Abstract
Therapies for patients with advanced esophageal squamous cell carcinoma (ESCC) are limited and accompanied by dismal prognosis. Here we use ESCC cell line K30 and TE-1 to investigate the antitumor efficacy of cisplatin plus anti-PD-1 antibody. Enhanced antitumor effects and increased CD8+ tumor-infiltrating lymphocytes of combination therapy were observed in TE-1 cells bearing humanized mice model. Lower cell viability and more cell apoptosis were found in the combination therapy in vitro. We next analyzed clinical data from patients with advanced ESCC received cisplatin-based chemotherapy plus an anti-PD-1 antibody (Tislelizumab or Sintilimab) as first line therapy from two clinical trials (NCT03469557, NCT03748134). With the response rate of 81.8%, duration of response of 15.2 months, median progression-free survival of 15.5 months, median overall survival of 21.5 months and manageable toxicity in patients with advanced ESCC, we demonstrated that cisplatin-based chemotherapy plus anti-PD-1 antibody is an effective and safe option. We further confirmed sublethal cisplatin could induce PD-L1 expression in ESCC cells and cisplatin-treated ESCC cells suppressed the activation and function of immune cells while the addition of sintilimab prevented this process. These results highlight the effectiveness of cisplatin combining with anti-PD-1 antibody in patients with advanced ESCC, revealed its capability to promote the PD-L1 expression in ESCC cells and act synergistically with anti-PD-1 antibody to restore exhausted immune cells activities, thus providing a theoretical basis for further explorations in the mechanism of the combination treatment of cisplatin-based chemotherapy with immune checkpoint inhibitors in ESCC.
Keywords: Esophageal squamous cell carcinoma, immunotherapy, chemotherapy, cisplatin, PD-1
Introduction
Esophageal cancer ranked in the top sixth leading cause of cancer-related death, with an estimated 604,000 new esophageal cancer cases and 544,000 deaths occurred in 2020 worldwide [1]. Compared to western countries where the most esophageal cancer is adenocarcinoma (EAC), the subtype of squamous cell carcinoma exceeds more than 90% of esophageal cancer in Asia, where bearing 70% globule burden of esophageal cancer [2,3]. And patients with ESCC have poorer survival than those with EAC [4]. The standard first line chemotherapeutic regimen for advanced ESCC contained cisplatin (CDDP) plus 5-fluorouracil (5-FU) or paclitaxel. However, the advantages of standard treatments are limited and it often leads to low qualities of life for patients [5]. Thus, new effective therapeutic approaches for advanced ESCC are urgently needed.
Immune checkpoint PD-1 or its ligand PD-L1 inhibitors have demonstrated robust and durable antitumor efficiency in numerous advanced tumors. Accumulating evidence indicates that cytotoxic chemotherapy can improve the effects of immune checkpoint inhibitors such as anti-PD-1/PD-L1 antibodies though the promotion of tumor cellular immunogenicity and suppression of immunosuppressive circuitries [6,7]. Thus, the combination of chemotherapy may synergistically improve antitumor activities of immune checkpoint inhibitors [6-8]. Ongoing clinical trials KEYNOTE-590 and CheckMate 648 are currently investigating the combination of cisplatin-based chemotherapy and pembrolizumab or nivolumab as first-line treatment in patients with advanced ESCC [9,10]. One phase II clinical trial (NCT03469557) assessed combination therapy of tislelizumab (an anti-PD-1 antibody) and cisplatin-based chemotherapy as first line treatment of advanced ESCC suggest this combination therapy indicated promising antitumor activities with manageable safety profiles in this patient population [11]. However, let alone the single arm clinical trial it is, with only 15 patients with advanced ESCC, the results are not so convincing. In addition, the mechanism of improved efficacy in patients received the combination therapy were not investigated.
Tisleizumab [12] and Sintilimab [13] are two fully human monoclonal antibody targeting immune checkpoint PD-1. Compared with nivolumab and pembrolizumab, another two well-investigated anti-PD-1 antibodies, tislelizumab has been reported for difference in structure [14]. Sintilimab exhibited a similar antitumor activity, a better tolerability when compared with nivolumab and pembrolizumab [15]. One clinical trial [16] (NCT03116152) using sintilimab (200 mg administrated intravenously every 21 days) and two clinical trials [17,18] (NCT02407990, CTR20160872) using tisleizumab (200 mg administrated intravenously every 21 days) indicated sintilimab and tisleizumab demonstrated manageable safety profiles and durable responses in patients with advanced solid tumors, including advanced ESCC.
Here, we established humanized mice and observed cisplatin plus sintilimab enhanced the antitumor effects and increased CD8+ tumor-infiltrating lymphocytes (TILs) in ESCC. We also found the combination of cisplatin and sintilimab could induce much more cell death in ESCC cells when compared to monotherapy either by cisplatin or sintilimab alone in vitro through ESCC cells/PBMCs co-culture system. In addition, we collected results from 11 patients from two clinical trials with advanced ESCC which was unresectable or incurable by radiotherapy received cisplatin-based chemotherapy (cisplatin+5-fluorouracil (5-FU) or paclitaxel) plus sintilimab or tislelizumab as first line treatment. Results in this patient population showed the combination therapy had promising antitumor activities and manageable adverse events. Further, we observed that sublethal doses of cisplatin could promote PD-L1 expression in ESCC cells, regulate immune cells activities and help provide a favorable tumor microenvironment (TME) for immune checkpoint blockade (ICB) therapies.
Material and methods
Cell culture
The K30 and TE-1 human esophageal squamous cell carcinoma cell lines were purchased from the American Type Culture Collection (Manassas, Virginia) and grown in RPMI medium supplemented with 10% fetal bovine serum (FBS). Human peripheral blood mononuclear cells (PBMCs) (LP202006) were obtained from ALLCELLS (Shanghai, China) and was grown in RPMI medium supplemented with 10% heat-inactivated FBS.
Chemicals and reagents
Cisplatin (S1166) was obtained from Selleck chemicals (Houston, USA). Anti PD-1 antibody sintilimab was provided by Innovent Biologics (Jiangsu, China). Cell counting kit 8 (CCK8) assay (K1018) came from APExBIO (Houston, USA). For Immunohistochemistry staining, Anti-human CD8 (85336S) antibody was obtained from cell signaling technology (Danvers, USA). Anti-human CD3 (B329667) and anti-human CD28 (B332380) antibodies for activation of T cells in PBMCs were bought from BioLegend (San Diego, USA). Human IL-2 (072012) was obtained from Peprotech (Rocky Hill, USA). For flow cytometry analysis, human CD25-PE (302606), human CD8-BV421 (8296990), human CD274 (PD-L1)-PE (B262099), human CD45-BV421 (304031) were bought from BioLegend (San Diego, USA). FITC-Annexin V apoptosis detection kit (556547) was obtained from BD Biosciences (Franklin Lakes, USA). For western blot analysis, anti-PD-L1 antibody was gained from cell signaling technology (Danvers, USA). For immunohistochemistry (IHC), anti-PD-L1 antibody (SK006) was purchased from Dako (Hovedstaden, Denmark). Human IL-2 ELISA kit (431807) and Human IFN-γ ELISA kit (430107) were obtained from BioLegend (San Diego, USA).
In vivo mouse studies
Four-week-old female NSG mice were purchased from Model Organisms (Shanghai, China). All procedures were performed in accordance with institutionally approved IACUC protocols. Four-week-old NSG female mice were subcutaneously injected in the right flank with 1×106 TE-1 ESCC cells. Mice were tail vein injected with 5×106 PBMCs when the tumor size reached approximately 50 mm3 (calculated by the formula: (L×W2)/2). After confirming that the percentages of human CD45 in peripheral blood of mice lager than 20% and tumor size reached 90-100 mm3, mice were randomly divided into five groups with a comparable average tumor size and administered intraperitoneally every 4 days with: vehicle (PBS), IgG isotype control antibody (1 mg/kg), cisplatin (cis-diamminedichloroplatinum (II): CDDP, 5 mg/kg), anti-PD-1 antibody sintilimab (1 mg/kg) or the combination CDDP and anti-PD-1. Tumors were measured every 3 to 4 days.
Patients
Patients aged ≥18 years with a diagnosis of unresectable advanced or recurrent ESCC (AJCC, 8th edition) who had received cisplatin-based chemotherapy plus tislelizumab or sintilimab as fist-line therapy from two clinical trials (NCT03469557; NCT03748134) in the first affiliated hospital of Zhejiang University from December 15th, 2017 to September 27th were collected. None of patients received previous chemotherapy or radiotherapy. TNM staging manual (8th edition) was used for staging. Patient demographics and clinical data were obtained from the institution’s digital archives. The investigations were performed in compliance with the study protocol, which was approved by the Clinical Research Ethics Committee of the first affiliated hospital of Zhejiang University with the reference number of 2021473. All authors have access to the data and participated in the written, reviewed or edit the draft of the article and ensure the integrity of the accuracy data analysis.
Therapeutic regimen
Patients in FP+T cohort received 5-fluorouracil (750 mg/m2, days 1-5) and cisplatin (75 mg/m2, day 1) plus tislelizumab (200 mg i.v., day 1) in each 21-day cycle. Patients in TP+S cohort received paclitaxel (175 mg/m2 i.v., days 1) and cisplatin (75 mg/m2, day 1) plus sintilimab (200 mg i.v., day 1) in each 21-day cycle. Treatment continued for up to six cycles, followed by maintenance therapy with sintilimab (200 mg i.v., every 21 days) or tislelizumab (200 mg i.v., every 21 days) until progression, death or unacceptable toxicity, or for up to 24 months. Patients could continue treatment after the initial disease progression based on RECIST v1.1, to confirm the disease progression status, as taking into account of the potential pseudoprogression.
Assessments
Response was assessed per RECIST v1.1 and per irRC by investigator. Progression free survival (PFS) was calculated as the time between day 1 (treatment started) and disease progression defined by RECIST v1.1 or last follow-up date. Patients were followed up for survival until death or study closure. Adverse events (AEs) were assessed for up to 30 days after treatment (90 days for serious AEs) and were graded according to Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Safety and efficacy analysis were performed in all patients. The response rate was assessed by point estimate. Duration of response (DoR), progression-free survival (PFS), and overall survival (OS) was estimated using the Kaplan-Meier method. 95% CIs were calculated using the Clopper-Pearson method. Statistical calculation was performed by GraphPad Prism Software V.8.0; T-test was used for factor change comparisons and two-way analysis of variance (ANOVA) was used for groups comparisons, P value <0.05 considered significant.
Results
Cisplatin plus anti-PD-1 antibody enhanced the antitumor effects and increased CD8+ TILs in ESCC in humanized mice model
Humanized mice were established and commonly used ESCC cell line TE-1 were inoculated. Schematic diagram of experiment was shown in Figure 1A. Humanized mice bearing TE-1 cells were divided into five treatment groups, and the tumor volumes of mice treated with PBS (control), isotope IgG antibody, CDDP, sintilimab or combined CDDP and sintilimab were measured and plotted (Figure 1B). Mice were euthanized and tumor weight were measured at day 15 (Figure 1C). Tumors in the control and the isotope IgG groups have no significant difference in tumor growth rate and tumor weight. In the cisplatin and anti-PD-1 antibody groups, tumor volume was reduced by 22% and 44% respectively as compared to control (1113.5±189 and 801.4±151 vs 1425.0±215 mm3). In the combination treatment group, the tumor volume was significantly reduced which the reduction reached 65% as compared to control (502.2±131 vs 1425.0±215 mm3) (Figure 1B). The differences among groups in terms of tumor weight were consistent with the differences showed in the tumor volume and these data were summarized in Figure 1C. Next, we evaluated the CD8+ TILs in ESCC from humanized mice of each group. We calculated CD8 positive area (brown area) on images at the same magnification and observed that the combination treatment exhibited the highest area of CD8+ lymphocytes infiltration (13568.4±2543 μm2) when compared to either control group (2663.1±450 μm2), IgG group (2613.6±530 μm2) or monotherapy groups (CDDP: 4111.2±1090; αPD-1: 8888.6±1860 μm2; Figure 1D, 1E). Thus, these results suggest cisplatin plus anti-PD-1 antibody could improve the therapeutic effects and increased CD8+ TILs in ESCC in animal models.
Figure 1.
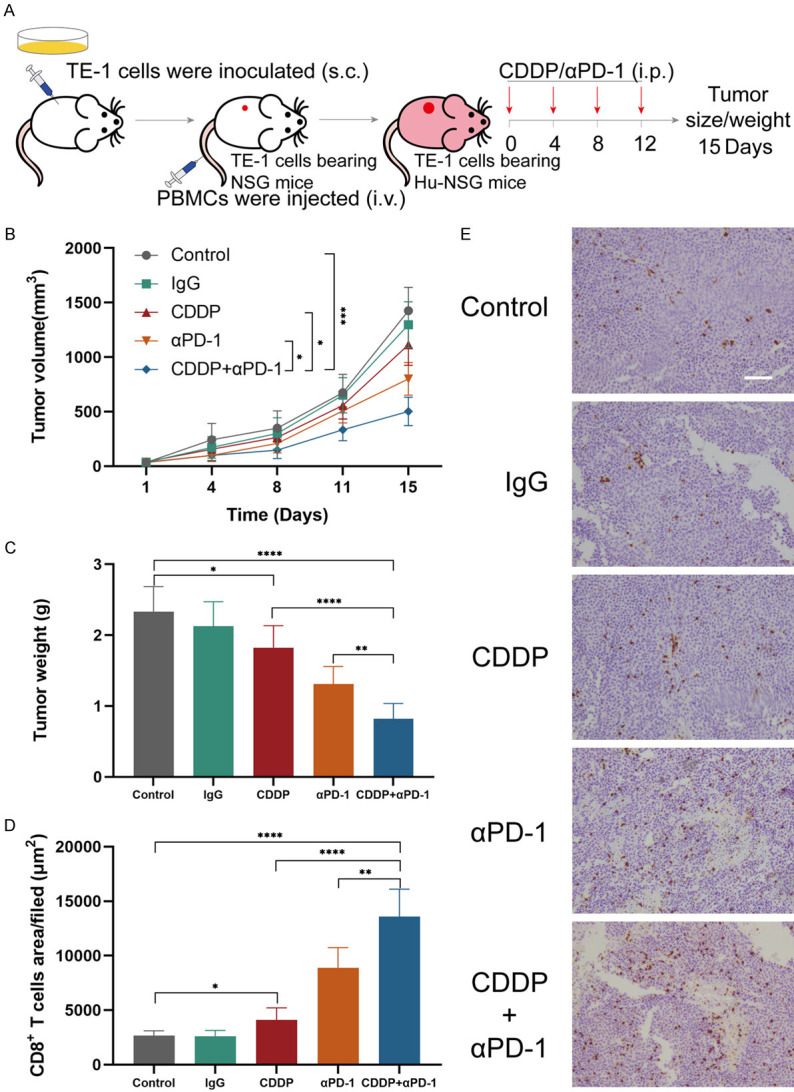
CDDP plus anti-PD-1 antibody enhanced the antitumor effects and increased CD8+ TILs in ESCC in humanized mice model. (A) Schematic diagram of humanized mice establishment and experimental design. (B) Average tumor volumes. (C) Weights of tumors on day 15. (D) Average percentages of CD8+ TILs area in tumors per filed. (E) Representative immunohistochemistry images of tumors stained for CD8 (brown). Scale bar, 100 μm. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001; two-way analysis of variance (ANOVA) in (B), two tailed unpaired Student’s t test in (C) and (D). Data from two independent experiments were shown (means ± SEM).
Cisplatin sensitizes K30 and TE-1 cells to activated PBMCs and the killing ability of immune cells can be enhanced by PD1 blockade
As CD8+ lymphocytes are the major cytotoxic immune cells to eliminate tumor cells and it increased the most significantly in the combination treatment group in ESCC in animal models, we suspected the increased CD8+ TILs played a role in tumor reduction and want to explore whether cisplatin could influence the sensitivity of ESCC cells to cytotoxic immune cells. We established an ESCC cells/PBMCs co-culture system in vitro. The schematic diagram is shown in Figure 2. Before the assays, PBMCs were in vitro expanded and activated with 100 U/ml IL-2 and 5 μg/ml anti-CD3, anti-CD28 antibodies for 7-10 days. The activation was confirmed by flow cytometry analysis of the increased expression of CD25 (a T cell activation marker) in CD8+ lymphocytes (Figure 3A, 3B). Cell viability of K30 and TE-1 cells in responding to different dose of CDDP were determined. In order to avoid cisplatin triggering massive cell death, sublethal doses (1 μg/ml) of cisplatin was used to treat ESCC cells in the following experiments. K30 or TE-1 cells were cultured with cisplatin (1 µg/ml) for 48 hours. Thereafter, untreated or CDDP-treated K30 or TE-1 cells (5×104 cells) were cultured with activated PBMCs (with 0.1 mg/ml IgG or with 0.1 mg/ml sintilimab) in 96-well round plates for 24 hours, the ratio of effector cells to target cells was 5:1 (E:T=5:1). Supernatant was discarded and PBMCs were washed away. Cell viability of K30 and TE-1 cells was measured by CCK8 assay. As a result, compared to untreated cells, cisplatin treated K30 or TE-1 cells were more susceptible to activated PBMCs and the addition of sintilimab could further lower the relative cell survival rate (Figure 3D, 3E). In order to further verify the killing effect of immune cells on tumor cells, we digested the whole cells in the co-culture system, and then stained with anti-CD45-BV421, followed by PI and annexin V-FITC. CD45 negative cells which represent tumor cells were gated and analyzed for apoptosis (Figure S1). The similar phenotype was observed, activated PBMCs induced more apoptosis on cisplatin treated K30 or TE-1 cells when compared to untreated cells and the addition of sintilimab enhanced the cell killing ability of immune cells. These data are summarized in Figure 4. These results indicated that cisplatin treatment could increase the sensitivity of K30 and TE-1 cells to cytotoxic immune cells, and the killing ability of immune cells could be enhanced by PD1 blockade.
Figure 2.
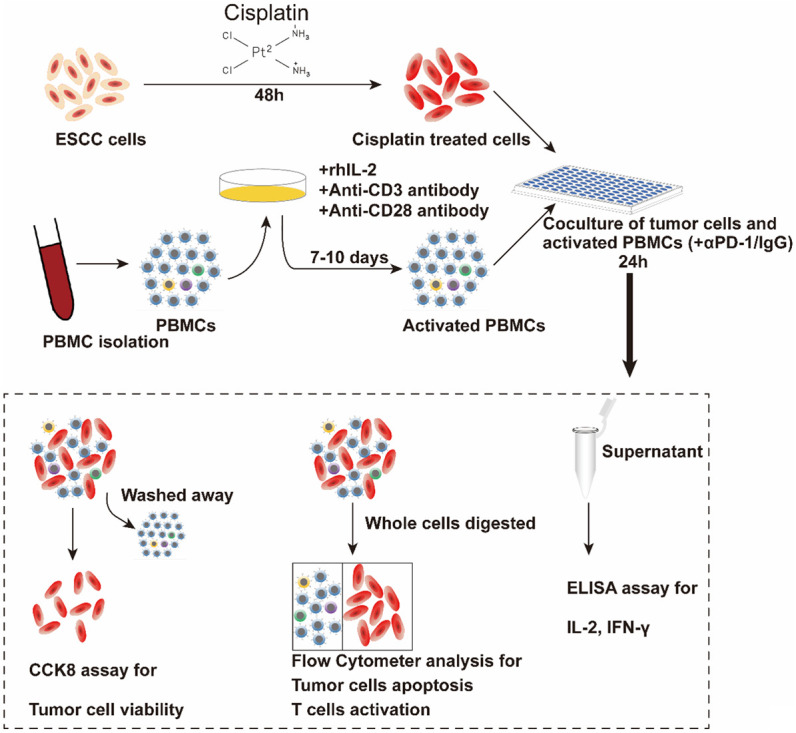
Schematic diagram of co-culturing ESCC cell lines and PBMCs. K30 or TE-1 cells were cultured with CDDP (1 μg/ml) for 48 h. Thereafter, untreated or CDDP-treated K30 or TE-1 cells (5×104 cells) were cultured with activated PBMCs (with 0.1 mg/ml IgG or with 0.1 mg/ml sintilimab) in 96-well round plates for 24 h, E:T=5:1. Subsequently, cell viability was detected by CCK8 assay and flow cytometry. The levels of IL-2 and IFN-γ in supernatants were measured.
Figure 3.
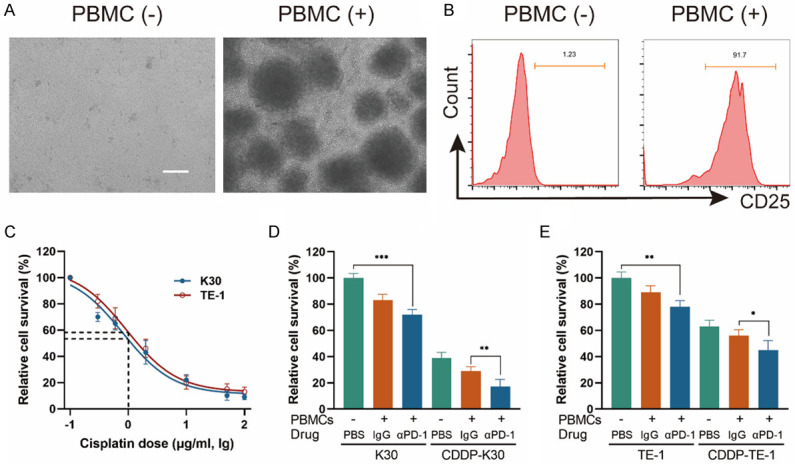
CDDP plus anti-PD-1 antibody significantly inhibited the growth of ESCC cells in the ESCC cells/PBMCs co-culture system. A. Representative images of PBMCs before and after stimulation. B. FACS showing the ratio of CD25+ cells in CD8+ lymphocytes before and after stimulation. C. CCK8 assay showing K30 and TE-1 cells viability in responding to different dose of CDDP and plot against lg μg/ml of CDDP. In the concentration of 1 µg/ml (lg dose =0), the relative cell survival rate of K30 and TE-1 cells were between 50% and 60%. D and E. ESCC cells/PBMCs co-culture system was established. After co-culturing, supernatant was discarded and PBMCs were washed away. Cell viability of adherent K30 and TE-1 cells was measured by CCK8 assay. Data from three independent experiments were shown (means ± SEM). Scale bar, 100 μm. *P<0.05, **P<0.01, ***P<0.001.
Figure 4.
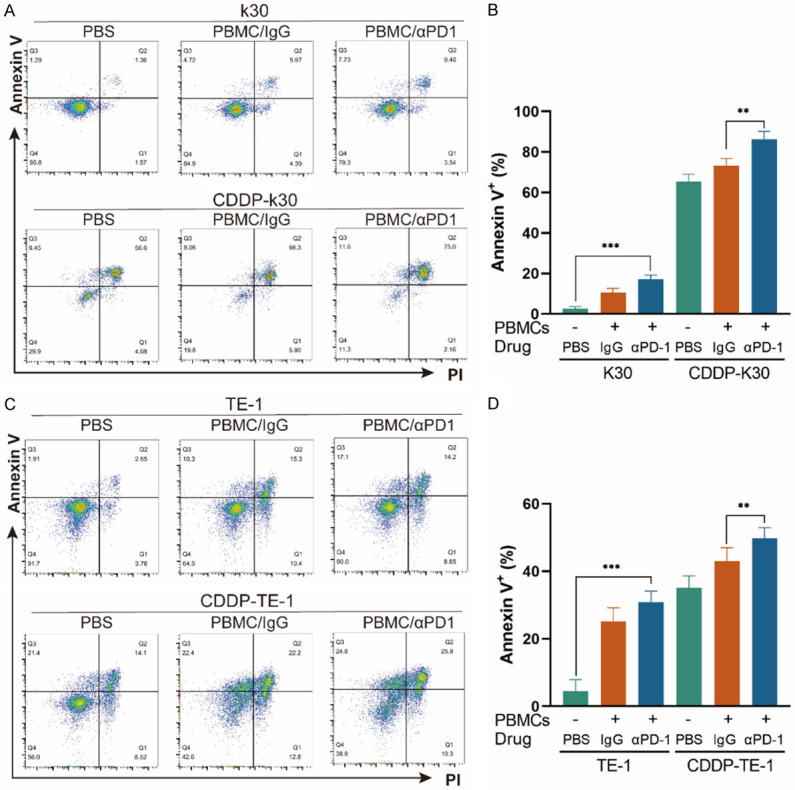
CDDP sensitizes K30 and TE-1 cells to PBMCs and the cell killing ability of immune cells can be enhanced by PD1 blockade. ESCC cells/PBMCs co-culture system was established. After co-culturing, whole cells were harvested and stained with anti-CD45-BV421, followed by PI and annexin V-FITC. CD45 negative cells were analyzed for apoptosis. (A and C) Representative dot plots of K30 (A) and TE-1 (C) cells from flow cytometry. (B and D) Percentages of annexin V+ of K30 (B) and TE-1 (D) cells. Data from three independent experiments were shown (means ± SEM). **P<0.01, ***P<0.001.
Antitumor activities of cisplatin-based chemotherapy plus anti-PD-1 antibody in patients with advanced ESCC
As the addition of anti-PD-1 antibody enhanced antitumor efficacy of cisplatin in vitro and in vivo, we next want to see the effect of the combination therapy in patients. From December 15th, 2017, to September 27th, 2019, 11 patients with advanced ESCC which was unresectable or incurable by radiotherapy from two clinical trials were collected. Baseline characteristics were list in Table 1. All patients received cisplatin-based chemotherapy plus an anti-PD-1 antibody as first line therapy. The regimen for 6 patients (NCT03469557) was 5-FU + cisplatin + tislelizumab (FP+T), and the other 5 patients (NCT03748134) was paclitaxel + cisplatin + sintilimab (TP+S), according which, patients were divided into FP+T cohort (n=6) and TP+S (n=5) cohort. The most common adverse events in both cohorts were decreased neutrophil count, decreased white blood cells, alopecia, asthenia and gastrointestinal reaction including decreased appetite, nausea and vomiting, details were summarized in Table S1. No patient died as a result of TRAEs. The median duration of followup time was 20.9 months (range, 6.0-39.6 months), 19.9 months (range, 6.0-39.6 months) and 20.9 months (range, 7-23.3 months), for all patients, patients in FP+T and TP+S cohort respectively, as data cutoff of June 15th, 2021. Two patients remained on treatment (n=2, 18.2%) as data cutoff. Nine patients [FP+T, n=6 (100%); TP+S, n=3 (60%)] discontinued the study treatment as disease progression (n=9, 81.8%) (Figure 5). The objective response rate (ORR) by central review per International Working Group 2007 criteria was 81.8% in total patients (95% CI, 48.2% to 97.7%; FP+T, 83.3%; TP+S, 80%). And the disease control rate (DCR) reached 100% in total patients (95% CI, 71.5%-100%; FP+T, 100%; TP+S, 100%). Overall, in FP+T cohort (n=6), 3 patients (50%) achieved a complete response (CR) and 2 patients (33.3%) achieved a partial response (PR), 1 patient (16.7%) achieved a stable disease (SD). In TP+S cohort (n=5), 4 patients (80%) achieved a PR, 1 patient (20%) achieved a SD by Lugano criteria (Table 2). Early and durable responses were observed in both the cohorts (Figure 7), the median time to response was 6 weeks in both the FP+T and TP+S cohorts. The median DoR of these patients was 15.2 months in total patients (range, 10.0-26.6 months; FP+T, 15.2 months; TP+S, 18.5 months; Table 2). The median PFS was 15.5 months in total patients (FP+T, 15.5 months, TP+S, 16.9 months), and the median OS was 21.5 months in total patients (FP+T, 18.3 months; Table 2; Figure 6). The median OS in TP+S was not reached, although was anticipated to extend beyond 20.9 months (Figure 6). Nine (81.8%) of 11 evaluable patients had target lesion size reduced from baseline (5/6 in FP+T cohort; 4/5 in TP+S cohort; Figure 7). Some representative computer tomography (CT) scan images of primary or metastatic lesions before and after treatment in FP+T or TP+S cohort are shown in Figures 8 and 9. Kaplan-Meier estimates of PFS and OS for patients in each cohort were showed in Figure 6. Collectively, these results indicated cisplatin-based chemotherapy plus anti-PD-1 antibody demonstrated promising antitumor activities in patients with advanced ESCC.
Table 1.
Baseline characteristics
| FP+T (n=6) | TP+S (n=5) | Overall (n=11) | |
|---|---|---|---|
| Age, years, median (range) | 64 (48-67) | 62 (61-67) | 63 (48-67) |
| Sex, n (%) | |||
| male | 6 (100) | 5 (100) | |
| ECOG status, n (%) | |||
| 0 | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| 1 | 5 (83.3) | 3 (60.0) | 8 (72.7) |
| 2 | 0 | 1 (20.0) | 1 (9.1) |
| TNM stage | |||
| Iva | 2 (33.3) | 2 (40.0) | 4 (36.4) |
| Ivb | 4 (66.7) | 3 (60.0) | 7 (63.6) |
| Histologic grade of primary tumor, n (%) | |||
| Gx | 0 | 1 (20.0) | 1 (9.1) |
| G1 | 0 | 1 (20.0) | 1 (9.1) |
| G2 | 1 (16.7) | 2 (40.0) | 3 (27.3) |
| G3 | 5 (83.3) | 1 (20.0) | 6 (54.5) |
| Tumor location | |||
| Upper thoracic | 1 (16.7) | 1 (20.0) | 2 (18.2) |
| Mid-thoracic | 0 | 2 (40.0) | 2 (18.2) |
| Lower thoracic | 5 (83.3) | 2 (40.0) | 7 (63.6) |
Abbreviation: ECOG PS, Eastern cooperative oncology group performance status.
Figure 5.
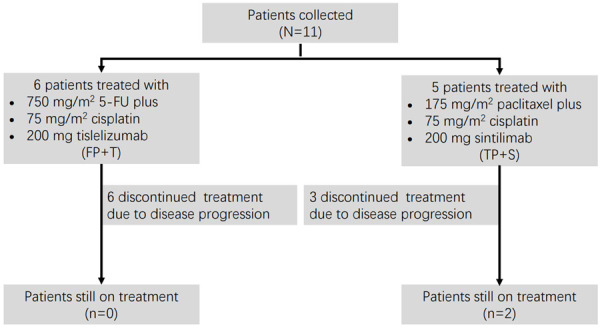
Treatment regimens.
Table 2.
Antitumor activity
| FP+T | TP+S | Total | |
|---|---|---|---|
| Response, % (95% CI) | |||
| Overall response | 83.3 (35.9-99.6) | 80 (28.4-99.5) | 81.8 (48.2-97.7) |
| Complete response | 50 (11.8-88.2) | 0 | 27.3 (6.0-61.0) |
| Partial response | 33.3 (4.3-77.7) | 80 (28.4-99.5) | 54.5 (23.4-83.3) |
| Stable disease | 16.7 (0.4-64.1) | 20 (0.5-71.6) | 17.4 (5.0-38.8) |
| Disease control rate | 100 (54.1-100) | 100 (47.8-100) | 100 (71.5-100) |
| Progressive disease | 0 | 0 | 0 |
| Median time to response, weeks | 6 | 6 | 6 |
| Median duration of response, months (range) | 15.2 (10.0-26.6) | 18.5 (13.7-21.1) | 15.2 (10.0-26.6) |
| Median PFS, months | 15.5 | 16.9 | 15.5 |
| Median OS, months | 18.3 | Not reached | 21.5 |
| 12 months disease control rate n(%) | 4 (66.7) | 4 (80.0) | 8 (72.7) |
Abbreviation: PFS, Progression-free survival; OS, Overall survival.
Figure 7.
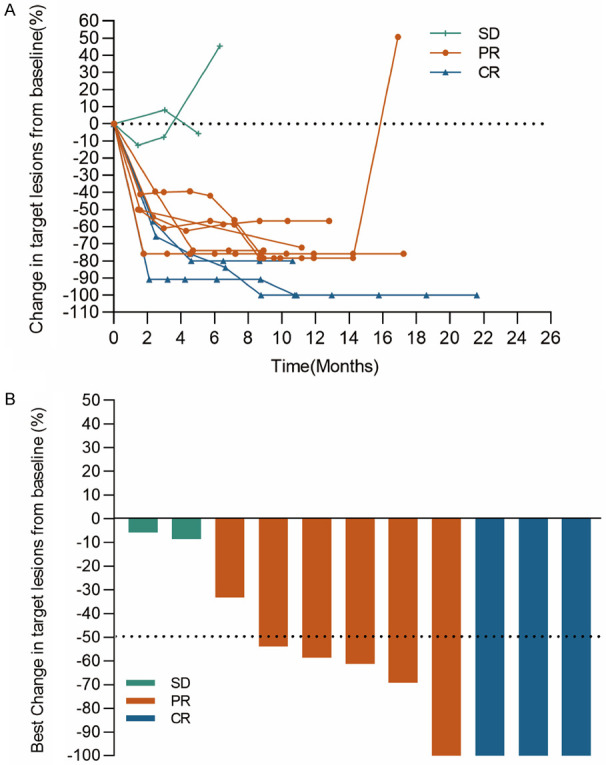
Change in sum of target lesion diameters over time for total patients (A). Best change from baseline in target lesion size for total patients (B).
Figure 6.
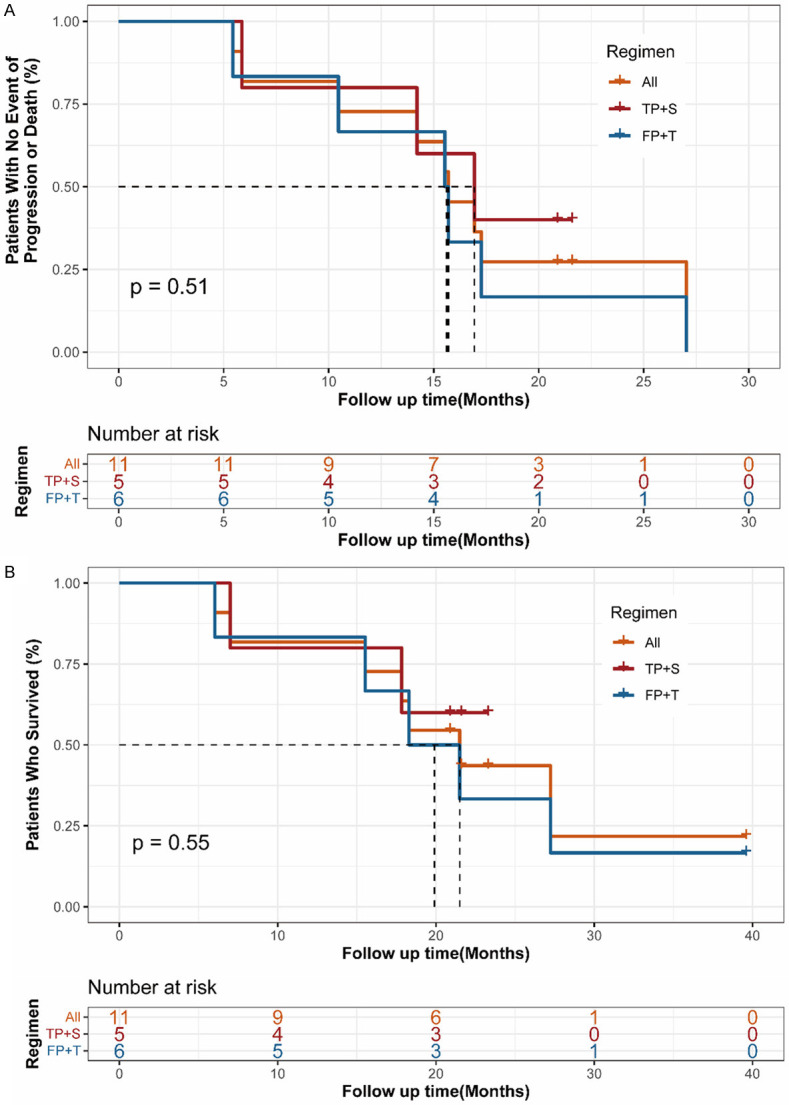
Kaplan-Meier plots of median progression-free survival (A) and overall survival (B).
Figure 8.
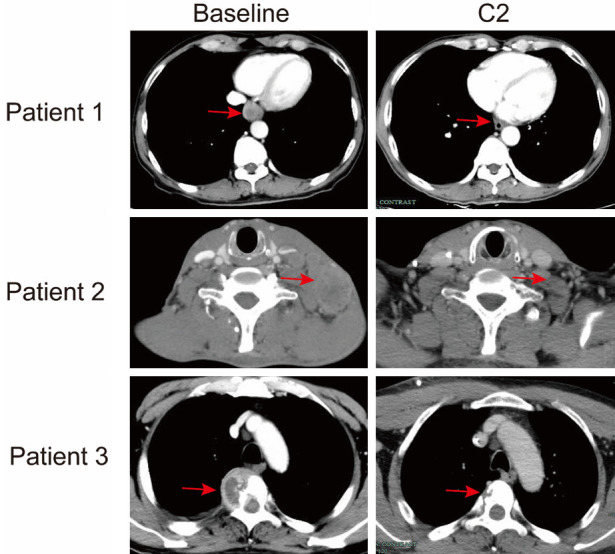
Representative CT scan images of primary lesion (patient 1), lymph nodes (patient 2) and spine (patient 3) metastatic lesions after two cycles of FP+T regimen. Red arrows indicate the primary or metastatic lesions.
Figure 9.
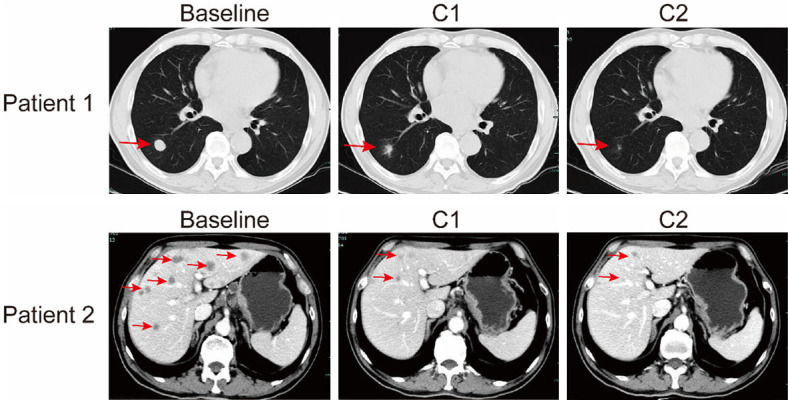
Representative CT scan images of lung (patient 1) and liver (patient 2) metastatic lesions after one and two cycles of TP+S regimen. Red arrows indicate the metastatic lesions.
Cisplatin increased PD-L1 expression in ESCC cells
Researches revealed that cisplatin could induce significant levels of PD-L1 upregulation in several tumors including lung cancer and ovarian cancer [19,20]. As previous studies showing that the addition of sintilimab enhanced the killing rate of cisplatin treated ESCC cells by activated PBMCs, we hypothesized cisplatin could also promote the levels of PD-L1 in ESCC cells, thus synergized with sintilimab to induce tumor cells killing by immune cells. After treatment by 1 µg/ml cisplatin for 48 hours, the PD-L1 expression of ESCC cells were found elevated in the protein levels as showed by western blot analysis (Figure 10C, 10F). We further detected it by a flow cytometer antibody targeting PD-L1, and observed the increased signals on cell surface of ESCC cells (Figure 10A-E), which proved that these elevated PD-L1 expression was targetable and functional. We next analyzed the PD-L1 expression in ESCC from humanized mice model by immunohistochemistry staining and western blot, it also showed the increased expression level of PD-L1 after treated by cisplatin (Figure 10G, 10I). In sum up, these results indicated that the subtoxic dose of cisplatin could promote the PD-L1 expression of ESCC cells in vitro and in vivo.
Figure 10.
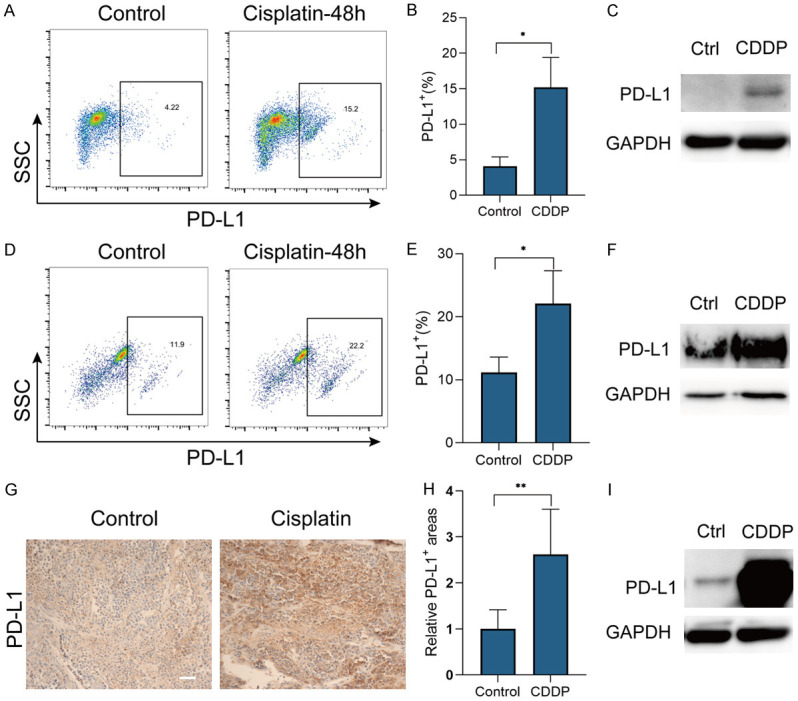
CDDP increased ESCC cells PD-L1 expression. (A-E) PD-L1 expression of K30 (A) and TE-1 (D) cells, treated with CDDP (1 μg/ml) or PBS (control) for 48 h, detected by flow cytometry. Gates were set according to isotype control. Percentages of PD-L1 positive K30 (B) and TE-1 (E) cells from three independent experiments were shown (means ± SEM). (C, F) PD-L1 expression of K30 (C) and TE-1 (F) cells, treated with CDDP (1 μg/ml) or PBS (control) for 48 h, detected by western blot. (G-I) PD-L1 expression in ESCC from humanized mice treated by CDDP (5 mg/kg) for 15 days, detected by IHC staining (G) and western blot (I). Relative PD-L1 positive area (H) from two experiments were shown (means ± SEM). Scale bar, 50 μm. *P<0.05, **P<0.01.
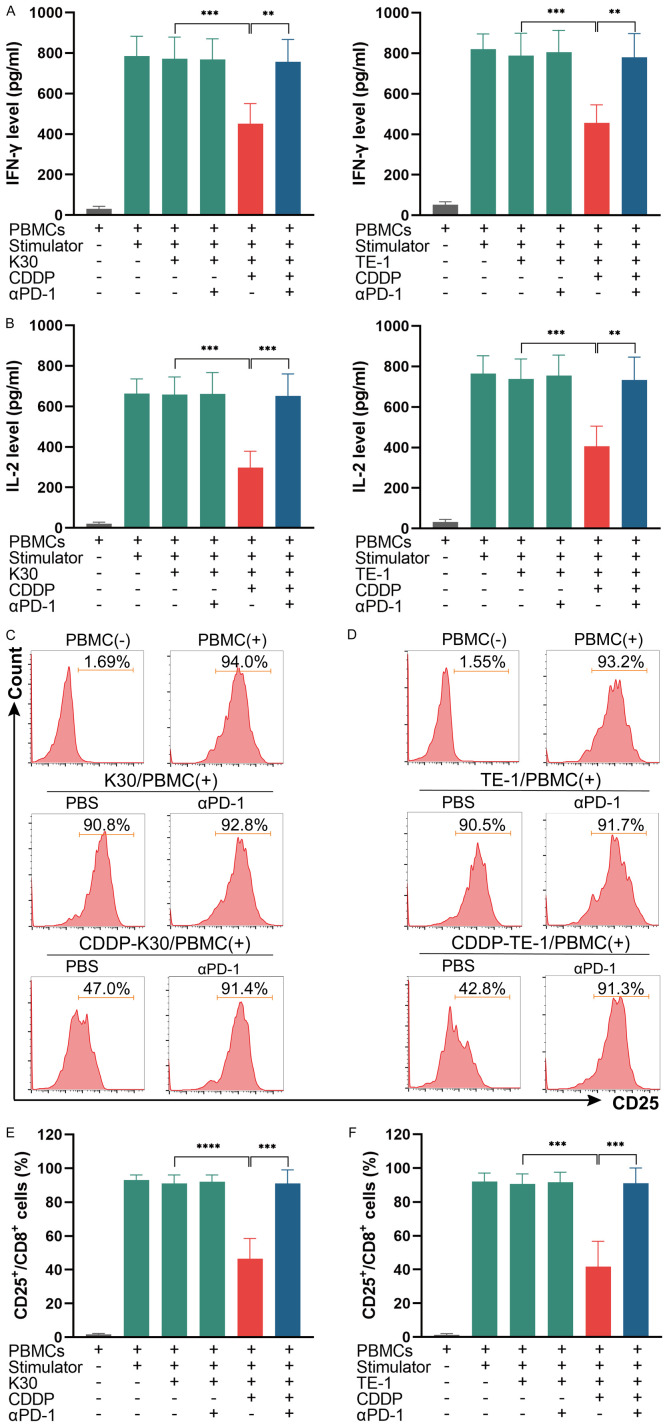
The activation of CD8+ lymphocytes as well as secretion of IFN-γ and IL-2 can be suppressed by cisplatin-treated ESCC cells while the addition of anti-PD-1 antibody prevent this process
As immune cells’ function can be inhibited by PD-L1 expressed on cancer cells, we next want to explore the effect of cisplatin-treated cancer cells on immune cells in the coculture system, including its activation and function. The co-culture system was established as mentioned previously (Figure 2). After co-culturing, the levels of IFN-γ and IL-2 reflecting the function of immune cells in cell culture supernatants were measured by ELISA. The results indicated that CDDP-treated ESCC cells suppressed immune cells function in the secretion of IFN-γ and IL-2, while the addition of the anti-PD1 antibody sintilimab in CDDP-treated ESCC cells/PBMCs co-culture system restored immune cells function (Figure 11A, 11B). We next detected the expression of CD25 (a marker of lymphocytes activation) in CD8+ lymphocytes to further examine the effect of cisplatin-treated ESCC cells on CD8+ lymphocytes’ activation. PBMCs were collected from the co-culture system and stained with CD8 and CD25 flow cytometer antibodies, the ration of CD25+ cells in CD8+ lymphocytes were analyzed. The results suggest that the ration of CD25+ cells in CD8+ lymphocytes was significantly decreased by CDDP-treated ESCC cells as compared to ESCC cells itself, while the addition of sintilimab in CDDP-treated ESCC cells/PBMCs co-culture system restored the activation of CD8+ lymphocytes (Figure 11C-F). Collectively, these results revealed that the activation of CD8+ lymphocytes as well as immune cells function in secretion of IFN-γ and IL-2 could be suppressed by cisplatin-treated ESCC cells in the co-culture system while the addition of anti-PD1 antibody prevent this process.
Figure 11.
The activation of CD8+ lymphocytes as well as secretion of IFN-γ and IL-2 can be suppressed by CDDP-treated ESCC cells in the co-culture system while the addition of anti-PD-1 antibody prevent this process. (A and B) ESCC cells/PBMCs co-culture system was established. After co-culturing, the levels of IL-2 and IFN-γ in supernatants were measured by ELISA. Data from three independent experiments were shown (means ± SEM). (C-F) PBMCs from K30 cells/PBMCs co-culture system (C) and TE-1 cells/PBMCs co-culture system (D) were collected and stained with CD8-PB and CD25-PE, the ratio of CD25+ cells in CD8+ lymphocytes were detected by flow cytometry. Percentages of CD25+ cells in CD8+ lymphocytes in K30 cells/PBMCs co-culture system (E) and TE-1 cells/PBMCs co-culture system (F) from three independent experiments were shown (means ± SEM). **P<0.01, ***P<0.001 and ****P<0.0001.
Discussion
For advanced ESCC, standard regimens is cisplatin plus paclitaxel or 5-FU [21]. The ORR in first line chemotherapy of advanced ESCC are approximately 37%-58%, while the DoR is only 4-7 months [17,22-24]. New effective therapeutic approaches for advanced ESCC are urgently needed. In our study, the enhanced antitumor effects of cisplatin plus sintilimab compared to single agent regimen were obtained in vivo and in vitro. In analysis of 11 patients with advanced ESCC which is unresectable or incurable by radiotherapy, we observed the clinical benefit of cisplatin-based chemotherapy plus anti-PD-1 antibody (tislelizumab or sintilimab) in this patient population. The safety profiles of this combination were acceptable and manageable. The response rate was up to 81.8% (9 of 11) with median DoR of 15.2 months (10-26.6 months), 3 patients (27.3%) achieved a complete response and 6 patients (54.5%) achieved a partial response. Although it is difficult to make direct comparisons among several trials since which had some different clinicopathological patient characteristics, the ORR was 33% and the median DoR was 5.75 months of standard chemotherapy (cisplatin+5-FU) in Japanese patients with advanced ESCC [25]. As data cutoff of June 15th, 2021, the median PFS was 15.5 months, the median OS was 21.5 months in our study while a media PFS of 4.8-7.9 months and median OS of 10.4-13.5 months [17,22-24] had been reported for first line chemotherapy across several clinical trials. In addition, preliminary results of an ongoing clinical trial (KEYNOTE-590) suggest promising antitumor activities of pembrolizumab plus cisplatin-based chemotherapy and median OS was 12.6 months in patients with combination therapy while which was 9.8 months in patients with chemotherapy alone [10]. Another ongoing clinical trial (CheckMate 648) investigating nivolumab plus cisplatin-based chemotherapy obtained the median OS of 13.2 months which is superior than the median OS of 10.7 months in patients with chemotherapy alone [9]. Another anti-PD-1 antibody camrelizumb plus cisplatin-based chemotherapy in advanced ESCC also showed promising antitumor activities with a manageable safety profiles [26]. Taken together, these results demonstrated compared to chemotherapy alone, cisplatin-based chemotherapy plus anti-PD-1 antibody had more potent antitumor efficiency in patients with advanced ESCC.
Current researches demonstrated that facilitating the lymphocytes activation and/or infiltration in the TME is the key factor for tumor responding to immune checkpoint PD-1 antibodies [27]. Yoshihiro observed neoadjuvant chemotherapy of 5-FU+cisplatin increased CD8+ TILs in ESCC [28]. In our study, we also found a significantly increased CD8+ TILs in ESCC in the combination group indicating cisplatin may turning the tumor immune microenvironment into a favorable one for ICB therapies. With high frequency of neoantigens, ESCC is theoretically suitable for immunotherapy and the neoantigens caused by platinum salts can be added to de novo mutations caused by tobacco smoke and other carcinogens in ESCC [29-31], thus enhanced the immunotherapy effects. Researches revealed that chemothrapuetic treatment of 5-FU+cisplatin increased PD-L1 expression in ESCC [32]. Here, we confirmed the ability of cisplatin in promoting ESCC cells PD-L1 expression. Some authors demonstrated that with lack of PD-L1 expression, tumors usually exhibit phenotype characterized as ‘cold’ tumors which shows a low level of immune cells infiltration in the surrounding microenvironment [33,34]. The elevated PD-L1 expression in tumors may play a significant role in transforming ESCC into “hot” tumor and partly explained the enhanced antitumor activities in the co-culture system by addition of sintilimab. IFN-γ and IL-2 can be secreted by lymphocytes in PBMCs, while which could be extinguished due to lymphocytes exhaustion [35,36]. Interestingly, in this study, we observed cisplatin-treated ESCC cells inhibited the activation of CD8+ lymphocytes and suppressed the secretion of IFN-γ and IL-2 in the co-culture system while which could be restored by the addition of sintilimab. These results demonstrated that the beneficial effect of cisplatin was mediated, at least partly, by its capability to promote PD-L1 expression in ESCC cells and act synergistically with anti-PD-1 antibody to restore exhausted lymphocytes activities.
One of the limitations in our study is that we did not follow up with biomarkers to predict the effect of this combination therapy. Preliminary results of KEYNOTE-590 and CheckMate 648 showed patients with high PD-L1 expression achieved longer median OS in pembrolizumab/nivolumab + chemotherapy regimen when compared to chemotherapy alone, however, superior clinical benefits were also obtained for OS, PFS and ORR in all ESCC patients regardless of PD-L1 expression in tumors [9,10]. In addition, another clinical trial testing tislelizumab plus chemotherapy in ESCC and gastric/gastroesophageal junction adenocarcinoma also demonstrated tislelizumab exhibited durable clinical responses regardless of PD-L1 status in these tumors [11]. Combined with the results from ESCC cells/PBMCs co-culture system we did, we assumed that cisplatin-based chemotherapy may upregulate the expression of PD- L1 in tumors, help providing a favorable TME for ICB therapies and act synergistically with anti-PD-1 antibody to restore exhausted T-cells activities, thus enhanced antitumor activities. Further investigation should be done to analyze the potential biomarkers for this combination therapy.
Conclusion
In summary, we found cisplatin plus anti-PD-1 antibody enhanced the antitumor effects and increased CD8+ TILs in ESCC in humanized mice model. In vitro co-culture system, we observed that cisplatin sensitized K30 and TE-1 cells to activated PBMCs and the killing ability of immune cells could be enhanced by PD1 blockade. We also provided clinical data in our center responding to the regimen cisplatin-based chemotherapy plus an anti-PD-1 antibody (tislelizumab or sintilimab) of 11 patients with advanced ESCC and demonstrated that treatment of cisplatin-based chemotherapy plus tislelizumab or sintilimab is an effective and safe option in patients with advanced ESCC. We further confirmed cisplatin could increase the expression of PD-L1 in ESCC cells and this capability act synergistically with anti-PD-1 antibody to restore exhausted T-cells activities, which partly explained the beneficial effect of the combination treatment. This study will hopefully provide a solid and reliable basis for further explorations in the mechanism of the combination treatment of cisplatin-based chemotherapy with immune checkpoint inhibitors in ESCC.
Acknowledgements
This study was supported by the project of the Regional Diagnosis and Treatment Center of the Health Planning Committee (No. JBZX-201903); Zhejiang Science and Technology Planning Project (No. 2021C03119) and Clinical Research Fund Project of Zhejiang Medical Association (2018ZYC-A18).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Edgren G, Adami HO, Vainio EW, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 4.Njei B, Mccarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–1146. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26:1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 9.Chau I, Doki Y, Ajani JA, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MIFM, Holtved E, Xynos I, Liu X, Lei M, Kondo K, Kato K, Kitagawa Y. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J. Clin. Oncol. 2021;39:LBA4001–LBA4001. [Google Scholar]
- 10.Kato K, Sun JM, Shah MA, Enzinger PC, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BCC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Liu Q, Shah S, Bhagia P, et al. LBA8_PR pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192–S1193. [Google Scholar]
- 11.Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, Li X, Wang X, Yuan X. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26:4542–4550. doi: 10.1158/1078-0432.CCR-19-3561. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, Zhang Y, Zhou X, Wang Z, Wang Y, Shi Y, Bai H, Liu N, Yang X, Cui X, Cao Y, Liu Q, Song J, Li Y, Tang Z, Guo M, Wang L, Li K. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy SM. Sintilimab: first global approval. Drugs. 2019;79:341–346. doi: 10.1007/s40265-019-1066-z. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Hong Y, Sun H, Zhang B, Wu H, Li K, Liu X, Liu Y. Abstract 2383: the molecular binding mechanism of tislelizumab, an investigational anti-PD-1 antibody, is differentiated from pembrolizumab and nivolumab. Published Online. 2019:2383. [Google Scholar]
- 15.Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol. 2020;10:594558. doi: 10.3389/fonc.2020.594558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Li Y, Fan Q, Shu Y, Wu Z, Cui T, Gu K, Tao M, Wang X, Cui C, Xu N, Xiao J, Gao Q, Liu Y, Zhang T, Zhou H, Wang Y, Xu L, Ma Z, Wang Y. Sintilimab in patients with advanced esophageal squamous cell carcinoma refractory to previous chemotherapy: a randomized, open-label phase II trial (ORIENT-2) J. Clin. Oncol. 2020;38:4511. [Google Scholar]
- 17.Lee SJ, Kim S, Kim M, Lee J, Park YH, Im YH, Park SH. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: a randomized phase II study. BMC Cancer. 2015;15:693. doi: 10.1186/s12885-015-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen L, Guo J, Zhang Q, Pan H, Yuan Y, Bai Y, Liu T, Zhou Q, Zhao J, Shu Y, Huang X, Wang S, Wang J, Zhou A, Ye D, Sun T, Gao Y, Yang S, Wang Z, Li J, Wu YL. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8:e000437. doi: 10.1136/jitc-2019-000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournel L, Wu Z, Stadler N, Damotte D, Lococo F, Boulle G, Ségal-Bendirdjian E, Bobbio A, Icard P, Trédaniel J, Alifano M, Forgez P. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019;464:5–14. doi: 10.1016/j.canlet.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Wang Y, Xie Y, Dai T, Fan M, Li C, Zou Y. Cisplatin remodels the tumor immune microenvironment via the transcription factor EB in ovarian cancer. Cell Death Discov. 2021;7:136. doi: 10.1038/s41420-021-00519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 22.Ilson DH, Ajani J, Bhalla K, Forastiere A, Huang Y, Patel P, Martin L, Donegan J, Pazdur R, Reed C, Kelsen DP. Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J. Clin. Oncol. 1998;16:1826–1834. doi: 10.1200/JCO.1998.16.5.1826. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Ren Z, Yuan L, Xu S, Yao Z, Qiao L, Li K. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am J Cancer Res. 2016;6:2345–2350. [PMC free article] [PubMed] [Google Scholar]
- 24.Hiramoto S, Kato K, Shoji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, Boku N. A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol. 2018;23:466–472. doi: 10.1007/s10147-018-1239-x. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, Takiyama W, Ishida K, Isono K, Makuuchi H, Imamura M, Shinoda M, Ikeuchi S, Kabuto T, Yamana H, Fukuda H. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) trial (JCOG9407) Jpn J Clin Oncol. 2001;31:419–423. doi: 10.1093/jjco/hye090. [DOI] [PubMed] [Google Scholar]
- 26.Xu R, Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Zhu Y, Yang Q, Zou J. ESCORT-1st: a randomized, double-blind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy versus chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC) J. Clin. Oncol. 2021;39:4000. [Google Scholar]
- 27.Van Der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, De Vires IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, Fujita M, Takiguchi G, Matsuda T, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y. Neoadjuvant chemotherapy increases PD-L1 expression and CD8+ tumor-infiltrating lymphocytes in esophageal squamous cell carcinoma. Anticancer Res. 2019;39:4539–4548. doi: 10.21873/anticanres.13631. [DOI] [PubMed] [Google Scholar]
- 29.Murugaesu N, Wilson GA, Birkbak NJ, Watkins TBK, Mcgranahan N, Kumar S, Abbassi-ghadi N, Salm M, Mitter R, Horswell S, Rowan A, Phillimore B, Biggs J, Begum S, Matthews N, Hochhauser D, Hanna GB, Swanton C. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov. 2015;5:821–831. doi: 10.1158/2159-8290.CD-15-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mimura K, Yamada L, Ujiie D, Hayase S, Tada T, Hanayama H, Kyi A, Min T, Shibata M, Momma T, Saze Z, Ohki S, Kono K. Immunotherapy for esophageal squamous cell carcinoma: a review. Fukushima J Med Sci. 2018;64:46–53. doi: 10.5387/fms.2018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng HY, Li J, Tao L, Lam AK, Chan KW, Ko JMY, Yu VZ, Wong M, Li B, Lung ML. Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation. Transl Oncol. 2018;11:1323–1333. doi: 10.1016/j.tranon.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 34.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-akita H. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 35.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 36.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.