Abstract
This study aimed to investigate outcomes and recurrence patterns after hepatectomy for hepatocellular carcinoma (HCC) patients with different China Liver Cancer staging (CNLC), and then analyze the risk factors of different recurrence patterns. A total of 731 HCC patients undergoing curative resection were reviewed from 6 independent institutions. Data on preoperative and clinicopathological parameters, operation and tumor recurrence information, recurrence management and long-term outcomes were analyzed. Our results showed that 1-, 3-, and 5-year OS rate for Ia was 96.6%, 88.5%, and 77.4%, while 1-, 3-, and 5-year of Ib was 84.2%, 65.5%, and 51.3%, respectively. Compared to Ia, the patients in IIa and IIb staging had poorer 1-, 3-, and 5-year OS and DFS. Furthermore, the 1-, 3-, and 5-year OS rate in IIIa was 59.3%, 37.3%, and 27.7%, while the 1-, 3-, and 5-year OS of IIIb was 25.6%, 12.8%, and 0%, respectively. The mostly site of recurrence after liver surgery was intrahepatic recurrence (CNLC Ia: 89.4%; Ib: 65.9%; IIa: 68.9%; IIb: 91.7%; IIIa: 63.8%). However, the CNLC IIIb patients have higher percentage of extrahepatic recurrence (56.5%). The main recurrence pattern of time course was late recurrence in CNLC Ia patients (61.1%). However, the rate of early recurrence in Ib, IIa, IIb, IIIa, IIIb patients was 69.0%, 62.2%, 62.5%, 78.3% and 95.7% respectively. In conclusion, the outcomes and recurrence patterns of HCC patients after resection vary with different CNLC staging, which defined the prognosis of patients with HCC after resection. The HCC patients with CNLC IIIa can also benefit from liver resection. The CNLC staging could be considered in forming management strategies, treatment choice and surveillance for HCC patients.
Keywords: Hepatocellular carcinoma, recurrence patterns, CNLC staging, risk factors, OS
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the third leading cause of cancer-related death worldwide [1]. According to Global Cancer Observatory (GCO), over 40% of new cases occurred in China [2]. Hepatectomy is one of curative treatment options for HCC patients [3]. However, long-term survival after curative resection remained unsatisfactory [4]. Tumor recurrence after hepatectomy was common, with 5-year recurrence rate reaching 70% post-surgical resection [4]. To date, a quantity of staging system has been used to define the prognosis of HCC patients, including the Milan criteria, the American Joint Committee on Cancer (AJCC), Barcelona Clinic Liver Cancer (BCLC) staging system. The BCLC system has been widely used by surgeons to guide treatment decisions and determine patient prognosis [5]. According to these guidelines, some HCC patients in China will not have the opportunity for surgery due to these patients have reached the middle and late staging. However, several surgeons have reported that some patients with BCLC B or even BCLC C staging HCC benefited from liver resection [6]. Therefore, Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China and CNLC staging were proposed in 2017 and edited in 2019 according to the patient’s general state of health, liver function, tumor characteristics [7,8]. There were some similarities and differences between CNLC and BCLC staging. CNLC-Ia: a single HCC≤5 cm; CNLC-Ib: a single HCC>5 cm or up to 3 tumors all less than 3 cm; CNLC-IIa: up to 3 tumors and more than 3 cm; CNLC-IIb: tumors ≥4; CNLC-IIIa: With Vascular invasion; CNLC-IIIb: With extrahepatic metastasis; CNLC-IV: Child-Pugh C or PS 3-4. However, the outcomes and recurrence patterns following curative hepatectomy for HCC patients with different CNLC staging have not been clear.
The recurrence patterns were thought to play significant roles in the overall prognosis of HCC patients and follow up treatment of recurrent HCC patients [9]. Several investigators had studied recurrence patterns of HCC after curative resection [10-12]. The recurrence patterns were mainly assessed from three aspects: (1) Location of the HCC recurrence: intrahepatic recurrence or extrahepatic metastasis. (2) Number of recurrent tumors: solitary intrahepatic recurrence and multiple intrahepatic recurrence. (3) Time course of HCC recurrence: early or late tumor recurrence. Furthermore, the patterns of recurrence were also conducted into with Milan criteria and beyond Milan criteria according to recurrent tumor size, number and location [13,14]. Risk factors related with tumor recurrence after hepatectomy for HCC patients were studied in plenty of studies, including hepatitis virus infection status, AFP level, tumor size and number, vascular invasion, TNM stage and so on [15,16]. However, the importance and risk factors of different recurrence patterns have not been clarified yet.
In this study, we firstly explored the outcomes and recurrence patterns following curative hepatectomy for HCC patients with different CNLC staging. And then analyzed the characteristics and risk factors of different recurrence patterns. Furthermore, we also analyzed the long-term survival of recurrent HCC patients underwent different treatment strategy.
Materials and methods
Patient population
From Jun 2009 to Dec 2019, 731 HCC patients underwent hepatectomy were retrospectively reviewed from 6 independent institutions in The First Affiliated Hospital of Nanjing Medical University, Yancheng No. 1 people’s Hospital, Taizhou people’s Hospital, The Affiliated Suqian First People’s Hospital of Nanjing Medical University, The Affiliated Hospital of Xuzhou Medical University, The First People’s Hospital of Changzhou, China. Tree diagram of HCC recurrence was showed in Figure 1. The patients with mixed cholangiocarcinoma/HCC and recurrent HCC were excluded. The institutional review boards of all participating institutions approved this retrospective study, and consent was obtained from every patient.
Figure 1.
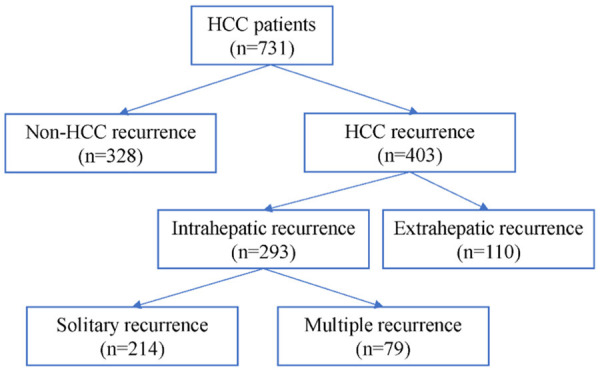
Tree diagram of HCC recurrence.
Data collection
Demographic information was recorded for each patient. Preoperative parameters such as AFP, preoperative liver function and bilirubin levels were also collected. Pathologic data included tumor size, tumor nodule, TNM stage, BCLC stage, CNLC stage, tumor differentiation and thrombus were collected based on the pathologic assessment. Furthermore, the type of hepatectomy and the information during operation were also recorded. The postoperative complications were assessed until 30 days after the operation.
Patient follow-up
The postoperative follow-up schedule consisted of every 3 months during the first year after surgery, every 6 months within 2-5 years and every year thereafter. In each visit, liver function, AFP, physical examination, and radiographic imaging were included. Tumor recurrence after resection based on the radiological evidence plus an AFP level or biopsy-proven recurrent lesion. The location, tumor size and number of recurrent HCC were evaluated through computed tomography, or magnetic resonance of postoperative follow-up.
The patterns and time of HCC recurrence
The recurrence patterns were assessed from three aspects: (1) Location of recurrent tumors: intrahepatic recurrence (IHR), extrahepatic metastasis (EHR), and intrahepatic plus extrahepatic metastasis (IHR+EHR). (2) Number of recurrent tumors: patients with intrahepatic recurrence were classified into 2 groups according to the number of recurrent tumors: solitary intrahepatic recurrence and multiple intrahepatic recurrence. (3) Recurrence course: the early tumor recurrence was defined 1 year after liver resection.
Treatment after recurrence
The treatments of patients with recurrent HCC in our institution were decided after multi-disciplinary team discussion based on the comprehensive consideration of the patient’s general condition and recurrence patterns. The treatment options for intrahepatic recurrence are diverse including liver transplantation, repeat liver resection, ablative therapy, and transarterial therapy. However, patients exhibited extrahepatic lesion(s) are mainly applied to systemic therapy. The curative treatments of recurrent HCC main include liver resection, liver transplantation and ablative therapy. TACE, chemoradiotherapy, targeted therapy and immunotherapy belong to non-curative treatment.
Statistical methods
Comparative survival analysis was performed by Kaplan-Meier and the log-rank test. Factors associated with DFS and OS were identified using univariable and multivariable Cox proportional hazards regression models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated. For all tests, P value <0.05 was considered to indicate statistical significance. All analyses were performed using SPSS version 21.0.
Results
Comparison of the variables in HCC patients with different CNLC staging
A total of 731 HCC patients who underwent hepatectomy were reviewed in the analytic cohort. Most patients were male (n=618, 84.5%). Roughly four-fifth of patients (n=564, 77.2%) had HBV infection. 59.1% patients (n=432) received non-anatomical resection. Median operation time was 188.5 min (50-771 min). Roughly one-third of patients (n=246, 33.7%) have microvascular invasion, while 15.6% (n=114) had macrovascular invasion.
Among 731 patients, 284 (38.9%) patients belong to CNLC Ia, while 227 (31.1%) patients belong to CNLC Ib. Furthermore, 64 (8.7%) patients with CNLC IIa, 32 (4.4%) patients with CNLC IIb and 97 (13.3%) patients with CNLC IIIa. Compared to patients with CNLC Ia, CNLC II-III patients had poorer preoperative liver function. Furthermore, the operation time and intraoperative blood loss were also higher in CNLC Ib and CNLC II-III patients. The baseline characteristics of patients with different CNLC staging were summarized in Table 1.
Table 1.
Comparison of the variables in HCC patients with different CNLC stage
| Variables | Total | CNLC Ia | CNLC Ib | CNLC IIa | CNLC IIb | CNLC IIIa | CNLC IIIb |
|---|---|---|---|---|---|---|---|
| (n=731) | (n=284) | (n=227) | (n=64) | (n=32) | (n=97) | (n=27) | |
| Demographics | |||||||
| Age, year, n (%) | |||||||
| ≤60 | 454 (62.1) | 174 (61.3) | 139 (61.2) | 40 (62.5) | 21 (65.6) | 65 (67) | 15 (55.6) |
| >60 | 277 (37.9) | 110 (38.7) | 88 (38.8) | 24 (37.5) | 11 (34.4) | 32 (33) | 12 (44.4) |
| Sex, male, n (%) | 618 (84.5) | 237 (83.5) | 199 (87.7) | 53 (82.8) | 31 (96.9) | 78 (80.4) | 20 (74.1) |
| Preoperative variables | |||||||
| ALB, g/L, median (range) | 41.2 (17.5-52.7) | 42.2 (17.5-51.6) | 41.2 (27.3-50) | 40.3 (29.2-50.1) | 40.9 (33.1-51.2) | 39.3 (25.6-48.6) | 39.3 (29.8-52.7) |
| ALT, U/L, median (range) | 35.8 (7.8-502.9) | 29.7 (10.1-502.9) | 36.5 (9.1-314) | 41.4 (10.8-116.6) | 40.2 (18.9-193.2) | 42.5 (7.8-234.6) | 41 (8.9-299.6) |
| AST, U/L, median (range) | 37 (9-614.4) | 28.1 (11-229.7) | 38.3 (9-614.4) | 45.3 (14.4-177.5) | 46.1 (24.9-182) | 56.2 (16.4-323.2) | 45.4 (20.1-211.8) |
| TBIL, μmol/L, median (range) | 14.5 (2.6-209.9) | 14.3 (2.6-42) | 14.8 (5.9-54.6) | 13.2 (5.1-26) | 12.5 (6.7-51.3) | 15.9 (5.7-150.8) | 16.6 (6.4-209.9) |
| PT, s, median (range) | 12.3 (8.6-21) | 12.2 (9.2-21) | 12.4 (8.6-17) | 12.4 (10-18.4) | 12.3 (10.5-13.7) | 12.3 (9.9-17.8) | 12.7 (9.5-19.2) |
| AFP, ng/mL, n (%) | |||||||
| ≤200 | 388 (53.1) | 181 (63.7) | 121 (53.3) | 32 (50) | 16 (50) | 30 (30.9) | 8 (29.6) |
| >200 | 343 (46.9) | 103 (36.3) | 106 (46.7) | 32 (50) | 16 (50) | 67 (69.1) | 19 (70.4) |
| HbsAg (+), n (%) | 564 (77.2) | 221 (77.8) | 170 (74.9) | 52 (81.3) | 28 (87.5) | 77 (79.4) | 16 (59.3) |
| Ascites (+), n (%) | 64 (8.8) | 9 (3.2) | 22 (9.7) | 9 (14.1) | 3 (9.4) | 21 (21.6) | 0 (0.0) |
| Hepatic cirrhosis, n (%) | |||||||
| YES | 498 (68.1) | 194 (68.3) | 143 (63.0) | 49 (76.6) | 27 (84.4) | 71 (73.2) | 14 (51.9) |
| No | 233 (31.9) | 90 (31.7) | 84 (37.0) | 15 (23.4) | 5 (15.6) | 26 (26.8) | 13 (48.1) |
| Child-Pugh grade, n (%) | |||||||
| A | 690 (94.4) | 276 (97.2) | 212 (93.4) | 60 (93.8) | 31 (96.9) | 87 (89.7) | 24 (88.9) |
| B | 41 (5.6) | 8 (2.8) | 15 (6.6) | 4 (6.3) | 1 (3.1) | 10 (10.3) | 3 (11.1) |
| ALBI grade, n (%) | |||||||
| I | 469 (64.2) | 206 (72.5) | 148 (65.2) | 35 (54.7) | 24 (75) | 44 (45.4) | 12 (44.4) |
| II | 262 (35.8) | 78 (27.5) | 79 (34.8) | 29 (45.3) | 8 (25) | 53 (54.6) | 15 (55.6) |
| Tumor and operative variables | |||||||
| Type of hepatectomy | |||||||
| Anatomical | 299 (40.9) | 71 (25) | 109 (48) | 25 (39.1) | 12 (37.5) | 67 (69.1) | 15 (55.6) |
| Non-anatomical | 432 (59.1) | 213 (75) | 118 (52) | 39 (60.9) | 20 (62.5) | 30 (30.9) | 12 (44.4) |
| Maximum tumor size, cm, n (%) | |||||||
| ≤5 | 382 (52.3) | 284 (100) | 36 (15.9) | 28 (43.8) | 10 (31.3) | 19 (19.6) | 5 (18.5) |
| >5 | 349 (47.7) | 0 (0) | 191 (84.1) | 36 (56.3) | 22 (68.8) | 78 (80.4) | 22 (81.5) |
| Tumor numbers, n (%) | |||||||
| 1 | 558 (76.3) | 284 (100) | 192 (84.6) | 0 (0) | 0 (0) | 66 (68) | 16 (59.3) |
| 2-3 | 122 (16.7) | 0 (0) | 35 (15.4) | 64 (100) | 0 (0) | 16 (16.5) | 6 (22.2) |
| ≥4 | 51 (7.0) | 0 (0) | 0 (0) | 0 (0) | 32 (100) | 15 (15.5) | 5 (18.5) |
| Operation time, min, median (range) | 188.5 (50-771) | 150 (50-480) | 198 (65-771) | 217.5 (60-400) | 202 (110-680) | 240 (110-510) | 300 (140-750) |
| Intraoperative blood loss, ml, median (range) | 200 (0-7000) | 150 (0-2500) | 300 (50-5300) | 280 (0-3600) | 300 (100-2500) | 500 (50-7000) | 700 (50-3000) |
| Intraoperative blood transfusion, n (%) | 186 (25.4) | 28 (9.9) | 59 (26) | 22 (34.4) | 8 (25) | 52 (53.6) | 17 (63) |
| Histological grade, n (%) | |||||||
| Well to moderately differentiated | 75 (10.3) | 43 (15.1) | 25 (11) | 2 (3.1) | 1 (3.1) | 4 (4.1) | 0 (0) |
| Poorly to undifferentiated | 656 (89.7) | 241 (84.9) | 202 (89) | 62 (96.9) | 31 (96.9) | 93 (95.9) | 27 (100.0) |
| Satellite nodules positive, n (%) | 82 (11.2) | 6 (2.1) | 14 (6.2) | 17 (27) | 21 (67.7) | 18 (18.6) | 6 (22.2) |
| Macrovascular invasion positive, n (%) | 114 (15.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 97 (100) | 16 (59.3) |
| Microvascular invasion positive, n (%) | 246 (33.7) | 44 (15.5) | 71 (31.3) | 32 (50) | 17 (53.1) | 68 (70.1) | 14 (51.9) |
| Postoperative variables | |||||||
| Postoperative complication, n (%) | 134 (18.3) | 32 (11.3) | 37 (16.3) | 18 (28.1) | 9 (28.1) | 27 (27.8) | 11 (40.7) |
Recurrence patterns and outcomes of HCC patients in all cohort patients
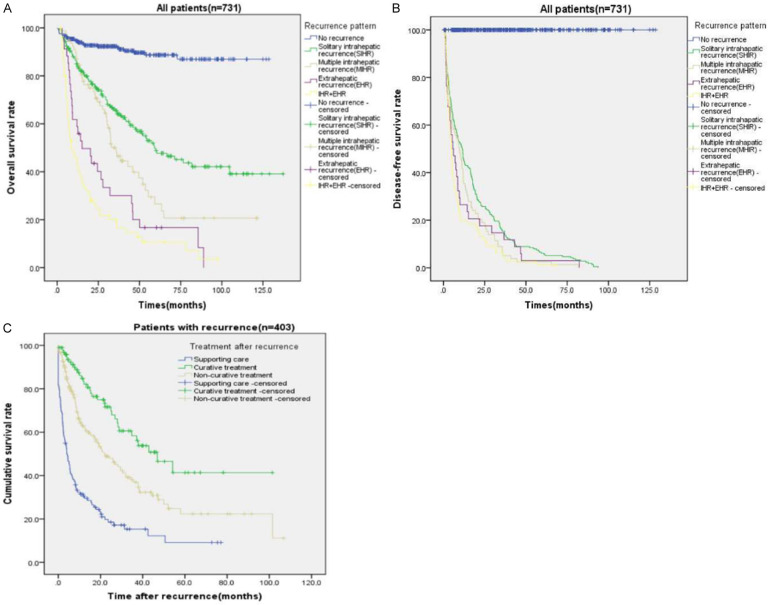
With a median follow-up of 29.6 months, the 1-, 3-, and 5-year OS for the entire cohort was 83.6%, 67.5%, and 55.2% (Figure 2A), while 1-, 3-, and 5-year DFS was 64.1%, 45.5%, and 37.1%, respectively (Figure 2B). 403 (55.1%) patients experienced tumor recurrence, while 328 (44.9%) patients had no recurrence evidence at the time of last follow-up. Among the 403 patients who recurred, 293 patients (72.7%) developed only intrahepatic recurrence (IHR). In contrast, 34 (8.4%) patients had only extrahepatic recurrence while 76 (18.9%) patients developed both extrahepatic and intrahepatic recurrence. Among the patients developed intrahepatic recurrence, 214 patients (73.0%) developed solitary intrahepatic recurrence, while 79 (27.0%) have multiple intrahepatic recurrence. The recurrence patterns after surgery were showed in Table 2.
Figure 2.
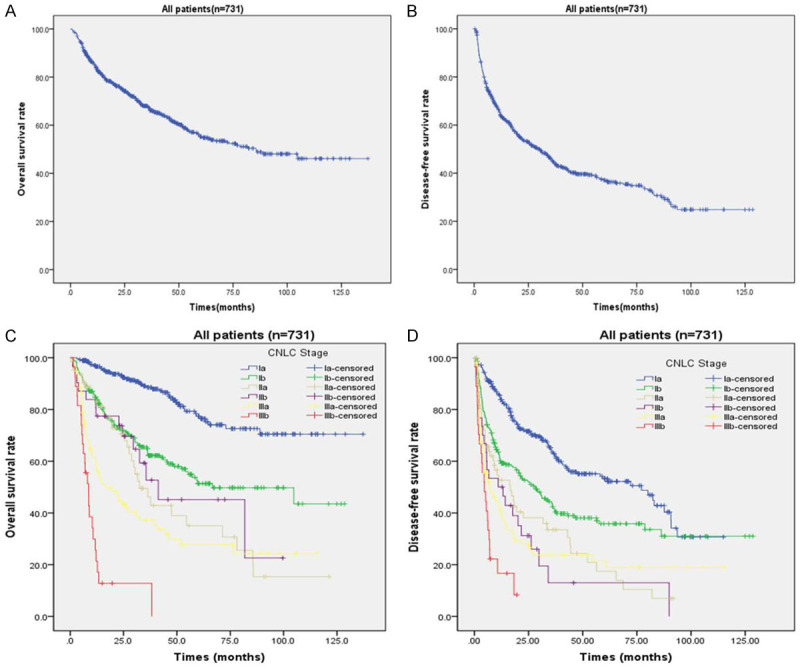
Overall survival and Disease-free survival curves for HCC patients following curative hepatectomy. A. Overall survival curve for the entire cohort; B. Disease-free survival curve for the entire cohort; C. Comparison of overall survival curves for HCC patients with different CNLC staging; D. Comparison of disease-free survival curves for HCC patients with different CNLC staging.
Table 2.
Recurrence Pattern in HCC patients with different CNLC staging
| Variables | Total | CNLC Ia | CNLC Ib | CNLC IIa | CNLC IIb | CNLC IIIa | CNLC IIIb |
|---|---|---|---|---|---|---|---|
| (n=731) | (n=284) | (n=227) | (n=64) | (n=32) | (n=97) | (n=27) | |
| Recurrence | |||||||
| YES | 403 (55.1) | 113 (39.8) | 129 (56.8) | 45 (70.3) | 24 (75) | 69 (71.1) | 23 (85.2) |
| NO | 328 (44.9) | 171 (60.2) | 98 (43.2) | 19 (29.7) | 8 (25) | 28 (28.9) | 4 (14.8) |
| Recurrence site | |||||||
| EHR (with or without IHR) | 110 (27.3) | 12 (10.6) | 44 (34.1) | 14 (31.1) | 2 (8.3) | 25 (36.2) | 13 (56.5) |
| IHR | 293 (72.7) | 101 (89.4) | 85 (65.9) | 31 (68.9) | 22 (91.7) | 44 (63.8) | 10 (43.5) |
| IHR type | |||||||
| Solitary IHR | 214 (73.0) | 75 (74.3) | 66 (77.6) | 21 (67.7) | 15 (68.2) | 29 (65.9) | 8 (80) |
| Multiple IHR | 79 (27.0) | 26 (25.7) | 19 (22.4) | 10 (32.3) | 7 (31.8) | 15 (34.1) | 2 (20) |
| Time to recurrence, mouths | |||||||
| ≤12 (early recurrence) | 252 (62.5) | 44 (38.9) | 89 (69.0) | 28 (62.2) | 15 (62.5) | 54 (78.3) | 22 (95.7) |
| >12 (late recurrence) | 151 (37.5) | 69 (61.1) | 40 (31.0) | 17 (37.8) | 9 (37.5) | 15 (21.7) | 1 (4.3) |
Among the 403 patients underwent tumor recurrence, 252 (62.5%) patients experienced early recurrence (tumor recurrence within 1 year) after surgery (Table 2). The recurrence location differed according to the recurrence time (Figure 3). 78.2% (86) extrahepatic recurrence occurred within the first 1 year after resection. Compared to late recurrence group, the incidence of extrahepatic recurrence with or without intrahepatic recurrence was higher in early recurrence group (34.1% vs. 15.9%). 54.2% (116) solitary intrahepatic recurrence and 63.3% (50) multiple intrahepatic recurrence occurred within 1 year after surgery. The incidence of solitary intrahepatic recurrence in late recurrence group was much higher than that in early recurrence group (64.9% vs. 46.0%).
Figure 3.
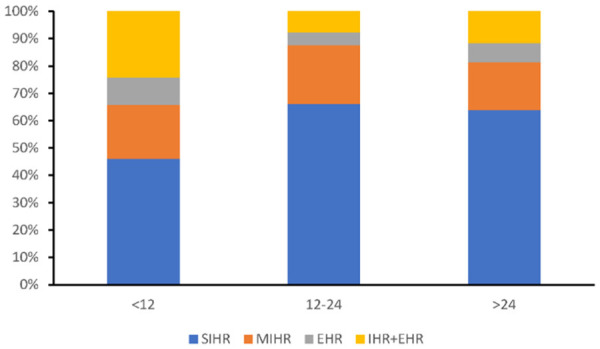
The recurrence location differed according to the time of recurrence. Extrahepatic recurrence of HCC was main in early recurrence group. The incidence of solitary intrahepatic recurrence in late recurrence group was much higher than that in early recurrence group.
Outcomes and recurrence patterns of HCC patients with different CNLC staging
Among patients undergoing hepatectomy, the 1-, 3-, and 5-year OS rate of Ia was 96.6%, 88.5%, and 77.4%, while 1-, 3-, and 5-year of Ib was 84.2%, 65.5%, and 51.3%, respectively. The 1-, 3-, and 5-year DFS rate in Ia was 83.8%, 63.5%, and 53.2%, while 1-, 3-, and 5-year DFS of Ib was 59.7%, 43.7%, and 35.8%, respectively. Compared to Ia, the patients in IIa, IIb staging had poorer 1-, 3-, and 5-year OS and DFS. The 1-, 3-, and 5-year OS rate in IIa was 83.1%, 49.5%, and 35.1%, while 1-, 3-, and 5-year of IIb was 80.6%, 52.7%, and 45.2%, respectively. According to BCLC staging system, the patients of IIIa and IIIb staging will not have the opportunity for surgery. In our cohort, the 1-, 3-, and 5-year OS rate in IIIa was 59.3%, 37.3%, and 27.7%, while 1-, 3-, and 5-year DFS of IIIa was 41.0%, 23.7%, and 21.4%, respectively. In the IIIb patients, the 1-, 3-, and 5-year OS of IIIb was 25.6%, 12.8%, and 0%, while 1-year DFS of IIIb was 16.7%. The 1-, 3-, and 5-year OS and DFS rates of HCC patients with different CNLC staging were showed in Figure 2C, 2D.
Recurrence patterns of HCC patients with different CNLC staging were showed in Table 2. The mostly site of recurrence after liver surgery was intrahepatic recurrence (CNLC Ia: 89.4%; Ib: 65.9%; IIa: 68.9%; IIb: 91.7%; IIIa: 63.8%). However, the CNLC IIIb patients have higher percentage of extrahepatic recurrence (56.5%). Among the intrahepatic recurrence, the percentage of recurrence in solitary intrahepatic recurrence was higher than patients with multinodular intrahepatic recurrence in all CNLC staging. The main recurrence pattern of time course was late recurrence in CNLC Ia patients (61.1%), while early recurrence rate was (38.9%). However, the rate of early recurrence in Ib, IIa, IIb, IIIa, IIIb patients was 69.0%, 62.2%, 62.5%, 78.3% and 95.7% respectively.
Overall survival and disease-free survival in the patients with different recurrence patterns
The OS and DFS of patients varied with recurrence patterns (Figure 4A, 4B). The 1-, 3- and 5-year OS of patients with intrahepatic recurrence was better than patients who experienced extrahepatic recurrence (84.8%, 60.6%, 42.6% vs. 49.0%, 23.1%, 12.5%, P<0.05, Figure 4A). Correspondingly, the 1-, 3-, and 5-year DFS rates of intrahepatic recurrence were also better than patients experienced extrahepatic recurrence (43.3%, 13.0%, 5.1% vs. 21.8%, 9.1%, 1.8%, P<0.05, Figure 4B). The patients with both intrahepatic and extrahepatic recurrence have the worst OS and DFS. Among patients who underwent intrahepatic recurrence after surgery, patients who experienced solitary intrahepatic recurrence had longer median OS and DFS than these patients with multinodular intrahepatic recurrence. Furthermore, patients with early recurrence had worse OS and DFS than patients with recurrence after 1 year.
Figure 4.
Overall survival and Disease-free survival curves for HCC patients with different recurrence patterns. A. Comparison of overall survival curves for HCC patients with different recurrence patterns; B. Comparison of disease-free survival curves for HCC patients with different recurrence patterns; C. Comparison of cumulative survival curves for HCC patients following different treatment after recurrence.
Risk factor analysis for OS and DFS of HCC patients with different recurrence patterns
Multivariable analysis showed that AST, AFP, ALBI, tumor size, tumor number and differentiation, microvascular and macrovascular invasion and postoperative complication were the independent risk factors for OS in all cohort patients. Furthermore, preoperative serum AST and AFP, intraoperative blood transfusion, tumor number and size, microvascular invasion were independently associated with the risk of recurrence after hepatectomy (Table 3).
Table 3.
Univariate and multivariate analysis for OS and tumor recurrence
| Characteristic | Overall survival (OS) | Disease-free survival (DFS) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | |
| Sex (female vs. male) | 0.424 | 0.866 (0.609-1.232) | 0.126 | 0.801 (0.602-1.064) | ||||
| Age (≤60 vs. >60) | 0.191 | 1.187 (0.918-1.534) | 0.021 | 1.278 (1.038-1.572) | ||||
| ALB | 0.000 | 0.946 (0.925-0.968) | 0.004 | 0.972 (0.953-0.991) | ||||
| ALT | 0.000 | 1.007 (1.004-1.009) | 0.000 | 1.006 (1.003-1.008) | ||||
| AST | 0.000 | 1.007 (1.006-1.009) | 0.000 | 1.005 (1.002-1.007) | 0.000 | 1.007 (1.006-1.009) | 0.000 | 1.005 (1.003-1.007) |
| TBIL | 0.001 | 1.013 (1.005-1.02) | 0.004 | 1.010 (1.003-1.016) | ||||
| PT | 0.003 | 1.140 (1.047-1.242) | 0.002 | 1.116 (1.04-1.197) | 0.061 | 1.075 (0.997-1.159) | ||
| AFP (≤200 vs. >200) | 0.000 | 0.520 (0.406-0.666) | 0.011 | 0.701 (0.533-0.923) | 0.000 | 0.558 (0.458-0.68) | 0.000 | 0.634 (0.512-0.786) |
| HBV infection (NO vs. YES) | 0.688 | 0.942 (0.703-1.262) | 0.06 | 0.791 (0.62-1.01) | ||||
| Ascites (NO vs. YES) | 0.000 | 0.453 (0.312-0.657) | 0.002 | 0.595 (0.43-0.823) | ||||
| Hepatic cirrhosis (NO vs. YES) | 0.066 | 0.774 (0.589-1.017) | 0.09 | 0.830 (0.67-1.029) | ||||
| Child-Pugh grade (A vs. B) | 0.000 | 0.416 (0.273-0.634) | 0.009 | 0.598 (0.407-0.879) | ||||
| ALBI grade (I vs. II) | 0.000 | 0.481 (0.377-0.613) | 0.003 | 0.661 (0.503-0.869) | 0.001 | 0.711 (0.582-0.867) | ||
| Type of hepatectomy (Non-anatomic vs. Anatomic) | 0.000 | 0.566 (0.443-0.722) | 0.001 | 0.726 (0.596-0.883) | ||||
| Intraoperative blood loss | 0.000 | 1.000 (1.000-1.000) | 0.000 | 1.000 (1.000-1.000) | 0.109 | 1.000 (1.000-1.000) | ||
| Intraoperative blood transfusion (NO vs. YES) | 0.000 | 0.446 (0.346-0.575) | 0.000 | 0.477 (0.387-0.588) | 0.000 | 0.594 (0.456-0.774) | ||
| Maximum tumor size (≤5 vs. >5) | 0.000 | 0.325 (0.25-0.422) | 0.000 | 0.542 (0.403-0.729) | 0.000 | 0.496 (0.407-0.604) | 0.015 | 0.751 (0.596-0.947) |
| Tumor number (Single vs. Multiple) | 0.000 | 0.474 (0.365-0.616) | 0.000 | 0.574 (0.434-0.759) | 0.000 | 0.497 (0.401-0.617) | 0.000 | 0.582 (0.462-0.733) |
| Tumor differentiation (Moderate-well vs. Poor) | 0.000 | 0.315 (0.177-0.563) | 0.013 | 0.453 (0.242-0.849) | 0.001 | 0.537 (0.372-0.775) | 0.094 | 0.708 (0.473-1.061) |
| Satellite nodules (NO vs. YES) | 0.000 | 0.492 (0.348-0.696) | 0.000 | 0.537 (0.404-0.715) | ||||
| Macrovascular invasion (NO vs. YES) | 0.000 | 0.290 (0.221-0.38) | 0.010 | 0.662 (0.484-0.907) | 0.000 | 0.435 (0.341-0.555) | ||
| Microvascular invasion (NO vs. YES) | 0.000 | 0.330 (0.258-0.423) | 0.001 | 0.631 (0.477-0.834) | 0.000 | 0.379 (0.31-0.463) | 0.000 | 0.599 (0.475-0.756) |
| Postoperative complication (NO vs. YES) | 0.000 | 0.454 (0.342-0.603) | 0.032 | 0.714 (0.525-0.972) | 0.000 | 0.571 (0.448-0.729) | ||
The risk factors related with specific recurrence patterns were further analyzed (Tables 4, 5 and 6). The multivariable analysis showed that AFP (≤200, HR 0.536 [0.355 0.809], P=0.003), intraoperative blood transfusion (NO, HR 0.622 [0.412 0.939], P=0.024), tumor size (≤5, HR 0.248 [0.147 0.420], P<0.001), tumor number (single, HR 0.541 [0.353 0.829], P=0.005) and microvascular invasion (NO, HR 0.619 [0.405 0.945], P=0.026) were the independent risk factors for extrahepatic recurrence after liver resection (Table 4). In addition, multivariable analysis showed that intraoperative blood transfusion, tumor number and microvascular invasion were the independent risk factors of multiple intrahepatic recurrence (Table 5). Furthermore, multivariable analysis showed that age (≤60, HR 1.409 [1.071 1.852], P=0.014), AFP level (≤200, HR 0.628 [0.479 0.823], P=0.001), tumor size (≤5, HR 0.536 [0.402 0.715], P<0.001) and number (single, HR 0.544 [0.417 0.712], P<0.001), intraoperative blood transfusion (NO, HR 0.607 [0.463 0.796], P<0.001) and microvascular invasion (NO, HR 0.513 [0.389 0.676], P<0.001) were the independent risk factors of early tumor recurrence (Table 6).
Table 4.
Risk factors of extrahepatic recurrence after liver resection
| Characteristic | EHR (n=110) | |||
|---|---|---|---|---|
|
| ||||
| Univariate | Multivariate | |||
|
|
|
|||
| P | HR (95% CI) | P | HR (95% CI) | |
| Sex (male vs. female) | 0.832 | 0.949 (0.584-1.541) | ||
| Age (≤60 vs. >60) | 0.009 | 1.746 (1.152-2.648) | ||
| AFP (≤200 vs. >200) | 0.000 | 0.339 (0.229-0.503) | 0.003 | 0.536 (0.355-0.809) |
| HBV infection (NO vs. YES) | 0.209 | 0.745 (0.47-1.18) | ||
| Ascites (NO vs. YES) | 0.000 | 0.396 (0.238-0.658) | ||
| Hepatic cirrhosis (NO vs. YES) | 0.19 | 0.759 (0.503-1.146) | ||
| Child-Pugh grade (A vs. B) | 0.013 | 0.436 (0.227-0.837) | ||
| ALBI grade (I vs. II) | 0.000 | 0.507 (0.348-0.738) | 0.097 | 0.717 (0.483-1.063) |
| Type of hepatectomy (Non-anatomic vs. Anatomic) | 0.000 | 0.371 (0.252-0.548) | ||
| Intraoperative blood loss | 0.000 | 1 (1-1) | ||
| Intraoperative blood transfusion (NO vs. YES) | 0.000 | 0.32 (0.219-0.469) | 0.024 | 0.622 (0.412-0.939) |
| Maximum tumor size (≤5 vs. >5) | 0.000 | 0.153 (0.094-0.249) | 0.000 | 0.248 (0.147-0.420) |
| Tumor number (Single vs. Multiple) | 0.000 | 0.422 (0.281-0.634) | 0.005 | 0.541 (0.353-0.829) |
| Tumor differentiation (Moderate-well vs. Poor) | 0.004 | 0.183 (0.058-0.577) | 0.072 | 0.338 (0.104-1.103) |
| Satellite nodules (NO vs. YES) | 0.003 | 0.452 (0.269-0.76) | ||
| Macrovascular invasion (NO vs. YES) | 0.000 | 0.28 (0.185-0.424) | ||
| Microvascular invasion (NO vs. YES) | 0.000 | 0.248 (0.17-0.362) | 0.026 | 0.619 (0.405-0.945) |
| Postoperative complication (NO vs. YES) | 0.004 | 0.518 (0.333-0.806) | ||
Table 5.
Risk factors of multiple intrahepatic recurrence after liver resection
| Characteristic | Multiple IHR (n=79) | |||
|---|---|---|---|---|
|
| ||||
| Univariate | Multivariate | |||
|
|
|
|||
| P | HR (95% CI) | P | HR (95% CI) | |
| Sex (male vs. female) | 0.053 | 2.150 (0.989-4.674) | ||
| Age (≤60 vs. >60) | 0.174 | 1.381 (0.867-2.200) | ||
| AFP (≤200 vs. >200) | 0.020 | 0.593 (0.381-0.922) | ||
| HBV infection (NO vs. YES) | 0.263 | 0.731 (0.422-1.266) | ||
| Ascites (NO vs. YES) | 0.126 | 0.564 (0.271-1.175) | ||
| Hepatic cirrhosis (NO vs. YES) | 0.709 | 0.915 (0.575-1.458) | ||
| Child-Pugh grade (A vs. B) | 0.087 | 0.482 (0.209-1.111) | ||
| ALBI grade (I vs. II) | 0.039 | 0.625 (0.399-0.977) | ||
| Type of hepatectomy (Non-anatomic vs. Anatomic) | 0.930 | 0.980 (0.620-1.549) | ||
| Intraoperative blood loss | 0.089 | 1.000 (1.000-1.000) | ||
| Intraoperative blood transfusion (NO vs. YES) | 0.000 | 0.342 (0.215-0.544) | 0.000 | 0.428 (0.266-0.687) |
| Maximum tumor size (≤5 vs. >5) | 0.034 | 0.62 (0.398-0.965) | ||
| Tumor number (Single vs. Multiple) | 0.000 | 0.328 (0.207-0.522) | 0.000 | 0.424 (0.263-0.684) |
| Tumor differentiation (Moderate-well vs. Poor) | 0.054 | 0.41 (0.166-1.014) | ||
| Satellite nodules (NO vs. YES) | 0.060 | 0.529 (0.272-1.028) | ||
| Macrovascular invasion (NO vs. YES) | 0.001 | 0.385 (0.222-0.667) | ||
| Microvascular invasion (NO vs. YES) | 0.000 | 0.35 (0.223-0.55) | 0.001 | 0.445 (0.279-0.709) |
| Postoperative complication (NO vs. YES) | 0.014 | 0.509 (0.296-0.872) | ||
Table 6.
Risk factors of early recurrence after liver resection
| Characteristic | Early recurrence (n=252) | |||
|---|---|---|---|---|
|
| ||||
| Univariate | Multivariate | |||
|
|
|
|||
| P | HR (95% CI) | P | HR (95% CI) | |
| Sex (male vs. female) | 0.315 | 1.198 (0.842-1.706) | ||
| Age (≤60 vs. >60) | 0.002 | 1.537 (1.178-2.006) | 0.014 | 1.409 (1.071-1.852) |
| AFP (≤200 vs. >200) | 0.000 | 0.433 (0.336-0.557) | 0.001 | 0.628 (0.479-0.823) |
| HBV infection (NO vs. YES) | 0.041 | 0.726 (0.534-0.987) | ||
| Ascites (NO vs. YES) | 0.011 | 0.608 (0.415-0.89) | ||
| Hepatic cirrhosis (NO vs. YES) | 0.119 | 0.806 (0.615-1.057) | ||
| Child-Pugh grade (A vs. B) | 0.029 | 0.595 (0.372-0.949) | ||
| ALBI grade (I vs. II) | 0.002 | 0.667 (0.519-0.858) | ||
| Type of hepatectomy (Non-anatomic vs. Anatomic) | 0.000 | 0.588 (0.459-0.752) | ||
| Intraoperative blood loss | 0.000 | 1.000 (1.000-1.000) | ||
| Intraoperative blood transfusion (NO vs. YES) | 0.000 | 0.409 (0.317-0.527) | 0.000 | 0.607 (0.463-0.796) |
| Maximum tumor size (≤5 vs. >5) | 0.000 | 0.365 (0.281-0.474) | 0.000 | 0.536 (0.402-0.715) |
| Tumor number (Single vs. Multiple) | 0.000 | 0.450 (0.347-0.585) | 0.000 | 0.544 (0.417-0.712) |
| Tumor differentiation (Moderate-well vs. Poor) | 0.001 | 0.383 (0.219-0.669) | ||
| Satellite nodules (NO vs. YES) | 0.000 | 0.480 (0.342-0.674) | ||
| Macrovascular invasion (NO vs. YES) | 0.000 | 0.365 (0.276-0.483) | ||
| Microvascular invasion (NO vs. YES) | 0.000 | 0.318 (0.247-0.408) | 0.000 | 0.513 (0.389-0.676) |
| Postoperative complication (NO vs. YES) | 0.000 | 0.567 (0.427-0.754) | ||
The treatment and survival of patients with HCC recurrence after resection
The median survival of patients with recurrence was 24.89 months. Among all patients, 98 patients received re-resection or local ablation therapy such as microwave ablation or radiofrequency ablation for recurrent HCC, 180 patients received non-curative therapy such as transcatheter TACE or chemoradiotherapy. Patients in IHR group had more opportunity to have curative/non-curative treatment than in EHR group (31.4%/47.4% vs. 5.5%/37.3%). And not surprisingly, the recurrent patients receiving curative-intent treatment had better survival than the patients underwent non-curative treatments (median survival: 46.9 months vs. 22.0 months, Figure 4C). Furthermore, the OS of patients received only supporting care was lower than patients received curative/non-curative treatments.
Discussion
In the current era of multidisciplinary patient care, liver resection is still first choice for the treatment of selected HCC [17,18]. However, tumor recurrence is one of the major issues resulting in poor outcome after curative-intent surgical resection. The BCLC staging system has widely been applied in the West. However, many HCC patients in China will not have the opportunity for surgery according to these guidelines. Because these patients have reached the middle and late staging at the time of diagnosis. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China suggested that hepatectomy is the first choice for the treatment of stage Ia, Ib and IIa patients, while the stage of IIb and IIIa patients were also suitable for liver resection after strict preoperative evaluation. However, the outcomes and recurrence pattern in different CNLC staging have not been clarified yet.
To our knowledge, this study was the first to explore the recurrence patterns and its risk factors in HCC patients with different CNLC staging after hepatectomy. Similar to BCLC staging, CNLC staging was also associated with postoperative OS and guide treatment of patients with HCC. To the early staging (CNLC Ia-Ib or BCLC A) patients, all guidelines suggested that hepatectomy is the first choice for the treatment. Early staging (CNLC Ia and Ib) patients have better OS and DFS than late staging (CNLC II-III) patients. The 1-, 3-, and 5-year OS rate of Ia was 96.6%, 88.5%, and 77.4%, while 1-, 3-, and 5-year of Ib was 84.2%, 65.5%, and 51.3%, respectively. To CNLC IIa-IIb or BCLC B patients, there is some controversy in treatment options. CNLC staging system recommends hepatectomy or TACE as the treatment choice of IIa-IIb patients, while BCLC B was recommended to receive the TACE treatment base on BCLC staging system. However, not all BCLC B patients benefited from TACE treatment. The reported OS after TACE for BCLC B patients was variable, ranging from 11-45 months [19,20]. Another study showed that 5-year OS of BCLC B patients after liver resection was 63%, while patients receiving nonsurgical treatment was only 22% [21]. Consistent with previous report, our results also demonstrated that liver resection is the good treatment choice for CNLC IIa-IIb, verified by satisfactory prognosis after resection [22]. Our data showed that the 1-, 3-, and 5-year OS rate in IIa was 83.1%, 49.5%, and 35.1%, while 1-, 3-, and 5-year of IIb was 80.6%, 52.7%, and 45.2%, respectively. According to Guidelines for CNLC staging system that hepatectomy is also the choice for the treatment of stage of IIIa patients after strict preoperative evaluation. Our data showed that the 1-, 3-, and 5-year OS rate in IIIa was 59.3%, 37.3%, and 27.7%, while 1-, 3-, and 5-year DFS of IIIa was 41.0%, 23.7%, and 21.4%, respectively. Recently, several investigators have supported the resection for the middle and late staging patients such as BCLC B and even BCLC C patients. Diamantis I et al. showed that the 5-year survival of BCLC B/C patients was 51.6% after liver resection [9]. According to BCLC and CNLC staging system, the patients of IIIb staging will not have the opportunity for surgery. For IIIb class patients with extrahepatic disease, they are not suitable for liver resection in theory. In our cohort, most of IIIb patients also received the systemic therapies, while only 27 patients in this study received hepatectomy. The 1-, 3-, and 5-year survival of IIIb was 25.6%, 12.8%, and 0%, respectively. These results suggested that the patients with late phase HCC patients with CNLC IIIa staging can also benefit from liver resection.
The different recurrence patterns after liver resection play significant roles in the prognosis of HCC patients and follow up treatment of recurrent HCC patients [23]. Our results showed that recurrence patterns vary with CNLC staging, were related with the survival. In our cohort, the main recurrence pattern after liver resection was intrahepatic recurrence, especially solitary intrahepatic recurrence. Compared to CNLC Ia patients, the CNLC III patients have higher percentages of extrahepatic recurrence and multinodular intrahepatic recurrence. The risk factors related with different recurrence patterns have not been clear. Our analysis showed that AFP, intraoperative blood transfusion, tumor size, tumor number and microvascular invasion were the independent risk factors of extrahepatic recurrence. Many studies also demonstrated that AFP level was associated with extrahepatic tumor recurrence and poor prognosis [23,24]. Several reports described that blood transfusion exerts immunomodulatory effects and affects the prognoses of HCC patients [25]. ST Fan et al. study showed that the circulating HCC stem cell level was a predictor of extrahepatic recurrence after liver resection [26]. Moreover, compared to the HBV-related HCC, HCV-related HCC had a higher incidence of multinodular intrahepatic recurrences after resection [27]. However, the surgical procedure such as anatomic or non-anatomic resection could not affect the recurrence patterns [10,28]. These results suggested that patients with high-risk for extrahepatic recurrence need careful follow-up, and aggressive management for extrahepatic recurrence.
Except to the location of HCC recurrence, the time course of recurrence was also an important risk factor associated with OS. Some research including ours confirmed that early recurrence after curative hepatectomy for HCC has been associated with poor survival [12]. The main recurrence pattern was late recurrence in CNLC Ia patients, while the recurrence pattern of time course was early recurrence in the Ib and II-III groups. Our results demonstrated that age, AFP level, tumor size and number, intraoperative blood transfusion and microvascular invasion were the risk factors for early recurrence. Several studies also reported that Milan criteria status, MVI and multiple tumors are the risk factors for early recurrence [29,30]. Furthermore, ICG15 was related to early recurrence after hepatectomy [31]. These results suggested that patients with risk factors for early recurrence should be reconsidered the indication for surgery and receive aggressive adjuvant treatment after liver resection.
Some studies have described that treatment variables were related with the prognosis of recurrent HCC patients [12]. Some investigators have highlighted the importance of repeat liver resection for intrahepatic recurrence [32], which was also proved by our findings. A recent study reported that recurrent HCC patients may benefit from surgery for not only intrahepatic recurrence but also for partial extrahepatic recurrence patients [33]. Liver transplantation is also a choice for patients with intrahepatic recurrence, but not all patients are candidates due to numerous reasons such as absence of donor, older age, and beyond Millan criteria [34].
In conclusion, outcomes and recurrence patterns of HCC patients after hepatectomy vary with different CNLC staging, which defined the prognosis of HCC patients after resection. With the development of targeted therapy and immunotherapy, the treatment of HCC has developed into a comprehensive treatment mode. To improve the long-term survival of HCC patients, the treatment plan should be formulated according to different individuals, such as surgical treatment, ablation, embolization, targeted therapy, immunotherapy and combined/sequential application of that comprehensive treatment. At present, surgery is still the best cure for HCC patients, especially for early-stage patients (CNLC I, IIa and IIb). The patients with late phase HCC patients with CNLC IIIa can also benefit from liver resection after strict screening. The CNLC staging could be considered in forming management strategies, treatment choice, and surveillance for HCC patients. We hope this study is helpful to determine therapeutic strategy and postoperative surveillance for HCC patients.
Acknowledgements
This study was supported by WU JIEPING Medical Foundation (320.2710.1820).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F, Ciarleglio FA, Bridda A, D’Amico DF. Prospective validation of the Barcelona clinic liver cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Moris D, Felekouras E. Ignore reality but not the consequences of its ignorance: broaden guidelines in surgery of hepatocellular carcinoma. Hepatology. 2017;65:1772–1773. doi: 10.1002/hep.28984. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition) Liver Cancer. 2018;7:235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Medical Administration, National Health and Health Commission of the People’s Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition) Zhonghua Gan Zang Bing Za Zhi. 2020;28:112–128. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Tsilimigras DI, Bagante F, Moris D, Hyer JM, Sahara K, Paredes AZ, Mehta R, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the Barcelona clinic liver cancer criteria. Ann Surg Oncol. 2020;22:2321–2331. doi: 10.1245/s10434-020-08452-3. [DOI] [PubMed] [Google Scholar]
- 10.Marubashi S, Gotoh K, Akita H, Takahashi H, Sugimura K, Miyoshi N, Motoori M, Kishi K, Noura S, Fujiwara Y, Ohue M, Nakazawa T, Nakanishi K, Ito Y, Yano M, Ishikawa O, Sakon M. Analysis of recurrence patterns after anatomical or non-anatomical resection for hepatocellular carcinoma. Ann Surg Oncol. 2015;22:2243–2252. doi: 10.1245/s10434-014-4214-4. [DOI] [PubMed] [Google Scholar]
- 11.Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154:209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 13.Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, Lui WY, Wu WC, Lin HC, Wu JC. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg. 2013;17:702–711. doi: 10.1007/s11605-012-2087-z. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, Iida T, Taura K, Yasuchika K, Uemoto S. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012;19:156–162. doi: 10.1245/s10434-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 15.Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61:526–536. doi: 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- 16.Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, Roayaie S, Florman S, Schwartz ME. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg. 2014;260:650–656. doi: 10.1097/SLA.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 19.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Ahn SW, Hong SK, Yoon KC, Kim HS, Choi YR, Lee HW, Yi NJ, Lee KW, Suh KS Korean Liver Cancer Association. Survival benefit of liver resection for Barcelona clinic liver cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104:1045–1052. doi: 10.1002/bjs.10541. [DOI] [PubMed] [Google Scholar]
- 22.Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26:3693–3700. doi: 10.1245/s10434-019-07580-9. [DOI] [PubMed] [Google Scholar]
- 23.Byeon J, Cho EH, Kim SB, Choi DW. Extrahepatic recurrence of hepatocellular carcinoma after curative hepatic resection. Korean J Hepatobiliary Pancreat Surg. 2012;16:93–97. doi: 10.14701/kjhbps.2012.16.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 25.Shiba H, Ishida Y, Wakiyama S, Iida T, Matsumoto M, Sakamoto T, Ito R, Gocho T, Furukawa K, Fujiwara Y, Hirohara S, Misawa T, Yanaga K. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg. 2009;13:1636–1642. doi: 10.1007/s11605-009-0963-y. [DOI] [PubMed] [Google Scholar]
- 26.Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254:569–576. doi: 10.1097/SLA.0b013e3182300a1d. [DOI] [PubMed] [Google Scholar]
- 27.Naito S, Imamura H, Tukada A, Matsuyama Y, Yoshimoto J, Sugo H, Ishizaki Y, Kawasaki S. Postoperative recurrence pattern and prognosis of patients with hepatocellular carcinoma, with particular reference to the hepatitis viral infection status. Liver Int. 2014;34:802–813. doi: 10.1111/liv.12447. [DOI] [PubMed] [Google Scholar]
- 28.Famularo S, Di Sandro S, Giani A, Lauterio A, Sandini M, De Carlis R, Buscemi V, Uggeri F, Romano F, Gianotti L, De Carlis L. Recurrence patterns after anatomic or parenchyma-sparing liver resection for hepatocarcinoma in a western population of cirrhotic patients. Ann Surg Oncol. 2018;25:3974–3981. doi: 10.1245/s10434-018-6730-0. [DOI] [PubMed] [Google Scholar]
- 29.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207–1215. doi: 10.3748/wjg.v21.i4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol. 2016;25:24–29. doi: 10.1016/j.suronc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi: 10.1002/bjs.10597. [DOI] [PubMed] [Google Scholar]
- 33.Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for recurrent hepatocellular carcinoma: achieving long-term survival. Ann Surg. 2021;273:792–799. doi: 10.1097/SLA.0000000000003358. [DOI] [PubMed] [Google Scholar]
- 34.Lim C, Shinkawa H, Hasegawa K, Bhangui P, Salloum C, Gomez Gavara C, Lahat E, Omichi K, Arita J, Sakamoto Y, Compagnon P, Feray C, Kokudo N, Azoulay D. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: an intent-to-treat analysis. Liver Transpl. 2017;23:1553–1563. doi: 10.1002/lt.24952. [DOI] [PubMed] [Google Scholar]