Abstract
BACKGROUND/OBJECTIVES:
There is increasing recognition that place of death is an important component of quality of end-of-life care (EOLC) and quality of death. This study examined where older persons with and without cognitive impairment die in the United States, what factors contribute to place of death, and whether place of death influences satisfaction with EOLC.
DESIGN:
Cross-sectional secondary data analysis.
SETTING:
In-person interviews with community-dwelling proxy respondents.
PARTICIPANTS:
Data were collected from 1,500 proxies for deceased participants in the National Health and Aging Trends Study (NHATS), a nationally-representative sample of community-dwelling Medicare beneficiaries aged 65 and older.
MEASUREMENTS:
Study variables were obtained from the NHATS “last month of life” interview data. Survey weights were applied to all analyses.
RESULTS:
Persons with cognitive impairment (CI) most often died at home, while cognitively healthy persons (CHP) were equally likely to die at home or in a hospital. Persons with CI who utilized the Medicare Hospice Benefit were 14.5 times more likely to die at home than in a hospital, and 3.4 times more likely to die at home than a nursing home. CHP who use this benefit were over six times more likely to die at home than in a hospital, and more than twice as likely to die at home than a nursing home. Place of death for CHP was also associated with age and race. Proxies of persons with CI who died at home rated EOLC as more favorable, while proxies of CHP rated in-home and hospital care equally.
CONCLUSION:
Findings add to the scant literature identifying factors associated with place of death for older adults with and without CI and results suggest that place of death is a quality of care indicator for these populations. These findings may inform EOLC planning and policy-making and facilitate greater well-being at end-of-life.
Keywords: dementia, Medicare, place of death, end-of-life, caregiver, NHATS
INTRODUCTION
In recent years, there has been a growing awareness in end-of-life care research that place of death may be an indicator of quality of care.1–3 Interest in this relationship was largely prompted by the finding that, while older adults prefer to die at home,1,4 the majority of older Americans die in hospitals or nursing homes.5 However, there is a dearth of research on place of death and satisfaction with care, particularly in United States populations. This is unfortunate, as such findings would inform advance care planning, such as completing advance directives and planning for end-of-life care (EOLC) services, and would assist health professionals in delivering high-quality palliative care across settings and terminal diagnoses.4,6,7
The low levels of congruence between preferred and actual place of death may be explained, in part, by the culture of EOLC in the United States. For example, almost one-third of persons receiving EOLC are admitted to the ICU during the last month of life, while nearly two-thirds of all dying Medicare patients are hospitalized during the last 90 days of life.8 There is a continued need for more community palliative services to care for individuals in their homes during the last 3–12 months of life.8 Additionally, a cultural shift among physicians in the United States is needed to recognize and accept when it is appropriate to cease curative efforts.8
Receiving EOLC in-home or in long-term care residences and avoiding unnecessary hospitalization may be particularly important for persons living with dementia (PLWD) and cognitive impairment.3,4,9 Hospital deaths for PLWD are associated with a higher incidence of delirium,10 falls,11 infectious diseases,4 aspiration,4 renal failure,4 or other adverse events11,12 as compared to deaths at other sites.4 In addition, when people die outside of the home in non-hospice environments, both formal and informal caregivers have poorer perceptions of the care provided at the end of life.9,13–15 However, PLWD are less likely to use hospice services at end-of-life than persons without a dementia diagnosis.1,6
An important factor that may increase the likelihood of receiving in-home or non-hospital EOLC for persons with and without cognitive impairment is the receipt of Medicare.8,16 The Medicare Hospice Benefit, enacted in 1986, is designed to provide palliative care to beneficiaries with terminal illnesses who are approaching end-of-life.17 In 2015, more than half of Medicare decedents received hospice care, with an increasing number of persons receiving continuous hospice care for at least 30 days.8 Furthermore, terminally ill persons receiving Medicare tend to experience fewer transfers from longterm care to hospital settings.8
This study aimed to coalesce and build upon the literature on the need for more research on place of death and associated quality of care in the United States. From the available literature there were several notable findings. First, that persons using the Medicare Hospice Benefit are more likely to receive hospice care (and thus, more likely to be satisfied with EOLC), and second, that PLWD are more likely to receive EOLC outside of the home, though home deaths are increasing. Using data from the National Health and Aging Trends Study (NHATS), we examined the following research questions:
Where do older adults with and without cognitive impairment die in the United States, and is there a difference?
Are there identifiable factors contributing to place of death?
Is satisfaction with end-of-life care associated with place of death?
It is important to note that the cause of death and impact of specific health conditions on both place of death and satisfaction with end-of-life care were not available. Consequently, we approached our research questions from a descriptive rather than a causal lens.
METHODS
Sample
This study utilized data from 1,500 participants in rounds 1 to 6 of the National Health and Aging Trends Study (NHATS), a large, nationally representative sample of Medicare beneficiaries ages 65 and older.18 Beginning in 2011, each round of the NHATS collects detailed information on participants’ physical and cognitive capacity, participation in daily activities, living arrangements, economic status, and well-being. The NHATS sample design, which is drawn from the Medicare enrollment file, oversamples persons at older ages and Black individuals. Further details regarding study design are described elsewhere.18,19 As this study focused on end-of-life experiences of persons with and without cognitive impairment, data are largely based on proxy report following the death of the NHATS participants.
Measures
Demographics
Demographic variables assessed included age, race/ethnicity (White, Black, Hispanic/Latino, other) marital status (married/partnered, divorced/separated, widowed, single/never married), gender (male, female), highest level of education (no schooling, 1st–8th grade, 9th–12th grade, high school diploma, vocational/tech/business/trade school, some college, associate’s degree, Bachelor’s degree, advanced degree), health status (poor, fair, good, very good, excellent), residential care status (community, residential care, nursing home), utilization of hospice care at end-of-life (yes/no), and relationship of proxy to the decedent (spouse/partner, child, other relative, other non-relative).
Cognitive status
As reported by Kasper et al,20 the NHATS classifies the dyad participants, hereafter referred to as the decedent and proxy, into three cognitive categories – probable dementia, possible dementia, and no dementia. Probable dementia was identified through three methods: (1) a formal diagnosis of dementia or Alzheimer’s disease by a physician (as reported by the decedent or proxy); (2) proxy responses to the AD8 screener that met criteria for likely dementia; or 3) impairment (defined as scores ≤ 1.5 SDs from the mean) in at least two of three domains of cognitive functioning (memory, orientation, executive functioning).20 Possible dementia was indicated by impairment in at least one cognitive domain.20 For this study, we collapsed the categories of probable dementia (n = 111) and possible dementia (n = 361) into one category of “cognitively impaired” (n = 472, weighted n = 1.5 million) and compared them to those without a probable or possible dementia diagnosis (n = 1,028, weighted n = 4.0 million). These categories were collapsed due to evidence of significant functional impairment by multiple measures, for example, as measured by the NHATS-Activities of Daily Living (NHATS-ADL)21 and Lawton Independent Activities of Daily Living (IADL)22 assessments in the sample of persons with possible dementia.
Place of death
The place of death of the NHATS participant was identified by proxy as being in the home, hospital, nursing home, hospice residence, or other. The response option of “in transit” was combined with the “other” category due to low frequency of occurrence.
Satisfaction with care
Proxies rated overall quality of care (poor, fair, good, very good, excellent) in the month before the death of the NHATS participant.
Analytic Strategy
The Pearson chi-square test assessed for differences in place of death for persons with and without cognitive impairment. The data were weighted for frequency. Statistical significance was set at P < .05. Post-hoc pairwise comparisons were completed using the Fisher’s exact test, controlling for Type I error across tests with the Bonferroni adjustment. Five separate binomial multivariate logistic regression analyses were performed to compare the chances of participants dying at home with those of them dying in the hospital, dying at home with dying in a nursing home, dying at home with dying in a hospice residence, dying in the hospital with dying in a nursing home, and dying in a hospital with dying in a hospice residence. Age, sex, race, marital status, cognitive status, and use of the Medicare Hospice Benefit were input using the Enter method to examine to what extent these variables were associated with place of death. Finally, bivariate correlations and crosstabulation assessed the association of place of death with proxy-rated satisfaction with end-of-life care. All analyses applied survey weights to take into account the complex survey design of the NHATS. SPSS 25.0 (SPSS, Inc., Chicago, IL) and R version 3.3.3 were used for the analyses.
RESULTS
Sample Characteristics
The sample included 472 persons with cognitive impairment and 1,028 cognitively healthy persons (CHP). As shown in Table 1, cognitively impaired participants were predominantly female (58%), Hispanic/Latino (43%), widowed (54.2%), and had a mean age of 87 years (SD = 7.14). They were primarily in fair (26.3%) or good (24.2%) health, with an average of 4.2 chronic conditions (SD = 1.77). The most frequent proxy respondent for persons with cognitive impairment was an adult child (53.2%).
Table 1.
Sociodemographic and Functional Characteristics of Sample
| Total Sample | Cog Impairment | No Cog Impairment | |
|---|---|---|---|
| (N = 1,500) | (N = 472) | (N = 1,028) | |
| Characteristics | (Weighted n = 1.5 Million) | (Weighted n = 4.0 Million) | |
| Age (M, SD) | 81.93 (.21) | 85.13 (.40) | 80.72 (.25) |
|
| |||
| Race | |||
| White | 47.8 | 32.9 | 43.5 |
| Black | 8.4 | 10 | 7.8 |
| Hispanic/Latino | 40 | 52.9 | 35.1 |
| Other | 3.8 | 4.2 | 3.7 |
|
| |||
| Gender (%) | |||
| Male | 46.5 | 43.1 | 47.8 |
| Female | 53.5 | 56.9 | 52.2 |
|
| |||
| Marital status (%) | |||
| Married/partnered | 40 | 33.8 | 42.4 |
| Widowed | 44.7 | 52.8 | 41.6 |
| Divorced/separated | 11.2 | 9.6 | 11.8 |
| Single, nm | 4.1 | 3.8 | 4.2 |
|
| |||
| Veteran status | |||
| Veteran | 63.8 | 25.2 | 30.4 |
| Non-veteran | 36.2 | 18.6 | 13.7 |
|
| |||
| Place of residence | |||
| Community | 63.3 | 57.8 | 65.8 |
| Residential care | 12.4 | 17.6 | 10 |
| Nursing home | 5.9 | 11.9 | 3.2 |
|
| |||
| #Chronic health conditions | 4.09 (0.06) | 4.16 (1.77) | 4.11 (1.55) |
|
| |||
| Overall health | |||
| Poor | 19.2 | 17.1 | 20.2 |
| Fair | 32.7 | 30.3 | 33.7 |
| Good | 27.7 | 30 | 26.7 |
| Very good | 16.6 | 17.8 | 16 |
| Excellent | 3.8 | 4.9 | 3.4 |
|
| |||
| Proxy relationship to pt | |||
| Spouse | 32.5 | 25.3 | 36.2 |
| Adult child | 41 | 44.8 | 39.1 |
| Other relative | 14.6 | 14.8 | 14.5 |
| Non-relative | 11.9 | 15.1 | 10.3 |
Abbreviations: Cog, cognitive; nm, never married; Pt, patient.
Cognitively healthy persons were predominantly female (52.8%), white (47.7%), widowed (44.1%), and had a mean age of 83 years (SD = 7.67). They were primarily in fair (28%) or good (21.8%) health, with an average of 4.1 chronic conditions (SD = 1.55). The most frequent proxy respondent for CHP was an adult child (50.3%).
Place of Death
Where do older adults with and without cognitive impairment die, and is there a difference?
Place of death was recorded as in-home, hospital, nursing home, hospice residence, or other location. Observed frequencies and percentages of place of death for persons with and without cognitive impairment are presented in Figure 1. Persons with cognitive impairment most often died at home (33.5%), while CHP were equally likely to die at home (36.6%) or in a hospital (36.8%). Cognitively healthy persons were significantly more likely than those with cognitive impairment to die in a hospital (36.8% vs 28.4%, P < .01), while persons with cognitive impairment were significantly more likely to die in a nursing home (25.6% vs 15.4%, P < .001). Approximately 9% of persons with cognitive impairment and 7.8% of CHP died in a hospice residence. The “other” category represented the site of 4.0% of deaths of persons with cognitive impairment and 3.4% of deaths of CHP.
Figure 1.
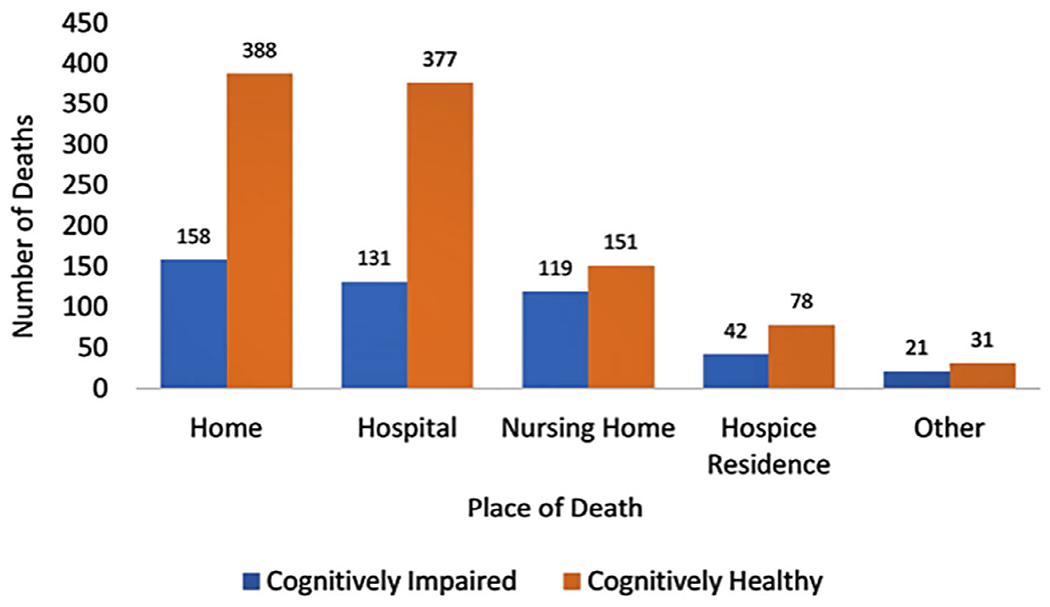
Crosstabulation of cognitive status and place of death. Cognitively impaired N = 472 (weighted n = 1.5 million), cognitively healthy N = 1,024 (weighted n = 4.0 million); home p = .171, hospital p = .002, nursing home p < .001, hospice residence p = .384, other p = .484
A chi-square test of independence was conducted between place of death and cognitive status. All expected cell frequencies were greater than five. There was a statistically significant association between place of death and cognitive status, χ2(4) = 27.180, P < .001, although this association was small,23 Cramer’s V = .135, P < .001. A total of 10 pairwise comparisons revealed a statistically significant difference between frequency of death in the home versus nursing home (Fisher’s exact P < .001) and hospital versus nursing home (P < .001). No other pairwise comparisons were significant.
Factors associated with place of death
Figure 2 summarizes factors associated with place of death by cognitive status. For persons with cognitive impairment, multivariate analysis showed that the strongest predictor of place of death was use of the Medicare Hospice Benefit (Supplementary Table S1). Persons with cognitive impairment who used this benefit were 14.5 times more likely to die at home than in a hospital, and 3.4 times more likely to die at home than in a nursing home. Age, race, gender, and marital status were not significantly related to place of death.
Figure 2.
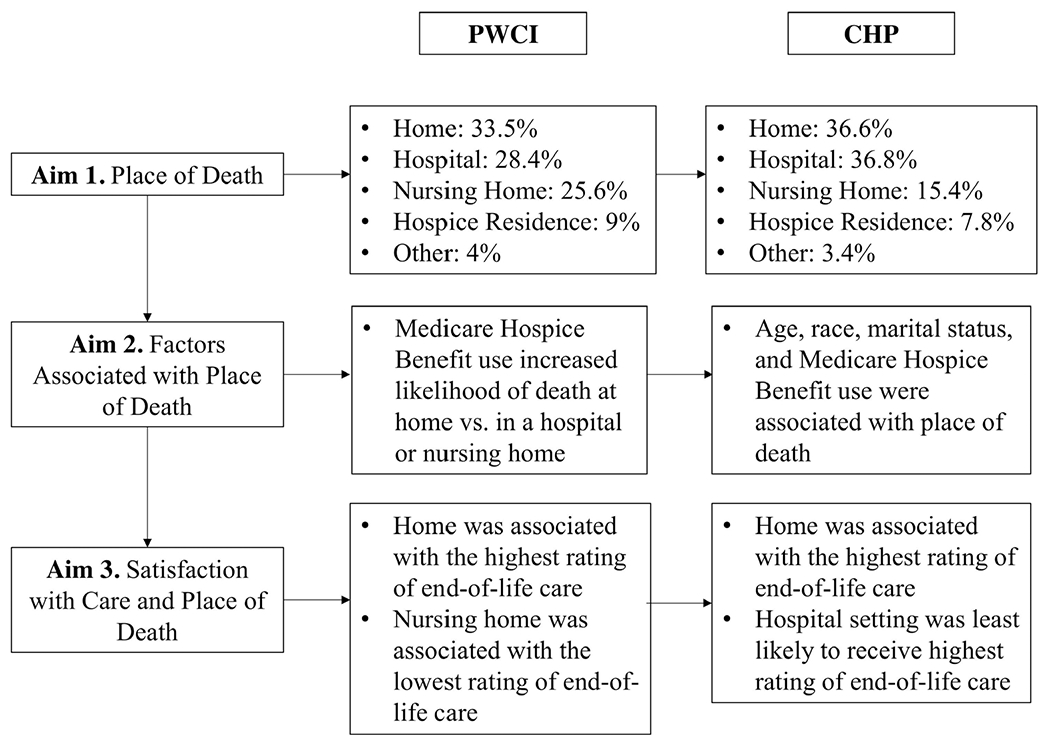
Outcomes of study aims by cognitive status. CHP, cognitively healthy persons; PWCI, persons with cognitive impairment
For CHP, place of death was associated with age, race, and use of the Medicare Hospice Benefit. Cognitively healthy persons aged 90 and above were twice as likely to die in a nursing home than in a hospice residence. The youngest participants, ages 65 to 69, were more than 4.5 times as likely to die at home and five times more likely to die in a hospital than in a nursing home. Participants ages 70 to 79 were 2.5 times more likely to die at home and twice as likely to die in a hospital than in a nursing home.
Black CHP were nearly twice as likely to die at home and 2.5 times more likely to die in a hospital than in a nursing home, as compared to all other racial/ethnic groups. Persons who were Hispanic/Latino were half as likely to die in a hospital than in a nursing home, as compared to all other racial/ethnic groups. Confidence intervals examining the impact of White or other racial/ethnic groups were not significant.
Cognitively healthy persons who were married or partnered were twice as likely to die at home or in a hospital as in a nursing home, and half as likely to die in a nursing home as a hospice residence. Widowed participants were nearly 40% less likely to die at home or in the hospital than in a nursing home.
Cognitively healthy persons who utilized the Medicare Hospice Benefit were more than six times more likely to die at home than in a hospital, and more than twice as likely to die at home than in a nursing home. No other confidence intervals were statistically significant.
Satisfaction with Care and Place of Death
Figure 2 summarizes proxy satisfaction with EOLC by cognitive status. Bivariate correlations and the crosstabulation procedure suggest an association with place of death (dummy-coded) and proxy report of satisfaction with end-of-life care (also dummy-coded). Proxies of persons with cognitive impairment gave the highest-quality rating of EOLC when their care recipient died at home (r = .167, P < .001), and were more likely to rate care in settings other than a hospital as “excellent” (r = −.101, P = .030). However, proxies of persons with cognitive impairment were more likely to rate hospital care as “good” as compared to all other settings (r = −.113, P = .016). These proxies gave the lowest-quality rating of EOLC (“poor”) when their care recipient died in a nursing home (r = .141, P = .002). No other significant correlations were found.
Proxies of CHP gave the highest-quality rating of EOLC when their care recipient died at home (r = .108, P = .001), and were more likely to rate care in settings other than a hospital as “excellent” (r = −.109, P = .001). No other significant correlations were found.
DISCUSSION
For nearly two decades, there has been increasing recognition that place of death is an important component of the quality of end-of-life care (EOLC) and quality of death.24 However, despite this almost 20-year push for awareness of the implications of place of death, little research has been done in the United States to provide detailed information on place of death and its correlates. Such information, as well as findings on how place of death has changed over time, would inform EOLC planning and policy-making and may facilitate greater well-being at end-of-life.
Using the NHATS dataset, this study identified place of death for older adults with and without cognitive impairment in the United States. As such, this study is distinguished from previous studies25,26 in that it examined cognitive impairment in the context of all-cause mortality and focused on the relationship between place of death and cognitive impairment. We deemed this important because we intended to describe broad relationships without the potential distraction of a focus on comorbidities. Cognitive impairment is often included as a secondary variable due to its complexity of definition and documentation. Having thus discovered relationships between cognitive impairment and place of death on several levels, further research can now be undertaken to examine these relationships in light of other variables, including comorbidities.
We found a significant relationship between place of death and cognitive status (Figure 1). The most frequent place of death for persons with cognitive impairment was in the home (32.8%); 28% of persons with cognitive impairment died in a hospital, 25.6% died in a nursing home, and 9.1% died in a hospice residence. Our findings are congruent with a recent study indicating that home deaths for PLWD are on the rise in the United States.27 This marks a significant change in the United States over the past two decades, shifting from nursing homes as the most common site of death6,28 to the home for those with dementia or cognitive impairment. Findings are similar to trends seen in Finland, where EOLC for PLWD is increasingly moving to more non-institutional settings.29 However, in contrast to our findings, hospitals or nursing homes continue to most frequently be the last place of care for individuals with dementia in the majority of European countries studied.1,25
Interestingly, cognitively healthy persons (CHP) were equally as likely to die in-home (36.6%) or in the hospital (36.8%). Proxies reported that 15.4% died in a nursing home and 7.8% died in a hospice residence. This is somewhat consistent with 2001 data6 that found the hospital to be the most common site of non- dementia-related deaths, although the rise in at-home deaths seen in our sample may signal a shift in the United States towards not only aging-in-place, but perhaps dying-in-place as well.
The results of this study suggest that a substantial number of persons with cognitive impairment and CHP continue to die in nursing homes, although this place of death is significantly more common for cognitively impaired older adults than their cognitively healthy peers (Figure 1). Unfortunately, palliative care has struggled to establish itself as a foundational component of care in nursing home settings.30 This may be, in part, because of the difficulty in providing adequate training and resource support for nursing and paraprofessional staff in long-term care. Staffing is frequently perpetually stretched in many of these settings and there is a pervasive perception that palliative care requires more time and attention than staff can give. Though recognized as a national priority in the United States, resources to support palliative care integration are infrequently directed to long-term care institutions.31 Furthermore, baseline knowledge of nursing home staff regarding palliative care is low.32 Greater investment in specialized staff training and support is needed, and it is important to consider whether and how it might be possible to move towards structural change that will support long-term integration of palliative and hospice care into nursing facilities.
One potential policy implication of the disparity between place of death and resource allocation for end-of-life care is that reimbursement for palliative care in nursing homes should be revisited. Administrative support is essential to the establishment and maintenance of palliative care as a priority in long-term care.30 As administrative structures are intrinsically linked to fiscal constraints, it is imperative that policies supporting payment for end-of-life care be implemented as part of a larger repurposing of health care funds. This priority is supported by findings such as ours, which highlight the significance of the Medicare Hospice Benefit as an important factor in where older adults die, regardless of cognitive status.
Our findings differed from previous studies that have explored EOLC at home for persons living with dementia,33,34 as caregivers in our sample were more satisfied with care in the home as compared to the hospital. Prior studies suggest that, while maintaining home care through the end of life may align with the patient’s wishes, caregivers may feel more distant from healthcare professionals and therefore less supported.34 In addition, family caregivers experience strain associated with the time and skill demands when attempting to facilitate dying-in-place for loved ones with cognitive impairment, and have ambivalent attitudes and beliefs about the level of appropriate treatment for end-of-life care35 that may only be available in a hospital setting. Higher caregiver burden is also associated with worsening behavioral and psychiatric problems resulting in transfers from home to hospital or nursing home at the end of life.36,37 Consequently, it is important to identify ways to maximize support for family caregivers as they endeavor to achieve their loved one’s wishes for end-of-life.
Limitations and Strengths
Several potential limitations should be noted. First, the NHATS “last month of life” data was provided by proxies, and there is no way to assess how closely proxy report reflects the experience of the decedents. Second, the authors were limited by the available variables in terms of the depth of analyses that could be conducted and conclusions that could be drawn. For example, the medical details surrounding participants’ cause of death were not available, and it is unknown how long persons receiving hospice care were provided with those services. Finally, given the available variables in the NHATS dataset, the specific cause of death for participants both with and without cognitive impairment was unknown. Similarly, the individual contributions of participants’ health conditions to place of death and proxy satisfaction with EOLC could not be determined from the dataset.
There are several noteworthy strengths of this study. First, the NHATS sample is nationally-representative of the 65-and-older Medicare population. Second, findings contribute to the literature on factors associated with place of death for older adults with and without cognitive impairment that is, at present, quite small. Third, data on where people die in the United States provides useful information for the development of EOLC policies and services that meet the needs of those with and without cognitive impairment and their families. Finally, our results provide insights to help support appropriate policy decision-making for persons with cognitive impairment.
Future research should include a greater focus on incorporating palliative care into dementia care strategies and addressing the barriers to providing palliative care within this population.4,38–40 It is also critical to examine how more resources might go towards the integration of palliative care into long-term care facilities and the training and support of long-term care staff. In addition, as these variables were not available within the current data set, it would be useful for future studies to better understand the specific aspects of home end-of-life care that were associated with caregiver satisfaction.
CONCLUSION
Given the paucity of literature surrounding the relationship between place of death and associated quality of care, particularly in the United States, it is likely that awareness of this important aspect of EOLC is lacking. Consequently, this topic may not be incorporated into advance care planning with patients, family, and caregivers. This is unfortunate, as the findings of this study indicate that place of death should be a central component of EOLC discussions for persons with and without cognitive impairment. As there is frequently incongruence between preferred and actual place of death,5 it is critical that providers in hospitals, long-term care, and palliative care settings are trained to provide person-centered care,41 particularly as provider knowledge of preferred place of death is associated with achieving that goal.42 It is also important that, in light of this incongruence, older adults allow for a degree of flexibility in end-of-life serving planning.43 At present, there remains a long way to go towards ensuring that persons at end-of-life, particularly those with cognitive impairment, receive adequate EOLC in accordance with their needs and preferences.
Supplementary Material
Supplementary Table S1 Adjusted Odds Ratios of Place of Death
Key Points
Place of death for older adults in the United States was associated with cognitive status.
The strongest predictor of place of death, regardless of cognitive status, was utilization of hospice care.
Satisfaction with end-of-life care was rated highest for persons with and without cognitive impairment who died at home.
Why Does This Paper Matter?
These findings add to the small body of research on place of death for persons with and without cognitive impairment in the United States. Understanding predictors and outcomes of place of death may inform end-of-life care planning and policymaking, and facilitate greater well-being at end-of-life.
Financial Disclosure:
Authors of this publication received support from the National Institute on Aging of the National Institutes of Health (NIH) under Award Number K23AG058809-011 (NGR), the Robert Wood Johnson (RJW) Harold Amos Medical Faculty Program (JLT), and the Cambia Foundation (VTC, RJW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the RWJ Foundation or the Cambia Foundation.
Sponsor’s Role:
No sponsor played a role in this article’s design, methods, data analysis, and preparation. As this was a secondary data analysis of The National Health and Aging Trends Study, original data collection and subject recruitment was sponsored by the National Institute on Aging (U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Dasch B, Bausewein C, Feddersen B. Place of death in patients with dementia and the association with comorbidities: a retrospective population-based observational study in Germany. BMC Palliat Care. 2018;17(80):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins PC, Garrido MM, Prigerson HG. Factors predicting bereaved caregiver perception of quality of care in the final week of life: implications for health care providers. J Palliat Med. 2015;18(10):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyniers T, Houttekier D, Cohen J, Pasman HR, Deliens L. The acute hospital setting as a place of death and final care: a qualitative study on perspectives of family physicians, nurses and family carers. Health Place. 2014;27C:77–83. [DOI] [PubMed] [Google Scholar]
- 4.Badrakalimuthu V, Barclay S. Do people with dementia die at their preferred location of death? A systematic literature review and narrative synthesis. Age Ageing. 2014;43(1):13–19. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. QuickStats: percentage distribution of deaths, by place of death — United States, 2000–2014. Morbid Mortal Weekly Rep. 2016;65(13):357. [Google Scholar]
- 6.Mitchell SL, Teno JM, Miller SC, Mor V. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53:299–305. [DOI] [PubMed] [Google Scholar]
- 7.Yung VY, Walling AM, Min L, Wenger NS, Ganz DA. Documentation of advance care planning for community-dwelling elders. J Palliat Med. 2010;13(7):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuel EJ. The status of end-of-life care in the United States: the glass is half full. JAMA. 2018;320(3):239–241. [DOI] [PubMed] [Google Scholar]
- 9.Pinzon LC, Claus M, Perrar KM, Zepf KI, Letzel S, Weber M. Dying with dementia: symptom burden, quality of care, and place of death. Dtsch Arztebl Int. 2013;110(12):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia. J Am Geriatr Soc. 2002;50(10):1723–1732. [DOI] [PubMed] [Google Scholar]
- 11.Watkin L, Blanchard MR, Tookman A, Sampson EL. Prospective cohort study of adverse events in older people admitted to the acute general hospital: risk factors and the impact of dementia. Int J Geriatr Psychiatry. 2012;27(1):76–82. [DOI] [PubMed] [Google Scholar]
- 12.Marengoni A, Corrao S, Nobili A, et al. In-hospital death according to dementia diagnosis in acutely ill elderly patients: the REPOSI study. Int J Geriatr Psychiatry. 2011;26(9):930–936. [DOI] [PubMed] [Google Scholar]
- 13.Borbasi S, Jones J, Lockwood C, Emden C. Health professionals’ perspectives of providing care to people with dementia in the acute setting: toward better practice. Geriatr Nurs. 2006;27(5):300–308. [DOI] [PubMed] [Google Scholar]
- 14.Cowdell F Care of older people with dementia in an acute hospital setting. Nurs Stand. 2010;24(23):42–48. [DOI] [PubMed] [Google Scholar]
- 15.Jurgens FJ, Clissett P, Gladman JR, Harwood RH. Why are family carers of people with dementia dissatisfied with general hospital care? A qualitative study. BMC Geriatr. 2012;12(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RAND Corporation. Hospice Care in Medicare: Recent Trends and A Review of the Issues http://67.59.137.244/publications/congressional_reports/June04_ch6.pdf. Accessed May 31, 2020.
- 18.Kasper JD, Freedman VA. Findings from the 1st round of the National Health and aging trends study (NHATS): introduction to a special issue. J Gerontol B Psychol Sci Soc Sci. 2014;69(7):S1–S7. [DOI] [PubMed] [Google Scholar]
- 19.Montaquila J, Freedman VA, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Collection: NHATS Technical Paper #1. https://www.nhats.org/scripts/sampling/NHATS_Round1_Sample_Design_05_10_12.pdf. Accessed May 31, 2020.
- 20.Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the National Health and Aging Trends Study. https://nhatspubdemo.westat.com/scripts/documents/NHATS_Dementia_Technical_Paper_5_Jul2013.pdf. Accessed May 31, 2020.
- 21.Frochen S, Mehdizadeh S. Functional status and adaptation: measuring activities of daily living and device use in the National Health and aging trends study. J Aging Health. 2018;30(7):1136–1155. [DOI] [PubMed] [Google Scholar]
- 22.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 23.Cohen J Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. https://www.taylorfrancis.com/books/9780203771587. [Google Scholar]
- 24.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88–93. [DOI] [PubMed] [Google Scholar]
- 25.Houttekier D, Cohen J, Bilsen J, Addington-Hall J, Onwuteaka-Philipsen BD, Deliens L. Place of death of older persons with dementia: a study in five European countries. J Am Geriatr Soc. 2010;58:751–756. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Wu C, Fletcher J. Assessment of changes in place of death of older adults who died from dementia in the United States, 2000-2014: a time-series cross-sectional analysis. BMC Pub Health. 2020;20:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross SH, Kaufman BG, Taylor DH, Kamal AF, Warraich HJ. Trends and factors associated with place of death for individuals with dementia in the United States. J Am Geriatr Soc. 2020;8(2):250–255. [DOI] [PubMed] [Google Scholar]
- 28.Reyniers T, Deliens L, Pasman R, et al. International variation in place of death of older people who died from dementia in 14 European and non-European countries. JAMDA. 2015;16(2):165–171. [DOI] [PubMed] [Google Scholar]
- 29.Masuchi Y, Jylhä M, Raitanen J, Aaltonen M. Changes in place of death among people with dementia in Finland between 1998 and 2013: a register study. Alzheimers Dement. 2017;10(2018):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norton SA, Ladwig S, Caprio TV, Quill TE, Temkin-Greener H. Staff experiences forming and sustaining palliative care teams in nursing homes. Gerontologist. 2018;58(4):e218–e225. [DOI] [PubMed] [Google Scholar]
- 31.Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff. 2017;36(7):1265–1273. [DOI] [PubMed] [Google Scholar]
- 32.Unroe KT, Cagle JG, Lane KA, Callahan CM, Miller SC. Nursing home staff palliative care knowledge and practices: results of a large survey of frontline workers. J Pain Symptom Manage. 2015;50(5):622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogan C, Lloyd-Williams M, Harrison-Dening K, Dowrick C. The facilitators and challenges of dying at home with dementia: a narrative synthesis. Palliat Med. 2018;32(6):1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps EJ, Braitman LE. Family caregiver satisfaction with care at end of life: report from the cultural variations study (CVAS). Am J Hosp Palliat Care. 2004;21(5):340–342. [DOI] [PubMed] [Google Scholar]
- 35.Davies N, Maio L, Rait G, Iliffe S. Quality end-of-life care for dementia: what have family carers told us so far? A narrative synthesis. Palliat Med. 2014;28(7):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treloar A, Crugel M, Adamis D. Palliative and end of life care of dementia at home is feasible and rewarding. Dement. 2009;8(3):335–347. [Google Scholar]
- 37.Volicer L, Hurley AC, Blasi ZV. Characteristics of dementia end-of-life care across care settings. Am J Hospice Palliat Med. 2003;20(3):191–200. [DOI] [PubMed] [Google Scholar]
- 38.Bamford C, Lee R, McLellan E, et al. What enables good end of life care for people with dementia? A multi-method qualitative study with key stakeholders. BMC Geriatr. 2018;18:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erel M, Marcus EL, Dekeyser-Ganz F. Barriers to palliative care for advanced dementia: a scoping review. Ann Palliat Med. 2017;6:365–379. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi M, Nakashima T, Shindo Y, et al. An evaluation of palliative care contents in national dementia strategies in reference to the European Association for Palliative Care white paper. Int Psychogeriatr. 2015;27:1551–1561. [DOI] [PubMed] [Google Scholar]
- 41.Berghout M, van Exel J, Leensvaart L, Cramm JC. Healthcare professionals’ views on patient-centered care in hospitals. BMC Health Serv Res. 2015;15:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiggins N, Droney J, Mohammed K, Riley J, Sleeman KE. Understanding the factors associated with patients with dementia achieving their preferred place of death: a retrospective cohort study. Age Ageing. 2019;48(3):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Adjusted Odds Ratios of Place of Death


