Abstract
Lymphovascular invasion (LVI) is a key step in breast cancer (BC) metastasis. Targeting the molecular drivers of LVI can improve BC patients’ management. However, the underlying molecular mechanisms of LVI are complex and interconnected with various carcinogenesis pathways. This study aimed to identify the key regulatory gene associated with LVI and to investigate its mechanisms of action and prognostic significance. Artificial neural network (ANN) was applied to two large transcriptomic datasets of BC with well-characterised LVI status. Cyclin B2 (CCNB2) was identified in the top genes associated with LVI positivity. In vitro functional assays were carried out to assess the role of CCNB2 in tumour cell behaviour and their interactions with endothelial cells using a panel of BC cell lines. Large annotated BC cohorts were used to assess the clinical and prognostic role of CCNB2 at the transcriptomic and protein levels. Knockdown (KD) of CCNB2 mRNA reduced BC cell migration, inhibited proliferation, blocked the G2/M transition during the cell cycle and increased the number of apoptotic cells. Importantly, KD of CCNB2 reduced BC cell lines adherence and transmigration across endothelial cell lines. High CCNB2 protein expression was independently associated with LVI positivity in addition to other features of aggressive behaviour, including larger tumour size, higher histological grade, hormonal receptor-negativity, and HER2-positivity, and with shorter survival. We conclude that CCNB2 plays a crucial role in LVI development in BC, implying that CCNB2 could confer a promising therapeutic target to inhibit LVI and reduce metastatic events.
Keywords: Breast cancer, CCNB2, prognosis, progression, LVI
Introduction
Breast cancer (BC) is the second most common cause of cancer-related mortality in women. Despite the advances in BC treatment, a considerable proportion of patients develop disease metastasis even within early-stage BC [1]. Lymphovascular invasion (LVI) is a key initial event of the metastatic cascade and serves as a prognostic factor in BC [2]. The lack of targeted therapy to inhibit LVI and prevent metastasis typically stems from the complex underlying molecular mechanisms of LVI, which interact with other carcinogenesis pathways and the subjectivity of assessing LVI in clinical BC cohorts. Thus, deciphering the underlying molecular mechanisms of LVI and the identification of the key genes controlling LVI in BC are warranted.
Machine learning (ML) is an innovative tool that has various applications, including running bioinformatical analysis of large datasets such as proteomic and transcriptomic data. ML depends on using sets of algorithms to analyse such data to obtain specific results that solve a given complex question [3,4]. Artificial neural networks (ANNs) are the framework of various ML algorithms to process complex datasets [3]. Previous studies have successfully used ML in discovering novel biomarkers associated with specific clinical outcomes in various cancers [5,6], including BC [7].
In this study, we hypothesised that the power of ANN in large annotated BC cohorts with well-characterised LVI status can identify genes strongly associated with LVI in BC. As association with LVI can be a secondary event, the direct role of the selected gene should be confirmed both ex vivo and in vitro. Mechanistic studies were carried out using a panel of cell lines to decipher its role in the tumour cell carcinogenesis processes and the interaction of BC cells with endothelial cells. Clinical samples were used to evaluate its association with the clinicopathological features and outcome.
Materials and methods
ML for the biomarker set enrichment
ANN was used to identify the differentially expressed gene(s) between LVI positivity and negativity in two large BC datasets; the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC; n=1,980) and The Cancer Genome Atlas (TCGA; n=854) [8,9]. LVI status was previously assessed for 1565 patients, including the Nottingham subset (n=285) from the METABRIC cohort, through histological analysis of H&E-stained paraffin embedded tissues. The LVI status was also defined in the Nottingham subset through the use of immunohistochemistry (IHC) staining for CD31, CD34, and D2-40 in addition to the H&E [10]. The LVI was assessed in the TCGA by the evaluation of histological slides stained with H&E as no IHC marker status was available for these cases. The definition of LVI positive cases was based on the presence of LVI in H&E-stained sections in both cohorts. Positive staining in IHC markers was also used to identify LVI positive cases in the Nottingham subgroup of the METABRIC cohort. The positive LVI in that cohort was based on both H&E and the stain of IHC markers. The ANN-based neural data mining was performed on the genomic expression data obtained from both datasets to locate an enriched set of concordant biomarkers linked to LVI. In this case, we executed a ML strategy grounded in an ANN and integrated with concordance analysis performed across the multiple splits of Monte Carlo data based on the methodology indicated previously [7], which has initially proven to be efficient in eradicating over-fitting and false discovery while enhancing the generalisation of the identified biomarkers. The data were classified into five random groups based on the LVI status, and the ANN algorithm was run separately for each group, with each run consisting of 20 loops. The results were filtered to identify the concordant transcripts with the lowest test errors that were present in multiple loops for each group, and the results for all groups were compared to identify similar transcripts that were present in the various groups. Cyclin B2 (CCNB2) was detected in the top ranked genes associated with LVI positivity on both METABRIC and TCGA cohorts, thus we decided to decipher its role in LVI development and BC outcome in both preclinical and clinical settings.
In vitro assessment of CCNB2
Pre-clinical studies were carried out to investigate the potential role of CCNB2 in LVI and other biological functions using the following cells and assays:
BC tumour cells
According to the protein expression levels, CCNB2 is expressed in the luminal and HER2 enriched cell lines. MCF-7, ZR-75-1 and SK-BR-3 BC cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MCF-7 and ZR-75-1 were cultured in RPMI 1640 medium with L-glutamine (SH30027.FS; Cytiva, UK) supplemented with 10% Foetal Bovine Serum (FBS). McCoy’s 5A medium modified with L-glutamine and sodium bicarbonate liquid (M9309; Sigma, UK) supplemented with 10% FBS was used to culture SK-BR-3.
Endothelial cells
Primary human umbilical vein endothelial cells (HUVECs) and dermal lymphatic endothelial cells (DLECs) were purchased from Promocell (Heidelberg, Germany). HUVECs and DLECs were cultured in endothelial cell growth medium MV2 (C-22022, Promocell, Germany). All cell cultures were performed under a sterile environment in a class II cabinet, and cells were incubated with 5% CO2 at 37°C.
Transfection of CCNB2 using siRNA
To investigate possible functional consequences of CCNB2 depletion and study its role in BC progression, with emphasising its role in LVI, we used a siRNA-based approach using BC cell lines. Differential expression of CCNB2 was carried out using western blotting (WB) and cells with high expression of CCNB2 were chosen.
Using pre-validated silencer select siRNA constructs mainly for designed CCNB2 or scrambled negative control siRNA (Silencer® Select siRNA, ThermoFisher Scientific), MCF-7, ZR-75-1 and SK-BR-3 were transfected with a mixture of Opti-MEM medium, 25 pmol siRNA and Lipofectamine™ RNAiMAX (13778150; ThermoFisher Scientific, Loughborough, UK) using the forward transfection method according to the manufacturer’s protocol. The sequence of CCNB2-siRNA is as follows: (5’->3’) GGCGAACUGUUUUAGAAGAtt.
Wound healing assay
In the scratching assay to study the effect of CCNB2 knockdown (KD) on cell migration, the wound repair rate of CCNB2 KD and control cells was observed by measuring the width of the gap left unhealed. A Culture-Inserts 2 well (Thistle Scientific Ltd, IB-81176), which has a built-in gap, was used to assess the migration ability of the cells according to the manufacture protocol. The wounds were observed by taking images several times via light microscopy (Lecia DMI 3000B, Leica microsystems, Germany) at the following time points: T0, T24, T48 and T72 h. Image J software (1.52 version) was used to measure the wound area and calculate the percentage of wound closure.
Proliferation assay
The effect of CCNB2 KD on the proliferation of tumour cells was assessed via 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTS) assay (Promega, (G3580); CellTitre 96 Aqueous One Solution Cell Proliferation Assay) as described by the manufacturer.
Clonogenic assays
The cells were seeded in six-well plates at 800 cells/well for MCF-7, ZR-75-1 and SK-BR-3. Cells were left in the incubator in the cultured medium for 14 days, following incubation; colonies were washed with phosphate buffered saline (PBS), fixed for 30 min with methanol and stained with crystal violet. Finally, the cloned cells were counted by using a microscope.
Cell cycle and apoptosis assays
The cells were harvested on the fifth day after transfection to detect and analyse the cell cycle distribution via the quantification of DNA content using a Propidium iodide flow cytometry kit (ab139418, Abcam, UK). Moreover, on the same day, cells were stained for the detection of apoptosis using an Annexin V detection kit (ab14082, Abcam, UK). Samples were analysed using a MACSQuant® analyzer flow cytometer (Miltenyi Biotec, Bergisch Gldbach, Germany) and the data were analysed by FlowJo software (version 14.0.0.0., USA).
Static adhesion assay
Endothelial cells (HUVECs and DLECs) were seeded to be confluent in a 24-well plate. Tumour cell adhesion was assessed following cell labelling with 1 μM Cell Tracker Green CMFDA (Invitrogen, C2925) and incubation at 37°C for 30 min. After labelling, tumour cell media supplemented with serum was used to re-suspend the tumour cells and incubated with endothelial cell monolayers for 35 min at 37°C. Non-adherent cells were washed with tumour cell media, and the adherent tumour cells were counted using a fluorescence microscope (Lecia DMI 3000B, Leica microsystems, Germany) at 10× magnification. In the central area of the well, which was marked manually with grid lines on the bottom of the plate, five fields of view were counted. The results were expressed as the absolute number of cells adhering to the endothelial layer and as the percentage of cells adhering with respect to the control.
Transmigration assay
A confluent endothelial cell monolayer was grown on hanging transwell inserts (Sigma, MCEP24H48). The confluency and integrity of the endothelial cell barrier were shown by preventing lucifer yellow leakage (Invitrogen, L453). After labelling with 1 μM of Cell Tracker Green CMFDA (Invitrogen, C2925), tumour cell transmigration was assessed. After 16 h, transmigration was monitored by counting cells on the bottom of the chamber using a fluorescence microscope (Lecia DMI 3000B, Leica microsystems, Germany).
For further assessment of CCNB2 in BC outcome and its association with LVI, we have used large cohorts obtained from BC patients to investigate the role of CCNB2 at the transcriptomic and proteomic levels.
CCNB2 mRNA expression
Two datasets, the METABRIC [8] and TCGA breast carcinoma [9], were used to assess CCNB2 mRNA expression and its association with clinicopathological parameters and patient outcome. In the METABRIC, to analyse the extracted mRNA obtained from primary tumour samples, the Illumina Human HT-12 v3 platforms (Illumina, Inc., San Diego, USA) were used. In TCGA, cBioPortal website was used for information about RNASeqV2 data and clinicopathological parameters [11,12].
CCNB2 protein expression
Tissue samples were derived from the well-characterised Nottingham invasive BC cohort (n=3,173). For each patient, robust clinicopathological profile was readily available, including age at diagnosis, primary tumour size, tumour stage, histological grade, Nottingham Prognostic Index (NPI), and LVI status (Supplementary Table 1). Data on breast cancer specific survival (BCSS) and time to distant metastasis (TTDM) were also available. The BCSS for each patient was calculated in months from the date of the primary surgical treatment to the time of death from BC, while TTDM for each patient was calculated in months from surgery to the occurrence of the first distant metastasis. None of the patients in this study received neoadjuvant therapy as previously described [13].
This cohort had data on oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2 (HER2) and Ki67 [14-17]. BC molecular subtypes were defined using IHC profiles: luminal A (ER+/HER2-; Ki67 <10%), luminal B: (ER+/HER2-; Ki67 ≥10%), HER2-positive class (HER2 positive regard-less of ER or Ki67 status), and TNBC (ER-, PR- and HER2-). To further understand the molecular interactions of CCNB2, the association with epithelial mesenchymal transition (EMT) related markers, including E-cadherin, N-cadherin, P-cadherin, TGFβ1, and TWIST2, which are available in our database [18,19], was investigated.
For IHC staining of the primary rabbit monoclonal anti-CCNB2 antibody (ab185622, Abcam, UK), tissue microarrays (TMAs) of the study cohort were prepared using a TMA Grand Master® (3D HISTECH®, Budapest, Hungary) [20,43]. As per the manufacturer’s recommendations, antigen retrieval was performed (citrate buffer pH 6.0 at 1000 W for 20 min using microwave energy). The expression of CCNB2 protein was evaluated by Novocastra Novolink Polymer Detection Systems kit (Code: RE7280-K, Leica, Biosystems, UK), which involved incubating 4 µm sections with CCNB2 antibody (dilution 1:50) in Leica antibody diluent (RE AR9352, Leica, Biosystems, UK) for 60 minutes. Normal kidney tissue was used as a negative control, while normal colon tissue was used as the positive control (Figure 1A, 1B). High resolution digital scanned images of the stained TMAs were obtained by a NanoZoomer scanner (NanoZoomer; Hamamatsu Photonics, Welwyn Garden City, UK) at 20× magnification and viewed using Xplore software (Philips, UK) to assess CCNB2 expression. Immunoreactivity staining was evaluated using a modified histochemical score (H-score) based on a semi-quantitative scoring. The entire field inspection was scored, and the cytoplasmic staining intensity was grouped as follows: score 0= negative, score 1= weak staining, score 2= moderate staining and score 3= strong staining. The percentage of each group was estimated (0-100%). Multiplying the intensity of staining and the percentage of staining provides an H-score, which has a range of 0-300 [13]. The TMAs were scored by two observers, and the interclass correlation coefficient (ICC) test was used to evaluate the concordance rate of the CCNB2.
Figure 1.
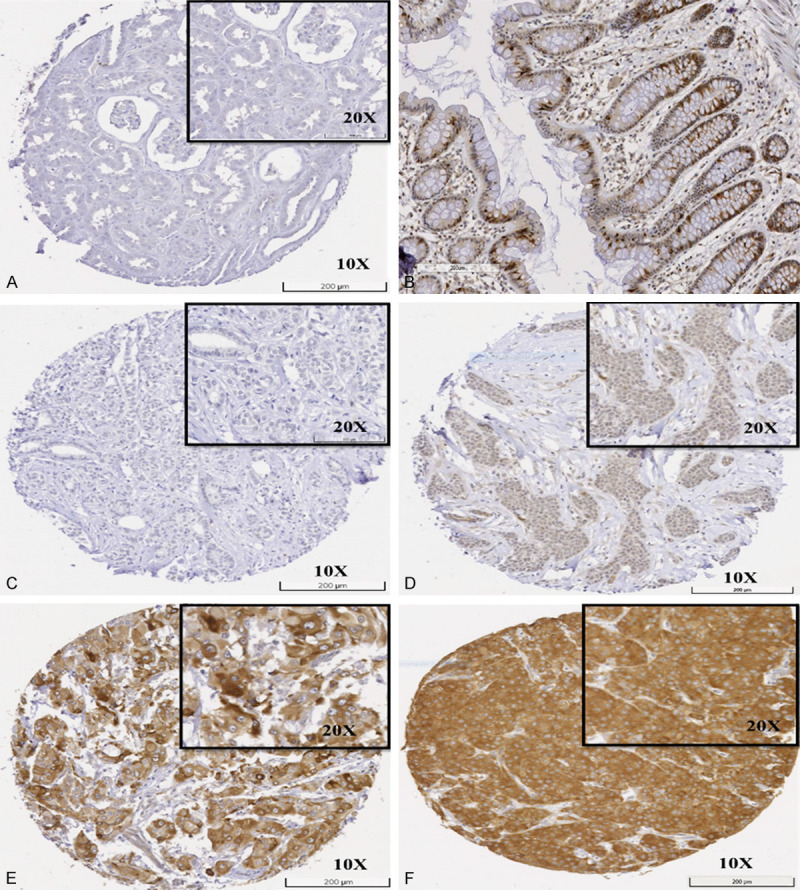
Immunohistochemistry expression of CCNB2 in tissue. A. Negative control of normal kidney tissue; B. Positive control of normal colon tissue; C. Negative expression in invasive breast carcinoma; D. Low expression in invasive breast carcinoma; E. Moderate expression in invasive breast carcinoma; F. High expression in invasive breast carcinoma.
Statistical analysis
GraphPad Prism software version 3.02 and SPSS version 24 (Chicago, IL, USA) were used for statistical analysis. In vitro data were represented as the mean ± standard error of the mean (SEM) of three independent experiments conducted in triplicate. A student’s t-test was performed to determine the significant differences between the control and KD of CCNB2.
The association with clinicopathological parameters was evaluated using continuous data on CCNB2 mRNA and protein level. To investigate the differences between three or more groups, one-way analysis of variance (ANOVA) with the post-hoc Tukey multiple comparison test (for parametric data) or Kruskal-Wallis test (for non-parametric distribution) was used. Student t-test (parametric data) or Mann-Whitney test (non-parametric distribution) was used to analyse the differences between two groups. The median was used to categorise the expression of CCNB2 mRNA and protein, with H-score of 20 was used for protein. The association between dependent and independent variables was analysed using logistic regression analysis. Correlation analysis was performed using the Spearman correlation test. The Log-rank and Kaplan-Meier curve tests were used for univariate survival analysis, whilst the Cox Regression model was used for multivariate survival analysis. Statistical significance was defined as P-value <0.05.
This study followed the reporting recommendations for tumour markers prognostic studies (REMARK) criteria [21] (Supplementary Table 2).
Results
ANN analysis of BC cohorts to identify LVI associated gene
The result of the analysis was ranked in an order based on the high predictive accuracies and lowest test errors. This resulted in a final model containing the top 100 transcripts that most accurately classified based on LVI status (Table 1). CCNB2 was chosen in this study as it was highly ranked with LVI positivity in both the METABRIC and TCGA cohorts. Other top ranked genes were either associated with LVI positivity in one cohort or associated with LVI negativity. Literature review revealed that CCNB2 is a key gene controlling cell proliferation and its expression is altered in many cancers [22-24].
Table 1.
Top 100 concordant genes by ANN from the METABRIC and TCGA breast cancer cohorts
| Concordant top 100 genes | ||
|---|---|---|
| DSCC1 | AK124197 | ATAD2 |
| CCNE2 | TCTN3 | ATP2A2 |
| YWHAZ | GPR34 | ATP6V1C1 |
| CCNB2 | TNRC6A | AURKA |
| LRCH3 | AGAP6 | AZIN1 |
| BX116720 | AK129555 | BBS5 |
| EIF4H | LIN52 | BDH2 |
| CCNB1 | ACIN1 | BRI3BP |
| AK022140 | CLASP1 | C1orf91 |
| DPRXP4 | AL359560 | C1RL |
| AK130741 | PHKA2 | CASC5 |
| AK130706 | ANAPC10 | CCDC130 |
| AK025793 | BX093900 | CDC25A |
| BX101409 | DA572426 | CDC6 |
| BX111162 | EBLN2 | CDCA5 |
| AL133627 | PTBP1 | CENPI |
| AX747098 | ZNF493 | CKAP5 |
| RRM2 | TYK2 | CLK1 |
| MLL4 | BU624523 | CLK4 |
| SPTLC1 | PUM1 | CPNE3 |
| MLL | DLC1 | CSE1L |
| ERP29 | CXorf56 | DCAF13 |
| NKTR | DNAH1 | DERL1 |
| PML | DR979451 | DFNB59 |
| RBM33 | ARPC3 | DTL |
| ASXL2 | TRPV1 | ECHDC2 |
| CD237904 | EPG5 | ESRP1 |
| CRYGS | AP1S1 | FAM83D |
| BROX | C11orf73 | FANK1 |
| SLC25A3 | C1R | FBXW4 |
| ALDOB | SOLH | FES |
| MAT2A | AI655567 | FLVCR1 |
| AK129699 | ADC | |
| SRRT | ANKRD10 | |
Preclinical studies (in vitro investigation of the role of CCNB2 in LVI-related biological processes)
Repression of CCNB2 expression using siRNA
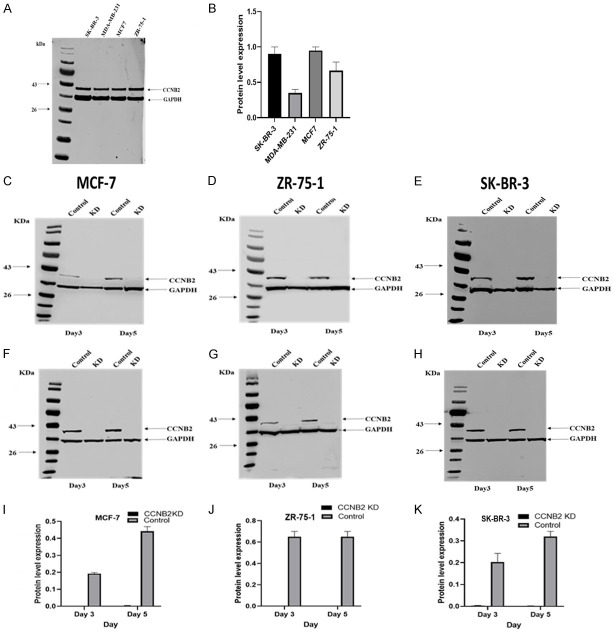
Based on the expression level, MCF-7 (ER+/PR+/HER2-), ZR-75-1 (ER+/PR±/HER2-) and SK-BR-3 (HER2+) BC cell lines will be used in this study (Figure 2A, 2B). MCF-7 and ZR-75-1 represent luminal molecular classes, while SK-BR-3 represents HER2+ tumour, which are associated with the highest incidence of LVI [25]. To test the efficacy of CCNB2 KD, two independent siRNAs targeting CCNB2 (IDs: s17446 and s17447) were used and compared to a non-targeting scrambled control siRNA (s10873). Hence, the negative control represents the effect of the overexpression of CCNB2, whereas the CCNB2 siRNA represents the downregulation effect of CCNB2 on the selected BC cell lines. Similar KD was observed with both siRNA targeting (Figure 2C-H), and siRNA (s17446) was prioritised for the subsequent functional assessments. Using WB, complete loss of CCNB2 protein expression in MCF-7, ZR-75-1 and SK-BR-3 (Figure 2I-K) was observed when comparing CCNB2 KD expression relative to GAPDH expression.
Figure 2.
CCNB2 protein expression in breast cancer cell lines (A) Evaluation of differential expression of CCNB2 in BC cell lines by western blotting (B) quantification of CCNB2 protein level expression in, SKBR-3, MDA-MB-231, MCF-7, and ZR-75-1. Using two pre-validated silencer select siRNAs constructs mainly for CCNB2 or scrambled negative control siRNA, silencing CCNB2 using the forward transfection method (IDs: s17447) relative to a non-targeting scrambled control siRNA (s10873) showed complete knockdown in (C) MCF-7 (D) ZR-75-1 and (E) SK-BR-3. Silencing CCNB2 using siRNA (IDs: s17446) relative to a non-targeting scramble control siRNA (s10873) showed complete knockdown in (F) MCF-7, (G) in ZR-75-1 and (H) SK-BR-3. Quantification of CCNB2 protein level expression in the transfected cell and the negative control by densitometry and normalized to GAPDH levels revealed complete loss of CCNB2 protein expression in (I) MCF-7, (J) ZR-75-1 and (K) SK-BR-3. The figures are representative of three or more independent experiments.
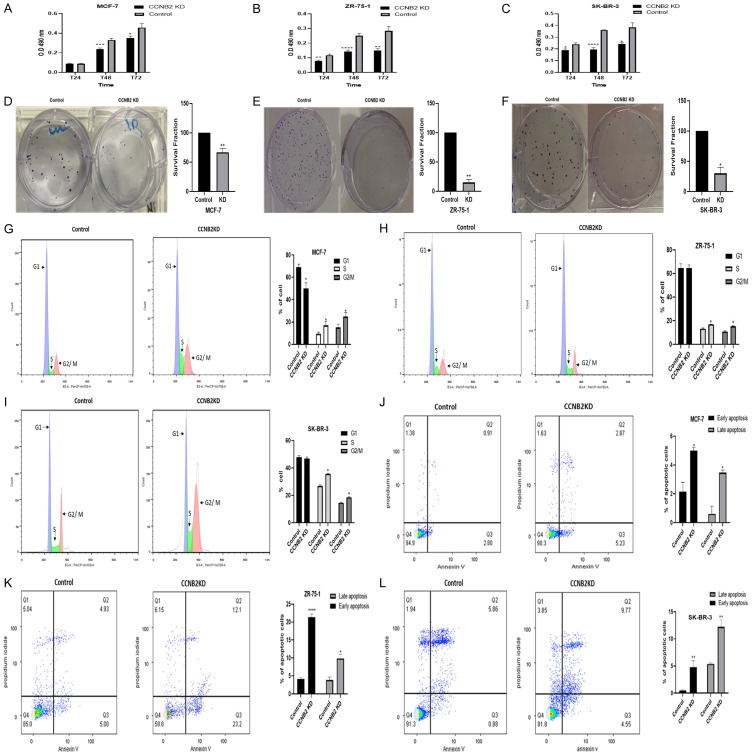
Downregulation of CCNB2 suppresses cell migration, proliferation and cell growth
KD of CCNB2 showed a larger unhealed wound compared to the negative controls (NC) in MCF-7, ZR-75-1 and SK-BR-3 (P=0.0377, P=0.0028 & P=0.0344, respectively, Figure 3A-C). This supported the role of CCNB2 in promoting cell migration.
Figure 3.
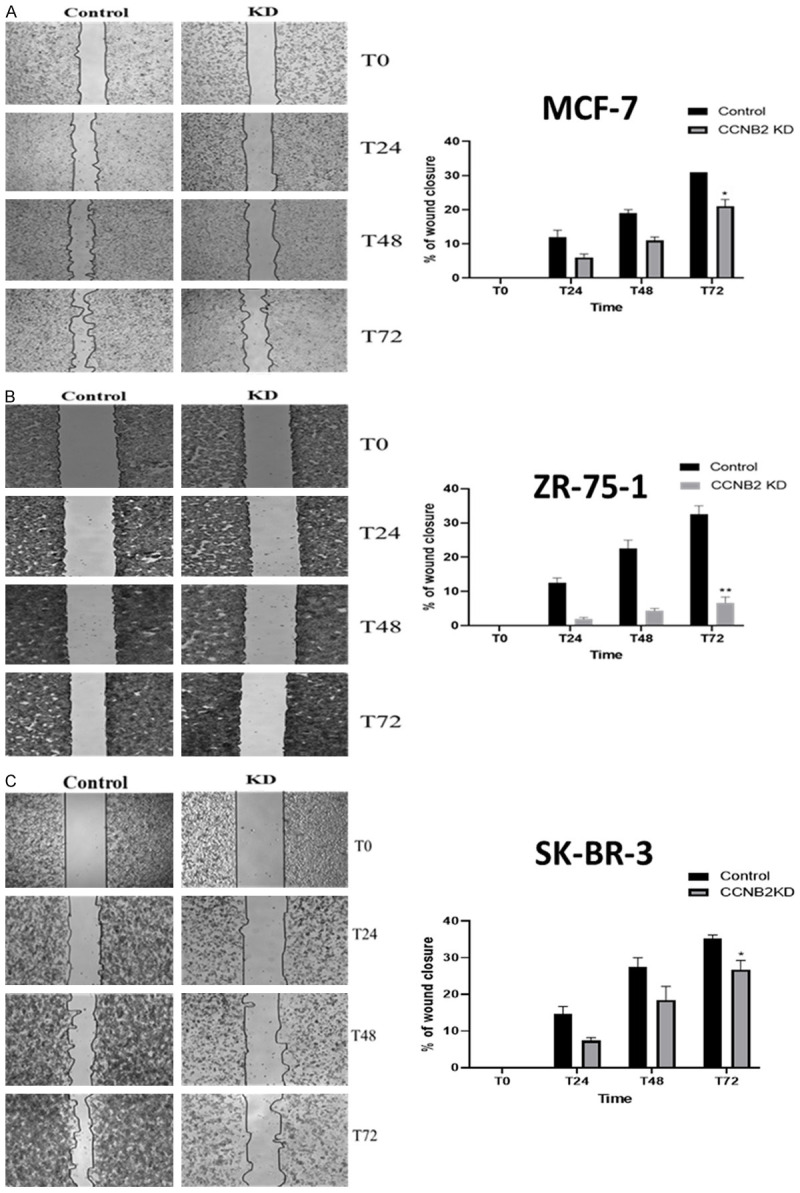
The effect of knockdown (KD) CCNB2 by siRNA on cell migration in (A) MCF-7, (B) ZR-75-1 and (C) SK-BR-3 cells. The wound repair rate of CCNB2 KD and control cells was observed by measuring the width of the gap left unhealed at T0, T24, T48 and T72. (A-C) silencing CCNB2 was significantly reduced the migration rate in BC cell lines as detected by wound healing assay. Results shown are mean ± standard error of the mean (SEM) of three independent experiments. The P-values *<0.05, **<0.01, ***<0.001 and ****<0.0001.
CCNB2 KD significantly reduced the proliferation rate in MCF-7 (Figure 4A; P=0.0003 (T48) & P=0.0343 (T72)), ZR-75-1 (Figure 4B; P=0.0016 (T24), P<0.0001 (T48) & P=0.0015 (T72)) and SK-BR-3 (Figure 4C; P=0.0296 (T24), P<0.0001 (T48) & P=0.0202 (T72)) compared to the control. Additionally, the ability of single cell colony to survive after it was exposed to the transfection agent was significantly weaker than the control on MCF-7 (Figure 4D; P=0.0075), ZR-75-1 (Figure 4E; P=0.0034) and SK-BR-3 (Figure 4F; P=0.0198).
Figure 4.
The effect of knockdown (KD) CCNB2 by siRNA on cell proliferation in (A) MCF-7, (B) ZR-75-1 and (C) SK-BR-3 cells. (A-C) Cell proliferation was significantly reduced after KD in BC cell lines as detected by MTS assay. CCNB2 siRNA transfection reduced the ability of BC cell lines to colonise in (D) MCF-7, (E) ZR-75-1 and (F) SK-BR-3 as detected by colony formation assay. The effect of knockdown (KD) CCNB2 by siRNA on cell cycle and apoptosis. Data of BC cells showed arrest in S phase of the cell cycle of (G) MCF-7, (H) ZR-75-1 cells and (I) SK-BR-3. (G-I) Silencing CCNB2 all BC cell lines increased the S phase as detected by flow cytometry. Data of BC cells showed KD of CCNB2 enhanced apoptosis in (J) MCF-7, (K) ZR-75-1 cells and (L) SK-BR-3. (J-L) More apoptotic cells in the transfected cells were observed as detected by flow cytometry. Results shown are mean ± standard error of the mean (SEM) of three independent experiments. The P-values *<0.05, **<0.01, ***<0.001 and ****<0.0001.
Downregulation of CCNB2 arrests cells in S phase and enhances cell apoptosis
KD of CCNB2 caused S phase arrest in MCF-7, ZR-75-1 and SK-BR-3, inhibiting the transition to the G2/M phase during cell cycle. The percentage of MCF-7 cells in the S phase were significantly increased in the CCNB2 KD cells (G1 50.5%, S 22%, and G2/M 25.4%) compared with the NC (G1 69.2%, S 10.5%, and G2/M 13.4%) (P=0.032). Similar observations were observed with ZR-75-1 (G1 70.3%, S 15%, and G2/M 14%) compared with control (G1 68.3%, S 8.7%, and G2/M 12.3%) (P=0.9916 in G1 phase, P=0.0248 in S phase, P=0.0309 in G2/M phase). Similarly, the percentage of SK-BR-3 cells in the S phase were significantly increased in the CCNB2 KD cells (G1 46%, S 35.2%, and G2/M 18.5%) compared with the NC (G1 51%, S 26.5%, and G2/M 15.7%) (P=0.6413 in G1 phase, P=0.0102 in S phase, P=0.0299 in G2/M phase) (Figure 4G-I). The number of apoptotic cells in CCNB2 KD cells increased significantly in comparison with NC in early and late apoptosis as follows: early apoptosis (KD 5.23 vs NC 2.80%); (KD 23.2 vs NC 5%); (KD 4.55 vs NC 0.88%) while in late apoptosis (KD 2.87 vs NC 0.91%); (KD 12.1 vs NC 4.93%); (KD 9.77 vs NC 5.86%) in MCF-7 P=0.0307 (early apoptosis); P=0.0241 (late apoptosis); ZR-75-1 P<0.0001 (early apoptosis); P=0.0132 (late apoptosis) and SK-BR-3 P=0.0035 (early apoptosis); P=0.0100 (late apoptosis) respectively (Figure 4J-L).
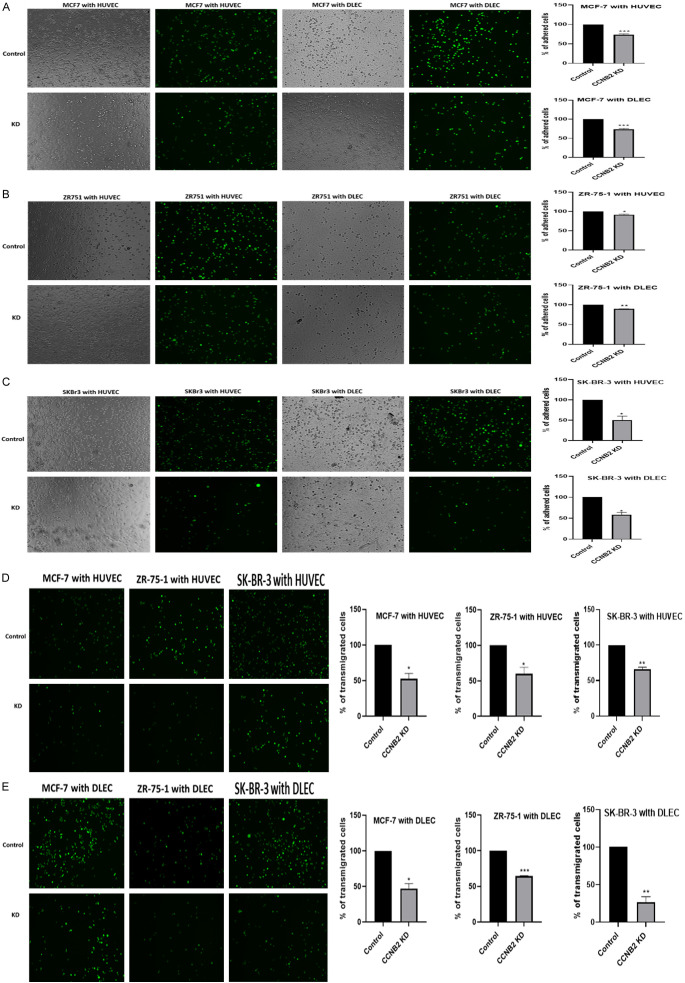
CCNB2 enhances BC cell adhesion and transmigration to the endothelial cells
All MCF-7, ZR-75-1 and SK-BR-3 cells lines showed higher adherence percentage to both HUVECs and DLECs compared to the CCNB2 transfected cells (MCF-7 both with HUVEC and DLEC P=0.0003), (ZR-75-1 with HUVEC P=0.0298; with DLEC P=0.0026) and (SK-BR-3 with HUVEC P=0.0377; with DLEC P=0.0171) (Figure 5A-C). Similar results were observed in the transmigration assay. The number of the tumour cells transmigrated across endothelial cells in the NC group was higher than the KD group ((MCF-7 with HUVEC P=0.0240; with DLEC P=0.0170), (ZR-75-1 with HUVEC P=0.0471; with DLEC P=0.0002) and (SK-BR-3 with HUVEC P=0.0077; with DLEC P=0.0091)) (Figure 5D, 5E).
Figure 5.
Representative photomicrographs of tumour cell adhesion across vascular and lymphatic endothelial cells (HUVECs, DLECs) (A) MCF-7, (B) ZR-75-1 cells and (C) SK-BR-3. (A-C) Silencing CCNB2 decreased the number of all BC cell lines adhered with HUVECs and DLECs. Representative photomicrographs of tumour cell (MCF-7, ZR-75-1 and SK-BR-3) transmigration across (D) HUVECs and (E) DLECs. (D, E) The number of tumour cells transmigrated across HUVECs and DLECs was higher in the control group compared to the KD group. Results shown are mean ± standard error of the mean (SEM) of three independent experiments. The P-values *<0.05, **<0.01, ***<0.001 and ****<0.0001.
The role of CCNB2 in LVI and BC outcome in the clinical BC cohorts
CCNB2 mRNA and protein expression
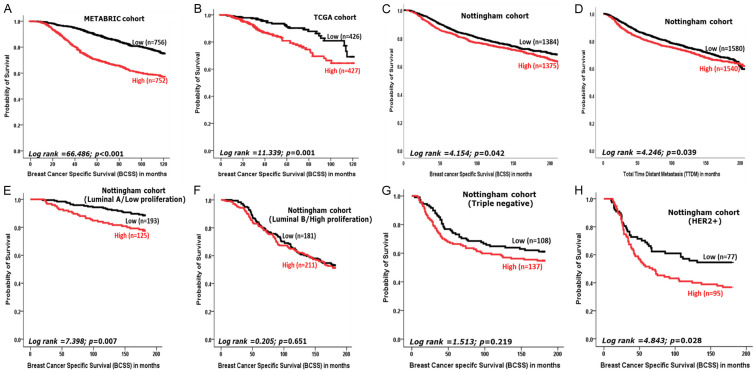
Univariate and multivariate analysis in the METABRIC & TCGA datasets, demonstrated a significant association between high CCNB2 mRNA expression and LVI-positivity (P<0.0001). High CCNB2 mRNA expression was also correlated with features of aggressive tumour behaviour in both datasets. These included high histological grade, large tumour size, hormone receptor negativity (all; P<0.0001), and HER2+ (P<0.0001 in METABRIC and P=0.002 in TCGA). In the METABRIC cohort, high expression of CCNB2 mRNA was significantly associated with the luminal type and HER2 enriched molecular classes (P<0.0001) (Table 2). BCSS of BC patients with high CCNB2 mRNA expression was significantly shorter than those with low expression in the METABRIC cohort, (P<0.0001) (Figure 6A) and TCGA dataset (P=0.001) (Figure 6B).
Table 2.
Statistical associations between CCNB2 mRNA expression and clinic-pathological parameters in the METABRIC (n=1980) and TCGA (n=854) breast carcinoma datasets
| Parameters | CCNB2 mRNA (METABRIC) | CCNB2 mRNA (TCGA) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Number (%) | Mean Rank | P-value | Number (%) | Mean Rank | P-value | |
| Patient Age (year) | ||||||
| ≤50 | 424 (21.4) | 8.25 | <0.0001 | 231 (27) | 466.3 | 0.005 |
| >50 | 1556 (78.6) | 7.95 | 623 (73) | 413.1 | ||
| Tumour Size | ||||||
| ≤2 cm | 622 (31.7) | 7.58 | <0.0001 | 239 (28) | 362.5 | <0.0001 |
| >2 cm | 1338 (68.3) | 8.09 | 615 (72) | 452.8 | ||
| Tumour Grade | ||||||
| 1 | 170 (9) | 7.16 | <0.0001 | 89 (11) | 198.5 | <0.0001 |
| 2 | 770 (40.6) | 7.66 | 375 (46) | 308.3 | ||
| 3 | 952 (50.4) | 8.50 | 352 (43) | 568.4 | ||
| Nodal Status | ||||||
| Negative | 1035 (52.5) | 7.90 | <0.0001 | 426 (51) | 418.5 | 0.439 |
| Positive | 938 (47.5) | 8.14 | 423 (49) | 431.5 | ||
| Lympho-vascular Invasion | ||||||
| Negative | 930 (59.4) | 7.93 | <0.0001 | 559 (65) | 392.5 | <0.0001 |
| Positive | 635 (40.6) | 8.10 | 295 (35) | 493.8 | ||
| Oestrogen Receptor | ||||||
| Negative | 474 (23.9) | 8.77 | <0.0001 | 185 (22) | 625.7 | <0.0001 |
| Positive | 1506 (76.1) | 7.78 | 639 (78) | 350.8 | ||
| Progesterone Receptor | ||||||
| Negative | 940 (47.4) | 8.53 | <0.0001 | 272 (33) | 552.1 | <0.0001 |
| Positive | 1040 (52.6) | 7.71 | 546 (67) | 338.4 | ||
| HER2 Status | ||||||
| Negative | 1733 (87.5) | 7.94 | <0.0001 | 567 (81) | 339.1 | 0.002 |
| Positive | 247 (12.5) | 8.52 | 133 (19) | 399.1 | ||
| Immunohistochemistry subtypes | ||||||
| ER+/HER2- Low Proliferation | 368 (36.9) | 8.74 | <0.0001 | Not available | ||
| ER+/HER2- High Proliferation | 368 (36.9) | 8.37 | ||||
| Triple Negative | 151 (15.1) | 7.19 | ||||
| HER2+ | 110 (11.1) | 8.52 | ||||
Significant P values are in bold.
Figure 6.
Kaplan-Meier survival plots showing the association between CCNB2 (A) mRNA expression (METABRIC) (B) mRNA expression (TCGA) (C) protein expression in the Nottingham cohort and breast cancer specific survival (BCSS) (D) the protein expression and total time distant metastasis (TTDM) (E) protein expression in luminal A and BCSS (F) protein expression in luminal B and BCSS (G) protein expression in triple negative and BCSS and (H) protein expression in HER2+ and BCSS.
CCNB2 protein expression was observed in the cytoplasm of BC cells, with expression levels varying from negative to strong (Figure 1C-F). Good concordance was observed between the two scorers in CCNB2 immuno-scoring (ICC=0.861, P<0.0001). High CCNB2 protein expression was observed in 506/1046 (53%) of LVI positive cases.
Similar to the transcriptomic results, high CCNB2 protein expression was significantly associated with clinicopathological parameters characteristic of aggressive behaviour, including higher histological grade, poorer NPI (all P<0.0001), larger tumour size (P=0.032), ER negativity (P=0.001) PR negativity (P=0.007) and HER2 positivity (P<0.0001) (Table 3). Positive LVI status was associated with high CCNB2 expression, which was an independent of other clinical parameters (P=0.038) (Table 4).
Table 3.
Statistical associations between CCNB2 protein expression and the clinic-pathological factors in Nottingham breast cancer cohort (n=3178)
| Nottingham Breast Cancer Cohort | |||
|---|---|---|---|
|
| |||
| Parameters | CCNB2 protein | ||
|
| |||
| Number % | Mean Rank | P-value | |
| Patient Age (year) | |||
| ≤50 | 1042 (33) | 1672.3 | <0.0001 |
| >50 | 2131 (67) | 1545.3 | |
| Tumour Size | |||
| ≤2 cm | 1768 (56) | 1555.6 | 0.032 |
| >2 cm | 1403 (44) | 1624.3 | |
| Tumour Grade | |||
| 1 | 543 (17) | 1314.0 | <0.0001 |
| 2 | 1131 (36) | 1503.9 | |
| 3 | 1496 (47) | 1745.7 | |
| Mitosis | |||
| 1 | 1284(42) | 1361.82 | <0.0001 |
| 2 | 598 (19) | 1597.05 | |
| 3 | 1211 (39) | 1718.63 | |
| Pleomorphism | |||
| 1 | 58 (1.8) | 1213.87 | <0.0001 |
| 2 | 1084 (35) | 1368.07 | |
| 3 | 1949 (36.2) | 1654.48 | |
| Tubular Formation | |||
| 1 | 205 (6.6) | 1282.29 | <0.0001 |
| 2 | 984 (31.7) | 1510.29 | |
| 3 | 1906 (61.7) | 1596.05 | |
| Lympho-vascular Invasion | |||
| Negative | 2205 (70) | 1549.6 | 0.004 |
| Positive | 954 (30) | 1650.1 | |
| Axillary Nodal Stage | |||
| 1 | 1989 (63) | 1563.5 | 0.136 |
| 2 | 903 (28) | 1607.3 | |
| 3 | 277 (9) | 1666.6 | |
| Nottingham Prognostic Index | |||
| Good | 1041 (33) | 1416.0 | <0.0001 |
| Moderate | 1632 (51) | 1659.3 | |
| Poor | 496 (16) | 1695.0 | |
| Oestrogen Receptor | |||
| Negative | 704 (22) | 1677.0 | 0.001 |
| Positive | 2453 (78) | 1550.9 | |
| Progesterone Receptor | |||
| Negative | 1256 (41) | 1585.5 | 0.007 |
| Positive | 1813 (59) | 1500.0 | |
| HER2 Status | |||
| Negative | 2677 (87) | 1513.8 | <0.0001 |
| Positive | 402 (13) | 1714.3 | |
| Triple Negative | |||
| Negative | 2630 (84) | 1538.7 | 0.007 |
| Positive | 483 (16) | 1656.5 | |
Significant P values are in bold.
Table 4.
Binary regression for predictors of CCNB2 protein expression in Nottingham breast cancer cohort and other variables
| Nottingham Breast Cancer Cohort | ||||
|---|---|---|---|---|
|
| ||||
| Parameters | Hazard ratio (HR) | 95% confident interval (CI) | Significance P-value | |
|
| ||||
| Lower | Upper | |||
| CCNB2 Protein Expression | 1.190 | 1.010 | 1.402 | 0.038 |
| Tumour Size | 1.889 | 1.598 | 2.232 | <0.001 |
| Lymph Nodal Stage | 2.793 | 2.465 | 3.166 | <0.001 |
Significant P values are in bold.
Survival analysis revealed that higher CCNB2 protein expression was associated with shorter BCSS (P=0.042, Figure 6C) and TTDM (P=0.039, Figure 6D). When we stratified the cohort into molecular subtypes, high expression of CCNB2 was significantly associated with poor outcome in the ER positive (luminal A) tumours (P=0.007, Figure 6E) and HER2 positive tumours (P=0.028, Figure 6H) but not in the TNBC (P=0.219, Figure 6G). Multivariate survival analysis revealed that high expression of CCNB2 is an independent marker of shorter survival (P=0.045) regardless of the other variables, including tumour size, histological grade, lymph nodal stage and LVI (Table 5).
Table 5.
Multivariate Cox regression for predictors of breast cancer specific survival (BCSS) and CCNB2 protein expression in Nottingham breast cancer cohort
| Nottingham Breast Cancer Cohort | ||||
|---|---|---|---|---|
|
| ||||
| Parameters | Hazard ratio (HR) | 95% confident interval (CI) | Significance P-value | |
|
| ||||
| Lower | Upper | |||
| CCNB2 Protein Expression | 1.001 | 1.000 | 1.002 | 0.045 |
| Tumour Size | 1.511 | 1.311 | 1.743 | <0.001 |
| Lymph Nodal Stage | 1.700 | 1.540 | 1.876 | <0.001 |
| Tumour Grade | 1.533 | 1.377 | 1.708 | <0.001 |
| Lymphovascular Invasion Status | 1.491 | 1.291 | 1.721 | <0.001 |
Significant P values are in bold.
Correlation between CCNB2 expression and other related biomarkers
To further evaluate the role of CCNB2 in BC and its interaction with other genes related to the various LVI related process, we interrogated the METABRIC and TCGA datasets for the correlation between CCNB2 mRNA and other genes involved in invasion, EMT and adhesion. This showed a correlation between CCNB2 and many genes involved the EMT and stromal degradations biomarkers, including E-cadherin, N-cadherin, P-cadherin, and GSK3B (all P<0.0001). Moreover, there was a significant correlation between CCNB2 and matrix metalloproteinase (MMPs), including MMP1, MMP7, MMP9, MMP12 and MMP20 (all P<0.0001) (Table 6). At the protein level, there was a positive correlation between CCNB2 with E-cadherin, (P=0.0001), N-cadherin (P=0.048), P-cadherin, (P=0.030), TGFβ1 (P=0.008) and TWIST2 (P=0.004), which are already available in our cohort (Table 6).
Table 6.
Correlations of CCNB2 expression with mRNA and protein expression of epithelial mesenchymal transition (EMT) and matrix metalloproteinases (MMPs) related genes
| Gene names | METABRIC cohort | TCGA cohort | Nottingham cohort | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Correlation value | P value | Correlation value | P value | Correlation value | P value | |
| EMT related genes | ||||||
| E-cadherin | -0.125 | <0.0001 | -0.140 | <0.0001 | -0.108 | <0.0001 |
| N-cadherin | 0.151 | <0.0001 | 0.133 | <0.0001 | 0.095 | 0.048 |
| P-cadherin | 0.322 | <0.0001 | 0.280 | <0.0001 | 0.261 | 0.030 |
| TGFB1 | 0.025 | 0.260 | 0.252 | <0.0001 | 0.080 | 0.008 |
| TWIST1 | 0.045 | 0.045 | 0.131 | <0.0001 | Not available | |
| TWIST2 | 0.270 | <0.0001 | 0.214 | <0.0001 | 0.087 | 0.004 |
| ZEB1 | 0.281 | <0.0001 | 0.420 | <0.0001 | Not available | |
| ZEB2 | 0.143 | <0.0001 | 0.214 | <0.0001 | ||
| NFKB1 | 0.176 | <0.0001 | 0.254 | <0.0001 | ||
| GSK3B | 0.330 | <0.0001 | 0.129 | <0.0001 | ||
| CTNNB1 | 0.073 | 0.001 | 0.172 | <0.0001 | ||
| MMPs related genes | ||||||
| MMP1 | 0.328 | <0.0001 | 0.428 | <0.0001 | Not available | |
| MMP7 | 0.134 | <0.0001 | 0.190 | <0.0001 | ||
| MMP9 | 0.308 | <0.0001 | 0.227 | <0.0001 | ||
| MMP11 | 0.051 | 0.022 | 0.064 | 0.060 | ||
| MMP12 | 0.382 | <0.0001 | 0.350 | <0.0001 | ||
| MMP15 | 0.199 | <0.0001 | 0.104 | 0.002 | ||
| MMP20 | 0.092 | <0.0001 | 0.134 | <0.0001 | ||
| MMP25 | 0.165 | <0.0001 | 0.073 | 0.034 | ||
Significant P values are in bold.
Using the GeneMANIA database (https://genemania.org/), a network was created to illustrate the molecular interactions between CCNB2 and EMT-related markers. The network revealed that CCNB2 genetically interacted with some EMT-related markers such as CTTNB1 and was co-expressed with NFKB1, which has an interaction with other EMT-related markers including E-cadherin, N-cadherin, P-cadherin, and TWIST1 (Figure 7).
Figure 7.
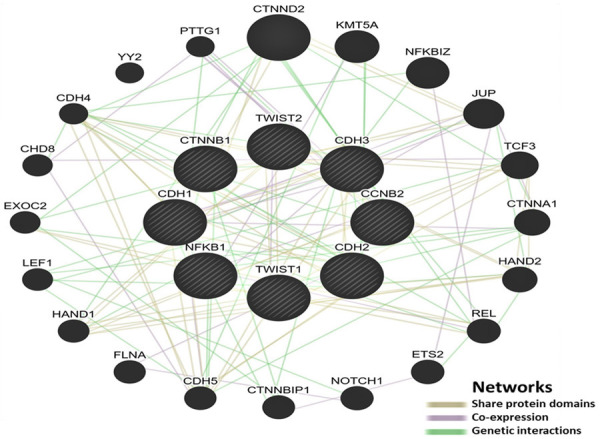
A schematic network illustrating the molecular interactions between CCNB2 and EMT-related markers generated by GeneMANIA database.
Discussion
Metastasis is the main cause of BC-related mortality [26]. Thus, early detection of metastatic potential in BC patients can be critical in reducing cancer-related mortality. LVI is an early event in the development of metastasis and a potent prognostic factor [27]. LVI in BC, similar to other cancers, has attracted attention not only for its prognostic role but also for being a potential therapeutic target. However, the complex molecular mechanism of LVI and its overlap with other carcinogenesis pathways contribute to the difficulty in identifying the key driver gene of LVI that can be targeted. In our study, we identified a strong association between LVI and CCNB2 utilising large cohorts of BC and ANN algorithms, which is a powerful technique with low-test errors. Likewise, recent studies using different bioinformatics and co-expression analyses confirmed the association of CCNB2 with human cancer progression [22,28,29].
Cyclins are crucial elements of the cell-division cycle, and various studies, including Stamatakos et al., [30] have discovered that defects in their functionality can lead to carcinogenesis. Although evidence is still emerging, recent studies have revealed more information regarding the mechanistic pathways through which cyclins can influence the oncogenic potential of cells [22,23]. Cyclins are indispensable core cell cycle regulators modulating the activation of cyclin dependent kinases (CDKs) complexes that propel cells through the cell cycle. Many of the cyclins are also identified as established oncogenes in several human tumours [30]. There are several different cyclins that are active in different parts of the cell cycle and that contribute to various other functions. The CCNB2 gene, which is a member of the cyclin B family, has a role in the G2/M transition through cell division control (CDC2) activation, and its inhibition leads to cell cycle arrest [31,32]. CCNB2 is a critical part of cell cycle regulation. It is one of the central protein kinases, and when activated, it becomes a master regulator during M-phase transition, phosphorylation, and the activation of other protein kinases [33,34]. The expression of CCNB2 is normally tightly regulated [22]. However, high expression of CCNB2 has been found in human tumours, including lung [24], liver [22], bladder [35] and BC [23]. CCNB2 may act as an oncogene and could be a potential biomarker for unfavourable outcomes [23,36]. This suggests that CCNB2 may also be involved in BC progression. The role of CCNB2 in LVI is unclear, and to our knowledge, this is the first study to report on the role of CCNB2 in LVI.
In our preclinical studies, we have revealed that knocking down CCNB2 significantly inhibits cell proliferation and reduces cell growth. As expected, silencing CCNB2 arrested the S phase of the cell cycle, which inhibited the cells from entering the G2/M transition phase during the cell cycle in BC cell lines. Similarly, knocking down CCNB2 promoted cell apoptosis. The failure to remove the dead cells via the inhibition of cell apoptosis causes the malignant proliferation of cancer cells [37]. A study showed that this occurs through the interference of proteolysis and the obstruction of normal intracellular trafficking [35]. In Lei et al.’s investigation of its role in bladder cancer, CCNB2’s tumourigenic function was linked to the dysregulation and abhorrent expression of cell-cycle associated proteins [35]. Li et al. investigated the effect of CCNB2 in liver cancer and arrived at a similar view, concluding that CCNB2 may promote cell apoptosis by causing S phase arrest and instigating the functional failure of the G2/M DNA/genetic damage checkpoint during the cell division cycle [22]. Checkpoints are important in repairing damaged DNA, as well as maintaining genomic integrity. Defects in this domain can lead to mutations and oncogenesis [38]. It is essential for the primary tumour cells to proliferate to invade the surrounding tissue and thus establish metastasis cascades, which can lead to the degradation of the basement membrane and LVI. Proliferation continues until the invasion of vascular or lymphatic channels occur. At this stage, tumour cells can evade apoptosis [39]. Therefore, CCNB2 is one of the essential regulators in cell proliferation, and a necessary prerequisite step in the development of LVI and, eventually, distant metastasis.
Although the role of CCNB2 in the cell cycle and proliferation is well known, its role in driving LVI in BC is not characterised. Our results revealed that the silencing of CCNB2 suppressed the migration of the cells and reduced the number of tumour cells adhered and transmigrated across the vascular and lymphatic endothelial cell lines. This was supported by the positive correlation between EMT and MMPs related markers. N-cadherin is linked to the EMT, which plays a vital role in the invasion and intravasation into the bloodstream and in the degradation of the extracellular matrix (ECM) caused by the production of proteases. Thus, an invasion into the stroma is a result of the loss of the linkage between the epithelial of the BC cell and other epithelial cells by the up-regulation of N-cadherin [40]. Moreover, P-cadherin, an important molecule that can activate integrin molecules, allows cancer cells to attach to ECM and activates the invasion of cancer cells [41], which could explain the role of CCNB2 in LVI as it has a positive correlation with P-cadherin. To invade the tissue, tumour cells must penetrate the tissue boundaries by breaking down the ECM through MMPs and the urokinase plasminogen activator (uPA) system. Inhibition of uPA halts the invasion and expression of MMP9 [42]. The LVI tumour microenvironment is strongly correlated with MMPs, specifically MMP9 and MMP1, expression. These expressions are responsible for cancer cell intravasation and metastasis in BC [25]. Thus, our in vitro assessment indicated the contribution of the upregulation of CCNB2 expression in migration, adhesion and transmigration through endothelial cell lines, which induced the LVI process. This is also consistent with a previous study of bladder cancer that reported that the silencing of CCNB2 can restrain migration and invasion leading to inhibition of tumour metastasis and prolonging survival time [35].
Our study has explored the prognostic significance of CCNB2 expression using multiple well-annotated BC cohorts. Overexpression is associated with aggressive clinicopathological BC features, including LVI positivity, high histological grade, large tumour size, hormonal receptor negativity and HER2 positivity, and worse clinical outcomes.
Because CCNB2 is a cell cycle regulator, the cell cycle could be one of the mechanisms contributing to LVI in BC. However, the molecular mechanisms that cause BC tumour cells to invade and disseminate in vascular spaces are still largely unknown and require more research. Cancer treatment strategies that induce cell cycle arrest in cancer cells are effective [43]. This suggests that CCNB2 is a key gene in BC, particularly given our findings regarding lymphatic invasion, which is a prerequisite for metastasis cascade. Thus, CCNB2 may represent a potential target for LVI in BC patients.
Our study provides evidence that CCNB2 is a key modulator of LVI in BC and could confer as a potential therapeutic target to suppress LVI and improve patient’s outcome. However, in vitro and/or in vivo experiments investigating the differential gene expression/pathways observed in CCNB2 knockdown cells are necessary.
Acknowledgements
We would like to acknowledge Dr. Omar Mohammed for his help and encouragement during this study. Abrar Aljohani is supported and funded by Taif University; Kingdom of Saudi Arabia. The authors are part of the PathLAKE digital pathology consortium. These new Centres are supported by a £ 50 m investment from the Data to Early Diagnosis and Precision Medicine strand of the government’s Industrial Strategy Challenge Fund, managed and delivered by UK Research and Innovation (UKRI).
Informed consent was obtained from all individuals prior to surgery to use their tissue materials in research.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ganz PA, Goodwin PJ. Breast cancer survivorship: where are we today? Adv Exp Med Biol. 2015;862:1–8. doi: 10.1007/978-3-319-16366-6_1. [DOI] [PubMed] [Google Scholar]
- 2.Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 3.Zafeiris D, Rutella S, Ball GR. An artificial neural network integrated pipeline for biomarker discovery using alzheimer’s disease as a case study. Comput Struct Biotechnol J. 2018;16:77–87. doi: 10.1016/j.csbj.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nevin L PLOS Medicine Editors. Advancing the beneficial use of machine learning in health care and medicine: toward a community understanding. PLoS Med. 2018;15:e1002708. doi: 10.1371/journal.pmed.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swan AL, Stekel DJ, Hodgman C, Allaway D, Alqahtani MH, Mobasheri A, Bacardit J. A machine learning heuristic to identify biologically relevant and minimal biomarker panels from omics data. BMC Genomics. 2015;16(Suppl 1):S2. doi: 10.1186/1471-2164-16-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libbrecht MW, Noble WS. Machine learning applications in genetics and genomics. Nat Rev Genet. 2015;16:321–332. doi: 10.1038/nrg3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Fatah TMA, Agarwal D, Liu DX, Russell R, Rueda OM, Liu K, Xu B, Moseley PM, Green AR, Pockley AG, Rees RC, Caldas C, Ellis IO, Ball GR, Chan SYT. SPAG5 as a prognostic biomarker and chemotherapy sensitivity predictor in breast cancer: a retrospective, integrated genomic, transcriptomic, and protein analysis. Lancet Oncol. 2016;17:1004–1018. doi: 10.1016/S1470-2045(16)00174-1. [DOI] [PubMed] [Google Scholar]
- 8.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S METABRIC Group. Langerød A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Børresen-Dale AL, Brenton JD, Tavaré S, Caldas C, Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA TCGA Research Network. Perou CM. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, Ellis IO. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011;223:358–365. doi: 10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
- 11.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aljohani AI, Joseph C, Kurozumi S, Mohammed OJ, Miligy IM, Green AR, Rakha EA. Myxovirus resistance 1 (MX1) is an independent predictor of poor outcome in invasive breast cancer. Breast Cancer Res Treat. 2020;181:541–551. doi: 10.1007/s10549-020-05646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abd El-Rehim DM, Pinder SE, Paish CE, Bell JA, Rampaul RS, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer. 2004;91:1532–1542. doi: 10.1038/sj.bjc.6602184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Lee AHS, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127:99–108. doi: 10.1007/s10549-010-0987-8. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 17.Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet JS, Akslen LA, Evans AJ, Blamey R, Reis-Filho JS, Foulkes WD, Ellis IO. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- 18.Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JFR, Macmillan D, Blamey RW, Ellis IO. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer. 2005;116:340–350. doi: 10.1002/ijc.21004. [DOI] [PubMed] [Google Scholar]
- 19.Rakha EA, Abd El Rehim D, Pinder SE, Lewis SA, Ellis IO. E-cadherin expression in invasive non-lobular carcinoma of the breast and its prognostic significance. Histopathology. 2005;46:685–693. doi: 10.1111/j.1365-2559.2005.02156.x. [DOI] [PubMed] [Google Scholar]
- 20.Sonbul SN, Aleskandarany MA, Kurozumi S, Joseph C, Toss MS, Diez-Rodriguez M, Nolan CC, Mukherjee A, Martin S, Caldas C, Ellis IO, Green AR, Rakha EA. Saccharomyces cerevisiae-like 1 (SEC14L1) is a prognostic factor in breast cancer associated with lymphovascular invasion. Mod Pathol. 2018;31:1675–1682. doi: 10.1038/s41379-018-0092-9. [DOI] [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumour maker prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Jiang X, Zhang Y, Wang S, Chen X, Yu X, Ma J, Huang X. Cyclin B2 overexpression in human hepatocellular carcinoma is associated with poor prognosis. Arch Med Res. 2019;50:10–17. doi: 10.1016/j.arcmed.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Shubbar E, Kovács A, Hajizadeh S, Parris TZ, Nemes S, Gunnarsdóttir K, Einbeigi Z, Karlsson P, Helou K. Elevated cyclin B2 expression in invasive breast carcinoma is associated with unfavorable clinical outcome. BMC Cancer. 2013;13:1. doi: 10.1186/1471-2407-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian X, Song X, He Y, Yang Z, Sun T, Wang J, Zhu G, Xing W, You C. CCNB2 overexpression is a poor prognostic biomarker in Chinese NSCLC patients. Biomed Pharmacother. 2015;74:222–227. doi: 10.1016/j.biopha.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Aleskandarany MA, Sonbul SN, Mukherjee A, Rakha EA. Molecular mechanisms underlying lymphovascular invasion in invasive breast cancer. Pathobiology. 2015;82:113–123. doi: 10.1159/000433583. [DOI] [PubMed] [Google Scholar]
- 26.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 27.Debled M, De Mascarel I, Brouste V, Mauriac L, Macgrogan G. Re: population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2010;102:275–276. doi: 10.1093/jnci/djp490. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, Kong D, Cui Q, Wang K, Zhang D, Gong Y, Wu G. Prognostic genes of breast cancer identified by gene co-expression network analysis. Front Oncol. 2018;8:374. doi: 10.3389/fonc.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng R, Wang Y, Mao L, Fang F, Guan H. Identification of core genes involved in the metastasis of clear cell renal cell carcinoma. Cancer Manag Res. 2020;12:13437–13449. doi: 10.2147/CMAR.S276818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamatakos M, Palla V, Karaiskos I, Xiromeritis K, Alexiou I, Pateras I, Kontzoglou K. Cell cyclins: triggering elements of cancer or not? World J Surg Onc. 2010;8:111. doi: 10.1186/1477-7819-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petri ET, Errico A, Escobedo L, Hunt T, Basavappa R. The crystal structure of human cyclin B. Cell Cycle. 2007;6:1342–1349. doi: 10.4161/cc.6.11.4297. [DOI] [PubMed] [Google Scholar]
- 32.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 33.Bellanger S, de Gramont A, Sobczak-Thépot J. Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2. Oncogene. 2007;26:7175–7184. doi: 10.1038/sj.onc.1210539. [DOI] [PubMed] [Google Scholar]
- 34.Wu T, Zhang X, Huang X, Yang Y, Hua X. Regulation of cyclin B2 expression and cell cycle G2/M transition by menin. J Biol Chem. 2010;285:18291–18300. doi: 10.1074/jbc.M110.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei CY, Wang W, Zhu YT, Fang WY, Tan WL. The decrease of cyclin B2 expression inhibits invasion and metastasis of bladder cancer. Urol Oncol. 2016;34:237, e1–10. doi: 10.1016/j.urolonc.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Bolognese F, Wasner M, Dohna CL, Gurtner A, Ronchi A, Muller H, Manni I, Mossner J, Piaggio G, Mantovani R, Engeland K. The cyclin B2 promoter depends on NF-Y, a trimer whose CCAAT-binding activity is cell-cycle regulated. Oncogene. 1999;18:1845–1853. doi: 10.1038/sj.onc.1202494. [DOI] [PubMed] [Google Scholar]
- 37.Tang D, Lotze MT, Kang R, Zeh HJ. Apoptosis promotes early tumorigenesis. Oncogene. 2011;30:1851–1854. doi: 10.1038/onc.2010.573. [DOI] [PubMed] [Google Scholar]
- 38.Ghelli Luserna di Rora’ A, Iacobucci I, Martinelli G. The cell cycle checkpoint inhibitors in the treatment of leukemias. J Hematol Oncol. 2017;10:77. doi: 10.1186/s13045-017-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlowski J, Kozlowska A, Kocki J. Breast cancer metastasis-insight into selected molecular mechanisms of the phenomenon. Postepy Hig Med Dosw (Online) 2015;69:447–451. doi: 10.5604/17322693.1148710. [DOI] [PubMed] [Google Scholar]
- 41.Vieira AF, Paredes J. P-cadherin and the journey to cancer metastasis. Mol Cancer. 2015;14:178. doi: 10.1186/s12943-015-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 43.Han YH, Mun JG, Jeon HD, Kee JY, Hong SH. Betulin inhibits lung metastasis by inducing cell cycle arrest, autophagy, and apoptosis of metastatic colorectal cancer cells. Nutrients. 2020;12:66. doi: 10.3390/nu12010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.