Abstract
Objective:
Although the sensitivity and specificity of bilateral inferior petrosal sinus sampling (BIPSS) were shown to be quite high in adult patients, pediatric studies are limited in number and have conflicting results, since BIPSS is much less commonly performed in children. The aim of this study was to assess the role of BIPSS in the detection and accuracy of lateralization of pituitary adenomas in pediatric patients with Cushing disease (CD) and its possible advantage over other diagnostic methods.
Methods:
This was a multicenter, nationwide, web-based study. The diagnostic value of BIPSS in 16 patients, aged between four and 16.5 years with a confirmed diagnosis of CD, was evaluated retrospectively. The sensitivity and specificity of BIPSS and magnetic resonance imaging (MRI) were calculated, and compared statistically.
Results:
Standard tests, except for morning cortisol level, were effective in proving the presence of Cushing syndrome. While MRI findings were consistent with microadenoma in eight cases (50%), CD presence and lateralization was successfully predicted in 14 of 16 patients using BIPSS. BIPSS compared with MRI examination was significantly more accurate, both in pre-stimulation and post-stimulation results (p=0.047 and p=0.041, respectively). BIPSS showed a significantly higher sensitivity (92.8%) than MRI in detecting the pituitary source of adrenocorticotropic hormone secretion.
Conclusion:
These results suggest that BIPSS is superior to MRI for diagnostic work-up to confirm the diagnosis of CD. Moreover, in line with previous studies, BIPSS was shown to provide better information about adenoma location, which is vital for possible surgical intervention.
Keywords: Cushing’s disease, pituitary adenoma, petrosal sinus sampling, sensitivity, lateralization
What is already known on this topic?
Although the sensitivity and specificity of bilateral inferior petrosal sinus sampling (BIPSS) were shown to be high in adult patients, studies in children are limited in number and have conflicting results since it is much less common in this population.
What this study adds?
Our study supports that BIPSS is a superior diagnostic work-up than MRI to confirm the diagnosis of Cushing disease. Moreover, BIPSS was shown to provide better information about adenoma localization.
Introduction
Cushing syndrome (CS) arises from chronic exposure to excess amount of exogenous or endogenous glucocorticoids. Exogenous administration of steroids is the most common cause of CS in children. The incidence of endogenous CS is 0.7-2.4/1,000,000 people/year, and approximately 10% of cases are children (1). Endogenous causes of CS are rare and can be either adrenocorticotropic hormone (ACTH)-dependent (75-90%) or ACTH-independent (15-20%). ACTH-dependent CS results from overproduction of ACTH from the pituitary by ectopic secretion of ACTH or corticotropin-releasing hormone (CRH) (2).
Once CS is suspected based upon clinical manifestations, diagnostic evaluation requires the administration of several tests (3). Loss of the cortisol circadian rhythm is the earliest biochemical marker of endogenous hypercortisolism (4). The sensitivity of 24-hour urinary cortisol measurement in children was found to be 88%, and serial measurements were recommended in pediatric practice (5). A spot morning plasma ACTH level may be an alternative, which has a sensitivity of 70%, with a cut-off value above 29 pg/mL in identifying children with ACTH dependent syndrome (6,7). Moreover, some patients with pituitary disease may present with ACTH levels in a low-to-normal range, and conversely, some patients with adrenal forms can present with ACTH levels that are not fully suppressed (8). A low-dose dexamethasone suppression test can be used to evaluate the lack of negative feedback of cortisol on the hypothalamic-pituitary-adrenal axis. A late-night serum cortisol value above 1.8 μg/dL is considered to be abnormal, with a sensitivity higher than 95% and a specificity of 80% (9). The standard high-dose dexamethasone suppression test is used to differentiate Cushing disease (CD) from ectopic ACTH secretion and adrenal causes of CS. Batista et al (7) reported that in a pediatric population, cortisol suppression of 20% from baseline had a sensitivity and specificity of 97.5% and 100%, respectively. Several tests have been used in the differential diagnosis of CS, but none of them can precisely differentiate the source of ACTH. Bilateral inferior petrosal sinus (IPS) sampling (BIPSS) is the gold standard for determining the source of ACTH to reveal whether the source is pituitary or ectopic. Although the sensitivity and specificity of BIPSS were shown to be quite high in adult patients (10,11), studies in children are limited in number and have conflicting results, since BIPSS is much less commonly performed in this population (11,12,13,14,15,16).
Many studies have shown that CRH increases the sensitivity of BIPSS by stimulating the secretion of ACTH from pituitary adenomas in adults and children (10,11,17,18). Desmopressin is administered in adult patients as a cheaper and feasible alternative to CRH (19).
In addition to the differentiation of CD from ectopic ACTH sources, the ACTH ratio between left and right veins is also useful in determining the location/lateralization of pituitary microadenomas (15,20), and thus guiding the neurosurgeon during surgery. A pituitary magnetic resonance imaging (MRI) should be performed in all patients with a suspicion of ACTH-dependent CS, but this does not identify pituitary adenoma in 36-78% of the cases in series (21,22,23). Sensitivity in determining the lateralization of tumor by BIPSS was reported to be up to 60-90% before and after CRH stimulation in children, despite limited data (24,25,26).
In the hands of an experienced interventional radiologist, BIPSS is a safe procedure with few but significant complications, such as inguinal hematoma or brainstem hemorrhage, or non-hemorrhagic brainstem infarctions or thromboembolic events (27).
Our study aimed to assess the role of BIPSS in the detection and accuracy of lateralization of pituitary adenomas and compare this to other diagnostic methods.
Methods
This retrospective study was conducted as a multicenter, nationwide, web-based exercise, and an electronic recording form (ERF) to collect the demographic data and clinical and laboratory findings of the patients with CD was used. All centers providing data to this study were university hospitals. Users reached the ERF from CEDD.net Web Registration System website (https://cedd.saglik-network.org/).
Data of 32 patients with ACTH-dependent CS were recorded (Figure 1). Of these 32 patients, 16 had BIPSS performed. Fourteen were confirmed to have CD histopathologically, and the remaining two cases responded to medical therapy with regression of clinical and laboratory findings.
Figure 1.
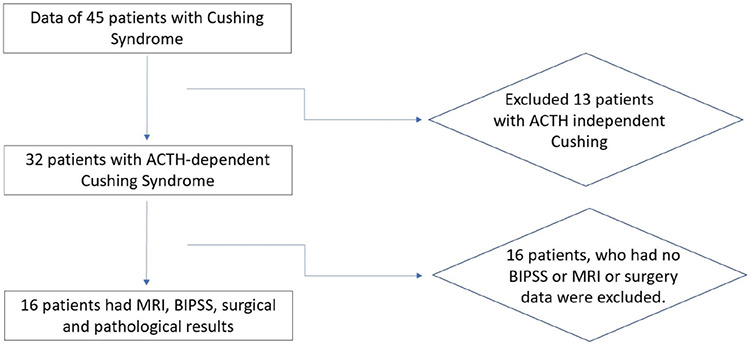
Flow chart
MRI: magnetic resonance imaging, BIPSS: bilateral inferior petrosal sinus sampling, ACTH: adrenocorticotropic hormone
In the overnight dexamethasone suppression test procedure, dexamethasone (1 mg, orally) was administered at 11:00 p.m., and blood samples for ACTH and cortisol measurement were obtained in the following morning. For the low-dose dexamethasone suppression test, 0.5 mg of dexamethasone was given at six-hour intervals for two days, with the cortisol level measured six hours after the final dose was given. For the high-dose dexamethasone suppression test, 2 mg of dexamethasone was given at six-hour intervals for two days, with the cortisol level measured six hours after the final dose was given. Gadolinium-enhanced MRI of the pituitary gland was performed on a 1.5 Tesla (15 patients) or a 3 Tesla (1 patient) MRI system in imaging studies. A microadenoma was described as a pituitary tumor of less than 1 cm in diameter and a macroadenoma was described as a tumor above 1 cm in diameter. Patients were sent for BIPSS, especially when pituitary MRI was negative or suspicious for adenoma, or when clinical and imaging results were inconsistent. The BIPSS procedures were performed by radiologists based on the technique described by Doppman et al (28). Blood samples were collected from the peripheral veins and left and right IPS. CRH stimulation test was conducted with 100 μg of CRH intravenously after catheterization. Sampling lateralization was used to determine which side of the pituitary gland involved a tumor responsible for the overproduction of ACTH.
IPS to peripheric vein ACTH ratio of >2 before CRH stimulation and IPS to peripheric vein ACTH ratio >3 post-stimulation were accepted as diagnostic for CD (29). The lateralization of the adenoma was considered in patients with the intergradient difference of the right and left petrosal sinuses of more than 1.4 (29).
All patients underwent transsphenoidal surgery. The lateralization of the tumor was also recorded during surgery. Suspicious tumor tissue was resected for histological assessment, including immunohistological staining for ACTH. The diagnosis of CD was confirmed with positive immunohistological staining for ACTH in histopathological studies in 14 patients.
The exclusion criteria of the study were: cases with findings of CS but not with a definitive diagnosis of CS; or cases with insufficient or missing data.
The study protocol was approved by the Ethical Committee of İzmir Tepecik Training and Research Hospital (decision no: 10, date: 04.02.2015). Informed consent was obtained from all patients and/or parents at admission to hospital.
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences v.21 for Windows (IBM Inc., Chicago, IL, USA). Data are presented as mean±standard deviation for parametric data and median (minimum-maximum) for non-parametric data. The sensitivity and specificity of tests were calculated according to standard statistical formulae. Descriptive statistics were used to analyze the data. Mann-Whitney U or Kruskal-Wallis tests were conducted to compare the parameters.
Results
MRI findings, laboratory tests, histopathological evaluation, and treatment results of 16 patients who underwent BIPSS with a pre-diagnosis of Cushing’s disease at eight centers were assessed. There were eight boys and eight girls with a mean age of 12.1±3.76 years, ranging from 4.23 to 16.5 years.
Baseline early morning cortisol levels of the cohort ranged between 12.3 and 59.8 mg/dL and seven were in the normal range. All patients had high late-night cortisol levels (>7.5 mg/dL). Twenty-four-hour urinary free cortisol excretion of 14/16 was increased. All 16 patients had ACTH levels above 20 pg/dL. Baseline characteristics of these 16 children and adolescents with CD are shown in Table 1. In 13 patients, 1 mg dexamethasone suppression test was performed overnight; none of them were suppressed. A low dose dexamethasone suppression test was performed in 10 of 16 patients and none of them were suppressed. A high-dose dexamethasone suppression test was performed in 13 of 16 patients, and suppression of serum cortisol level over 50% was achieved in all 13 patients. On MRI, findings compatible with microadenoma were detected in 8 (50%), while no finding supporting CD was detected in the remaining eight cases (Table 2).
Table 1. Baseline characteristics of 16 children and adolescents with Cushing disease.
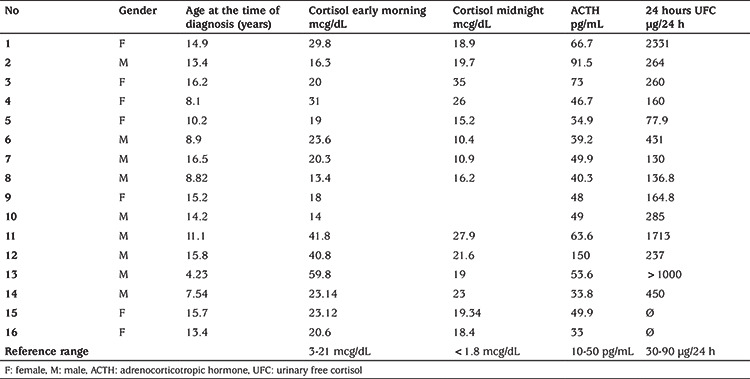
Table 2. MRI, BIPSS and histopathological examination data of 16 patients.
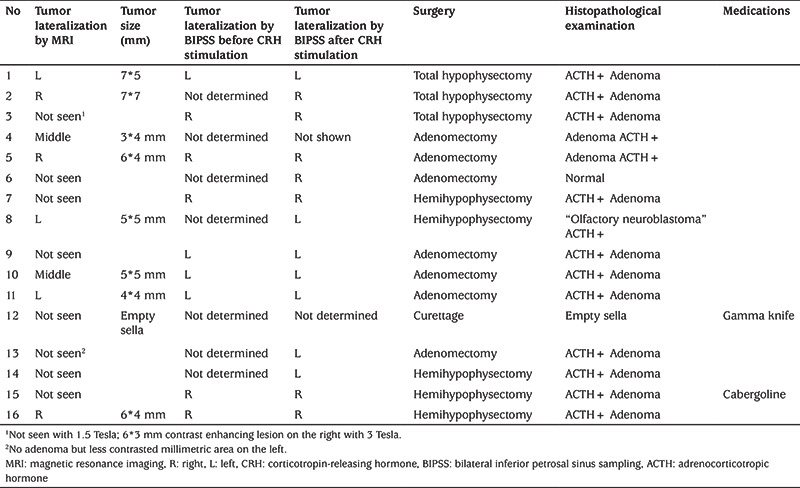
Severe adverse effects were not observed in any of 16 patients who underwent BIPPS protocol during or after the procedure. The percentage of predicting CD was 81% (13/16) pre-stimulation and 87.5% (14/16) after stimulation with CRH (Table 2). There was no statistically significant difference between before and after stimulation (p=0.106). When compared accuracy of BIPSS to MRI examination, a statistical significance was obtained both in pre-stimulation and post-stimulation results (p=0.047 and p=0.041, respectively). BIPSS showed a significantly greater sensitivity 92.8% than MRI (sensitivity, 53.3%; specificity, 100%) in detecting the pituitary source of ACTH secretion. Overall, lateralization of ACTH levels by a baseline inter-petrosal sinus gradient (IPSG) ≥1.4 was compatible with the surgical location of the pituitary corticotropinoma in nine of the cases in which a tumor was located by surgery. After CRH stimulation, an IPSG of ≥1.4 predicted the site of the pituitary lesion in 14 of the cases.
All patients underwent surgical intervention (Table 2). After the operation, empty sella was detected in one of the two patients whose clinical findings did not regress. Thus, curettage was performed, but clinical findings regressed only after gamma-knife application. Clinical findings of the other patient with failed surgery regressed with cabergoline treatment. Except for these two patients, the diagnosis of CD was confirmed by histopathological studies in 14 patients, and clinically with the regression of CD/CS findings in all patients.
Discussion
Different studies have reported the results of children and adolescents with CD undergoing BIPPS from different countries, and the role of BIPSS in diagnosis and lateralization has been evaluated and a significant difference between MRI and BIPSS in terms of success in detecting CD was found (3,14,16,17,30,31). Herein, we present the first report of a series of children and adolescent with CD who underwent BIPSS from Turkey. The results suggest that BIPSS is a superior diagnostic tool compared to MRI for diagnosing CD and determining lateralization.
Since cortisol is secreted in a circadian rhythm, basal cortisol value is not used in the diagnosis of hypercortisolemia, and morning cortisol level is also not recommended to be useful for the diagnosis of CS because it may cause false-positive results due to the increase in morning cortisol values (32,33,34). In addition, 80% of cortisol is bound to cortisol-binding globulin, and since assays measure total protein-bound globulin, serum cortisol levels change as serum protein decreases. In our study, the finding that 50% of 16 patients with confirmed CD diagnosis had normal morning cortisol levels support the view that it cannot be used in diagnosis. However, in our study, it was also found that standard tests, except for the morning cortisol level, were useful for proving the presence of CS.
MRI is commonly used to investigate CD and to identify pituitary adenomas non-invasively (21). ACTH-secreting microadenomas are commonly not visible on MRI in patients with CD. This may be in part related to their small sizes, or it could be related to the fact that these lesions have a signal and enhancing characteristics similar to those seen in the normal pituitary gland. In a case series of 200 patients, Lonser et al (3) detected adenoma in 97 patients (50%) on MRI, and lateralization was achieved in 96 patients with MRI. Chen et al (30) reported that lateralization by MRI was found to be consistent with operation in 80% of patients. In our study in 8 (57%) of 14 surgically proven CD cases, findings consistent with microadenoma were detected and lateralization could be achieved in 7 of these 14 cases (50%) on MRI. When BIPSS was compared with MRI examination, statistical significance was obtained for both pre-stimulation and post-stimulation results in terms of CD diagnosis. BIPSS is a highly specialized and invasive technique and is routinely used in adults, to distinguish CD from ectopic ACTH syndrome (EAS), and for lateralization of the pituitary microadenoma. Although BIPSS is a routinely used technique to distinguish CD from EAS, in our study, there were no cases of EAS. To date, a total of 453 children, who underwent BIPSS, have been reported by different studies, but only 12 were found to have EAS (3,14,35).
In our cases, the accuracy of BIPSS for predicting CD was 81% (13/16) pre-stimulation and was 87.5% (14/16) after stimulation with CRH (p=0.106). BIPSS showed a sensitivity of 92.8% and specificity of 100% in detecting the pituitary source. In a series with a large number of adult cases, the sensitivity of CRH stimulation was reported as >90% (36). In 2019, Chen et al (30) reported the sensitivity of BIPSS without stimulation as only 64.7% for the diagnosis of CD in children and adolescents, while the sensitivity with desmopressin stimulation was 83.3%. The difference between this study and our study was the use of desmopressin for stimulation, rather than CRH.
Since EAS is very rare in children, the main use of BIPSS in the pediatric age group is for accurate location of the pituitary microadenoma. However, few studies have investigated the usefulness of BIPSS in predicting the location of pituitary adenoma in the pediatric population (14,15,17,31). As reported previously in children and adolescents, rates of identifying the laterality of the tumor by BIPSS ranged from 73.7 to 100%, and the consistency of these predictions with the actual tumor lateralization was 58.7-100% (3,13,18,26,30,37). In Lonser’s series (3), BIPSS accurately predicted the lateralization of the adenoma in 57 of the 82 patients (70%) in whom an adenoma located off midline was found at surgery. In 2006, Batista et al (17) published the second largest series in the literature and evaluated the results of 43 patients, reporting that BIPSS was a poor predictor of the site of a microadenoma in children. Lateralization estimation (confirmed by surgery) in Magiakou et al’s (14) series of 50 patients before and after stimulation with CRH was 67% and 76%, respectively. In a study published by the same center in 2013, the lateralization rate was reported as 88% in the evaluation of 140 pediatric patients (3). In the current study, while the lateralization rate before CRH stimulation was 56.25%, it increased to 87.5% after CRH. These percentages are comparable to those previously reported (3,14,26,30). It was suggested that in the previous studies, the centers being tertiary referral centers may have caused a selection bias, i.e., referral of the cases with no signs on MRI, with a history of an unsuccessful surgery or mildly affected cases to those centers may have underestimated the lateralization rates. The centers our patients were referred to were also reference centers. In addition, different results are most likely to occur due to the different number of cases. Lienhardt et al (13) evaluated seven patients, reporting a lateralization rate of 91%, but commented that this rate would decrease as the number of cases increased. Another factor affecting the results is that when calculating the lateralization rate in the studies, some of them only included tumors with lateralization, while others included midline tumors. Different results may be obtained with smaller age groups and depending on the experience of the surgeon. It was reported that anatomical variations of the IPS were reported to influence the venous drainage of the pituitary or hinder the correct positioning of the catheter, which might lead to misleading results from BIPSS (38,39). Such variations are reported to be common (40). In a quarter of cases, the IPS was plexiform (40), but in most cases, this did not cause diagnostic errors (36).
Study Limitations
The limitation of our study was that MRI reports sent from different centers, taken with different quality devices, and interpreted by different radiologists, may have affected our false-negative results.
Conclusion
In our study, we found that standard tests, except for morning cortisol level, were effective in proving the presence of CS. The reliability of the high-dose dexamethasone suppression test was confirmed in our cases, since CD was found in all cases with a positive response to this test. These results support that BIPSS is a superior diagnostic work-up compared with MRI to confirm the diagnosis of CD. Moreover, in line with previous studies, BIPSS was shown to provide better information about adenoma location, which is vital for possible surgical intervention.
Footnotes
Ethics
Ethics Committee Approval: The study were approved by the Tepecik Training and Research Hospital of Local Ethics Committee (decision no: 10, date: 04.05.2015).
Informed Consent: Retrospective study.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: Oya Ercan, Bumin Dündar, Gönül Çatlı, Design: Oya Ercan, Bumin Dündar, Gönül Çatlı, Hande Turan, Data Collection or Processing: Hande Turan, Gönül Çatlı, Aslı Derya Kardelen, Ece Böber, Ayşehan Akıncı, Semra Çetinkaya, Özgecan Demirbaş, Eren Er, Saadet Olcay Evliyaoğlu, Bumin Dündar, Oya Ercan, Analysis or Interpretation: Hande Turan, Oya Ercan, Literature Search: Oya Ercan, Bumin Dündar, Gönül Çatlı, Hande Turan, Writing: Hande Turan, Gönül Çatlı, Bumin Dündar, Oya Ercan.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Stratakis CA. Cushing Syndrome in Pediatrics. Endocrinol Metab Clin North Am. 2012;41:793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386:913–927. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 3.Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing’s disease. J Clin Endocrinol Metab. 2013;98:892–901. doi: 10.1210/jc.2012-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev. 2017;38:3–45. doi: 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro L, Elahi S, Riddoch F, Perry LA, Martin L, Akker SA, Monson JP, Drake WM, Grossman AB, Savage MO, Storr HL. Investigation for Paediatric Cushing’s Syndrome Using Twenty-Four-Hour Urinary Free Cortisol Determination. Horm Res Paediatr. 2016;86:21–26. doi: 10.1159/000446913. [DOI] [PubMed] [Google Scholar]
- 6.Stratakis CA. Cushing Syndrome in Pediatrics. Endocrinol Metab Clin North Am. 2012;41:793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic Tests for Children Who Are Referred for the Investigation of Cushing Syndrome. Pediatrics. 2007;120:575–586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 8.Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94:3121–3131. doi: 10.1210/jc.2009-0612. [DOI] [PubMed] [Google Scholar]
- 9.Findling JW, Raff H, Aron DC. The Low-Dose Dexamethasone Suppression Test: A Reevaluation in Patients with Cushing’s Syndrome. J Clin Endocrinol Metab. 2004;89:1222–1226. doi: 10.1210/jc.2003-030207. [DOI] [PubMed] [Google Scholar]
- 10.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB Jr, Loriaux DL. Petrosal Sinus Sampling with and without Corticotropin-Releasing Hormone for the Differential Diagnosis of Cushing’s Syndrome. N Engl J Med. 991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 11.Pecori Giraldi F, Cavallo LM, Tortora F, Pivonello R, Colao A, Cappabianca P, Mantero F; Altogether to Beat Cushing’s Syndrome Group. The role of inferior petrosal sinus sampling in ACTH-dependent Cushing’s syndrome: review and joint opinion statement by members of the Italian Society for Endocrinology, Italian Society for Neurosurgery, and Italian Society for Neuroradiology. Neurosurg Focus. 2015;38:5. doi: 10.3171/2014.11.FOCUS14766. [DOI] [PubMed] [Google Scholar]
- 12.Zampetti B, Grossrubatscher E, Dalino Ciaramella P, Boccardi E, Loli P. Bilateral inferior petrosal sinus sampling. Endocr Connect. 2016;5:12–25. doi: 10.1530/EC-16-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lienhardt A, Grossman AB, Dacie JE, Evanson J, Huebner A, Afshar F, Plowman PN, Besser GM, Savage MO. Relative contributions of inferior petrosal sinus sampling and pituitary imaging in the investigation of children and adolescents with ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2001;86:5711–5714. doi: 10.1210/jcem.86.12.8086. [DOI] [PubMed] [Google Scholar]
- 14.Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB Jr, Nieman LK, Chrousos GP. Cushing’s Syndrome in Children and Adolescents. Presentation, Diagnosis, and Therapy. N Engl J Med. 1994;331:629–636. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 15.Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J Clin Endocrinol Metab. 2013;98:2285–2293. doi: 10.1210/jc.2012-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moszczyńska E, Marczak E, Szalecki M, Kądziołka K, Roszkowski M, Zagata-Lesnicka P. The Effects of Sampling Lateralization on Bilateral Inferior Petrosal Sinus Sampling for Pediatric Cushing’s Disease-A Single Endocrinology Centre Experience and Review of the Literature. Front Endocrinol. 2021;12:650967. doi: 10.3389/fendo.2021.650967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batista D, Gennari M, Riar J, Chang R, Keil MF, Oldfield EH, Stratakis CA. An Assessment of Petrosal Sinus Sampling for Localization of Pituitary Microadenomas in Children with Cushing Disease. J Clin Endocrinol Metab. 2006;91:221–224. doi: 10.1210/jc.2005-1096. [DOI] [PubMed] [Google Scholar]
- 18.Storr HL, Alexandraki KI, Martin L, Isidori AM, Kaltsas GA, Monson JP, Besser GM, Matson M, Evanson J, Afshar F, Sabin I, Savage MO, Grossman AB. Comparisons in the epidemiology, diagnostic features and cure rate by transsphenoidal surgery between paediatric and adult-onset Cushing’s disease. Eur J Endocrinol. 2011;164:667–674. doi: 10.1530/EJE-10-1120. [DOI] [PubMed] [Google Scholar]
- 19.Deipolyi AR, Alexander B, Rho J, Hirsch JA, Oklu R. Bilateral inferior petrosal sinus sampling using desmopressin or corticotropic-releasing hormone: a single-center experience. J Neurointerv Surg. 2015;7:690–693. doi: 10.1136/neurintsurg-2014-011262. [DOI] [PubMed] [Google Scholar]
- 20.Feng M, Liu Z, Liu X, Zhang X, Bao X, Yao Y, Deng K, Xing B, Lian W, Zhu H, Lu L, Wang R. Tumour lateralization in Cushing’s disease by inferior petrosal sinus sampling with desmopressin. Clin Endocrinol (Oxf) 2018;88:251–257. doi: 10.1111/cen.13505. [DOI] [PubMed] [Google Scholar]
- 21.Kaskarelis IS, Tsatalou EG, Benakis SV, Malagari K, Komninos I, Vassiliadi D, Tsagarakis S, Thalassinos N. Bilateral inferior petrosal sinuses sampling in the routine investigation of Cushing’s syndrome: a comparison with MRI. AJR Am J Roentgenol. 2006;187:562–570. doi: 10.2214/ajr.05.0557. [DOI] [PubMed] [Google Scholar]
- 22.Tabarin A, Laurent F, Catargi B, Olivier-Puel F, Lescene R, Berge J, Galli FS, Drouillard J, Roger P, Guerin J. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing’s disease. Clin Endocrinol (Oxf) 1998;49:293–300. doi: 10.1046/j.1365-2265.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 23.T Testa RM, Albiger N, Occhi G, Sanguin F, Scanarini M, Berlucchi S, Gardiman MP, Carollo C, Mantero F, Scaroni C. The usefulness of combined biochemical tests in the diagnosis of Cushing’s disease with negative pituitary magnetic resonance imaging. Eur J Endocrinol. 2007;156:241–248. doi: 10.1530/eje.1.02332. [DOI] [PubMed] [Google Scholar]
- 24.Colao A, Faggiano A, Pivonello R, Pecori Giraldi F, Cavagnini F, Lombardi G; Study Group of the Italian Endocrinology Society on the Pathophsiology of the Hypothalamic-Pituitary-Adrenal Axis. Inferior petrosal sinus sampling in the differential diagnosis of Cushing’s syndrome: Results of an Italian multicenter study. Eur J Endocrinol. 2001;144:499–507. doi: 10.1530/eje.0.1440499. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli FS, Huston J, Carpenter PC, Erickson D, Young WF Jr, Meyer FB. Adrenocorticotropic hormone-dependent Cushing’s syndrome: Sensitivity and specificity of inferior petrosal sinus sampling. AJNR Am J Neuroradiol. 2000;21:690–696. [PMC free article] [PubMed] [Google Scholar]
- 26.Moszczyńska E, Marczak E, Szalecki M, Kądziołka K, Roszkowski M, Zagata-Lesnicka P. The Effects of Sampling Lateralization on Bilateral Inferior Petrosal Sinus Sampling for Pediatric Cushing’s Disease—A Single Endocrinology Centre Experience and Review of the Literature. Front Endocrinol 2021;12. Available from: [Internet] https://www.frontiersin.org/articles/10.3389/fendo.2021.650967/full. [DOI] [PMC free article] [PubMed]
- 27.Deipolyi A, Oklu R. Bilateral inferior petrosal sinus sampling in the diagnosis of Cushing disease. Journal of Vascular Diagnostics. 2015;3:1–7. [Google Scholar]
- 28.Doppman JL, Oldfield E, Krudy AG, Chrousos GP, Schulte HM, Schaaf M, Loriaux DL. Petrosal sinus sampling for Cushing syndrome: anatomical and technical considerations. Work in progress. Radiology. 1984;150:99–103. doi: 10.1148/radiology.150.1.6316418. [DOI] [PubMed] [Google Scholar]
- 29.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB Jr, Loriaux DL. Petrosal Sinus Sampling with and without Corticotropin-Releasing Hormone for the Differential Diagnosis of Cushing’s Syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Chen K, Lu L, Zhang X, Tong A, Pan H, Zhu H, Lu Z. The effects of sampling lateralization on bilateral inferior petrosal sinus sampling and desmopressin stimulation test for pediatric Cushing’s disease. Endocrine. 2019;63:582–591. doi: 10.1007/s12020-018-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalcante LBCP, Freitas TC, Musolino NRC, Cescato VAS, Silva GO, Fragoso MCBV, Puglia P Jr, Bronstein MD, Machado MC. High accuracy of bilateral and simultaneous petrosal sinus sampling with desmopressin for the differential diagnosis of pediatric ACTH-dependent Cushing’s syndrome. Pituitary. 2020;23:507–514. doi: 10.1007/s11102-020-01051-1. [DOI] [PubMed] [Google Scholar]
- 32.Machado MC, Fragoso MC, Moreira AC, Boguszewski CL, Vieira L Neto, Naves LA, Vilar L, Araújo LA, Czepielewski MA, Gadelha MR, Musolino NR, Miranda PA, Bronstein MD, Ribeiro-Oliveira A Jr. Recommendations of the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism for the diagnosis of Cushing’s disease in Brazil. Arch Endocrinol Metab. 2016;60:267–286. doi: 10.1590/2359-3997000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barros APT, Lamback EB, Coelho MCA, Neto LV. Limitations of Basal Cortisol in the Diagnosis of Cushing Syndrome. AACE Clin Case Rep. 2019;5:91–94. doi: 10.4158/ACCR-2018-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.More J, Young J, Reznik Y, Raverot G, Borson-Chazot F, Rohmer V, Baudin E, Coutant R, Tabarin A; Groupe Français des Tumeurs Endocrines (GTE) Ectopic ACTH Syndrome in Children and Adolescents. J Clin Endocrinol Metab. 2011;96:1213–1222. doi: 10.1210/jc.2010-2276. [DOI] [PubMed] [Google Scholar]
- 36.Swearingen B, Katznelson L, Miller K, Grinspoon S, Waltman A, Dorer DJ, Klibanski A, Biller BM. Diagnostic Errors after Inferior Petrosal Sinus Sampling. J Clin Endocrinol Metab. 2004;89:3752–3763. doi: 10.1210/jc.2003-032249. [DOI] [PubMed] [Google Scholar]
- 37.Shah NS, George J, Acharya SV, Lila AR, Sarathi V, Bandgar TR, Jalali R, Goel AH, Menon P. Cushing Disease in Children and Adolescents: Twenty Years’ Experience in A Tertiary Care Center in India. Endocr Pract. 2011;17:369–376. doi: 10.4158/EP10143.OR. [DOI] [PubMed] [Google Scholar]
- 38.Mamelak AN, Dowd CF, Tyrrell JB, McDonald JF, Wilson CB. Venous angiography is needed to interpret inferior petrosal sinus and cavernous sinus sampling data for lateralizing adrenocorticotropin-secreting adenomas. J Clin Endocrinol Metab. 1996;81:475–481. doi: 10.1210/jcem.81.2.8636253. [DOI] [PubMed] [Google Scholar]
- 39.Doppman JL, Chang R, Oldfield EH, Chrousos G, Stratakis CA, Nieman LK. The Hypoplastic Inferior Petrosal Sinus: A Potential Source of False-Negative Results in Petrosal Sampling for Cushing’s Disease. J Clin Endocrinol Metab. 1999;84:533–540. doi: 10.1210/jcem.84.2.5475. [DOI] [PubMed] [Google Scholar]
- 40.Miller DL, Doppman JL. Petrosal sinus sampling: technique and rationale. Radiology. 1991;178:37–47. doi: 10.1148/radiology.178.1.1845785. [DOI] [PubMed] [Google Scholar]


