Abstract
It has been established that blacks have higher overall incidence and prevalence of hypertension compared to their white counterparts. However, the maximum blood pressure (BP) response of blacks to exercise has not been characterized. A total of 5996 apparently healthy men from the Fitness Registry and Importance of Exercise: A National Database (FRIEND) who underwent maximum cardiopulmonary exercise tests on a cycle ergometer were included in this analysis. Of these participants, 1245 (21%) self-identified as black while the remaining 4751 (79%) identified as white. All subjects had a respiratory exchange ratio (RER) of ≥1.0 and had no reports of cardiovascular or pulmonary disease. Systolic BP (BP) response to exercise was indexed according to increase in workload (SBP/MET-slope). Both racial groups were subdivided into age groups by decade. Black men had higher peak SBP and higher SBP/MET-slopes compared to white men across all age groups (p < 0.001). Resting SBP was not different between blacks and whites except within the 18–29-year age group. The differences in peak SBP and SBP/MET-slope between age and race groups indicate that black men have an exaggerated BP response to exercise irrespective of resting BP values. Further investigation is warranted to determine the underlying mechanisms responsible and clinical implications for this exaggerated BP response to exercise.
Introduction
The hemodynamic response to exercise is an integral component of cardiopulmonary exercise testing (CPX) [1, 2]. One of the main values of exercise testing is that the added physiological stress of exercise can uncover masked cardiovascular and hemodynamic pathologies that otherwise would remain unidentified. Furthermore, it has been shown that the hemodynamic response to exercise can provide useful clinical information, both diagnostic and prognostic, independent of resting blood pressure (BP) [3–7].
The expected physiological BP response to dynamic upright exercise is an increase in systolic BP (SBP), no change or a slight drop in diastolic BP (DBP), and thus widening of the pulse pressure. During dynamic upright exercise, there is a rapid increase in cardiac output (CO), to meet the increase in metabolic demand, and a drop in total peripheral resistance (TPR) due to vasodilation. However, with increases in exercise intensity the gain in CO is greater than the decrease in TPR and thus SBP begins to rise. The average increase in SBP from rest to maximal exercise is generally from 55 to 65 mmHg in men and from 45 to 60 mmHg in women; however, these values vary considerably across the lifespan, and among other factors [8]. For DBP, women tend to show greater changes from baseline DBP than men during exercise (ΔDBP), with ΔDBP also increasing in magnitude along the lifespan [8].
Racial differences in prevalence and incidence of hypertension (HTN) have been widely appreciated. In the United States, the prevalence of HTN among blacks is significantly greater compared to their white counterparts. In 2011–2014, the age-adjusted prevalence of HTN was 45 and 46% in black males and females, respectively [9]. This amounts to about a 10–12% higher rate in blacks compared to whites [10]. Even when adjusting for variables such as body mass index (BMI), cigarette smoking, cardiorespiratory fitness (CRF), alcohol use, etc., blacks have a 1.5–2 times higher risk for HTN than whites [11].
From a prognostic standpoint, individuals who exhibit an exaggerated BP response to exercise, also known as exercise HTN, have been shown to possess a 1.4–3.0-fold higher relative risk for cardiovascular events compared to subjects with a normal BP response to exercise [12]. As such, it becomes essential to appreciate that many factors contribute to the overall BP response to exercise. These include resting BP levels, obesity, sex, race/ethnicity, and mode of exercise (treadmill vs cycle ergometer), among other factors. To enhance the clinical application of exercise BP as a screening tool, there is a need to determine normative BP values that consider these factors. While the normative exercise BP values in a predominately white cohort of North American men and women undergoing symptom-limited CPX on a treadmill was recently published, the characterization of exercise BPs in black men has not previously been addressed [8]. Therefore, the purpose of the current study was to determine the normative BP response to exercise in a cohort of black and white North American men using cycle ergometer exercise. In addition, we sought to test the hypothesis that black men have an exaggerated BP response during graded exercise test compared to white men across the age lifespan.
Methods
The Fitness Registry and Importance of Exercise: A National Database (FRIEND) was established in response to an American Heart Association Policy Statement [13] as a multi-institute initiative with the purpose of establishing normative CRF values in the United States across the adult lifespan. Details of the FRIEND registry have been previously published [14, 15]. Data were gathered from multiple CPX laboratories across the United States (see “Acknowledgments”). All exercise testing laboratories contributing to the FRIEND database were performing CPX and employing experienced qualified personnel to conduct exercise tests, consistent with recommendations provided in recently published guidelines [16, 17].
Participating laboratories were responsible for obtaining and presenting local institutional review board (IRB) approval for inclusion in the FRIEND registry before submitting de-identified, coded data to the FRIEND Core Data Coordinating Center at Ball State University prior to transferring the data to the core CPX laboratory housed at the University of Illinois at Chicago (UIC). IRB approval for the core CPX laboratory was also obtained at UIC.
All submitted data were reviewed for uniformity prior to inclusion in the registry as previously described [14]. The core laboratory housed at UIC ensured that all data points were within physiologic ranges. In the event data were not within the expected normal range, or other errors were identified, the CPX laboratory submitting the data was contacted for clarification, validation, and correction if needed.
Cohort and study data points
The current FRIEND database included a total of 105,253 exercise tests from multiple geographic locations. We applied strict exclusion criteria to only include participants free from any apparent disease that racially identified as black or white. All other racial groups were excluded for the purpose of this analysis. We only included exercise tests performed on the cycle ergometer (Fig. 1). Exercise tests were indicated for determination of CRF either in the clinical or research setting. Participant screening for testing contraindications and risk stratification were specific for each laboratory [14, 18]. Inclusion criteria were: (1) black and white participants age 18–75 years; (2) maximal CPX performed on a cycle ergometer; and (3) peak SBP values available; (4) peak V̇O2 values available; (5) peak respiratory exchange ratio (RER) of ≥1.00 [14]. Exclusion criteria included: (1) any tests that were terminated for abnormal clinical findings prior to achieving voluntary maximal effort; (2) the presence of CV or pulmonary disease (e.g., HTN, coronary artery disease, peripheral artery disease, heart failure, pulmonary disease, etc.); (3) the presence of other disease (e.g., diabetes, cancer); (4) use of any medication (e.g., ß-blockers or calcium-channel blockers, etc.); and (5) any drop in SBP during exercise (Fig. 1) [14]. Participants with BMI ≥ 30 were not excluded. Peak SBP values submitted to the FRIEND registry were collected in the final stage of exercise according to guidelines. Baseline hemodynamic variables were collected in the seated position after a brief period of rest, according to guidelines. All BP measurements were collected manually using standard auscultatory methods according to current guidelines [16, 19]. All CPX tests were conducted between September 2010 and February 2019. Due to the small sample size of females after applying exclusion criteria (white females, n = 1046; black females, n = 189) the analysis was made exclusive to male participants. The final sample for the current analysis included 5996 cycle ergometer exercise tests from multiple geographic locations within the United States (see “Acknowledgments”).
Fig. 1. Study flowchart.
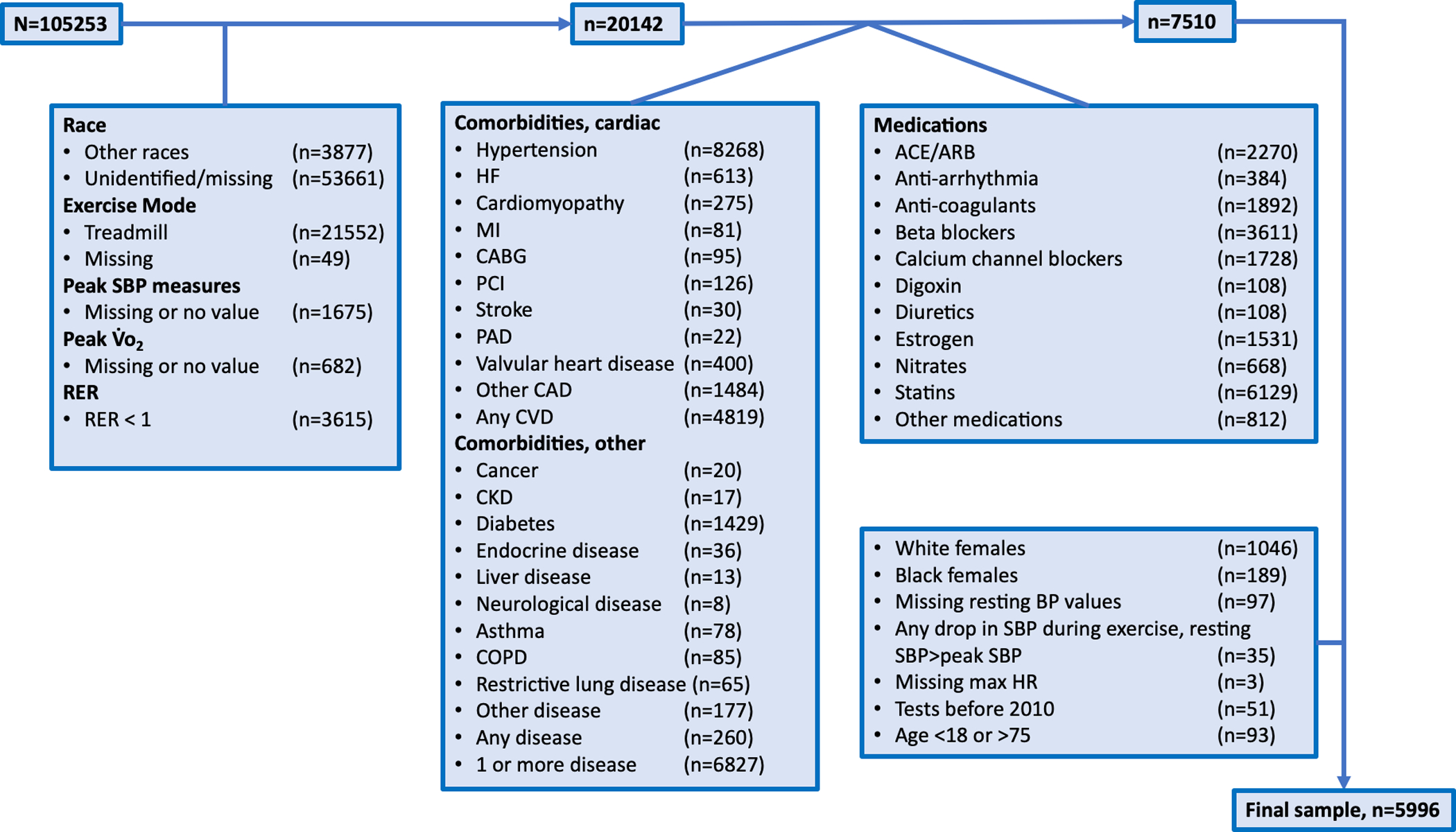
Boxes indicate specific exclusions and their numbers. RER respiratory exchange ratio; HF heart failure; MI myocardial infarction; CABG coronary artery bypass graft; PCI percutaneous coronary intervention; PAD peripheral artery disease; CAD coronary artery disease; CVD cardiovascular disease; ACE angiotensin converting enzyme inhibitors; ARB angiotensin-receptor blockers; BP blood pressure; SBP systolic BP; HR heart rate.
Peak SBP, peak V̇O2, and the SBP/MET-slope were the primary variables used for analysis. The SBP/MET-slope was calculated as the change in SBP (ΔSBP) from rest to peak values and divided by the increase in METs from rest to peak [(peak SBP—resting SBP)/(peak MET-1)]. The MET value was calculated directly from derived peak V̇O2 rather than estimated using prediction equations.
Participants without resting BP or peak SBP reported from the CPX were not included in the analysis. The testing protocol used variable adjustments in workload based upon each individual’s estimated fitness level. The goal was to elicit a maximal effort within 8–12 min in accordance with guideline recommendations [20].
Statistical analysis
Data are presented as mean ± standard deviation (SD) unless noted otherwise. Independent t-tests were used to compare means between blacks and whites. Two-way analysis of variance (ANOVA) was used to compare differences in BP values between race and across age groups. Age groups were presented by decades and 15-year intervals at the tails to allow for sufficient sample sizes within each group. When significant interactions were detected by ANOVA, analysis of simple main effects for race and age group were performed with a Bonferroni adjustment accepted at the p < 0.025 level. To understand the role of multiple covariates on the BP response a hierarchical multiple regression analysis was performed. SPSS statistical software, version 26.0 (SPSS Inc.) was used for all analyses and R studio (version 1.1.453) was used for generating graphs. All tests with a p < 0.05 were considered statistically significant.
Results
The current cohort included a total of 5996 male participants between the ages of 18 and 75 years. Of these participants, 1245 (21%) self-identified as black while the remaining 4751 (79%) identified as white. Descriptive statistics of the cohort are presented in Table 1. Descriptive statistics as well as cardiovascular and pulmonary responses to maximal exercise grouped by race and age groups are presented in Table 2. Range of peak SBP values was between 100 and 280 mmHg. Race and age-group specific percentiles for peak SBP and SBP/MET-slope are presented in Supplemental Table 1.
Table 1.
Descriptive statistics for baseline variables, hemodynamic, and cardiorespiratory fitness responses to maximum exercise on a cycle ergometer for black and white American men. Data presented as mean ± SD along with results of independent t tests.
| Blacks (n = 1245) |
Whites (n = 4751) |
||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p |
| Age (y) | 42.2 | 10.8 | 42.9 | 11.3 | 0.052 |
| Height (cm) | 178.9 | 7.6 | 179.8 | 6.9 | <.001* |
| Weight (kg) | 89.1 | 14.3 | 88.9 | 14.1 | 0.667 |
| BMI (kg/m2) | 27.8 | 3.9 | 27.5 | 3.9 | .01* |
| Resting HR (bpm) | 74.8 | 11.5 | 75.2 | 12.2 | 0.389 |
| Resting SBP (mmHg) | 120.2 | 13.3 | 119.5 | 11.9 | 0.073 |
| Resting DBP (mmHg) | 78.9 | 9 | 77.5 | 8.4 | <.001* |
| Max HR (bpm) | 151.2 | 17.3 | 156.4 | 16.5 | <.001* |
| Peak SBP (mmHg) | 179 | 26.9 | 174.7 | 22.8 | <.001* |
| Δ SBP (mmHg) | 58.7 | 23.3 | 55.2 | 20.8 | <.001* |
| Absolute V̇o2peak (L·min−1) | 2.08 | 0.49 | 2.53 | 0.61 | <.001* |
| Relative V̇o2peak (ml·kg−1·min−1) | 23.6 | 5.3 | 28.8 | 7.2 | <.001* |
| Max RER (V̇co2/V̇o2) | 1.15 | 0.1 | 1.13 | 0.08 | <.001* |
| Peak O2 Pulse (ml/beat) | 13.8 | 2.92 | 16.2 | 3.5 | <.001* |
| SBP/MET-slope (mmHg/MET) | 10.8 | 5.1 | 8.2 | 3.7 | <.001* |
Table 2.
Descriptive, baseline variables, hemodynamic, and cardiorespiratory fitness responses to maximum exercise on a cycle ergometer for black and white American mean stratified by race and age group. Data presented as mean ± SD along with results of two-way ANOVA.
| Age (y) | 24.4 ± 3.5 | 24.6 ± 3.2 | 34.9 ± 2.8 | 34.8 ± 2.8 | 44.5 ± 2.7 | 44.6 ± 2.8 | 53.6 ± 2.8 | 53.7 ± 2.8 | 63.7 ± 3.5 | 64.3 ± 3.6 | |
| Interaction (p=.674) | |||||||||||
| Height (cm) | 178.3 ± 7.7 | 180.1 ± 7.2 | 178.6 ± 7.7 | 179.8 ± 7.0 | 178.7 ± 7.6 | 179.8 ± 6.8 | 179.5 ± 7.1 | 179.5 ± 6.7 | 181.4 ± 8.6 | 179.7 ± 6.4 | |
| Interaction (p=.007)* | |||||||||||
| Weight (kg) | 83.1 ± 15.7 | 85.1 ± 15.8 | 89.5 ± 14.5 | 89.6 ± 14.3 | 91.4 ± 13.4 | 90.4 ± 13.9 | 89.7 ± 13.7 | 89.2 ± 12.8 | 90.0 ± 11.4 | 85.8 ± 12.6 | |
| Interaction (p=.052) | |||||||||||
| BMI (kg/m2) | 26.1 ± 4.4 | 26.2 ± 4.4 | 28.0 ± 3.9 | 27.7 ± 3.8 | 28.6 ± 3.6 | 27.9 ± 3.8 | 27.8 ± 3.7 | 27.8 ± 3.7 | 27.3 ± 3.0 | 27.3 ± 3.0 | |
| Interaction (p=.202) | |||||||||||
| Resting SBP (mmHg) | 115 ± 12 | 117 ± 11 | 119 ± 12 | 118 ± 11 | 120 ± 13 | 120 ± 12 | 123 ± 14 | 122 ± 13 | 128 ± 14 | 124 ± 13 | |
| Interaction (p=.02)* | |||||||||||
| Resting DBP (mmHg) | 76 ± 9 | 75 ± 8 | 78 ± 9 | 77 ± 8 | 80 ± 9 | 78 ± 9 | 80 ± 9 | 79 ± 8 | 81 ± 8 | 78 ± 8 | |
| Interaction (p=.14) | |||||||||||
| Resting HR (bpm) | 75 ± 11 | 78 ± 13 | 76 ± 12 | 75 ± 12 | 75 ± 12 | 75 ± 12 | 74 ± 12 | 75 ± 12 | 72 ± 11 | 72 ± 12 | |
| Interaction (p=.035)* | |||||||||||
| Peak SBP (mmHg) | 167 ± 24 | 167 ± 21 | 176 ± 26 | 171 ± 21 | 182 ± 26 | 176 ± 22 | 184 ± 27 | 180 ± 24 | 187 ± 27 | 182 ± 24 | |
| Interaction (p=.12) | |||||||||||
| Δ SBP (mmHg) | 52 ± 21 | 50 ± 20 | 57 ± 23 | 53 ± 21 | 62 ± 24 | 56 ± 21 | 61 ± 23 | 59 ± 22 | 58 ± 25 | 58 ± 20 | |
| Interaction (p=.208) | |||||||||||
| Max HR (bpm) | 158 ± 16 | 168 ± 15 | 155 ± 17 | 162 ± 14 | 150 ± 17 | 155 ± 15 | 147 ± 16 | 150 ± 15 | 139 ± 17 | 143 ± 16 | |
| Interaction (p<.001)* | |||||||||||
| Absolute V̇o2 (L·min−1) | 2.23 ± 0.55 | 2.74 ± 0.67 | 2.19 ± 0.52 | 2.7 ± 0.62 | 2.07 ± 0.46 | 2.53 ± 0.56 | 1.96 ± 0.42 | 2.34 ± 0.53 | 1.8 ± 0.39 | 2.11 ± 0.49 | |
| Interaction (p=.016)* | |||||||||||
| Relative V̇o2 (ml·kg−1·min−1) | 27.0 ± 5.8 | 32.8 ± 8.2 | 24.6 ± 5.4 | 30.5 ± 7.0 | 22.8 ± 4.7 | 28.3 ± 6.6 | 22.0± 4.4 | 26.5 ± 6.1 | 20.2 ± 4.6 | 24.9 ± 6.0 | |
| Interaction (p=.124) | |||||||||||
| Max RER | 1.13 ± 0.09 | 1.13 ± 0.08 | 1.16 ± 0.1 | 1.13 ± 0.07 | 1.16 ± 0.1 | 1.13 ± 0.07 | 1.15 ± 0.09 | 1.13 ± 0.08 | 1.15 ± 0.1 | 1.12 ± 0.08 | |
| Interaction (p=.001)* | |||||||||||
| Peak O2 Pulse (ml/beat) | 14.1 ± 3.1 | 16.3 ± 3.7 | 14.1 ± 3.3 | 16.7 ± 3.7 | 13.8 ± 2.8 | 16.4 ± 3.5 | 13.4 ± 2.6 | 15.6 ± 3.3 | 12.9 ± 2.4 | 14.7 ± 3.0 | Age Group (p<.001)* |
| Race (p<.001)* | |||||||||||
| Interaction (p=.321) | |||||||||||
| SBP/MET-slope (mmHg/MET) |
8.07 ± 3.82 | 6.35 ± 2.93 | 9.81 ± 4.55 | 7.25 ± 3.25 | 11.63 ± 4.92 | 8.38 ± 3.59 | 12.14 ± 5.51 | 9.36 ± 3.93 | 13.02 ± 6.41 | 10.1 ± 4.18 | |
| Interaction (p=.002)* |
Results of the two-way ANOVA indicated a significant interaction between race and age group on resting SBP (p = 0.02). Analysis of simple main effects for race indicated that differences between both groups only existed in the 60–75-year age group (p = 0.003). Differences between all other groups were nonsignificant. Pairwise comparisons revealed that blacks had a 5 mmHg (95% CI, 2–8 mmHg) SBP value greater than whites in this age group (60–75 years) (Supplemental Table 2). There was also a significant interaction of race and age group on resting HR (p = 0.035) with simple main effects analysis showing differences only within the 18–29-year age group (p = 0.002). Blacks had resting HR values that were 3 bpm (95% CI, 1–5 bpm) lower than whites within this age group (Supplemental Table 2). Similarly, there was a significant interaction on maximum HR (p < 0.001) with simple main effects analysis showing significant differences with the 18–29, 30–39, 40–49, and 50–59-year age groups (Supplemental Table 2). There were no significant interactions for peak SBP or ΔSBP (p = 0.12 and p = 0.208, respectively); however, there were significant mains effects for race on both variables (p < 0.001) (Table 2). Significant interactions were noted for absolute V̇O2peak between groups (p = 0.016). Analysis of simple main effects revealed that there were significant differences in absolute V̇O2peak between both races in all five age groups (p < 0.001) (Supplemental Table 2). Although there were no significant interactions for relative V̇O2peak between groups, there was a significant main effect for race (p < 0.001) (Table 2). There was also a significant interaction for max RER between groups (p = 0.001) with differences between both races within the 30–39, 40–49, 50–59, and 60–75-year age groups (Supplemental Table 2). When considering the SBP/MET-slope a positive interaction between race and age-group was found (p = 0.002). Analysis of simple main effects revealed significant differences between all five age groups (p < 0.001). Pairwise comparisons for all significant interactions are presented in (Supplemental Table 2). Adjusted marginal means for hemodynamic and CRF variables are presented in (Supplemental Table 3) and graphically represented in Fig. 2.
Fig. 2. Blood pressure responses at rest and maximum exercise using a cycle ergometer in black and white men.
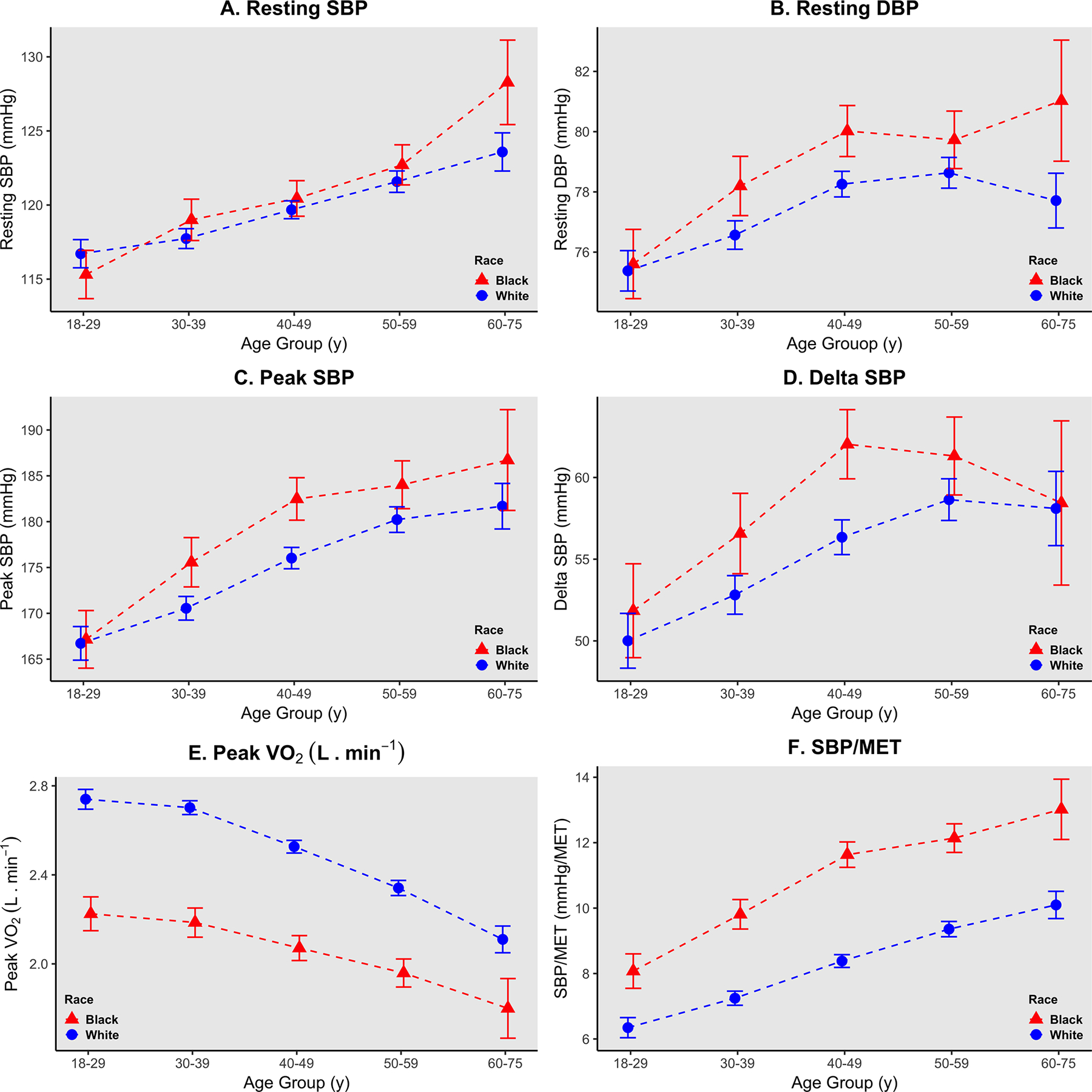
Data points indicate adjusted marginal mean values and error bars indicate 95% confidence intervals. a, b Resting systolic and diastolic blood pressures (SBP, DBP, mmHg). c Peak exercise systolic blood pressures (SBP, mmHg). d Delta systolic blood pressure defined as (peak value—resting value, mmHg). e peak oxygen consumption (L·min−1) in black and white American men. f SBP/MET-slope (mmHg/MET).
Hierarchical multiple regression models were run to determine if the addition of BMI, resting SBP, resting DBP, max HR, V̇O2peak (L·min−1), and race obtained from CPX improved the prediction of peak SBP or SBP/MET-slope over and above age alone (Table 3). For peak SBP, the full model of age, BMI, resting SBP, resting DBP, absolute V̇O2peak, max HR, and race was significant (R2 = 0.283, p < 0.001; adjusted R2 = 0.282). All added variables were found to be statistically significant (p < 0.001). Black men were found to have peak SBP that were 8 mmHg (95% CI, 6–9 mmHg) higher than their white counterparts. When predicting the SBP/MET relationship using the same models, the final model was also significant (R2 = 0.317, p < 0.001; adjusted R2 = 0.316). All added variables were found to be statistically significant (p < 0.001). Black men were found to have an SBP/MET-slope that is 1.63 mmHg/MET (95% CI, 1.4–1.86 mmHg/MET) higher than their white counterparts.
Table 3.
Summary of hierarchical multiple regression models using peak SBP and SBP/MET-slope as dependent variables. B, unstandardized coefficients.
| Peak SBP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |||||
| Constant | 156.230 | 153.942 | 158.518 | 48.794 | 42.563 | 55.025 | 3.785 | −4.615 | 12.186 | −1.127 | −9.488 | 7.234 |
| Age | 0.453 | 0.402 | 0.505 | 0.280 | 0.233 | 0.328 | 0.468 | 0.417 | 0.519 | 0.505 | 0.454 | 0.555 |
| Resting SBP | 0.670 | 0.615 | 0.724 | 0.646 | 0.593 | 0.699 | 0.646 | 0.594 | 0.699 | |||
| Resting DBP | 0.240 | 0.163 | 0.317 | 0.251 | 0.175 | 0.326 | 0.233 | 0.159 | 0.308 | |||
| BMI | 0.582 | 0.440 | 0.723 | 0.490 | 0.349 | 0.632 | 0.425 | 0.285 | 0.566 | |||
| Max HR | 0.192 | 0.155 | 0.228 | 0.197 | 0.161 | 0.233 | ||||||
| V̇o2peak (L·min−1) | 4.845 | 3.883 | 5.807 | 6.487 | 5.492 | 7.482 | ||||||
| Race (Black=1) | 7.565 | 6.234 | 8.897 | |||||||||
| R 2 | 0.047 | 0.227 | 0.268 | 0.283 | ||||||||
| Adj. R2 | 0.047 | 0.227 | 0.267 | 0.282 | ||||||||
| SBP/MET-slope | ||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |||||
| Constant | 4.230 | 3.832 | 4.627 | −2.623 | −3.762 | −1.483 | 1.245 | −0.214 | 2.704 | 0.185 | −1.258 | 1.629 |
| Age | 0.105 | 0.096 | 0.114 | 0.103 | 0.094 | 0.112 | 0.073 | 0.064 | 0.082 | 0.081 | 0.072 | 0.089 |
| Resting SBP | −0.054 | −0.064 | −0.044 | −0.049 | −0.059 | −0.040 | −0.049 | −0.058 | −0.040 | |||
| Resting DBP | 0.066 | 0.052 | 0.080 | 0.050 | 0.037 | 0.063 | 0.046 | 0.033 | 0.059 | |||
| BMI | 0.299 | 0.273 | 0.325 | 0.382 | 0.357 | 0.406 | 0.368 | 0.343 | 0.392 | |||
| Max HR | 0.015 | 0.008 | 0.021 | 0.016 | 0.010 | 0.022 | ||||||
| V̇o2peak (L·min−1) | −2.637 | −2.804 | −2.470 | −2.283 | −2.455 | −2.111 | ||||||
| Race (Black=1) | 1.632 | 1.402 | 1.862 | |||||||||
| R 2 | 0.08 | 0.174 | 0.294 | 0.317 | ||||||||
| Adj. R2 | 0.08 | 0.173 | 0.294 | 0.316 | ||||||||
Discussion
The present study provides a current reference and comparative analysis of SBP at peak exercise in black and white American men. We show that black men have higher peak SBP responses to cycle ergometer exercise than their white counterparts. This was true despite there being no differences in resting SBP between blacks and whites except within the 60–75 years age group (Supplemental Table 2). This is also evident from the multiple regression models predicting an 8 mmHg difference in peak SBP when controlling for other factors such as age, BMI, resting BP, maximum HR, and CRF. However, our model only accounted for 28% of the variability (R2 = 0.283). We also showed that black men have a higher SBP/MET-slope across all age groups with a predicted difference of 1.63 mmHg/MET when controlling for age, BMI, resting BP, maximum HR, and CRF between both race groups. The model accounted for 32% of the variability (R2 = 0.317). Although race was found to be significant in all models, the small effect size suggests that there are other variables unaccounted for that are likely behind these differences.
In our cohort, without considering age group, black men showed statistically significant differences in height, BMI, and resting DBP (Table 1). However, when considering race and age group together, height was only different between races in the first three age groups (Supplemental Table 2). There was no interaction for BMI and resting DBP between groups, but there were significant main effects for race (Table 2). CRF levels indicated by V̇O2peak were also significantly different across both racial groups (Table 2). This is in agreement with previously published data [11, 21] that showed blacks to have higher rates of modifiable risk factors such as higher BMI and lower CRF levels than whites. This held true in our cohort as black men had slightly higher BMI values (27.8 ± 3.9 kg/m2) compared to white men (27.5 ± 3.9 kg/m2) (p = 0.01). BMI is a known risk factor and culprit for HTN [22, 23]; however, it is questionable whether the mean difference in BMI (0.3 kg/m2) observed in this cohort, albeit statistically significant, is physiologically or clinically significant.
The exact mechanisms behind the disparity in the BP response to exercise between blacks and whites seem to be multifactorial. Berry et al. [21] determined that blacks and whites had similar CV lifetime risk when their risk profiles were similar. They concluded that it is the presence or absence of traditional risk factors, and not race, which is the main determinant of long-term CVD risk. Recently published data from the CARDIA study [11] also found blacks to have higher rates of modifiable risk factors than whites. However, after multivariable adjustment blacks had 1.5–2 times the risk for HTN than whites, which indicates that other physiological mechanisms beside modifiable risk factors are contributing to the development of HTN in this population [11].
Pathophysiological mechanisms behind the high prevalence and incidence of HTN in blacks are multiple, but it has been shown that blacks have higher levels of endothelial dysfunction, oxidative stress, sympathetic overactivity and reduced functional sympatholysis, arterial stiffness, and lower nitric oxide bioavailability than whites [22, 24–30]. The vascular response to exercise training is also not identical between blacks and whites. It has been hypothesized that blacks can achieve greater reductions in central BP and improved arterial function with resistance exercise training than aerobic training while white individuals show greater improvements with aerobic training. Even so, Bond et al. [31] demonstrated that aerobic exercise training was successful in decreasing an exaggerated exercise BP response in 21 normotensive young African-American men. This reduction in the BP response was coupled with reductions in TPR supporting improved vasodilation or vascular compliance. It should be noted that the authors defined an exaggerated exercise BP response as a calculated change in SBP from rest to 50% V̇O2peak of greater than or equal to 50 mmHg [31]. A detailed exercise prescription guideline for the prevention of HTN and reduction of CV risk that takes into consideration racial and ethnic differences is not yet available and is an area of active research.
Despite blacks having higher average peak SBP values, we also show that white men had greater absolute and relative V̇O2peak values than black men. In our cohort black men expressed an average increase in SBP of 10.8 ± 5.1 mmHg/MET compared to an increase of 8.2 ± 3.7 mmHg/MET in white men (Table 1). Current guidelines consider the average increase in SBP to be 10 mmHg/MET [20, 32]. This finding indicates that the BP response per unit of metabolic rate is considerably higher in black than white men. The higher BP response per unit of metabolic rate observed in black men may be due to increases in TPR that are compensated by increases in BP to maintain adequate CO necessary for the increased workload. Both impaired vasodilation secondary to endothelial dysfunction [28] and reduced vascular compliance [30, 33] have been found to be significantly greater in blacks than whites, and would explain the greater BP response per unit of metabolic rate observed in our study. Generally speaking, functional sympatholysis has also been found to be reduced in patients with HTN [29], which is more predominant in blacks, and may possibly explain the greater BP response per unit of metabolic rate. In a group (n = 20) of young healthy black and white men, Barbosa et al. [34] showed that black men indeed had attenuated increases in forearm conductance with incremental forearm exercise. This was also true for forearm blood flow; however, mean arterial pressure was similar between groups. In the current study, blacks also showed lower peak O2 pulse values (a surrogate for stroke volume) [35] compared to whites (blacks: 13.8 ± 2.92 mL/beat, whites: 16.2 ± 3.5 mL/beat; p < 0.001). This finding could result from greater afterload and would be indicative of reduced peak stroke volume and thus reduced CO. Furthermore, the higher peak RER in blacks reported in our study (Table 1) is indicative of greater metabolic acidosis, and together with the reduced O2 pulse values may explain the lower peak V̇O2peak values as reported here. However, it has recently been shown that antihypertensive medication fails to abolish the exaggerated BP response to exercise in adults with HTN, even when resting levels are well controlled [36]. Whether BP-lowering medications would effectively reduce an exaggerated BP response in black men with normal resting BP remains to be determined. It is important to mention that the BP response to exercise indexed by workload was found to be a superior predictor of all-cause mortality than the peak SBP response [5, 6]. Hedman et al. recently showed that an SBP/MET-slope greater than 10 mmHg/MET was associated with 69% higher risk of 20-year all-cause mortality in unadjusted analysis and 20% higher risk in adjusted analyses [6]. In the current study, this value was surpassed starting from the 30–39-year age group and on in black men and was not achieved in white men until the 60–75-year age group. Given that there were no differences in resting SBP between these groups, the SBP response to exercise expressed as the SBP/MET-slope can potentially provide a useful prognostic indicator.
An exaggerated BP response to exercise has been defined as a reading that is ≥90th percentile from relative normative data [8, 37]. Other available definitions include readings ≥95th percentile, absolute values >210 mmHg for men and >190 mmHg for women, and increases >12 mmHg/MET [5]. However, these values appear to be arbitrarily defined and usually do not appreciate differences in sex, race and ethnicity, population, or exercise modality. Furthermore, 95% or more of normative data used to derive these values come from white or Caucasian populations [8, 38, 39]. Given the differences in the BP response to exercise between whites and blacks presented in this study; using most of the above mentioned definitions for an exaggerated BP response to exercise will entail that a greater number of blacks fall under the classification of exercise HTN. This would be in accordance with what we already know regarding the higher prevalence and incidence of HTN in blacks. Nonetheless, it is still to be determined if using these normative values obtained from predominantly white cohorts yield similar prognostic outcomes in blacks as they do in whites. The differences between blacks and whites presented in this study also stress the importance of establishing race-specific normative data for hemodynamic responses to exercise. In addition, further consideration must be given when interpreting results of nonwhite individuals. Furthermore, the results of this study emphasize the importance of monitoring and assessing exercise BP in black men during exercise testing.
Limitations
There were several limitations of our study. First, our cohort only included male participants and thus a study of female responses to maximal exercise across racial groups is still needed. Second, the current study only included participants who are under 75 years of age and did not include an analysis of BP responses of older individuals. Additionally, we only had 68 black participants in the 60–75-year age group. Third, values reported here only pertain to cycle ergometer exercise and should not be generalized to other forms of exercise. Lastly, while data included in the present study came from established and experienced CPX laboratories, the accuracy of manual auscultatory methods during exercise is subject to some degree of measurement error.
Conclusion
We believe that racial differences pose significant nuances that need to be considered when interpreting physiological data. Here we show that the BP response to maximal exercise is different between black and white American men. Black men show higher values of peak SBP as well as greater increases in SBP per workload during exercise (SBP/MET-slope) that are independent of resting BP values. Further investigation is warranted to determine the underlying mechanisms responsible for the exaggerated BP response to exercise in black compared to white men and the prognostic implications of these higher values in blacks compared to whites.
Supplementary Material
Summary table.
What is known about this topic
Black individuals have higher incidence and prevalence rates of hypertension compared to their white counterparts.
The peak BP response to exercise can serve as a prognostic marker of future CV disease.
What this study adds
The peak BP response to exercise is higher in black men compared to white men independent of resting BP values.
The SBP response per workload (SBP/MET-slope) is also higher in black men compared to white men independent of resting BP values.
Acknowledgements
FRIEND Consortium Contributors are as follows: Ball State University (Leonard Kaminsky, Matthew Harber), MET-TEST (Sundeep Chaudhry), Massachusetts General Hospital (Gregory Lewis). This study was supported in part by an NIH training grant to Ahmad Sabbahi (T32-HL-139439).
Footnotes
Supplementary information The online version of this article (https://doi.org/10.1038/s41371-020-00411-5) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133:e694–711. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. http://circ.ahajournals.org/content/126/18/2261%5Cnhttp://circ.ahajournals.org//subscriptions/%5Cnhttp://circ.ahajournals.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010;122:1637–48. [DOI] [PubMed] [Google Scholar]
- 4.Schultz MG, La Gerche A, Sharman JE. Blood pressure response to exercise and cardiovascular disease. Curr Hypertens Rep. 2017;19:89. http://link.springer.com/10.1007/s11906-017-0787-1. [DOI] [PubMed] [Google Scholar]
- 5.Currie KD, Floras JS, La Gerche A, Goodman JM. Exercise blood pressure guidelines: time to re-evaluate what is normal and exaggerated? Sport Med. 2018;48:1763–71. http://link.springer.com/10.1007/s40279-018-0900-x. [DOI] [PubMed] [Google Scholar]
- 6.Hedman K, Cauwenberghs N, Christle JW, Kuznetsova T, Haddad F, Myers J. Workload-indexed blood pressure response is superior to peak systolic blood pressure in predicting all-cause mortality. Eur J Prev Cardiol [Internet]. 2019. Sep 30;2047487319877268. 10.1177/2047487319877268. [DOI] [PubMed] [Google Scholar]
- 7.Jae SY, Kurl S, Kunutsor SK, Franklin BA, Laukkanen JA. Relation of maximal systolic blood pressure during exercise testing to the risk of sudden cardiac death in men with and without cardiovascular disease. Eur J Prev Cardiol. 2019;2047487319880031. https://pubmed.ncbi.nlm.nih.gov/31604381/. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Sabbahi A, Arena R, Kaminsky LA, Myers J, Phillips SA. Peak blood pressure responses during maximum cardiopulmonary exercise testing. Hypertension. 2018;71:229–36. http://hyper.ahajournals.org/lookup/doi/10.1161/HYPERTENSIONAHA.117.10116. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492. 20 http://www.ncbi.nlm.nih.gov/pubmed/29386200. [DOI] [PubMed] [Google Scholar]
- 10.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- 11.Thomas SJ, Booth JN, Dai C, Li X, Allen N, Calhoun D, et al. Cumulative incidence of hypertension by 55 years of age in blacks and whites: the CARDIA Study. J Am Heart Assoc. 2018;7: e007988. http://www.ncbi.nlm.nih.gov/pubmed/29997132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller K, Stelzer K, Ostad MA, Post F. Impact of exaggerated blood pressure response in normotensive individuals on future hypertension and prognosis: systematic review according to PRISMA guideline. Adv Med Sci. 2017;62:317–29. http://ac.els-cdn.com.proxy.cc.uic.edu/S1896112617300159/1-s2.0-S1896112617300159-main.pdf?_tid=21dd363e-3c44-11e7-a5f5-00000aab0f27&acdnat=1495165078_fe250c8926dd4ccbe225bf9c00feadc5. [DOI] [PubMed] [Google Scholar]
- 13.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, et al. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the american heart association. Circulation. 2013;127:652–62. [DOI] [PubMed] [Google Scholar]
- 14.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing data from the fitness registry and the importance of exercise national database. Mayo Clin Proc. 2015;90:1515–23. 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference Standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: Data From the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin Proc. 2017;92:228–33. [DOI] [PubMed] [Google Scholar]
- 16.Myers J, Arena R, Franklin B, Pina I, Kraus WE, McInnis K, et al. Recommendations for clinical exercise laboratories: a scientific statement from the american heart association. Circulation. 2009;119:3144–61. [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Forman DE, Balady GJ, Franklin BA, Nelson-Worel J, Martin B-JJ, et al. Supervision of exercise testing by non-physicians: a scientific statement from the American Heart Association. Circulation. 2014;130:1014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arena R, Myers J, Kaminsky LA. Revisiting age-predicted maximal heart rate: can it be used as a valid measure of effort? Am Heart J. 2016;173:49–56. 10.1016/j.ahj.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens. 2015;29:351–8. http://www.nature.com/articles/jhh201484. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 21.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–9. http://www.nejm.org/doi/pdf/10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, et al. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38:379–83. http://hyper.ahajournals.org/lookup/doi/10.1161/01.HYP.38.3.379. [DOI] [PubMed] [Google Scholar]
- 23.Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, et al. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension. 2011;58: 1029–35. [DOI] [PubMed] [Google Scholar]
- 24.Feairheller DL, Park J-Y, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K, Hurr C, Patik JC, Matthew Brothers R. Attenuated cutaneous microvascular function in healthy young African Americans: role of intradermal l-arginine supplementation. Microvasc Res. 2018;118:1–6. https://www-sciencedirect-com.proxy.cc.uic.edu/science/article/pii/S0026286217302339. [DOI] [PubMed] [Google Scholar]
- 26.Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, et al. Asymmetric Dimethylarginine and reduced nitric oxide bioavailability in young black African men. Hypertension. 2007;49:873–7. http://hyper.ahajournals.org/cgi/doi/10.1161/01.HYP.0000258405.25330.80. [DOI] [PubMed] [Google Scholar]
- 27.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–7. http://www.circulationaha.org. [DOI] [PubMed] [Google Scholar]
- 28.Ozkor MA, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A, et al. Differences in vascular nitric oxide and endothelium-derived hyperpolarizing factor bioavailability in blacks and whites. Arterioscler Thromb Vasc Biol. 2014;34:1320–7. http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.303136/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, et al. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–20. https://physoc-onlinelibrary-wiley-com.proxy.cc.uic.edu/doi/abs/10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Circ Physiol. 2008;295: H2380–7. http://www.physiology.org/doi/10.1152/ajpheart.00902.2008. [DOI] [PubMed] [Google Scholar]
- 31.Bond V, Stephens Q, Adams RG, Vaccaro P, Demeersman R, Williams D, et al. Aerobic exercise attenuates an exaggerated exercise blood pressure response in normotensive young adult African-American men. Blood Press. 2002;11:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riebe D, Ehrman JK, Liguori G, Magal M, (eds) ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018. [Google Scholar]
- 33.Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, et al. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Circ Physiol. 2014;306:H60–8. http://www.physiology.org/doi/10.1152/ajpheart.00710.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbosa TC, Kaur J, Stephens BY, Akins JD, Keller DM, Brothers RM, et al. Attenuated forearm vascular conductance responses to rhythmic handgrip in young African-American compared with Caucasian-American men. Am J Physiol Circ Physiol. 2018;315:H1316–21. https://www.physiology.org/doi/10.1152/ajpheart.00387.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. http://circ.ahajournals.org/cgi/content/long/122/2/191. [DOI] [PubMed] [Google Scholar]
- 36.Chant B, Bakali M, Hinton T, Burchell AE, Nightingale AK, Paton JFR, et al. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension. 2018;72:102–9. [DOI] [PubMed] [Google Scholar]
- 37.Schultz MG, Sharman JE. Exercise hypertension. Pulse. 2013;1:161–76. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4315351&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation.2010;121:2109–16. http://circ.ahajournals.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clin Proc. 1996;71: 445–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


