Release, 112 (2006) 15–25. [DOI] [PubMed] [Google Scholar]
- [178].Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N, T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes, Int J Pharm, 392 (2010) 218–223. [DOI] [PubMed] [Google Scholar]
- [179].Tagami T, Nakamura K, Shimizu T, Ishida T, Kiwada H, Effect of siRNA in PEG-coated siRNAlipoplex on anti-PEG IgM production, J Control Release, 137 (2009) 234–240. [DOI] [PubMed] [Google Scholar]
- [180].Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H, CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes, J Control Release, 142 (2010) 160–166. [DOI] [PubMed] [Google Scholar]
- [181].Tagami T, Uehara Y, Moriyoshi N, Ishida T, Kiwada H, Anti-PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA, J Control Release, 151 (2011) 149–154. [DOI] [PubMed] [Google Scholar]
- [182].Laverman P, Brouwers AH, Dams ET, Oyen WJ, Storm G, van Rooijen N, Corstens FH, Boerman OC, Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose, J Pharmacol Exp Ther, 293 (2000) 996–1001. [PubMed] [Google Scholar]
- [183].Laverman P, Carstens MG, Boerman OC, Dams ET, Oyen WJ, van Rooijen N, Corstens FH, Storm G, Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection, J Pharmacol Exp Ther, 298 (2001) 607–612. [PubMed] [Google Scholar]
- [184].Kaminskas LM, McLeod VM, Porter CJ, Boyd BJ, Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance, J Pharm Sci, 100 (2011) 5069–5077. [DOI] [PubMed] [Google Scholar]
- [185].Wang XY, Ishida T, Ichihara M, Kiwada H, Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats, J Control Release, 104 (2005) 91–102. [DOI] [PubMed] [Google Scholar]
- [186].Koide H, Asai T, Hatanaka K, Urakami T, Ishii T, Kenjo E, Nishihara M, Yokoyama M, Ishida T, Kiwada H, Oku N, Particle size-dependent triggering of accelerated blood clearance phenomenon, Int J Pharm, 362 (2008) 197–200. [DOI] [PubMed] [Google Scholar]
- [187].Ishida T, Maeda R, Ichihara M, Irimura K, Kiwada H, Accelerated clearance of PEGylated liposomes in rats after repeated injections, J Control Release, 88 (2003) 35–42. [DOI] [PubMed] [Google Scholar]
- [188].Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H, Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes, J Control Release, 105 (2005) 305–317. [DOI] [PubMed] [Google Scholar]
- [189].Ishida T, Atobe K, Wang X, Kiwada H, Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection, J Control Release, 115 (2006) 251–258. [DOI] [PubMed] [Google Scholar]
- [190].Ishida T, Ichikawa T, Ichihara M, Sadzuka Y, Kiwada H, Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice, J Control Release, 95 (2004) 403–412. [DOI] [PubMed] [Google Scholar]
- [191].Ishida T, Kashima S, Kiwada H, The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats, J Control Release, 126 (2008) 162–165. [DOI] [PubMed] [Google Scholar]
- [192].Ishida T, Kiwada H, Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes, Int J Pharm, 354 (2008) 56–62. [DOI] [PubMed] [Google Scholar]
- [193].Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H, PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner, J Control Release, 122 (2007) 349–355. [DOI] [PubMed] [Google Scholar]
- [194].Ishihara T, Maeda T, Sakamoto H, Takasaki N, Shigyo M, Ishida T, Kiwada H, Mizushima Y, Mizushima T, Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers, Biomacromolecules, 11 (2010) 2700–2706. [DOI] [PubMed] [Google Scholar]
- [195].Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, Yuki K, Tanaka K, Takenaga M, Igarashi R, Maeda T, Yamakawa N, Okamoto Y, Otsuka M, Ishida T, Kiwada H, Mizushima Y, Mizushima T, Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles, Pharm Res, 26 (2009) 2270–2279. [DOI] [PubMed] [Google Scholar]
- [196].Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC, Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes, J Pharmacol Exp Ther, 292 (2000) 1071–1079. [PubMed] [Google Scholar]
- [197].Coins B, Philips WT, Klipper R, Repeat Injection Studies of Technetium-99M-Labeled Peg-Liposomes in the Same Animal, J Liposome Research, 8 (1998) 265–281. [Google Scholar]
- [198].Arima Y, Toda M, Iwata H, Complement activation on surfaces modified with ethylene glycol units, Biomaterials, 29 (2008) 551–560. [DOI] [PubMed] [Google Scholar]
- [199].Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MG, Role of the methoxy group in immune responses to mPEG-protein conjugates, Bioconjug Chem, 23 (2012) 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Francis GE, Fisher D, Delgado C, Malik F, Gardiner A, Neale D, PEGylation of cytokines and other therapeutic proteins and peptides: the importance of biological optimisation of coupling techniques, Int J Hematol, 68 (1998) 1–18. [DOI] [PubMed] [Google Scholar]
- [201].Richter AW, Akerblom E, Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors, Int Arch Allergy Appl Immunol, 74 (1984) 36–39. [DOI] [PubMed] [Google Scholar]
- [202].Leger RM, Arndt P, Garrathy, Normal donor sera contain antibodies tp polyethyleneglycol (PEG), Transfusion, 41 (2001). [Google Scholar]
- [203].Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G, Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients, Cancer, 110 (2007) 103–111. [DOI] [PubMed] [Google Scholar]
- [204].Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS, Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase, Arthritis Res Ther, 8 (2006) R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Armstrong JK, Leger RM, Wanby RB, Meiselman HJ, Garratty G, Fisher TC, Occurance of an antibody to poly(ethylene glycol) in normal donors, Blood, 102 (2003). [Google Scholar]
- [206].Chen E, Chen BM, Su YC, Chang YC, Cheng TL, Barenholz Y, Roffler SR, Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex, ACS nano, ( 10.1021/acsnano.9b07218) (2020) () [DOI] [PubMed] [Google Scholar]
- [207].Henry CE, Wang YY, Yang Q, Hoang T, Chattopadhyay S, Hoen T, Ensign LM, Nunn KL, Schroeder H, McCallen J, Moench T, Cone R, Roffler SR, Lai SK, Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus, Acta Biomater, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP, The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation, Polymers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zhang P, Sun F, Liu S, Jiang S, Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation, J Control Release, 244 (2016) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Borresen B, Henriksen JR, Clergeaud G, Jorgensen JS, Melander F, Elema DR, Szebeni J, Engelholm SA, Kristensen AT, Kjaer A, Andresen TL, Hansen AE, Theranostic Imaging May Vaccinate against the Therapeutic Benefit of Long Circulating PEGylated Liposomes and Change Cargo Pharmacokinetics, ACS nano, 12 (2018) 11386–11398. [DOI] [PubMed] [Google Scholar]
- [211].Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, Szebeni J, Ishida T, PEGylated liposomes: immunological responses, Sci Technol Adv Mater, 20 (2019) 710–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Browne EK, Moore C, Sykes A, Lu Z, Jeha S, Mandrell BN, Clinical Characteristics of Intravenous PEG-Asparaginase Hypersensitivity Reactions in Patients Undergoing Treatment for Acute Lymphoblastic Leukemia [Formula: see text], J Pediatr Oncol Nurs, 35 (2018) 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Henriksen LT, Harila-Saari A, Ruud E, Abrahamsson J, Pruunsild K, Vaitkeviciene G, Jonsson OG, Schmiegelow K, Heyman M, Schroder H, Albertsen BK, Nordic H Society of Paediatric, g. Oncology, PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol, Pediatr Blood Cancer, 62 (2015) 427–433. [DOI] [PubMed] [Google Scholar]
- [214].Dadla A, Tannenbaum S, Yates B, Holle L, Delayed hypersensitivity reaction related to the use of pegfilgrastim, Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners, 21 (2015) 474–477. [DOI] [PubMed] [Google Scholar]
- [215].Fischbach M, Wuhl E, Reigner SCM, Morgan Z, Schaefer F, Efficacy and Long-Term Safety of C.E.R.A. Maintenance in Pediatric Hemodialysis Patients with Anemia of CKD, Clin J Am Soc Nephrol, 13 (2018) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Larimore K, Nguyen T, Badillo B, Lau K, Zori R, Shepherd G, Zoog SJ, Weng HH, Gupta S, Depletion of interfering IgG and IgM is critical to determine the role of IgE in pegvaliase-associated hypersensitivity, J Immunol Methods, 468 (2019) 20–28. [DOI] [PubMed] [Google Scholar]
- [217].Weinhandl ED, Gilbertson DT, Collins AJ, Foley RN, Relative safety of peginesatide and epoetin alfa, Pharmacoepidemiol Drug Saf, 23 (2014) 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Calabrese LH, Kavanaugh A, Yeo AE, Lipsky PE, Frequency, distribution and immunologic nature of infusion reactions in subjects receiving pegloticase for chronic refractory gout, Arthritis Res Ther, 19 (2017) 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, Hershfield MS, Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer, J Allergy Clin Immunol, 137 (2016) 1610–1613 e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Povsic TJ, Lawrence MG, Lincoff AM, Mehran R, Rusconi CP, Zelenkofske SL, Huang Z, Sailstad J, Armstrong PW, Steg PG, Bode C, Becker RC, Alexander JH, Adkinson NF, Levinson AI, Investigators R-P, Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer, J Allergy Clin Immunol, 138 (2016) 1712–1715. [DOI] [PubMed] [Google Scholar]
- [221].Povsic TJ, Vavalle JP, Aberle LH, Kasprzak JD, Cohen MG, Mehran R, Bode C, Buller CE, Montalescot G, Cornel JH, Rynkiewicz A, Ring ME, Zeymer U, Natarajan M, Delarche N, Zelenkofske SL, Becker RC, Alexander JH, Investigators R, A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial, Eur Heart J, 34 (2013) 2481–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Staudacher DL, Putz V, Heger L, Reinohl J, Hortmann M, Zelenkofske SL, Becker RC, Rusconi CP, Bode C, Ahrens I, Direct factor IXa inhibition with the RNA-aptamer pegnivacogin reduces platelet reactivity in vitro and residual platelet aggregation in patients with acute coronary syndromes, Eur Heart J Acute Cardiovasc Care, (2017) 2048872617703065. [DOI] [PubMed] [Google Scholar]
- [223].Carrol J, Top 10 Phase III disasters of 2014, Fierce Biotech, 2014. [Google Scholar]
- [224].Sundy JS, Becker MA, Baraf HS, Barkhuizen A, Moreland LW, Huang W, Waltrip RW 2nd, Maroli AN, Horowitz Z, I. Pegloticase Phase 2 Study, Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study, Arthritis Rheum, 58 (2008) 2882–2891. [DOI] [PubMed] [Google Scholar]
- [225].Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS, Induced and preexisting anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients, Arthritis Res Ther, 16 (2014) R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Prescrire, Pegloticase: withdrawal of its European marketing authorisation is welcome, Prescrire Int, 26 (2017) 71. [PubMed] [Google Scholar]
- [227].Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S, The incidence and management of infusion reactions to infliximab: a large center experience, Am.J Gastroenterol, 98 (2003) 1315–1324. [DOI] [PubMed] [Google Scholar]
- [228].Lenz HJ, Management and preparedness for infusion and hypersensitivity reactions, Oncologist, 12 (2007) 601–609. [DOI] [PubMed] [Google Scholar]
- [229].Mayer L, Young Y, Infusion reactions and their management, Gastroenterol.Clin.North Am, 35 (2006) 857–866. [DOI] [PubMed] [Google Scholar]
- [230].Vogel WH, Infusion reactions: Diagnosing, Asessment and management, Clinical Journal of Oncology Nursing, 14 (2010) E10–E 21. [DOI] [PubMed] [Google Scholar]
- [231].Gamazo C, García-Azpíroz M, Souza Rebouças J, Gastaminza G, Ferrer M, Irache JM, Oral immunotherapy using polymeric nanoparticles loaded with peanut proteins in a murine model of fatal anaphylaxis, Immunotherapy, 9 (2017) 1205–1217. [DOI] [PubMed] [Google Scholar]
- [232].Dezsi L, Fulop T, Meszaros T, Szenasi G, Urbanics R, Vazsonyi C, Orfi E, Rosivall L, Nemes R, Kok RJ, Metselaar JM, Storm G, Szebeni J, Features of complement activation-related pseudoallergy to liposomes with different surface charge and PEGylation: comparison of the porcine and rat responses, J Control Release, 195 (2014) 2–10. [DOI] [PubMed] [Google Scholar]
- [233].Dezsi L, Meszaros T, Orfi E, Fulop TG, Hennies M, Rosivall L, Hamar P, Szebeni J, Szenasi G, Complement Activation-Related Pathophysiological Changes in Anesthetized Rats: Activator-Dependent Variations of Symptoms and Mediators of Pseudoallergy, Molecules, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Mészáros T, Szénási G, Rosivall L, Szebeni J, Dézsi L, Paradoxical rise of hemolytic complement in the blood of mice during zymosan- and liposome induced CARPA: a pilot study, Eur. J. Nanomed , 7 (2015) 257–262. [Google Scholar]
- [235].Orfi E, Meszaros T, Hennies M, Fulop T, Dezsi L, Nardocci A, Rosivall L, Hamar P, Neun BW, Dobrovolskaia MA, Szebeni J, Szenasi G, Acute physiological changes caused by complement activators and amphotericin B-containing liposomes in mice, Int J Nanomedicine, 14 (2019) 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Szebeni J, Complement activation-related pseudoallergy caused by amphiphilic drug carriers: the role of lipoproteins, Current Drug Delivery, 2 (2005) 443–449. [DOI] [PubMed] [Google Scholar]
- [237].Szebeni J, Complement mediated hypersensitivity to nanomedicines, Molecular Immunology, 48 (2011) 1715. [Google Scholar]
- [238].Szebeni J, Complement activation-related pseudoallergy: A stress reaction in blood triggered by nanomedicines and biologicals, Mol. Immunol, 61 (2014) 163–173. [DOI] [PubMed] [Google Scholar]
- [239].Szebeni J, Alving CR, Complement-mediated acute effects of liposome-encapsulated hemoglobin, Artif Cells Blood Substit Immobil Biotechnol, 27 (1999) 23–41. [DOI] [PubMed] [Google Scholar]
- [240].Szebeni J, Bedocs P, Csukas D, Rosivall L, Bunger R, Urbanics R, A porcine model of complement-mediated infusion reactions to drug carrier nanosystems and other medicines, Adv Drug Deliv Rev, 64 (2012) 1706–1716. [DOI] [PubMed] [Google Scholar]
- [241].Szebeni J, Bedőcs P, Dézsi L, Urbanics R, A porcine model of complement activation-related pseudoallergy to nano-pharmaceuticals: Pros and cons of translation to a preclinical safety test, Prec. Nanomed , 1 (2018) 63–73. [Google Scholar]
- [242].Dézsi L, Szénási G, Urbanics R, Rosivall L, Szebeni J, Cardiopulmonary and hemodynamic changes in complement activation-related pseudoallergy, (2013). [Google Scholar]
- [243].Hugli TE, Morgan EL, Mechanisms of leukocyte regulation by complement-derived factors., Contemp Top Immunobiol, 14 (1984) 109–153. [DOI] [PubMed] [Google Scholar]
- [244].Hugli TE, Stimler NP, Gerard C, Moon KE, Possible role of serum anaphylatoxins in hypersensitivity reactions, Int Arch Allergy Appl Immunol, 66 Suppl 1 (1981) 113–120. [DOI] [PubMed] [Google Scholar]
- [245].Heideman M, Hugli TE, Anaphylatoxin generation in multisystem organ failure., J Trauma, 24 (1984) 1038–1043. [DOI] [PubMed] [Google Scholar]
- [246].Lundberg C, Gardinali M, Hugli TE, Complement activation and membrane lipids in lung vascular injury, Am Rev Respir Dis, 136 (1987) 459–462. [DOI] [PubMed] [Google Scholar]
- [247].Lundberg C, Marceau F, Hugli TE, C5a-induced hemodynamic and hematologic changes in the rabbit. Role of cyclooxygenase products and polymorphonuclear leukocytes, Am J Pathol, 128 (1987) 471–483. [PMC free article] [PubMed] [Google Scholar]
- [248].Marceau F, Lundberg C, Hugli TE, Effects of anaphylatoxins on circulation, Immunopharmacol, 14 (1987) 67–84. [DOI] [PubMed] [Google Scholar]
- [249].Szebeni J, The interaction of liposomes with the complement system., Crit Rev Ther Drug Carrier Syst, 15 (1998) 57–88. [PubMed] [Google Scholar]
- [250].Szebeni J, The Complement System: Novel Roles in Health and Disease, Kluwer, Boston, 2004, pp. 512. [Google Scholar]
- [251].Szebeni J, Complement Activation-Related Pseudoallergy, The Complement System, (2004) 361–396. [Google Scholar]
- [252].Szebeni J, Nanomedicine: application of nanotechnology in medicine. Opportunities in neuropsychiatry, Neuropsychopharmacol. Hung, 13 (2011) 15–24. [PubMed] [Google Scholar]
- [253].Szebeni J, Jiskoot W, Immunological Issues with Nanomedicines: Immunogenicity, Hypersensitivity, Accelerated Clearance and Immune Suppression, in: Torchillin V (Ed.) Handbook of Nanobiomedical Research, World Scientific, Singapore, 2014, pp. 45–73. [Google Scholar]
- [254].Szebeni J, Muggia F, Gabizon A, Barenholz Y, Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention, Adv Drug Deliv Rev, 63 (2011) 1020–1030. [DOI] [PubMed] [Google Scholar]
- [255].Kozma GT, Meszaros T, Vashegyi I, Fulop T, Orfi E, Dezsi L, Rosivall L, Bavli Y, Urbanics R, Mollnes TE, Barenholz Y, Szebeni J, Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions, ACS nano, 13 (2019) 9315–9324. [DOI] [PubMed] [Google Scholar]
- [256].Milosevits G, Meszaros T, Orfi E, Bakos T, Garami M, Kovacs G, Dezsi L, Hamar P, Gyorffy B, Szabo A, Szenasi G, Szebeni J, Complement-mediated hypersensitivity reactions to an amphotericin Bcontaining lipid complex (Abelcet) in pediatric patients and anesthetized rats: Benefits of slow infusion, Nanomedicine, 34 (2021) 102366. [DOI] [PubMed] [Google Scholar]
- [257].SZEBENI J, The Complement System and the Kidney: Interplays in Health and Disease, Nephrology, Hypertension, Dialysis, Transplantation, (2006) 123. [Google Scholar]
- [258].Szebeni J, Alving CR, Baranyi L, Bunger R, Interactions of Liposomes with Complement Leading to Adverse Reactions, Liposome Technology, Volume III: Interactions of Liposomes with the Biological Milieu, 3 (2006) 1. [Google Scholar]
- [259].Szebeni J, Baranyi L, Savay S, Bodo M, Milosevits J, Alving CR, Bunger R, Complement activation-related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function, Am J Physiol Heart Circ Physiol, 290 (2006) H1050–1058. [DOI] [PubMed] [Google Scholar]
- [260].Szebeni J, Juhasz B, Koltai C, Szabo Z, Szekely L, Rakoczy G, Rosivall L, Horkay F, Complement activation during heart surgery with cardiopulmonary bypass: lack of correlation between soluble terminal complex formation and inflammatory cell response, MOLECULAR IMMUNOLOGY, PERGAMON-ELSEVIER SCIENCE LTD THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLAND, 2006, pp. 171–171. [Google Scholar]
- [261].Castells MC, Phillips EJ, Maintaining Safety with SARS-CoV-2 Vaccines. Reply, N Engl J Med, 384 (2021) e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Hatziantoniou S, Maltezou HC, Tsakris A, Poland GA, Anastassopoulou C, Anaphylactic reactions to mRNA COVID-19 vaccines: A call for further study, Vaccine, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Kelso JM, Anaphylactic reactions to novel mRNA SARS-CoV-2/COVID-19 vaccines, Vaccine, 39 (2021) 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Klimek L, Bergmann KC, Brehler R, Practical handling of allergic reactions to COVID-19 vaccines, Allergo J Int, 10.1007/s40629-021-00165-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Rodriguez-Nava G, Egoryan G, Trelles-Garcia DP, Yanez-Bello MA, Murguia-Fuentes R, Disproportionality Analysis of Anaphylactic Reactions after Vaccination with mRNA COVID-19 Vaccines in the United States, Ann Allergy Asthma Immunol, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Kauffman KJ, Webber MJ, Anderson DG, Materials for non-viral intracellular delivery of messenger RNA therapeutics, J Control Release, 240 (2016) 227–234. [DOI] [PubMed] [Google Scholar]
- [267].Rönnau AC, Wulferink M, Gleichmann E, Unver E, Ruzicka T, Krutmann J, Grewe M, Anaphylaxis to polyvinylpyrrolidone in an analgesic preparation, Br J Dermatol, 143 (2000) 1055–1058. [DOI] [PubMed] [Google Scholar]
- [268].Palacios Castaño MI, Venturini Díaz M, Lobera Labairu T, González Mahave I, Del Pozo Gil MD, Blasco Sarramián A, Anaphylaxis Due to the Excipient Polysorbate 80, J Investig Allergol Clin Immunol, 26 (2016) 394–396. [DOI] [PubMed] [Google Scholar]
- [269].Fairhurst D, Wilkinson M, Independent sensitization to polidocanol and trometamol or glycerol within same product, Contact dermatitis, 56 (2007) 179. [DOI] [PubMed] [Google Scholar]
- [270].Tamagawa-Mineoka R, Katoh N, Kishimoto S, Allergic contact dermatitis due to 1,3-butylene glycol and glycerol, Contact dermatitis, 56 (2007) 297–298. [DOI] [PubMed] [Google Scholar]
- [271].Washizaki K, Kanto H, Yazaki S, Ito M, A case of allergic contact dermatitis to polyglyceryl laurate, Contact dermatitis, 58 (2008) 187–188. [DOI] [PubMed] [Google Scholar]
- [272].He Z, Wan X, Schulz A, Bludau H, Dobrovolskaia MA, Stern ST, Montgomery SA, Yuan H, Li Z, Alakhova D, Sokolsky M, Darr DB, Perou CM, Jordan R, Luxenhofer R, Kabanov AV, A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity, Biomaterials, 101 (2016) 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Tavano R, Gabrielli L, Lubian E, Fedeli C, Visentin S, Polverino De Laureto P, Arrigoni G, Geffner-Smith A, Chen F, Simberg D, Morgese G, Benetti EM, Wu L, Moghimi SM, Mancin F, Papini E, C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes, ACS Nano, 12 (2018) 5834–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Kierstead PH, Okochi H, Venditto VJ, Chuong TC, Kivimae S, Fréchet JMJ, Szoka FC, The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes, J Control Release, 213 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Bakaltcheva I, Ganong JP, Holtz BL, Peat RA, Reid T, Effects of high-molecular-weight cryoprotectants on platelets and the coagulation system, Cryobiology, 40 (2000) 283–293. [DOI] [PubMed] [Google Scholar]
- [276].Bakaltcheva I, Reid T, Effects of blood product storage protectants on blood coagulation, Transfus Med Rev, 17 (2003) 263–271. [DOI] [PubMed] [Google Scholar]
- [277].Mulloy B, Hogwood J, Gray E, Lever R, Page CP, Pharmacology of Heparin and Related Drugs, Pharmacol Rev, 68 (2016) 76–141. [DOI] [PubMed] [Google Scholar]
- [278].FDA, ImmunogenicityRelated Considerations for Low Molecular Weight Heparin Guidance for Industry, 2016. [Google Scholar]
- [279].Arepally GM, Ortel TL, Heparin-induced thrombocytopenia, Annu Rev Med, 61 (2010) 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Fan X, Dong M, Li T, Ma Q, Yin Y, Two Cases of Adverse Reactions of Hyaluronic Acid-based Filler Injections, Plast Reconstr Surg Glob Open, 4 (2016) e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Rowland-Warmann MJ, Hypersensitivity reaction to Hyaluronic Acid Dermal filler following novel Coronavirus infection - a case report, J Cosmet Dermatol, 20 (2021) 1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Turkmani MG, De Boulle K, Philipp-Dormston WG, Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness, Clin Cosmet Investig Dermatol, 12 (2019) 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Elsabahy M, Li A, Zhang F, Sultan D, Liu Y, Wooley KL, Differential immunotoxicities of poly(ethylene glycol)- vs. poly(carboxybetaine)-coated nanoparticles, J Control Release, 172 (2013) 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Li B, Yuan Z, Hung HC, Ma J, Jain P, Tsao C, Xie J, Zhang P, Lin X, Wu K, Jiang S, Revealing the Immunogenic Risk of Polymers, Angew Chem Int Ed Engl, 57 (2018) 13873–13876. [DOI] [PubMed] [Google Scholar]
- [285].Karabasz A, Szczepanowicz K, Cierniak A, Mezyk-Kopec R, Dyduch G, Szczęch M, Bereta J, Bzowska M, In vivo Studies on Pharmacokinetics, Toxicity and Immunogenicity of Polyelectrolyte Nanocapsules Functionalized with Two Different Polymers: Poly-L-Glutamic Acid or PEG, Int J Nanomedicine, 14 (2019) 9587–9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Inomata N, Nomura Y, Ikezawa Z, Involvement of poly (γ-glutamic acid) as an allergen in late-onset anaphylaxis due to fermented soybeans (natto), J Dermatol, 39 (2012) 409–412. [DOI] [PubMed] [Google Scholar]
- [287].Inomata N, Chin K, Nagashima M, Ikezawa Z, Late-onset anaphylaxis due to poly (γ-glutamic acid) in the soup of commercial cold Chinese noodles in a patient with allergy to fermented soybeans (natto), Allergol Int, 60 (2011) 393–396. [DOI] [PubMed] [Google Scholar]
- [288].Wang X, Uto T, Akagi T, Akashi M, Baba M, Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccine, J Med Virol, 80 (2008) 11–19. [DOI] [PubMed] [Google Scholar]
- [289].Fernandes AI, Gregoriadis G, The effect of polysialylation on the immunogenicity and antigenicity of asparaginase: implication in its pharmacokinetics, Int J Pharm, 217 (2001) 215–224. [DOI] [PubMed] [Google Scholar]
- [290].Kim YH, Min KH, Wang Z, Kim J, Jacobson O, Huang P, Zhu G, Liu Y, Yung B, Niu G, Chen X, Development of Sialic Acid-coated Nanoparticles for Targeting Cancer and Efficient Evasion of the Immune System, Theranostics, 7 (2017) 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T, Structural basis for sialic acid-mediated self-recognition by complement factor H, Nat Chem Biol, 11 (2015) 77–82. [DOI] [PubMed] [Google Scholar]
- [292].Shade KC, Conroy ME, Washburn N, Kitaoka M, Huynh DJ, Laprise E, Patil SU, Shreffler WG, Anthony RM, Sialylation of immunoglobulin E is a determinant of allergic pathogenicity, Nature, 582 (2020) 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Watzlawik JO, Kahoud RJ, Ng S, Painter MM, Papke LM, Zoecklein L, Wootla B, Warrington AE, Carey WA, Rodriguez M, Polysialic acid as an antigen for monoclonal antibody HIgM12 to treat multiple sclerosis and other neurodegenerative disorders, J Neurochem, 134 (2015) 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Lambert J, Matthieu L, Dockx P, Contact dermatitis from acrylamide, Contact dermatitis, 19 (1988) 65. [DOI] [PubMed] [Google Scholar]
- [295].Gorman M, Chim YH, Hart A, Riehle MO, Urquhart AJ, Poly(N-acryloylmorpholine): a simple hydrogel system for temporal and spatial control over cell adhesion, J Biomed Mater Res A, 102 (2014) 1809–1815. [DOI] [PubMed] [Google Scholar]
- [296].PubChem, PubChem Compound Summary for CID 98723, 4-Acryloylmorpholine, in: N.C.f.B.I. National Library of Medicine (US) (Ed.)Bethesda (MD), 2021. [Google Scholar]
- [297].Gebauer M, Skerra A, Prospects of PASylation® for the design of protein and peptide therapeutics with extended half-life and enhanced action, Bioorg Med Chem, 26 (2018) 2882–2887. [DOI] [PubMed] [Google Scholar]
- [298].Anand R, Vallooran J,, 11 - Polypeptides: PASylation and XTEN,, in: Parambath A (Ed.) Engineering of Biomaterials for Drug Delivery Systems, Woodhead Publishing; 2018, pp. 299–315. [Google Scholar]
- [299].León A, Reuquen P, Garín C, Segura R, Vargas P, Zapata P, Orihuela P, FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2Methoxyestradiol, Applied Sciences, 7 (2017) 49. [Google Scholar]
- [300].Li Y-P, Pei Y-Y, Zhang X-Y, Gu Z-H, Zhou Z-H, Yuan W-F, Zhou J-J, Zhu J-H, Gao X-J, PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats, Journal of Controlled Release, 71 (2001) 203–211. [DOI] [PubMed] [Google Scholar]
- [301].Hong M, Zhu S, Jiang Y, Tang G, Sun C, Fang C, Shi B, Pei Y, Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles, Journal of Controlled Release, 141 (2010) 22–29. [DOI] [PubMed] [Google Scholar]
- [302].Ülkü Ö, Atakay M, Kohneshahri MY, Uzun C, Salih B, Site-Specific characterization of peptide-polymer conjugates in various stoichiometries by MALDI-Tandem mass spectrometry, Microchemical Journal, 152 (2020) 104467. [Google Scholar]
- [303].Trathnigg B, Maier B, Schulz G, Krüger R-P, Just U, Characterization of polyethers using different methods: SFC, LAC and SEC with different detectors, and MALDI-TOF-MS, Macromolecular Symposia, 110 (1996) 231–240. [Google Scholar]
- [304].Conway U, Warren AD, Arthur CJ, Gates PJ, A study of the application of graphite MALDI to the analysis of short-chain polyethylene glycols, Polymer Chemistry, 12 (2021) 439–448. [Google Scholar]
- [305].Gan CW, Feng S-S, Transferrin-conjugated nanoparticles of Poly(lactide)-d-α-Tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood–brain barrier, Biomaterials, 31 (2010) 7748–7757. [DOI] [PubMed] [Google Scholar]
- [306].Wöll S, Schiller S, Bachran C, Swee LK, Scherließ R, Pentaglycine lipid derivates – rp-HPLC analytics for bioorthogonal anchor molecules in targeted, multiple-composite liposomal drug delivery systems, International Journal of Pharmaceutics, 547 (2018) 602–610. [DOI] [PubMed] [Google Scholar]
- [307].Xie Y, Ding Y, Sun D, Wang G, Yang H, Xu H, Wang Z, Chen J, An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery, International Journal of Nanomedicine, (2015) 6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Ahmed OAA, El-Bassossy HM, El-Sayed HM, El-Hay SSA, Rp-HPLC Determination of Quercetin in a Novel D-α-Tocopherol Polyethylene Glycol 1000 Succinate Based SNEDDS Formulation: Pharmacokinetics in Rat Plasma, Molecules, 26 (2021) 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Heald CR, Stolnik S, Kujawinski KS, De Matteis C, Garnett MC, Illum L, Davis SS, Purkiss SC, Barlow RJ, Gellert PR, Poly(lactic acid)−Poly(ethylene oxide) (PLA−PEG) Nanoparticles: NMR Studies of the Central Solidlike PLA Core and the Liquid PEG Corona, Langmuir, 18 (2002) 3669–3675. [Google Scholar]
- [310].Bootz A, Vogel V, Schubert D, Kreuter J, Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 57 (2004) 369–375. [DOI] [PubMed] [Google Scholar]
- [311].Crawford R, Dogdas B, Keough E, Haas RM, Wepukhulu W, Krotzer S, Burke PA, Sepp-Lorenzino L, Bagchi A, Howell BJ, Analysis of lipid nanoparticles by Cryo-EM for characterizing siRNA delivery vehicles, International Journal of Pharmaceutics, 403 (2011) 237–244. [DOI] [PubMed] [Google Scholar]
- [312].Kuhlbrandt W, The Resolution Revolution, Science, 343 (2014) 1443–1444. [DOI] [PubMed] [Google Scholar]
- [313].Zamboni WC, Szebeni J, Kozlov SV, Lucas AT, Piscitelli JA, Dobrovolskaia MA, Animal models for analysis of immunological responses to nanomaterials: Challenges and considerations, Adv Drug Deliv Rev, (2018). [DOI] [PubMed] [Google Scholar]
- [314].Szebeni J, Simberg D, Gonzalez-Fernandez A, Barenholz Y, Dobrovolskaia MA, Roadmap and strategy for overcoming infusion reactions to nanomedicines, Nat Nanotechnol, 13 (2018) 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Bedőcs P, Szebeni J, The Critical Choice of Animal Models in Nanomedicine Safety Assessment: A Lesson Learned From Hemoglobin-Based Oxygen Carriers, Front Immunol, 11 (2020) 584966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Fülöp T, Nemes R, Mészáros T, Urbanics R, Kok RJ, Jackman JA, Cho NJ, Storm G, Szebeni J, Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: Correlation with physicochemical features and clinical information, J Control Release, 270 (2018) 268–274. [DOI] [PubMed] [Google Scholar]
- [317].Neun B, Dobrovolskaia MA, NCL Method ITA-36: Detection of naturally occurring antibodies to PEG and PEGylated liposomes in plasma of human donor volunteers, 2020. [PubMed] [Google Scholar]
- [318].Costabile M, Measuring the 50% haemolytic complement (CH50) activity of serum, J Vis Exp, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Neun B, Cedrone E, Dobrovolskaia M, NCL Method ITA-5.2: Analysis of complement activation by single-plex EIA or multiplex ELISA. , ELISA https://ncl.cancer.gov/resources/assaycascade-protocols (2020). [PubMed] [Google Scholar]
- [320].Jaskowski TD, Martins TB, Litwin CM, Hill HR, Comparison of three different methods for measuring classical pathway complement activity, Clin Diagn Lab Immunol, 6 (1999) 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Cedrone E, Neun BW, Rodriguez J, Vermilya A, Clogston JD, McNeil SE, Barenholz Y, Szebeni J, Dobrovolskaia MA, Anticoagulants Influence the Performance of In Vitro Assays Intended for Characterization of Nanotechnology-Based Formulations, Molecules, 23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Braley-Mullen H, Regulation of IgG memory responses by helper and suppressor T cells activated by the type 2 antigen, polyvinylpyrrolidone, J Exp Med, 161 (1985) 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Braley-Mullen H, Milligan GN, Van Buskirk AM, Requirement for B cell-derived immunoglobulin for Th activation by the type 2 antigen polyvinylpyrrolidone, Int Immunol, 6 (1994) 805–815. [DOI] [PubMed] [Google Scholar]
- [324].Van Buskirk AM, Braley-Mullen H, In vitro activation of specific helper and suppressor T cells by the type 2 antigen polyvinylpyrrolidone (PVP), J Immunol, 139 (1987) 1400–1405. [PubMed] [Google Scholar]
- [325].Van Buskirk AM, Braley-Mullen H, Characterization of helper T cells induced by the type 2 antigen polyvinylpyrrolidone, J Immunol, 138 (1987) 1031–1037. [PubMed] [Google Scholar]
- [326].Dobrovolskaia MA, McNeil SE, Strategy for selecting nanotechnology carriers to overcome immunological and hematological toxicities challenging clinical translation of nucleic acid-based therapeutics, Expert Opin Drug Deliv, 12 (2015) 1163–1175. [DOI] [PubMed] [Google Scholar]
- [327].Dobrovolskaia MA, Shurin M, Shvedova AA, Current understanding of interactions between nanoparticles and the immune system, Toxicol Appl Pharmacol, 299 (2015) 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Abbas AK, Lichtman AH, Pober JS, Effector mechanisms of immunoglobulin E-initiated immune reactions, in: Abbas AK, Lichtman AH, Pober JS (Eds.) Cellular and Molecular Immunology, W.B. Saunders Co., Philadelphia, 1994, pp. 279–292. [Google Scholar]
- [329].Yang QL, S.K., Anti-PEG immunity: emergence, characteristics, and unaddressed questions, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7 (2015) 655–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Abbas AK, Lichtman AH, Pillai S, Cellular and molecular immunology, Elsevier, Philadelphia, PA, 2018. [Google Scholar]
- [331].Sethu S, Govindappa K, Alhaidari M, Pirmohamed M, Park K, Sathish J, Immunogenicity to biologics: mechanisms, prediction and reduction, Arch Immunol Ther Exp (Warsz), 60 (2012) 331–344. [DOI] [PubMed] [Google Scholar]
- [332].Labcorp, Allergen panels, 2021. [Google Scholar]
- [333].Veronese FM, Pasut G, PEGylation: Posttranslational bioengineering of protein biotherapeutics, Drug Discovery Today: Technologies, 5 (2008) e57–e64. [DOI] [PubMed] [Google Scholar]


